Abstract
Acute pancreatitis (AP) is associated with multiple cellular mechanisms that trigger and or are triggered by the inflammatory injury and death of the acinar cells. One of the key mechanisms is the endoplasmic reticulum (ER) stress, which manifests as an accumulation of misfolded proteins within ER, an event that has proinflammatory and proapoptotic consequences. Hence, the degree of cell insult during AP could considerably depend on the signaling pathways that are upregulated during ER stress and its resulting dyshomeostasis such as C/EBP homologous protein (CHOP), cJUN NH2-terminal kinase (JNK), nuclear factor kappa B (NF-κB), and NOD-like receptor protein 3 (NLRP3) inflammasome. Exploring these molecular pathways is an interesting area for translational medicine as it may lead to identifying new therapeutic targets in AP. This review of the literature aims to shed light on the different roles of ER stress in the etiopathogenesis and pathogenesis of AP. Then, it specifically focuses on the therapeutic implications of ER stress in this context.
Keywords: Acute pancreatitis, Endoplasmic reticulum stress, UPR, Pyroptosis, CHOP, NF-κB
Introduction
Acute pancreatitis (AP) is the most common disorder of the pancreas worldwide.1 It is the condition where there is an inflammation of the acinar exocrine cells, which can lead to extensive tissular necrosis and systemic inflammatory response syndrome with potential death.2 AP can occur most frequently as a result of the passage of small gallbladder stones or biliary sludge to the adjacent pancreatic duct or the ampulla of Vater leading to acute biliary (obstructive) pancreatitis. This condition is favored by anomalies in pancreatic and biliary ductal anatomy, gallstone‑related factors, biliopancreatic reflux, duodenal bile, and pancreatic juice exclusion.3 The obstruction of the pancreatic duct causes the pancreatic juices to accumulate locally with likely a premature activation of pancreatic enzymes (autodigestion) inducing pancreatitis. The other most common etiology of AP is alcoholism which when metabolized by the pancreas, provokes by its turn the premature activation of intracellular digestive enzymes thereby promoting autodigestive injury.4 The other less frequent etiologies include but are not exclusively hypertriglyceridemia, postendoscopic retrograde cholangiopancreatography, malignancy (obstructive), drugs, trauma, hypercalcemia, and infection. In contrast, AP can be idiopathic in a high proportion of patients.5, 6
Two of upper abdominal pain, amylase/lipase ≥3 ×upper limit of normal, and/or cross-sectional imaging findings in favor of AP are required to establish the diagnosis.7 The management of AP is mainly supportive as to date, there is no available drug to treat the disease or reduce its severity.8 In a pathological sense, the severity of AP depends on two key pathogenic factors: the extent of cellular necrosis and the amount of inflammation. Both of these factors are in turn correlated with the degree of organelles dysfunction, the latter being triggered by the different AP inducers such as alcoholism and smoking.2 One of the main organelles involved in the pathophysiology of AP is the endoplasmic reticulum (ER), which is prone to profound functional alterations generally referred to as ER stress. The recognition of ER stress implications in AP has driven attention to its possible utility as a therapeutic target.9 Based on this, various AP research have investigated the beneficial effects of pharmacological agents acting primarily on ER stress, demonstrating interesting findings.10, 11, 12, 13, 14, 15 This literature review highlights first the main contributions of ER stress and proteins misfolding to the pathogenesis of AP. Afterward, it focuses on the therapeutic implications of ER stress by summarizing evidence on drugs demonstrating ER stress activity in AP models. In this section, potential pharmacological targets in AP-induced ER stress are also discussed.
ER stress and unfolded protein response
Following translation, synthesized structural or secretory proteins are translocated into the ER lumen where post-translational modification occurs to ensure the formation of properly folded and mature proteins. The ER function requires multiple chaperones and folding enzymes and has the key role of ensuring quality control before the proteins are released by the chaperones to reach their site of action. Notably, due to their detrimental effects, misfolded proteins undergo correction or degradation by a process called ER-associated degradation via the ubiquitin-proteasome pathway.16, 17 The ER should ensure the folding of the synthesized proteins, however, if this capacity is saturated ER stress develops.18 ER stress triggers a compensatory mechanism known as unfolded protein response (UPR). Such a response would aim to increase the protein-folding capacity in ER.19
Three ER sensors initiate the UPR including inositol-requiring enzyme 1 (IRE1), double-stranded RNA-activated protein kinase R (PKR)-like ER kinase (PERK), and activating transcription factor 6 (ATF6). Under normal circumstances, these sensors are coupled with an ER chaperone called BiP, which keeps them inactive, whereas the presence of unfolded proteins during ER stress generates their activation through a competitive binding of the unfolded protein with BiP due to a higher affinity.20 The ability of the UPR to increase the protein-folding capacity is critical in determining the fate of the cell undergoing ER stress. An adaptive UPR occurs in case of mild ER stress to promote cell survival, and results in the expansion of the ER size and lumen through increased protein and lipid biogenesis. Moreover, the transcription of the genes encoding for ER chaperones and ER-resident enzymes increases, which enhances the removal and degradation of the locally accumulated misfolded proteins.21 These genes encode for ER chaperone proteins such as BiP/GRP78 and GRP94, folding enzymes such as disulfide isomerase (PDI) and peptidyl-prolyl isomerase (PPI), as well as structural components of the ER including sarcoplasmic ER Ca2+-ATPase 2 (SERCA2).19 Nevertheless, during severe ER stress, IRE1α becomes hyperactivated and undergoes sustained homo-oligomerization. The latter causes the IRE1α’s RNase to cleave repressors (ie, micro-RNAs) of proapoptotic proteins, thereby, inducing caspase-1 activation, and IL-1β and IL-18 production eventually promoting cell death.21
The X-box binding protein 1 (XBP1s) is an important regulator of UPR. Hence, when spliced by the activated stress sensor IRE1α, XBP1s induces the transcription of UPR target genes encoding ER molecular chaperones, folding enzymes, and ER-associated degradation (ERAD) components.22 In cells where UPR is ineffective, apoptosis is triggered to protect the organism by eliminating the cells with severely damaged ER. Among the main ER stress-mediated proapoptotic pathways is the C/EBP homologous protein (CHOP), in addition to cJUN NH2-terminal kinase (JNK), and caspase-12 (in mouse).19 CHOP has pro‑apoptotic and pro‑inflammatory properties. Hence, the silencing of CHOP expression markedly inhibited apoptosis and ER stress, reducing the activation of nuclear factor‑κB (NF-κB) and inflammation injury in AP.23
ER stress role in the pathophysiology of AP
Pancreatic acinar cells are susceptible to ER stress due to their unique rate of protein turnover, synthesis, and secretion, which particularly exposes them to ER dysfunction and dyshomeostasis. These anomalies occur at the early stages of pancreatitis, regardless of trypsinogen activation.24 ER stress appears to play a major role in both the etiopathogenesis and pathogenesis of AP. On an etiopathogenic level, different AP triggers were shown to induce pancreatic cell insult via aberrant ER response. For instance, alcoholism may promote the development of AP by ER stress, especially in patients with genetic polymorphism favoring ER dyshomeostasis and UPR component accumulation.25 Genetically determined preexisting interhuman disparities in the adaptive UPR/anti-ER stress mechanisms may explain more susceptibility to alcoholic pancreatitis in some alcohol consumers than others.26 The combined exposure to ethanol and cigarette smoke extract increased ER stress in acinar cells via the suppression of the XBP1s-associated adaptive UPR signaling pathway.27 Paradoxically, when the UPR is adaptive it protects against alcohol-induced pancreatic damage.28 Furthermore, in one experiment, the components of UPR including GRP78/Bip, XBP1, and GADD153/CHOP were upregulated in hypertriglyceridemic AP rats model.29 Another study demonstrated that incubation of rat primary acinar cells with palmitic acid resulted in enhanced UPR with subsequent local inflammation through ER stress-induced activation of the CCAAT-enhancer-binding protein β and α.30 Saturated free fatty acids induced ER stress via ER Ca2+ depletion.31 Iatrogenic pancreatitis such as that induced by secretagogues and methimazole was also found to be promoted by aberrant ER responses.32, 33 Experimentally arginine-induced AP was also shown to manifest with an early activation of ER stress.34
Main consequences of ER stress in AP
The contribution of ER stress to pathologic/premature activation of trypsinogen is unclear.24 In a mechanistic model proposed by Habtezion et al.,2 the etiopathogenic factors of AP such as alcohol, smoking, hypertriglyceridemia, gallstones, and/or genetic mutations (CFTR mutation) can elicit disruptions in the organelles’ function within acini cells by excessive Ca2+ entry and other cellular damaging stimuli. This causes ER stress, mitochondrial failure, and autophagy impairment with each of them aggravating the other. Eventually, there is a premature trypsinogen activation, along with proinflammatory and proapoptotic pathways upregulation resulting in diffuse inflammation and insult to the pancreatic tissue.2 Figure 1 summarizes the main deleterious effects of ER stress on acinar cells during the development of AP. Of note, ER stress was also shown to induce a regenerative response in the exocrine pancreas following acinar-specific disruption of XBP1 in mice.35 This suggests that ER stress and UPR are initially beneficial for tissular recovery during AP; however, when the cellular damage is severe or persistent, ER stress becomes part of an aberrant response promoting cell death.
Fig. 1.
Consequences of endoplasmic reticulum (ER) stress on acinar cells during acute pancreatitis. The accumulation of misfolded proteins and activation of ER stress pathways within acinar cells results in the initiation of multiple cytoplasmic and nuclear processes: (i) autophagy: which occurs in response to JNK activation and mTOR inhibition; (ii) cell death: five types of programmed and nonprogrammed cell death mechanisms can develop through ER stress including apoptosis, pyroptosis, necroptosis, ferroptosis (iron homeostasis-dependent death), and necrosis; (iii) inflammation: ER stress and unfolded proteins response trigger the activation of NFκB via ER overload response. Ultimately, NFκB acts by inducing the transcriptional expression of NOD-like receptor protein 3, which, in turn, induces caspase-mediated activation of pro-IL-1β and pro-IL-18. Once activated, these cytokines stimulate the recruitment and proliferation of inflammatory cells, thus, favoring cell insults. Abbreviations used: JNK, cJUN NH2-terminal kinase; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor kappa B; NLRP3, NOD-like receptor protein 3.
Autophagy
Macroautophagy (hereafter referred to as autophagy) is a stress-induced intracellular process during which there is a lysosome-mediated bulk degradation pathway aiming to recycle and eliminate worn-out proteins, protein aggregates, and damaged organelles. It is activated as a novel signaling pathway in response to ER stress.36 The sequestered damaged cytoplasmic components are degraded by the lysosomal enzymes after lysosome fusion with double-membrane bounded vacuoles called autophagosomes.37 The induction of autophagy by ER stress is through IRE1α mediated TNF Receptor Associated Factor 2 (TRAF2) and apoptosis signal-regulating kinase 1 (ASK1) recruitment, and subsequent JNK activation.16 Also, studies have shown the involvement of autophagy related 12 (ATG12) expression and mammalian target of rapamycin (mTOR) inhibition in the activation of the autophagic process by ER stress.38 The fate of the cell is then determined depending on the local cellular circumstances with either cell survival, nonapoptotic cell death when apoptosis is experimentally blocked, or caspase-independent autophagic cell death triggered by specific treatments.39 Drugs acting on ER stress such as melatonin and rapamycin were shown to activate autophagy in AP cells in a way of promoting their apoptosis.40, 41 In the pancreatic β cells, autophagy prevents ER stress-induced death.42 The same effects are replicated in acinar cells. Autophagy prevents ER stress and the resulting lethal changes in the exocrine pancreas. Thus, deficiency in canonical and noncanonical autophagy proteins (autophagy-related protein 7, and CCPG1, respectively) had led to disruption of ER homeostasis and proteostasis, with subsequent ER stress and trypsinogen activation, resulting in severe acinar cell degeneration and spontaneous pancreatitis in the mouse pancreas.43, 44
Cell death
Once ER stress develops in response to different AP triggers, it acts as a crucial exacerbator of the acini cells’ proapoptotic signals. Thus, following the onset of AP, there is an increase in the ER stress molecules (e.g., PERK pathway), which activates several proapoptotic proteins such as caspase-1 and cathepsin B, thus further favoring cell death.45, 46 Apoptosis occurs through intrinsic (mitochondrial) and extrinsic pathways which both lead to the activation of the executioner molecules caspases. These molecules commit cells to death in an irreversible manner. The intrinsic apoptosis is triggered by the activation of the B-cell Lymphoma 2 (BCL2) family of proapoptotic effectors notably BAX, BAK, and possibly BOK. This causes the mitochondria to undergo outer membrane permeabilization (MOMP) with subsequent caspase activation.47 The extrinsic apoptosis develops following the TNF-α-induced expression of death receptors including TNF receptor type 1-associated death domain protein/Fas-associated protein with death domain (FADD) or receptor-interacting protein kinase 1 (RIP1)/RIP3, ultimately eliciting caspase-8 activation.48 These pathways are both promoted by ER stress.49
In the context of AP, another prominent mechanism of cell death is pyroptosis due to the high participation of inflammation in the activation of lethal signals. Although pyroptosis is a recently discovered mechanism of programmed cell death, numerous studies have supported the substantial involvement of NOD-like receptor protein 3 (NLRP3) inflammasome-dependent pyroptosis in the pathogenesis of AP.50 One recent study demonstrated that the PERK pathway in ER stress can drive acinar cells to undergo pyroptosis by activating caspase-1, ultimately aggravating AP.46 ER stress can also induce pyroptosis by increasing IRE1α and cytosolic Ca2+.51
A third mode of cell death is necroptosis which resembles pyroptosis in being an inflammatory programmed death process but with different implicated pathways. Thus, while pyroptosis is activated by the gasdermin D pathway, necroptosis is triggered by TNF-α activation of the receptor-interacting protein kinase 1(RIPK1) and receptor-interacting protein kinase 3 followed by RIP3 binding to and activation of mixed lineage kinase domain-like (MLKL), a newly defined executioner molecule.52, 48 During AP conditions, ER stress was found to promote acinar cell necroptosis through cathepsin B-mediated activating protein-1 (AP-1) activation.45
A fourth mode is ferroptosis, which is a special type of programmed cell death driven by intracellular iron accumulation and peroxidation of polyunsaturated fatty acids. This form differs from apoptosis and other nonapoptotic cell death by the absence of apoptotic bodies or chromatin condensation, cell shrinkage, or cell membrane rupture.53 ER stress is responsible for ferroptosis by several mechanisms including dysregulating lipid metabolism, redox balance, and iron homeostasis anomalies, and also by activating the transcription factor p53.54, 55, 56 There is a substantial body of evidence that supports the involvement of ferroptosis in AP development and exacerbation.57, 58, 59, 60 ER stress participates in AP-associated ferroptosis via the BiP/p-EIF2α/CHOP signaling pathway which is, in turn, iron-dependent.61, 62
Finally, necrosis is the natural consequence of ER stress stimulation of the recruitment of inflammatory cells which induce necrotic injury of acinar cells by releasing cell breakdown molecules. In contrast to apoptosis which predominates in mild forms, necrosis is particularly observed in severe forms of AP.63
Inflammatory reaction
The ER stress within acini cells generates a robust immune response mainly through the activation of NFκB transcription factor which is known as a central mediator of immune and antiapoptotic responses.19 This ER stress-induced NFκB activation (ie, through IκB degradation) is particularly triggered by the membrane proteins aggregates in the ER, consequently, the pathway is called ER overload response. For the ER overload response to occur, it was suggested that there is a need for the release of Ca2+ from the ER and the subsequent production of reactive oxygen intermediates.64 ER stress is a potent activator of NF-κB through IκBα kinase, which, by phosphorylating and degrading IκBα, induces NF-κB activation. Moreover, UPR initiators notably IRE1 and PERK also contribute to activating NF-κB.65 When the NF-κB is activated, it triggers the NLRP3 inflammasome, which develops as follows: NLRP3 activates caspase-1, which, in turn, converts IL-1β and IL-18 to their bioactive forms. These two cytokines along with others (IL6, IL8, TNFα, and chemokine MCP-1) mediate leukocytes proliferation, migration, and maturation during pancreatitis.66, 67 The NLRP3 inflammasome can also be initiated by the CHOP, IRE1α, ROS/TXNIP, and PERK–CHOP signaling pathways.68
Investigated drugs with ER stress protective function
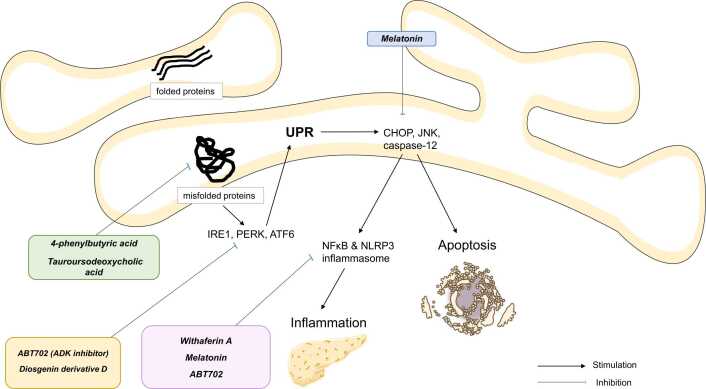
Currently, the main ER stress inhibitors that have been investigated for AP are the following (Figure 2).
Fig. 2.
Main current drugs showing potential activity on endoplasmic reticulum stress in acute pancreatitis models.
ER chaperones
Drugs that act as ER chaperones could be effective in reducing the progression of AP. The Food and Drug Administration (FDA)-approved 4-phenylbutyric acid (4-PBA) is a low molecular weight ER chaperone that decreases the aggregation of misfolded proteins and corrects their trafficking, hence alleviating ER stress. Notably, the hydrophobic regions of 4-PBA interact with the exposed hydrophobic segments of the unfolded protein, resulting in the restoration of protein folding.69, 70 Evidence from in vitro and in vivo studies suggests the efficiency of 4-PBA as an ER-stress inhibitor in AP models. In one study, 4-PBA enhanced the enzymatic secretion in rat acini cells stimulated with supraphysiological cholecystokinin, thus suppressing trypsin activation, while inhibiting UPR and proapoptotic pathways.12 In another experiment, using a rat model of severe AP, Hong et al.10 investigated the effects of 4-PBA on injured pancreatic beta cells. The drug was able to attenuate histopathological injuries of the pancreatic islets. Additionally, the authors noted a reduction in, TNF-α, IL-1β, and insulin levels, with an increase in glucose levels, in addition to re-establishment of beta-cell ultrastructure and ER hemostasis. The latter was accomplished by the suppression of BiP, ORP150, and CHOP, but also caspase-3, suggesting antiapoptotic potentials of 4-PBA.
Additional targets were found in Han et al.45 experiment. In this study, 4-PBA exerted an in vivo anti-ER stress activity through the inhibition of cJun pathway and cathepsin B/activating protein 1, which is involved in the necroptosis of pancreatic acinar cells. Further, preincubation of acinar cells with 4-PBA led to opposing the activation of CCAAT-enhancer-binding protein α and CCAAT-enhancer-binding protein β in palmitic acid-induced AP in rats. This resulted in the prevention of the inflammatory responses triggered by ER stress in acinar cells.30 Interestingly, 4-PBA administration rescued damaged pancreas and vital organs including lungs, liver, and kidneys in rats with sodium taurocholate-induced AP.71 This effect was driven by ER stress relief and systemic inflammation inhibition as evidenced by reductions in neutrophils and macrophage infiltration, as well as proinflammatory TNF-α and IL-1β levels. Moreover, 4-PBA displayed an antiapoptotic action in the above vital organs. The weak chaperoning ability of 4-PBA could be a pharmacodynamics disadvantage. Nonetheless, this can be overcome with the development of 4-PBA analogs such as 2-POAA-OMe, 2-POAA-NO2 and 2-NOAA that display much stronger ER stress elements notably IRE1 and ATF6.72 Another ER stress inhibitor with chaperone activity is tauroursodeoxycholic acid (TUDCA) which is approved by the FDA for the treatment of primary biliary cholangitis.73 TUDCA when used in AP has also decreased ER stress, acinar cell damage/necrosis, trypsin activation, and systemic inflammation.13, 14
Melatonin
Melatonin has well-established anti-inflammatory properties.74, 75 Furthermore, its ER stress opposing action has been demonstrated.77, 76, 78 These effects were replicated in animal models of AP.40, 15, 23 Thus, rats and AR42J cells treated with melatonin were found to resist pancreatic tissue injury secondary to experimentally induced AP. On a molecular level, melatonin therapy resulted in the inhibition of IRE1α-mediated JNK/NF-κB pathway-related proteins which translated to downregulation of ER stress and proinflammatory cytokines expression with subsequent prevention of cells apoptosis and tissue damage.15 The CHOP pathway of ER stress is also antagonized by pretreatment with melatonin in rats during AP, as shown by Zhao et al.23, 79 study. The latter suggested that melatonin's anti‑inflammatory and antiapoptotic effects are partially related to its CHOP counteraction. Thus, the knockdown of CHOP expression was responsible for marked inhibition of the NF-κB pathway, inflammation, and apoptosis following the induction of AP. These effects were seen with CHOP downregulation by melatonin.23, 79
NF-κB/NLRP3 inflammasome inhibitors: Withaferin A
NF-κB/NLRP3 agents were found to reduce AP severity with several mechanisms among which ER stress relief.80 One interesting study explored the potential of a small molecule inhibitor of NF-κB called withaferin A in preventing the progression of chronic pancreatitis. Mice received the drug before or after the induction of pancreatitis using intraperitoneal injections of cerulein. Withaferin A was effective for both the prevention and reversal of pancreatic parenchyma injury and local inflammatory cell infiltration as shown by histology analysis and serum amylase levels, which decreased significantly compared to pretreatment. Further, the mice treated with withaferin A were protected from the morphological consequences of repetitive AP, including acinar tissue atrophy, leukocyte infiltrations, ductal metaplasia, and hemorrhage, thus showing no tendency to evolve into chronic disease. Besides inhibiting NFκB activation (also NLRP3 inflammasome) and the resulting proinflammatory and proapoptotic gene expression, withaferin A resisted ER stress stimulation by downregulating CHOP, XBP1s, and transcription factor 4. This suggests that the drug may target the initiators (ie, CHOP) as well as the effectors (ie, NFκB) in ER stress-induced pathways.11
Others
Adenosine kinase (ADK) is an enzyme that was shown to participate in the pathophysiology of various pancreatic disorders.81, 82, 83 Sun et al.84 carried out a unique work in which they uncovered the implication of ADK in the development of AP-related ER stress. Notably, the activation (ie, phosphorylation) of ER stress proteins, notably PERK and eIF-2α in cerulein-induced AP, was both abolished and prevented by the ADK inhibitor ABT702. The treatment elicited normalization of the pancreatic tissue histopathology, prevention of inflammation (via downregulation of phosphorylation NF-κB), and blockade of acinar cell necroptosis.84 Zhang et al.85 investigated the curative effect of Diosgenin derivative D (extracted from Dioscorea zingiberensison plant) in in vivo and in vitro rat models of L-arginine-induced AP. Thus, the treatment led to a dose-dependent attenuation of acinar cell necrosis and ER stress-associated proteins (ie, p-IRE1α) and inflammation. The ER stress alleviation was accomplished through the inhibition of TXNIP/HIF-1α pathway, the latter triggers the accumulation of the propyroptotic protein gasdermin D in the ER, thus favoring ER stress and acinar cell death.85 Furthermore, genistein a natural dietary belonging to isoflavones was capable of reducing the severity of AP in rats by stimulating ER stress regulators (GRP78, PERK, and eIF2α), and enhancing the proapoptotic caspase-12 and CHOP activity via a genomic mechanism, consequently promoting injured acinar cells death.86 Additional agents such as emodin and rapamycin also succeeded in suppressing ER stress in AP models.41, 87
Potential therapeutic targets for AP-associated ER stress
Multiple regulators of ER stress have shown their possible utility as therapeutic targets for AP. A study by He et al.88 revealed that mice with silenced sulfiredoxin-1 gene were susceptible to AP. Hence, in mice with experimental AP, the inhibition of sulfiredoxin-1 (a physiological antioxidant) promoted ROS-induced ER stress, apoptosis with cathepsin B activation, and pathological trypsinogen conversion to trypsin. As a result, histological score and inflammatory responses increased in sulfiredoxin-1 knockout mice. Whereas, overexpression of sulfiredoxin-1 achieved by adeno-associated virus led to AP attenuation and ROS/ER stress/cathepsin B axis inhibition with subsequent prevention of acinar cells apoptosis.88 Recently, the antioxidant liproxstatin-1 was also found to mitigate ferroptosis and ER stress to resist hypertriglyceridemic pancreatitis in rats.62
Remarkably, Najenson et al.89 identified the presence of atrial natriuretic peptide (ANP)’s mRNA and zymogen/protein in the acinar cells suggesting that this tissue is another extracardiac source of ANP secretion besides the kidney and liver.89 Such findings could stress the role of ANP in pancreatic diseases including AP. In line with this, treatment of rats with ANP enabled the attenuation of ER stress in AP. On one hand, ANP induced the downregulation of Bip and p-eIF2α expression, thus modulating UPR. On the other hand, it enhanced the expression of CHOP, and proapoptotic proteins Bax and Bak as well as caspase-2, ultimately promoting ER-dependent apoptosis. Consistently, after ANP administration, the AP-induced alterations in ER ultrastructure notably organelle swelling and ribosomal loss were all reversed.90 The induction of cell apoptosis by ANP may limit inflammatory responses in AP as previously reported.91
Irisin is a thermogenic adipomyokine involved in the browning of adipose tissue and appears to play roles in multiple disorders.92 Importantly, low serum levels of irisin are correlated with worse outcomes in AP patients.93 During an experiment on a murine model of chronic pancreatitis, irisin showed potential therapeutic benefits by reducing acinar cell apoptosis and fibrosis, oxidative stress, and ER stress.94 In AP mice, irisin could further alleviate intestinal injury while resisting local cell apoptosis, oxidative and ER stress, and tissue inflammation. These effects were achieved in a dose-dependent manner.95 The UPR in AP was modulated by irisin which induced the activation of ATF6 and XBP1 and the inhibition of CHOP, favoring acinar cell survival.96 Besides protection against ER stress, irisin improved mitochondrial function by upregulating the expression of uncoupling protein 2 (UCP2).93 In a different study, the suppression of ER stress and NF-κB activation was possible in AP mice by the administration of another human hormone called Tumor Necrosis Factor-α-Stimulated Gene/Protein-6 (TSG-6) that mediates multiple beneficial properties of the mesenchymal stem/stromal cells.97, 98
An additional possible target is translocation-associated membrane protein (TRAM1). This protein has an antagonizing action of the ER stress-JNK pathway in HepG2 cells.99 In pancreatic AR42J cells, the silencing of TRAM1 expression resulted in upregulation of the UPR-associated chaperone GRP78 and the ER-stress proapoptotic CHOP. Moreover, the suppression of TRAM1 activity further induced proinflammatory cytokines release and was linked with increased cellular injury markers notably LDH.100
Conclusion
ER stress is a major mechanism of acinar cell death and pancreatic tissue inflammation during AP regardless of the triggering factor. The currently available animal models of AP have allowed us to understand the molecular pathways included in ER stress and proteasome dyshomeostasis, notably the UPR initiators IRE1, PERK, and ATF6, and their subsequent proapoptotic and proinflammatory pathways mediated by CHOP, JNK, and caspase-12. Insights have also been expanded regarding the crucial implication of ER stress in activating the proinflammatory pathway NF-κB/NLRP3 inflammasome which is the primary stimulator of the AP-associated immune response. Drugs targeting these pathways such as the ER chaperones 4-PBA and TUDCA, the well-known molecule melatonin, NF-κB/NLRP3 inflammasome modulator Withaferin A, and other agents may represent a promising and worthy investigation strategy to prevent or reverse the progression of AP. Moreover, multiple therapeutic targets that interfere with AP-associated ER stress have been identified including sulfiredoxin-1, liproxstatin-1, ANP, irisin, TSG-6, and TRAM1. Therefore, more animal and human studies are warranted to further explore the therapeutic advantages of ER stress/UPR modulators in reducing the clinical burden of AP.
Ethics statement
Not applicable.
Funding and support
The authors did not receive any funding.
Author contributions
All authors participated in the manuscript conceptualization, drafting, and editing.
CRediT authorship contribution statement
Zhang Haiwei: Supervision. Ji LiJuan: Writing – review & editing, Writing – original draft. Xu Chenchen: Writing – original draft. Zhang Xiaoliang: Writing – review & editing, Writing – original draft, Visualization, Conceptualization.
Declarations of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None.
Data availability statement
No data were used for the research described in the article.
References
- 1.Xiao A.Y., Tan M.L.Y., Wu L.M., et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45–55. doi: 10.1016/S2468-1253(16)30004-8. [DOI] [PubMed] [Google Scholar]
- 2.Habtezion A., Gukovskaya A.S., Pandol S.J. Acute pancreatitis: a multifaceted set of organelle and cellular interactions. Gastroenterology. 2019;156:1941–1950. doi: 10.1053/j.gastro.2018.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Geenen E.J.M., van der Peet D.L., Bhagirath P., et al. Etiology and diagnosis of acute biliary pancreatitis. Nat Rev Gastroenterol Hepatol. 2010;7:495–502. doi: 10.1038/nrgastro.2010.114. [DOI] [PubMed] [Google Scholar]
- 4.Apte M.V., Pirola R.C., Wilson J.S. Mechanisms of alcoholic pancreatitis. J Gastroenterol Hepatol. 2010;25:1816–1826. doi: 10.1111/j.1440-1746.2010.06445.x. [DOI] [PubMed] [Google Scholar]
- 5.Zheng Y., Zhou Z., Li H., et al. A multicenter study on etiology of acute pancreatitis in beijing during 5 years. Pancreas. 2015;44:409–414. doi: 10.1097/MPA.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 6.Zilio M.B., Eyff T.F., Azeredo-Da-Silva A.L.F., et al. A systematic review and meta-analysis of the aetiology of acute pancreatitis. HPB. 2019;21:259–267. doi: 10.1016/j.hpb.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Szatmary P., Grammatikopoulos T., Cai W., et al. Acute pancreatitis: diagnosis and treatment. Drugs. 2022;82:1251–1276. doi: 10.1007/s40265-022-01766-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vege S.S., DiMagno M.J., Forsmark C.E., et al. Initial medical treatment of acute pancreatitis: American Gastroenterological Association Institute Technical Review. Gastroenterology. 2018;154:1103–1139. doi: 10.1053/j.gastro.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 9.Borrello M.T., Martin M.B., Pin C.L., et al. The unfolded protein response: an emerging therapeutic target for pancreatitis and pancreatic ductal adenocarcinoma. 2022;22:148–159. doi: 10.1016/j.pan.2021.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Hong Y., Guo W., Wang W., et al. 4-Phenylbutyric acid attenuates pancreatic beta-cell injury in rats with experimental severe acute pancreatitis. Int J Endocrinol. 2016;2016 doi: 10.1155/2016/4592346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanak M.A., Shahbazov R., Yoshimatsu G., et al. A small molecule inhibitor of NFκB blocks ER stress and the NLRP3 inflammasome and prevents progression of pancreatitis. J Gastroenterol. 2017;52:352–365. doi: 10.1007/s00535-016-1238-5. [DOI] [PubMed] [Google Scholar]
- 12.Malo A., Krüger B., Göke B., Kubisch C.H. 4-Phenylbutyric acid reduces endoplasmic reticulum stress, trypsin activation, and acinar cell apoptosis while increasing secretion in rat pancreatic acini. Pancreas. 2013;42:92–101. doi: 10.1097/MPA.0b013e318259f6ca. [DOI] [PubMed] [Google Scholar]
- 13.Malo A., Krüger B., Seyhun E., et al. Tauroursodeoxycholic acid reduces endoplasmic reticulum stress, trypsin activation, and acinar cell apoptosis while increasing secretion in rat pancreatic acini. Am J Physiol Gastrointest Liver Physiol. 2010;299:G877–G886. doi: 10.1152/ajpgi.00423.2009. [DOI] [PubMed] [Google Scholar]
- 14.Seyhun E., Malo A., Schäfer C., et al. Tauroursodeoxycholic acid reduces endoplasmic reticulum stress, acinar cell damage, and systemic inflammation in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2011;301:G773–G782. doi: 10.1152/ajpgi.00483.2010. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Q., Tang X., Huang J., et al. Melatonin attenuates endoplasmic reticulum stress in acute pancreatitis. Pancreas. 2018;47:884–891. doi: 10.1097/MPA.0000000000001082. [DOI] [PubMed] [Google Scholar]
- 16.Almanza A., Carlesso A., Chintha C., et al. Endoplasmic reticulum stress signalling - from basic mechanisms to clinical applications. FEBS J. 2019;286:241–278. doi: 10.1111/febs.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarz D.S., Blower M.D. The endoplasmic reticulum: structure, function and response to cellular signaling. Cell Mol Life Sci. 2016;73:79–94. doi: 10.1007/s00018-015-2052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin J.H., Walter P., Yen T.S.B. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol. 2008;3:399–425. doi: 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oyadomari S., Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 20.Chen X., Shi C., He M., et al. Endoplasmic reticulum stress: molecular mechanism and therapeutic targets. Signal Transduct Target Ther. 2023;8:352. doi: 10.1038/s41392-023-01570-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oakes S.A., Papa F.R. The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol Mech Dis. 2015;10:173–194. doi: 10.1146/annurev-pathol-012513-104649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park S.-M., Kang T.-I., So J.-S. Roles of XBP1s in transcriptional regulation of target genes. Biomedicines. 2021;9:791. doi: 10.3390/biomedicines9070791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Q., Zhang H., Huang J., et al. Melatonin attenuates the inflammatory response via inhibiting the C/EBP homologous protein-mediated pathway in taurocholate-induced acute pancreatitis. Int J Mol Med. 2018;42:3513–3521. doi: 10.3892/ijmm.2018.3920. [DOI] [PubMed] [Google Scholar]
- 24.Saluja A., Dudeja V., Dawra R., Sah R.P. Early intra-acinar events in pathogenesis of pancreatitis. Gastroenterology. 2019;156:1979–1993. doi: 10.1053/j.gastro.2019.01.268. [DOI] [PubMed] [Google Scholar]
- 25.Li H., Wen W., Luo J. Targeting endoplasmic reticulum stress as an effective treatment for alcoholic pancreatitis. Biomedicines. 2022;10:108. doi: 10.3390/biomedicines10010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandol S.J., Gorelick F.S., Gerloff A., Lugea A. Alcohol abuse, endoplasmic reticulum stress and pancreatitis. Dig Dis. 2010;28:776–782. doi: 10.1159/000327212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lugea A., Gerloff A., Su H.-Y., et al. The combination of alcohol and cigarette smoke induces endoplasmic reticulum stress and cell death in pancreatic acinar cells. Gastroenterology. 2017;153:1674–1686. doi: 10.1053/j.gastro.2017.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lugea A., Tischler D., Nguyen J., et al. Adaptive unfolded protein response attenuates alcohol-induced pancreatic damage. Gastroenterology. 2011;140:987–997.e8. doi: 10.1053/j.gastro.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng Y., Wang X., Zhang W., et al. Hypertriglyceridemia aggravates ER stress and pathogenesis of acute pancreatitis. Hepatogastroenterology. 2012;59:2318–2326. doi: 10.5754/hge12042. [DOI] [PubMed] [Google Scholar]
- 30.Wu J., Hu G., Lu Y., et al. Palmitic acid aggravates inflammation of pancreatic acinar cells by enhancing unfolded protein response induced CCAAT-enhancer-binding protein β-CCAAT-enhancer-binding protein α activation. Int J Biochem Cell Biol. 2016;79:181–193. doi: 10.1016/j.biocel.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 31.Cunha D.A., Hekerman P., Ladrière L., et al. Initiation and execution of lipotoxic ER stress in pancreatic β-cells. J Cell Sci. 2008;121:2308–2318. doi: 10.1242/jcs.026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubisch C.H., Logsdon C.D. Secretagogues differentially activate endoplasmic reticulum stress responses in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1804–G1812. doi: 10.1152/ajpgi.00078.2007. [DOI] [PubMed] [Google Scholar]
- 33.Yazıcı Ö., Kara M., Boran T., Ozhan G. The role of endoplasmic reticulum stress in cell injury induced by methimazole on pancreatic cells. Adv Pharm Bull. 2023;13:196–201. doi: 10.34172/apb.2023.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kubisch C.H., Sans M.D., Arumugam T., et al. Early activation of endoplasmic reticulum stress is associated with arginine-induced acute pancreatitis. Am J Physiol Liver Physiol. 2006;291:G238–G245. doi: 10.1152/ajpgi.00471.2005. [DOI] [PubMed] [Google Scholar]
- 35.Hess D.A., Humphrey S.E., Ishibashi J., et al. Extensive pancreas regeneration following acinar-specific disruption of Xbp1 in mice. Gastroenterology. 2011;141:1463–1472. doi: 10.1053/j.gastro.2011.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rashid H.-O., Yadav R.K., Kim H.-R., Chae H.-J. ER stress: autophagy induction, inhibition and selection. Autophagy. 2015;11:1956–1977. doi: 10.1080/15548627.2015.1091141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chipurupalli S., Samavedam U., Robinson N. Crosstalk between ER stress, autophagy and inflammation. Front Med. 2021;8 doi: 10.3389/fmed.2021.758311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quan W., Lim Y.-M., Lee M.-S. Role of autophagy in diabetes and endoplasmic reticulum stress of pancreatic β-cells. Exp Mol Med. 2012;44:81–88. doi: 10.3858/emm.2012.44.2.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Høyer-Hansen M., Jäättelä M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007;14:1576–1582. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y., Zhang J., Zhao Q., et al. Melatonin induces anti-inflammatory effects to play a protective role via endoplasmic reticulum stress in acute pancreatitis. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2016;40:1094–1104. doi: 10.1159/000453164. [DOI] [PubMed] [Google Scholar]
- 41.Mei Q., Zeng Y., Huang C., et al. Rapamycin alleviates hypertriglyceridemia-related acute pancreatitis via restoring autophagy flux and inhibiting endoplasmic reticulum stress. Inflammation. 2020;43:1510–1523. doi: 10.1007/s10753-020-01228-7. [DOI] [PubMed] [Google Scholar]
- 42.Bartolome A., Guillen C., Benito M. Autophagy plays a protective role in endoplasmic reticulum stress-mediated pancreatic β cell death. Autophagy. 2012;8:1757–1768. doi: 10.4161/auto.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antonucci L., Fagman J.B., Kim J.Y., et al. Basal autophagy maintains pancreatic acinar cell homeostasis and protein synthesis and prevents ER stress. Proc Natl Acad Sci USA. 2015;112:E6166–E6174. doi: 10.1073/pnas.1519384112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith M.D., Harley M.E., Kemp A.J., et al. CCPG1 is a non-canonical autophagy cargo receptor essential for ER-phagy and pancreatic ER proteostasis. Dev Cell. 2018;44:217–232.e11. doi: 10.1016/j.devcel.2017.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han X., Li B., Bao J., et al. Endoplasmic reticulum stress promoted acinar cell necroptosis in acute pancreatitis through cathepsin B-mediated AP-1 activation. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.968639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan C., Ma Y., Li H., et al. Endoplasmic reticulum stress promotes caspase-1-dependent acinar cell pyroptosis through the PERK pathway to aggravate acute pancreatitis. Int Immunopharmacol. 2023;120 doi: 10.1016/j.intimp.2023.110293. [DOI] [PubMed] [Google Scholar]
- 47.Vitale I., Pietrocola F., Guilbaud E., et al. Apoptotic cell death in disease—current understanding of the NCCD 2023. Cell Death Differ. 2023;30:1097–1154. doi: 10.1038/s41418-023-01153-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe T., Kudo M., Strober W. Immunopathogenesis of pancreatitis. Mucosal Immunol. 2017;10:283–298. doi: 10.1038/mi.2016.101. [DOI] [PubMed] [Google Scholar]
- 49.Iurlaro R., Muñoz-Pinedo C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016;283:2640–2652. doi: 10.1111/febs.13598. [DOI] [PubMed] [Google Scholar]
- 50.Al Mamun A., Suchi S.A., Aziz M.A., et al. Pyroptosis in acute pancreatitis and its therapeutic regulation. Apoptosis. 2022;27:465–481. doi: 10.1007/s10495-022-01729-w. [DOI] [PubMed] [Google Scholar]
- 51.An Y., Wang X., Guan X., et al. Endoplasmic reticulum stress-mediated cell death in cardiovascular disease. Cell Stress Chaperones. 2024;29:158–174. doi: 10.1016/j.cstres.2023.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang L., Liu S., Li S., et al. Induction mechanism of ferroptosis, necroptosis, and pyroptosis: a novel therapeutic target in nervous system diseases. Int J Mol Sci. 2023;24 doi: 10.3390/ijms241210127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu J., Zhang C., Wang J., et al. The regulation of ferroptosis by tumor suppressor p53 and its pathway. Int J Mol Sci. 2020;21:1–19. doi: 10.3390/ijms21218387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo Z., Chi R., Peng Y., et al. The role and interactive mechanism of endoplasmic reticulum stress and ferroptosis in musculoskeletal disorders. Biomolecules. 2024;14:1369. doi: 10.3390/biom14111369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He Z., Shen P., Feng L., et al. Cadmium induces liver dysfunction and ferroptosis through the endoplasmic stress-ferritinophagy axis. Ecotoxicol Environ Saf. 2022;245 doi: 10.1016/j.ecoenv.2022.114123. [DOI] [PubMed] [Google Scholar]
- 56.Zhao C., Yu D., He Z., et al. Endoplasmic reticulum stress-mediated autophagy activation is involved in cadmium-induced ferroptosis of renal tubular epithelial cells. Free Radic Biol Med. 2021;175:236–248. doi: 10.1016/j.freeradbiomed.2021.09.008. [DOI] [PubMed] [Google Scholar]
- 57.Gu X., Huang Z., Ying X., et al. Ferroptosis exacerbates hyperlipidemic acute pancreatitis by enhancing lipid peroxidation and modulating the immune microenvironment. Cell Death Discov. 2024;10:242. doi: 10.1038/s41420-024-02007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li H., Lin Y., Zhang L., et al. Ferroptosis and its emerging roles in acute pancreatitis. Chin Med J. 2022;135:2026–2034. doi: 10.1097/CM9.0000000000002096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li H., Wu D., Zhang H., et al. Autophagy-mediated ferroptosis is involved in development of severe acute pancreatitis. BMC Gastroenterol. 2024;24:245. doi: 10.1186/s12876-024-03345-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu K., Liu J., Zou B., et al. Trypsin-mediated sensitization to ferroptosis increases the severity of pancreatitis in mice. Cell Mol Gastroenterol Hepatol. 2022;13:483–500. doi: 10.1016/j.jcmgh.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deng L., Mo M.-Q., Zhong J., et al. Iron overload induces islet β cell ferroptosis by activating ASK1/P-P38/CHOP signaling pathway. PeerJ. 2023;11 doi: 10.7717/peerj.15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiang X., Xu M., Liu L., et al. Liproxstatin-1 attenuates acute hypertriglyceridemic pancreatitis through inhibiting ferroptosis in rats. Sci Rep. 2024;14:9548. doi: 10.1038/s41598-024-60159-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhatia M. Apoptosis versus necrosis in acute pancreatitis. Am J Physiol Liver Physiol. 2004;286:G189–G196. doi: 10.1152/ajpgi.00304.2003. [DOI] [PubMed] [Google Scholar]
- 64.Pahl H.L., Baeuerle P.A. The ER-overload response: activation of NF-kappa B. Trends Biochem Sci. 1997;22:63–67. doi: 10.1016/s0968-0004(96)10073-6. [DOI] [PubMed] [Google Scholar]
- 65.Tam A.B., Mercado E.L., Hoffmann A., Niwa M. ER stress activates NF-κB by integrating functions of basal IKK activity, IRE1 and PERK. PLoS One. 2012;7 doi: 10.1371/journal.pone.0045078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim S., Joe Y., Jeong S.O., et al. Endoplasmic reticulum stress is sufficient for the induction of IL-1β production via activation of the NF-κB and inflammasome pathways. Innate Immun. 2013;20:799–815. doi: 10.1177/1753425913508593. [DOI] [PubMed] [Google Scholar]
- 67.Liu T., Wang Q., Du Z., et al. The trigger for pancreatic disease: NLRP3 inflammasome. Cell Death Discov. 2023;9:246. doi: 10.1038/s41420-023-01550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu X., Huang H., Fu X., et al. The role of endoplasmic reticulum stress and NLRP3 inflammasome in liver disorders. Int J Mol Sci. 2022;23:3528. doi: 10.3390/ijms23073528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iannitti T., Palmieri B. Clinical and experimental applications of sodium phenylbutyrate. Drugs R D. 2011;11:227–249. doi: 10.2165/11591280-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kolb P.S., Ayaub E.A., Zhou W., et al. The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis. Int J Biochem Cell Biol. 2015;61:45–52. doi: 10.1016/j.biocel.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 71.Hong Y., Deng W., Guo W., et al. Inhibition of endoplasmic reticulum stress by 4-phenylbutyric acid prevents vital organ injury in rat acute pancreatitis. Am J Physiol Liver Physiol. 2018;315:G838–G847. doi: 10.1152/ajpgi.00102.2018. [DOI] [PubMed] [Google Scholar]
- 72.Zhang H., Nakajima S., Kato H., et al. Selective, potent blockade of the IRE1 and ATF6 pathways by 4-phenylbutyric acid analogues. Br J Pharmacol. 2013;170:822–834. doi: 10.1111/bph.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kusaczuk M. Tauroursodeoxycholate-bile acid with chaperoning activity: molecular and cellular effects and therapeutic perspectives. Cells. 2019;8:1471. doi: 10.3390/cells8121471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cho J.H., Bhutani S., Kim C.H., Irwin M.R. Anti-inflammatory effects of melatonin: a systematic review and meta-analysis of clinical trials. Brain Behav Immun. 2021;93:245–253. doi: 10.1016/j.bbi.2021.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reiter R.J., Calvo J.R., Karbownik M., et al. Melatonin and its relation to the immune system and inflammation. Ann N Y Acad Sci. 2000;917:376–386. doi: 10.1111/j.1749-6632.2000.tb05402.x. [DOI] [PubMed] [Google Scholar]
- 76.Aouichat S., Navarro-Alarcon M., Alarcón-Guijo P., et al. Melatonin improves endoplasmic reticulum stress-mediated IRE1α pathway in Zücker diabetic fatty rat. Pharmaceuticals. 2021;14:232. doi: 10.3390/ph14030232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fan C., Feng J., Tang C., et al. Melatonin suppresses ER stress-dependent proapoptotic effects via AMPK in bone mesenchymal stem cells during mitochondrial oxidative damage. Stem Cell Res Ther. 2020;11:442. doi: 10.1186/s13287-020-01948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Song Q., He Z., Li B., et al. Melatonin inhibits oxalate-induced endoplasmic reticulum stress and apoptosis in HK-2 cells by activating the AMPK pathway. Cell Cycle. 2020;19:2600–2610. doi: 10.1080/15384101.2020.1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao Q., Zhang H., Wu J., et al. Melatonin inhibits the endoplasmic reticulum stress‑induced, C/EBP homologous protein‑mediated pathway in acute pancreatitis. Mol Med Rep. 2020;22:1647–1655. doi: 10.3892/mmr.2020.11219. [DOI] [PubMed] [Google Scholar]
- 80.Ferrero-Andrés A., Panisello-Roselló A., Roselló-Catafau J., Folch-Puy E. NLRP3 inflammasome-mediated inflammation in acute pancreatitis. Int J Mol Sci. 2020;21:5386. doi: 10.3390/ijms21155386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahmed Abdalhamid Osman M., Sun Y.-J., Li R.-J., et al. Deletion of pancreatic β-cell adenosine kinase improves glucose homeostasis in young mice and ameliorates streptozotocin-induced hyperglycaemia. J Cell Mol Med. 2019;23:4653–4665. doi: 10.1111/jcmm.14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen T., Yu J., Guo X., et al. Adenosine kinase inhibits β-cell proliferation by upregulating DNA methyltransferase 3A expression. Diabetes, Obes Metab. 2024;26:2956–2968. doi: 10.1111/dom.15621. [DOI] [PubMed] [Google Scholar]
- 83.Navarro G., Abdolazimi Y., Zhao Z., et al. Genetic disruption of adenosine kinase in mouse pancreatic β-cells protects against high-fat diet–induced glucose intolerance. Diabetes. 2017;66:1928–1938. doi: 10.2337/db16-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun S., Han Y., Zhang C., et al. Adenosine kinase inhibition prevents severe acute pancreatitis via suppressing inflammation and acinar cell necroptosis. Front Cell Dev Biol. 2022;10:1–10. doi: 10.3389/fcell.2022.827714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang C., Niu H., Wan C., et al. Drug D, a diosgenin derive, inhibits L-arginine-induced acute pancreatitis through meditating GSDMD in the endoplasmic reticulum via the TXNIP/HIF-1α pathway. Nutrients. 2022;14:2591. doi: 10.3390/nu14132591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xia S., Wang J., Kalionis B., et al. Genistein protects against acute pancreatitis via activation of an apoptotic pathway mediated through endoplasmic reticulum stress in rats. Biochem Biophys Res Commun. 2019;509:421–428. doi: 10.1016/j.bbrc.2018.12.108. [DOI] [PubMed] [Google Scholar]
- 87.Wu L., Cai B., Zheng S., et al. Effect of emodin on endoplasmic reticulum stress in rats with severe acute pancreatitis. Inflammation. 2013;36:1020–1029. doi: 10.1007/s10753-013-9634-y. [DOI] [PubMed] [Google Scholar]
- 88.He J., Ma M., Li D., et al. Sulfiredoxin-1 attenuates injury and inflammation in acute pancreatitis through the ROS/ER stress/Cathepsin B axis. Cell Death Dis. 2021;12:626. doi: 10.1038/s41419-021-03923-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Najenson A.C., Bianchi M., Courreges A.P., et al. The exocrine pancreas is an extracardiac source of atrial natriuretic peptide. Pflugers Arch. 2019;471:915–924. doi: 10.1007/s00424-018-02247-y. [DOI] [PubMed] [Google Scholar]
- 90.Courreges A.P., Najenson A.C., Vatta M.S., Bianciotti L.G. Atrial natriuretic peptide attenuates endoplasmic reticulum stress in experimental acute pancreatitis. Biochim Biophys acta Mol basis Dis. 2019;1865:485–493. doi: 10.1016/j.bbadis.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 91.Najenson A.C., Courreges A.P., Perazzo J.C., et al. Atrial natriuretic peptide reduces inflammation and enhances apoptosis in rat acute pancreatitis. Acta Physiol. 2018;222 doi: 10.1111/apha.12992. [DOI] [PubMed] [Google Scholar]
- 92.Arhire L.I., Mihalache L., Covasa M. Irisin: a Hope in Understanding and Managing Obesity and Metabolic Syndrome. Front Endocrinol. 2019;10:524. doi: 10.3389/fendo.2019.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ren Y., Qiu M., Zhang J., et al. Low serum irisin concentration is associated with poor outcomes in patients with acute pancreatitis, and irisin administration protects against experimental acute pancreatitis. Antioxid Redox Signal. 2019;31:771–785. doi: 10.1089/ars.2019.7731. [DOI] [PubMed] [Google Scholar]
- 94.Ren Y., Zhang J., Wang M., et al. Identification of irisin as a therapeutic agent that inhibits oxidative stress and fibrosis in a murine model of chronic pancreatitis. Biomed Pharmacother. 2020;126 doi: 10.1016/j.biopha.2020.110101. [DOI] [PubMed] [Google Scholar]
- 95.Ren Y.-F., Wang M.-Z., Bi J.-B., et al. Irisin attenuates intestinal injury, oxidative and endoplasmic reticulum stress in mice with L-arginine-induced acute pancreatitis. World J Gastroenterol. 2019;25:6653–6667. doi: 10.3748/wjg.v25.i45.6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Horwitz A., Birk R. Irisin ameliorate acute pancreatitis and acinar cell viability through modulation of the unfolded protein response (UPR) and PPARγ-PGC1α-FNDC5 pathways. Biomolecules. 2024;14:643. doi: 10.3390/biom14060643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Day A.J., Milner C.M. TSG-6: a multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. 2019;78–79:60–83. doi: 10.1016/j.matbio.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 98.Li Q., Song W.-J., Ryu M.-O., et al. TSG-6 secreted by human adipose tissue-derived mesenchymal stem cells ameliorates severe acute pancreatitis via ER stress downregulation in mice. Stem Cell Res Ther. 2018;9:255. doi: 10.1186/s13287-018-1009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tang Z., Zhang W., Wan C., et al. TRAM1 protect HepG2 cells from palmitate induced insulin resistance through ER stress-JNK pathway. Biochem Biophys Res Commun. 2015;457:578–584. doi: 10.1016/j.bbrc.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 100.Cai Y., Shen Y., Xu G., et al. TRAM1 protects AR42J cells from caerulein-induced acute pancreatitis through ER stress-apoptosis pathway. In Vitro Cell Dev Biol Anim. 2016;52:530–536. doi: 10.1007/s11626-016-0011-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used for the research described in the article.