Abstract
Chimeric antigen receptor T-cell (CAR-T) therapies are one of the main approaches among targeted cellular therapies. Despite the potential benefit and durable responses observed in some patients receiving CAR-T therapies, serious and potentially fatal toxicities remain a major challenge. The most common CAR-T-associated toxicities include cytokine release syndrome (CRS), neurotoxicity, cytopenias, and infections. While CRS and neurotoxicity are generally managed with tocilizumab and corticosteroids, respectively, high-grade toxicities can be life-threatening. Close postinfusion monitoring and assessment of clinical laboratory parameters, patient-related and clinical risk factors (e.g., age, tumor burden, comorbidities, baseline laboratory parameters, and underlying abnormalities), and therapy-related risk factors (e.g., CAR-T type, dose, and CAR-T-induced toxicity) are effective strategies to mitigate the toxicities. Clinical laboratory parameters, including various cytokines, have been identified for CRS (interleukin [IL]-1, IL-2, IL-5, IL-6, IL-8, IL-10, C-reactive protein [CRP], interferon [IFN]-γ, ferritin, granulocyte-macrophage colony-stimulating factor [GM-CSF], and monocyte chemoattractant protein-1), neurotoxicity (IL-1, IL-2, IL-6, IL-15, tumor necrosis factor [TNF]-α, GM-CSF, and IFN-γ), cytopenias (IL-2, IL-4, IL-6, IL-10, IFN-γ, ferritin, and CRP), and infections (IL-8, IL-1β, CRP, IFN-γ, and procalcitonin). CAR-T-associated toxicities can be monitored and treated to mitigate the risk to patients. Assessment of alterations in clinical laboratory parameter values that are correlated with CAR-T-associated toxicities may predict development and/or severity of a given toxicity, which can improve patient management strategies and ultimately enable the patients to better tolerate these therapies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40264-025-01538-5.
Plain Language Summary
Chimeric antigen receptor T-cell (CAR-T) therapies are used in the treatment of various aggressive blood cancers. These therapies use a patient’s immune cells (T cells) that are genetically modified to fight cancer. In this article, we focus on the adverse effects associated with CAR-T therapies and discuss how they can be managed. The most common CAR-T-associated adverse effects include cytokine release syndrome (a rapid release of signaling proteins [cytokines] from affected immune cells), neurotoxicity (toxic effects on the nervous system), cytopenias (lower-than-normal blood cell levels), and infections. Patients receiving CAR-T therapies need to be closely monitored for signs of any adverse effects. Some of these effects can be prevented or treated with medications. However, current efforts focus on making the adverse effects less severe, and on identifying risk factors that may predict the likelihood and onset of a potential adverse effect. When an adverse effect occurs, the levels of certain molecules in the blood change. These changes can help physicians determine the type of adverse effect and select the best treatment to combat it. Some patient features (e.g., age, medical conditions, the size and spread of the tumor, and levels of certain molecules in the blood) and treatment-related factors (e.g., therapy type and dose) should be considered before starting a CAR-T therapy. Adverse effects are monitored and treated to reduce the risk to patients. Evaluating the levels of certain parameters in the blood can improve patient management strategies and help patients better tolerate CAR-T therapies.
Graphical Abstract

Supplementary Information
The online version contains supplementary material available at 10.1007/s40264-025-01538-5.
Key Points
| Potentially life-threatening toxicities associated with chimeric antigen receptor T-cell (CAR-T) therapies can be monitored and treated to minimize the risks to oncology patients. |
| We focus on management and mitigation strategies for the most common CAR-T-associated toxicities: cytokine release syndrome, neurotoxicity, cytopenias, and infections. |
| Assessment of clinical laboratory parameter values that are correlated with CAR-T-associated toxicities may improve patient management during and after CAR-T infusion and help the patients better tolerate these lifesaving therapies. |
Introduction
Immunotherapy has significantly improved the care of cancer patients. Among immunotherapies, adoptive cell therapies (ACTs) rely on the transfer of ex vivo-expanded autologous or allogeneic immune cells, mostly conventional alpha beta T cells [1, 2]. ACT using T cells that are genetically modified and ex vivo-activated to suppress T-cell inhibition in the tumor microenvironment and eradicate tumor cells remains the current focus of research [3–6]. Within these therapies, chimeric antigen receptor T-cell (CAR-T) therapy, consisting of genetically engineered T cells modified to express a receptor directed against a tumor antigen, represents one of the main approaches in the current development of targeted cellular therapies. CAR-T therapy utilizes genetically engineered artificial receptors that recognize tumor cell surface antigens independently of the major histocompatibility complex [7]. CAR-T therapy can potentially be used for the treatment of cancer, infectious diseases, autoimmune diseases, cardiac diseases, and others [8, 9]. Furthermore, CAR technology has recently been used to develop novel ACTs with other types of immune cells, including natural killer cells and their subtypes, macrophages, or gamma delta T cells [10–12].
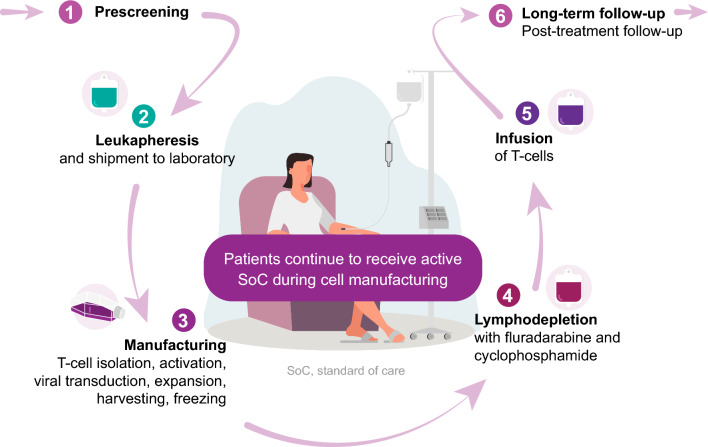
Following isolation of T cells and their genetic modification and expansion, the cells are injected back into the patient, where they exert their cytotoxic activity and help to mount a sustained immune response (Fig. 1).
Fig. 1.
T-cell therapy patient journey. (1) Before receiving T-cell therapy, patient eligibility for treatment is assessed by a multi-disciplinary team; initial evaluations include medical history, performance status, screening laboratory tests, and imaging. (2) Following leukapheresis, T cells are shipped to a laboratory for processing. (3) T cells are isolated and activated. Activated T cells are genetically engineered, expanded, and then cryopreserved before being reintroduced into the patient. (4) Prior to infusion, the patient undergoes lymphodepletion to ensure effective CAR-T expansion and cytotoxic impact. Lymphodepletion is routinely performed with a combination of fludarabine and cyclophosphamide. (5) Modified T cells are injected back into the patient via infusion. (6) Following the infusion, patients are closely monitored for 2–4 weeks for potential toxicities with regular medical assessments thereafter
To date, six CAR-T therapies have been approved by the US Food and Drug Administration (FDA) for the treatment of various hematologic cancers (Table 1).
Table 1.
| Product name (brand name, manufacturer) | Indication |
|---|---|
| Tisagenlecleucel (Kymriah, Novartis Pharmaceuticals Corporation) | Acute lymphoblastic leukemia [14], B-cell lymphoma [15], and follicular lymphoma [16] |
| Axicabtagene ciloleucel (Yescarta, Kite Pharma Inc.) | B-cell lymphoma [17] and follicular lymphoma [18] |
| Brexucabtagene autoleucel (Tecartus, Kite Pharma Inc.) | Mantle cell lymphoma [19] and acute lymphoblastic leukemia [20] |
| Lisocabtagene maraleucel (Breyanzi, Juno Therapeutics, Inc., a Bristol-Myers Squibb Company) | Large B-cell lymphoma [21] |
| Idecabtagene vicleucel (Abecma, Celgene Corporation, a Bristol-Myers Squibb Company) | Multiple myeloma [22] |
| Ciltacabtagene autoleucel (Carvykti, Janssen Biotech, Inc.) | Multiple myeloma [23] |
CAR-T, chimeric antigen receptor T cell
Long-term efficacy and remission potential with CAR-T therapy has been observed in clinical trials [24–35], as well as in postmarketing studies [36–41]. For instance, data from a postmarketing study with axicabtagene ciloleucel indicate an overall response rate of 70% and a complete response rate of 52% in patients with large B-cell lymphoma [37].
Despite the potential benefit of CAR-T therapies, they are associated with life-threatening toxicities [42, 43]. In addition, the US FDA has recently highlighted the risk of T-cell malignancies based on data from clinical trials and/or postmarketing studies with CAR-T therapies [44]. Effective mitigation and clinical management of these toxicities can reduce life-threatening risks to patients and afford them the potentially life-sustaining benefits of these therapies.
Initial preclinical models with CAR-T therapies primarily aimed to assess the efficacy of these therapies but failed to predict toxic complications in humans [43]. Toxicities associated with these therapies pose safety risks and are major barriers for their wider clinical application. Thus, the adoption of a benefit–risk-based approach in clinical development of these therapies is of crucial importance to assure the safety of patients. Furthermore, given the risk of toxicities associated with CAR-T therapies, effective mitigation and management strategies are needed to enable a broader use of these therapies to benefit patients who have limited choices for effective therapies. In this paper, we discuss the use of clinical laboratory assessments to better predict toxicities, as well as management and mitigation strategies for CAR-T-related toxicities in the clinic.
CAR-T-Associated Toxicities
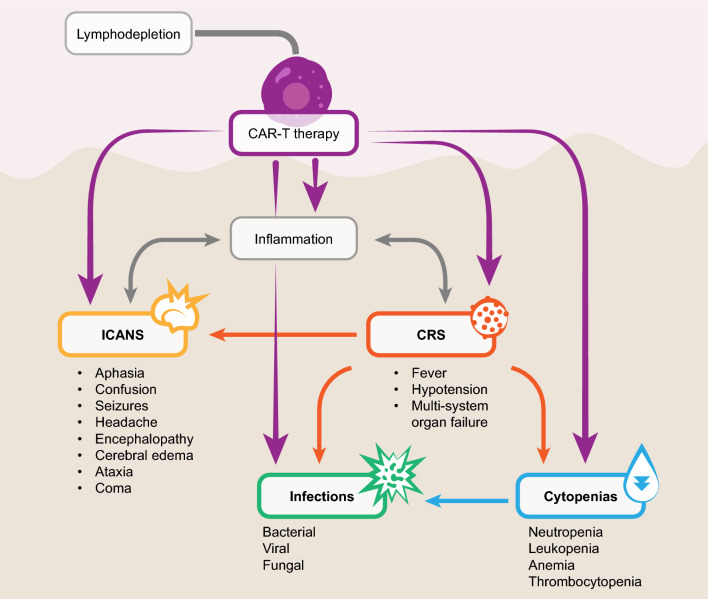
The most common CAR-T-related toxicities include cytokine release syndrome (CRS) resulting from immune activation; neurotoxicity, also referred to as immune effector cell-associated neurotoxicity syndrome (ICANS); cytopenias; and infections (Fig. 2). Less common toxicities include secondary T-cell malignancies and “on-target/off-tumor” (i.e., recognition of the target antigen on normal cells) and “off-target/off-tumor” (i.e., recognition of an unrelated antigen; cross-reactivity) toxicities [43, 45–48].
Fig. 2.
Overview of the main toxicities associated with CAR-T therapies. CAR-T; chimeric antigen receptor T cell; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome
CAR-T infusion can lead to CRS, ICANS, cytopenias, and infections. CAR-T causes inflammation that can lead to CRS and ICANS. CRS may lead to ICANS, cytopenias, and infections. In addition, lymphodepletion may lead to cytopenias and infections as well.
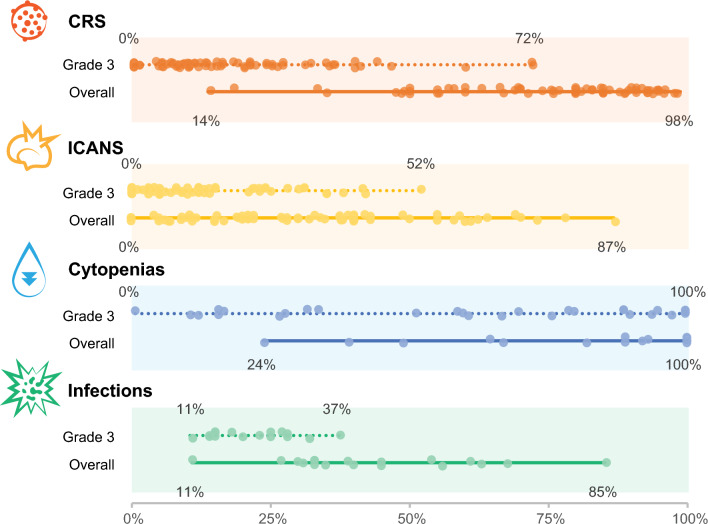
The incidence and severity of toxicities associated with CAR-T therapies vary largely between studies, likely due to various CAR-T types, the infusion time and dose, and co-administration of other therapies (Fig. 3).
Fig. 3.
Incidence and severity of the most common toxicities associated with CAR-T therapies. The circles represent the incidence reported in individual studies. Data sources are listed in Electronic Supplementary Material 1; the individual studies were published between 2014 and 2023 for CRS and ICANS, and between 2017 and 2023 for cytopenias and infections. CAR-T; chimeric antigen receptor T cell; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome
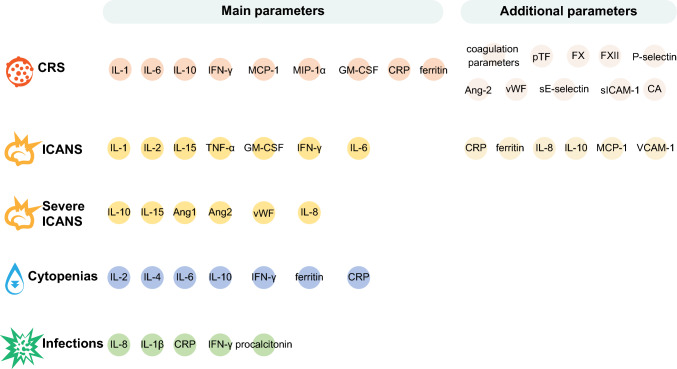
Figure 4 provides an overview of clinical laboratory parameters that are associated with the risk of developing each of these toxicities.
Fig. 4.
Clinical laboratory parameters correlated with the risk of developing CAR-T-associated toxicities. Ang, angiopoietin; CA, catecholamines; CAR-T, chimeric antigen receptor T cell; CRP, C-reactive protein; CRS, cytokine release syndrome; F, factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; ICANS, immune effector cell-associated neurotoxicity syndrome; IFN, interferon; IL, interleukin; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; pTF, plasma tissue factor; sE-selectin, soluble E-selectin; sICAM, soluble intercellular adhesion molecule; TNF, tumor necrosis factor; VCAM, vascular-cell adhesion molecule; vWF, von Willebrand factor
CRS
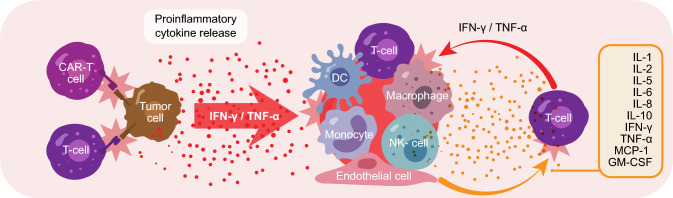
CRS is one of the most common life-threatening toxicities associated with engineered CAR-T therapies that was not observed in preclinical models, but first noted in subsequent clinical studies [43]. CRS is a systemic inflammatory response that can be induced by the binding of CAR-T cells to a specific antigen on the surface of target cells, inducing the release of cytokines such as interferon (IFN)-γ or tumor necrosis factor (TNF)-α. Subsequent activation of bystander immune and non-immune cells, such as monocytes, macrophages, dendritic cells, and endothelial cells, results in the hypersecretion of proinflammatory cytokines, initiating a cascade of events leading to CRS (Fig. 5) [49, 50].
Fig. 5.
Sequence of events leading to cytokine release syndrome, adapted from Cosenza et al. Int J Mol Sci. 2021;22:7652 [49]. CAR-T cells bind to the tumor cells and induce the release of cytokines such as IFN-γ or TNF-α, leading to the activation of bystander immune and non-immune cells, which further release proinflammatory cytokines, triggering a cascade reaction in which high levels of released IL-6 activate T cells and other immune cells, leading to a cytokine storm. CAR-T, chimeric antigen receptor T cell; DC, dendritic cell; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; MCP, monocyte chemoattractant protein; NK, natural killer; TNF, tumor necrosis factor
The incidence and severity of CRS vary depending on the CAR-T product, dose, and disease burden at the time of infusion [51, 52]. Symptoms of CRS may occur immediately after the administration of CAR-T cells or may be delayed until days or weeks after treatment, with a median time of onset of 2–3 days following CAR-T infusion [51, 53, 54]. The severity of CRS ranges from mild, flu-like symptoms, including fever and chills, to severe and life-threatening symptoms, including hypotension, tachycardia, pleural effusion, pulmonary edema, capillary leak syndrome, and hypoxia, which may ultimately lead to multisystem organ failure and death [51, 55].
Management and Mitigation Strategies
When treated effectively, CRS is manageable. Nevertheless, following CAR-T infusion, patients should be closely monitored at the hospital for at least 7 days, including daily assessments of biochemistry and blood counts, review of organ systems and physical exam, and assessment of vital signs at least every 4 h [56–58]. The Society for Immunotherapy of Cancer recommends daily monitoring of clinical laboratory values associated with CRS for a postinfusion period related to a specific CAR-T product (typically several weeks); in addition, patients with a high disease burden require specific attention, including cardiac function monitoring [59].
The current gold standard of CRS treatment is anti-cytokine therapy that is initiated as soon as symptoms of CRS begin to occur to prevent progression to severe CRS [53]. Tocilizumab, an IL-6 antagonist, has been approved by the US FDA for the treatment of CRS occurring after CAR-T therapy [14, 53] and is commonly used in the management of CRS [60, 61]. Prophylactic tocilizumab prior to administration of T-cell therapies has also been evaluated. For instance, in a recent single-center study, the use of prophylactic tocilizumab prior to the administration of another T-cell redirecting therapy, based on the bispecific antibody teclistamab, decreased the incidence and severity of CRS; CRS occurred in 26.3% of patients who received prophylactic tocilizumab versus 73.3% of patients who did not receive prophylactic tocilizumab [62]. In another study, among 20 patients with non-Hodgkin lymphoma who received prophylactic tocilizumab 1 h prior to infusion of anti-CD19 CAR-T cells, only low-grade CRS was observed in 50% of patients, indicating that prophylactic tocilizumab is a viable option to mitigate high grade CRS [63]. Another anti-IL-6 monoclonal antibody, siltuximab, has also been used, although it has not been approved by the US FDA for treatment of CRS following CAR-T therapy [53, 64, 65]. Other agents for the treatment of CRS include corticosteroids, dasatinib, A3 adenosine receptor agonists, JAK/STAT pathway inhibitors, or lenzilumab [53, 66].
CRS should be managed according to the toxicity grade [59, 67, 68]. The American Society of Clinical Oncology (ASCO) guidelines recommend mostly supportive care for grade 1 CRS and tocilizumab for higher grades; steroids may be considered earlier in treatment depending on the CAR-T product [67].
Several clinical trials focusing on CRS treatment are ongoing (Electronic Supplementary Material 2).
Clinical Laboratory Assessments
The broad use of CAR-T therapy necessitates the identification of clinical laboratory parameter values associated with the risk of developing severe CRS. Several studies showed that severe CRS following CAR-T therapy is associated with an increase in certain cytokine levels [69–73]. Such cytokine activation profiles could be established following CAR-T infusion to help mitigate the severe effects, and close monitoring of those levels is suggested to be a valuable tool in assessing the risk of CRS [43]. The main cytokines implicated in the pathogenesis of CRS include IL-1, IL-2, IL-5, IL-6, IL-8, IL-10, IFN-γ, monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein-1α, and granulocyte-macrophage colony-stimulating factor (GM-CSF) (Fig. 4) [51, 74–78]. Higher levels of these cytokines have been shown to be associated with severe CRS, indicating their potential as clinically relevant diagnostic predictors for high-grade CRS [70, 71, 79–81]. Among these, high serum IL-6 levels are detected in nearly all patients with CRS [49], and several studies found these levels to be associated with the severity of CRS after CAR-T therapy [69, 70, 72, 73, 79, 82–85]. Furthermore, an increase in C-reactive protein (CRP) levels correlating with increased IL-6 levels has also been detected in patients with CRS [68, 69, 82, 86–89]; elevated ferritin levels have also been shown to correlate with CRS [72, 90, 91] (Fig. 4). Surrogate markers of systemic inflammation could identify patients with CRS and potentially be used to guide intervention to either suppress cytokine release or eliminate CAR-T cells in the event of severe toxicity. While the results of a full cytokine panel may not be promptly available in many hospitals, CRP and ferritin may serve as surrogate markers of CRS. In addition, monitoring lactate dehydrogenase levels, complete blood count, coagulation, and uric acid levels is also recommended [51, 92].
Other, less common clinical laboratory parameters have also been shown to be predictive of the occurrence and severity of CRS, including coagulation parameters; levels of the plasma tissue factor, Factor X, Factor XII, and P-selectin [93]; angiopoietin (Ang)-2 and von Willebrand factor (vWF); soluble E‐selectin and soluble intercellular adhesion molecule-1 [71, 94]; or catecholamines [95]. Furthermore, a regression modeling study predicted a 3-cytokine signature (i.e., IFN-γ, IL-6, and soluble IL-2 receptor α) associated with progression to severe CRS [70].
Clinical, Patient-, and Treatment-Related Risk Factors
Clinical factors that increase the risk of developing higher-grade CRS include disease burden and marrow involvement, common lymphodepletion regimens such as fludarabine and/or cyclophosphamide, and higher CAR-T infusion doses. Patient-specific factors include age, bulky disease, comorbidities, early-onset CRS (within 3 days of cell infusion), pre-existent inflammation, and baseline thrombocytopenia [53, 56, 69, 72, 86, 96]. Of note, high tumor burden (≥ 40% lymphoblasts) in the bone marrow has been identified as the major risk factor for severe CRS [72, 97]. Although elevated levels of the abovementioned cytokines in the blood of patients following CAR-T infusion are generally considered predictors of CRS, individual patient factors might affect these levels, making it difficult to predict the grade of CRS in these individuals. Furthermore, a certain level of IL-6, for instance, might be associated with grade 3 CRS in one patient but not in another, indicating that other individual patient factors need to be considered. Such factors, as well as the factors contributing to the extent of cytokine release, e.g., the CAR-T type, dose, and their abundance in the patient, may help with predicting the effects of cytokine release.
As patients with a higher baseline disease burden are likely to have more severe CRS, imaging techniques such as positron emission tomography–computed tomography (PET-CT) can be useful to assess the tumor burden before CAR-T administration [98]. Furthermore, abnormal imaging findings based on radiography, computed tomography (CT), or magnetic resonance imaging (MRI) have been shown to correlate with higher-grade CRS following CAR-T infusion [99]. Such abnormalities included pleural effusions and pulmonary edema; therefore, thoracic imaging (chest radiography or CT) is often performed in patients experiencing CRS, especially grade 2 or higher CRS, and in patients experiencing fever, cough, and hypoxia following the CAR-T infusion [99]. Pleural effusion with atelectasis and pulmonary edema are common complications of CAR-T-cell therapy occurring during CRS that can be detected through CT; other, less common complications include organizing pneumonia patterns and CAR-T-cell infiltration [100]. Of note, patients with grade ≥ 2 CRS were more likely to have chest CT scans or radiographs with abnormal findings and had a higher incidence of pleural effusions and atelectasis [99]. While patients with CRS may not be routinely imaged, thoracic imaging may provide a useful tool to capture early signs of pulmonary toxicity and guide physicians in managing this toxicity.
Mechanistic models capturing various factors related to the drug (i.e., structure, selectivity, and binding affinity to the tumor target), target (i.e., density of target antigen on the tumor cell surface and accessibility), and disease (i.e., tumor load) have been used to predict the extent of cytokine release. These, however, do not account for individual patient factors, such as comorbidities, received treatment, or their outcomes, and are thus not useful in predicting CRS severity. Combining mechanistic modeling with real-world data and patient databases is currently being investigated [101].
Strategies to mitigate CRS include detailed assessment of individual patient factors and tumor characteristics prior to treatment, and assessments of clinical laboratory parameters and product-related factors. Prompt clinical management of symptoms related to CRS can ensure patient safety and allow patients to realize the benefit of the therapy.
Neurotoxicity
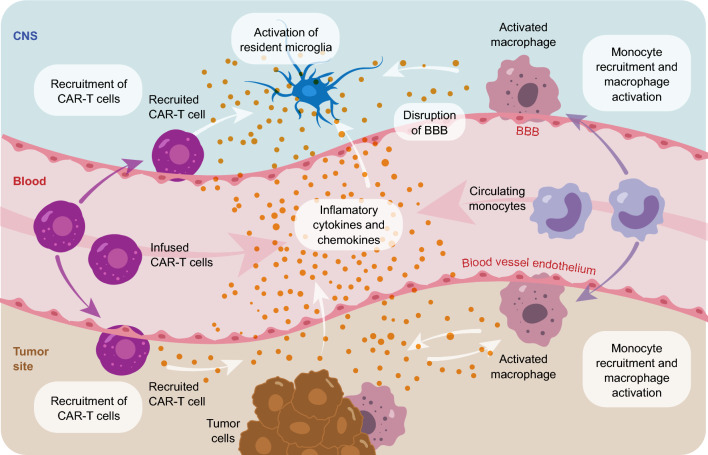
Alongside CRS, CAR-T-associated neurotoxicity or ICANS is another common toxicity observed with CAR-T therapies. ICANS presents as a toxic encephalopathy manifested with aphasia, headache, and confusion but can progress to a depressed level of consciousness, seizures, motor weakness, cerebral edema, and coma [102]. Typically, ICANS occurs following peak CRS severity, when patients begin to recover from CRS; however, delayed onset of ICANS has also been reported [103, 104]. Pathophysiology of ICANS involves peripheral immune overactivation and massive cytokine release, which induce endothelial activation and dysfunction of the blood–brain barrier with increased permeability, facilitating infiltration of CAR-T cells, as well as immune cells and cytokines, leading to central nervous system (CNS) inflammation [51, 102, 103, 105–107] (Fig. 6). The main cytokines involved in the pathophysiology of ICANS include IL-1, IL-2, IL-6, IL-15, IFN-γ, TNF-α, and GM-CSF [107, 108].
Fig. 6.
Pathophysiology of immune effector cell-associated neurotoxicity syndrome, adapted from Morris et al. Nat Rev Immunol. 2022; 22(2):85-96 [105]. Infused CAR-T cells and other activated host immune cells release proinflammatory cytokines leading to activation of endothelial cells and disruption of the BBB; increased permeability of the BBB facilitates infiltration of CAR-T cells and cytokines into the CNS, triggering inflammation and microglial activation, leading to abnormal neuronal function. BBB, blood–brain barrier; CAR-T, chimeric antigen receptor T cell; CNS, central nervous system
Management and Mitigation Strategies
Prophylactic treatment can be used to prevent and manage ICANS. According to the ASCO guidelines, the mainstay of ICANS treatment includes supportive care and corticosteroids. The choice of treatment depends on the grade of the event and the presence of concurrent CRS [67].
Unlike for CRS, in which IL-6 is one of the critical regulators, IL-6 inhibitors are less efficacious against ICANS [57], although still recommended for ICANS with concurrent CRS [67, 109]. Owing to their broad systemic anti-inflammatory function by blocking cytokine signaling, corticosteroids are commonly used to manage ICANS [51, 108]. However, to limit the undesirable effects of corticosteroids and considering IL-1 plays a central role in ICANS, blockade with an IL-1 receptor antagonist may be recommended [110, 111]. As an example, a recent study demonstrated a low incidence of ICANS (19% all grades; 9.7% severe ICANS) in patients who received a prophylactic IL-1 receptor antagonist (anakinra) from day 2 for at least 10 days post-CAR-T infusion [112].
Clinical Laboratory Assessments
Similar to CRS, ICANS is also associated with elevated levels of blood cytokines, including IL-1, IL-2, IL-15, TNF-α, GM-CSF, IFN-γ, and IL-6 [57, 81, 107, 108, 113–116] (Fig. 4). In addition, increased CRP and ferritin levels, as well as IL-8, IL-10, MCP-1, and vascular-cell adhesion molecule 1 were also shown to be associated with high-grade neurotoxicity following CAR-T therapy [57, 81, 117]. Among these cytokines, IL-1 plays a crucial role in the pathophysiology of ICANS [74, 107, 110]. Cytokine levels may be used as predictive markers to aid in the clinical management of symptoms associated with ICANS. For instance, serum concentrations of IL-6 and IFN-γ correlate with the severity of toxicity and have a predictive value in identifying patients who subsequently developed severe ICANS [116]. Concentrations of IL-10 and IL-15 have also been shown to correlate with a risk of developing severe ICANS [57].
Although elevated cytokine levels can be detected in both serum and cerebrospinal fluid (CSF), ICANS is generally associated with CNS-specific cytokine production [57]. Therefore, the analysis of CSF is considered to be a more suitable method for the diagnosis of ICANS [107]. Elevated levels of IL‐1, IL‐6, IL‐10, IFN‐γ, TNF‐α, MCP-1, C-X-C motif chemokine ligand 10, and granzyme B, as well as GFAP (marker of astrocyte injury) and S100b (marker of astrocyte activation), in the CSF are typically observed in patients with neurotoxicity [57, 105, 107, 108]. In addition, decreased levels of Ang1 and increased levels of Ang2, vWF, and IL-8 were suggested to be unique markers in patients with severe ICANS [105, 118, 119].
Furthermore, patients who experience ICANS following CAR-T infusion often undergo imaging analysis (head CT or MRI), especially those with altered mental status and headache [99]. While typically there are no abnormal imaging findings in most ICANS cases, T2-weighted fluid-attenuated inversion recovery (T2/FLAIR ) hyperintensity, vasogenic edema, leptomeningeal enhancement, multifocal microhemorrhages, cortical diffusion restriction, and transient corpus callosum lesions have been observed in some patients [99, 100, 120].
Risk Factors
As ICANS is significantly associated with early systemic inflammation and CRS, mitigation or prevention of severe CRS may decrease the incidence of severe ICANS. The onset of ICANS is correlated with presence and severity of CRS and elevation of CRP and ferritin levels [57, 114, 115, 121]. Early onset and higher severity of CRS, higher pretreatment tumor burden, pre-existing neurological conditions (headache, seizures, cognitive impairment, peripheral neuropathies, and intracranial hemorrhages), older age, acute postinfusion brain MRI findings or brain electroencephalogram changes, lower baseline platelet count, fever, and higher CAR-T dose are main risk factors for ICANS [57, 107, 114, 120–126].
Based on these findings, both clinical laboratory assessments and clinical factors have been used to develop a variety of predictive tools or models for a patient’s risk to develop ICANS [121, 127–131]. For instance, the endothelial activation and stress index (EASIX) score, a surrogate marker of endothelial activation defined as (creatinine × lactate dehydrogenase )/platelets, combined with the inflammatory markers ferritin and CRP, can be used to predict the risk of developing ICANS [127]. Similarly, a modified EASIX, which replaces creatinine with CRP, has been used to predict the risk of developing severe ICANS [128]. Furthermore, on the basis of predictive factors for ICANS, a prognostic score was developed, calculated from values of parameters measured during the first 5 days following a CAR-T infusion. In that model, every parameter (e.g., age, histologic subtype, CRP level, and ferritin level) was assigned a score of 0–3; the total score could range from 0 to 14, with 14 indicating the highest probability of developing ICANS [121]. Another model based on clinical predictors such as maximum daily temperature, CRP, IL-6, and procalcitonin was shown to predict with high accuracy the day of onset of ICANS, and which patients would develop ICANS and with which severity, based on data from the first few days following CAR-T infusion [130]. In addition, a recent model demonstrating a strong association between ferritin levels at day 3 after CAR-T infusion and severe ICANS was validated in an external cohort of patients, further highlighting the key role of ferritin in the development of ICANS after CAR-T infusion [131]. Altogether, these models allow physicians to identify high-risk patients and closely monitor and effectively manage patients who may experience ICANS, thereby reducing the occurrence of serious toxicities in these patients. However, these models need to be further optimized and/or validated before broader implementation in clinical practice.
Cytopenias
Cytopenias are common toxicities associated with CAR-T therapy. Early cytopenias occur up to 30 days post-CAR-T transfusion, prolonged cytopenias occur from 30 to 90 days post-transfusion, and late cytopenias may persist beyond 90 days [132, 133]. Most cases of cytopenia occur immediately following the infusion and are linked to the lymphodepletion chemotherapy [132, 134]. Factors related to prolonged cytopenias are less well understood, although baseline cytopenia is likely associated with prolonged cytopenias post-CAR-T therapy [133, 135–137]. Neutropenia seems to be the most common among cytopenias following a CAR-T infusion [138]. Sustained cytopenia predisposes for severe infectious complications; thus, mitigation and early management of severe cytopenia is crucial [139].
Management and Mitigation Strategies
Management of cytopenias includes supportive care measures such as blood transfusions, prophylactic antibiotics, corticosteroids, antifungal agents, thrombopoietin receptor agonists, and growth factors [59, 67, 140].
Certain baseline clinical characteristics have been shown to have potential prognostic value for predicting severe cytopenia, including baseline hemoglobin and blood cell count, baseline ferritin, CRP and IL-6 levels, lines of prior therapy, response to pre-CAR-T treatment, and the CAR-T product [141, 142]. High-grade CRS and/or neurotoxicity are also associated with prolonged and late cytopenia [71, 132, 143]; furthermore, grade ≥ 3 cytopenia is associated with severe CRS, suggesting that CRS severity can be used as an independent predictor for cytopenia [141, 143, 144]. Consistent with this observation, CRS-associated laboratory parameters, including CRP, ferritin, IL-2, IL-4, IL-6, IL-10, and IFN-γ, have been shown to be associated with cytopenia (Fig. 4) [133, 144]. Thus, clinicians should closely monitor cytopenias following CAR-T cell infusion, especially in patients with suspected or confirmed CRS.
Risk factors associated with prolonged cytopenia include baseline cytopenia, the baseline inflammatory state (elevated serum CRP and ferritin levels), elevated IL-6 levels, pretreatment bone marrow disease burden, severe CRS, and allogeneic hematopoietic stem cell transplantation [60, 141, 145, 146].
Identifying patients at risk for cytopenias and the magnitude of the change in the blood cell levels is crucial considering the complications of cytopenias can be life-threatening. A recently described CAR-HEMATOTOX model evaluates the association of the baseline clinical markers and the risk of developing cytopenia following CAR-T therapy [146]. In addition, this model can be used to predict duration of cytopenia and duration of hospitalization [146, 147].
Infections
Patients receiving CAR-T therapy are typically immunosuppressed due to prior treatments, leading to prolonged cytopenia; moreover, the use of IL-6 blockers and corticosteroids to treat CRS and neurotoxicity further increases the risk of infection. Several studies demonstrated an association of neutropenia [148], higher grade ICANS [149], and CRS [150] with higher rates of infections. Furthermore, prolonged cytopenias can lead to a higher risk of late infections [30, 140].
Overall, most infections are bacterial or viral, while fungal infections are less common [151–155]. The majority of early infections are bacterial, while most late infections are viral and include predominantly upper respiratory tract infections [139, 154, 156].
Management and Mitigation Strategies
According to the ASCO guidelines, full blood count and blood cultures should be obtained along with other relevant diagnostic tests in the management of infections. Broad-spectrum antibiotics should be initiated according to fever and neutropenia guidelines. Antivirals should be initiated per institutional guidelines, and antifungals should be considered for high-risk patients [67]. Physicians treating patients receiving CAR-T therapy should be aware of the various risk factors and available diagnostic tests mitigate the infections early.
To prevent severe infections in patients after CAR-T therapies, determining their immune status is of utmost importance as many patients considered for CAR-T receive prior hematopoietic cell transplantation and are immunocompromised.
To mitigate the risk of infection, antiviral prophylaxis for a minimum of 60–100 days postinfusion (or longer for high-risk patients) is recommended; antibacterial and antifungal prophylaxis should be considered until complete recovery of neutrophil count [140, 157]. Furthermore, screening for certain pathogens should be done prior to CAR-T administration, as latent infections can be reactivated in patients treated with corticosteroids for CRS and ICANS. All patients should be screened for viral (e.g., human immunodeficiency virus and hepatitis B and C viruses, herpes simplex virus, varicella-zoster virus) and bacterial infections (e.g., Treponema pallidum [syphilis], Toxoplasma gondii, or Mycobacterium tuberculosis), as well as Strongyloides stercoralis, and parasites in feces; other tests can also be recommended according to the geographical area [154, 157]. In addition to prophylactic antibiotics, prophylactic granulocyte colony stimulating factor can be considered for neutropenic patients [158, 159].
Risk factors for the development of infection following CAR-T therapy include patient-related factors, i.e., baseline leukopenia, acute lymphocytic leukemia, history of infection prior to CAR-T therapy, bridging chemotherapy (lasting from the decision to treat until T-cell infusion), impaired performance status, history of prior hematopoietic cell transplant, comorbidities, and CAR-T-related factors, i.e., CAR-T-induced cytopenia, CRS, and ICANS [141, 156, 160–162]. The latter two toxicities appear as the major risk factors for infections after CAR-T therapy [139, 150, 154, 160]; grade 3 or higher CRS is a predictor of subsequent infection within 6 months after CD-19-directed CAR-T infusion [150]. Furthermore, the use of tocilizumab and steroids in patients with CRS/ICANS is likely to also contribute to a higher infection risk in such patients [156].
Clinical laboratory values such as elevated CRP levels and elevated procalcitonin levels (≥ 0.4 ng/mL) [163, 164] have been identified as being predictive for the occurrence of infections following CAR-T therapy.
As CRS and infection have overlapping clinical presentations, specific clinical laboratory values allowing for differentiation of these events are particularly useful to determine the appropriate treatment. In one study, a secondary increase in IL-6 levels in patients with CRS was associated with severe infection, and a 3-cytokine signature (IL-8, IL-1β, and IFN-γ) associated with infections was identified [165]. Furthermore, in another study, elevated IFN-γ levels in combination with low IL-1β was associated with CRS, while normal to mildly elevated IFN-γ in combination with elevated IL-1β was associated with sepsis [166].
Secondary Malignancies
CAR-T products carry a potential risk of oncogenesis owing to the genetic modifications used for their generation. To date, several cases of secondary T-cell cancers have been reported, including T-cell lymphoma and leukemia [167, 168]. Thus, the US FDA currently recommends the lifetime monitoring for the development of new cancers in patients undergoing CAR-T therapies [44]. In addition, all CAR-T products are now required to include a boxed warning of the risk of secondary cancer development [169]. Nevertheless, considering the low number of currently reported cases, the risk of developing secondary cancers following CAR-T therapy remains low and the overall benefits of these products continue to outweigh their potential risks for their approved uses [168–170].
On-Target/Off-Tumor and Off-Target/Off-Tumor Toxicities
The targets recognized by CAR-T cells are tumor-associated antigens, which may be also present in normal tissue; on-target/off-tumor toxicity is a CAR-T-mediated cytotoxicity against non-malignant tissues expressing the target antigen [171]. Such effects reported with CAR-T included liver toxicity [172], lung toxicity [173–175], sustained pyrexia and dermal toxicities [176], mucosal/cutaneous toxicities including oral mucositis, oral ulcers, gastrointestinal hemorrhage, desquamation, and pruritus, as well as acute respiratory distress [177]. In patients with hematological malignancies, the most common on-target/off-tumor toxicity is B-cell aplasia [48, 87]; however, rare cases of fatal cerebral edema and movement disorder have also been reported [178, 179].
Various strategies have been explored to mitigate these on-target/off-tumor toxicities, including the incorporation of suicide gene switches, inhibitory receptors in CAR-Ts to provide a self-regulating safety switch, intra-tumoral injection of CAR-Ts, use of reduced-affinity CAR-Ts, or use of hypoxia-inducible CAR-Ts [180].
Off-target/off-tumor toxicity, i.e., cross-reactivity with an unexpected target expressed on a healthy tissue, has mostly been observed in patients following T-cell receptor T-cell therapies but, to date, has not been reported following CAR-T therapies [181].
Conclusion
Although CAR-T therapies are associated with life-threatening toxicities, their potential benefit to patients has clearly emerged. Common toxicities such as CRS, ICANS, cytopenias, and infections result in significant morbidity but can be monitored and treated to mitigate the risk to patients. Assessment of clinical laboratory parameter values, as well as patient-related, treatment-related, and clinical risk factors appears as an effective strategy to mitigate the toxicities. These risk factors and clinical laboratory assessments can be used to select the therapies with the best benefit–risk profile, improving patient management strategies and enabling them to better tolerate these therapies and thus realize their benefits.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the Akkodis Belgium platform for writing and editorial assistance, manuscript coordination, and design support, on behalf of GSK. Urszula Miecielica, Ph.D., provided medical writing support.
Declarations
Funding
This work and the related publication were sponsored by GSK.
Conflicts of Interest
All authors are employees of GSK. J.R. and H.S. hold financial equities in GSK.
Ethics Approval
Not applicable; no data were analyzed in this opinion paper.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
Code Availability
Not applicable.
Authors’ Contribution
All authors contributed to the concept of this manuscript, and all authors reviewed the manuscript and provided final approval for publication.
References
- 1.Parsonidis P, Papasotiriou I. Adoptive cellular transfer immunotherapies for cancer. Cancer Treat Res Commun. 2022;32: 100575. 10.1016/j.ctarc.2022.100575. [DOI] [PubMed] [Google Scholar]
- 2.Fang KK, Lee JB, Zhang L. Adoptive cell therapy for t-cell malignancies. Cancers (Basel). 2022;15:94. 10.3390/cancers15010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim WA, June CH. The principles of engineering immune cells to treat cancer. Cell. 2017;168:724–40. 10.1016/j.cell.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao L, Cao YJ. Engineered T cell therapy for cancer in the clinic. Front Immunol. 2019;10:2250. 10.3389/fimmu.2019.02250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim GB, Riley JL, Levine BL. Engineering T cells to survive and thrive in the hostile tumor microenvironment. Curr Opin Biomed Eng. 2022;21:100360. 10.1016/j.cobme.2021.100360. [Google Scholar]
- 6.Li D, Li X, Zhou W-L, Huang Y, Liang X, Jiang L, et al. Genetically engineered T cells for cancer immunotherapy. Signal Transduct Target Ther. 2019;4:35. 10.1038/s41392-019-0070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagar G, Gupta A, Masoodi T, Nisar S, Merhi M, Hashem S, et al. Harnessing the potential of CAR-T cell therapy: progress, challenges, and future directions in hematological and solid tumor treatments. J Transl Med. 2023;21:449. 10.1186/s12967-023-04292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maldini CR, Ellis GI, Riley JL. CAR T cells for infection, autoimmunity and allotransplantation. Nat Rev Immunol. 2018;18:605–16. 10.1038/s41577-018-0042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdalla AME, Miao Y, Ahmed AIM, Meng N, Ouyang C. CAR-T cell therapeutic avenue for fighting cardiac fibrosis: Roadblocks and perspectives. Cell Biochem Funct. 2024;42: e3955. 10.1002/cbf.3955. [DOI] [PubMed] [Google Scholar]
- 10.Zhang P, Zhang G, Wan X. Challenges and new technologies in adoptive cell therapy. J Hematol Oncol. 2023;16:97. 10.1186/s13045-023-01492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber EW, Maus MV, Mackall CL. The emerging landscape of immune cell therapies. Cell. 2020;181:46–62. 10.1016/j.cell.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kent A, Crump LS, Davila E. Beyond αβ T cells: NK, iNKT, and γδT cell biology in leukemic patients and potential for off-the-shelf adoptive cell therapies for AML. Front Immunol. 2023;14:1202950. 10.3389/fimmu.2023.1202950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YJ, Abila B, Mostafa KY. CAR-T: what is next? Cancers (Basel). 2023;15:663. 10.3390/cancers15030663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Food and Drug Administration. FDA approves tisagenlecleucel for B-cell ALL and tocilizumab for cytokine release syndrome 2017. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisagenlecleucel-b-cell-all-and-tocilizumab-cytokine-release-syndrome. Accessed: 30 Apr 2024.
- 15.Food and Drug Administration. FDA approves tisagenlecleucel for adults with relapsed or refractory large B-cell lymphoma 2018. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisagenlecleucel-adults-relapsed-or-refractory-large-b-cell-lymphoma. Accessed: 30 Apr 2024.
- 16.Food and Drug Administration. FDA approves tisagenlecleucel for relapsed or refractory follicular lymphoma 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisagenlecleucel-relapsed-or-refractory-follicular-lymphoma#:~:text=On%20May%2027%2C%202022%2C%20the,more%20lines%20of%20systemic%20therapy. Accessed: 30 Apr 2024.
- 17.Food and Drug Administration. FDA approves axicabtagene ciloleucel for second-line treatment of large B-cell lymphoma 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-axicabtagene-ciloleucel-second-line-treatment-large-b-cell-lymphoma#:~:text=On%20April%201%2C%202022%2C%20the,months%20of%20first%2Dline%20chemoimmunotherapy. Accessed: 30 Apr 2024.
- 18.Food and Drug Administration. FDA grants accelerated approval to axicabtagene ciloleucel for relapsed or refractory follicular lymphoma 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-axicabtagene-ciloleucel-relapsed-or-refractory-follicular-lymphoma. Accessed: 30 Apr 2024.
- 19.Food and Drug Administration. FDA approves brexucabtagene autoleucel for relapsed or refractory mantle cell lymphoma 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-brexucabtagene-autoleucel-relapsed-or-refractory-mantle-cell-lymphoma. Accessed: 30 Apr 2024.
- 20.Food and Drug Administration. FDA approves brexucabtagene autoleucel for relapsed or refractory B-cell precursor acute lymphoblastic leukemia 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-brexucabtagene-autoleucel-relapsed-or-refractory-b-cell-precursor-acute-lymphoblastic. Accessed: 30 Apr 2024.
- 21.Food and Drug Administration. FDA approves lisocabtagene maraleucel for relapsed or refractory large B-cell lymphoma 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lisocabtagene-maraleucel-relapsed-or-refractory-large-b-cell-lymphoma. Accessed: 30 Apr 2024.
- 22.Food and Drug Administration. FDA approves idecabtagene vicleucel for multiple myeloma 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-idecabtagene-vicleucel-multiple-myeloma. Accessed: 30 Apr 2024.
- 23.Food and Drug Administration. FDA approves ciltacabtagene autoleucel for relapsed or refractory multiple myeloma 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ciltacabtagene-autoleucel-relapsed-or-refractory-multiple-myeloma. Accessed: 30 Apr 2024.
- 24.Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20:31–42. 10.1016/s1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qu X, An G, Sui W, Wang T, Zhang X, Yang J, et al. Phase 1 study of C-CAR088, a novel humanized anti-BCMA CAR T-cell therapy in relapsed/refractory multiple myeloma. J Immunother Cancer. 2022;10: e005145. 10.1136/jitc-2022-005145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi X, Yan L, Shang J, Kang L, Yan Z, Jin S, et al. Anti-CD19 and anti-BCMA CAR T cell therapy followed by lenalidomide maintenance after autologous stem-cell transplantation for high-risk newly diagnosed multiple myeloma. Am J Hematol. 2022;97:537–47. 10.1002/ajh.26486. [DOI] [PubMed] [Google Scholar]
- 27.Yang Q, Li X, Zhang F, Yang Q, Zhou W, Liu J. Efficacy and safety of CAR-T therapy for relapse or refractory multiple myeloma: a systematic review and meta-analysis. Int J Med Sci. 2021;18:1786–97. 10.7150/ijms.46811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neelapu SS, Dickinson M, Munoz J, Ulrickson ML, Thieblemont C, Oluwole OO, et al. Axicabtagene ciloleucel as first-line therapy in high-risk large B-cell lymphoma: the phase 2 ZUMA-12 trial. Nat Med. 2022;28:735–42. 10.1038/s41591-022-01731-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neelapu SS, Jacobson CA, Ghobadi A, Miklos DB, Lekakis LJ, Oluwole OO, et al. Five-year follow-up of ZUMA-1 supports the curative potential of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood. 2023;141:2307–15. 10.1182/blood.2022018893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-Cell Lymphoblastic Leukemia. New Engl J Med. 2018;378:439–48. 10.1056/nejmoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Otero P, Ailawadhi S, Arnulf B, Patel K, Cavo M, Nooka AK, et al. Ide-cel or standard regimens in relapsed and refractory multiple myeloma. N Engl J Med. 2023;388:1002–14. 10.1056/NEJMoa2213614. [DOI] [PubMed] [Google Scholar]
- 32.Li C, Wang D, Song Y, Huang H, Li J, Chen B, et al. CT103A, a novel fully human BCMA-targeting CAR-T cells, in patients with relapsed/refractory multiple myeloma: updated results of phase 1b/2 study (FUMANBA-1). J Clin Oncol. 2023;41:8025. 10.1200/JCO.2023.41.16_suppl.8025. [Google Scholar]
- 33.Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. Three-year follow-up of KTE-X19 in patients with relapsed/refractory mantle cell lymphoma, including high-risk subgroups, in the ZUMA-2 Study. J Clin Oncol. 2023;41:555–67. 10.1200/jco.21.02370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Tang Y, Huang Z. Efficacy and safety of chimeric antigen receptor (CAR)-T cell therapy in the treatment of relapsed and refractory multiple myeloma: a systematic-review and meta-analysis of clinical trials. Transl Cancer Res. 2022;11:569–79. 10.21037/tcr-22-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cappell KM, Kochenderfer JN. Long-term outcomes following CAR T cell therapy: what we know so far. Nat Rev Clin Oncol. 2023;20:359–71. 10.1038/s41571-023-00754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasquini MC, Hu ZH, Curran K, Laetsch T, Locke F, Rouce R, et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv. 2020;4:5414–24. 10.1182/bloodadvances.2020003092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pasquini MC, Locke FL, Herrera AF, Siddiqi T, Ghobadi A, Komanduri KV, et al. Post-marketing use outcomes of an anti-CD19 chimeric antigen receptor (CAR) T cell therapy, axicabtagene ciloleucel (Axi-Cel), for the treatment of large B cell lymphoma (LBCL) in the United States (US). Blood. 2019;134:764. 10.1182/blood-2019-124750. [Google Scholar]
- 38.Schultz LM, Baggott C, Prabhu S, Pacenta HL, Phillips CL, Rossoff J, et al. Disease burden affects outcomes in pediatric and young adult B-cell lymphoblastic leukemia after commercial tisagenlecleucel: a pediatric real-world chimeric antigen receptor consortium report. J Clin Oncol. 2022;40:945–55. 10.1200/jco.20.03585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bader P, Rossig C, Hutter M, Ayuk FA, Baldus CD, Bücklein VL, et al. CD19 CAR T cells are an effective therapy for posttransplant relapse in patients with B-lineage ALL: real-world data from Germany. Blood Adv. 2023;7:2436–48. 10.1182/bloodadvances.2022008981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barsan V, Li Y, Prabhu S, Baggott C, Nguyen K, Pacenta H, et al. Tisagenlecleucel utilisation and outcomes across refractory, first relapse and multiply relapsed B-cell acute lymphoblastic leukemia: a retrospective analysis of real-world patterns. EClinicalMedicine. 2023;65: 102268. 10.1016/j.eclinm.2023.102268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cappell KM, Sherry RM, Yang JC, Goff SL, Vanasse DA, McIntyre L, et al. Long-term follow-up of anti-CD19 chimeric antigen receptor T-cell therapy. J Clin Oncol. 2020;38:3805–15. 10.1200/jco.20.01467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baulu E, Gardet C, Chuvin N, Depil S. TCR-engineered T cell therapy in solid tumors: state of the art and perspectives. Sci Adv. 2023;9: eadf3700. 10.1126/sciadv.adf3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donnadieu E, Luu M, Alb M, Anliker B, Arcangeli S, Bonini C, et al. Time to evolve: predicting engineered T cell-associated toxicity with next-generation models. J Immunother Cancer. 2022;10: e003486. 10.1136/jitc-2021-003486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Food and Drug Administration. FDA investigating serious risk of T-cell malignancy following BCMA-directed or CD19-directed autologous himeric antigen receptor (CAR) T cell immunotherapies 2023. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/fda-investigating-serious-risk-t-cell-malignancy-following-bcma-directed-or-cd19-directed-autologous. Accessed: 30 Apr 2024.
- 45.Jin Y, Dong Y, Zhang J, Sun J, Liu Y, Chen Y. The toxicity of cell therapy: mechanism, manifestations, and challenges. J Appl Toxicol. 2021;41:659–67. 10.1002/jat.4100. [DOI] [PubMed] [Google Scholar]
- 46.Hiltensperger M, Krackhardt AM. Current and future concepts for the generation and application of genetically engineered CAR-T and TCR-T cells. Front Immunol. 2023;14:1121030. 10.3389/fimmu.2023.1121030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.American Society of Hematology. T-cell malignancies represent small fraction of the reported secondary cancers following CAR-T in the FDA’s Adverse Event Reporting System 2024. https://www.hematology.org/newsroom/press-releases/2023/t-cell-malignancies-represent-small-fraction-of-the-reported-secondary-cancers-following-car-t#:~:text=(WASHINGTON%2C%20March%2014%2C%202024,)%2C%20with%20T%2Dcell%20malignancies. Accessed: 26 Apr 2024.
- 48.Müller A. Rare adverse events of CAR T-cell therapy. Healthbook TIMES Onco Hema. 2024;19:42–9. 10.36000/HBT.OH.2024.19.138. [Google Scholar]
- 49.Cosenza M, Sacchi S, Pozzi S. Cytokine release syndrome associated with T-cell-based therapies for hematological malignancies: pathophysiology, clinical presentation, and treatment. Int J Mol Sci. 2021;22:7652. 10.3390/ijms22147652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larson RC, Maus MV. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat Rev Cancer. 2021;21:145–61. 10.1038/s41568-020-00323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Freyer CW, Porter DL. Cytokine release syndrome and neurotoxicity following CAR T-cell therapy for hematologic malignancies. J Allergy Clin Immunol. 2020;146:940–8. 10.1016/j.jaci.2020.07.025. [DOI] [PubMed] [Google Scholar]
- 52.Chou CK, Turtle CJ. Assessment and management of cytokine release syndrome and neurotoxicity following CD19 CAR-T cell therapy. Expert Opin Biol Ther. 2020;20:653–64. 10.1080/14712598.2020.1729735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murthy H, Iqbal M, Chavez JC, Kharfan-Dabaja MA. Cytokine release syndrome: current perspectives. Immunotargets Ther. 2019;8:43–52. 10.2147/itt.S202015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frey N, Porter D. Cytokine release syndrome with chimeric antigen receptor T cell therapy. Biol Blood Marrow Transplant. 2019;25:e123–7. 10.1016/j.bbmt.2018.12.756. [DOI] [PubMed] [Google Scholar]
- 55.Sheth VS, Gauthier J. Taming the beast: CRS and ICANS after CAR T-cell therapy for ALL. Bone Marrow Transplant. 2021;56:552–66. 10.1038/s41409-020-01134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62. 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Santomasso BD, Park JH, Salloum D, Riviere I, Flynn J, Mead E, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8:958–71. 10.1158/2159-8290.Cd-17-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yáñez L, Sánchez-Escamilla M, Perales MA. CAR T cell toxicity: current management and future directions. Hemasphere. 2019;3: e186. 10.1097/hs9.0000000000000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maus MV, Alexander S, Bishop MR, Brudno JN, Callahan C, Davila ML, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J Immunother Cancer. 2020;8: e001511. 10.1136/jitc-2020-001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hayden PJ, Roddie C, Bader P, Basak GW, Bonig H, Bonini C, et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA). Ann Oncol. 2022;33:259–75. 10.1016/j.annonc.2021.12.003. [DOI] [PubMed] [Google Scholar]
- 61.Si S, Teachey DT. Spotlight on tocilizumab in the treatment of CAR-T-cell-induced cytokine release syndrome: clinical evidence to date. Ther Clin Risk Manag. 2020;16:705–14. 10.2147/tcrm.S223468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scott SA, Marin EM, Maples KT, Joseph NS, Hofmeister CC, Gupta VA, et al. Prophylactic tocilizumab to prevent cytokine release syndrome (CRS) with teclistamab: a single-center experience. Blood Cancer J. 2023;13:191. 10.1038/s41408-023-00963-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caimi PF, Pacheco Sanchez G, Sharma A, Otegbeye F, Ahmed N, Rojas P, et al. Prophylactic tocilizumab prior to anti-CD19 CAR-T cell therapy for non-Hodgkin lymphoma. Front Immunol. 2021;12: 745320. 10.3389/fimmu.2021.745320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bajwa A, Zhao Q, Geer MJ, Mian A, Lin C, Frame D, et al. Efficacy of siltuximab for chimeric antigen receptor T-cell therapy toxicities—a multicenter retrospective analysis. Transplant Cel Ther. 2024;30:S201–2. 10.1016/j.jtct.2023.12.261. [Google Scholar]
- 65.Patel S, Cenin D, Corrigan D, Hamilton BK, Kalaycio M, Sobecks RM, et al. Siltuximab for first-line treatment of cytokine release syndrome: a response to the national shortage of tocilizumab. Blood. 2022;140:5073–4. 10.1182/blood-2022-169809. [Google Scholar]
- 66.Leclercq G, Steinhoff N, Haegel H, De Marco D, Bacac M, Klein C. Novel strategies for the mitigation of cytokine release syndrome induced by T cell engaging therapies with a focus on the use of kinase inhibitors. Oncoimmunology. 2022;11:2083479. 10.1080/2162402x.2022.2083479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Santomasso BD, Nastoupil LJ, Adkins S, Lacchetti C, Schneider BJ, Anadkat M, et al. Management of guideline immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO. J Clin Oncol. 2021;39:3978–92. 10.1200/jco.21.01992. [DOI] [PubMed] [Google Scholar]
- 68.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–95. 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224–5. 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6:664–79. 10.1158/2159-8290.Cd-16-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hay KA, Hanafi LA, Li D, Gust J, Liles WC, Wurfel MM, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130:2295–306. 10.1182/blood-2017-06-793141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17. 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-Cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44. 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24:739–48. 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 75.Wang Z, Han W. Biomarkers of cytokine release syndrome and neurotoxicity related to CAR-T cell therapy. Biomark Res. 2018;6:4. 10.1186/s40364-018-0116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang L, Li J, Yang J, Zhang X, Zhang M, He J, et al. Safety and efficacy of humanized versus murinized CD19 and CD22 CAR T-cell cocktail therapy for refractory/relapsed B-cell lymphoma. Cells. 2022;11:4085. 10.3390/cells11244085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lu W, Wei Y, Cao Y, Xiao X, Li Q, Lyu H, et al. CD19 CAR-T cell treatment conferred sustained remission in B-ALL patients with minimal residual disease. Cancer Immunol Immunother. 2021;70:3501–11. 10.1007/s00262-021-02941-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24:731–8. 10.1038/s41591-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heng G, Jia J, Li S, Fu G, Wang M, Qin D, et al. Sustained therapeutic efficacy of humanized anti-CD19 chimeric antigen receptor T cells in relapsed/refractory acute lymphoblastic leukemia. Clin Cancer Res. 2020;26:1606–15. 10.1158/1078-0432.Ccr-19-1339. [DOI] [PubMed] [Google Scholar]
- 80.Ma F, Ho JY, Du H, Xuan F, Wu X, Wang Q, et al. Evidence of long-lasting anti-CD19 activity of engrafted CD19 chimeric antigen receptor-modified T cells in a phase I study targeting pediatrics with acute lymphoblastic leukemia. Hematol Oncol. 2019;37:601–8. 10.1002/hon.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331–42. 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Turtle CJ, Hanafi LA, Berger C, Hudecek M, Pender B, Robinson E, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8: 355ra116. 10.1126/scitranslmed.aaf8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu J, Chen LJ, Yang SS, Sun Y, Wu W, Liu YF, et al. Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proc Natl Acad Sci U S A. 2019;116:9543–51. 10.1073/pnas.1819745116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ortíz-Maldonado V, Rives S, Castellà M, Alonso-Saladrigues A, Benítez-Ribas D, Caballero-Baños M, et al. CART19-BE-01: a multicenter trial of ARI-0001 cell therapy in patients with CD19(+) relapsed/refractory malignancies. Mol Ther. 2021;29:636–44. 10.1016/j.ymthe.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Song F, Hu Y, Zhang Y, Zhang M, Yang T, Wu W, et al. Safety and efficacy of autologous and allogeneic humanized CD19-targeted CAR-T cell therapy for patients with relapsed/refractory B-ALL. J Immunother Cancer. 2023;11: e005701. 10.1136/jitc-2022-005701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–28. 10.1016/s0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics. 2016;3:16011. 10.1038/mto.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Y, Wang Y, Liu Y, Tong C, Wang C, Guo Y, et al. Long-term activity of tandem CD19/CD20 CAR therapy in refractory/relapsed B-cell lymphoma: a single-arm, phase 1–2 trial. Leukemia. 2022;36:189–96. 10.1038/s41375-021-01345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou X, Tu S, Wang C, Huang R, Deng L, Song C, et al. Phase I trial of fourth-generation anti-CD19 chimeric antigen receptor T cells against relapsed or refractory B cell non-Hodgkin lymphomas. Front Immunol. 2020;11: 564099. 10.3389/fimmu.2020.564099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cohen AD, Garfall AL, Stadtmauer EA, Melenhorst JJ, Lacey SF, Lancaster E, et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J Clin Invest. 2019;129:2210–21. 10.1172/jci126397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu J, Meng F, Cao Y, Zhang Y, Zhu X, Wang N, et al. Sequential CD19/22 CAR T-cell immunotherapy following autologous stem cell transplantation for central nervous system lymphoma. Blood Cancer J. 2021;11:131. 10.1038/s41408-021-00523-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Y, Qin D, Shou AC, Liu Y, Wang Y, Zhou L. Exploring CAR-T cell therapy side effects: mechanisms and management strategies. J Clin Med. 2023;12:6124. 10.3390/jcm12196124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shao M, Yu Q, Teng X, Guo X, Wei G, Xu H, et al. CRS-related coagulopathy in BCMA targeted CAR-T therapy: a retrospective analysis in a phase I/II clinical trial. Bone Marrow Transplant. 2021;56:1642–50. 10.1038/s41409-021-01226-9. [DOI] [PubMed] [Google Scholar]
- 94.Hong F, Shi M, Cao J, Wang Y, Gong Y, Gao H, et al. Predictive role of endothelial cell activation in cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukaemia. J Cell Mol Med. 2021;25:11063–74. 10.1111/jcmm.17029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Staedtke V, Bai RY, Kim K, Darvas M, Davila ML, Riggins GJ, et al. Disruption of a self-amplifying catecholamine loop reduces cytokine release syndrome. Nature. 2018;564:273–7. 10.1038/s41586-018-0774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lickefett B, Chu L, Ortiz-Maldonado V, Warmuth L, Barba P, Doglio M, et al. Lymphodepletion—an essential but undervalued part of the chimeric antigen receptor T-cell therapy cycle. Front Immunol. 2023;14:1303935. 10.3389/fimmu.2023.1303935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yan Z, Zhang H, Cao J, Zhang C, Liu H, Huang H, et al. Characteristics and risk factors of cytokine release syndrome in chimeric antigen receptor T cell treatment. Front Immunol. 2021;12: 611366. 10.3389/fimmu.2021.611366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang J, Hu Y, Yang S, Wei G, Zhao X, Wu W, et al. Role of Fluorodeoxyglucose positron emission tomography/computed tomography in predicting the adverse effects of chimeric antigen receptor T cell therapy in patients with non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2019;25:1092–8. 10.1016/j.bbmt.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 99.Smith DA, Kikano E, Tirumani SH, de Lima M, Caimi P, Ramaiya NH. Imaging-based toxicity and response pattern assessment following CAR T-cell therapy. Radiology. 2022;302:438–45. 10.1148/radiol.2021210760. [DOI] [PubMed] [Google Scholar]
- 100.Pozzessere C, Mazini B, Omoumi P, Jreige M, Noirez L, Digklia A, et al. Immune-related adverse events induced by immune checkpoint inhibitors and CAR-T cell therapy: a comprehensive imaging-based review. Cancers (Basel). 2024;16:2585. 10.3390/cancers16142585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Van De Vyver AJ, Marrer-Berger E, Wang K, Lehr T, Walz AC. Cytokine release syndrome by T-cell-redirecting therapies: can we predict and modulate patient risk? Clin Cancer Res. 2021;27:6083–94. 10.1158/1078-0432.Ccr-21-0470. [DOI] [PubMed] [Google Scholar]
- 102.Velasco R, Mussetti A, Villagrán-García M, Sureda A. CAR T-cell-associated neurotoxicity in central nervous system hematologic disease: is it still a concern? Front Neurol. 2023;14:1144414. 10.3389/fneur.2023.1144414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hunter BD, Jacobson CA. CAR T-cell associated neurotoxicity: mechanisms, clinicopathologic correlates, and future directions. J Natl Cancer Inst. 2019;111:646–54. 10.1093/jnci/djz017. [DOI] [PubMed] [Google Scholar]
- 104.Danish H, Santomasso BD. Neurotoxicity biology and management. Cancer J. 2021;27:126–33. 10.1097/ppo.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 105.Morris EC, Neelapu SS, Giavridis T, Sadelain M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol. 2022;22:85–96. 10.1038/s41577-021-00547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xiao X, Huang S, Chen S, Wang Y, Sun Q, Xu X, et al. Mechanisms of cytokine release syndrome and neurotoxicity of CAR T-cell therapy and associated prevention and management strategies. J Exp Clin Cancer Res. 2021;40:367. 10.1186/s13046-021-02148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gu T, Hu K, Si X, Hu Y, Huang H. Mechanisms of immune effector cell-associated neurotoxicity syndrome after CAR-T treatment. WIREs Mech Dis. 2022;14: e1576. 10.1002/wsbm.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gust J, Ponce R, Liles WC, Garden GA, Turtle CJ. Cytokines in CAR T cell-associated neurotoxicity. Front Immunol. 2020;11: 577027. 10.3389/fimmu.2020.577027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thompson JA, Schneider BJ, Brahmer J, Achufusi A, Armand P, Berkenstock MK, et al. Management of immunotherapy-related toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:387–405. 10.6004/jnccn.2022.0020. [DOI] [PubMed] [Google Scholar]
- 110.Genoud V, Migliorini D. Novel pathophysiological insights into CAR-T cell associated neurotoxicity. Front Neurol. 2023;14:1108297. 10.3389/fneur.2023.1108297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jain MD, Smith M, Shah NN. How I treat refractory CRS and ICANS after CAR T-cell therapy. Blood. 2023;141:2430–42. 10.1182/blood.2022017414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Park JH, Nath K, Devlin SM, Sauter CS, Palomba ML, Shah G, et al. CD19 CAR T-cell therapy and prophylactic anakinra in relapsed or refractory lymphoma: phase 2 trial interim results. Nat Med. 2023;29:1710–7. 10.1038/s41591-023-02404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stolz S, Roncador M, Rösler W, Zenz T, Manz MG, Müller AMS, et al. Introducing innovative cellular therapies into the clinic: a 2-year retrospective experience of a chimeric antigen receptor T-cell programme at a single centre in Switzerland. Swiss Med Wkly. 2022;152: w30186. 10.4414/smw.2022.w30186. [DOI] [PubMed] [Google Scholar]
- 114.Grant SJ, Grimshaw AA, Silberstein J, Murdaugh D, Wildes TM, Rosko AE, et al. Clinical presentation, risk factors, and outcomes of immune effector cell-associated neurotoxicity syndrome following chimeric antigen receptor T cell therapy: a systematic review. Transplant Cell Ther. 2022;28:294–302. 10.1016/j.jtct.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shalabi H, Martin S, Yates B, Wolters PL, Kaplan C, Smith H, et al. Neurotoxicity following CD19/CD28ζ CAR T-cells in children and young adults with B-cell malignancies. Neuro Oncol. 2022;24:1584–97. 10.1093/neuonc/noac034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126:2123–38. 10.1172/jci85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jacobson CA, Hunter BD, Redd R, Rodig SJ, Chen PH, Wright K, et al. Axicabtagene ciloleucel in the non-trial setting: outcomes and correlates of response, resistance, and toxicity. J Clin Oncol. 2020;38:3095–106. 10.1200/jco.19.02103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mackall CL, Miklos DB. CNS endothelial cell activation emerges as a driver of CAR T cell-associated neurotoxicity. Cancer Discov. 2017;7:1371–3. 10.1158/2159-8290.Cd-17-1084. [DOI] [PubMed] [Google Scholar]
- 119.Siegler EL, Kenderian SS. Neurotoxicity and cytokine release syndrome after chimeric antigen receptor t cell therapy: insights into mechanisms and novel therapies. Front Immunol. 2020;11:1973. 10.3389/fimmu.2020.01973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7:1404–19. 10.1158/2159-8290.Cd-17-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rubin DB, Al Jarrah A, Li K, LaRose S, Monk AD, Ali AB, et al. Clinical predictors of neurotoxicity after chimeric antigen receptor T-cell therapy. JAMA Neurol. 2020;77:1536–42. 10.1001/jamaneurol.2020.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Karschnia P, Jordan JT, Forst DA, Arrillaga-Romany IC, Batchelor TT, Baehring JM, et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood. 2019;133:2212–21. 10.1182/blood-2018-12-893396. [DOI] [PubMed] [Google Scholar]
- 123.Fontanelli L, Pizzanelli C, Milano C, Cassano Cassano R, Galimberti S, Rossini MI, et al. Pre-existing frontal lobe dysfunction signs as predictors of subsequent neurotoxicity in CAR T cell therapy: insights from a case series. Neurol Sci. 2023;44:3291–7. 10.1007/s10072-023-06841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pensato U, Amore G, Muccioli L, Sammali S, Rondelli F, Rinaldi R, et al. CAR t-cell therapy in Bologna-Neurotoxicity Treatment and Assessment in Lymphoma (CARBON-NEUTRAL): proposed protocol and results from an Italian study. J Neurol. 2023;270:2659–73. 10.1007/s00415-023-11595-4. [DOI] [PubMed] [Google Scholar]
- 125.Butt OH, Zhou AY, Ances BM, DiPersio JF, Ghobadi A. A systematic framework for predictive biomarkers in immune effector cell-associated neurotoxicity syndrome. Front Neurol. 2023;14:1110647. 10.3389/fneur.2023.1110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nie E, Su Y-J, Baird J, Agarwal N, Bharadwaj S, Weng W-K, et al. Clinical and neuroradiological features of immune effector cell-associated neurotoxicity syndrome (ICANS) following CD19 CAR T-cell therapy in mantle cell lymphoma (MCL) (S23.007). Neurology. 2024;102:6545. 10.1212/WNL.0000000000206540. [Google Scholar]
- 127.Greenbaum U, Strati P, Saliba RM, Torres J, Rondon G, Nieto Y, et al. CRP and ferritin in addition to the EASIX score predict CAR-T-related toxicity. Blood Adv. 2021;5:2799–806. 10.1182/bloodadvances.2021004575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pennisi M, Sanchez-Escamilla M, Flynn JR, Shouval R, Alarcon Tomas A, Silverberg ML, et al. Modified EASIX predicts severe cytokine release syndrome and neurotoxicity after chimeric antigen receptor T cells. Blood Adv. 2021;5:3397–406. 10.1182/bloodadvances.2020003885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.de Boer JW, Keijzer K, Pennings ERA, van Doesum JA, Spanjaart AM, Jak M, et al. Population-based external validation of the EASIX scores to predict CAR T-cell-related toxicities. Cancers (Basel). 2023;15:5443. 10.3390/cancers15225443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Amidi Y, Eckhardt CA, Quadri SA, Malik P, Firme MS, Jones DK, et al. Forecasting immune effector cell-associated neurotoxicity syndrome after chimeric antigen receptor T-cell therapy. J Immunother Cancer. 2022;10: e005459. 10.1136/jitc-2022-005459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huang JJ, Liang EC, Albittar A, Portuguese AJ, Wuliji N, Torkelson A, et al. Early prediction of severe ICANS after CD19 CAR T-cell therapy based on serum ferritin levels. Transplant Cell Ther. 2024;30:s193–4. 10.1016/j.jtct.2023.12.251. [Google Scholar]
- 132.Taneja A, Jain T. CAR-T-OPENIA: chimeric antigen receptor T-cell therapy-associated cytopenias. EJHaem. 2022;3:32–8. 10.1002/jha2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jain T, Olson TS, Locke FL. How I treat cytopenias after CAR T-cell therapy. Blood. 2023;141:2460–9. 10.1182/blood.2022017415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Logue JM, Peres LC, Hashmi H, Colin-Leitzinger CM, Shrewsbury AM, Hosoya H, et al. Early cytopenias and infections after standard of care idecabtagene vicleucel in relapsed or refractory multiple myeloma. Blood Adv. 2022;6:6109–19. 10.1182/bloodadvances.2022008320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sharma N, Reagan PM, Liesveld JL. Cytopenia after CAR-t cell therapy—a brief review of a complex problem. Cancers (Basel). 2022;14:1501. 10.3390/cancers14061501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schuster SJ, Tam CS, Borchmann P, Worel N, McGuirk JP, Holte H, et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22:1403–15. 10.1016/s1470-2045(21)00375-2. [DOI] [PubMed] [Google Scholar]
- 137.Jain T, Knezevic A, Pennisi M, Chen Y, Ruiz JD, Purdon TJ, et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies. Blood Adv. 2020;4:3776–87. 10.1182/bloodadvances.2020002509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xia Y, Zhang J, Li J, Zhang L, Li J, Fan L, et al. Cytopenias following anti-CD19 chimeric antigen receptor (CAR) T cell therapy: a systematic analysis for contributing factors. Ann Med. 2022;54:2951–65. 10.1080/07853890.2022.2136748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stewart AG, Henden AS. Infectious complications of CAR T-cell therapy: a clinical update. Ther Adv Infect Dis. 2021;8:20499361211036772. 10.1177/20499361211036773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chakraborty R, Hill BT, Majeed A, Majhail NS. Late Effects after chimeric antigen receptor T cell therapy for lymphoid malignancies. Transplant Cell Ther. 2021;27:222–9. 10.1016/j.jtct.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhou J, Zhang Y, Shan M, Zong X, Geng H, Li J, et al. Cytopenia after chimeric antigen receptor T cell immunotherapy in relapsed or refractory lymphoma. Front Immunol. 2022;13: 997589. 10.3389/fimmu.2022.997589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Penack O, Peczynski C, Koenecke C, Polge E, Kuhnl A, Fegueux N, et al. Severe cytopenia after CD19 CAR T-cell therapy: a retrospective study from the EBMT Transplant Complications Working Party. J Immunother Cancer. 2023;11: e006406. 10.1136/jitc-2022-006406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fried S, Avigdor A, Bielorai B, Meir A, Besser MJ, Schachter J, et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transplant. 2019;54:1643–50. 10.1038/s41409-019-0487-3. [DOI] [PubMed] [Google Scholar]
- 144.Wang L, Hong R, Zhou L, Ni F, Zhang M, Zhao H, et al. New-onset severe cytopenia after CAR-T cell therapy: analysis of 76 patients with relapsed or refractory acute lymphoblastic leukemia. Front Oncol. 2021;11: 702644. 10.3389/fonc.2021.702644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Qiu T, Hu L, Zhang Y, Wang Y, Ma S, Li D, et al. Cytopenia after CAR-T cell therapy: analysis of 63 patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Oncol Lett. 2023;26:338. 10.3892/ol.2023.13924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rejeski K, Perez A, Sesques P, Hoster E, Berger C, Jentzsch L, et al. CAR-HEMATOTOX: a model for CAR T-cell-related hematologic toxicity in relapsed/refractory large B-cell lymphoma. Blood. 2021;138:2499–513. 10.1182/blood.2020010543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rejeski K, Hansen DK, Bansal R, Sesques P, Ailawadhi S, Logue JM, et al. The CAR-HEMATOTOX score as a prognostic model of toxicity and response in patients receiving BCMA-directed CAR-T for relapsed/refractory multiple myeloma. J Hematol Oncol. 2023;16:88. 10.1186/s13045-023-01465-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wittmann Dayagi T, Sherman G, Bielorai B, Adam E, Besser MJ, Shimoni A, et al. Characteristics and risk factors of infections following CD28-based CD19 CAR-T cells. Leuk Lymphoma. 2021;62:1692–701. 10.1080/10428194.2021.1881506. [DOI] [PubMed] [Google Scholar]
- 149.Wudhikarn K, Palomba ML, Pennisi M, Garcia-Recio M, Flynn JR, Devlin SM, et al. Infection during the first year in patients treated with CD19 CAR T cells for diffuse large B cell lymphoma. Blood Cancer J. 2020;10:79. 10.1038/s41408-020-00346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Park JH, Romero FA, Taur Y, Sadelain M, Brentjens RJ, Hohl TM, et al. Cytokine release syndrome grade as a predictive marker for infections in patients with relapsed or refractory B-Cell acute lymphoblastic leukemia treated with chimeric antigen receptor T cells. Clin Infect Dis. 2018;67:533–40. 10.1093/cid/ciy152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mercadal S, Gomez CA, Lee CJ, Couriel DR. Infectious complications following CAR-t cell therapy for B cell non-Hodgkin lymphoma: a single-center experience and review of the literature. Ann Hematol. 2023;102:1837–43. 10.1007/s00277-023-05131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cordeiro A, Bezerra ED, Hirayama AV, Hill JA, Wu QV, Voutsinas J, et al. Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol Blood Marrow Transplant. 2020;26:26–33. 10.1016/j.bbmt.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Baird JH, Epstein DJ, Tamaresis JS, Ehlinger Z, Spiegel JY, Craig J, et al. Immune reconstitution and infectious complications following axicabtagene ciloleucel therapy for large B-cell lymphoma. Blood Adv. 2021;5:143–55. 10.1182/bloodadvances.2020002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hill JA, Seo SK. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood. 2020;136:925–35. 10.1182/blood.2019004000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.McGann M, Velayati A, Roubal K, Granger K, Davis JA, Gaffney KJ, et al. Early cytopenias and infections following chimeric antigen receptor T-Cell therapy for hematologic malignancies. Leuk Lymphoma. 2023;64:1592–5. 10.1080/10428194.2023.2220455. [DOI] [PubMed] [Google Scholar]
- 156.Wudhikarn K, Perales MA. Infectious complications, immune reconstitution, and infection prophylaxis after CD19 chimeric antigen receptor T-cell therapy. Bone Marrow Transplant. 2022;57:1477–88. 10.1038/s41409-022-01756-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Los-Arcos I, Iacoboni G, Aguilar-Guisado M, Alsina-Manrique L, Díaz de Heredia C, Fortuny-Guasch C, et al. Recommendations for screening, monitoring, prevention, and prophylaxis of infections in adult and pediatric patients receiving CAR T-cell therapy: a position paper. Infection. 2021;49:215–31. 10.1007/s15010-020-01521-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Haroon A, Muhsen IN, Abid MB, Albabtain A, Alahmari A, Ahmed SO, et al. Infectious complications and preventative strategies following chimeric antigen receptor T-cells (CAR-T cells) therapy for B-cell malignancies. Hematol Oncol Stem Cell Ther. 2022;15:153–8. 10.56875/2589-0646.1049. [DOI] [PubMed] [Google Scholar]
- 159.Liévin R, Di Blasi R, Morin F, Galli E, Allain V, De Jorna R, et al. Effect of early granulocyte-colony-stimulating factor administration in the prevention of febrile neutropenia and impact on toxicity and efficacy of anti-CD19 CAR-T in patients with relapsed/refractory B-cell lymphoma. Bone Marrow Transplant. 2022;57:431–9. 10.1038/s41409-021-01526-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Meir J, Abid MA, Abid MB. State of the CAR-T: risk of infections with chimeric antigen receptor T-cell therapy and determinants of SARS-CoV-2 vaccine responses. Transplant Cell Ther. 2021;27:973–87. 10.1016/j.jtct.2021.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Perica K, Flynn J, Curran KJ, Rivere I, Wang X, Senechal B, et al. Impact of bridging chemotherapy on clinical outcome of CD19 CAR T therapy in adult acute lymphoblastic leukemia. Leukemia. 2021;35:3268–71. 10.1038/s41375-021-01196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kampouri E, Little JS, Rejeski K, Manuel O, Hammond SP, Hill JA. Infections after chimeric antigen receptor (CAR)-T-cell therapy for hematologic malignancies. Transpl Infect Dis. 2023;25(Suppl 1): e14157. 10.1111/tid.14157. [DOI] [PubMed] [Google Scholar]
- 163.Powell MZ, Mara KC, Bansal R, Hathcock MA, Khurana A, Bennani NN, et al. Procalcitonin as a biomarker for predicting bacterial infection in chimeric antigen receptor T-cell therapy recipients. Cancer Med. 2023;12:9228–35. 10.1002/cam4.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rejeski K, Blumenberg V, Iacoboni G, Lopez-Corral L, Kharboutli S, Hernani R, et al. Identifying early infections in the setting of CRS with routine and exploratory serum proteomics and the HT10 score following CD19 CAR-T for relapsed/refractory B-NHL. Hemasphere. 2023;7: e858. 10.1097/hs9.0000000000000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Luo H, Wang N, Huang L, Zhou X, Jin J, Li C, et al. Inflammatory signatures for quick diagnosis of life-threatening infection during the CAR T-cell therapy. J Immunother Cancer. 2019;7:271. 10.1186/s40425-019-0767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Diorio C, Shaw PA, Pequignot E, Orlenko A, Chen F, Aplenc R, et al. Diagnostic biomarkers to differentiate sepsis from cytokine release syndrome in critically ill children. Blood Adv. 2020;4:5174–83. 10.1182/bloodadvances.2020002592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Verdun N, Marks P. Secondary cancers after chimeric antigen receptor T-cell therapy. N Engl J Med. 2024;390:584–6. 10.1056/NEJMp2400209. [DOI] [PubMed] [Google Scholar]
- 168.Ghilardi G, Fraietta JA, Gerson JN, Van Deerlin VM, Morrissette JJD, Caponetti GC, et al. T cell lymphoma and secondary primary malignancy risk after commercial CAR T cell therapy. Nat Med. 2024;30:984–9. 10.1038/s41591-024-02826-w. [DOI] [PubMed] [Google Scholar]
- 169.Suran M. FDA adds boxed warning to CAR T-cell therapies, but says benefits outweigh risks of secondary cancers. JAMA. 2024;331:818–20. 10.1001/jama.2024.1011. [DOI] [PubMed] [Google Scholar]
- 170.Levine BL, Pasquini MC, Connolly JE, Porter DL, Gustafson MP, Boelens JJ, et al. Unanswered questions following reports of secondary malignancies after CAR-T cell therapy. Nat Med. 2024;30:338–41. 10.1038/s41591-023-02767-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sun S, Hao H, Yang G, Zhang Y, Fu Y. Immunotherapy with CAR-modified T cells: toxicities and overcoming strategies. J Immunol Res. 2018;2018:2386187. 10.1155/2018/2386187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lamers CHJ, Sleijfer S, van Steenbergen S, van Elzakker P, van Krimpen B, Groot C, et al. Treatment of Metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21:904–12. 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–51. 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu Y, Guo Y, Wu Z, Feng K, Tong C, Wang Y, et al. Anti-EGFR chimeric antigen receptor-modified T cells in metastatic pancreatic carcinoma: a phase I clinical trial. Cytotherapy. 2020;22:573–80. 10.1016/j.jcyt.2020.04.088. [DOI] [PubMed] [Google Scholar]
- 175.Thistlethwaite FC, Gilham DE, Guest RD, Rothwell DG, Pillai M, Burt DJ, et al. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol Immunother. 2017;66:1425–36. 10.1007/s00262-017-2034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Feng KC, Guo YL, Liu Y, Dai HR, Wang Y, Lv HY, et al. Cocktail treatment with EGFR-specific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma. J Hematol Oncol. 2017;10:4. 10.1186/s13045-016-0378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Guo Y, Feng K, Liu Y, Wu Z, Dai H, Yang Q, et al. Phase I study of chimeric antigen receptor-modified T cells in patients with EGFR-positive advanced biliary tract cancers. Clin Cancer Res. 2018;24:1277–86. 10.1158/1078-0432.CCR-17-0432. [DOI] [PubMed] [Google Scholar]
- 178.Parker KR, Migliorini D, Perkey E, Yost KE, Bhaduri A, Bagga P, et al. Single-cell analyses identify brain mural cells expressing CD19 as Potential off-tumor targets for CAR-T immunotherapies. Cell. 2020;183:126-42.e17. 10.1016/j.cell.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Van Oekelen O, Aleman A, Upadhyaya B, Schnakenberg S, Madduri D, Gavane S, et al. Neurocognitive and hypokinetic movement disorder with features of parkinsonism after BCMA-targeting CAR-T cell therapy. Nat Med. 2021;27:2099–103. 10.1038/s41591-021-01564-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Flugel CL, Majzner RG, Krenciute G, Dotti G, Riddell SR, Wagner DL, et al. Overcoming on-target, off-tumour toxicity of CAR T cell therapy for solid tumours. Nat Rev Clin Oncol. 2023;20:49–62. 10.1038/s41571-022-00704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shimabukuro-Vornhagen A, Böll B, Schellongowski P, Valade S, Metaxa V, Azoulay E, et al. Critical care management of chimeric antigen receptor T-cell therapy recipients. CA Cancer J Clin. 2022;72:78–93. 10.3322/caac.21702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.