Abstract
Although endothelial cell (EC) dysfunction contributes to the etiology of more diseases than any other tissue in the body, EC metabolism is an understudied therapeutic target. Evidence regarding the important role of autophagy in maintaining EC homeostasis is accumulating rapidly. Here we focus on advances over the past two years regarding how EC autophagy mediates EC nitric oxide generation in the context of aging and cardiovascular complications including coronary artery disease, aneurysm, and stroke. In addition, insight concerning the efficacy of maneuvers designed to boost EC autophagy in an effort to combat cardiovascular complications associated with repressed EC autophagy is discussed.
Keywords: Aging, coronary artery disease, aneurysm, stroke, atherosclerosis
Introduction
Autophagy is a highly conserved trafficking system whereby intracellular cargo is delivered to lysosomes for degradation by acid hydrolases, producing substrates that can be recycled for use in new biosynthetic reactions, or diverted to metabolic pathways that generate ATP [1, 2]. Defective autophagy leads to the accumulation of misfolded proteins or damaged organelles, thereby driving pathologies such as neurodegeneration. Challenging the existing paradigms about how autophagy controls cellular homeostasis, recent advances indicate endothelial cell (EC) autophagy influences EC synthesis and release of vasoactive molecules which contributes to vascular smooth muscle cell tone. Of the factors that cause smooth muscle cells to relax, myriad studies indicate decreased synthesis of nitric oxide (NO) by EC NO synthase (eNOS) and / or increased destruction of NO contributes importantly to vascular dysfunction associated with aging and cardiovascular diseases, but precise molecular mechanisms are unclear. Here we highlight new insight into how EC autophagy impacts EC NO generation in the context of aging and cardiovascular disease (Figure 1).
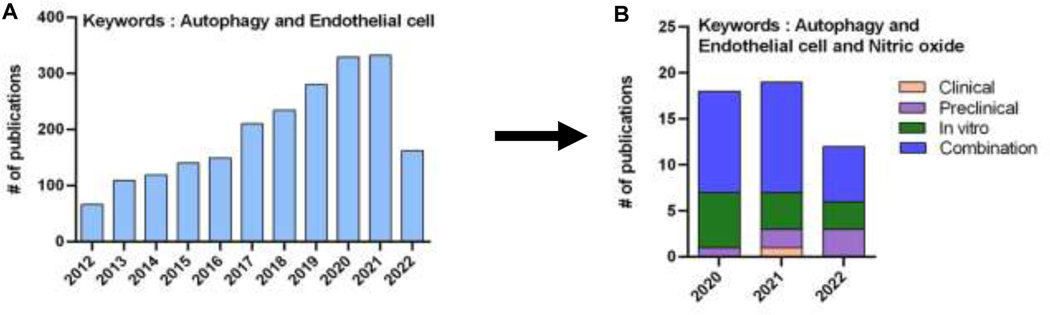
Figure 1. Recent research.
The number of publications identified by keywords “autophagy and endothelial cell” has grown over the past decade (A). This review focused upon publications selected from 41 that were identified by keywords “autophagy and endothelial cell and nitric oxide” from 2020, 2021, and between January and July 2022 (B).
Autophagy and EC NO generation and aging
LaRocca et al. first reported that steady-state autophagy is compromised at baseline in primary arterial ECs from older vs. adult male subjects that subsequently displayed impaired brachial artery vasodilation upon acetylcholine infusion [3]. Because additional risk factors associated with aging have potential to impair eNOS activity, Bharath et al. discerned whether a lack of EC autophagy per se was causal. Indeed, activating phosphorylation of eNOS at Ser1177 (p-eNOSS1177) and NO generation were prevented, whereas reactive oxygen species (ROS) production and inflammatory responses were amplified, in bovine aortic endothelial cells (BAECs) exposed to shear stress after siRNA-mediated knockdown of Atg3, a protein necessary for formation of autophagosomes [4]. These findings were confirmed by others [5, 6], and evidence supporting a molecular mechanism was revealed [7]. Impaired EC autophagy suppresses glucose transporter 1 expression and EC glycolysis, resulting in deficient ATP synthesis and release, and defective purinergic 2Y1-receptor (P2Y1-R) mediated signaling to eNOS via protein kinase C δ.
Autophagy is a dynamic process and it is instructive to differentiate between trafficking of the autophagosome to the lysosome e.g., autophagic flux, and lysosomal degradation of the autophagosome e.g., lysosomal function. Bafilomycin A1 (BAF A1) is a V-ATPase inhibitor that blocks the terminal phases of autophagy by impairing autophagosome-lysosome fusion, thereby preventing degradation of autophagolysosome contents. Increased accumulation of LC3-II and p62 in the presence vs. the absence of BAF A1 indicates increased autophagosome formation, and is an estimate of autophagic flux.
Cho et al., determined the impact of aging on autophagic flux [8*]. Compared to the respective vehicle treatment, shear-stress increased LC3-II and p62 to a greater extent in ECs isolated from adult but not older mice after treatment with BAF A1. This strongly suggests that aging represses EC autophagic flux. In addition to these findings, and congruent with an earlier study wherein autophagy was genetically repressed, shear-stress increased glycolysis, ATP production, p-eNOSS1177, and NO generation in ECs from adult but not older mice [8*].
Translating findings from ECs exposed to shear-stress in vitro, to ECs exposed to active hyperemia, the same authors demonstrated that autophagy and p-eNOSS1177 activation were robust in arterial ECs from adult but not older mice after 60-min treadmill-running [8*]. Providing translational relevance from mice to humans, Cho et al. substantiated that steady state autophagy is compromised in primary arterial ECs from older vs. adult male volunteers that exhibit impaired peripheral arterial function [8*, 9]. Importantly, even though active hyperemia evoked by rhythmic handgrip exercise elevated radial artery shear rate similarly from baseline between groups, autophagy initiation, p-eNOSS1177 activation, and NO generation occurred in radial artery ECs obtained from adult but not older subjects.
The in vitro and in vivo findings from ECs isolated from older mice, coupled with results from primary ECs obtained from older humans, suggest an inability to upregulate EC autophagy might contribute to the age-associated repression of peripheral arterial function. Accordingly, compromised intraluminal flow-mediated vasodilation displayed by femoral arteries from older mice was recapitulated in vessels from adult animals by : (i) NO synthase inhibition; (ii) acute autophagy impairment using 3-methyladenine (3-MA); (iii) inducible disruption of Atg3 specifically in ECs; (iv) P2Y1-R blockade; and (v) germline depletion of P2Y1-Rs. Importantly, P2Y1-R activation using 2-methylthio-ADP improved vasodilatory capacity in arteries from : (i) adult mice treated with 3-MA; (ii) adult Atg3EC−/− mice; and (iii) older animals with repressed EC autophagy [8*]. Collectively, NO-mediated arterial dysfunction evoked by EC autophagy compromise is improved by activating P2Y1-Rs. G protein-coupled P2Y receptors might represent a target to manipulate endothelial dysfunction that is secondary to or associated with defective EC autophagy in the context of aging. [10]
Autophagy and EC NO generation and coronary artery disease
The Beyer / Gutterman group established that shear-induced EC release of NO and endothelium-derived hyperpolarizing factors (EDHFs) stimulate flow-mediated vasodilation of adipose arterioles from healthy adult volunteers. In older individuals [11] and coronary artery disease (CAD) patients [12], however, a shift toward mitochondrial generated hydrogen peroxide (H2O2) governs this response. Based on findings that : (i) EC autophagy promotes EC NO formation and limits ROS generation; [4–8*, 13] (ii) telomerase reverse transcriptase (TERT; the catalytic subunit of telomerase) inhibits mitochondrial-derived ROS and induces a switch in the mechanism of dilation from H2O2 to NO in adipose arterioles from CAD patients [14–16] and (iii) overexpression of TERT in mouse embryonic fibroblasts increases LC3-I to LC3-II conversion, whereas inhibiting TERT reduces LC3-I to LC3-II [17], Hughes et al. tested the hypothesis that telomerase activity influences autophagy to an extent that impacts NO-mediated, flow-induced dilation in the microvasculature [18**].
As predicted, human adipose arterioles displayed increased autophagic flux and NO-mediated vasodilation that switched to H2O2-mediated vasodilation upon lysosomal inhibition or Atg3 siRNA. Further, arterioles obtained from CAD patients were refractory to shear-induced autophagy, but vasodilatory mechanisms could be reverted from mitochondrial generated H2O2 to EC mediated NO in response to autophagy activation using the histone deacetylase inhibitor trichostatin-A (TSA) or the disaccharide trehalose. Implicating autophagy as “downstream” from telomerase, neither gain nor loss of autophagy impacted TERT activity in either group. Underscoring the importance of telomerase, TERT inhibition of non-CAD arterioles limited shear-induced autophagic flux that could be rejuvenated by concurrent treatment with TSA. Congruent with these findings, TERT activation of CAD arterioles restored autophagic flux that could be subsequently abrogated by autophagy inhibition using BAF A1. In support of the original hypothesis, autophagy activation maintained NO as the primary mechanism of vasodilation in non-CAD arterioles despite TERT inhibition, whereas increasing TERT expression in the presence of autophagy inhibition maintained H2O2 as the vasodilatory mechanism of CAD arterioles. The authors concluded that autophagy acts downstream of telomerase to determine NO-mediated, flow-induced dilation in human adipose arterioles [18**].
The ability for aspirin to reduce cardiovascular risk and attenuate EC dysfunction is well-known. Using human coronary arteries ECs (HCAECs), Chen et al. showed that aspirin dose-dependently : (i) increased steady-state autophagy (e.g., ↑ LC3-II / LC3-I, ↓ p-62) and autophagic flux (tandem mRFP-GFP-tagged LC3); and (ii) heightened p-eNOSS1177 and NO generation, to an extent that reduced protein expression of p-p38, p-NF-kB, sICAM-1, and sVCAM-1, which are otherwise elevated by treatment with oxidized low-density lipoprotein, angiotensin-II, or high glucose. Remarkably, the ability of aspirin to attenuate cell injury in response to each intervention was lost in HCAECs transfected with beclin-1 vs. scrambled siRNA. Determining the translational relevance of these interesting findings using a relevant preclinical model is warranted [19].
Autophagy and EC NO generation and aneurysm
Impaired NO bioavailability and heightened oxidative stress directly contribute to vascular smooth muscle cell growth, proliferation, contraction, and differentiation, which are major determinants of aneurysm development [20]. Implicating a contribution from defective autophagy, Atg5 deficiency increases aortic dissection [21] whereas Atg7 depletion promotes aortic aneurysm induced by high cholesterol [22]. Iraci et al. determined the translational relevance of these in vitro findings by comparing aortic tissue and blood samples from patients undergoing surgery to repair thoracic aortic aneurysm (TAA) to patients referred for aortic valve replacement or repair (control) [23*]. Protein expression of LC3-II, Atg5, and Atg7 was repressed, whereas p62 was elevated, in aortic lysates from TAA vs. controls, but the source of the defect i.e., EC or vascular smooth muscle, was not discerned. Because mRNA abundance for these markers was similar between groups, the authors concluded that post-translational modifications contributed to lowering vascular autophagy in TAA patients but mechanisms were not evaluated. Of note, aortic lysates from aneurysm patients with repressed vascular autophagy exhibited lower circulating and tissue nitrite (estimates of NO bioavailability), coupled with NADPH oxidase 2 activation and elevated H2O2, (estimates of oxidative stress). The authors acknowledge the observational nature of their study and relatively low sample size, but nevertheless this report provides translational evidence that contexts involving repressed vascular autophagy couple with reduced NO bioavailability and amplified oxidative stress in the setting of aneurysm.
Autophagy and EC NO generation and stroke
Acute ischemic stroke (AIS) limits delivery of oxygen and glucose to cerebral artery ECs. Upon detecting a nutrient-stress, the activity of mammalian target of rapamycin (mTOR) complex 1 (mTORC1) decreases, stimulating EC autophagy to support basal metabolism [24, 25]. In addition to canonical roles for EC autophagy in this context [26], evidence exists that EC autophagy enables NO-mediated cerebral arterial vasodilation. In this regard, low-dose mTORC1 inhibition using rapamycin might increase collateral perfusion during and reperfusion following AIS, to an extent that reduces infarct volume and improves neurological outcomes in normotensive [27**, 28] and hypertensive rats [27**]. Underscoring the importance of ECs, rapamycin-induced dilation of leptomeningeal anastomoses (i.e., collaterals) from normotensive and hypertensive rats was abolished by NO synthase inhibition ex vivo [27**]. The authors speculated that benefits of rapamycin on cerebral perfusion might be secondary to induction of autophagy and activation of p-eNOSS1177 on penetrating arterioles [27**].
mTORC1 inhibition might improve stroke outcome via alternative NO-mediated mechanisms. For example, mTORC1 diminution reduces inflammation secondary to cyclooxygenase repression in the context of oxygen-glucose deprivation or AIS, thus shifting the balance toward epoxyeicosatrienoic acid (EET) production. EET generation might be protective in two ways. First, 14,15-EET activates sirtuin (SIRT) 1-mediated mitophagy in brain microvascular ECs [29]. Second, 14,15-EET induces NO generation by human microvascular ECs [30]. Either mechanism has potential to improve stroke outcome and warrants further pursuit.
The Sciarretta laboratory demonstrated that directly re-activating EC autophagy via pharmacological [31**] or nutraceutical [32–34*] maneuvers is protective in the context of hemorrhagic stroke [31**]. ECs isolated from brains of stroke-prone hypertensive rats (SP-SHRs) prior to stroke-onset exhibit depressed autophagy and greater cell death, that associates with downregulation of the mitochondrial complex 1 subunit NDUFC2, mitochondrial dysfunction, and NAD+ depletion upon exposure to salt. Remarkably, treating primary ECs from SP-SHRs in vitro with the peptide autophagy agonist Tat-D11 [35] improved their viability, and administering Tat-D11 to SP-SHRs in vivo reduced stroke occurrence [31**]. Of interest, this study demonstrated translational relevance. Endothelial progenitor cells derived from individuals harboring a variant of NDUFC2 that associates with increased incidence of juvenile ischemic stroke [31**] exhibit depressed steady-state autophagy and ineffective autophagic responses to salt exposure. Underscoring the importance of autophagy, Tat-D11 treatment reduced indexes of cell senescence in mutant progenitors exposed to salt.
Mitochondrial complex I maintains the NAD+ / NADH ratio, and since NAD+ supplementation stimulates autophagy [36], Forte et al. sought to determine whether nicotinamide mononucleotide (NMN) treatment could be restorative in vascular smooth muscle cells (i.e., A10 cells). Indeed, NMN treatment improves NAD+ levels that associate with heightened autophagy and mitophagy, and this intervention reduced stroke occurrence in SP-SHRs. Although not studied directly, NMN-induced upregulation of EC autophagy and EC NO generation could have contributed partly to the observed benefits. NMN treatment increases NAD+ levels in ECs [37], optimizes NAD+ / NADH in aortic tissue [38], improves NO-dependent vasodilation in carotid arteries from older mice [39], possibly by sirtuin (e.g., SIRT1) -mediated upregulation of eNOS [40], and direct administration of nicotinamide to human umbilical vein endothelial cells (HUVECs) treated concurrently with H2O2 improves NO bioavailability and cell viability [34*]. In the latter study, trehalose exerted benefits similar to nicotinamide in HUVECs exposed to oxidative stress. The same group showed increased acetylcholine-evoked mesenteric artery vasorelaxation in SP-SHRs that display reduced stroke occurrence [32], providing proof of concept that this approach might improve stroke outcome by preserving cerebral arterial function and subsequent perfusion. Taken together, amplifying EC autophagy has potential to benefit stroke outcomes in the context of AIS and / or hypertensive stroke by mediating EC NO generation.
Directions for future study
First, our review is “NO-centric” by design and we acknowledge that additional work is justified concerning the impact of EC autophagy on other vasoactive molecules. Underscoring this point, McCarthy et al. tested whether rejuvenating whole-body autophagy resolves dysregulated vasomotion displayed by mesenteric resistance arteries (MRAs) from spontaneously hypertensive rats (SHRs). Arteries from SHRs administered trehalose exhibited: (i) decreased p62 protein abundance; (ii) reduced cyclooxygenase 1 and cyclooxygenase 2 expression; and (iii) lower dihydroethidium fluorescence vs. vehicle-treated SHRs, that altogether associated with diminished vasocontraction to high-dose acetylcholine in a manner could be recapitulated by indomethacin or tempol. Notably, although boosting autophagy did not impact NOS activity in this study, the potassium channel inhibitor tetraethylammonium impaired acetylcholine-evoked vasorelaxation to a greater extent in MRAs from trehalose vs. vehicle-treated SHRs. These findings support that whole-body autophagy amplification via trehalose in the context of systemic hypertension restrains acetylcholine-stimulated generation of endothelium-derived vasoconstrictor prostaglandins and reactive oxygen species, while increasing endothelium-dependent hyperpolarization, in MRAs from SHRs. Future studies investigating the impact of EC autophagy on EDRFs other than NO together with EDCFs are warranted. Second, to translate in vitro [19, 31**, 34*] and ex vivo [3, 18**, 27**, 28, 32] “restoration of EC autophagy” interventions to pre-clinical models, studies using EC specific amplification procedures are required. Underscoring this need, trehalose ingestion increased stiffness and fibrosis in arteries from Wistar rats that exhibit intact autophagy [41]. Third, although physiological maneuvers, even if initiated late-in-life, can restore autophagic flux in cardiomyocytes that associates with improved myocardial function [42], the extent to which this and other (e.g., time-restricted feeding) lifestyle interventions improves EC autophagic flux and NO generation is unknown. Finally, whether sex differences exist concerning EC autophagy and EC NO generation have not been explored. Progress toward these and other issues will certainly be made in the coming years.
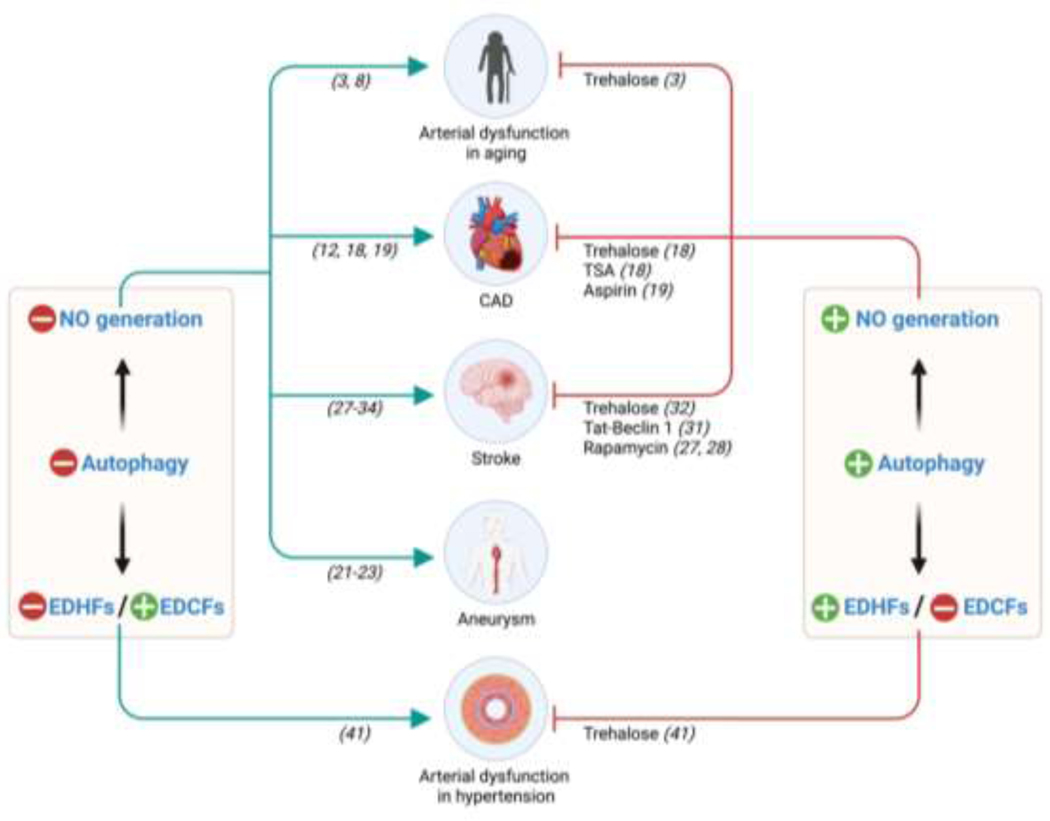
Figure 2. EC autophagy impacts EC NO generation.
Evidence exists that EC autophagy is repressed in the context of aging and cardiovascular complications including coronary artery disease, aneurysm, and stroke. Amplifying whole body or arterial EC autophagy might improve outcomes in each context by improving nitric oxide (NO) bioavailability. The numbers in brackets refer to the specific citation. EDHF, endothelium-derived hyperpolarizing factor; EDCF, endothelium-derived constricting factor; TSA, trichostatin A; NMN: nicotinamide mononucleotide.
Table 1.
Autophagy activators.
| Activator | Target cells or tissues | Disease model | Treatment | Verification | References |
|---|---|---|---|---|---|
| Trehalose | Aorta | Aging | 2% trehalose in drink water for 4 weeks in old mice | Arterial Beclin 1, WIPI-1, LC3-II, LAMP-2A, and p62 protein levels | [3] |
| Trehalose | Artery | CAD | 10 mmol/L trehalose for overnight in adipose and atrial resistance arterioles from CAD patients | No verification | [18**] |
| Trehalose | Rat brain | Stroke | 2% trehalose in drinking water for 4 weeks in SHRSP† received a JD‡ | LC3-II and p62 protein expression levels | [32] |
| Trehalose | Mesenteric artery | Hypertension | 2% trehalose in drink water for 4 weeks in SHRΨ | p62 protein expression | [41] |
| TSA | Artery | CAD | 100 mmol/L TSA for 30 min or overnight in adipose and atrial resistance arterioles from CAD patients | Lysotracker Red DND-99 Fluorescence in artery | [18**] |
| Aspirin | HCAECs# | CAD | 2.5 mM Aspirin for 16 h in HCAECs | LC3-II and p62 protein expression levels and mRFP-GFP-tagged LC3 | [19] |
| Tat-Beclin1 | CECsΩ | Stroke | 15 mg/kg/d of Tat-Beclin1 D11 in SHRSP receiving JD for 3 weeks | LC3-II protein expression | [31**] |
| Rapamycin | Rat brain | Stroke | 250 ug/kg rapamycin after 30 min of MCAO in SHR | No verification | [27] |
| Rapamycin | Mouse/Rat brain | Stroke | 10 mg/kg rapamycin after MCAO in SD rats or C57BL/6 mice | No verification | [28] |
| Nutraceutical | HUVECs | Stroke | Combination of trehalose (10 uM), cathechin (10 uM), epicatechin (10 uM), spermidine (5 uM) in HUVECs | No verification | [34*] |
SHRSP: Stroke-prone spontaneously hypertensive rat
JD: Japanese-style diet
HCAECs: Human Coronary Artery Endothelial Cells
CECs: Cerebral Endothelial Cells
SHR: Spontaneous hypertensive rat; Nutraceutical
Nutraceutical : 10 uM trehalose, 10 uM catechin, 10 uM epicatechin, and 5 uM spermidine for Mix 1. 10 uM trehalose, 5 uM spermidine, 5 uM nicotinamide for mix 2
Acknowledgments
SKP by AHA 17POST33670663; JMC by a University of Utah (UU) Graduate Research Fellowship and American Heart Association (AHA) 20PRE35110066; PWP by R00 HL140106, R01 AG073230, Alzheimer’s Association AARGD-21-850835; JDS by AHA16GRNT31050004, NIH R03AGO52848, NIH R01HL141540, and R01HL153244.
Footnotes
Conflict of interest statement
All authors have nothing to declare.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as: *of special interest; **of outstanding interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dikic I, Elazar Z, Mechanism and medical implications of mammalian autophagy, Nat. Rev. Mol. Cell Biol 19(6) (2018) 349–364. [DOI] [PubMed] [Google Scholar]
- [2].Morishita H, Mizushima N, Diverse Cellular Roles of Autophagy, Annu Rev Cell Dev Biol 35 (2019) 453–475. [DOI] [PubMed] [Google Scholar]
- [3].Larocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce GL, Seals DR, Translational evidence that impaired autophagy contributes to arterial ageing, J. Physiol July 15(590 (Pt 14)) (2012) 3305–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bharath LP, Mueller R, Li Y, Ruan T, Kunz D, Goodrich R, Mills T, Deeter L, Sargsyan A, Anandh Babu PV, Graham TE, Symons JD, Impairment of autophagy in endothelial cells prevents shear-stress-induced increases in nitric oxide bioavailability, Can. J. Physiol Pharmacol 92(7) (2014) 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Guo F, Li X, Peng J, Tang Y, Yang Q, Liu L, Wang Z, Jiang Z, Xiao M, Ni C, Chen R, Wei D, Wang GX, Autophagy regulates vascular endothelial cell eNOS and ET-1 expression induced by laminar shear stress in an ex vivo perfused system, Ann. Biomed. Eng 42(9) (2014) 1978–1988. [DOI] [PubMed] [Google Scholar]
- [6].Liu J, Bi X, Chen T, Zhang Q, Wang SX, Chiu JJ, Liu GS, Zhang Y, Bu P, Jiang F, Shear stress regulates endothelial cell autophagy via redox regulation and Sirt1 expression, Cell Death Dis 6 (2015) e1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bharath LP, Cho JM, Park SK, Ruan T, Li Y, Mueller R, Bean T, Reese V, Richardson RS, Cai J, Sargsyan A, Pires K, Anandh Babu PV, Boudina S, Graham TE, Symons JD, Endothelial cell autophagy maintains shear-stress-induced nitric oxide generation via glycolysis-dependent purinergic signaling to eNOS, Arterioscler. Thromb. Vasc. Biol 37(9) (2017) 1646–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cho JM, Park S-K, Kwon OS, La Salle DT, Cerbie J, Fermoyle CC, Morgan D, Nelson A, Bledsoe A, Bharath LP, Tandar M, Kunapuli SP, Richardson RS, Anandh Babu PV, Mookherjee S, Kishore BK, Wang F, Yang T, Boudina S, Trinity JD, Symons JD, Activating P2Y1 receptors improves function in arteries with repressed autophagy, Cardiovascular Research cvac061 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Park SK, La Salle DT, Cerbie J, Cho JM, Bledsoe A, Nelson A, Morgan DE, Richardson RS, Shiu YT, Boudina S, Trinity JD, Symons JD, Elevated arterial shear rate increases indexes of endothelial cell autophagy and nitric oxide synthase activation in humans, Am J Physiol Heart Circ Physiol 316(1) (2019) H106–H112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Abdellatif M, Sedej S, Carmona-Gutierrez D, Madeo F, Kroemer G, Autophagy in Cardiovascular Aging, Circ Res 123(7) (2018) 803–824. [DOI] [PubMed] [Google Scholar]
- [11].Beyer AM, Zinkevich N, Miller B, Liu Y, Wittenburg AL, Mitchell M, Galdieri R, Sorokin A, Gutterman DD, Transition in the mechanism of flow-mediated dilation with aging and development of coronary artery disease, Basic Res Cardiol 112(1) (2017) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Phillips SA, Hatoum OA, Gutterman DD, The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during CAD, Am J Physiol Heart Circ Physiol 292(1) (2007) H93–100. [DOI] [PubMed] [Google Scholar]
- [13].Velayutham ABP, Kim EM, Bharath LP, Muthuswamy V, Jalili T, Soorappan RN, Symons JD, Jia Z, Berry anthocyanins and their metabolite ameliorate high glucose-induced adhesion of monocytes to human aortic endothelial cells by modulating the cross-talk between eNOS and NFkB signaling., Arterioscler Thromb Vasc Biol (2014). [Google Scholar]
- [14].Beyer AM, Freed JK, Durand MJ, Riedel M, Ait-Aissa K, Green P, Hockenberry JC, Morgan RG, Donato AJ, Peleg R, Gasparri M, Rokkas CK, Santos JH, Priel E, Gutterman DD, Critical Role for Telomerase in the Mechanism of Flow-Mediated Dilation in the Human Microcirculation, Circ Res 118(5) (2016) 856–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ait-Aissa K, Heisner JS, Norwood Toro LE, Bruemmer D, Doyon G, Harmann L, Geurts A, Camara AKS, Beyer AM, Telomerase Deficiency Predisposes to Heart Failure and Ischemia-Reperfusion Injury, Front Cardiovasc Med 6 (2019) 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ait-Aissa K, Kadlec AO, Hockenberry J, Gutterman DD, Beyer AM, Telomerase reverse transcriptase protects against angiotensin II-induced microvascular endothelial dysfunction, Am J Physiol Heart Circ Physiol 314(5) (2018) H1053–H1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ali M, Devkota S, Roh JI, Lee J, Lee HW, Telomerase reverse transcriptase induces basal and amino acid starvation-induced autophagy through mTORC1, Biochem Biophys Res Commun 478(3) (2016) 1198–204. [DOI] [PubMed] [Google Scholar]
- [18].Hughes WE, Chabowski DS, Ait-Aissa K, Fetterman JL, Hockenberry J, Beyer AM, Gutterman DD, Critical Interaction Between Telomerase and Autophagy in Mediating Flow-Induced Human Arteriolar Vasodilation, Arterioscler Thromb Vasc Biol 41(1) (2021) 446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen J, Wang L, Liu WH, Shi J, Zhong Y, Liu SJ, Liu SM, Aspirin protects human coronary artery endothelial cells by inducing autophagy, Physiol Int 107(2) (2020) 294–305. [DOI] [PubMed] [Google Scholar]
- [20].Siasos G, Mourouzis K, Oikonomou E, Tsalamandris S, Tsigkou V, Vlasis K, Vavuranakis M, Zografos T, Dimitropoulos S, Papaioannou TG, Kalampogias A, Stefanadis C, Papavassiliou AG, Tousoulis D, The Role of Endothelial Dysfunction in Aortic Aneurysms, Curr Pharm Des 21(28) (2015) 4016–34. [DOI] [PubMed] [Google Scholar]
- [21].Clement M, Chappell J, Raffort J, Lareyre F, Vandestienne M, Taylor AL, Finigan A, Harrison J, Bennett MR, Bruneval P, Taleb S, Jorgensen HF, Mallat Z, Vascular Smooth Muscle Cell Plasticity and Autophagy in Dissecting Aortic Aneurysms, Arterioscler Thromb Vasc Biol 39(6) (2019) 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Osonoi Y, Mita T, Azuma K, Nakajima K, Masuyama A, Goto H, Nishida Y, Miyatsuka T, Fujitani Y, Koike M, Mitsumata M, Watada H, Defective autophagy in vascular smooth muscle cells enhances cell death and atherosclerosis, Autophagy 14(11) (2018) 1991–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Irace FG, Cammisotto V, Valenti V, Forte M, Schirone L, Bartimoccia S, Iaccarino A, Peruzzi M, Schiavon S, Morelli A, Marullo AGM, Miraldi F, Nocella C, De Paulis R, Benedetto U, Greco E, Biondi-Zoccai G, Sciarretta S, Carnevale R, Frati G, Role of Oxidative Stress and Autophagy in Thoracic Aortic Aneurysms, JACC Basic Transl Sci 6(9–10) (2021) 719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Beard DJ, Hadley G, Thurley N, Howells DW, Sutherland BA, Buchan AM, The effect of rapamycin treatment on cerebral ischemia: A systematic review and meta-analysis of animal model studies, Int J Stroke 14(2) (2019) 137–145. [DOI] [PubMed] [Google Scholar]
- [25].Papadakis M, Hadley G, Xilouri M, Hoyte LC, Nagel S, McMenamin MM, Tsaknakis G, Watt SM, Drakesmith CW, Chen R, Wood MJ, Zhao Z, Kessler B, Vekrellis K, Buchan AM, Tsc1 (hamartin) confers neuroprotection against ischemia by inducing autophagy, Nat Med 19(3) (2013) 351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ghosh R, Vinod V, Symons JD, Boudina S, Protein and Mitochondria Quality Control Mechanisms and Cardiac Aging, Cells 9(4) (2020) 933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Beard DJ, Li Z, Schneider AM, Couch Y, Cipolla MJ, Buchan AM, Rapamycin Induces an eNOS (Endothelial Nitric Oxide Synthase) Dependent Increase in Brain Collateral Perfusion in Wistar and Spontaneously Hypertensive Rats, Stroke 51(9) (2020) 2834–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang J, Lin X, Mu Z, Shen F, Zhang L, Xie Q, Tang Y, Wang Y, Zhang Z, Yang GY, Rapamycin Increases Collateral Circulation in Rodent Brain after Focal Ischemia as detected by Multiple Modality Dynamic Imaging, Theranostics 9(17) (2019) 4923–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Qu Y, Cao J, Wang D, Wang S, Li Y, Zhu Y, 14,15-Epoxyeicosatrienoic Acid Protect Against Glucose Deprivation and Reperfusion-Induced Cerebral Microvascular Endothelial Cells Injury by Modulating Mitochondrial Autophagy via SIRT1/FOXO3a Signaling Pathway and TSPO Protein, Front Cell Neurosci 16 (2022) 888836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Su KH, Lee KI, Shyue SK, Chen HY, Wei J, Lee TS, Implication of transient receptor potential vanilloid type 1 in 14,15-epoxyeicosatrienoic acid-induced angiogenesis, Int J Biol Sci 10(9) (2014) 990–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Forte M, Bianchi F, Cotugno M, Marchitti S, De Falco E, Raffa S, Stanzione R, Di Nonno F, Chimenti I, Palmerio S, Pagano F, Petrozza V, Micaloni A, Madonna M, Relucenti M, Torrisi MR, Frati G, Volpe M, Rubattu S, Sciarretta S, Pharmacological restoration of autophagy reduces hypertension-related stroke occurrence, Autophagy 16(8) (2020) 1468–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Forte M, Marchitti S, Cotugno M, Di Nonno F, Stanzione R, Bianchi F, Schirone L, Schiavon S, Vecchio D, Sarto G, Scioli M, Raffa S, Tocci G, Relucenti M, Torrisi MR, Valenti V, Versaci F, Vecchione C, Volpe M, Frati G, Rubattu S, Sciarretta S, Trehalose, a natural disaccharide, reduces stroke occurrence in the stroke-prone spontaneously hypertensive rat, Pharmacol Res 173 (2021) 105875. [DOI] [PubMed] [Google Scholar]
- [33].Sciarretta S, Forte M, Castoldi F, Frati G, Versaci F, Sadoshima J, Kroemer G, Maiuri MC, Caloric restriction mimetics for the treatment of cardiovascular diseases, Cardiovasc Res 117(6) (2021) 1434–1449. [DOI] [PubMed] [Google Scholar]
- [34].Carnevale R, Nocella C, Schiavon S, Cammisotto V, Cotugno M, Forte M, Valenti V, Marchitti S, Vecchio D, Biondi Zoccai G, Rubattu S, Martinelli O, Pignatelli P, Violi F, Volpe M, Versaci F, Frati L, Frati G, Sciarretta S, Beneficial effects of a combination of natural product activators of autophagy on endothelial cells and platelets, Br J Pharmacol 178(10) (2021) 2146–2159. [DOI] [PubMed] [Google Scholar]
- [35].Shoji-Kawata S, Sumpter R, Leveno M, Campbell GR, Zou Z, Kinch L, Wilkins AD, Sun Q, Pallauf K, MacDuff D, Huerta C, Virgin HW, Helms JB, Eerland R, Tooze SA, Xavier R, Lenschow DJ, Yamamoto A, King D, Lichtarge O, Grishin NV, Spector SA, Kaloyanova DV, Levine B, Identification of a candidate therapeutic autophagy-inducing peptide, Nature 494(7436) (2013) 201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Thomas HE, Zhang Y, Stefely JA, Veiga SR, Thomas G, Kozma SC, Mercer CA, Mitochondrial Complex I Activity Is Required for Maximal Autophagy, Cell Rep 24(9) (2018) 2404–2417 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hughes-Large JM, Pang DK, Robson DL, Chan P, Toma J, Borradaile NM, Niacin receptor activation improves human microvascular endothelial cell angiogenic function during lipotoxicity, Atherosclerosis 237(2) (2014) 696–704. [DOI] [PubMed] [Google Scholar]
- [38].Marcu R, Kotha S, Zhi Z, Qin W, Neeley CK, Wang RK, Zheng Y, Hawkins BJ, The mitochondrial permeability transition pore regulates endothelial bioenergetics and angiogenesis, Circ Res 116(8) (2015) 1336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].de Picciotto NE, Gano LB, Johnson LC, Martens CR, Sindler AL, Mills KF, Imai S, Seals DR, Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice, Aging Cell 15(3) (2016) 522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ministrini S, Puspitasari YM, Beer G, Liberale L, Montecucco F, Camici GG, Sirtuin 1 in Endothelial Dysfunction and Cardiovascular Aging, Front Physiol 12 (2021) 733696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].McCarthy CG, Wenceslau CF, Calmasini FB, Klee NS, Brands MW, Joe B, Webb RC, Reconstitution of autophagy ameliorates vascular function and arterial stiffening in spontaneously hypertensive rats, Am J Physiol Heart Circ Physiol 317(5) (2019) H1013–H1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cho JM, Park SK, Ghosh R, Ly K, Ramous C, Thompson L, Hansen M, Mattera M, Pires KM, Ferhat M, Mookherjee S, Whitehead KJ, Carter K, Buffolo M, Boudina S, Symons JD, Late-in-life treadmill training rejuvenates autophagy, protein aggregate clearance, and function in mouse hearts, Aging Cell 20(10) (2021) e13467. [DOI] [PMC free article] [PubMed] [Google Scholar]