Abstract
Hypereosinophilic syndrome (HES) was first described in 1968 by Hardy and Anderson. It is a group of rare, multisystemic and heterogeneous pathologies, characterized by significant morbidity and mortality. The occurrence of clonal hypereosinophilic syndrome associated with FIP 1L1-PDGFRA+ is estimated to range between 0.31 and 6.3 cases per million individuals. The organs most commonly impacted are the heart and spleen, with the lungs being the next most affected. Clonal hypereosinophilic syndromes with exclusive pulmonary involvement are exceptional. Due to the rarity of clonal HES, this report aims to not only describe the patient's clinical, biological, and radiological manifestations of clonal HES but also enrich the literature to ameliorate the management of this uncommon syndrome.
we report the case of a patient with past medical history of obstructive bronchopneumopathy who was presented with cough and dyspnea. Investigations revealed peripheral blood hypereosinophilia (between 4000 and 9000/mm3) which lead us to suspect clonal hypereosinophilic syndrome (HES). This diagnosis was confirmed by cytogenetics/fluorescence in situ hybridization (FISH) which demonstrated a positive FIP 1L1-PDGFRA rearrangement. The CTAP confirmed isolated lung involvement with interstitial infiltrate of the subpleural territories of both lung bases and the bronchoalveolar lavage showed eosinophil count elevated at 15%. The patient was treated by imatinib at a dose of 100 mg/day was initiated. The patient follow-up showed a reduction in eosinophils count to 7500/mm3 at two months of treatment. A molecular evaluation is scheduled in 3 months to assess the response to imatinib.
Keywords: Hypereosinophilic syndome, Clonal, Lungs, PDGFRA, Imatinib
1. Introduction
Hypereosinophilic syndromes (HES) constitute a constellation of rare and heterogeneous disorders. Defined by stringent criteria, including a blood eosinophil count surpassing 1500 cells/mm3 and discernible signs of eosinophilic organ involvement, these syndromes arise from a complex interplay of etiological factors, encompassing infectious (parasitic), allergic, rheumatologic, neoplastic, and clonal contributors.
Clonal hypereosinophilic syndromes have been found to be linked with a rare deletion on chromosome 4q12, which leads to the activation of a tyrosine kinase, as observed in the FIP 1L1-PDGFRA rearrangement. The occurrence of clonal hypereosinophilic syndrome associated with FIP 1L1-PDGFRA+ is estimated to range between 0.31 and 6.3 cases per million individuals. The organs most commonly impacted are the heart and spleen, with the lungs being the next most affected Myeloid/lymphoid neoplasms with eosinophilia and rearrangement of PDGFRA, PDGFRB, or FGFR1, or with PCM1-JAK2 associated with exclusive pulmonary involvement are exceptional.
Herein, we report an uncommon case involving a patient presenting with Myeloid neoplasm with eosinophilia and rearrangement of PDGFRA associated with eosinophFilic pulmonary disease, demonstrating successful therapeutic response to imatinib.
2. Case presentation
A 76-year-old Tunisian male patient, presented in december 2023, at the pneumology Department of the Hospital of Fattouma Bourguiba in Monastir, with complaints of cough associated with dyspnea and fatigue on moderate and mild exertion, starting two months earlier and progressively worsening. When describing medical history, the patient reported suffering from obstructive broncho-pneumopathy treated with bronchodilator and budesonide; he refuted the presence of other comorbidities or the continuous use of other medication.
Investigations revealed peripheral blood hypereosinophilia (between 4000 and 9000/mm3).
The patient has undergone a thorough infectious and allergic check-up with negative results.
A thoracic CT scan showed diffuse pulmonary emphysema, a pleural cap with a sequelae-like appearance, bilateral hilobasal bronchiectasis and an interstitial infiltrate of the subpleural territories of both bases.
Bronchoalveolar lavage showed no cells suspicious of malignancy; but the eosinophil count was elevated at 15%, exceeding the normal reference range.
Moreover the patient presented an unexplained persistant normochromic normocytic aregenerative anemia with a hemoglobin level of 9 g/dl with MCV of 85 um3, therefore the suspicion arosed regarding a potential clonal HES. So the patient was transferred to the hematology department in el Omrane hospital in Monastir for further explorations .
During the clinical examination, his vital signs were as follows: temperature= 37.6 °C; Blood Pressure = 137/84 mm Hg; heart rate = 105 beats/min; respiratory rate= 40 breaths/min; and oxygen saturation = 94% on room air. Chest examination was clear to auscultation of diffuse crepitations bilaterally. Abdomen examination showed normal bowel sounds and no plapable spleen nor liver. Skin examination was normal.
As for the laboratory findings, The WBC count was 12,370/ mm3, hemoglobin level was 9 g/dL, and platelet count was 314,000/ m L. The WBC differential absolute counts showed 590/mm3 neutrophils, 2000/mm3 lymphocytes, and 9210/mm3 eosinophils. The vitamin B12 level was normal at 520 pg/ml (normal range between 191 and 663pg/ml).
A myelogram was performed and revealed a marrow of normal richness; with an erythroblastic lineage showing many signs of dyserythropiesis; hyperplasia of the eosinophilic granular lineage at various stages of maturation showing rare dystrophic granulations (Fig. 1).
Fig. 1.
Bone Marrow Aspirate Findings: Hyperplasia of the Eosinophilic Lineage with Dyserythropoiesis and Rare Dystrophic Granulations.
The search for FIP 1L1-PDGFRA rearrangement by cytogenetics/fluorescence in situ hybridization (FISH) performed on bone marrow was positive, confirming our initial suspicion of diagnosis (Fig. 2).
Fig. 2.
Molecular biology confirming the FIP 1L1-PDGFRA rearrangement.
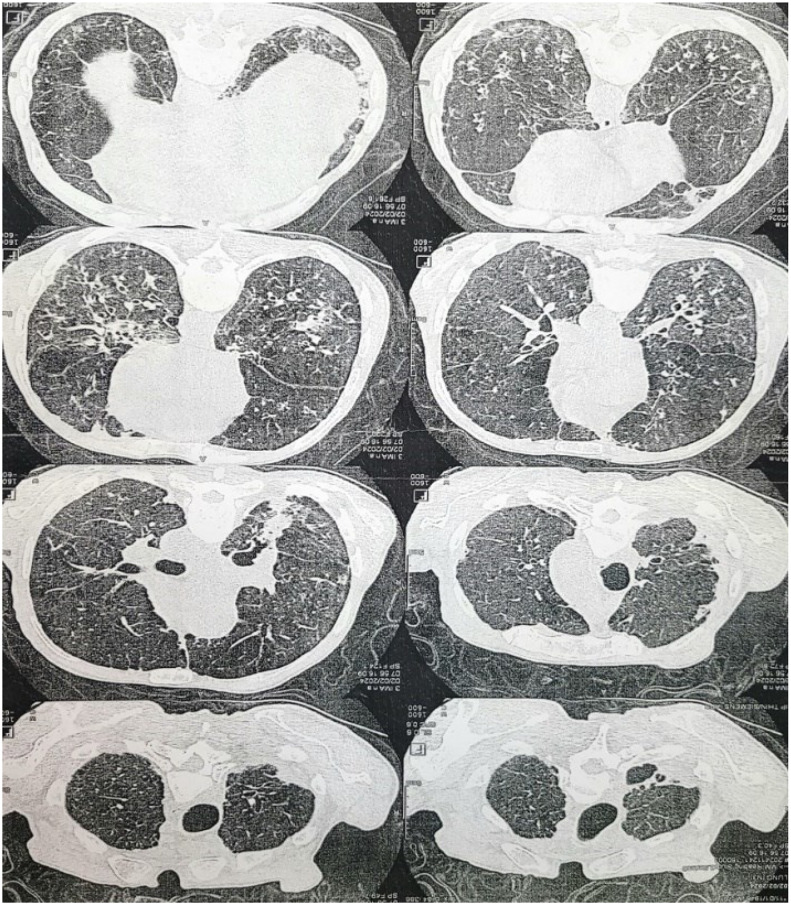
Regarding the extension assessment of this syndrome, a cardiac ultrasound was conducted, revealing no abnormalities. Additionally, troponin and pro-BNP assays returned negative results. A CTAP scan was ordered, which confirmed isolated lung involvement with interstitial infiltrate of the subpleural territories of both lung bases (Fig. 3).
Fig. 3.
CTAP scan showing an emphysematous lung with retractive condensations of both upper lobes and sequellar thickening of the right apical cap.
The patient was diagnosed for Myeloid neoplasm with eosinophilia and rearrangement of PDGFRA with associated exclusive lung involvment.
Treatment with imatinib 100 mg daily was initiated. He tolerated treatment without complications and had a rapid hematologic response with resolution of peripheral hypereosinophilia and anemia within two months. A molecular evaluation is scheduled in 3 months to assess the response to imatinib (Table 1).
Table 1.
Summary of Laboratory, Radiological and Therapeutic Findings Supporting Bone Marrow and molecular biology Images.
| Category | Details |
|---|---|
| Laboratory Data |
|
| Radiological Data |
|
| Therapeutic Regimen |
|
| Bone Marrow Aspirate |
|
| molecular biology |
|
3. Discussion
Hypereosinophilia (HE) is defined by eosinophil count > 1.5 × 109/mm3 on two occasions one month apart and/or tissue eosinophilia . Hypereosinophilic syndrome (HES) is defined by blood HE and organ damage or dysfunction caused by tissue eosinophils and exclusion of other possible causes of organ dysfunction [1].
The severity of hypereosinophilia is conventionally categorized based on the AEC (Absolute Eosinophil Count): mild when it's ranging from the upper limit of normal to 1.5× 10⁹/mm3, moderate: when it's between 1.5 and 5 × 10⁹/mm3 and severe when the AEC is exceeding 5 × 10⁹/mm3.
3.1. What diagnostic approach should be taken in the case of hypereosinophilia?
The classification of eosinophilic diseases was revised in the 2008 WHO scheme of myeloid neoplasms and reaffirmed in 2016 and 2022 [2].
Our initial evaluation should focus on identifying potential secondary causes of eosinophilia which arises from a wide spectrum of underlying conditions, often necessitating a comprehensive diagnostic workup by specialists from various fields. In developing nations, infectious diseases, especially those caused by tissue-invasive parasites, are the most frequent culprits [3]. Within a germane clinical context, possible causative factors encompass allergic/atopic and hypersensitivity reactions,collagen vascular diseases,eosinophilic gastroenteritis,and even metabolic derangements like adrenal insufficiency [1,4].
Following the exclusion of secondary causes for eosinophilia, the diagnostic workup should shift towards evaluating a possible primary bone marrow disorder:
Initial laboratory evaluation of primary eosinophilia should commence with peripheral blood screening for the FIP 1L1-PDGFRA fusion gene. Fluorescent in situ hybridization (FISH) probes targeting the chromosomal region encompassing the FIP 1L1 and PDGFRA loci can effectively detect the cytogenetically occult 800-kb deletion on chromosome 4q12, which is the underlying driver of the FIP 1L1-PDGFRA fusion event [5,6].
If the FIP 1L1-PDGFRA gene fusion is not detected, further evaluation for other primary eosinophilias linked to recurring genetic abnormalities is recommended. Typically, the presence of a PDGFRA, PDGFRB, FGFR1, JAK2, FLT3 or ETV6 ABL1 fusion gene identified through molecular testing will be accompanied by a corresponding chromosomal abnormality detectable through cytogenetic analysis [7].
These chromosomal abnormalities often involve translocations in specific chromosomal regions: 4q12 (for PDGFRA fusions other than FIP 1L1), 5q31∼33 (for PDGFRB fusions), 8p11∼12 (for FGFR1 fusions), 9p24 (for JAK2 fusions), 13q12 (for FLT3 fusions), t(9;12)(q34.1;p13.2) (for ETV6::ABL1 fusions) [7,8].
If we confirm the presence of a myeloid (MPN-like or MDS/MPN-like or AML/myeloid sarcoma) and/or lymphoid neoplasm (B- or T-cell ALL), or MPAL, usually with prominent eosinophilia, and sometimes with neutrophilia or monocytosis associated with one gene rearrangement PDGFRA, PDGFRB, FGFR1, JAK 2, FLT3 or ETV6 ABL1 by fish we ascertain the diagnosis of Myeloid/lymphoid neoplasms with eosinophilia and tyrosine kinase gene fusion” (MLN-eo-TK) [8].
If testing for the FIP 1L1-PDGFRA gene fusion isn't available, high blood levels of tryptase and vitamin B12 and an increased number of mast cells can be helpful clues. These markers often appear together in patients with the FIP 1L1-PDGFRA abnormality (found in 79% of patients in one study) and myeloproliferative variants of hypereosinophilic syndrome [9].
If testing for the previously mentioned fusion tyrosine kinases associated with eosinophilia is negative, then chronic eosinophilic leukemia (CEL) becomes a possible diagnosis [10]. This pathology is defined by Sustained hypereosinophilia (eosinophil count ≥1.5 × 109/mm3, and ≥10% eosinophilsa) for 4 weeks, The presence of a clonal abnormality (by cytogenetics and/or somatic mutation by NGS) (Abnormal bone marrow morphology (e.g., megakaryocytic and/or erythroid dysplasia) Or The presence of increased blasts (≥5% in the bone marrow and/or ≥2% in the peripheral blood), Blasts <20% in peripheral blood and bone marrow and not meeting WHO criteria for another myeloid neoplasm [8].
After ruling out all potential underlying conditions that might be causing primary or secondary hypereosinophily as well as a specific type called lymphocyte-variant HE, which involves abnormal immune T-cells producing excessive cytokines, idiopathic hypereosinophilic syndrome (HES) becomes the most likely diagnosis.
3.2. What is the typical management approach for a patient confirmed to have clonal hypereosinophilic syndrome?
Clonal HES can cause a range of tissue damage mediated by eosinophils. This damage can manifest as cardiomyopathy, pneumonitis, dermatitis, sinusitis, central nervous system or peripheral neuropathy, gastrointestinal inflammation, thromboembolic complications, and other organ involvement [11].
The decision to treat hypereosinophilia with medication depends heavily on whether organ damage is present. Therefore, the initial evaluation prioritizes tests that assess potential damage: a complete blood count checks, a chest X-ray looks for lung abnormalities, echocardiography evaluates heart function and structure for cardiomyopathy, and serum troponin levels, can be particularly helpful in identifying cardiomyopathy in HES patients [12,13].
Additional tests may be tailored to the specific organs involved. These could include pulmonary function tests for respiratory symptoms, gastrointestinal endoscopy for digestive issues, skin biopsy for suggestive skin lesions, sinus radiography for sinusitis, and neuroimaging studies (MRI or CT scan) for neurological concerns [14].
In cases of clonal eosinophilia linked with imatinib mesylate-sensitive molecular markers such as FIP 1L1-PDGFRA, PDGFRB rearrangement, or BCR-ABL, early intervention is prudent. This is because the onset of symptoms or progression to aggressive disease is likely unavoidable, and targeted therapy can lead to both complete clinical and molecular remission, potentially averting complications like leukemic transformation [15,16].
Longitudinal investigations into patient outcomes have demonstrated a robust complete hematological response rate exceeding 90% . Despite the absence of standardized protocols governing the duration and cessation of imatinib treatment, empirical evidence from select studies suggests a median relapse interval ranging between 17 and 30 months post-imatinib discontinuation [17].
4. Clinical course
In this case, the persistent eosinophilia, along with the negative investigation results and the presence of unexplained normochromic normocytic anemia, suggested the likelihood of a clonal marrow disorder. When the marrow findings revealed a primary eosinophilic proliferation, the marrow sample was specifically sent for fluorescence in situ hybridization (FISH) analysis to check for the PDGFRA rearrangement. Following the identification of the FIP 1L1-PDGFRA fusion, the patient was promptly started on imatinib at a dosage of 100 mg daily. Within two months of beginning imatinib treatment, the patient experienced a rapid resolution of eosinophilia, with an absolute eosinophil count of 400/mL. His symptoms also improved quickly. Radiological and molecular assessments are planned after three months of ongoing imatinib therapy.
5. Conclusion
In conclusion, persistent hyperosinophilia may silently lead to substantial and often irreversible tissue damage, making it essential to investigate both primary and secondary causes [18]. Although uncommon, Myeloid/lymphoid neoplasms with eosinophilia can be swiftly diagnosed using FISH on peripheral blood to detect the FIP 1L1-PDGFRA gene fusion, and this test should be conducted early in the evaluation of HES [14].
Over time, a clearer understanding has developed regarding various aspects of the fusion, including its incidence, biological characteristics, and the clinical profile of patients with this molecular rearrangement. Several prospective trials have more precisely outlined the natural history of FIP 1L1–PDGFRA-positive patients treated with imatinib, leading to a few key conclusions: the prognosis is excellent, acquired resistance is extremely rare, but ongoing imatinib therapy is likely necessary to prevent relapse [19].
Informed consent
I, R. Mohamed, hereby give my full and informed consent for the publication of my medical case report in a medical journal. I understand that my anonymized medical information, including diagnosis, treatment, clinical course, and any relevant images or test results, may be used for educational and scientific purposes.
I acknowledge that I have been fully informed about the nature of the publication and the process involved. I approve the use of my anonymized medical data and images for this case report, with the understanding that all personal identifying information will be removed.
I confirm that my participation is entirely voluntary and that I can withdraw my consent at any time without affecting my medical care.
CRediT authorship contribution statement
Zaineb Mlayah: Writing – review & editing, Validation, Supervision, Conceptualization. Inés Ben-Rekaya: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Conceptualization. Inaam Bizid: Validation, Supervision. Nader Slama: Validation, Supervision. Sara Boukhris: Validation, Supervision. Mohamed-Adnene Laatiri: Validation, Supervision.
Declaration of competing interest
The authors declare no competing interests regarding the publication of this case report and literature review on a rare clonal eosinophilia with exclusive pulmonary involvement driven by PDGFRA rearrangement treated with imatinib.
Contributor Information
Inés Ben-Rekaya, Email: benrekayaines@gmail.com.
Mohamed-Adnene Laatiri, Email: adnenelaatiri2@yahoo.fr.
References
- 1.Valent P., et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J. Allergy. Clin. Immunol. 2012;130(3):607–612.e9. doi: 10.1016/j.jaci.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shomali W., Gotlib J. World Health Organization and International Consensus Classification of eosinophilic disorders: 2024 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2024;99(5):946–968. doi: 10.1002/ajh.27287. [DOI] [PubMed] [Google Scholar]
- 3.Tefferi A., Patnaik M.M., Pardanani A. Eosinophilia: secondary, clonal and idiopathic. Br. J. Haematol. 2006;133(5):468–492. doi: 10.1111/j.1365-2141.2006.06038.x. [DOI] [PubMed] [Google Scholar]
- 4.Arber D.A., Orazi A., Hasserjian R., et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. Blood, 2016. 128(3): p. 462-463. [DOI] [PubMed] [Google Scholar]
- 5.Cools J., et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N. Engl. J. Med. 2003;348(13):1201–1214. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- 6.Pardanani A., et al. CHIC2 deletion, a surrogate for FIP1L1-PDGFRA fusion, occurs in systemic mastocytosis associated with eosinophilia and predicts response to imatinib mesylate therapy. Blood. 2003;102(9):3093–3096. doi: 10.1182/blood-2003-05-1627. [DOI] [PubMed] [Google Scholar]
- 7.Arber D.A., et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 8.Khoury J.D., et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36(7):1703–1719. doi: 10.1038/s41375-022-01613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohmer J., et al. Epidemiology, clinical picture and long-term outcomes of FIP1L1-PDGFRA-positive myeloid neoplasm with eosinophilia: data from 151 patients. Am. J. Hematol. 2020;95(11):1314–1323. doi: 10.1002/ajh.25945. [DOI] [PubMed] [Google Scholar]
- 10.Bain B.J. Myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB or FGFR1. Haematologica. 2010;95(5):696–698. doi: 10.3324/haematol.2009.021675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gleich G.J., Leiferman K.M. The hypereosinophilic syndromes: current concepts and treatments. Br. J. Haematol. 2009;145(3):271–285. doi: 10.1111/j.1365-2141.2009.07599.x. [DOI] [PubMed] [Google Scholar]
- 12.Sato Y., et al. Measurement of serum concentrations of cardiac troponin T in patients with hypereosinophilic syndrome: a sensitive non-invasive marker of cardiac disorder. Intern. Med. 2000;39(4):350. doi: 10.2169/internalmedicine.39.350. [DOI] [PubMed] [Google Scholar]
- 13.Pitini V., et al. Serum concentration of cardiac Troponin T in patients with hypereosinophilic syndrome treated with imatinib is predictive of adverse outcomes. Blood. 2003;102(9):3456–3457. doi: 10.1182/blood-2003-07-2393. author reply 3457. [DOI] [PubMed] [Google Scholar]
- 14.Tefferi A., Gotlib J., Pardanani A. Hypereosinophilic syndrome and clonal eosinophilia: point-of-care diagnostic algorithm and treatment update. Mayo Clin. Proc. 2010;85(2):158–164. doi: 10.4065/mcp.2009.0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klion A.D., et al. Molecular remission and reversal of myelofibrosis in response to imatinib mesylate treatment in patients with the myeloproliferative variant of hypereosinophilic syndrome. Blood. 2004;103(2):473–478. doi: 10.1182/blood-2003-08-2798. [DOI] [PubMed] [Google Scholar]
- 16.Ascione L., et al. Reversal of cardiac abnormalities in a young man with idiopathic hypereosinophilic syndrome using a tyrosine kinase inhibitor. Eur. J. Echocardiogr. 2004;5(5):386–390. doi: 10.1016/j.euje.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Locke M., et al. FIP1L1-PDGFRA clonal hypereosinophilic syndrome with eosinophilic myocarditis and intracardiac thrombus. Cureus. 2023;15(8):e43138. doi: 10.7759/cureus.43138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voeller J., DeNapoli T., Griffin T.C. Two pediatric oncologic cases of hypereosinophilic syndrome and review of the literature. Cancer Rep. (Hoboken) 2022;5(11):e1710. doi: 10.1002/cnr2.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gotlib J., Cools J. Five years since the discovery of FIP1L1-PDGFRA: what we have learned about the fusion and other molecularly defined eosinophilias. Leukemia. 2008;22(11):1999–2010. doi: 10.1038/leu.2008.287. [DOI] [PubMed] [Google Scholar]