Abstract
Introduction
Risankizumab and secukinumab are effective treatment options for patients with moderate to severe psoriasis.
Objectives
We sought to estimate the efficacy and the cost per responder of risankizumab and secukinumab by comparing these two drugs in a real-life setting.
Methods
A multicentric retrospective study was conducted in patients from the Lazio region of Italy affected by moderate-to-severe psoriasis who initiated risankizumab or secukinumab between September 2020 to September 2022. Psoriasis Area and Severity Index (PASI) was measured at baseline and after 16, 52, and 78 weeks. Clinical responses were evaluated by PASI90 and PASI100 responses at the same timepoints. The cost per responder at week 16 and 52 was adopted as a cost-effectiveness indicator.
Results
Included were 141 patients, 74 (52.5%) treated with risankizumab and 67 (47.5%) treated with secukinumab. PASI90 responses in risankizumab-treated patients were higher than those observed in patients treated with secukinumab at both weeks 16 and 52 (79.7% versus 64.2% (P = 0.041) and 98.6% versus 83.6% (P = 0.003), respectively). Risankizumab also showed superior PASI100 rates at week 52 (85.5% versus 65.6%, P = 0.009). No statistically significant differences were observed in PASI90 and PASI100 rates between the 2 groups at week 78. The cost per PASI90 and PASI100 responder for risankizumab was lower at both weeks 16 (€5833.66 and €8394.78, compared to €8747.18 and €10746.53 for secukinumab) and 52 (€11798.90 and €13598.73 vs €15347.70 and €19568.31, respectively).
Conclusions
Risankizumab showed superior efficacy than secukinumab and a lower cost per responder.
Keywords: Efficacy, Cost Per Responder, Risankizumab, Secukinumab, Psoriasis
Introduction
Psoriasis is a chronic, inflammatory, immune-mediated disease with high prevalence in the Italian population, ranging from 1.8% to 4.8% [1,2] and affecting over 100 million people worldwide, with plaque psoriasis being the most common variant [3]. The concept of psoriasis as a systemic inflammatory disease is now well established [4,5], with a complex network of dendritic cells, T-cells and cytokines eventually leading to inflammation, neutrophilic chemotaxis and keratinocytes proliferation [6]. While previously considered a Th1-mediated disease, the most recent findings regarding psoriasis pathogenesis highlight the central role of the IL23/Th17 axis, with IL17 and IL23 being key mediators in establishing psoriasis inflammatory cascade [7,8]. As a result of the growing understanding of the pathogenesis of psoriasis, there has been a steady development of more targeted and effective therapies. The introduction of anti-interleukin (IL) drugs, inhibiting the action of IL17 or IL23 at different levels of psoriasis inflammatory network [9], has set a new standard in the treatment of moderate-to-severe forms of psoriasis, offering superior efficacy and safety profiles when compared to conventional systemic therapies (i.e., methotrexate and cyclosporine) and anti-TNF drugs (etanercept, infliximab, adalimumab, certolizumab) [6]. Secukinumab, a fully human monoclonal antibody targeting interleukin-17A (IL17A), was the first anti-IL17 drug to receive the FDA and EMA approval in 2015 for the treatment of moderate-to-severe forms of psoriasis [10]. In two phase III trials, secukinumab showed superior efficacy and similar safety when compared to etanercept [11]. Secukinumab also proved superior in a “head-to-head” comparison with ustekinumab (an anti-IL12/IL23 drug), showing higher rates of skin clearance up to 1 year of treatment [12]. Risankizumab, a humanized monoclonal antibody that binds with high affinity to the p19 subunit of IL23, also showed favorable efficacy and safety profiles in several phase III trials [13], with higher rates of skin clearance than both placebo and ustekinumab and received FDA and EMA approval in 2019 [14].
Of note, in the IMMerge trial, a “head-to-head” comparison of risankizumab vs secukinumab showed superior efficacy for the former, along with similar safety and a more convenient dosing schedule [15]. Although the efficacy and safety of these drugs are well established in randomized clinical trials (RCTs), there is little data regarding a direct comparison of risankizumab and secukinumab in a real-life setting, except for indirect comparisons between different anti-interleukin agents in drug-survival studies [16,17]. Moreover, there are few data comparing the economic impact of using secukinumab or risankizumab to treat moderate-to-severe psoriasis. In 2021, Gisondi et al showed a more favorable economic profile for risankizumab, in addition to its greater efficacy, in an analysis aimed at establishing and comparing the cost per responder (ie. the evaluation of the cost-effectiveness of a certain drug in treating a specific condition) of these two drugs, on the basis of data retrieved from IMMerge trial [18]. Further data regarding cost-effectiveness analyses on risankizumab and secukinumab can be found in comparative studies of anti-interleukin agents based on efficacy data from other registrational studies or network meta-analyses [19,20], without any experience in a real-world setting.
Objectives
The objective of this retrospective, multicenter study is to perform a real-world analysis to estimate the efficacy and the cost per responder of risankizumab and secukinumab by comparing these two drugs in a population of patients from the Lazio region of Italy affected by moderate-to-severe psoriasis.
Methods
A retrospective analysis was performed in a cohort of patients affected by chronic plaque psoriasis who started treatment with risankizumab or secukinumab between September 2020 and September 2022. The study population consisted of patients attending the outpatient clinics of the 5 participating centers (Fondazione Policlinico Universitario A. Gemelli IRCCS; Fondazione Policlinico Tor Vergata; Policlinico Umberto I; A.O.U. Sant’Andrea – Sapienza, Università di Roma; P.O. Centro “A. Fiorini” of Terracina - Sapienza, Università di Roma - Polo Pontino) in Lazio, Italy.
All enrolled patients were >18 years old. Patients affected by generalized or palmoplantar pustular psoriasis, erythrodermic psoriasis or who had started treatment within a clinical trial were excluded, as well as patients concurrently treated with other systemic therapies. Moreover, in order to avoid influence of previous therapies, patients treated with secukinumab and previously treated with risankizumab or other IL23 inhibitors were excluded, as well as patients treated with risankizumab and previously treated with secukinumab or other IL17 inhibitors. Risankizumab and secukinumab were administered at EMA approved dosage, and no dose or frequency variations were permitted. The dosing regimen adopted for the two treatments in the first year of observation includes the induction phase (300 mg and 2100 mg for risankizumab and secukinumab, respectively, over a period of 16 weeks) followed by the maintenance phase, with one administration (2 75 mg pens, total 150 mg) every 12 weeks for risankizumab and one administration (2 150 mg pens, total 300 mg) every 4 weeks for secukinumab. For each patient, demographic and clinical data (age, sex, weight, age of onset and duration of psoriasis), history of previous biological therapies and baseline disease severity were collected at the time of initiation of risankizumab or secukinumab. Data regarding the treatment status and potential drug withdrawal were also collected. The severity of psoriasis was estimated by Psoriasis Area and Severity Index (PASI) [21] measured at baseline and after 16, 52 and 78 weeks, respectively. Data were recorded on potential safety issues and adverse events (AEs), including both serious and mild AEs. The entire study was conducted according to the principles of the Helsinki Declaration.
Statistical Analysis
Descriptive data were reported using absolute and relative (%) frequencies for categorical variables, and mean values and standard deviations when appropriate. Efficacy analysis was performed by assessing the percentage of achieving a PASI90 or PASI100 response (i.e. whether patients reached an improvement of 90% or 100% from baseline to weeks 16, 52 or 78) in the study population. Student t-test was used to establish clinical significance of PASI variations. The cost per responder at week 16 and 52 was adopted as a cost-effectiveness indicator. Due to the smaller number of patients reaching week 78, the cost per responder was not evaluated at this time-point. A purchase price of €1162.79 for a 75-mg pen of risankizumab and €400.99 for a 150-mg pen of secukinumab, resulting from the average of the purchase prices provided by the hospital pharmacies of the respective participating centers, was considered. The cost per responder was calculated by dividing the total cost of treatment after 16 and 52 weeks by the relative efficacy data (PASI90, PASI100) collected in the retrospective real-world analysis. Statistical significance was set at P value <0.05. Analyses were performed by using STATA 13.0 Software (StataCorp, Texas).
Results
A total of 141 patients were enrolled in the study, 74 (52.5%) treated with risankizumab and 67 (47.5%) treated with secukinumab. Mean age was 51.2 ± 16.4 and 50.9 ± 14.3 for the risankizumab and secukinumab group, respectively. The percentage of patients with history of previous biological therapies was 77.0% for risankizumab and 41.8% for secukinumab (P = 0.00005), with a mean number of prior biological therapies of 1.3 ± 1.3 and 0.6 ± 0.89, respectively (P = 0.001). Baseline PASI was similar between the two subgroups (17.0 and 17.2 for risankizumab and secukinumab, respectively). Further demographic and clinical data of enrolled patients are reported in Table 1.
Table 1.
Clinical and Demographic Characteristics of the Study Population.
| Characteristics | Risankizumab | Secukinumab | P Value |
|---|---|---|---|
| Patients (N) | 74 | 67 | |
| Mean age (years ± SD) | 51.2 ± 16.4 | 50.9 ± 14.3 | 0.862 |
| Gender (% male) | 55.4% | 67.2% | 0.137 |
| Weight (kg ± SD) | 77.8 ± 15.8 | 78.8 ± 15.7 | 0.400 |
| Duration of disease (years ± SD) | 21.1 ± 14.8 | 17.3 ± 11.6 | 0.079 |
| Bio-exposed (%) | 77.0% | 41.8% | 0.00005a |
| N. previous biologics (mean ± SD) | 1.3 ± 1.2 | 0.6 ± 0.8 | 0.001 a |
| Baseline PASI (mean ± SD) | 17.0 ± 6.8 | 17.2 ± 8.4 | 0.899 |
PASI = Psoriasis Area and Severity Index; SD = standard deviation.
statistically significant
Patients were followed at weeks 16, 52 and 78 for risankizumab and secukinumab. Treatment suspension was recorded in 2 (2.9%) secukinumab-treated patients; both subjects experienced primary inefficacy and suspended the treatment at weeks 17 and 19, respectively. No mild or serious safety issues or discontinuations related to AEs were reported.
Clinical Efficacy
In our population, 79.7% versus 64.2% of patients treated respectively with risankizumab or secukinumab achieved a PASI90 response at week 16 (P = 0.041), while the difference in PASI100 at week 16 was not statistically significant between the 2 groups. At week 52, PASI 90 was achieved by 98.6% of patients in the risankizumab group and by 83.6% of patients treated with secukinumab (P = 0.003). Risankizumab also showed superior PASI100 rates at week 52 (85.5% versus 65.6%, P=0.009). No statistically significant differences were observed in PASI90 and PASI100 rates between the 2 groups at week 78, although risankizumab showed higher rates of PASI90 and PASI100 (95.16% and 82.26% versus 89.83% and 72.88%, respectively). The proportions of patients reaching PASI90 and PASI100 response at the different time-points are represented in Table 2 and Figure 1.
Table 2.
PASI90 and PASI100 rates at weeks 16, 52 and 78.
| PASI | Risankizumab | Secukinumab | Delta risankizumab vs secukinumab | P Value |
|---|---|---|---|---|
| Patients (N) | 74 | 67 | ||
| PASI 90 - week 16 | 79.7% | 64.2% | 15.6% | 0.041 a |
| PASI 100 - week 16 | 55.4% | 52.2% | 3.2% | 0.707 |
| Patients (N) | 69 | 61 | ||
| PASI 90 - week 52 | 98.6% | 83.6% | 14.9% | 0.003 a |
| PASI 100 - week 52 | 85.5% | 65.6% | 19.9% | 0.009 a |
| Patients (N) | 62 | 59 | ||
| PASI 90 - week 78 | 95.16% | 89.83% | 5.3% | 0.266 |
| PASI 100 - week78 | 82.26% | 72.88% | 9.4% | 0.218 |
PASI = Psoriasis Area and Severity Index.
statistically significant
Figure 1.
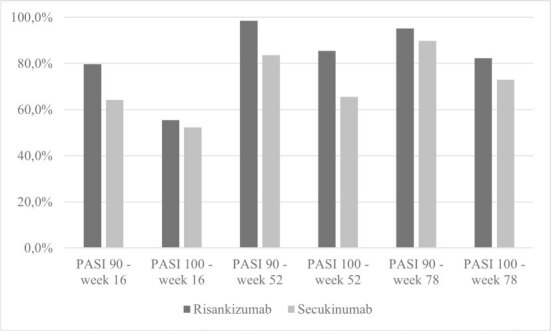
Proportions (%) of patients achieving Psoriasis Area and Severity Index (PASI)90 and PASI100 responses at weeks 16, 52 and 78.
Cost per Responder Analysis
The cost per responder for risankizumab and secukinumab was calculated using PASI90 and PASI100 rates at weeks 16 and 52, respectively (Table 3). At week 16, the cost per responder calculated using PASI90 index was €5833.66 for risankizumab and €8747.18 for secukinumab, whereas the cost per responder calculated with PASI100 index was €8394.78 for risankizumab and €10746.53 for secukinumab. Risankizumab also had lower costs per responder than secukinumab at week 52, with PASI90 and PASI100 costs per responder of €11798.90 and €13598.73 versus €15347.70 and €19568.31, respectively. The results of the cost per responder analysis are shown in Figure 2.
Table 3.
Cost per PASI90 and PASI100 Responder at Weeks 16 and 52.
| Cost per Responder | Risankizumab a | Secukinumabb | Delta Risankizumab versus secukinumab |
|---|---|---|---|
| PASI 90 - week 16 | € 5833.66 | € 8747.18 | −€ 2913.52 |
| PASI 100 - week 16 | € 8394.78 | € 10746.53 | −€ 2351.76 |
| PASI 90 - week 52 | € 11798.90 | € 15347.70 | −€ 3548.80 |
| PASI 100 - week 52 | € 13598.73 | € 19568.31 | −€ 5969.58 |
A purchase price of €1162.79 for a 75-mg pen of risankizumab was considered
A purchase price of €400.99 for a 150-mg pen of secukinumab was considered
PASI = Psoriasis Area and Severity Index.
Figure 2.

Cost per Psoriasis Area and Severity Index (PASI)90 and PASI100 responder patient at weeks 16 and 52.
Conclusions
This retrospective, multicenter study analyzed a cohort of patients from the Lazio region of Italy affected by moderate-to-severe psoriasis and treated with risankizumab or secukinumab. The maximum follow-up period was 78 weeks. The results of the study confirm the efficacy and safety of both risankizumab and secukinumab in patients with psoriasis, consistent with the results of the RCTs. Significant reductions in PASI scores were observed at the time points of interest, and no safety issues were reported. In our study population, risankizumab PASI90 and PASI100 responses rates at week 16 (79.7% and 55.4%) and week 52 (98.6% and 85.5%) are similar to those reported in RCTs, although slightly higher at week 52 in our study respect to data described in UltIMMA-1 and -2 trials [13]. The small sample size of our study compared to RCTs may be a possible explanation for this discrepancy. On the other hand, the secukinumab-treated population showed PASI90 and PASI100 rates of 64.2% and 52.2% at week 16 and 83.6% and 65.6% at week 52, similar to those reported in ERA-SURE and FIXTURE studies [11]. Several real-world studies have also evaluated the efficacy of risankizumab and secukinumab, highlighting their overall superiority over anti-TNFs in terms of disease activity reduction [22–25]. However, as previously stated, few data are available regarding a direct comparison of efficacy and safety of risankizumab and secukinumab in a real-life setting, except for data from comparisons between classes of drugs (eg anti-IL23 vs. anti-IL17) in single-center experiences [26,27] and drug survival studies [16,17]. In our real-life comparison, risankizumab outperformed secukinumab, showing higher PASI90 and PASI100 rates at every time point examined. In particular, at over 1 year of treatment, 98.6% of the risankizumab-treated population achieved a PASI90 response, in contrast with the secukinumab-treated population in which 83.6% of patients met this efficacy endpoint. These results are consistent with those reported in the IMMerge study, where a head-to-head comparison between risankizumab and secukinumab showed superiority in the short and long term for the IL23 inhibitor, with similar safety profiles. Notably, in a subsequent subgroup analysis of the population enrolled in the IMMerge trial, risankizumab demonstrated superior efficacy rates over secukinumab in different categories of patients, unaffected by variables such as BMI, prior therapies or disease activity at baseline [28]. Although a univariate analysis to determine the influence, if any, of patient or disease-related variables on the clinical efficacy of the two drugs was not performed in our study, it should be noted that the risankizumab-treated population had a significantly higher percentage of patients previously treated with biologic agents than the secukinumab-treated population (77.0% versus 41.8%, respectively), which could represent a potential negative predictive factor of the efficacy of a biological drug [29,30]. Despite this difference, risankizumab offered higher PASI90 and PASI100 rates than secukinumab, in line with the literature data highlighting the low incidence of extraneous variables on its efficacy [22,31]. Moreover, in our study, risankizumab demonstrated a faster onset of action than secukinumab and was superior in terms of efficacy after only 16 weeks of treatment, confirming that anti-IL23 inhibitors may be equal to or superior to anti-IL17 inhibitors in terms of rapidity in inducing a clinical response [15].
From a pharmaco-economic perspective, risankizumab had a more favorable cost-effectiveness ratio than secukinumab in our cost-per-responder analysis based on the clinical efficacy data just reported. This is consistent with the results reported by Gisondi et al [18] in which risankizumab showed better clinical and cost-effectiveness performances than secukinumab over an observation period of up to 2 years of treatment. It should be noted, however, that this analysis was based on clinical data derived from the population enrolled in the IMMerge trial. Population of randomized clinical trials (RCTs) may not be fully representative of daily clinical practice, in which a greater variability in the demographic and clinical characteristics of treated patients can be observed. Our results are also in line with a recent Japanese study in which the authors compared the cost-effectiveness of risankizumab with other biologic agents (adalimumab, infliximab, ustekinumab, secukinumab, brodalumab, ixekizumab, and guselkumab). In this context, risankizumab showed a significantly more favorable pharmaco-economic profile than the comparators [32]. However, even in this case, much of the efficacy data on which the cost-effectiveness estimates were based were obtained from registry studies, while our study was able to confirm these findings in a real-life setting.
The difference in purchase price per unit should also be considered in the comparison of the two drugs. Secukinumab, being on the market since its approval in 2015, to date has a lower cost per single 150 mg pen than a 75 mg pen of risankizumab (€400.99 versus €1162.79, respectively) in the Italian healthcare system. However, risankizumab and secukinumab differ significantly in their respective dosing regimens. Risankizumab has a significantly lower total number of annual administrations than the IL17 inhibitors, similar to the other IL23 inhibitors [33]. This certainly represents a pharmaco-economic advantage, as it allows for a lower net annual cost of therapy and should be taken into consideration when making therapeutic decisions. Indeed, in several countries, especially after the recent introduction of their biosimilars, anti-TNF drugs are often recommended as first-line treatment in patients with moderate-to-severe psoriasis for economic reasons (eg significantly lower acquisition costs than anti-interleukins). In any case, it should be taken into account that the choice of an anti-TNF drug cannot be applied indiscriminately to each and every patient. Several real-life studies have shown that certain categories of patients are unlikely to benefit from the use of such molecules [34,35] making therapy with an anti-interleukin drug more appropriate. In this context, the availability of data from cost-effectiveness analyses, as the one performed in our study, can be an important decision-making tool to guide clinician therapeutic choices. However, it can also be valuable for decision-makers in pharmaco-economic policy to comprehend how to optimize patient treatment while minimizing unnecessary expenses.
Limitations
The limitations of this study are mostly represented by the retrospective method of data collection (i.e. potential reporting bias) and the relatively small sample size, suggesting the need for a future analysis on a larger population, possibly with a prospective method, in order to be fully representative of daily clinical practice. Such an approach might also encompass a sub-analysis of indirect costs associated with the treatment and the disease itself. It should be noted that the cost per responder analysis was limited to 52 weeks, and therefore only covered the first year of treatment including the induction period of the two drugs, as not all patients in the study reached week 78 of treatment. Considering the optimal drug survival data available in literature for both the drugs analyzed, we aim in the near future to expand this analysis over a longer period of observation in order to fully capture the long-term cost-effectiveness of the treatments. Moreover, differences in drug prices between different countries (both European and extra-European) should be considered. In this study, the analysis was geographically limited to one single region in Italy and therefore the results cannot be applied to every pharmaco-economic scenario. Further national and international multi-center studies should investigate these aspects.
In conclusion, risankizumab proved superior clinical efficacy than secukinumab in a real-life setting and showed a lower cost per PASI90 and PASI100 responder in each time point considered, suggesting that it is a cost-effective treatment option for moderate to severe psoriasis in Italy. This could aid in personalizing biologic therapy, leading to better outcomes and increased patient satisfaction while also optimizing costs.
Footnotes
Key message: Risankizumab proved superior efficacy and cost-effectiveness versus secukinumab in patients with psoriasis in a real-life, multicenter study
Competing Interests: None.
Authorship: All authors have contributed significantly to this publication.
Funding: None.
References
- 1.Prignano F, Rogai V, Cavallucci E, Bitossi A, Hammen V, Cantini F. Epidemiology of Psoriasis and Psoriatic Arthritis in Italy-a Systematic Review. Curr Rheumatol Rep. 2018;20(7):43. doi: 10.1007/s11926-018-0753-1. [DOI] [PubMed] [Google Scholar]
- 2.Gianfredi V, Casu G, Bricchi L, Kacerik E, Rongioletti F, Signorelli C. Epidemiology of psoriasis in Italy: burden, cost, comorbidities and patients’ satisfaction. A systematic review. Acta Biomed. 2022;93(6):e2022332. doi: 10.23750/abm.v93i6.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parisi R, Iskandar IYK, Kontopantelis E, Augustin M, Griffiths CEM, Ashcroft DM Global Psoriasis Atlas. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020 May 28;369:m1590. doi: 10.1136/bmj.m1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong AW, Read C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA. 2020;323(19):1945–1960. doi: 10.1001/jama.2020.4006. [DOI] [PubMed] [Google Scholar]
- 5.Alwan W, Nestle FO. Pathogenesis and treatment of psoriasis: exploiting pathophysiological pathways for precision medicine. Clin Exp Rheumatol. 2015;33(5 Suppl 93):S2–S6. [PubMed] [Google Scholar]
- 6.Kim J, Krueger JG. Highly Effective New Treatments for Psoriasis Target the IL-23/Type 17 T Cell Autoimmune Axis. Annu Rev Med. 2017 Jan 14;68:255–269. doi: 10.1146/annurev-med-042915-103905. [DOI] [PubMed] [Google Scholar]
- 7.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009 Jun;129(6):1339–50. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 8.Rendon A, Schäkel K. Psoriasis Pathogenesis and Treatment. Int J Mol Sci. 2019 Mar 23;20(6):1475. doi: 10.3390/ijms20061475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tokuyama M, Mabuchi T. New Treatment Addressing the Pathogenesis of Psoriasis. Int J Mol Sci. 2020 Oct 11;21(20):7488. doi: 10.3390/ijms21207488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanford M, McKeage K. Secukinumab: first global approval. Drugs. 2015;75(3):329–338. doi: 10.1007/s40265-015-0359-0. [DOI] [PubMed] [Google Scholar]
- 11.Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 12.Blauvelt A, Reich K, Tsai TF, Tyring S, Vanaclocha F, Kingo K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017 Jan;76(1):60–69.e9. doi: 10.1016/j.jaad.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, Papp KA, Sofen H, Puig L, Foley P, Ohtsuki M, Flack M, Geng Z, Gu Y, Valdes JM, Thompson EHZ, Bachelez H. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018 Aug 25;392(10148):650–661. doi: 10.1016/S0140-6736(18)31713-6. [DOI] [PubMed] [Google Scholar]
- 14.McKeage K, Duggan S. Risankizumab: First Global Approval. Drugs. 2019;79(8):893–900. doi: 10.1007/s40265-019-01136-7. [DOI] [PubMed] [Google Scholar]
- 15.Warren RB, Blauvelt A, Poulin Y, Beeck S, Kelly M, Wu T, Geng Z, Paul C. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021 Jan;184(1):50–59. doi: 10.1111/bjd.19341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mastorino L, Dapavo P, Susca S, et al. Drug survival and clinical effectiveness of secukinumab, ixekizumab, brodalumab, guselkumab, risankizumab, tildrakizumab for psoriasis treatment. J Dtsch Dermatol Ges. doi: 10.1111/ddg.15251. Published online November 5, 2023. [DOI] [PubMed] [Google Scholar]
- 17.Torres T, Puig L, Vender R, et al. Drug Survival of Interleukin (IL)-17 and IL-23 Inhibitors for the Treatment of Psoriasis: A Retrospective Multi-country, Multicentric Cohort Study. Am J Clin Dermatol. 2022;23(6):891–904. doi: 10.1007/s40257-022-00722-y. [DOI] [PubMed] [Google Scholar]
- 18.Gisondi P, Loconsole F, Raimondo P, Ravasio R. Costo per responder di risankizumab e secukinumab nel trattamento della psoriasi a placche da moderata a grave in Italia. Glob Reg Health Technol Assess. 2021;8:120–130. doi: 10.33393/grhta.2021.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu JJ, Jia X, Zhao Y, et al. Comparative cost-effectiveness of tildrakizumab and other commonly used treatments for moderate-to-severe psoriasis. J Dermatolog Treat. 2021;32(7):693–700. doi: 10.1080/09546634.2019.1698700. [DOI] [PubMed] [Google Scholar]
- 20.Nyholm N, Schnack H, Danø A, Skowron F. Cost per responder of biologic drugs used in the treatment of moderate-to-severe plaque psoriasis in France and Germany. Curr Med Res Opin. 2023;39(6):833–842. doi: 10.1080/03007995.2023.2214046. [DOI] [PubMed] [Google Scholar]
- 21.Fredriksson T, Pettersson U. Severe psoriasis--oral therapy with a new retinoid. Dermatologica. 1978;157(4):238–44. doi: 10.1159/000250839. [DOI] [PubMed] [Google Scholar]
- 22.Caldarola G, Zangrilli A, Bernardini N, et al. Risankizumab for the treatment of moderate-to-severe psoriasis: A multicenter, retrospective, 1 year real-life study. Dermatol Ther. 2022;35(6):e15489. doi: 10.1111/dth.15489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunasso AMG, Burlando M, Amoruso F, et al. Risankizumab: Daily Practice Experience of High Need Patients. Biomedicines. 2023;11(6):1769. doi: 10.3390/biomedicines11061769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dastoli S, Passante M, Loconsole F, et al. Long-term efficacy and safety of secukinumab in real life: a 240 weeks multicenter study from Southern Italy. J Dermatolog Treat. 2023;34(1):2200868. doi: 10.1080/09546634.2023.2200868. [DOI] [PubMed] [Google Scholar]
- 25.Thaçi D, Körber A, von Kiedrowski R, et al. Secukinumab is effective in treatment of moderate-to-severe plaque psoriasis: real-life effectiveness and safety from the PROSPECT study. J Eur Acad Dermatol Venereol. 2020;34(2):310–318. doi: 10.1111/jdv.15962. [DOI] [PubMed] [Google Scholar]
- 26.Narcisi A, Valenti M, Cortese A, et al. Anti-IL17 and anti-IL23 biologic drugs for scalp psoriasis: A single-center retrospective comparative study. Dermatol Ther. 2022;35(2):e15228. doi: 10.1111/dth.15228. [DOI] [PubMed] [Google Scholar]
- 27.Cortese A, Gargiulo L, Ibba L, et al. Anti-interleukin-17 and anti-interleukin-23 biologic drugs for genital psoriasis: a single-center retrospective comparative study. Dermatol Reports. 2023;15(3):9692. doi: 10.4081/dr.2023.9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crowley JJ, Langley RG, Gordon KB, et al. Efficacy of Risankizumab versus Secukinumab in Patients with Moderate-to-Severe Psoriasis: Subgroup Analysis from the IMMerge Study. Dermatol Ther (Heidelb) 2022;12(2):561–575. doi: 10.1007/s13555-021-00679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papp KA, Gordon KB, Langley RG, et al. Impact of previous biologic use on the efficacy and safety of brodalumab and ustekinumab in patients with moderate-to-severe plaque psoriasis: integrated analysis of the randomized controlled trials AMAGINE-2 and AMAGINE-3. Br J Dermatol. 2018;179(2):320–328. doi: 10.1111/bjd.16464. [DOI] [PubMed] [Google Scholar]
- 30.García-Doval I, Pérez-Zafrilla B, Ferrandiz C, et al. Development of clinical prediction models for good or bad response to classic systemic drugs, anti-TNFs, and ustekinumab in psoriasis, based on the BIOBADADERM cohort. J Dermatolog Treat. 2016;27(3):203–209. doi: 10.3109/09546634.2015.1088130. [DOI] [PubMed] [Google Scholar]
- 31.Gkalpakiotis S, Cetkovska P, Arenberger P, Dolezal T, Arenbergerova M, Velackova B, Fialova J, Kojanova M BIOREP study group. Risankizumab for the Treatment of Moderate-to-Severe Psoriasis: Real-Life Multicenter Experience from the Czech Republic. Dermatol Ther (Heidelb) 2021 Jun 5;:1–11. doi: 10.1007/s13555-021-00556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saeki H, Ishii K, Joshi A, Bensimon AG, Yang H, Kawaguchi I. An economic evaluation of risankizumab versus other biologic treatments of moderate to severe plaque psoriasis in Japan. J Dermatolog Treat. 2022;33(1):229–239. doi: 10.1080/09546634.2020.1744505. [DOI] [PubMed] [Google Scholar]
- 33.Norden A, Moon JY, Javadi SS, Munawar L, Maul JT, Wu JJ. Anti-drug antibodies of IL-23 inhibitors for psoriasis: a systematic review. J Eur Acad Dermatol Venereol. 2022;36(8):1171–1177. doi: 10.1111/jdv.18042. [DOI] [PubMed] [Google Scholar]
- 34.De Simone C, Caldarola G, Maiorino A, et al. Clinical predictors of nonresponse to anti-TNF-α agents in psoriatic patients: A retrospective study. Dermatol Ther. 2016;29(5):372–376. doi: 10.1111/dth.12364. [DOI] [PubMed] [Google Scholar]
- 35.Mourad A, Straube S, Armijo-Olivo S, Gniadecki R. Factors predicting persistence of biologic drugs in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2019;181(3):450–458. doi: 10.1111/bjd.17738. [DOI] [PubMed] [Google Scholar]


