This randomized clinical trial investigates if reldesemtiv slows disease progression in amyotrophic lateral sclerosis.
Key Points
Question
Does orally administered reldesemtiv slow disease progression in amyotrophic lateral sclerosis (ALS)?
Findings
A Study to Evaluate the Efficacy and Safety of Reldesemtiv in Patients With Amyotrophic Lateral Sclerosis (COURAGE-ALS) was a multicenter international trial that included 486 participants. The trial was terminated due to futility after the second planned interim analysis.
Meaning
Results demonstrate that reldesemtiv, a fast skeletal troponin activator, was not effective in slowing progression in ALS.
Abstract
Importance
Treatment options for amyotrophic lateral sclerosis (ALS) remain suboptimal. Results from a phase 2 study of reldesemtiv in ALS suggested that it may slow disease progression.
Objective
To assess the effect of reldesemtiv vs placebo on functional outcomes in ALS.
Design, Setting, and Participants
A Study to Evaluate the Efficacy and Safety of Reldesemtiv in Patients With Amyotrophic Lateral Sclerosis (COURAGE-ALS) was a double-blind, placebo-controlled phase 3 randomized clinical trial conducted at 83 ALS centers in 16 countries from August 2021 to July 2023. The first 24-week period was placebo controlled vs reldesemtiv. All participants received reldesemtiv during the second 24-week period with a 4-week follow-up. Two interim analyses were planned, the first for futility and the second for futility and possible resizing. This was a hybrid decentralized trial with approximately half the trial visits performed remotely and the remaining visits in the clinic. Eligible participants met criteria for definite, probable, or possible ALS with lower motor neuron signs by modified El Escorial Criteria, ALS symptoms for 24 months or less, ALS Functional Rating Scale–Revised (ALSFRS-R) total score of 44 or less, and forced vital capacity of greater than or equal to 65% of predicted.
Interventions
Oral reldesemtiv, 300 mg, or placebo twice daily.
Main Outcomes and Measures
The primary end point was change in ALSFRS-R total score from baseline to week 24.
Results
Of the 696 participants screened, 207 were screen failures. A total of 486 participants (mean [SD] age, 59.4 [10.9] years; 309 male [63.6%]) were randomized to reldesemtiv (n = 325) or placebo (n = 161); 3 randomized patients were not dosed. The second interim analysis at 24 weeks after randomization included 256 participants. The data monitoring committee recommended that the trial should end due to futility, and the sponsor agreed. The mean (SE) group difference in the ALSFRS-R score from baseline to week 24 was −1.1 (0.53; 95% CI, −2.17 to −0.08; P = .04, favoring placebo). Given excess missing data from early termination, the combined assessment assumed greater importance; it, too, failed to show a benefit from treatment with reldesemtiv (win probability was 0.44 for reldesemtiv and 0.49 for placebo, with a win ratio of 0.91; 95% CI of win ratio, 0.77-1.10; P = .11).
Conclusions and Relevance
This randomized clinical trial failed to demonstrate efficacy for reldesemtiv in slowing functional decline in ALS.
Trial Registration
ClinicalTrials.gov Identifier: NCT04944784
Introduction
Targets for therapeutic intervention in amyotrophic lateral sclerosis (ALS) have focused on central and peripheral motor neurons, the primary sites of degeneration as disease progresses.1 However, as the end organ for weakness in ALS, skeletal muscle has also been considered a potential target. Strategies for ameliorating skeletal muscle atrophy have been studied,2,3,4 as well as approaches to improve skeletal muscle function.5,6 Fast skeletal troponin activators (FSTAs) increase the efficiency of skeletal muscle contraction such that muscles stimulated by motor neuron activation show increased force over a wide range of stimulation frequencies in both animal models7 and healthy participants,8 suggesting the possibility that such agents could be functionally important and therapeutically useful in the treatment of people with ALS.
Tirasemtiv, a first-generation FSTA, was studied in phase 2 and 3 trials in people with ALS.9,10,11 Despite encouraging trends, tolerability was poor.9
Reldesemtiv is a second-generation FSTA with improved potency regarding muscle force and was well tolerated in phase 1 studies.8 FORTITUDE-ALS (A Phase 2, Double-Blind, Randomized, Dose-Ranging Trial of Reldesemtiv in Patients With ALS), a large phase 2 study of reldesemtiv in the treatment of ALS, reported trends favoring reldesemtiv, but the primary and secondary end points did not meet statistical significance.12 However, a post hoc analysis combining all reldesemtiv doses compared with placebo showed a 25% reduction in functional decline as measured by ALS Functional Rating Scale–Revised (ALSFRS-R), as well as a 27% reduction in decline of slow vital capacity (SVC).12 In post hoc subgroup analyses, effect appeared greatest in those with prestudy estimates of disease progression in the intermediate or fastest progressing tertiles13; inclusion criteria were modified in the COURAGE-ALS (A Study to Evaluate the Efficacy and Safety of Reldesemtiv in Patients With Amyotrophic Lateral Sclerosis) study accordingly to enrich for this population. COURAGE-ALS, a phase 3 study evaluating the effectiveness of reldesemtiv over 24 weeks of placebo-controlled treatment, was designed to confirm the results of the FORTITUDE-ALS trial.13
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
All participants in the COURAGE-ALS study provided written informed consent, and institutional review board approvals were received at all sites before enrollment. The study was conducted in accordance with the Declaration of Helsinki. An independent data and safety monitoring committee (DMC) oversaw safety, assessed unblinded efficacy for the planned interim analyses, and made recommendations on study conduct. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Participants
Adults aged 18 to 80 years with sporadic or familial laboratory-supported probable, probable, or definite ALS in accordance with the revised El Escorial Criteria14 were enrolled at 83 sites in 16 countries in North America, Europe, and Australia between August 2021 and July 2023. Those meeting El Escorial criteria for possible ALS were eligible only if they had lower motor neuron findings; purely upper motor neuron findings were exclusionary even though criteria for possible ALS were met. Inclusion criteria required ALS symptom onset of 24 months or less, ALSFRS-R score of 44 or less, and predicted forced vital capacity (FVC) greater than or equal to 65% using the Global Lung Initiative equation,15 with less than 10% variability between the 2 highest results.
Trial Design and Assessment
The COURAGE-ALS trial design was previously published (Supplement 1)13 and briefly described here. After a screening period of 21 days or less, eligible participants were randomized 2:1 to oral reldesemtiv, 300 mg, twice daily or matching placebo tablets for a 24-week double-blind period followed by 24 weeks during which all participants knowingly received reldesemtiv. Participants, sponsor, and site staff remained blinded to the original double-blind treatment assignment. Randomization was stratified according to riluzole and edaravone use. Study flow is described in Shefner et al.13 Screening, day 1, and weeks 4, 12, 24, 36, 48, and 52 were scheduled as in-clinic visits. Remote visits using video telemedicine (RV-TM) with a mobile phone provided by the sponsor took place at weeks 8, 16, 20, 28, 32, 40, and 44. Collection of blood and urine samples also took place remotely at weeks 2 and 26. For RV-TM assessments, a trained evaluator performed the ALSFRS-R evaluation and observed and coached the participant as they performed the FVC using a trial-provided mobile spirometer. Additional FVCs were completed during RV-TM and performed by a trained evaluator a few days around the time of in-clinic visits. After randomization, in-clinic visits could be converted to RV-TM, and vice versa, if deemed in the participant’s best interest. Participants received standard of care for ALS for the local region, as determined by the treating physician in discussion with the participant. Participants self-identified with respect to race and ethnicity. Race classifications were American Indian or Alaska Native, Black or African American, Native Hawaiian or Other Pacific Islander, Northeast Asian, Southeast Asian, White, or other, which includes all categories not listed previously. Ethnicity classifications were Hispanic or Latino vs non-Hispanic or non-Latino. A list of COURAGE-ALS trial investigators is available in eTable 3 in Supplement 2.
The primary end point was the change from baseline to week 24 in ALSFRS-R total score. Secondary end points in hierarchal order were the combined assessment of change in ALSFRS-R total score, time to respiratory insufficiency, survival up to week 24, change from baseline to week 24 in percentage predicted FVC, 40-item ALS Assessment Questionnaire (ALSAQ-40) total score, and handgrip strength (average of both hands).
Statistical Analysis
An initial sample size of approximately 555 people with ALS was required to achieve at least 90% power to detect a 1.8-point treatment difference between reldesemtiv and placebo in the change from baseline to week 24 in ALSFRS-R total score. This calculation was for 2:1 randomization ratio of reldesemtiv to placebo groups, respectively, and based on a 2-sample t test with 2-sided α at .05 level, a common SD of 5.5 points, and accounting for 20 of missing data and early treatment termination.
The primary analysis was conducted using a mixed model for repeated measures (MMRM) with a restricted maximum-likelihood method. The model terms include treatment group, baseline ALSFRS-R total score, visit, baseline riluzole use, baseline edaravone use, prestudy disease progression rate, age, and baseline percentage predicted FVC, as well as the following interaction terms: baseline ALSFRS-R total score-by-visit and treatment group-by-visit interactions.
Missing ALSFRS-R total scores were imputed for the primary analysis (MMRM), with the assumption that data were missing at random. This was felt appropriate if missing data were sparse. However, as the study was terminated due to futility, missing data at week 24 was greater than originally expected, such that the missing at random assumption no longer held. Under this circumstance, the first secondary end point, Combined Assessment of Function and Survival (CAFS), was elevated in importance as the CAFS analysis approach is based on a nonparametric ranking method that does not rely on specific assumptions about the data distribution and would be less impacted by the large percentage of missing data.
The first secondary end point (combined assessment of change in ALSFRS-R total score, time to dependence on assisted ventilation, and survival time up to week 24) was analyzed using the joint rank comparison method.16 A stratified Wilcoxon test was used to compare the joint ranks between reldesemtiv and placebo groups, adjusting for baseline riluzole use and baseline edaravone use. The win probabilities and win ratio between treatment groups were also calculated. The remaining secondary end points were analyzed using MMRM with the following model terms: treatment group, baseline ALSFRS-R total score, visit, riluzole use at baseline, and edaravone use at baseline, as well as baseline-by-visit and treatment group-by-visit interactions.
Two interim analyses (IAs) were planned and conducted; the first IA was for futility, and the second was for both futility and possible resizing. The DMC reviewed data related to the first and second IAs and provided recommendations based on the results. The first IA occurred 12 weeks after approximately one-third of participants were randomized. Futility would be met if the treatment difference for change from baseline to week 12 in ALSFRS-R total score had a 1-sided P ≥.5 (ie, the estimated treatment difference favors placebo). The second IA, planned for 24 weeks following randomization of at least one-third of the participants, occurred after approximately 46% were randomized. The adaptive method using promising zones with the conditional power under the current trend17 was applied for the primary end point to evaluate whether resizing was required to ensure sufficient statistical power for the final analysis or the study was futile at the interim analysis. Three additional zones—efficacy, unfavorable, and favorable—were also possible based on the conditional power.17 Data were analyzed using SAS software, version 9.4 (SAS Institute).
Results
Patient Disposition
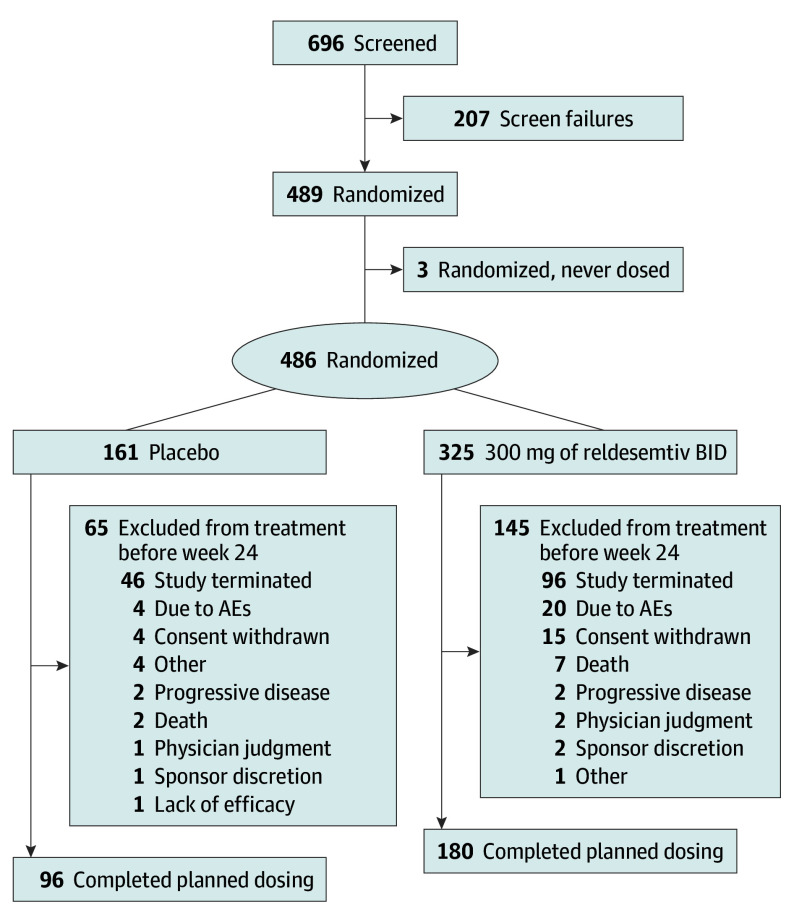
The COURAGE-ALS study was conducted between August 2021 and July 2023 (last patient, last visit). There were 696 participants screened for eligibility, 486 were randomized (mean [SD] age, 59.4 (10.9) years; 177 female [36.4%]; 309 male [63.6%]) and received either reldesemtiv (n = 325) or placebo (n = 161), and 3 were randomized but not dosed (2 participants not dosed due to eligibility, 1 because study was stopped before dosing) (Figure 1). Additional information on inclusion criteria is available in the eMethods in Supplement 2. Based on recommendations made by the DMC, the study continued after the first IA. After the second IA (which occurred at 24 weeks after randomization and included 256 participants), the DMC recommended stopping due to futility and, on March 31, 2023, the sponsor notified sites to inform participants to stop dosing. Plasma pharmacokinetic analysis through week 24 confirmed appropriate treatment assignment and expected plasma levels of reldesemtiv. One participant (0.3%) in the reldesemtiv group was American Indian or Alaska Native, 3 participants (1.9%) in the placebo group and 1 (0.3%) in the reldesemtiv group reported being Asian, 4 participants (2.5%) in the placebo group and 1 (0.3%) in the reldesemtiv group reported being Black or African American, 149 participants (92.5%) in the placebo group and 308 (94.8%) in the reldesemtiv group reported their race as White, and 5 participants (3.1%) in the placebo group and 9 (2.8%) in the reldesemtiv group reported their race as other. With regard to ethnicity, 12 participants (7.5%) in the placebo group and 15 (4.6%) in the reldesentiv group identified as Hispanic or Latino, 132 participants (82%) in the placebo group and 278 (85.5%) in the reldesemtiv group identified as not Hispanic or Latino, 2 participants (1.2%) in the placebo group and 5 (1.5%) in the reldesemtiv group reported unknown ethnicity, and 15 participants (9.3%) in the placebo group and 27 (8.3%) in the reldesemtiv group did not report their ethnicity.
Figure 1. Consolidated Standards of Reporting Trials Diagram of Participant Disposition.
AE indicates adverse event; BID, twice daily.
There were 207 screen failures; the most common reason was FVC less than 65% (90 participants [43.5%]). Screen failures included 25 participants in screening at the time the trial was stopped due to futility. Of those participants randomized to reldesemtiv, 180 (56.4%) completed dosing through week 24, and 96 (59.6%) randomized to placebo completed dosing during this time frame. The most common reason for early termination in both groups was the trial ending due to futility (96 participants [29.5%] receiving reldesemtiv; 46 participants [28.6%] receiving placebo). Four participants (2 each in the reldesemtiv and placebo groups) did not have any postbaseline efficacy assessments and were excluded from the efficacy analyses. Participant disposition is shown in Shefner et al.13
Participant Characteristics
Baseline demographics and disease characteristics are summarized in the Table. All baseline characteristics were similar between treatment arms, including use of riluzole, edaravone, and sodium phenylbutyrate/taurursodiol.
Table. Key Baseline Characteristics.
| Characteristic | Placebo (n = 161) | Reldesemtiv (n = 325) |
|---|---|---|
| Age, mean (SD), y | 60.0 (10.8) | 59.2 (11.0) |
| Sex, No. (%) | ||
| Female | 64 (39.8) | 113 (34.8) |
| Male | 155 (96.3) | 212 (65.2) |
| Race and ethnicity, No. (%) | ||
| American Indian or Alaska Native | 0 | 1 (0.3) |
| Black or African American | 4 (2.5) | 1 (0.3) |
| Hispanic or Latino | 12 (7.5) | 15 (4.6) |
| Non-Hispanic or non-Latino | 132 (82.0) | 278 (85.5) |
| Native Hawaiian or Other Pacific Islander | 0 | 0 |
| Northeast Asian | 1 (0.6) | 3 (0.9) |
| Southeast Asian | 2 (1.2) | 3 (0.9) |
| White | 149 (92.5) | 308 (94.8) |
| Othera | 5 (3.1) | 9 (2.8) |
| Weight, mean (SD), kg | 78.0 (15.1) | 79.9 (19.1) |
| BMI, mean (SD)b | 26.4 (4.4) | 26.9 (5.7) |
| FVC, mean (SD), % predicted | 85.7 (14.2) | 84.4 (14.7) |
| ALSFRS-R score, mean (SD) | 36.6 (5.4) | 37.2 (5.0) |
| ALSAQ-40 score, mean (SD) | 31.7 (16.8) | 29.2 (15.5) |
| No riluzole, edaravone, or AMX0035, No. (%) | 16 (9.9) | 27 (8.3) |
| Riluzole alone, No. (%) | 117 (72.7) | 238 (73.2) |
| Edaravone alone, No. (%) | 0 | 3 (0.9) |
| AMX0035 alone, No. (%) | 1 (0.6) | 1 (0.3) |
| Both riluzole + edaravone, No. (%) | 15 (9.3) | 27 (8.3) |
| Both riluzole + AMX0035, No. (%) | 7 (4.4) | 12 (3.7) |
| Both edaravone + AMX0035, No. (%) | 0 | 0 |
| Riluzole + edaravone + AMX0035, No. (%) | 5 (3.1) | 17 (5.2) |
| El Escorial criteria for ALS, definite, No. (%) | 36 (22.4) | 87 (26.8) |
| Time since ALS symptom onset, mean (SD), mo | 16.1 (5.4) | 15.6 (5.3) |
| Time since ALS diagnosis, mean (SD), mo | 6.6 (4.6) | 6.4 (4.6) |
| Prestudy disease progression rate, No. (%)c | 0.8 (0.6) | 0.8 (0.5) |
| Site of ALS onset, bulbar, No. (%) | 30 (18.6) | 58 (17.8) |
| Site of ALS onset, upper limb, No. (%) | 69 (42.9) | 116 (35.7) |
| Site of ALS onset, lower limb, No. (%) | 60 (37.3) | 147 (45.2) |
| Site of ALS onset, respiratory, No. (%) | 1 (0.6) | 3 (0.9) |
Abbreviations: ALS, amyotrophic lateral sclerosis; ALSAQ-40, ALS Assessment Questionnaire 40; ALSFRS-R, ALS Functional Rating Scale-Revised; BMI, body mass index; FVC, forced vital capacity.
Other includes all categories not listed previously.
Calculated as weight in kilograms divided by height in meters squared.
Calculated as 48 − baseline ALSFRS-R total score/symptom duration in months.
Although genetic testing was not required, family history of ALS and results of previously performed genetic testing were recorded. Of participants who were randomized and dosed, family history was reported in 6% (31 of 486) of randomized and dosed participants. Genetic testing was reported in 267 participants (55%), including 26 of 35 (84%) with family history of ALS. A potentially causal gene variant was found in 29 participants (11%) of those tested, including 6 variants of uncertain significance. Extent of testing ranged from a single gene to a 26-gene panel. Genes identified in more than 2 participants were C9orf72 (18 [7% of tested participants]) and TARDP (3 [1% of tested participants]).
Efficacy
The primary end point was the change in the total score of the ALSFRS-R from baseline to week 24; the mean (SE) change was −1.1 (0.53; 95% CI, −2.17 to −0.08; P = .04 favoring placebo) (Figure 2A). Given the number of missing data due to stopping for futility, the assumptions underlying the MMRM were not met. Therefore, the joint rank test assumed extra importance for the study. The win probability was 0.44 for reldesemtiv and 0.49 for placebo, with a win ratio of 0.91 (95% CI, 0.77-1.10; P = .11). At the time of discontinuation, the conditional power for the MMRM analysis of ALSFRS-R was 8.4%, and less than 1% for the CAFS. The change in FVC, ALSAQ-40, and handgrip strength from baseline through week 24 failed to show any slowing of disease progression for those assigned reldesemtiv compared with placebo (Figure 2B-D).
Figure 2. Change From Baseline Through Week 24 .
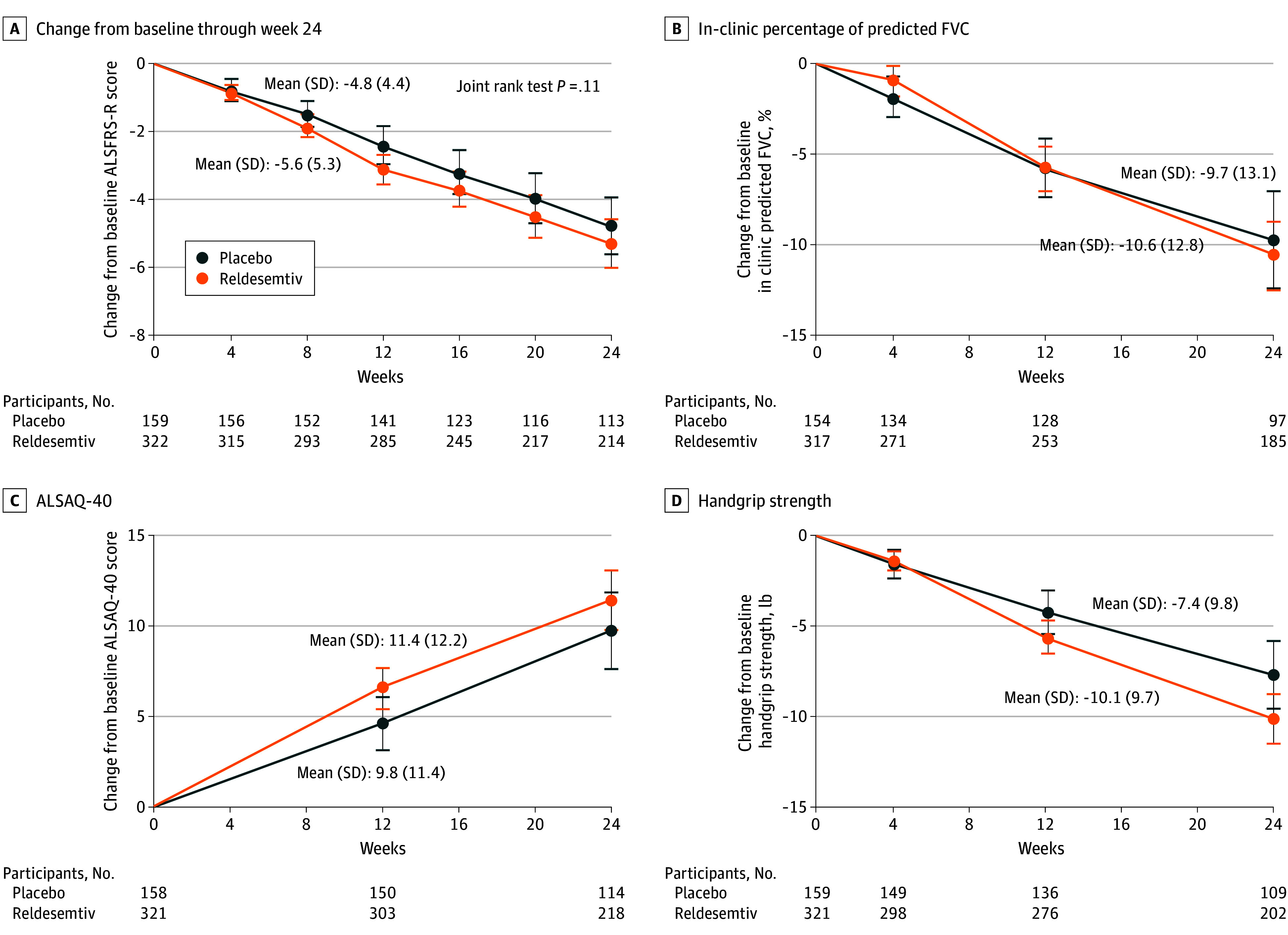
A, Amyotrophic Lateral Sclerosis (ALS) Functional Rating Scale–Revised (ALSFRS-R). B, In-clinic percentage predicted forced vital capacity (FVC). C, Forty-item ALS Assessment Questionnaire (ALSAQ-40). D, Handgrip strength.
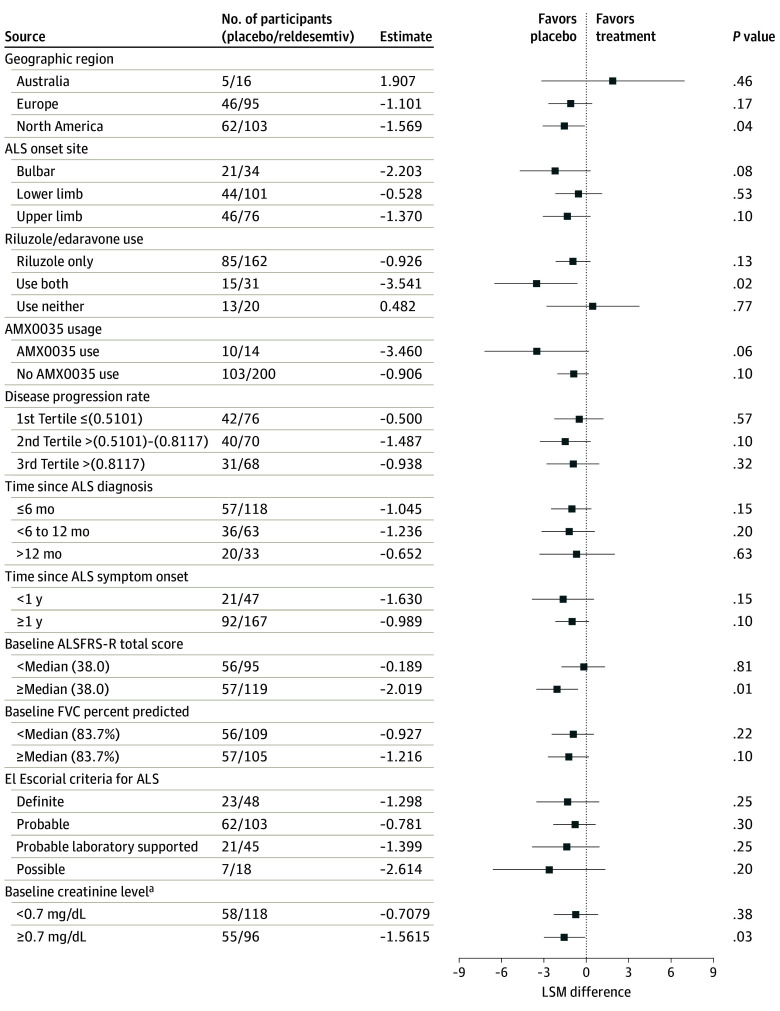
Preplanned subgroup analyses comparing clinical and demographic characteristics failed to identify any subgroups that favored reldesemtiv regarding rate of disease progression as measured by the ALSFRS-R. To investigate the possibility that reduced muscle mass may have contributed to the negative trial results given the mechanism of action for reldesemtiv, post hoc analyses of creatinine, a biomarker for muscle mass,18 were performed. Participants in the reldesemtiv and placebo groups were similar regarding creatinine levels, defined as normal (≥0.7 mg/dL; to convert to micromoles per liter, multiply by 88.4) and low (<0.7 mg/dL); subgroup analysis did not support a better outcome for reldesemtiv for those with normal baseline creatinine (Figure 3).
Figure 3. Change in Amyotrophic Lateral Sclerosis (ALS) Functional Rating Scale–Revised (ALSFRS-R) at Week 24 by Subgroup.
FVC indicates forced vital capacity; LSM, least squares mean.
aDetermined post hoc.
During the double-blind, placebo-controlled period of the trial, there were 15 deaths, 9 (2.8%) in the reldesemtiv group and 6 (3.7%) in the placebo group. Through weeks 24 to 48, 15 additional deaths (4.6%) occurred in those originally assigned reldesemtiv, and 6 deaths (3.7%) occurred in those originally assigned placebo. The most common cause of death was respiratory failure/arrest (12 [2.5%]), followed by assisted suicide/euthanasia (9 [1.9%]) and ALS (7 [1.4%]). No deaths were attributed to the study drug.
Safety
During the double-blind, placebo-controlled period, there were 66 participants who experienced a serious adverse event (AE): 41 (12.6%) in the reldesemtiv group and 25 (15.5%) in the placebo group (eTable 1 in Supplement 2). Correspondingly, 258 participants (79.4%) vs 125 participants (77.6%) experienced 1 or more treatment-emergent AE (TEAE) (eTable 2 in Supplement 2). The most common AEs with reldesemtiv vs placebo were falls (58 [17.8%] vs 23 [14.3%]), skin and subcutaneous tissue disorder (33 [10.2%] vs 19 [11.8%]), and COVID-19 infections (30 [9.2%] vs 13 [8.1%]). During the double-blind period, specific TEAEs occurred with similar frequencies in both treatment groups, except for investigations related to elevation of alanine transaminase (ALT) and aspartate transaminase (AST). Elevations of ALT level were found in 6.5% of participants (21 of 325) taking reldesemtiv vs 1.9% (3 of 161) taking placebo and elevations of AST in 5.5% of participants (18 of 325) taking reldesemtiv vs 0.6% (1 of 161) taking placebo. Dose interruptions related to AST and or ALT elevations occurred more frequently in the reldesemtiv group (5 of 325 [1.5%]) compared with the placebo group (0), as did permanent discontinuation of study drug (6 [1.8%] vs 1 [0.6%]). A dose-dependent decline in estimated glomerular filtration rate cystatin C (eGFRCysC; Chronic Kidney Disease Epidemiology Collaboration cystatin C equation) reported in the FORTITUDE-ALS trial reversed after stopping treatment.12 A similar pattern was seen in the COURAGE-ALS trial. Two participants, 1 each assigned to reldesemtiv and placebo, permanently discontinued study drug due to decline in eGFRCysC.
Discussion
COURAGE-ALS, a phase 3 study, was designed and implemented to replicate and extend the results of its phase 2 predecessor, the FORTITUDE-ALS trial.12 However, the COURAGE-ALS study was halted after the second IA, at a time when 489 participants had been randomized and 276 participants had completed 24 weeks of double-blind, placebo-controlled dosing. Reldesemtiv did not show evidence of efficacy in ALS. Given excess missing data, the original primary analysis is problematic. The combined assessment of function and mortality was considered valid in dealing with missing data, and it, too, failed to show a benefit from reldesemtiv treatment. Although statistical testing terminated after the primary analysis, secondary end points trended numerically in favor of placebo. Reldesemtiv was well tolerated, with a similar percentage of participants continuing active treatment in the reldesemtiv and placebo groups. Like the FORTITUDE-ALS trial, reldesemtiv was associated with a higher incidence of elevated transaminase levels compared with placebo in the COURAGE-ALS study. Preplanned subgroup analyses failed to suggest that any factors were associated with either better or worse impact of study drug on disease progression.
Several modifications were implemented in the COURAGE-ALS study compared with the FORTIDUDE-ALS trial, with the goal of maximizing the probability of observing treatment benefit. The FORTITUDE-ALS trial studied 3 reldesemtiv dose levels (150 mg, 300 mg, and 450 mg administered orally twice daily), the COURAGE-ALS study evaluated 1 dose (300 mg administered twice daily), and both compared with placebo. The dose in the COURAGE-ALS study was chosen due to its safety profile, and because all 3 doses in the FORTITUDE-ALS study were nearly equally beneficial. Second, a subgroup analysis performed on the FORTITUDE-ALS data suggested a larger point estimate of effect in those with prestudy estimates of disease progression that were in the intermediate or fastest progressing tertiles. Therefore, modified inclusion criteria included only participants within 24 months of first symptom onset (rather than 24 months since disease diagnosis), and an ALSFRS-R upper limit of 44 at screening. These modifications were successful in recruiting participants with higher prebaseline progression rates in the COURAGE-ALS study compared with FORTITUDE-ALS trial. However, the on-study progression rate in the placebo group in the COURAGE-ALS study was lower (0.80 ALSFRS-R points per month) than that seen in participants meeting these modified criteria in the shorter, phase 2 FORTITUDE-ALS study (1.17 ALSFRS-R points per month). Based on the subgroup analyses of participants by prestudy progression rates, it is unlikely that this difference accounted for the failure to detect efficacy. Enriching enrollment of participants with more rapidly progressive disease likely also resulted in a population with greater loss of muscle. Given the mechanism of action for reldesemtiv, this raised the possibility that the change in eligibility criteria may have contributed to the negative results. Post hoc subgroup analysis of those with normal vs low baseline creatinine did not suggest this played a role in the negative results.
The planned IA proved important in reducing the time participants were exposed to an agent that had no efficacy. The study was stopped before 300 participants had completed 6 months of study and after 489 participants had been randomized instead of the planned 555 participants. The independent DMC had clear guidelines that informed their decision, which the study management team supported after their review of the data. To our knowledge, this was the first ALS trial that implemented a second IA based on the promising zone design using the CDL method.
The results reported here parallel findings from other clinical trials testing agents intended to improve skeletal muscle function, including levosimendan5,6 and ozanezumab.19 Although muscle-directed strategies may prove effective in static or slowly progressive processes, our data and those of others suggest this is unlikely to be an effective strategy in ALS or other conditions with ongoing denervation.
Limitations
Overall, the negative results of the COURAGE-ALS study may have 2 critically important implications. First, although promising trends were discerned in all phases of the tirasemtiv development program and in the phase 2 study of reldesemtiv, none of these studies met their primary efficacy end point. In the phase 2 study of tirasemtiv, no efficacy signal was seen in ALSFRS-R, although a key secondary end point, SVC, showed a nominally significant benefit.9,11,12 SVC was the primary end point of the phase 3 tirasemtiv study; negative results were believed to reflect poor tolerability, as a per protocol post hoc analysis again suggested a benefit.11 In the FORTITUDE-ALS trial, the phase 2 study of reldesemtiv, SVC was the primary outcome, with ALSFRS-R secondary; neither end point reached statistical significance, although combined analysis of all reldesemtiv treated participants compared to placebo showed 27% slower progression in SVC and 25% slower progression in ALSFRS-R.12 This contrasts to the findings in the current study, in which ALSFRS-R scores fell 17% faster in the reldesemtiv treated group.
Conclusions
In the COURAGE-ALS randomized clinical trial, the overall development of FSTAs evaluated an important clinical hypothesis with great rigor. The results, although not supportive of future development for ALS, are clear and important.
Trial Protocol and Statistical Analysis Plan.
eMethods. Additional Information for the Inclusion Criteria
eTable 1. Serious Adverse Events (Through Week 24)
eTable 2. Treatment-Emergent Adverse Events (≥10 in Any Treatment Group) Through Week 24
eTable 3. COURAGE-ALS Investigators
Nonauthor Collaborators. COURAGE-ALS Study Group.
Data Sharing Statement.
References
- 1.Brown RH, Al-Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med. 2017;377(2):162-172. doi: 10.1056/NEJMra1603471 [DOI] [PubMed] [Google Scholar]
- 2.Holzbaur EL, Howland DS, Weber N, et al. Myostatin inhibition slows muscle atrophy in rodent models of amyotrophic lateral sclerosis. Neurobiol Dis. 2006;23(3):697-707. doi: 10.1016/j.nbd.2006.05.009 [DOI] [PubMed] [Google Scholar]
- 3.Morrison BM, Lachey JL, Warsing LC, et al. A soluble activin type IIB receptor improves function in a mouse model of amyotrophic lateral sclerosis. Exp Neurol. 2009;217(2):258-268. doi: 10.1016/j.expneurol.2009.02.017 [DOI] [PubMed] [Google Scholar]
- 4.Harrison C. Neuromuscular disorders: troponin activator improves muscle function. Nat Rev Drug Discov. 2012;11(4):272. doi: 10.1038/nrd3703 [DOI] [PubMed] [Google Scholar]
- 5.Al-Chalabi A, Shaw P, Leigh PN, et al. Oral levosimendan in amyotrophic lateral sclerosis: a phase II multicenter, randomised, double-blind, placebo-controlled trial. J Neurol Neurosurg Psychiatry. 2019;90(10):1165-1170. doi: 10.1136/jnnp-2018-320288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cudkowicz M, Genge A, Maragakis N, et al. ; REFALS investigators . Safety and efficacy of oral levosimendan in people with amyotrophic lateral sclerosis (the REFALS study): a randomized, double-blind, placebo-controlled phase 3 trial. Lancet Neurol. 2021;20(10):821-831. doi: 10.1016/S1474-4422(21)00242-8 [DOI] [PubMed] [Google Scholar]
- 7.Russell AJ, Hartman JJ, Hinken AC, et al. Activation of fast skeletal muscle troponin as a potential therapeutic approach for treating neuromuscular diseases. Nat Med. 2012;18(3):452-455. doi: 10.1038/nm.2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrews JA, Miller TM, Vijayakumar V, et al. CK-2127107 amplifies skeletal muscle response to nerve activation in humans. Muscle Nerve. 2018;57(5):729-734. doi: 10.1002/mus.26017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shefner JM, Cudkowicz ME, Hardiman O, et al. ; VITALITY-ALS Study Group . A phase III trial of tirasemtiv as a potential treatment for amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2019;0(0):1-11. doi: 10.1080/21678421.2019.1612922 [DOI] [PubMed] [Google Scholar]
- 10.Shefner JM, Wolff AA, Meng L. The relationship between tirasemtiv serum concentration and functional outcomes in patients with ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(7-8):582-585. doi: 10.3109/21678421.2013.817587 [DOI] [PubMed] [Google Scholar]
- 11.Shefner JM, Wolff AA, Meng L, et al. ; BENEFIT-ALS Study Group . A randomized, placebo-controlled, double-blind phase IIb trial evaluating the safety and efficacy of tirasemtiv in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17(5-6):426-435. doi: 10.3109/21678421.2016.1148169 [DOI] [PubMed] [Google Scholar]
- 12.Shefner JM, Andrews JA, Genge A, et al. A phase 2, double-blind, randomized, dose-ranging trial of reldesemtiv in patients with ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2021;22(3-4):287-299. doi: 10.1080/21678421.2020.1822410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shefner JM, Al-Chalabi A, Andrews JA, et al. COURAGE-ALS: a randomized, double-blind phase 3 study designed to improve participant experience and increase the probability of success. Amyotroph Lateral Scler Frontotemporal Degener. 2023;24(5-6):523-534. doi: 10.1080/21678421.2023.2216223 [DOI] [PubMed] [Google Scholar]
- 14.Brooks BR, Miller RG, Swash M, Munsat TL; World Federation of Neurology Research Group on Motor Neuron Diseases . El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293-299. doi: 10.1080/146608200300079536 [DOI] [PubMed] [Google Scholar]
- 15.Quanjer PH, Stanojevic S, Cole TJ, et al. ; ERS Global Lung Function Initiative . Multiethnic reference values for spirometry for the 3- to 95-year age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324-1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berry JD, Miller R, Moore DH, et al. The Combined Assessment of Function and Survival (CAFS): a new end point for ALS clinical trials. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(3):162-168. doi: 10.3109/21678421.2012.762930 [DOI] [PubMed] [Google Scholar]
- 17.Chen YH, DeMets DL, Lan KK. Increasing the sample size when the unblinded interim result is promising. Stat Med. 2004;23(7):1023-1038. doi: 10.1002/sim.1688 [DOI] [PubMed] [Google Scholar]
- 18.Barp A, Ferrero A, Casagrande S, Morini R, Zuccarino R. Circulating biomarkers in neuromuscular disorders: what is known, what is new. Biomolecules. 2021;11(8):1246. doi: 10.3390/biom11081246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meininger V, Genge A, van den Berg LH, et al. ; NOG112264 Study Group . Safety and efficacy of ozanezumab in patients with amyotrophic lateral sclerosis: a randomized, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16(3):208-216. doi: 10.1016/S1474-4422(16)30399-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan.
eMethods. Additional Information for the Inclusion Criteria
eTable 1. Serious Adverse Events (Through Week 24)
eTable 2. Treatment-Emergent Adverse Events (≥10 in Any Treatment Group) Through Week 24
eTable 3. COURAGE-ALS Investigators
Nonauthor Collaborators. COURAGE-ALS Study Group.
Data Sharing Statement.