Abstract
Lung cancer remains the leading cause of cancer-related mortality worldwide. Since 2024, the non–small-cell lung cancer (NSCLC) landscape has undergone a transformative shift, driven by 11 FDA approvals. Recent advances in molecular profiling, targeted therapies, and immunotherapies have revolutionized NSCLC management, ushering in an era of personalized treatment with improved patient outcomes. The increased adoption of low-dose computed tomography (LDCT) for screening has enhanced early detection, enabling intervention at more curable stages. Molecular diagnostics now play a pivotal role in guiding treatment strategies, with actionable genomic alterations (AGAs) informing the use of EGFR, ALK, ROS1, KRAS, NRG1, and other targeted inhibitors in both early and advanced settings. For instance, targeted therapies are increasingly being integrated into early-stage management, with adjuvant osimertinib for EGFR-mutated NSCLC and alectinib for ALK-positive NSCLC demonstrating substantial survival benefits. Immunotherapy, particularly immune checkpoint inhibitors, has become a cornerstone of treatment for AGA-negative NSCLC, either as monotherapy or in combination with chemotherapy, and is increasingly being utilized in the perioperative setting. Furthermore, emerging therapies such as bispecific antibodies, antibody–drug conjugates (ADCs), and novel immunotherapeutic agents show promise in addressing resistance mechanisms and improving outcomes in advanced-stage disease. Although new challenges arise, the evolving NSCLC treatment paradigm continues to prioritize precision medicine, offering hope for prolonged survival and enhanced quality of life for patients.
Keywords: NSCLC, Lung Cancer Management, Immunotherapy, Targeted Therapy, Actionable Genomic Alterations
Introduction
Lung cancer has remained the leading cause of cancer-related mortality worldwide since the 1950s, posing a significant global health challenge [1]. NSCLC, which constitutes the majority of lung cancer cases, has historically been associated with poor outcomes due to its often late diagnosis and limited treatment options [2]. However, the landscape of NSCLC management has undergone exponential growth in recent years, driven by groundbreaking advancements in targeted therapies and immunotherapies. These innovations have ushered in a new era of precision medicine, allowing treatments to be tailored to the unique molecular and immunological profiles of patients, thereby improving efficacy and survival outcomes. Additionally, these advancements have redefined the standard of care, offering renewed hope to patients and clinicians alike. This review provides a comprehensive update on the evolving management strategies for NSCLC, with a focus on recent developments in screening, diagnostics, and therapeutic advancements in early-stage and advanced disease.
Lung Cancer Screening
Lung cancer screening with LDCT plays a pivotal role in detecting lung cancer at earlier, more treatable stages, as evidenced by a 20% reduction in mortality demonstrated in the National Lung Screening Trial (NLST) in 2013 [3]. This resulted in a Grade B recommendation by the United States Preventive Services Task Force (USPSTF) in 2013 for annual lung cancer screening in adults aged 55–80 years with a 30-pack-year smoking history, who are either current smokers or have quit within the last 15 years [4] and a similar recommendation from the American Cancer Society (ACS) for adults aged 55–74 years old [5]. In 2020, the NELSON study in the Netherlands and Belgium published a large, randomized lung cancer screening trial which not only confirmed the relative reduction in death rate by 20% but also reinforced the importance of early detection.
These studies ultimately led to updated guidelines (Table 1) by the USPSTF in March 2021 and the ACS in November 2023, which have expanded eligibility for screening, targeting individuals aged 50–80 years with a 20 pack-year smoking history who are currently smoking or have quit within the past 15 years [6, 7]. These updates specifically aim to include underserved and lower socioeconomic status (SES) groups, as well as women and minorities who may face a heightened risk of lung cancer despite lower daily cigarette use. By reducing barriers to screening and enhancing early detection, these changes have resulted in a 61.1% relative increase in eligibility for the lowest SES quintile compared to a 49.6% increase for the highest SES quintile. However, further efforts are needed to allocate resources for tailored community outreach strategies and to address persistent barriers to screening for individuals from lower SES backgrounds. The ACS guidelines further emphasize shared decision-making and smoking cessation support to complement screening efforts, aiming to reduce lung cancer mortality and morbidity across diverse populations [7].
Table 1.
Comparison of ACS and USPSTF lung cancer screening guideline
| Criteria | ACS (2023)[7] | USPSTF (2021)[6] |
|---|---|---|
| Age Range | 50–80 years | 50–80 years |
| Smoking History | ≥ 20 pack-years | ≥ 20 pack-years |
| Time Since Quitting | No requirement | Within the last 15 years |
| Screening Method | Annual LDCT | Annual LDCT |
| Key Change | Removed "years since quitting" criterion | Lowered age and pack-year thresholds |
While screening efforts focus on high-risk individuals with a smoking history, the increasing prevalence of lung cancer among never-smokers presents a critical area for future research and a significant public health consideration. Current risk prediction models inadequately address this population [8], necessitating the development of tools incorporating factors such as family history, second-hand smoke, occupational and environmental exposures, and genetic predispositions. Validating these models and assessing their cost-effectiveness will be essential for expanding screening efforts to never-smokers, potentially reducing lung cancer mortality in this growing demographic [8].
Diagnostic Work-Up
Staging
The staging of NSCLC is continually refined through evidence-based iterations led by the International Association for the Study of Lung Cancer (IASLC). Following the widespread adoption of the 8th edition since 2017 [9], which introduced more granular tumor (T) descriptors and refined stratification of metastatic (M) disease, the recently developed 9th edition has further enhanced the accuracy and clinical utility of NSCLC staging [10]. Key modifications from the 8th to the 9th edition include the reclassification of stage groups based on a more refined nodal (N) category, as well as more nuanced M descriptors that better distinguish distinct patterns of metastatic spread. These refinements reflect an expanded global database, the incorporation of more nuanced prognostic data, and the consideration of emerging treatment modalities.
Looking ahead, with the 10th edition already underway, efforts aim to build upon this trajectory by integrating emerging data sources, advanced imaging modalities, and molecular profiling tools, ultimately refining staging accuracy and better reflecting its prognostic value.
Molecular Profiling
Molecular profiling has emerged as a cornerstone in the management of NSCLC, revolutionizing the approach to diagnosis, prognosis, and personalized treatment. Advances in molecular testing based on next-generation sequencing (NGS) have enabled the identification of key driver mutations and genetic alterations, such as EGFR, ALK, ROS1, BRAF, MET, RET, NTRK, NRG, KRAS, and ERBB2, that are critical for selecting targeted therapies [11]. Additionally, the discovery of biomarkers such as PD-L1 expression and tumor mutational burden has expanded the landscape of immunotherapy in NSCLC, although their role as predictive biomarkers needs further investigation [11]. Nonetheless, molecular profiling not only guides therapeutic decision-making but also provides insights into mechanisms of resistance, facilitating the development of novel treatment strategies [12]. As molecular diagnostic technologies continue to evolve, their integration into clinical practice is essential for refining prognostic accuracy, optimizing treatment outcomes, and advancing the field of precision oncology in NSCLC. Additionally, emerging evidence suggests that circulating tumor DNA analysis may aid in minimal residual disease detection, though further validation is needed.
AGA Negative NSCLC Treatment Approaches
Immunotherapy with or without chemotherapy has been a fundamental aspect of the management of metastatic NSCLC without AGA. In recent years, immunotherapy has expanded from late-stage treatment to earlier stages of disease, leading to FDA approvals in the perioperative and adjuvant settings (Table 2).
Table 2.
Perioperative systemic therapy for resectable NSCLC with negative actionable mutations
| Drug/ Trial | Eligible NSCLC stage (AJCC edition) | Study design | Study size | Median EFS/DFS; EFS/DFS (%); HR (95% CI) | pCR: | Other outcomes | EFS based on PD-L1 score HR (95% CI) | FDA approval |
|---|---|---|---|---|---|---|---|---|
| Preoperative | ||||||||
|
Nivolumab CheckMate 816[16] |
Resectable stage IB-IIIA (7th) | Neoadjuvant nivolumab + CT vs. CT for 3 cycles | 179 vs. 179 |
31.6 mo vs. 20.8 mo; 63.8% vs. 45.3% at 24 mo; 0.63 (0.45–0.87) |
24% vs. 2.2% | OS: 82.7% vs. 70.6% at 24 mo; HR: 0.57 (0.30–1.07) |
< 1%: 0.85 (0.54–1.32) 1–49%: 0.58 (0.30–1.12) ≥ 50%: 0.24 (0.10–0.61) |
Mar 2022 |
| Perioperative | ||||||||
|
Pembrolizumab KEYNOTE-671[17] |
Resectable stage II-IIIB (N2 stage; 8th) | Neoadj pembrolizumab + CT vs. placebo + CTx every 3 weeks for 4 cycles → surgery → adjuvant pembrolizumab vs. placebo every 3 weeks up to 13 cycles | 397 vs. 400 | NE vs. 17 mo; 62.4% vs. 40.6% at 24 mo; 0.58 (0.46–0.72) | 18.1% vs. 4.0% | OS: 80.9% vs. 77.6% at 24 mo; HR: 0.73 (0.54–0.99) |
< 1%: 0.77 (0.55–1.07) 1–49%: 0.51 (0.34–0.75) ≥ 50%: 0.42 (0.28–0.65) |
Oct 2023 |
|
Durvalumab AEGEAN[18] |
Resectable stage IIA-IIIB (N2 stage; 8th) | Neoadjuvant durvalumab + CTx vs. placebo + CTx every 3 weeks for 4 cycles → surgery → adjuvant durvalumab vs. placebo every 4 weeks for 12 cycles | 400 vs. 402 | NE vs. 25.9 mo; 63.3% vs. 52.4% at 24 mo; 0.68 (0.53–0.88) | 13.0% vs. 4.3% | OS: not yet available |
< 1%: 0.76 (0.49–1.17) 1–49%: 0.70 (0.46–1.05) ≥ 50%: 0.60 (0.35–1.01) |
Aug 2024 |
|
Nivolumab CheckMate 77 T[19] |
Resectable stage IIA-IIIB (8th) | Neoadjuvant nivolumab + CTx vs. placebo + CTx every 3 weeks for 3 cycles → surgery → adjuvant nivolumab vs. placebo every 4 weeks for 1 year | 229 vs. 232 | NE vs. 18.4 mo; 70.2% vs. 50.0% at 18 mo; 0.58 (0.42–0.81) | 25.3% vs. 4.7% | OS: not yet available |
< 1%: 0.73 (0.47–1.15) 1–49%: 0.76 (0.46–1.25) ≥ 50%: 0.26 (0.12–0.55) |
Oct 2024 |
| Postoperative | ||||||||
|
Atezolizumab |
Resected IB-IIIA (7th) | Surgery → 1–4 cycles of CTx → atezolimumab every 3 weeks for 16 cycles or 1 year vs. best supportive care (regular scans) | 507 vs. 498 | 42.3 mo vs. 35.3 mo; 70.2% vs. 61.6% at 24 mo; 0.79 (0.64–0.96) | NA | OS: 81.1% vs. 79.3% at 36 mo; HR: 0.995 (0.78–1.28) |
< 1%: 0.97 (0.72–1.31) 1–49%: 0.87 (0.60–1.26) ≥ 50%: 0.43 (0.27–0.68) |
Oct 2021 |
|
Pembrolizumab PEARLS/ KEYNOTE-091[15] |
Resected stage IB-IIIA (7th) | Surgery → adjuvant pembrolizumab vs. placebo every 3 weeks for up to 18 cycles; adjuvant CTx allowed; RTx not permitted | 590 vs. 587 | 53.6 mo vs. 42.0 mo; 67% vs. 59% at 24 mo; 0.76 (0.63–0.91) | NA | OS: 89% vs. 88% at 24 mo; HR: 0.87 (0.67–1.15) |
< 1%: 0.78 (0.58–1.03) 1–49%: 0.67 (0.48–0.92) ≥ 50%: 0.82 (0.57–1.18) |
Jan 2023 |
EFS, event-free survival; DFS, disease-free survival; pCR, pathologic complete response; HR, hazard ratio; CI, confidence interval; CT, chemotherapy; RT, radiation therapy; mo, months; OS, overall survival; NE, not estimable; mPR, major pathological response rate; *All preoperative and perioperative trials excluded patients with known EGFR or ALK alterations
Early-Stage Resectable NSCLC
In early-stage resectable NSCLC, two pivotal studies first demonstrated the benefit of adjuvant immunotherapy. The IMpower010 trial assessed atezolizumab as adjuvant therapy after adjuvant chemotherapy in resected stage IB-IIIA NSCLC. The result showed that, in the intention-to-treat population, atezolizumab improved disease-free survival (DFS) compared to best supportive care (HR, 0.81; 95% CI, 0.67–0.99; P = 0.04). The greatest benefit was observed in the stage II-IIIA population with PD-L1 ≥ 1% (HR 0.66; 95% CI, 0.50–0.88; P = 0.0039) [13]. Notably, stage II-IIIA patients with PD-L1 < 1% did not derive a DFS benefit with atezolizumab[13]. The updated overall survival (OS) data from IMpower010 revealed an OS benefit of atezolizumab only in stage II-IIIA patients with PD-L1 ≥ 50% (HR 0.43; 95% CI, 0.24–0.78), while other subgroups did not show significant improvement [14].
The PEARLS/KEYNOTE-091 compared pembrolizumab with placebo as adjuvant therapy in resected stage IB-IIIA NSCLC. In the overall population, pembrolizumab improved DFS compared to placebo (53.6 m vs. 42.0 m; HR 0.76; 95% CI, 0.63–0.91; P = 0.0014) [15]. Unlike the IMpower010 study, the PEARLS/KEYNOTE-091 study demonstrated a DFS benefit of pembrolizumab across all PD-L1 groups. The OS data for PEARLS/KEYNOTE-091 are still pending.
Neoadjuvant immunotherapy was subsequently explored in resectable NSCLC without AGA due to several key advantages over adjuvant approaches: early control of micrometastatic disease, enhanced immune response, improved pathologic responses and reduced treatment delays. The CheckMate 816 is an open-label, randomized phase III trial that assessed neoadjuvant nivolumab plus chemotherapy versus chemotherapy alone in resectable stage IB to IIIA NSCLC. Adding nivolumab to chemotherapy prolonged event-free survival (EFS) (31.6 m vs. 20.8 m; HR 0.63; 97.38% CI, 0.43 to 0.91; P = 0.005) and resulted in a higher pathologic complete response (pCR) (24% vs. 2.2%; OR 13.94; 99% CI, 3.49 to 55.75; P < 0.001) [16].
Following the establishment of neoadjuvant chemoimmunotherapy as a standard of care for resectable NSCLC based on the results of the CheckMate 816 trial, several phase III randomized trials have further evaluated the role of immunotherapy combined with chemotherapy in the perioperative setting.
The CheckMate 77 T trial evaluated neoadjuvant nivolumab and chemotherapy compared to chemotherapy, followed by surgery and adjuvant nivolumab or chemotherapy for up to 1 year in resectable stage IIA and IIIB NSCLC. The result revealed significantly better median EFS (NR vs. 18.4 m; HR 0.58; 97.36% CI, 0.42–0.81; P < 0.001), pCR (25.3% vs. 4.7%; OR 6.64; 95% CI, 3.40–12.97) and major pathologic response (MPR) (35.4% vs.12.1%; OR 4.01; 95% CI, 2.48–6.49) in the nivolumab arm [19].
The Keynote-671 trial demonstrated that perioperative pembrolizumab plus chemotherapy in resectable stage IIA-IIIB resulted in significantly longer 24-month EFS (62.4% vs. 40.6%; HR 0.58; 95% CI, 0.46–0.72; P < 0.001), pCR (18.1% vs.4.0%; difference 14.2%; 95% CI, 10.1–18.7; P < 0.0001) and MPR (30.2% vs. 11.0%; difference 19.2%; 95% CI, 13.9–24.7; P < 0.0001) were observed. The EFS benefit favored pembrolizumab and chemotherapy across all subgroups [17].
The Neotorch trial assessed toripalimab versus placebo in combination with chemotherapy in the perioperative setting for stage II and III resectable NSCLC. The study demonstrated a benefit in the toripalimab arm with longer EFS (not estimable vs. 15.1 m; HR 0.40; 95% CI, 0.28–0.57; P < 0.001) and higher percentage of MPR (48.5% vs. 8.4%; difference 40.2%; 95% CI, 32.2–48.1; P < 0.001) as well as pCR (24.8% vs 1.0%; difference 23.7%; 95% CI, 17.6–29.8) [20].
The AEGEAN trial evaluated durvalumab vs. placebo in combination with chemotherapy in the perioperative setting for resectable stage II to IIIB NSCLC. The EFS was significantly longer in the durvalumab arm compared to placebo (HR 0.68; 95% CI, 0.53–0.88; P = 0.004). The pCR was also significantly higher, favoring durvalumab (17.2% vs. 4.3%; difference 13.0%; 95% CI, 8.7–17.6; P < 0.001) [18].
These studies collectively reinforce the efficacy of integrating immunotherapy into the perioperative setting and offer a multimodal strategy that requires early multidisciplinary discussions for resectable NSCLC. However, several important unanswered questions remain that require further investigation. For instance, determining the optimal patient selection for adjuvant therapy, including identifying prognostic factors such as pCR and MPR, is essential. In addition, there is a need to establish the correlation between surrogate endpoint and overall survival, which will require long-term follow-up for validation.
Stage III Unresectable NSCLC
For unresectable stage III NSCLC, concurrent chemoradiotherapy remains the standard of care as a definitive treatment. The PACIFIC trial demonstrated the survival benefit of durvalumab consolidation for up to 12 months post-chemoradiation and it was approved by the FDA in 2018. The 5-year updated results were consistent with the primary analysis. Median OS was significantly longer in the durvalumab arm (47.5 m vs. 29.1 m; HR 0.72; 95% CI, 0.59–0.89), with an estimated 5-year OS rate of 42.9% for durvalumab, compared to 33.4% for placebo. Median EFS was also longer in the durvalumab arm compared to placebo (16.9 m vs. 5.6 m; HR 0.55; 95% CI, 0.45–0.68), and 5-year PFS rate was 33.1% for durvalumab compared to 19.0% for placebo. Of note, the OS benefit favored durvalumab across most subgroups, but was less clear for the PD-L1 negative cohort (HR 1.15; 95% CI, 0.75–1.75) highlighting an unmet need in this population [21, 22].
Locally Advanced/Metastatic NSCLC
For metastatic NSCLC without AGAs, immunotherapy, either alone or with chemotherapy, has remained the standard of care for frontline treatment. Updated long-term follow-up results from several landmark trials have demonstrated significant and durable survival benefits in this population.
Immunotherapy: Monotherapy and Dual Combinations
In the KEYNOTE-024 and KEYNOTE-042 studies, patients with PD-L1 ≥ 50% and PD-L ≥ 1%, respectively, and without EGFR or ALK alterations were randomized to receive pembrolizumab monotherapy versus platinum-based chemotherapy as frontline therapy. In KEYNOTE-024, the median OS was 26.3 months for pembrolizumab compared to 13.4 months for chemotherapy (HR 0.62; 95% CI, 0.48–0.81). The 5-year OS was estimated to be 31.9% for pembrolizumab versus 16.3% for the chemotherapy group [23]. In KEYNOTE-042, the OS favored pembrolizumab over chemotherapy in patients with PD-L1 ≥ 50% (HR 0.68; 95% CI, 0.57–0.81), PD-L1 ≥ 20% (HR 0.75; 95% CI, 0.64–0.87), and PD-L1 ≥ 1% (HR 0.79; 95% CI, 0.70–0.89). The estimated 5-year OS was 16.6%-21.9% compared to 8.5%-10.1% across different PD-L1 groups. In the exploratory analysis, patients with PD-L1 score of 1–49% did not achieve a statistically significant OS benefit, indicating that additional treatment strategies are needed in this population[24].
A similar OS benefit was observed with cemiplimab in metastatic NSCLC with PD-L1 ≥ 50% and without EGFR/ALK/ROS1 alterations in the frontline setting [25]. Likewise, atezolizumab demonstrated OS superiority in metastatic non-squamous and squamous NSCLC with PD-L1 ≥ 1% and EGFR/ALK wild-type status [26]. Long-term follow-up data are awaited to confirm the durability of these results.
The combination of dual immunotherapy with ipilimumab and nivolumab was evaluated in the CheckMate 227 trial as a frontline treatment and included patients across all PD-L1 TPS scores. The 5-year follow-up revealed a significant survival benefit. In patients with PD-L1 ≥ 1%, the 5-year OS rate was 24% for ipilimumab plus nivolumab compared to 14% for chemotherapy (HR 0.77; 95% CI, 0.66–0.91). In PD-L1 negative patients, ipilimumab plus nivolumab also demonstrated an improved 5-year OS rates (19% vs. 7%; HR 0.65; 95% CI, 0.52–0.81) [27].
Immunotherapy in Combination with Chemotherapy
Long-term follow-up data from key chemoimmunotherapy trials—KEYNOTE-189, IMpower150, KEYNOTE-407, and CheckMate 9LA—have further established the role of immunotherapy combined with chemotherapy as a standard of care with durable benefit in metastatic NSCLC without AGA.
In KEYNOTE-189, patients with untreated metastatic non-squamous NSCLC without EGFR or ALK alterations were randomized to receive pembrolizumab or placebo in combination with platinum and pemetrexed chemotherapy. The 5-year OS rate was 19.4 months for pembrolizumab plus chemotherapy versus 11.3 months for placebo plus chemotherapy (HR 0.60; 95% CI, 0.50–0.72). The OS benefit favored pembrolizumab plus chemotherapy across all PD-L1 groups [28].
In the IMpower150 trial, patients with metastatic non-squamous NSCLC without EGFR or ALK alterations were randomized to receive atezolizumab-carboplatin-paclitaxel (ACP), atezolizumab-bevacizumab-carboplatin-paclitaxel (ABCP), or bevacizumab-carboplatin-paclitaxel (BCP). The ABCP regimen showed an OS benefit compared to the BCP regimen (19.5mo vs. 14.7mo; HR 0.80; 95% CI, 0.67–0.95). Exploratory analysis revealed that both ABCP and ACP demonstrated significantly longer OS compared to BCP in the PD-L1-high (30.0 m vs. 26.3 m vs.15.0 m) and PD-L1-positive (22.5mo vs. 24.4mo vs. 16.0mo) subgroups, but not the PD-L1-negative group. Notably, unlike most other trials where immunotherapy was continued for a maximum of 2 years, atezolizumab was continued until disease progression or unacceptable toxicity, suggesting a flexible approach without a definitive stopping point [29].
For metastatic squamous NSCLC, the KEYNOTE-407 trial randomized patients to receive pembrolizumab or placebo in combination with carboplatin and paclitaxel or nab-paclitaxel for four cycles, followed by pembrolizumab or placebo. The estimated 5-year OS rates were 18.4% vs 9.7% (HR 0.71; 95% CI, 0.0.59–0.85), and the OS benefit favored pembrolizumab plus chemotherapy across all PD-L1 subgroups [30].
In the CheckMate 9LA trial, metastatic NSCLC patients without sensitizing EGFR or ALK alterations were randomized to receive ipilimumab and nivolumab in combination with chemotherapy or chemotherapy alone. The 5-year OS favored the ipilimumab plus nivolumab plus chemotherapy regardless of PD-L1 status (5-year OS rates: 18% vs. 11%; HR 0.73; 95% CI, 062–0.85) [31].
AGA Directed Therapy in NSCLC
Targeted therapies have revolutionized the management of NSCLC, most notably in the metastatic setting, and are now increasingly being studied for incorporation into earlier stages, including the neoadjuvant and adjuvant settings [32]. We will explore how AGA-directed therapies are integrated into clinical decision-making across the spectrum of NSCLC and then examine the key genetic alterations guiding precision treatment in the metastatic setting.
Early-Stage Resectable NSCLC with AGA
To date, the FDA has approved two targeted agents as adjuvant therapy —osimertinib for EGFR-mutated and alectinib for ALK-mutated tumors—for resectable, early-stage NSCLC with AGA. In the ADAURA trial, patients with stage IB–IIIA (AJCC 7th edition) NSCLC harboring EGFR exon 19 deletion or exon 21 L858R mutations who had undergone surgical resection were randomized to receive three years of osimertinib versus placebo. The osimertinib arm demonstrated a marked improvement in DFS, reaching 65.8 months compared to 28.1 months in the placebo group (HR 0.27; 95% CI 0.21–0.34), and a 5-year OS of 88% versus 78% (HR 0.49; 95% CI, 0.34–0.70) [33]. Similarly, the ALINA trial evaluated patients with resected stage IB–IIIA (AJCC 7th edition) ALK-positive NSCLC randomized to receive either two years of alectinib or four cycles of platinum-based chemotherapy. At three years, DFS in the alectinib arm was 88.7%, compared to 54.0% in the platinum-based chemotherapy group (HR 0.24; 95% CI, 0.13–0.43) [34]. Although immunotherapy has improved outcomes in many subsets of NSCLC, it is generally considered less effective in tumors harboring driver genomic alterations such as EGFR and ALK, further emphasizing the importance of molecularly targeted therapies in these settings.
Stage III Unresectable NSCLC with AGA
The LAURA study investigated the use of osimertinib until disease progression versus placebo in patients with unresectable stage III NSCLC harboring EGFR exon 19 deletion or exon 21 L858R mutations [35]. Notably, PFS was significantly improved with osimertinib (39.1 m vs. 5.6 m; HR 0.16; 95% CI, 0.10–0.24), and at three years, OS was 84% versus 74% (HR 0.81; 95% CI, 0.42–1.56), findings that led to FDA approval of osimertinib in the adjuvant setting after chemoradiation [35]. Although the indefinite treatment duration may be unsettling, these results highlight substantial patient benefit given the poor PFS in the placebo arm and the high incidence of distant metastases, including CNS involvement. Nevertheless, further efforts are needed in this adjuvant space to achieve a truly curative outcome.
Locally Advanced/Metastatic NSCLC with AGA
As molecular profiling becomes increasingly nuanced and comprehensive, a growing number of phase III trials have demonstrated the efficacy of targeted therapies for locally advanced and metastatic NSCLC, both as monotherapy and in combination with chemotherapy or other targeted agents (Table 3). These advancements extend beyond traditional tyrosine kinase inhibitors (TKI) to include emerging treatment modalities such as bispecific antibodies and ADCs further expanding the therapeutic arsenal for patients with AGA.
Table 3.
Key trials supporting FDA-approved targeted therapies for advanced-stage NSCLC with actionable genomic alterations
| Drug/ Major Trial (phase) | Study population | Study design | Study size | Primary endpoints | Median PFS (mo); PFS (%); HR (95% CI) |
Median OS; OS (%); HR (95% CI) | Other outcomes | FDA approval month |
|---|---|---|---|---|---|---|---|---|
| EGFR mutation | ||||||||
|
Amivantamab, Lazertinib MARIPOSA (III)[36] |
Advanced untreated with EGFR Ex19del/L858R |
Av + La vs Osi vs. La |
429 vs. 429 vs. 216 | PFS (Av + La vs. Os) |
23.7 vs. 16.6 mo; 48 vs. 34% at 24mo; 0.70 (0.58–0.85) |
NE vs. NE; 74 vs. 69% at 24mo; 0.80 (0.61–1.05) |
ORR: 86 vs. 85% | Aug 2024 |
|
Amivantamab, Lazertinib MARIPOSA-2 (III)[37] |
Advanced pretreated with Osimertinib with EGFR Ex19del/L858R | Av + La + CT vs. Av + CT vs. CT | 263 vs. 263 vs. 131 | PFS |
8.3 vs. 6.3 vs. 4.2 mo; 37 vs. 22 vs.13% at 12 mo; Av + La + CT vs. CT: 0.48 (0.36–0.64), Av + CT vs. CT: 0.44 (0.35–0.56) |
Median OS and % NR; Av + La + CT vs. CT: 0.77 (0.49–1.21), Av + CT vs. CT: 0.96 (0.67–1.35) |
ORR: 63 vs. 64 vs. 36% |
Sep 2024 |
|
Amivantamab PAPILLON (III)[38] |
Advanced untreated with EGFR Ex20ins | Amivantamab + CT vs. CT only | 153 vs. 155 | PFS |
11.4 mo vs. 6.7 mo; 31 vs. 3% at 18 mo; 0.40 (0.30–0.53) |
NE vs. 24.4%; 72 vs. 54% at 24 mo; 0.67 (0.42–1.09) |
ORR: 73 vs. 47% | Mar 2024 |
|
Osimertinib FLAURA-2 (III)[39] |
Advanced untreated EGFR Ex19del/L858R | Osi + CT vs. Osi | 279 vs. 278 | PFS |
25.5 vs. 16.7 mo; 57 vs. 41% at 24 mo; 0.62 (0.49–0.79) |
NE vs. 36.7 mo; 80 vs. 72% at 24 mo; 0.75 (0.57–0.97) |
ORR: 83 vs. 76% | Feb 2024 |
| ALK fusion | ||||||||
|
Alectinib ALEX (III)[40] |
Advanced untreated with ALK | Alectinib vs. crizotinib | 152 vs. 151 | PFS |
34.8 vs. 10.9 mo; % NR; 0.43 (0.32–0.58) |
NE vs. 57.4 mo; 62.5 vs. 45.5% at 60 mo; 0.67 (0.46–0.98) |
ORR: 82.9 vs. 75.5% | Nov 2017 |
|
Brigatinib ALTA-1L (III)[41] |
Advanced untreated with ALK | Brigatinib vs. crizotinib | 137 vs. 138 | PFS | 24.0 vs. 11.1 mo; 43% vs. 19% at 36 mo; 0.48 (0.35–0.66) |
NE vs. NE; 66 vs. 60% at 48 mo; 0.81 (0.53–1.22) |
ORR: 71 vs. 60% | May 2020 |
|
Ensartinib eXALT3 (III)[42] |
Advanced untreated with ALK | Ensartinib vs. crizotinib | 143 vs. 147 | PFS | 25.8 vs. 12.7 mo; 0.51 (0.35–0.72) |
NE vs. NE; 78 vs. 78% at 24 mo; 0.91 (0.54–1.54) |
ORR: 74 vs. 67% | Dec 2024 |
|
Lorlatinib CROWN (III)[43] |
Advanced untreated with ALK | Lorlatinib vs. crizotinib | 149 vs. 147 | PFS |
NE vs. 9.1 mo; 60 vs. 8% at 60 mo; 0.19 (0.13–0.27) |
NE vs. NE; % NR; 0.72 (0.41–1.25) |
ORR: 76 vs. 58% | Mar 2021 |
| ROS1 fusion | ||||||||
|
Crizotinib PROFILE 1001 (I)[44] |
Locally advanced or metastatic ROS1 rearrangement-positive NSCLC | Study arm | 53 | ORR | 19.3 mo; % NR | 51.4 mo; 51% at 48 mo | ORR: 72% | Mar 2016 |
|
Entrectinib ALKA-372–001 (I), STARTRK-1 (I), STARTRK-2 (II)[45] |
Advanced or metastatic ROS1 fusion-positive NSCLC receiving at least 600 mg of entrectinib every day for at least 12 mo follow-up | Study arm | 53 | ORR | 19.0 mo; % NR | NE; 82% at 18 mo | ORR: 77% | Aug 2019 |
|
Repotrectinib TRIDENT-1 (I/II)[46] |
Advanced untreated and treated with ROS1 | ROS1-TKI untreated vs. treated vs. no CT | 71 vs. 56 | ORR |
35.7 vs. 9.0 mo; 77 vs. 41% at 12 mo |
NE vs. 25.1 mo; 91 vs. 69% at 12 mo |
ORR: 79 vs. 38% | Nov 2023 |
| BRAFV600E mutation | ||||||||
|
Dabrafenib + trametinib NCT01336634 (II)[47] |
Advanced untreated with BRAFV600E | Study arm | 36 | ORR | 10.9 mo; 72% at 6 mo | 24.6 mo; 51% at 24 mo | ORR: 64% | Jun 2017 |
|
Dabrafenib + trametinib NCT01336634 (II)[48] |
Advanced treated with BRAFV600E | Study arm | 57 | ORR | 9.7 mo; 65% at 6 mo | NE; 82% at 6 mo | ORR: 63.2% | Jun 2017 |
|
Encorafenib + binimetinib PHAROS (II)[49] |
Advanced untreated and treated with BRAFV600E | Study arm untreated and treated | 98 | ORR | NE untreated, 9.3 mo in previously treated group; % NR |
NE in both group; % NR |
ORR: 75% untreated, 46% in treated group | Oct 2023 |
| MET mutation and amplification | ||||||||
|
Capmatinib GEOMETRY mono-1 (II)[50] |
Advanced with MET ex14 mutation or amplification | Study arm untreated and treated by MET status | 364 | ORR |
4.2 untreated, 4.1 mo in treated group; % NR |
OS NR | ORR: 40% untreated, 29% in treated group | May 2020 |
|
Tepotinib VISION (II)[51] |
Advanced with MET ex14 mutation | Study arm | 99 | ORR | 8.5 mo; % NR | 17.1 mo; % NR | ORR: 46% | Feb 2024 |
| RET fusion | ||||||||
|
Selpercatinib LIBRETTO-431 (III)[52] |
Advanced untreated with RET | Selpercatinib vs. CT ± pembrolizumab | 129 vs. 83 | PFS |
24.8 vs. 11.2 mo; % NR; 0.46 (0.31–0.70) |
NE vs. NE; % NR; 1.04 (0.58–1.87) |
ORR: 84 vs. 65% | Sep 2022 |
|
Pralsetinib ARROW (II)[53] |
Advanced with RET | Study arm | 121 | ORR and safety | 9.1 untreated, 17.1 mo treated group; % NR |
NE in both groups; % NR |
ORR: 70% untreated, 61% prior CT | Aug 2023 |
| NTRK fusion | ||||||||
|
Entrectinib ALKA-372–001 (I), STARTRK-1 (I), STARTRK-2 (II)[54] |
Advanced with NTRK 600 mg of entrectinib | Study arm | 54 | ORR/DOR | 11.2 mo; % NR | 21 mo; % NR | ORR: 57% | Aug 2019 |
|
Larotrectinib NCT02122913 (I), SCOUT (I/II), NAVIGATE (II)[55] |
Non-CNS primary Advanced with NTRK | Study arm | 159 (12 lung cancer) | ORR |
28.3 mo; 6 7% at 12 mo |
44.4 mo; 88% at 12 mo |
ORR: 79% | Nov 2018 |
| KRASG12C mutation | ||||||||
|
Sotorasib CodeBreaK 200 (III)[56] |
Advanced treated with KRASG12C without AGA | Sotorasib vs. CT | 171 vs. 174 | PFS |
5.6 vs. 4.5 mo; 24.8 vs.10.1% at 12 mo; 0.66(0.51–0.86) |
10.6 vs. 11.3 mo; % NR; 1.01 (0.77–1.33) |
ORR: 28.1 vs. 13.2% | AA only in May 2021 |
|
Adagrasib KRYYSTAL-1 (I/II)[57] |
Advanced treated with KRASG12C | Study arm | 116 | ORR |
6.5 mo; 29% at 12 mo |
12.6 mo; 51% at 12 mo |
ORR: 42.9% | Dec 2022 |
| HER2 (ERBB2) alteration | ||||||||
|
Trastuzumab deruxtecan DESTINY-Lung01[58] |
Advanced treated with HER2 | Study arm | 91 | ORR | 8.2 mo; % NR | 17.8 mo; % NR | ORR: 55% | AA Apr 2024 |
| NRG1 fusion | ||||||||
|
Zenocutuzumab-zbco |
Advanced treated with NRG1 | Study arm | 64 NSCLC | ORR/DOR | NR | NR |
ORR: 33% DOR: 7.4mo |
AA Dec 2024 |
Abbreviations: AA, accelerated approval; Av, amivantamab; DOR, duration of response; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; CTx, chemotherapy; La, lazertinib; Osi, osimertinib; RTx, radiation therapy; mo, months; OS, overall survival; NE, not estimable; NR, not reported; ORR, objective response rate
EGFR-Targeted Therapy in Advanced NSCLC
Osimertinib, an irreversible EGFR TKI, has emerged as the mainstay of first-line therapy for EGFR-mutated (exon 19 deletion or L858R) advanced NSCLC. Following the full approval of osimertinib based on the AURA3 study [61] in patients who had progressed on a prior EGFR TKI with an exon 20 T790M mutation, the FLAURA study demonstrated superiority over earlier-generation EGFR TKIs (gefitinib and erlotinib) with an OS benefit of 38.6 months in the osimertinib group compared to 31.8 months in the comparator group (HR 0.8; 95% CI, 0.64–1) [62]. The trial also demonstrated a PFS benefit of 18.9 months in the osimertinib arm versus 10.2 months in the gefitinib or erlotinib arm (HR 0.46; 95%CI, 0.37–0.57) [63]. More recently, the FLAURA-2 study found that adding chemotherapy to osimertinib improved PFS from 16.7 to 25.5 months (HR 0.62).
Beyond osimertinib, amivantamab—an EGFR-MET bispecific antibody—has shown promise in EGFR exon 20 insertion–positive disease: the PAPILLON study reported a PFS of 11.4 vs. 6.7 months (HR 0.4; 95%CI, 0.3–0.53) for amivantamab plus chemotherapy compared to chemotherapy alone as first-line therapy [38]. Amivantamab plus lazertinib, a third generation of EGFR TKI, was also compared head-to-head with osimertinib in the MARIPOSA study which showed a PFS benefit of 23.7 vs. 16.6 months (HR 0.7; 95%CI, 0.58–0.85) [36]. However, a higher toxicity profile was observed in the amivantamab in combination plus lazertinib arm, with 75% vs. 43% of patients experiencing grade 3 or higher adverse events, leading to more frequent interruptions, dose adjustments, and treatment discontinuation [36]. Additionally, the MARIPOSA-2 study explored amivantamab with or without lazertinib in combination with chemotherapy after osimertinib progression, reporting PFS of 8.3, 6.3, and 4.2 months for the triple combination, double combination (amivantamab and chemotherapy), and chemotherapy alone, respectively [37]. These findings underscore the evolving treatment paradigm, with increasingly sophisticated EGFR-directed strategies and combination approaches improving outcomes for patients with EGFR-mutated advanced NSCLC.
ALK-Targeted Therapy in Advanced NSCLC
In ALK-rearranged advanced NSCLC, multiple first-line options have been approved, including crizotinib, alectinib, brigatinib, lorlatinib, and most recently, ensartinib [42]. Crizotinib’s use has diminished over time due to its limited CNS penetration, making newer-generation ALK inhibitors more attractive. Notably, the CROWN study demonstrated that lorlatinib delivered a median PFS that was not yet reached at five years, compared to 9.1 months in the crizotinib arm (HR 0.19; 95% CI, 0.13–0.27) [43]. Similarly, ensartinib has also recently been FDA-approved in the first-line setting, showing a PFS of 25.8 months versus 12.7 months with crizotinib (HR 0.51; 95% CI, 0.35–0.72). As the treatment landscape evolves, the diverse efficacy and side effect profiles of ALK-targeted agents—including the impressive results demonstrated by lorlatinib—will play a pivotal role in guiding personalized therapy decisions and optimizing outcomes for each patient.
Emerging Therapies for Other AGA
Repotrectinib has expanded the treatment repertoire for ROS1-rearranged advanced NSCLC, joining entrectinib and crizotinib as an FDA-approved option. In the TRIDENT-1 phase I/II study, repotrectinib demonstrated a remarkable PFS of 35.7 months compared to 9 months for crizotinib [46], underscoring its potential as a highly effective therapy in this molecularly defined patient population.
Encorafenib and binimetinib offer another therapeutic option for BRAF V600E-mutated NSCLC, demonstrating a 75% overall response rate (ORR) in untreated patients. However, treatment-related adverse events occurred in 24% of patients, leading to dose reductions or discontinuations, underscoring the need for larger studies to better understand tolerability and long-term outcomes [49].
Tepotinib was approved in February 2024, demonstrating a PFS of 8.5 months and an OS of 17.1 months, showing promise for patients with MET exon 14 skipping mutations [51].
Selpercatinib received approval based on the LIBRETTO-001 study with an ORR of 84% compared to 65% in the control group [52], and pralsetinib has also gained approval for RET-altered NSCLC with an ORR of 70% in the treatment naïve group [53].
Entrectinib and larotrectinib remain viable options for patients harboring NTRK fusions, either as an initial treatment when no other alternatives exist or after prior therapies [54, 55].
Sotorasib and adagrasib have been approved based on the CodeBreaK200 [56] and KRYSTAL-1 [57] studies, respectively. However, sotorasib did not receive full approval in 2023 after its accelerated approval because, despite meeting the PFS endpoint (5.6 vs. 4.5 months), the OS was not improved (10.6 vs. 11.3 months), and concerns were raised regarding patient dropout in the docetaxel arm and early crossover to sotorasib before disease progression [56].
Trastuzumab deruxtecan-nxki (TDxd) received tumor-agnostic approval for HER2-positive (IHC 3 +) solid tumors. This HER2-directed ADCs deliver deruxtecan payload, a topoisomerase I inhibitor, directly to HER2-expressing tumor cells. In the DESTINY-Lung01 phase II study, TDxd demonstrated an ORR of 55%, a median DOR of 9.3 months, and a PFS of 8.2 months [58], highlighting its efficacy in this subset of patients and offering an alternative in second line treatment.
The recent approval of zenocutuzumab-zbco (zeno), based on the eNRGy study, presents an advancement for previously treated advanced NSCLC with NRG1 fusion. Zeno is a bispecific antibody targeting HER2/HER3 signaling, effectively inhibiting NRG1 binding. While the full publication is pending, the FDA has reported an ORR of 33% and a median DOR of 7.4 months in a cohort of 64 NSCLC patients [60]. Previous abstract presentation showed 41 patients with NSCLC with an ORR 35% with good tolerability [64].
Conclusions
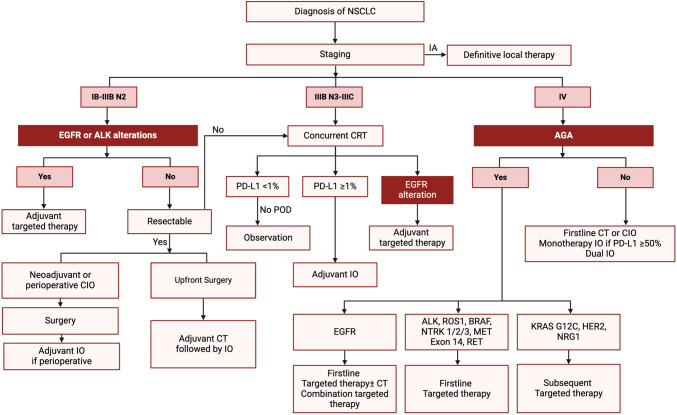
The management of NSCLC has evolved remarkably over the past decade, reflecting a paradigm shift driven by advances in screening, molecular diagnostics, and targeted therapeutics. Enhanced screening protocols have enabled earlier detection, while ongoing refinements in staging ensure patients receive the most appropriate treatments based on precise risk stratification. The incorporation of molecular profiling and immunotherapy into both early-stage and advanced disease settings has expanded treatment options, improved survival outcomes, and set the stage for more personalized care (Fig. 1). Targeted therapies for EGFR, ALK, ROS1, and other actionable alterations have revolutionized the therapeutic landscape, and novel approaches—ranging from bispecific antibodies to ADCs—continue to emerge. Although challenges remain, the future of NSCLC management is increasingly defined by innovation and collaboration.
Fig. 1.
Simplified schematic of initial management of NSCLC. AGA actionable genomic alterations, CIO chemoimmunotherapy, CT chemotherapy, IO immunotherapy, NSCLC non-small-cell lung cancer
Abbreviations
- ABCP
Atezolizumab-bevacizumab-carboplatin-paclitaxel
- ACP
Atezolizumab-carboplatin-paclitaxel
- ADCs
Antibody–drug conjugates
- AGA
Actionable genomic alterations
- ACS
American Cancer Society
- BCP
Bevacizumab-carboplatin-paclitaxel
- DFS
Disease-free survival
- EFS
Event-free survival
- ITT
Intention to treat
- IASLC
International Association for the Study of Lung Cancer
- LDCT
Low-dose computed tomography
- MPR
Major pathologic response
- NLST
National Lung Screening Trial
- NSCLC
Non-small-cell lung cancer
- OS
Overall survival
- ORR
Overall response rate
- pCR
Pathologic complete response
- SES
Socioeconomic status
- TDxd
Trastuzumab deruxtecan-nxki
- TKI
Tyrosine kinase inhibitors
- USPSTF
United States Preventive Services Taskforce
- zeno
Zenocutuzumab-zbco
Author Contributions
H.J., J.S., and H.C. contributed to the literature review and conceptualization. The initial draft was prepared by H.J., S.W., J.S., H.G., and H.C., with all authors reviewing, editing, and approving the final version of the manuscript. H.C. provided overall supervision for the manuscript.
Funding
The authors declare that no funds, grants, or other support was received during the preparation of this manuscript.
Data Availability
No datasets were generated or analyzed during the current study.
Declarations
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A: Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2024, 74(3):229–263. [DOI] [PubMed]
- 2.Siegel RL, Miller KD, Wagle NS, Jemal A: Cancer statistics, 2023. CA: A Cancer Journal for Clinicians 2023, 73(1):17–48. [DOI] [PubMed]
- 3.Team TNLSTR: Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. New England Journal of Medicine 2011, 365(5):395–409. [DOI] [PMC free article] [PubMed]
- 4.Moyer VA: Screening for Lung Cancer: U.S. Preventive Services Task Force Recommendation Statement. Annals of Internal Medicine 2014, 160(5):330–338. [DOI] [PubMed]
- 5.Wender R, Fontham ETH, Barrera E, Colditz GA, Church TR, Ettinger DS, Etzioni R, Flowers CR, Scott Gazelle G, Kelsey DK et al: American Cancer Society lung cancer screening guidelines. CA: A Cancer Journal for Clinicians 2013, 63(2):106–117. [DOI] [PMC free article] [PubMed]
- 6.Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Kubik M et al (2021) Screening for Lung Cancer. JAMA 325(10):962 [DOI] [PubMed] [Google Scholar]
- 7.Wolf AMD, Oeffinger KC, Shih TYC, Walter LC, Church TR, Fontham ETH, Elkin EB, Etzioni RD, Guerra CE, Perkins RB et al: Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA: A Cancer Journal for Clinicians 2024, 74(1):50–81. [DOI] [PubMed]
- 8.Kerpel-Fronius A, Tammemägi M, Cavic M, Henschke C, Jiang L, Kazerooni E, Lee C-T, Ventura L, Yang D, Lam S et al (2022) Screening for Lung Cancer in Individuals Who Never Smoked: An International Association for the Study of Lung Cancer Early Detection and Screening Committee Report. J Thorac Oncol 17(1):56–66 [DOI] [PubMed] [Google Scholar]
- 9.Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WEE, Nicholson AG, Groome P, Mitchell A, Bolejack V et al (2016) The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 11(1):39–51 [DOI] [PubMed] [Google Scholar]
- 10.Rami-Porta R, Nishimura KK, Giroux DJ, Detterbeck F, Cardillo G, Edwards JG, Fong KM, Giuliani M, Huang J, Kernstine KH et al (2024) The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groups in the Forthcoming (Ninth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 19(7):1007–1027 [DOI] [PubMed] [Google Scholar]
- 11.Odintsov I, Sholl LM (2024) Prognostic and predictive biomarkers in non-small cell lung carcinoma. Pathology 56(2):192–204 [DOI] [PubMed] [Google Scholar]
- 12.Ferro A, Marinato GM, Mulargiu C, Marino M, Pasello G, Guarneri V, Bonanno L (2024) The study of primary and acquired resistance to first-line osimertinib to improve the outcome of EGFR-mutated advanced Non-small cell lung cancer patients: the challenge is open for new therapeutic strategies. Crit Rev Oncol Hematol 196:104295 [DOI] [PubMed] [Google Scholar]
- 13.Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, Luft A, Akopov A, Martinez-Marti A, Kenmotsu H et al (2021) Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. The Lancet 398(10308):1344–1357 [DOI] [PubMed] [Google Scholar]
- 14.Felip E, Altorki N, Zhou C, Vallières E, Martínez-Martí A, Rittmeyer A, Chella A, Reck M, Goloborodko O, Huang M et al (2023) Overall survival with adjuvant atezolizumab after chemotherapy in resected stage II-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase III trial. Ann Oncol 34(10):907–919 [DOI] [PubMed] [Google Scholar]
- 15.O’Brien M, Paz-Ares L, Marreaud S, Dafni U, Oselin K, Havel L, Esteban E, Isla D, Martinez-Marti A, Faehling M et al (2022) Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB–IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol 23(10):1274–1286 [DOI] [PubMed] [Google Scholar]
- 16.Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson SJ et al (2022) Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med 386(21):1973–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wakelee H, Liberman M, Kato T, Tsuboi M, Lee S-H, Gao S, Chen K-N, Dooms C, Majem M, Eigendorff E et al (2023) Perioperative Pembrolizumab for Early-Stage Non–Small-Cell Lung Cancer. N Engl J Med 389(6):491–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heymach JV, Harpole D, Mitsudomi T, Taube JM, Galffy G, Hochmair M, Winder T, Zukov R, Garbaos G, Gao S et al (2023) Perioperative Durvalumab for Resectable Non–Small-Cell Lung Cancer. N Engl J Med 389(18):1672–1684 [DOI] [PubMed] [Google Scholar]
- 19.Cascone T, Awad MM, Spicer JD, He J, Lu S, Sepesi B, Tanaka F, Taube JM, Cornelissen R, Havel L et al (2024) Perioperative Nivolumab in Resectable Lung Cancer. N Engl J Med 390(19):1756–1769 [DOI] [PubMed] [Google Scholar]
- 20.Lu S, Zhang W, Wu L, Wang W, Zhang P, Fang W, Xing W, Chen Q, Yang L, Mei J et al (2024) Perioperative Toripalimab Plus Chemotherapy for Patients With Resectable Non-Small Cell Lung Cancer. JAMA 331(3):201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, Vansteenkiste JF, Garassino MC, Hui R, Quantin X et al (2022) Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. J Clin Oncol 40(12):1301–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, De Wit M et al (2017) Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N Engl J Med 377(20):1919–1929 [DOI] [PubMed] [Google Scholar]
- 23.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S et al (2021) Five-Year Outcomes With Pembrolizumab Versus Chemotherapy for Metastatic Non–Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50%. J Clin Oncol 39(21):2339–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Castro G, Kudaba I, Wu Y-L, Lopes G, Kowalski DM, Turna HZ, Caglevic C, Zhang L, Karaszewska B, Laktionov KK et al (2023) Five-Year Outcomes With Pembrolizumab Versus Chemotherapy as First-Line Therapy in Patients With Non–Small-Cell Lung Cancer and Programmed Death Ligand-1 Tumor Proportion Score ≥ 1% in the KEYNOTE-042 Study. J Clin Oncol 41(11):1986–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, Turk HM, Cicin I, Bentsion D, Gladkov O et al (2021) Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. The Lancet 397(10274):592–604 [DOI] [PubMed] [Google Scholar]
- 26.Herbst RS, Giaccone G, De Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, Morise M, Felip E, Andric Z, Geater S et al (2020) Atezolizumab for First-Line Treatment of PD-L1–Selected Patients with NSCLC. N Engl J Med 383(14):1328–1339 [DOI] [PubMed] [Google Scholar]
- 27.Brahmer JR, Lee J-S, Ciuleanu T-E, Bernabe Caro R, Nishio M, Urban L, Audigier-Valette C, Lupinacci L, Sangha R, Pluzanski A et al (2023) Five-Year Survival Outcomes With Nivolumab Plus Ipilimumab Versus Chemotherapy as First-Line Treatment for Metastatic Non–Small-Cell Lung Cancer in CheckMate 227. J Clin Oncol 41(6):1200–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garassino MC, Gadgeel S, Speranza G, Felip E, Esteban E, Dómine M, Hochmair MJ, Powell SF, Bischoff HG, Peled N et al (2023) Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non–Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J Clin Oncol 41(11):1992–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Socinski MA, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA et al (2021) IMpower150 Final Overall Survival Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in First-Line Metastatic Nonsquamous NSCLC. J Thorac Oncol 16(11):1909–1924 [DOI] [PubMed] [Google Scholar]
- 30.Novello S, Kowalski DM, Luft A, Gümüş M, Vicente D, Mazières J, Rodríguez-Cid J, Tafreshi A, Cheng Y, Lee KH et al (2023) Pembrolizumab Plus Chemotherapy in Squamous Non–Small-Cell Lung Cancer: 5-Year Update of the Phase III KEYNOTE-407 Study. J Clin Oncol 41(11):1999–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reck M, Ciuleanu T-E, Schenker M, Bordenave S, Cobo M, Juan-Vidal O, Reinmuth N, Richardet E, Felip E, Menezes J et al (2024) Five-year outcomes with first-line nivolumab plus ipilimumab with 2 cycles of chemotherapy versus 4 cycles of chemotherapy alone in patients with metastatic non-small cell lung cancer in the randomized CheckMate 9LA trial. Eur J Cancer 211:114296 [DOI] [PubMed] [Google Scholar]
- 32.Tsuboi M, Weder W, Escriu C, Blakely C, He J, Dacic S, Yatabe Y, Zeng L, Walding A, Chaft JE (2021) Neoadjuvant Osimertinib With/Without Chemotherapy Versus Chemotherapy Alone for EGFR -Mutated Resectable Non-Small-Cell Lung Cancer: NeoADAURA. Future Oncol 17(31):4045–4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuboi M, Herbst RS, John T, Kato T, Majem M, Grohé C, Wang J, Goldman JW, Lu S, Su W-C et al (2023) Overall Survival with Osimertinib in Resected EGFR -Mutated NSCLC. N Engl J Med 389(2):137–147 [DOI] [PubMed] [Google Scholar]
- 34.Wu Y-L, Dziadziuszko R, Ahn JS, Barlesi F, Nishio M, Lee DH, Lee J-S, Zhong W, Horinouchi H, Mao W et al (2024) Alectinib in Resected ALK -Positive Non–Small-Cell Lung Cancer. N Engl J Med 390(14):1265–1276 [DOI] [PubMed] [Google Scholar]
- 35.Lu S, Kato T, Dong X, Ahn M-J, Quang L-V, Soparattanapaisarn N, Inoue T, Wang C-L, Huang M, Yang JC-H et al: Osimertinib after Chemoradiotherapy in Stage III EGFR -Mutated NSCLC. New England Journal of Medicine 2024, 391(7):585–597. [DOI] [PubMed]
- 36.Cho BC, Lu S, Felip E, Spira AI, Girard N, Lee J-S, Lee S-H, Ostapenko Y, Danchaivijitr P, Liu B et al (2024) Amivantamab plus Lazertinib in Previously Untreated EGFR -Mutated Advanced NSCLC. N Engl J Med 391(16):1486–1498 [DOI] [PubMed] [Google Scholar]
- 37.Passaro A, Wang J, Wang Y, Lee SH, Melosky B, Shih JY, Wang J, Azuma K, Juan-Vidal O, Cobo M et al (2024) Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: primary results from the phase III MARIPOSA-2 study. Ann Oncol 35(1):77–90 [DOI] [PubMed] [Google Scholar]
- 38.Zhou C, Tang K-J, Cho BC, Liu B, Paz-Ares L, Cheng S, Kitazono S, Thiagarajan M, Goldman JW, Sabari JK et al (2023) Amivantamab plus Chemotherapy in NSCLC with EGFR Exon 20 Insertions. N Engl J Med 389(22):2039–2051 [DOI] [PubMed] [Google Scholar]
- 39.Planchard D, Jänne PA, Cheng Y, Yang JCH, Yanagitani N, Kim S-W, Sugawara S, Yu Y, Fan Y, Geater SL et al (2023) Osimertinib with or without Chemotherapy in EGFR -Mutated Advanced NSCLC. N Engl J Med 389(21):1935–1948 [DOI] [PubMed] [Google Scholar]
- 40.Mok T, Camidge DR, Gadgeel SM, Rosell R, Dziadziuszko R, Kim DW, Pérol M, Ou SHI, Ahn JS, Shaw AT et al (2020) Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol 31(8):1056–1064 [DOI] [PubMed] [Google Scholar]
- 41.Camidge DR, Kim HR, Ahn M-J, Yang JCH, Han J-Y, Hochmair MJ, Lee KH, Delmonte A, Garcia Campelo MR, Kim D-W et al (2021) Brigatinib Versus Crizotinib in ALK Inhibitor-Naive Advanced ALK-Positive NSCLC: Final Results of Phase 3 ALTA-1L Trial. J Thorac Oncol 16(12):2091–2108 [DOI] [PubMed] [Google Scholar]
- 42.Horn L, Wang Z, Wu G, Poddubskaya E, Mok T, Reck M, Wakelee H, Chiappori AA, Lee DH, Breder V et al (2021) Ensartinib vs Crizotinib for Patients With Anaplastic Lymphoma Kinase−Positive Non-Small Cell Lung Cancer. JAMA Oncol 7(11):1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solomon BJ, Liu G, Felip E, Mok TSK, Soo RA, Mazieres J, Shaw AT, De Marinis F, Goto Y, Wu Y-L et al (2024) Lorlatinib Versus Crizotinib in Patients With Advanced ALK -Positive Non-Small Cell Lung Cancer: 5-Year Outcomes From the Phase III CROWN Study. J Clin Oncol 42(29):3400–3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaw AT, Riely GJ, Bang YJ, Kim DW, Camidge DR, Solomon BJ, Varella-Garcia M, Iafrate AJ, Shapiro GI, Usari T et al (2019) Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol 30(7):1121–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drilon A, Siena S, Dziadziuszko R, Barlesi F, Krebs MG, Shaw AT, De Braud F, Rolfo C, Ahn M-J, Wolf J et al (2020) Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1–2 trials. Lancet Oncol 21(2):261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drilon A, Camidge DR, Lin JJ, Kim S-W, Solomon BJ, Dziadziuszko R, Besse B, Goto K, De Langen AJ, Wolf J et al (2024) Repotrectinib in ROS1 Fusion-Positive Non–Small-Cell Lung Cancer. N Engl J Med 390(2):118–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Planchard D, Smit EF, Groen HJM, Mazieres J, Besse B, Helland Å, Giannone V, D’Amelio AM, Zhang P, Mookerjee B et al (2017) Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 18(10):1307–1316 [DOI] [PubMed] [Google Scholar]
- 48.Planchard D, Besse B, Groen HJM, Souquet P-J, Quoix E, Baik CS, Barlesi F, Kim TM, Mazieres J, Novello S et al (2016) Dabrafenib plus trametinib in patients with previously treated BRAFV600E-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 17(7):984–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riely GJ, Smit EF, Ahn M-J, Felip E, Ramalingam SS, Tsao A, Johnson M, Gelsomino F, Esper R, Nadal E et al (2023) Phase II, Open-Label Study of Encorafenib Plus Binimetinib in Patients With BRAFV600-Mutant Metastatic Non–Small-Cell Lung Cancer. J Clin Oncol 41(21):3700–3711 [DOI] [PubMed] [Google Scholar]
- 50.Wolf J, Seto T, Han J-Y, Reguart N, Garon EB, Groen HJM, Tan DSW, Hida T, De Jonge M, Orlov SV et al (2020) Capmatinib inMETExon 14–Mutated orMET-Amplified Non–Small-Cell Lung Cancer. N Engl J Med 383(10):944–957 [DOI] [PubMed] [Google Scholar]
- 51.Paik PK, Felip E, Veillon R, Sakai H, Cortot AB, Garassino MC, Mazieres J, Viteri S, Senellart H, Van Meerbeeck J et al (2020) Tepotinib in Non–Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N Engl J Med 383(10):931–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou C, Solomon B, Loong HH, Park K, Pérol M, Arriola E, Novello S, Han B, Zhou J, Ardizzoni A et al (2023) First-Line Selpercatinib or Chemotherapy and Pembrolizumab in RET Fusion-Positive NSCLC. N Engl J Med 389(20):1839–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gainor JF, Curigliano G, Kim D-W, Lee DH, Besse B, Baik CS, Doebele RC, Cassier PA, Lopes G, Tan DSW et al (2021) Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol 22(7):959–969 [DOI] [PubMed] [Google Scholar]
- 54.Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, Blakely CM, Seto T, Cho BC, Tosi D et al (2020) Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol 21(2):271–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong DS, Dubois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, Van Tilburg CM, Nagasubramanian R, Berlin JD, Federman N et al (2020) Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol 21(4):531–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Langen AJ, Johnson ML, Mazieres J, Dingemans A-MC, Mountzios G, Pless M, Wolf J, Schuler M, Lena H, Skoulidis F et al: Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRASG12C mutation: a randomised, open-label, phase 3 trial. The Lancet 2023, 401(10378):733–746. [DOI] [PubMed]
- 57.Jänne PA, Riely GJ, Gadgeel SM, Heist RS, Ou S-HI, Pacheco JM, Johnson ML, Sabari JK, Leventakos K, Yau E et al: Adagrasib in Non–Small-Cell Lung Cancer Harboring a KRASG12C Mutation. New England Journal of Medicine 2022, 387(2):120–131. [DOI] [PubMed]
- 58.Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazières J, Nagasaka M, Bazhenova L, Saltos AN, Felip E et al (2022) Trastuzumab Deruxtecan in HER2-Mutant Non–Small-Cell Lung Cancer. N Engl J Med 386(3):241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim D-W, Schram AM, Hollebecque A, Nishino K, Macarulla T, Rha SY, Duruisseaux M, Liu SV, Al Hallak MN, Umemoto K et al (2024) The phase I/II eNRGy trial: Zenocutuzumab in patients with cancers harboring NRG1gene fusions. Future Oncol 20(16):1057–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.FDA grants accelerated approval to zenocutuzumab-zbco for non-small cell lung cancer and pancreatic adenocarcinoma
- 61.Papadimitrakopoulou VA, Mok TS, Han JY, Ahn MJ, Delmonte A, Ramalingam SS, Kim SW, Shepherd FA, Laskin J, He Y et al (2020) Osimertinib versus platinum–pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann Oncol 31(11):1536–1544 [DOI] [PubMed] [Google Scholar]
- 62.Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y, Chewaskulyong B et al (2020) Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 382(1):41–50 [DOI] [PubMed] [Google Scholar]
- 63.Soria J-C, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T et al (2018) Osimertinib in Untreated EGFR -Mutated Advanced Non–Small-Cell Lung Cancer. N Engl J Med 378(2):113–125 [DOI] [PubMed] [Google Scholar]
- 64.Schram AM, Goto K, Kim D-W, Martin-Romano P, Ou S-HI, O’Kane GM, O’Reilly EM, Umemoto K, Duruisseaux M, Neuzillet C et al: Efficacy and safety of zenocutuzumab, a HER2 x HER3 bispecific antibody, across advanced NRG1 fusion (NRG1+) cancers. Journal of Clinical Oncology 2022, 40(16_suppl):105–105.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.