Abstract
Background
Diabetic nephropathy (DN) is a leading cause of end-stage renal disease (ESRD) among individuals with diabetes, highlighting the urgent need for effective therapeutic strategies to combat this condition. Prior research has indicated that T. spiralis possesses hypoglycemic properties. In this investigation, we aimed to evaluate the efficacy of T. spiralis antigens, derived from both adult and larval forms, in treating diabetic nephropathy in alloxan-induced diabetic mice (AIDM).
Methods
A total of forty Swiss albino mice were allocated into four groups, each consisting of ten mice. Diabetes was induced in three of the groups using alloxan, while one group served as a control without diabetes. Two diabetic groups received treatment with either crude larva (CLA) antigen or adult worm antigen (AWA), while one group remained untreated. The study assessed various parameters, including fasting blood glucose levels, blood urea, serum creatinine, and serum albumin across all groups. Additionally, histopathological examinations of the kidneys were conducted.
Results
The results indicated that treatment with CLA or AWA antigens led to a significant reduction in blood glucose, serum creatinine, and blood urea levels, alongside an increase in serum albumin. Notably, the administration of AWA antigens resulted in substantial improvements in renal pathological changes induced by diabetes, as evidenced by hematoxylin and eosin staining and Masson trichrome staining, which also demonstrated a reduction in fibrosis.
Conclusions
The findings suggest that T. spiralis antigens may mitigate renal damage in diabetic mice by alleviating hyperglycemia-induced inflammation and oxidative stress, warranting further investigation into their potential role in preventing DN in diabetic patients.
Keywords: Antigens, Fibrosis, Diabetic nephropathy, Trichinella spiralis
Introduction
Infection with parasitic helminths has been demonstrated to influence the progression of various diseases, including bacterial, viral, parasitic, and autoimmune conditions; however, such infections are less common in developed nations [1, 2]. The “hygiene hypothesis” posits that the increased incidence of autoimmune diseases, such as Type 1 Diabetes Mellitus (T1DM) in these countries, may be linked to the decline in helminth infections [3]. Supporting this theory, numerous studies involving animal models infected with helminth parasites have indicated that these infections can inhibit the onset of several autoimmune diseases, including T1DM [4, 5].
The mechanisms underlying these protective effects of helminth infections may involve the direct activation of regulatory T (TReg) cells or modifications in the movement of autoreactive T cells [3]. Additionally, helminth infections are known to robustly stimulate T helper 2 (Th2) cell-mediated immune responses, which include eosinophilia, the production of specific cytokines (notably interleukins IL-4, IL-5, and IL-13), and the induction of immunoglobulin E (IgE) [6]. Alongside these Th2 responses, regulatory immune responses have also been observed following exposure to antigens from parasitic helminths [1, 6].
Trichinella spiralis is a unique parasitic nematode. Its life cycle comprises three primary stages: adult worms (Ad), newborn larvae (NBL) and muscle larvae (ML) [7]. Throughout the progression of trichinella infection, the immune system of the host is activated by various antigens, which may either be linked to the parasite’s cuticle or be secreted by the parasite itself. This activation prompts a transition from an inflammatory immune response to an anti-inflammatory one, a shift that has been shown to be crucial for the survival of the parasite [8]. The induction of this anti-inflammatory regulatory response, which includes regulatory T cells (TReg), alternatively activated macrophages (AAMs), and regulatory B cells (BReg), influences dendritic cells (DCs) and T cells, resulting in the downregulation of adaptive immune responses [8].
In light of the aforementioned findings, T. spiralis infection has been proposed by various research groups as a potential therapeutic approach for conditions such as airway diseases [9], inflammatory bowel disorders [10], autoimmune encephalitis [11], and rheumatoid arthritis [12] and Type 1 Diabetes Mellitus (T1DM) [13].
Diabetic nephropathy (DN) represents a prevalent and serious consequence of diabetes mellitus, characterized by significant morbidity and mortality rates [14]. Research indicates that the primary factor contributing to DN is damage to the microvascular endothelium, which can ultimately result in end-stage renal disease (ESRD) and renal failure [15, 16]. Notable pathological alterations linked to DN encompass mesangial hypertrophy, increased thickness of the glomerular basement membrane (GBM), accumulation of extracellular matrix, and loss of glomerular epithelial cells [17]. Additionally, changes within the glomeruli, such as apoptosis of mesangial cells and fibrosis, are critical in the progression of DN [18]. The adverse impact of diabetes on renal function underscores the urgent need for the development of novel strategies and pharmacological interventions aimed at protecting the kidneys from the detrimental effects of diabetic nephropathy.
Inflammation and oxidative stress associated with hyperglycemia in diabetes mellitus are critical factors contributing to diabetic nephropathy, with antioxidant enzymes playing a protective role against its progression [19]. Notably, research has indicated that mice infected with T. spiralis exhibited reduced blood glucose levels [20]. The authors proposed that the dynamics of blood glucose levels during the infection are related to the development of muscle larvae and the encystment of infected muscle cells [20]. Furthermore, the infected subjects demonstrated significantly elevated levels of superoxide dismutase (SOD), glutathione-S-transferase, and peroxidase, alongside an increase in antioxidant levels, including Vitamin E [21]. Consequently, this study aimed to explore the potential protective effects of T. spiralis antigens against diabetic nephropathy in alloxan-induced diabetic mice (AIDM).
Materials and methods
The present study took place in the department of Medical Parasitology, Faculty of Medicine, South Valley University, Qena, Egypt. All animal experiments were conducted according to the guidelines of the Declaration of Helsinki. The Faculty of Medicine’s Institutional Review Board and Ethics Committee at South Valley University, Qena, Egypt, approved all animal experiments (Protocol approval number: SVU-MED-PAR008-4-22-8-425).
T. spiralis was maintained in Swiss albino mice at the Department of Parasitology, Faculty of Medicine, Assiut University, Assiut, Egypt. Ten laboratory-bred male Swiss albino mice infected with T. spiralis adult worms were used to prepare adult worm antigens (AWA), on the 3rd day postinfection (dpi), whereas 10 mice were used to prepare crude larvae antigens (CLA) on the 49th dpi.
.
Preparation of T. Spiralis crude larva antigen (CLA)
Muscle tissues from infected mice, which were euthanized on the 49th day post-infection, were subjected to artificial digestion to extract larvae, following established methodologies [22]. To prepare CLA, the isolated larvae were suspended in phosphate-buffered saline (PBS), then homogenized using a Braun S Potter homogenizer. This mixture was subsequently sonicated five times in an ice-water bath. The resulting homogenate was centrifuged at 3,000 g for 30 min at 4 °C to obtain the supernatant, which was then ultra-centrifuged, aliquoted, and stored at -20 °C.
Preparation of T. Spiralis adult worm antigen (AWA)
On the 3rd day post-infection, ten mice infected with T. spiralis were euthanized, and their small intestines were removed to isolate the adult worms [23, 24]. For the preparation of AWA, the adult worms were homogenized. Following the centrifugation of the homogenate, the supernatant was collected [25, 26], and the protein concentration was determined using the Bradford assay.
Animals Experiments
Forty male Swiss albino mice, aged four to six weeks and weighing approximately 25 g, were utilized for the study. Alloxan was administered intraperitoneally at a dosage of 80 mg/kg to thirty of the mice to induce diabetes. Mice exhibited fasting blood glucose levels of 11.0 mmol/L or higher, measured 48 h post-alloxan injection, were classified as diabetic [27, 28].
The diabetic mice were subsequently divided into three groups, each containing ten mice. Two groups received intradermal injections over the sternum with either AWA or CLA at a dosage of 70 mg/kg [12]. After a two-week interval, both antigens were administered again [12]. The control groups, which included both diabetic and nondiabetic mice, were injected with saline in an equivalent volume. One week after the final treatment, the animals were euthanized, and blood and tissue samples were collected for biochemical analysis and histopathological examination.
Blood Glucose, Serum Albumin and Kidney Function Measurement
Blood samples underwent centrifugation at 3,000 rpm to facilitate serum separation. The quantification of blood glucose (Spinreact, Spain), serum creatinine (Abcam, Cambridge, MA, USA), blood urea (Abcam, Cambridge, MA, USA), and serum albumin (Sigma-Aldrich, Darmstadt, Germany) was performed using a Beckman Coulter automated chemistry analyzer along with commercial kits.
Antioxidant Enzyme Activity in Kidney Homogenate
The activity of Superoxide dismutase (SOD) was assessed following established methodologies. One unit of the enzyme was defined as the amount that results in 50% inhibition of epinephrine autooxidation [29]. The level of Reduced Glutathione (GSH) were measured using the technique described by Chatuphonprasert et al. [30]. The GSH concentrations were expressed in mmol/mg protein or nmol/mg protein, based on the comparison with the GSH standard curve slope [31].
Histological Analyses
Hematoxylin and Eosin (H&E) Staining
The kidneys were extracted from various groups of mice and subsequently fixed in 10% formalin. They underwent dehydration through a series of increasing alcohol concentrations and were ultimately embedded in paraffin to prepare H&E stained sections. The assessment of tubular and glomerular damage was conducted as previously outlined [32].
Masson Trichrome Staining
To evaluate the extent of fibrosis, Masson’s trichrome (MT) staining was performed using the trichrome Gomori One-Step Aniline Blue Stain kit from Newcomer Supply, located in Middleton, WI, USA. Imaging was conducted with a Leica microscope (model CH9435 Hee56rbrugg) from Leica Microsystems, Switzerland.Scoring of fibrosis results by determination of reaction area percent in 10 microscopic fields using image J 1.53t, Wayne Rasband and contributors, National Institutes of Health, USA.
Statistical Analysis
All data were expressed as mean ± standard deviation (SD). The differences between groups were analyzed using one-way ANOVA and LSD post hoc tests. Differences were considered statistically significant at p value < 0.05.
Results
Administration of T. Spiralis Antigens Significantly Reduced Blood Glucose in AIDM
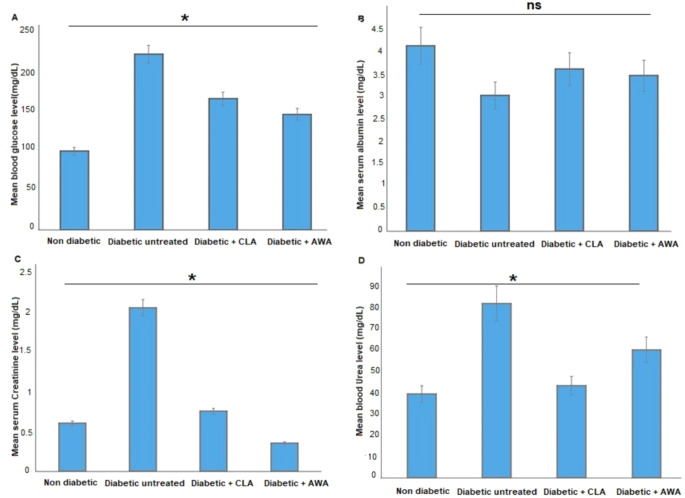
As illustrated in Fig. 1A, untreated diabetic mice (group II) exhibited a significant increase (p < 0.05) in blood glucose levels. The administration of T. spiralis antigens (AWA or CLA) to these diabetic mice resulted in a significant decrease in blood glucose levels (p = 0.002 and 0.006, respectively), as shown in Fig. 1A. Furthermore, untreated diabetic mice displayed markedly lower serum albumin levels compared to control mice, which increased following treatment with AWA or CLA, although this change did not reach statistical significance (P > 0.05, Fig. 1B).
Fig. 1.
Effect of AWA or CLA treatments on blood glucose (A), serum albumin (B), serum creatinine (C), and blood urea (D) of diabetic mice. Asterisks (*) indicate statistically significant difference; p < 0.05, whereas “ns” indicates non-significant difference when compared to diabetic untreated mice group.
Additionally, we observed significantly elevated levels of serum creatinine and blood urea in untreated diabetic mice when compared to control nondiabetic mice. Nevertheless, treatment with AWA or CLA led to a significant reduction in both serum creatinine (Fig. 1C) and blood urea (Fig. 1D) levels in the diabetic mice.
Treatment with AWA or CLA Antigens Increased Antioxidant Enzyme Activities in the Kidneys of AIDM
Subsequently, we sought to evaluate the protective effects of CLA or AWA administration on the kidneys of AIDM. Our findings showed that the activity of the SOD and GSH antioxidant enzymes was reduced in the kidneys of diabetic mice when compared to control nondiabetic mice. Nevertheless, treatment with CLA or AWA antigens reinstated SOD and GSH enzymes activity, ultimately reaching levels comparable to those observed in nondiabetic mice (Fig. 2).
Fig. 2.
CLA or AWA administration restored SOD ans GSH enzyme activity in kidneys of diabetic mice. Diabetic untreated mice showed marked reduction of SOD and GSH level in comparison with normal group. Higher levels of SOD and GSH were reported in treated animals compared to diabetic untreated group with no statistical difference (ns); p > 0.05
Treatment with CLA or AWA Antigens Protected the Kidneys of AIDM against DN
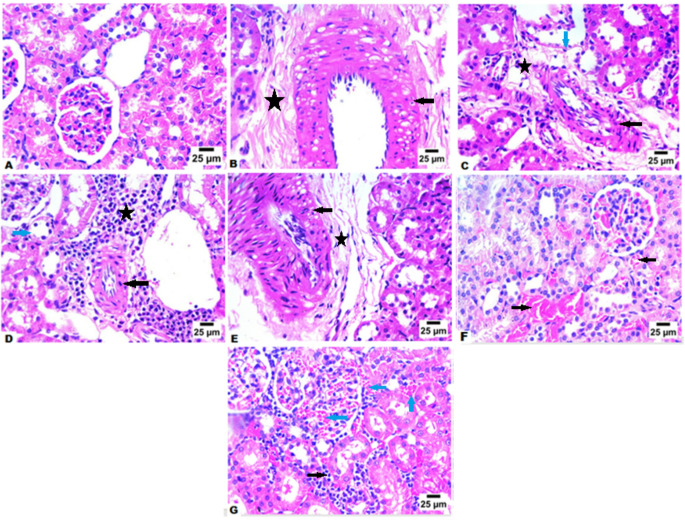
We conducted an examination of the kidneys from various groups of mice to assess the protective effects of AWA or CLA administration on the kidneys of diabetic mice. Histological analysis using H&E staining of kidney tissue sections from control nondiabetic mice revealed glomeruli exhibiting normal structure, volume, and mesangial matrix, along with a clear and patent tubular lumen (Fig. 3A). In contrast, kidney tissue sections from untreated diabetic mice showed pronounced hyalinization in blood vessels (black arrow, Fig. 3B and 3C) and perivascular fibrosis, with infiltration by mononuclear inflammatory cells (star, Fig. 3B and 3C) and degenerative changes in renal tubules (blue arrow, Fig. 3C). Notably, these pathological changes were mitigated in mice treated with AWA and CLA . Renal sections from CLA-treated animals also showed hyalinization in blood vessels (black arrow, Fig. 3D and 3E) and perivascular fibrosis, with infiltration primarily by lymphocytes and eosinophils (star, Fig. 3D and 3E), as well as degenerative changes in certain renal tubules (blue arrows, Fig. 3D). On the other hand, we recognized better therapeutic potential in AWA treated mice group. Renal sections from AWA-treated animals exhibited congestion in preglomerular and pretubular blood vessels (black arrows, Fig. 3F and blue arrowsFig. 3G) and infiltration of interstitial tissue by mononuclear inflammatory cells (black arrow, Fig. 3G), in comparison to untreated diabetic mice. Furthermore, no hyalinization in blood vessels or tubular injury was observed in AWA-treated mice group.
Fig. 3.
CLA or AWA treatments alleviated diabetic nephropathy in diabetic mice. (A) Representative image of H&E-stained kidney tissue of control nondiabetic mice showing normal hitological structure of glomeruli and renal tubules (B) Representative image of H&E-stained kidney tissue of diabetic untreated mice showing marked hyalinzation in blood vessels 54and presence of perivascular fibrosis with infiltration by few number of mononuclear inflammatory cells (star). (C) Representative image of H&E-stained kidney tissue of diabetic untreated mice showing marked hyalinzation in blood vessels (black arrow) and presence of perivascular fibrosis with infiltration by few number of mononuclear inflammatory cells (star) and degenerative changes in some renal tubules (blue arrow) (D) Representative image of H&E-stained kidney tissue of diabetic mice treated with CLA showing marked hyalinzation in blood vessels (black arrow) and presence of perivascular fibrosis with infiltration by inflammatory cells mainly lymphocytes and eosinophils (star) and degenerative changes in some renal tubules (blue arrow). (E) Representative image of H&E-stained kidney tissue of diabetic mice treated with CLA showing marked hyalinzation in blood vessels [5 4and presence of perivascular fibrosis (star). (F) Representative image of H&E-stained kidney tissue of diabetic mice treated with AWA showing preglomerular and pretubular blood vessels congestion ( black arrows). (G) Representative image of H&E-stained kidney tissue of diabetic mice treated with AWA showing preglomerular and pretubular blood vessels congestion (blue arrows)and infiltration of interstitial tissue by mononuclear inflammatory cells (black arrow). Magnification is 400X
Treatment with CLA or AWA Antigens Significantly Reduced Fibrosis in Kidneys of AIDM
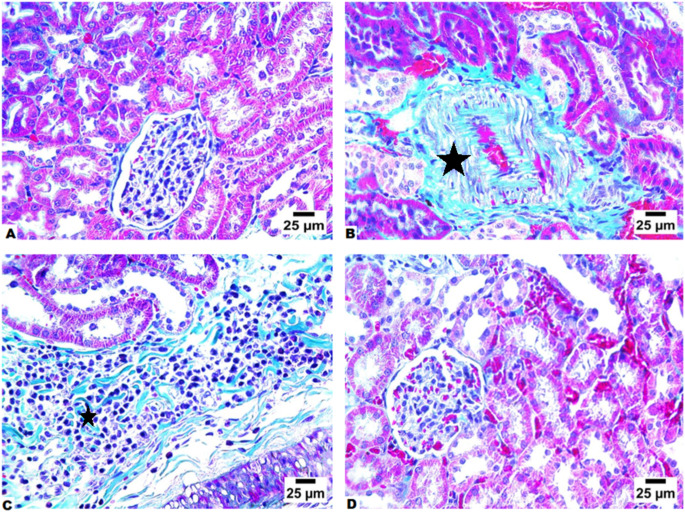
We subsequently evaluated the extent of fibrosis across various groups of mice by examining MT-stained kidney sections. The non-diabetic control mice group showed normal histological architecture of the renal tubules and glomeruli with minimal collagen deposition (Fig. 4A). In contrast, the renal tissues of untreated diabetic mice displayed significant peritubular collagen deposition, quantified at 20.6%, which was statistically significant when compared to the non-diabetic control mice group (Fig. 4B). Conversely, in the treated animal groups, there was a notable reduction in collagen fibrous tissue deposition, particularly surrounding the congested blood vessels (Fig. 4C and D). A comparison between the treated and untreated animal groups revealed a significant decrease in the fibrosis score, with untreated diabetic animals showing 20.6% fibrosis, while the CLA-treated group exhibited 16.3%, and the AWA-treated group demonstrated only 0.76%. .
Fig. 4.
Treatment with CLA or AWA antigens ameliorated renal fibrosis in diabetic mice. (A) Representative image of MT-stained renal tissues of control nondiabetic mice showing showing normal histological structure of renal tubules and glomeruli without fibrous connective tissue formation. (B) Representative image of MT-stained renal tissues of diabetic untreated mice showing extensive showing pretubular fibrosis (star). (C) Representative image of MT-stained renal tissues of CLA-treated diabetic mice showing perivascular fibrosis (star). (D) Representative image of MT-stained renal tissues of AWA-treated diabetic mice showing no fibrous connective tissue formation. Magnification is 400X
Discussion
Recent estimates indicate that approximately 37.3 million individuals in the United States are affected by diabetes mellitus [33]. DN is recognized as one of the most serious complications associated with DM, contributing significantly to the morbidity and mortality rates among diabetic individuals [14]. Research has demonstrated that trichinellosis can lead to reduced blood glucose levels in humans, as well as in experimentally infected dogs and mice [34]. This reduction is believed to be linked to the glucose consumption by the developing larvae of Trichinella spiralis [20].
Our study sought to determine whether the administration of T. spiralis antigens could mitigate DN in AIDM, and our findings indicated that treatment with AWA and CLA antigens provided protection against DN. It has been well documented that serum urea and serum creatinine significantly increased in DN mice compared to control group [35]. This is consistent with our findings that diabetic mice have increased serum urea and serum creatinine and reduced serum albumin which confirms DN in these mice. Interestingly, AWA or CLA antigenic treatments lowered blood glucose levels of diabetic mice and improved the renal function as evidenced by reduction in serum blood urea and serum creatinine and an increase in serum albumin levels compared to diabetic untreated mice. This is consistent with increase in renal SOD activity in renal tissues of AWA or CLA-treated mice compared to diabetic untreated mice. The improvement of renal function and the antioxidant enzymes activity was aligned with the improvement in DN associated renal pathological alterations in AWA or CLA-treated mice.
Histopathological examination of renal tissue of animals, in our study, revealed significant pathological alterations in kidneys of diabetic mice compared to control mice which provide evidence of DN. We detected glomerular (mesangial hypercellularity and expansion of glomeruli with reduced Bowman’s space and thickened basement membrane) and tubular injuries (tubular dilatation, attenuation, vacuolation, and desquamation of tubular epithelial cells with necrotic material in the tubular lumen) in renal tissues of diabetic mice. Similar results were previously reported in renal tissues of alloxan induced-diabetic rats with DN [36]. The treatment of diabetic mice with AWA or CLA ameliorated these histopathological alterations and reversed fibrosis as shown by reduced collagen deposition in glomeruli, perivascular, and peritubular regions of renal tissues compared to diabetic untreated mice..
Several mechanisms have been proposed to elucidate the protective effects of infections on autoimmune disorders. The two primary mechanisms are competition and immunoregulation [37]. The first mechanism posits that a robust immune response against the antigens of infectious agents may inhibit responses to “weaker” antigens, such as auto-antigens and allergens [38]. The second mechanism pertains to the function of regulatory T cells, which can suppress immune responses not only to infectious agents but also to bystander antigens [37]. Helminth parasites, known to induce predominantly Th2 and regulatory responses, have been demonstrated to affect the progression of autoimmune and allergic diseases [2, 39–42]. Numerous experiments conducted on animal models of human autoimmune diseases have shown that helminth infections or the administration of helminth-derived products can either prevent the onset or alleviate the symptoms of autoimmune diseases [2, 43–50].
In the present study, the therapeutic potential of antigens derived from T. spiralis adult worm (AWA) in treatment of DN was better than that resulted from treatment of diabetic mice by antigens derived form T. spiralis muscle larvae (CLA). This can be explained by findings reported by previous studies [51]. The authors investigated the impact of T. spiralis infection on the differentiation of CD4 + T cells at different stages of infection. Notable increases in Th2 CD4 + T cells, which express IL-4, as well as Treg cells were observed during the early intestinal phase (3–6 days) and the NBL migration phase (at day 15). The enhanced production of IL-4 correlating with the helminth-induced Th2 suppression of autoimmune disease [5, 45]. Following the expulsion of adult worms from the intestine and the migration of newborn larvae to muscle tissue to form encapsulated muscle larvae, the levels of Th1, Th2, and Treg cells returned to baseline [51]. The authors concluded that T. spiralis infection primarily promotes Th2 and Treg responses during the initial stages of infection, and that the persistence of these cell types requires ongoing stimulation from the presence of the worms. Once the adult worms were expelled and the muscle larvae encapsulated, there was no observable host cellular immune response [51]. Although, both T. spiralis adults and larvae trigger Th2 immune response, the adult worm is different in its ability to stimulate the production of IL-17 which was found to be elevated in the intestines of animals experimentally infected with T. spiralis [52, 53].
We have shown that antigens from T. spiralis can mitigate diabetic nephropathy (DN) in an animal model of diabetes mellitus. The administration of CLA or AWA antigens resulted in decreased blood glucose levels, serum urea and creatinine and offered protection to mice against renal histopathological changes, as indicated by diminished tissue damage, reduced collagen deposition, and less fibrosis in the renal tissues. These results suggest that T. spiralis antigens may represent a promising therapeutic approach for combating DN. The application of autoclaved parasitic antigens eliminates the harmful consequences associated with inducing helminthic infections through live parasites. Nevertheless, more comprehensive research is required to confirm their effectiveness in diabetic patients and to identify the specific molecule(s) that contribute to immune modulation.
Acknowledgements
. This project was funded by The Central Research Laboratory/ Research Institute/ Center Supporting Program (RICSP-25-3)King Saud University, Riyadh, Saudi Arabia.
Author Contributions
Author Contributions: A.M.E and H.A.E contributed to the conceptualization, experimental design, data analysis. A.M.E, H.H.A, M.H.W, S.A.A, K.M, E.A.A, H.E, D.H, H.A.E. contributed to writing, review, and editing of the manuscript. All authors contributed to the submitted version of the manuscript.
Funding
No funding was received.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Informed Consent
Not applicable.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Asmaa M. El-kady, Email: asmaa.elkady@med.svu.edu.eg
Hatem A. Elshabrawy, Email: hatem.elshabrawy@shsu.edu
References
- 1.Yazdanbakhsh M, Kremsner PG, van Ree R (2002) Allergy, parasites, and the hygiene hypothesis. Sci (New York N Y) 296(5567):490–494 [DOI] [PubMed] [Google Scholar]
- 2.Zaccone P, Fehérvári Z, Jones FM, Sidobre S, Kronenberg M, Dunne DW, Cooke A (2003) Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur J Immunol 33(5):1439–1449 [DOI] [PubMed] [Google Scholar]
- 3.Cooke A, Zaccone P, Raine T, Phillips JM, Dunne DW (2004) Infection and autoimmunity: are we winning the war, only to lose the peace? Trends Parasitol 20(7):316–321 [DOI] [PubMed] [Google Scholar]
- 4.El-Wakil HS, Aboushousha TS, El Haddad O, Gamil NB, Mansour T, El-Said H (2002) Effect of schistosoma mansoni egg deposition on multiple low doses streptozotocin induced insulin dependent diabetes. J Egypt Soc Parasitol 32(3):987–1002 [PubMed] [Google Scholar]
- 5.Saunders KA, Raine T, Cooke A, Lawrence CE (2007) Inhibition of autoimmune type 1 diabetes by Gastrointestinal helminth infection. Infect Immun 75(1):397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawrence CE (2003) Is there a common mechanism of Gastrointestinal nematode expulsion? Parasite Immunol 25(5):271–281 [DOI] [PubMed] [Google Scholar]
- 7.Rajapakse R, Mousli M, Pfaff AW, Uring-Lambert B, Marcellin L, Bronner C, Jeanblanc M, Villard O, Letscher-Bru V, Klein JP, Candolfi E (2005) 1,25-Dihydroxyvitamin D3 induces splenocyte apoptosis and enhances BALB/c mice sensitivity to toxoplasmosis. J Steroid Biochem Mol Biol 96(2):179–185 [DOI] [PubMed] [Google Scholar]
- 8.Campbell W (2012) Trichinella and trichinosis. Springer Science & Business Media
- 9.Park H-K, Cho MK, Choi SH, Kim YS (2011) H.S.J.E.p. Yu, Trichinella spiralis: infection reduces airway allergic inflammation in mice. 127(2):539–544 [DOI] [PubMed]
- 10.Ashour D, Othman A, Shareef M, Gaballah H (2014) W.J.J.o.h. Mayah, interactions between Trichinella spiralis infection and induced colitis in mice. 88(2):210–218 [DOI] [PubMed]
- 11.Gruden-Movsesijan A, Ilic N, Mostarica-Stojkovic M, Stosic-Grujicic S, Milic M.J.P.I., Lj S-M (2010) Mechanisms of modulation of experimental autoimmune encephalomyelitis by chronic Trichinella spiralis infection in dark Agouti rats. 32:450–459 [DOI] [PubMed]
- 12.Eissa MM, Mostafa DK, Ghazy AA, El Azzouni MZ, Boulos LM, Younis LK (2016) Anti-Arthritic activity of Schistosoma mansoni and Trichinella spiralis Derived-Antigens in adjuvant arthritis in rats: role of FOXP3 + Treg cells. PLoS ONE 11(11):e0165916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruschi F, Dupouy-Camet J Helminth infections and their impact on global public health, Springer2014
- 14.Zhang XX, Kong J, Yun K (2020) Prevalence of Diabetic Nephropathy among Patients with Type 2 Diabetes Mellitus in China: A Meta-Analysis of Observational Studies, J Diabetes Res (2020) 2315607 [DOI] [PMC free article] [PubMed]
- 15.Kim Y (2017) C.W.J.T.K.j.o.i.m. Park. New Therapeutic Agents Diabet Nephrop 32(1):11 [Google Scholar]
- 16.Lai X, Tong D, Ai X, Wu J, Luo Y, Zuo F, Wei Z, Li Y, Huang W, Wang W, Jiang Q, Meng X, Zeng Y, Wang P (2018) Amelioration of diabetic nephropathy in Db/db mice treated with Tibetan medicine formula Siwei Jianghuang Decoction powder extract. Sci Rep 8(1):16707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armani R, Ramezani A, Yasir A, Sharama S, Canziani M (2017) D.J.C.h.r. Raj. Gut Microbiome Chronic Kidney Disease 19(4):1–8 [DOI] [PubMed] [Google Scholar]
- 18.Oh YS, Bae GD, Baek DJ, Park E-Y, Jun H-S J.F.i.e. (2018) Fatty acid-induced lipotoxicity in pancreatic beta-cells during development of type 2 diabetes. 9:384 [DOI] [PMC free article] [PubMed]
- 19.Čolak E, Majkić-Singh N, Stanković S, Srecković‐Dimitrijević V, Djordjević PB, Lalić K, Lalić N (2005) Parameters of antioxidative defense in type 2 diabetic patients with cardiovascular complications. Ann Med 37(8):613–620 [DOI] [PubMed] [Google Scholar]
- 20.Wu Z, Nagano I, Kajita K, Nishina M, Takahashi Y (2009) Hypoglycaemia induced by Trichinella infection is due to the increase of glucose uptake in infected muscle cells. Int J Parasitol 39(4):427–434 [DOI] [PubMed] [Google Scholar]
- 21.Derda M, Wandurska-Nowak E, Hadas E (2004) Changes in the level of antioxidants in the blood from mice infected with Trichinella spiralis. Parasitol Res 93:207–210 [DOI] [PubMed] [Google Scholar]
- 22.Dea-Ayuela MA, Rama-Iñiguez S, Bolas-Fernández F (2007) Contrasting effects of Trichinella spiralis and trichuris muris antigens on the infection by leishmania infantum in BALB/c mice. Acta Trop 103(3):212–221 [DOI] [PubMed] [Google Scholar]
- 23.Long SR, Wang ZQ, Liu RD, Liu LN, Li LG, Jiang P, Zhang X, Zhang ZF, Shi HN, Cui J (2014) Molecular identification of Trichinella spiralis nudix hydrolase and its induced protective immunity against trichinellosis in BALB/c mice. Parasites Vectors 7(1):1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J, Pan W, Sun X, Zhao X, Yuan G, Sun Q, Huang J, Zhu X (2015) Immunoproteomic profile of Trichinella spiralis adult worm proteins recognized by early infection Sera. Parasites Vectors 8(1):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui J, Wang Z, Xu B (2011) The epidemiology of human trichinellosis in China during 2004–2009. Acta Trop 118(1):1–5 [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, jun Wen Y, Cai YN, Vallée I, Boireau P, Liu MY, Cheng SP (2015) Serine proteases of parasitic helminths. Korean J Parasitol 53(1):1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bari MW, Islam MM, Khatun M, Sultana MJ, Ahmed R, Islam A, Hossain MI, Rahman MM, Islam MA (2020) Antidiabetic effect of Wedelia chinensis leaf extract in Alloxan induced Swiss albino diabetic mice. Clin Phytoscience 6(1):4–11 [Google Scholar]
- 28.Miao MS, Tian S, Guo L (2014) Effect of Curcumin on diabetes in an Alloxan mice model. Adv Mater Res 1051(030321):363–367 [Google Scholar]
- 29.Afolabi OB, Oloyede OI (2014) Antioxidant properties of the extracts of talinum triangulare and its effect on antioxidant enzymes in tissue homogenate of Swiss albino rat. Toxicol Int 21(3):307–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatuphonprasert W, Udomsuk L, Monthakantirat O, Churikhit Y, Putalun W, Jarukamjorn K (2013) Effects of pueraria Mirifica and Miroestrol on the antioxidation-related enzymes in ovariectomized mice. J Pharm Pharmacol 65(3):447–456 [DOI] [PubMed] [Google Scholar]
- 31.Kondo S, Chatuphonprasert W, Jaruchotikamol A, Sakuma T, Nemoto N (2011) Cellular glutathione content modulates the effect of Andrographolide on β-naphthoflavone-induced CYP1A1 mRNA expression in mouse hepatocytes. Toxicology 280(1–2):18–23 [DOI] [PubMed] [Google Scholar]
- 32.Pieters TT, Falke LL, Nguyen TQ, Verhaar MC, Florquin S, Bemelman FJ, Kers J, Vanhove T, Kuypers D, Goldschmeding R, Rookmaaker MB (2019) Histological characteristics of acute tubular injury during delayed graft function predict renal function after renal transplantation. Physiological Rep 7(5):e14000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmad FB, Anderson RN (2021) The leading causes of death in the US for 2020. JAMA 325(18):1829–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishina M, Suzuki M, Matsushita K (2004) Trichinella spiralis: activity of the cerebral pyruvate recycling pathway of the host (mouse) in hypoglycemia induced by the infection. Exp Parasitol 106(1–2):62–65 [DOI] [PubMed] [Google Scholar]
- 35.Ranjbar A, Ghasemi H, Hatami M, Dadras F, Shayesteh T, Khoshjou F (2016) Tempol effects on diabetic nephropathy in male rats. J Ren Injury Prev 5:74–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaffie NM, Morsy FA, Ali AG, Sharaf HA (2010) Effect of Craway, coriander and fennel on the structure of kidney and Islets of Langerhan in Alloxan-Induced diabetic rats. Histological and Histochemical Study
- 37.Bach JF (2005) Six questions about the hygiene hypothesis. Cell Immunol 233(2):158–161 [DOI] [PubMed] [Google Scholar]
- 38.Okada H, Kuhn C, Feillet H, Bach JF (2010) The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol 160(1):1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Correale J, Farez M (2007) Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol 61(2):97–108 [DOI] [PubMed] [Google Scholar]
- 40.Zaccone P, Burton OT, Cooke A (2008) Interplay of parasite-driven immune responses and autoimmunity. Trends Parasitol 24(1):35–42 [DOI] [PubMed] [Google Scholar]
- 41.Maizels RM (2005) Infections and allergy - helminths, hygiene and host immune regulation. Curr Opin Immunol 17(6):656–661 [DOI] [PubMed] [Google Scholar]
- 42.Smits HH, Yazdanbakhsh M (2007) Chronic helminth infections modulate allergen-specific immune responses: protection against development of allergic disorders? Ann Med 39(6):428–439 [DOI] [PubMed] [Google Scholar]
- 43.Cooke A, Tonks P, Jones FM, O’Shea H, Hutchings P, Fulford AJ, Dunne DW (1999) Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol 21(4):169–176 [DOI] [PubMed] [Google Scholar]
- 44.La Flamme AC, Ruddenklau K, Bäckström BT (2003) Schistosomiasis decreases central nervous system inflammation and alters the progression of experimental autoimmune encephalomyelitis. Infect Immun 71(9):4996–5004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sewell D, Qing Z, Reinke E, Elliot D, Weinstock J, Sandor M, Fabry Z (2003) Immunomodulation of experimental autoimmune encephalomyelitis by helminth Ova immunization. Int Immunol 15(1):59–69 [DOI] [PubMed] [Google Scholar]
- 46.Elliott DE, Li J, Blum A, Metwali A, Qadir K, Urban JF Jr., Weinstock JV (2003) Exposure to schistosome eggs protects mice from TNBS-induced colitis, American journal of physiology. Gastrointest Liver Physiol 284(3):G385–G391 [DOI] [PubMed] [Google Scholar]
- 47.Reardon C, Sanchez A, Hogaboam CM, McKay DM (2001) Tapeworm infection reduces epithelial ion transport abnormalities in murine dextran sulfate sodium-induced colitis. Infect Immun 69(7):4417–4423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McInnes IB, Leung BP, Harnett M, Gracie JA, Liew FY, Harnett W (2003) A novel therapeutic approach targeting articular inflammation using the filarial nematode-derived phosphorylcholine-containing glycoprotein ES-62. J Immunol (Baltim Md 1950) 171(4):2127–2133 [DOI] [PubMed] [Google Scholar]
- 49.Summers RW, Elliott DE, Urban JF Jr., Thompson R, Weinstock JV (2005) Trichuris suis therapy in Crohn’s disease. Gut 54(1):87–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elliott DE, Setiawan T, Metwali A, Blum A, Urban JF Jr., Weinstock JV (2004) Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur J Immunol 34(10):2690–2698 [DOI] [PubMed] [Google Scholar]
- 51.Sun X-M, Guo K, Hao C-Y, Zhan B, Huang J-J, Zhu X (2019) Trichinella spiralis Excretory–Secretory Products Stimulate Host Regulatory T Cell Differentiation through Activating Dendritic Cells, Cells [DOI] [PMC free article] [PubMed]
- 52.Fu Y, Wang W, Tong J, Pan Q, Long Y, Qian W, Hou X (2009) Th17: a new participant in gut dysfunction in mice infected with Trichinella spiralis, mediators of inflammation 2009 517052 [DOI] [PMC free article] [PubMed]
- 53.Steel N, Faniyi AA, Rahman S, Swietlik S, Czajkowska BI, Chan BT, Hardgrave A, Steel A, Sparwasser TD, Assas MB, Grencis RK, Travis MA, Worthington JJ (2019) TGFβ-activation by dendritic cells drives Th17 induction and intestinal contractility and augments the expulsion of the parasite Trichinella spiralis in mice. PLoS Pathog 15(4):e1007657 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.