Abstract
The European Commission asked EFSA to provide an opinion according to Article 23(6) of Regulation (EC) No 1107/2009, in conjunction with Article 29 of Regulation (EC) No 178/2002, regarding the approved plant protection uses of chitosan and chitosan hydrochloride as basic substances. The Panel on Plant Protection Products and their Residues (PPR) was not provided with new dossiers but collated available scientific and technical knowledge and used a weight of evidence approach and experts' judgement for its appraisal. The statement has considered the possibility for extrapolation of the toxicological properties between chitosan and chitosan hydrochloride, and whether both substances can be expected to be of no toxicological concern; a comparison between the estimated levels of chitosan and chitosan hydrochloride resulting from the approved uses as basic substances and the level of chitosan expected to naturally occur in the environment. This last comparison served to verify whether the approved uses as basic substances might lead to an exceedance of the expected natural background levels in any of the environmental compartments (quantitative for the soil compartment and (semi)quantitative for the freshwater compartment); and accordingly, whether there was a need to advise on the safety of chitosan and chitosan hydrochloride to non‐target species occurring in the impacted environmental compartments. Overall, the PPR Panel concluded that toxicological properties can be extrapolated between chitosan and chitosan hydrochloride and that no toxicological concerns were identified. The estimated levels of chitosan and chitosan hydrochloride in the environment following application in accordance with their approved uses as basic substances would be within the same range, or below, the expected natural background exposure levels in soil and freshwaters. Considering the available ecotoxicological data and the environmental fate assessment, further consideration in relation to the safety to non‐target organisms was considered not necessary. Missing information alongside related uncertainties have been identified and considered in the overall weight of the evidence.
Keywords: chitin, basic substances, chitosan, chitosan hydrochloride, mammalian toxicology, environmental fate, natural waters, pesticide, soil
SUMMARY
Chitosan and Chitosan Hydrochloride are approved as basic substances for use in plant protection as laid down in Commission Implementing Regulation (EU) 2022/456 and Commission Implementing Regulation 563/2014, respectively.
EFSA evaluated these substances previously, in accordance with Article 23(4) of Regulation (EC) No 1107/2009, and issued the results of its work in the form of a Technical Report first for chitosan hydrochloride (in 2013) and then for chitosan (in 2020).
In 2022, following the request of two Member States to review the approvals of chitosan and chitosan hydrochloride, as in their view there was an insufficient level of information to conclude that the uses as basic substances would not lead to any risks for human or animal health, nor any unacceptable risk to the environment, EFSA was requested by the European Commission to provide an opinion according to Article 23(6) of Regulation (EC) No 1107/2009, in conjunction with Article 29 of Regulation (EC) No 178/2002. The request concerned specifically:
to verify whether the toxicological properties can be extrapolated between chitosan and chitosan hydrochloride and, consequently, advise whether it can be expected that there is no toxicological concern as indicated in the EFSA Technical Report of 2020 for chitosan hydrochloride (Term of Reference, ToR 1);
to advise to which extent it could be expected that the estimated levels of chitosan and chitosan hydrochloride resulting from the approved uses as basic substance would exceed the level expected to occur in the environment, either naturally or derived from other uses (ToR 2);
in addition, in cases the environmental levels of chitosan and chitosan hydrochloride resulting from the approved uses as basic substance would exceed significantly the levels expected to occur in the environmental compartments, to advise on the safety of chitosan and chitosan hydrochloride to non‐target species occurring in these compartments (ToR 3).
By using a weight of evidence approach and experts' judgement, the PPR Panel derived the following overall conclusions:
For the ToR 1, the PPR Panel concluded that considering the chemical structures of chitosan and chitosan hydrochloride, the history of safe use of chitosan and its salts, the poor absorption in the gastrointestinal tract of these molecules independent of their chemical form (salt or free) and the absence of toxicological concern from the available and recent literature, the two molecules are similar and the toxicological properties can be extrapolated between chitosan and chitosan hydrochloride. Overall, based on the evidence considered to address the current mandate, a toxicological concern was not identified. However, different uncertainties have been considered in the overall weight of the evidence.
For the ToR 2, the PPR Panel concluded that the estimated levels of chitosan and chitosan hydrochloride in the environment following application in accordance with their approved uses as basic substances, conservatively calculated as predicted environmental concentrations (PECs) for soil and freshwater 1 compartments, would be within the same range or below the expected natural background exposure levels of chitosan 2 in soil and natural freshwaters. A quantitative comparison for the soil compartment was carried out by the PPR Panel, while for the freshwater compartment only a semi‐quantitative comparison was possible. Moreover, the PPR Panel took note of the extensive body of knowledge of the uses of chitosan and chitosan hydrochloride other than the use as basic substance, in the first instance, uses falling under the Regulation (EU) 2019/1009 on fertilising products. The PPR Panel acknowledged that these uses shall be considered as constituting a major potential input for the overall environmental exposure, even though these uses could not be accounted for in the comparison PECs vs expected natural background exposure level due to a lack of accurate quantitative data applicable at the EU‐level. 3
For the ToR 3, the PPR Panel concluded that based on the available information in ecotoxicology and the environmental fate properties of chitosan, no further assessment in relation to the safety to non‐target soil, and surface water and sediment organisms was deemed necessary since the exposure levels resulting from the use of chitosan and chitosan hydrochloride as basic substances, in comparison to the natural background exposure levels, are not considered sufficient to trigger any risk for non‐target organisms.
1. INTRODUCTION
Chitosan was approved as a basic substance for use in plant protection by Commission Implementing Regulation (EU) 2022/456, 4 following the submission of an application by the company Kitozyme on 19 December 2018, on the basis of a Technical Report published on 22 July 2020 by the European Food Safety Authority (EFSA, 2020b). This application originally concerned an extension of use of chitosan hydrochloride, however, based on the Technical Report by EFSA and the documentation provided by the applicant, the scope of the application was re‐defined and the subsequent approval was granted for the basic substance ‘chitosan’.
Chitosan hydrochloride was approved as a basic substance by Commission Implementing Regulation (EU) 563/2014, 5 on the basis of a Technical Report published in 2013 EFSA (EFSA, 2013). In 2021, the Commission review report for chitosan hydrochloride was amended to include, in its Appendix II, additional uses as a basic substance. 6 Furthermore, later in 2020 and following years, other applications for extension of use of chitosan hydrochloride have been submitted. 7
In March 2022, two Member States requested the Commission to review the approvals of chitosan and chitosan hydrochloride in accordance with Article 23(6) of Regulation (EU) No 1107/2009. 8 In their view, the available level of information did not allow to conclude that the uses as basic substances would not lead to any risks for human or animal health, nor any unacceptable risk to the environment.
Considering the request for a review of the approval, the arguments discussed at the Standing Committee on Plants Animals, Food and Feed, and the EFSA Technical Reports from 2013 and 2020, the European Commission considered that there were indications that chitosan and chitosan hydrochloride no longer satisfy the criteria provided for in paragraphs 1 to 3 of Article 23 of Regulation (EU) No 1107/2009. Accordingly, in accordance with Article 23(6) of Regulation (EU) 1107/2009, the European Commission invited the applicants to submit any relevant information or comments on the eligibility of chitosan and/or chitosan hydrochloride to be approved as basic substances. Two applicants submitted their comments. These comments were made available to the Member States and EFSA, who were then also invited to comment. EFSA submitted its comments on 17 April 2023, and one Member State submitted its comments on 3 May 2023.
On 23 January 2024 EFSA was requested by the European Commission to provide an opinion according to Article 23(6) of Regulation (EC) No 1107/2009, in conjunction with Article 29 of Regulation (EC) No 178/2002, concerning the basic substances chitosan and chitosan hydrochloride taking into account: (i) already existing European and other regulatory or governmental assessments; 9 (ii) the dossiers of both substances submitted for their approvals 10 as well as the dossiers for the subsequent extensions of use, (iii) information submitted by the applicants in the context of the current review of approval, (iv) other scientific and technical knowledge.
The specific requests of the present mandate are presented in the following section.
1.1. Terms of Reference (ToR) as provided by the requestor
In accordance with Article 23(6) of Regulation (EC) No 1107/2009 in conjunction with Article 29 of Regulation (EC) No 178/2002, EFSA is requested to provide an opinion regarding the approved use(s) 11 of the substances chitosan and chitosan hydrochloride as basic substances.
Specifically, by using a weight of evidence approach and under consideration of expert judgement regarding the occurrence in the environment of chitosan and chitosan hydrochloride, the Commission asks EFSA to:
-
–
verify whether the toxicological properties can be considered similar and extrapolated between chitosan and chitosan hydrochloride and, consequently, advise whether it can be expected that both substances are of no toxicological concern as indicated in the EFSA Technical Report of 2020 for chitosan hydrochloride;
-
–
advise to which extent it could be expected that the estimated levels of chitosan and chitosan hydrochloride resulting from uses as basic substance 12 (currently approved at application rates 100–800 g/ha) would exceed levels expected to occur in the environment, either naturally or derived from other uses, also taking into account the structural similarity between chitin and chitosan (and its hydrochloride), the natural abundance of chitin 13 in the environment, the route of degradation of chitin which can be the precursor of chitosan 14 as well as the degradation/persistency of chitosan and chitosan hydrochloride;
-
–
In addition, in cases the environmental levels of chitosan and chitosan hydrochloride resulting from the conditions of use as defined by the existing approvals as basic substance would exceed significantly the levels expected to occur in the environmental compartments (see previous point), advise on the safety of chitosan and chitosan hydrochloride to non‐target species occurring in these compartments.
1.2. Interpretation of the Terms of Reference
EFSA deemed it appropriate to request the PPR to provide its opinion, in the form of a statement, to the European Commission regarding the approved uses of chitosan and chitosan hydrochloride as basic substances, according to Article 23(6) of Regulation (EC) No 1107/2009. Accordingly, a Working Group (WG) was established under the PPR Panel 15 to elaborate this assessment.
The PPR Panel interpreted that the ToR required the Panel to provide advice on:
- the comparison of chitosan and chitosan hydrochloride chemical structures, and whether the mammalian toxicological properties can be extrapolated between them. The Panel will also indicate whether both substances can be expected of being of no mammalian toxicological concern.
- estimates of the expected natural background exposure levels of chitosan in soil and, possibly, freshwaters, by considering chitosan and chitin abundance and their route of degradation in the environment.
- the possibility of comparing the estimated levels of chitosan/chitosan hydrochloride resulting from the approved uses as basic substance (with a maximum yearly application rate of 8 × 800 g/ha) against the expected natural background exposure levels in soil and, possibly, freshwaters.
- the collection of qualitative/quantitative information on uses of (chitin)/chitosan and chitosan hydrochloride, other than the use as basic substances, in particular as improvers of the quality of soil and as animal feed material(s).
If triggered by the outcome of ToR 2, (b), the Panel would present, if possible, a risk assessment and/or ecotoxicological consideration for the non‐target species occurring in the pertinent environmental compartments in which the exposure to chitosan/chitosan hydrochloride following application in accordance with their approved uses as basic substances is judged to significantly exceed the expected/estimated natural background exposure levels and thus, whether that possible exceedance could impact non‐target organisms in any possible way.
The PPR Panel also noted that the outcome of the consultation process organised by EFSA 16 and EFSA's scientific views on the individual comments received for the application dossiers on chitosan hydrochloride and its extension of use had been presented in the EFSA Technical Reports issued in 2013 (EFSA, 2013) and 2020 (EFSA, 2020b) respectively. Accordingly, these applications were not intended to be reassessed by the PPR Panel in this output.
2. PROBLEM FORMULATION
In line with the ToRs and the draft framework for protocol development for EFSA's scientific assessments (EFSA, 2020a), the following tasks were addressed:
For the ToR 1, in order to verify whether the toxicological properties can be extrapolated between chitosan and chitosan hydrochloride and that there is no toxicological concern:
1a. A comparison of the chemical structures of chitosan and chitosan hydrochloride.
1b. Verification of the availability of chitosan salt products to the general public (e.g. medicines and cosmetics) and their presence in foods as food constituents.
1c. Evaluation of the available absorption, distribution, metabolism and excretion (ADME) and toxicological data from basic substance applications and their extension of uses, of the relevant studies from other regulatory frameworks and of the evidence retrieved on chitosan hydrochloride from public literature and any other evidence regarding chitosan (review only).
For ToR 2:
2a. It was considered that the compound of environmental interest would be chitosan in its dry (free) or eventually ‘cationic’ form, depending on the environmental conditions, and not any possible ‘anionic’ counterions (e.g. chlorides, acetates) usually employed to get the salt form, such as chitosan hydrochloride. Accordingly, an estimation of the expected natural background exposure levels of chitosan only in soil and, possibly, freshwaters would be performed by considering:
-
–
the natural abundance of chitin and chitosan: their average occurrence in biomass found in fungi, insects, nematodes and crustaceans;
-
–
the route of degradation of chitin into chitosan: chitosan derived from the microbial degradation of chitin by considering the metabolism process and the related enzymes involved;
-
–
chitosan degradation: degradation and biodegradability of chitosan and its behaviour under environmental conditions.
2b. Possible semi‐quantitative comparison of the estimated levels of chitosan/chitosan hydrochloride following application in accordance with their approved uses as basic substances (up to a max application rate of 8 × 800 g ha−1) against the estimated natural background exposure levels of chitosan in soil and possibly in freshwaters (see bullet point 2a above). Accordingly, to enable this semi‐quantitative comparison, a direct comparison by using the application rates and the PECs for the selected environmental compartments (i.e. soil, surface water and sediment) resulting from the approved uses as basic substances will be carried out by the Panel. The estimation of the PECs will be made based on the available evidence and the parameters reported in the respective Good Agricultural Practice (GAP) tables as available in the respective EC Review reports for chitosan and chitosan hydrochloride (European Commission, 2023a, 2023b; see also Appendix E).
A comparison for the groundwater compartment will not be provided considering the expected natural biodegradation of chitosan, and because chitosan is insoluble at the pH relevant for groundwater used for potable uses (Drinking Water Directive, 2020 17 ).
2c. Collection of quali−/quantitative information on uses of (chitin)/chitosan and chitosan hydrochloride other than the uses as basic substances, mainly as improvers of the quality of soil and as animal feed materials, to better understand the potential contribution of these additional uses to the overall environmental exposure of these substances. However, a total environmental load taking into account these additional uses will not be considered, and the comparisons under task 2b will not take into account the potential exposure derived from the environmental load of chitosan by these other non‐natural sources, including uses as fertilisers/soil improvers or feed materials.
For ToR 3:
3a. Should the semi‐quantitative comparison and further environmental fate and behaviour considerations conducted under task 2b trigger the need for ecotoxicological consideration for certain taxa/non‐target species, a risk assessment would be made based on the available evidence.
3. DEFINITION OF THE METHODS
The following methodology was identified by the PPR Panel to address the problem formulation questions:
- Tools for data collection:
- –
-
–Available regulatory and/or governmental assessments pertinent to and/or supporting the present assessment of chitosan/chitosan hydrochloride were investigated by EFSA staff from Pesticides Peer Review (PREV) unit and Environment, Plants & Ecotoxicology (PLANTS) unit.
-
–EFSA staff from PREV and PLANTS units collated the available evidence from application dossiers and information submitted in the context of the former evaluations of chitosan and chitosan hydrochloride to support their approval as basic substances or in the context of peer‐review processes of chitosan‐based active substances (see Section 4.1).
-
–Hearing expert with academic and regulatory experience in chitosan research and EFSA staff from PLANTS unit with predominant expertise in (micro)biology were consulted.
-
–Papers provided by the PPR Panel WG experts.
-
–Collaboration with EFSA Panel on Food Additives and Flavourings (FAF) and Panel on Nutrition, Novel Foods and Food Allergens (NDA) and their related WGs dealing with cross‐cutting chitosan applications. 18
- Tools for data validation and evidence appraisal:
-
–Distiller SR and EndNote for title and abstract screening of the retrieved papers from the literature search (see Appendix A).
-
–Environmental fate and exposure modelling in use at European level for the estimation of the predicted environmental concentrations of pesticides in soil, surface water and sediment (see Appendix E).
-
–
- Tools for uncertainty analysis:
-
–listed sources of uncertainties (qualitative only), following the principles of the EFSA Guidance on Uncertainty Analysis in Scientific Assessments (EFSA Scientific Committee, 2018).
-
–
4. DATA AND METHODOLOGIES
The current assessment was performed by the EFSA PPR Panel in accordance with Article 23(6) of Regulation (EC) No 1107/2009 and in conjunction with Article 29 of Regulation (EC) No 178/2002. The PPR Panel collected and selected available data and information, appraised the relevant evidence, and analysed and integrated the evidence in a weight of evidence approach and under consideration of experts' judgement to enable drawing conclusions forming the basis for the present statement.
4.1. Data
The PPR Panel was not provided with newly submitted application dossiers 20 for chitosan and chitosan hydrochloride.
The Panel based its assessment on relevant information submitted to EFSA in the context of:
the evaluation, in accordance with Article 23 of Regulation (EC) No 1107/2009, of an application for approval of chitosan hydrochloride as a basic substance (EFSA, 2013);
the evaluation, in accordance with Article 23 of Regulation (EC) No 1107/2009, of an application for extension of use of chitosan hydrochloride as a basic substance (EFSA, 2020b);
the call to applicants to submit any relevant information or comments on the eligibility of chitosan and/or chitosan hydrochloride to be approved as a basic substance;
additional applications for extension of use(s) of chitosan/chitosan hydrochloride submitted by applicants 21 ;
peer‐review processes on chitosan‐based active substances COS‐OGA (EFSA, 2014) and hydrolysed chitosan, hydrochloride (polimerisation degree n = 25–55; average acetylation degree 0%–20%) (also named ‘OptiCHOs’) 22 .
In addition, the Panel also considered available EU/non‐EU regulatory or governmental assessments and other scientific and technical knowledge. All references and information considered in the present assessment are summarised in Appendices B and C along with an indication of the respective sources.
4.2. Methodologies
Based on the latest available knowledge and pertinent risk assessment methodologies when weighing the available evidence, the PPR Panel addressed the topics as presented and discussed in Section 2 Problem formulation. In this respect, an evidence‐based approach for the data retrieval, compilation and integration in a weight of evidence (WoE) was used. The PPR Panel collected the data as reported in Section 3.
The internal validity of the study design was included as part of the expert judgement while evaluating the evidence retrieved. No pre‐defined critical appraisal tools were applied for the studies included.
For task 1c, a targeted literature review was performed. New evidence from the years 2013–2024 on chitosan hydrochloride was retrieved from bibliographic databases using a fit‐for‐purpose search strategy (see Appendix A.1, search #1). The selection of the scientific studies for inclusion or exclusion was carried out by two WG experts by following these steps: (1) Title & Abstract (Ti&Ab) screening and (2) full‐text screening to further identify references to be excluded or included based on criteria related to test item and study type (i.e. whether the reference is informative of toxicological properties). Distiller SR has been used for title and abstract screening of the retrieved papers. The studies that passed the full‐text screening (see Appendix A.1.1) are reported in tables and excel spreadsheets (Appendix B) and, when relevant, discussed in the corresponding sections of the statement (Section 5.3).
New evidence from the years 2019 to 2024 on chitosan was retrieved from bibliographic databases using a fit‐for‐purpose search strategy (see Appendix A.1, search #2). Due to the large number of papers available in the public domain (more than 10,000 references), only review papers were selected for informing the assessment and the search period was limited to 5 years also considering the year of the submission of the application on chitosan (December 2018). The selection of the scientific studies for inclusion or exclusion was conducted by two WG experts by following these steps: (1) Ti&Ab screening and (2) full‐text screening to further identify references to be excluded or included based on criteria related to test item and study type (i.e. whether the reference is informative of toxicological properties). Distiller SR has been used for title and abstract screening of the retrieved papers. The studies that passed the full‐text screening (see Appendix A.1.2) are reported in tables and excel spreadsheets (Appendix B) and, when relevant, they are also discussed in the corresponding section of the statement (Section 5.3).
The evidence identified was appraised through a structured review and collegial discussion within the WG and it was synthesised and integrated using a qualitative approach.
For tasks 2a and 2c, evidence on chitin and chitosan was retrieved from bibliographic databases using three different search strategies targeted for specific purposes (see Appendix A.2):
natural occurrence and environmental fate (degradation) of chitin/chitosan,
agricultural uses of chitosan/chitosan hydrochloride other than the use as basic substances (e.g. to improve the quality of agriculture soils),
use of chitosan/chitosan hydrochloride as feed additive/material.
For all three searches, the selection of the scientific studies for inclusion or exclusion was conducted by the WG experts by following these steps: (1) Ti&Ab screening 23 and (2) full‐text screening to further identify references to be included or excluded based on criteria related to test item and study type. The studies that passed the full‐text screening are reported in Appendix C and, when relevant, they are also discussed in the corresponding section of the statement (Section 5.4).
For search #1, due to the large number of papers available in the public domain even after an optimised targeted search (more than 5000 references), only review papers, without limitation as to year of publication, 24 were selected for the first screening (Ti&Ab). Moreover, specifically for addressing task 2a, a targeted estimation of the chitosan content from natural biological sources, based on the findings of the Scientific Report on chitin estimation in agricultural soils (EFSA, 2025), was provided. Accordingly, this estimation enabled, at least for the soil compartment, a quantitative estimation of chitosan natural background exposure levels. However, chitin/chitosan ‘natural loads’ occurring in the freshwater compartments could not be quantitatively investigated. This was due to a lack of necessary information to perform a quantitative assessment considering the different variables (mainly seasonal) that play a key role in estimating concentrations in small edge‐of‐field water bodies.
For search #2 and #3, the search was extended to reviews and individual original papers and limited to the timeframe 2018–2024 (see Appendix A.2).
For task 2b, the estimation of chitosan concentrations in the selected environmental compartments, i.e. soil, surface water and sediment, was provided by the calculation of predicted environmental concentrations (PECsoil, PECsw and PECsed) following the guidance documents and using the pesticide fate models currently employed for regulatory purposes in line with the applicable legislation 25 and Commission Communications. 26 The input parameters for the PEC modelling were selected by the PPR Panel from the relevant evidence collected from the literature and available dossiers (see Section 5.4.2 and Appendix E). The PECs were calculated for both a single application at the highest rate of application and for the maximum number of applications at the shortest interval and highest rates of application (see GAP tables in Appendix E). The exposure assessment was not conducted for each approved use available in the GAP tables, but only for the worst‐case exposure scenarios, which are assumed to also cover scenarios with lower exposure (risk envelope approach 27 ). The PECsw and PECsed were estimated acknowledging the difficulties encountered obtaining reliable values, mainly due to the lack of substance property data needed as input parameters for the aquatic compartments (see details in Appendix E).
The comparison between the estimated levels of chitosan/chitosan hydrochloride resulting from the approved uses as basic substance with the expected natural background exposure levels was provided quantitatively for the soil compartment. Whereas for the aquatic compartments, the comparison was made semi‐quantitatively based on an evidence‐based approach and by using experts' judgement.
For all tasks described above, an overall qualitative uncertainty analysis was carried out to address the main sources of uncertainty identified across the scientific assessment process (see Section 7).
5. ASSESSMENT
5.1. Identity of the basic substances
Chitin & Chitosan (and its salts)
Chitin is a linear polymer (polysaccharide) of N‐acetyl‐d‐glucosamine. Chitin is the second most abundant natural polymer in the environment, after cellulose and it is found in organisms belonging to the Kingdoms Fungi, Animalia and, to a lesser extent, Protista (e.g. certain protozoans) and Chromista (e.g. some algae). It is not found in vertebrate animals and higher plants (Kurita, 2006; Sharp, 2013); but it is a basic component of the exoskeleton of arthropods (including crustaceans, insects and arachnids) and of the cell walls of fungi. (see further information on chitin sources in Section 5.4.1) (Figure 1).
FIGURE 1.
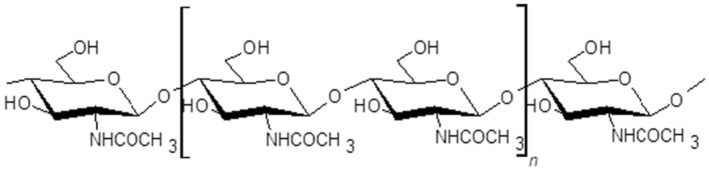
Chitin chemical structure (CAS number: 1398‐61‐4) (fully acetylated).
Commercially produced chitosan is a linear polysaccharide derived from the (partial or total) deacetylation of chitin (i.e. deacetylation of its amino groups) and it is composed of two units of randomly distributed d‐glucosamine (GlcN) and N‐acetyl‐d‐glucosamine (GlcNAc), linked by β (1,4) glycosidic bonds. Chitosan is also naturally occurring, produced mainly by certain fungi belonging to the phyla Zoopagomycota and Mucoroycota. 28 The latter phylum, contains various moulds which, notably, produce considerable amounts of chitosan (see further information on chitosan sources in Section 5.4.1).
In nature, chitin can have various degrees of deacetylation and therefore the distinction from chitosan is not strict (Mouyna et al., 2020). In this regard, it is noted that polymers with a degree of deacetylation of 50% or higher, are generally termed as ‘chitosan’, while those with lower deacetylation levels are commonly referred to as ‘chitin’. For instance, the PPR Panel noted that the commercial preparations of chitosan, assessed by the EFSA Panel of Dietetic Products, Nutrition and Allergies (NDA) for the substantiation of health claims (EFSA NDA Panel, 2011), had a degree of deacetylation (DD) ranging from 60% to 100% (EFSA NDA Panel, 2011).
Accordingly, it is acknowledged that the term ‘chitosan’ does not refer to a single polymer with a strict molecular definition, but to a family of molecules with differences in their composition, size and monomer distribution (Aranaz et al., 2021). Overall, the PPR Panel observed that the terms chitin, chitosan and chitosan oligomers are often used interchangeably in literature (see Uncertainty No. 1, Section 7).
The pattern of acetylated/amino moieties in the polymer chains of chitin/chitosan affects the physical–chemical properties of these polymers, including their solubility. Chitin, as a result, is insoluble in water and in most organic solvents due to its amino acetylated groups. Conversely, chitosan, having free (deacetylated) primary amino groups, is a weak basic cationic polyelectrolyte, which is soluble in aqueous acidic solutions (pH < 6.5), with higher solubility as the pH of the aqueous media decreases due to the protonation of the amino group. The transition between more soluble and insoluble polymers occurs around the value of the pKa that is typically between 6.0 and 6.5 (Aranaz et al., 2021) according to the degree of deacetylation, which is one determinant of this property of the polymer. The best solvent for chitosan is considered to be aqueous formic acid, while the most commonly used ones are aqueous acetic acid (1% with a pH close to 4; see also Table 2), hydrochloric acid (1%), lactic acid and nitric acid (Romanazzi et al., 2009). The solubility decreases when pH rises (from physiological values, i.e. those typically occurring in organism tissues) to alkaline values, with the increase of ionic strength of the media, with the molecular weight, with the degree of deacetylation and with the degree of polymerisation (Casadidio et al., 2019).
TABLE 2.
Identity and biological properties of chitosan as reported in the EC review report (SANTE/10594/2021 Rev. 2, European Commission, 2023a).
| Common name (ISO) | Chitosan |
| Chemical name (IUPAC, not CA) | poly[4‐O‐(2‐acetamido‐2‐deoxy‐β‐d‐glucopyranosyl)‐2‐amino‐2‐deoxy‐β‐d‐glucopyranose] |
| Common names | poly‐d‐glucosamine, Poliglusam |
| CAS No | 9012‐76‐4 |
| CIPAC No and EEC No | 618‐480‐0 (EC) |
| FAO SPECIFICATION | None |
| Minimum purity |
≥ 85% chitosan Heavy metals: max. 20 mg/kg Food grade, meeting the specifications for ‘chitosan extract from fungi’ as set out in Commission Implementing Regulation (EU) 2017/2470. |
| Molecular mass and structural formula | polycationic polysaccharide |
| Origin |
Aspergillus niger The strain of Aspergillus niger used in production of chitosan for plant protection purposes must be the strain that is used in food production and not produce mycotoxins, in particular ochratoxin A. |
| Mode of Use |
Low‐medium volume sprayer Post‐harvest treatment by immersion/dipping |
| Preparation to be used |
Soluble powder (SP) to be diluted in compliance with rate of application reported in Appendix II. 30 Preparation 1: chitosan powder should be added to a half‐filled water tank, making sure the powder is evenly distributed over the water surface to avoid aggregation. The mixture should be stirred vigorously while adding the remaining water. The mixture should be used as soon as possible. Preparation 2: Chitosan powder can be dissolved in water with pH < 5. The pH of water should be regulated by adding 7 mL vinegar (8% of acetic acid) per 1 L of water). |
| Function of plant protection | Plant elicitor |
Overall, the PPR Panel took note that as ‘chitosan materials’ are typically not water soluble at basic/neutral pH, chitosan needs to be in an acidic media or transformed into its soluble salt variant (e.g. chitosan hydrochloride or acetate) to dissolve in water, thus to enable a dilution to be prepared for making applications for plant protection purposes (see Figure 3, Tables 1 and 2 below, entry ‘Preparation to be used’).
FIGURE 3.
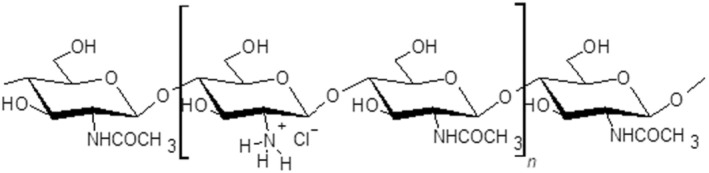
Chitosan hydrochloride chemical structure (CAS number: 70694‐72‐3).
TABLE 1.
Identity and biological properties of chitosan hydrochloride as reported in the EC review report (SANCO/12388/2013– rev. 5. European Commission, 2023b).
| Common name (ISO) | Not relevant |
| Chemical name (IUPAC) | Not relevant |
| Chemical Name (CA) | Not relevant |
| Common names |
Chitosan hydrochloride Hydrochloride of linear polysaccharide composed of randomly distributed 1–4 linked d‐glucosamine and N‐acetyl‐d‐glucosamine produced by deacetylation of chitin. |
| CAS No | 70694‐72‐3 |
| CIPAC No and EEC No | Not relevant |
| FAO SPECIFICATION | Not relevant |
| Minimum purity |
European Pharmacopeia Chitosan hydrochloride being a product of animal origin must be in compliance with the requirements of Regulation (EC) No 1069/2009 and Commission Regulation (EU) No 142/2011. |
| Molecular formula | Not relevant |
| Relevant impurities | Max content of heavy metals: 40 ppm |
| Molecular mass and structural formula | Not relevant |
| Mode of Use | Chitosan hydrochloride to be used in water solution for application on various crops or for seed treatment. |
| Preparation to be used | Chitosan hydrochloride to be diluted in compliance with rate of application reported in Appendix II. 29 |
| Function of plant protection | Elicitor, having a fungicide and bactericide effect via the stimulation of natural defence mechanisms. |
5.2. Specifications for chitosan and chitosan hydrochloride as approved basic substances
5.2.1. Chitosan hydrochloride
Chitosan hydrochloride was approved as a basic substance as laid down in Commission Implementing Regulation (EU) No 563/2014 of 23 May 2014. A summary of the identity and biological properties of the basic substance chitosan hydrochloride is presented in Table 1.
5.2.2. Chitosan
Chitosan was approved as a basic substance as laid down in Commission Implementing Regulation (EU) No 456/2022 of 21 March 2022. A summary of the identity and biological properties of the basic substance chitosan is presented in Table 2.
5.2.3. Overview of identity properties and conditions of use of chitosan and chitosan hydrochloride
Chitosan (from KytoZyme application, see Documentation provided to EFSA Nr: (1) and chitosan hydrochloride (from ChiPro application, see Documentation provided to EFSA Nr: (2) approved as basic substances and subjects of the current statement, are produced in the form of a SP and a water‐soluble powder for seed treatment (SS):
chitosan hydrochloride is soluble in neutral aqueous environment, and it is added as such by the end user in water at 1% w/w.
chitosan has to be dissolved in acidic solution (e.g. by adding natural vinegar) prior to application.
As outlined in Section 5.1, chitosan, when used for plant protection purposes, either needs to be in the dissociated form dissolved in acidic aqueous solution or in the form of a salt (e.g. chitosan hydrochloride or acetate) that following dilution in water dissociates to the cation chitosan and related counterions in the water including the chloride anion.
5.3. Human health
5.3.1. Toxicological consideration based on chemical structure of chitosan and chitosan hydrochloride (task 1a)
The PPR Panel has performed a comparison of the chemical structures of chitosan and chitosan hydrochloride (reported in Sections 5.1 and 5.2) to verify whether the toxicological properties can be extrapolated between the two basic substances.
The chemical structure of the two basic substances differs for the chloride ion. The addition of hydrogen chloride to the chitosan molecule has the technological function to improve the water solubility of chitosan. In fact, chitosan, when used for plant protection purposes, either needs to be in the dissociated form (e.g. dissolved in acidic aqueous solution) or in the form of a salt (e.g. chitosan hydrochloride or acetate) that following dilution in water dissociates to the cation chitosan and related counterions (including the chloride anion).
To further support the comparison between chitosan and chitosan hydrochloride and to clarify the toxicity of hydrochloride per se, the PPR Panel considered the pharmaceutical experience in drug development where hydrochlorides and chlorides have been by far the most frequent choice for salts of basic drugs due to their easy availability and physiological tolerability.
Stahl (2011) investigated the safety of salts‐forming ions used for pharmaceutical purposes and classified them in first‐class, second‐class and third‐class. The first‐class are the salts that can be used without restriction because they form physiologically ubiquitous ions or because they occur as intermediate metabolites in biochemical pathways. The hydrochloride salts, as well as chlorides and sodium salts, are falling under this first‐class being of substances of very low toxicity (no acceptable daily intake (ADI) specified and having generally recognised as safe (GRAS) notifications granted by the US Food and Drug Administration (21 CFR 182.1057).
Stahl (2011) also reviewed the impact of salt formation on the safety of the pharmaceutical product, underlining that the relative toxicity of the different salts reflects their aqueous solubility as long as the unwanted action of the parent compound is limited to local effects (irritation) e.g. lower solubility will exhibit less irritative effects. However, the relationship between increased solubility and toxicity cannot be generalised. It is also reported by the same authors that hydrochloride salts of active pharmaceutical ingredients (APIs) frequently exhibit less than desirable solubility in gastric and other physiological fluids because of the abundance of Cl− ion in such media due to the law of mass action (common‐ion effect).
Other information on MW and DDA have been considered. However, the available specifications provided in the applications presented for the basic substance approval as well as in the literature considered for the current mandate are too sparse to allow a direct comparison of these properties for the two basic substances (see Uncertainty No. 1, Section 7).
5.3.2. Use of chitosan salts, including hydrochloride, under other regulatory frameworks with relevance for toxicological considerations (task 1b)
Chitosan has a wide range of agricultural, biopharmaceutical, biomedical, cosmetic, textile and food additive uses (reviewed by Bellich et al., 2016; reviewed by Hamed et al., 2016; US EPA, 2007).
Chitosan derivatives have been produced aiming to improve chitosan's properties, such as solubility or biodegradability, or to introduce new functions or properties. For instance, solubility has been improved in aqueous media by deacetylation, de‐polymerisation or quaternisation among other processes (Aranaz et al., 2010).
In the various regulations authorising the uses reported above, the chemical form of chitosan is rarely clearly mentioned. In fact, it is reported that even if the two molecules i.e. chitosan and chitosan hydrochloride, are identified by different CAS numbers, in their industrial use they are seen as interchangeable (Romanazzi et al., 2009). Also, in registration dossiers, studies with chitosan and/or chitosan salts (including chitosan hydrochloride) are used often mutually, or no clear information is provided on the salt used in the studies. For example, medical devices known to have therapeutic and osteoarthritis preventing abilities are commercially available in form of hydrochloric and sulfate salts (Crolle & D'este, 1980; Kajimoto, 1998) as well as the European Pharmacopoeia (EP) monograph having been prepared for the hydrochloride salt of chitosan (European Pharmacopoeia, 2019) . The 29th edition of the United States Pharmacopeia (USP) 34‐NF that included chitosan as is, was almost 10 years later.
As polymers for human use, both chitosan hydrochloride and chitosan‐valeric acid‐hydrocolloid are reported in IRIS, 32 the online platform of the European Medicines Agency (EMA).
In other cases (e.g. medical devices, biomedical and tissue‐engineered medical product applications), the use is regulated, more generically, either for chitosan and chitosan salts/derivatives (see Appendix B for the uses of chitosan/chitosan salts under other regulatory framework with relevance for the toxicological assessment).
5.3.3. Toxicological properties (task 1c)
General considerations
Chitosan and chitosan hydrochloride were previously assessed in the respective EFSA technical reports (EFSA, 2020b, 2013) pursuant to Article 23 (4) of Regulation (EC) No 1107/2009.
Papers from the different sources (see Section 4.1), were included in the current assessment. In addition, a targeted literature review of the recent literature on chitosan hydrochloride and chitosan, as explained in Section 4.2, was conducted by the PPR Panel.
The list of all the studies considered in the current assessment is available as Appendix to this statement (see Appendix B); in the following paragraphs only a summary of relevant evidence and/or information is reported.
It is noted that the specifications of chitosan and chitosan hydrochloride include polymers ranging from 10 to 1500 kDa MW and with a DD in the range of 70%–100% (see Table 3), therefore papers testing chitosan/chitosan hydrochloride with similar specifications were considered relevant in the current evaluation. In the open literature, information on chitosan oligomers with a MW < 10 kDa is also available, and this is also considered in the current assessment due to the possible degradation of longer chitosan polymers into smaller oligomers. Moreover, the PPR Panel took note that the possible presence of nanoparticles in chitosan materials, or its other derivatives, cannot be excluded. Further considerations in this regard may be addressed in the context of the other ongoing EFSA assessments on chitosan 33 (see also Section 7).
TABLE 3.
Overview of identity properties and conditions of use of chitosan and chitosan hydrochloride (European Commission, 2023a, 2023b; documentation provided to EFSA nr: 1 and 2).
| Chitosan | Chitosan hydrochloride |
|---|---|
|
Chemical structure of Poly‐d‐Glucosamine (Chitosan), shown as dimer. Picture as provided in the KitoZyme Basic Substance Application (2020). |
Chemical structure of Poly‐d‐Glucosamine Hydrochloride (Chitosan Hydrochloride), shown as dimer. Picture as provided in the ChiPro Basic Substance Application (2011). |
| Degree of deacetylation (DDA) = 70–100% |
Degree of deacetylation (DDA) = 80–95%; Degree of acetylation (DA) = 19% |
| Molecular weight (MW): 10kDa‐1500kDa |
MW = 182–207 kDa Mn 31 = 47,000 to 65,000 g/mol |
| Solubility = water must have a pH < 6 | Solubility = 100% in water leading to an acidic pH |
| Maximum concentration of chitosan in the product = 0.6 g/L. | Maximum concentration of chitosan hydrochloride in the product = 10 g/L in aqueous solution. |
Absorption, Distribution, Metabolism, Excretion (ADME)
Chitosan's systemic absorption and distribution is largely dependent on its MW, DD and on the water solubility of the polysaccharide. It is generally recognised that molecules above 1 kDa are poorly absorbed in the gastrointestinal tract and therefore are not considered to present a toxicological risk (EFSA CEF Panel, 2016).
Zeng et al. (2008) studied the absorption profiles of fluorescein isothiocyanate (FITC)‐labelled chitosans with different MW after single oral (gavage) administration of 500 mg/kg chitosan in mice (strain not reported). The absorption of chitosan increased with decreasing MW (see Table 4). Water‐soluble chitosan (WSC, MW = 39.1 kDa) is absorbed in a greater amount compared to non‐water‐soluble counterparts with similar MW (Zeng et al., 2008). The results of the study suggested that the absorbed chitosan might consist of water‐soluble small molecules that could be degraded (chemically or enzymatically) before absorption in the intestine. Computational assessment also supports that smaller and deacetylated chitosan oligomers could be better absorbed at gastrointestinal level (Roman et al., 2019); however, the scientific validity and the reliability of this study cannot be assessed due to the paucity of data reported in the publication.
TABLE 4.
Maximum plasma concentration (Cmax, mg/g) achieved after single oral administration of 500 mg/kg of chitosan (with different MW) in female mice (adapted from Zeng et al., 2008).
| Chitosan MW | C max (mg/g) |
|---|---|
| WSC 39.1 kDa | 0.28 |
| COS 0.99 kDa | 0.68 |
| MCS 32.7 kDa | 0.15 |
| HCS 760 kDa | 0.08 |
Abbreviations: COS, chitosan‐oligosaccharides; HCS, high‐MW chitosan; MCS, mid‐MW chitosan; WSC, water‐soluble chitosan.
The water solubility of chitosan depends on the pH of the solution; high‐molecular weight chitosan (HCS) almost precipitates at pH > 6.2 but could be degraded in the stomach, where hydrogen ions (H+) bind with the amino group (–NH2) forming a cationic tertiary amino group (–NH3+). Thus, chitosan becomes a soluble salt in the stomach environment where it interacts with hydrochloric acid to form a cationic gel (Ahmadi et al., 2015). These soluble chitosan molecules could be degraded by the enzymes in the upper intestine; however, macromolecules would gradually precipitate with increasing pH in the lower intestine, which makes the chitosan, especially the HCS proportion, less well degraded by the enzymes and so probably less well absorbed by the intestine. Therefore, chitosan polymers with higher molecular weights behave like other dietary fibre and are excreted unchanged in faeces (Zeng et al., 2008).
The study author reported that chitosan oligomers can be absorbed in a certain amount, and since the definition of oligomers varied between the different publications, it was not possible to precisely define MW ranges for what would be absorbed. However, overall chitosan oligomers have a MW in the range between 1 and 32 kDa.
Due to its use as a drug carrier and its uses in wound healing processes, there are different studies where the in vitro and in vivo (after intraperitoneal and intravenous administration) biotransformation of chitosan was investigated (see Appendix B). In vitro studies showed that chitosan is transformed mainly by lysozymes and other enzymes normally produced by bacteria in the colon. In mammals, chitinases hydrolyze the β‐1, 4 glycoside bonds of chitin. In humans, two distinct chitinases belonging to glycosyl hydrolase family 18 (GH18), i.e. chitotriosidase (CHIT1) and acidic mammalian chitinase (AMCase) and one belonging to the GH22, i.e. human lysozyme, have shown chitinolytic activity against chitin and chitosan polymers (Hellmann et al., 2025). It is reported that these enzymes play a major protective role against chitin/chitosan‐containing pathogens (Hellmann et al., 2025). The process of degradation provides shorter chitosan polymers and/or chitosan monomeric residues, i.e. d‐glucosamine (GlcN) and N‐acetyl‐d‐glucosamine (GlcNAc), that are quickly absorbed and distributed into other body compartments. GlcN and GlcNAc are synthesised in the body from glucose and are present at high concentrations in joint tissues, moreover they have an approved use in the treatment of osteoarthritis (Houpt et al., 1999). It is reported that glucosamine of exogenous origin is transported into cells by glucose transporters. The safety in animals and humans of this monosaccharide component: of chitosan was reviewed by Anderson et al. (2005), who concluded that the lethal dose (LD)50 in animals (rats, mice, rabbits) is ~8000 mg/kg with no adverse effects up to 2700 mg/kg bw per day when glucosamine is administered in different species, including rats, mice, dogs and rabbits, and in studies of variable duration i.e. up to 12 months. Safety concerns have not been reported in humans when the substance was administered in clinical trials.
Chitosans WSC, MCS and HCS are distributed in liver and kidney after oral administration in mice (Zeng et al., 2008) and HCS polymers accumulate in liver. Similarly, chitosan (hydrochloride) with MW > 10 kDa accumulates in liver after intravenous administration (Richardson et al., 1999).
Only one study (Chae et al., 2005) investigated the intestinal absorption of a chitosan salt i.e. chitosan lactate. FITC‐labelled water‐soluble chitosan (WSC i.e. chitosan lactate) with low MW 3.8 kDa (88.4% degree of deacetylation ‐ DD) had the greatest plasma concentration after administering 20 mg/kg chitosan lactate via oral (gavage) in Sprague Dawley rats (see Table 5). At increasing MW the plasma concentration, reflecting the intestinal absorption of the compound, decreased. In addition, the WSC transport through the CaCo2 cell layer is also dependant on the MW. Higher apparent permeability coefficient (Papp) and transepithelial electric resistance (TERR) values were measured with oligomeric forms of chitosan (WSC; MWs = 3.8 kDa and 7.5 kDa), whereas mid‐ and high‐MW chitosan (≥ 13 kDa) showed a time‐dependent reduction of TEER values. Chitosan was bound at intestinal cell surfaces, opened the cell membrane tight junctions and increased paracellular permeability. This characteristic of chitosan was also studied by Schipper et al. (1996, 1997) in the context of the intestine absorption‐enhancing properties of chitosan (chloride salt). The MW and the degree of acetylation (DA) also influenced the absorption‐enhancing properties of chitosan i.e. under the condition of Schipper's study, low DA and high‐MW seems to be necessary to increase epithelial permeability. This is because the positive charge of chitosan makes a charge interaction possible between chitosan and the negatively charged surface of the epithelial layer.
TABLE 5.
Maximum plasma concentrations (C max, μg/mL) and the areas under the curve (AUC, μg/mL h) values achieved after single oral administration of 20 mg/kg of chitosan (with different MWs) in Sprague Dawley male rats. The apparent permeability coefficient (Papp, cm/sec) is calculated after exposing human intestinal CaCo2 cells to chitosan lactate (adapted from Chae et al., 2005).
| Chitosan lactate MW | C max (μg/mL) | AUC (μg/mL h) | Papp (cm/s) |
|---|---|---|---|
| 3.8 kDa | 20.23 | 24.13 | 3.306*10−6 |
| 7.5 kDa | 9.30 | 11.55 | 1.825*10−6 |
| 13 kDa | 5.86 | 8.71 | 1.363*10−6 |
| 22 kDa | 4.32 | 5.59 | 0.870*10−6 |
| 230 kDa | < 0.5 | 0.97 | 8.290*10−8 |
Information on the ADME properties of chitosan and chitosan hydrochloride after dermal or inhalation exposure was not found in the applications, or in the subsequent literature searches. However, on the basis of information on the chemical characteristics of the substances (polymers), internal exposure through these compartments is not expected and if there was any dermal or inhalation exposure these are not expected to have an impact on the assessment based upon the oral route of exposure.
Acute toxicity
The table below reports an overview of the oral lethal dose (LD) 50 from the available studies relevant to this statement (Table 6).
TABLE 6.
Oral LD 50. Summary of the acute toxicity of chitosan, chitosan oligomers and chitosan derivatives following oral administration:
| Test item | MW | DD a | Species | LD50 | Reference b |
|---|---|---|---|---|---|
| Chitosan free, chitosan acetate, chitosan formate | N/R | N/R | Mice | 16,000 mg/kg | Arai et al. (1968) c |
| Chitosan | 350 kDa | 96.2% | SD b Rats M | 10,000 mg/kg | Zhang et al. (2012) |
| Chitosan | 309 kDa | 83% | Wistar Rats F | > 2000 mg/kg | Lagarto et al. (2015) |
| Water‐soluble chitosan | 210 kDa | 85% | SD Rats M | 10,000 mg/kg | Zhang et al. (2012) |
| Low‐molecular weight chitosan | 110 kDa | 84% | SD Rats F | > 5000 mg/kg | Chang et al. (2014) |
| Chitosan | N/R | N/R | SD Rats F | 2000 mg/kg | KitoZyme (2008) |
| Chitosan oligosaccharide | N/R | N/R | Wistar Rats F | 1500 mg/kg | Eisa et al. (2018) |
| Chitosan oligosaccharide | 1.2 and 5.3 kDa | 80–85% | Balb/c Mice M | > 1000 mg/kg | Fernandes et al. (2010) |
| Chitosan oligosaccharide | 1.86 kDa | 85% | Kunming Mice M,F | > 10,000 mg/kg | Qin et al. (2006) |
| Chitosan catechol | 50–190 kDa | N/R | Swiss albino Mice M,F | > 2000 mg/kg | Kaur et al. (2020) |
Abbreviations: F, female; M, male; N/R, not reported; SD, Sprague Dawley.
Degree of deacetylation.
Complete reference available in Appendix B.
Paper in Chinese, only abstract available in English.
For what concerns the other potential exposure routes i.e. dermal and inhalation, in the study conducted by Rao and Sharma (1997), chitosan showed no skin or eye irritating potential 34 when tested in guinea pigs and rabbits, respectively. Moreover, chitosan is used in cosmetics applications (review by Aranaz et al., 2018), in wound dressings and as a drug delivery system in the treatment of eye diseases (review by Shariatinia, 2019) and no adverse effects were reported in the recent literature (see Appendix A.1 and Appendix B). No local damage to pulmonary tissue was observed in rats after intratracheal administration of chitosan (MW: 31kDa, DD: ≥ 75%; de Jesús Valle et al., 2008) and chitosan oligomers (MW range: 0.340–96 kDa, DD: 87%–100%; Yamada et al., 2005).
Chitosan hydrochloride (MW: 160 kDa; DD% 87%) is well tolerated after topical administration onto the corneal surface of rabbits (Felt et al., 1999).
Genotoxicity
There is no indication of chromosomal aberration in the in vitro study conducted by Chang et al. (2014) (Chitosan from crab shell; MW: 1100 kDa, DD: 84%). The in vivo micronucleus assay conducted by the same authors and reported in the same paper confirmed this finding; however, it was not reported whether the bone marrow was sufficiently exposed.
Other studies conducted with low‐molecular weight chitosan (Fernandes et al., 2011), chitosan oligosaccharides (Fernandes et al., 2011; Nam et al., 2001; Qin et al., 2006; Yoon et al., 2005) and on the monosaccharide glucosamine (the monomer of chitosan) (review by Anderson et al., 2005) were already considered as part of the basic substance applications. It is noted that the absence of genotoxic potential of these compounds is considered a sufficiently conservative approach for addressing the concern for genetic toxicity of chitosan polymer with larger MW (Bolognesi et al., 2017; EFSA CEF Panel, 2016).
Developmental toxicity
In a prenatal developmental toxicity study (Cheng et al., 2013), high‐molecular weight chitosan was administered via single dose intraperitoneal injection in pregnant ICR mice at gestation day (GD) 6. In F0 dams, signs of maternal toxicity i.e. reduced body weight and food consumption, diarrhoea, vaginal bleeding and clinical adverse effects, were observed at doses above 500 mg/kg bw per day, the development of F1 and F2 animals was not affected. Statistically significant reduced numbers of live foetuses and increased number of early resorptions have been observed at 500 and 2000 mg/kg bw per day in F0 dams, in addition, also reduced body weights of F1 and F2 was observed and statistically significant at the top dose. The author concluded that the study had limitations, among which were the unclear mode of action leading to vaginal bleeding and diarrhoea in dams. Moreover, the route of exposure i.e. intraperitoneal, is not considered relevant to the current evaluation.
Repeated‐dose toxicity
Most of the repeated‐dose toxicity studies on chitosan and its salts or derivatives were aimed at evaluating the beneficial effects of chitosan, especially in animal models of hyperlipidaemia and obesity, therefore findings were limited to effects on body weight, behavioural signs, when reported, triglycerides and cholesterol levels and histopathology of mainly the liver and kidney.
In a few cases, a LOEL or LOAEL/NOAEL were identified for chitosan (see Table 7) from oral toxicity studies. No adverse effects have been reported for chitosan acetate and chitosan lactate.
TABLE 7.
Overview of NOAEL/LOAEL from oral repeated‐dose toxicity studies.
| Test item, MW, DD | Study design | NOAEL/LOAEL | Effect (as reported by the study author) | Reference a |
|---|---|---|---|---|
| Chitosan | ||||
|
Chitosan – C 309 g/mol, 83% Chitosan Acetate – CA (48.9%) Chitosan Lactate ‐ CL (57.1%) |
28‐days oral Wistar Rats/F n = 7 animals/group DOSES: C: 0, 100, 300, 1000 mg/kg bw per day CA: 0, 700 mg/kg bw per day CL: 0, 1000 mg/kg bw per day |
C: 1000 mg/kg bw per day CA and CL: N/R |
No signs of toxicity | Lagarto et al. (2015) |
| LMW Chitosan 110 kDa, 84% |
28‐days oral Wistar Rats/M‐F n = 10 animals/sex/group DOSES: 0, 0.2, 0.5, 1.0 g/kg bw per day |
1000 mg/kg bw | No signs of toxicity | Chang et al. (2014) |
|
Chitosan 82 kDa, 86.5% |
6 months oral Sprague Dawley Rats/M‐F n = 10 animals/sex/group DOSES: 0%, 1%, 3% or 9% corresponding to M/F: 450/650, 1500/1800, 5200/6000 mg chitosan/kg bw per day. |
LOEL: 450 mg/kg bw per day (M); 6000 mg/kg bw per day (F) | ↓ levels of serum vitamin A and serum and hepatic vitamin E and increased levels of serum 1,25 (OH)2 vitamin D. | NTP (2017) |
| Other test items | ||||
|
N‐acetylglucosamine MW and DD not reported |
52 and 104 weeks, oral Fisher Rat/M/F n = 10 animals/sex/group DOSES: Chronic > 0% (control), 1.25%, 2.5% or 5% equivalent to M/F: 580/647, 1159/1269, 2323/2545 mg/kg bw per day. Carcinogenic: 0; 2.5%, 5% equivalent to M/F: 964/ 1106 1935/2244 mg/kg bw per day. |
2323 (M)/2545 (F) mg/kg bw per day No carcinogenic potential was found in male and female. |
No signs of toxicity | Takahashi et al. (2009) |
| Chitosan oligosaccharide 1.86 kDa, 85% |
30‐day, oral Sprague Dawley Rat M/F (N = 10 animals/sex/group) DOSES: 0.75, 1.5, 3.0 g/kg bw per day |
No systemic toxicity up to 3.0 g/kg bw per day. | No signs of toxicity | Qin et al. (2006) |
|
Chitosan oligosaccharide < 1 kDa, DD not reported |
28‐day, oral Sprague Dawley Rat (N = 36 animals/sex) DOSES: 0, 500, 1000 and 2000 mg/kg bw per day |
NOAEL > 2000 mg/kg bw per day. | No signs of toxicity | Kim et al. (2001) |
|
OAG (oligo‐N‐acetyl‐glucosamine) < 1 kDa, DD not reported |
90‐day, oral Fisher Rat/M/F n = 10 animals/sex group DOSES: 0.2%, 1%, 5% equivalent to: not determined, 0.6411, 3.64 g/kg bw per day |
641 (M)/3640 (F) mg/kg bw per day | No signs of toxicity | Tago et al. (2007) |
|
Oligoglucosamine (OG) MW and DD not reported |
90‐day, oral Fisher F344 Rats M/F N = 20 animals/group DOSES: 0, 0.04, 0.2 or 1.0 (w/w)% equivalent to 0, 24.5/26.6, 124.0/142.0, 653.1/719.8 mg/kg bw per day |
124.0/142.0 mg/kg bw per day 653.1/719.8 mg/kg bw per day |
↑ plasma creatinine, proteinuria, abnormal testes. Additionally, several changes due to systemic malnutrition | Naito et al. (2007) |
|
Chitosan oligomers 5 kDa, > 90% |
50d, oral C57BL/6 male mice N = 15 animals/group DOSES: 0, 20, 100, 500 mg/kg bw/day |
// | No systemic toxicity up to 500 mg/kg bw per day | Mattaveewong et al. (2016) |
|
N‐acetylglucosamine (GlcNAc); MW and DD not reported |
90‐days, oral F344/DuCrj rats (n = 10 animals/sex/group) DOSES: M/F:: 0, 301.7/350.9, 587.8/694.9, 1218.0/1412.1, 2475.6/2833.6 mg/kg bw per day |
2476 and 2834 mg/kg bw per day for male and female rats | No signs of toxicity | Lee et al. (2004) |
complete reference available in Appendix B.
Repeated‐dose toxicity studies were also available on chitin‐glucan and chitin/chitosan oligosaccharides, showing no or low toxicity for chitosan oligosaccharides. Only in one oral toxicity study in Fischer F344 Rats (Naito et al., 2007), conducted with oligoglucosamine (i.e. chitosan oligosaccharide) (permitted as Food Additive in Japan 35 ), were increased plasma creatinine, proteinuria and abnormal testes reported; changes due to systemic malnutrition were also reported, with a decrease of 36% of body weight at the highest dose tested. Therefore, clinical chemistry and testicular effects are considered secondary to the poor systemic condition and not the expression of target organ toxicity.
In the study by Minami et al., 1996, mongrel dogs exposed via the subcutaneous route to chitosan (mean particle size 5 μm) suffered from severe pneumonia symptoms; the correlation between chitosan exposure and the observed effect was not clearly investigated in the study and a number of flaws were identified in the study i.e. local effects at the injection site were not reported and this could have been relevant for the systemic response of the animals (clinical chemistry and haematology). Therefore, this repeated‐dose toxicity study conducted via subcutaneous (Minami et al., 1996) route of administration was not considered relevant for the current evaluation.
Human studies
Although the main aim of the available studies in humans was to investigate the efficacy of chitosan as a dietary supplement to improve weight loss and reduce cholesterol levels, there was no evidence of adversities when chitosan was administered as a dietary supplement up to 6.75 g/day in a healthy population and up to 3 g/day in subjects with clinical conditions associated with obesity (e.g. hypercholesterolemia, hyperlipidaemia, type 2 diabetes) (see Appendix B). In the available studies (n = 28, see Appendix B) the test item was always referred to as chitosan, and there is no information on whether a salt had been used.
It is noted that chitosan and its salts, including hydrochloride, due to their absorption‐enhancing properties, are widely used as drug carriers in different medical treatments without safety concerns having been reported in pharmacovigilance. In addition, chitosan is subject to ongoing evaluations as a novel food 36 and food additive, 37 and it is frequently consumed as a dietary supplement.
Other studies– In vitro toxicity and allergenicity
In vitro toxicity
Chitosan and chitosan salts are used in biomedical applications, therefore there are several studies that have evaluated cell viability as an initial screening test for biocompatibility.
The toxicity of chitosan and its derivates (chitosan oligosaccharides and chitosan salts) on different cell types from mice (i.e. murine melanoma cell line – B16F10 and murine macrophages cell lines – RAW 264.7 and J774), chicken (i.e. ganglion neurons) and humans (i.e. immortalised cell line of human colorectal adenocarcinoma (CaCo2), human embryonic lung (L132), human lung cancer (A549), human lymphoblastic leukaemia (CCRF‐CEM), human pancreatic carcinoma (COLO‐357), human kidney carcinoma (KCC853)) was investigated in a number of studies. Also, studies on the effect of chitosan on cell morphology (in CaCo2 cells) and haemocompatibility properties of chitosan (red blood cells (RBC) from rats) were available.
The available studies underlined that the toxic effect of chitosan on the cells is highly dependent on the concentration of chitosan used, on its MW, on its deacetylation degree (DD) and finally on the salt form, modifications or derivatives. From the available in vitro evidence, Carreno‐Gomez and Duncan (1997) found chitosan hydrochloride salt to be cytotoxic (inhibitory concentration (IC50) 0.21 ± 0.04 mg/mL) against B16F10 (murine melanoma) cells. All the chitosan salts tested (chitosan hydrochloride CL110 and CL210, chitosan hydroglutamate G110, and G210 and chitosan hydrolactate L110 and L210) caused/showed concentration‐dependent cytotoxicity. Chitosan cytotoxicity could be ranked in the order as follows: chitosan hydrochloride > hydroglutamate > glycol chitosan > hydrolactate. Polymer MW also influenced the cytotoxicity, and all chitosans that had MW of > 100 kDa were more cytotoxic towards B16F10 cells. When tested on red blood cells, all the chitosans had a lytic effect in a time‐ and molecular weight‐dependent manner. At 1 h, relatively little lysis was seen, except for the hydroglutamate chitosans, G 110 (75% lysis), G 210 (60% lysis) and hydrochloride CL210 (12%). However, this study appears to have used solutions of the chitosan salts at the pH formed when dissolved, for chitosan hydrochloride in phosphate buffered saline (PBS) (10 mg/mL), pH = 5.8. At this pH the 3‐[4,5‐dimethylthiazol‐2‐yl]‐2,5‐diphenyl tetrazolium bromide (MTT) assay is not appropriate, and cell viability is compromised (reviewed by Kean & Thanou, 2010).
Richardson et al. (1999) found chitosan (probably chitosan hydrochloride, as reported in one picture but not confirmed in the text) of low MW to be slightly cytotoxic against CCRF‐CEM and L132 cells (IC50 > 1 mg/mL). However, chitosan is not haemolytic (15% lysis after 1 and 5 h).
According to Schipper et al. (1996), the toxicity of chitosan chloride salts appears to be related to the positive charge density of the polymer, and this is in line with the well‐known cell lytic and toxic properties of cationic polymers such as poly‐L lysine. In fact, the results of the study showed that the higher the MW (large molecule with high charge density) and the lower the DA, the more toxic chitosan is to cells (CaCo2 cells). The IC50 was not calculated by the authors; however, from the graphical representation (Figure 2 in Schipper et al., 1996), it seems that enzyme activity decreases by 50% when cells are treated with chitosan chloride salt concentrations between 0.01 and 0.1 mg/mL. Chitosans with low degree of acetylation (DA) (1%) also lead to change in number of microvilli and organisation of the terminal web. However, it has to be noted that the test system does not fully represent physiological conditions, i.e. the absence of mucus on the gastrointestinal epithelium, that could be relevant for the biological and/or toxicological effects of chitosan (binding to mucus may be important).
FIGURE 2.
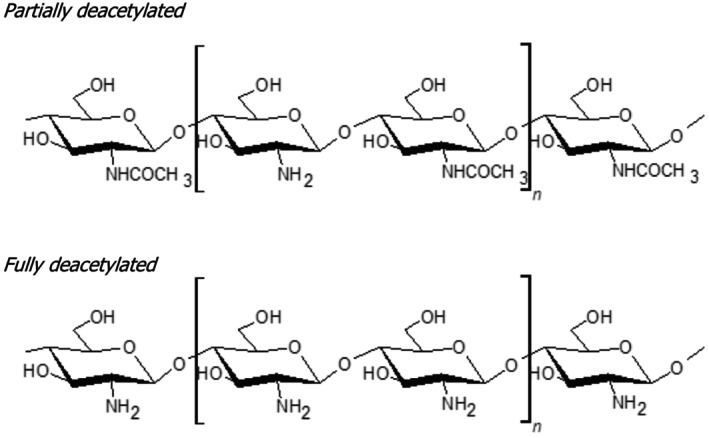
Chitosan chemical structure (CAS number: 9012‐76‐4).
Chae et al. (2005) showed that CaCo2 cell viability is highly dependent on the concentration and on the MW of chitosan (WSC = Chitosan Lactate). With increasing concentrations of chitosan lactate, the cytotoxic effect is affected by the MW. EC50 (effective concentration for 50% cell viability) values of each chitosan were evaluated. EC50 of 3.2 and 4.5 mg/mL were measured after treating CaCo2 cells with high and middle MW water‐soluble chitosan, respectively (i.e. 230 kDa and 22 kDa).
A comprehensive overview of the IC50 of chitosan, chitosan salts and chitosan derivatives was reported in the review by Kean and Thanou (2010) (Table 8). It has to be noted that a number of studies were not submitted as part of the basic substance applications. Among these, the study by Opanasopit et al. (2007) investigating the cytotoxic effect of different chitosan salts, including chitosan hydrochloride, on CaCo2 cells. The results reported by Opanasopit are in line with the ones from Carreno‐Gomez and Duncan (1997); chitosan hydrochloride has higher cytotoxicity compared to the other salts with an IC50 of 0.22 ± 0.06 mg/mL (for chitosan hydrochloride with MW 45 kDa).
TABLE 8.
Summary of cytotoxicity (IC50) of chitosan and chitosan derivatives to different cells (adapted from Kean & Thanou, 2010).
| Chitosan details (DD, MW) | Modification | Assessment | IC50 | Reference |
|---|---|---|---|---|
| 95% DD, 18.7 kDa | Steric acid conjugation micelle | In vitro, A549 cells | 369 ± 27 μg/mL | Ye, Y. Q., Chen, F. Y., Wu, Q. A., Hu, F. Q., Du, Y. Z., Yuan, H., and Yu, H. Y. (2009). Enhanced cytotoxicity of core modified chitosan‐based polymeric micelles for doxorubicin delivery, Journal of Pharmacology Science, 98, 704–712. |
| 95% DD, 18.7 kDa | Steric acid conjugation and entrapment in micelle | In vitro, A549 cells | 234 ± 9 μg/mL | |
| 97% DD, 65 kDa | N‐octyl‐O‐sulfate | In vitro, primary rat hepatocytes | > 200 mg/mL | Zhang, C., Qu, G., Sun, Y., Yang, T., Yao, Z., Shen, W., Shen, Z., Ding, Q., Zhou, H., and Ping, Q. (2008). Biological evaluation of n‐octyl‐o‐sulfate chitosan as a new nano‐carrier of intravenous drugs. European Journal of Pharmacology Science, 33, 415–423. |
| 87% DD, 20, 45, 200, 460 kDa | None, aspartic acid salt | In vitro, Caco‐2 cells, pH 6.2 | 0.67 ± 0.24, 0.61 ± 0.10, 0.65 ± 0.20, 0.72 ± 0.16 mg/mL | Opanasopit, P., Aumklad, P., Kowapradit, J., Ngawhiranpat, J., Apirakaramwong, A., Rojanarata, T., and Puttipipatkhachorn, S. (2007). Effect of salt forms and molecular weight of chitosans on in vitro permeability enhancement in intestinal epithelial cells (caco‐2), Pharmaceutical Development & Technology, 12, 447–455. |
| 87% DD, 20, 45, 200, 460 kDa | None, glutamic acid salt | 0.56 ± 0.10, 0.48 ± 0.07, 0.35 ± 0.06,0.46 ± 0.06 mg/mL | ||
| 87% DD, 20, 45, 200, 460 kDa | None, Lactic acid salt | 0.38 ± 0.13, 0.31 ± 0.06, 0.34 ± 0.04, 0.37 ± 0.08 mg/mL | ||
| 87% DD, 20, 45, 200, 460 kDa | None, hydrochloride salt | 0.23 ± 0.13, 0.22 ± 0.06, 0.27 ± 0.08, 0.23 ± 0.08 mg/mL | ||
| 78% DD, < 50 kDa | None, lactic acid salt | In vitro B16F10 cells | 2.50 mg/mL | Carreño‐Gómez, B., and Duncan, R. (1997). Evaluation of the biological properties of soluble chitosan and chitosan microspheres. International Journal of Pharmaceutics, 148, 231–240. |
| 82% DD, 150–170 kDa | None, lactic acid salt | In vitro B16F10 cells | 2.00 ± 0.18 mg/mL | |
| > 80% DD, 60–90 kDa | None, glutamic acid salt | In vitro B16F10 cells | 2.47 ± 0.14 mg/mL | |
| 77% DD, 180–230 kDa | None, lactic acid salt | In vitro B16F10 cells | 1.73 ± 1.39 mg/mL | |
| 85% DD, 60–90 kDa | None, hydrochloric acid salt | In vitro B16F10 cells | 2.24 ± 0.16 mg/mL | |
| 81% DD, 100–130 kDa | None, hydrochloric acid salt | In vitro B16F10 cells | 0.21 ± 0.04 mg/mL | |
| 100% DD, 152 kDa | Glycol chitosan | In vitro B16F10 cells | 2.47 ± 0.15 mg/mL | |
| 100% DD, 3–6 kDa | 20, 44, 55% Trimethyl chitosan, chloride salt | In vitro, MCF7 and COS7 cells, 6 and 24 h | > 10 mg/mL | Kean, T., Roth, S., and Thanou, M. (2005). Trimethylated chitosans as non‐viral gene delivery vectors: Cytotoxicity and transfection efficiency. Journal of Control Release, 103, 643–653. |
| 100% DD, 3–6 kDa | 94% Trimethyl chitosan, chloride salt | In vitro, MCF7, 6 h | 1.402 ± 0.210 mg/mL | |
| 100% DD, 3–6 kDa | 94% Trimethyl chitosan, chloride salt | In vitro, COS7, 6 h | 2.207 ± 0.381 mg/mL | |
| 100% DD, 100 kDa | 36% Trimethyl chitosan, chloride salt | In vitro, MCF7, 6 h | 0.823 ± 0.324 mg/mL | |
| 100% DD, 100 kDa | 36% Trimethyl chitosan, chloride salt | In vitro, COS7, 6 h | > 10 mg/mL | |
| 84.7% DD, 400, 100, 50, 25,5 kDa | 40% Trimethyl chitosan | In vitro, L929 cells, 3 h | 30, 70, 90, 270, > 1000 μg/mL | Mao, S., Shuai, X., Unger, F., Wittmar, M., Xie, M., and Kissel, T. (2005). Synthesis, characterisation and cytotoxicity of poly(ethylene glycol)‐graft‐trimethyl chitosan block copolymers, Biomaterials, 26, 6343–6356. |
| 84.7% DD, 1.89 MDa | 12% PEG modified 40% trimethyl chitosan | In vitro, L929 cells, 3 h | 220 μg/mL | |
| 84.7% DD, 3.6 MDa | 25.7% PEG modified 40% trimethyl chitosan | In vitro, L929 cells, 3 h | 370 μg/mL | |
| 84.7% DD, 300 kDa | 6.44% PEG modified 40% trimethyl chitosan (and all PEG modified TMC with lower M w) | In vitro, L929 cells, 3 h | > 500 μg/mL | |
| 97% DD, 65 kDa | N‐octyl‐O‐sulfate | In vivo, IV, mice | 102.59 mg/kg | Zhang, C., Qu, G., Sun, Y., Yang, T., Yao, Z., Shen, W., Shen, Z., Ding, Q., Zhou, H., Ping, Q. (2008). Biological evaluation of n‐octyl‐o‐sulfate chitosan as a new nano‐carrier of intravenous drugs. European Journal of Pharmaceutical Sciences, 33, 415–423. |
| 97% DD, 65 kDa | N‐octyl‐O‐sulfate | In vivo, IP, mice | 130.53 mg/kg |
Zubareva et al. (2017), showed that unmodified chitosan is slightly toxic (5%–10% at 100 μg/mL) to cells compared to the quaternised chitosan. The cytotoxicity of chitosan (2% w/v in 6% v/v CH3COOH, 100 mL) on A549 Cells was investigated by Huang et al. (2004), who reported that cell viability (A549 Cells) was not affected at concentration lower than 0.741 mg/mL. Wiegand et al. (2010) found that chitosan (MW: 120 kDa, DD: 85%, source and manufacturing process not reported) and chitosan oligosaccharide lactate (MW: 5 kDa, DD: > 90%) caused MW‐dependent cytotoxicity, measured as intracellular ATP content, on human keratinocytes when tested in vitro (at 0.5% and 1% w/v), no positive control was included in the study.
Allergenicity
Chitosan and chitosan hydrochloride are not included in the list of substances or products causing allergies or intolerances (Annex II, Regulation (EU) No 1169/2011); however, since chitosan may derive from sources included in the list (e.g. crustaceans), a dedicated chapter has been included in the current evaluation.
Chitosan is unlikely to pose allergenicity concerns when industrially processed, provided that all animal proteins are removed during extraction and purification processes. The manufacturing process, involving demineralisation with hydrochloric acid, protein removal with sodium hydroxide and extraction with organic solvents is considered effective in removing or denaturing proteins, fats and other potentially allergenic or toxic contaminants (US EPA, 2019). Since strong acid/alkali are used to obtain chitosan from chitin, it is reasonable to assume that no protein will remain that can induce an allergenic reaction.
While there has been research into other methods of manufacturing chitosan, this process is understood to be the industry standard and other methods have not been shown to be viable on the scale required to produce chitosan at its current level of demand.
Waibel et al. (2011) investigated the safety of ‘HemCon®’ bandages that were introduced in 2005 for US soldiers. Patients who reported shellfish allergy were recruited. Initial assessment included a detailed history, IgE skin prick testing (SPT) and serum testing to shellfish allergens. Participants who demonstrated specific shellfish IgE underwent a bandage challenge. It was reported that of the 19 participants who were enrolled, 10 completed the study. No participant had a positive SPT to chitosan powder or experienced an adverse reaction during bandage challenges (Waibel et al., 2011).
5.4. Environmental fate and behaviour
As outlined in the chemistry and human health sections, it is relevant to recall even in the context of the environmental fate and behaviour that the chemical structure of chitosan and its salt variant chitosan hydrochloride, the two basic substances in subject, differs for the chloride ion. The addition of hydrogen chloride to the chitosan molecule has the technological function to improve the water solubility of chitosan. In this respect it is noted that chitosan, when used as elicitor for plant protection purposes, either needs to be in the dissociated form (e.g. dissolved in acidic aqueous solution) or in the form of a salt (e.g. chitosan hydrochloride or acetate) that following dilution in water dissociates to the cation chitosan and related counterions (including the chloride anion). Accordingly, chitosan in its dry (free) or eventually ‘cationic’ form depending on the environmental conditions, and not any other possible ‘anionic’ counterions (e.g. chlorides, acetates), is the compound of environmental (and ecotoxicological) interest.
5.4.1. Chitosan and chitin abundance and their route of degradation in the environment (task 2a)
5.4.1.1. Chitin abundance
The majority of the literature retrieved reported that chitin is the second most abundant natural polysaccharide found in the environment after cellulose, with annual production worldwide ranging from 1011 to 1014 tonnes in the biosphere. Although this is a recurrently reported background information in the literature, it is not supported by a quantitative estimation, especially when considering specific environments like the soil and freshwater compartments. In general, quantitative data on chitin contents are still incomplete, and estimations are suggested to be interpreted with care, especially for the different quantification methods used and the varying parts of the biomass considered for the calculations (Broek & Boeriu, 2019).
An estimation of the chitin content from biological sources in soil has been presented in the EFSA Scientific Report on ‘Chitin estimation in agricultural soils’ (EFSA, 2025). Some considerations were tailored towards agricultural soil when this information was available in the literature, while others refer to soil more generally. All information was obtained from the review of scientific literature integrated with expert knowledge. The biological producers of chitin that were considered in this scientific report were fungi, insects and nematodes. To estimate the amount of chitin in the topsoil (in two layers, from 0 to 5 cm and 0 to 20 cm depth), a calculation was performed based on the formulas outlined in the report. Each parameter was estimated separately and finally combined to obtain the ultimate result. Two different scenarios were developed to encompass the unavoidable uncertainties, one of a high‐soil chitin content and one of a low‐soil chitin content. Fungi are considered the main chitin producer since their cell wall constitutively contains chitin. Insects are also considered as chitin producers due to the presence of this polymer in their cuticles, exoskeleton and exuviae. Finally, nematodes are considered in this report because their eggshell and pharynx contain chitin. The estimation of the fungal chitin content has been performed by dividing the members of this group into subgroups that differ by size, relative abundance and chitin content. In particular, three groups were considered as being dominant in soil and different enough to be treated separately: Ascomycota (with a further distinction between yeast and filamentous fungi), Basidiomycetes and Zygomycetes (old phyla maintained in this report, although the data used for this estimation were taken from the order of Mucorales, now belonging to the new phyla Mucoromycota). The amount of chitin for each cell type was estimated by assessing the average cell biomass (wet weight or dry weight), the percentage of dry matter constituting the cell wall and finally the percentage of chitin in the cell wall. Considering agricultural soil as the reference biome, the relative abundance of a typical fungal community composition was estimated based on metabarcoding studies retrieved in the literature (EFSA, 2025). In this way Ascomycota represent the dominant phyla, followed by Basidiomycota and Mucoromycota (formerly placed in Zygomycota). Finally, based on the existing literature, the fungal population size was estimated considering a range of uncertainty of one order of magnitude (106–107 fungal cells/gram of soil). Insects were treated in a similar manner distinguishing between microarthropods and large arthropods, which greatly differ in biomass and population size. Also in this case, a range of uncertainty was considered assuming the insect population spanning from 107 to 108 individuals per hectare. Finally, the estimation of chitin in Nematodes was performed in two different ways based on the available information and providing the range of uncertainty computed in the model for the high and low content of chitin in soil. To conclude, chitin in soil was estimated to range from 31 to 320 kg ha −1 in the first 0–5 cm layer and 113 to 1038 kg ha −1 in the 0–20 cm layer. Fungi are the main producers, followed by insects and their respective population size is the strongest predictor of chitin content in agricultural soil. It is highlighted that the bulk density of the average soils considered for these calculations is adjusted to 1.5 g cm−3 to be consistent with the estimation of the PECs according to the FOCUS (1997) Guidance.
Soil crustaceans were not considered in the assessment due to the lack of information collected and the variability of their presence. The population, when present, is estimated to be around 101–102 per m3, for instance, in the aquatic compartment, making the distribution on a large landscape level a challenge. The same considerations would need to be applied to the surrounding fields, where the size of the population could be even lower. The biomass considering crustaceans is larger, with expected values of 15%–20% of its dry weight made of chitin. Although the size of the crustacean population is difficult to estimate, it will likely constitute a small addition to the overall amount of chitin predicted in soils.
The only quantitative estimation of chitin in soil, from the review paper by Dhillon et al. (2013), reported higher values compared to the calculations above, though they also concluded that fungi provide the highest amount of chitin in the soil, i.e. 500–5000 kg ha−1. However, uncertainties were noticed since the primary study referred to in the review, i.e. Shahidi and Abuzaytoun (2005), did not report a clear methodology for this quantitative estimation. It is also noted that the reported data are probably higher because different types of soils are considered, rather than only agricultural soils. Further details on the results of the EFSA Scientific Report (EFSA, 2025) and the qualitative assessment from the references collected within this Statement are provided in Appendix E.
The assessment of the chitin content from biological sources in the freshwater aquatic environment has not resulted in a quantitative estimation. This was mainly due to a lack of necessary information to perform a quantitative assessment, considering the different variables (mainly seasonal) that play a key role in estimating concentration in small edge‐of‐field water bodies, e.g. small rivers, streams, irrigation channels, ditches and ponds. This variability reflects the challenge in estimating the taxonomy and distribution of aquatic species on a landscape level. However, it is assumed that the major contribution of chitin in freshwater aquatic ecosystems is provided by arthropods (crustaceans and insects), at least in terms of biomass and it is considered that the contribution of fungi and yeasts is lower compared to the soil compartment. In support of this assumption, Cauchie (2002) estimated the chitin production by crustaceans and insects in the total hydrosphere, considering all continental and marine waters worldwide. In this paper, it was reported that as per the literature data on chitin production by arthropods, the total annual chitin production in aquatic environments could be estimated with a methodological approach at 27.7 × 106 tonnes chitin/year for freshwater ecosystems (15.8 × 106 tonnes chitin/year for pelagic communities and 11.9 × 106 tonnes chitin/year for benthic communities), acknowledging that the chitin production in marine ecosystems, not considered by the PPR Panel in the assessment for the present statement, is more than two orders of magnitude higher compared to freshwater ecosystems. These data are underestimated, since the studies taken into account do not consider the production of exuviae (moult), that could be multiple in the lifecycle of an organism. Conversely, the same review reported that the annual production in natural aquatic ecosystem of fungi was rarely estimated, the reported studies referred to at least 3.4 g chitin m−2 per year in freshwater streams. Several other studies stated chitin content in the average range of 5%–20% for freshwater invertebrates (crustaceans and insects), e.g. Tharanathan and Kittur (2003), Kaya et al. (2014), Anggraeni et al. (2022). Chitin in crustacean biomass is typically in the range 2%–12% of the total body mass and the yearly production of chitin from freshwater crustaceans is estimated as 600 million tonnes according to Rkhaila et al. (2021). This data is supported by the Ali et al. (2024) review, which stated that chitin is available in a large quantity as an aquatic polysaccharide and is the second most abundant natural polymer, after cellulose, also in the aquatic systems, with an annual production in ecosystems of 1600 and 600 million tonnes in marine waters and freshwater, respectively. However, most of the crustaceans investigated as potential sources of chitin and chitosan are derived from the marine environment, marine organism waste being the primary large‐scale source of chitin and chitosan used commercially. Overall, there is a lack of studies to determine the content of chitin in numerous species of soil and freshwater crustaceans (Iber et al., 2022). This is the reason why soil crustaceans were not considered in the aforementioned assessment and in the above‐mentioned EFSA scientific report (EFSA, 2025).
5.4.1.2. Chitosan abundance
Although far less frequent than chitin in nature (Aranaz et al., 2021), chitosan can be natively present in some types of biomasses. Several papers reported the identification of chitosan chains in fungal communities as an important structural component of the cell walls of fungi at various stages of their life cycle (Abo Elsoud & El Kady, 2019; Gooday, 1990; Muzzarelli et al., 2012; Pochanavanich & Suntornsuk, 2002; Rkhaila et al., 2021), and in certain bacteria species able to convert chitin into chitosan using enzymatic deacetylation (Broek & Boeriu, 2019; Dhillon et al., 2013).
The biosynthesis of chitosan in nature is reported in Figure 4, starting from the fructose‐6‐phosphate and glutamine, derived from the carbon and nitrogen metabolisms, respectively.
FIGURE 4.
Presumed route of bioshynthesis of chitosan (adapted from Ghormade et al., 2017). (‘EC’ referring to the ‘Enzyme Commission’ number).
In the chitosan‐containing fungal species, chitosan can be directly extracted from the cell wall without the need of an acetyl group cleavage. The former Zygomycota phylum is the most abundant source of chitosan in nature, with higher amounts having been detected in genera like Absidia, Mucor, Rhizopus, Gongronella and in the order of Mucorales (Alemu et al., 2023). Mucorales have been shown to generate chitosan during the vegetative growth phase and Mucor rouxii is reported to be the species where chitosan is the most abundant component of the cell wall, up to 27.9%–32.7% according to Muzzarelli et al. (2012). In Mucor rouxii, chitosan is the principal fibrous polymer of the cell wall in addition to chitin and the same review reported that among the Zygomycetes classes, the Mucorales order is the only one that has both chitin and chitosan in considerable amounts in the cell walls. Chitosan compounds isolated from Mucorales typically show a molecular weight in the range 4 × 105–1.2 × 106, i.e. 400–1200 kDa (Dhillon et al., 2013). Tan et al. (1996) also provided an estimation of chitosan extracted from 13 strains of Zygomycetes demonstrating that the extractable chitosan content varied widely among fungi and even considering species of the same genus with values from 2.25% to 7.14% of chitosan/biomass w/w. Among all species, Gongronella butleri and Cunninghamella echinulata produced the highest amount of extractable chitosan, at up to 93.4 mg/200 mL substrate. Ghormade et al. (2017) reported that the content of chitosan in Zygomycetes vary from 1% to 10% of the dry weight of cells and organisms such as Absidia coerulea, Benjaminiella poitrasii, Cunnighamella blackesleeanus, Gongrenella butleri, Mortierella isabelina, Rhizopus delemar, Rhizopus stolonifer have contents between 6.7% and 10.4% with a > 87% DD.
Regarding the occurrence, Mucorales are commonly found in the soil and in the surface of decaying vegetation (Mousavi et al. (2018). Most species are saprotrophic and grow on organic substrates, while some are considered pathogens of animals, plants and fungi. It is the biggest order of Zygomycetes fungi with more than 300 species. Mousavi et al. (2018) reported that although Mucorales seem to be distributed worldwide, few studies focused on their occurrence. This study evaluated the presence and frequency of different species of Mucorales in soils sampled in France. A total number of 170 soil samples were collected in different regions in France between 2013 and 2015, 38 the presence of Mucorales was detected in 22% of the collected soil samples. Ziaee et al. (2016) investigated the Mucoromycotina which constitute a subphylum historically within the former phylum Zygomycota, as a large group of soil fungi. 340 soil samples were collected from seven public parks in 14 municipal districts in Iran. 400 pure colonies, belonging to the orders Mucorales and Mortierellales were identified. The genus Rhizopus (35.5%) was the most frequent isolate, followed by Mucor (32.25%) and Rhizomucor (27.5%). Considering soils characteristics and climate conditions, the study concluded that Mucoromycotina are recognised as ubiquitous organisms in the soil all over the world.
Zygomycetes is also the most studied phylum of fungi for commercial chitosan production; in this regard, several papers retrieved were related to the optimisation of the fermenting and cultivation conditions of these fungi, to maximise the production of chitosan (Araújo et al., 2020; Crognale et al., 2022; Dhillon et al., 2013; Huq et al., 2022; Logesh et al., 2012; Pellis et al., 2022; Tharanathan & Kittur, 2003). These papers are acknowledged by the PPR Panel as supporting the case that chitosan is readily found in nature.
Other fungal phyla with notable chitin/chitosan content are also Ascomycota, Basidiomycota and Deuteromycota. Ascomycota is mostly associated to the natural occurrence of chitin, as already reported, that could be viewed as wide source of chitosan, while only one report was found on the presence of chitosan in a Basidiomycetes, Lentinus edodes (Shiitake mushroom) (Dhillon et al., 2013). Other studies confirmed that the spore wall glucosamine exists mainly as unacetylated β‐(1,4)‐linked polymer, meaning chitosan, in yeast Saccharomyces cerevisiae (Briza et al., 1988) is only formed during the sporulation stage in its life cycle (Dhillon et al., 2013). Recent observations suggested that non zygote plant‐ and insect‐ pathogenic fungi also have a high proportion of chitosan in their cell walls, e.g. Metarhizium ansiopliae that grows naturally in soil (Ghormade et al., 2017).
Many bacteria, e.g. Streptomyces genus, can use auto‐produced chitosan as a single source of carbon. Streptomyces is the largest group of Actinomycetes, and it is widespread in aquatic and terrestrial ecosystems (El‐Naggar, 2021). According to Chater et al. (2010), bacteria of the genus Streptomyces are abundant in soil, being soil dwelling mycelial bacteria giving rise to branching hyphal filaments under suitable conditions.
Moreover, some microalgae species can possess both native chitin and chitosan in their cell walls. The isolation and physical–chemical characterisation of chitin and different lengths of chitosans are well‐documented from cell wall of Chlorophycean algae Chlorella spp. and the enzymatic deacetylation mechanism underlying chlorella's natural development of chitosan was investigated with a focus on identifying active chitin deacetyl deacetylases enzymes (Davis & Eveleigh, 1984; Iber et al., 2022; Thadathil & Velappan, 2014). It is acknowledged that chlorella is a genus of different species of single‐celled green algae which are ubiquitous in both freshwaters and marine waters.
It is also reported that chitin deacetylation occurs in arthropods and it seems to be related to chitinous structures that undergo subsequent expansion, examples being the abdominal cuticle of physogastric queen termites and eye‐lens cuticles (Gooday, 1990).
The Panel also noted that a large fraction of the retrieved papers from the scientific literature discussed extraction of chitosan from several different organisms containing chitin (Bolat et al., 2010; Kaya et al., 2014; Younes & Rinaudo, 2015; Sharif et al., 2018; Zainol Abidin et al., 2020; Hahn et al., 2020; Mohan et al., 2020; Hasan et al., 2022; Ma et al., 2022; Sáenz‐Mendoza et al., 2023; see Appendix C). Such production of chitosan is normally achieved via chemical deacetylation of chitin (using alkaline solutions at high temperature) or via controlled fermentation and enzymatic deacetylation. Although collected in Appendix C, this quantitative information on chitosan production was not considered relevant for the present assessment by the Panel, as it does not relate to chitosan being formed under natural conditions.
5.4.1.3. Route of degradation of chitin into chitosan
Although chitosan in most natural waters and chitin polymers are insoluble, information was not found in the literature on their quantitative accumulation in nature, including in soil and freshwater compartments. The rate of the degradation of these natural polysaccharides is influenced by several factors, mainly environmental conditions such as: temperature, pH, O2 levels and the organism communities in the ecosystem, but also by the particle size and the chain length distribution of the polymer. The degradation and consequent mineralisation of chitin is primarily a microbial process, in both aerobic and anaerobic conditions (Wieczorek et al., 2014), and it is reported that the high estimated annual production of chitin is well balanced by an equal rate of recycling (Gooday, 1990). This might be explained by the efficient enzymatic degradation systems in many living organisms. The different enzymes involved in the complex process of synthesis, degradation and deacetylation of chitin and chitosan occur in several living organisms, from bacteria to higher animals (Gooday, 1990) contributing to the carbon (C) and nitrogen (N) cycles in terrestrial ecosystems (see Figure 5).
FIGURE 5.
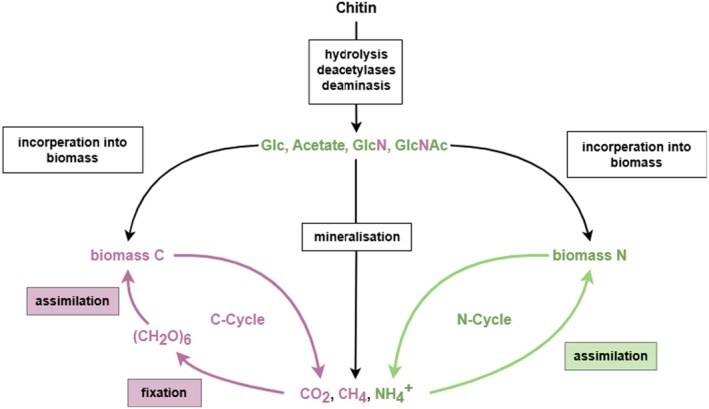
General scheme of chitin degradation and contribution to the global N and C‐cycles (adapted from Beier & Bertilsson, 2013).
The occurrence of chitinases, i.e. the hydrolytic enzymes that break down glycosidic bonds in chitin, in bacteria is common among phyla and the production of chitinolytic enzymes is a widespread process in several individual bacterial strains (Beier & Bertilsson, 2013).
The PPR Panel noted that none of the papers assessed reported that accumulation of chitin and/or chitosan was expected to occur in the environment. Davis and Eveleigh (1984) first reviewed the pathway of degradation of chitin. Two major routes were identified as being responsible for the degradation of chitin: a chitinoclastic mechanism via chitinase enzymes action, the predominant pathway and a deacetylation mechanism via chitin deacetylase enzyme action, to form chitosan, that is subsequently degraded by chitosanase enzymes and/or deaminases. This finding was also confirmed by later publications, although some studies suggested that the hydrolysis via initial deacetylation and chitosan formation might be important in freshwater systems (Sato et al., 2010) and even predominant in estuarine sediments (Wieczorek et al., 2014). The final metabolite for both routes of degradation of chitin is d‐glucosamine (GlcN), i.e. the monomer constituent of chitosan, meaning that both pathways implicitly host an intermediate step with a reaction of deacetylation. GlcN is then expected to be further degraded by natural organisms to carbon dioxide, methane and ammonium ions, with carbon and nitrogen subsequently considered feed sources for bacteria, thus entering in the respective natural cycles (see Figure 5 above, from Beier & Bertilsson, 2013). The pathways of degradation of chitin by these two routes of degradation is shown in Figure 6.
FIGURE 6.
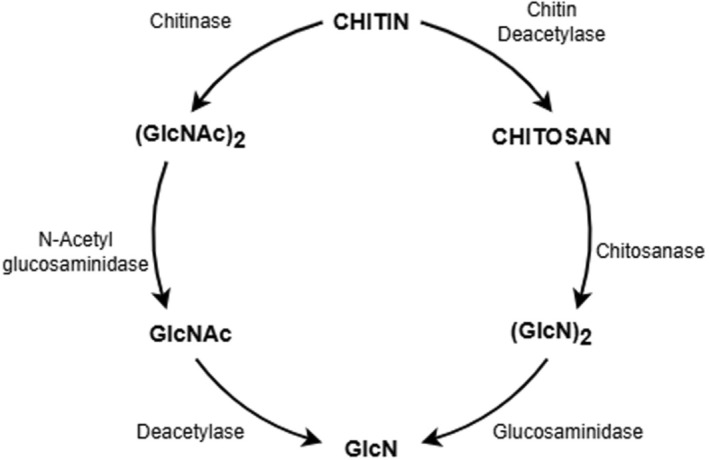
Presumed scheme of microbial degradation of chitin (adapted from Gooday, 1990). GlcNAc, N‐acetyl‐d‐glucosamine.
The specific chitin and chitosan extracellular degrading enzymes involved are: (i) endo‐ and exo‐chitinases, with the function of directly hydrolysing chitin into N‐acetyl‐d‐glucosamine (GlcNAc) oligomers and monomer, (ii) chitin deacetylases that convert chitin into chitosan and (iii) chitosanases, with the function of directly hydrolysing chitosan into GlcN oligomers. Nonetheless, many other less‐specific enzymes contribute to the degradation of both chitin and chitosan. All these less‐specific enzymes have different degradation patterns, but the main effect is the hydrolysis of the polymers/oligomers and the cleavage to shorter chains to obtain GlnNAc and GlcN monosaccharides. Some less‐specific enzymes can also have a more efficient hydrolysis action compared to chitinases and chitosanases, especially for the soluble form of chitosan, as it is more accessible than insoluble chitin (Poshina et al., 2018). The less‐specific hydrolytic enzymes reported included proteases, lipases, a large number of glycosyl hydrolases, dextranases, pectinases, cellulases, hemicellulases, as well as pepsin, papain, pancreatin and lysozyme (Aranaz et al., 2021; Rkhaila et al., 2020; Rkhaila et al., 2021; Thakur, Bairwa, et al., 2023; Thakur, Chauhan, et al., 2023).
Kumeta et al. (2018) reported the rate of degradation of chitin when added to brown forest soils (60 g kg−1 of soil). The chitin was found to decrease exponentially within days with values being below the limit of detection (< LOD) 90 days after the start of the incubation. As a comparison, chitosan powder was also added to the soils and again a fast exponential decline was observed in soil.
Also in the aquatic compartment, chitin was rapidly recycled in most environments and accumulation of chitin in sediment is not foreseen. Cauchie (2002) reported that in marine environments about 90% of chitin produced in the water column is digested within 150 h, while in marine sediments complete digestion took at least 1 year.
According to Dhillon et al. (2013), the formation of chitosan in cell walls of fungi is the result of the complex synergistic action of the chitin synthase and the chitin deacetylase enzymes and ‘chitosan is not directly synthesised, rather the deacetylase enzyme can act efficiently to convert chitin to chitosan. N‐deacetylation is a common step in the modification of sugar moieties, which may confer resistance to lysozyme action’.
Chitin deacetylase enzymes have been identified in different organisms, including bacteria, fungi and some insects where they are heavily involved during moulting growth stages. Many microbial genomes include different genes encoding chitinolytic enzymes and microorganisms produce numerous hydrolytic enzymes, among which there are chitinases (Veliz et al., 2017). Soil microbial populations were found to contain chitosan‐degrading bacteria, with these accounting for 0.5%–7% of the total soil heterotrophic bacteria (Davis & Eveleigh, 1984). However, soil microbial communities were better adapted to chitin as substrate compared to chitosan and this observation agrees with expectations, as chitin is more abundant than chitosan in nature (Wieczorek et al., 2014).
Chitin and its hydrolysed products, i.e. low molecular weight chitin chains, if added to soil, generally affect the bacterial and fungal communities, increasing their population with a simultaneous inhibitory effect on the growth of pathogenic strains, e.g. Fusarium oxysporum (Ootsuka et al., 2021). The increase of the population in response to the chitin added seems related to the capability of chitinolytic microorganisms in hydrolysing the chitinous hyphae of pathogenic fungi, resulting in pathogen suppression (Cretoiu et al., 2013). Sharp (2013) reported that: ‘addition of chitin alters the environmental conditions in the rhizosphere and phyllosphere to shift the microbial balance in favour of beneficial organisms and to the detriment of plant pathogens. Chitinolytic microbes produce extracellular chitinase enzymes to degrade chitin‐rich tissues of other organisms. Chitinases are also used by organisms for reasons other than to utilise chitin as a food source. Firstly, chitin‐containing organisms (both beneficial and pathogenic) use chitinases to regulate their growth and development by controlling the synthesis and lysis of cell walls and skeletons. Secondly, chitinases are also produced in organisms that do not produce chitin themselves, such as higher plants, bacteria and vertebrates, as well as viruses, where they are used for detecting, consuming and interacting with chitin‐containing organisms’. It is further reported that soil bacteria like Serratia marcescens and common soil‐borne fungi such as the Aspergillus and Trichoderma genera have strong chitinolytic activity.
Moreover, the PPR Panel took note of some references discussing the estimated natural occurrence of GlcN in EU agricultural soils (see Appendix C). The analysis of GlcN, mainly performed by HPLC, was provided to indirectly quantify chitin/chitosan and their degradation products, as the degradation of chitin and chitosan is the main source of the formation of this amino sugar in the environment. GlcN is considered a precursor in the biochemical synthesis of all nitrogen‐containing sugars, glycosylated proteins and lipids, and is one of the most abundant monosaccharides found in nature, however its degradation in soil is also quick, with a half‐life estimated at 1–3 h (Roberts et al., 2007). Some examples of estimated natural occurrence of GlcN in samples from Austria, Germany and Switzerland are reported below (see Table 13 in paragraph 5.4.3.1), ranging from 575.5 to 1400 mg kg−1 depending on the depth and type of agricultural soil tested (Appuhn et al., 2004; Joergensen, 2018; Sae‐Tun et al., 2022; OptiCHOS 39 ). It is also reported that, in general, arable and cropland soils show lower concentrations of GlcN than grassland and forest soils, where background levels measured with gas chromatography (GC) technique can reach 5164 mg kg−1 dry weight (Zhang & Amelung, 1996). It is worth noticing then that GlcN contributes up to 68% of the total amino sugar content of soils (Joergensen, 2018).
TABLE 13.
Comparison of maximum PECsoil against the expected natural background exposure level of chitosan in soil, as calculated by EFSA (2025), and the estimated natural occurrence of GlcN as retrieved from the scientific literature.
| Max PECsoil [mg kg−1 chitosan/chitosan hydrochloride]* | Expected natural background exposure level in soil § [mg kg−1 chitosan] | GlcN estimated natural occurrence [mg kg−1 GlcN] | ||
|---|---|---|---|---|
| 1.1–4.2 |
Single/multiple DT50 = 14.1 days 0–5 cm |
4.1–42.7 3.1–32 kg ha −1 (0–5 cm) |
575.5–958.3 a | AT agriculture soils, different tillage (0–15 cm) |
| 1.1–8.3 |
Single/multiple DT50 = 381 days 0–5 cm |
980–1400 b | DE agriculture soil (0–10 cm) | |
| 0.27–1.1 |
Single/multiple DT50 = 14.1 days 0–20 cm |
3.8–34.6 11.3–103.8 kg ha −1 (0–20 cm) |
830 c | DE agriculture soil (0–10 cm) |
| 0.27–2.1 |
Single/multiple DT50 = 381 days 0–20 cm |
660 d | CH agriculture soil (0–5 cm) | |
| > 5000 e | Forest | |||
Sae‐Tun et al. (2022).
Indorf et al. (2011).
Appuhn et al. (2004).
Joergensen (2018).
Zhang and Amelung (1996).
Ranges as reported in Table 10 considering the values of all representative crops for both chitosan and chitosan hydrochloride.
Assuming a soil bulk density of 1.5 kg m−3 (see also Section 5.4.1.4).
However, the PPR Panel was not able to find a quantitative estimation as to which extent the predominant pathway of degradation via direct hydrolysis of chitin is utilised compared to the secondary route via chitosan production (see scheme above in Figure 6).
Based on the experts' judgement, it was proposed to assume, as a conservative approach, that 90% of the chitin is degraded through hydrolysis (chitinase pathway) as the most frequent route of degradation, while only 10% is degraded via chitosan production (chitin deacetylase pathway). This proportion of 1/10 for the less predominant route was then used to estimate the chitosan content from biological sources in soil, by considering 10% of the quantitative estimation of chitin produced in an average agricultural soil. If chitin in soil ranges from 31 to 320 kg ha−1 in the first 0–5 cm layer and from 113 to 1038 kg ha−1 in the 0–20 cm layer (EFSA, 2025), then an estimation of 3.1 to 32 kg ha −1 in the first 0–5 cm layer and of 11.3 to 103.8 kg ha −1 in the 0–20 cm layer can be suggested for chitosan.
It is noted that the chitosan estimation in the soil compartment above does not consider the amount of chitosan directly produced by Zygomycetes in soil. This decision was made due to the general uncertainty on the reported values in the scientific literature; notably, the lack of clarity regarding whether the chitosan estimations are referring to chitosan as already occurring in natural conditions or rather being yielded in experimental conditions after chitin extraction, and thus not mimicking its natural behaviour. Nevertheless, the Panel considered that this contribution could only partially influence the total estimation of chitosan in the soil compartment, as Zygomycetes correspond only to 10% (coefficient of 0.1 in the Scientific Report (EFSA, 2025)) of the overall fungal distribution in an average agricultural soil.
5.4.1.4. Chitosan degradation
The PPR Panel extracted information on the degradation of chitosan under natural conditions from the available scientific literature. Accordingly, references that discussed chitosan degradation under laboratory conditions that did not reflect natural environmental conditions, such as increased temperatures or strong reactive conditions, were not considered relevant and were excluded. In the retained relevant literature, it was noted that the type of degradation was generally not specified, and therefore the Panel formulated a definition of what it assumed as the ‘degradation of chitosan’. The Panel considered the following processes to be those leading to the degradation of chitosan. They may act alone or in combination:
deacetylation from chitosan polymers into chitosan chains with lower degrees of acetylation;
transformation from polymer chains into oligomers and monomers (degree of polymerisation from above 100 to below 100);
transformation from oligomers into GlcNAc and GlcN monosaccharides. 40
It needs to be noted that the ultimate transformation into inorganic products is generally not regarded as chitosan degradation in the studies, except for the readily biodegradability studies, where degradation is typically expressed as percentage of theoretical CO2 production or oxygen demand.
Moreover, the literature available to the Panel encompassed a large variety of types of chitosan materials (e.g. flakes, films, powder) that degraded in various environmental compartments, such as different soil types or surface waters. In nearly all studies kinetic fitting of the degradation pattern was not carried out. Therefore, where possible, the Panel estimated an indicative DT50 from each of the relevant studies, specifying as much as possible the type of chitosan material, its type of transformation and the conditions in the studied environmental compartment (see details in Appendix E). It is underlined that the DT50 values estimated are approximate values only. This is because in the first instance all loss of weight was attributed to chitosan degradation, and secondly, because often a slow initial phase of degradation followed by a faster phase after adaptation of the microbial community was observed. However, the Panel still used single first order (SFO) kinetics as the decline pattern for the estimations, as the number of data points available were too few to estimate two rate constants when fitting the data.
It is generally agreed that MW and DD are the dominant factors determining the biodegradation of chitosan (Abdel‐Ghany & Salem, 2020; Beier & Bertilsson, 2013; Jiang et al., 2014; Matica & Menghiu, 2017). Chitosan degradation usually starts with de‐polymerisation, resulting in a decreasing MW, followed by deacetylation, leading to an increase in DD (Szymańska & Winnicka, 2015). For similar DD, chitosan with higher MW degraded more slowly than chitosan with lower MW (Ali et al., 2024; Rkhaila et al., 2021).
In soil the degradation rate was found to vary strongly, depending on the nature of chitosan, type of soil and the environmental conditions. Several examples retrieved from the literature are reported in Table 9 with data compared also to chitin (in italics) if tested in the same study (see Appendices C and E for summaries and details of the specific studies).
TABLE 9.
Examples of degradation rate of a number of different chitosan materials. (Entries for chitin are given in italics).
| Chitosan material | Estimated DT50 (days) at specified T (°C) | Compartment | Reference |
|---|---|---|---|
| 0.25–0.35 mm polyethylene‐chitin films | 60 days; 25°C | Soil lab | Makarios‐Laham and Lee (1995) |
| 45 days; n.a. | Field | ||
| 0.25–0.35 mm polyethylene‐chitosan films | 120 days; 25°C | Soil lab | |
| 60 days; n.a. | Field | ||
| Chitin membrane (40 × 0.3 mm) | (100% in 60 days) | Sand dune (Tottori, Japan) | Hirano ( 1996 ) |
| Chitosan membranes (40 × 0.3 mm) | – | ||
| 50–55 μm S‐form (acetic acid bound by salt bonds) chitosan film | – | Soil (sifted peat with organic fertilisers and lime; pH = 5.5–6; moisture content = 20%) | Vikhoreva et al. (2002) |
| 50–55 μm B‐form (removed acetic acid and water‐insoluble polybase) chitosan film | – | ||
| Bioplastic chitosan strips (6 × 6 cm) | 243 days; 25°C | Sandy soil | Mostafa et al. (2010) |
| 69 days; 25°C | Sandy loam soil | ||
| 51 days; 25°C | Loamy soil | ||
| Chitin (DA 94%) and chitosan (DA 38%) flakes | 136 days; n.a. | Soil (experimental farm Shinshu University) | Sato et al. (2010) |
| < 136 days; n.a. | River | ||
| < 136 days; n.a. | Moat | ||
| Powdered chitosan | No degradation; 25°C | Sand soil | Sawaguchi et al. (2015) |
| 7–8 days; 25°C | Silty soil | ||
| Chitosan powder | – | Red latosol | Moretti Angelo et al. (2021) |
| Chitin | 43 days; n.a. | Sediment | Hillman et al. (1989) |
| Chitosan | 75 days; n.a. | Sediment | |
| Commercial chitosan flakes | n.d.; 23, 30, 36, 40 and 50°C | Active sludge from the wastewater of a cellulose plant | Ratajska et al. (2003) |
| Commercial chitosan flakes | n.a.; 24–25°C | Active sludge from the wastewater of a cellulose plant | Boryniec et al. (1996) |
| Chitosan 80 μm films | 1 days; 22°C | Ready biodegrad. aqueous solution | Bonilla et al. (2020) |
| 14 days; 22°C | Ready biodegrad. aqueous solution | ||
| 60 days; n.a. | River | ||
| Chitosan oligomers | 19.5 days; n.a. | Activated sludge from a water treatment plant | Timmer (2019) |
Abbreviation: n.a., not available.
In general, soil microorganisms exhibited an initial lag phase in their ability to degrade before they adapted to chitosan as a substrate, thereafter the biodegradation rate increases (e.g. Hirano, 1996; Vikhoreva et al., 2002). This demonstrated that the types of bacteria and microorganism communities, and thus the composition of the microbial biomass, plays a key role in the degradation of chitosan.
In freshwater aquatic systems, a DT50 of 75 days in the sediment of an estuary in Scotland was found by Hillman et al. (1989), and no other quantified degradation half‐lives were found in the literature the PPR panel assessed. Similarly to soils, microorganisms in the aquatic environment required an adaptation/induction period, before a subsequent increased biodegradation occurred (Ratajska et al., 2003).
Overall, chitosan with different MW and DD and under various experimental conditions, was generally evaluated as being readily biodegradable, as demonstrated in various specific tests, including studies performed following the OECD TG 301 and conducted in the guideline‐defined aerobic aqueous media (Bonilla et al., 2020; Boryniec et al., 1996; Moretti Angelo et al., 2021; Timmer, 2019).
A full overview of the degradation studies discussed above is available in Appendices C and E.
5.4.2. Chitosan and chitosan hydrochloride PECs calculations (task 2b)
An estimation of the PECs for chitosan and chitosan hydrochloride was carried out by the Panel by considering the approved uses as basic substances (i.e. application rates 100–800 g ha−1, see details in Appendix D).
The calculations of the PEC used have been based on the pesticide fate models currently in use for regulatory purposes and in line with the provisions of Commission Regulation (EU) No 284/2013. 41 The input parameters for the models were selected by the Panel according to the available relevant evidence and using the tools foreseen by the pertinent guidance documents in force (for details on PEC methodologies see Section 4.2).
The maximum total application rates considering the maximum number of applications, according to the applicable GAPs, are set out below for the following intended uses:
CHITOSAN (concentration 100% chitosan hydrochloride 42 ): 0.8 kg a.i. ha −1 for eight applications (14 days interval between applications) for small fruit crops resulting in 6.4 kg a.i. ha −1 if considered as a single total load application.
CHITOSAN HYDROCHLORIDE (concentration 100% chitosan hydrochloride 43 ): 0.8 kg a.i. ha −1 for eight applications for ornamental herbaceous plants/bulbous plants/beet crops (5 days minimum interval between applications) resulting in 6.4 kg a.i. ha −1 if considered as a single total load application.
PECs soils
A conservative assumption of no crop interception (0%) was selected by the Panel for all relevant crops assessed (small fruit crops, ornamental herbaceous plants/bulbous plants and beet crops). The calculations were provided using a DT50 of 14.1 days considering chitosan as readily biodegradable with the proposed default value of 30 days at 12°C normalised to 20°C (ECHA, 2017). As recommended by ECHA (2017) in the Biocidal Products Regulation, the DT50 value of 30 days for soil can be applied in the PEC calculations and is commonly assumed for readily biodegradable substances during the EFSA peer‐review process when assessing active substances and reliable experimental endpoints are not available. To be complete, the calculations were also repeated using the most conservative DT50 of 381 days, 44 calculated from the available literature that had been assessed. The Panel acknowledges that this value of degradation rate is overly conservative being only based upon the initial, generally slower, phase of degradation, when the microbial community has not yet adapted itself to chitosan (see Section 5.4.1.4 Chitosan degradation and Appendix E). However, other reliable and/or more realistic kinetic information was not available in the publication that was the source of this value. Moreover, it is worth noting that in the US‐EPA documents in support of the addition of chitosan to the list of Minimum Risk Pesticides (EPA, 2022), the value of DT50 in soil of 10 days from Sawaguchi et al. (2015) was used, a value lower than the two selected for the PEC calculations in this statement.
The max PECsoil values are reported below in the Table 10.
TABLE 10.
Maximum PECsoil values for chitosan and chitosan hydrochloride.
| Basic substance | Crop | Application | DT50 [d] | Max PEC 0–5 cm [mg kg−1] | Max PEC 0–20 cm [mg kg−1] |
|---|---|---|---|---|---|
| Chitosan | Small fruit crops | Single application |
14.1 381 |
1.067 1.067 |
0.267 0.267 |
| Multiple application |
14.1 381 |
2.135 7.819 |
0.532 1.955 |
||
| Single total load application * |
14.1 381 |
8.533 8.533 |
2.133 2.133 |
||
| Single application |
14.1 381 |
1.067 1.067 |
0.267 0.267 |
||
| Chitosan Hydrochloride | Beet crops (arable) | Multiple application |
14.1 381 |
4.210 8.268 |
1.053 2.067 |
| Single total load application * |
14.1 381 |
8.533 8.533 |
2.133 2.133 |
||
| Single application |
14.1 381 |
1.067 1.067 |
0.267 0.267 |
||
| Chitosan Hydrochloride | Bulbus plants | Multiple application |
14.1 381 |
4.210 8.268 |
1.053 2.067 |
| Single total load application * |
14.1 381 |
8.533 8.533 |
2.133 2.133 |
Results reported only to complete the information, since more refined multiple applications have also been provided.
As reported in the table above, the max PECsoil calculated for single and multiple applications ranges from 1.067 to 8.268 mg kg−1 (0–5 cm soil depth) and from 0.267 to 2.067 mg kg−1 (0–20 cm soil depth), depending on the DT50 selected. It shall be noted that the use of the most conservative (worst‐case) degradation rate found in literature gave a normalised value > of 1 year, which usually triggers the calculation of the PECplateau and PECaccumulation. These further calculations were provided just for completeness, although an accumulation in soil was considered unlikely by the Panel, supported also by the evidence in the literature. Full details of the PECsoil calculations are provided in Appendix E.
PEC surface water (PECsw) and sediment (PECsed)
For the aquatic freshwater compartments (surface water and sediment) standard FOCUS STEPS 1–2 (version 3.2) calculations have been carried out, for both chitosan hydrochloride and chitosan.
As recommended by ECHA (2017) in the Biocidal Products Regulation, DT50 values of 30 days for soil/sediments and 15 days for water at 12°C (corresponding to 14.1 and 7.1 days, respectively at 20°C) can be applied in the PEC calculations, as commonly assumed for readily biodegradable substances and as standard practice during the EFSA peer‐review process when assessing active substances and no reliable experimental endpoints are available. Note that due to the uncertainty in the DT50 values found, and chitosan properties, the Panel opted for using the DT50 value of 14.1 days at 20°C for the total water‐sediment system DT50 for FOCUS STEP 1 and, as more conservative approach, also for both the DT50 in water and in the sediment for PEC calculations in FOCUS STEP 2. 45 A DT50 of 14.1 days, as well as the very conservative DT50 of 381 days, in all compartments (soil, water/sediment whole system, water and sediment) were then used as input parameters for the degradation rate.
The maximum PECsw and PECsed calculated with FOCUS STEP 2, obtained for ornamental bulbous plants (interval between applications of 5 days) for chitosan hydrochloride and for small fruit crops (interval between applications of 14 days) for chitosan/chitosan hydrochloride, were calculated considering the default values of K OC of 1 and 10,000 L kg−1 (see below Table 11) as the highest calculated values for the max application rates. The use of a high default K OC is considered appropriate and reasonable given the strong adsorption capacity of chitosan (being also used for wastewater treatment, see details in Section 5.4.4.3) and consequently its limited mobility in aquatic environments. In all eight scenarios (for each of the two DT50 values of 14.1 and 381 days and considering the two extreme K OC values for both chitosan and chitosan hydrochloride), the calculated PECs resulted from the multiple, sequential applications and not from a single application (as indicated in the STEP 2 calculator version 3.2 (FOCUS, 2001).
TABLE 11.
Maximum PECsw and PECsed values of FOCUS STEP 2 for chitosan hydrochloride and chitosan.
| Basic: Substance | Crop and crop growth stage | Application (total and number × rate) [g ha−1] | K oc: [kg L−1] | DT50 a [days] | Max PECsw [μg L−1] | Max PECsed [μg kg−1] |
|---|---|---|---|---|---|---|
| Chitosan hydrochloride | Ornamental bulbous plants (BBCH 10–92) | 6400 (8 × 800) | 1 |
14.1 381 |
323.89 b 768.71 |
3.2 7.67 |
| Chitosan hydrochloride | Ornamental bulbous plants (BBCH 10–92) | 6400 (8 × 800) | 10,000 |
14.1 381 |
23.06 54.71 |
2260 5360 |
| Chitosan | Small fruit crops (BBCH 10–79) | 6400 (8 × 800) | 1 |
14.1 381 |
237.58 1050 |
2.26 10.49 |
| Chitosan | Small fruit crops (BBCH 10–79) | 6400 (8 × 800) | 10,000 |
14.1 381 |
77.86 97.28 |
1630 7330 |
The DT50 value is used as input parameter for the soil, water and sediment compartments, i.e. DT50,soil, DT50,water, DT50,sed.
The values in bold refer to the lowest and highest values using the DT50 of 14.1 days and the values in bold italic to those using the DT50 of 381 days.
As reported in the table above, combining the results for chitosan and chitosan hydrochloride and for K OC of 1 and 10,000 L kg−1, the maximum PEC sw ranged from 23.06 to 323.89 μg L−1 (DT50 of 14.1 days) and from 54.71 to 1050 μg L−1 (DT50 of 381 days), having so a difference of a factor of around 14 to 20 between the minimum and maximum values.
The maximum PEC sed ranged from 2.26 to 2260 μg kg−1 (DT50 of 14.1 days) and from 7.67 to 7330 μg kg−1 (DT50 of 381 days), so, having a difference of a factor of around 1000. For comprehensiveness, PECsw and PECsed were also calculated for crops with 8 applications but with similar/lower application rates and/or larger application windows resulting in lower maximum PECsw or PECsed: fruit crops (other than small fruit crops and grapevine) (max 0.4 kg a.i. ha−1 with 14 days minimum interval between applications), beet crops (max 0.8 kg a.i. ha−1 with 5 days minimum interval), grass (max 0.4 kg a.i. ha−1 with 14 days minimum interval), grapevine (max 0.6 kg a.i. ha−1 with 14 days minimum interval) and cereals (max 0.4 kg a.i. ha−1 with 14 days minimum interval). A full explanation of the selected endpoints and the detailed max PECsw and PECsed calculations performed at Step 1 (not reported in the table above) and Step 2, are provided in Appendix E.
Furthermore, to mimic the precipitation of insoluble chitosan on top of the freshwater sediment, an additional calculation was included for the case with the highest PECsed obtained, considering both STEP 1 and STEP 2 results (Table E2, in Appendix E). The worst‐case scenario identified was for the ornamental bulbous plants at a growth stage described as ‘Leaf Development – Senescence (BBCH 10‐92)’ with eight applications of 800 g ha−1 and the minimum application interval of 5 days (assuming chitosan instead of chitosan hydrochloride). This additional calculation for an increased K oc value of 100,000 L kg−1 resulted in a STEP 2 PECsed of 2410 μg kg−1, instead of 2260 μg kg−1 for the K oc of 10,000 L kg−1 (Table E2 in Appendix E), i.e. an increase of less than 10% compared to 2260 μg kg−1. Note that the PECsed value of 2410 μg kg−1 is still considerably lower than the PECsed of 7330 μg kg−1 obtained by using the DT50 value of 381 days, demonstrating that for multiple applications the DT50 value is an important parameter.
An attempt to calculate the PECsw/sed for a higher tier (Step 3) was not carried out as too many experimental input parameters were not available and thus, a refinement could not be well justified even using default values. Already at STEP 1–2, the quantitative estimation should be considered with a certain level of uncertainty, although less input parameters are required in those models. However, the PECsw/sed were tentatively estimated acknowledging the difficulties to obtain reliable values mainly due to the lack of data as input parameters for the aquatic compartment and the uncertainty related to the identity of the basic substances (see Section 7).
Other PECs calculations
Some of the intended uses provided in the GAPs relate to seed treatments before sowing at BBCH 00, i.e. cereal crops, potatoes and sugar beet for both chitosan and chitosan hydrochloride. An estimation of the PEC soil for seed treatment was performed following the pertinent guidance in use for the national authorisation in The Netherlands (CTGB, 2020). The estimation for small seeds (< 0.5 cm) like sugar beet and cereal crops were covered by the risk envelope approach with the PEC soil calculations above 46 ) considering that the uses were covered by the spray application with deposition to soil (soluble powder). A tentative estimation for large seeds (> 0.5 cm), like potatoes (rate of 50–100 g hL−1 as single application), failed even when considering the standard assumptions set out in the guidance for planting rate and calculating seed potato volume and average density (see Appendix E for details). In this regard, it is noted that the information outlined in the GAP tables, as given in the EC review reports for both chitosan and chitosan hydrochloride, report only the application rate as ‘mass of a.s. per hectoliter’ and not ‘as mg of active substance per kg of potatoes’. Without this information, it was not possible to estimate the exposure derived from these specific uses as seed treatment. Moreover, for one application of chitosan hydrochloride on ornamental bulbous plants, it was specified a use as dipping and drenching, i.e. before planting. For this specific use, PECsw and PECsed were further calculated, resulting in any case in values lower than the max PECsw/sed reported in Table 11 (see Appendix E for details).
The exposure for the groundwater (gw) compartment, i.e. PECgw calculations, was not completed considering the following:
-
–
expected natural biodegradation of chitosan: proven action of several specific and non‐specific enzymes involved in the degradation, thus suggesting low persistence of chitosan/chitin and strong adsorption capacity of chitosan materials.
-
–
chitosan insolubility at pH relevant for groundwater used for potable uses, i.e. pH within the range 6.5–9.5 (Drinking Water Directive, 2020 47 ), in which pH range the insoluble chitosan is expected to precipitate and be retained by the upper layers of soil.
-
–
Expected limited mobility in aquatic environments given the strong adsorption capacity of chitosan (being also used in the treatment of oily wastewaters or for modifying the soil properties to enhance erosion stability, see details in Section 5.4.4.3).
Likewise, the exposure for the air compartment is not considered necessary as both chitosan and chitosan hydrochloride polymers are not volatile.
For the post‐harvest uses, i.e. at BBCH above 89, of chitosan (immersion/dipping of fruits in a max 2% w/v chitosan solution for max 60 s) and an estimation to max 0.02% of the fruit weight, PECs in the different environmental compartments are considered not necessary as the treatment is normally carried out in confined environment (building) and not in the field.
The representative uses in protected crops (greenhouses and crops grown under cover), reported in the two GAP tables, were considered covered by the assumptions and evidence provided and discussed for the open field uses.
Uncertainties on the above PEC estimations are mainly related to the complexity of the polymeric structure of chitosan (and chitosan hydrochloride). The length of the polymeric chain, the degree of acetylation/deacetylation and the pattern of the deacetylated monomers in the molecules of chitosan influence not only the molecular weights, but also their physical–chemical properties and consequently the endpoints to be selected as input parameters in the models, such as the degradation rates or the K OC.
The broad range of MW and DD were already reflected on the identity properties and conditions of use of the approved basic substances (European Commission, 2023a, 2023b; documentation provided to EFSA nr: 1 and 2) reported also in Table 3 where chitosan has a DD of 70%–100% with MW 10–1500 kDa, while chitosan hydrochloride a DD of 80%–95% with MW of 47–65 kDa. Due to the lack of specific experimental data for the basic substances in subject, the Panel decided to use default values when an endpoint was not available, as well as to treat the formulation as 100% pure chitosan, this being a conservative approach when PEC are calculated for the chitosan hydrochloride (i.e. mass of chitosan in chitosan hydrochloride is lower than in chitosan formulation when the two basic substances are compared at the same application rates). However, for PECsoil and the Steps 1–2 tool where PEC for metabolites are not calculated, the molecular weights have no effect on the PEC calculated noting that for Steps 1–2 calculations volatilisation is not calculated. The substance solubility value is also not influencing these PEC calculations as the value input in the Steps 1–2 tool is only used to flag a warning when the PECsw is higher than the water solubility value that was input in the tool.
5.4.3. Comparison with natural background level
5.4.3.1. Soil
A first evaluation can be performed by directly comparing the total annual load (max as single application and max as multiple applications considering the total number of applications) in the field from the approved uses as basic substance with the expected natural background exposure level in soil from biological sources as proposed in Section 5.4.1.2.
Both approaches proposed, for the use as basic substance and for the expected natural background exposure level, considered conservative assumptions. This first preliminary comparison reported in Table 12 is only based on the application rate and does not take into account the degradation of chitosan in soil. These values are proposed to maximise the evaluation of the natural occurrence with the overall load derived from the maximum application rate. As regards the expected natural background exposure level in soil of chitosan and chitin, several uncertainties are identified, as discussed in the former chapters and for this reason a range of values is given.
TABLE 12.
Comparison between the total annual load (max application rate) derived from the approved uses of chitosan and chitosan hydrochloride as basic substances and the expected natural background exposure level of chitosan and chitin in soil, as calculated by EFSA (2025).
| Use as basic substance [kg ha−1 year−1] | Expected natural background exposure level in soil* [kg ha−1] |
|---|---|
|
0.8 (chitosan for max single application) 6.4 (chitosan for max total application) 0.8 (chitosan hydrochloride for max single application) 6.4 (chitosan hydrochloride for max total application) |
3.1–32 (chitosan in the first 0–5 cm) 31–320 (chitin in the first 0–5 cm) 11.3–103.8 (chitosan in the 0–20 cm) 113–1038 (chitin in the 0–20 cm) |
Values considering the normalisation to a bulk density of 1.5 g cm−3 (see also Section 5.4.1.4).
Therefore, the Panel also provided a more accurate and refined comparison by considering the PECsoil calculations, where also the interval between applications, the degradation rates, the types of crops involved and the characteristics of an average soil have been taken into account. Precisely, the PPR Panel took the max PECsoil values obtained from the approved uses with the highest application rates to compare them against the expected natural background exposure levels of chitosan (Table 13). Moreover, this comparison was also integrated with information retrieved from the scientific literature on the estimated natural occurrence of GlcN, it being the chitosan monomer and the terminal metabolite of both chitin and chitosan degradation before the complete mineralisation to inorganic compounds, and thus relevant for the purpose of this comparison even if the physical–chemical properties of the monomer GlcN differ considerably from those of the polymeric chains of chitosan and chitosan hydrochloride. All values reported below are expressed in dry weight and the literature assessed is captured in Appendix C.
The Panel observed that the estimated range of the natural exposure background level in soil, (see Section 5.4.1) covers around one order of magnitude. Considering an average soil depth of 20 cm and the worst‐case DT50 of 381 days with the hypothesis of SFO degradation, i.e. the most conservative assumption, the resulting maximum PECs soil for chitosan/chitosan hydrochloride (2.1 mg kg −1 ) is lower than the lowest concentration value of the estimated natural background exposure level (3.8 mg kg −1 ) (see Table 12). Moreover, it is also noted that, when considering only the first 5 cm of topsoil layer, the resulting maximum PECs soil (8.3 mg kg −1 ) is within the same order of magnitude of the lowest concentration value of the estimated background exposure level (4.1 mg kg −1 ) (see Table 13).
As further evidence, the estimated natural occurrence of GlcN, also reported in the table above, confirmed these findings. Indeed, considering the molecular weight of the single GlcN monomer of 179.17 g mol−1, i.e. roughly 0.2 kDa, the estimated soil PECs 48 result at least two/four orders of magnitude lower than GlcN concentrations in agricultural soils.
5.4.3.2. Freshwaters (surface water + sediment)
Contrary to soil, the comparison of the annual load ending up in surface water/sediment systems from the use of chitosan/chitosan hydrochloride as basic substances with their expected natural background exposure level from biological sources in surface water systems is more challenging.
On one hand, only part of the annual load applied on soil ends up in the adjacent surface water. This fraction can be estimated with the support of the FOCUS STEP 2 scenarios (FOCUS, 2001).
For arable crops this raises to an annual load of the maximum 8 applications with each 1.5% for spray drift deposition, i.e. 1.5% of the total annual application, plus a maximum of 5% of the residue in soil 4 days after the last application, for a runoff/drainage loading, mounting to a total estimated maximum annual load of about 2%–3% of the total applied mass.
For fruit trees this may raise up to eight applications with each 22.2% spray drift deposition plus 5% of the soil residue 4 days after the last application, for a runoff/drainage loading, so a total estimated maximum annual load of not more than around 24%–25% (Section 2.4 FOCUS, 2001). This would correspond to 0.03, resp. 0.10 times the maximally applied 6.4 kg ha−1, i.e. 0.2 resp. 1.6 kg ha −1.
On the other hand, an attempt to provide an expected natural background exposure level of chitin/chitosan in soil from biological sources is provided in Section 5.4.1, but it was also noted that it is difficult to make similar calculations for the surface water compartment, because of less data that are in addition more variable and uncertain. Comparing the maximum annual loads by application of chitosan/chitosan hydrochloride as a basic substance of 0.2–1.6 kg ha−1 to the mentioned natural background exposure level of 11.3–103.8 kg ha−1 for the upper 20 cm soil (see Table 12), though surface waters are generally deeper than 20 cm, the applied mass appeared to be significantly lower than the natural background exposure level in surface water systems.
Moreover, there is evidence that chitosan is insoluble in natural waters, which commonly have a pH above 6.5. Therefore, even if all the chitosan/chitosan hydrochloride mass as calculated above would precipitate on top of the sediment, and next be mixed in the upper layer by bioturbation and settling of organic debris, the applied mass would be estimated to be significantly lower than the natural background exposure level mentioned for soils.
In addition, at pH > 6.3–6.5, chitosan precipitates to the first layer of sediment and then bacterial degradation occurs. Microorganisms which degrade chitin and chitosan are widely present in the environment and prevent the accumulation in soils and sediments of these polysaccharides after deposition of dead fungi and animals (Beier & Bertilsson, 2013; Cauchie, 2002; Gooday, 1990; Somashekar & Joseph, 1996; Thakur, Bairwa, et al., 2023). Some studies also suggested a degradation of chitin via initial deacetylation, with consequent chitosan production, as likely being the predominant pathway in estuarine sediments (Gooday et al., 1991; Hillman et al., 1989; Wieczorek et al., 2014). Accordingly, the Panel concurred that this indicates that the maximum annual loads calculated above overestimate the chitosan mass in sediment, as they do not take degradation into account.
The maximum calculated exposure level in the upper 1‐cm sediment layer was 2260 μg kg−1 (DT50 of 14.1 days, Appendix E). Based upon the characteristics of the STEP 2 FOCUS waterbody (1 cm of sediment effective sorption depth with a sediment bulk density of 800 kg m−3), this exposure corresponds to 18.08 mg m−2 surface area sediment, i.e. 0.18 kg ha−1. For the worst‐case DT50 of 381 days this was 7330 μg kg−1 in the upper 1‐cm sediment layer, corresponding to 0.59 kg ha−1. Compared to the expected background exposure level in the upper 5 cm soil of chitosan of 3.1–32 kg ha−1, these exposure levels in sediment are approximately at least a factor 5 to 50 lower.
In the literature assessed, no reliable quantitative data were found considering the natural background level of chitin or chitosan, nevertheless accumulation of these amino polysaccharides in surface waters was not reported.
In addition to a DT50 of 381 days, the Panel further remarked that the calculations provided in STEP 1–2 are using also other conservative input parameters, e.g. K oc values, to highlight a possible worst‐case approach, thus most likely resulting in an overestimation of the results that in any case are considered in a semi‐quantitative way.
Moreover, the Panel acknowledged that all the comparisons above do not take into account the potential exposure derived from the environmental load of chitosan by other different non‐natural sources, including uses as fertilisers/soil improvers or feed materials. In particular, the use of chitin and chitosan/chitosan hydrochloride according to the EU fertiliser regulation, although not quantitatively reflected in the above comparisons, should be acknowledged as a major potential input of these saccharides in the agricultural environment (see next paragraph).
5.4.4. Other possible sources of (chitin)/chitosan and chitosan hydrochloride with relevance for environmental considerations (task 2c)
5.4.4.1. Agricultural and horticultural uses
Possible agricultural uses in EU (and non‐EU) of chitin and chitosan, and other possible derivatives, other than the approved uses as basic substances are extensively documented and discussed in the peer‐reviewed scientific literature. These may encompass uses as fertilisers, 49 soil improvers 50 and plant biostimulants. 51 These uses are regulated in the EU under Regulation (EU) 2019/1009 52 on fertilising products. The regulation sets out requirements on contaminants (heavy metal) limits and for microbial pathogens, where relevant; minimum content of nutrients and other relevant characteristics depending on the category of the product; and labelling. For substances falling in the scope of Regulation (EU) 2019/1009, there is no EU risk assessment conducted, but the legislation lays down a common legal framework for fertiliser products in the form of a ‘conformity assessment mechanism’ conducted by a notified body. 53 Also, it is noted that there is no dedicated EU Union list for these substance categories covered by Regulation (EU) 2019/1009. However, authorisation of a given substance can be granted at the national level, and there might be national authorisation lists. 54
The scientific literature concurs and reports on chitin/chitosan capacity as plant resistance inducer and as an elicitor for numerous defence responses related to both biotic and abiotic stresses (Grammenou et al., 2023; Hidangmayum et al., 2019; Shahrajabian et al., 2021). Biostimulants effects are described for ‘chitosan and other biopolymer’ in the review by Grammenou et al. (2023): ‘they enhance defense and resistance against pathogens, they chelate nutrients and increase their uptake, they increase chlorophyll concentration and photosynthetic rate, they ameliorate biomass production’. Shahrajabian et al. (2021) report the main effects of chitin and chitosan as being to ‘protect and stimulate seed germination, induce plant's growth and development, act as resistance elicitor, improve soil properties and prevent nutrient leaching, mitigate negative effects of abiotic stress, improve shelf‐life of vegetable products, increase crop yield and quality, chelate heavy metals, effectiveness against pest and pathogens e.g. fungi, bacteria, nematodes insects and viruses’. Malerba & Cerana (2020) also report on ‘[R]esistance against Phytophthora capsica, accumulation of defense‐related enzymes and phenolic compounds’ and, notably for abiotic stress, on ‘[D]rought stress tolerance, salt stress tolerance, heat stress tolerance, poor fertilisation tolerance’.
This ‘dual’ capacity of chitin/chitosan of improving tolerance both to biotic stress, pertaining to plant protection products and to abiotic stress, instead pertaining to fertiliser products (biostimulants category) makes chitosan fall in scope both of Regulation EU 1107/2009 and Regulation (EU) 2019/1009. In this respect, the Panel noted that chitin 55 is listed, without any specified limits, in Annex II of Commission Implementing Regulation (EU) 2021/1165, 56 as amended, reporting authorised fertilisers, soil conditioners and nutrients for use in organic production. Also, the Panel acknowledged the use of chitosan/chitin‐based products as biostimulants in Belgium; their specific properties and the high‐nitrogen content ensure increased absorption of nutrients, better crop quality, higher growth and yield, increased resistance to abiotic stress (derogation EM028.C, EM624, EM721.N).
The Panel also took note of the fact that the mode of action of chitosan in plants is not fully understood (Hidangmayum et al., 2019), and a deeper understanding of its mode of action might lead to a reconsideration of its regulatory status by risk managers.
Moreover, it is also observed that most of the literature papers discussing the use of chitin/chitosan in plant protection against biotic and abiotic stresses have been conducted at laboratory/research level only and not tested in e.g. field experiment set‐ups. However, the Panel took note of a long‐term 57 in field experiment in The Netherlands, on the effect of chitin addition to soil, in which 20 tons ha −1of shrimp waste chitin (previously treated with NaOH and HCl) were applied (Cretoiu et al., 2013).
Chitosan hydrochloride and chitosan 58 are also listed in Annex I (part I) of Commission Implementing Regulation (EU) No 2021/1165, as amended, listing the active substances contained in plant protection products authorised for use in organic production. Moreover, chitosan derived from Aspergillus niger is listed in Annex V part D (authorised products and substances for the production and conservation of organic grapevine products of the wine sector) of the same regulation.
In addition, uses of chitin/chitosan as carrier of fertiliser products are reported in the scientific literature (Liu, Xu, et al., 2023; Mujtaba et al., 2020; Riseh et al., 2023; Sharif et al., 2018).
As collateral information, the Panel also noted that fertilisers and soil amendments containing chitosan are used in other non‐EU countries. Some examples from the United States are cited in the dossier submitted to support the approval of OptiCHOS 59 : BioGan 6–8‐0 fertiliser from BioOregon Protein (US), with recommended uses rate up to 1800 lbs. per acre year−1, with a 20% chitin content in the product, would correspond to 440 kg chitin ha−1 year−1; NutriBoom Crab Meal Fertiliser from Intergro applied at rates from 300 lbs. per acre (corresponding to approximately 325 kg chitin ha−1); and fertiliser for fruits and vegetables from Coast of Maine containing 21% chitin and with recommended dilution rate of 1 ounce to 1 gallon of water resulting in a spray dilution of 1.64%.
The Panel took note of the extensive body of knowledge, alongside the related continuous research, available on the agricultural uses of chitin/chitosan and chitosan hydrochloride preparations and of their regulated EU uses. The authorised uses of chitosan and chitosan hydrochloride under Annex I of Commission Implementing Regulation (EU) No 2021/1165 for organic production shall be in accordance with the uses, conditions and restrictions set in the relevant review reports for their approval as basic substances (European Commission, 2023a, 2023b) 60 and therefore corresponding to the approved Good Agricultural Practice (GAPs) as basic substances (see Appendix E). The authorised use of chitin, under Annex II of Commission Implementing Regulation (EU) No 2021/1165, does not report specific GAPs and/or other specified limits. However, as the concerned use is ‘as fertilisers, soil conditioners and nutrients’, applications with higher orders of magnitude compared to the uses as plant protection products are expected. In this respect, for instance, the national authorisations for chitosan/chitin‐based biostimulants in Belgium having application rates up to 300 kg ha −1 1–2 times per month are noted. Literature papers generally do not report thorough quantitative information on the expected/recommended amounts of these substances for the agricultural uses investigated (e.g. application rates, total yearly concentration amount).
The contribution of these agricultural applications, regulated under EU/national legislations and extensively described in the literature, to the overall addition of chitin/chitosan and chitosan hydrochloride to the environment (mainly soil and freshwaters compartments) is fully acknowledged by the Panel. However, it is not taken into account for the comparison against the natural background levels (see Section 5.4.3) given the scarcity of accurate quantitative data and the lack of applicability at the EU‐level (authorisation of chitin/chitosan as plant biostimulant might be at the national level only). Nevertheless, these uses shall still be considered as constituting a major potential input for the overall environmental exposure to these substances.
A full overview of the pertinent references considered by the Panel alongside their main findings is available in Appendix C.
5.4.4.2. Feed material and animal nutrition
Chitosan (and chitin) does not need authorisation as a feed additive under EC Regulation No 1831/2003 61 because it is listed in the EU register of feed materials. 62 ‘Feed materials’ are described in Regulation (EC) No 767/2009 63 (Article 3.2(g)) as ‘products of vegetable or animal origin, whose principal purpose is to meet animals' nutritional needs, in their natural state, fresh or preserved, and products derived from the industrial processing thereof, and organic or inorganic substances, whether or not containing feed additives, which are intended for use in oral animal‐feeding either directly as such, or after processing, or in the preparation of compound feed, or as carrier of premixtures’.
No quantitative information is reported in the EU register for feed materials. Feed materials are not subject to evaluation by the EFSA Scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) unless EFSA receives a specific mandate from the European Commission. Accordingly, feed materials are not systematically assessed at the EU‐level.
Notwithstanding, in the peer‐reviewed scientific literature and in the regulatory context there is a well‐documented body of knowledge on the use of chitin/chitosan (and its salts or other derivatives) in the feed sector, as well as on their possible beneficial effects on the environment. In this respect, the PPR Panel noted that chitosan (described as ‘Chitosan FG 95’) is referenced in an EFSA supporting publication reviewing substances/agents that have direct beneficial effects on the environment, as having potential environmental benefits for reducing ruminant ammonia and methane production (Lewis et al., 2013).
The scientific literature of the last 5 years has clearly reported and concurs on the advantages and importance of chitin/chitosan and its derivates as feed supplements in poultry, pig, rabbit and fish nutrition. A large variety of positive effects are described. To list a few: improving growth indicators and intestinal health, nutrient digestibility and utilisation efficiency, immuno‐protective, anti‐inflammatory and antioxidative properties (Al‐Sagheer et al., 2023; Ayman et al., 2022; Dalmoro et al., 2023; Elnesr et al., 2022; Farivar et al., 2022; Hossain & Bhuiyan, 2023; Ibitoye et al., 2019; Lokman et al., 2019; Park et al., 2023; Pereira et al., 2020; Santos et al., 2021; Tao et al., 2022; Tufan & Arslan, 2020; Xu et al., 2018; Xu et al., 2020; Zhang et al., 2020; Zheng et al., 2021). Also, chitosan potential for reducing enteric methane emissions is well described in the literature (Anggraeni et al., 2022; Harahap et al., 2022; Kamal et al., 2023; Lan et al., 2024; Shah et al., 2022; Thao et al., 2022; Tong et al., 2020).
As collateral information, it is also noted that the Food and Drug Administration (FDA) in 1983 granted GRAS status to chitosan used as an animal feed component.
In addition, the literature discusses the re‐use of waste from insect production for animal consumption in the context of circular economy, e.g. insect frass containing chitin used as plant fertiliser (Chavez & Uchanski, 2021). For further discussion of these agricultural uses, see the section above.
Overall, the PPR Panel acknowledged the chitin/chitosan uses as a feed material and its role in animal nutrition. Some quantitative information on chitosan/chitin feed supplementation is described in the experimental designs tested in the retrieved literature references (see Appendix C). In this regard, the Panel noted that the maximum doses tested were ranging between 2 to 5 mg kg−1 body weight for chicken (Lokman et al., 2019) and mammals (cows and pigs), and up to 2 g kg−1 body weight for fish (Ahmed et al., 2021). 64 The possible contribution of these uses of chitin/chitosan to the overall environmental exposure was judged by the Panel as being minimal, if not even virtually absent, considering that there is an animal metabolism before the compound(s) would be excreted and accordingly no direct environmental exposure of chitin/chitosan as such is anticipated via this route. Considering the uses for fish nutrition (Abdel‐Ghany & Salem, 2020; Abdel‐Tawwab et al., 2019), some potential residues may remain in the treated waters, derived not only from excretion faeces, but also from possible feed spills. However, sufficient quantitative information is not available to enable an estimation of the feed amount that may reside in the treated waters and in any case potential exposure to waters would be restricted to aquaculture systems only, noting that these can include cages in natural fresh and marine water bodies.
A full overview of the pertinent references considered by the Panel alongside their main findings are available in Appendix C.
5.4.4.3. Other uses
The PPR Panel also noted possible uses of chitin/chitosan for wastewater treatment discussed in the peer‐reviewed scientific literature, as well as the 1986 US EPA approval of chitosan for the purification of potable water. In particular, the flocculant action of chitosan when used in the treatment of oily wastewaters or for modifying the soil properties to enhance erosion stability (Wang et al., 2016; Orts et al., 2000, Malerba & Cerana, 2020; Gao et al., 2022) highlighted chitosan affinity to different substrates. This showed the strong adsorption capacity of chitosan and consequently its limited mobility in aquatic environments. In this regard the Panel noted that, given these physio‐chemical properties of chitosan, the use of a high default K oc as an input parameter for the calculations of the PEC in aquatic systems, and the waiving of the calculation of PEC in groundwater, are appropriate and reasonable (see Section 5.4.2).
Moreover, chitosan has been approved as a wine processing aid in the European Union, 65 in Argentina, Australia and Chile (public dossier OptiCHOS). Also, the Panel took note that the recent literature reports of chitosan (EU) applications for improvement of grapevine performance and wine quality (Soares et al., 2023). Chitosan is also listed as processing aid in the Codex General Standard for Fruit Juices and Nectars (FAO, 2005).
These reported other uses are not intended to be a comprehensive list, and they mainly focus on uses with a potential for environmental exposure. In this regard, the Panel acknowledged the large variety of uses of chitin/chitosan (and/or its salt and derivatives) for industrial applications (e.g. bio‐plastics, cosmetics, food packaging, aquaculture, wastewater treatment) and in the pharmaceutical and biomedical sectors (drug and gene delivery, antimicrobial, tissue engineering, chromatography; see also Section 5.2) extensively discussed in the peer‐reviewed literature (Pandit et al., 2021). Likewise, the EU and non‐EU dietary/food applications of chitosan are recognised (FEMA No. 4946, GRAS as food additive, 66 EU novel food, used in South Korea).
5.5. Ecotoxicology
Based on the available information and the data collection exercise related to ToR 2, it was concluded that the environmental levels of chitosan and chitosan hydrochloride resulting from the conditions of use as defined by the existing approvals as basic substance are, under worst‐case assumptions, in the same range of the levels expected to occur (see Section 5.4.1) in soil and surface water, including sediment.
Although the amount reaching the different and relevant environmental matrices could be considered as something added on top of the existing natural background exposure levels, the resulting exposure levels would still be ranging in the same order of magnitude and not considered enough to trigger any risk for non‐target organisms, owing to the environmental fate properties of the substance, in particular the absence of accumulation and the beneficial effect it may have on some physiological functions of non‐target organisms, as also explained below. Thus, no further assessment in relation to the safety to non‐target soil (Žabka & Pavela, 2021) and surface water and sediment organisms was deemed necessary. Moreover, all the considerations related to the chemical specification explained in Section 5.3 are also relevant for non‐target organisms.
Although a quantitative comparison of the natural background level in surface water and sediment was not possible, it could still be concluded that a quantitative risk assessment for aquatic and sediment dwelling organisms is not necessary, considering that chitosan is insoluble in water at pH normally expected in natural waters and once it reaches sediment, it will be degraded quickly by microorganisms present in sediments (Beier & Bertilsson, 2013; Cauchie, 2002; Gooday, 1990; Somashekar & Joseph, 1996; Thakur, Bairwa, et al., 2023; Thakur, Chauhan, et al., 2023). Moreover, available toxicity studies do not seem to show major concern in relation to potential toxicity of free chitosan. As a consequence of the low or no toxicity of chitosan on fish, it is also used as food supplement since it has been demonstrated that up to a certain dose it has beneficial impact on a number of fish physiological functions such as growth (Abdel‐Ghany & Salem, 2020; Yu et al., 2023). The doses where the beneficial effects as food supplement may be weakened towards more inhibitory effects are nevertheless very high (> 4 g kg−1) and not considered to be reached after exposure to chitosan/chitosan hydrochloride following its use as a basic substance. From the available information, it seems that chitosan acetate and chitosan hydrochloride may damage or induce obstruction of the branchial epithelium in the gill (Bullock et al., 2000). However, that study presented several shortcomings, e.g. no physical–chemical parameters were measured, no analytical verification was conducted and information on the administration of the test substance was insufficient. Moreover, the limited information available does not allow to understand whether that effect can be considered a direct toxicity of the substance, or a consequence of an acidification of the water (low pH) used in the toxicity test following the spiking of the acid‐solubilised form to enable dissolution in water medium used for testing. In relation to terrestrial non‐target organisms, other than soil organisms, with different routes of exposure, e.g. residues in plants, pollen and nectar, although a quantitative estimation of the natural background level could not be done in those matrices, based on qualitative considerations, safety concerns are not anticipated.
It has been demonstrated that chitosan, when administered to broilers and hens through the diet up to 1.4 g chitosan kg−1 of body weight, has no adverse effects (Hirano, 1996). Moreover, chitosan, when used as feed supplement in poultry, has shown beneficial properties like immunomodulatory, antioxidant, antimicrobial and hypocholesterolemic effects (Swiatkiewicz et al., 2015). These benefits were reflected in improved body weight and enhanced nutrient digestibility (Huang et al., 2005). The findings showed improvements in growth performance, laying performance and immune responses (Meng et al., 2010; Yan et al., 2010). Additionally, chitin is a natural component of many birds' diets, especially insectivorous species.
Based on the nature of the basic substance it is not expected that chitosan will cause adverse effects on honeybee and other non‐target arthropods. Chitin is naturally produced and found as a structural component for strength and reinforcement of arthropods (Fabritius et al., 2011). Chitosan has shown several beneficial effects in plants, such as enhancing resistance to dehydration (Iriti et al., 2009) and diseases (Hadwiger, 2020; Malerba & Cerana, 2020), as well as promoting growth and yield (Sajid et al., 2020). Chitosan and chitosan oligomers are found in honeybees' and other non‐target arthropods natural environment.
In addition, no safety concerns are anticipated for wild mammals based on the conclusion for the ToR 1.
6. DISCUSSION AND CONCLUSIONS
6.1. Human health
The PPR Panel concluded that the toxicological properties can be extrapolated between chitosan and chitosan hydrochloride. This conclusion has been reached considering the following lines of evidence in the WoE in order to address the ToR 1 of the mandate.
Chemical structures
-
–
The addition of hydrogen chloride has the purpose to improve the water solubility of chitosan and it is expected that the salt readily dissociates to a cation and related counter‐ion.
-
–
Moreover, chitosan has to be dissolved in acidic solution (e.g. by adding vinegar) prior to application for plant protection purposes; therefore, the same cationic form is present in solution independently that the substance is a hydrochloride salt or not.
History of safe uses
-
–
Chitosan has a wide range of applications in agricultural, biopharmaceutical, biomedical, cosmetic, textile and food additive fields for which no distinction has been made between chitosan and its salts and adverse effects are not known/have not been reported for humans from any of these uses. In addition, the US EPA office of pollution prevention (OPPTS) has recently added chitosan and its salts (including chitosan hydrochloride) to the list of ‘minimum risk pesticides (MRPs)’.
Toxicological profile
-
–
It is reported that the ADME properties and toxicity of chitosan are strongly influenced by its molecular weight and by the degree of deacetylation and it is generally recognised that molecules above 1 kDa are poorly absorbed in the gastrointestinal tract (EFSA, 2016). According to the current specifications (see Table 3), chitosan hydrochloride has a MW in the range 182–207 kDa (calculated), whereas for chitosan this is not clearly specified, and the range varies between 10 and 1500 kDa.
-
–
According to the available evidence, chitosan oligomers (MW up to 30 kDa) could potentially be absorbed at low extent and in this case are expected to be degraded to monosaccharides, i.e. d‐glucosamine (GlcN) and N‐acetyl‐d‐glucosamine (GlcNAc). GlcN and GlcNAc are natural components of human tissues and have an approved use in the treatment of osteoarthritis, and therefore might be considered safe, though their oral consumption has to be managed/controlled in patients with diabetes.
-
–
According to the available evidence, chitosan and its salts (i.e. chitosan acetate and chitosan lactate) with different MWs are non‐toxic i.e. LD 50 is > 1000 mg kg−1 and no critical adverse effects were observed in the repeated‐dose toxicity studies.
There is no evidence in the recent scientific public literature suggesting that chitosan or chitosan hydrochloride would have adverse effects in mammals. Considering the chemical structures of chitosan and chitosan hydrochloride, the history of safe use of chitosan and its salts, the poor absorption in the gastrointestinal tract of these molecules independently from their chemical form (salt or free) and the absence of toxicological concern from the available and recent literature, the PPR Panel concluded that the two molecules are similar and that the toxicological properties can be extrapolated. Overall, in the evidence considered to address the current mandate, no toxicological concern has been identified. However, different uncertainties were considered in the overall WoE (see Section 7 ).
6.2. Environmental fate
The PPR Panel considered the following lines of evidence in the experts' judgement and weight of evidence approach used to address the ToR 2 of the mandate.
Chitin and chitosan occurrence
-
–
Chitosan is a naturally occurring substance, derived from specific organisms like fungi (former Zygomycota phylum) and from the natural process of deacetylation of chitin.
-
–
Chitin occurrence in nature, and in the different environmental compartments, is much higher than that of chitosan. Chitin is widely abundant in nature.
-
–
Although the production of chitosan is not the preferential pathway in the microbial degradation of chitin, a considerable amount of chitosan is naturally formed in the different soil and freshwater aquatic compartments. The expected natural background exposure level of chitosan in soil was estimated and quantified from the assessment of the natural content of chitin in an average agricultural soil.
-
–
The ubiquity of chitosan‐degrading organisms in soil may at first seem surprising, given the limited amount of chitosan in nature compared to chitin. The widespread occurrence of chitosan‐degrading organisms, fungi and bacteria, is reflected in the distribution of their substrate.
-
–
The degradation of chitin and chitosan is mainly microbial, their final metabolite is the monomer d‐glucosamine (GlcN), that is further mineralised to inorganic compounds and their atoms of carbon and nitrogen are prone to entering the respective natural C and N cycles.
Chitosan degradation
-
–
Chitosan generally undergoes fast degradation and accumulation of chitin and/or chitosan in the environment is not foreseen.
-
–
The following processes were considered as contributing to the degradation of chitosan, acting alone or in combination: (i) deacetylation from chitosan polymers into chitosan chains with lower degrees of acetylation; (ii) transformation from polymer chains into oligomers and monomers; (iii) transformation from oligomers into GlcNAc and GlcN monosaccharides.
-
–
The ultimate transformation into inorganic products is generally not regarded as chitosan degradation in the studies, except for the ready biodegradability. However, many publications confirmed that chitosan and chitosan oligomers are readily biodegradable.
-
–
It is generally agreed that molecular weight, degree and pattern of deacetylation, nature of the polymeric form and environmental conditions, including the microbial biomass content, are the factors determining the biodegradation of chitosan.
-
–
Generally, soil microbial organisms involved in the degradation exhibit an initial lag phase to adapt to the substrate of chitosan, thereafter the biodegradation rate increases exponentially, as chitosan is considered a valuable source of nitrogen for the microorganisms.
PECs
-
–
PEC calculations have been provided according to the specifications available in the GAP tables and, applying the risk envelope approach limited to the highest application rates.
-
–
Estimations were provided quantitatively for the soil compartment, and for the aquatic (surface water and sediment) compartments up to FOCUS STEP 1–2, typically considering the uncertainties encountered, using a conservative approach for the selection of the substance endpoints.
-
–
The PEC values were provided considering that chitosan is a readily biodegradable substance (ECHA, 2017), but also, for comprehensiveness, using the most conservative degradation rate endpoints that might be assumed from the literature assessed.
Comparison of the use as basic substances with the expected natural background exposure levels
-
–
The quantitative comparison of the maximum PECs soil with the expected natural background exposure levels in soil showed that the overall annual use of chitosan/chitosan hydrochloride as basic substances does not significantly exceed the estimated natural background exposure level (see Section 5.4.1). In this respect, it is noted that: the maximum PECs soil based upon overly conservative assumptions, 67 result in lower: or within the same order of magnitude, concentration values (2.1 mg kg −1 /8.3 mg kg −1 ) compared to the lowest concentration values of the estimated natural background exposure levels in soil (3.8 mg kg −1 /4.1 mg kg −1 ) (see Table 13 of Section 5.4.3).
It is acknowledged that any addition of chitosan/chitosan hydrochloride following application in accordance with their approved uses as basic substances may increase the environmental load. Notwithstanding, this ‘addition’ would be less than an order of magnitude compared to the natural background exposure level and thus not resulting in an overall disturbance of the (chitin)/chitosan already present under natural conditions.
-
–
Only a semi‐quantitative comparison of the max calculated PECs (PECsw and PECsed) with the expected natural background exposure levels in freshwater systems could be provided, as quantitative data on the natural occurrence of chitosan in edge‐of‐field waterbodies are missing. However, evidence from the collected information indicated that the applied mass reaching edge‐of‐field waterbodies, appeared to be lower than the semi‐quantitatively estimated natural background exposure level in surface water systems. Moreover, chitosan is insoluble in natural waters, commonly having a pH above 6.5. So, even if all chitosan/chitosan hydrochloride mass as calculated would precipitate on top of the aquatic sediment, the applied mass would appear to be significantly lower than the natural background exposure level mentioned for soils.
-
–
It was acknowledged that all the comparisons made have not taken into account the potential exposure derived from other environmental loadings and the contribution of chitosan by other different non‐natural sources, including uses as fertilisers/soil improvers. Nevertheless, the Panel considered that these other uses shall be considered as constituting a major potential input for the overall environmental exposure to these substances.
6.3. Ecotoxicology
The PPR Panel concluded that based on the available information in ecotoxicology and environmental fate properties of chitosan, no further assessment in relation to the safety to non‐target soil, and surface water and sediment organisms was deemed necessary since the exposure levels resulting from the use of chitosan and chitosan hydrochloride as basic substances, in comparison to the natural background exposure levels, are not considered sufficient to trigger any risk for non‐target organisms.
7. UNCERTAINTIES INCLUDING MISSING INFORMATION
In the present assessment missing information alongside related uncertainties were identified by the Panel and duly explained in the pertinent sections above. Below the Panel provided an overview of all identified uncertainties clustered by relevance for human health and environmental and fate behaviour, or for both.
Uncertainties relevant both for human health and environmental and fate behaviour:
Chitin/chitosan identity and relevance of the tested materials compared to chitosan/chitosan hydrochloride as approved basic substances.
-
–It is acknowledged that the toxicological and environmental fate and behaviour properties of chitosan are largely dependent on its MW, degree of (de)acetylation (DD or DA) and pattern of the (de)acetylation. However, there is no consensus within the literature/scientific community on:
-
○A clear ‘cut‐off’ between what is ‘chitin’ and what is ‘chitosan’. The terms chitin, chitosan and chitosan oligomers are often used interchangeably in literature.Several papers, e.g. Alemu et al. (2023), Casadidio et al. (2019), combined and use both terms chitin and chitosan to identify the same natural polymer despite the degree of deacetylation. Zheng et al. (2021) reported that chitosan is a collective name for ‘biochitin polymers’ that are partially deacetylated. Shah et al. (2022) also reported that according to the nomenclature of the European Chitin Society (EUCHIS), 68 chitosan and chitin should be divided by the solubility and insolubility in 0.1 M acetic acid. As another example, Matica and Menghiu (2017) stated that chitin should be at least 60% deacetylated to be called chitosan.
-
○The definition of ‘high’, ‘mid’ and ‘low’ molecular weight.
-
○
-
–
Lack of reporting of MW, DD (or DA) and pattern of DD (or DA) is frequent in the literature studies reviewed under the present mandate and therefore there is an overall uncertainty on how the materials tested in the toxicological and environmental studies relate to chitosan/chitosan hydrochloride approved as basic substances. In turn, it is also noted that the available specifications for chitosan and chitosan hydrochloride approved as basic substances are not very precise and accurate, i.e. giving broad ranges of MW as well as ranges of DD (or DA), no information on the pattern of DD (or DA) (see section Sections 5.2 and 5.3.1.).
-
–
The process of obtaining chitosan (from crustaceans, fungi or insects) and the subsequent purification steps is poorly reported in the available literature studies. It is noted that such information would be needed to better identify the possible sensitising potential of chitosan.
-
2
Possible presence of nanoparticles
The PPR Panel took note that the possible presence of nanoparticles in chitosan materials, or its other derivatives, cannot be excluded. However, this aspect has not been further explored in the context of the present mandate due to a lack of specific data requirements for characterising particle size under the applicable regulatory framework for basic substances, 69 and also considering the general uncertainty on the accurate characterisation of chitosan and chitosan hydrochloride approved as basic substances (see point 1 below).
In addition, the PPR Panel acknowledged that safety evaluations of chitosan applications as proposed food additive 70 and novel food 71 are currently ongoing under the EFSA FAF and NDA Panels, respectively. In the frame of these assessments, considerations as to confirming whether the conventional risk assessment should be complemented with nano‐specific considerations, or not, may be given and this aspect on nanoparticles possibly further elaborated.
Uncertainties relevant for environmental and fate behaviour:
Natural occurrence and species population
- There is a general lack of experimental data related to the determination of the natural occurrence of chitin and chitosan in the different environmental compartments. Most of the literature reviewed reported that chitin is the second most abundant natural polysaccharide found in the environment, derived mainly from fungi and arthropods, being biocompatible and biodegradable. Though this is recurring background information, it is not supported by a quantitative estimation, especially when considering specific environments like soil and freshwater compartments. Accordingly, a quantitative estimation of chitin in EU agricultural soils has been proposed by EFSA and presented in a separate scientific report (EFSA, 2025), which also served to the estimation of chitosan natural occurrence. The calculations provided for the estimation of the chitin/chitosan content from biological sources in soil accounted for general uncertainties such as:
-
○the population variability, thus having a scenario of ‘high content’ and one of ‘low content’ of chitin in soil;
-
○the fungal population size was estimated considering a range of uncertainty of one order of magnitude (106–107 fungal cells/gram of soil);
-
○the insect population was spanning from 107 to 108 individuals per hectare;
-
○the pathway of degradation of chitin to chitosan was assumed by the Panel, as conservative approach, not the predominant route of degradation, but considering that only 10% of the chitosan is produced directly from chitin.
-
○
-
2
Degradation of chitosan
In the available studies/literature considered for the degradation of chitosan, the type of degradation(s) was not specified and there was no clear ‘definition’ of chitosan degradation. This is one of the reasons for uncertainty in the estimated degradation rates, along with attributing all chitosan weight loss to degradation and the use of SFO kinetics instead of two‐phased degradation rates. Moreover, a large variety of chitosan materials and different environmental compartments needed to be considered.
Although from the literature reviewed an accumulation of chitin and/or chitosan in the environment is clearly not foreseen, implications of this lack of information are also reflected in the selection of the proper endpoints to estimate the degradation rate from the environmental degradation studies reviewed, and consequently in a proper selection of the DT50 as an input parameter for the PEC calculations.
-
3
Analytical challenges
Analytical difficulties for the determination and quantification of chitosan in an environmental compartment (e.g. estimation in soil or in freshwater) are known, especially when considering polymeric materials with not well‐defined identity and properties. A way to by‐pass these analytical issues was provided by some publications reviewed that measured the content of GlcN in the different compartments, as final metabolite before the complete mineralisation to inorganic compounds. As already reported, the estimated natural occurrence of GlcN was measured in different soils, including agricultural soil with analytical techniques like GC (Zhang & Amelung, 1996) and HPLC (Appuhn et al., 2004; Indorf et al. 2011). However, the determination of the final metabolite GlcN is not able to distinguish its origin, making it impossible to discriminate if the parent compound was chitin or chitosan.
-
4
Other uses
Chitosan and chitosan hydrochloride are already on the market as approved basic substances under the plant protection product regulation and as different categories of products under the fertiliser regulation. Thus, they can already be bought and used, but it is not known which quantities are being employed for which purposes, e.g. eliciting plant defences to control plant disease or other functions (such as soil improvers and plant biostimulants both under the fertiliser regulations and animal feeds).
8. DOCUMENTATION AS PROVIDED TO EFSA
KitoZyme Basic Substance Application (2020): https://open.efsa.europa.eu/questions/EFSA‐Q‐2020‐00339
ChiPro Basic Substance Application (2011): https://open.efsa.europa.eu/questions/EFSA‐Q‐2013‐00306
Applications for extension of use(s) submitted to the European Commission (see also footnote 21): https://open.efsa.europa.eu/questions?search=chitosan+hydrochloride
Information submitted by applicants in the context of the EC call for comments following the onset of the review of approval of chitosan and chitosan hydrochloride as basic substances pursuant to Article 23(6) of Regulation (EC) No 1107/2009. See Appendices B and C.
ABBREVIATIONS
- ADI
acceptable daily intake
- ADME
absorption, distribution, metabolism and excretion
- AUC
areas under the curve
- bw
body weight
- CIPAC
No and EEC
- COS
chitosan‐oligosaccharides
- DA
degree of acetylation
- DDA
Degree of deacetylation
- EC
emulsifiable concentrate
- EMA
European Medicines Agency
- FAF
EFSA Panel on Food Additives and Flavourings
- FAO
Food and Agriculture Organization
- FDA
Food and Drug Administration
- FITC
fluorescein isothiocyanate
- GAP
Good Agricultural Practices
- GC
gas chromatography
- GlcN
d‐glucosamine
- GlcNAc
N‐acetyl‐d‐glucosamine
- GR
granule
- GRAS
generally recognized as safe
- HCS
high‐MW chitosan
- LD
lethal dose
- LOD
limit of detection
- MCS
mid‐MW chitosan
- MESE
Methodology and Scientific Support
- NDA
EFSA Panel on Nutrition, Novel Foods and Food Allergens
- Papp
apparent permeability coefficient
- PECs
predicted environmental concentrations
- PPR
EFSA Panel on Plant Protection Products and their Residues
- SFO
single first order
- SPT
skin prick testing
- TERR
transepithelial electric resistance
- ToR
Terms of Reference
- USP
United States Pharmacopeia
- WG
Working Group
- WoE
Weight of Evidence
- WP
wettable powder
- WSC
water soluble chitosan
REQUESTOR
European Commission
QUESTION NUMBER
EFSA‐Q‐2024‐00037
COPYRIGHT FOR NON‐EFSA CONTENT
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
PANEL MEMBERS
EFSA Panel on Plant Protection Products and their Residues (PPR Panel), Tamara Coja, Pauline Adriaanse, Judy Choi, Antonio Finizio, Maeva Giraudo, Thomas Kuhl, Francesca Metruccio, Emily McVey, Martin Paparella, Silvia Pieper, Eugenio Scanziani, Ivana Teodorovic, Paul Van der Brink and Martin Wilks.
Supporting information
Literature searches
Distiller_Search#1_Chitosan_Hydrochloride
Distiller_Search#2_Chitosan_Review
Literature search for chitosan and chitosan hydrochloride under ToR 2
Data collection for toxicological endpoints
Data collection for environmental endpoints
Fate additional information and PEC calculations
ACKNOWLEDGEMENTS
The Panel on Plant Protection Products and their Residues wishes to thank the following for the support provided to this scientific output: the hearing expert Gianfranco Romanazzi; the EFSA staff: Maria Arena, Marco Binaglia, Isabelle Delaunois, Christopher Lythgo and Tunde Molnar; the EFSA trainees: Amarela Becirovic and Kyriaki Koukoutsi.
APPENDIX A. Literature searches
Appendix A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2025.9318
Human health
1.
APPENDIX A.1.1. Distiller_Search#1_Chitosan_Hydrochloride.
APPENDIX A.1.2. Distiller_Search#2_Chitosan_Review.
Environmental fate
APPENDIX A.2.1. Literature search for chitosan and chitosan hydrochloride under ToR 2.
APPENDIX B. Data collection for toxicological endpoints
Appendix B can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2025.9318
APPENDIX C. Data collection for environmental endpoints
Appendix C can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2025.9318
APPENDIX D. GAP tables (as reported in the respective EC review reports 72 )
CHITOSAN ‐ List of (extension) of uses
| Crop and/or situation a | F G or I b | Pests or group of pests controlled c | Formulation | Application | Application rate | PHI (days) m | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type d , e , f | Conc of a.i. g/kg i | Method kind f , g , h | Growth stage & season j | No. of application min/max k | Interval between applications (min) | g a.i./hL min max (g/hL) | Water L/ha min max | Total rate each application g a.i./ha min max (g/ha) l | |||||
|
Olive trees Olea europaea OLVEU |
FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
≥ 85% Chitosan |
Low‐Medium volume spraying | BBCH 10 ‐ BBCH 79 | 4–8 | 2 weeks | 50–100 | 200–400 | 100–400 | Not applicable | Chitosan can be prepared for use following any of the two recipes provided in Appendix I (preparation for use). |
|
Grass (Lawns) Grassland English ryegrass Lolium perenne Italian ryegrass Lolium multiflorum Timotheegrass Phleum pratense ‘Ornamental’ grasses 3AMGC including Miscanthus × giganteus |
FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
≥ 85% Chitosan |
Low‐Medium volume spraying | BBCH 09 ‐ BBCH 89 | 4–8 | 2 weeks | 50–100 | 200–400 | 100–400 | Not applicable | Chitosan can be prepared for use following any of the two recipes provided in Appendix I (preparation for use). |
| Grass (Sport fields, Golf courses) | FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
≥ 85% Chitosan |
Low‐Medium volume spraying | BBCH 09 ‐BBCH 89 | 4–8 | 2 weeks | 50–100 | 200–400 | 100–400 | Not applicable | Chitosan can be prepared for use following any of the two recipes provided in Appendix I (preparation for use). |
|
Ornamentals plants (Wood spurge Euphorbia amygdaloides subsp. Robbiae, EPHRO Magnolia, 1MAGG Griffith's spurge Euphorbia griffithii, EPHGH Philadelphus, 1PHIG Beech Fagus sylvatica, FAUSY Poplar tree Populus spp., 1POPG Hebe Hebe spp., 1HBEG Prunus sp., 1PRNG Wintergreen Gaultheria, 1GAHG Pear tree Pyrus sp., 1PYUG Maple Acer, 1ACRG Rose Rosa, 1ROSG Cotoneaster, 1CTTG Blackberry Rubus, 1RUBG Euonymus, 1EUOG Lilac Syringa, 1SYRG Forsythia, 1FOSG Blueberry Vaccinium, 1VACG) |
FGI | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
≥ 85% Chitosan |
Low‐Medium volume spraying | BBCH 09 ‐BBCH 89 | 4–8 | 2 weeks | 50–100 | 200–400 | 100–400 | Not applicable | Chitosan can be prepared for use following any of the two recipes provided in Appendix I (preparation for use). |
| Post‐harvest fruit treatment (peelable fruit: Banana Musa × paradisiaca, MUBPA Kiwis Actinidia chinensis, ATICH Avocado Persea americana, PEBAM Melon Mango Mangifera indica, MNGIN Pineapple Ananas comosus, ANHCO Citrus sp. 1CIDG) | FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
≥ 85% Chitosan |
Immersion | Post‐harvest BBCH 89+ | 1 | – | 1 | – | – | Immersion/dipping of the fruit in a max of 2% (wt:vol) chitosan solution for a very short time (from a few second to 60 s) before being air dried, leading to a very thin film coating on the surface of the fruit (estimated to max ~0.02% of the fruit weight). | |
| Small fruit crops (3SMFC) | FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
100% Chitosan hydrochloride |
Low‐Medium volume spraying | Leaf development ‐ development of fruit (BBCH 10–79) | 4–8 | 2 weeks | 50–200 | 200–400 | 100–800 | 0 | Chitosan can be prepared for use following any of the two recipes provided in Appendix I (preparation for use). |
| Grapevine Vitis vinifera (VITVI) | FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
100% chitosan hydrochloride | Low‐Medium volume spraying | BBCH 10–BBCH 79 | 4–8 | 2 weeks | 50–100 | 200–600 | 100–600 | 0 | Chitosan can be prepared for use following any of the two recipes provided in Appendix I (preparation for use). |
| Fruit crops (3FRUC) Other than small fruit crops and grapevine | FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
100% chitosan hydrochloride | Low‐Medium volume spraying | BBCH 10–BBCH 79 | 4–8 | 2 weeks | 50–100 | 200–400 | 100–400 | 0 |
Chitosan can be prepared for use following any of the two recipes provided in Appendix I (preparation for use). Specific application rates are defined for small fruit crops and grapevine as set out above in this table. |
| Vegetables | FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
≥ 85% Chitosan |
Low‐Medium volume spraying | BBCH 09–BBCH 79 | 4–8 | 2 weeks | 50–100 | 200–400 | 100–400 | Not applicable | Chitosan can be prepared for use following any of the two recipes provided in Appendix I (preparation for use). |
| Cereals | FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
≥ 85% Chitosan |
Low‐Medium volume spraying | BBCH 09–BBCH 79 | 4–8 | 2 weeks | 50–100 | 200–400 | 100–400 | Not applicable | Chitosan can be prepared for use following any of the two recipes provided in Appendix I (preparation for use). |
| Spices | FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
≥ 85% Chitosan |
Low‐Medium volume spraying | BBCH 09–BBCH 79 | 4–8 | 2 weeks | 50–100 | 200–400 | 100–400 | Not applicable | Chitosan can be prepared for use following any of the two recipes provided in Appendix I (preparation for use). |
| Crops for animal feed | FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
≥ 85% Chitosan |
Low‐Medium volume spraying | BBCH 09–BBCH 79 | 4–8 | 2 weeks | 50–100 | 200–400 | 100–400 | Not applicable | Chitosan can be prepared for use following any of the two recipes provided in Appendix I (preparation for use). |
|
Cereals Seed treatment |
FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
≥ 85% Chitosan |
Low volume spraying | Before sowing (BBCH 00) | 1 | Not applicable | 50–100 | Not applicable | Not applicable | Not applicable | Chitosan can be prepared for use following any of the two recipes provided in Appendix I (preparation for use). |
|
Potatoes Seed treatment |
FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
≥ 85% Chitosan |
Low volume Spraying/dipping |
Before sowing (BBCH 00) | 1 | Not applicable | 50–100 | Not applicable | Not applicable | Not applicable | Chitosan can be prepared for use following any of the two recipes provided in Appendix I (preparation for use). |
|
Sugar beet Seed treatment |
FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
≥ 85% Chitosan |
Low volume spraying | Before sowing (BBCH 00) | 1 | Not applicable | 50–100 | Not applicable | Not applicable | Not applicable | Chitosan can be prepared for use following any of the two recipes provided in Appendix I (preparation for use). |
For crops, the EU and Codex classification (both) should be taken into account; where relevant, the use situation should be described (e.g. fumigation of a structure).
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
e.g. pests as biting and suckling insects, soil born insects, foliar fungi, weeds or plant elicitor.
e.g. wettable powder (WP), emulsifiable concentrate (EC), granule (GR) etc.
GCPF Codes – GIFAP Technical Monograph No 2, 1989.
All abbreviations used must be explained.
Method, e.g. high volume spraying, low volume spraying, spreading, dusting, drench.
Kind, e.g. overall, broadcast, aerial spraying, row, individual plant, between the plant – type of equipment used must be indicated.
g/kg or g/L. Normally the rate should be given for the substance (according to ISO).
Growth stage at last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including where relevant, information on season at time of application.
Indicate the minimum and maximum number of application possible under practical conditions of use.
The values should be given in g or kg whatever gives the more manageable number (e.g. 200 kg/ha instead of 200,000 g/ha or 12.5 g/ha instead of 0.0125 kg/ha.
PHI – minimum pre‐harvest interval.
CHITOSAN HYDROCHLORIDE – List of (extension) of uses
| Crop and/or situation a | F G or I b | Pests or group of pests controlled c | Formulation | Application | Application rate | PHI (days) m | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type d , e , f | Conc of a.i. g/kg i | Method kind f , g , h | Growth stage and season j | No. of application min/max k | Interval between applications (min) | g a.i./hL min max (g/hL) | Water L/ha min max | Total rate each application g a.i./ha min max (g/ha) l | |||||
| Small fruit crops (3SMFC) | FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
100% chitosan hydrochloride | Low‐Medium volume spraying | Leaf development ‐ development of fruit (BBCH 10‐79) | 4–8 | 2 weeks | 50–200 | 200–400 | 100–800 | 0 | |
| Grapevine Vitis vinifera (VITVI) | FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
100% chitosan hydrochloride | Low‐Medium volume spraying | BBCH 10 ‐ BBCH 79 | 4–8 | 2 weeks | 50–100 | 200–600 | 100–600 | 0 | |
| Fruit crops (3FRUC) Other than small fruit crops and grapevine | FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
100% chitosan hydrochloride | Low‐Medium volume spraying | BBCH 10 ‐ BBCH 79 | 4–8 | 2 weeks | 50–100 | 200–400 | 100–400 | 0 | Specific application rates are defined for small fruit crops and grapevine as set out in rows 1 and 2 of this table. |
| Vegetable crops (3VEGC) | FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
100% chitosan hydrochloride | Low‐Medium volume spraying | Leaf development ‐ development of fruit (BBCH 10‐79) | 4–8 | 2 weeks | 50–100 | 200–400 | 100–400 | 0 | |
| Cereal crops (3CERC) | FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
100% chitosan hydrochloride | Low‐Medium volume spraying | Leaf development ‐ development of fruit (BBCH 10‐79) | 4–8 | 2 weeks | 50–100 | 200–400 | 100–400 | 0 | |
| Spice crops (3SPIC) | FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
100% chitosan hydrochloride | Low‐Medium volume spraying | Leaf development ‐ development of fruit (BBCH 10‐79) | 4–8 | 2 weeks | 50–100 | 200–400 | 100–400 | 0 | |
| Crops grown for animal consumption | FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
100% chitosan hydrochloride | Low‐Medium volume spraying | Leaf development ‐ development of fruit (BBCH 10‐79) | 4–8 | 2 weeks | 50–100 | 200–400 | 100–400 | 0 | |
|
Cereal crops (3CERC) Seed treatment |
FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
100% chitosan hydrochloride | Low volume spraying | Before sowing | 1 | Not applicable | 50–100 | Not applicable | Not applicable | 0 | |
|
Potato (SOLTU) Seed treatment |
FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
100% chitosan hydrochloride | Low volume spraying/dipping | Before sowing | 1 | Not applicable | 50–200 | Not applicable | Not applicable | 0 | |
|
Sugar beet (BEAVA) Seed treatment |
FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
100% chitosan hydrochloride | Bulb treatment – Dipping/drenching | Germination (BBCH 00–01) | 1 | Not applicable | 50–100 | 200–800 | 100–800 | n.a. | |
|
Ornamentals herbaceous plants – bulbous plants Bulb treatment |
FGI | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
Low volume spraying/dipping | Bulb treatment – Dipping/drenching | Leaf development – Senscence (BBCH 10–92) | 1–8 | 5–7 days | 50–200 | 200–400 | 100–800 | 0 | |
| Arable crops ‐ Beet crops (3BEEC) | FG | Plant elicitor, plant resistance against pathogenic fungi and bacteria |
SP Soluble Powder |
Low volume spraying/dipping | Bulb treatment – Dipping/drenching | Leaf development – Senscence (BBCH 10‐92) | 1–8 | 5–7 days | 50–200 | 200–400 | 100–800 | 0 | |
For crops, the EU and Codex classification (both) should be taken into account; where relevant, the use situation should be described (e.g. fumigation of a structure).
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
e.g. pests as biting and suckling insects, soil born insects, foliar fungi, weeds or plant elicitor.
e.g. wettable powder (WP), emulsifiable concentrate (EC), granule (GR) etc.
GCPF Codes – GIFAP Technical Monograph No 2, 1989.
All abbreviations used must be explained.
Method, e.g. high volume spraying, low volume spraying, spreading, dusting, drench.
Kind, e.g. overall, broadcast, aerial spraying, row, individual plant, between the plant – type of equipment used must be indicated.
g/kg or g/L. Normally the rate should be given for the substance (according to ISO).
Growth stage at last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including where relevant, information on season at time of application.
Indicate the minimum and maximum number of application possible under practical conditions of use.
The values should be given in g or kg whatever gives the more manageable number (e.g. 200 kg/ha instead of 200,000 g/ha or 12.5 g/ha instead of 0.0125 kg/ha.
PHI – minimum pre‐harvest interval.
APPENDIX E. Fate additional information and PEC calculations
Appendix E can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2025.9318
EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) , Coja, T. , Adriaanse, P. , Choi, J. , Finizio, A. , Giraudo, M. , Kuhl, T. , Metruccio, F. , McVey, E. , Paparella, M. , Pieper, S. , Scanziani, E. , Teodorovic, I. , Van der Brink, P. , Wilks, M. , Marinovich, M. , Ferilli, F. , Gobbi, A. , Panzarea, M. , Vianello, G. , & Lava, R. (2025). Statement concerning the review of the approval of the basic substances chitosan and chitosan hydrochloride when used in plant protection. EFSA Journal, 23(4), e9318. 10.2903/j.efsa.2025.9318
Adopted: 26 February 2025
Amended: 4 April 2025
The declarations of interest of all scientific experts active in EFSA's work are available at https://open.efsa.europa.eu/experts
Appendices A–C and Appendix E are available under the Supporting Information section .
Notes
Surface water and sediments.
Estimated considering chitin and chitosan natural abundance, route of degradation of chitin into chitosan and chitosan degradation.
There is no nominated EU Union list for substance categories covered by Regulation (EU) 2019/1009. Authorisation of a given substance can be granted at national level and there might be national authorisation lists only.
Commission Implementing Regulation (EU) 2022/456 of 21 March 2022 approving the basic substance chitosan. OJ L 93, 22.3.2022, p. 138–141.
Commission Implementing Regulation (EU) No 563/2014 of 23 May 2014 approving the basic substance chitosan hydrochloride. OJ L 156, 24.5.2014, p. 5–7.
For details, please see the Review report for the basic substance chitosan hydrochloride available via EU Pesticides Database at https://ec.europa.eu/food/plant/pesticides/eu‐pesticides‐database/start/screen/active‐substances/details/1193.
See pertinent EFSA question numbers (at intake status) and related public details and supporting documents in OpenEFSA: https://open.efsa.europa.eu/questions?search=chitosan+hydrochloride.
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC.
Please see in particular “Pesticides: Addition of Chitosan (Including Chitosan Salts) to the List of Active Ingredients Permitted in Exempted Minimum Risk Pesticide Products”; US Environmental Protection Agency; Federal Register/Vol. 87, No. 215/Tuesday, November 8, 2022/Rules and Regulations; https://downloads.regulations.gov/EPA‐HQ‐OPP‐2019‐0701‐0026/content.pdf.
See in OpenEFSA: https://open.efsa.europa.eu/questions/EFSA‐Q‐2013‐00306?search=EFSA‐2013‐00306; https://open.efsa.europa.eu/questions/EFSA‐Q‐2020‐00339?search=EFSA‐Q‐2020‐00339.
As specified in the review report for the basic substance chitosan SANTE/10594/2021 Rev. 2 from 25 May 2023 and chitosan hydrochloride SANCO/12388/2013 Rev. 5 from 23 March 2023 available via EU Pesticides Database at https://ec.europa.eu/food/plant/pesticides/eu‐pesticides‐database/start/screen/active‐substances.
Review report for the basic substance chitosan (SANTE/10594/2021 Rev. 2; 25 May 2023) and chitosan hydrochloride (SANCO/12388/2013 Rev. 5; 23 March 2023) available via EU Pesticides Database at https://ec.europa.eu/food/plant/pesticides/eu‐pesticides‐database/start/screen/active‐substances.
The degree of deacetylation of chitin in nature seems to vary, and therefore the distinction from chitosan, is not strict (Beier et al., 2013; Frontiers Microbiology, Sec. Terrestrial Microbiology, Vol 4. https://www.frontiersin.org/articles/10.3389/fmicb.2013.00149/full).
One of the chitin degradation pathway in nature might be via chitosan (involving enzymes chitin deacetylases). (De Tender, et al., 2019; Scientific Reports Vol 9, Article number: 9890 (2019) available via https://www.nature.com/articles/s41598‐019‐46106‐x).
As according to SANCO Guidance document on the procedure for application of basic substances to be approved in compliance with Article 23 of Regulation (EC) No 1107/2009 (SANCO/10363/2012).
Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption (recast). OJ L 435, 23.12.2020, p. 1–62.
Under FAF Panel see EFSA‐Q‐2023‐00904: https://open.efsa.europa.eu/questions/EFSA‐Q‐2023‐00904; and under NDA Panel see EFSA‐Q‐2019‐00324: https://open.efsa.europa.eu/questions/EFSA‐Q‐2019‐00324?search=chitosan.
Commission Communication in the framework of the implementation of Commission Regulation (EU) No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market.
See chapter 2.2.2. of SANCO Guidance SANCO/10363/2012.
Applications currently at EC level under validity check or at EFSA intake phase (see OpenEFSA: https://open.efsa.europa.eu/questions?search=chitosan+hydrochloride), see chapter 2.2. of SANCO Guidance SANCO/10363/2012 for the overview of the approval procedure.
See details in OpenEFSA: https://open.efsa.europa.eu/questions/EFSA‐Q‐2020‐00668.
For search #1 it was carried out in DistillerSR, while for search #2 and #3 all the screening steps were carried out via the use of EndNote (see Appendix A).
For the main databases consulted or except for Agricola database, see full details in Appendix A.2.1.
Commission Regulation (EU) No 284/2013 setting out the data requirements for plant protection products, in accordance with Regulation (EC) No 1107/2009.
Communication (EU) No 2023/C 344/01: https://eur‐lex.europa.eu/legal‐content/EN/TXT/PDF/?uri=OJ:C:2023:344:FULL.
See the Guidance document on the preparation and submission of dossiers for plant protection products according to the ‘risk envelope approach’ SANCO/11244/2011. https://webgate.ec.europa.eu/dyna2/pgd/.
Formerly unique phylum called ‘Zygomycota’.
Available in the review report on chitosan hydrochloride (SANCO/12388/2013– rev. 5.).
Available in the review report on chitosan (SANTE/10594/2021 ‐ Rev. 2).
Number average molecular weight. This is the statistical average molecular weight of all the polymer chains in a sample.
Available at: https://iris.ema.europa.eu/substances/.
Under EFSA FAF Panel see EFSA‐Q‐2023‐00904: https://open.efsa.europa.eu/questions/EFSA‐Q‐2023‐00904 and Under NDA Panel see EFSA‐Q‐2019‐00324: https://open.efsa.europa.eu/questions/EFSA‐Q‐2019‐00324?search=chitosan.
Some notified classifications for chitosan are available, though it is noted the reduced number of notifiers: https://echa.europa.eu/it/information‐on‐chemicals/cl‐inventory‐database/‐/discli/details/79723.
Please refer to: http://www.mhlw.go.jp/english/topics/foodsafety/foodadditives/Index.html.
See EFSA‐Q‐2019‐00324: https://open.efsa.europa.eu/questions/EFSA‐Q‐2019‐00324.
See EFSA‐Q‐2023‐00904: https://open.efsa.europa.eu/questions/EFSA‐Q‐2023‐00904.
Including 67 cultivated fields, 52 non‐cultivated fields, 10 forests, 13 flower beds and 28 other types of soil.
Public summary dossier available in OpenEFSA: https://open.efsa.europa.eu/questions/EFSA‐Q‐2020‐00668.
If assuming chitosan having 100% deacetylation degree, then the resulting monomers would be only GlcN monosaccharides.
Commission Regulation (EU) No 284/2013 of 1 March 2013 setting out the data requirements for plant protection products, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market.
As specified in GAP table (formulation column) in Appendix D.
As specified in GAP table (formulation column) in Appendix D.
DT50 derived from the value of 242.5 days in soil at 25°C (k = 0.0028583 day−1 assuming SFO degradation kinetic fit) and 70% relative humidity after normalisation at 20°C (Mostafa et al., 2010).
Value of DT50 normalised at 20°C and assuming for sediment a Kp ≤ 100 L kg−1, i.e. measured Kp value is not available, but an estimated value can be derived from the Kow parameter reported in the OptiCHOS (2024) documents.
Max application rate of 200 g hL−1 as single application for sugar beet.
Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption. OJ L 435, 23.12.2020, p. 1–62.
It is recalled the generic molecular weight of chitosan and chitosan hydrochloride: 10–1500 kDa and 47–65 kDa, respectively.
A fertiliser shall be an EU fertilising product the function of which is to provide nutrients to plants or mushrooms (Regulation (EU) 2019/1009).
A soil improver shall be an EU fertilising product the function of which is to maintain, improve or protect the physical or chemical properties, the structure or the biological activity of the soil to which it is added (Regulation (EU) 2019/1009).
A plant biostimulant shall be an EU fertilising product the function of which is to stimulate plant nutrition processes independently of the product's nutrient content with the sole aim of improving one or more of the following characteristics of the plant or the plant rhizosphere: (a) nutrient use efficiency, (b) tolerance to abiotic stress, (c) quality traits or (d) availability of confined nutrients in the soil or rhizosphere (Regulation (EU) 2019/1009).
Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003.
List of conformity assessment bodies notified available at the following link: https://webgate.ec.europa.eu/single‐market‐compliance‐space/home.
See as an example the Belgian dedicated website informing about products authorised in agriculture: https://fytoweb.be/en/fertilising‐products.
Obtained from organic aquaculture or from sustainable fisheries, in accordance with Article 2 of Regulation (EU) No 1380/2013.
Commission Implementing Regulation (EU) 2021/1165 of 15 July 2021 authorising certain products and substances for use in organic production and establishing their lists. OJ L 253, 16.7.2021, p. 13–48.
8‐year time monitoring.
Both chitosan hydrochloride and chitosan obtained from Aspergillus or organic aquaculture or from sustainable fisheries, as defined in Article 2 of Regulation (EU) No 1380/2013 of the European Parliament and of the Council.
Peer‐review process under EFSA‐Q‐2020‐00668, public summary dossier available in OpenEFSA: https://open.efsa.europa.eu/questions/EFSA‐Q‐2020‐00668.
Available in the EU Pesticides database: https://ec.europa.eu/food/plant/pesticides/eu‐pesticides‐database/start/screen/active‐substances/details/1490 (chitosan) and https://ec.europa.eu/food/plant/pesticides/eu‐pesticides‐database/start/screen/active‐substances/details/1193 (chitosan hydrochloride).
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition.
Regulation (EC) No 767/2009 of the European Parliament and of the Council of 13 July 2009 on the placing on the market and use of feed, OJ L 229, 1.9.2009.
No more precise concentration values could be extracted from the retrieved literature papers because of differences among feed characteristics (e.g. chitosan nanoparticles, or estimations based on dry weight or calculated based on body weight or feed weight or otherwise).
Commission Delegated Regulation (EU) 2019/934 of 12 March 2019 supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as regards wine‐growing areas where the alcoholic strength may be increased, authorised oenological practices and restrictions applicable to the production and conservation of grapevine products, the minimum percentage of alcohol for by‐products and their disposal. OJ L 149, 7.6.2019, p. 1–52.
Very high degradation half‐life of chitosan in soil, no crop interception, low occurrence of chitin producing organisms in the soil, low expected degradation of chitin into chitosan (see also Sections 5.4.1 and 5.4.2).
Commission Regulation (EU) No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009.
Under FAF Panel see EFSA‐Q‐2023‐00904: https://open.efsa.europa.eu/questions/EFSA‐Q‐2023‐00904.
Under NDA Panel see EFSA‐Q‐2019‐00324: https://open.efsa.europa.eu/questions/EFSA‐Q‐2019‐00324.
REFERENCES
- Abdel‐Ghany, H. M. , & Salem, M. E.‐S. (2020). Effects of dietary chitosan supplementation on farmed fish; a review. Reviews in Aquaculture, 12, 438–452. 10.1111/raq.12326 [DOI] [Google Scholar]
- Abdel‐Tawwab, M. , Razek, N. A. , & Abdel‐Rahman, A. M. (2019). Immunostimulatory effect of dietary chitosan nanoparticles on the performance of Nile tilapia, Oreochromis niloticus (L.). Fish & Shellfish Immunology, 88, 254–258. 10.1016/j.fsi.2019.02.063 [DOI] [PubMed] [Google Scholar]
- Abo Elsoud, M. M. , & El Kady, E. M. (2019). Current trends in fungal biosynthesis of chitin and chitosan. Bulletin of the National Research Centre, 43, 59. 10.1186/s42269-019-0105-y [DOI] [Google Scholar]
- Ahmadi, F. , Oveisi, Z. , Samani, S. M. , & Amoozgar, Z. (2015). Chitosan based hydrogels: Characteristics and pharmaceutical applications. Research in Pharmaceutical Sciences, 10(1), 1–16. [PMC free article] [PubMed] [Google Scholar]
- Ahmed, F. , Soliman, F. M. , Adly, M. A. , Soliman, H. A. M. , El‐Matbouli, M. , & Saleh, M. (2021). Dietary chitosan nanoparticles: Potential role in modulation of rainbow trout (Oncorhynchus mykiss) antibacterial defense and intestinal immunity against enteric Redmouth disease. Marine Drugs, 19, 72. 10.3390/md19020072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemu, D. , Getachew, E. , & Mondal, A. K. (2023). Study on the physicochemical properties of chitosan and their applications in the biomedical sector. International Journal of Polymeric Science, 2023, 1–13. 10.1155/2023/5025341 [DOI] [Google Scholar]
- Ali, G. , Sharma, M. , Salama, E. S. , Ling, Z. , & Li, X. (2024). Applications of chitin and chitosan as natural biopolymer: Potential sources, pretreatments, and degradation pathways. Biomass Conversion and Biorefinery, 14, 4567–4581. 10.1007/s13399-022-02684-x [DOI] [Google Scholar]
- Al‐Sagheer, A. A. , Abdel‐Rahman, G. , El‐Moniem, E. A. A. , Mahgoub, S. , & Ayyat, M. S. (2023). Influence of dietary chitosan supplementation on growth indicators, nutrient digestibility, immunity, Cecal microbiota, and intestinal morphology of growing male rabbits. Annals of Animal Science, 23, 4. 10.2478/aoas-2023-0031 [DOI] [Google Scholar]
- Anderson, J. W. , Nicolosi, R. J. , & Borzelleca, J. F. (2005). Glucosamine effects in humans: A review of effects on glucose metabolism, side effects, safety considerations and efficacy. Food and Chemical Toxicology, 43(2), 187–201. [DOI] [PubMed] [Google Scholar]
- Anggraeni, A. S. , Jayanegara, A. , Laconi, E. B. , Kumalasari, N. R. , & Sofyan, A. (2022). Marine by‐products and insects as a potential chitosan source for ruminant feed additives. Czech Journal of Animal Science, 67(8), 295–317. 10.17221/42/2022-CJAS [DOI] [Google Scholar]
- Appuhn, A. , Joergensen, R. G. , Raubuch, M. , Scheller, E. , & Wilke, B. (2004). The automated determination of glucosamine, galactosamine, muramic acid, and mannosamine in soil and root hydrolysates by HPLC. Zeitschrift für Pflanzenernährung und Bodenkunde, 167(1), 17–21. 10.1002/jpln.200321302 [DOI] [Google Scholar]
- Arai, K. , Kinumaki, T. , & Fujita, T. (1968). Toxicity of chitosan. Bulletin of Tokai Regional Fisheries Research Laboratory, 56, 89–94. [Google Scholar]
- Aranaz, I. , Acosta, N. , Civera, C. , Elorza, B. , Mingo, J. , Castro, C. , Gandía, M. L. L. , & Heras Caballero, A. (2018). Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers, 10(2), 213. 10.3390/polym10020213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranaz, I. , Alcántara, A. R. , Civera, M. C. , Arias, C. , Elorza, B. , Heras Caballero, A. , & Acosta, N. (2021). Chitosan: An overview of its properties and applications. Polymers, 13, 3256. 10.3390/polym13193256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranaz, I. , Harris, R. , & Heras, A. (2010). Chitosan amphiphilic derivatives. Chemistry and applications. Current Organic Chemistry, 14(3), 308–330. [Google Scholar]
- Araújo, D. F. , Ferreira, I. , Torres, C. , Neves, L. , & Freitas, F. (2020). Chitinous polymers: Extraction from fungal sources, characterization and processing towards value‐added applications. Journal of Chemical Technology & Biotechnology, 95(5), 1277–1289. 10.1002/jctb.6325 [DOI] [Google Scholar]
- Ayman, U. , Akter, L. , Islam, R. , Bhakta, S. , Rahman, M. A. , Islam, M. R. , Sultana, N. , Sharif, A. , Jahan, M. R. , Rahman, M. S. , & Haque, Z. (2022). Dietary chitosan oligosaccharides improves health status in broilers for safe poultry meat production. Annals of Agricultural Science, 67(1), 90–98. 10.1016/j.aoas.2022.05.003 [DOI] [Google Scholar]
- Beier, S. , & Bertilsson, S. (2013). Bacterial chitin degradation—Mechanisms and Ecophysiological strategies. Frontiers in Microbiology, 4, 149. 10.3389/fmicb.2013.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellich, B. , D'Agostino, I. , Semeraro, S. , Gamini, A. , & Cesaro, A. (2016). “The good, the bad and the ugly” of Chitosans. Marine Drugs, 14(5), 99. 10.3390/md14050099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolat, Y. , Bilgin, Ș. , Günlü, A. , Izci, L. , Koca, S. B. , Çetinkaya, S. , & Koca, H. U. (2010). Chitin‐chitosan yield of freshwater crab (Potamon potamios, Olivier 1804) shell. Pakistan Veterinary Journal, 30(4), 227–231. [Google Scholar]
- Bolognesi, C. , Castoldi, A. F. , Crebelli, R. , Barthélémy, E. , Maurici, D. , Wölfle, D. , Volk, K. , & Castle, L. (2017). Genotoxicity testing approaches for the safety assessment of substances used in food contact materials prior to their authorization in the European Union. Environmental and Molecular Mutagenesis, 58, 361–374. 10.1002/em.22094 [DOI] [PubMed] [Google Scholar]
- Bonilla, J. , Paiano, R. B. , Lourenço, R. V. , Bittante, A. M. Q. B. , & Sobral, P. J. A. (2020). Biodegradability in aquatic systems of thin materials based on chitosan, PBAT, and HDPE polymers: Respirometric and physical‐chemical analysis. International Journal of Biological Macromolecules, 164, 1399–1412. 10.1016/j.ijbiomac.2020.07.309 [DOI] [PubMed] [Google Scholar]
- Boryniec, S. , Ratajska, M. , & Strobin, G. (1996). Biodegradation of chitosan. Polimery, 41(10), 564–567. https://archiwum.ichp.vot.pl/1996/rok_1996_10_art_03.pdf [Google Scholar]
- Briza, P. , Ellinger, A. , Winkler, G. , & Breitenbach, M. (1988). Chemical composition of the yeast Ascospore Wall. The second outer layer consists of chitosan. The Journal of Biological Chemistry, 263(23), 11569–11574. 10.1016/s0021-9258(18)37997-3 [DOI] [PubMed] [Google Scholar]
- van den Broek, L. A. M. , & Boeriu, C. G. (2019). Chitin and chitosan: Properties and applications. Wiley. 10.1002/9781119450467 [DOI] [Google Scholar]
- Bullock, G. , Blazer, V. , Tsukuda, S. , & Summerfelt, S. (2000). Toxicity of acidified chitosan for cultured rainbow trout (Oncorhynchus mykiss). Aquaculture, 185(3–4), 273–280. [Google Scholar]
- Carreno‐Gomez, B. , & Duncan, R. (1997). Evaluation of the biological properties of soluble chitosan and chitosan microspheres. International Journal of Pharmaceutics, 148(2), 231–240. [Google Scholar]
- Casadidio, C. , Vargas Peregrina, D. , Gigliobianco, M. R. , Deng, S. , Censi, R. , & Di Martino, P. (2019). Chitin and Chitosans: Characteristics, eco‐friendly processes, and applications in cosmetic science. Marine Drugs, 17(6), 369. 10.3390/md17060369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauchie, H. M. (2002). Chitin production by arthropods in the hydrosphere. Hydrobiologia, 470, 63–95. 10.1023/A:1015615819301 [DOI] [Google Scholar]
- Chae, S. Y. , Jang, M. K. , & Nah, J. W. (2005). Influence of molecular weight on oral absorption of water soluble chitosans. Journal of Controlled Release, 102(2), 383–394. [DOI] [PubMed] [Google Scholar]
- Chang, Y. M. , Lee, Y. J. , Liao, J. W. , Jhan, J. K. , Chang, C. T. , & Chung, Y. C. (2014). In vitro and in vivo safety evaluation of low molecular weight chitosans prepared by hydrolyzing crab shell chitosans with bamboo shoots chitosanase. Food and Chemical Toxicology, 71, 10–16. [DOI] [PubMed] [Google Scholar]
- Chater, K. F. , Biró, S. , Lee, K. J. , Palmer, T. , & Schrempf, H. (2010). The complex extracellular biology of Streptomyces. FEMS Microbiology Reviews, 34(2), 171–198. 10.1111/j.1574-6976.2009.00206.x [DOI] [PubMed] [Google Scholar]
- Chavez, M. , & Uchanski, M. (2021). Insect left‐over substrate as plant Fertiliser. Journal of Insects as Food and Feed, 7, 683–694. 10.3920/JIFF2020.0063 [DOI] [Google Scholar]
- Cheng, Q. , Zhang, J. , & Xia, W. (2013). Prenatal and developmental effect of high molecular weight chitosan (HMWCS) to mice. Regulatory Toxicology and Pharmacology, 65(3), 294–303. [DOI] [PubMed] [Google Scholar]
- Council of Europe . (2019). European Pharmacopoeia (10th ed.). European Directorate for the Quality of Medicines Healthcare. [Google Scholar]
- Cretoiu, M. S. , Korthals, G. W. , Visser, J. H. M. , & van Elsas, J. D. (2013). Chitin amendment increases soil Suppressiveness toward plant pathogens and modulates the Actinobacterial and Oxalobacteraceal communities in an experimental agricultural field. Applied and Environmental Microbiology, 79(6), 1726–1736. 10.1128/AEM.01361-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crognale, S. , Russo, C. , Petruccioli, M. , & D'Annibale, A. (2022). Chitosan production by fungi: Current state of knowledge, future opportunities, and constraints. Fermentation, 8(2), 76. 10.3390/fermentation8020076 [DOI] [Google Scholar]
- Crolle, G. , & D'este, E. (1980). Glucosamine sulphate for the management of arthrosis: A controlled clinical investigation. Current Medical Research and Opinion, 7(2), 104–109. 10.1185/03007998009112035 [DOI] [PubMed] [Google Scholar]
- CTGB . (2020). Board for the Authorisation of plant protection products and biocides. Evaluation Manual for the Authorisation of plant protection products according to Regulation (EC) No 1107/2009, EU part Plant protection products, Chapter 6: Fate and behaviour in the environment; behaviour in soil; persistence. Version 2.3 (Jan 2020).
- Dalmoro, Y. K. , Franceschi, C. H. , & Stefanello, C. (2023). A systematic review and Metanalysis on the use of Hermetia illucens and Tenebrio molitor in diets for poultry. Veterinary Sciences, 10, 702. 10.3390/vetsci10120702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, B. , & Eveleigh, D. E. (1984). Chitosanases: Occurrence, production and immobilization. In Zikakis J. P. (Ed.), Chitin, chitosan, and related enzymes (pp. 161–179). Academic Press. 10.1016/B978-0-12-780950-2.50016-2 [DOI] [Google Scholar]
- de Jesús Valle, M. J. , Dinis‐Oliveira, R. J. , Carvalho, F. , Bastos, M. L. , & Navarro, A. S. (2008). Toxicological evaluation of lactose and chitosan delivered by inhalation. Journal of Biomaterials Science, Polymer Edition, 19(3), 387–397. [DOI] [PubMed] [Google Scholar]
- Dhillon, G. S. , Kaur, S. , Brar, S. K. , & Verma, M. (2013). Green synthesis approach: Extraction of chitosan from fungus mycelia. Critical Reviews in Biotechnology, 33(4), 379–403. 10.3109/07388551.2012.717217 [DOI] [PubMed] [Google Scholar]
- ECHA (European Chemical Agency) . (2017). Guidance on Biocidal Products Regulation: Volume IV Environment ‐ Assessment and Evaluation (Parts B+C), October 2017. Reference: ECHA‐17‐G‐23‐EN; Cat. Number: ED‐01‐17‐897‐EN‐N; ISBN: 978‐92‐9020‐151‐9. 10.2823/033935 [DOI]
- EFSA (European Food Safety Authority) . (2013). Outcome of the consultation with Member States and EFSA on the basic substance application for chitosan hydrochloride and the conclusions drawn by EFSA on the specific points raised. EFSA Supporting Publications, 10(6), EN‐426. 10.2903/sp.efsa.2013.EN-426 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2014). Conclusion on the peer review of the pesticide risk assessment of the active substance COS‐OGA. EFSA Journal, 12(10), 3868. 10.2903/j.efsa.2014.3868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2020a). Draft framework for protocol development for EFSA's scientific assessments. EFSA Supporting Publications, 17(4), EN‐1843. 10.2903/sp.efsa.2020.EN-1843 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2020b). Technical report on the outcome of the consultation with Member States and EFSA on the basic substance application for approval of chitosan hydrochloride for the extension of use in plant protection as an elicitor in horticulture, olive trees, grapes, grass and post‐harvest fruit treatment. EFSA Supporting Publications, 17(7), EN‐1900. 10.2903/sp.efsa.2020.EN-1900 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2025). Scientific Report on chitin estimation on agricultural soils. EFSA Journal, e9313. 10.2903/j.efsa.2025.9313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) . (2016). Scientific opinion on recent developments in the risk assessment of chemicals in food and their potential impact on the safety assessment of substances used in food contact materials. EFSA Journal, 14(1), 4357. 10.2903/j.efsa.2016.4357 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) . (2011). Scientific opinion on the substantiation of health claims related to chitosan and reduction in body weight (ID 679, 1499), maintenance of normal blood LDL‐cholesterol concentrations (ID 4663), reduction of intestinal transit time (ID 4664) and reduction of inflammation (ID 1985) pursuant to article 13(1) of regulation (EC) No 1924/2006. EFSA Journal, 9(6), 2214. 10.2903/j.efsa.2011.2214 [DOI] [Google Scholar]
- EFSA Scientific Committee . (2017). Scientific Opinion on the guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal, 15(8), 4971. 10.2903/j.efsa.2017.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee . (2018). Guidance on uncertainty analysis in scientific assessments. EFSA Journal, 16(1), 5123. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Naggar, N. E.‐A. (2021). Streptomyces‐based cell factories for production of biomolecules and bioactive metabolites. In Singh V. (Ed.), Microbial cell factories engineering for production of biomolecules (pp. 183–234). Academic Press. 10.1016/B978-0-12-821477-0.00011-8 [DOI] [Google Scholar]
- Elnesr, S. S. , Elwan, H. A. M. , El Sabry, M. I. , Shehata, A. M. , & Alagawany, M. (2022). Impact of chitosan on productive and physiological performance and gut health of poultry. World's Poultry Science Journal, 78, 483–498. 10.1080/00439339.2022.2041992 [DOI] [Google Scholar]
- Eisa, A. A. A. , Aboelghar, G. E. , Ammar, I. M. , Metwally, H. G. , & Arafa, S. S. (2018). Teratogenic effects induced by chitosan oligosaccharide in Wistar female rat Rattus norvegicus. Environmental Science and Pollution Research International, 25(10), 9371–9379. 10.1007/s11356-018-1199-8 [DOI] [PubMed] [Google Scholar]
- EPA (Envinroment Protection Agency) memorandum . (2022). Addendum to the science review in support of the addition of chitosan (Poly‐DGlucosamine) to the list of minimum risk pesticides (MRPs) contained in 40 CFR 152.25(f). https://downloads.regulations.gov/EPA‐HQ‐OPP‐2019‐0701‐0029/content.pdf
- European Commission . (2023a). Final Review report for the basic substance chitosan finalised by the Standing Committee on Plants, Animals, Food and Feed on 28 January 2022 in view of the approval of chitosan as basic substance in accordance with Regulation (EC) No 1107/2009. Chitosan SANTE/10594/2021 Rev. 2. https://ec.europa.eu/food/plant/pesticides/eu‐pesticides‐database/start/screen/active‐substances/details/1490
- European Commission . (2023b). Final Review report for the basic substance chitosan hydrochloride finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 20 March 2014 in view of the approval of chitosan hydrochloride as basic substance in accordance with Regulation (EC) No 1107/2009, amended in the Standing Committee on Plants, Animals, Food and Feed at its meeting on 25 January 2021 and corrected on 5 July 2021 and on 23 March 2023. Chitosan hydrochloride SANCO/12388/2013– rev. 5 23 March 2023. https://ec.europa.eu/food/plant/pesticides/eu‐pesticides‐database/start/screen/active‐substances/details/1193
- FAO (Food and Agriculture Organisation) . (2005). Codex Alimentarius International Food Standard. General standard for fruit juices and nectar, CXS 247‐2005. Adopted in 2005. Amended in 2022.
- Farivar, A. , Atay, A. , Şahan, Z. , Serbester, U. , Yenilmez, F. , Tekeli, A. , Küçükgülmez, A. , Kadak, A. E. , Celik, M. , Uzun, Y. , Kutlu, H. R. , & Baykal Çelik, L. (2022). Effects of different degrees of Deacetylation and levels of chitosan on performance, egg traits and serum biochemistry of laying hens. Archives of Animal Nutrition, 76, 112–124. 10.1080/1745039X.2022.2082908 [DOI] [PubMed] [Google Scholar]
- Fabritius, H. , Sachs, C. , Raabe, D. , Nikolov, S. , Friák, M. , & Neugebauer, J. (2011). Chitin in the exoskeletons of Arthropoda: from ancient design to novel materials science. In Gupta N. (Ed.), Chitin. Topics in Geobiology (Vol. 34). Springer. 10.1007/978-90-481-9684-5_2 [DOI] [Google Scholar]
- Felt, O. , Furrer, P. , Mayer, J. M. , Plazonnet, B. , Buri, P. , & Gurny, R. J. I. J. O. P. (1999). Topical use of chitosan in ophthalmology: Tolerance assessment and evaluation of precorneal retention. International Journal of Pharmaceutics, 180(2), 185–193. [DOI] [PubMed] [Google Scholar]
- Fernandes, J. C. , Borges, M. , Nascimento, H. , Bronze‐da‐Rocha, E. , Ramos, O. S. , Pintado, M. E. , Malcata, F. X. , & Santos‐Silva, A. (2011). Cytotoxicity and genotoxicity of chitooligosaccharides upon lymphocytes. International Journal of Biological Macromolecules, 49(3), 433–438. 10.1016/j.ijbiomac.2011.05.032 [DOI] [PubMed] [Google Scholar]
- Fernandes, J. C. , Spindola, H. , de Sousa, V. , Santos‐Silva, A. , Pintado, M. E. , Malcata, F. X. , & Carvalho, J. E. (2010). Anti‐inflammatory activity of chitooligosaccharides in vivo. Marine Drugs, 8(6), 1763–1768. 10.3390/md8061763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) . (1997). Soil persistence models and EU registration. The final report of the work of the Soil Modelling Work group of FOCUS. Version 29.2.97.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) . (2001). FOCUS surface water scenarios in the EU evaluation process under 91/414/EEC. Report of the FOCUS Working Group on Surface Water Scenarios. EC Document Reference SANCO/4802/2001‐rev.2, 245 pp., as updated by Generic guidance for FOCUS surface water scenarios, v. 1.4, May 2015.
- Gao, N. , Du, W. , Zhang, M. , Ling, G. , & Zhang, P. (2022). Chitosan‐modified biochar: Preparation, modifications, mechanisms and applications. International Journal of Biological Macromolecules, 209, 31–49. 10.1016/j.ijbiomac.2022.04.006 [DOI] [PubMed] [Google Scholar]
- Ghormade, V. , Pathan, E. K. , & Deshpande, M. V. (2017). Can fungi compete with marine sources for chitosan production? International Journal of Biological Macromolecules, 104, 1415–1421. 10.1016/j.ijbiomac.2017.01.112 [DOI] [PubMed] [Google Scholar]
- Gooday, G. W. (1990). Physiology of microbial degradation of chitin and chitosan. Biodegradation, 1, 177–190. 10.1007/BF00058835 [DOI] [Google Scholar]
- Gooday, G. W. , Prosser, J. I. , Hillman, K. , & Cross, M. G. (1991). Mineralization of chitin in an estuarine sediment: The importance of the chitosan pathway. Biochemical Systematics and Ecology, 19(5), 395–400. 10.1016/0305-1978(91)90056-6 [DOI] [Google Scholar]
- Grammenou, A. , Petropoulos, S. A. , Thalassinos, G. , Rinklebe, J. , Shaheen, S. M. , & Antoniadis, V. (2023). Biostimulants in the soil–plant Interface: Agro‐environmental implications—A review. Earth Systems and Environment, 7(3), 583–600. 10.1007/s41748-023-00349-x [DOI] [Google Scholar]
- Hadwiger, L. A. (2020). Nonhost disease resistance in pea: Chitosan's suggested role in DNA minor groove actions relative to Phytoalexin‐eliciting anti‐cancer compounds. Molecules, 25(24), 5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn, T. , Tafi, E. , Paul, A. , Salvia, R. , Falabella, P. , & Zibek, S. (2020). Current state of chitin purification and chitosan production from insects. Journal of Chemical Technology and Biotechnology, 95(11), 2775–2795. 10.1002/jctb.6533 [DOI] [Google Scholar]
- Hamed, I. , Ozogul, F. , & Regenstein, J. M. (2016). Industrial applications of crustacean by‐products (chitin, chitosan, and chitooligosaccharides): A review. Trends in Food Science & Technology, 48, 40–50. [Google Scholar]
- Harahap, R. P. , Suharti, S. , Ridla, M. , Laconi, E. B. , Nahrowi, N. , Irawan, A. , Kondo, M. , Obitsu, T. , & Jayanegara, A. (2022). Meta‐analysis of dietary chitosan effects on performance, nutrient utilization, and product characteristics of ruminants. Animal Science Journal, 93, e13676. 10.1111/asj.13676 [DOI] [PubMed] [Google Scholar]
- Hasan, S. , Boddu, V. , Viswanath, D. , & Ghosh, T. (2022). Chitin and chitosan: Science and engineering. Springer Nature. 10.1007/978-3-031-01229-7 [DOI] [Google Scholar]
- Hellmann, M. J. , Marongiu, G. L. , Gorzelanny, C. , Moerschbacher, B. M. , & Cord‐Landwehr, S. (2025). Hydrolysis of chitin and chitosans by the human chitinolytic enzymes: Chitotriosidase, acidic mammalian chitinase, and lysozyme. International Journal of Biological Macromolecules, 297, 139789. 10.1016/j.ijbiomac.2025.139789 [DOI] [PubMed] [Google Scholar]
- Hidangmayum, A. , Dwivedi, P. , Katiyar, D. , & Hemantaranjan, A. (2019). Application of chitosan on plant responses with special reference to abiotic stress. Physiology and Molecular Biology of Plants, 25, 313–326. 10.1007/s12298-018-0633-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman, K. , Gooday, G. W. , & Prosser, J. I. (1989). The mineralization of chitin in the sediments of the Ythan estuary, Aberdeenshire, Scotland. Estuarine, Coastal and Shelf Science, 29(6), 601–612. 10.1016/0272-7714(89)90013-9 [DOI] [Google Scholar]
- Hirano, S. (1996). Chitin biotechnology applications. Biotechnology Annual Review, 2, 237–258. 10.1016/s1387-2656(08)70012-7 [DOI] [PubMed] [Google Scholar]
- Hossain, M. A. , & Bhuiyan, M. J. U. (2023). Black soldier Fly (Hermetia illucens): A Proteinous substitution of soybean and fish meal for broiler and layer chicken: A review. Poultry Science Journal, 11, 133–147. 10.22069/psj.2023.20508.1851 [DOI] [Google Scholar]
- Houpt, J. B. , McMillan, R. , Wein, C. , & Paget‐Dellio, S. D. (1999). Effect of glucosamine hydrochloride in the treatment of pain of osteoarthritis of the knee. The Journal of Rheumatology, 26(11), 2423–2430. [PubMed] [Google Scholar]
- Huang, M. , Khor, E. , & Lim, L. Y. (2004). Uptake and cytotoxicity of chitosan molecules and nanoparticles: Effects of molecular weight and degree of deacetylation. Pharmaceutical Research, 21, 344–353. [DOI] [PubMed] [Google Scholar]
- Huang, R. L. , Yin, Y. L. , Wu, G. Y. , Zhang, Y. G. , Li, T. J. , Li, L. L. , Li, M. X. , Tang, Z. R. , Zhang, J. , Wang, B. , He, J. H. , & Nie, X. Z. (2005). Effect of dietary oligochitosan supplementation on ileal digestibility of nutrients and performance in broilers. Poultry Science, 84(9), 1383–1388. 10.1093/ps/84.9.1383 [DOI] [PubMed] [Google Scholar]
- Huq, T. , Khan, A. , Brown, D. , Dhayagude, N. , He, Z. , & Ni, Y. (2022). Sources, production and commercial applications of fungal chitosan: A review. Journal of Bioresources and Bioproducts, 7(2), 85–98. 10.1016/j.jobab.2022.01.002 [DOI] [Google Scholar]
- Iber, B. T. , Kasan, N. A. , Torsabo, D. , & Omuwa, J. W. (2022). A review of various sources of chitin and chitosan in nature. Journal of Renewable Materials, 10(4), 1097–1123. 10.32604/jrm.2022.018142 [DOI] [Google Scholar]
- Ibitoye, E. B. , Lokman, I. H. , Hezmee, M. N. M. , Goh, Y. M. , Zuki, A. B. Z. , Jimoh, A. A. , Danmaigoro, A. , & Nicholas, N. P. (2019). Gut health and serum growth hormone levels of broiler chickens fed dietary chitin and chitosan from cricket and shrimp. Poultry Science, 98, 745–752. 10.3382/ps/pey419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indorf, C. , Dyckmans, J. , Khan, K. S. , et al. (2011). Optimisation of amino sugar quantification by HPLC in soil and plant hydrolysates. Biology and Fertility of Soils, 47, 387–396. 10.1007/s00374-011-0545-5 [DOI] [Google Scholar]
- Iriti, M. , Picchi, V. , Rossoni, M. , Gomarasca, S. , Ludwig, N. , Gargano, M. , & Faoro, F. (2009). Chitosan antitranspirant activity is due to abscisic acid‐dependent stomatal closure. Environmental and Experimental Botany, 66(3), 493–500. [Google Scholar]
- Jiang, T. , James, R. , Kumbar, S. G. , & Laurencin, C. T. (2014). Chapter 5 ‐ chitosan as a biomaterial: Structure, properties, and applications in tissue engineering and drug delivery. In Kumbar S. G., Laurencin C. T., & Deng M. (Eds.), Natural and synthetic biomedical polymers (pp. 91–113). Elsevier. 10.1016/B978-0-12-396983-5.00005-3 [DOI] [Google Scholar]
- Joergensen, R. G. (2018). Amino sugars as specific indices for fungal and bacterial residues in soil. Biology and Fertility of Soils, 54, 559–568. 10.1007/s00374-018-1288-3 [DOI] [Google Scholar]
- Kajimoto, O. (1998). Therapeutic activity of oral glucosamine hydrochloride in osteoarthritis of the knee: A placebo‐controlled, double‐blind, cross‐over study. Nippon Rinsho Eiyo Gakkaishi, 20, 41–47. [Google Scholar]
- Kamal, M. , Youssef, I. M. , Khalil, H. A. , Ayoub, M. A. , & Hashem, N. M. (2023). Multifunctional role of chitosan in farm animals: A comprehensive review. Annals of Animal Science, 23, 69–86. 10.2478/aoas-2022-0054 [DOI] [Google Scholar]
- Kaur, L. , Raj, R. , Thakur, A. K. , & Singh, I. (2020). Development of chitosan‐catechol conjugates as mucoadhesive polymer: assessment of acute oral toxicity in mice. Environmental Analysis Health and Toxicology, 35(3), e2020014. 10.5620/eaht.2020014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya, M. , Baran, T. , Mentes, A. , Asaroglu, M. , Sezen, G. , & Tozak, K. O. (2014). Extraction and characterization of α‐chitin and chitosan from six different aquatic invertebrates. Food Biophysics, 9, 145–157. 10.1007/s11483-013-9327-y [DOI] [Google Scholar]
- Kean, T. , & Thanou, M. (2010). Biodegradation, biodistribution and toxicity of chitosan. Advanced Drug Delivery Reviews, 62(1), 3–11. [DOI] [PubMed] [Google Scholar]
- Kim, S. K. , Park, P. J. , Yang, H. P. , & Han, S. S. (2001). Subacute toxicity of chitosan oligosaccharide in Sprague‐Dawley rats. Arzneimittel‐Forschung, 51(9), 769–774. 10.1055/s-0031-1300113 [DOI] [PubMed] [Google Scholar]
- KitoZyme . (2008). Acute oral toxicity of mushroom derived chitosan (OECD 423); unpublished study report.
- Kumeta, Y. , Inami, K. , Ishimaru, K. , Yamazaki, Y. , Sameshima‐Saito, R. , & Saito, A. (2018). Thermogravimetric evaluation of chitin degradation in soil: Implication for the enhancement of ammonification of native organic nitrogen by chitin addition. Soil Science and Plant Nutrition, 64(4), 512–519. 10.1080/00380768.2018.1457408 [DOI] [Google Scholar]
- Kurita, K. (2006). Chitin and Chitosan: Functional biopolymers from marine crustaceans. Marine Biotechnology, 8, 203–226. 10.1007/s10126-005-0097-5 [DOI] [PubMed] [Google Scholar]
- Lan, R. , Wu, F. , Wang, Y. , Lin, Z. , Wang, H. , Zhang, J. , & Zhao, Z. (2024). Chitosan oligosaccharide improves intestinal function by promoting intestinal development, alleviating intestinal inflammatory response, and enhancing antioxidant capacity in broilers aged D 1 to 14. Poultry Science, 103, 103381. 10.1016/j.psj.2023.103381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarto, A. , Merino, N. , Valdes, O. , Dominguez, J. , Spencer, E. , de la Paz, N. , & Aparicio, G. (2015). Safety evaluation of chitosan and chitosan acid salts from Panurilus argus lobster. International Journal of Biological Macromolecules, 72, 1343–1350. 10.1016/j.ijbiomac.2014.10.030 [DOI] [PubMed] [Google Scholar]
- Lee, K. Y. , Shibutani, M. , Takagi, H. , Arimura, T. , Takigami, S. , Uneyama, C. , Kato, N. , & Hirose, M. (2004). Subchronic toxicity study of dietary N‐acetylglucosamine in F344 rats. Food and Chemical Toxicology, 42(4), 687–695. [DOI] [PubMed] [Google Scholar]
- Lewis, K. A. , Tzilivakis, J. , Green, A. , Warner, D. J. , Stedman, A. , & Naseby, D. (2013). Review of substances/agents that have direct beneficial effect on the environment: mode of action and assessment of efficacy. EFSA Supporting Publications, 10(6), EN‐440. 10.2903/sp.efsa.2013.EN-440 [DOI] [Google Scholar]
- Liu, H. , Xu, Z. , Han, Y. , Li, T. , Sun, A. , Chen, S. , Li, X. , Chen, J. , Ren, L. , Zou, H. , & Zhang, Y. (2023). Synergistic effects of bio‐available heavy metals and antibiotics on the fate of antibiotic resistance genes and the elimination of their compound pollution by chitosan during composting. Journal of Soils and Sediments, 23, 2234–2243. 10.1007/s11368-023-03443-9 [DOI] [Google Scholar]
- Logesh, A. R. , Thillaimaharani, K. A. , Sharmila, K. , Kalaiselvam, M. , & Raffi, S. M. (2012). Production of chitosan from Endolichenic fungi isolated from mangrove environment and its antagonistic activity. Asian Pacific Journal of Tropical Biomedicine, 2(2), 140–143. 10.1016/S2221-1691(11)60208-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokman, I. H. , Ibitoye, E. B. , Hezmee, M. N. M. , Goh, Y. M. , Zuki, A. B. Z. , & Jimoh, A. A. (2019). Effects of chitin and chitosan from cricket and shrimp on growth and carcass performance of broiler chickens. Tropical Animal Health and Production, 51, 2219–2225. 10.1007/s11250-019-01936-9 [DOI] [PubMed] [Google Scholar]
- Ma, J. , Faqir, Y. , Tan, C. , & Khaliq, G. (2022). Terrestrial insects as promising source of chitosan and recent developments in its application for various industries. Food Chemistry, 373, 131407. 10.1016/j.foodchem.2021.131407 [DOI] [PubMed] [Google Scholar]
- Makarios‐Laham, I. , & Lee, T. C. (1995). Biodegradability of chitin‐ and chitosan‐containing films in soil environment. Journal of Environmental Polymer Degradation, 3, 31–36. 10.1007/BF02067791 [DOI] [Google Scholar]
- Malerba, M. , & Cerana, R. (2020). Chitin‐ and chitosan‐based derivatives in plant protection against biotic and abiotic stresses and in recovery of contaminated soil and water. Polysaccharides, 1(1), 21–30. 10.3390/polysaccharides1010003 [DOI] [Google Scholar]
- Matica, M. , & Menghiu, G. (2017). Biodegradability of chitosan‐based products. New Frontiers in Chemistry, 26(1), 75–86. http://newfrontchem.iqstorm.ro/upload/08_NFC‐26‐1_Matica%20et%20al.pdf [Google Scholar]
- Meng, Q. W. , Yan, L. , Ao, X. , Jang, H. D. , Cho, J. H. , & Kim, I. H. (2010). Effects of chito‐oligosaccharide supplementation on egg production, nutrient digestibility, egg quality and blood profiles in laying hens. Asian‐Australasian Journal of Animal Sciences, 23(11), 1476–1481. [Google Scholar]
- Minami, S. , Oh‐Oka, M. , Okamoto, Y. , Miyatake, K. , Matsuhashi, A. , Shigemasa, Y. , & Fukumoto, Y. (1996). Chitosan‐inducing hemorrhagic pneumonia in dogs. Carbohydrate Polymers, 29(3), 241–246. [Google Scholar]
- Mohan, K. , Ganesan, A. R. , Muralisankar, T. , Jayakumar, R. , Sathishkumar, P. , Uthayakumar, V. , Chandirasekar, R. , & Revathi, N. (2020). Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends in Food Science & Technology, 105, 17–42. 10.1016/j.tifs.2020.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti Angelo, L. M. , França, D. , & Faez, R. (2021). Biodegradation and viability of chitosan‐based microencapsulated fertilizers. Carbohydrate Polymers, 257, 117635. 10.1016/j.carbpol.2021.117635 [DOI] [PubMed] [Google Scholar]
- Mostafa, H. M. , Sourell, H. , & Bockisch, F. J. (2010). Mechanical properties of some bioplastics under different soil types used as biodegradable drip tubes. Agricultural Engineering International: CIGR Journal, 12(1), 12–21. [Google Scholar]
- Mousavi, B. , Costa, J. M. , Arné, P. , Guillot, J. , Chermette, R. , Botterel, F. , & Dannaoui, E. (2018). Occurrence and species distribution of pathogenic Mucorales in unselected soil samples from France. Medical Mycology, 56(3), 315–321. 10.1093/mmy/myx051 [DOI] [PubMed] [Google Scholar]
- Mouyna, I. , Dellière, S. , Beauvais, A. , Gravelat, F. , Snarr, B. , Lehoux, M. , Zacharias, C. , Sun, Y. , de Jesus Carrion, S. , Pearlman, E. , Sheppard, D. C. , & Latgé, J.‐P. (2020). What are the functions of chitin deacetylases in aspergillus fumigatus? Frontiers in Cellular and Infection Microbiology, 10, 28. 10.3389/fcimb.2020.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujtaba, M. , Khawar, K. M. , Camara, M. C. , Carvalho, L. B. , Fraceto, L. F. , Morsi, R. E. , Elsabee, M. Z. , Kaya, M. , Labidi, J. , Ullah, H. , & Wang, D. (2020). Chitosan‐based delivery systems for plants: A brief overview of recent advances and future directions. International Journal of Biological Macromolecules, 154, 683–697. 10.1016/j.ijbiomac.2020.03.128 [DOI] [PubMed] [Google Scholar]
- Muzzarelli, R. A. A. , Boudrant, J. , Meyer, D. , Manno, N. , DeMarchis, M. , & Paoletti, M. G. (2012). Current views on fungal chitin/chitosan, human Chitinases, food preservation, Glucans, Pectins and inulin: A tribute to Henri Braconnot, precursor of the carbohydrate polymers science, on the chitin bicentennial. Carbohydrate Polymers, 87(2), 995–1012. 10.1016/j.carbpol.2011.09.063 [DOI] [Google Scholar]
- Naito, Y. , Tago, K. , Nagata, T. , Furuya, M. , Seki, T. , Kato, H. , Morimura, T. , & Ohara, N. (2007). A 90‐day ad libitum administration toxicity study of oligoglucosamine in F344 rats. Food and Chemical Toxicology, 45(9), 1575–1587. 10.1016/j.fct.2007.02.018 [DOI] [PubMed] [Google Scholar]
- Nam, K. S. , Choi, Y. R. , & Shon, Y. H. (2001). Evaluation of the antimutagenic potential of chitosan oligosaccharide: Rec, Ames and Umu tests. Biotechnology Letters, 23, 971–975. [Google Scholar]
- National Toxicology Program (NTP) . (2017). Technical Report on the Toxicity Study of Chitosan (CASRN 9012‐76‐4) Administered in Feed to Sprague Dawley [Crl:CD(SD)] Rats. Toxicity Report 93. https://www.ncbi.nlm.nih.gov/books/NBK552709/ [DOI] [PMC free article] [PubMed]
- Ootsuka, E. , Iwasaki, Y. , Takagi, K. , & Saito, A. (2021). LMC60, a material containing low‐molecular weight chitin: Degradation and effects on soil microorganisms in incubated upland soil. Soil Science and Plant Nutrition, 67(4), 389–399. 10.1080/00380768.2021.1947734 [DOI] [Google Scholar]
- Opanasopit, P. , Aumklad, P. , Kowapradit, J. , Ngawhiranpat, T. , Apirakaramwong, A. , Rojanarata, T. , & Puttipipatkhachorn, S. (2007). Effect of salt forms and molecular weight of chitosans on in vitro permeability enhancement in intestinal epithelial cells (Caco‐2). Pharmaceutical Development and Technology, 12(5), 447–455. [DOI] [PubMed] [Google Scholar]
- Orts, W. J. , Sojka, R. E. , & Glenn, G. M. (2000). Biopolymer additives to reduce erosion‐induced soil losses during irrigation. Industrial Crops and Products, 11(1), 19–29. 10.1016/S0926-6690(99)00030-8 [DOI] [Google Scholar]
- Pandit, A. , Indurkar, A. , Deshpande, C. , Jain, R. , & Dandekar, P. (2021). A systematic review of physical techniques for chitosan degradation. Carbohydrate Polymers, 2, 100033. 10.1016/j.carpta.2021.100033 [DOI] [Google Scholar]
- Park, B. M. , Lee, J. , Park, Y. K. , Yang, Y. C. , Jung, B. G. , & Lee, B. J. (2023). Immune‐enhancing effects of chitosan‐fermented feed additive on broiler chickens and subsequent protection conferred against experimental infection with salmonella Gallinarum. The Journal of Poultry Science, 60, 2023016. 10.2141/jpsa.2023016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellis, A. , Guebitz, G. M. , & Nyanhongo, G. S. (2022). Chitosan: Sources, processing and modification techniques. Gels, 8(7), 393. 10.3390/gels8070393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, T. L. , Fernandes, A. R. M. , Oliveira, E. R. , Cônsolo, N. R. B. , Marques, O. F. C. , Maciel, T. P. , Pordeus, N. M. , Barbosa, L. C. G. S. , Buarque, V. L. M. , Padilla, A. R. H. , Colnago, L. A. , & Gandra, J. R. (2020). Serum Metabolomic fingerprints of lambs fed chitosan and its association with performance and meat quality traits. Animals, 14, 1987–1998. 10.1017/S1751731120000749 [DOI] [PubMed] [Google Scholar]
- Pochanavanich, P. , & Suntornsuk, W. (2002). Fungal chitosan production and its characterization. Letters in Applied Microbiology, 35(1), 17–21. 10.1046/j.1472-765X.2002.01118.x [DOI] [PubMed] [Google Scholar]
- Poshina, D. , Raik, S. , Poshin, A. , & Skorik, Y. (2018). Accessibility of chitin and chitosan in enzymatic hydrolysis: A review. Polymer Degradation and Stability, 156, 101–116. 10.1016/j.polymdegradstab.2018.09.005 [DOI] [Google Scholar]
- Qin, C. , Gao, J. , Wang, L. , Zeng, L. , & Liu, Y. (2006). Safety evaluation of short‐term exposure to chitooligomers from enzymic preparation. Food and Chemical Toxicology, 44(6), 855–861. [DOI] [PubMed] [Google Scholar]
- Rao, S. B. , & Sharma, C. P. (1997). Use of chitosan as a biomaterial: Studies on its safety and hemostatic potential. Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials and The Japanese Society for Biomaterials, 34(1), 21–28. [DOI] [PubMed] [Google Scholar]
- Ratajska, M. , Strobin, G. , Wiśniewska‐Wrona, M. , Ciechańska, D. , Struszczyk, H. , Boryniec, S. , Binias, D. , & Biniaś, W. (2003). Studies on the biodegradation of chitosan in an aqueous medium. Fibres & Textiles in Eastern Europe, 11, 75–79. [Google Scholar]
- Richardson, S. W. , Kolbe, H. J. , & Duncan, R. (1999). Potential of low molecular mass chitosan as a DNA delivery system: Biocompatibility, body distribution and ability to complex and protect DNA. International Journal of Pharmaceutics, 178(2), 231–243. [DOI] [PubMed] [Google Scholar]
- Riseh, R. S. , Vazvani, M. G. , & Kennedy, J. F. (2023). The application of chitosan as a carrier for fertilizer: A review. International Journal of Biological Macromolecules, 252, 126483. 10.1016/j.ijbiomac.2023.126483 [DOI] [PubMed] [Google Scholar]
- Rkhaila, A. , Chtouki, T. , Erguig, H. , El Haloui, N. , & Ounine, K. (2021). Chemical proprieties of biopolymers (chitin/chitosan) and their synergic effects with Endophytic bacillus species: Unlimited applications in agriculture. Molecules, 26(4), 1117. 10.3390/molecules26041117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rkhaila, A. , Elhartiti, A. , Bouziani Idrissi, M. , & Ounine, K. (2020). The amendment with chitin and/or chitosan improves the germination and growth of Lycopersicon esculentum L., Capsicum annuum L., and Solanum melongena L. Indian Journal Of Agricultural Research, 54(4), 432–440. 10.18805/IJARe.A-452 [DOI] [Google Scholar]
- Roberts, P. , Bol, R. , & Jones, D. (2007). Free amino sugar reactions in soil in relation to soil carbon and nitrogen cycling. Soil Biology and Biochemistry, 39, 3081–3092. 10.1016/j.soilbio.2007.07.001 [DOI] [Google Scholar]
- Roman, D. L. , Roman, M. , Som, C. , Schmutz, M. , Hernandez, E. , Wick, P. , Casalini, T. , Perale, G. , Ostafe, V. , & Isvoran, A. (2019). Computational assessment of the pharmacological profiles of degradation products of chitosan. Frontiers in Bioengineering and Biotechnology, 7, 214. 10.3389/fbioe.2019.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanazzi, G. , Gabler, F. M. , Margosan, D. , Mackey, E. , & Smilanich, J. L. (2009). Effect of chitosan dissolved in different acids on its ability to control postharvest gray Mold of table grape. Phytopathology, 99(9), 1028–1036. 10.1094/PHYTO-99-9-1028 [DOI] [PubMed] [Google Scholar]
- Sáenz‐Mendoza, A. , Zamudio‐Flores, P. , Tirado‐Gallegos, J. , García‐Anaya, M. , Rios, C. , Acosta, C. , Díaz, M. , González, M. , Vela‐Gutiérrez, G. , Delgado, R. , Villalobos, R. , & Ortega, A. (2023). Insects as a potential source of chitin and chitosan: Physicochemical, morphological, and structural characterization—A review. Emirates Journal of Food and Agriculture, 35(5), 388–407. 10.9755/ejfa.2023.v35.i5.3095 [DOI] [Google Scholar]
- Sae‐Tun, O. , Bodner, G. , Rosinger, C. , Zechmeister‐Boltenstern, S. , Mentler, A. , & Keiblinger, K. (2022). Fungal biomass and microbial necromass facilitate soil carbon sequestration and aggregate stability under different soil tillage intensities. Applied Soil Ecology, 179, 104599. 10.1016/j.apsoil.2022.104599 [DOI] [Google Scholar]
- Santos, M. V. D. , Goes, R. H. T. B. , Takiya, C. S. , Cabral, L.d. S. , Mombach, M. A. , Oliveira, R. T. , Silva, N. G. d. , Anschau, D. G. , Freitas Júnior, J. E. d. , de Araújo, M. L. G. M. L. , & Gandra, J. R. (2021). Effect of increasing doses of chitosan to grazing beef steers on the relative population and transcript abundance of archaea and cellulolytic and amylolytic bacterias. Animal Biotechnology, 34(2), 246–252. 10.1080/10495398.2021.1954936 [DOI] [PubMed] [Google Scholar]
- Sajid, M. , Basit, A. , Ullah, Z. , et al. (2020). Chitosan‐based foliar application modulated the yield and biochemical attributes of peach (Prunus persica L.) cv. Early Grand. Bulletin of the National Research Centre, 44, 150. 10.1186/s42269-020-00405-w [DOI] [Google Scholar]
- Sato, K. , Azama, Y. , Nogawa, M. , Taguchi, G. , & Shimosaka, M. (2010). Analysis of a change in bacterial Community in Different Environments with addition of chitin or chitosan. Journal of Bioscience and Bioengineering, 109(5), 472–478. 10.1016/j.jbiosc.2009.10.021 [DOI] [PubMed] [Google Scholar]
- Sawaguchi, A. , Ono, S. , Oomura, M. , Inami, K. , Kumeta, Y. , Honda, K. , & Saito, A. (2015). Chitosan degradation and associated changes in bacterial community structures in two contrasting soils. Soil Science and Plant Nutrition, 61(3), 471–480. 10.1080/00380768.2014.1003965 [DOI] [Google Scholar]
- Schipper, N. G. , Olsson, S. , Hoogstraate, J. A. , deBoer, A. G. , Vårum, K. M. , & Artursson, P. (1997). Chitosans as absorption enhancers for poorly absorbable drugs 2: Mechanism of absorption enhancement. Pharmaceutical Research, 14(7), 923–929. 10.1023/a:1012160102740 [DOI] [PubMed] [Google Scholar]
- Schipper, N. G. , Vårum, K. M. , & Artursson, P. (1996). Chitosans as absorption enhancers for poorly absorbable drugs. 1: Influence of molecular weight and degree of acetylation on drug transport across human intestinal epithelial (Caco‐2) cells. Pharmaceutical Research, 13, 1686–1692. [DOI] [PubMed] [Google Scholar]
- Shah, A. M. , Qazi, I. H. , Matra, M. , & Wanapat, M. (2022). Role of chitin and chitosan in ruminant diets and their impact on digestibility, microbiota and performance of ruminants. Fermentation, 8(10), 549. 10.3390/fermentation8100549 [DOI] [Google Scholar]
- Shahidi, F. , & Abuzaytoun, R. (2005). Chitin, chitosan, and Co‐products: Chemistry, production, applications, and health effects. Advances in Food and Nutrition Research, 49, 93–135. 10.1016/S1043-4526(05)49003-8 [DOI] [PubMed] [Google Scholar]
- Shahrajabian, M. H. , Chaski, C. , Polyzos, N. , Tzortzakis, N. , & Petropoulos, S. A. (2021). Sustainable agriculture systems in vegetable production using chitin and chitosan as plant biostimulants. Biomolecules, 11(6), 819. 10.3390/biom11060819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariatinia, Z. (2019). Pharmaceutical applications of chitosan. Advances in Colloid and Interface Science, 263, 131–194. [DOI] [PubMed] [Google Scholar]
- Sharif, R. , Mujtaba, M. , Ur Rahman, M. , Shalmani, A. , Ahmad, H. , Anwar, T. , Tianchan, D. , & Wang, X. (2018). The multifunctional role of chitosan in horticultural crops: A review. Molecules, 23(4), 872. 10.3390/molecules23040872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp, R. G. (2013). A review of the applications of chitin and its derivatives in agriculture to modify plant‐microbial interactions and improve crop yields. Agronomy, 3(4), 757–793. 10.3390/agronomy3040757 [DOI] [Google Scholar]
- Soares, B. , Barbosa, C. , & Oliveira, M. J. (2023). Chitosan application towards the improvement of grapevine performance and wine quality. Ciência e Técnica Vitivinícola, 38, 43–59. 10.1051/ctv/ctv20233801043 [DOI] [Google Scholar]
- Somashekar, D. , & Joseph, R. (1996). Chitosanases—Properties and applications: A review. Bioresource Technology, 55(1), 35–45. 10.1016/0960-8524(95)00144-1 [DOI] [Google Scholar]
- Stahl, P. H. (2011). Handbook of pharmaceutical salts: Properties, selection, and use. Wiley‐VCH. [Google Scholar]
- Swiatkiewicz, S. , Swiatkiewicz, M. , Arczewska‐Wlosek, A. , & Jozefiak, D. (2015). Chitosan and its oligosaccharide derivatives (chito‐oligosaccharides) as feed supplements in poultry and swine nutrition. Journal of Animal Physiology and Animal Nutrition, 99(1), 1–12. [DOI] [PubMed] [Google Scholar]
- Szymańska, E. , & Winnicka, K. (2015). Stability of chitosan—A challenge for pharmaceutical and biomedical applications. Marine Drugs, 13(4), 1819–1846. 10.3390/md13041819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, S. C. , Tan, T. K. , Wong, S. M. , & Khor, E. (1996). The chitosan yield of Zygomycetes at their optimum harvesting time. Carbohydrate Polymers, 30(4), 239–242. 10.1016/S0144-8617(96)00052-5 [DOI] [Google Scholar]
- Tago, K. , Naito, Y. , Nagata, T. , Morimura, T. , Furuya, M. , Seki, T. , Kato, H. , & Ohara, N. (2007). A ninety‐day feeding, subchronic toxicity study of oligo‐N‐acetylglucosamine in Fischer 344 rats. Food and Chemical Toxicology, 45(7), 1186–1193. 10.1016/j.fct.2006.12.027 [DOI] [PubMed] [Google Scholar]
- Tao, W. , Jin, F. , Fan, Q. , Zhao, N. , Wang, G. , Du, E. , Chen, F. , Guo, W. , Huang, S. , Chen, M. , & Wei, J. (2022). Effects of chitosan oligosaccharide on production performance, egg quality and ovarian function in laying hens with fatty liver syndrome. Animals, 12, 2465. 10.3390/ani12182465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, M. , Inoue, K. , Yoshida, M. , Morikawa, T. , Shibutani, M. , & Nishikawa, A. (2009). Lack of chronic toxicity or carcinogenicity of dietary N‐acetylglucosamine in F344 rats. Food and Chemical Toxicology, 47(2), 462–471. 10.1016/j.fct.2008.12.002 [DOI] [PubMed] [Google Scholar]
- Thadathil, N. , & Velappan, S. P. (2014). Recent developments in Chitosanase research and its biotechnological applications: A review. Food Chemistry, 150, 392–399. 10.1016/j.foodchem.2013.10.083 [DOI] [PubMed] [Google Scholar]
- Thakur, D. , Bairwa, A. , Dipta, B. , & Chauhan, A. (2023). An overview of fungal Chitinases and their potential applications. Protoplasma, 260, 1031–1046. 10.1007/s00709-023-01839-5 [DOI] [PubMed] [Google Scholar]
- Thakur, D. , Chauhan, A. , Jhilta, P. , & Dipta, B. (2023). Microbial Chitinases and their relevance in various industries. Folia Microbiologica, 68, 29–53. 10.1007/s12223-022-00999-w [DOI] [PubMed] [Google Scholar]
- Thao, N. T. , Phesatcha, K. , Matra, M. , Phesatcha, B. , & Wanapat, M. (2022). Sources of rumen enhancers including nitrate, chitosan extract and shrimp Shell meal could modulate nutrient degradability and in vitro gas fermentation. Journal of Applied Animal Research, 50, 394–399. 10.1080/09712119.2022.2088540 [DOI] [Google Scholar]
- Tharanathan, R. N. , & Kittur, F. S. (2003). Chitin—The undisputed biomolecule of great potential. Critical Reviews in Food Science and Nutrition, 43(1), 61–87. 10.1080/10408690390826455 [DOI] [PubMed] [Google Scholar]
- Timmer, N. (2019). Determination of ‘Ready’ Biodegradability: Carbon Dioxide (CO2) Evaluation Test (modified Sturm Test) of OptiCHOS. https://open.efsa.europa.eu/questions/EFSA‐Q‐2020‐00668
- Tong, J. J. , Zhang, H. , Wang, J. , Liu, Y. , Mao, S. Y. , Xiong, B. H. , & Jiang, L. S. (2020). Effects of different molecular weights of chitosan on methane production and bacterial community structure in vitro. Journal of Integrative Agriculture, 19, 1644–1655. 10.1016/S2095-3119(20)63174-4 [DOI] [Google Scholar]
- Tufan, T. , & Arslan, C. (2020). Dietary supplementation with chitosan oligosaccharide affects serum lipids and nutrient digestibility in broilers. South African Journal of Animal Science, 50(5), 663–671. 10.4314/sajas.v50i5.3 [DOI] [Google Scholar]
- US EPA . (2007). Chitin and Chitosan Summary Document, Registration Review: Initial Docket September 2007. Docket Number: EPA‐HQ‐EPA‐2006‐056.
- US EPA . (2019). Science review in support of the addition of Chitosan (Poly‐D‐Glucosamine) to the list of minimum risk pesticides (MRPs) contained in 40 CFR 152.25(f).
- Veliz, E. A. , Martínez‐Hidalgo, P. , & Hirsch, A. M. (2017). Chitinase‐producing bacteria and their role in biocontrol. AIMS Microbiology, 3(3), 689–705. 10.3934/microbiol.2017.3.689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikhoreva, G. A. , Kil'deeva, N. R. , Ustinov, M. Y. , et al. (2002). Fabrication and study of the degradability of chitosan films. Fibre Chemistry, 34, 407–411. 10.1023/A:1022904023526 [DOI] [Google Scholar]
- Waibel, K. H. , Haney, B. , Moore, M. , Whisman, B. , & Gomez, R. (2011). Safety of chitosan bandages in shellfish allergic patients. Military Medicine, 176(10), 1153–1156. 10.7205/milmed-d-11-00150 [DOI] [PubMed] [Google Scholar]
- Wang, Z. , Zhang, H. , & Pan, G. (2016). Ecotoxicological assessment of Flocculant modified soil for Lake restoration using an integrated biotic toxicity index. Water Research, 97, 133–141. 10.1016/j.watres.2015.08.033 [DOI] [PubMed] [Google Scholar]
- Wieczorek, A. S. , Hetz, S. A. , & Kolb, S. (2014). Microbial responses to chitin and chitosan in Oxic and anoxic agricultural soil slurries. Biogeosciences, 11, 3339–3352. 10.5194/bg-11-3339-2014 [DOI] [Google Scholar]
- Wiegand, C. , Winter, D. , & Hipler, U. C. (2010). Molecular‐weight‐dependent toxic effects of chitosans on the human keratinocyte cell line HaCaT. Skin Pharmacology and Physiology, 23(3), 164–170. 10.1159/000276996 [DOI] [PubMed] [Google Scholar]
- Xu, Q. , Qu, C. , Wan, J. , Cheng, G. , Yang, W. , Gong, C. , He, J. , & Du, Y. (2018). Effect of dietary chitosan oligosaccharide supplementation on the pig ovary transcriptome. RSC Advances, 8, 13266–13273. 10.1039/c7ra10172d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y. , Mao, H. , Yang, C. , Du, H. , Wang, H. , & Tu, J. (2020). Effects of chitosan nanoparticle supplementation on growth performance, humoral immunity, gut microbiota and immune responses after lipopolysaccharide challenge in weaned pigs. Journal of Animal Physiology and Animal Nutrition, 104, 597–605. 10.1111/jpn.13283 [DOI] [PubMed] [Google Scholar]
- Yamada, K. , Odomi, M. , Okada, N. , Fujita, T. , & Yamamoto, A. (2005). Chitosan oligomers as potential and safe absorption enhancers for improving the pulmonary absorption of interferon‐α in rats. Journal of Pharmaceutical Sciences, 94(11), 2432–2440. [DOI] [PubMed] [Google Scholar]
- Yan, L. , Lee, J. H. , Meng, Q. W. , Ao, X. , & Kim, I. H. (2010). Evaluation of dietary supplementation of delta‐aminolevulinic acid and chito‐oligosaccharide on production performance, egg quality and hematological characteristics in laying hens. Asian‐Australasian Journal of Animal Sciences, 23(8), 1028–1033. [Google Scholar]
- Yoon, H. J. , Park, H. S. , Bom, H. S. , Roh, Y. B. , Kim, J. S. , & Kim, Y. H. (2005). Chitosan oligosaccharide inhibits 203 HgCl 2‐induced genotoxicity in mice: Micronuclei occurrence and chromosomal aberration. Archives of Pharmacal Research, 28, 1079–1085. [DOI] [PubMed] [Google Scholar]
- Younes, I. , & Rinaudo, M. (2015). Chitin and chitosan preparation from marine sources. Structure, properties and applications. Marine Drugs, 13(3), 1133–1174. 10.3390/md13031133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, H. , Zhou, Q. , He, D. , Yang, J. , Wu, K. , Chai, X. , Xiang, Y. , Duan, X. , & Wu, X. (2023). Enhanced mechanical and functional properties of chitosan/polyvinyl alcohol/hydroxypropyl methylcellulose/alizarin composite film by incorporating cinnamon essential oil and tea polyphenols. International Journal of Biological Macromolecules, 253(Pt 4), 126859. 10.1016/j.ijbiomac.2023.126859 [DOI] [PubMed] [Google Scholar]
- Žabka, M. , & Pavela, R. (2021). The dominance of chitosan hydrochloride over modern natural agents or basic substances in efficacy against Phytophthora infestans, and its safety for the non‐target model species Eisenia fetida. Horticulturae, 7(10), 366. 10.3390/horticulturae7100366 [DOI] [Google Scholar]
- Zainol Abidin, N. A. , Kormin, F. , Zainol Abidin, N. A. , Mohamed Anuar, N. A. F. , & Abu Bakar, M. F. (2020). The potential of insects as alternative sources of chitin: An overview on the chemical method of extraction from various sources. International Journal of Molecular Sciences, 21(14), 4978. 10.3390/ijms21144978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, L. , Qin, C. , Wang, W. , Chi, W. , & Li, W. (2008). Absorption and distribution of chitosan in mice after oral administration. Carbohydrate Polymers, 71(3), 435–440. [Google Scholar]
- Zhang, H. L. , Zhong, X. B. , Tao, Y. , Wu, S. H. , & Su, Z. Q. (2012). Effects of chitosan and water‐soluble chitosan micro‐ and nanoparticles in obese rats fed a high‐fat diet. International Journal of Nanomedicine, 7, 4069–4076. 10.2147/IJN.S33830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M. , Zhang, F. , Li, C. , An, H. , Wan, T. , & Zhang, P. (2020). Application of chitosan and its derivative polymers in clinical medicine and agriculture. Polymers, 14(5), 958. 10.3390/polym14050958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , & Amelung, W. (1996). Gas chromatographic determination of muramic acid, glucosamine, mannosamine, and galactosamine in soils. Soil Biology and Biochemistry, 28(9), 1201–1206. 10.1016/0038-0717(96)00117-4 [DOI] [Google Scholar]
- Zheng, Y. G. , Zhang, B. Q. , Qi, J. Y. , Guo, X. Y. , Shi, B. L. , & Yan, S. M. (2021). Dietary supplementation of chitosan affects milk performance, markers of inflammatory response and antioxidant status in dairy cows. Animal Feed Science and Technology, 277(2021), 114952. 10.1016/j.anifeedsci.2021.114952 [DOI] [Google Scholar]
- Ziaee, A. , Zia, M. , Bayat, M. , & Hashemi, J. (2016). Identification of Mucorales isolates from soil using morphological and molecular methods. Current Medical Mycology, 2(1), 13–19. 10.18869/acadpub.cmm.2.1.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubareva, A. , Shagdarova, B. , Varlamov, V. , Kashirina, E. , & Svirshchevskaya, E. (2017). Penetration and toxicity of chitosan and its derivatives. European Polymer Journal, 93, 743–749. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Literature searches
Distiller_Search#1_Chitosan_Hydrochloride
Distiller_Search#2_Chitosan_Review
Literature search for chitosan and chitosan hydrochloride under ToR 2
Data collection for toxicological endpoints
Data collection for environmental endpoints
Fate additional information and PEC calculations




