Abstract
Rationale:
Self-improving collodion baby (SICB) is a rare subtype of autosomal recessive congenital ichthyosis with distinct clinical features and generally favorable prognosis. This study aims to enhance understanding of SICB by examining its clinical characteristics and recent developments in diagnosis and management. Our findings provide insights that may aid in the etiological diagnosis and treatment of congenital ichthyosis.
Patient concerns:
We present a case of SICB treated at Peking University Shenzhen Hospital, characterized by the appearance of a collodion membrane at birth. The primary approach involved intensive moisturizing care to manage skin abnormalities.
Diagnoses:
Based on clinical examination and genetic testing, a diagnosis of SICB was confirmed, with mutations identified in genes commonly associated with autosomal recessive congenital ichthyosis, such as ALOX12B, TGM1, ALOXE3, CYP4F22, and PNPLA1.
Outcomes:
The patient showed significant improvement following targeted supportive care, consistent with the generally positive prognosis for SICB.
Lessons:
A comprehensive literature review of 31 SICB cases from 18 studies highlighted that the condition typically presents at birth with a collodion membrane. Intensive moisturizing is the main treatment, and early genetic testing is recommended to facilitate timely diagnosis and intervention. Early diagnosis can support effective genetic counseling and improve outcomes for newborns with ichthyosis.
Keywords: autosomal recessive congenital ichthyosis, collodion baby, newborn, self-improving collodion ichthyosis, whole exome sequencing
1. Introduction
Self-improving collodion ichthyosis (SICI) is a rare subtype of autosomal recessive congenital ichthyosis (ARCI), characterized by the presence of typical collodion membrane features at birth, with almost complete resolution of scaling within 3 months to 1 year.[1,2] At birth, infants with SICI are encased in a thick, tight, smooth, and shiny shell, formed by an overgrowth of the stratum corneum that resembles a collodion-like membrane.[3]
This hardened shell, resulting from significant thickening and tension in the skin, can cause various complications, including ectropion, lip deformities, and underdevelopment of nasal and ear cartilage.[4] More severely, the tight membrane may interfere with critical neonatal functions such as sucking, breathing, and ventilation, leading to dehydration, malnutrition, hypoxia, and potentially life-threatening pulmonary infections.[1]
Although SICI’s clinical manifestations have been reported, its incidence and pathogenesis remain incompletely understood, likely involving multiple gene mutations.[5] This study reports a typical case of neonatal SICI and systematically analyzes existing literature to enhance clinicians’ understanding of the condition. Improved recognition and knowledge of SICI can aid in the diagnosis, treatment, and management of similar cases in the future, offering valuable insights for medical professionals.
This manuscript is presented following the CARE reporting checklist.
2. Case presentation
2.1. Data of the present case
2.1.1. Clinical materials
A female infant was admitted 30 minutes after birth due to “generalized edema with skin fissures.” The infant was born to a primiparous mother at 38+3 weeks of gestation age via spontaneous vaginal delivery, with a birth weight of 3050 g and no history of asphyxia. After birth, she was breastfed, had strong sucking ability, was mentally alert, showed no lethargy, and was not irritable or agitated. Jaundice appeared on the second day of life, and meconium was passed within 24 hours, with normal quantity. There was no history of consanguineous marriage, and the mother had regular antenatal checkups with no significant abnormalities. There was no similar family history.
All procedures in this study complied with the ethical standards of the Peking University Shenzhen Hospital Ethics Committee (Reference No. [2024] 119) and the revised Declaration of Helsinki (2013). The patient’s guardian provided written informed consent.
2.1.2. Physical examination at admission
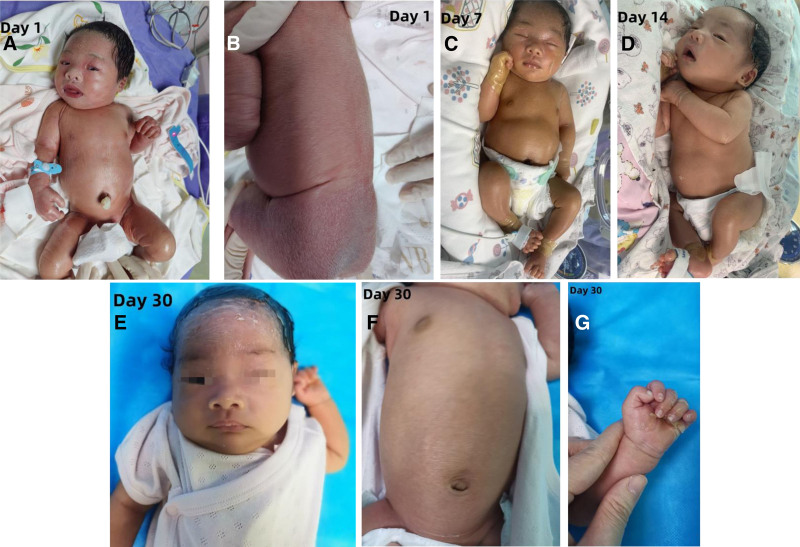
Upon admission, the infant’s vital signs were as follows: temperature 36.7 °C, pulse rate 126 beats per minute, respiratory rate 55 breaths per minute, weight 3.05 kg, length 50 cm, head circumference 33.5 cm, and chest circumference 32 cm. The infant was alert with a strong cry, and the anterior fontanel was flat and soft. The skin was erythematous and shiny, covered by a tense, transparent, plastic-like membrane. Notable desquamation was observed on the limbs and buttocks, with erosions present in the groin area. The back had dry scales, both eyelids were everted, the ears were stiff, and there was swelling and fissuring of the palms. The soles were pale and edematous, and the lips were everted. Respiratory sounds were symmetrical in both lungs, without rales, and heart sounds were strong, with a regular rhythm and no murmurs. The abdomen was soft, with normal limb muscle tone and a capillary refill time of <2 seconds (see Fig. 1A and B).
Figure 1.
Skin evolution from birth to 30 days in this case.
2.1.3. Laboratory examinations
After admission, test results indicated normal levels of myocardial enzymes, liver and kidney function, electrolytes, complete blood count, C-reactive protein, and procalcitonin. Torch IgM antibodies were negative, and both blood glucose and blood gas levels were within normal ranges. Ultrasound examinations of the brain, liver, gallbladder, and spleen showed no abnormalities. Cardiac ultrasound revealed a 2.4 mm oblique fissure in the middle of the atrial septum, and a 2 to 3 mm discontinuity near the apex of the interventricular septum, with no pericardial effusion observed. X-rays of the hands and feet showed mild soft tissue swelling in both hands, with no abnormalities in the bones of the feet. The patient’s 25-hydroxyvitamin D level was 16.15 ng/mL, G6PD activity was normal, and blood ammonia was 46.6 μmol/L.
2.1.4. Diagnosis and treatment
2.1.4.1. Diagnosis: SICI
Treatment: the infant was placed in a humidified incubator with continuous monitoring of vital signs to prevent fluid and electrolyte imbalances. Intensive skin moisturizing care was provided by applying a mixture of petroleum jelly and medical moisturizing cream every hour to the entire body to prevent scaling and secondary infections. Erythromycin ointment was applied to the areas of erosion, and erythromycin eye ointment was applied every night to prevent eye infections. Both the infant and the parents underwent multi-gene sequencing for hereditary skin disorders. Oral administration of vitamin A (2000 IU) and vitamin D (700 IU) was initiated, and skin care continued throughout the treatment. One week after birth, the infant began to shed skin (Fig. 1C), with peeling mostly completed on the face and trunk by the second week (Fig. 1D). After 15 days of treatment, the infant’s condition stabilized, and by discharge, the skin had returned to normal, except for the head and extremities (Fig. 1E–G).
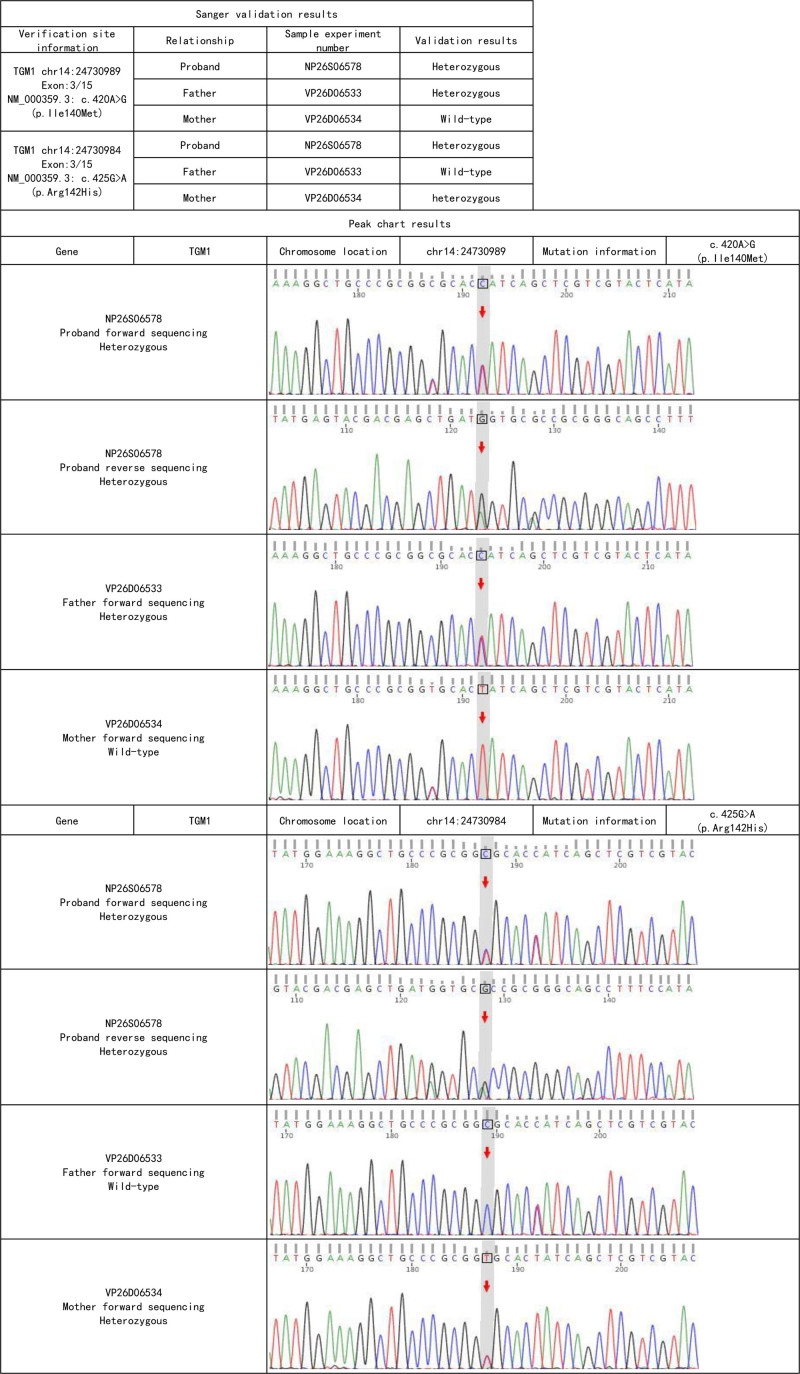
2.1.5. Results of gene sequencing
The proband and her parents underwent whole exome sequencing of anticoagulated peripheral venous blood, followed by Sanger sequencing to validate the suspected pathogenic variants. According to the ACMG classification guidelines for genetic mutations, the patient was identified as having compound heterozygous pathogenic variants in the TGM1 gene (see Table 1). The patient carries the c.425G > A (p. Arg142His) variant inherited from her mother, which has been reported to reduce TGase activity (PMID:19278426) and is classified as a likely pathogenic variant. The patient also carries the c.420A > G (p. Ile140Met) variant inherited from her father, which has been previously observed in compound heterozygous ichthyosis patients and is also classified as a likely pathogenic variant (see Fig. 2).
Table 1.
Pathogenic or likely pathogenic single nucleotide variants (SNVs) and small insertion/deletion variants (Indels) in the WES results that can explain the phenotype of the examined individual.
| Gene | Chromosomal location | Variant information | Zygosity type | Disease name | Inheritance pattern | Variant origin | Variant classification |
|---|---|---|---|---|---|---|---|
| TGM1 | chr14:24730984 | NM 000359.3:c.425G > A(p.Arg142His) | Heterozygous | ARCI type 1 [MIM:242300] | AR | Mother | Suspected pathogenic variant |
| TGM1 | chr14:24730989 | NM 000359.3:c.420A > G(p.lle140Met) | Heterozygous | ARCI type 1 [MIM:242300] | AR | Father | Suspected pathogenic variant |
A = adenine, AR = autosomal recessive, ARCI = autosomal recessive congenital ichthyosis type 1, Arg = arginine, G = guanine, His = histidine, Ile = isoleucine, Met = methionine, NM = refers to the RefSeq (NCBI Reference Sequence) accession number for a specific mRNA transcript.
Figure 2.
Sanger sequencing chart.
2.1.6. Literature review
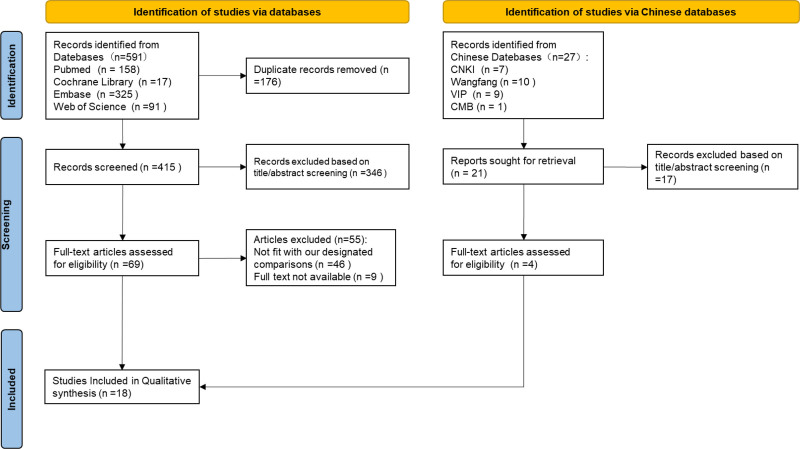
A literature search was conducted using the keywords “collodion baby,” “self-improving collodion ichthyosis,” “autosomal recessive congenital ichthyosis,” and “neonates” in databases including CNKI, Wanfang Database, VIP Database, Chinese Medical Journal Full-Text Database, PubMed, Embase, Web of Science, and Cochrane Library, with data up to July 27, 2024. The search focused on studies involving neonates diagnosed with self-improving collodion ichthyosis and with genetic testing results, excluding cases with additional diseases. All identified records were downloaded to Endnote X9, and duplicates were removed both automatically and manually. Two independent researchers screened the studies by title and abstract, selected eligible cases, and obtained full texts for further review. From the 618 publications identified, 436 articles remained after removing duplicates. Of these, 363 were excluded based on title and abstract evaluation. Among the remaining 73 studies, 55 did not meet the inclusion criteria, and 9 were excluded due to the unavailability of full texts. Ultimately, 18 studies[6–23] were included, as shown in Figure 3 and Table S1, Supplemental Digital Content, http://links.lww.com/MD/O608. Of these, 14 were in English and 4 in Chinese. After removing duplicate cases, a total of 31 patients were analyzed.
Figure 3.
Flow chart for the selection of studies.
The clinical manifestations of self-improving collodion ichthyosis in neonates were summarized. Among the 31 patients, 16 were female (51.6%), and 22 were full-term infants (71.0%). At birth, all infants were covered with a thick, varying-layer collodion membrane. Symptomatic supportive treatments, including localized or systemic skin moisturizing and infection prevention, were applied. The membrane naturally peeled off within 2 to 4 weeks, revealing near-normal skin, and most patients had nearly normal skin by 2 to 4 months, with only a few showing mild ichthyosis characteristics later. Genetic analysis revealed common mutations including ALOX12B (51.61%, 16/31), TGM1 (22.58%, 7/31), ALOXE3 (12.90%, 4/31), CYP4F22 (9.68%, 3/31), and PNPLA1 (3.23%, 1/31). The clinical prognosis was generally good, consistent with the commonly reported symptoms of self-improving collodion ichthyosis in the literature (see Table 2).
Table 2.
Characteristics of studies included in the literature review.
| Author (year) | Number of cases (n) | Sex | Full-term/premature | Postnatal clinical manifestations | Treatment | Mutated gene | Prognosis |
|---|---|---|---|---|---|---|---|
| Hao (2024) [6] | 1 | F | Full-term | Collodion baby, eyelid eversion, diffuse skin scaling with erythema | ABC | TGM1 | b |
| Chen (2024) [7] | 1 | M | Premature | Diffuse skin scaling with erythema | ABC | ALOXE3 | b |
| Yu (2023) [8] | 1 | M | Full-term | Collodion baby, eyelid eversion | ABC | ALOX12B | a |
| Amoedo (2023) [9] | 1 | M | Full-term | Collodion baby, eyelid edema and eversion | ABC | ALOX12B | b |
| Zhu (2023) [10] | 1 | F | Full-term | Collodion baby | ABC | ALOX12B | a |
| Li (2022) [11] | 1 | F | Premature | Collodion baby, eyelid eversion, syndactyly | ABC | PNPLA1 | a |
| Zhang (2022) [12] | 1 | M | Full-term | Collodion baby, erythroderma with multiple fissures on the skin, eyelid edema and eversion, deformities of the external ear and auricle | ABC | ALOX12B | a |
| Takeichi (2022) [13] | 1 | M | Full-term | Collodion baby, moderate fissures at the joints | ABC | CYP4F22 | b |
| Yang (2021) [14] | 3 | 2 M, 1 F | 2 Full-term, 1 premature | Collodion baby, eyelid eversion, auricular forward folding, limb swelling with restricted movement | ABC | ALOX12B | b |
| Noguera-Morel (2016) [15] | 2 | 2 M, 1 F | 1 Full-term, 1 premature | Collodion baby | ABC | CYP4F22 | b |
| Santesteban (2016) [16] | 1 | M | Premature | Collodion baby, eyelid eversion, nail deformities | ABC | ALOX12B | b |
| Tanahashi (2014) [17] | 1 | F | Full-term | Collodion baby | ABC | TGM1 | a |
| Bourrat (2012) [18] | 1 | M | Full-term | Collodion baby | ABC | TGM1 | a |
| Theiler (2010) [19] | 1 | F | Full-term | Collodion baby | ABC | TGM1 | a |
| Vahlquist (2010) [20] | 9 | 2 M, 7 F | Premature | Collodion baby | ABC | 6ALOX12B, 3 ALOXE3 |
b |
| Mazereeuw-Hautier (2009) [21] | 1 | F | 7 Full-term, 2 premature | Collodion baby | ABC | TGM1 | a |
| Harting (2008) [22] | 2 | M | Premature | Collodion baby | ABC | ALOX12B | a |
| Raghunath (2003) [23] | 2 | F | Full-term | Collodion baby | ABC | TGM1 | a |
A: symptomatic support; B: skin moisturizing; C: infection prevention; a: skin completely normal; b: localized skin has a few thin scales.
3. Discussion
This study systematically reviews and summarizes the genetic mutations associated with SICI, revealing that mutations in the ALOX12B gene are a major cause of this transient ARCI, accounting for 51.61% of the 31 genotyped cases. These findings are consistent with previous research. The ALOX12B gene encodes 12R-lipoxygenase, an enzyme essential for skin barrier formation and keratinocyte differentiation. Mutations in the ALOX12B gene impair lipid metabolism, leading to skin barrier dysfunction and clinical manifestations characteristic of collodion baby in neonates.[24]
Additionally, mutations in the TGM1 gene are identified as the second most common cause of SICI. The TGM1 gene encodes an enzyme crucial for the terminal differentiation of skin keratinocytes, catalyzing the cross-linking reactions of keratins to form a robust extracellular matrix that maintains skin barrier integrity.[25] Whole exome sequencing confirmed TGM1 mutations in the patient, identifying 2 suspected pathogenic variants: c.425G > A (p. Arg142His) and c.420A > G (p. Ile140Met), inherited from the mother and father, respectively, resulting in compound heterozygous mutations. Although these mutations reduce TGM1 enzyme activity, they do not completely eliminate its function. The p. Arg142His and p. Ile140Met mutations may impact different domains or functional regions of the enzyme. Although these mutations impair the skin barrier at birth, environmental factors and compensatory differentiation pathways in keratinocytes contribute to gradual skin improvement. At birth, the patient’s skin was covered with a tense, glossy, transparent membrane, consistent with the clinical presentation of SICI. Typically, this collodion-like membrane peels off within weeks, and the skin gradually returns to normal or exhibits only minor damage. Consequently, SICI is also referred to as self-healing collodion baby. In this case, the clinical presentation was consistent with the genetic findings, confirming the diagnosis of SICI. It is noteworthy that not all SICI patients exhibit identical locations of skin damage, which may be associated with different pathogenic gene mutations.
We also identified ALOXE3 gene mutations as the third most common cause of SICI. The ALOXE3 gene encodes epidermal lipoxygenase 3, which is critical for skin barrier formation by oxidizing lipids in the stratum corneum and facilitating epidermal barrier maturation. Compound heterozygous mutations in ALOXE3 can affect the activity of epidermal lipoxygenase 3, resulting in initial defects in stratum corneum structure. However, with the turnover of stratum corneum cells and the dynamic reconstruction of the skin barrier, the skin gradually adapts to these mutations, leading to improved function.[26]
In this study, CYP4F22 mutations were found to be a less common cause of SICI, accounting for 9.68% of the 31 genotyped cases. CYP4F22 encodes a cytochrome P450 enzyme that is essential for the metabolism of fatty acids in the stratum corneum, contributing to the production of very long-chain fatty acids and keratinization. Compound heterozygous or homozygous mutations in CYP4F22 can reduce enzyme activity, impairing lipid metabolism and resulting in a collodion-like appearance.[27]
Among the SICI cases in our study, only 1 patient had compound heterozygous PNPLA1 mutations. The PNPLA1 gene encodes a protein from the patatin-like phospholipase family, responsible for producing ceramide fatty acids, which are crucial for maintaining skin barrier integrity. PNPLA1 mutations may alter the composition of stratum corneum lipids at birth, resulting in a collodion-like appearance.[28] However, with the turnover of stratum corneum cells and the reconstruction of lipid composition, skin function gradually recovers. Spontaneous adjustments in lipid metabolism partially compensate for the defects caused by the mutation, promoting skin self-healing.
The strength of this study lies in its comprehensive literature search, which includes major English and Chinese databases, thereby minimizing the risk of omitting important published reports. To our knowledge, this is the largest study on the etiology of SICI conducted to date. The phenotypes of SICI patients vary according to different mutation types, and the precise genotype–phenotype correlation remains to be fully elucidated.
In conclusion, SICI exemplifies the genetic diversity and clinical overlap among various ARCI subtypes. Only through meticulous analysis of clinical and genetic findings can the SICI subtype of ARCI be clearly delineated. For clinicians, understanding ichthyosis cases that transition from severe to mild, along with their recessive genetic characteristics, is crucial for delivering precise genetic counseling.
Acknowledgments
Thanks to all authors for their contributions to this study.
Author contributions
Conceptualization: Yanping Guo.
Data curation: Yanping Guo, Zhihao Xiao, Xiaoyan Hu, Ying Liu, Guobing Chen.
Supervision: Guobing Chen.
Writing – original draft: Yanping Guo.
Writing – review & editing: Yanping Guo.
Supplementary Material
Abbreviations:
- ARCI
- autosomal recessive congenital ichthyosis
- SICB
- self-improving collodion baby
Informed consent was obtained from the patient for publication of this case report details.
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the Peking University Shenzhen Hospital Ethics Committee (Reference No. [2024] 119) and the revised Declaration of Helsinki (2013).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Supplemental Digital Content is available for this article.
How to cite this article: Guo Y, Xiao Z, Hu X, Liu Y, Chen G. A case report and literature review of self-improving collodion baby in the newborn. Medicine 2025;104:14(e42045).
Contributor Information
Zhihao Xiao, Email: a2822063@qq.com.
Xiaoyan Hu, Email: xyhu77@126.com.
Ying Liu, Email: 13902992158@139.com.
Guobing Chen, Email: guobingchen2002@163.com.
References
- [1].Hake L, Süssmuth K, Komlosi K, et al. Quality of life and clinical characteristics of self-improving congenital ichthyosis within the disease spectrum of autosomal-recessive congenital ichthyosis. J Eur Acad Dermatol Venereol. 2022;36:582–91. [DOI] [PubMed] [Google Scholar]
- [2].Zdraveska N, Kostovski A, Sofijanova A, Jancevska S, Damevska K. Collodion phenotype remains a challenge for neonatologists: a rare case of self-healing collodion baby. Clin Case Rep. 2022;10:e6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Galeazzo B, Valerio E, Cutrone M. Acral self-healing collodion baby. Arch Dis Child Fetal Neonatal Ed. 2017;102:F542–3. [DOI] [PubMed] [Google Scholar]
- [4].Cuperus E, Bolling MC, de Graaf M, et al. Collodion babies: A 15-year retrospective multicenter study in The Netherlands – Evaluation of severity scores to predict the underlying disease. J Am Acad Dermatol. 2021;84:1111–3. [DOI] [PubMed] [Google Scholar]
- [5].de Jonge CJE, van der Smagt JJ, van Gijn ME, De Graaf M. Self-healing collodion baby: a new mutation. Nederlands Tijdschrift voor Dermatologie en Venereologie. 2019;29:72–4. [Google Scholar]
- [6].Hao J, Zeng L, Xia L, et al. Congenital autosomal recessive ichthyosis. J Clin Dermatol. 2024;53:343–6. [Google Scholar]
- [7].Chen KQ, Wang D, Lin YT, et al. Clinical and genetic analysis of a case of congenital ichthyosis caused by compound heterozygous mutations in the ALOXE3 Gene. Chin J Eugenics Genetics. 2023;31:605–8. [Google Scholar]
- [8].Yu YQ, Zhou Z. Clinical manifestations and ALOX12B gene mutation detection in a case of self-improving collodion ichthyosis. Chin J Dermatol Venereolo. 2023;37:1363–7. [Google Scholar]
- [9].Amoedo P, Cerejeira A, Pacheco J, Cruz MJ, Mota A. A case of self-improving collodion ichthyosis associated with a rare variant of the ALOX12B gene. Dermatol Online J. 2023;29:1–3. [DOI] [PubMed] [Google Scholar]
- [10].Zhu SY, Jiang YZ, Shen N, Yin HJ, Qiao JB. Case report of self-improving collodion ichthyosis in the newborn. J Int Med Res. 2023;51:1– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li H, Qian LJ, Xu N, Huang L, Qiao LX. Novel pathogenic mutation of PNPLA1 identified in autosomal recessive congenital ichthyosis: A case report. Chin Med Sci J. 2022;37:349–52. [DOI] [PubMed] [Google Scholar]
- [12].Zhang L, Hu Y, Lu J, et al. Identification of the first congenital ichthyosis case caused by a homozygous deletion in the ALOX12B gene due to chromosome 17 mixed uniparental disomy. Front Genet. 2022;13:1– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Takeichi T, Ohno Y, Tanahashi K, et al. Ceramide analysis in combination with genetic testing may provide a precise diagnosis for self-healing collodion babies. J Lipid Res. 2022;63:100308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang Z, Xu Z, Ma L. Analysis of 12R-lipoxygenase gene mutations in three families with self-improving collodion ichthyosis. Chin J Dermatol. 2021;54:397–401. [Google Scholar]
- [15].Noguera-Morel L, Feito-Rodríguez M, Maldonado-Cid P, et al. Two cases of autosomal recessive congenital ichthyosis due to CYP4F22 mutations: expanding the genotype of self-healing collodion baby. Pediatr Dermatol. 2016;33:e48–51. [DOI] [PubMed] [Google Scholar]
- [16].Santesteban MR, Larumbe IA, Yanguas BI, Ramos AM. Self-healing collodion baby: a new mutation in the ALOX12B Gene. Actas Dermosifiliogr. 2016;107:433–5. [DOI] [PubMed] [Google Scholar]
- [17].Tanahashi K, Sugiura K, Asagoe K, Aoyama Y, Iwatsuki K, Akiyama M. Novel TGM1 missense mutation p. Arg727Gln in a case of self-healing collodion baby. Acta Derm Venereol. 2014;94:589–90. [DOI] [PubMed] [Google Scholar]
- [18].Bourrat E, Blanchet-Bardon C, Derbois C, Cure S, Fischer J. Specific TGM1 mutation profiles in bathing suit and self-improving collodion ichthyoses: phenotypic and genotypic data from 9 patients with dynamic phenotypes of autosomal recessive congenital ichthyosis. Arch Dermatol. 2012;148:1191–5. [DOI] [PubMed] [Google Scholar]
- [19].Theiler M, Mann C, Weibel L. Self-healing collodion baby. J Pediatr. 2010;157:169–169.e1. [DOI] [PubMed] [Google Scholar]
- [20].Vahlquist A, Bygum A, Gånemo A, et al. Genotypic and clinical spectrum of self-improving collodion ichthyosis: ALOX12B, ALOXE3, and TGM1 mutations in Scandinavian patients. J Invest Dermatol. 2010;130:438–43. [DOI] [PubMed] [Google Scholar]
- [21].Mazereeuw-Hautier J, Aufenvenne K, Deraison C, et al. Acral self-healing collodion baby: report of a new clinical phenotype caused by a novel TGM1 mutation. Br J Dermatol. 2009;161:456–63. [DOI] [PubMed] [Google Scholar]
- [22].Harting M, Brunetti-Pierri N, Chan CS, et al. Self-healing collodion membrane and mild nonbullous congenital ichthyosiform erythroderma due to 2 novel mutations in the ALOX12B gene. Arch Dermatol. 2008;144:351–6. [DOI] [PubMed] [Google Scholar]
- [23].Raghunath M, Hennies HC, Ahvazi B, et al. Self-healing collodion baby: a dynamic phenotype explained by a particular transglutaminase-1 mutation. J Invest Dermatol. 2003;120:224–8. [DOI] [PubMed] [Google Scholar]
- [24].Krieg P, Dick A, Latzko S, et al. Conditional Alox12b Knockout: degradation of the corneocyte lipid envelope in a mouse model of autosomal recessive congenital ichthyoses. J Invest Dermatol. 2020;140:249–53.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Almazroea A, Ijaz A, Aziz A, et al. Identification and in silico analysis of a homozygous nonsense variant in TGM1 gene segregating with congenital ichthyosis in a consanguineous family. Medicina (Kaunas). 2023;59:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hotz A, Kopp J, Bourrat E, et al. Meta-analysis of mutations in ALOX12B or ALOXE3 identified in a large cohort of 224 Patients. Genes (Basel). 2021;12:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Miyamoto M, Itoh N, Sawai M, Sassa T, Kihara A. Severe skin permeability barrier dysfunction in knockout mice deficient in a Fatty Acid ω-Hydroxylase crucial to acylceramide production. J Invest Dermatol. 2020;140:319–26.e4. [DOI] [PubMed] [Google Scholar]
- [28].Meyer JM, Boeglin WE, Brash AR. Recombinant PNPLA1 catalyzes the synthesis of acylceramides and acyl acids with selective incorporation of linoleic acid. J Lipid Res. 2023;64:100379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.