Abstract
Gestational diabetes mellitus (GDM) is one of the most common medical complications of pregnancy. It is generally defined as glucose intolerance with onset or first recognition during pregnancy. The pathogenesis of GDM has long been attributed to inadequate pancreatic β-cell compensation for the physiological insulin resistance of pregnancy. This defect is thought to resolve after pregnancy but become manifest in later life as an increased risk of diabetes. Examination of mechanisms underlying GDM does not support this commonly held picture. In this Perspective, we present evidence that, like diabetes outside of pregnancy, GDM has no single etiology. It results from multiple causes of a common physiological manifestation, inadequate β-cell function, which leads to a common clinical manifestation, elevated glucose levels. We provide evidence that GDM often represents detection of chronic and progressive β-cell dysfunction that is temporally but not mechanistically related to pregnancy. We provide detailed characterization of the β-cell defect in one high-risk group, Hispanic Americans. Finally, we address some of the clinical and research implications of these findings.
Article Highlights
Gestational diabetes mellitus (GDM) is not one disease but many that share inadequate β-cell function as a common cause for elevated glucose levels.
Inadequate β-cell function may result from factors that occur outside of pregnancy, such as autoimmunity, monogenic disorders, obesity, and insulin resistance. Pregnancy-specific causes may exist as well but remain to be defined.
Detailed physiological studies in women with obesity reveal that inadequate β-cell function is likely a chronic condition that is detected by routine glucose screening in pregnancy and that worsens over time, leading to diabetes in later life.
The authors’ studies in Hispanic patients identify obesity and insulin resistance as important causes of β-cell dysfunction, providing a rationale for treating both to prevent diabetes after GDM. Additional work is needed to define the full breadth of underlying causes of GDM as the basis for precision management during and, especially, after pregnancy.
Gestational Diabetes Mellitus: Population Screening for Glucose Intolerance
Despite major efforts at standardization, there are many approaches to detecting gestational diabetes mellitus (GDM). They have one thing in common: they represent broad-based screening for glucose levels at the upper end of the population distribution in relatively young women. If such screening were done outside of pregnancy, it would detect hyperglycemia of many etiologies, including autoimmune, monogenic, and those related to insulin resistance. That same pattern is observed with GDM. A small minority of patients have evidence for pancreatic autoimmunity (1–3), and even fewer have monogenic forms of hyperglycemia that were not known prior to pregnancy (2,4–6). Most have phenotypic characteristics that are typical of type 2 diabetes, which is itself mechanistically heterogenous (7). Based on studies to date, there appears to be considerable phenotypic and genetic heterogeneity in GDM within and across racial and ethnic groups. These facts suggest that, from the standpoint of etiology, GDM is not one disease. It is important to keep this fact in mind as we discuss the mechanisms underlying GDM, as those mechanisms likely vary among different racial, ethnic, and phenotypic subtypes of GDM.
GDM: A β-Cell Disease Not Always Specific to Pregnancy
The interplay between insulin supply and demand is a critical determinant of circulating glucose. Supply is largely determined by pancreatic β-cell responses to nutrients, modified for peripheral tissues by hepatic insulin extraction. Demand is the result of a complex set of metabolic, hormonal, and neural influences that determine the efficiency (i.e., insulin sensitivity) with which insulin suppresses endogenous glucose production and promotes glucose clearance. Glucose uptake independent of insulin also contributes to glucose regulation but is quantitively less important. Bergman et al. (8) demonstrated that the insulin supply-demand relationship conforms to a hyperbola in which the product of supply and demand is a constant across a broad range of each parameter. The product, the disposition index, is an established way to quantify pancreatic β-cell compensation for insulin resistance, a critical determinant of glycemia.
Insulin sensitivity declines markedly during normal pregnancies, and insulin responses increase reciprocally (Fig. 1B–D, Normal). In this context, if GDM resulted from a failure of β-cells to compensate for the insulin resistance of pregnancy, women would develop GDM by moving from one sensitivity-secretion hyperbola (one disposition index) when not pregnant to a lower one during pregnancy (Fig. 1A). However, that is not what studies have shown (9–11). Women with GDM from each of three different racial and ethnic groups (Fig. 1B–D, GDM) moved up and down a single sensitivity-secretion hyperbola just as do women without GDM. However, the hyperbolae in GDM groups reflected reduced β-cell compensation whether or not they were pregnant. Thus, impaired β-cell compensation in GDM was neither specific to nor dependent on pregnancy. Rather, the deficit in compensation appeared to be chronic and, as we will see below, often progressive. This pattern suggests that many cases of GDM represent detection of a chronic, often progressive loss of β-cell compensation that neither develops during pregnancy nor depends on pregnancy to become manifest. Oral glucose tolerance tests (OGTTs) from the study of Catalano et al. (10) reflect the same concept in plasma glucose levels (Fig. 2). Comparing the sum of OGTT glucose levels between individuals with or without GDM revealed that a very large fraction of third-trimester glucose elevations in GDM was already present before pregnancy (77% for the full 3 h, 88% for the 2-h period currently used to diagnose GDM). Thus, while pregnancy increased glucose elevations slightly in women with GDM, most of their hyperglycemia preceded pregnancy.
Figure 1.
A: Theoretical diagram of conventional wisdom that GDM develops during pregnancy when β-cells fail to compensate for increasing insulin resistance. This pathogenesis predicts that women would move from a normal to an abnormal sensitivity-secretion relationship during pregnancy. The upper arrow represents reaching a maximum capacity for β-cell compensation. The middle arrow represents an inadequate rate of increasing compensation. The lower arrow indicates inability to increase compensation. B–D: Actual results from three studies (9–11) in which women with normal glucose tolerance and women with GDM had insulin sensitivity and insulin secretion (B) or responses (C and D) measured in the third trimester and when they were not pregnant. Curved lines represent the product of mean insulin sensitivity (x-axis) and mean insulin response (y-axis) in nonpregnant women with normal glucose tolerance or participants with GDM. Percentages next to GDM symbols represent fractions of analogous (third trimester or not pregnant) disposition index in women with normal glucose tolerance. In all three studies, women with GDM had similar reductions in β-cell compensation for insulin resistance whether pregnant or not, in contrast to conventional wisdom for the pathogenesis of GDM depicted in A. MINMOD SI, minimal model assessment of insulin sensitivity. B and C are reproduced from Buchanan et al. (11). D was created using results published in Catalano et al. (10).
Figure 2.
Plasma glucose levels during OGTTs conducted before pregnancy (75-g test) (A) and during the third trimester (100-g tests) (B) of pregnancies involving normal glucose tolerance (NGT) or pregnancies complicated by GDM. Differences in mean glucose levels between groups at each time point appear below curves. The sum of those differences is depicted in C, which demonstrates that most glucose differences in the third trimester were already present before pregnancy (77% for 3 h, 88% for the 2-h interval currently used to diagnose GDM). Figure was created using results published in Catalano et al. (10).
It is important to note that these studies involved women who, on average, had obesity. In keeping with the theme of heterogeneity in GDM, Catalano et al. (12) found in women with a BMI in the normal prepregnancy range that only ∼45% of differences in OGTT glucose sums observed in the third trimester were present before pregnancy. Similarly, Thaweethai et al. (13) found that only 58% of third-trimester differences in OGTT glucose areas were present at 12 weeks of gestation. These findings are consistent with a condition that is worsened by pregnancy, providing one of many examples of heterogeneity in GDM.
GDM: Is It Insulin Sensitivity, Insulin Secretion, or Both?
Some groups have tried to separate insulin sensitivity and secretion when studying the pathogenesis of GDM. That approach often leads to erroneous conclusions. For example, Homko et al. (9) assessed insulin sensitivity and insulin secretion separately (Fig. 3A and B). They found the biggest difference between control participants and GDM patients during pregnancy was in insulin secretion (Fig. 3A). In contrast, the biggest difference after pregnancy was in insulin sensitivity (Fig. 3B). They concluded that “women with GDM had a major β-cell defect that made it impossible for them to compensate for their increased level of insulin resistance which occurred during late pregnancy.” However, analysis of the two parameters together (Fig. 1B) demonstrates the same β-cell deficit in the GDM group during and after pregnancy.
Figure 3.
A and B: Insulin sensitivity and secretion from hyperglycemic clamps conducted in the third trimester or after pregnancy in women who had normal glucose tolerance (n = 8) or GDM (n = 7) during pregnancy. P values reflect differences between normal glucose-tolerant and GDM groups under each condition. C: Insulin sensitivity estimated from by the Matsuda index and β-cell response measured by the Stumvoll first-phase index from 75-g OGTTs administered to 809 women (8.3% with GDM) between 24 and 30 weeks’ gestation. The GDM group was divided into physiological subtypes who had insulin responses (Secretion), insulin sensitivity (Sensitivity), or both (Mixed) below the 25th percentile for the respective distributions in normal glucose-tolerant pregnant women. All three GDM subtypes have the same reduction in β-cell compensation for insulin resistance. They differ in insulin sensitivity as well as BMI. DI, disposition index. A and B were created using results published in Homko et al. (9). C was created using results published in Powe et al. (14).
In the study by Powe et al. (14), women with GDM were divided into subgroups with predominant defects in insulin sensitivity, insulin secretion, or both based on OGTT-derived parameters below the 25th percentile for pregnant women with normal glucose tolerance. As can be seen in Fig. 3C, all three subgroups had essentially the same disposition index, reduced compared with that of women with normal glucose tolerance. They differed primarily in their degree of insulin resistance, which is a major determinant of insulin responses (Fig. 1). Thus, while β-cell dysfunction in the three subgroups may have developed over years under different degrees of insulin resistance, all groups had the same degree of β-cell dysfunction during pregnancy. As discussed below, this is a common, perhaps universal finding in GDM, because of the degree of glucose elevation required for the diagnosis. Of course, many patients and subgroups remain to be carefully studied.
What Is the Nature of the β-Cell Defect?
There is no single explanation of the nature of the β-cell defect in GDM. GDM is simply hyperglycemia that is detected during pregnancy. There are many underlying causes, some reflecting causes of diabetes outside of pregnancy and some specific to pregnancy. A complete review of relevant studies is beyond the scope of this Perspective. Rather, as an example, we review a large body of work done by our University of Southern California Gestational Diabetes Study Group over nearly three decades with clinical, physiological, prevention, offspring, and genetic cohorts totaling nearly 3,000 Hispanic American participants in Los Angeles. The work advances our understanding of the fundamental biology of GDM in this very-high-risk group.
Clinical Cohort
The clinical cohort consisted of 671 women with GDM (criteria of the National Diabetes Data Group, 1979) who did not have diabetes 4–16 weeks postpartum and were followed with annual glucose tolerance testing for 5–7 years. The cumulative incidence of diabetes was 47% after 5 years. The degrees of hyperglycemia at diagnosis of GDM and shortly after delivery were the strongest predictors of diabetes during follow-up (15). A simple explanation for this common finding is that women whose glucose levels are close to that indicating diabetes require relatively little deterioration to cross the line into diabetes. That finding may be useful clinically, but it provides very little insight into potential causes of deteriorating glucose regulation. More revealing was the observation that weight gain, an additional pregnancy, and use of progestin-only contraception independently increased the risk of developing diabetes after adjustment for initial glucose levels (16,17). This pattern suggested a role of insulin resistance in accelerating deterioration to diabetes.
Physiological Cohort
The physiological cohort consisted of 150 women of Mexican or Central American ancestry with GDM (criteria of the Third International Workshop Conference on GDM) and 25 age-, BMI-, and parity-matched women who had normal 1-h glucose screening tests during pregnancy. All were negative for anti-islet cell antibodies. They were studied with hyperinsulinemic-euglycemic clamps with labeled glucose, intravenous glucose tolerance tests (IVGTTs), and body composition studies during the third trimester (18). Clamps revealed slightly greater insulin resistance for glucose clearance, glucose production, and fatty acid levels in the GDM group. IVGTTs revealed a 67% reduction in β-cell compensation (disposition index) compared with normal levels. Thus, during late pregnancy, the women with GDM had slightly greater insulin resistance in muscle, liver, and fat and much worse β-cell compensation compared with pregnant women with normal glucose tolerance.
A subset of 72 women with GDM participated in OGTTs, IVGTTs, and body composition studies every 12–15 months until they developed diabetes or reached the 12-year study end. Median follow-up was 6 years, and the average annual diabetes incidence rate was 7.2% (19). A low disposition index at baseline and falling disposition index during follow-up were associated with development of diabetes (Fig. 4). Figure 4A demonstrates that women who developed diabetes within 5 years had a very low baseline disposition index compared with women who developed diabetes later. The earlier cases also had higher glucose at baseline. Figure 4B demonstrates falling disposition index in both groups but a more rapid fall in women who developed diabetes. Together, these patterns reveal that being closer to diabetes at baseline (lower DI, higher glucose) and having faster loss of β-cell compensation during follow-up each predicted a relatively short time to diabetes. Of course, these two features tend to be related in a chronic condition like GDM, because people whose disposition index is falling faster are more likely to be closer to diabetes when GDM is diagnosed.
Figure 4.
A: Mean (95% CI) disposition index at baseline in physiological cohort of Hispanic women with prior GDM who remained diabetes free or developed diabetes during up to 135 months after the index pregnancy, grouped according to whether and when they developed diabetes. B: Disposition index over time in the same cohort. Results are plotted relative to final study visit because follow-up ended with development of diabetes. Numbers by symbols are sample sizes. Reproduced from Xiang et al. (19).
Analysis of the women in the GDM group during the first 5 years of follow-up revealed two important features of diabetes development. First, in multivariate analysis, baseline fasting and OGTT glucose levels were not associated with subsequent rates of change in β-cell compensation, speaking against glucose toxicity as a major cause of β-cell decline (20). Instead, gain in body weight or fat was the strongest correlate of falling β-cell compensation. Covariate modeling revealed that increasing insulin resistance, rising C-reactive protein, and falling adiponectin contributed independently to the association between changes in obesity and changes in β-cell compensation. Together, these three factors accounted for 70% of that association.
Second, there was a nonlinear relationship between disposition index and circulating glucose, which rose very slowly across a wide range of falling β-cell compensation levels and accelerated to diabetes only when the disposition index reached ∼20% of normal (Fig. 5). This pattern, which was also observed for 2-h glucose levels from 75-g OGTTs (21), demonstrates a very long period of declining β-cell function reflected in relatively subtle glycemic changes. This is the period during which diabetes prevention is possible (more on that below). Deterioration is detectable by rising glucose levels rather than by any specific glucose threshold.
Figure 5.
Relationship between disposition index and fasting glucose in women described in Fig. 4 during first 5 years after index pregnancies. Solid horizontal lines denote thresholds for impaired and diabetic glucose levels. Arrows denote direction of change over time. Round symbols represent means for women with prior GDM, and the star denotes the mean from 30 nonpregnant women of age, parity, and BMI similar to those of women in the GDM group but who had normal 1-h, 50-g glucose screen during pregnancy. Adapted from Xiang et al. (21).
Also apparent in Fig. 5 is the fact that β-cell dysfunction in GDM does not resolve after delivery, even if glucose levels reach the normal range. Average fasting glucose in GDM participants who did not develop diabetes by year 5 was in the normal range at their first postpartum visit. This was true for 2-h glucose as well (21), but their β-cell function was less than half that of control participants with normal functioning. This pattern can be explained by the cross-sectional observation of Ferrannini et al. (22) that there is substantial loss of β-cell compensation across the normal range of glucose tolerance.
The findings from this physiological cohort define a pattern of chronically falling β-cell compensation, worsened by obesity and insulin resistance, that leads to a slowly accelerating rise in glycemia. Given the information presented above that GDM often represents a chronic condition detected during pregnancy, we speculate that this process starts long (perhaps 10–15 years) before GDM is first diagnosed and is how GDM develops.
Intervention Cohort
The intervention cohort participated in a mechanistic clinical trial to test whether chronic amelioration of insulin resistance can preserve pancreatic β-cell function and delay or prevent diabetes (23,24). A total of 266 Hispanic women with prior GDM and high-risk glucose levels on OGTTs were randomized to receive the insulin-sensitizing thiazolidinedione drug troglitazone or placebo. OGTTs were performed at baseline and annually to test for diabetes. IVGTTs were performed at baseline and 3 months later to examine how treatment affected insulin sensitivity and β-cell function early on and then relate any changes to subsequent diabetes rates.
Troglitazone reduced the diabetes risk by 56% during a median of 30 months on blinded treatment (24). Metformin and lifestyle interventions had similar impact on post hoc analysis of prior GDM participants in the Diabetes Prevention Program (25). In our study, protection from diabetes persisted 8 months after blinded treatment was stopped, demonstrating true modification of the natural history of diabetes. Of all changes observed between baseline and 3 months on treatment, the one most closely related to subsequent diabetes risk was the change in IVGTT total insulin area. We view this as a measure of β-cell secretory loading rather than dynamic β-cell function. A reduction in insulin area was associated with reduced diabetes risk.
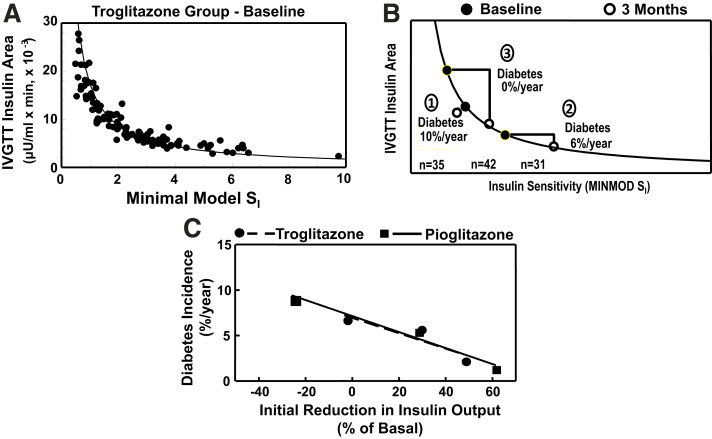
Important mechanistic insights are provided in Fig. 6A and B. Figure 6A shows the baseline relationship between insulin sensitivity and total insulin area for individuals in the active treatment group. Figure 6B shows initial changes in insulin sensitivity and insulin area in three subgroups. Group 1 had no increase in insulin sensitivity between baseline and 3 months despite good medication compliance. Their insulin area did not change significantly, and their average annual diabetes rate was 10%, similar to the 12% rate in the placebo group. Women in groups 2 and 3 had similar reductions in insulin resistance at 3 months. Their β-cell responses were qualitatively identical, namely, reduced insulin output to maintain stable β-cell compensation and glucose levels (not shown). This is the same pattern that was observed in other individuals with GDM in response to changing (Fig. 1B–D) or different (Fig. 3C) insulin sensitivities. However, because of the hyperbolic shape of the sensitivity-secretion relationship, group 2 had a 25% reduction in total insulin output compared with baseline, while group 3 had a 50% reduction. Their subsequent annual diabetes rates were markedly different, 6% and 0%, respectively.
Figure 6.
A: Relationship between insulin sensitivity (minimal model SI [MINMOD SI], where SI is insulin sensitivity) and total area under the insulin curve during tolbutamide-modified IVGTTs at baseline in women in the intervention cohort (defined in the text) who were randomized to the troglitazone arm. Symbols represent individual participants; curved line is the mean disposition index (SI × insulin area) for all women. B: Changes in insulin sensitivity and insulin area between baseline and 3 months on treatment in the active treatment arm. The three subgroups are indicated by circled numbers: group 1, women who did not have an increase in insulin sensitivity; group 2, women who had an increase in insulin sensitivity and a change in insulin area below the median; and group 3, women who had an increase in insulin sensitivity and a change in insulin area above the median. “Diabetes” denotes annual average incidence rates. C: Relationship between early change from baseline in total IVGTT insulin area and average annual diabetes incidence in the three subgroups in the active troglitazone treatment arm depicted in B and analogous subgroups from the follow-on study of open-label pioglitazone treatment in participants who did not have diabetes after washout in the troglitazone study. Symbols represent tertiles of change from baseline in IVGTT area in each study, and lines represent linear fit of tertile values in each study. A and B were adapted from Buchanan et al. (24), and C was adapted from Xiang et al. (26).
Figure 6C depicts the very tight relationship between the percent change in IVGTT insulin area at 3 months and subsequent annual diabetes rates in the three troglitazone subgroups. Troglitazone was removed from the market near the end of the trial. After the 8-month washout period, participants without diabetes were invited to an open-label study with another thiazolidinedione drug, pioglitazone (26). That drug maintained a low rate of diabetes and stable β-cell function and a low rate of development of diabetes. The slope of the relationship between the early change in total insulin area (after 1 year in this study) and annual diabetes rates was identical to the slope in the troglitazone study (Fig. 6C).
Two features in Fig. 6 provide specific clues about the heterogeneity of β-cell defects in this patient group. First, it was the relative rather than absolute early change in β-cell loading that was associated with reduction in diabetes risk. Second, at the end of 3 months on troglitazone, group 3 had a higher secretory load than group 2 but went on to have a much lower rate of diabetes. These two findings suggest that there is important individual variability in the susceptibility of β-cells to deteriorate under secretory loading, which itself varies widely among individuals. It follows that the interplay between β-cell susceptibility and ambient insulin resistance is a critical determinant of whether and how fast β-cells fail over time and, thus, the risk of both GDM and diabetes after GDM, at least in Hispanic women.
Viewed in the broader population sense, GDM is at once highly uniform in its pathophysiology and highly heterogenous in its etiology. Uniformity is a function of GDM diagnostic criteria, which select women whose glucose tolerance, and thus disposition index, are in a relatively narrow range at one point in time (Fig. 6A). Heterogeneity is a function of the many ways to arrive at a disposition index in the GDM range during reproductive years. Some ways may be short-term and specific to pregnancy while others reflect chronic, often progressive β-cell defects that are detected during pregnancy. For patients experiencing the progressive decline in β-cell compensation that we observed in Hispanic patients, having a disposition index in the GDM range logically depends on three factors: initial β-cell compensation, rate of decline in compensation, and the duration of decline. Initial β-cell compensation cannot be determined retrospectively, but our offspring studies, described below, suggest that it may have been normal at a young age, before the onset of obesity. The rate of decline in Hispanic patients appears to be determined individually by the susceptibility of β-cells to deteriorate under secretory loading and the degree of loading, which can vary over time due to factors such as pregnancies and weight change.
This scenario has implications for who has GDM at any point in time and who does not. Women in our intervention cohort (Fig. 6) had the right combination of β-cell susceptibility and secretory loading for the right period of time to have GDM during pregnancy. Group 2 did so with relatively mild loading and relatively high β-cell susceptibility to loading conditions. Group 3 did so with relatively severe secretory loading but lower susceptibility. This concept is depicted schematically in Fig. 7 by two arrows that end in the GDM range. Figure 7 also depicts women with low probability of GDM. One group has high β-cell susceptibility, like group 2 in Fig. 6, and a high β-cell loading, like group 3. They are predicted to lose β-cell compensation rapidly and progress to diabetes before pregnancy, perhaps even in youth if obesity and β-cell loading are severe. In contrast, women with low β-cell susceptibility, like group 3, and mild β-cell loading, like group 2, would not have progressed to GDM by the time they became pregnant. Some might never develop diabetes, while others might do so later in life. In all cases, GDM is just a snapshot in time of evolving glucose regulation. This whole scenario is one example of the complex interactions that lead to GDM. Based on these findings, we believe that a deeper understanding of the mechanisms that determine β-cell susceptibility will enable more precise approaches to GDM and diabetes prevention in Hispanic women. Much work is needed to idenepsy both short-term and chronic mechanisms underlying GDM in other patient groups.
Figure 7.
Conceptual diagram of the interplay between β-cell secretory loading (insulin output) and susceptibility or resistance of β-cells to deteriorate under such loading, based on evidence from Hispanic women who develop GDM. Open arrows represent β-cells that are relatively susceptible to loading, and closed arrows represent β-cells that are relatively resistant to loading. In each case, increased loading accelerates the rate of deterioration; the effect is more prominent for susceptible β-cells. Women with resistant β-cells and low loading should have the slowest rate of deterioration and may not develop GDM during reproductive years. Women with susceptible β-cells and high loading should deteriorate most rapidly and may develop diabetes before they become pregnant. While the diagram depicts two states for each variable, they are likely continuously distributed, with many possible combinations.
Offspring Cohort
Women with GDM have established defects in β-cell compensation, often with a background of insulin resistance and obesity. It would be useful to know the sequence of development of these three core conditions. Given evidence that exposure to maternal hyperglycemia in utero is associated with obesity and hyperglycemia in offspring (27–29), we are conducting the longitudinal BrainChild Study to characterize children born to mothers with or without GDM. The study focuses on appetite regulation, body composition, insulin resistance, and β-cell function in offspring, assessed with OGTTs, neuroimaging, anthropometrics, and measures of diet and physical activity. Initial observations from children aged 7–11 years reveal several key findings (30–34). Most relevant to this Perspective is the observation that offspring exposed to GDM before 26 weeks of gestation had greater hypothalamic activation to glucose, higher brain reward activation to palatable food cues, and increased total caloric intake at a time when their BMI, total body fat, and glucose levels were similar to those of children not exposed to GDM (31). Importantly, the hypothalamic changes predicted weight gain over the subsequent year. We are currently assessing changes in other metabolic parameters. For now, our findings suggest that some aspect of GDM alters brain appetite regulation in offspring in a way that is a precursor to the obesity, insulin resistance, and β-cell dysfunction we have seen in mothers.
These observations differ from results of the transgenerational Pune Maternal Nutrition Study (35), where mothers had mean BMI (18.1 kg/m2) that was much lower than that in BrainChild (30.1 kg/m2) and had few individuals with GDM. At age 18 years, 28% of offspring had prediabetes, often with low or normal BMI, and they had been exposed to higher maternal glucose levels in pregnancy, even within the normal range. Importantly, their fasting glucose levels and disposition index from OGTTs were already trending higher at ages 6–12 years, a time when GDM-exposed and control offspring in BrainChild had similar glucose levels. These studies highlight racial and ethnic as well as phenotypic variability that may influence the etiology of glucose dysregulation leading to GDM. Genetic factors affecting β-cell function and adipose biology may contribute to GDM in South Asians (36).
Genetic Cohort
We have examined a number of genotype-phenotype relationships in a cohort of 1,759 Mexican Americans from 523 families of probands with or without GDM (37–41). Of general interest is that some of the associations with IVGTT-based insulin sensitivity or secretion were dependent on the level of body fat (37,38), consistent with the effect of adiposity in our earlier studies (15,16,20) and those of Powe et al. (14). We also showed that variation in MTNR1B set the absolute level of β-cell compensation for insulin resistance but was not associated with rates of change in compensation over a 5-year follow-up period (41). Variation at MTNR1B rs10830963 may also modify the effectiveness of lifestyle interventions in pregnancy that are designed to reduce the incidence of GDM (42). This represents one form of heterogeneity in determinants of β-cell compensation.
More broadly, genome-wide genotype- and sequence-based studies have identified hundreds of loci associated with type 2 diabetes. Similar studies of genetic architecture in GDM are fewer in number (43–49) and have smaller sample sizes. Combined with numerous SNP association studies, the existing evidence suggests GDM shares a number of risk loci with type 2 diabetes but may also have unique loci contributing to risk (43,48,49). The loci with the strongest evidence for association appear to be related to insulin secretion or biology of pancreatic β-cells but with effect sizes that are stronger than those observed in type 2 diabetes (43–49). There is also clear evidence for heterogeneity within the genetics/genomics of GDM (48–50) that is reflected in both transcriptomic and metabolic analyses (49–51). Analyses based on polygenic risk scores suggest that women with GDM have elevated risk for type 2 diabetes (52,53). All of this supports the concept that many cases of GDM represent type 2 diabetes in evolution, as we have seen in Hispanic patients. The overlap with type 2 diabetes may reflect the relatively high frequency of the disease in most populations, but there are other genetic associations for GDM, consistent with the concept that it is not one unique disease.
Summary and Implications
GDM is not one disease. β-Cell dysfunction appears to be a universal feature, but the causes appear to be as varied as they are for diabetes outside of pregnancy. The long-held idea that GDM results from short-term inability of β-cells to compensate for physiological insulin resistance of pregnancy is not supported by studies in White, Black, and Hispanic patients with obesity. Instead, their reductions in β-cell compensation are similar before, during, and after pregnancy, strongly supporting the concept that their GDM results from a chronic defect in β-cell compensation that is detected by routine glucose testing in pregnancy. Early abnormal glucose metabolism in pregnancy is likely part of this continuum (54). Other studies in lean patients demonstrate more dependency on pregnancy for glucose elevations. Our detailed studies in Hispanic women demonstrate that their β-cell defect does not resolve after delivery, even if glucose levels regress into the normal range. In fact, β-cell compensation tends to worsen progressively over time, leading eventually to diabetes in many patients. Increased secretory loading of β-cells by chronic obesity and insulin resistance appears to be an important cause of declining β-cell compensation, but there is considerable interindividual variability in the susceptibility of β-cells to deterioration under different degrees of loading. Our ongoing studies in offspring of Hispanic women with GDM indicate that alterations in central appetite regulation may be one of the earliest abnormalities that proceeds the development of weight gain, insulin resistance, and declining β-cell function, which may bring about GDM in the next generation. Of course, this is just one of many possible scenarios by which GDM develops.
These concepts have implications for clinical care and research. First, the fact that the physiological abnormalities that cause GDM likely antedate pregnancy by many years may explain why short-term interventions to prevent GDM have had only modest success (55), especially compared with long-term approaches to prevent type 2 diabetes. Second, animal models characterized by failure of β-cell expansion during pregnancy have limited application to most human cases of GDM. Third, our findings support efforts to screen for and diagnose GDM early in pregnancy, although diagnostic criteria specific to that window may be required. Fourth, attempts to predict GDM based on non-glucose measurements made in early pregnancy may represent early detection rather than prediction of GDM. Finally, and perhaps most importantly, understanding that GDM is not one disease should lead to careful characterizations of etiological subtypes that can inform precision approaches to treatment of the condition during pregnancy and prevention of diabetes in mothers and their offspring.
Article Information
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Funding Statement
Prior work conducted by the authors and presented here was supported by grants R01DK46334, R01DK46374, R01DK61628, R01DK134079, and R01DK116858 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (NIH); Distinguished Clinical Scientist Award 7-04-DCS-03, Clinical/Translation Research Award 7-09-CT, and Pathway Accelerator Award 1-14-ACE-36 from the American Diabetes Association; grants M01 RR00043 from the National Center for Research Resources, NIH, and UL1-TR001855 from National Center for Advancing Translational Sciences, NIH; and investigator-initiated research grants from Parke-Davis Pharmaceutical Research, Takeda Pharmaceuticals North America, and Merck & Co.
References
- 1. Petersen JS, Dyrberg T, Damm P, Kühl C, Mølsted-Pedersen L, Buschard K. GAD65 autoantibodies in women with gestational or insulin dependent diabetes mellitus diagnosed during pregnancy. Diabetologia 1996;39:1329–1333 [DOI] [PubMed] [Google Scholar]
- 2. Weng J, Ekelund M, Lehto M, et al. Screening for MODY mutations, GAD antibodies, and type 1 diabetes–associated HLA genotypes in women with gestational diabetes mellitus. Diabetes Care 2002;25:68–71 [DOI] [PubMed] [Google Scholar]
- 3. Löbner K, Knopff A, Baumgarten A, et al. Predictors of postpartum diabetes in women with gestational diabetes mellitus. Diabetes 2006;55:792–797 [DOI] [PubMed] [Google Scholar]
- 4. Kousta E, Ellard S, Allen LI, et al. Glucokinase mutations in a phenotypically selected multiethnic group of women with a history of gestational diabetes. Diabet Med 2001;18:683–684 [DOI] [PubMed] [Google Scholar]
- 5. Ellard S, Beards F, Allen LI, et al. A high prevalence of glucokinase mutations in gestational diabetic subjects selected by clinical criteria. Diabetologia 2000;43:250–253 [DOI] [PubMed] [Google Scholar]
- 6. Chen Y, Liao WX, Roy AC, Loganath A, Ng SC. Mitochondrial gene mutations in gestational diabetes mellitus. Diabetes Res Clin Pract 2000;48:29–35 [DOI] [PubMed] [Google Scholar]
- 7. Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 2018;6:361–369 [DOI] [PubMed] [Google Scholar]
- 8. Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 1981;68:1456–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Homko C, Sivan E, Chen X, Reece EA, Boden G. Insulin secretion during and after pregnancy in patients with gestational diabetes mellitus. J Clin Endocrinol Metab 2001;86:568–573 [DOI] [PubMed] [Google Scholar]
- 10. Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol 1999;180:903–916 [DOI] [PubMed] [Google Scholar]
- 11. Buchanan TA, Xiang A, Kjos SL, Watanabe R. What is gestational diabetes? Diabetes Care 2007;30(Suppl 2):S105–S111 [DOI] [PubMed] [Google Scholar]
- 12. Catalano PM, Tyzbir ED, Wolfe RR, et al. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am J Physiol 1993;264:E60–E67 [DOI] [PubMed] [Google Scholar]
- 13. Thaweethai T, Soetan Z, James K, Florez JC, Powe CE. Distinct insulin physiology trajectories in euglycemic pregnancy and gestational diabetes mellitus. Diabetes Care 2023;46:2137–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Powe CE, Allard C, Battista M-C, et al. Heterogeneous contribution of insulin sensitivity and secretion defects to gestational diabetes mellitus. Diabetes Care 2016;39:1052–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kjos SL, Peters RK, Xiang A, Henry OA, Montoro M, Buchanan TA. Predicting future diabetes in Latino women with gestational diabetes. Utility of early postpartum glucose tolerance testing. Diabetes 1995;44:586–591 [DOI] [PubMed] [Google Scholar]
- 16. Peters RK, Kjos SL, Xiang A, Buchanan TA. Long-term diabetogenic effect of single pregnancy in women with previous gestational diabetes mellitus. Lancet 1996;347:227–230 [DOI] [PubMed] [Google Scholar]
- 17. Kjos SL, Peters RK, Xiang A, Thomas D, Schaefer U, Buchanan TA. Contraception and the risk of type 2 diabetes mellitus in Latina women with prior gestational diabetes mellitus. JAMA 1998;280:533–538 [DOI] [PubMed] [Google Scholar]
- 18. Xiang AH, Peters RK, Trigo E, Kjos SL, Lee WP, Buchanan TA. Multiple metabolic defects during late pregnancy in women at high risk for type 2 diabetes. Diabetes 1999;48:848–854 [DOI] [PubMed] [Google Scholar]
- 19. Xiang AH, Kjos SL, Takayanagi M, Trigo E, Buchanan TA. Detailed physiological characterization of the development of type 2 diabetes in Hispanic women with prior gestational diabetes mellitus. Diabetes 2010;59:2625–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiang AH, Kawakubo M, Trigo E, Kjos SL, Buchanan TA. Declining beta-cell compensation for insulin resistance in Hispanic women with recent gestational diabetes mellitus: association with changes in weight, adiponectin, and C-reactive protein. Diabetes Care 2010;33:396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xiang AH, Wang C, Peters RK, Trigo E, Kjos SL, Buchanan TA. Coordinate changes in plasma glucose and pancreatic beta-cell function in Latino women at high risk for type 2 diabetes. Diabetes 2006;55:1074–1079 [DOI] [PubMed] [Google Scholar]
- 22. Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Beta-cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 2005;90:493–500 [DOI] [PubMed] [Google Scholar]
- 23. Azen SP, Peters RK, Berkowitz K, Kjos S, Xiang A, Buchanan TA. TRIPOD (TRoglitazone In the Prevention Of Diabetes): a randomized, placebo-controlled trial of troglitazone in women with prior gestational diabetes mellitus. Control Clin Trials 1998;19:217–231 [DOI] [PubMed] [Google Scholar]
- 24. Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes 2002;51:2796–2803 [DOI] [PubMed] [Google Scholar]
- 25. Ratner RE, Christophi CA, Metzger BE, et al.; Diabetes Prevention Program Research Group . Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab 2008;93:4774–4779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xiang AH, Peters RK, Kjos SL, et al. Effect of pioglitazone on pancreatic beta-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes 2006;55:517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pettitt DJ, Baird HR, Aleck KA, Bennett PH, Knowler WC. Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N Engl J Med 1983;308:242–245 [DOI] [PubMed] [Google Scholar]
- 28. Silverman BL, Rizzo TA, Cho NH, Metzger BE. Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diabetes Care 1998;21(Suppl 2):B142–B149 [PubMed] [Google Scholar]
- 29. Dabelea D, Mayer-Davis EJ, Lamichhane AP, et al. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case-Control Study. Diabetes Care 2008;31:1422–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Page KA, Romero A, Buchanan TA, Xiang AH. Gestational diabetes mellitus, maternal obesity, and adiposity in offspring. J Pediatr 2014;164:807–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Page KA, Luo S, Wang X, et al. Children exposed to maternal obesity or gestational diabetes mellitus during early fetal development have hypothalamic alterations that predict future weight gain. Diabetes Care 2019;42:1473–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chandrasekaran S, Melhorn S, Olerich KLW, et al. Exposure to gestational diabetes mellitus prior to 26 weeks is related to the presence of mediobasal hypothalamic gliosis in children. Diabetes 2022;71:2552–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lynch KM, Alves JM, Chow T, et al. Selective morphological and volumetric alterations in the hippocampus of children exposed in utero to gestational diabetes mellitus. Hum Brain Mapp 2021;42:2583–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luo S, Angelo BC, Chow T, et al. Erratum. Associations between exposure to gestational diabetes mellitus in utero and daily energy intake, brain responses to food cues, and adiposity in children. Diabetes Care 2021;44:2447–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yajnik CS, Bandopadhyay S, Bhalerao A, et al. Poor in utero growth, and reduced β-cell compensation and high fasting glucose from childhood, are harbingers of glucose intolerance in young Indians. Diabetes Care 2021;44:2747–2757 [DOI] [PubMed] [Google Scholar]
- 36. Hodgson S, Williamson A, Bigossi M, et al.; Genes & Health Research Team . Genetic basis of early onset and progression of type 2 diabetes in South Asians. Nat Med 2025;31:323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Watanabe RM, Allayee H, Xiang AH, et al. Transcription factor 7-like 2 (TCF7L2) is associated with gestational diabetes mellitus and interacts with adiposity to alter insulin secretion in Mexican Americans. Diabetes 2007;56:1481–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li X, Allayee H, Xiang AH, et al. Variation in IGF2BP2 interacts with adiposity to alter insulin sensitivity in Mexican Americans. Obesity (Silver Spring) 2009;17:729–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li X, Shu Y-H, Xiang AH, et al. Additive effects of genetic variation in GCK and G6PC2 on insulin secretion and fasting glucose. Diabetes 2009;58:2946–2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shu Y-H, Hartiala J, Xiang AH, et al. Evidence for sex-specific associations between variation in acid phosphatase locus 1 (ACP1) and insulin sensitivity in Mexican-Americans. J Clin Endocrinol Metab 2009;94:4094–4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ren J, Xiang AH, Trigo E, et al. Genetic variation in MTNR1B is associated with gestational diabetes mellitus and contributes only to the absolute level of beta cell compensation in Mexican Americans. Diabetologia 2014;57:1391–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grotenfelt NE, Wasenius NS, Rönö K, et al. Interaction between rs10830963 polymorphism in MTNR1B and lifestyle intervention on occurrence of gestational diabetes. Diabetologia 2016;59:1655–1658 [DOI] [PubMed] [Google Scholar]
- 43. Powe CE, Udler MS, Hsu S, et al. Genetic loci and physiologic pathways involved in gestational diabetes mellitus implicated through clustering. Diabetes 2021;70:268–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kwak SH, Kim S-H, Cho YM, et al. A genome-wide association study of gestational diabetes mellitus in Korean women. Diabetes 2012;61:531–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu N-N, Zhao D, Ma W, et al. A genome-wide association study of gestational diabetes mellitus in Chinese women. J Matern Fetal Neonatal Med 2021;34:1557–1564 [DOI] [PubMed] [Google Scholar]
- 46. Yue S, Pei L, Lai F, et al. Genome-wide analysis study of gestational diabetes mellitus and related pathogenic factors in a Chinese Han population. BMC Pregnancy Childbirth 2023;23:856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhen J, Gu Y, Wang P, et al. Genome-wide association and Mendelian randomisation analysis among 30,699 Chinese pregnant women identifies novel genetic and molecular risk factors for gestational diabetes and glycaemic traits. Diabetologia 2024;67:703–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Elliott A, Walters RK, Pirinen M, et al.; FinnGen . Distinct and shared genetic architectures of gestational diabetes mellitus and type 2 diabetes. Nat Genet 2024;56:377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee K, Kuang A, Bain JR, et al. Metabolomic and genetic architecture of gestational diabetes subtypes. Diabetologia 2024;67:895–907 [DOI] [PubMed] [Google Scholar]
- 50. Benny P, Ahn HJ, Burlingame J, et al. Genetic risk factors associated with gestational diabetes in a multi-ethnic population. PLoS One 2021;16:e0261137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fuller H, Iles MM, Moore JB, Zulyniak MA. Metabolic drivers of dysglycemia in pregnancy: ethnic-specific GWAS of 146 metabolites and 1-sample Mendelian randomization analyses in a UK multi-ethnic birth cohort. Front Endocrinol (Lausanne) 2023;14:1157416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li Y, Yang M, Yuan L, Li T, Zhong X, Guo Y. Associations between a polygenic risk score and the risk of gestational diabetes mellitus in a Chinese population: a case-control study. Endocr J 2023;70:1159–1168 [DOI] [PubMed] [Google Scholar]
- 53. Choi J, Lee H, Kuang A, et al. Genome-wide polygenic risk score predicts incident type 2 diabetes in women with history of gestational diabetes. Diabetes Care 2024;47:1622–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. American Diabetes Association Professional Practice Committee . 2. Diagnosis and classification of diabetes: Standards of Care in Diabetes—2025. Diabetes Care 2025;48(Suppl. 1):S27–S49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Takele WW, Vesco KK, Josefson J, et al.; ADA/EASD PMDI . Effective interventions in preventing gestational diabetes mellitus: a systematic review and meta-analysis. Commun Med (Lond) 2024;4:75. [DOI] [PMC free article] [PubMed] [Google Scholar]