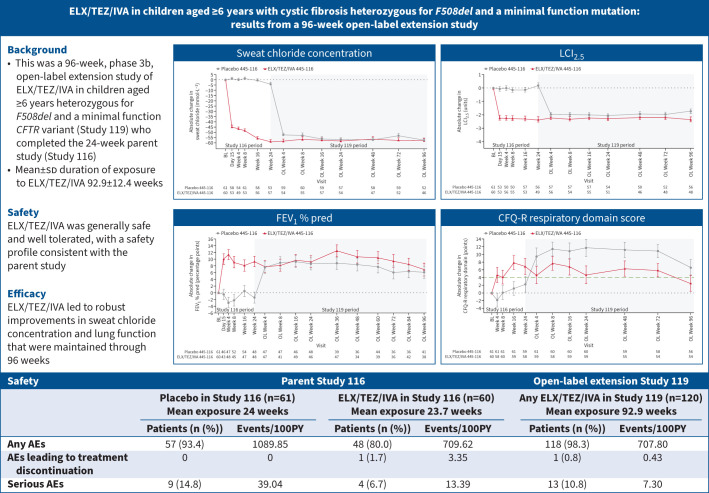
Graphical abstract
Overview of the study. ELX: elexacaftor; TEZ: tezacaftor; IVA: ivacaftor; CFTR: cystic fibrosis transmembrane conductance regulator; BL: baseline; OL: open-label; LCI2.5: lung clearance index; FEV1: forced expiratory volume in 1 s; CFQ-R: Cystic Fibrosis Questionnaire-Revised (minimal clinically important difference shown as a green dashed line); AE: adverse event; events/100PY: number of events per 100 patient-years (336 days=48 weeks per year)=number of events/total duration of treatment-emergent period for each study in 100PY. Data in the graphs are presented as least squares mean±se.
Abstract
Background
Elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) was efficacious and safe in children aged 6–11 years with cystic fibrosis (CF) heterozygous for F508del and a minimal function CF transmembrane conductance regulator (CFTR) variant (F/MF genotypes) in a 24-week, placebo-controlled trial. We conducted a 96-week open-label extension study for children who completed the 24-week parent study.
Methods
In this phase 3b extension study, dosing was based on weight and age, with children weighing <30 kg and aged <12 years receiving ELX 100 mg once daily, TEZ 50 mg once daily and IVA 75 mg every 12 h, and children ≥30 kg or ≥12 years receiving ELX 200 mg once daily, TEZ 100 mg once daily and IVA 150 mg every 12 h. The primary end-point was safety and tolerability. Secondary and other efficacy end-points included absolute changes from parent study baseline in sweat chloride concentration, lung clearance index (LCI2.5), percentage predicted forced expiratory volume in 1 s (FEV1) and Cystic Fibrosis Questionnaire-Revised (CFQ-R) respiratory domain score.
Results
A total of 120 children were enrolled and dosed. 118 children (98.3%) had adverse events (AEs), which for most were mild (43.3%) or moderate (48.3%) in severity. The most common AEs (≥20% of children) were COVID-19 (58.3%), cough (51.7%), nasopharyngitis (45.0%), pyrexia (40.0%), headache (37.5%), upper respiratory tract infection (30.8%), oropharyngeal pain (26.7%), rhinitis (24.2%), abdominal pain (22.5%) and vomiting (20.0%). Children who transitioned from the placebo and ELX/TEZ/IVA groups of the parent study had improvements from parent study baseline at Week 96 in mean sweat chloride concentration (−57.3 (95% CI −61.6– −52.9) and −57.5 (95% CI −62.0– −53.0) mmol·L−1), LCI2.5 (−1.74 (95% CI −2.09– −1.38) and −2.35 (95% CI −2.72– −1.97) units), FEV1 % pred (6.1 (95% CI 2.6–9.7) and 6.9 (95% CI 3.2–10.5) percentage points) and CFQ-R respiratory domain score (6.6 (95% CI 2.5–10.8) and 2.6 (95% CI −1.6–6.8) points).
Conclusions
ELX/TEZ/IVA treatment was generally safe and well tolerated, with a safety profile consistent with the parent study and older age groups. After starting ELX/TEZ/IVA, children had robust improvements in sweat chloride concentration and lung function that were maintained through 96 weeks. These results demonstrate the safety and durable efficacy of ELX/TEZ/IVA in this paediatric population.
Shareable abstract
In this 96-week open-label extension study in children with CF aged ≥6 years with F/MF genotypes, ELX/TEZ/IVA treatment remained generally safe and well tolerated, and led to sustained improvements in lung function, CFTR function and respiratory symptoms https://bit.ly/3DP9tXX
Introduction
Cystic fibrosis (CF) is an autosomal recessive disease affecting more than 105 000 people worldwide [1–3]. CF is the result of pathogenic variants in the CF transmembrane conductance regulator (CFTR) gene leading to mucus buildup in the lungs, pancreas and other secretory organs due to impairment of ion and water transport across cell membranes [1, 4]. Early diagnosis and treatment interventions can limit organ damage and improve long-term outcomes for children with CF [2, 5–7].
Ivacaftor (IVA) is a small-molecule therapeutic designed to improve the flow of chloride by improving CFTR gating [8, 9], while tezacaftor (TEZ) and elexacaftor (ELX) facilitate trafficking of CFTR proteins to the cell membrane thereby increasing cell surface expression of CFTR proteins [10, 11]. In pivotal phase 3 trials in adolescents and adults with CF ≥12 years of age with at least one F508del allele, the triple combination ELX/TEZ/IVA was shown to be safe and effective, improving lung function, respiratory symptoms and CFTR function [12, 13]. Globally, F508del is the most commonly occurring CFTR pathogenic variant amongst people with CF of Northern European ancestry [14]. Although the prevalence of this variant is lower in other populations, it is estimated that ELX/TEZ/IVA has the potential to treat ∼70–90% of patients with CF [14, 15].
Given the importance of early intervention in changing the trajectory of CF disease, an open-label phase 3 study (VX18-445-106) was conducted to assess the safety, pharmacokinetics and efficacy of ELX/TEZ/IVA in children aged 6–11 years heterozygous for F508del and a minimal function CFTR variant (F/MF) or homozygous for F508del (F/F). That study showed that, consistent with trials in adolescents and adults, ELX/TEZ/IVA was safe in this paediatric group and led to improvements in percentage predicted forced expiratory volume in 1 s (FEV1), Cystic Fibrosis Questionnaire-Revised (CFQ-R) respiratory domain score, lung clearance index (LCI2.5) and sweat chloride concentration [16]. To further elucidate the impact of ELX/TEZ/IVA in this paediatric population, a 24-week placebo-controlled phase 3b trial was conducted to assess the efficacy and safety in children with F/MF genotypes. Significant improvements in lung function along with robust improvements in respiratory symptoms and CFTR function were reported [17]. These results suggest that, consistent with adolescents and adults, ELX/TEZ/IVA has the potential to change CF disease trajectory in children.
Children who completed the 24-week placebo-controlled trial were given the opportunity to enrol in a 96-week extension study to assess the safety and efficacy of ELX/TEZ/IVA. Here we report the results from this open-label extension study. Some results were previously reported in a conference abstract (Deutschen Mukoviszidose Tagung (DMT), 23–25 November 2023, Wurzburg, Germany).
Methods
Patients, trial design and oversight
This study (VX20-445-119 (Study 119); ClinicalTrials.gov: NCT04545515; EudraCT: 2020-001404-42) was a phase 3b, multicentre, 96-week open-label extension study for children aged ≥6 years with CF, heterozygous for F508del and a minimal function CFTR variant (F/MF) who completed the 24-week parent study (VX19-445-116 (Study 116) for children aged 6–11 years; a phase 3b, randomised, double-blind, placebo-controlled, multicentre trial) and met the eligibility criteria. The definition of a minimal function variant along with a list of qualifying minimal function variants can be found in the supplementary methods and supplementary table S1. A schematic of the study design is shown in supplementary figure S1.
Dosing was based on age and weight: children aged ≥6– <12 years weighing <30 kg at study entry received ELX 100 mg once daily, TEZ 50 mg once daily and IVA 75 mg every 12 h, while children weighing ≥30 kg or aged ≥12 years received ELX 200 mg once daily, TEZ 100 mg once daily and IVA 150 mg every 12 h (adult dose).
In collaboration with the authors, the trial was designed by Vertex Pharmaceuticals Incorporated. Written informed consent was obtained from the child's legal representative/guardian and assent obtained per local guidelines. Safety was monitored by an independent data safety monitoring committee. Vertex Pharmaceuticals Incorporated performed data collection and analysis. All authors had complete access to the data, reviewed the manuscript and approved it for submission. The study investigators assure accuracy and completeness of the data, and the investigators and Vertex Pharmaceuticals Incorporated assure the fidelity of the trial to the study protocol.
Because parts of this trial overlapped with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, a global protocol addendum was implemented to enable children to continue in the study while ensuring safety by minimising risk to SARS-CoV-2 exposure. Measures were adapted based on country and local regulations and site-level considerations. These measures included shipment of study drug from site to home, telephone/video calls for safety assessments, in-home assessments, use of local laboratories for safety assessments, at-home pregnancy testing, verbal re-consenting, remote re-consenting, remote monitoring/source data verification and electronic data capture visit type forms.
Outcome measures
The primary end-point was safety and tolerability as assessed by adverse events (AEs), clinical laboratory values, ECG, vital signs and pulse oximetry. Secondary end-points were absolute change in sweat chloride concentration and standard LCI at 2.5% of the starting gas concentration (LCI2.5) from parent study baseline. Nitrogen multiple-breath washout testing was performed with an Exhalyzer D using Spiroware version 3.1.6 (Eco Medics, Duernten, Switzerland). This version of Spiroware has since been updated to correct for cross-sensitivity issues associated with the oxygen and carbon dioxide sensors in the device that can cause overestimation of nitrogen concentrations [18]. A study by Robinson et al. [19] that reassessed 1036 previous tests using the updated software found that although the corrected algorithm did result in lower LCI2.5 values, the interpretations and significance of observed treatment effects did not change. Other end-points were absolute change in FEV1 % pred and CFQ-R respiratory domain score from parent baseline.
Statistical analysis
Safety and efficacy analyses included all participants who received at least one dose of study drug. Analysis of safety data was descriptive, and there was no statistical testing. Secondary and other efficacy end-points (absolute change in sweat chloride concentration, LCI2.5, FEV1 % pred and CFQ-R respiratory domain score from baseline) were analysed using a mixed effects model for repeated measures with absolute change from baseline at each post-baseline visit as the dependent variable. The model included parent study treatment group, visit and parent study treatment-by-visit interaction as fixed effects with continuous parent study baseline LCI2.5 and weight at screening visit of parent study (<30 versus ≥30 kg) as covariates. The least squares (LS) mean changes from parent study baseline and two-sided 95% confidence intervals at each visit were estimated for each treatment group.
Results
Population
The trial took place at 34 sites in Australia, Canada, Denmark, France, Germany, Israel, the Netherlands, Spain, Switzerland and the UK between 11 January 2021 and 24 March 2023. A total of 120 children were enrolled and received at least one dose of study drug. Demographics and clinical characteristics at parent study baseline are presented in table 1.
TABLE 1.
Demographic and clinical characteristics at parent study baseline#
| Placebo in Study 116 (n=61) |
ELX/TEZ/IVA in Study 116 (n=59) |
Study 119 (n=120) |
|
|---|---|---|---|
| Female | 35 (57.4) | 34 (57.6) | 69 (57.5) |
| Age at baseline (years) | 9.2±1.7 | 9.0±1.8 | 9.1±1.7 |
| Race | |||
| White | 42 (68.9) | 45 (76.3) | 87 (72.5) |
| Black or African American | 0 | 1 (1.7) | 1 (0.8) |
| Asian | 0 | 1 (1.7) | 1 (0.8) |
| American Indian or Alaska Native | 0 | 1 (1.7) | 1 (0.8) |
| Native Hawaiian or Other Pacific Islander | 0 | 0 | 0 |
| Other | 1 (1.6) | 0 | 1 (0.8) |
| Not collected per local regulations | 18 (29.5) | 10 (16.9) | 28 (23.3) |
| Multiracial | 0 | 1 (1.7) | 1 (0.8) |
| Ethnicity | |||
| Hispanic or Latino | 0 | 1 (1.7) | 1 (0.8) |
| Not Hispanic or Latino | 42 (68.9) | 48 (81.4) | 90 (75.0) |
| Not collected per local regulations | 19 (31.1) | 10 (16.9) | 29 (24.2) |
| Sweat chloride (mmol·L−1) | 102.6±8.6 | 102.8±10.0 | 102.7±9.3 |
| LCI2.5 (units) | 9.75±1.95 | 10.21±2.20 | 9.98±2.08 |
| FEV1 % pred category | |||
| <70% pred | 10 (16.4) | 4 (6.8) | 14 (11.7) |
| ≥70– ≤90% pred | 23 (37.7) | 19 (32.2) | 42 (35.0) |
| >90% pred | 28 (45.9) | 36 (61.0) | 64 (53.3) |
| FEV1 % pred at baseline | 87.2±15.8 | 91.6±13.8 | 89.4±15.0 |
| CFQ-R respiratory domain score (Child version) (points) | 82.7±14.1 | 86.0±11.5 | 84.3±13.0 |
Data are presented as n (%) or mean±sd. ELX: elexacaftor; TEZ: tezacaftor; IVA: ivacaftor; LCI2.5: lung clearance index; FEV1: forced expiratory volume in 1 s; CFQ-R: Cystic Fibrosis Questionnaire-Revised. #: baseline was defined as the most recent non-missing measurement before the first dose of study drug in the treatment period of the parent study.
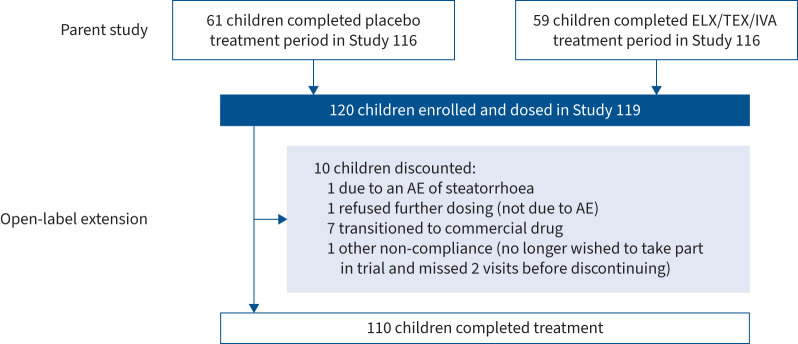
The mean±sd exposure to ELX/TEZ/IVA in this extension study was 92.9±12.4 weeks (supplementary table S2), representing 232.2 patient-years of exposure. 10 children discontinued the study (adverse event (AE) of steatorrhoea (n=1), refused further dosing not due to AE (n=1), transitioned to commercial drug (n=7) or other due to non-compliance (n=1)) (figure 1).
FIGURE 1.
Participant disposition. ELX: elexacaftor; TEZ: tezacaftor; IVA: ivacaftor; AE: adverse event.
Safety
118 (98.3%) children had at least one AE (table 2). The majority of children experienced AEs that were mild (43.3%) or moderate (48.3%) in severity. 13 (10.8%) children had at least one serious AE (SAE). The only SAE reported in more than one child was infective pulmonary exacerbation of CF which occurred in two children (1.7%) (supplementary table S4). One child (0.8%) discontinued study drug due to a SAE of steatorrhoea that subsequently resolved and was assessed as related to study drug. Alanine aminotransferase (ALT) or aspartate aminotransferase (AST) >3× and >5× upper limit of normal (ULN) occurred in 11 (9.2%) and six (5.0%) children, respectively; no children had ALT or AST >8×ULN (supplementary table S5). There were no children who had ALT or AST >3×ULN concurrent with total bilirubin elevation >2×ULN. Overall, 11 children (9.2%) had at least one elevated transaminase event (supplementary table S6). All elevated transaminase events were mild or moderate in severity, and none were serious. Rash events occurred in 13 children (10.8%), all of which were mild or moderate in severity, and none were serious (supplementary table S7). Overall, 10 out of 69 female children (14.5%) and three out of 51 male children (5.9%) had rash events. Of the 13 children who had rash events in this extension study, 10 had a single rash event and three had more than one rash event. There were five children who had a rash event within 1 month of starting ELX/TEZ/IVA, two of which were considered not related or unlikely to be related to ELX/TEZ/IVA. There were no trends observed in creatine kinase concentration levels, haematology parameters, coagulation, urinalysis, ECG or vital signs. Changes in blood pressure remained stable across the parent and open-label extension studies (supplementary table S8). Three children (2.5%) had an AE of cataract and one child (0.8%) had an AE of lenticular opacities, all of which were mild and non-serious, did not lead to change in study drug dosing, and were not considered clinically significant by investigators. None of the children had a history of lens opacity or cataract, or had any relevant findings at the baseline ophthalmological exam. All events of cataracts were ongoing at the time of study completion. Overall, AEs were generally consistent with common manifestations of CF disease or with common illnesses in children with CF aged ≥6 years. The exposure-adjusted AE rates in the open-label extension study were similar to the active arm of the parent study, and lower than the placebo arm of the parent study (table 2).
TABLE 2.
Adverse events (AEs)
| Parent Study 116 | Open-label extension Study 119 | |||||
|---|---|---|---|---|---|---|
| Placebo in Study 116 (n=61) Mean exposure 24 weeks |
ELX/TEZ/IVA in Study 116 (n=60) Mean exposure 23.7 weeks |
Any ELX/TEZ/IVA in Study 119 (n=120) Mean exposure 92.9 weeks |
||||
| n (%)# | Events/100PY | n (%)# | Events/100PY | n (%)# | Events/100PY | |
| Any AEs | 57 (93.4) | 1089.85 | 48 (80.0) | 709.62 | 118 (98.3) | 707.80 |
| AEs by maximum severity | ||||||
| Mild | 26 (42.6) | NA | 30 (50.0) | NA | 52 (43.3) | NA |
| Moderate | 29 (47.5) | NA | 16 (26.7) | NA | 58 (48.3) | NA |
| Severe | 2 (3.3) | NA | 2 (3.3) | NA | 8 (6.7) | NA |
| Life-threatening AEs | 0 | NA | 0 | NA | 0 | NA |
| AEs leading to treatment discontinuation | 0 | 0 | 1 (1.7) | 3.35 | 1 (0.8) | 0.43 |
| AEs leading to treatment interruption | 0 | 0 | 7 (11.7) | 33.47 | 10 (8.3) | 6.01 |
| Serious AEs | 9 (14.8) | 39.04 | 4 (6.7) | 13.39 | 13 (10.8) | 7.30 |
| AEs occurring in at least 20% of children (PT) in Study 119 | ||||||
| COVID-19 | NA | NA | NA | NA | 70 (58.3) | 33.50 |
| Cough | 26 (42.6) | 130.13 | 14 (23.3) | 56.90 | 62 (51.7) | 70.01 |
| Nasopharyngitis | 9 (14.8) | 39.04 | 7 (11.7) | 26.78 | 54 (45.0) | 49.39 |
| Pyrexia | 3 (4.9) | 9.76 | 1 (1.7) | 3.35 | 48 (40.0) | 36.51 |
| Headache | 12 (19.7) | 65.07 | 18 (30.0) | 73.64 | 45 (37.5) | 45.53 |
| Upper respiratory tract infection | 5 (8.2) | 26.03 | 3 (5.0) | 13.39 | 37 (30.8) | 27.92 |
| Oropharyngeal pain | 12 (19.7) | 45.55 | 3 (5.0) | 13.39 | 32 (26.7) | 21.90 |
| Rhinitis | 5 (8.2) | 16.27 | 3 (5.0) | 10.04 | 29 (24.2) | 21.47 |
| Abdominal pain | 17 (27.9) | 87.84 | 5 (8.3) | 30.13 | 27 (22.5) | 18.90 |
| Vomiting | 4 (6.6) | 13.01 | 3 (5.0) | 13.39 | 24 (20.0) | 18.04 |
ELX: elexacaftor; TEZ: tezacaftor; IVA: ivacaftor; events/100PY: number of events per 100 patient-years (336 days=48 weeks per year)=number of events/total duration of treatment-emergent period for each study in 100PY; PT: Preferred Term (MedDRA version 25.1 used for Study 119 and MedDRA 24.0 used for Study 116); NA: not applicable. #: when summarising the number and percentage of patients, a patient with multiple occurrences of the same AE or a continuing AE was counted once.
Efficacy
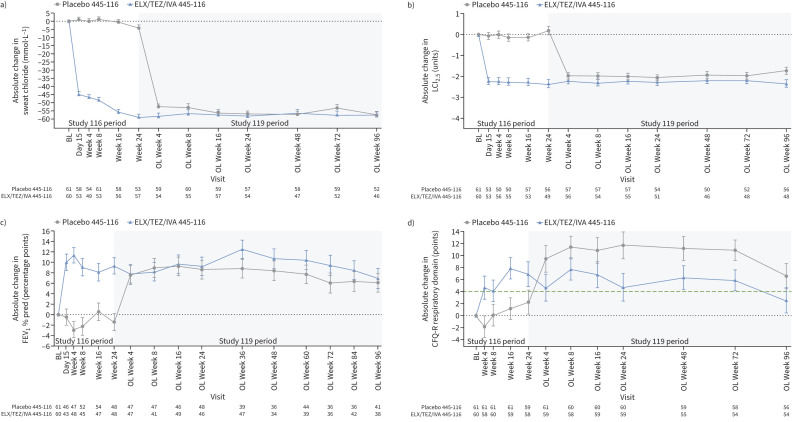
At Week 96, LS mean change in sweat chloride concentration from parent study baseline was −57.3 (95% CI −61.6– −52.9) and −57.5 (95% CI −62.0– −53.0) mmol·L−1 for children from the placebo and ELX/TEZ/IVA groups of the parent study, respectively (figure 2a and table 3). The LS mean change in LCI2.5 from parent study baseline was −1.74 (95% CI −2.09– −1.38) and −2.35 (95% CI −2.72– −1.97) units for children from the placebo and ELX/TEZ/IVA groups of the parent study, respectively, at Week 96 (figure 2b and table 3). The LS mean change in FEV1 % pred from parent study baseline was 6.1 (95% CI 2.6–9.7) and 6.9 (95% CI 3.2–10.5) percentage points for children from the placebo and ELX/TEZ/IVA groups of the parent study, respectively, at Week 96 (figure 2c and table 3). For CFQ-R respiratory domain score, an LS mean change from parent study baseline of 6.6 (95% CI 2.5–10.8) and 2.6 (95% CI −1.6–6.8) points was observed at Week 96 for subjects from the placebo and ELX/TEZ/IVA groups of the parent study, respectively (figure 2d and table 3). LS mean change ranged from 6.6 to 11.7 points for the placebo group of the parent study and from 2.6 to 7.7 points for the ELX/TEZ/IVA group of the parent study. Overall, CFQ-R respiratory domain scores, which were high at baseline, increased following initiation of ELX/TEZ/IVA and were maintained through the 96-week open-label extension period (supplementary figure S2 and supplementary table S3).
FIGURE 2.
Absolute change from parent study baseline in a) sweat chloride, b) lung clearance index (LCI2.5), c) percentage predicted forced expiratory volume in 1 s (FEV1) and d) Cystic Fibrosis Questionnaire-Revised (CFQ-R) respiratory domain score at each visit. Parent study baseline was defined as the most recent non-missing measurement before the first dose of study drug in the treatment period of the parent study. Data are presented as least squares mean±se. The minimal clinically important difference for CFQ-R respiratory domain score (4.0 points) is shown as a green dashed line in d). BL: baseline; OL: open-label.
TABLE 3.
Absolute change in sweat chloride, lung clearance index (LCI2.5), percentage predicted forced expiratory volume in 1 s (FEV1) and Cystic Fibrosis Questionnaire-Revised (CFQ-R) respiratory domain from parent study baseline# at Week 96
| Placebo–ELX/TEZ/IVA (n=61) | ELX/TEZ/IVA–ELX/TEZ/IVA (n=59) | |
|---|---|---|
| Absolute change in sweat chloride (mmol·L−1) (secondary end-point) | ||
| n | 52 | 46 |
| LS mean±se | −57.3±2.2 | −57.5±2.3 |
| 95% CI of LS mean | −61.6– −52.9 | −62.0– −53.0 |
| Absolute change in LCI2.5 (units) (secondary end-point) | ||
| n | 56 | 48 |
| LS mean±se | −1.74±0.18 | −2.35±0.19 |
| 95% CI of LS mean | −2.09– −1.38 | −2.72– −1.97 |
| Absolute change in FEV1 % pred (percentage points) (other end-point) | ||
| n | 41 | 38 |
| LS mean±se | 6.1±1.8 | 6.9±1.9 |
| 95% CI of LS mean | 2.6–9.7 | 3.2–10.5 |
| Absolute change in CFQ-R respiratory domain score (points) (other end-point) | ||
| n | 56 | 54 |
| LS mean±se | 6.6±2.1 | 2.6±2.1 |
| 95% CI of LS mean | 2.5–10.8 | −1.6–6.8 |
ELX: elexacaftor; TEZ: tezacaftor; IVA: ivacaftor; LS: least squares. #: parent study baseline was defined as the most recent non-missing measurement before the first dose of study drug in the treatment period of the parent study.
Discussion
We evaluated the safety and efficacy of ELX/TEZ/IVA in children with CF aged ≥6 years who had an F/MF genotype over a 2-year period. ELX/TEZ/IVA was generally safe and well tolerated, with a safety profile consistent with prior studies [16, 17]. The significant improvements in lung function and robust decreases in sweat chloride concentration previously reported for children given ELX/TEZ/IVA in the parent study were maintained over the period of this extension study. Children who received placebo in the parent study had similar efficacy improvements after starting ELX/TEZ/IVA treatment in this open-label extension study, and these improvements were maintained.
CFTR modulator therapies, such as ELX/TEZ/IVA, require ongoing administration, making studies of long-term safety and efficacy imperative to understanding the clinical benefits of extended use [20]. In the current study, children aged ≥6 years who had previously received ELX/TEZ/IVA in a 24-week, phase 3b, randomised clinical trial, received ELX/TEZ/IVA for an additional 96 weeks. The majority of AEs observed were mild or moderate in severity, and generally consistent with common manifestations of CF in this age group. There was a higher incidence of AEs related to respiratory and upper respiratory tract infection in this extension study than reported in the parent study, most likely the result of symptomatic COVID-19 infections, which were not reported in the parent study that was completed during the early part of the COVID-19 pandemic, but which were the most common AEs in this study (58.3%). Overall, the exposure-adjusted rate of AEs was lower in this extension study than in the parent study. There was a low rate of SAEs (10.8%) and, similar to other AEs, SAEs were generally consistent with common manifestations of CF and complications for children in this age group (e.g. COVID-19, cough, nasopharyngitis, pyrexia, headache, upper respiratory tract infection, oropharyngeal pain, rhinitis, abdominal pain and vomiting). One child experienced a SAE of steatorrhoea that was assessed by the study investigators as being related to study drug and resolved after study drug discontinuation. The incidence of transaminase events (9.2%) and rash events (10.8%) was consistent with the parent study and with previous studies of ELX/TEZ/IVA in children aged 6–11 years. Overall, ELX/TEZ/IVA treatment for an additional 96 weeks remained safe and well tolerated, with no new safety findings.
Sweat chloride concentration is an established biomarker of CFTR function, with decreases in sweat chloride concentration suggestive of improved CFTR function in patients with CF [21]. Previous studies, including the parent study of this extension study, showed initiation of ELX/TEZ/IVA treatment led to rapid and robust decreases in sweat chloride concentrations in children aged 6–11 years with CF [16, 17]. Consistent with these previous observations, a robust decrease in mean sweat chloride concentration was seen in children who initiated ELX/TEZ/IVA in this extension study after previously receiving placebo in the parent study. The decreases in sweat chloride concentration following initiation of ELX/TEZ/IVA in both treatment groups were maintained throughout the 96-week treatment period of this extension study. These results suggest that ELX/TEZ/IVA leads to rapid and durable improvements in CFTR function in children with CF.
Significant improvements in lung function and respiratory symptoms were also previously reported in children treated with ELX/TEZ/IVA [17]. While spirometry is typically used to detect impairment in lung function, values for FEV1 % pred are often close to the normal range in younger children with CF despite evidence of early structural lung damage [22]. In the current study, robust increases in mean FEV1 % pred were seen in children who began ELX/TEZ/IVA treatment in this extension study, consistent with the increases in children given ELX/TEZ/IVA in the parent study, and were maintained through this extension study. To further elucidate the impact of ELX/TEZ/IVA treatment on lung function in this paediatric population, changes in LCI2.5 were also assessed. Measures of LCI2.5 can provide a more sensitive means to detect early changes in lung function and are considered an appropriate efficacy measurement for younger children with airway diseases [23, 24]. A natural history study reported that children 3–18 years of age with CF not treated with a CFTR modulator had an unadjusted mean annual increase in LCI2.5 of 0.29 units [25], as would be expected in a disease characterised by progressive loss of lung function. In contrast, robust decreases in mean LCI2.5 were observed in children after beginning treatment with ELX/TEZ/IVA. The improvements in mean LCI2.5 seen in both groups after starting ELX/TEZ/IVA were maintained through the 96-week treatment period. Children also had improvements in their respiratory symptoms following the initiation of ELX/TEZ/IVA, with increases in CFQ-R respiratory domain scores. The mean changes from parent study baseline in CFQ-R respiratory domain score ranged from 6.6 to 11.7 points in children who received placebo in the parent study and 2.6 to 7.7 points in those who received ELX/TEZ/IVA in the parent study. The differences in change from baseline between the two treatment groups following start of ELX/TEZ/IVA are likely attributable to a ceiling effect, with smaller changes from baseline observed in the group who previously received ELX/TEZ/IVA in the parent study and had a higher baseline mean CFQ-R respiratory domain score (85.7 versus 82.7 points). Despite variability in the change from baseline in mean CFQ-R respiratory domain score at study visits, and a mean change at Week 96 of 2.6 points in children who previously received ELX/TEZ/IVA which was below the established minimal clinically important difference in people with CF and stable disease of 4 points [26], both groups generally maintained improvements in CFQ-R respiratory domain score throughout the extension study and had similar scores at Week 96 (89.6 and 89.2 points) that were in the normal range. Taken together, these results demonstrate that ELX/TEZ/IVA leads to durable improvements in lung function and respiratory symptoms in children with CF.
It should be noted that as this was an open-label study, the lack of a direct comparator group does limit the ability to interpret the safety and efficacy data.
ELX/TEZ/IVA was generally safe and well tolerated for up to 96 weeks of treatment in children ≥6 years, with a safety profile generally consistent with the established safety profile of ELX/TEZ/IVA. The sustained improvements in lung function, respiratory symptoms and CFTR function seen in the parent study were maintained during this open-label extension study for children who received ELX/TEZ/IVA in the parent study. Similarly, children who received placebo in the parent study had efficacy improvements upon initiating ELX/TEZ/IVA that were maintained throughout the open-label extension treatment period. These results confirm the favourable safety profile and durable clinical benefits of ELX/TEZ/IVA in this paediatric population.
Shareable PDF
Acknowledgements
The authors would like to thank the participants and their families, the study investigators and coordinators for their role in the study. This study was supported by Vertex Pharmaceuticals Incorporated. Medical writing support was provided by Nathan Blow and Lorilei Richardson. Graphics support was provided by Alexandra Battaglia. N. Blow, L. Richardson and A. Battaglia are employees of Vertex Pharmaceuticals Incorporated (Boston, MA, USA) and may own stock or stock options in that company.
Footnotes
This clinical trial is prospectively registered with ClinicalTrials.gov as NCT04545515.
Ethics statement: The study was be conducted in accordance with the current International Council for Harmonisation E6 Good Clinical Practice Guidelines, which are consistent with the ethical principles founded in the Declaration of Helsinki, and in accordance with local applicable laws and regulations. The institutional review board/independent ethics committee reviewed all appropriate study documentation to safeguard the rights, safety and wellbeing of the subjects. The study was conducted only at sites where ethics committee approval was obtained. The protocol, investigator's brochure, sample informed consent form, advertisements (if applicable), written information given to the subjects (including diary cards), safety updates and annual progress reports, and any revisions to these documents, were provided to the review board by the investigator or Vertex, as allowable by local applicable laws and regulations.
Author contributions: The study sponsor (Vertex Pharmaceuticals Incorporated) designed the protocol in collaboration with the academic authors. Site investigators collected the data, which were analysed by the sponsor. All authors had full access to the study data. All authors participated in the development and subsequent revisions of the manuscript. All authors approved the final version of the manuscript submitted for publication.
This article has an editorial commentary: https://doi.org/10.1183/13993003.00793-2025
Conflict of interest: M.A. Mall reports grants from Vertex Pharmaceuticals Incorporated, Boehringer Ingelheim, German Innovation Fund, German Ministry for Education and Research (BMBF) and German Research Foundation (DFG), travel support from Vertex Pharmaceuticals Incorporated and Boehringer Ingelheim, consulting fees from AbbVie, Boehringer Ingelheim, Enterprise Therapeutics, Kither Biotec, Prieris, Recode, Splisense and Vertex Pharmaceuticals Incorporated, is a Fellow of the European Respiratory Society (FERS), and serves on the advisory board for AbbVie, Boehringer Ingelheim, Enterprise Therapeutics, Kither Biotec, Pari and Vertex Pharmaceuticals Incorporated. C.E. Wainwright received honoraria from Vertex Pharmaceuticals Incorporated and personal fees on a per-participant basis to institutions derived from pharmaceutical studies conducted, and served as Deputy Editor for Thorax and Associate Editor for Respirology, and on the advisory board to Vertex Pharmaceuticals Incorporated. J. Legg reports payment or honoraria for lectures, presentations, manuscript writing or educational events from the Vertex Pharmaceuticals Incorporated cystic fibrosis advisory board. M. Chilvers reports support for the current manuscript, consulting fees and payment or honoraria for educational events from Vertex Pharmaceuticals Incorporated, and serving as Chair of the Health Care Advisory Committee for Cystic Fibrosis Canada and Chair of the CF Working Group for the Canadian Thoracic Society. S. Gartner reports honoraria for lectures from Vertex Pharmaceuticals Incorporated. A-M. Dittrich reports grants or funding from Vertex Pharmaceuticals Incorporated (for execution of clinical studies) and German Federal Ministry of Education and Research (German Lung Center (DZL); for clinical research), payment or honoraria from Vertex Pharmaceutical Incorporated (for an article on the long-term effects of CFTR modulators and an online education forum Coliquio), StreamedUP (for an online educational presentation) and the Christiane Herzog Foundation (for a medical book on CF care), payment for expert testimony from the Austrian Society for Rare Disease, and leadership or fiduciary roles as a member of the German CF patient advocacy board, member of the German CF Clinical Trial Network Executive Committee, and as part of the ECFS CTN Executive Committee. F. Stehling reports clinical trial sponsorship and honoraria for lectures from Vertex Pharmaceuticals Incorporated. S. Connor, S. Grant, N. Suresh and T.G. Weinstock are employees of Vertex Pharmaceuticals Incorporated and may own stock or stock options in that company. J.C. Davies reports research grants from UK Cystic Fibrosis Trust, US CF Foundation, Cystic Fibrosis Ireland and EPSRC, clinical trial leadership and/or advisory board and speaking roles with Vertex Pharmaceuticals Incorporated, Boehringer Ingelheim, Eloxx, AlgiPharma, AbbVie, Arcturus, Enterprise Therapeutics, Recode, LifeArc, Genentech and Tavanta, and is Deputy Editor for the Journal of Cystic Fibrosis and President of the European Cystic Fibrosis Society.
Support statement: This study was funded by Vertex Pharmaceuticals Incorporated. Supported by the National Institute of Health and Care Research through the Imperial Biomedical Research Centre, the Brompton Clinical Research Facility and a Senior Investigator Award (to J.C. Davies). Funding information for this article has been deposited with the Crossref Funder Registry.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material
ERJ-02435-2024.Supplement
Data availability
Details on Vertex data sharing criteria and process for requesting access can be found at: www.vrtx.com/independent-research/clinical-trial-data-sharing
References
- 1.Endres TM, Konstan MW. What is cystic fibrosis? JAMA 2022; 327: 191. doi: 10.1001/jama.2021.23280 [DOI] [PubMed] [Google Scholar]
- 2.Grasemann H, Ratjen F. Cystic fibrosis. N Engl J Med 2023; 389: 1693–1707. doi: 10.1056/NEJMra2216474 [DOI] [PubMed] [Google Scholar]
- 3.Cystic Fibrosis Foundation . About Cystic Fibrosis. 2024. www.cff.org/intro-cf/about-cystic-fibrosis Date last accessed: 13 March 2024.
- 4.Mall MA, Burgel PR, Castellani C, et al. Cystic fibrosis. Nat Rev Dis Primers 2024; 10: 53. doi: 10.1038/s41572-024-00538-6 [DOI] [PubMed] [Google Scholar]
- 5.Myer H, Chupita S, Jnah A. Cystic fibrosis: back to the basics. Neonatal Netw 2023; 42: 23–30. doi: 10.1891/NN-2022-0007 [DOI] [PubMed] [Google Scholar]
- 6.VanDevanter DR, Kahle JS, O'Sullivan AK, et al. Cystic fibrosis in young children: a review of disease manifestation, progression, and response to early treatment. J Cyst Fibros 2016; 15: 147–157. doi: 10.1016/j.jcf.2015.09.008 [DOI] [PubMed] [Google Scholar]
- 7.Stahl M, Steinke E, Graeber SY, et al. Magnetic resonance imaging detects progression of lung disease and impact of newborn screening in preschool children with cystic fibrosis. Am J Respir Crit Care Med 2021; 204: 943–953. doi: 10.1164/rccm.202102-0278OC [DOI] [PubMed] [Google Scholar]
- 8.Solomon GM, Marshall SG, Ramsey BW, et al. Breakthrough therapies: cystic fibrosis (CF) potentiators and correctors. Pediatr Pulmonol 2015; 50: Suppl. 40, S3–S13. doi: 10.1002/ppul.23240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mall MA, Mayer-Hamblett N, Rowe SM. Cystic fibrosis: emergence of highly effective targeted therapeutics and potential clinical implications. Am J Respir Crit Care Med 2020; 201: 1193–1208. doi: 10.1164/rccm.201910-1943SO [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiferaw D, Faruqi S. Profile of tezacaftor/ivacaftor combination and its potential in the treatment of cystic fibrosis. Ther Clin Risk Manag 2019; 15: 1029–1040. doi: 10.2147/TCRM.S165027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graeber SY, Mall MA. The future of cystic fibrosis treatment: from disease mechanisms to novel therapeutic approaches. Lancet 2023; 402: 1185–1198. doi: 10.1016/S0140-6736(23)01608-2 [DOI] [PubMed] [Google Scholar]
- 12.Middleton PG, Mall MA, Drevinek P, et al. Elexacaftor–tezacaftor–ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med 2019; 381: 1809–1819. doi: 10.1056/NEJMoa1908639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heijerman HGM, McKone EF, Downey DG, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet 2019; 394: 1940–1948. doi: 10.1016/S0140-6736(19)32597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ideozu JE, Liu M, Riley-Gillis BM, et al. Diversity of CFTR variants across ancestries characterized using 454,727 UK biobank whole exome sequences. Genome Med 2024; 16: 43. doi: 10.1186/s13073-024-01316-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ong T, Ramsey BW. Cystic fibrosis: a review. JAMA 2023; 329: 1859–1871. doi: 10.1001/jama.2023.8120 [DOI] [PubMed] [Google Scholar]
- 16.Zemanick ET, Taylor-Cousar JL, Davies J, et al. A phase 3 open-label study of elexacaftor/tezacaftor/ivacaftor in children 6 through 11 years of age with cystic fibrosis and at least one F508del allele. Am J Respir Crit Care Med 2021; 203: 1522–1532. doi: 10.1164/rccm.202102-0509OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mall MA, Brugha R, Gartner S, et al. Efficacy and safety of elexacaftor/tezacaftor/ivacaftor in children 6 through 11 years of age with cystic fibrosis heterozygous for F508del and a minimal function mutation: a phase 3b, randomized, placebo-controlled study. Am J Respir Crit Care Med 2022; 206: 1361–1369. doi: 10.1164/rccm.202202-0392OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wyler F, Oestreich MA, Frauchiger BS, et al. Correction of sensor crosstalk error in Exhalyzer D multiple-breath washout device significantly impacts outcomes in children with cystic fibrosis. J Appl Physiol 2021; 131: 1148–1156. doi: 10.1152/japplphysiol.00338.2021 [DOI] [PubMed] [Google Scholar]
- 19.Robinson PD, Jensen R, Seeto RA, et al. Impact of cross-sensitivity error correction on representative nitrogen-based multiple breath washout data from clinical trials. J Cyst Fibros 2021; 21: e204–e207. doi: 10.1016/j.jcf.2021.08.033 [DOI] [PubMed] [Google Scholar]
- 20.Cuzick J. The importance of long-term follow up of participants in clinical trials. Br J Cancer 2023; 128: 432–438. doi: 10.1038/s41416-022-02038-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCague AF, Raraigh KS, Pellicore MJ, et al. Correlating cystic fibrosis transmembrane conductance regulator function with clinical features to inform precision treatment of cystic fibrosis. Am J Respir Crit Care Med 2019; 199: 1116–1126. doi: 10.1164/rccm.201901-0145OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lombardi E, Gambazza S, Pradal U, et al. Lung clearance index in subjects with cystic fibrosis in Italy. Ital J Pediatr 2019; 45: 56. doi: 10.1186/s13052-019-0647-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraemer R, Blum A, Schibler A, et al. Ventilation inhomogeneities in relation to standard lung function in patients with cystic fibrosis. Am J Respir Crit Care Med 2005; 171: 371–378. doi: 10.1164/rccm.200407-948OC [DOI] [PubMed] [Google Scholar]
- 24.Stahl M, Wielputz MO, Graeber SY, et al. Comparison of lung clearance index and magnetic resonance imaging for assessment of lung disease in children with cystic fibrosis. Am J Respir Crit Care Med 2017; 195: 349–359. doi: 10.1164/rccm.201604-0893OC [DOI] [PubMed] [Google Scholar]
- 25.Frauchiger BS, Binggeli S, Yammine S, et al. Longitudinal course of clinical lung clearance index in children with cystic fibrosis. Eur Respir J 2021; 58: 2002686. doi: 10.1183/13993003.02686-2020 [DOI] [PubMed] [Google Scholar]
- 26.Quittner AL, Modi AC, Wainwright C, et al. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire-Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009; 135: 1610–1618. doi: 10.1378/chest.08-1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This PDF extract can be shared freely online.
Shareable PDF
ERJ-02435-2024.Shareable
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material
ERJ-02435-2024.Supplement
Data Availability Statement
Details on Vertex data sharing criteria and process for requesting access can be found at: www.vrtx.com/independent-research/clinical-trial-data-sharing