Summary
The implementation of genetic medicine services in African healthcare systems is a complex process that presents both challenges and opportunities. The primary objective of this study was to provide evidence-based recommendations to policymakers and healthcare organizations to help ensure the successful integration of genetic services into African healthcare systems. To achieve this goal, we conducted a scoping review of peer-reviewed studies published between 2003 and 2023. The studies were sourced from PubMed, Scopus, and Africa-wide information databases. We based their findings on eight relevant research studies conducted between 2016 and 2023, covering a wide range of genetic topics in six African countries, including Cameroon, Kenya, Nigeria, Rwanda, South Africa, and Tanzania. The studies identified several challenges associated with the implementation of genetic services in African healthcare systems. These challenges include a lack of awareness and education about genetic diseases, barriers to genetic testing, resource limitations, ethical dilemmas, and challenges in follow-up and retention. However, the authors also highlighted opportunities and strategies for successful implementation, emphasizing preventive measures through community engagement, advocacy, and supportive networks. Apart from the potential to improve healthcare outcomes, implementing genetic services in Africa presents opportunities and challenges for healthcare and biotech companies. To address gaps in disease awareness, we recommend that healthcare providers should invest in education, collaborate with local institutions, and utilize digital platforms. Furthermore, businesses should explore innovative, cost-effective genetic testing models and create dialog platforms like online forums to positively impact African health care.
Keywords: genetic services, Africa, health care, challenges, opportunities, community engagement
This study systematically reviews challenges and opportunities in integrating genetic medicine services into African healthcare systems. The main barriers include limited awareness, education, and access to genetic services. Community engagement, advocacy, and digital innovations can enhance medical curricula, research development, genetic medicine services, infrastructures, and healthcare outcomes.
Introduction
Genetic services, which include genetic testing (GT), genetic counseling (GC), and personalized medicine (PM), are reshaping modern health care. They offer remarkable insights and personalized approaches to patient care.1,2 GT involves analyzing an individual’s DNA to detect genetic variations associated with specific health conditions, thus providing critical information for diagnosis and risk assessment.3 This is complemented by GC, which equips individuals and families with knowledge of genetic risks, inheritance patterns, and the significance of test results, enabling informed decision-making.4 PM advances this effort by tailoring treatment plans, identifying risk factors, and implementing preventive measures based on genetic profiles, further advancing the potential of genetic services by aligning health care with each patient’s unique genetic makeup.5 Together, these components enable a more holistic and personalized approach to health care, which has been shown to improve patient outcomes.
The concept of digital phenotyping, which involves the real-time monitoring of human behavior through personal digital devices, has significant potential to enhance genetic services. It can improve the early detection of genetic disorders and support customized interventions based on both genetic and phenotypic profiles. For instance, smartphone data have demonstrated an impressive 83% accuracy in predicting schizophrenia relapse, illustrating how digital biomarkers can enrich genetic risk assessments in mental health.6 Additionally, monitoring speech and motor activity through mobile technology can facilitate the early diagnosis of neurodegenerative diseases like Parkinson disease, which often have genetic connections.7 However, it is crucial to validate these findings across diverse populations, especially in low-resource settings where access to smartphones and digital literacy can vary greatly.8
In the African healthcare context, the integration of genetic services holds transformative potential for enhancing the quality and scope of health care across the continent. Genetic insights play an essential role in understanding, diagnosing, and treating various diseases, making the incorporation of genetic services into African healthcare systems a vital step toward providing precise diagnoses and personalized treatments.9,10 Notably, early detection and prevention of genetic disorders are among the primary benefits of genetic integration. By identifying at-risk individuals, healthcare providers can offer timely counseling and interventions, ultimately reducing the long-term burden on healthcare systems and improving patient outcomes.11,12,13
Despite these potential benefits, the use of genetic services in African healthcare systems faces significant challenges. Much of the current research in digital phenotyping has originated from high-income countries, raising questions about its applicability to diverse genetic backgrounds, particularly in Africa, where populations have the highest human genomic diversity.14 A systematic review by Mikat-Stevens et al. in 2015 revealed that while studies in Europe and the United States have extensively explored genetic services, the African literature remains underrepresented.15 Resource limitations, insufficient training of healthcare personnel, and ethical issues concerning privacy and consent are well-documented barriers, but their application to the African context requires careful adaptation to diverse cultural, socioeconomic, and healthcare settings.16
Cameroon, a lower- to middle-income country in central Africa, with a population of 28,372,687 in 2023,17 operates a mixed public-private healthcare system structured into central, intermediary, and peripheral levels.18 Despite ongoing improvements, the system faces significant challenges, including underfunding, a shortage of specialized personnel, and disparities in healthcare access between urban and rural areas.18 The burden of infectious and non-communicable diseases further strains healthcare resources, while access to specialized services like genetic healthcare remains limited. Wonkam et al. highlighted a lack of genetic knowledge among Cameroonian healthcare professionals, reflecting a regional issue also observed in South Africa, where Kromberg et al. reported a critical shortage of genetic healthcare providers.19,20 This scarcity underscores the urgent need for capacity building in medical genetics to improve the diagnosis and management of genetic disorders and congenital conditions in Cameroon and other low- and middle-income countries.
Genetic services are foundational to precision medicine, yet their adoption in Africa remains constrained by infrastructural, financial, and educational hurdles.21 While some African nations have made significant strides in developing genetic programs, Cameroon, in particular, still lacks a structured framework for delivering these essential services. By learning from existing research and successful models across the continent, there is a real opportunity to address the unique challenges faced. This scoping review aims to synthesize insights from genetic service initiatives in Africa, offering tailored recommendations that can enable African countries like Cameroon to establish an effective and sustainable genetic service model.
Material and methods
Research design
This study employed a scoping review methodology, aiming to capture a comprehensive view of genetic service integration within African healthcare systems by including both qualitative and quantitative studies, along with case studies and interviews with key stakeholders in the field. The review time frame spanned from 2003 to 2023, enabling a broad understanding of historical and recent advancements.
Search strategy
Following a multi-faceted search approach,22 we conducted an exhaustive review using three key databases: PubMed, Scopus, and African Wide Information. A structured search in February 2024 applied key words and phrases related to “genetic services” and “Africa,” ensuring the retrieval of relevant literature across different study designs.
The search was limited to English-language publications from the period 2003–2023, focusing specifically on research relevant to Africa. To capture a diverse range of studies, our search terms included all African countries and a wide spectrum of genetic services, such as pediatric GC, newborn screening follow-up, genetic service provision and delivery models, genetic healthcare services, diagnostic services, PM, and precision medicine. These terms were carefully selected to ensure the retrieval of relevant literature reflecting the landscape of genetic services across the continent.
Study selection
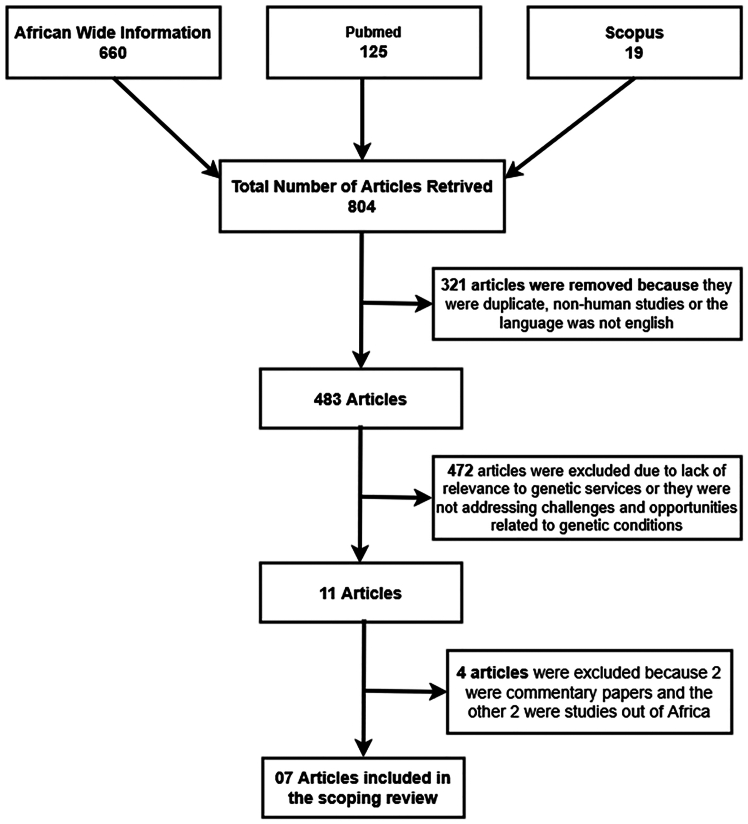
A well-defined inclusion and exclusion criterion was established to streamline the selection process and ensure accuracy. We focused on primary, peer-reviewed studies that addressed our research question on genetic service integration within African healthcare systems. Unrelated studies or those conducted outside the African context were excluded. Only studies meeting predefined quality standards were included, ensuring rigor in the selection process. The search and selection process, conducted in February 2024, is illustrated in Figure 1.
Figure 1.
Study selection process
Data extraction and analysis
For data extraction, we developed a comprehensive form to capture key study characteristics, methodologies, findings, challenges, opportunities, and strategies.23 NVIVO 14 was used to analyze data, employing framework analysis to derive themes and subthemes for a narrative synthesis of findings. To enhance rigor and quality, a third-party review (S.N.) was performed to scrutinize themes and provide input on areas of disagreement, reaching a consensus on the inclusion of studies in the final review.
Results
Description of study strategy
We conducted a thorough search and found 7 studies on genetics in 6 African countries between 2016 and 2023. These studies explored different aspects of GC and testing, primarily focusing on addressing challenges and possible opportunities (Table 1).
Table 1.
Description of the key research articles
| First author’s name et al. (year of publication) | Country of study | Study design | Genetic service | Results/findings |
|---|---|---|---|---|
| Silverstein et al. (2016)24 | Rwanda | review | GT for cancer | genomic medicine is underutilized in low-income areas, contributing to health disparities; limited research on the genetic determinants of breast cancer in African populations hinders tailored treatment; strengthening research and integrating genomic medicine into breast cancer care is crucial to improving outcomes, despite systemic barriers24 |
| Tahir et al. (2018)25 | Nigeria | review | prenatal diagnosis for SCD | PDT is growing in Nigeria, enabling early detection of genetic disorders; however, ethical concerns arise around counseling, sample collection, informed consent, and decisions regarding affected pregnancies; this paper examines these ethical dilemmas in the context of the fetus, pregnant women, and society25 |
| Zhong et al. (2021)26,27 | Kenya | qualitative study | GC and testing | Kenya faces a shortage of licensed genetic counselors and limited access to GT; this study found that key barriers include education, training, costs, and counseling challenges, while opportunities lie in growing demand, multidisciplinary collaboration, and improved laboratory infrastructure; addressing these factors can enhance genetic service delivery27 |
| Nkya et al. (2022)28 | Tanzania | quantitative, prospective | neonatal diagnosis of SCD | establishing SCD birth cohorts supports early disease management, but such cohorts are rare in Africa; this study followed 341 babies for 3 years, collecting clinical and demographic data; key insights highlight the importance of integrating cohorts with existing programs for sustainability28 |
| Galadanci et al. (2023)29 | Nigeria | qualitative study | premarital GC for SCD | premarital GC for SCD is unavailable in Nigeria, with barriers including limited awareness, testing facilities, transportation, and stigma; however, community willingness for premarital testing exists; addressing these challenges can enhance access to GC29 |
| Zingela et al. (2023)30 | South Africa | review | GC for schizophrenia | GC for schizophrenia poses ethical challenges, requiring clear communication and adherence to principles like autonomy, beneficence, and informed consent; lessons from South African studies highlight the need for culturally sensitive approaches and community advisory boards; building expertise through funding and resources is essential for ethical and effective counseling30 |
| Mapoko et al. (2023)31 | Cameroon | quantitative study | GC and testing for cancer | at Yaoundé General Hospital, 97.5% of cancer patients were willing to undergo and pay for genetic cancer screening; however, barriers included cost, lack of equipment, and anticipated anxiety; the findings support establishing a cancer risk assessment clinic to improve screening programs in Cameroon31 |
Our findings reveal a diverse array of studies focused on the implementation, challenges, and ethical considerations surrounding genetic services across various African countries, including Nigeria, Rwanda, Tanzania, South Africa, Cameroon, and Kenya. Research spans a range of genetic conditions, such as sickle cell disease (SCD), breast cancer, and schizophrenia, with services including prenatal genetic diagnosis, premarital counseling, and cancer GT. Studies employ various methodologies—qualitative, quantitative, and reviews—highlighting both systemic barriers and unique opportunities to advance genetic services in Africa. Key findings underscore critical issues such as limited access to GC, ethical concerns in prenatal testing, community attitudes toward genetic conditions, and the need for increased research capacity. This body of literature provides insight into the gaps and potential strategies for improving genetic services, emphasizing the importance of culturally relevant approaches, resource allocation, and policy support to enhance healthcare outcomes through genetic services across the continent (see Table 1).
Themes identified
The findings from the review reveal key themes and subthemes related to the challenges and opportunities in implementing genetic services within African healthcare systems (Table 2). Challenges include a widespread lack of disease awareness and education, as well as significant barriers to GT, such as limited access to resources and testing facilities. Ethical dilemmas surrounding informed consent and cultural considerations also present challenges in integrating these services. Additional difficulties include follow-up and retention of patients, which impact continuity of care. However, the studies identify promising opportunities for improving genetic services in Africa. Prevention strategies, community engagement, advocacy, and supportive networks emerge as critical facilitators, providing a foundation for awareness building and fostering partnerships to support service delivery. Collectively, these findings highlight both the obstacles and actionable pathways toward enhancing genetic services for better healthcare outcomes in Africa (see Table 2).
Table 2.
Description of the different themes identified: Challenges and opportunities
| Main theme | Subtheme | References |
|---|---|---|
| Challenges of implementing genetic services | lack of disease awareness and education | Galadanci et al. (2023)29; Mapoko et al. (2023)31; Nkya et al. (2022)28; Silverstein et al. (2016)24; Tahir et al. (2018)25; Zhong et al. (2021)26,27 |
| barriers to testing | Galadanci et al. (2023)29; Mapoko et al. (2023)31; Silverstein et al. (2016)24; Tahir et al. (2018)25; Zhong et al. (2021)26,27 | |
| recourse, limitations, and access to services | Nkya et al. (2022)28; Silverstein et al. (2016)24; Zhong et al. (2021)26,27; Zingela et al. (2023)30 | |
| ethical dilemmas | Tahir et al. (2018)25; Zingela et al. (2023)30 | |
| follow-up and retention challenges | Nkya et al. (2022)28 | |
| Opportunities to implementing genetic services | prevention | Galadanci et al. (2023)29; Mapoko et al. (2023)31; Silverstein et al. (2016)24; Tahir et al. (2018)25; Zingela et al. (2023)30 |
| presence of supportive networks | Nkya et al. (2022)28; Silverstein et al. (2016)24; Zhong et al. (2021)26,27; Zingela et al. (2023)30 | |
| advocacy | Silverstein et al. (2016)24; Zhong et al. (2021)26,27 | |
| community engagement | Silverstein et al. (2016)24; Zingela et al. (2023)30 |
Discussion
From this study, the challenges confronting the implementation of genetic services in Africa are extensively documented. The findings of this review highlight the disparities in genetic service implementation across Africa and provide a foundation for tailoring a genetic service model specific to African healthcare systems. While countries like Tanzania, Nigeria, and South Africa have made significant progress, some other countries like Kenya, Cameroon, and Rwanda have yet to establish structured genetic services, despite the high burden of genetic disorders such as SCD and hereditary cancers. Notable efforts were reported in Cameroon, where initiation of prenatal diagnosis of SCD and chromosomal anomalies were used as points of entry for a medical genetic service.32,33,34 By analyzing the strategies employed in other African nations, this study offers a roadmap for policymakers, clinicians, and researchers in many African countries to develop a sustainable, context-specific approach to genetic healthcare.
Despite the enormous potential of genomic medicine to address health disparities and improve clinical outcomes, its application remains underutilized in many low-income regions.35 This is especially apparent in the case of cancer care, where the lack of research on genetic determinants in African populations limits the development of tailored treatments. As demonstrated by studies in Cameroon, where a high percentage of cancer patients expressed willingness to undergo genetic screening, there is a clear demand for genetic services.31 However, systemic barriers such as cost, lack of infrastructure, and limited access to resources remain significant hurdles to be addressed. These findings underscore the need to strengthen research efforts and build capacity for genomic medicine, particularly in underrepresented regions like Africa.36,37 Additionally, integrating genetic services into cancer care through the establishment of risk assessment clinics could provide accessible and affordable genetic cancer screening, helping to bridge the gap in early detection and treatment options.38
A critical area that has garnered increasing attention is the integration of genomic medicine into prenatal care. As seen in Nigeria, where prenatal diagnostic testing (PDT) is growing, the ability to detect genetic disorders early holds promise for reducing the burden of disease and improving maternal and child health outcomes. However, ethical concerns about counseling, informed consent, and decision-making in the context of prenatal testing must be navigated carefully.25 Similar challenges were reported in Cameroon, Tanzania, and Ghana regarding the development of prenatal genetic services for countries with SCD.39,40 In particular, in Cameroon, while authors observed a high acceptance of the principle of prenatal diagnosis in most participants and stakeholders, including doctors, adult patients, and parents with affected children, the attitude toward the termination of affected pregnancy varied (lower among doctors and higher among parents), indicating a potential ethical conflict between various segment of societies.41,42,43 These concerns highlight the importance of developing frameworks that ensure that ethical guidelines are adhered to while also empowering individuals and families to make informed choices about their reproductive health. Given the sensitive nature of GT, it is essential to provide appropriate education and support for pregnant women and their families, taking into consideration cultural perspectives, local norms, and legal implications.26,44 Ethical challenges related to GC and testing are not unique to prenatal care, as evidenced by studies on schizophrenia and SCD, where cultural sensitivity and effective communication strategies are paramount for building trust and ensuring that individuals fully understand the implications of genetic tests.30,45 Additionally, Blackshaw argued that a pro-choice stance could widen the scope of permissible selective abortion, while Al Shamsi et al. (2020) highlighted the importance of addressing language barriers in GC to enhance communication between healthcare providers and patients.46,47
While the evidence points to significant gaps in genetic service delivery in several African countries, there are also important opportunities to enhance access to these services and improve their quality. In Kenya, for example, the shortage of licensed genetic counselors and limited access to GT presents both challenges and opportunities.27 There is a clear need for increased training and education to build a skilled workforce of genetic counselors.48 Moreover, addressing the high costs of GT and improving access to genetic services in rural and underserved areas are crucial steps in making genomic medicine more accessible. As highlighted in the Nigerian context, community willingness for premarital GC for SCD exists, but logistical and sociocultural barriers hinder widespread adoption.29,49,50 Increasing awareness, improving access to testing facilities, and addressing stigma are critical factors that can pave the way for broader implementation of genetic services in Africa.51,52 Furthermore, leveraging successful models from countries like South Africa could provide insight into effective strategies for improving access and sustainability of genetic services.52 Community engagement and education are also vital for raising awareness about genetic services and dispelling myths.53 Chapple et al. noted that limited access to information hampers the ability to utilize genetic services, underscoring the necessity for increased educational efforts.54
Despite the challenges faced in the implementation of genomic services, there are promising avenues for growth. Establishing birth cohorts for diseases such as SCD, integrating genetic services into existing healthcare programs, and expanding research on the genetic determinants of diseases common in African populations are essential steps toward improving genetic health care in the region.28 Moreover, the positive findings from Cameroon regarding cancer screening suggest that with proper infrastructure, a high level of patient engagement and willingness to participate in GT exists.31 This presents an important opportunity to scale up genetic services in the region, particularly through the establishment of risk assessment clinics that could provide accessible and affordable genetic cancer screening to more individuals. Hamilton et al. emphasized the need for a comprehensive approach to addressing the obstacles of genetic service implementation, including disease awareness, poor healthcare infrastructure, and cultural stigmas.55 Establishing community advisory boards can help bridge the gap between healthcare providers and communities, ensuring that genetic services address specific needs. O’Daniel et al. found that intermediaries can improve communication and satisfaction in healthcare settings.56 Furthermore, advocacy is crucial for driving policy changes and securing resources. Martinez et al. highlighted the importance of national registries for SCD patients, illustrating the role of patient advocacy in enhancing genetic healthcare access.57 Collaborative, multidisciplinary efforts are essential for fostering culturally sensitive genetic service implementation.58
Implications
Implementing genetic services in Africa presents challenges and opportunities that have significant business implications for healthcare, biotechnology, and genetic services companies. Understanding these implications is crucial for developing effective business strategies and contributing positively to the healthcare landscape in Africa.
One promising solution for overcoming some of the barriers to genetic service delivery is the use of digital phenotyping. Digital phenotyping, the use of digital devices and mobile applications to collect data on health status and behavior, offers a novel way to integrate genetic services into healthcare systems, particularly in resource-limited settings. This approach can enable remote data collection, facilitate access to GC through telemedicine, and improve the accessibility of GT by reducing the need for physical infrastructure. For example, mobile health applications could be used to gather health data for prenatal testing or for managing chronic conditions such as SCD. Furthermore, digital phenotyping can also help in tracking patient outcomes, promoting adherence to treatment plans, and providing real-time support, thus improving the quality and reach of genetic services. As digital technologies continue to advance, there is tremendous potential for these tools to bridge gaps in access and enhance the delivery of genomic medicine across Africa.59,60 However, we may face several ethical challenges when collecting digital data, especially concerning data ownership, informed consent, and adherence to global regulatory frameworks like the General Data Protection Regulation and the Health Insurance Portability and Accountability Act .61,62 Therefore, it is essential for us as researchers to consider these ethical implications when planning to incorporate digital phenotyping in the implementation of genetic services in African contexts. Additionally, businesses can explore innovative and cost-effective models for GT to overcome the barriers related to high costs and limited accessibility. This might involve developing affordable testing kits, partnering with local healthcare providers, or implementing telemedicine solutions to reach underserved populations.
Furthermore, healthcare providers and scholars can play a pivotal role in addressing the lack of disease awareness and education by investing in educational programs targeting healthcare professionals and communities. This could involve collaborations with local institutions, creating informative materials, and leveraging digital platforms to disseminate educational content. For instance, a 2-week intensive GC training program for healthcare providers in Nigeria significantly improved knowledge and counseling competencies in SCD management.48,63 Expanding such initiatives across other genetic conditions could enhance access to GC services, particularly in rural and underserved communities. Additionally, integrating GC modules into existing medical and nursing curricula would equip healthcare workers with foundational knowledge, reducing reliance on specialized genetic counsellors.
Limitations of the study
This scoping review has some limitations that might impact our findings. One of these limitations is the search strategy that we used, which concentrated solely on studies published in English. Other pertinent literature may have been published in different languages and may have been overlooked in this review. This could restrict the extent and thoroughness of our review and have an impact on the precision of its conclusions. As a result, it is essential to bear in mind this limitation while interpreting the outcomes of this review.
Another limitation is the relatively small number of studies that met the inclusion criteria. This reflects the broader challenge of limited research on genetic service implementation in Africa. Additionally, while the review synthesizes data from various African countries, direct applicability to specific African countries remains uncertain due to differences in healthcare infrastructure, regulatory environments, and cultural attitudes toward GT. Future research should focus on primary studies assessing the feasibility and acceptability of genetic services, incorporating perspectives from healthcare providers, patients, and policymakers to develop a tailored genetic service framework.
Conclusion
While challenges persist in implementing genetic services in Africa, the identified opportunities and strategies underscore the potential for positive change. Prioritizing prevention, leveraging supportive networks, engaging communities, and advocating for resource allocation are crucial components of a comprehensive approach to address the complexities of genetic services in the African context. These findings emphasize the need for tailored interventions that align with cultural values, involve diverse stakeholders, and prioritize education and community engagement to foster the successful integration of genetic services in African healthcare systems. Businesses must adopt a holistic and socially responsible approach to address the challenges and leverage the opportunities to implement genetic services in Africa. By aligning with the needs and values of the local communities, businesses can contribute to advancing genetic services while building sustainable and impactful operations in the African healthcare landscape.
Data and code availability
The data supporting this scoping review’s findings are derived from publicly available literature, and no original datasets were generated or analyzed during the review. All references and relevant data sources used in this study are cited in the manuscript. The review protocol and data extraction framework are available upon request from the corresponding author.
Acknowledgments
This research was funded by the National Institutes of Health (NIH), grant nos. NIH U54HG009790, U24HL135600, and U01MH127692, to A.W. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
K.K.K.: study design, literature search, interpretation, and writing. P.F.M.: literature search and writing. S.N. and A.W.: interpretation and writing.
Declaration of interests
The authors declare no competing interests.
References
- 1.Stoll K., Kubendran S., Cohen S.A., editors. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. Wiley Online Library; 2018. The past, present and future of service delivery in genetic counseling: Keeping up in the era of precision medicine. [DOI] [PubMed] [Google Scholar]
- 2.Turbitt E., Jacobs C., McEwen A. Special Issue: “Genetic Counseling and Genetic Testing in Precision Medicine”. J. Personalized Med. 2023;13:1192. doi: 10.3390/jpm13081192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alliance G., Genetic NY-M-ACf. Services NS . Understanding Genetics: a New York, Mid-Atlantic Guide for Patients and Health Professionals. 2009. APPENDIX G: GENETIC TESTING. [PubMed] [Google Scholar]
- 4.NHGRI Genetic Counseling. 2024. https://www.genome.gov/genetics-glossary/Genetic-Counseling
- 5.NHGRI Personalized medicine. 2024. https://www.genome.gov/genetics-glossary/Personalized-Medicine
- 6.Barnett I., Torous J., Staples P., Sandoval L., Keshavan M., Onnela J.-P. Relapse prediction in schizophrenia through digital phenotyping: a pilot study. Neuropsychopharmacology. 2018;43:1660–1666. doi: 10.1038/s41386-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torous J., Larsen M.E., Depp C., Cosco T.D., Barnett I., Nock M.K., Firth J. Smartphones, sensors, and machine learning to advance real-time prediction and interventions for suicide prevention: a review of current progress and next steps. Curr. Psychiatry Rep. 2018;20:51. doi: 10.1007/s11920-018-0914-y. [DOI] [PubMed] [Google Scholar]
- 8.Bzdok D., Meyer-Lindenberg A. Machine learning for precision psychiatry: opportunities and challenges. Biol. Psychiatry. Cogn. Neurosci. Neuroimaging. 2018;3:223–230. doi: 10.1016/j.bpsc.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Moyo E., Moyo P., Mashe T., Dzobo M., Chitungo I., Dzinamarira T. Implementation of Public Health Genomics in Africa: Lessons from the COVID-19 pandemic, challenges, and recommendations. J. Med. Virol. 2023;95 doi: 10.1002/jmv.28295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owolabi P., Adam Y., Adebiyi E. Personalizing medicine in Africa: current state, progress and challenges. Front. Genet. 2023;14 doi: 10.3389/fgene.2023.1233338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emery J., Hayflick S. The challenge of integrating genetic medicine into primary care. Bmj. 2001;322:1027–1030. doi: 10.1136/bmj.322.7293.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khoury M.J., Gwinn M., Yoon P.W., Dowling N., Moore C.A., Bradley L. The continuum of translation research in genomic medicine: how can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genet. Med. 2007;9:665–674. doi: 10.1097/GIM.0b013e31815699d0. [DOI] [PubMed] [Google Scholar]
- 13.Alwan A., Modell B. Recommendations for introducing genetics services in developing countries. Nat. Rev. Genet. 2003;4:61–68. doi: 10.1038/nrg978. [DOI] [PubMed] [Google Scholar]
- 14.Obermeyer Z., Powers B., Vogeli C., Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366:447–453. doi: 10.1126/science.aax234. [DOI] [PubMed] [Google Scholar]
- 15.Mikat-Stevens N.A., Larson I.A., Tarini B.A. Primary-care providers’ perceived barriers to integration of genetics services: a systematic review of the literature. Genet. Med. 2015;17:169–176. doi: 10.1038/gim.2014.101. [DOI] [PubMed] [Google Scholar]
- 16.Taylor R.J., Chatters L.M., Cross C.J. Taking diversity seriously: Within-group heterogeneity in African American extended family support networks. J. Marriage Fam. 2021;83:1349–1372. doi: 10.1111/jomf.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The World Bank Group Cameroon, Population total. 2025. https://data.worldbank.org/country/cameroon?view=chart
- 18.Tandi T.E., Cho Y., Akam A.J.-C., Afoh C.O., Ryu S.H., Choi M.S., Kim K., Choi J.W. Cameroon public health sector: shortage and inequalities in geographic distribution of health personnel. Int. J. Equity Health. 2015;14:43. doi: 10.1186/s12939-015-0172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kromberg J.G.R., Sizer E.B., Christianson A.L. Genetic services and testing in South Africa. J. Community Genet. 2013;4:413–423. doi: 10.1007/s12687-012-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wonkam A., Njamnshi A.K., Angwafo F.F., 3rd Knowledge and attitudes concerning medical genetics amongst physicians and medical students in Cameroon (sub-Saharan Africa) Genet. Med. 2006;8:331–338. doi: 10.1097/01.gim.0000223542.97262.21. [DOI] [PubMed] [Google Scholar]
- 21.Wonkam A. Sequence three million genomes across Africa. Nature. 2021;590:209–211. doi: 10.1038/d41586-021-00313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toronto C.E., Remington R. Springer; 2020. A Step-by-step Guide to Conducting an Integrative Review. [Google Scholar]
- 23.Büchter R.B., Rombey T., Mathes T., Khalil H., Lunny C., Pollock D., Puljak L., Tricco A.C., Pieper D. Systematic reviewers used various approaches to data extraction and expressed several research needs: A survey. J. Clin. Epidemiol. 2023;159:214–224. doi: 10.1016/j.jclinepi.2023.05.027. [DOI] [PubMed] [Google Scholar]
- 24.Silverstein A., Sood R., Costas-Chavarri A. Breast Cancer in Africa: Limitations and Opportunities for Application of Genomic Medicine. Int. J. Breast Cancer. 2016;2016 doi: 10.1155/2016/4792865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tahir M.I., Ahmad A.E., Suleiman A.B. Anticipated ethical challenges with growing molecular prenatal diagnosis in Nigeria. Bayero J. Pure App. Sci. 2018;10:188–191. doi: 10.4314/bajopas.v10i1.38S. [DOI] [Google Scholar]
- 26.Zhong A., Darren B., Loiseau B., He L.Q.B., Chang T., Hill J., Dimaras H. Ethical, social, and cultural issues related to clinical genetic testing and counseling in low-and middle-income countries: a systematic review. Genet. Med. 2021;23:2270–2280. doi: 10.1038/s41436-018-0090-9. [DOI] [PubMed] [Google Scholar]
- 27.Zhong A., Xia K., Hadjis Z., Lifman G., Njambi L., Dimaras H. Opportunities and barriers for genetic service delivery in Kenya from a health personnel perspective. J. Community Genet. 2021;12:525–538. doi: 10.1007/s12687-021-00532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nkya S., Njiro B.J., Ngowi D., Solomon D., Kaywanger F., Nyangasa S., Ndoje G., Marco E., Moses M., Makani J. Building research capacity for sickle cell disease in Africa: Lessons and challenges from establishing a birth cohort in Tanzania. Front. Pediatr. 2022;10 doi: 10.3389/fped.2022.826199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galadanci A.A., Estepp J.H., Khan H., Farouk Z.L., Caroll Y., Hodges J., Yarima S., Ibrahim U.A., Idris I.M., Gambo A., et al. Barriers and Facilitators of Premarital Genetic Counseling for Sickle Cell Disease in Northern Nigeria. J. Pediatr. Hematol. Oncol. 2023;45:e716–e722. doi: 10.1097/MPH.0000000000002702. [DOI] [PubMed] [Google Scholar]
- 30.Zingela Z., Sokudela F., Thungana Y., van Wyk S. Ethical principles, challenges and opportunities when conducting genetic counselling for schizophrenia. Front. Psychiatr. 2023;14 doi: 10.3389/fpsyt.2023.1040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mapoko B.S.E., Ndi K.C., Tabola L., Mouaye V., Douanla P., Nsangou N., Nkeng G., Vanvolkenburgh C., Dzekem B., Huo D., et al. Feasibility of cancer genetic counselling and screening in Cameroon: perceived benefits and barriers. ecancermedicalscience. 2023;17:1588. doi: 10.3332/ecancer.2023.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wonkam A., Ngongang Tekendo C., Zambo H., Morris M.A. Initiation of prenatal genetic diagnosis of sickle cell anaemia in Cameroon (sub-Saharan Africa) Prenat. Diagn. 2011;31:1210–1212. doi: 10.1002/pd.2896. [DOI] [PubMed] [Google Scholar]
- 33.Wonkam A., Tekendo C.N., Sama D.J., Zambo H., Dahoun S., Béna F., Morris M.A. Initiation of a medical genetics service in sub-Saharan Africa: experience of prenatal diagnosis in Cameroon. Eur. J. Med. Genet. 2011;54:e399–e404. doi: 10.1016/j.ejmg.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Wonkam A., Zambo H., Tekendo C.N., Béna F., Dahoun S. Initiation of prenatal genetic diagnosis of chromosomal anomalies in Cameroon. Int. J. Gynaecol. Obstet. 2012;116:174–175. doi: 10.1016/j.ijgo.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Addie S., Alper J., Beachy S.H. Vol. 10. National Academies Press; Washington, DC: 2018. (Understanding Disparities in Access to Genomic Medicine). [PubMed] [Google Scholar]
- 36.Adebamowo S.N., Francis V., Tambo E., Diallo S.H., Landouré G., Nembaware V., Dareng E., Muhamed B., Odutola M., Akeredolu T., et al. Implementation of genomics research in Africa: challenges and recommendations. Glob. Health Action. 2018;11 doi: 10.1080/16549716.2017.1419033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rotimi C., Abayomi A., Abimiku A., Adabayeri V.M., Adebamowo C., Adebiyi E., Ademola A.D., Adeyemo A., Adu D., et al. H3Africa Consortium Enabling the genomic revolution in Africa: H3Africa is developing capacity for health-related genomics research in Africa. Science. 2014;344:1346–1348. doi: 10.1126/science.1251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fashoyin-Aje L., Sanghavi K., Bjornard K., Bodurtha J. Integrating genetic and genomic information into effective cancer care in diverse populations. Ann. Oncol. 2013;24:vii48–vii54. doi: 10.1093/annonc/mdt264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munung N.S., Kamga K.K., Treadwell M.J., Dennis-Antwi J., Anie K.A., Bukini D., Makani J., Wonkam A. Perceptions and preferences for genetic testing for sickle cell disease or trait: a qualitative study in Cameroon, Ghana and Tanzania. Eur. J. Hum. Genet. 2024;32:1307–1313. doi: 10.1038/s41431-024-01553-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munung N.S., Treadwell M., Kamga K.K., Dennis-Antwi J., Anie K., Bukini D., Makani J., Wonkam A. Caught between pity, explicit bias, and discrimination: a qualitative study on the impact of stigma on the quality of life of persons living with sickle cell disease in three African countries. Qual. Life Res. 2024;33:423–432. doi: 10.1007/s11136-023-03533-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wonkam A., de Vries J., Royal C.D., Ramesar R., Angwafo F.F., 3rd Would you terminate a pregnancy affected by sickle cell disease? Analysis of views of patients in Cameroon. J. Med. Ethics. 2014;40:615–620. doi: 10.1136/medethics-2013-101392. [DOI] [PubMed] [Google Scholar]
- 42.Wonkam A., Mba C.Z., Mbanya D., Ngogang J., Ramesar R., Angwafo F.F., 3rd Psychosocial stressors of sickle cell disease on adult patients in Cameroon. J. Genet. Counsel. 2014;23:948–956. doi: 10.1007/s10897-014-9701-z. [DOI] [PubMed] [Google Scholar]
- 43.Wonkam A., Hurst S. A call for policy action in sub-Saharan Africa to rethink diagnostics for pregnancy affected by sickle cell disease: differential views of medical doctors, parents and adult patients predict value conflicts in Cameroon. OMICS. 2014;18:472–480. doi: 10.1089/omi.2013.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andrews L.B., Fullarton J.E., Holtzman N.A., Motulsky A.G. National Academy of Sciences; 1994. Assessing Genetic Risks. Implications for Health and Social Policy. [PubMed] [Google Scholar]
- 45.Muthuswamy V. Ethical issues in genetic counselling with special reference to haemoglobinopathies. Indian J. Med. Res. 2011;134:547–551. [PMC free article] [PubMed] [Google Scholar]
- 46.Blackshaw B.P. Genetic selective abortion: Still a matter of choice. Ethical Theory Moral Pract. 2020;23:445–455. doi: 10.1007/s10677-020-10080-5. [DOI] [Google Scholar]
- 47.Al Shamsi H., Almutairi A.G., Al Mashrafi S., Al Kalbani T. Implications of language barriers for healthcare: a systematic review. Oman Med. J. 2020;35 doi: 10.5001/omj.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Treadwell M.J., Anie K.A., Grant A.M., Ofori-Acquah S.F., Ohene-Frempong K. Using formative research to develop a counselor training program for newborn screening in Ghana. J. Genet. Counsel. 2015;24:267–277. doi: 10.1007/s10897-014-9759-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oludare G.O., Ogili M.C. Knowledge, attitude and practice of premarital counseling for sickle cell disease among youth in Yaba, Nigeria. Afr. J. Reprod. Health. 2013;17:175–182. [PubMed] [Google Scholar]
- 50.Oluwole E.O., Okoye C.D., Ogunyemi A.O., Olowoselu O.F., Oyedeji O.A. Knowledge, attitude and premarital screening practices for sickle cell disease among young unmarried adults in an urban community in Lagos, Nigeria. Pan Afr. Med. J. 2022;42 doi: 10.11604/pamj.2022.42.8.27705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim N., Kong S.-Y., Yoo J., Kim D.-H., Seo S.H., Kim J. Current issues, challenges, and future perspectives of genetic counseling in Korea. Ann. Lab. Med. 2022;42:314–320. doi: 10.3343/alm.2022.42.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wessels T.-M., Greenberg J.L., Fourie K., Kromberg J.G.R. Genetic counseling in South Africa: a growing profession. Genet. Med. Open. 2024;2 doi: 10.1016/j.gimo.2024.101862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maio M., Carrion P., Yaremco E., Austin J.C. Awareness of genetic counseling and perceptions of its purpose: a survey of the Canadian public. J. Genet. Counsel. 2013;22:762–770. doi: 10.1007/s10897-013-9633-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chapple A., May C., Campion P. Lay understanding of genetic disease: A British study of families attending a genetic counseling service. J. Genet. Counsel. 1995;4:281–300. doi: 10.1007/BF01408074. [DOI] [PubMed] [Google Scholar]
- 55.Hamilton A.B., Oishi S., Yano E.M., Gammage C.E., Marshall N.J., Scheuner M.T. Factors influencing organizational adoption and implementation of clinical genetic services. Genet. Med. 2014;16:238–245. doi: 10.1038/gim.2013.101. [DOI] [PubMed] [Google Scholar]
- 56.O’Daniel J.M., Ackerman S., Desrosiers L.R., Rego S., Knight S.J., Mollison L., Byfield G., Anderson K.P., Danila M.I., Horowitz C.R., et al. Integration of stakeholder engagement from development to dissemination in genomic medicine research: Approaches and outcomes from the CSER Consortium. Genet. Med. 2022;24:1108–1119. doi: 10.1016/j.gim.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martinez R.M., Osei-Anto H.A., McCormick M., National Academies of Sciences E, Medicine . National Academies Press (US); 2020. Community Engagement and Patient Advocacy. Addressing Sickle Cell Disease: A Strategic Plan and Blueprint for Action. [PubMed] [Google Scholar]
- 58.Battista R.N., Blancquaert I., Laberge A.-M., Van Schendel N., Leduc N. Genetics in health care: an overview of current and emerging models. Public Health Genom. 2012;15:34–45. doi: 10.1159/000328846. [DOI] [PubMed] [Google Scholar]
- 59.Alaran M.A., Lawal S.K., Jiya M.H., Egya S.A., Ahmed M.M., Abdulsalam A., Haruna U.A., Musa M.K., Lucero-Prisno D.E., 3rd Challenges and opportunities of artificial intelligence in African health space. Digit. Health. 2025;11 doi: 10.1177/20552076241305915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ansah M.R., Ugo H.C., Aboagye I.A., Sowah N.L., Osei G., Balapangu S.S., Kwofie S.K. In: Examining the Rapid Advance of Digital Technology in Africa. Amoah L.G.A., editor. IGI Global; 2024. Artificial Intelligence and Health in Africa: Opportunities, Challenges, and Ethical; pp. 103–123. [DOI] [Google Scholar]
- 61.Alhoussari H. Securing Health Data in the Digital Age: Challenges, Regulatory Frameworks, and Strategic Solutions in Saudi Arabia. JoE. 2025;4:2310–2319. doi: 10.62754/joe.v4i1.6052. [DOI] [Google Scholar]
- 62.Sallstrom L., Morris O., Mehta H. Artificial intelligence in Africa’s healthcare: ethical considerations. ORF Issue Brief. 2019;312:1–11. [Google Scholar]
- 63.Sickle Cell Foundation N . Sickle cell Disease foundation; 2025. Genetic Counselling Programme P.O Box 3463, Surulere, Lagos, Nigeria info@sicklecellfoundation.com.https://www.sicklecellfoundation.com/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this scoping review’s findings are derived from publicly available literature, and no original datasets were generated or analyzed during the review. All references and relevant data sources used in this study are cited in the manuscript. The review protocol and data extraction framework are available upon request from the corresponding author.