Abstract
Objective
To explore the impact of menopause for age at diagnosis (AAD) of glaucoma in women and illustrate its interaction with the apolipoprotein E (APOE) E4 allele.
Design
A retrospective, case-only analysis using the UK Biobank participants with complete data (2006–2010) for analysis.
Participants
One thousand three hundred fifty-eight female glaucoma patients.
Methods
Multivariable-adjusted associations of AAD of glaucoma, APOE E4 allele, age of menopause, and hormone replacement therapy (HRT) were analyzed by linear mixed model (LMM) analyses across groups stratified by whether glaucoma developed before or after menopause and whether or not HRT was used.
Main Outcome Measures
Age at diagnosis of glaucoma, age of menopause, APOE E4 allele, and HRT information.
Results
The age-adjusted univariate LMM showed that later menopause was significantly associated with an older AAD of glaucoma in both the overall cohort and subgroups where glaucoma developed before or after menopause (model 1, all P < 0.05). The age-adjusted multivariate LMM found that carrying the APOE E4 allele combined with later menopause significantly increased the AAD of glaucoma in patients diagnosed before menopause (model 3: βage of menopause = 0.711 ± 0.074, P < 0.001; βe4 = 1.406 ± 0.596, P = 0.019; model 1 vs. model 3: P = 0.018). No similar association was observed in patients diagnosed after menopause (P > 0.05). Additionally, the age-adjusted univariate LMM showed that HRT was associated with an older AAD of glaucoma (model 4: βHRT = 1.239 ± 0.368, P = 0.001), with this effect being more pronounced in patients with later menopause (model 5: βHRT = 1.625 ± 0.356, P < 0.001; βage of menopause = 0.301 ± 0.033, P < 0.001; model 4 vs. model 5: P < 0.001).
Conclusions
Later menopause was associated with an older AAD of glaucoma, with the APOE E4 allele providing increased protection against glaucoma in those diagnosed before, but not after, menopause. The protective effect of later menopause was also enhanced by HRT use after menopause. These findings underscore the interaction of hormonal status and APOE genotype in glaucoma onset, potentially guiding the prevention or management of glaucoma and other age-related health conditions in women.
Financial Disclosure(s)
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Keywords: Menopause, APOE E4 allele, Age at diagnosis of glaucoma, Alzheimer's disease, UK Biobank
Glaucoma is one of the leading causes of irreversible blindness globally.1 It is a multifactorial condition characterized by the progressive loss of visual function and retinal ganglion cells (RGCs). Recent research has highlighted pathogenic links between neurodegeneration in the brain, optic nerve, and inner retinal layers, reinforcing the view of the eye as an extension of the central nervous system.2 These findings align with the shared neurodegenerative processes in primary open-angle glaucoma (POAG) and Alzheimer's disease (AD).
Indeed, older age is already established as a primary risk factor for both POAG and AD, with sex-specific factors also emerging.3,4 Women are disproportionately affected by AD, showing more severe pathology and faster degeneration, with the apolipoprotein E (APOE) E4 allele posing a higher risk, particularly for heterozygous carriers.5, 6, 7, 8 Sex is not yet recognized as a risk factor for glaucoma, despite women making up 59% of glaucoma cases. Existing evidence has already indicated greater visual impairment and lower treatment-seeking behavior among women, reinforcing the idea that glaucoma may also have a more severe impact on women and that the number of affected women may be underreported.4 Mechanically, estrogen is thought to influence aging in the optic nerve and brain. The complex interaction between estrogen and APOE E4 genotype in women would further suggest that genetic predispositions and hormonal states may influence susceptibility to both glaucoma and AD.4,9 Thus, menopause, as a significant hormonal turning point in the female reproductive aging process, potentially acts as a gender-specific risk factor for glaucoma and AD. Although excluding female animals from preclinical studies hinders our understanding of the underlying mechanisms, recent clinical studies have shown that late menopause and postmenopausal hormone replacement therapy (HRT) are linked to a later age at diagnosis (AAD) of glaucoma.10,11 This study seeks to delve into these associations in female glaucoma by examining their interaction with the APOE E4 allele through data from the UK Biobank (UKBB), potentially providing valuable insights for future research on menopause and HRT in relation to age-related health issues in women.
Methods
Ethics Statement
The research conducted using the UKBB adheres to the tenets of the Declaration of Helsinki. The UKBB received approval from the North West Multi-centre Research Ethics Committee. Our access to the resource was approved by UKBB, conforming to their access procedures and ethics. All participants provided written, informed consent, and the data were fully deidentified.
Study Population
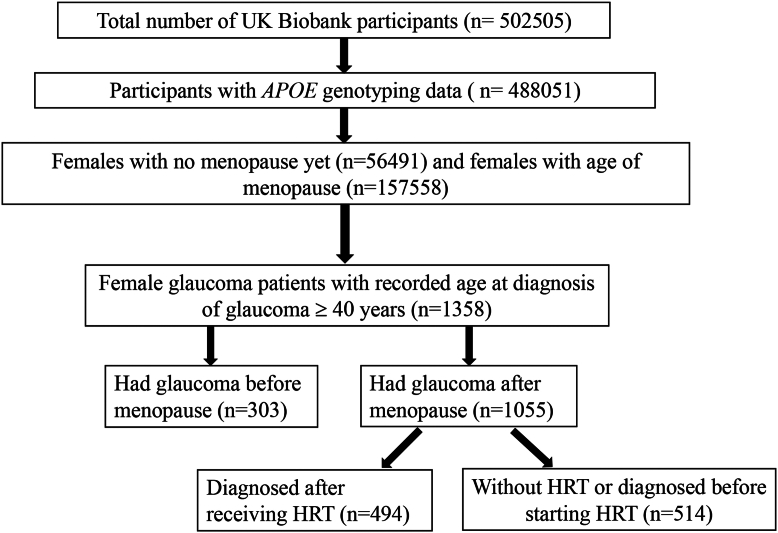
The diagram illustrating the sample cohort selection is shown in Figure 1. The UKBB is a large, population-wide cohort of 502 505 participants aged between 39 and 73 at recruitment from 2006 through 2010 across the United Kingdom, of whom 488 051 had established APOE alleles. With menopause as the primary prognostic factor for glaucoma, we refined the study population to include only female participants who either had documented ages at menopause (n = 157 558) or had not yet reached menopause (n = 56 491). Age at menopause was determined through self-reporting (data field 2724, “Have you had your menopause (periods stopped)?” and data field 3581, “How old were you when your periods stopped?”). To ensure data integrity, UKBB participants were surveyed periodically and asked to follow up and confirm their responses. Entries with menopause age >70 years were also rejected due to concerns of data reliability.
Figure 1.
Cohort selection diagram illustrating the stratification of the UK Biobank dataset into subsets to analyze female-specific risk factors for glaucoma. Menopause status and age at glaucoma diagnosis were selected. The diagram depicts the grouping of female participants into those who had glaucoma before or after menopause, further stratified by whether they received hormone replacement therapy (HRT) and the timing relative to menopause onset when HRT was received. APOE = apolipoprotein E.
Further narrowing of the cohort set was performed to include those with AAD for glaucoma. Glaucoma patients were identified through a combination of self-reporting (data field: 6148) and the International Classification of Diseases, 10th Revision (data field: 41270; H40.1 and H40.2). The AAD of glaucoma patients was also determined through self-reported surveys (data field: 4689, “What was your age when glaucoma was first diagnosed?”). Within the initial UKBB cohort, the affected population set was composed of 1358 female glaucoma patients, after excluding individuals without accurate AAD of glaucoma information and those diagnosed before 40 years of age. This exclusion targeted cases of childhood and juvenile open-angle glaucoma, which are predominantly influenced by genetic factors like CYP1B1 and myocilin mutations, rather than the natural aging process.12 Although other types of glaucoma diagnosed after 40 years of age, such as POAG, primary angle-closure glaucoma, and normal-tension glaucoma, are age-related,13 their cohort sizes were limited by the number of International Classification of Diseases, 10th Revision definitive diagnoses and were not clearly identified in the self-reported survey. Thus, we opted not to exclude other types of glaucoma from the study. While the majority of the analyses in this study were performed on individuals affected with glaucoma, stratified by onset age, APOE E4, HRT, and menopause status, a normal control cohort for the glaucoma subset was also generated. Normal control was constructed using individuals whose International Classification of Diseases, 10th Revision glaucoma diagnosis (data field: 41270; H40.1 and H40.2) and self-reporting (data field: 6148) were both negative. To investigate the associations between menopause and AAD of glaucoma, glaucoma patients were stratified based on whether their diagnosis occurred before menopause (n = 232) or after menopause (n = 1054). Hormone replacement therapy is a known therapeutic strategy for postmenopausal women, aimed at ameliorating some of the vasomotor, cardiovascular, and genitourinary effects of menopause by helping regulate some of the body's natural hormonal levels.14 We, therefore, tracked 2 HRT metrics among our cohort: history of HRT use (data field 2814, “Have you ever used HRT?”) and age at the start of HRT (data field 3536). The cohort was further filtered to exclude individuals without HRT information, creating additional subgroups: those diagnosed after receiving HRT (n = 494) and those either without HRT or diagnosed before starting HRT (n = 514).
Genotyping
DNA microarray genotyping was generated using Axiom arrays (including the UK Biobank Lung Exome Variant Evaluation [BiLEVE] and UKBB arrays; Thermo Fisher) for the UKBB. Apolipoprotein E alleles (E1, E2, E3, and E4) were determined from 2 relevant single-nucleotide polymorphisms (SNPs) within the APOE gene (rs429358 and rs7412; GRCh38 reference genome). Due to the rarity of the E1 allele and, thus, the lack of a statistically significant cohort size, E1-associated genotypes (E1E2 and E1E4) were excluded from the analysis to eliminate confounding variables. E1E3 genotypes, which are most likely to represent incorrect attribution of 2 variants to the same allele rather than alternate common variants on separate alleles, were relabeled as E2E4. Apolipoprotein SNPs were measured directly from Axiom arrays. Because the relevant APOE SNPs were not included on the Illumina arrays, they were imputed in Minimac3 using Haplotype Reference Consortium r1.1 as a reference panel (rs429358 imputation R2 ¼ 0.93; rs7412 imputation R2 ¼ 0.92).15
Statistical Analysis
Statistical analyses were conducted using R (version 4.2.1) with publicly available packages. Demographic parameters were initially compared through chi-square tests and independent sample t tests. The association between the age of menopause and the AAD of glaucoma was examined using linear mixed model (LMM, model 1) analysis, adjusting for age. For the allelic regression, an indicator variable for E4 status defined by the presence or absence of the relevant allele and its association with AAD of glaucoma was analyzed using LMM (model 2), adjusting for age. In this context, the βe4 represents the impact of the E4 allele on the AAD of glaucoma, indicating a value of 1 or 0 to represent its presence or absence, respectively. Multivariate LMMs (model 3) were further conducted, incorporating both age of menopause and APOE E4 status as variables, adjusting for age. Analysis of variance was used to compare the difference between model 1 and model 3. Given the rarity of APOE E4E4 in our glaucoma cohort, the dosage effect of E4 alleles [0–2] was not addressed in this study. The association between HRT status and the AAD of glaucoma was examined using LMM (model 4), adjusting for age. Multivariate LMMs adjusted for age were further conducted using age of menopause and HRT status as variables (model 5) or incorporating age of menopause, APOE E4 status, and HRT status as variables (model 6). Analysis of variance was used to compare the difference between model 4, model 5, and model 6. The threshold for statistical significance (α) was set at P < 0.05.
Results
Comparison of Demographics and Age of Menopause between Groups
Table 1 presents a comparison of demographics and age of menopause based on (1) had or had no glaucoma, (2) had or had no menopause, (3) the presence or absence of the APOE E4 allele, and (4) with or without HRT. Overall, women who had experienced menopause exhibited a significantly higher prevalence of glaucoma compared with those who had not yet gone through menopause (P < 0.001). Women with glaucoma experienced menopause earlier than those without glaucoma (P < 0.001). Apolipoprotein E E4 carriers had a slightly earlier menopause (P = 0.037) than noncarriers, while the prevalence of glaucoma was not different between APOE E4 carriers and noncarriers (P = 0.457). Women who received HRT were older and experienced menopause earlier than those without HRT (both P < 0.001). The prevalence of glaucoma was higher in women with HRT compared with those without it (P < 0.001).
Table 1.
Comparison of Demographic and Age of Menopause between Groups
| Without Glaucoma | With Glaucoma | P | Had No Menopause | Had Menopause | P | Without APOE E4 | With APOE E4 | P | Without HRT | With HRT | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total sample, n (%) | 67 461 (95.42) | 3235 (4.58) | <0.001 | 56 491 (25.16) | 168 061 (74.84) | <0.001† | 112 883 (71.65) | 44 675 (28.35) | <0.001† | 86 342 (54.92) | 70 874 (45.08) | <0.001† |
| Age (yrs; mean ± SD) | 58.98 ± 6.36 | 62.22 ± 5.17 | 0.076 | 46.12 ± 4.34 | 59.76 ± 5.90 | <0.001† | 59.66 ± 5.90 | 59.51 ± 5.94 | <0.001† | 58.53 ± 6.11 | 60.94 ± 5.36 | <0.001† |
| Age of menopause | 50.07 ± 5.14 | 49.90 ± 5.50 | <0.001 | - | 49.81 ± 5.15 | - | 49.83 ± 5.14 | 49.77 ± 5.18 | 0.037‡ | 50.38 ± 4.40 | 49.13 ± 5.86 | <0.001† |
| Glaucoma (n, %, +) | - | - | - | 471 (2.62) | 5618 (8.80) | <0.001† | 1446 (8.46) | 3721 (8.65) | 0.457 | 1239 (1.43) | 1374 (1.94) | <0.001† |
| APOE E4 carriers∗ (n, %) | 19 106 (28.32) | 911 (28.16) | 0.857 | 16 188 (28.66) | 47 717 (28.39) | 0.232 | - | - | - | 24 643 (30.86) | 19 922 (28.11) | 0.059 |
APOE = apolipoprotein E; HRT = hormone replacement therapy.
APOE E4 carriers: carrying ≥1 copy (E24 + E34 + E44).
P < 0.001.
P < 0.05.
Comparison of Demographics and APOE E4 Status among Groups with Different Ages at Diagnosis of Glaucoma
In female glaucoma patients diagnosed after menopause, menopause occurred earlier than in those diagnosed before menopause (P < 0.001). For those diagnosed with glaucoma before menopause, the AAD was younger compared with those diagnosed after menopause (P < 0.001). In patients diagnosed after menopause, those who had undergone HRT were older at the time of glaucoma diagnosis than those who had not received HRT (P < 0.001). The prevalence of APOE E4 status did not differ between female glaucoma patients diagnosed before or after menopause nor did it differ between those with and without HRT (all P > 0.05) (Table 2).
Table 2.
Comparison of Demographics and APOE E4 Status among Groups with Different Age at Diagnosis of Glaucoma
| Total | Before Menopause | After Menopause | P | After Menopause without HRT† | After Menopause and HRT | P | |
|---|---|---|---|---|---|---|---|
| Total sample, n (%) | 1358 | 303 | 1055 | <0.001‡ | 514 | 494 | 0.529 |
| Age (yrs; mean ± SD) | 60.92 ± 6.10 | 56.77 ± 7.22 | 62.11 ± 5.15 | <0.001‡ | 61.19 ± 5.64 | 62.91 ± 4.53 | <0.001‡ |
| Age of menopause | 57.43 ± 8.34 | 52.78 ± 3.54 | 49.89 ± 5.48 | <0.001‡ | 50.40 ± 4.64 | 49.51 ± 6.08 | 0.010§ |
| Age at diagnosis of glaucoma | 58.05 ± 8.05 | 46.80 ± 4.83 | 60.49 ± 6.41 | <0.001‡ | 59.20 ± 6.25 | 61.91 ± 6.22 | <0.001‡ |
| APOE E4 carriers∗ (n, %) | 980:378 (27.84) | 218:85 (28.05) | 762:293 (27.77) | 0.982 | 365:149 (28.99) | 359:135 (27.33) | 0.606 |
APOE = apolipoprotein E; HRT = hormone replacement therapy; SD = standard deviation.
APOE E4 status: 0 copy: noncarriers = E22 + E23 + E33; ≥1 copy: carriers = E24 + E34 + E44.
Included female glaucoma patients without HRT or diagnosed before having HRT.
P < 0.001.
P < 0.05.
Factors Associated with Age at Diagnosis of Glaucoma
The univariate LMM adjusted by age showed that later menopause was significantly associated with an older AAD of glaucoma in the overall glaucoma cohort (model 1: β = 0.116 ± 0.039, P < 0.003). This association remained significant when stratifying based on whether glaucoma developed before menopause (model 1, β = 0.699 ± 0.075, P < 0.001) or after menopause (model 1, β = 0.288 ± 0.032, P < 0.001) (Table 3). Although APOE E4 status alone was not associated with AAD of glaucoma in the univariate LMM adjusted by age in both the general and subgroup analyses (model 2, all P > 0.05), the age-adjusted multivariate LMM found that carrying the APOE E4 allele combined with later menopause significantly increased the AAD of glaucoma in patients diagnosed before menopause (model 3: βage of menopause = 0.711 ± 0.074, P < 0.001; βe4 = 1.406 ± 0.596, P = 0.019; model 1 vs. model 3: P=0.018). No such association was found in the general unstratified glaucoma cohort or in patients diagnosed after menopause (all P > 0.05).
Table 3.
Factors Associated with Age at Diagnosis of Glaucoma (Linear Mixed Regression)
| Total (n = 1287) |
Had Glaucoma before Menopause (n = 232)† |
Had Glaucoma after Menopause (n = 1055) |
||||
|---|---|---|---|---|---|---|
| β Coefficient | P | β Coefficient | P | β Coefficient | P | |
| Model 1: age of menopause | 0.116 ± 0.039 | 0.003§ | 0.699 ± 0.075 | <0.001‡ | 0.288 ± 0.032 | <0.001‡ |
| Model 2: APOE E4 (0/1: without/with) | 0.029 ± 0.464 | 0.950 | 1.017 ± 0.702 | 0.145 | −0.484 ± 0.402 | 0.229 |
| Model 3: age of menopause + APOE E4 | Model 3 vs. model 1 | 0.919 | Model 3 vs. model 1 | 0.018§ | Model 3 vs. model 1 | 0.233 |
| Age of menopause | 0.116 ± 0.039 | 0.003§ | 0.711 ± 0.074 | <0.001‡ | 0.288 ± 0.032 | <0.001‡ |
| APOE E4 status 0: ≥1 copy (%, ≥1 copy)∗ | 0.048 ± 0.463 | 0.918 | 1.406 ± 0.596 | 0.019§ | −0.462 ± 0.388 | 0.233 |
APOE = apolipoprotein E.
APOE E4 status: 0 copy: noncarriers = E22 + E23 + E33; ≥1 copy: carriers = E24 + E34 + E44.
Excluded those who had no menopause yet.
P < 0.001.
P < 0.05.
Factors Associated with AAD of Glaucoma in Women with or without HRT after Menopause
Table 4 displays factors associated with the AAD of glaucoma in women with or without HRT after menopause. The univariate LMM adjusted for age revealed HRT was associated with an older AAD of glaucoma (model 4: βHRT = 1.239 ± 0.368, P = 0.001), and this effect was more pronounced in patients with later menopause (model 5: βHRT = 1.625 ± 0.356, P < 0.001; βage of menopause = 0.301 ± 0.033, P < 0.001; model 4 vs. model 5: P < 0.001). This association remained unchanged even when APOE E4 status was included as a covariable (model 5 vs. model 6: P = 0.295).
Table 4.
Factors Associated with Age at Diagnosis of Glaucoma in Women with or without HRT after Menopause (lmer Regression)
| Female Glaucoma with HRT Information (n = 1008) |
||
|---|---|---|
| β Coefficient | P | |
| Model 4: HRT | ||
| HRT | 1.239 ± 0.368 | 0.001‡ |
| Model 5: age of menopause + HRT | Model 4 vs. model 5 | <0.001† |
| Age of menopause | 0.301 ± 0.033 | <0.001† |
| HRT | 1.625 ± 0.356 | <0.001† |
| Model 6: age of menopause + APOE E4 + HRT | Model 6 vs. model 5 | 0.295 |
| Age of menopause | 0.301 ± 0.033 | <0.001† |
| APOE E4∗ (0/≥1: without/with) | −0.275 ± 0.389 | 0.480 |
| HRT | 1.616 ± 0.357 | <0.001† |
APOE = apolipoprotein E; HRT = hormone replacement therapy.
APOE E4 status: 0 copy: noncarriers = E22 + E23 + E33; ≥1 copy: carriers = E24 + E34 + E44.
P < 0.001.
P < 0.05.
Discussion
In the context of glaucoma, the role of the APOE E4 allele as a risk factor remains a subject of debate, with numerous studies having previously reported positive16,17 and negative18, 19, 20 associations. In our previous investigation using the UKBB dataset, we found that APOE E4 presence initially delayed but later accelerated the development of glaucoma in women around the transition period of 50 years, which aligns with the average age of menopause.21 This suggests that menopause may play a key role in how APOE E4 alleles influence the development of glaucoma in women, making it a potential female-specific risk factor.4 To our knowledge, this study is the most extensive analysis of menopause and APOE as risk factors in female glaucoma. We found a significant association between earlier menopause and a younger AAD of glaucoma in a large female cohort, with distinct interactions involving APOE E4 status and the influence of HRT. Our stratification of findings by menopause onset status supports a more nuanced understanding of the interaction between hormonal and genetic factors in the mechanisms of neurodegenerative diseases involving APOE alleles.
Menopause is a significant hormonal turning point in the female aging process, beginning with perimenopause (typically starting in a woman's 40s) and progressing to postmenopause (often referred to as menopause, with a median onset age between 50 and 52 years, although this is known to vary slightly based on sociodemographic factors).22 It is known to directly influence life expectancy and increase a woman's risk for various conditions, including cardiovascular disease, strokes, osteoporosis, and diabetes.23, 24, 25, 26, 27 Identifying menopause as a standalone risk factor for glaucoma has been challenging, partly because of the difficulty of distinguishing between the impacts of aging and menopause in large clinical populations.28, 29, 30 However, similar to cardiovascular disease studies, several clinical studies suggest that the age of menopause onset is related to the risk of developing glaucoma.24,29,31, 32, 33 Additionally, basic science research has linked menopause to changes in inflammatory mediators, cell survival in the retina, and biomechanical properties in the eye—paralleling changes seen in the cardiovascular and musculoskeletal systems (cartilage and bone).34, 35, 36, 37, 38 Our research, along with that of others10, has discovered that early menopause was associated with early AAD of glaucoma, suggesting that the aging process might be accelerated in women during this transition period, potentially accelerating age-related neurodegeneration. This explanation may better account for the overall higher prevalence of glaucoma among middle-aged women (aged 40–59 years).4
The mechanisms underlying the association between menopause and glaucoma have not been thoroughly investigated. Genetic determinants of age at natural menopause are unlikely to explain the previously reported link between age at natural menopause and glaucoma development in the Nurses' Health Study (7143 women).39 Other research has shown that postmenopausal women have a 1.5 to 3.5 mmHg higher intraocular pressure (IOP) compared with age-matched premenopausal women,40,41 and postmenopausal women receiving HRT containing estrogen had a 0.5–3 mmHg lower IOP than those not on HRT.42, 43, 44, 45 We acknowledge that while differences may be minor, sustained changes in IOP related to menopause might still be a potential factor to consider in female glaucoma risk. In the UKBB dataset, however, IOP measurements were not recorded at the time of diagnosis and may have been affected by treatment; thus, further scrutiny of IOP was beyond the scope of our study. A more plausible explanation is the association between estrogen homeostasis and the development of glaucoma in women. A larger study using the Glaucoma Genes and Environment study and the National Eye Institute Glaucoma Human Genetics Collaboration consortium examined the relationship between sex, estrogen metabolism SNPs, and POAG.9 The study found that SNPs along the estrogen metabolic pathway were associated with an increased risk of POAG in women, but not in men.9 Mechanistically, estrogen is known to exert neuroprotective effects by regulating key pathways responsible for enhancing neurotrophin expression, reducing oxidative stress, modulating apoptosis, and improving mitochondrial function.46, 47, 48 Estrogen's effects in women are crucial for maintaining neuronal health and function, specifically by enhancing the expression of neuroprotective factors and improving synaptic function.49,50 Estrogen is protective against RGC loss in multiple experimental models of RGC injury, and various studies have suggested that estrogen could be a promising therapeutic target for mitigating the onset of neurodegenerative diseases like glaucoma and AD.51, 52, 53 Thus, early menopause, which suggests an earlier loss of estrogen, might therefore make the optic nerve more vulnerable to damage from other risk factors, such as aging and high IOP.
Previous studies on AD have suggested that the bioenergetic shift during the perimenopause to menopause transition, unique to women, may increase the lifetime risk of AD in women by exacerbating the effects of the APOE E4 allele.50,54 This heightened risk could be attributed to changes in synaptic integrity and cerebrovascular health.55,56 However, the relatively young age of participants in the UKBB cohort (capped at 73 years) limits the number (n = 290) of AD cases (with the average onset of AD being 75 years of age), restricting our ability to fully analyze the association between menopause, the APOE E4 allele, and AD. It was reported that the APOE E4 allele could impair the immunomodulatory functions of microglia, contributing to chronic neuroinflammation and thus AD onset.57,58 However, estrogen has been demonstrated to play a critical role in reducing microglial inflammation and supporting neuroprotection in various neurodegenerative conditions, including both glaucoma and AD.59, 60, 61, 62 Therefore, in premenopausal individuals, the impaired activation of APOE E4-expressing microglia may have a homeostatic function in the presence of estrogen, preventing neurodegenerative phenotypes. However, as estrogen levels decline postmenopause, the deleterious potential of APOE E4 in its ability to impair microglial response to injury or disease may result in greater, chronic, proinflammatory effects, potentially leading to an exacerbation in neurodegenerative rates. This aligns with the APOE E4 allele being the strongest genetic risk factor for late-onset AD, which typically has an onset age of 75 years.63 In contrast, a previous study has demonstrated that the presence of APOE E4 impairs microglial activation in the context of glaucoma, where it reduces microglial response as well as expression of neurodegenerative genes such as Galectin-3 (Lgals3), a key mediator of microglial neurodegeneration, leading to protection against RGC loss despite elevated IOP.64 Additionally, the protective effect of APOE E4 on glaucoma risk has been observed in certain populations.18, 19, 20 However, other studies have reported inverse associations or found no significant relationship, highlighting variability in these findings.21 In our glaucoma cohorts, the APOE E4 allele only showed a protective effect on glaucoma development before menopause, and this protective effect was less pronounced in patients with early-onset menopause and was completely lost in those who had already gone through menopause. One explanation for this phenomenon is that earlier onset of menopause, representing lower estrogen levels before menopause,65 might reduce the protective effect of the APOE E4 allele. The hormonal decline during menopause, then, may entirely eliminate this protective effect, suggesting that the protective effect of APOE E4 allele against glaucoma is especially sensitive to changes in estrogen levels. Our findings with APOE E4 in glaucoma align with existing AD findings, where younger individuals with the E4 allele have shown greater neural efficiency in episodic memory tasks, demonstrated by a quicker decline in blood-oxygen-level-dependent response during learning trials, leading to more efficient memory use. They also exhibit better performance in processing speed, attention, and verbal fluency, with these cognitive advantages extending into middle age (45–55 years). In fact, a study comparing individuals aged 45 to 57 and 58 to 68 years of age with age-matched controls found that the 45 to 57 years of age APOE E4 group outperformed the control group in verbal memory tests, though this advantage was lost in the 58 to 68 group.66 Additionally, older adults with the E4 allele showed increased functional magnetic resonance imaging activation in the association cortex during memory challenges and tasks involving verbal fluency compared with non-E4 age-matched controls, as well as altered connectivity in the default mode network.66,67 All these findings suggest that the APOE E4 allele might be an example of antagonistic pleiotropy—a gene that provides advantages during one period of life but later becomes a disadvantage. Based on our findings, we speculate that this effect may be mediated by changes in estrogen levels throughout different life stages. Although more research is warranted, understanding the interaction between hormonal and genetic factors in glaucoma mechanisms involving APOE alleles is particularly important. Retinal ganglion cells are highly metabolically active neurons with unmyelinated axons extending into the optic nerve; these cells are generally more vulnerable to the convergence of vascular, lipid/cholesterol, mitochondrial, and inflammatory dysregulation,68,69 leading to a typical degenerative onset earlier than other central nervous system–associated conditions.70 Thus, RGC vulnerability may precede cognitive symptoms, and analyzing the interactions between hormonal and genetic factors in RGC degeneration with APOE alleles could deepen our understanding of their roles in age-related neurodegenerative diseases, potentially providing a critical window for early intervention and treatment.
Meanwhile, previous studies regarding the role of estrogen pathways in women's health also suggested HRT could be a potential treatment after menopause,71, 72, 73, 74, 75 and estrogen therapy has been found to be neuroprotective, preserving RGCs following ocular hypertension in ovariectomized rats. In our study, we found that although the incidence of glaucoma was higher in women with HRT compared with those without it—likely influenced by early menopause in patients receiving HRT—multivariate LMM analysis revealed that, in women who have already gone through menopause, HRT could delay the AAD of glaucoma, aligning with findings from a recent study.11 In an existing clinical study, estrogen therapy was shown to improve insulin sensitivity,76 potentially providing protective effects before menopause. Additionally, estrogen can upregulate APOE gene expression by increasing APOE mRNA, leading to greater protection in cognition and heart health in women until menopause, when estrogen levels decline sharply.77 However, the impact of estrogen therapy in postmenopausal women is complex. Apolipoprotein E E4-negative women receiving estrogen or hormone therapy showed the highest level of cognitive performance, whereas APOE E4-positive women on therapy performed worse than those not receiving therapy.78 In our glaucoma cohort, we did not observe an interaction between APOE E4 and HRT. Also, we did not differentiate between different types of HRT formulations or routes of administration, which suggested by other studies that HRT-containing estrogen (e.g., oral conjugated equine estrogens) reduced the risk of developing glaucoma, but HRT-containing estrogen and progesterone (e.g., oral conjugated equine estrogens and medroxyprogesterone acetate) was not beneficial.11 Although the effects of HRT on glaucoma and other aging-related neurodegenerative conditions remain relatively understudied, our results still suggest promising potential for HRT in delaying glaucoma progression.
We acknowledge several limitations in our study. First, the primary limitation of this study stems from its reliance on the UKBB, where much of the critical data are self-reported. This raises the likelihood of undiagnosed glaucoma cases in the comparison group, potentially impacting the results. Additionally, participants self-reported the onset of menopause, which can be challenging to pinpoint accurately, as perimenopause and menopause often span several years. Similarly, the AAD of glaucoma and the use of HRT were also self-reported, with no information available on the specific types of HRT used. These limitations are inherent to the study design and dataset, making them difficult to address. Ideally, a prospective, large, population-based cohort with detailed information would provide a more robust dataset for such investigations. Second, the recorded AAD does not necessarily reflect the true age of disease onset. Age at diagnosis can be influenced by various factors, such as the absence of symptoms in the early stages of the disease and differences in health care utilization or access to care. As a result, AAD primarily indicates the age at which the condition was diagnosed rather than when it actually began. Third, this study's lack of specific biomarker or neuroimaging data, which would be challenging to obtain for a dataset of this size, and the inherent limitations of self-reported health data are significant constraints. Future prospective longitudinal studies incorporating multimodal biomarkers and quantitative structural/functional neuroimaging could help clarify the specific mechanistic links between menopause, APOE, HRT, and retinal/central neurodegeneration. Four, while POAG is the most common glaucoma subtype in Europeans, the inclusion of other glaucoma subtypes in our cohorts could introduce potential confounders. Lastly, although European ancestry was the predominant ethnic group in this study cohort, we did not address ethnic variations, which limits the analysis's applicability to diverse ethnic groups.
In this study, we leveraged the population-wide data of the UKBB to study APOE4 and menopause as risk factors for developing glaucoma. We found that later menopause was associated with older AAD of glaucoma, with the APOE E4 allele providing increased protection against glaucoma in those diagnosed before, but not after, menopause. The protective effect of later menopause was also enhanced by HRT use after menopause. The differential effect of the APOE E4 allele based on menopause status found in this study suggests that glaucoma pathogenesis may be partially regulated by hormonal homeostasis, underscoring the importance of considering endocrine factors when assessing genetic susceptibility to glaucoma, and potentially leading to more personalized and effective strategies for managing glaucoma and other age-related health conditions in women.
Acknowledgments
The authors acknowledge that this research used data from the UK Biobank Resource: Application 19416.
Manuscript no. XOPS-D-24-00419.
Footnotes
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
This work was supported by the National Natural Science Foundation of China (Grant No. 82201171 and Grant No. 82171050). The sponsor or funding organization had no role in the design or conduct of this research.
HUMAN SUBJECTS: Human subjects were included in this study. The research conducted using the UK Biobank (UKBB) adheres to the tenets of the Declaration of Helsinki. The UKBB received approval from the North West Multi-centre Research Ethics Committee. Our access to the resource was approved by UKBB, conforming to their access procedures and ethics. All participants provided written, informed consent, and the data were fully deidentified.
No animal subjects were used in this study.
Author Contributions:
Conception and design: Shi, Qiu, Zhang, Fan
Data collection: Shi, Liu, Zhang, Fan
Analysis and interpretation: Shi, Liu, Hu, Qiu, He, Gao
Obtained funding: Shi, Fan
Overall responsibility: Shi, Liu, Zhang, Fan
References
- 1.Bourne R.R.A., Jonas J.B., Friedman D., et al. Global estimates on the number of people blind or visually impaired by glaucoma: a meta-analysis from 2000 to 2020. Eye. 2024;38:2036–2046. doi: 10.1038/s41433-024-02995-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guidoboni G., Sacco R., Szopos M., et al. Neurodegenerative disorders of the eye and of the brain: a perspective on their fluid-dynamical connections and the potential of mechanism-driven modeling. Front Neurosci. 2020;14 doi: 10.3389/fnins.2020.566428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou Y., Dan X., Babbar M., et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15:565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 4.Douglass A., Dattilo M., Feola A.J. Evidence for menopause as a sex-specific risk factor for glaucoma. Cell Mol Neurobiol. 2023;43:79–97. doi: 10.1007/s10571-021-01179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Payami H., Montee K.R., Kaye J.A., et al. Alzheimer's disease, apolipoprotein E4, and gender. JAMA. 1994;271:1316–1317. [PubMed] [Google Scholar]
- 6.Altmann A., Tian L., Henderson V.W., Greicius M.D. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. 2014;75:563–573. doi: 10.1002/ana.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrer L.A., Cupples L.A., Haines J.L., et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer disease meta-analysis consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 8.Damoiseaux J.S., Seeley W.W., Zhou J., et al. Gender modulates the APOE ε4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. J Neurosci. 2012;32:8254–8262. doi: 10.1523/JNEUROSCI.0305-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasquale L.R., Loomis S.J., Weinreb R.N., et al. Estrogen pathway polymorphisms in relation to primary open angle glaucoma: an analysis accounting for gender from the United States. Mol Vis. 2013;19:1471–1481. [PMC free article] [PubMed] [Google Scholar]
- 10.Hogan K., Cui X., Giangiacomo A., Feola A.J. Association of age of menopause and glaucoma diagnosis in female veterans. Invest Ophthalmol Vis Sci. 2024;65:32. doi: 10.1167/iovs.65.10.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hogan K., Cui X., Giangiacomo A., Feola A.J. Postmenopausal hormone therapy was associated with later age of onset among glaucoma cases. Invest Ophthalmol Vis Sci. 2024;65:31. doi: 10.1167/iovs.65.10.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selvan H., Gupta S., Wiggs J., Gupta Juvenile-onset open-angle glaucoma - A clinical and genetic update. Surv Ophthalmol. 2022;67:1099–1117. doi: 10.1016/j.survophthal.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan E.W., Li X., Tham Y., et al. Glaucoma in Asia: regional prevalence variations and future projections. Br J Ophthalmol. 2016;100:78–85. doi: 10.1136/bjophthalmol-2014-306102. [DOI] [PubMed] [Google Scholar]
- 14.Flores V.A., Pal L., Manson J.E. Hormone therapy in menopause: concepts, controversies, and approach to treatment. Endocr Rev. 2021;42:720–752. doi: 10.1210/endrev/bnab011. [DOI] [PubMed] [Google Scholar]
- 15.Das S., Forer L., Schönherr S., et al. Next-generation genotype imputation service and methods. Nat Genet. 2016;48:1284–1287. doi: 10.1038/ng.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Dabbagh N.M., Al-Dohayan N., Arfin M., Tariq M. Apolipoprotein E polymorphisms and primary glaucoma in Saudis. Mol Vis. 2009;15:912–919. [PMC free article] [PubMed] [Google Scholar]
- 17.Vickers J.C., Craig J.E., Stankovich J., et al. The apolipoprotein epsilon4 gene is associated with elevated risk of normal tension glaucoma. Mol Vis. 2002;8:389–393. [PubMed] [Google Scholar]
- 18.Margeta M.A., Letcher S.M., Igo R.P., Jr., et al. Association of APOE with primary open-angle glaucoma suggests a protective effect for APOE ε4. Invest Ophthalmol Vis Sci. 2020;61:3. doi: 10.1167/iovs.61.8.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam C.Y., Fan B.J., Wang D.Y., et al. Association of apolipoprotein E polymorphisms with normal tension glaucoma in a Chinese population. J Glaucoma. 2006;15:218–222. doi: 10.1097/01.ijg.0000212217.19804.a7. [DOI] [PubMed] [Google Scholar]
- 20.Mabuchi F., Tang S., Ando D., et al. The apolipoprotein E gene polymorphism is associated with open angle glaucoma in the Japanese population. Mol Vis. 2005;11:609–612. [PubMed] [Google Scholar]
- 21.Shi Y., Hu J., Liu W., et al. Female-specific association between the apolipoprotein E E4 allele and age at diagnosis of glaucoma in UK biobank. Ophthalmol Glaucoma. 2024;8:53–62. doi: 10.1016/j.ogla.2024.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Gold E.B. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am. 2011;38:425–440. doi: 10.1016/j.ogc.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inayat K., Danish N., Hassan L. Symptoms of menopause in peri and postmenopausal women and their attitude towards them. J Ayub Med Coll Abbottabad. 2017;29:477–480. [PubMed] [Google Scholar]
- 24.Su H., Jiang C., Zhang W., et al. Natural menopausal age and cardiovascular disease risk factors in older Chinese women: guangzhou biobank cohort study. Menopause. 2021;28:1410–1417. doi: 10.1097/GME.0000000000001856. [DOI] [PubMed] [Google Scholar]
- 25.Scarabin P.Y. Premature menopause and risk for cardiovascular disease. JAMA. 2020;323:1616. doi: 10.1001/jama.2020.2533. [DOI] [PubMed] [Google Scholar]
- 26.Muka T., Oliver-Williams C., Kunutsor S., et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol. 2016;1:767–776. doi: 10.1001/jamacardio.2016.2415. [DOI] [PubMed] [Google Scholar]
- 27.Anagnostis P., Theocharis P., Lallas K., et al. Early menopause is associated with increased risk of arterial hypertension: a systematic review and meta-analysis. Maturitas. 2020;135:74–79. doi: 10.1016/j.maturitas.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Klein B.E., Klein R., Sponsel W.E., et al. Prevalence of glaucoma. the beaver dam eye study. Ophthalmology. 1992;99:1499–1504. doi: 10.1016/s0161-6420(92)31774-9. [DOI] [PubMed] [Google Scholar]
- 29.Vajaranant T.S., Nayak S., Wilensky J.T., Joslin C.E. Gender and glaucoma: what we know and what we need to know. Curr Opin Ophthalmol. 2010;21:91–99. doi: 10.1097/ICU.0b013e3283360b7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tielsch J.M., Sommer A., Katz J., et al. Racial variations in the prevalence of primary open-angle glaucoma. the Baltimore eye survey. JAMA. 1991;266:369–374. [PubMed] [Google Scholar]
- 31.Hogan K., Cui X., Giangiacomo A., Feola A.J. Menopause is associated with age of developing glaucoma: a retrospective study of female veterans. Invest Ophthalmol Vis Sci. 2023;64:2909. [Google Scholar]
- 32.Hulsman C.A., Westendorp I.C., Ramrattan R.S., et al. Is open-angle glaucoma associated with early menopause? the Rotterdam study. Am J Epidemiol. 2001;154:138–144. doi: 10.1093/aje/154.2.138. [DOI] [PubMed] [Google Scholar]
- 33.Lam J.S.H., Tay W.T., Aung T., et al. Female reproductive factors and major eye diseases in Asian women -the Singapore Malay eye study. Ophthalmic Epidemiol. 2014;21:92–98. doi: 10.3109/09286586.2014.884602. [DOI] [PubMed] [Google Scholar]
- 34.Knowlton A.A., Lee A.R. Estrogen and the cardiovascular system. Pharmacol Ther. 2012;135:54–70. doi: 10.1016/j.pharmthera.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cetinkaya D.B., Uyar Y., Ozbilgin K., Köse C. Effect of raloxifene and atorvastatin in atherosclerotic process in ovariectomized rats. J Obstet Gynaecol Res. 2013;39:229–236. doi: 10.1111/j.1447-0756.2012.01969.x. [DOI] [PubMed] [Google Scholar]
- 36.Lin Y., Chen J., Wu X., et al. Combined effects of 17β-estradiol and exercise training on cardiac apoptosis in ovariectomized rats. PLoS One. 2018;13 doi: 10.1371/journal.pone.0208633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rachoń D. [Role of tumor necrosis factor (TNF) and interleukin-6 (IL-6) in the pathogenesis of late complications of menopause. effects of hormone replacement therapy on TNF and IL-6 expression] Pol Merkur Lekarski. 2005;18:724–727. [PubMed] [Google Scholar]
- 38.Chung H.Y., Cesari M., Anton S., et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pasquale L.R., Kang J.H. Female reproductive factors and primary open-angle glaucoma in the nurses' health study. Eye (Lond) 2011;25:633–641. doi: 10.1038/eye.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pai S.R., Shenoy J.P., J S., Kole S.B. Postmenopausal intraocular pressure changes in south Indian females. J Clin Diagn Res. 2013;7:1322–1324. doi: 10.7860/JCDR/2013/5325.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qureshi I.A. Ocular hypertensive effect of menopause with and without systemic hypertension. Acta Obstet Gynecol Scand. 1996;75:266–269. doi: 10.3109/00016349609047099. [DOI] [PubMed] [Google Scholar]
- 42.Affinito P., Di Spiezio Sardo A., Di Carlo C., et al. Effects of hormone replacement therapy on ocular function in postmenopause. Menopause. 2003;10:482–487. doi: 10.1097/01.GME.0000063568.84134.35. [DOI] [PubMed] [Google Scholar]
- 43.Vajaranant T.S., Maki P.M., Pasquale L.R., et al. Effects of hormone therapy on intraocular pressure: the women's health initiative-sight exam study. Am J Ophthalmol. 2016;165:115–124. doi: 10.1016/j.ajo.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Altintaş O., Caglar Y., Yüksel N., et al. The effects of menopause and hormone replacement therapy on quality and quantity of tear, intraocular pressure and ocular blood flow. Ophthalmologica. 2004;218:120–129. doi: 10.1159/000076148. [DOI] [PubMed] [Google Scholar]
- 45.Vajaranant T.S., Pasquale L.R. Estrogen deficiency accelerates aging of the optic nerve. Menopause. 2012;19:942–947. doi: 10.1097/gme.0b013e3182443137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hösli E., Jurasin K., Rühl W., et al. Colocalization of androgen, estrogen and cholinergic receptors on cultured astrocytes of rat central nervous system. Int J Dev Neurosci. 2001;19:11–19. doi: 10.1016/s0736-5748(00)00082-4. [DOI] [PubMed] [Google Scholar]
- 47.Dubal D.B., Wise P.M. Estrogen and neuroprotection: from clinical observations to molecular mechanisms. Dialogues Clin Neurosci. 2002;4:149–161. doi: 10.31887/DCNS.2002.4.2/ddubal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nilsen J., Brinton R.D. Mitochondria as therapeutic targets of estrogen action in the central nervous system. Curr Drug Targets CNS Neurol Disord. 2004;3:297–313. doi: 10.2174/1568007043337193. [DOI] [PubMed] [Google Scholar]
- 49.Brinton R.D., Yao J., Yin F., et al. Perimenopause as a neurological transition state. Nat Rev Endocrinol. 2015;11:393–405. doi: 10.1038/nrendo.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mosconi L., Berti V., Guyara-Quinn C., et al. Perimenopause and emergence of an Alzheimer's bioenergetic phenotype in brain and periphery. PLoS One. 2017;12 doi: 10.1371/journal.pone.0185926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prokai-Tatrai K., Xin H., Nguyen V., et al. 17β-estradiol eye drops protect the retinal ganglion cell layer and preserve visual function in an in vivo model of glaucoma. Mol Pharm. 2013;10:3253–3261. doi: 10.1021/mp400313u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou X., Li F., Ge J., et al. Retinal ganglion cell protection by 17-beta-estradiol in a mouse model of inherited glaucoma. Dev Neurobiol. 2007;67:603–616. doi: 10.1002/dneu.20373. [DOI] [PubMed] [Google Scholar]
- 53.Russo R., Cavaliere F., Watanabe C., et al. 17Beta-estradiol prevents retinal ganglion cell loss induced by acute rise of intraocular pressure in rat. Prog Brain Res. 2008;173:583–590. doi: 10.1016/S0079-6123(08)01144-8. [DOI] [PubMed] [Google Scholar]
- 54.Scheyer O., Rahman A., Hristov H., et al. Female sex and Alzheimer's risk: the menopause connection. J Prev Alzheimers Dis. 2018;5:225–230. doi: 10.14283/jpad.2018.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moir M.E., Corkery A.T., Senese K.A., et al. Age at natural menopause impacts cerebrovascular reactivity and brain structure. Am J Physiol Regul Integr Comp Physiol. 2023;324:R207–R215. doi: 10.1152/ajpregu.00228.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yin F., Yao J., Sancheti H., et al. The perimenopausal aging transition in the female rat brain: decline in bioenergetic systems and synaptic plasticity. Neurobiol Aging. 2015;36:2282–2295. doi: 10.1016/j.neurobiolaging.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parhizkar S., Holtzman D.M. APOE mediated neuroinflammation and neurodegeneration in Alzheimer's disease. Semin Immunol. 2022;59 doi: 10.1016/j.smim.2022.101594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yin Z., Rosenzweig N., Kleemann K.L., et al. APOE4 impairs the microglial response in Alzheimer's disease by inducing TGFβ-mediated checkpoints. Nat Immunol. 2023;24:1839–1853. doi: 10.1038/s41590-023-01627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baker A.E., Brautigam V.M., Watters J.J. Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor β. Endocrinology. 2004;145:5021–5032. doi: 10.1210/en.2004-0619. [DOI] [PubMed] [Google Scholar]
- 60.Valencia-Olvera A.C., Maldonado Weng J., Christensen A., et al. Role of estrogen in women's Alzheimer's disease risk as modified by APOE. J Neuroendocrinol. 2023;35 doi: 10.1111/jne.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dewundara S.S., Wiggs J.L., Sullivan D.A., Pasquale L.R. Is estrogen a therapeutic target for glaucoma? Semin Ophthalmol. 2016;31:140–146. doi: 10.3109/08820538.2015.1114845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Villa A., Vegeto E., Poletti A., Maggi A. Estrogens, neuroinflammation, and neurodegeneration. Endocr Rev. 2016;37:372–402. doi: 10.1210/er.2016-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He L., Loika Y., Park Y., et al. Exome-wide age-of-onset analysis reveals exonic variants in ERN1 and SPPL2C associated with Alzheimer's disease. Transl Psychiatry. 2021;11:146. doi: 10.1038/s41398-021-01263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Margeta M.A., Yin Z., Madore C., et al. Apolipoprotein E4 impairs the response of neurodegenerative retinal microglia and prevents neuronal loss in glaucoma. Immunity. 2022;55:1627–1644.e7. doi: 10.1016/j.immuni.2022.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sullivan S.D., Sarrel P.M., Nelson L.M. Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. Fertil Steril. 2016;106:1588–1599. doi: 10.1016/j.fertnstert.2016.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rabipour S., Rajagopal S., Yu E., et al. APOE4 status is related to differences in memory-related brain function in asymptomatic older adults with family history of Alzheimer's disease: baseline analysis of the PREVENT-AD task functional MRI dataset. J Alzheimers Dis. 2020;76:97–119. doi: 10.3233/JAD-191292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evans S., Dowell N.G., Tabet N., et al. Cognitive and neural signatures of the APOE E4 allele in mid-aged adults. Neurobiol Aging. 2014;35:1615–1623. doi: 10.1016/j.neurobiolaging.2014.01.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lightman S., McDonald W.I., Bird A.C., et al. Retinal venous sheathing in optic neuritis. its significance for the pathogenesis of multiple sclerosis. Brain. 1987;110:405–414. doi: 10.1093/brain/110.2.405. [DOI] [PubMed] [Google Scholar]
- 69.Ba-Ali S., Lund-Andersen H. Pupillometric evaluation of the melanopsin containing retinal ganglion cells in mitochondrial and non-mitochondrial optic neuropathies. Mitochondrion. 2017;36:124–129. doi: 10.1016/j.mito.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 70.Bevan R.J., Hughes T.R., Williams P.A., et al. Retinal ganglion cell degeneration correlates with hippocampal spine loss in experimental Alzheimer's disease. Acta Neuropathol Commun. 2020;8:216. doi: 10.1186/s40478-020-01094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Allen R.S., Sayeed I., Oumarbaeva Y., et al. Progesterone treatment shows greater protection in brain vs. retina in a rat model of middle cerebral artery occlusion: progesterone receptor levels may play an important role. Restor Neurol Neurosci. 2016;34:947–963. doi: 10.3233/RNN-160672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cutler S.M., Cekic M., Miller D.M., et al. Progesterone improves acute recovery after traumatic brain injury in the aged rat. J Neurotrauma. 2007;24:1475–1486. doi: 10.1089/neu.2007.0294. [DOI] [PubMed] [Google Scholar]
- 73.Lobo R.A. Hormone-replacement therapy: current thinking. Nat Rev Endocrinol. 2017;13:220–231. doi: 10.1038/nrendo.2016.164. [DOI] [PubMed] [Google Scholar]
- 74.Thaung Zaw J.J., Howe P.R.C., Wong R.H.X. Postmenopausal health interventions: time to move on from the women's health initiative? Ageing Res Rev. 2018;48:79–86. doi: 10.1016/j.arr.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 75.Hulley S.B., Grady D. The WHI estrogen-alone trial--do things look any better? JAMA. 2004;291:1769–1771. doi: 10.1001/jama.291.14.1769. [DOI] [PubMed] [Google Scholar]
- 76.Yan H., Yang W., Zhou F., et al. Estrogen improves insulin sensitivity and suppresses gluconeogenesis via the transcription factor Foxo1. Diabetes. 2018;68:291–304. doi: 10.2337/db18-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Srivastava R.A., Srivastava N., Averna M., et al. Estrogen up-regulates apolipoprotein E (ApoE) gene expression by increasing ApoE mRNA in the translating pool via the estrogen receptor alpha-mediated pathway. J Biol Chem. 1997;272:33360–33366. doi: 10.1074/jbc.272.52.33360. [DOI] [PubMed] [Google Scholar]
- 78.Yaffe K., Haan M., Byers A., et al. Estrogen use, APOE, and cognitive decline: evidence of gene-environment interaction. Neurology. 2000;54:1949–1954. doi: 10.1212/wnl.54.10.1949. [DOI] [PubMed] [Google Scholar]