Abstract
Post-stroke depression (PSD) poses a serious impact on patients’ life quality. Effective drugs to treat this annoying disease are still being sought. Yi-nao-jie-yu (YNJY) prescription has been found to relieve PSD; however, the underlying mechanisms remain unelucidated. This work elucidated the therapeutic effects and mechanisms underlying YNJY prescription in PSD. PSD rat model was treated with YNJY prescription and ML385. Depression-like behaviors of rats was appraised. Hematoxylin–eosin, Nissl, and NeuN immunofluorescence staining were performed to observe hippocampal neuronal damage. Transmission electron microscopy was used to assess hippocampal mitochondrial damage. Commercial kits and western blotting were adopted to research ferroptosis-related factors and Nrf2/GPX4/SLC7A11 signals. In vitro experiments were performed using rat hippocampal neurons to explore the mechanism by which YNJY prescription relieves PSD. In PSD rats, YNJY prescription relieved depression-like behaviors, attenuated hippocampal neuronal damage, mitigated hippocampal ferroptosis and mitochondrial damage, and activated hippocampal Nrf2/GPX4/SLC7A11 pathway. By in vitro experiments, erastin treatment exacerbated hippocampal neuronal damage, ferroptosis, mitochondrial damage, and lipid peroxidation; however, YNJY prescription abolished these erastin-induced effects. In the erastin-treated hippocampal neuronal model of PSD, ML385 treatment increased ferroptosis, hippocampal neuronal damage, and lipid peroxidation; however, YNJY prescription counteracted these ML385-induced effects. By in vivo study, ML385 reversed the relief of YNJY prescription on depressive-like behaviors of PSD rats, and the inhibition on ferroptosis in PSD rats’ hippocampus. YNJY prescription relieves PSD by blocking ferroptosis via activating the Nrf2/GPX4/SLC7A11 pathway. It may be a promising agent for treating PSD clinically.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11481-024-10167-1.
Keywords: Yi-nao-jie-yu prescription, Post-stroke depression, Hippocampal neurons, Ferroptosis, Nrf2/GPX4/SLC7A11
Introduction
Post-stroke depression (PSD) is a common and severe psychiatric complication after a stroke. The major triggering factors for PSD include stroke severity and mental illness history (Guo et al. 2022). The common manifestations in patients with PSD are depressive symptoms such as anxiety, hopelessness, sleep disturbances, and lack of interest (Wijeratne et al. 2022). PSD seriously threatens the long-term recovery and life quality of patients and poses a heavy financial and emotional burden on the family and society.
Pharmacological and polypharmacological interventions form the basis for current treatment strategies for PSD; in contrast, poor adherence to antidepressants, hypotension, blurry vision, severe insomnia, and sexual dysfunction are the major reasons for treatment failure (Krivoy et al. 2017). Therefore, drugs that are more effective in treating PSD should be discovered to promote functional recovery and improve life quality of patients to a considerable extent. Because of concerns such as the poor tolerability of current antidepressants, recently, researchers have shifted their focus to studying the feasibility of traditional Chinese herbal medicines for PSD treatment. Multiple traditional Chinese herbal medicines have exhibited effectiveness and safety in treating PSD, with decreased neurological- and gastrointestinal-associated adverse events (Zhang et al. 2021a, b, c, 2023; Kwon et al. 2018). Therefore, traditional Chinese herbal medicines exhibit better prospects for PSD treatment. The Yi-nao-jie-yu (YNJY) prescription is a herbal formula comprising five different Chinese medical herbs: Acanthopanax senticosus, Curcuma longa, Fructus Gardeniae sp., Schisandra chinensis, and Bupleurum. These five Chinese medical herbs of the YNJY prescription are well-known Chinese traditional medicine with multiple pharmacological functions, such as neuroprotective effect, anti-inflammation, and anti-depression, and have shown favorable safety and efficacy for the treatment of stroke or PSD (Wang et al. 2014, 2020a, b, 2021; Xie et al. 2018; Liu et al. 2021; Min et al. 2022). Recently, YNJY prescription has exerted good therapeutic effects in treating animals with post-stroke psychiatric disorders, which significantly alleviated anxiety symptoms and hippocampal neuronal damage in rats with post-stroke anxiety by maintaining neuroendocrine–immune system balance via decreasing GABAAR expression in the hippocampus (Zhang et al. 2016). Similarly, in rats with PSD, treatment with YNJY prescription alleviated depression-like behaviors and positively affected the regeneration of hippocampal neurons by activating the Notch signaling pathway in rats with PSD (Tian et al. 2018). Simultaneously, YNJY prescription treatment not only improved microcirculation in the hippocampus as well as prefrontal cortex of rats with PSD, but also repaired microstructural damage in both these brain regions. Furthermore, treatment with YNJY prescription mitigated abnormal changes in brain metabolite levels by regulating membrane phospholipid metabolism and the glutamatergic system. Therefore, the alleviating effects of YNJY prescription on depressive symptoms in rats with PSD may be attributed to these functions (Zhao et al. 2020). Collectively, the abovementioned findings preliminary indicate the effectiveness of YNJY prescription for managing PSD. However, to widely apply YNJY prescription for treating PSD in clinical settings, additional evidence regarding its efficacy is warranted; in particular, the molecular mechanisms underlying this process should be investigated. Thus, this paper was to investigate the therapeutic effect of YNJY prescription on PSD; more importantly, the molecular mechanism of YNJY prescription in treating PSD was investigated deeply in order to provide reliable basis for its use in clinical treatment of PSD.
Ferroptosis is a newly proposed iron-dependent type of cell death that is primarily characterized by the hyperactivation of lipid peroxidation, oxidative stress, and mitochondrial disruption (Mou et al. 2019). Proteomics analysis of the hippocampus has revealed the hyperactivation of ferroptosis in the hippocampus of chronically unpredictable mild stress (CUMS)-induced mice with depression (Cao et al. 2021). Interestingly, the traditional Chinese herbal medicine Xiaoyaosan exerts an antidepressant effect in CUMS-induced mice with depression by suppressing ferroptosis in the hippocampal neurons (Jiao et al. 2021). Therefore, in the present work, we established a PSD rat model through inducing post-stroke CUMS to elucidate the therapeutic effect of YNJY prescription on PSD. Simultaneously, a rat hippocampal neuronal model of PSD was established to elucidate whether YNJY prescription plays a therapeutic role in PSD by regulating ferroptosis. More importantly, the molecular mechanisms by which YNJY prescription affects ferroptosis in treating PSD were further explored. To the best of our knowledge, this was the first time that ferroptosis and its molecular mechanism have been used as a clue to explore the role of YNJY prescription in the treatment of PSD.
Materials and Methods
Preparation of the YNJY Prescription
YNJY prescription was purchased from Kangrentang Pharmaceutical Co. (Beijing, China). It comprises five different Chinese medical herbs: 20 g of Acanthopanax senticosus, 13 g of Curcuma longa, 10 g of Fructus Gardeniae sp., 10 g of Schisandra chinensis, and 10 g of Bupleurum. Before using YNJY, the granules were dissolved in 100 mL of distilled water and then stored at 4 °C.
Animals
Sixty male Sprague Dawley (SD) rats (weighing 250–280 g) were supplied by Si Pei Fu Biotechnology Co. (Beijing, China). Before the experiments, the rats were subjected to adaptive feeding for 1 week in a 22 °C ± 2 °C room with a 12-h day/night cycle. All of these rats were given ad libitum access to food and water. The Animal Committee of the MDKN Biotech Co. (Beijing, China) approved all animal studies (Approval No.: MDKN-2023–010). Furthermore, all animal experiments were conducted according to the “Guiding Opinions on the Good Treatment of Laboratory Animals” issued by the Ministry of Science and Technology of the People’s Republic of China.
Construction of the PSD Rat Model
Stroke following CUMS stimulation is the common method for the construction of PSD (Li et al. 2021; Zhang et al. 2021a, b, c). Thus, in this work, the PSD rat model was established via two steps: developing the intracerebral hemorrhage (ICH) model and then the PSD model. The procedure was implemented as previously reported (Li et al. 2021; Zhang et al. 2021a, b, c; Liu et al. 2022).
To develop the ICH model, collagenase was injected into the rats via the caudate nucleus. Specifically, the rats were subjected to deep anesthesia via 2.5% isoflurane (Reward Life Technology Co., Shenzhen, China) inhalation. Thereafter, they were fixed on a brain stereotaxic apparatus (Yuyan Scientific Instrument Co., Shanghai, China). Head hair was shaved, and the skin of the exposed head was sterilized and incised. At the site of the caudate nucleus ( the injection coordinates were 3 mm to the right of the breggma, 1 mm posterior to the breggma, and 5 mm in depth, with breggma as the zero point), a 5-mm deep hole was drilled into the skull. Then, a microsyringe containing 0.6 µL of collagenase type VII (Sigma, St Louis, MO, USA) was directly inserted into the caudate nucleus; the microsyringe was withdrawn 5 min after the injection. At last, the head skin was sutured and then sterilized. After the surgery, rats were intraperitoneally injected with penicillin (800,000 IU/kg/d; North China Pharmaceutical Group Co., Shijiazhuang, China) to prevent infection. Based on the Berderson scoring criteria (Desland et al. 2014), rats without stroke signs or those still not awake 2 h after the surgery were excluded from this study.
Next, rats with ICH were subjected to CUMS stimulation and solitary rearing to develop the PSD model. After the ICH surgery, rats were routinely housed for 1 week for recovery. In the subsequent 4 weeks, they were subjected to CUMS stimulation every day. Seven stimulation methods were included to stimulate CUMS, with one method conducted daily per week. The CUMS stimulation methods were as follows: (1) rats were subjected to fasting from food and water from 7:00 to 7:00 the next day; (2) 250 mL of water was poured into the rat bedding and the rats were left in the damp bedding for 24 h; (3) rats were treated with circadian inversion; (4) rats were placed in cold water (4 °C) for swimming for 5 min; (5) rats were placed in warm water (40 °C) for swimming for 5 min; (6) rats were kept in a bottle with its tail exposed; the tail was clamped with a clip for 1 min at 1 cm from the tail root; and (7) rats were placed in a fixator (20 cm long) for 2 h.
Animal Treatment
Sixty SD rats were randomly divided into six groups: Sham, ICH, PSD, low-dose YNJY (YNJY-L), high-dose YNJY (YNJY-H), and YNJY-H + ML385 groups. There were 10 rats in each group. ML385 is an inhibitor of Nrf2, which was used to block the Nrf2 pathway in rats in this study. The dose of ML385 used in rats was referred to the study of Chen et al. (Chen et al. 2023a, b).
The rats in the Sham group were subjected to ICH surgery; notably, 0.6 µL of saline (rather than collagenase type VII) was injected via the caudate nucleus. After the surgery, rats were routinely housed and not subjected to any other interventions or treatments.
The rats in the ICH group were only subjected to ICH surgery but not any other interventions or treatments.
The rats in the PSD group underwent ICH surgery and PSD model construction. However, no other interventions or treatments were performed.
The rats in the YNJY-L and YNJY-H groups underwent ICH surgery and PSD model construction. During CUMS stimulation, low-dose (4.19 g/kg/day, equivalent to normal clinical minimum dosage) and high-dose (8.38 g/kg/day, equivalent to normal clinical minimum dosage) YNJY were administered to the rats in the YNJY-L and YNJY-H groups, respectively, via gavage.
The rats in the YNJY-H + ML385 group were subjected to ICH surgery and construction of PSD. During CUMS stimulation, high-dose (8.38 g/kg/day) YNJY administration combined with intraperitoneal injection of 30 mg/kg/day ML385 (Chen et al. 2023a, b) (Nrf2 inhibitor) were given to rats.
After surgery, mice were injected subcutaneously with meloxicam (dose: 2 mg/kg/d) for 3 days for analgesia. During the last week of the experiment, the rats in each group were subjected to behavioral tests to evaluate depression-like behaviors. At the end of the entire experiment, the rats were weighed and then euthanized to collect whole brains. The hippocampus was isolated and preserved in a refrigerator at − 80 °C.
Sucrose Preference Test
Two identical water bottles containing 1% sucrose solution were placed in the cages one day before the sucrose preference test for habituation. The next day, rats were deprived of water for 4 h. Subsequently, one bottle was filled with 1% sucrose solution, whereas the other one was filled with pure water. To avoid the rats from developing positional preference, the two bottles were displaced after 12 h. After 24 h, sucrose preference was evaluated using the following formula: Sucrose preference = (sucrose water intake/the total of pure water and sucrose water intake) × 100%.
Tail Suspension Test (TST)
The tail of each rat was secured with tape and then hung upside down on a stand. The distance of the tape from the tail tip was 1 cm, and the head of the rat was 15 cm from the ground. After 6 min, the immobility time of the rats was recorded. The entire experiment was recorded using a digital camera (Nikon, Tokyo, Japan).
Forced Swimming Test (FST)
A transparent glass cylinder (diameter of 15 cm and height of 30 cm) was prepared. The cylinder was filled with water at 23 °C to a depth of 15 cm. Then, the rats were placed into this cylinder and kept for 6 min. The immobility time was recorded. The entire experiment was recorded using a digital camera (Nikon).
Open-Field Test (OFT)
Each rat was placed in a box (100 cm long, 100 cm wide, and 50 cm height) for 5 min. The center of the box was a square that was 50 cm long and 50 cm wide. After the test, the counts of rearing and crossing the center were recorded. The entire experiment was recorded using a digital camera (Nikon).
Hematoxylin–eosin (HE) and Nissl Staining
The hippocampus of each rat was fixed with 4% paraformaldehyde (Beyotime Biotechnology Co., Shanghai, China) for 48 h at 4 °C. After dehydrating the hippocampal samples and embedding them in paraffin, 5-µm thick slices were prepared. Then, the slices were treated with xylene and gradient alcohol to dewax and rehydrate them, respectively.
For HE staining, hematoxylin and eosin solutions (both from Lianmai Biological Engineering Co., Shanghai, China) were sequentially added to the sections for staining for 5 and 2 min, respectively. For Nissl staining, the sections were stained with Nissl solution (Yiyan Biotechnology Co., Shanghai, China) for 5 min. After dehydration using gradient alcohol and transparency using xylene, the dried sections were sealed with neutral resin for histopathological and neuronal observations under an optical microscope (CX41, Olympus, Tokyo, Japan).
Immunofluorescence Staining
The dewaxed and rehydrated 5-µm thick hippocampal tissue sections were permeabilized with 0.5% Triton X-100 (Beyotime Biotechnology Co.) for 30 min at room temperature. Then, the sections were blocked with 5% normal goat serum (Beyotime Biotechnology Co.) for 30 min at room temperature. To measure neuronal nuclear antigen (NeuN) expression, the sections were treated with mouse anti-NeuN (1:200, ab104224, Abcam, Shanghai, China) primary antibodies overnight at 4 °C. Then, the sections were treated with Alexa Fluor 488-conjugated goat anti-mouse (1:200, SY0683, Biolab Technology Co., Beijing, China) secondary antibodies for 2 h at room temperature. Further, to detect the co-localization of NeuN and nuclear factor erythroid 2-related factor-2 (Nrf2), the sections were incubated with both mouse anti-NeuN (1:200, ab104224, Abcam) and rabbit anti-Nrf2 (1:200, ab62352, Abcam) primary antibodies overnight at 4 °C. Thereafter, the sections were treated with both Alexa Fluor 594-conjugated goat anti-mouse secondary antibody (1:200, SY0688, Biolab Technology Co.) and Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody (1:200, SY0669, Biolab Technology Co.) for 2 h at room temperature. Then, 4’, 6-diamidino-2-phenylindole (DAPI) (Beyotime Biotechnology Co.) was used to stain the sections for 5 min in the dark. After drying and treating the sections with neutral resin, they were observed and imaged under a fluorescence microscope (IX70, Olympus).
Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from rat hippocampus using TRIzol reagent (Lianmai Biological Engineering Co.). Total RNA was reverse-transcribed into cDNA through adopting the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) based on the manufacturer’s instructions. Then, quantitative PCR was performed using the cDNA template with the SYBR Green Master Mix (Applied Biosystems) on the 7500 Real-Time PCR System (Applied Biosystems). The cycling conditions were as follows: 94 °C for 3 min; 39 cycles at 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 35 s; and 72 °C for 5 min. The primers used were as follows: Nrf2: sense, 5′-GCCTTCCTCTGCTGCCATTAGTC-3′, and antisense, 5′-TGCCTTCAGTGTGCTTCTGGTTG-3′; glutathione peroxidase (GPX4): sense, 5′-CCAGCAACAGCCACGAGTTCC-3′, and antisense, 5′-CACACGCAACCCCTGTACTTATCC-3′; solute carrier family 7 member 11 (SLC7A11): sense, 5′-CCATCATCATCGGCACCGTCATC-3′, and antisense, 5′-TACTCCACAGGCAGACCAGAACAC-3′; and glyceraldehyde-3-phosphate dehydrogenase (GAPDH): sense, 5′-AGTGCCAGCCTCGTCTCATA-3′, and antisense, 5′-TGAACTTGCCGTGGGTAGAG-3′. The relative mRNA expression of Nrf2, GPX4, and SLC7A11 was calculated using the 2−ΔΔCt method and normalized using GAPDH.
Isolation and Identification of Primary Rat Hippocampal Neurons
Primary hippocampal neurons were isolated from newborn SD rats (Kaixue Biotechnology, Shanghai, China) using a previously described method (Combs et al. 2021). Briefly, the hippocampal samples isolated from 10 newborn SD rats were sliced into small pieces. To dissociate the hippocampal neurons, these small hippocampal pieces were incubated with 0.125% trypsin (Lianmai Biological Engineering Co.) for 15 min at 37 °C. The dissociated hippocampal neurons were cultured in neurobasal medium (Yanhui Biotechnology Co., Shanghai, China) supplemented with 2% B-27 (Yanhui Biotechnology Co.) and 1% GlutaMAX (Kemin Biotechnology Co., Shanghai, China) at 37 °C and 5% CO2 in 6-well plates precoated with poly-D-Lysine (Beyotime Biotechnology Co.).
After culturing for 5 days, the isolated primary hippocampal neurons were identified via immunofluorescence staining for NeuN and microtubule-associated protein2 (MAP2). Hippocampal neurons were fixed in 4% paraformaldehyde for 15 min, permeabilized in 0.5% Triton X-100 for 10 min, and then blocked in 5% normal goat serum for 30 min at room temperature. After probing with both mouse anti-NeuN (1:200, ab104224, Abcam) and rabbit anti-matrix metalloproteinase 2 (MMP2) (1:200, ab235167, Abcam) primary antibodies overnight at 4 °C, these hippocampal neurons were treated with both Alexa Fluor 488-conjugated goat anti-mouse (1:200, SY0683, Biolab Technology Co.) and Alexa Fluor 594-conjugated goat anti-rabbit (1:200, SY0673, Biolab Technology Co.) secondary antibodies for 2 h at room temperature. Then, DAPI solution was added dropwise, and the hippocampal neurons were incubated for 5 min in the dark. Finally, hippocampal neurons were observed and imaged under a fluorescence microscope (IX70, Olympus).
Transfection of Hippocampal Neurons
Hippocampal neurons were plated into the 6-well plates with 1 × 105 cells in 1 mL serum-free neurobasal medium. Short interfering RNA (siRNA) against Nrf2 and the corresponding siRNA negative control (NC) were both commercially supplied by GeneChem (Shanghai, China). The two kinds of siRNA were separately transfected into hippocampal neurons by using Lipofectamine 3000 (Thermo Fisher Scientific, San Jose, CA USA). After transfection, hippocampal neurons were cultivated in normal neurobasal medium at 37 °C and 5% CO2.
Treatment of Primary Hippocampal Neurons
To determine the safe and effective concentrations of the YNJY prescription, hippocampal neurons were treated with neurobasal medium supplemented with different concentrations of YNJY prescription (1 µg/mL, 10 µg/mL, 100 µg/mL, 500 µg/mL, 1 mg/mL, 5 mg/mL, 10 mg/mL, and 100 mg/mL) for 48 h at 37 °C and 5% CO2.
To induce ferroptosis, erastin (Beyotime Biotechnology Co.) was dispersed to the neurobasal medium at a final concentration of 10 µM (Dahlmanns et al. 2017) and hippocampal neurons were pretreated for 8 h. Then, these hippocampal neurons were incubated with the neurobasal medium in the presence or absence of YNJY prescription for 48 h at 37 °C and 5% CO2.
To inactivate Nrf2, hippocampal neurons were pretreated with ML385 (Nrf2 inhibitor) at a final concentration of 1 µM (Chen et al. 2023a, b) for 1 h. Furthermore, to cotreat erastin and ML385, hippocampal neurons were pretreated with erastin for 8 h and then with ML385 for 1 h. All subsequent experiments were performed by culturing these hippocampal neurons in the presence or absence of YNJY prescription for 48 h at 37 °C and 5% CO2. Hippocampal neurons cultured under normal conditions were used as a control.
Cell Counting Kit-8 (CCK-8) Assay
The hippocampal neurons were inoculated in 96-well plates at a density of 5 × 103 cells/mL. Five replicates were prepared in each group. After the relevant treatment, the CCK-8 solution (Beyotime Biotechnology Co.) was dispersed into the wells and incubated for 2 h at 37 °C. Finally, the optical density (OD) was measured using a microplate reader (BioTek, Winooski, VT, USA).
Lactate Dehydrogenase (LDH) Assay
The culture medium of the hippocampal neurons isolated from each group was collected and centrifuged at 4 °C and 120,000 rpm for 10 min. The supernatant was collected. The LDH activity in each supernatant sample was measured using the LDH activity assay kit (Sangon Biological Engineering Co., Shanghai, China) according to the manufacturer’s instructions.
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick end Labeling (TUNEL) Staining
After the relevant treatments, hippocampal neurons in 6-well plates were fixed with 4% paraformaldehyde for 10 min and then permeated with 0.1% Triton X-100 for 2 min. Then, the neurons were reacted with the TUNEL reaction solution (Beyotime Biotechnology Co.) for 40 min in the dark, followed by treatment with DAPI for 5 min at room temperature. The residual staining solution was removed using phosphate buffer saline (Beyotime Biotechnology Co.). Finally, hippocampal neurons were observed under a fluorescence microscope (IX70, Olympus) with green fluorescence indicating apoptotic neurons.
Detection of Ferroptosis-Related Factors
The levels of ferroptosis-related factors in the hippocampus and hippocampal neurons of rats were measured, including iron, malondialdehyde (MDA), superoxide dismutase (SOD), glutathione (GSH), and reactive oxygen species (ROS). The hippocampus and hippocampal neurons were collected from each group and lysed with lysis buffer (Kanglang Biotechnology Co., Shanghai, China) for 30 min on ice. The composition of the lysis buffer included 20 mM Tris (pH 7.5), 150 mM NaCl, 1% Triton X-100, and 100 mM phenylmethanesulfonyl fluoride. The supernatant was obtained by centrifuging the samples at 4 °C and 12,000 rpm for 10 min. The levels of these ferroptosis-related factors were measured by using the iron assay kit, MDA assay kit, SOD assay kit, GSH assay kit, and ROS assay kit according to the manufacturers’ instructions. All these kits were commercially supplied by Yubo Biotechnology Co. (Shanghai, China).
Transmission Electron Microscopy (TEM)
After fixed the hippocampal tissues with 4% paraformaldehyde for 48 h, they were routinely dehydrated, paraffin-embedded, and sliced into 60-nm thick sections. Furthermore, after relevant treatments, the hippocampal neurons were collected from each group, fixed with 4% paraformaldehyde for 2 h, paraffin-embedded, and then sliced into 60-nm-thick sections. These hippocampal tissue and neuronal sections were routinely dewaxed and dehydrated and then transferred to copper grids. After staining with uranyl acetate (Kanglang Biotechnology Co.) for 10 min and with lead citrate (Head Biotechnology Co., Beijing, China) for 2 min, the sections were imaged under a transmission electron microscope (HT7700, Hitachi, Tokyo, Japan) at an accelerating voltage of 80 kV.
JC-1 Staining
To measure mitochondrial membrane potential, hippocampal neurons were cultured under specific conditions in 6-well plates and then fixed with 4% paraformaldehyde for 2 h. Next, the neurons were stained with JC-1 solution (Beyotime Biotechnology Co.) for 30 min in the dark. After staining the nucleus with DAPI, the hippocampal neurons from each group were imaged under a fluorescence microscope (IX70, Olympus). The mitochondrial membrane potential was indicated as the ratio of JC-1 aggregate/JC-1 monomer.
C11-BODIPY Staining
Hippocampal neurons were cultured in 6-well plates under relevant conditions and then fixed with 4% paraformaldehyde for 2 h. To measure lipid peroxidation, the C11-BODIPY 581/591 probe (27086–1, AmyJet Scientific, Wuhan, China) was added into the wells and incubated for 30 min in the dark. After DAPI staining, the hippocampal neurons were observed and imaged under a fluorescence microscope (IX70, Olympus). In the presence of lipid peroxidation, the color of C11-BODIPY 5 shifted from red fluorescence to green fluorescence.
Mito-Tracker Red Staining
Hippocampal neurons were cultured under the relevant conditions in 6-well plates and then fixed with 4% paraformaldehyde for 2 h. Thereafter, Mito-Tracker Red solution (Beyotime Biotechnology Co.) was added to stain the neurons and incubated in the dark for 30 min at 37 °C. DAPI solution was added for routine nuclear staining. The mitochondria length were observed and imaged under a fluorescence microscope (IX70, Olympus) and analyzed by Mitochondria Analyzer software.
Western Blotting
Total proteins were extracted from the hippocampus and hippocampal neurons using the radioimmunoprecipitation assay lysis buffer (Kanglang Biotechnology Co.). The lysed samples were centrifuged at 4 °C and 120,000 rpm for 15 min. The supernatant of each sample was collected to measure the total protein concentration using a BCA kit (Biolab Technology Co.). Total proteins were then separated via sodium dodecyl sulfate–polyacrylamide gel electrophoresis. After transferring the gels onto polyvinylidene fluoride (PVDF) membranes (Lianshuo Biotechnology Co., Shanghai, China), the proteins were subjected to blocking with 5% skimmed milk for 2 h at room temperature. Then, the following primary antibodies were added onto the PVDF membranes to treat the proteins overnight at 4 °C: rabbit anti-Nrf2 (1:1000, ab92946, Abcam), rabbit anti-GPX4 (1:1000, AF7020, Beyotime Biotechnology Co.), rabbit anti-SLC7A11 (1:1000, PAB14937, Kemin Biotechnology Co., Wuhan, China), and rabbit anti-GAPDH (1:1000, ab37168, Abcam). The remaining primary antibodies were removed using Tris-buffered saline with 0.1% Tween 20 (TBST). Horseradish peroxidase-labeled goat anti-rabbit secondary antibody (1:3000, ab97051, Abcam) was added onto the PVDF membranes and allowed to react for 2 h at room temperature. After washing with TBST, the PVDF membranes were treated with the enhanced chemiluminescent reagent (Absin Biotechnology Co., Shanghai, China) to visualize protein blots. Protein blots were quantified using Image J software (NIH, Bethesda, Maryland, USA). The relative protein expression of Nrf2, GPX4, and SLC7A11 was normalized to that of GAPDH.
Statistical Analysis
Data were represented as mean ± standard deviation and analyzed using GraphPad Prism 8.0 software (GraphPad Software, San Diego, CA, USA). In this work, data were shown to be normally distributed by the Kolmogorov–Smirnov and the Shapiro–Wilk tests. One-way analysis of variance combined with Tukey’s post-hoc test was conducted to analyze statistical differences in multiple groups (at least three groups). A P-value of < 0.05 was considered statistically significant.
Results
YNJY Prescription Relieved the Depression-Like Behaviors of Rats with PSD
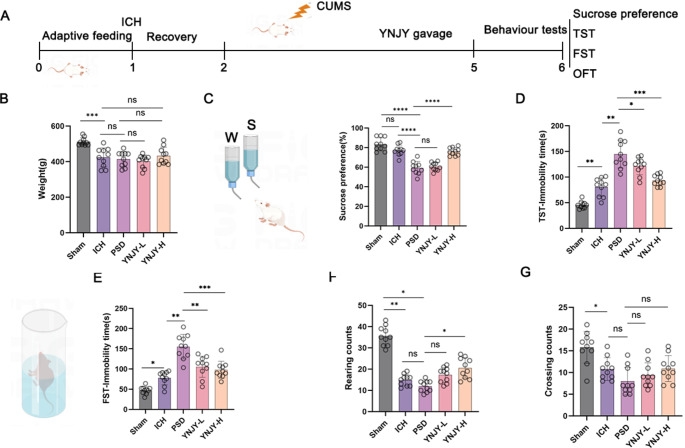
The experimental procedure of this study was shown in Fig. 1A. At the end of the experiment, the body weight of the rats was measured. The rats in the ICH group had lower body weight than those in the Sham group (P < 0.001). The differences in body weight were not obvious between the ICH group and the PSD group, between the PSD group and the YNJY-L group, between the ICH group and the YNJY-H group, as well as between the PSD group and the YNJY-H group (Fig. 1B).
Fig. 1.
YNJY prescription relieved the depression-like behaviors of rats with PSD. (A) Design of the animal experiment. (B) Body weight of the rats at the end of the experiment. N = 10 per group. (C) Effect of YNJY prescription on the sucrose preference of rats in the sucrose preference test. N = 10 per group. (D) Effect of YNJY prescription on the immobility time of rats among the five groups in TST. N = 10 per group. (E) Effect of YNJY prescription on the immobility time of rats in FST. N = 10 per group. (F and G) Effect of YNJY prescription on the rearing and crossing counts of rats in OFT. N = 10 per group. * P < 0.05, ** P < 0.01, *** P < 0.001, and ***** P < 0.0001. The symbol “ns” indicated that the difference was not statistically significant
The sucrose preference test revealed the similar sucrose preference of the rats between the ICH group and the Sham group. However, when refered to the Shan group, rats of the PSD group exhibited distinctly lower sucrose preference (P < 0.0001). The rats in the PSD group exhibited distinctly decreased sucrose preference, compared with those in the ICH group (P < 0.0001). The difference in sucrose preference was not obvious in rats between the PSD group and the YNJY-L group. Compared with the rats in the PSD group, those in the YNJY-H group had significantly increased sucrose preference (P < 0.0001) (Fig. 1C). In TST (Fig. 1D) and FST (Fig. 1E), the rats in the ICH group had a longer immobility time than those in the Sham group (P < 0.05 and P < 0.01). Besides, compared with the rats in the ICH group, those in the PSD group showed a much longer immobility time (P < 0.001). In contrast, YNJY treatment (the YNJY-L and YNJY-H groups) shortened the immobility time, matched to those in the PSD group (P < 0.05, P < 0.01, and P < 0.001, respectively).
OFT (Fig. 1F and G) revealed that the rats in the ICH group displayed prominently decreasing rearing and crossing counts compared with those in the Sham group (P < 0.05 and P < 0.01). Simultaneously, lower rearing counts in rats of the PSD group was found, as refered to the Sham group (P < 0.05). However, in comparison to the rats in the PSD group, those in the YNJY-H group displayed a higher rearing count (P < 0.05). Collectively, the findings of these behavioral tests suggest that YNJY prescription effectively ameliorates the depression-like behaviors of rats with PSD.
YNJY Prescription Mitigated Hippocampal Neuronal Damage in Rats with PSD
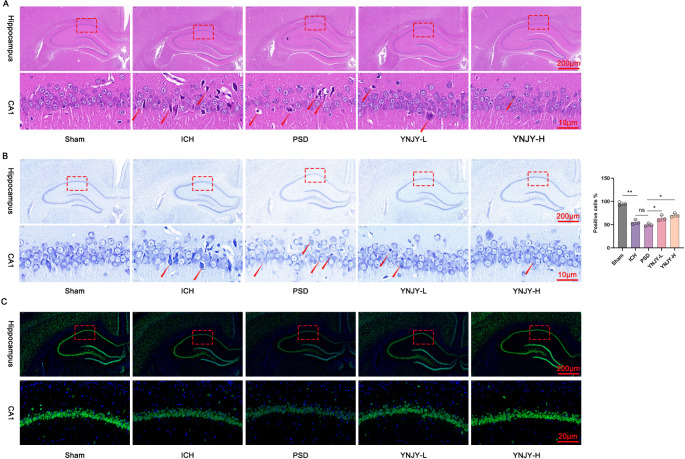
To elucidate the effect of YNJY prescription on hippocampal damage in rats with PSD, hippocampal samples were subjected to HE staining. In the hippocampus of the rats in the Sham group, the neurons were structurally intact and tightly arranged with full nuclei. However, severe hippocampal damage, including loosely and disorderly arranged neurons, increased space between adjacent neurons, and nuclear atrophy, was observed in the rats in the ICH group, particularly in those in the PSD group. Notably, compared with the rats in the PSD group, the abovementioned pathological characteristics of the hippocampus were obviously relieved in the rats in the YNJY-L and YNJY-H groups, particularly in the YNJY-H group (Fig. 2A).
Fig. 2.
YNJY prescription weakened neuronal damage in the hippocampus of rats with PSD. (A) Hematoxylin and eosin staining demonstrating hippocampal damage in the rats in the five groups. The red arrows indicated the damaged neurons. N = 3 per group. (B) Nissl staining showing hippocampal neuron loss in the rats in the five groups. The red arrows indicated the damaged neurons. N = 3 per group. (C) NeuN immunofluorescence staining showing hippocampal neuronal damage in the rats in the five groups. N = 3 per group. * P < 0.05 and ** P < 0.01. The symbol “ns” indicated that the difference was not statistically significant
To elucidate neuron loss, rat hippocampal samples were subjected to Nissl staining. Abundant Nissl bodies and clear neuron outlines were observed in the hippocampal samples of the rats in the Sham group; however, as relative to the Sham group, in the rats in the ICH group, particularly in those in the PSD group, Nissl bodies were significantly decreased (P < 0.01), the cytoplasm and nucleus were atrophied, neurons were loosely arranged, and neuron outlines were unclear. Interestingly, YNJY prescription (the YNJY-L and YNJY-H groups) remarkably increased the number of Nissl bodies (P < 0.05) and improved intact neurons in the hippocampus of rats with PSD, when matched to the rats in the PSD group (Fig. 2B).
NeuN immunofluorescence staining revealed the presence of abundant NeuN-labeled hippocampal neurons; however, the intensity of NeuN immunofluorescence staining in hippocampus was considerably decreased in the rats in the ICH group, particularly in those in the PSD group. Interestingly, YNJY prescription considerably intensified NeuN immunofluorescence staining in hippocampus in rats with PSD (Fig. 2C). Collectively, these findings suggest that YNJY prescription can effectively attenuate hippocampal damage and neuronal loss in rats with PSD.
YNJY Prescription Attenuated Ferroptosis in the Hippocampus of PSD Rats
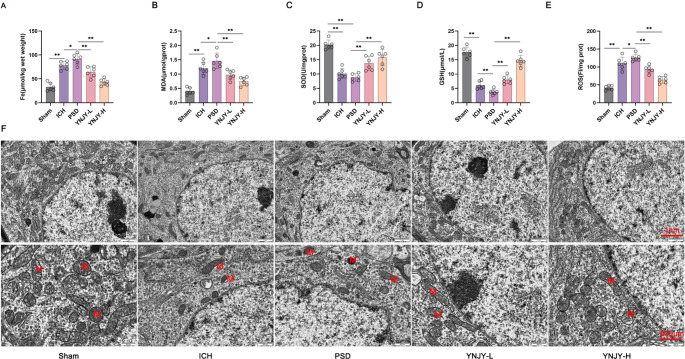
Ferroptosis is overactivated in the hippocampus of mice with depression-like behaviors (Jiao et al. 2021). Therefore, we evaluated the effect of YNJY prescription on ferroptosis in the hippocampus of rats by measuring the levels of several ferroptosis-related factors. Figure 3A, B, C and D, and 3E illustrate that, compared with the rat hippocampus in the Sham group, the ICH group had higher Fe, MDA, and ROS levels but lower SOD and GSH levels (P < 0.01). Furthermore, relative to rat hippocampus in the ICH group, higher Fe, MDA, and ROS levels, and lower GSH levels were detected in rat hippocampus in the PSD group (P < 0.05 and P < 0.01). Treatment with YNJY prescription (YNJY-L and YNJY-H groups) dramatically decreased Fe, MDA, and ROS levels but increased SOD and GSH levels, when compared to the PSD group (P < 0.01). Collectively, these data suggest that YNJY prescription suppresses ferroptosis in the hippocampus of rats with PSD.
Fig. 3.
YNJY prescription attenuated ferroptosis in the hippocampus of rats with PSD. (A–E) Levels of ferroptosis-related factors in rat hippocampus tested using commercial kits. N = 6 per group. (F) TEM showing mitochondrial damage in rat hippocampus. N = 6 per group.* P < 0.05 and ** P < 0.01
Next, we performed TEM to observe mitochondrial damage in the hippocampus of rats. The rat hippocampus in the Sham group had normal mitochondria; however, severe mitochondrial damage, including morphological changes and damaged mitochondrial ridges, was observed in the rat hippocampus in the ICH group, particularly in the PSD group. YNJY prescription evidently relieved this damage, particularly YNJY-H (Fig. 3F). Therefore, YNJY prescription can effectively relieve mitochondrial damage in the hippocampus of rats with PSD.
YNJY Prescription Activated the Nrf2/GPX4/SLC7A11 Pathway in the Hippocampus of Rats with PSD
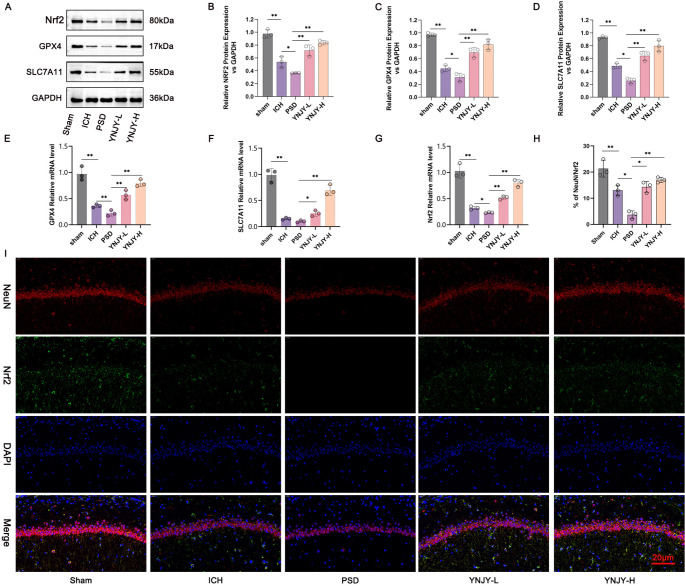
The Nrf2/GPX4/SLC7A11 pathway is an important pathway associated with ferroptosis. Therefore, we determined the effect of YNJY prescription on the Nrf2/GPX4/SLC7A11 pathway in rat hippocampus. Western blotting (Fig. 4A, B and C, and 4D) and qRT-PCR (Fig. 4E and F, and 4G) were performed to detect the mRNA and protein expression for NRF2, GPX4, and SLC7A11. The mRNA and protein expression for NRF2, GPX4, and SLC7A11 was lower in the rat hippocampus in the ICH group, as referring to that in the Sham group (P < 0.01). Simultaneously, the rats in the PSD group expressed less protein expression for NRF2, GPX4, and SLC7A11, as well as lower mRNA expression for GPX4 and NRF2 in their hippocampus, in contrast to that in the ICH group (P < 0.05 and P < 0.01). In contrast, YNJY prescription treatment (YNJY-L and YNJY-H groups) distantly enhanced the mRNA and protein expression of NRF2, GPX4, and SLC7A11, when compared to the rats in the PSD group (P < 0.05 and P < 0.01).
Fig. 4.
YNJY prescription activated the Nrf2/GPX4/SLC7A11 pathway in the hippocampus of rats with PSD. (A–D) Western blotting detecting the protein levels of Nrf2, GPX4, and SLC7A11 in rat hippocampus. N = 3 per group. (E–G) qRT-PCR showing the expression of mRNA for Nrf2, GPX4, and SLC7A11 in rat hippocampus. N = 3 per group. (H and I) NeuN/Nrf2 immunofluorescence staining of rat hippocampus. N = 3 per group. * P < 0.05 and ** P < 0.01
NeuN/Nrf2 immunofluorescence staining (Fig. 4H and I) revealed that the percentage of NeuN/Nrf2-positive hippocampal neurons was decreased in the rats in the ICH group compared with those in the Sham group (P < 0.01). At the same time, NeuN/Nrf2-positive hippocampal neurons were much reduced in the rats in the PSD group than in those in the ICH group (P < 0.05). However, by referring to the rats in the PSD group, those in the YNJY-H group presented more NeuN/Nrf2-positive hippocampal neurons in their hippocampus (P < 0.05). Collectively, these findings suggest that the Nrf2/GPX4/SLC7A11 pathway is abnormally suppressed in the hippocampus of rats with PSD. Interestingly, YNJY prescription activated this pathway.
YNJY Prescription Blocked Erastin-Induced Hippocampal Neuronal Damage and Ferroptosis
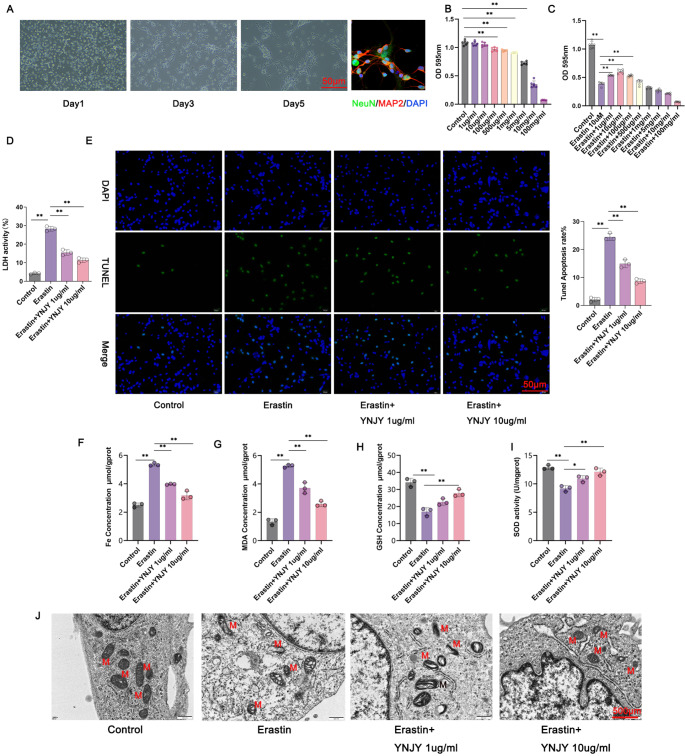
Hippocampal neurons were isolated from rats and then subjected to NeuN/MAP-2 immunofluorescence staining. The isolated hippocampal neurons expressed NeuN and MAP-2 proteins (Fig. 5A). This finding suggests the successful isolation of hippocampal neurons from rats.
Fig. 5.
YNJY prescription counteracted erastin-induced hippocampal neuronal damage and ferroptosis. (A) NeuN/MAP-2 immunofluorescence staining showing the hippocampal neurons isolated from rats. (B and C) CCK-8 assay showing the safe and effective concentration of the YNJY prescription. N = 6 per group. (D) LDH activity detection in the hippocampal neurons using a commercial kit. N = 3 per group. (E) TUNEL staining showing the apoptosis of hippocampal neurons. N = 3 per group. (F–I) Levels of ferroptosis-related factors were determined using commercial kits. N = 3 per group. (J) TEM showing mitochondrial damage in the hippocampal neurons. N = 3 per group. * P < 0.05 and ** P < 0.01
The isolated hippocampal neurons were subjected to the CCK-8 assay to determine the safe and effective concentration of the YNJY prescription. Figure 5B demonstrated that 1 and 10 µg/mL YNJY prescription did not exert toxicity against hippocampal neurons; however, when the concentration of YNJY prescription reached 100 µg/mL, the viability of the hippocampal neurons was significantly attenuated (P < 0.01). Subsequently, the hippocampal neurons were treated with 10 µM erastin (a ferroptosis activator) with or without different concentrations of the YNJY prescription. Figure 5C presents the results. Erastin (10 µM) treatment dramatically weakened the viability of the hippocampal neurons (P < 0.01), as relative to the Control group (P < 0.01). On the Other hand, compared with erastin (10 µM)-treated hippocampal neurons, the viability of those treated with erastin (10 µM) and 1, 10, and 100 µg/mL YNJY prescription was prominently enhanced (P < 0.01). Therefore, YNJY prescription at concentrations of 1 and 10 µg/mL was used in the subsequent experiments.
As demonstrated by Fig. 5D, erastin treatment markedly increased LDH activity in the hippocampal neurons (P < 0.01). However, compared with erastin treatment only, treatment with erastin and 1 and 10 µg/mL YNJY prescription markedly suppressed LDH activity in the hippocampal neurons (P < 0.01). TUNEL staining revealed that the proportion of apoptotic neurons was elevated in the erastin treatment group, when referring to the Control group (P < 0.01). In contrast, the two erastin combined with YNJY prescription (1 and 10 µg/mL) treatment groups exhibited less apoptotic neurons than the erastin treatment group (P < 0.01) (Fig. 5E).
Furthermore, in contrast to the Control group, higher Fe and MDA levels, and lower SOD and GSH levels were observed in the erastin treatment group (P < 0.01). In contrast, Fe and MDA levels were significantly decreased, but SOD and GSH levels were increased in the erastin combined with YNJY prescription (1 and 10 µg/mL) treatment group, relative to the erastin treatment group (P < 0.05 and P < 0.01) (Fig. 5F, G and H, and 5I). TEM revealed severe mitochondrial damage (such as morphological changes and damaged mitochondrial ridges) in erastin-treated hippocampal neurons compared with control neurons. In contrast, YNJY prescription (1 and 10 µg/mL) evidently relieved erastin-induced mitochondrial damage (Fig. 5J) in the hippocampal neurons. Collectively, YNJY prescription abolishes erastin-induced hippocampal neuronal damage.
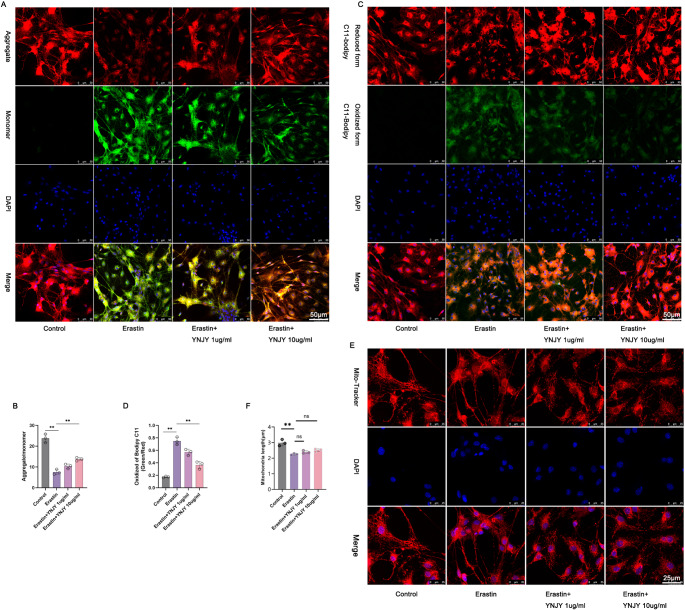
YNJY Prescription Reversed erastin-induced Mitochondrial Damage in the Hippocampal Neurons
To determine the effect of YNJY prescription on mitochondrial damage in the hippocampal neurons, JC-1 staining (Fig. 6A and B), C11-BODIPY staining (Fig. 6C and D), and Mito-Tracker Red staining (Fig. 6E and F) were performed to elucidate the changes in mitochondrial membrane potential, lipid peroxidation, and mitochondria length, respectively. The considerably decreased mitochondrial membrane potential, increased lipid peroxidation, and reduced mitochondria length were discovered in the erastin-treated hippocampal neurons compared with control hippocampal neurons (P < 0.01). However, the erastin-induced hippocampal neurons exhibited the elevated mitochondrial membrane potential along with the declined lipid peroxidation after treatment with YNJY prescription (10 µg/mL) (P < 0.01). YNJY prescription had no obvious influence on the mitochondria length in the erastin-induced hippocampal neurons. Therefore, YNJY prescription relieves erastin-induced mitochondrial damage in the hippocampal neurons.
Fig. 6.
YNJY prescription reversed erastin-induced mitochondrial damage. (A and B) JC-1 staining of the hippocampal neurons to evaluate mitochondrial membrane potential. N = 3 per group. (C and D) C11-BODIPY staining of the hippocampal neurons to evaluate lipid peroxidation. N = 3 per group. (E and F) Mito-Tracker Red staining of the hippocampal neurons to investigate mitochondria length. N = 3 per group. ** P < 0.01. The symbol “ns” indicated that the difference was not statistically significant
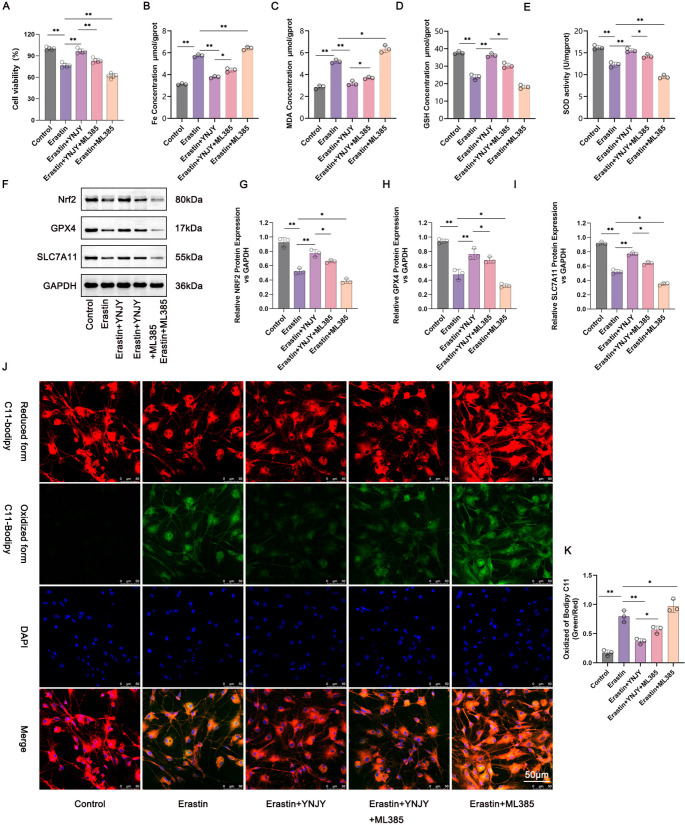
YNJY Prescription Mitigated Erastin-Induced Ferroptosis in the Hippocampal Neurons by Activating the Nrf2/GPX4/SLC7A11 Pathway
To verify whether YNJY prescription relives ferroptosis in the hippocampal neurons through activating the Nrf2/GPX4/SLC7A11 pathway, we treated erastin-treated hippocampal neurons with both YNJY prescription (10 µg/mL) and Nrf2 inhibitor (ML385, 1 µM). The CCK-8 assay was performed to measure the viability of the hippocampal neurons in each group. The hippocampal neurons in the erastin treatment group had lower viability than those in the Control group (P < 0.01). As relative to the Erastin group, hippocampal neurons in the Erastin + YNJY group possessed higher viability, but those in the Erastin + ML385 group exhibited the decreased viability (P < 0.01). In contrast to the Erastin + YNJY group, the attenuated viability was monitored in hippocampal neurons in the Erastin + YNJY + ML385 group (P < 0.01) (Fig. 7A). These findings suggest that ML385 treatment reverses the promoting effect of YNJY prescription on the viability of erastin-treated hippocampal neurons.
Fig. 7.
YNJY prescription mitigated erastin-induced ferroptosis in the hippocampal neurons by activating the Nrf2/GPX4/SLC7A11 pathway. (A) CCK-8 assay measuring the viability of the hippocampal neurons in each group. N = 5 per group. (B–E) Levels of the ferroptosis-related factors in the hippocampal neurons of each group were detected using commercial kits. N = 3 per group. (F–I) Western blotting showing the activity of the Nrf2/GPX4/SLC7A11 pathway in the hippocampal neurons of each group. N = 3 per group. (J and K) C11-BODIPY staining of the hippocampal neurons in each group to explore the degree of lipid peroxidation. N = 3 per group. * P < 0.05 and ** P < 0.01
Commercial kits were used to measure the ferroptosis-related factors in the hippocampal neurons of each group. As illustrated in Fig. 7B, C and D, and 7E, higher levels of Fe and MDA, and lower levels of SOD and GSH were detected in the hippocampal neurons in the Erastin group than in those in the Control group (P < 0.01). When matched to the Erastin group, hippocampal neurons in the Erastin + YNJY group showed lower levels of Fe and MDA and higher levels of SOD and GSH, and those in the Erastin + ML385 group displayed higher levels of Fe and MDA and lower levels of SOD and GSH (P < 0.05 and P < 0.01). Intriguingly, compared with the hippocampal neurons in the Erastin + YNJY group, those in the Erastin + YNJY + ML385 group had higher Fe and MDA levels but lower SOD and GSH levels (P < 0.05 and P < 0.01). Collectively, these data suggest that ML385 treatment abolishes the suppressing effect of YNJY prescription on erastin-induced ferroptosis in the hippocampal neurons.
Western blotting was performed to measure the activity of the Nrf2/GPX4/SLC7A11 pathway in the hippocampal neurons in each group. The results are illustrated in Fig. 7F, G and H, and 7I. The protein levels of Nrf2, GPX4, and SLC7A11 were lower in the hippocampal neurons in the Erastin group than in those in the Control group (P < 0.05). Furthermore, compared to the Erastin group, the protein levels of Nrf2, GPX4, and SLC7A11 were elevated in the Erastin + YNJY group, but decreased in the Erastin + ML385 group (P < 0.05 and P < 0.01). Additionally, compared with the Erastin + YNJY group, Nrf2, GPX4, and SLC7A11 protein levels were much declined in the Erastin + YNJY + ML385 group (P < 0.05). Therefore, ML385 treatment abrogates the activating effect of YNJY prescription on the Nrf2/GPX4/SLC7A11 pathway in erastin-treated hippocampal neurons.
Lastly, C11-BODIPY staining was performed to evaluate lipid peroxidation in the hippocampal neurons in each group. As illustrated in Fig. 7J and K, lipid peroxidation was markedly increased in hippocampal neurons in the Erastin group, in contrast to the Control group (P < 0.01). Lower lipid peroxidation in the Erastin + YNJY group, but higher in the Erastin + ML385 group, were discovered, as compared to hippocampal neurons in the Erastin group (P < 0.05 and P < 0.01). Simultaneously, lipid peroxidation was enhanced in the Erastin + YNJY + ML385 group compared with the Erastin + YNJY group (P < 0.05). Therefore, ML385 treatment counteracts the repressing effect of YNJY prescription on erastin-induced lipid peroxidation in hippocampal neurons. Collectively, the abovementioned findings suggest that YNJY prescription attenuates ferroptosis in erastin-induced hippocampal neurons by activating the Nrf2/GPX4/SLC7A11 pathway.
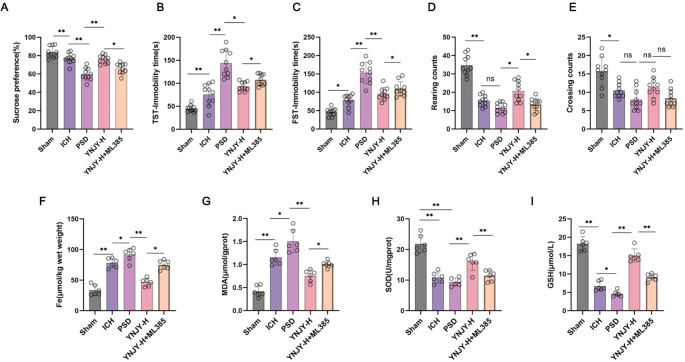
YNJY Prescription Relieved the Depression-Like Behaviors of PSD Rats by Alleviating Hippocampal Ferroptosis Via Activating the Nrf2/GPX4/SLC7A11 Pathway
In this work, ML385 (Nrf2 inhibitor) at a dose of 30 mg/kg/day was intraperitoneally injected into the high-dose YNJY-treated rats. The depression-like behaviors of the rats were appraised by a series of behavioral tests, including sucrose preference test (Fig. 8A), TST (Fig. 8B), FST (Fig. 8C), and OFT (Fig. 8D and E). As a result, by referring to the rats in the Sham group, the rats with ICH (the ICH group) displayed the attenuated sucrose preference, the increased immobility time in TST and FST, as well as the reduced rearing and crossing counts in OFT (P < 0.05 and P < 0.01). Further, PSD rats (the PSD group) had much decreased sucrose preference, as well as longer immobility time in TST and FST, in contrasted to the ICH group (P < 0.01). The difference in rearing and crossing counts in OFT was not obvious in the rats between the PSD group and the ICH group. In fact, a high-dose YNJY treatment (the YNJY-H group) pronouncedly enhanced sucrose preference, shortened immobility time in TST and FST, and increased rearing counts (rather than crossing counts) in OFT in PSD rats, as relative to the PSD rats without treatment (the PSD group) (P < 0.05 and P < 0.01). However, when referring to the YNJY-H group, the rats of the YNJY-H + ML385 group exhibited distinctly reduced sucrose preference, increased immobility time in TST and FST, as well as reduced rearing counts (rather than crossing counts) in OFT (P < 0.05).
Fig. 8.
YNJY prescription might mitigate the depression-like behaviors of PSD rats by alleviating hippocampal ferroptosis via activating the Nrf2/GPX4/SLC7A11 pathway. (A) Sucrose preference of rats to appraise the depression-like behaviors. (B) TST of rats to observe the immobility time. N = 10 per group. (C) FST of rats to monitor the immobility time. N = 10 per group. (D-E) OFT of rats to count rearing and crossing counts. N = 10 per group. (F-I) The contents of ferroptosis-related factors in the hippocampus of rats were detected by commercial kits. N = 6 per group.* P < 0.05 and ** P < 0.01. The symbol “ns” indicated that the difference was not statistically significant
In the following, the ferroptosis-related factors in the hippocampus of the rats were scrutinized, including the content of Fe, MDA, SOD and GSH. Data were presented in Fig. 8F, G, H and I. Higher levels of Fe and MDA, as well as lower SOD and GSH levels, were observed in the hippocampus of ICH rats, comparatively (the ICH group vs. the Sham group) (P < 0.01). Besides, as matched to the ICH group, the rats of the PSD group possessed distinctly higher Fe and MDA levels, as well as lower SOD and GSH levels (P < 0.05 and P < 0.01). By contrast, a high-dose YNJY treatment declined the content of Fe and MDA, and elevated SOD and GSH contents in PSD rats (the YNJY-H group vs. the PSD group) (P < 0.01). However, these effects of YNJY prescription on the contents of Fe, MDA, SOD and GSH in PSD rats were abrogated by ML385 treatment, comparatively (the YNJY-H + ML385 group vs. the YNJY-H group) (P < 0.05 and P < 0.01). Thereby, all of these data were indicative that YNJY prescription might mitigate the depression-like behaviors of PSD rats by alleviating hippocampal ferroptosis via activating the Nrf2/GPX4/SLC7A11 pathway.
Nrf2 Silencing Reversed the Suppression of YNJY Prescription on Ferroptosis in the Erastin-Induced Hippocampal Neurons
We transfected the in vitro hippocampal neurons with Nrf2 siRNA, and subsequently treated them with erastin and YNJY prescription (10 µg/mL). By CCK-8 assay, erastin treatment (the Erastin group) resulted in a much decrease in viability of hippocampal neurons, as refered to the Control (P < 0.01). Hippocampal neurons of the Erastin + YNJY + siNrf2 NC group showed a higher viability than those of the Erastin group (P < 0.01). However, when reltive to the Erastin + YNJY + siNrf2 NC group, a distinct reduction in viability of hippocampal neurons was found in the Erastin + YNJY + siNrf2 group (P < 0.01). Meanwhile, hippocampal neurons of the Erastin + siNrf2 group possessed lower hippocampal neuron viability than the Erastin group (P < 0.01) (Figure S1 A).
The ferroptosis-related factors in hippocampal neurons of each group was monitored, and the data were presented in Figure S1 B, C, D and E. Obvuiously, higher Fe and MDA levels, along with lower GSH and SOD levels, occurred in the erastin-treated hippocampal neurons (the Erastin group), as incomparison to the Control (P < 0.01). Interestingly, the reduced Fe and MDA levels, as well as the elevated GSH and SOD levels, were monitored in hippocampal neurons of the Erastin + YNJY + siNrf2 NC group, when matched to the Erastin group (P < 0.01). Conversely, by refered to the Erastin + YNJY + siNrf2 NC group, hippocampal neurons of the Erastin + YNJY + siNrf2 group exhibited the increased Fe and MDA levels, as well as the declined GSH and SOD levels (P < 0.05). When compared to hippocampal neurons in the Erastin group, those in the Erastin + siNrf2 group displayed higher MDA level and lower GSH and SOD levels (P < 0.05 and P < 0.01).
Western blot was responsible for the evaluation of the Nrf2/GPX4/SLC7A11 pathway activity. Erastin treatment (the Erastin group) led to an reduction in the expression of Nrf2, GPX4 and SLC7A11 proteins in hippocampal neurons, as matched to the Control (P < 0.001). In contrast to the Erastin group, hippocampal neurons of the Erastin + YNJY + siNrf2 NC group expression higher proteins for Nrf2, GPX4 and SLC7A11 (P < 0.001). However, a much reduction in Nrf2, GPX4 and SLC7A11 protein expression was observed in hippocampal neurons of the Erastin + YNJY + siNrf2 group, matched to the Erastin + YNJY + siNrf2 NC group (P < 0.01 and P < 0.001). In referring to the Erastin group, hippocampal neurons of the Erastin + siNrf2 group showed lower expression of Nrf2, GPX4 and SLC7A11 proteins (P < 0.05 and P < 0.01) (Figure S1 F, G, H and I).
C11-BODIPY staining (Figure S1 J and K) displayed the up-regulated lipid peroxidation level in the erastin-induced hippocampal neurons (the Erastin group), as relative to the Control (P < 0.01). However, a much decrease in lipid peroxidation level was discovered in hippocampal neurons of the Erastin + YNJY + siNrf2 NC group, in contrasted to the Erastin group (P < 0.01). Oppositely, hippocampal neurons of the Erastin + YNJY + siNrf2 group had higher lipid peroxidation level than the the Erastin + YNJY + siNrf2 NC group (P < 0.05). When matched to the Erastin group, hippocampal neurons of the Erastin + siNrf2 group possessed higher lipid peroxidation level (P < 0.05). Thus, Nrf2 silencing eliminated the suppression of YNJY prescription on the ferroptosis and the activation on the Nrf2/GPX4/SLC7A11 pathway in the erastin-induced hippocampal neurons. Taken together, in PSD, YNJY prescription might relieve the ferroptosis in hippocampal neurons through activating the Nrf2/GPX4/SLC7A11 pathway.
Discussion
In this study, we elucidated the efficacy and mechanism of action of YNJY prescription for PSD. As we know, not all stroke patients suffer from PSD. Thus, in this paper, the ICH group and the PSD group were set simultaneously in order to ensure that the rats did suffer from depression after ICH. Compared with rats with ICH, those with PSD exhibited evident depression-like behaviors, including decreased sucrose preference, increased immobility time in TST and FST, and decreased rearing and crossing counts in OFT. Therefore, the PSD rat model developed in this work is valid. Interestingly, these depression-like behaviors of PSD rats were alleviated after administering the YNJY prescription. Histological staining revealed that hippocampal and hippocampal neuronal damage was aggravated in rats with PSD; however, treatment with YNJY prescription evidently mitigated the extent of these damages. Therefore, YNJY prescription can alleviate depression-like behaviors and hippocampal and neuronal damage in PSD. These phenomena indicate the therapeutic effect of YNJY prescription in PSD. Our findings are consistent with those of previous studies (Tian et al. 2018; Zhao et al. 2020). As we know, NeuN protein has been identified to be neuron-specific marker; in fact, the loss of NeuN staining is not always associated with neuronal death, which is also influenced by other factors such as stimulus-induced phosphorylation or protein-protein interactions mediated by the epitope; nevertheless, the attenuated NeuN staining is still indicative of neuronal damage (Gusel’nikova and Korzhevskiy 2015). Thus, in addition to HE staining and Nissl staining, this study also implemented NeuN immunofluorescence staining to appraise hippocampal neuronal damage in rats.
Ferroptosis is a type of cell death that is characterized by massive iron accumulation and ROS, excessive lipid peroxidation, and GSH depletion (Tang et al. 2021). Some abnormal cytological changes occur in ferroptosis, including ruptured mitochondrial outer membrane, decreased or even vanished mitochondria cristae, and condensed mitochondrial membrane; these abnormalities are owing to the loss of the selective permeability of the membrane, which is induced by membrane lipid peroxidation and oxidative stress (Mou et al. 2019). SOD and GSH are two key members of the antioxidant defense system. As vital antioxidants and free radical scavengers, they can inhibit ferroptosis occurrence by attenuating the degree of oxidative stress in the cells (Wang et al. 2020a, b). MDA is a major end product of lipid peroxidation. Its level can reflect the degree of ferroptosis and damage to tissues and cells (Song et al. 2022). In the present study, we observed that Fe, ROS, and MDA were excessively accumulated; SOD and GSH levels were dramatically decreased; and mitochondrial damage was aggravated in the hippocampus of rats with PSD. These results suggest that ferroptosis was intensified in the hippocampus of rats with PSD. Interestingly, the abovementioned signs of ferroptosis were abolished after administering YNJY prescription to rats with PSD. Therefore, YNJY prescription can weaken ferroptosis in the hippocampus of rats with PSD.
Ferroptosis occurs in individuals with depressive disorder (Zhang et al. 2022). Ferroptosis has been found to play a crucial role in the pathogenesis of depressive disorder, and some antidepressant drugs alleviate depressive symptoms by blocking ferroptosis (Wang et al. 2023). In patinets with major depressive disorder, several ferroptosis-related genes have been discovered to be abnormally expressed (Chen et al. 2023a, b). Researchers have not only found evidence of ferroptosis in the hippocampus of animal models of depression, but have also found that ferroptosis severely impairs the survival of hippocampal neurons in major depression (Jiao et al. 2021; Ren et al. 2020; Zhang et al. 2021a, b, c). Harmful substances such as excessive alcohol can induce depression-like behaviors by stimulating ferroptosis in mouse hippocampus (Xu et al. 2022). Next, to determine whether YNJY prescription relieves PSD in rats by suppressing ferroptosis, we induced ferroptosis in rat hippocampal neurons via erastin induction and treated them with the YNJY prescription. Erastin treatment induced severe ferroptosis, including enhanced LDH activity, augmented lipid peroxidation, serious mitochondrial damage (such as the decreased mitochondrial membrane potential and mitochondria length), and changes in ferroptosis-related factors. Importantly, YNJY prescription counteracted these ferroptosis-related phenomena (but not included mitochondria length) in erastin-treated hippocampal neurons. Therefore, YNJY prescription may relieve PSD by attenuating ferroptosis in the hippocampus. A study has reported that some traditional Chinese herbal medicines can attenuate ferroptosis in animals with depression-like behaviors (Jiao et al. 2021). To the best of our knowledge, our study is the first to confirm that YNJY prescription can relieve PSD by suppressing ferroptosis.
The Nrf2/GPX4/SLC7A11 pathway exerts neuroprotective effects by suppressing ferroptosis in stroke- or neurodegenerative disease-induced cognitive impairment (Yang et al. 2023; Fu et al. 2022). In PSD, multiple factors can lead to a decrease of Nrf2 level. For instance, autophagy associated protein p62 involves in regulating the expression of Nrf2 by acting on the interaction domain of Kelch-like ECH-associated protein-1 (Keap1), while the conformationally altered Keap1 under the stress state is also involved in the regulation of Nrf2 expression (Liu et al. 2024). In stroke, the reduced brahma-related gene-1 protein can result in the decrease in Nrf2 expression (Guo et al. 2023). Besides, some protein kinase can degradated Nrf2 to down-regulated its level (Zuo et al. 2022). As a major regulator of antioxidant responses, Nrf2 dysregulation can decrease antioxidant expression; this has been implicated in depression pathogenesis (Robledinos-Antón et al. 2019; Bouvier et al. 2017). Nrf2 can regulate homeostasis and ferroptosis, and the antiferroptotic activity of Nrf2 is achieved by controlling the expression of its antioxidant target genes, including SLC7A11 and GPX4 (Anandhan et al. 2023). GPX4 is a vital ferroptosis inhibitor that can decrease the accumulation of lipid peroxidation products in ferroptosis (Ge et al. 2021). Furthermore, Nrf2 can induce GPX4 synthesis. Impaired GPX4 function can ultimately lead to ferroptosis (Dang et al. 2022; Huang et al. 2022). GSH produced by activating SLC7A11, a cystine–glutamate antitransporter, is indispensable for inducing GPX4 activity (Chen et al. 2021). In the present study, the activity of the Nrf2/GPX4/SLC7A11 pathway was suppressed in the hippocampus of rats with PSD; however, the administration of YNJY prescription activated this pathway. Therefore, YNJY prescription could activate the Nrf2/GPX4/SLC7A11 pathway in the hippocampus of rats with PSD.
Existing evidence suggests that activated Nrf2 can induce SLC7A11 and GPX4 expression and protect the neurons from ferroptosis-induced damage (Yuan et al. 2021). Therefore, we hypothesized that YNJY prescription can attenuate ferroptosis in the hippocampus of PSD by activating the Nrf2/GPX4/SLC7A11 pathway. To verify this hypothesis, in vitro rescue experiments were performed by treating erastin-induced hippocampal neurons with ML385 and the YNJY prescription. Surprisingly, ML385 treatment abrogated the suppressive role of YNJY prescription on ferroptosis in erastin-induced hippocampal neurons. Simultaneously, Nrf2 silencing counteracted the repression of YNJY prescription on the ferroptosis and the activation on the Nrf2/GPX4/SLC7A11 pathway in the erastin-induced hippocampal neurons. This suggests that YNJY prescription relieves PSD by weakening ferroptosis by activating the Nrf2/GPX4/SLC7A11 pathway. More importantly, by in vivo study, it was also noticed that ML385 treatment reversed the mitigating influence of YNJY prescription on the depressive-like behaviors of PSD rats, as well as the inhibition of it on ferroptosis in hippocampus of PSD rats. A previous study has suggested that YNJY prescription exerts a neuroprotective effect on PSD by activating the Notch signaling pathways (Tian et al. 2018). This was the first study to demonstrate that YNJY prescription can relieve PSD by inhibiting ferroptosis by activating the Nrf2/GPX4/SLC7A11 pathway. This finding provides reliable evidence for applying YNJY prescription as a useful agent for treating PSD in clinical settings.
Conclusion
In the present study, we revealed that the traditional Chinese medicine YNJY prescription is an effective agent for treating PSD. It can relieve depression-like behaviors and hippocampal neuronal damage in rats with PSD, possibly by inhibiting ferroptosis by activating the Nrf2/GPX4/SLC7A11 pathway. Therefore, YNJY prescription may be a useful agent for treating PSD in clinical settings.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- CCK-8
Cell counting kit-8
- CUMS
Chronically unpredictable mild stress
- DAPI
4’, 6-diamidino-2-phenylindole
- FST
Forced swimming test
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- GPX4
Glutathione peroxidase
- GSH
Glutathione
- HE
Hematoxylin–eosin
- ICH
Intracerebral hemorrhage
- LDH
Lactate dehydrogenase
- MAP2
Microtubule-associated protein2
- MDA
Malondialdehyde
- MMP2
Matrix metalloproteinase 2
- NeuN
Neuronal nuclear antigen
- Nrf2
Nuclear factor erythroid 2-related factor-2
- OFT
Open-field test
- PSD
Post-stroke depression
- PVDF
Polyvinylidene fluoride
- qRT-PCR
Real-time quantitative reverse transcription polymerase chain reaction
- ROS
Reactive oxygen species
- SD
Sprague Dawley
- SLC7A11
Solute carrier family 7 member 11
- SOD
Superoxide dismutase
- TBST
Tris-buffered saline with 0.1% Tween 20
- TEM
Transmission electron microscopy
- TST
Tail suspension test
- TUNEL
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling
- YNJY
Yi-nao-jie-yu
- YNJY-H
A high-dose of YNJY
- YNJY-L
A low-dose of YNJY
Author Contributions
Yuan Zhang desighed this study, performed the experiments, analysed the statistics, and wrote this paper; Hongwei Liu, Changmin Xu, Zechun Guo and Shuqing Liu supervised the experiments, guided the analysis of the statistics, and corrected the draft; Qisheng Tang and Jin Yao corrected the draft; Ruizhen Zhao guided the experiments and the draft writing. All authors have read and approved the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 82104816].
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Ethics Approval and Consent to Participate
The Animal Committee of the MDKN Biotech Co. (Beijing, China) approved all animal studies (Approval No.: MDKN-2023–010). Furthermore, all animal experiments were conducted according to the “Guiding Opinions on the Good Treatment of Laboratory Animals” issued by the Ministry of Science and Technology of the People’s Republic of China.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Anandhan A et al (2023) NRF2 controls iron homeostasis and ferroptosis through HERC2 and VAMP8. Sci Adv 9(5):eade9585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier E et al (2017) Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol Psychiatry 22(12):1701–1713 [DOI] [PubMed] [Google Scholar]
- Cao H et al (2021) Hippocampal proteomic analysis reveals activation of necroptosis and ferroptosis in a mouse model of chronic unpredictable mild stress-induced depression. Behav Brain Res 407:113261 [DOI] [PubMed] [Google Scholar]
- Chen X et al (2021) Ferroptosis: machinery and regulation. Autophagy 17(9):2054–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y et al (2023a) Srs11-92, a ferrostatin-1 analog, improves oxidative stress and neuroinflammation via Nrf2 signal following cerebral ischemia/reperfusion injury. CNS Neurosci Ther 29(6):1667–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J et al (2023b) Ferroptosis-related genes as diagnostic markers for major depressive disorder and their correlations with immune infiltration. Front Med (Lausanne) 10:1215180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs B et al (2021) Frontotemporal Lobar Dementia Mutant Tau Impairs Axonal Transport through a protein phosphatase 1γ-Dependent mechanism. J Neurosci 41(45):9431–9451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlmanns M et al (2017) Chemotherapeutic xCT inhibitors sorafenib and erastin unraveled with the synaptic optogenetic function analysis tool. Cell Death Discov 3:17030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang R et al (2022) Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J Neuroinflammation 19(1):41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desland FA et al (2014) Manual versus automated rodent behavioral Assessment: comparing efficacy and ease of Bederson and Garcia Neurological Deficit scores to an Open Field Video-Tracking System. J Cent Nerv Syst Dis 6:7–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C et al (2022) Rehmannioside A improves cognitive impairment and alleviates ferroptosis via activating PI3K/AKT/Nrf2 and SLC7A11/GPX4 signaling pathway after ischemia. J Ethnopharmacol 289:115021 [DOI] [PubMed] [Google Scholar]
- Ge MH et al (2021) Zinc attenuates ferroptosis and promotes functional recovery in contusion spinal cord injury by activating Nrf2/GPX4 defense pathway. CNS Neurosci Ther 27(9):1023–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J et al (2022) The advances of post-stroke depression: 2021 update. J Neurol 269(3):1236–1249 [DOI] [PubMed] [Google Scholar]
- Guo K et al (2023) BRG1 alleviates microglial activation by promoting the KEAP1-NRF2/HO-1 signaling pathway and minimizing oxidative damage in cerebral ischemia-reperfusion. Int Immunopharmacol 119:110201 [DOI] [PubMed] [Google Scholar]
- Gusel’nikova VV, Korzhevskiy DE (2015) NeuN as a neuronal Nuclear Antigen and Neuron differentiation marker. Acta Naturae 7(2):42–47 [PMC free article] [PubMed] [Google Scholar]
- Huang J et al (2022) Platycodin D regulates high glucose-induced ferroptosis of HK-2 cells through glutathione peroxidase 4 (GPX4). Bioengineered 13(3):6627–6637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao H et al (2021) Traditional Chinese Formula Xiaoyaosan alleviates depressive-like Behavior in CUMS mice by regulating PEBP1-GPX4-Mediated ferroptosis in the Hippocampus. Neuropsychiatr Dis Treat 17:1001–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivoy A et al (2017) Low adherence to antidepressants is associated with increased mortality following stroke: a large nationally representative cohort study. Eur Neuropsychopharmacol 27(10):970–976 [DOI] [PubMed] [Google Scholar]
- Kwon CY et al (2018) Herbal medicine Sihogayonggolmoryeo-Tang or Chai-Hu-Jia-Long-Gu-Mu-Li-Tang for the treatment of post-stroke depression: a protocol for a systematic review and meta-analysis. Med (Baltim) 97(38):e12384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z et al (2021) Morinda officinalis oligosaccharides alleviate depressive-like behaviors in post-stroke rats via suppressing NLRP3 inflammasome to inhibit hippocampal inflammation. CNS Neurosci Ther 27(12):1570–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H et al (2021) GJ-4 ameliorates memory impairment in focal cerebral ischemia/reperfusion of rats via inhibiting JAK2/STAT1-mediated neuroinflammation. J Ethnopharmacol 267:113491 [DOI] [PubMed] [Google Scholar]
- Liu C et al (2022) Progesterone attenuates neurological deficits and exerts a protective effect on damaged axons via the PI3K/AKT/mTOR-dependent pathway in a mouse model of intracerebral hemorrhage. Aging 14(6):2574–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H et al (2024) CRHR1 antagonist alleviated depression-like behavior by downregulating p62 in a rat model of post-stroke depression. Exp Neurol 378:114822 [DOI] [PubMed] [Google Scholar]
- Min X et al (2022) Gomisin J attenuates cerebral ischemia/reperfusion injury by inducing anti-apoptotic, anti-inflammatory, and antioxidant effects in rats. Bioengineered 13(3):6908–6918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou Y et al (2019) Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol 12(1):34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren JX et al (2020) Ferroptosis in neurological diseases. Front Cell Neurosci 14:218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledinos-Antón N et al (2019) Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxid Med Cell Longev, 2019: p. 9372182 [DOI] [PMC free article] [PubMed]
- Song JX et al (2022) Liraglutide attenuates hepatic iron levels and ferroptosis in db/db mice. Bioengineered 13(4):8334–8348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X et al (2021) Curcumin induces ferroptosis in non-small-cell lung cancer via activating autophagy. Thorac Cancer 12(8):1219–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H et al (2018) Yi-nao-Jie-Yu prescription exerts a positive effect on neurogenesis by regulating notch signals in the Hippocampus of Post-stroke Depression rats. Front Psychiatry 9:483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PR et al (2014) Neuroprotective effects of Huang-Lian-Jie-Du-Decoction on ischemic stroke rats revealed by (1)H NMR metabolomics approach. J Pharm Biomed Anal 88:106–116 [DOI] [PubMed] [Google Scholar]
- Wang Y et al (2020a) Study on the therapeutic material basis and effect of Acanthopanax senticosus (rupr. Et Maxim.) Harms leaves in the treatment of ischemic stroke by PK-PD analysis based on online microdialysis-LC-MS/MS method. Food Funct 11(3):2005–2016 [DOI] [PubMed] [Google Scholar]
- Wang C et al (2020b) Dexmedetomidine alleviated sepsis–induced myocardial ferroptosis and septic heart injury. Mol Med Rep 22(1):175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AR et al (2021) Saikosaponin A improved depression-like behavior and inhibited hippocampal neuronal apoptosis after cerebral ischemia through p-CREB/BDNF pathway. Behav Brain Res 403:113138 [DOI] [PubMed] [Google Scholar]
- Wang L et al (2023) Targeting the ferroptosis crosstalk: novel alternative strategies for the treatment of major depressive disorder. Gen Psychiatr 36(5):e101072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijeratne T, Sales C, Wijeratne C (2022) A narrative review on the non-pharmacologic interventions in Post-stroke Depression. Psychol Res Behav Manag 15:1689–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie CJ et al (2018) Curcumin protects neural cells against ischemic injury in N2a cells and mouse brain with ischemic stroke. Brain Behav 8(2):e00921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C et al (2022) Alcohol exposure induces depressive and anxiety-like behaviors via activating ferroptosis in mice. Int J Mol Sci, 23(22) [DOI] [PMC free article] [PubMed]
- Yang S et al (2023) Salidroside alleviates cognitive impairment by inhibiting ferroptosis via activation of the Nrf2/GPX4 axis in SAMP8 mice. Phytomedicine 114:154762 [DOI] [PubMed] [Google Scholar]
- Yuan Y et al (2021) Kaempferol Ameliorates Oxygen-Glucose Deprivation/Reoxygenation-Induced Neuronal Ferroptosis by Activating Nrf2/SLC7A11/GPX4 Axis. Biomolecules, 11(7) [DOI] [PMC free article] [PubMed]
- Zhang W et al (2016) Effect of Yi-nao-Jie-Yu decoction on γ-aminobutyric acid type A receptor in the hippocampus and serum inflammatory factors in a rat model of poststroke anxiety. Neuropsychiatr Dis Treat 12:2827–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Li M, Xu T (2021a) Therapeutic effect of Chinese herbal medicines for post-stroke depression: a meta-analysis of randomized controlled trials. Med (Baltim) 100(1):e24173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang L, Sui R (2021b) Ganoderic Acid A-Mediated modulation of Microglial polarization is involved in Depressive-Like behaviors and Neuroinflammation in a rat model of Post-stroke Depression. Neuropsychiatr Dis Treat 17:2671–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H et al (2021c) Combined exposure of alumina nanoparticles and chronic stress exacerbates hippocampal neuronal ferroptosis via activating IFN-γ/ASK1/JNK signaling pathway in rats. J Hazard Mater 411:125179 [DOI] [PubMed] [Google Scholar]
- Zhang M et al (2022) Ketamine may exert Rapid Antidepressant effects through Modulation of Neuroplasticity, Autophagy, and ferroptosis in the Habenular Nucleus. Neuroscience 506:29–37 [DOI] [PubMed] [Google Scholar]
- Zhang J et al (2023) Herbal medicine as adjunctive therapy with antidepressants for post-stroke depression: a systematic review and network meta-analysis of randomized controlled trials. Front Pharmacol 14:1180071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z et al (2020) Multimodal Magnetic Resonance Imaging and therapeutic intervention with Yi-nao-Jie-Yu Decoction in a rat model of Post-stroke Depression. Front Psychiatry 11:557423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo C et al (2022) Nrf2: an all-rounder in depression. Redox Biol 58:102522 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.