Abstract
This study aims to investigate the association and causality between osteoarthritis (OA) and chronic kidney disease (CKD) using data from the National Health and Nutrition Examination Survey (NHANES) and Mendelian Randomization (MR) analysis. Participants with OA, urinary albumin, urinary creatinine, urinary albumin-to-creatinine ratio (UACR), blood creatinine, and estimated glomerular filtration rate (eGFR) were selected from NHANES. CKD was calculated using the CKD-EPI equation, and logistic regression assessed by the OA-CKD association. A two-sample MR analysis was conducted using Genome-wide association studies (GWAS) data for OA, hip OA (HOA), knee OA (KOA), acute renal failure (ARF), chronic renal failure (CRF), cystatin C, serum creatinine (eGFRcrea), and microalbuminuria. The inverse variance weighted (IVW) method was used, with heterogeneity, sensitivity, and pleiotropy assessments. The cross-sectional analysis showed a significant positive association between OA and CKD [unadjusted OR: 2.398 (95% CI: 2.176–2.643), p < 0.001], which persisted after adjustment for demographic factors, socioeconomic status, lifestyle factors, and medical history [adjusted OR: 1.161 (95% CI: 1.029–1.310), p = 0.015]. The MR analysis revealed no significant causal relationship between overall OA and renal function markers but found a significant genetic association between HOA and cystatin C [IVW p = 0.0014, OR = 1.02, 95% CI: 1.01–1.03], and between KOA and cystatin C [IVW p < 0.0001, OR = 1.06, 95% CI: 1.04–1.08]. Our study indicates that HOA and KOA are risk factors for renal function injury, providing new insights for clinical OA management.
Keywords: Osteoarthritis, Chronic kidney disease, NHANES, MR, Causality
Subject terms: Genetics, Nephrology
Introduction
Osteoarthritis (OA) and chronic kidney disease (CKD) are two common chronic conditions that place a significant burden on global healthcare systems1,2. OA can affect nearly all joints, characterized by cartilage degeneration, synovial changes, and subchondral bone alterations. Its primary symptoms include joint pain, stiffness, and limited mobility3,4. With the global rise in aging populations, alongside the prevalence of metabolic disorders such as obesity and diabetes, the incidence of OA continues to increase5,6. It is estimated that over 240 million people worldwide, including more than 32 million in the United States, are affected by OA, particularly in the hip and knee joints. Hip and knee OA often lead to severe disability, significantly impairing mobility and quality of life. In advanced cases, total hip and knee replacement surgeries are frequently required to alleviate pain and restore function, highlighting the substantial burden of OA on individuals and healthcare systems7,8. However, OA is not confined solely to joint issues; it may also impact multiple organ systems through mechanisms such as systemic inflammation and metabolic dysfunction9,10.
CKD is another major global health threat, with a prevalence of approximately 11–13%11, and it continues to rise each year12. The progression of CKD can lead to renal failure, worsening cardiovascular disease, and an increased risk of mortality13. Growing evidence suggests that CKD is more prevalent among OA patients14,15, with studies reporting a prevalence of 61.9% (95% CI: 56.4–66.3) based on eGFR calculated using the Cockcroft-Gault equation16. although the underlying reasons remain unclear. This may be related to shared risk factors such as smoking, alcohol consumption, obesity, and type 2 diabetes. Therefore, we hypothesize that a potential pathophysiological link exists between OA and CKD. The underlying biological mechanisms may include systemic inflammation, oxidative stress, and the use of non-steroidal anti-inflammatory drugs (NSAIDs), which are commonly prescribed for OA management. These factors may contribute to accelerated renal damage by affecting kidney structure and function.
Given the complex interplay of these risk factors, investigating the relationship between OA and renal function impairment holds significant clinical importance. Cross-sectional studies based on data from the National Health and Nutrition Examination Survey (NHANES) can reveal the statistical correlation between OA and CKD, but they struggle to overcome the influence of confounding factors and the possibility of reverse causality. Therefore, this study further employs the Mendelian Randomization (MR) method, using genetic variants as instrumental variables to more reliably assess the causal effect of OA on renal function impairment. By integrating cross-sectional analysis with genetic epidemiology, this study aims to uncover the potential impact of OA on kidney function, providing a scientific basis for optimizing clinical management and early intervention strategies.
Materials and methods
Study design
This study employed two distinct analytical approaches to investigate the relationship between OA and renal function: (a)Cross-sectional analysis using NHANES data: We conducted a population-based cross-sectional study to assess the association between OA and CKD using data from NHANES cycles (2011–2020). This design allowed us to evaluate the prevalence of OA and CKD, as well as their association, in a large, representative sample of the U.S. population. (b)Two-sample Mendelian Randomization (TSMR) analysis using GWAS data: To explore potential causal relationships, we performed a two-sample MR analysis using summary-level data from published genome-wide association studies (GWAS). This approach leverages genetic variants as instrumental variables to infer causality between OA (including overall OA(AOA), hip OA(HOA), and knee OA(KOA)) and renal function markers, while minimizing confounding and reverse causality.
Cross-sectional study
Data source
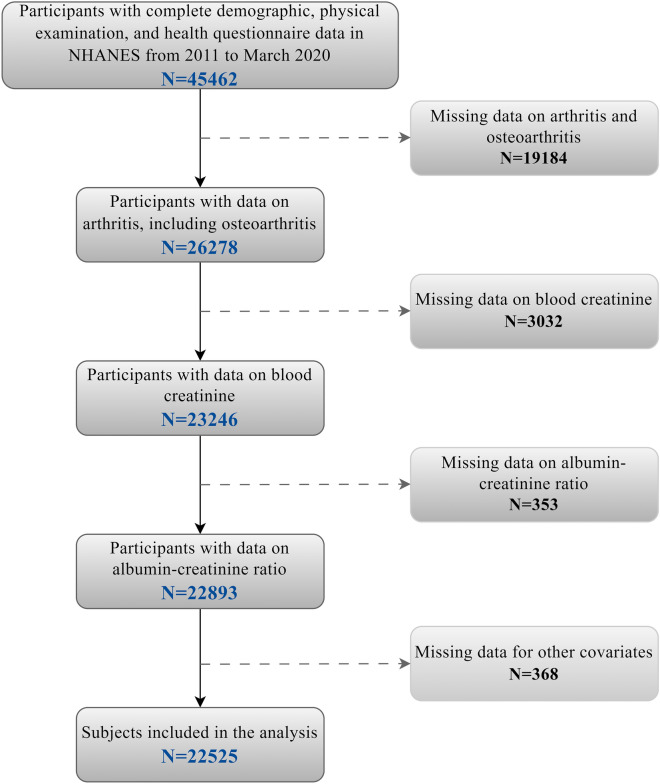
NHANES is a population-based cross-sectional survey designed to collect health and nutrition information from the U.S. population, including demographic data, lifestyle factors, and health and nutrition status17,18. In this study, we selected NHANES cycles from 2011 to 2020 because this time frame provides the most recent and comprehensive data on OA and CKD. These cycles include standardized measurements of key renal function indicators (e.g., urinary albumin, creatinine, and eGFR) and self-reported OA diagnoses, ensuring consistency and reliability in our analysis. Additionally, the inclusion of multiple cycles over this decade ensures a robust and representative sample, enhancing the generalizability of our findings. After merging demographic variables, questionnaire responses, and laboratory test data, a total of 45,462 participants aged over 20 were included from the five selected NHANES cycles. We further excluded participants with missing OA questionnaire data, relevant laboratory tests, or covariates. Ultimately, 22,525 participants were included in this study. Figure 1 illustrates the participant selection flowchart.
Fig. 1.
Flowchart of NHANCES in our study.
Measurements and definitions
Self-reported OA was defined through a questionnaire using the following two questions: "Have you ever been told by a doctor or other healthcare professional that you have arthritis?" and "What type of arthritis do you have?" Participants who answered “yes” were classified as having OA. The quality of the interviews and data entry process was ensured through a computer-assisted personal interview system19. Five continuous renal function indicators were derived from the NHANES laboratory data as dependent variables: urinary albumin, urinary creatinine, urinary albumin-to-creatinine ratio (UACR), blood creatinine, and estimated glomerular filtration rate (eGFR) based on creatinine levels. Notably, eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation20. CKD was further.
Covariates
In this study, we included sociodemographic factors (e.g., gender, age, race, poverty-income ratio (PIR), Physical activity, education level, body mass index (BMI)), lifestyle factors (e.g., smoking status, alcohol consumption), and medical history (e.g., diabetes) as covariates. These covariates were selected based on their established or potential influence on both OA and CKD. Sociodemographic factors such as PIR, education level, and marital status were included because they are closely associated with healthcare access, health behaviors, and disease prevalence. For instance, lower PIR and education levels are linked to reduced access to healthcare and higher rates of chronic conditions21,22, while marital status may influence social support and health-seeking behaviors23. Lifestyle factors like smoking and alcohol consumption were prioritized due to their well-documented roles in systemic inflammation and renal function injury. Smoking is a known risk factor for both OA progression and CKD24,25, while excessive alcohol consumption can exacerbate renal damage26. Additionally, medical history (e.g., diabetes) was included as it is a shared risk factor for both OA and CKD27,28, potentially confounding their relationship. Education level was categorized into three groups used by NHANES: less than high school, high school graduate or equivalent, and college or above. Participants were classified into four racial/ethnic groups: non-Hispanic White, non-Hispanic Black, Mexican American, and other races. PIR was divided into three categories: low (< 1.5), middle (1.5–3.5), and high (> 3.5). PIR was categorized into three groups: (1) Low income: PIR < 1.5, indicating income below 1.5 times the poverty threshold; (2) Middle income: PIR 1.5–3.5, indicating income between 1.5 and 3.5 times the poverty threshold; and (3) High income: PIR > 3.5, indicating income above 3.5 times the poverty threshold. Physical activity was categorized into three levels based on self-reported frequency: (a) Regular activities: participants who engaged in moderate or vigorous physical activity at least 3 times per week; (b) Occasional activities: participants who engaged in moderate or vigorous physical activity 1–2 times per week; and (c) Rarely active: participants who engaged in moderate or vigorous physical activity less than once per week. Marital status was defined in three groups: married and living with a partner, other statuses (including widowed, divorced, or separated), and never married. For lifestyle factors, individuals who had smoked more than 100 cigarettes in their lifetime were classified as smokers. Alcohol consumption was assessed using the questions "Did you have at least 12 drinks in the past year?" and "Have you ever had at least 12 drinks in your life?" Diabetes was defined as self-reported diabetes, the use of diabetes medications, or meeting the criteria established by the American Diabetes Association (ADA)29.
Mendelian randomization analysis
Summary-level GWAS data
Data for overall AOA, HOA, and KOA were obtained from the Genetics of Osteoarthritis (GO) Consortium, which conducted a genome-wide meta-analysis of 11 osteoarthritis phenotypes across 13 international cohorts from 9 populations, encompassing a total of 826,690 individuals (including 177,517 OA patients)30. For renal function outcomes, we selected serum creatinine, acute renal failure (ARF), chronic renal failure (CRF), microalbuminuria, and cystatin C as representative markers. Detailed information regarding the sample sizes and variables can be found in Appendix Table 131–34.
Table 1.
Demographic and baseline characteristics of OA group versus non-OA group.
| All participants (N = 22,525) |
In non-OA group (N = 16,453) |
In OA group (N = 6,072) |
p-value | |
|---|---|---|---|---|
| Age | 47.700 ± 16.978 | 43.378 ± 15.889 | 59.909 ± 13.691 | < 0.001 |
| Gender, % | < 0.001 | |||
| Man | 48.294 | 51.51 | 39.21 | |
| Women | 51.706 | 48.49 | 60.79 | |
| Race, % | < 0.001 | |||
| Mexican Americans | 8.593 | 10.008 | 4.596 | |
| Non-Hispanic whites | 65.127 | 61.87 | 74.327 | |
| Non-Hispanic blacks | 10.764 | 11.051 | 9.954 | |
| other | 15.515 | 17.071 | 11.123 | |
| Poverty to income ratio, % | 0.015 | |||
| < 1.5 | 25.873 | 25.856 | 25.922 | |
| 1.5–3.5 | 31.296 | 30.814 | 32.659 | |
| > 3.5 | 42.83 | 43.33 | 41.42 | |
| Educational level, % | < 0.001 | |||
| High school and lower | 13.52 | 13.053 | 14.837 | |
| High school and equivalent | 22.861 | 22.278 | 24.506 | |
| University and above | 63.619 | 64.668 | 60.656 | |
| Marital status, % | < 0.001 | |||
| Married and living with a partner | 62.446 | 62.682 | 61.781 | |
| Widowed, divorced or separated | 19.349 | 16.04 | 28.694 | |
| Never married | 18.205 | 21.278 | 9.526 | |
| Smoking status, % | < 0.001 | |||
| No | 56.776 | 60.144 | 47.266 | |
| Yes | 43.224 | 39.856 | 52.734 | |
| Alcohol consumption status, % | 0.001 | |||
| No | 53.425 | 52.781 | 55.244 | |
| Yes | 46.575 | 47.219 | 44.756 | |
| BMI | 29.356 ± 6.983 | 28.735 ± 6.678 | 31.110 ± 7.509 | < 0.001 |
| Diabetes status, % | < 0.001 | |||
| No | 89.61 | 92.4 | 81.73 | |
| Yes | 10.39 | 7.6 | 18.27 | |
| Physical activity, % | < 0.001 | |||
| Regular activities | 19.544 | 22.523 | 11.131 | |
| Occasional activities | 35.686 | 36.473 | 33.466 | |
| Rarely active | 44.77 | 41.005 | 55.403 | |
| Albumin, urine (ug/mL) | 34.267 ± 246.212 | 30.573 ± 236.211 | 44.701 ± 272.212 | 0.00015 |
| Urine creatinine (mg/dL) | 121.679 ± 80.652 | 124.572 ± 82.536 | 113.505 ± 74.473 | < 0.001 |
| Albumin-creatinine ratio (mg/g) | 33.647 ± 275.374 | 29.931 ± 283.148 | 44.141 ± 251.833 | 0.0007 |
| Creatinine-frozen serum (mg/dL) | 0.878 ± 0.340 | 0.868 ± 0.316 | 0.905 ± 0.397 | < 0.001 |
| eGFR | 92.672 ± 21.835 | 96.547 ± 20.881 | 81.727 ± 20.729 | < 0.001 |
| CKD | < 0.001 | |||
| No | 84.714 | 88.017 | 75.386 | |
| Yes | 15.286 | 11.983 | 24.614 |
BMI: body mass index; CKD: Chronic kidney disease; OA: Osteoarthritis; Values are presented as mean ± standard deviation (SD) unless otherwise specified.
Selection of instrument variables
In our MR study, AOA, HOA, and KOA were considered as exposure factors, while the renal function indicators served as outcome variables. We utilized single nucleotide polymorphisms (SNPs) that showed genome-wide significant associations with the exposure factors and exhibited no linkage disequilibrium (LD) (r2 < 0.001, kb = 10,000) as instrumental variables (P < 5E-08)35,36. Considering that smoking, alcohol consumption, obesity, hyperlipidemia, hypertension, and diabetes are risk factors for kidney disease, we searched for SNPs associated with these risk factors using the LDtrait website37, We identified rs1121980 and rs13107325 in AOA, rs67924081 in HOA, and rs1047891, rs10974438, and rs1426371 in KOA that were related to these risk factors, and consequently, we excluded these SNPs to minimize potential confounding effects. Additionally, we excluded SNPs with inconsistent alleles between exposure and outcome, as well as SNPs with intermediate allele frequencies that were palindromic, while avoiding the use of proxy SNPs. After applying these stringent selection criteria, we identified 23 SNPs associated with AOA, 41 SNPs associated with HOA, and 28 SNPs associated with KOA; only these rigorously selected SNPs were used for the final causal analysis.
Statistical analyses
Cross-sectional study
The NHANES data analysis followed the NHANES statistical tutorial, with sample weights applied according to the complex multistage probability sampling design. Continuous variables were presented as mean ± standard deviation, while categorical variables were reported as counts and percentages. Complex sample weights were used to estimate population characteristics, BMI, and the overall prevalence of diabetes and arthritis. Statistically significant covariates were then added to a multivariable logistic regression model for further analysis. In this study, OA was treated as the predictor variable, while CKD was the outcome variable in the association analysis. After adjusting for covariates, we further explored the relationship between OA and CKD, calculating the odds ratio (OR) and its 95% confidence intervals (CIs). To improve result accuracy, we performed subgroup analyses based on age, gender, and physical activity levels. A p-value of < 0.05 was considered statistically significant. All analyses were conducted using R software (version 4.2.3).
TSMR analysis
As an epidemiological statistical method, MR must satisfy three key assumptions: (a) Relevance assumption: The genetic variants used as instrumental variables must be strongly associated with the exposure. (b) Independence assumption: The genetic variants must be independent of any unmeasured confounding factors between the exposure and the outcome. (c) Exclusion restriction assumption: The genetic variants must influence the outcome only through the exposure, not directly38. In this study, the inverse variance weighted (IVW) method was used as the primary analytical approach, while MR-Egger regression and the weighted median estimator (WM) were employed as supplementary methods for two-sample MR analysis. When all selected SNPs are valid instrumental variables, the IVW method provides the most accurate estimate of causal effects39. When more than half of the SNPs are valid, the WM method can yield a reliable causal estimate40. In cases where all SNPs are considered invalid, the MR-Egger method can still provide a reliable causal estimate41.
To assess the reliability of MR results, we used the IVW and MR-Egger regression methods to detect heterogeneity, calculating Cochran’s Q statistic to quantify the degree of heterogeneity; heterogeneity was considered present when the p-value was less than 0.0542. We assessed horizontal pleiotropy by calculating the intercept of the MR-Egger regression; a p-value of less than 0.05 for the MR-Egger intercept indicates the presence of horizontal pleiotropy, making the MR results unreliable41. We employed the MR-PRESSO global test to identify outliers; a p-value of less than 0.05 in the MR-PRESSO global test indicates the presence of outliers, which should be excluded and the MR analysis repeated43. Additionally, leave-one-out analysis was used to evaluate whether the MR results were influenced by any single SNP. Finally, we used the F statistics to assess the strength of the genetic instruments. An F statistic greater than 10 (F = β2/SE2) indicates that the MR results are unlikely to be affected by weak instrumental variables44. All MR-related analyses were conducted in R (version 4.2.3) using the TwoSampleMR package45, and the MR analysis flow is illustrated in Fig. 2.
Fig. 2.
Flowchart of Mendelian randomization in our study.
Since multiple testing was conducted, we applied the Bonferroni method to adjust the significance threshold (0.05 / (3 × 5) = 0.003). Therefore, for a conclusion to be considered significant in this MR study, the following three criteria must be met: (a) The p-value of the IVW method is < 0.003; (b) The results of the weighted median and MR-Egger regression methods are consistent with the direction of the IVW results; (c) Sensitivity analyses indicate that there is no horizontal pleiotropy, and the MR results are not driven by a single SNP.
Result
Population-based study
This study included a total of 22,525 eligible participants with a mean age of 47.700 years (standard deviation ±16.978), of which 48.294% were male. Among them, 6,072 had OA and 4,359 had CKD. Table 1 presents the demographic and baseline characteristics of the OA and non-OA groups. Notably, the OA group was significantly older than the non-OA group (p < 0.001). The proportion of females with OA (51.706%) was higher than that of males (48.294%). The percentage of non-Hispanic Whites was higher among arthritis patients compared to those without arthritis (74.327% vs. 61.87%). Additionally, individuals with OA had higher rates of poverty, lower education levels, were more likely to be married, smoked, consumed alcohol, had higher BMI, were less physically active, and had higher rates of diabetes (all p < 0.001). Most importantly, the proportion of individuals with impaired renal function and CKD was significantly higher in the OA group compared to the non-OA group (24.614% vs. 11.983%, p < 0.001). Table 2 shows the results of the logistic regression analysis with OA as the independent variable and CKD as the outcome. Overall, OA was significantly positive associated with CKD [OR 2.398 (2.176, 2.643), p < 0.001]. This relationship remained significant after adjusting for demographic factors, socioeconomic status, BMI, smoking, alcohol consumption, and diabetes [OR 1.161 (1.029, 1.310), p = 0.015]. Table 3 displays the results of the stratified analysis, showing that the association between OA and CKD increased with age and decreased physical activity. The final model showed the following relationships between OA and CKD: in the ≥60 age group [OR = 1.162 (1.011, 1.336), p = 0.035], in the group with low physical activity [OR = 1.226 (1.055, 1.424), p = 0.008], and among females with OA [OR = 1.192 (1.015, 1.399), p = 0.032].
Table 2.
Association between chronic kidney disease status and osteoarthritis.
| CKD | Crude OR (95%CI) | Model1, OR (95%CI) | Model2, OR (95%CI) | Model3, OR (95%CI) |
|---|---|---|---|---|
| OA | 2.398(2.176,2.643) | 1.356(1.207,1.524) | 1.274(1.134,1.431) | 1.161(1.029,1.310) |
| Non-OA | Ref | Ref | Ref | Ref |
Logistic regression models:
Crude Model: Unadjusted model.
Model 1: Adjusted for age, gender and race.
Model 2: Further adjusted for socioeconomic factors including educational level, poverty to income ratio, physical activity and Marital status.
Model 3: Further adjusted for diabetes, alcohol using, smoking and BMI.
Table 3.
Subgroup analysis of the association of chronic kidney disease and osteoarthritis.
| OR (95%CI) | p-value | |
|---|---|---|
| Gender | ||
| Men | ||
| OA | 1.166(0.971, 1.400) | 0.100 |
| Non-OA | Ref | Ref |
| Women | ||
| OA | 1.192(1.015, 1.399) | 0.032 |
| Non-OA | Ref | Ref |
| Physical activity | ||
| Regular activities | ||
| OA | 1.189(0.779, 1.816) | 0.423 |
| Non-OA | Ref | Ref |
| Occasional activities | ||
| OA | 1.061(0.847, 1.329) | 0.604 |
| Non-OA | Ref | Ref |
| Rarely active | ||
| OA | 1.226(1.055, 1.424) | 0.008 |
| Non-OA | Ref | Ref |
| Age | ||
| < 60 | ||
| OA | 1.756(1.462, 2.108) | < 0.001 |
| Non-OA | Ref | Ref |
| ≥ 60 | ||
| OA | 1.162(1.011,1.336) | 0.035 |
| Non-OA | Ref | Ref |
All data were adjusted for gender, age, race, education level and poverty to income ratio, physical activity and Marital status, diabetes, alcohol using, smoking and BMI.
MR framework analyses
Causal effects of AOA, HOA, and KOA on renal function
When AOA, HOA, and KOA were used as exposure factors, and after controlling confounding factors such as hypertension, hyperlipidemia, diabetes, smoking, alcohol consumption, and obesity, we identified 23, 41, and 28 validated SNPs, respectively. According to Figure 3, no significant causal association was found between AOA and the various renal function indicators. However, a significant genetic association was observed between HOA and cystatin C [IVW p = 0.0014, OR = 1.02, 95% CI = 1.01–1.03], as well as between KOA and cystatin C [IVW p < 0.0001, OR = 1.06, 95% CI = 1.04–1.08]. No significant associations were found between HOA and ARF, CRF, serum creatinine (eGFRcrea), or microalbuminuria, nor between KOA and ARF, CRF, serum creatinine (eGFRcrea), or microalbuminuria. The results from the weighted media and MR-Egger methods were consistent with those of the IVW method, indicating an increased risk (scatter plots are available in the supplementary materials). The Cochran’s Q test for both IVW and MR-Egger methods yielded p-values greater than 0.05, indicating no heterogeneity (funnel plots are available in the supplementary materials). Similarly, the p-values from the MR-PRESSO and MR-Egger intercept tests were also greater than 0.05, suggesting no pleiotropy. Additionally, leave-one-out analysis showed that the associations between HOA, KOA, and cystatin C were not driven by any single SNP (leave-one-out plots are available in the supplementary materials).
Fig. 3.
MR estimates derived from each method of assessing the causal effect of OA on renal function indices.
Combining the results of NHANES and MR analysis, we observed a significant association between OA and CKD in a cross-sectional study, suggesting a potential link between these two diseases. However, in the MR analysis, significant associations were only observed between HOA and KOA and cystatin C. Therefore, we speculate that OA may be a potential risk factor for renal function injury, but further studies are needed to verify these findings and explore their causal relationship in depth.
Discussion
This study, using NHANES data combined with the MR method, is the first to explore the potential causal relationship between OA and renal function injury. The results revealed a significant positive statistical association between OA and CKD in the NHANES sample, suggesting that individuals with OA face a higher risk of CKD. However, this association does not directly prove causality between the two conditions. Further MR analysis indicated that not all types of OA are significantly associated with renal function impairment, but specific types, such as HOA and KOA, showed a significant causal relationship with kidney damage. This may be attributed to several factors. First, AOA encompasses a heterogeneous group of joint conditions, and the genetic variants associated with OA may not uniformly influence renal function. Second, the sample size for AOA in the GWAS data may have been insufficient to detect a causal effect, particularly if the genetic variants have weak or moderate effects on renal function. Future studies with larger sample sizes and more refined OA phenotypes are needed to further explore this relationship.
Although our study suggests a potential association between HOA and KOA with renal function impairment, it is important to recognize that the underlying mechanisms remain unclear. To further explore these mechanisms, we analyzed relevant literature to identify possible factors that increase the risk of renal damage in OA patients and provide new insights for the clinical management of OA. The mechanisms linking OA and renal function impairment are complex, involving multiple pathophysiological processes. Several hypotheses have been proposed by researchers. First, NSAIDs, commonly used in the clinical treatment of OA, may contribute to renal damage. However, this remains controversial. On one hand, NSAIDs inhibit prostaglandin synthesis, impairing renal blood flow regulation, which reduces renal blood flow and decreases glomerular filtration rate, potentially inducing or exacerbating kidney injury46. Some researchers have suggested that NSAIDs may be a potential risk factor for end-stage renal disease, though clear direct evidence is still lacking47. On the other hand, some studies indicate that topical NSAIDs are safe and effective for OA patients, without significant adverse renal effects48. Additionally, recent research shows that the long-term use of NSAIDs in arthritis patients has a limited impact on cardiovascular and renal events under modern medical conditions49. Second, the systemic inflammation hypothesis is another important mechanism linking OA to renal impairment. Inflammatory markers such as IL-6, IL-17, and TNF-α are commonly elevated in OA patients, contributing not only to local joint inflammation but also potentially damaging renal microvascular systems50,51. Furthermore, reduced physical activity is common among OA patients. Joint pain or fear of disease progression may lead patients to limit their exercise, which further promotes endothelial dysfunction and other pathological changes that exacerbate renal damage52, This finding is consistent with our study results. Finally, the oxidative stress hypothesis suggests that OA leads to elevated levels of reactive oxygen species (ROS), a key factor in kidney injury. ROS are produced in both OA and CKD, leading to renal cell damage and further affecting renal function53. Our findings are consistent with previous studies suggesting that OA is a potential risk factor for CKD, which further adds to the credibility of our findings54,55. In summary, osteoarthritis may contribute to renal impairment through multiple mechanisms, including the use of NSAIDs, systemic inflammation, reduced physical activity, and oxidative stress.
Although OA itself affects renal function through mechanisms such as systemic inflammation and oxidative stress, total hip and knee arthroplasty, as a common treatment for advanced OA, can also significantly impact renal function56–58. Therefore, it is essential to consider the potential effects of surgical intervention on kidney function as part of OA treatment. Analyzing the impact of surgery on renal function through relevant literature can provide guidance for the prevention of renal impairment in clinical settings. First, intraoperative hypotension is a critical factor affecting renal perfusion. Hemorrhage can lead to a reduction in overall blood volume, and when the mean arterial pressure falls below 65 mm Hg for an extended period, the kidney’s autoregulatory function may be compromised, potentially leading to postoperative acute kidney injury (AKI)59. Second, intraoperative blood transfusions may increase the risk of postoperative AKI; although the specific mechanisms are not fully understood, studies indicate an independent association between transfusion and the occurrence of AKI60. Furthermore, improper fluid management during surgery may negatively impact renal function, especially regarding the use of colloidal solutions, although the evidence remains debated61,62. The duration of surgery is also a significant factor; research has shown that when surgical time exceeds 120 min, the incidence of AKI nearly doubles 63, often associated with substantial intraoperative blood loss, coagulopathy, and the complexity of the surgical site. Additionally, while NSAIDs are widely used for postoperative analgesia, their potential nephrotoxic effects—through the inhibition of prostaglandin synthesis, reduction of renal blood flow, and decrease in eGFR—may increase the risk of AKI64. Postoperative hypoalbuminemia is also considered a risk factor for AKI65. Moreover, the use of antibiotic-impregnated bone cement may contribute to the risk of AKI. Jafari et al.66 pointed out that antibiotic-impregnated bone cement contains aminoglycosides and/or vancomycin, both of which are nephrotoxic. McGlothan et al.67 also found that AKI patients exhibited rapid recovery of renal function following the removal of high-dose antibiotic bone cement, suggesting that the use of high-dose cement may be a contributing factor to AKI. It is also noteworthy that pre-existing renal insufficiency is a risk factor for postoperative kidney damage68, particularly as elevated creatinine levels significantly affect renal filtration capacity69. Even a slight increase in plasma creatinine, if left unaddressed, can lead to progressive renal failure and increased mortality70. Studies have shown that patients with pre-existing CKD have a significantly higher incidence of AKI following surgery. For instance, Porter et al.71 reported that patients with a preoperative eGFR of less than 30 mL/min per 1.73 m2 have a 2.4-fold increased risk of developing AKI compared to those with normal renal function, and the incidence of AKI in stage 3 CKD patients can reach as high as 16.7%, significantly prolonging hospital stays72.
Based on the above findings, we propose several new insights and strategies for the clinical treatment of OA. Our study highlights the significant association between HOA and KOA with renal function injury, which has important implications for the screening and management of OA patients. First, clinicians should consider regular renal function screening for patients with HOA or KOA, particularly those with additional risk factors such as advanced age, obesity, or diabetes. Early detection of renal injury could facilitate timely interventions to slow disease progression. We recommend that OA treatment should be individualized73, taking into account the patient’s specific clinical characteristics, comorbidities, and risk factors for renal injury. An individualized strategy can be developed through a comprehensive assessment that includes: (a) patient history and comorbidities, such as diabetes, hypertension, or pre-existing CKD, which may influence treatment choices; (b) severity of OA symptoms, including pain levels and functional limitations, to determine the most appropriate therapeutic approach; (c) renal function status, assessed through routine monitoring of eGFR and urinary albumin-to-creatinine ratio (UACR), to guide the selection of medications with minimal renal toxicity; and (d) lifestyle factors, such as physical activity levels and dietary habits, which can be modified to improve overall health and reduce systemic inflammation. Based on this assessment, safer alternative medications or topical treatments can be selected to reduce renal burden. For example, in patients with mild OA and no significant renal injury, topical NSAIDs may be preferred over oral NSAIDs to minimize systemic exposure and renal risk. In patients with moderate to severe OA and impaired renal function, non-NSAID analgesics (e.g., acetaminophen) or disease-modifying agents (e.g., glucosamine) may be considered. Additionally, anti-inflammatory medications should be used judiciously to manage systemic inflammation, and moderate exercise should be encouraged to improve joint function and overall health. Increasing the intake of antioxidants, such as through dietary modifications or supplements, may also help mitigate oxidative stress and its impact on renal function. For patients with end-stage hip or knee osteoarthritis who require total hip or knee replacement, a preoperative renal function assessment is recommended, with particular attention to those with pre-existing renal insufficiency, allowing for targeted preventive measures. During surgery, blood pressure and blood volume should be closely monitored to avoid renal hypoperfusion due to intraoperative hypotension. The cautious use of antibiotic-impregnated bone cement is also necessary, given its potential nephrotoxicity. Moreover, efforts should be made to reduce operative time to lower the incidence of postoperative AKI. By implementing these measures, we aim to effectively manage the risk of renal function injury in OA patients.
However, this study has several limitations. First, due to the constraints of the NHANES data source, it was not possible to specifically investigate the correlation between total hip and knee replacement and CKD, nor the effects of different types of osteoarthritis on CKD. Second, the data on OA primarily relied on patient recall questionnaires, which may introduce recall bias and affect the accuracy of the data. Additionally, a substantial number of participants (n = 19,184) were excluded because they responded ‘unknown’ or had missing data to the arthritis-related questions, potentially limiting the generalizability of our findings. Additionally, the sample in this study was limited to European populations, which may restrict the generalizability of the results to other populations. Future research should consider including data from diverse population samples to enhance the generalizability of the findings and explore the relationship between OA and CKD across different ethnic groups. Furthermore, conducting prospective cohort studies would help overcome the limitations of the current research and provide more direct causal evidence, particularly regarding the association between total hip and knee replacement and CKD. Lastly, future studies should further investigate the underlying mechanisms linking OA and total hip and knee replacement to CKD, especially the pathophysiological processes affecting renal function, to provide stronger theoretical support for clinical interventions.
In summary, this study suggests that HOA and KOA may be risk factors for renal function injury, as indicated by both NHANES and MR analyses. These findings highlight the potential impact of total hip and knee replacement as a treatment for advanced OA on renal function. However, further research is needed to explore the underlying mechanisms and provide stronger evidence to support clinical prevention and treatment strategies.
Supplementary Information
Author contributions
P and KW were responsible for writing the manuscript, YZ was responsible for the production of Figs. 1-3, QW and ZZ were responsible for the production of Tables 1-3, and L and B were responsible for reviewing and revising the manuscript.
Funding
The effect of Bu Yang Hui Wu Tang combined with wrist and ankle acupuncture on the prevention of postoperative delirium after arthroplasty for hip fracture in elderly people, Funding Project Number :2022FSYYZQ22.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Competing interests
The authors declare no competing interests.
Moral declaration
Data from the NHANES database and the GAW database were used in this study. The NHANES data were collected in accordance with U.S. federal regulations and Centers for Disease Control and Prevention (CDC) ethical guidelines, and all participants provided informed consent. The GAW data were used in accordance with the appropriate data use protocols and privacy protections. The analysis of this study involved only de-identified data, ensuring participant privacy and data confidentiality.
Institutional review board statement
This study used summary data published by multiple GWAS; the review board statement can be found in the original work. Informed Consent Statement: Patient consent was obtained by corresponding studies, which have been cited in the manuscript.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-97756-z.
References
- 1.Glyn-Jones, S. et al. Osteoarthritis. Lancet.386(9991), 376–387 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Glassock, R. J., Warnock, D. G. & Delanaye, P. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat. Rev. Nephrol.13(2), 104–114 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Ji, P. et al. Non-apoptotic cell death in osteoarthritis: Recent advances and future. Biomed. Pharmacother.179, 117344 (2024). [DOI] [PubMed] [Google Scholar]
- 4.Woolf, A. D. & Pfleger, B. Burden of major musculoskeletal conditions. Bull World Health Organ.81(9), 646–656 (2003). [PMC free article] [PubMed] [Google Scholar]
- 5.Englund, M. Osteoarthritis, part of life or a curable disease?. A bird’s-eye view. J. Intern. Med.293(6), 681–693 (2023). [DOI] [PubMed] [Google Scholar]
- 6.Cao, F. et al. Trends and cross-country inequalities in the global burden of osteoarthritis, 1990–2019: A population-based study. Ageing Res. Rev.99, 102382 (2024). [DOI] [PubMed] [Google Scholar]
- 7.Katz, J. N., Arant, K. R. & Loeser, R. F. Diagnosis and treatment of hip and knee osteoarthritis: A review. JAMA325(6), 568–578 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madry, H. Surgical therapy in osteoarthritis. Osteoarthr. Cartil.30(8), 1019–1034 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Motta, F., Barone, E., Sica, A. & Selmi, C. Inflammaging and osteoarthritis. Clin. Rev. Allergy Immunol.64(2), 222–238 (2023). [DOI] [PubMed] [Google Scholar]
- 10.Sampath, S. J. P., Venkatesan, V., Ghosh, S. & Kotikalapudi, N. Obesity, metabolic syndrome, and osteoarthritis-an updated review. Curr. Obes Rep.12(3), 308–331 (2023). [DOI] [PubMed] [Google Scholar]
- 11.Hill, N. R. et al. Global prevalence of chronic kidney disease - A Systematic review and meta-analysis. PLoS ONE11(7), e0158765 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coresh, J. et al. Prevalence of chronic kidney disease in the United States. JAMA298(17), 2038–2047 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Gansevoort, R. T. et al. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet382(9889), 339–352 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Zemedikun, D. T. et al. Comorbidity phenotypes and risk of mortality in patients with osteoarthritis in the UK: A latent class analysis. Arthritis Res. Ther.24(1), 231 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swain, S. et al. Temporal relationship between osteoarthritis and comorbidities: a combined case control and cohort study in the UK primary care setting. Rheumatology (Oxford)60(9), 4327–4339 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muyodi, M. M., Bhatt, K. M. & Kayima, J. K. Prevalence of and factors associated with chronic kidney disease in osteoarthritis patients at Kenyatta National Hospital. East Afr. Orthop. J.14(2), 72–80 (2020). [Google Scholar]
- 17.Wang, M. et al. Coffee consumption and prostate cancer risk: Results from national health and nutrition examination survey 1999–2010 and mendelian randomization analyses. Nutrients13(7), 2317 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang, X., Seo, Y. A. & Park, S. K. Serum selenium and non-alcoholic fatty liver disease (NAFLD) in U.S. adults: National Health and Nutrition Examination Survey (NHANES) 2011-2016. Environ. Res.197, 111190 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zipf, G. et al. National health and nutrition examination survey: Plan and operations, 1999-2010. Vital Health Stat.56, 1–37 (2013). [PubMed] [Google Scholar]
- 20.Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern Med.150(9), 604–612 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gouri Suresh, S. S. & Schauder, S. A. Income segregation and access to healthy food. Am. J. Prev. Med.59(2), e31–e38 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Liao, W. et al. Associations between healthy lifestyle score and health-related quality of life among Chinese rural adults: Variations in age, sex, education level, and income. Qual. Life Res.32(1), 81–92 (2023). [DOI] [PubMed] [Google Scholar]
- 23.Robards, J., Evandrou, M., Falkingham, J. & Vlachantoni, A. Marital status, health and mortality. Maturitas73(4), 295–299 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang, R. et al. Smoking timing, healthy diet, and risk of incident CKD among smokers: Findings from UK biobank. Am. J. Kidney Dis.84(5), 593-600.e1 (2024). [DOI] [PubMed] [Google Scholar]
- 25.Zhu, S. et al. Association of smoking and osteoarthritis in US (NHANES 1999–2018). Sci. Rep.13(1), 3911 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumoto, A. et al. The association of alcohol and smoking with CKD in a Japanese nationwide cross-sectional survey. Hypertens. Res.40(8), 771–778 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Arruda, A. L. et al. Genetic underpinning of the comorbidity between type 2 diabetes and osteoarthritis. Am. J. Hum. Genet.110(8), 1304–1318 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas, M. C., Cooper, M. E. & Zimmet, P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat. Rev. Nephrol.12(2), 73–81 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Diabetes Care in the Hospital. Standards of medical care in diabetes-2022. Diabetes Care45(Suppl 1), S244–S253 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Boer, C. G. et al. Deciphering osteoarthritis genetics across 826,690 individuals from 9 populations. Cell184(24), 6003–6005 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kellum, J. A., Ronco, C. & Bellomo, R. Conceptual advances and evolving terminology in acute kidney disease. Nat. Rev. Nephrol.17(7), 493–502 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Ostermann, M. et al. Controversies in acute kidney injury: Conclusions from a kidney disease: Improving global outcomes (KDIGO). Kidney Int.98(2), 294–309 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Futrakul, N., Sridama, V. & Futrakul, P. Microalbuminuria–a biomarker of renal microvascular disease. Ren Fail.31(2), 140–143 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Li, C. et al. Association of cystatin C kidney function measures with long-term deficit-accumulation frailty trajectories and physical function decline. JAMA Netw Open.5(9), e2234208 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purcell, S. et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet.81(3), 559–575 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abecasis, G. R. et al. A map of human genome variation from population-scale sequencing. Nature467(7319), 1061–1073 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin, S. H., Brown, D. W. & Machiela, M. J. LDtrait: An online tool for identifying published phenotype associations in linkage disequilibrium. Cancer Res.80(16), 3443–3446 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davies, N. M., Holmes, M. V. & Davey, S. G. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ362, k601 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burgess, S., Bowden, J., Fall, T., Ingelsson, E. & Thompson, S. G. Sensitivity analyses for robust causal inference from mendelian randomization analyses with multiple genetic variants. Epidemiology28(1), 30–42 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol.40(4), 304–314 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowden, J., Davey Smith, G. & Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through egger regression. Int. J. Epidemiol.44(2), 512–525 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greco, M. F., Minelli, C., Sheehan, N. A. & Thompson, J. R. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med.34(21), 2926–2940 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Verbanck, M., Chen, C. Y., Neale, B. & Do, R. Publisher Correction: Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet.50(8), 1196 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Feng, R. et al. Pulmonary embolism and 529 human blood metabolites: genetic correlation and two-sample Mendelian randomization study. BMC Genom. Data.23(1), 69 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hemani, G. et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife10.7554/eLife.34408 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drożdżal, S. et al. Kidney damage from nonsteroidal anti-inflammatory drugs-myth or truth? Review of selected literature. Pharmacol. Res. Perspect.9(4), e00817 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang, X., Donnan, P. T., Bell, S. & Guthrie, B. Non-steroidal anti-inflammatory drug induced acute kidney injury in the community dwelling general population and people with chronic kidney disease: systematic review and meta-analysis. BMC Nephrol.18(1), 256 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng, C. et al. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: A systematic review and network meta-analysis of randomised controlled trials and observational studies. Br. J. Sports Med.52(10), 642–650 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Obeid, S. et al. Cardiorenal risk of celecoxib compared with naproxen or ibuprofen in arthritis patients: insights from the PRECISION trial. Eur. Heart J. Cardiovasc. Pharmacother.8(6), 611–621 (2022). [DOI] [PubMed] [Google Scholar]
- 50.Mazzaferro, S. et al. Inflammation, oxidative stress, and bone in chronic kidney disease in the osteoimmunology era. Calcif. Tissue Int.108(4), 452–460 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mihai, S. et al. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J. Immunol. Res.2018, 2180373 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Booth, F. W., Roberts, C. K. & Laye, M. J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol.2(2), 1143–1211 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rashid, H., Jali, A., Akhter, M. S. & Abdi, S. A. H. Molecular mechanisms of oxidative stress in acute kidney injury: Targeting the loci by resveratrol. Int. J. Mol. Sci.25(1), 3 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katsuno, T. et al. Burden of renal events associated with nonsteroidal anti-inflammatory drugs in patients with osteoarthritis and chronic low back pain: A retrospective database study. Pain Ther.10(1), 443–455 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Julovi, S. M. et al. Disease-modifying interactions between chronic kidney disease and osteoarthritis: A new comorbid mouse model. RMD Open9(3), e003109 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang, E. X. et al. Acute kidney disease after total hip and knee arthroplasty: Incidence and associated factors. J. Arthroplasty.32(8), 2381–2385 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Lee, Y. J. et al. Analysis of the risk factors of acute kidney injury after total hip or knee replacement surgery. Yeungnam Univ. J. Med.38(2), 136–141 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perregaard, H., Damholt, M. B., Solgaard, S. & Petersen, M. B. Renal function after elective total hip replacement. Acta Orthop.87(3), 235–238 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thongprayoon, C. et al. Acute kidney injury in patients undergoing total hip arthroplasty: A systematic review and meta-analysis. J. Clin. Med.8(1), 60 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weingarten, T. N. et al. Acute kidney injury following total joint arthroplasty: Retrospective analysis. Can. J. Anaesth.59(12), 1111–1118 (2012). [DOI] [PubMed] [Google Scholar]
- 61.Lewis, S. R. et al. Colloids versus crystalloids for fluid resuscitation in critically ill people. Cochrane Database Syst. Rev.10.1002/14651858.CD000567.pub7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Degoul, S. et al. lntraoperative administration of 6% hydroxyethyl starch 130/0.4 is not associated with acute kidney injury in elective non-cardiac surgery: A sequential and propensity-matched analysis. Anaesth. Crit. Care Pain Med.39(2), 199–206 (2020). [DOI] [PubMed] [Google Scholar]
- 63.Jämsä, P. et al. Risk factors associated with acute kidney injury in a cohort of 20,575 arthroplasty patients. Acta Orthop.88(4), 370–376 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ejaz, P., Bhojani, K. & Joshi, V. R. NSAIDs and kidney. J. Assoc. Physicians India.52, 632–640 (2004). [PubMed] [Google Scholar]
- 65.Shin, K. H. & Han, S. B. Early postoperative hypoalbuminemia is a risk factor for postoperative acute kidney injury following hip fracture surgery. Injury49(8), 1572–1576 (2018). [DOI] [PubMed] [Google Scholar]
- 66.Jafari, S. M., Huang, R., Joshi, A., Parvizi, J. & Hozack, W. J. Renal impairment following total joint arthroplasty: who is at risk?. J. Arthroplast.25(6), 49–53 (2010). [DOI] [PubMed] [Google Scholar]
- 67.McGlothan, K. R. & Gosmanova, E. O. A case report of acute interstitial nephritis associated with antibiotic-impregnated orthopedic bone-cement spacer. Tenn. Med.105(9), 37–40 (2012). [PubMed] [Google Scholar]
- 68.Novis, B. K., Roizen, M. F., Aronson, S. & Thisted, R. A. Association of preoperative risk factors with postoperative acute renal failure. Anesth. Analg.78(1), 143–149 (1994). [DOI] [PubMed] [Google Scholar]
- 69.Ma, Y. et al. Occurrence and predictive factors of acute renal injury following hip and knee arthroplasty. Clin. Exp. Nephrol.24(7), 598–605 (2020). [DOI] [PubMed] [Google Scholar]
- 70.Bihorac, A. et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann. Surg.249(5), 851–858 (2009). [DOI] [PubMed] [Google Scholar]
- 71.Porter, C. J. et al. Acute and chronic kidney disease in elderly patients with hip fracture: Prevalence, risk factors and outcome with development and validation of a risk prediction model for acute kidney injury. BMC Nephrol.18(1), 20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nowicka, A. & Selvaraj, T. Incidence of acute kidney injury after elective lower limb arthroplasty. J. Clin. Anesth.34, 520–523 (2016). [DOI] [PubMed] [Google Scholar]
- 73.Magni, A. et al. Management of osteoarthritis: Expert opinion on NSAIDs. Pain Ther.10(2), 783–808 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.