Abstract
Purpose of review
Genetic testing of patients with severe hypertriglyceridemia often identifies a single heterozygous pathogenic variant in the LPL gene. The complex and variable phenotype associated with this genotype is the topic of this review.
Recent findings
Previous research showed that heterozygosity for lipoprotein lipase deficiency is associated with reduced but variable post heparin lipolytic activity alongside inconsistent plasma lipid phenotypes ranging from normal to mild-to-moderate to severe hypertriglyceridemia. Recent research confirms and extends these observations, showing that a heterozygous individual can express a highly variable phenotype over time, depending on the presence of secondary factors. About 10% (range 8–20%) of patients with severe hypertriglyceridemia or multifactorial chylomicronemia syndrome are heterozygous for a rare pathogenic LPL variant, and a clinically relevant minority of these has recalcitrant or sustained hypertriglyceridemia.
Summary
Heterozygosity for lipoprotein lipase deficiency predisposes to hypertriglyceridemia, which is sometimes severe depending on secondary factors, but is typically quite responsive to routine interventions such as diet, lifestyle and existing lipid-lowering therapies. However, many heterozygotes for pathogenic variants in LPL have completely normal plasma lipids.
Keywords: chylomicronemia, familial chylomicronemia syndrome, hyperlipoproteinemia type 1, hyperlipoproteinemia type 4, hyperlipoproteinemia type 5, hypertriglyceridemia, lipoprotein lipase, lipoprotein lipase deficiency, multifactorial chylomicronemia syndrome, pathogenic variant
INTRODUCTION
Diagnostic next-generation DNA sequencing for dyslipidemia is becoming more widely available [1]. Interpretation of reported heterozygous rare pathogenic variants in the diagnosis of familial hypercholesterolemia is relatively straightforward [2]. However, severe hypertriglyceridemia is a more genetically complex trait [3]. While pathogenic variants in candidate genes in patients with hypertriglyceridemia are often reported, their meaning and relevance is not analogous to rare pathogenic variants reported in patients with suspected familial hypercholesterolemia [2]. In the context of severe hypertriglyceridemia, how should a clinician interpret a report of a heterozygous pathogenic rare variant in a candidate causal gene, most often LPL encoding lipoprotein lipase (LPL)?
Box 1.
no caption available
LIPOPROTEIN LIPASE GENETIC VARIATION IN HYPERTRIGLYCERIDEMIA
For this review, I will focus on rare pathogenic variants in LPL as defined by the American College of Medical Genetics and Genomics (ACMG) [4]. Criteria for pathogenicity include a low allele frequency in the population (e.g. < 0.5%); a qualitatively null variant (nonsense, frameshift or altering a canonical splice site); a missense variant with multiple lines of computational evidence supporting a deleterious effect; a missense variant located in mutational hotpot or critical functional domain; evidence for mendelian inheritance (autosomal recessive) in affected families; and well established functional studies clearly showing a damaging effect [4]. I will exclude common nonpathogenic LPL coding sequence polymorphisms with allele frequency more than 1% and small effects on plasma triglyceride levels that contribute to polygenic risk of hypertriglyceridemia, such as LPL p.Asp36Asn (formerly p.Asp9Asn) and p.Asn318Ser (formerly p.Asn291Ser), which are sometimes reported and can be confused with actual pathogenic variants.
WHY ARE VARIANTS IN LPL DETECTED IN PATIENTS WITH SEVERE HYPERTRIGLYCERIDEMIA?
When a clinician orders DNA sequencing on a patient with severe hypertriglyceridemia, defined as plasma triglyceride more than 10 mmol/l (> 880 mg/dl), the most commonly used technology is a targeted next-generation DNA sequencing panel [5]. The standard diagnostic panel for severe hypertriglyceridemia screens coding regions and intron-exon boundaries for five canonical genes that are causative for familial chylomicronemia syndrome (FCS), formerly designated as ‘Frederickson hyperlipoproteinemia type 1’ or ‘lipoprotein lipase deficiency’ [6].
FCS is an ultrarare autosomal recessive disorder primarily affecting children and adolescents that is caused by biallelic pathogenic variants in one of five genes – LPL, GPIHBP1, APOA5, APOC2 and LMF1 – leading to absent plasma lipolytic activity [6–8]. Biallelic LPL mutations underlie 60–80% of cases [9]. The five gene products play key roles in catabolism of the contents of triglyceride-rich lipoproteins such as chylomicrons and VLDL [3,7]. In patient cohorts with severe hypertriglyceridemia, only 1–6% have mendelian recessive FCS with biallelic (i.e. homozygous or compound heterozygous) variants in the five genes [3]. Remaining patients with severe hypertriglyceridemia have a more complicated etiology. The phenotype is sometimes referred to as multifactorial chylomicronemia syndrome (MCS) if there are clinical manifestations such as lipemia retinalis, eruptive xanthomas, hepatosplenomegaly and/or acute pancreatitis [6–8].
WHAT IS THE GENETIC BASIS OF SEVERE HYPERTRIGLYCERIDEMIA IN ADULTS?
Severe hypertriglyceridemia or MCS patients have triglyceride more than 10 mmol/l (> 880 mg/dl), but the lipoprotein disturbances are broader than the isolated hyperchylomicronemia in FCS [7,8]. In severe hypertriglyceridemia or MCS patients, there are in addition to pathological presence of chylomicrons elevated levels of VLDL [7,8]. Levels of LDL and HDL are variable but are often depressed. This complex biochemical phenotype was previously called ‘Frederickson hyperlipoproteinemia type 5’ or ‘mixed hyperlipoproteinemia’.
Severe hypertriglyceridemia is seen in 1 in 400–600 adults [10] and typically occurs in the presence of secondary factors such as obesity, poor diet, type 2 diabetes and use of alcohol or certain medications [7,8,10]. Because chylomicrons play a pathogenic role in acute pancreatitis, both FCS and MCS patients are at an increased risk of pancreatitis, although the relative and lifetime risk are much higher in FCS [11]. However, in absolute terms, there are many more patients with MCS, so statistically an adult who presents with the combination of severe hypertriglyceridemia, lipemic plasma and acute pancreatitis is perhaps 50 to 100-times more likely to have MCS than FCS [3,7,11].
Adults with severe hypertriglyceridemia or MCS have four possible genetic profiles [3,12▪▪]. First, about 15–25% are heterozygous for a pathogenic rare variant in one of the above five FCS genes, as discussed further below. The most prevalent are variants in LPL, accounting for 60–80% of all heterozygous pathogenic variants [9] and are found in 8–20% of adults with severe hypertriglyceridemia or MCS. Second, 35–50% of these patients have a high polygenic risk score for elevated triglycerides constructed by aggregating the small effects of common single nucleotide polymorphisms (SNPs) from many chromosomal loci across the genome [3,13]. The majority of these polygenic triglyceride-related SNPs are not FCS loci: only LPL and APOA5 genes harbor common SNPs that contribute to polygenic risk, while GPIHBP1, APOC2 and LMF1 do not [3,13]. Polygenic risk for hypertriglyceridemia is beyond the scope of this brief review. Third, some MCS patients have both a heterozygous rare variant and increased polygenic risk [12▪▪,13]. Fourth, some MCS patients have neither a heterozygous rare variant nor increased polygenic risk [12▪▪,13]. The undefined genetic risk in this latter group of patients includes putative new genes or mutation types, nonmendelian mechanisms such as epigenetics, mitochondrial DNA inheritance, or nonlinear gene-gene or gene-environment interactions.
HETEROZYGOUS LIPOPROTEIN LIPASE DEFICIENCY IS UNLIKE HETEROZYGOUS FAMILIAL HYPERCHOLESTEROLEMIA
Most targeted diagnostic DNA panels for severe hypertriglyceridemia are not designed to evaluate and report polygenic risk [14]. Instead, they are designed specifically to detect biallelic variants in the five FCS genes, LPL, GPIHBP1, APOA5, APOC2 and LMF1[13,14]. Because 15–25% of patients with severe hypertriglyceridemia or MCS has a heterozygous variant in one of these genes [15], this is a common reported finding among these patients.
However, historical and recent research shows that severe hypertriglyceridemia or MCS is only one of several phenotypes seen in patients who carry a single-copy heterozygous variant in LPL – or other FCS gene – as discussed below. Heterozygotes can also have mild-to-moderate hypertriglyceridemia or even completely normal lipids. Furthermore, the lipid phenotype in these patients can fluctuate over time. The variability and volatility of triglyceride levels in heterozygous LPL deficiency stands out in stark contrast to the more consistent and relatively invariant elevated LDL cholesterol levels in patients with untreated heterozygous familial hypercholesterolemia [2]. LDL cholesterol levels in heterozygous familial hypercholesterolemia are highly heritable and penetrant, and generally do not fluctuate over time and do not range between normal and the severely elevated levels characteristic of homozygous FH. Receptor pathobiology in familial hypercholesterolemia is not analogous to enzyme pathobiology in hypertriglyceridemia. Heterozygous familial hypercholesterolemia is an unsatisfactory model for the inconsistent inheritance and variable phenotypic expression in heterozygous LPL deficiency.
PRE-MILLENNIAL STUDIES OF HETEROZYGOUS LIPOPROTEIN LIPASE DEFICIENCY
The phenotype ranging from normal to mild-to-moderate to severe hypertriglyceridemia has been noted in historical studies of heterozygous LPL deficiency, with similar findings from different groups. For this review, normal, mild-to-moderate and severe hypertriglyceridemia phenotypes are defined as triglycerides less than 2 mmol/l, 2–9.9 mmol/l and at least 10 mmol/l, respectively.
In 1989, Babirak et al. [16] from Seattle reported 7 FCS children who were diagnosed biochemically with complete deficiency of LPL activity in postheparin plasma. No genotyping had been performed, so genetic status was inferred. Among the 14 obligate heterozygote parents of these FCS probands, 13 had LPL activity that was reduced by more than 2 standard deviations, although many values were more than 50% of normal and one heterozygote even had normal LPL activity. Five heterozygotes had normal triglycerides, while nine had mild-to-moderate hypertriglyceridemia with the highest level being 3.9 mmol/l. Three had LDL-cholesterol more than 4 mmol/l and nine had apolipoprotein (apo) B more than 1 g/l. The authors suggested that heterozygotes for LPL deficiency were predisposed to develop mild-to-moderate hypertriglyceridemia and possibly also combined hyperlipidemia (hyperlipoproteinemia type 2B) [16].
In 1990, Wilson et al. [17] from Utah reported an extended 126-member pedigree of an FCS proband who was homozygous for the classic pathogenic LPL p.Gly215Glu variant, known by previous nomenclature as LPL p.Gly188Glu. Among 29 heterozygotes, the authors found a mean 50% reduction in adipose tissue LPL activity, but with such wide variability that it was impossible to distinguish individual carriers from noncarriers [17]. Heterozygotes were susceptible to mainly mild-to-moderate hypertriglyceridemia with increased plasma concentrations of triglyceride, VLDL cholesterol and apo B, and decreased LDL and HDL cholesterol. However, expression of hypertriglyceridemia was age dependent. In those less than 40 years old, mean triglyceride level was not statistically different between carriers and noncarriers: 1.92 vs. 1.68 mmol/l, respectively. But in those more than 40 years old, mean triglyceride level was significantly higher in carriers compared to noncarriers: 4.98 vs. 2.20 mmol/l, respectively. Nine (31%), 18 (62%) and two (7%) heterozygotes had normal lipids, mild-to-moderate and severe hypertriglyceridemia, respectively. There was poor co-segregation of hypertriglyceridemia phenotype with the heterozygous LPL variant genotype. Indeed, there were numerous examples of siblings who had identical heterozygous LPL variant genotypes but had widely discordant triglyceride levels. The authors commented that obesity, diet and impaired glucose tolerance were associated with higher triglyceride levels in carriers [17].
In 1997, Julien et al. [18] from Quebec reported 43 heterozygotes for one of three rare pathogenic LPL variants, in contemporary nomenclature known as p.Gly215Glu, p.Prol234Leu and p.Asp277Asn. They noted that those with abdominal obesity had higher mean triglyceride levels vs. nonobese heterozygotes vs. normal controls: 7.1 vs. 3.5 vs. 1.5 mmol/l, respectively [18]. Similar to Wilson et al. [17], they also found that plasma LPL activity was reduced by half in heterozygotes but varied widely and could not reliably differentiate heterozygotes from normal controls. In a follow-up analysis of 90 heterozygotes for pathogenic LPL variants, these authors reported that 29% had normal triglyceride levels while 13% had severe hypertriglyceridemia [19].
In a report from 2000 of the extended family of a 5-year-old Austrian child with FCS due to compound heterozygous pathogenic LPL variants, eight heterozygotes were identified [20]. LPL activity in the postheparin plasma of the heterozygotes ranged from 49 to 79% of that from normal controls. Three heterozygotes (37.5%) had normal triglycerides, three (37.5%) had mild-to-moderate hypertriglyceridemia and two (25%) had severe hypertriglyceridemia.
Thus, findings from studies prior to 2000 of families and cohorts consistently showed that heterozygotes for LPL deficiency had reduced but widely variable LPL activity with no clear threshold to differentiate from the normal range; a wide range of untreated triglyceride levels, including normal triglycerides, mild-to-moderate and severe hypertriglyceridemia in 15–25, 35–65 and 10–25% of carriers, respectively; and an important influence of secondary factors such as increasing age, male sex, obesity, impaired glucose tolerance and diabetes on phenotype [16–20].
POST-GENOMIC STUDIES OF HETEROZYGOUS LIPOPROTEIN LIPASE DEFICIENCY IN COHORTS WITH HYPERTRIGLYCERIDEMIA
In 2007, we performed Sanger sequencing of coding regions and intron-exon boundaries of LPL along with APOA5 and APOC2 genes in 110 nondiabetic lipid clinic patients with severe hypertriglyceridemia compared with 472 age and sex-matched normolipidemic controls [21]. Among patients with severe hypertriglyceridemia, we found seven heterozygotes for pathogenic LPL variants (6.4%) compared to zero controls (P < 0.00001). We found that mean plasma triglyceride level was not different between LPL variant heterozygotes and the overall hypertriglyceridemia cohort (33.1 vs. 32.6 mmol/l, respectively), although triglyceride response to fibrates was reduced in heterozygotes [21].
In a follow-up Sanger sequencing project in 2010, we found that 10% of 438 individuals with mean TG 14.6 mmol/l and 2.4% of 327 normolipidemic controls with mean TG 1.2 mmol/l had a heterozygous rare variant in LPL (P < 0.0001) [22]. Heterozygote frequencies were 8.1 vs. 13.2% in severe and mild-to-moderate hypertriglyceridemia subgroups, respectively [23]. The findings indicated that heterozygous LPL pathogenic variants are present among healthy controls with normal triglyceride levels; and predispose to expression of both mild-to-moderate and severe hypertriglyceridemia. Consistent with this interpretation, an observational study from the Netherlands showed that 7/43 (16.3%) patients with severe hypertriglyceridemia (i.e. Frederickson type 5 hyperlipoproteinemia) had heterozygous pathogenic variants in LPL[15].
Subsequently, we used targeted next-generation DNA sequencing in 563 patients with severe hypertriglyceridemia and found that 33 (5.9%) had a heterozygous rare pathogenic variant in LPL compared to 0.6% (3/503) of normal controls (P < 0.0001) [24]. Consistent with these findings, in the COMPASS trial of volanesorsen, which enrolled patients with MCS (mean triglycerides of 14.2 mmol/l), 11.4% (13/114) of patients randomized had heterozygous rare pathogenic variants in LPL[25]. For context, 49% (55/114) of COMPASS patients had a high polygenic score for triglycerides compared to 5.0% of controls (P < 0.0001). There were no between-genotype differences in triglyceride reduction with volanesorsen, which was nearly 70% across all groups [25].
Bashir et al. [26▪▪] have recently reported observations from a United Kingdom cohort of 74 genetically or clinically defined FCS patients and 80 MCS patients with severe hypertriglyceridemia. Among the latter group, 20% (16/80) were heterozygous for a pathogenic or likely pathogenic variant in LPL, while 37.5% (30/80) were heterozygous for a pathogenic variant in any of the five FCS causal genes. MCS patients with heterozygous variants had no difference in maximal triglyceride levels compared to nonheterozygote MCS patients (39.9 vs. 39.6 mmol/l, respectively) [26▪▪]. However, heterozygotes tended to show more severe pancreatitis metrics, poorer response to lipid-lowering therapy and higher prevalence of both hypertension and diabetes. The authors concluded that the clinical and metabolic phenotype in MCS patients with heterozygous variants was intermediate in severity between variant-negative MCS patients and FCS patients [26▪▪].
Spagnuolo et al. [12▪▪] from our center recently reported results from 182 MCS patients with severe hypertriglyceridemia. Of these, 4.3% (8/182) were heterozygous for a pathogenic or likely pathogenic variant in LPL. Overall, 17.5% (32/182) had a heterozygous variant in one of the five FCS genes (i.e. the mono-allelic MCS subgroup), while 39.6% (72/182) had a high polygenic score for triglycerides [12▪▪]. MCS patients with heterozygous rare variants compared to polygenic MCS patients had higher mean peak triglycerides (34.5 vs. 22.7 mmol/l). Interestingly, 4.9% (9/182) of MCS patients had both a heterozygous variant and a high polygenic score. Although numbers were small, these individuals appeared to have a more severe hypertriglyceridemia phenotype than others. An important subgroup of this MCS cohort were 8.8% (16/182) with ‘refractory’ or ‘persistent’ hypertriglyceridemia, defined as having no recorded triglyceride value less than 10 mmol/l. Of these, 37.5% (6/16) had a heterozygous pathogenic variant in an FCS gene, compared with 15.7% (26/166) of other MCS subgroups (P = 0.04) [12▪▪]. Thus, MCS patients heterozygous LPL deficiency appear to be somewhat more severely affected clinically than other genetic subgroups.
LPL HETEROZYGOSITY IN OTHER DYSLIPIDEMIA PHENOTYPES
Heterozygotes for LPL deficiency are also seen in clinical cohorts with other types of dyslipidemia. For instance, in 3.0% (4/134) of patients with mild-to-moderate hypertriglyceridemia (mean triglyceride 4.7 mmol/l) had a heterozygous rare pathogenic variant in LPL compared to 0.6% (3/503) of normal controls (P < 0.01) [27]. Furthermore, 12/259 (4.6%) of patients with combined hyperlipidemia (mean total cholesterol of 7.3 mmol/l and mean triglyceride of 4.4 mmol/l) had a heterozygous rare pathogenic variant in LPL compared to 0.6% (3/503) of normal controls (P < 0.01) [28]. In addition, in a multiethnic cohort, we showed that Hispanic, East Asian and European severe HTG patients were 2.5 to 5-times more likely to carry a rare heterozygous FCS-related variant compared to ancestry-matched controls, with heterozygous LPL variants accounting for the majority [29]. Finally, patients with heterozygous large partial deletions of the LPL gene had a more severe clinical phenotype than those with heterozygous missense variants or polygenic risk [24].
LONGITUDINAL PHENOTYPE OF PATIENTS WITH HETEROZYGOUS LPL RARE VARIANTS
We evaluated inter-individual variability of triglycerides over time in 15 heterozygotes for pathogenic LPL variants who were followed in lipid clinic for a mean of 10.3 years [30▪▪]. One heterozygote had normal triglycerides for the entire observation period, while four had at least one normal triglyceride reading. Most heterozygotes fluctuated between mild-to-moderate and severe HTG: five patients had only mild-to-moderate hypertriglyceridemia, while six patients had at least one instance of severe hypertriglyceridemia [30▪▪]. Of the total of 203 triglyceride measurements from these 15 heterozygous patients, 14.8, 67.0 and 18.2% were in the normal, mild-to-moderate and severe hypertriglyceridemia ranges, respectively [30▪▪]. This confirmed that the heterozygous LPL deficiency phenotype varies widely both within and between patients. Heterozygosity confers susceptibility to a range of triglyceridemic phenotypes, with severity depending on secondary factors [30▪▪]. Similar findings were observed for individuals with heterozygous APOA5 variants who were followed longitudinally [31▪,32].
LPL VARIANT HETEROZYGOSITY AND ATHEROSCLEROSIS RISK
In addition to susceptibility to hypertriglyceridemia, heterozygotes for LPL deficiency may also be predisposed to atherosclerotic cardiovascular disease (ASCVD). For instance, in a population-based study, Khera et al. [33] evaluated 46 891 individuals (51% female, mean age 50 years) who had undergone LPL gene sequencing. Of these, 188 individuals (0.40%) were heterozygous for a pathogenic LPL variant. Compared with 46 703 noncarriers, the 188 heterozygotes had slightly higher plasma triglyceride levels by 0.22 mmol/l (P < 0.001) as well as a higher risk of coronary artery disease (odds ratio = 1.84; 95% confidence interval, 1.35–2.51; P < 0.001). The authors commented that further research is needed to validate and define causal mechanisms underlying increased ASCVD risk in heterozygous LPL deficiency [33].
WHAT HAPPENS MECHANISTICALLY IN HETEROZYGOUS LIPOPROTEIN LIPASE DEFICIENCY?
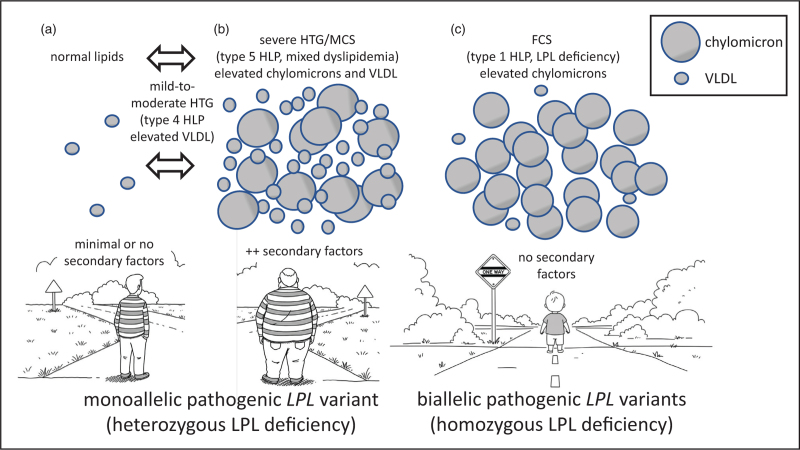
In contrast to biallelic FCS, the pathogenesis of chylomicronemia in heterozygous LPL deficiency is complex (See Fig. 1). In FCS, biallelic loss-of-function pathogenic variants in LPL or co-factors result in essentially absent plasma lipolytic capacity, which is easily saturated, for instance by the post prandial surge of chylomicrons. Because of this severe blockade in processing, remnant particles are not produced, resulting in deficiency of exogenous fat substrate for delivery to the liver for repackaging in hepatic triglyceride-rich lipoproteins, that is VLDL. This explains the relative deficiency in FCS patients of VLDL and its terminal product of remodeling, LDL [8]. Isolated chylomicron excess is the classic lipoprotein disturbance in FCS, previously called hyperlipoproteinemia type 1 or LPL deficiency [8].
FIGURE 1.
Schematic overview of biochemical consequences of heterozygous and homozygous LPL deficiency. (a) An adult carrier of a single copy of pathogenic LPL gene variant is shown, with heterozygosity signified by the striped sweater. In the absence of secondary factors – such as increasing age, obesity, poor diet, inactivity, alcohol use, impaired glucose tolerance, insulin resistance, diabetes or use of triglyceride-raising medications – such an individual often shows normal plasma triglyceride levels, carried within VLDL and without fasting chylomicronemia. In this scenario, the genetically compromised lipolytic capacity is unstressed and sufficient to manage the influx of triglyceride (TG)-rich lipoproteins. Bi-directional arrows signify that such individuals can transition to mild-to-moderate hypertriglyceridemia (HTG), formerly known as hyperlipoproteinemia (HLP) type 4 with increased VLDL but not chylomicrons, with increasing age and accumulation of secondary factors. The bidirectionality signifies that individuals in this state can revert to normal with lifestyle modification, diet and existing medications. But also possible is progression to (b), namely multifactorial severe HTG or multifactorial chylomicronemia syndrome (MCS), formerly known as hyperlipoproteinemia (HLP) type 5 with increased VLDL and chylomicrons. This phenotype occurs in a heterozygote who has significant secondary factors. Chylomicrons accumulate secondarily as compromised lipolytic capacity becomes saturated with liver-derived VLDL. (c) A young patient with biallelic pathogenic LPL variants (with homozygosity signified by the solid sweater) has familial chylomicronemia syndrome (FCS), formerly known as hyperlipoproteinemia (HLP) type 1 or LPL deficiency. Lipolytic capacity is essentially absent from birth. Chylomicrons cannot be catabolized and accumulate pathologically. The blockade prevents dietary triglyceride from reaching the liver, reducing production of VLDL.
In contrast, in heterozygous LPL deficiency, total lipolytic capacity is reduced but not absent and, in most circumstances, allows processing of triglyceride-rich lipoproteins of both exogenous (i.e. chylomicrons) and endogenous (i.e. VLDL) origin, producing remnants of each species that undergo normal removal [8]. However, in the presence of secondary stresses, such as age, poor diet, alcohol, obesity, insulin resistance, dysglycemia and diabetes, there is both hepatic over-production of triglyceride-rich lipoproteins and suppression of genetically compromised lipolysis. This results initially in accumulation of VLDL, which manifests as hyperlipoproteinemia type 4. If the metabolic stress is sufficiently severe, eventually the excessive VLDL will saturate LPL capacity producing a state that is functionally similar to the genetic deficiency of LPL seen in FCS [8]. As a result, chylomicrons begin to accumulate. The elevations in both chylomicrons and VLDL are characteristic of hyperlipoproteinemia type 5; the elevated VLDL is associated with elevated plasma apo B, which is a key feature that differentiates MCS from FCS, in which apo B is relatively low [34,35▪▪]. Control of secondary factors in the heterozygote often relieves lipase saturation, allowing the reduced but viable lipolytic capacity the opportunity to catabolize accumulated triglyceride-rich lipoproteins, with improvement in the lipid profile [8,36▪]. These pathogenic concepts are summarized in Fig. 1.
CONCLUSION
Research over the past four decades has shown that heterozygotes for LPL deficiency have increased risk of developing hypertriglyceridemia, but this is variable between individuals, even those with the identical genotype from the same family. The possible lipid profile ranges from normal to mild-to-moderate to severe hypertriglyceridemia in 15–25, 35–65 and 10–25% of carriers of heterozygous rare pathogenic variants in LPL, respectively. In longitudinal follow-up of patients with heterozygous LPL deficiency, the phenotype can vary from normal to severe in the same patient. In an adult with severe hypertriglyceridemia, it is more likely that a DNA sequencing report will reveal heterozygosity for a pathogenic LPL variant rather than biallelic (i.e. homozygous or compound heterozygous) variants that are diagnostic for FCS. Specifically, 15–25 vs. 1–6% of adults with severe hypertriglyceridemia will have monoallelic vs. biallelic variants, respectively, and 60–80% are in the LPL gene.
Because many patients with a heterozygous pathogenic variant in LPL have normal LPL activity, it could be argued that the term ‘heterozygous LPL deficiency’ is a misnomer. Heterozygosity for a pathogenic LPL variant is a genetic subtype of both mild-to-moderate and severe hypertriglyceridemia or MCS. Furthermore, compared to authentic monogenic FCS, the triglyceride level in MCS is responds better to diet, lifestyle and lipid-lowering drugs [8,36▪]. Nevertheless, a small but clinically relevant subgroup of severe hypertriglyceridemia or MCS individuals remains refractory to treatment [12▪▪]. In these patients with recalcitrant hypertriglyceridemia, more potent biological therapies such as those indicated for FCS might be appropriate to reduce pancreatitis risk [37–39]. Other phenotypes associated with heterozygosity for a pathogenic LPL variant might include combined hyperlipidemia [16,28] and also susceptibility to ASCVD [40], although more research is needed to confirm these associations.
Acknowledgements
None.
Financial support and sponsorship
R.A.H. is supported by the Jacob J. Wolfe Distinguished Medical Research Chair, the Edith Schulich Vinet Research Chair and the Martha G. Blackburn Chair in Cardiovascular Research.
Conflicts of interest
R.A.H. reports consulting fees from Akcea/Ionis, Amgen, Arrowhead, HLS Therapeutics, Medison, Novartis, Pfizer, Regeneron, Sanofi and Ultragenyx.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Brown EE, Sturm AC, Cuchel M, et al. Genetic testing in dyslipidemia: a scientific statement from the National Lipid Association. J Clin Lipidol 2020; 14:398–413. [DOI] [PubMed] [Google Scholar]
- 2.Berberich AJ, Hegele RA. The complex molecular genetics of familial hypercholesterolaemia. Nat Rev Cardiol 2019; 16:9–20. [DOI] [PubMed] [Google Scholar]
- 3.Dron JS, Hegele RA. Genetics of hypertriglyceridemia. Front Endocrinol (Lausanne) 2020; 11:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazarte J, Hegele RA. Can genetic testing help in the management of dyslipidaemias? Curr Opin Lipidol 2020; 31:187–193. [DOI] [PubMed] [Google Scholar]
- 6.Hegele RA, Borén J, Ginsberg HN, et al. Rare dyslipidaemias, from phenotype to genotype to management: a European Atherosclerosis Society task force consensus statement. Lancet Diabetes Endocrinol 2020; 8:50–67. [DOI] [PubMed] [Google Scholar]
- 7.Laufs U, Parhofer KG, Ginsberg HN, Hegele RA. Clinical review on triglycerides. Eur Heart J 2020; 41:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chait A, Eckel RH. The chylomicronemia syndrome is most often multifactorial: a narrative review of causes and treatment. Ann Intern Med 2019; 170:626–634. [DOI] [PubMed] [Google Scholar]
- 9.Hegele RA, Berberich AJ, Ban MR, et al. Clinical and biochemical features of different molecular etiologies of familial chylomicronemia. J Clin Lipidol 2018; 12:920–927. [DOI] [PubMed] [Google Scholar]
- 10.Berberich AJ, Ouédraogo AM, Shariff SZ, et al. Incidence, predictors and patterns of care of patients with very severe hypertriglyceridemia in Ontario, Canada: a population-based cohort study. Lipids Health Dis 2021; 20:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paquette M, Bernard S, Hegele RA, Baass A. Chylomicronemia: differences between familial chylomicronemia syndrome and multifactorial chylomicronemia. Atherosclerosis 2019; 283:137–142. [DOI] [PubMed] [Google Scholar]
- 12▪▪.Spagnuolo CM, Wang J, McIntyre AD, et al. Comparison of patients with familial chylomicronemia syndrome and multifactorial chylomicronemia syndrome. J Clin Endocrinol Metab 2024; dgae613.doi: 10.1210/clinem/dgae613. [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; An observational study comparing genetically proven FCS and MCS patients from Canada showing numerous differences in clinical, biochemical and genetic attributes.
- 13.Dron JS, Wang J, Cao H, et al. Severe hypertriglyceridemia is primarily polygenic. J Clin Lipidol 2019; 13:80–88. [DOI] [PubMed] [Google Scholar]
- 14.Dron JS, Wang J, McIntyre AD, et al. Six years’ experience with LipidSeq: clinical and research learnings from a hybrid, targeted sequencing panel for dyslipidemias. BMC Med Genomics 2020; 13:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Surendran RP, Visser ME, Heemelaar S, et al. Mutations in LPL, APOC2, APOA5, GPIHBP1 and LMF1 in patients with severe hypertriglyceridaemia. J Intern Med 2012; 272:185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babirak SP, Iverius PH, Fujimoto WY, Brunzell JD. Detection and characterization of the heterozygote state for lipoprotein lipase deficiency. Arteriosclerosis 1989; 9:326–334. [DOI] [PubMed] [Google Scholar]
- 17.Wilson DE, Emi M, Iverius PH, et al. Phenotypic expression of heterozygous lipoprotein lipase deficiency in the extended pedigree of a proband homozygous for a missense mutation. J Clin Invest 1990; 86:735–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Julien P, Vohl MC, Gaudet D, et al. Hyperinsulinemia and abdominal obesity affect the expression of hypertriglyceridemia in heterozygous familial lipoprotein lipase deficiency. Diabetes 1997; 46:2063–2068. [DOI] [PubMed] [Google Scholar]
- 19.Julien P, Gagné C, Murthy MR, et al. Dyslipidemias associated with heterozygous lipoprotein lipase mutations in the French-Canadian population. Hum Mutat 1998; Suppl 1:S148–S153. [DOI] [PubMed] [Google Scholar]
- 20.Hölzl B, Kraft HG, Wiebusch H, et al. Two novel mutations in the lipoprotein lipase gene in a family with marked hypertriglyceridemia in heterozygous carriers. Potential interaction with the polymorphic marker D1S104 on chromosome 1q21-q23. J Lipid Res 2000; 41:734–741. [PubMed] [Google Scholar]
- 21.Wang J, Cao H, Ban MR, et al. Resequencing genomic DNA of patients with severe hypertriglyceridemia (MIM 144650). Arterioscler Thromb Vasc Biol 2007; 27:2450–2455. [DOI] [PubMed] [Google Scholar]
- 22.Johansen CT, Wang J, Lanktree MB, et al. Excess of rare variants in genes identified by genome-wide association study of hypertriglyceridemia. Nat Genet 2010; 42:684–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansen CT, Wang J, Lanktree MB, et al. An increased burden of common and rare lipid-associated risk alleles contributes to the phenotypic spectrum of hypertriglyceridemia. Arterioscler Thromb Vasc Biol 2011; 31:1916–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dron JS, Wang J, McIntyre AD, et al. Partial LPL deletions: rare copy-number variants contributing towards severe hypertriglyceridemia. J Lipid Res 2019; 60:1953–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gouni-Berthold I, Alexander VJ, et al. Efficacy and safety of volanesorsen in patients with multifactorial chylomicronaemia (COMPASS): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 2021; 9:264–275. [DOI] [PubMed] [Google Scholar]
- 26▪▪.Bashir B, Downie P, Forrester N, et al. Ethnic diversity and distinctive features of familial versus multifactorial chylomicronemia syndrome: insights from the UK FCS National Registry. Arterioscler Thromb Vasc Biol 2024; 44:2334–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]; An important observational study of FCS patients in the UK and controls with MCS comparing clinical, biochemical and genetic attributes.
- 27.Dron JS, Wang J, McIntyre AD, et al. The polygenic nature of mild-to-moderate hypertriglyceridemia. J Clin Lipidol 2020; 14:28–34. [DOI] [PubMed] [Google Scholar]
- 28.Gill PK, Dron JS, Berberich AJ, et al. Combined hyperlipidemia is genetically similar to isolated hypertriglyceridemia. J Clin Lipidol 2021; 15:79–87. [DOI] [PubMed] [Google Scholar]
- 29.Gill PK, Dron JS, Dilliott AA, et al. Ancestry-specific profiles of genetic determinants of severe hypertriglyceridemia. J Clin Lipidol 2021; 15:88–96. [DOI] [PubMed] [Google Scholar]
- 30▪▪.Perera SD, Wang J, McIntyre AD, et al. The longitudinal triglyceride phenotype in heterozygotes with LPL pathogenic variants. J Clin Lipidol 2023; 17:87–93. [DOI] [PubMed] [Google Scholar]; An observational study of patients with heterozygous pathogenic variants in LPL showing wide intra and inter-individual variability in triglyceride levels over time.
- 31▪.Perera SD, Wang J, McIntyre AD, Hegele RA. Variability of longitudinal triglyceride phenotype in patients heterozygous for pathogenic APOA5 variants. J Clin Lipidol 2023; 17:659–665. [DOI] [PubMed] [Google Scholar]; An observational study of patients with heterozygous pathogenic variants in APOA5 showing wide intra and inter-individual variability in triglyceride levels over time.
- 32.Perera SD, Hegele RA. Genetic variation in apolipoprotein A-V in hypertriglyceridemia. Curr Opin Lipidol 2024; 35:66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khera AV, Won HH, Peloso GM, et al. Association of rare and common variation in the lipoprotein lipase gene with coronary artery disease. JAMA 2017; 317:937–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Dea LSL, MacDougall J, Alexander VJ, et al. Differentiating familial chylomicronemia syndrome from multifactorial severe hypertriglyceridemia by clinical profiles. J Endocr Soc 2019; 3:2397–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35▪▪.Hegele RA, Ahmad Z, Ashraf A, et al. Development and validation of clinical criteria to identify familial chylomicronemia syndrome (FCS) in North America. J Clin Lipidol 2024; S1933-2874:00251-4. [DOI] [PubMed] [Google Scholar]; A validated scoring system to differentiate genetically confirmed FCS patients from other forms of severe hypertriglyceridemia using easily available clinical variables to infer genotype.
- 36▪.Berberich AJ, Hegele RA. A modern approach to dyslipidemia. Endocr Rev 2022; 43:611–653. [DOI] [PMC free article] [PubMed] [Google Scholar]; A review of how modern concepts of pathophysiology, genetics, diagnosis and treatment can be integrated into management of patients with dyslipidemia.
- 37.Tramontano D, Bini S, D’Erasmo L, Arca M. Recent apolipoprotein CIII trials. Curr Opin Lipidol 2022; 33:309–318. [DOI] [PubMed] [Google Scholar]
- 38.Bashir B, Ferdousi M, Durrington P, Soran H. Pancreatic and cardiometabolic complications of severe hypertriglyceridaemia. Curr Opin Lipidol 2024; 35:208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan DC, Watts GF. ANGPTL3 and apoC-III inhibitors for treating hypertriglyceridemia in context: horses for courses? Curr Opin Lipidol 2024; 35:101–109. [DOI] [PubMed] [Google Scholar]
- 40.Ganda OP. Triglyceride-rich lipoproteins, remnant-cholesterol, and atherosclerotic cardiovascular disease. Curr Opin Lipidol 2023; 34:105–113. [DOI] [PubMed] [Google Scholar]