Abstract
Objective: To investigate the effects of different doses of dexmedetomidine on delirium and hemodynamics after plasma resection of adenoids in children. Methods: A retrospective analysis was conducted on the clinical data of 80 children who underwent plasma adenoidectomy of tonsil at the Pediatric Hospital of Fudan University from January 2022 to December 2023. The patients were divided into normal saline group, 0.1 μg/kg dexmedetomidine group, and 0.5 μg/kg dexmedetomidine group according to the dose of dexmedetomidine injected intravenously. Hemodynamic changes, modified Yale Preoperative Anxiety Scale (mYPAS-SF) scores, pharyngeal pain (at rest and during swallowing), coagulation function, and postoperative adverse reactions were compared at T0, 10 min after dexmedetomidine pumping (T1), extubation (T2), recovery (T3), 2 h after returning to ward (T4), 12 h after returning to ward (T5) and 24 h after returning to ward (T6), respectively. Results: There were no significant differences in extubation time, recovery time, or unguardianship time among the three groups (P>0.05). The incidence of postoperative delirium was significantly lower in the 0.1 μg/kg dexmedetomidine group and 0.5 μg/kg dexmedetomidine group compared to the normal saline group (P<0.05), with the 0.5 μg/kg group showing better results. At T2, heart rate (HR) and mean arterial pressure (MAP) levels were significantly lower in 0.1 μg/kg group and 0.5 μg/kg dexmedetomidine group than those in normal saline group (P<0.05). The mYPAS-SF score was significantly lower in the 0.1 μg/kg group and 0.5 μg/kg dexmedetomidine groups than that of the normal saline group at T3, T4 and T5 (P<0.05). The score of pharyngeal pain during swallowing was significantly lower in the 0.5 μg/kg dexmedetomidine group at T5 than that of the normal saline group and 0.1 μg/kg dexmedetomidine group (P<0.05). Coagulation values (PT, APTT, and TT) were significantly altered 36 hours post-surgery, with PT, APTT, and TT increasing, while fibrinogen (FIB) decreased (P<0.05). Postoperative nasopharyngeal hemorrhage occurred in one case and nausea/vomiting in two cases in the saline group. No anesthesia-related adverse reactions were observed in the dexmedetomidine group. Conclusion: Intravenous injection of 0.5 μg/kg dexmedetomidine during plasma resection of tonsillar adenoids can reduce the incidence of postoperative delirium, stabilize hemodynamics, relieve postoperative anxiety and pharyngeal pain, with minimal impacts on coagulation function. Additionally, it reduces the incidence of adverse reactions, making it a promising option for clinical use.
Keywords: Dexmedetomidine, plasma resection of tonsil adenoid, postoperative delirium
Introduction
Tonsil adenoidectomy is one of the most common surgical procedures in children [1]. This procedure is primarily performed to treat sleep-disordered breathing caused by tonsil and adenoid hypertrophy, recurrent throat inflammation, and other related complications. Sevoflurane, a fast acting and well-tolerated inhaled anesthetic, is commonly used in pediatric anesthesia due to its advantages, including stable hemodynamics, low liver toxicity, and a short anesthesia recovery period [2]. However, postoperative monitoring has shown that sevoflurane is associated with a high incidence of postoperative delirium in children, with reported rates ranging from 18% to as high as 80% [3,4]. Postoperative delirium is characterized by transient emotional agitation, irritability, inconsolable crying, disorientation, cognitive changes, memory impairment, hallucinations and delusions [5]. It is a common postoperative complication that can lead to prolonged hospital stays, increased medical costs, poor prognosis, and even chronic brain syndrome, which may increase the rate of disability and mortality in children. Dexmedetomidine, a highly selective α2-adrenergic receptor agonist, has analgesic, sedative and sympathetic inhibitory properties, serving as a new anesthetic drug widely used in clinical practice in recent years [6]. Previous studies have indicated that dexmedetomidine can help prevent postoperative delirium when used perioperatively [7]. However, the optimal intraoperative dose of dexmedetomidine remains unclear, limiting its broader clinical application. This study retrospectively analyzed the clinical data of children undergoing tonsillectomy and adenoidectomy to evaluate the effects of different doses of dexmedetomidine on postoperative delirium, analgesia, and hemodynamics. By identifying the optimal dose of dexmedetomidine, we hope to provide a more effective anesthetic management strategy, reduce the incidence of postoperative delirium, improve the quality of postoperative recovery, and reduce the economic burden on family and society.
Materials and methods
Case selection
The clinical data of 80 children who underwent plasma tonsil adenoidectomy at the Pediatric Hospital of Fudan University from January 2022 to December 2023 were retrospectively analyzed. Inclusion criteria: (1) All patients met the surgical indications for tonsil adenoidectomy; (2) Age between 4-10; (3) Preoperative anesthesia risk assessment classified as American Society of Anesthesiologists (ASA) [8] Grade I-II; (4) No known allergic reaction to dexmedetomidine. Exclusion criteria: (1) Contraindications to surgery or refusal by family members; (2) Active respiratory inflammation or congenital diseases that may affect surgery; (3) Intellectual development disorders; (4) Congenital heart disease.
The patients were divided into three groups according to the intravenous dose of dexmedetomidine administered: normal saline group (30 cases), 0.1 μg/kg dexmedetomidine group (25 cases), and 0.5 μg/kg dexmedetomidine group (25 cases). This study was reviewed and approved by the Ethics Committee of the Pediatric Hospital of Fudan University.
Methods
All children in the three groups were given routine preoperative preparation. After entering the operating room, 8% sevoflurane and 100% oxygen were administered, followed by an intravenous injection of 2 μg/kg fentanyl once access was established. Endotracheal intubation was performed when the minimum alveolar concentration (MAC) of sevoflurane reached 2.3. During the surgery, the normal saline group was injected with 10 ml of normal saline via intravenous pump, 0.1 μg/kg dexmedetomidine group received 0.1 μg/kg of dexmedetomidine intravenously (Jiangsu Hengrui Pharmaceutical Co., LTD., Approval number: National Medicine Approval number H20090248), and 0.5 μg/kg dexmedetomidine group was injected with dexmedetomidine at 0.5 μg/kg. The infusion was administered over 10 minutes. All patients were maintained with sevoflurane (1.0-2.0 MAC) and remifentanil (0.2 μg/(kg·min)) during the procedure. After the operation, patients were transferred to the recovery room, and the tracheal tube was removed once spontaneous breathing had fully recovered. Oxygen was administered by mask at 5 L/min.
Data collection and outcome measurement
The clinical data, including gender, age, and weight, were collected from the hospital’s electronic medical records.
Primary outcome measures
(1) The Pediatric Anesthesia Emergence Delirium (PAED) scale was used to evaluate postoperative delirium within 60 minutes. The scale includes five items, each rated on a 1-5 scale, resulting in a total score ranging from 0 to 20 (Table 1). A score of ≥10 indicates postoperative delirium, and a score ≥15 indicates severe delirium. (2) Heart rate (HR) and mean arterial pressure (MAP) were recorded at the following time points: operation room entering (T0), 10 min after dexmedetomidine infusion (T1), extubation (T2), and upon awakening (T3). Blood oxygen saturation (SpO2) was measured at T0, T2 and T3. SpO2 monitoring at T1 was not performed due to artificial ventilation.
Table 1.
Delirium scale during anesthesia recovery in children
| Items | Not at all | Just a little | Quite a bit | Very much | Extremely |
|---|---|---|---|---|---|
| Eye contact | 4 | 3 | 2 | 1 | 0 |
| Purposefulness of actions | 4 | 3 | 2 | 1 | 0 |
| Awareness of surroundings | 4 | 3 | 2 | 1 | 0 |
| Restlessness | 0 | 1 | 2 | 3 | 4 |
| Inconsolable | 0 | 1 | 2 | 3 | 4 |
Secondary outcome measures
(1) The time of postoperative extubation, recovery time, and unguardianship time were recorded. (2) Postoperative anxiety score: The modified Yale Preoperative Anxiety Scale (mYPAS-SF) was used to assess the anxiety at T0, T3, 2 h after returning to the ward (T4), and 12 h after returning to the ward (T5). The score range from 23 to 100, with higher scores indicating more severe anxiety. (3) The degree of pharyngeal pain was assessed using the Visual Analog Scale (VAS) [9] at T3, T4, T5, and 24 h post-ward transfer (T6). The VAS ranges from 0 (no pain) to 10 (severe pain), with higher scores indicating more severe pain. Pain was assessed both at rest and during swallowing. (4) Coagulation function: A 5 mL fasting venous blood sample was collected immediately after surgery and 36 h after surgery. Serum was separated and stored in a -80°C. Prothrombin time (PT), activated partial thrombin time (APTT), thrombin time (TT), and fibrinogen (FIB) were detected using ELISA. (5) Adverse reactions, including nausea and vomiting, delayed recovery, and nasopharyngeal hemorrhage, were recorded for each group.
Statistical methods
SPSS 22.0 software was used for statistical analysis. Normal distribution data were expressed as mean ± standard deviation (X̅±s). One-way analysis of variance (ANOVA) was used for multi-group comparison, and LSD test was used for pairwise comparisons. For data collected at different time points, repeated measures ANOVA was used. Counted data were expressed as n (%), and comparison between groups was analyzed using the chi-square test. P<0.05 was considered significant.
Results
Comparison of basic Information among groups
There were no significant differences in age, gender, or weight among the three groups (P>0.05), as shown in Table 2.
Table 2.
Comparison of baseline data
| Group | Gender | Age | Body weight (kg) | |
|---|---|---|---|---|
|
| ||||
| Male | Female | |||
| Normal saline group | 17 (56.67) | 13 (43.33) | 6.80±1.52 | 25.50±5.12 |
| 0.1 μg/kg dexmedetomidine group | 14 (56.00) | 11 (44.00) | 6.88±1.76 | 25.80±5.22 |
| 0.5 μg/kg dexmedetomidine group | 15 (60.00) | 10 (40.00) | 6.76±1.59 | 25.76±4.96 |
| F/χ2 value | 0.095 | 0.036 | 0.029 | |
| P value | 0.953 | 0.965 | 0.972 | |
Comparison of operational metrics among groups
There were no significant differences in extubation time, recovery time, or unguardianship time among the three groups (P>0.05), as shown in Figure 1.
Figure 1.
Comparison of surgical conditions among the three groups. A: Postoperative extubation time; B: Recovery time; C: Unguardianship time.
Comparison of postoperative delirium incidence among groups
As shown in Figure 2, a total of 20 cases developed postoperative delirium among all the children included in the study. Compared to the normal saline group, the incidence of postoperative delirium was lower in the 0.1 μg/kg and 0.5 μg/kg dexmedetomidine groups (P<0.05), and there was no significant difference between the 0.5 μg/kg and 0.1 μg/kg dexmedetomidine groups (P>0.05).
Figure 2.
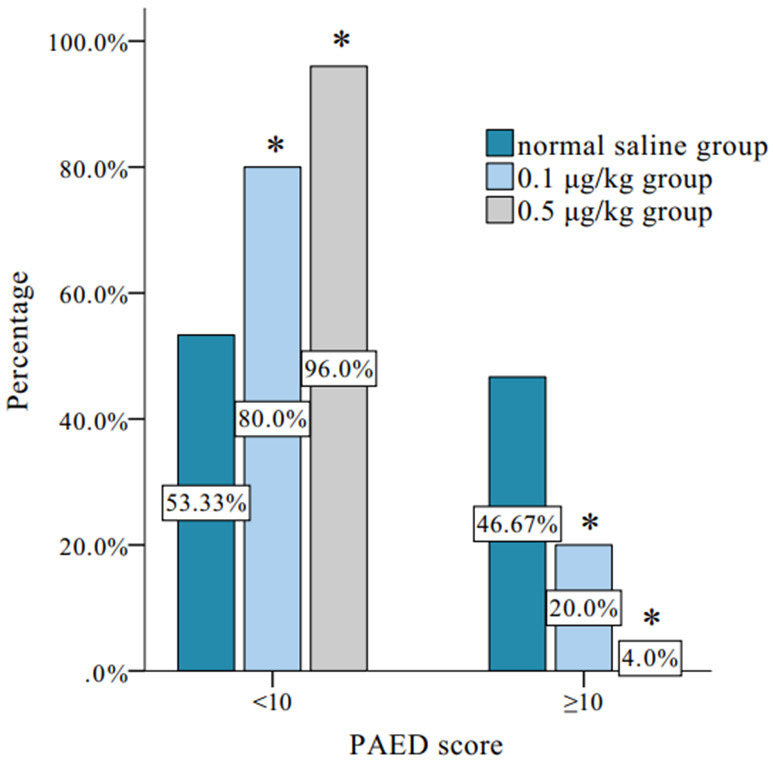
Comparison of incidence of postoperative delirium. Note: *Compared to the normal saline group, *P<0.05; PAED: pediatric anesthesia emergence delirium.
Comparison of hemodynamic parameters at different time points among groups
There were significant differences in HR and MAP among three groups (FIntergroup=5.534, 10.830, P<0.05), but there was no significant difference in the change trend over time (FTime=2.780, 2.995, P=0.064, 0.052). There was no significant interaction between time and group for HR and MAP (FInteraction=1.508, 0.890, P=0.175, 0.502).
There was no significant difference in SpO2 among the three groups (FIntergroup=2.292, P=0.103), and no significant difference in the trend of SPO2 over time (FTime=0.197, P=0.822). There was no significant interaction for SpO2 between the groups and time (Fintergroup=0.089, P=0.822) (P=0.986).
The levels of HR and MAP in 0.1 μg/kg and 0.5 μg/kg dexmedetomidine groups at T2 were significantly lower than those of the normal saline group (P<0.05), as shown in Figure 3.
Figure 3.

Comparison of hemodynamic indexes at different time points among the three groups. Note: A: HR (heart rate); B: MAP (Mean arterial pressure); C: SpO2 (Blood oxygen saturation); *Compared to the normal saline group, *P<0.05; T0: entering the operation room; T1: 10 min after pumping dexmedetomidine; T2: extubation; T3: awakening from anesthesia.
Comparison of mYPAS-SF scores at different time points among groups
There were significant differences in mYPAS-SF scores among the three groups (FIntergroup=63.570, P<0.001), as well as significant differences in the trends at different time points (FTime=29.120, P<0.001). There was a significant interaction between group and time (FInteraction=4.421, P=0.003). LSD test was revealed that the mYPAS-SF scores at T3, T4 and T5 was significantly lower in the 0.1 μg/kg and 0.5 μg/kg dexmedetomidine groups compared to the normal saline group. Additionally, the 0.5 μg/kg dexmedetomidine group had lower mYPAS-SF scores than the 0.1 μg/kg dexmedetomidine group (P<0.05), as shown in Figure 4.
Figure 4.
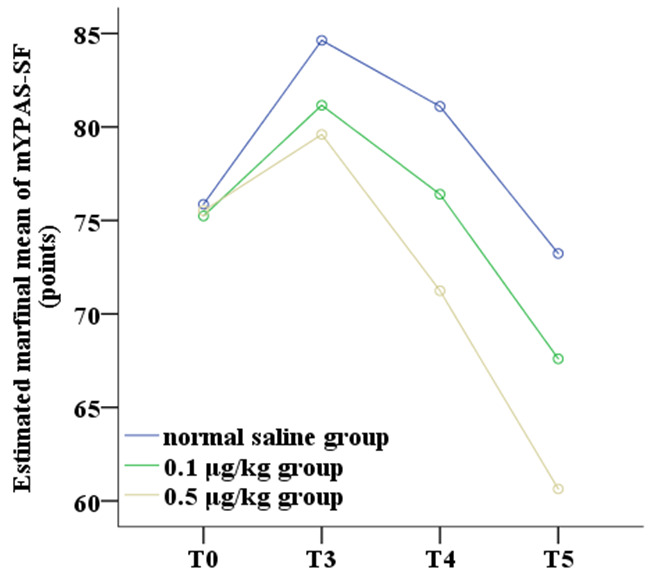
Comparison of mYPAS-SF scores at different time points among the three groups. Note: T0: entering the operation room; T3: awakening from anesthesia; T4: 2 hours post-return to the ward; T5: 12 hours post-return to the ward; mYPAS-SF: Simplified Chinese version of the Modified Yale Preoperative Anxiety Scale.
Comparison of pharyngeal pain scores at different time points among groups
There were no significant differences in pharyngeal pain at rest among the three groups (FIntergroup=0.211, P=0.973), but significant differences were observed in the trend of change over time (FTime=91.240, P<0.001), with an interaction between group and time (Finteraction=8.773, P=0.973, P<0.001) (P<0.001). Regarding pharyngeal pain during swallowing, there were significant differences among the groups (Fintergroup=2.135, P=0.049), and a significant difference in the trend of change over time (Ftime=32.84, P<0.001). A significant interaction between group and time was also observed (FInteraction=13.390, P<0.001). LSD test revealed that the pharyngeal pain score for swallowing in the 0.5 μg/kg dexmedetomidine group was significantly lower than in both the normal saline group and the 0.1 μg/kg dexmedetomidine group at T5 (P<0.05), as shown in Figure 5.
Figure 5.

Comparison of pharyngodynia pain at different time points among the three groups. Note: *Compared to the normal saline group, *P<0.05; A: VAS scores at resting; B: VAS scores during swallowing; T3: awakening from anesthesia; T4: 2 hours post-return to the ward; T5: 12 hours post-return to the ward; T6: 24 hours post-return to the ward; VAS: Visual analog rating scale.
Comparison of coagulation function indexes among groups
PT, APPT, and TT increased, while FIB decreased at 36 h after surgery in all three groups. Statistically significant differences were observed between the values immediately after surgery and those at 36 h after surgery (P<0.05) (Table 3).
Table 3.
Comparison of coagulation function indexes at different time points after operation
| Item | Normal saline group | 0.1 μg/kg dexmedetomidine group | 0.5 μg/kg dexmedetomidine group | F value | P value | |
|---|---|---|---|---|---|---|
| PT (s) | Immediately after surgery | 13.37±0.49 | 13.44±0.51 | 13.40±0.50 | 0.148 | 0.863 |
| 36 h after surgery | 15.37±0.49* | 15.40±0.50* | 15.48±0.51* | 0.362 | 0.697 | |
| APPT (s) | Immediately after surgery | 36.47±0.51 | 36.44±0.51 | 36.48±0.51 | 0.040 | 0.960 |
| 36 h after surgery | 39.53±0.51* | 39.48±0.51* | 39.60±0.50* | 0.353 | 0.704 | |
| TT (s) | Immediately after surgery | 17.43±0.50 | 17.48±0.51 | 17.60±0.50 | 0.772 | 0.465 |
| 36 h after surgery | 19.43±0.50* | 19.52±0.51* | 19.60±0.50* | 0.748 | 0.477 | |
| FIB (g/L) | Immediately after surgery | 3.60±0.50 | 3.60±0.50 | 3.64±0.49 | 0.056 | 0.946 |
| 36 h after surgery | 1.60±0.50* | 1.52±0.51* | 1.48±0.51* | 0.406 | 0.668 | |
Note: Compared to immediately after surgery;
P<0.05.
PT: prothrombin time; APTT: activated partial thrombin time; TT: thrombin time; FIB: fibrinogen.
Comparison of the occurrence of adverse reactions among groups
In the normal saline group, there were 1 case of nasopharyngeal hemorrhage and 2 cases of nausea, all of which recovered after symptomatic treatment. No significant adverse reactions were observed in the 0.1 μg/kg or 0.5 μg/kg dexmedetomidine groups (Table 4).
Table 4.
Comparison of the occurrence of adverse reactions among groups
| Group | Nasopharyngeal hemorrhage | Nausea | Delayed recovery | Total |
|---|---|---|---|---|
| Normal saline group | 1 (3.33) | 2 (6.67) | 0 (0.00) | 3 (10.00) |
| 0.1 μg/kg dexmedetomidine group | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| 0.5 μg/kg dexmedetomidine group | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| χ2 value | 3.526 | |||
| P value | 0.105 |
Discussion
Postoperative delirium is a common side effect of sevoflurane in pediatric anesthesia, with reported incidences as high as 80% [10,11]. Postoperative delirium is an acute brain dysfunction associated with multiple neurotransmitter abnormalities [12]. Factors such as dysregulation of the norepinephrine system, the use of anticholinergic drugs and opioids, and sleep deprivation may increase the risk of delirium by disrupting neurotransmitter balance [13,14]. In addition, hypoxemia and hypoxia of the central nervous system have been implicated in the development of postoperative delirium [15].
Dexmedetomidine, a highly selective α2-adrenergic receptor agonist, binds to transmembrane G-protein-bound adrenergic receptors in peripheral tissues (α2A), as well as the brain and spinal cord (α2B, α2C). It exerts dose-dependent effects, including analgesia, anti-anxiety, and anti-sympathetic activities, without causing respiratory depression. Furthermore, dexmedetomidine promotes a physiological sleep-wake cycle and offers neuroprotective benefits, with minimal anticholinergic effects [16]. These characteristics suggest that dexmedetomidine may be particularly advantageous in preventing postoperative delirium.
Based on the above findings, this study retrospectively analyzed the clinical data of 80 children undergoing plasma tonsil adenoidectomy to further explore the effects of different doses of dexmedetomidine on postoperative delirium, analgesia, and hemodynamics. The results showed no significant differences in extubation time, recovery time, or unguardianship time among the three groups. However, the pain score in the 0.5 μg/kg dexmedetomidine group was significantly lower than in the 0.1 μg/kg dexmedetomidine group and normal saline group. This result is consistent with the study of Yang et al. [17], which suggested that dexmedetomidine effectively provides an analgesic effect during tonsillar adenoidectomy under general anesthesia, facilitating smooth recovery without significantly affecting extubation, recovery, or release time. The analgesic effect of dexmedetomidine may be attributed to its activation of α2-adrenergic receptors in the dorsal horn of the spinal cord, reducing the release of substance P. In addition, the incidence of postoperative delirium was significantly lower in the dexmedetomidine groups that of the normal saline group, with the 0.5 μg/kg dexmedetomidine group showing the most favorable outcome. This aligns with the findings of Shi et al. [18], indicating that 0.5 μg/kg dexmedetomidine can effectively prevent postoperative delirium without affecting recovery or extubation times. The underlying mechanism may involve dexmedetomidine’s action on α2-adrenergic receptors, which helps regulate neurotransmitter levels in the brain, thereby reducing the occurrence of delirium. Meta-analysis studies [19,20] on dexmedetomidine for postoperative delirium prevention also support these results, showing that intraoperative use of dexmedetomidine reduces delirium incidence compared to other sedatives. In conclusion, the results of this study suggest that while dexmedetomidine does not significantly impact operation-related time measures, it can effectively reduce the incidence of postoperative delirium in pediatric patients undergoing tonsil adenoidectomy.
In this study, the hemodynamic values of children during and after plasma adenoidectomy were analyzed at different time points. The results revealed that at T2, both MAP and HR levels in the 0.1 μg/kg and 0. 5 μg/kg dexmedetomidine groups were significantly lower than those of the normal saline group. This can be explained by the fact that after dexmedetomidine infusion in dexmedetomidine groups, α2-adrenergic receptors in peripheral vascular smooth muscle were activated, leading to vasoconstriction. The mechanism may be that dexmedetomidine can simultaneously inhibit the release of α1 and α2-epinephrine. While α1-epinephrine is mainly distributed in the presynaptic membrane and vascular smooth muscle, and inhibition of α1-epinephrine-mediated norepinephrine release results in vasodilation [21], thereby reducing both MAP and HR in children. There was no significant difference in MAP or HR levels between 0.1 μg/kg and 0.5 μg/kg dexmedetomidine groups at T0, T1, T2 and T3, indicating that dexmedetomidine could effectively maintain hemodynamic stability during anesthesia. Furthermore, there were no significant differences in SpO2 among the three groups after operation. Therefore, the results of this study indicate that dexmedetomidine can effectively stabilize hemodynamics during anesthesia, which has important implications for perioperative hemodynamic management.
In this study, we observed distinct differences in the pharyngeal pain scores between the resting and swallowing states, indicating that these two types of pain should be evaluated separately when assessing postoperative pain. The pain scores peaked at 2 h after returning to the ward, and gradually decreased thereafter. This is consistent with a previous study [22], suggesting that dexmedetomide at different concentrations similarly improves resting pharyngeal pain in children. The results also showed that 0.5 μg/kg dexmedetomidine solution was more effective than both the normal saline group and 0.1 μg/kg dexmedetomidine group in reducing pharyngeal pain during swallowing. The study also found that the mYPAS-SF score changed over time, following a pattern of increasing and then decreasing. Early postoperative anxiety was significantly improved in the 0.5 μg/kg dexmedetomidine group. Therefore, the results of this study indicate that an appropriate dose of dexmedetomidine can not only prevent postoperative delirium, but also effectively relieve postoperative pain and anxiety, thereby improving the overall quality of recovery in children.
The monitoring results of postoperative coagulation function showed that, at 36 h after operation, PT, APTT, and TT were higher, and FIB was lower compared to the immediate postoperative values. There was no statistical difference in the coagulation function indexes among the three groups at either time point. This suggests that none of the treatments affected coagulation function, which aligns with the findings of Zhang Feng et al. [23]. In addition, the results of this study showed that no adverse reactions occurred in the two dexmedetomidine groups. In contrast, the normal saline group experienced 1 case of nasopharyngeal hemorrhage and 2 cases of nausea. This suggests that dexmedetomidine has a favorable safety profile, consistent with the findings of Devroe et al. [24].
Despite the promising results, there are some limitations to this study. First, as a retrospective analysis, there may have been selection and information bias. Second, the sample size was relatively small, which could have affected the generalizability and reliability of the results. Furthermore, the long-term effects of dexmedetomidine, particularly on neurocognitive development in children, were not assessed and warrant further investigation.
In summary, intravenous injection of 0.5 μg/kg dexmedetomidine during plasma adenoidectomy of tonsils significantly reduces the incidence of postoperative delirium, alleviates early postoperative pain, effectively maintains hemodynamic stability. It does not affect recovery time or respiratory function and has a low incidence of adverse reactions.
Disclosure of conflict of interest
None.
References
- 1.Hao X, Wang JZ, Qu H. Effect of resection of adenoids and/or tonsil on the immune indexes in children with obstructive sleep apnea hypopnea syndrome. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019;54:830–836. doi: 10.3760/cma.j.issn.1673-0860.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Abdallah BM, Elshoeibi AM, ElTantawi N, Arif M, Hourani RF, Akomolafe AF, Hamwi MN, Mahmood FR, Saracoglu KT, Saracoglu A, Chivese T. Comparison of postoperative pain in children after maintenance anaesthesia with propofol or sevoflurane: a systematic review and meta-analysis. Br J Anaesth. 2024;133:93–102. doi: 10.1016/j.bja.2024.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehrotra S. Postoperative anaesthetic concerns in children: Postoperative pain, emergence delirium and postoperative nausea and vomiting. Indian J Anaesth. 2019;63:763–770. doi: 10.4103/ija.IJA_391_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X, Lin C, Chen S, Huang Y, Cheng Q, Yao Y. Remimazolam for the prevention of emergence delirium in children following tonsillectomy and adenoidectomy under sevoflurane anesthesia: a randomized controlled study. Drug Des Devel Ther. 2022;16:3413–3420. doi: 10.2147/DDDT.S381611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urbánek L, Urbánková P, Satinský I, Trávníček T, Penka I, Hruda J. Postoperative delirium. Rozhl Chir. 2023;102:381–386. doi: 10.33699/PIS.2023.102.10.381-386. [DOI] [PubMed] [Google Scholar]
- 6.Eizaga Rebollar R, García Palacios MV, Fernández Riobó MC, Torres Morera LM. Dexmedetomidine and perioperative analgesia in children. Rev Esp Anestesiol Reanim (Engl Ed) 2022;69:487–492. doi: 10.1016/j.redare.2022.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Fondeur J, Escudero Mendez L, Srinivasan M, Hamouda RK, Ambedkar B, Arzoun H, Sahib I, Mohammed L. Dexmedetomidine in prevention of postoperative delirium: a systematic review. Cureus. 2022;14:e25639. doi: 10.7759/cureus.25639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apfelbaum JL, Hagberg CA, Connis RT, Abdelmalak BB, Agarkar M, Dutton RP, Fiadjoe JE, Greif R, Klock PA, Mercier D, Myatra SN, O’Sullivan EP, Rosenblatt WH, Sorbello M, Tung A. 2022 American society of anesthesiologists practice guidelines for management of the difficult airway. Anesthesiology. 2022;136:31–81. doi: 10.1097/ALN.0000000000004002. [DOI] [PubMed] [Google Scholar]
- 9.Goudman L, Pilitsis JG, Billet B, De Vos R, Hanssens K, Billot M, Roulaud M, Rigoard P, Moens M. The level of agreement between the numerical rating scale and visual analogue scale for assessing pain intensity in adults with chronic pain. Anaesthesia. 2024;79:128–138. doi: 10.1111/anae.16151. [DOI] [PubMed] [Google Scholar]
- 10.Menser C, Smith H. Emergence agitation and delirium: considerations for epidemiology and routine monitoring in pediatric patients. Local Reg Anesth. 2020;13:73–83. doi: 10.2147/LRA.S181459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu M, Yuan Q, Yang Q, Song W, Yu Y, Luo Y, Xiong X, Yu G. Risk factors and incidence of postoperative delirium after cardiac surgery in children: a systematic review and meta-analysis. Ital J Pediatr. 2024;50:24. doi: 10.1186/s13052-024-01603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudy M, Saller T. Postoperative delirium in the recovery room. Anaesthesiologie. 2023;72:459–466. doi: 10.1007/s00101-023-01281-5. [DOI] [PubMed] [Google Scholar]
- 13.Xiao MZ, Liu CX, Zhou LG, Yang Y, Wang Y. Postoperative delirium, neuroinflammation, and influencing factors of postoperative delirium: a review. Medicine (Baltimore) 2023;102:e32991. doi: 10.1097/MD.0000000000032991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mevorach L, Forookhi A, Farcomeni A, Romagnoli S, Bilotta F. Perioperative risk factors associated with increased incidence of postoperative delirium: systematic review, meta-analysis, and grading of recommendations assessment, development, and evaluation system report of clinical literature. Br J Anaesth. 2023;130:e254–e262. doi: 10.1016/j.bja.2022.05.032. [DOI] [PubMed] [Google Scholar]
- 15.Ahrens E, Tartler TM, Suleiman A, Wachtendorf LJ, Ma H, Chen G, Kendale SM, Kienbaum P, Subramaniam B, Wagner S, Schaefer MS. Dose-dependent relationship between intra-procedural hypoxaemia or hypocapnia and postoperative delirium in older patients. Br J Anaesth. 2023;130:e298–e306. doi: 10.1016/j.bja.2022.08.032. [DOI] [PubMed] [Google Scholar]
- 16.Abd Ellatif SE, Mowafy SMS, Shahin MA. Ketofol versus dexmedetomidine for preventing postoperative delirium in elderly patients undergoing intestinal obstruction surgeries: a randomized controlled study. BMC Anesthesiol. 2024;24:1. doi: 10.1186/s12871-023-02378-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang W, Liu S, Zhao RY, Cui XL, Hu J, Jin Z. Effects of different doses of dexmedetomidine on postoperative analgesia and hemodynamics after tonsillectomy in children. Chin J Med. 2019;54:331–334. [Google Scholar]
- 18.Shi M, Miao S, Gu T, Wang D, Zhang H, Liu J. Dexmedetomidine for the prevention of emergence delirium and postoperative behavioral changes in pediatric patients with sevoflurane anesthesia: a double-blind, randomized trial. Drug Des Devel Ther. 2019;13:897–905. doi: 10.2147/DDDT.S196075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li P, Li LX, Zhao ZZ, Xie J, Zhu CL, Deng XM, Wang JF. Dexmedetomidine reduces the incidence of postoperative delirium after cardiac surgery: a meta-analysis of randomized controlled trials. BMC Anesthesiol. 2021;21:153. doi: 10.1186/s12871-021-01370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petre MA, Levin DN, Englesakis M, Maynes JT, Pechlivanoglou P, Aoyama K. Dexmedetomidine vs. total intravenous anaesthesia in paediatric emergence delirium: a network meta-analysis. Eur J Anaesthesiol. 2021;38:1111–1123. doi: 10.1097/EJA.0000000000001490. [DOI] [PubMed] [Google Scholar]
- 21.He H, Cui Q, Chen H, Huang X, Wang S, Yu T, Feng J, Shao Y. The effect of intranasal dexmedetomidine on emergence delirium prevention in pediatric ambulatory dental rehabilitation under general anesthesia: a randomized clinical trial. Drug Des Devel Ther. 2023;17:3563–3570. doi: 10.2147/DDDT.S427291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou HG, Xie CL, Xue L, Xu HM, Li JY. Effect of different doses of dexmedetomidine on hemodynamics and postoperative sore throat in children undergoing tonsil adenoidectomy. Academic J Chin Pla Med School. 2020;41:988–991. 996. [Google Scholar]
- 23.Zhang F. Effects of flurbiprofen axetil and dexmedetomidine combined anesthesia on agitation and pain during recovery period of children undergoing tonsillectomy and adenoidectomy. J Molecular Diagnosis and Therapy. 2023;15:630–634. [Google Scholar]
- 24.Devroe S, Devriese L, Debuck F, Fieuws S, Cools B, Gewillig M, Van de Velde M, Rex S. Effect of xenon and dexmedetomidine as adjuncts for general anesthesia on postoperative emergence delirium after elective cardiac catheterization in children: study protocol for a randomized, controlled, pilot trial. Trials. 2020;21:310. doi: 10.1186/s13063-020-4231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


