Abstract
The oncomutation lysine 27-to-methionine in histone H3 (H3K27M) is frequently identified in tumors of patients with diffuse midline glioma-H3K27 altered (DMG-H3K27a). H3K27M inhibits the deposition of the histone mark H3K27me3, which affects the maintenance of transcriptional programs and cell identity. Cells expressing H3K27M are also characterized by defects in genome integrity, but the mechanisms linking expression of the oncohistone to DNA damage remain mostly unknown. In this study, we demonstrate that expression of H3.1K27M in the model plant Arabidopsis thaliana interferes with post-replicative chromatin maturation mediated by the H3.1K27 methyltransferases ATXR5 and ATXR6. As a result, H3.1 variants on nascent chromatin remain unmethylated at K27 (H3.1K27me0), leading to ectopic activity of TONSOKU (TSK/TONSL), which induces DNA damage and genomic alterations. Elimination of TSK activity suppresses the genome stability defects associated with H3.1K27M expression, while inactivation of specific DNA repair pathways prevents survival of H3.1K27M-expressing plants. Overall, our results suggest that H3.1K27M disrupts the chromatin-based mechanisms regulating TSK activity, which causes genomic instability and may contribute to the etiology of DMG-H3K27a.
Subject terms: Double-strand DNA breaks, Histone post-translational modifications
Cancer mutations in histone H3 cause genome instability, but the mechanisms remain to be elucidated. Here, the authors show in a plant model that the oncohistone H3.1K27M disrupts the TSK/TONSL-H3.1 DNA repair pathway.
Introduction
DMG-H3K27a, a type of brain cancer that mostly affects children, is characterized by a very poor prognosis with fewer than 10% of patients surviving more than two years after diagnosis1. Approximately 80% of DMG patients are carriers of a somatic K27M mutation in one of the histone H3 genes2,3. The H3K27M mutation can occur in genes encoding different histone H3 variants: replication-dependent H3.1 or H3.2 (H3.1/H3.2 variants hereafter referred to as H3.1), and replication-independent H3.33,4. Initial work has shown that expression of H3.1K27M or H3.3K27M leads to a decrease of histone H3 lysine 27 tri-methylation (H3K27me3) by inhibiting the activity of the H3K27 methyltransferase POLYCOMB REPRESSIVE COMPLEX 2 (PRC2)5–8. In cancer cells, H3.1K27M and H3.3K27M are both expressed amid a much larger contingent of wild-type H3.1 and H3.3 proteins, but the mutated histones inhibit PRC2 in a dominant-negative manner5,7, which accounts for the drastic loss of H3K27me3 observed in DMG-H3K27a cells.
A large majority of the work on oncogenic H3K27M mutations has centered on the consequences of decreased H3K27me3 levels and the subsequent effects on transcriptional regulation. However, the disruption of other cellular activities by H3K27M may have been overlooked, as PRC2 activity in mammals and Drosophila melanogaster is responsible for all levels of methylation at H3K279–11. Mono- and di-methylation at H3K27 (H3K27me1/2), which are together much more abundant than H3K27me3 in mouse embryonic stem cells10, have cellular functions that are independent of H3K27me310,12,13. In line with this, it has been confirmed that levels of H3K27me1/2 at specific loci are also reduced by the H3K27M oncomutation in H3K27M-expressing cell lines14. Consequently, there is a major increase in unmethylated histone H3 at K27 (H3K27me0), to the point where it can become the most dominant form of H3K2715. Importantly, PRC2 inhibition by H3K27M leads to a large increase of K27me0 on both H3.1 and H3.3 variants15.
Lysine 27 of newly synthesized H3.1 proteins is unmethylated prior to incorporation of H3.1 into chromatin during DNA replication16,17. Recently, it was shown in the model plant Arabidopsis thaliana (Arabidopsis) that H3.1K27me0 is specifically required for the recruitment of the conserved DNA repair protein TONSOKU (TSK; known as TONSL in animals) to replication forks18. In mammals, TONSL has been shown to initiate homologous recombination-mediated resolution of stalled or broken replication forks19–22. DNA repair via TSK/TONSL needs to be tightly regulated in dividing cells of plants and animals by histone methyltransferases, which methylate post-replicative chromatin to prevent TSK/TONSL activity outside of stalled or broken replication forks18,23. In the absence of the post-replicative chromatin maturation step that inhibits TSK/TONSL, genome instability is observed in a TSK/TONSL-dependent manner18. Chromatin-based regulation of TSK in plants has been shown to depend on the redundant activity of the H3.1K27 mono-methyltransferases ATXR5 and ATXR6 (ATXR5/6)24,25, which mono-methylate H3.1K27 to prevent binding of TSK to H3.1 through its conserved tetratricopeptide repeat (TPR) domain18,25. In Arabidopsis, where unregulated TSK activity has been extensively studied, this results in many phenotypes, including widespread genomic amplification of heterochromatic sequences (hereafter referred to as heterochromatin amplification), DNA damage, and loss of transcriptional silencing18,25–27.
Genomic instability is a defining characteristic of many cancer cells28, including H3.1K27M and H3.3K27M tumors29–33. The frequent co-occurrence of specific secondary mutations associated with H3.1K27M and H3.3K27M32, and the defects in genome integrity in cancer cells harboring these mutations, suggest a model where expression of H3K27M generates a DNA error-prone environment that can induce and/or accelerate tumorigenesis33. Accordingly, specific DNA repair pathways and DNA repair pathway choice have been shown to be affected by H3K27M30,33, but a direct link between the oncohistone and misregulation of DNA repair proteins has not yet been established. In this work, we demonstrate that expression of H3.1K27M disrupts the regulation of the TSK/TONSL-H3.1 DNA repair pathway. Using Arabidopsis as a model system for K-to-M mutations on H334, we show that expression of H3.1K27M induces DNA damage. This effect of H3.1K27M is due to its ability to block the activity of ATXR5/6, which results in an increase of H3.1K27me0 in chromatin. Similarly to atxr5/6 mutants, increased levels of H3.1K27me0 induce heterochromatin amplification and loss of transposon silencing. By inactivating TSK in H3.1K27M-expressing plants, we were able to confirm that ectopic activity of the TSK-H3.1 DNA repair pathway is responsible for disrupting genome integrity. In addition, loss of the DNA repair proteins MRE11 and RAD51 in H3.1K27M-expressing plants results in synthetic lethality, thus suggesting specific vulnerabilities in cells expressing H3.1K27M. We discuss the implications of these results obtained in the Arabidopsis model and how they could translate to a better understanding of the etiology of cancers characterized by H3.1K27M expression.
Results
H3.1K27M expression induces genomic instability in Arabidopsis
To explore the biological mechanisms affected by an H3K27M mutation, we created transgenic Arabidopsis plants expressing H3.1K27M under a native H3.1 promoter (H3.1K27M) in the wild-type Columbia-0 (Col-0) background, which also expresses a normal set of endogenous histone H3.1 from five H3.1 genes35. As a control, we also created transgenic plants expressing wild-type H3.1 (H3.1 WT). RNA-seq analysis showed that H3.1K27M transcripts corresponded to ~20% of all H3.1 transcripts (Fig. 1A), indicating that a majority of H3.1 proteins in the transgenic plants are wild type. This approach provides the opportunity to evaluate the phenotypic and molecular effects caused by H3.1K27M expression in a model system where H3-variant-specific effects on genome stability and DNA repair have been previously characterized18.
Fig. 1. H3.1K27M expression induces developmental defects and DNA damage in Arabidopsis.
A Relative abundance of H3.1 gene transcripts. B Morphological phenotypes of Col-0, H3.1 WT, and H3.1K27M plants. C Survival rates of Col-0, H3.1 WT, and H3.1K27M plants. D Representative comet assay images. E Quantification of DNA percentage in the comet tails. Horizontal bars indicate the mean. One-way ANOVA with Tukey’s multiple comparison test: ns = not significantly different (n = 28 nuclei). F Relative root length of seedlings grown on ½ MS plates with 100 µg/ml MMS compared to the average root length of seedlings grown on plates without MMS. Each dot represents one individual T1 plant (n = 18). Horizontal bars indicate the mean. SEM is shown. One-way ANOVA with Tukey’s multiple comparison test: ns = not significantly different. G Representative image of histochemical staining of reporter plants for GUS activity. Blue areas indicate that a functional reporter gene has been restored via a somatic recombination event. Three independent experiments were performed with similar results. H RNA-seq data showing relative transcript levels of 158 DNA damage response genes (Supplementary Data 1) measured by transcripts per million (TPM). The center line denotes the median value (50th percentile) and the box contains the 25th to 75th percentiles of the dataset. The whiskers mark the 5th and 95th percentiles. One-way ANOVA with Kruskal-Wallis test: ns = not significantly different. Source data are provided as a Source Data file.
The H3.1K27M transgenic plants demonstrated significant developmental defects compared to Col-0 and wild-type H3.1 controls, exhibiting stunted growth and much smaller and narrower leaves compared to the control plants (Fig. 1B and Supplementary Fig. 1A). The germination and survival rates of H3.1K27M plants were severely affected, with most plants dying before day 20 (Fig. 1C). In addition, the H3.1K27M plants displayed an increase in anthocyanin content, which is commonly associated with cellular stress (Supplementary Fig. 1B)36. To assess for the presence of DNA damage, we conducted comet assays and found a significant increase in tail DNA percentage for H3.1K27M-expressing plants compared to Col-0 and H3.1 WT controls, which is indicative of DNA breaks (Fig. 1D, E). We also found that H3.1K27M plants were hypersensitive to the genotoxic effects of methyl methanesulfonate (MMS) (Fig. 1F and Supplementary Fig. 1C). We then used the disrupted beta-glucuronidase (uidA/GUS) system to directly assess the frequency of homologous recombination events in our transgenic plants37. In this system, homology-directed repair is required to reconstitute a gene that can produce a functional GUS protein. Our results showed that GUS activity was much stronger in H3.1K27M transgenic plants, indicating higher levels of homology-directed repair at the GUS gene (Fig. 1G). In accord with these results, RNA-seq experiments in Col-0, H3.1 WT, and H3.1K27M plants showed that many DNA damage response genes are upregulated when H3.1K27M is expressed (Fig. 1H and Supplementary Data 1). Taken together, these results indicate that the expression of H3.1K27M in Arabidopsis generates DNA damage.
H3.1K27M expression inhibits the histone methyltransferase activity of PRC2 and ATXR5/6
In human cells, H3K27M mutations interfere with PRC2 in a dominant-negative manner, which prevents the spreading of H3K27me35,7. Given the high degree of conservation between plant and animal histone H3 variants and PRC2 complexes35,38, we hypothesized that expression of H3K27M in plants would also block PRC2 activity. To verify this, we performed immunostaining and observed a drastic decrease of H3K27me3 in nuclei from H3.1K27M-expressing plants compared to Col-0 and H3.1 WT (Fig. 2A). We also assessed H3K27me3 levels by chromatin immunoprecipitation sequencing combined with exogenous chromatin spike-in (ChIP-Rx) to normalize the H3K27me3 signal between samples. We observed a large decrease of H3K27me3 in H3.1K27M plants compared to Col-0 and H3.1 WT (Fig. 2B and Supplementary Fig. 2A). Together, these results suggest that expression of the oncohistone H3.1K27M disrupts PRC2 activity in plants similarly to human cells.
Fig. 2. H3.1K27M-expressing plants exhibit loss of ATXR5/6-mediated H3.1K27me1.
A Staining of leaf nuclei with an anti-H3K27me3 antibody and DAPI. Three independent experiments were performed with similar results. B ChIP-seq profiles and heatmaps of normalized H3K27me3 signal at H3K27me3-marked genes in reference-adjusted reads per million (RRPM). TSS transcription start site; TES transcription end site. C ChIP-Rx normalized H3K27me1 signal using 100 kb windows over chromosome 5. The pericentromeric region is shown in gray. Staining of leaf nuclei with anti-H3K27M and anti-H3K27me1 (D, E) or anti-H3K27me3 antibodies (F) and DAPI. Three independent experiments were performed with similar results. Relative Western blot quantification of H3K27me1 (G) and H3K27me3 (H) levels in leaf total histones. Mean and SEM are shown. One-way ANOVA with Tukey’s multiple comparison test: ns = not significantly different (n = 3 independent experiments). I Representative in vitro histone methyltranferase assay using ATXR6 and recombinant nucleosomes containing plant H3.1-Strep. Nucleosomes containing H3.1K27M or H3.1K27A (i.e., H3.1 inhibitor) were added to the reactions. J Quantification of the relative amount of 3H-SAM incorporated into the H3.1-Strep substrate. Bars represent the mean. SEM is shown. One-way ANOVA with Tukey’s multiple comparison test: ns = not significantly different (n = 3 independent experiments). Source data are provided as a Source Data file.
In plants, H3K27 methylation is also catalyzed by the SET domain-proteins ATXR5/6, which specifically mono-methylate the H3.1 variant24,25. K-to-M mutations on histone H3 have been shown to inhibit the activity of multiple histone methyltransferases39, so we predicted that expression of H3.1K27M in Arabidopsis may not only inhibit PRC2, but also ATXR5/6. To validate this hypothesis, we first performed ChIP-Rx analysis and observed a large decrease in H3K27me1 signal over heterochromatin in H3.1K27M-expressing plants compared to Col-0 and H3.1 WT plants (Fig. 2C and Supplementary Fig. 2B). Immunofluorescence staining using an H3K27M antibody showed that the mutated histone is enriched at chromocenters (Fig. 2D), as expected because H3.1 proteins are concentrated within heterochromatic domains in somatic cells of Arabidopsis40,41. Staining with an H3K27me1 antibody showed the characteristic enrichment of this histone mark at chromocenters in Col-0 and H3.1 WT plants42, but a drastic reduction in H3.1K27M-expressing plants (Fig. 2D). To demonstrate that the loss of H3K27me1 was due to ATXR5/6 inhibition, we generated transgenic plants expressing H3.1::H3.3K27M in the wild-type Col-0 background. As ATXR5/6 are unable to interact with H3.324, we hypothesized that they would not be inhibited by H3.3K27M, but that PRC2 complexes would be negatively impacted as they can interact with both H3.1 and H3.3 in plants. As expected, we found that the level of H3K27me1 in H3.1::H3.3K27M plants was similar to Col-0, indicating that the presence of H3.3K27M did not inhibit ATXR5/6 (Fig. 2E). We did, however, find that the level of H3K27me3 was reduced in these plants (Fig. 2F), thus confirming that PRC2 activity in Arabidopsis is disrupted by H3.3K27M. We also observed a large decrease of H3K27me1 in H3.1K27M-expressing plants, but not in H3.1::H3.3K27M plants, in total histone samples extracted from leaves (Fig. 2G and Supplementary Fig. 2C). In contrast, a reduction in H3K27me3 was seen in both H3.1K27M and H3.1::H3.3K27M plants (Fig. 2H and Supplementary Fig. 2C). Consistent with a global H3K27me3 decrease, H3.1::H3.3K27M plants have visible developmental growth defects, including small plant size and curled leaves (Supplementary Fig. 3A). However, they lack many of the phenotypes associated with H3.1K27M plants, such as low survival rate (Fig. 1C and Supplementary Fig. 3B), MMS hypersensitivity (Fig. 1F and Supplementary Fig. 3C, D), increased expression of the DNA repair gene BRCA1 (Supplementary Fig. 3E), and transcriptional de-repression of TSI (Supplementary Fig. 3F). These results suggest that the phenotypes are specific to expression of H3.1K27M.
Previous studies have shown that H3K27M acts in a dominant-negative manner by directly inhibiting the catalytic subunit (i.e., EZH2) of PRC25,7,43,44. Based on this, we hypothesized that the effect of H3.1K27M on H3K27me1 levels may work through a similar direct inhibitory mechanism on ATXR5/6. To test this, we performed in vitro histone methyltransferase (HMT) assays with and without H3.1K27M nucleosomes serving as potential ATXR6 inhibitors. Our results showed decreased levels of methylated H3.1 substrates when H3.1K27M nucleosomes were present in the reaction (Fig. 2I, J). In contrast, H3.1K27A (lysine 27-to-alanine) had a smaller inhibitory effect than H3.1K27M on ATXR6 (Fig. 2I, J), which is comparable to the effects of these two H3.1 mutants on mammalian PRC2 activity in vitro45. Overall, our data indicate that, in the presence of H3.1K27M, ATXR5/6-mediated mono-methylation of H3.1K27 is inhibited, leading to widespread loss of H3.1K27me1 in the Arabidopsis genome.
H3.1K27M induces genomic instability defects similar to atxr5/6 mutants
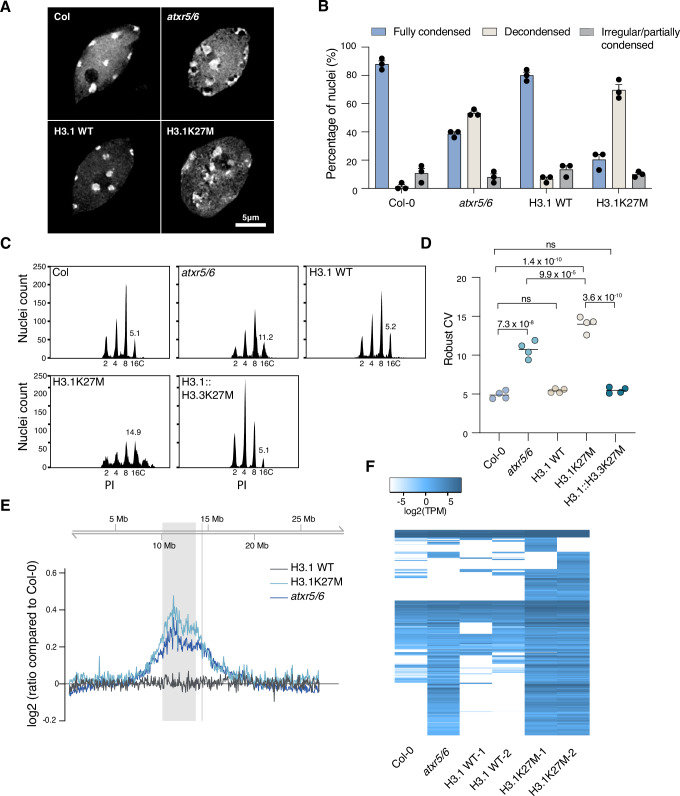
Loss of H3.1K27me1 in atxr5/6 mutants leads to various heterochromatic phenotypes, including chromocenter decondensation, heterochromatin amplification (i.e., an increase in the copy number of transposons and other repetitive elements) and loss of transposon silencing25–27. As H3.1K27M-expressing plants show a massive reduction of H3K27me1 (Fig. 2C, D), we hypothesized that they would have heterochromatic defects similar to atxr5/6 mutants. DAPI staining of nuclei from H3.1K27M-expressing plants revealed a hollowed sphere conformation of chromocenters (i.e., decondensed) characteristic of nuclei from atxr5/6 mutant plants (Fig. 3A, B)46. Flow cytometry analyses showed that H3.1K27M plants exhibit broader peaks corresponding to endoreduplicated nuclei, a phenotype associated with heterochromatin amplification and also observed in atxr5/6 mutants (Fig. 3C, D)26. In accord with this, whole-genome sequencing of H3.1K27M-expressing plants revealed that heterochromatic regions are amplified similarly to atxr5/6 mutants (Fig. 3E). Finally, RNA-seq analysis indicated transcriptional reactivation of transposable elements (TEs) normally silenced in a Col-0 background, like in atxr5/6 mutants (Fig. 3F).
Fig. 3. H3.1K27M induces heterochromatin defects similar to atxr5/6 mutants.
A Leaf interphase nuclei stained with DAPI. B Quantification of chromocenter appearance of nuclei from experiment in panel A. Mean and SEM are shown. Each dot represents a biological replicate for Col-0 and atxr5/6 and an independent T1 plant for H3.1 WT and H3.1K27M. For each replicate, 25 nuclei were assessed. C Flow cytometry profiles of leaf nuclei stained with propidium iodide (PI). Ploidy levels of the nuclei are shown below the peaks. The numbers above the 16C peaks indicate the robust coefficient of variation (CV). D Robust CV quantification of 16C peaks. Each dot represents a biological replicate. The mean is shown. One-way ANOVA with Tukey’s multiple comparison test: ns = not significantly different. E Chromosomal view (Chr 5) of DNA-seq reads. The pericentromeric region is highlighted in gray. F Heat map showing the relative expression levels of TEs induced in H3.1K27M plants (Supplementary Data 2). Source data are provided as a Source Data file.
In all our assays (Fig. 3A–F), we observed an increase in the magnitude of heterochromatic defects in H3.1K27M plants compared to atxr5/6 mutants. The well-characterized atxr5/6 double mutant used in our experiments is a hypomorphic mutant, as ATXR6 expression from the mutant allele is only reduced compared to wild-type plants25. We use this particular mutant for our studies because complete elimination of ATXR5 and ATXR6 activity is lethal in Arabidopsis47. The phenotypic variation between H3.1K27M-expressing plants and atxr5/6 mutants may be linked to the difference in H3.1K27me1 levels between the two genetic backgrounds. This hypothesis is supported by Western blot analyses showing that H3.1K27me1 levels are much lower in H3.1K27M-expressing plants than atxr5/6 mutants in two-week-old plants (Supplementary Fig. 4A, B). However, we should note that only H3.1K27me1 is impacted in atxr5/6 mutants, while both H3.1K27me1 and H3K27me3 are decreased in H3.1K27M plants. In summary, our results indicate that expression of H3.1K27M mimics the phenotypes of atxr5/6 mutants, and that in vivo levels of H3.1K27me1 may dictate the severity of these phenotypes.
H3.1K27M induces genomic instability in a TSK-dependent manner
In plants, ATXR5/6 methylate newly synthesized H3.1 variants inserted on chromatin to induce post-replicative chromatin maturation, a key regulatory step that prevents the activity of the DNA repair protein TSK by inhibiting its binding to chromatin18,24,25,48. TSK is recruited to nascent chromatin via its conserved TPR domain, which specifically interacts with H3.1 proteins unmethylated at K27 (H3.1K27me0)18. In the absence of post-replicative chromatin maturation via H3.1K27me1, TSK is thought to either remain associated with chromatin post-replication or interact with it de novo. Genomic instability in atxr5/6 mutants has been shown to be dependent on TSK activity18, thus suggesting that the phenotypes observed in H3.1K27M-expressing plants may similarly rely on this protein.
To investigate a potential link between TSK and the H3.1K27M-associated phenotypes, we introduced the H3.1K27M transgene into a tsk mutant background (T-DNA insertional mutant SALK_034207). We found that elimination of TSK activity partially suppressed the growth abnormalities and early lethality associated with H3.1K27M expression in the Col-0 background (Fig. 4A, B). Though the tsk/H3.1K27M plants remained smaller than Col-0, H3.1 WT, tsk and tsk/H3.1 WT plants, they were able to progress to the reproductive phase of growth, which was never observed when H3.1K27M was expressed in the Col-0 background (Supplementary Fig. 5A). Flow cytometry analyses of tsk/H3.1K27M plants showed suppression of heterochromatin amplification as represented by the loss of broad peaks and confirmed by whole-genome sequencing (Fig. 4C and Supplementary Fig. 5B, C). Chromocenter decondensation was also found to be suppressed in H3.1K27M-expressing plants lacking TSK (Fig. 4D). A possible mechanism of suppression is that H3K27me1 levels are restored to wild-type levels in a tsk mutant, however, this was ruled out by immunostaining and Western blot analyses (Fig. 4D, E and Supplementary Fig. 5D). We also tested if suppression of the phenotypes associated with loss of H3.1K27me1 was due to lower H3.1K27M transgene expression in tsk mutants. We performed RT-qPCR and found no significant difference between Col-0 and tsk (Supplementary Fig. 5E). It is interesting to note that complete suppression of the genomic instability phenotypes in tsk/H3.1K27M plants does not lead to plants morphologically resembling Col-0 (Fig. 4A and Supplementary Fig. 5A). This is likely due to the loss of H3K27me3 in these plants caused by inhibition of PRC2 (Fig. 4F). Similar phenotypes were observed in the H3.1::H3.3K27M and tsk/H3.1::H3.3K27M plants (Supplementary Fig. 5F, G), where H3K27me3 is reduced, but not H3K27me1 (Fig. 2E–H).
Fig. 4. H3.1K27M causes genomic instability by inducing ectopic TSK activity.
A Morphological phenotypes of Col-0, tsk, H3.1 WT, H3.1K27M, tsk/H3.1 WT, and tsk/H3.1K27M plants. B Survival rate curves. C Chromosomal view (Chr 5) of DNA-seq reads. The pericentromeric region is highlighted in gray. D Immunostaining of leaf nuclei with anti-H3K27M and anti-H3K27me1 antibodies. DNA is stained with DAPI. Three independent experiments were performed with similar results. E Western blot quantification showing relative H3K27me1 levels in total histones extracted from leaves. Mean and SEM are shown. One-way ANOVA with Tukey’s multiple comparison test: ns = not significantly different (n = 3 independent experiments). F Staining of leaf nuclei with an anti-H3K27me3 antibody and DAPI. Three independent experiments were performed with similar results. G Peptide pull-down assay using the TPR domain of TSK (TSKTPR) and GST-tagged histone peptides (aa 1 to 58). H Domain architecture of plant and animal TSK/TONSL. TPR Tetratricopeptide Repeats, ARD Ankyrin Repeat Domain, LRR Leucine-Rich Repeats. Pull-down assay with TPR + ARD domains of TONSL (TONSLTPR + ARD) (I) or the TONSL TPR domain only (TONSLTPR) (J) with GST-tagged histone peptides (aa 1 to 58). K Pull-down assay of TONSLTPR + ARD with biotinylated recombinant nucleosomes. All pull-down assays were performed at least three times with similar results. Source data are provided as a Source Data file.
Our findings suggest that H3.1K27M inhibits ATXR5/6 in a dominant-negative manner, which leads to an increase in K27me0 on wild-type H3.1 proteins. This likely results in chromatin binding of TSK outside of its normal spatial and temporal boundaries, and as a consequence, DNA damage. This model is supported by mass spectrometry analyses that showed an increase of H3.1K27me0 levels in plants expressing H3.1K27M compared to the H3.1 WT control (Supplementary Fig. 5H). It is also possible that H3.1K27M increases the interaction of TSK with H3.1, thus leading to DNA damage at genomic regions where H3.1K27M is inserted. Analysis of the structure of the TSKTPR-H3.1 complex does not support this mechanism, as H3.1K27 is positioned within a polar pocket that makes it unfavorable for binding hydrophobic entities such as the sidechain of methionine18. We verified this by performing in vitro binding assays and confirmed that the interaction of TSK with H3.1 is reduced when lysine 27 is replaced with methionine (Fig. 4G). We also conducted split-luciferase complementation assays and detected luminescence signals when TSKTPR-cLuc was coexpressed with H3.1 WT-nLuc, but not with H3.1K27M-nLuc (Supplementary Fig. 5I, J). Altogether, these findings support a model where, in plants, genomic instability is not due to a direct interaction between H3.1K27M nucleosomes and TSK, but instead arises from increased levels of H3.1K27me0 leading to ectopic TSK activity.
To assess if the inhibitory effect of H3.1K27M and H3.1K27 methylation on TSK binding is conserved in the mammalian TONSL ortholog, we performed binding assays using the complete histone interaction module of TONSL (TPR and ARD domains) or the TPR domain only (Fig. 4H). Interestingly, we found that mouse TONSLTPR+ARD and TONSLTPR displayed increased binding to H3.1K27M compared to wild-type H3.1 (Fig. 4I, J), and that the interaction of TONSLTPR+ARD with histones is restricted by H4K20me1, but not H3.1K27me1 (Fig. 4K). These results suggest that H3.1K27M insertion on chromatin leads to ectopic TSK/TONSL activity in both plants and animals, but via different mechanisms.
H3.1K27M-expressing plants are hypersensitive to the loss of specific DNA repair proteins
H3.1K27M expression has similar effects on genomic stability as atxr5/6 mutants. Our previous work showed that various phenotypes in atxr5/6 mutants are either enhanced or suppressed by the loss of specific DNA repair pathways18. For example, loss of RAD51 (involved in homologous recombination) or Pol θ (key player in theta-mediated end joining [TMEJ]) in atxr5/6 mutants enhances the growth defects of these plants. This is likely due to an increased reliance on repair of damaged DNA resulting from higher levels of H3.1K27me0 and unregulated TSK activity. Therefore, we hypothesized that plants expressing H3.1K27M may also be hypersensitive to the loss of DNA repair pathways due to high levels of DNA damage caused by the oncohistone. To test this, H3.1K27M was first introduced into heterozygous rad51 mutants (T0 plants), as homozygous rad51 mutants are sterile and therefore cannot be transformed by Agrobacterium using the flower dip method49. Transformation of heterozygous rad51 mutants with constructs expressing H3.1K27M or H3.1 WT resulted in 1% (1/95) and 26% (23/89) homozygous rad51 mutants (T1 plants), respectively (Fig. 5A). The near absence of homozygous rad51 mutants in the T1 generation of H3.1K27M plants strongly suggests that homologous recombination is a major contributor to DNA repair and plant/cell viability in H3.1K27M-expressing plants. We could not replicate this strategy to assess the contribution of TMEJ via Pol θ in H3.1K27M plants, as polq mutants cannot be transformed via Agrobacterium-mediated flower dipping50. As TMEJ relies on resected DNA as a substrate to initiate DNA repair51,52, we tested the effect of MRE11 in plants expressing H3.1K27M. MRE11 is a key component of the MRE11–RAD50–NBS1 (MRN) complex responsible for DNA end resection53. Similarly to the experiment using rad51, we introduced H3.1K27M and H3.1 WT into heterozygous T0 plants since homozygous mre11 mutants are sterile54. We only recovered 2% (1/59) homozygous mre11 mutants among the T1 progeny (Fig. 5B). In contrast, the transformation of H3.1 WT resulted in 23% (18/78) of the T1 transformants being homozygous mre11 mutant, which is close to the expected 25% for a transgene with no effect on plant survival. This result suggests that DNA resection mediated by the MRN complex specifically contributes to the survival of H3.1K27M plants. We also transformed H3.1::H3.3 WT and H3.1::H3.3K27M into heterozygous rad51 and mre11 plants and recovered approximately 25% homozygous rad51 and mre11, suggesting that plants with reduced H3K27me3 do not rely on these DNA repair genes for survival (Supplementary Fig. 6A–D). To further explore if H3.1K27M-expressing plants respond like atxr5/6 mutants to the absence of key DNA repair pathways, we tested non-homologous end joining (NHEJ) by directly transforming H3.1K27M or H3.1 WT into homozygous ku80 mutants. The absence of Ku80 in plants expressing H3.1K27M did not enhance growth defects or suppress heterochromatin amplification, which parallels the phenotypes observed in atxr5/6 ku80 triple mutants (Fig. 5C and Supplementary Fig. 6E)18. Finally, we assessed the impact of RAD17 in H3.1K27M-expressing plants. As observed in atxr5/6 rad17 triple mutants18, loss of RAD17 in H3.1K27M plants abolished heterochromatin amplification while having no measurable effects on plant growth (Fig. 5D and Supplementary Fig. 6F, G). We also tested for transgene expression in the ku80 and rad17 mutant plants and did not find any significant difference in expression compared to Col-0 (Supplementary Fig. 6H). Overall, these results demonstrate that the phenotypes of H3.1K27M-expressing plants and atxr5/6 mutants are modulated by the same DNA repair proteins, and that expression of the oncohistone in Arabidopsis generates a dependence on specific DNA repair pathways for survival.
Fig. 5. Differential effects of H3.1K27M expression in DNA repair mutants.
Genotypes of T1 transgenic plants resulting from transformation of H3.1 WT and H3.1K27M into rad51 heterozygous (rad51 [+/-]) (A) and mre11 heterozygous (mre11 [+/-]) (B) T0 plants. C, D Chromosomal view (Chr 5) of DNA-seq reads. The pericentromeric region is highlighted in gray. E Model depicting how H3.1K27M expression leads to genomic instability by increasing the levels of H3.1K27me0, which causes misregulation of TSK. Created in BioRender. Jacob, Y. (2025) https://BioRender.com/l39x793. Source data are provided as a Source Data file.
Discussion
Genomic instability is associated with DMG-H3K27a and the H3K27M oncomutation29–33. However, underlying mechanisms directly linking H3K27M to DNA damage in these cancers remain mostly unexplored. In this study, we showed using Arabidopsis as a model that expression of H3.1K27M replicates the genomic instability phenotypes observed in atxr5/6 mutants, which are caused by misregulation of the TSK-H3.1 DNA repair pathway18. Loss of H3.1K27me1 in atxr5/6 mutants or H3.1K27M-expressing plants prevents post-replicative chromatin maturation, leaving H3.1 variants unmethylated. As H3.1K27me0 serves to recruit TSK to chromatin, a model emerges where ectopic binding of TSK to chromatin in H3.1K27M-expressing cells, or its retention on chromatin post-replication, induces DNA damage leading to genomic instability (Fig. 5E). More work will be needed to understand the mechanism by which TSK induces DNA damage when it is present on chromatin outside of its normal path of entry and exit during chromatin replication.
An important question is whether the findings of this study in plants translate to human cells, and if they can help us understand the etiology of DMG-H3K27a. In mammals, post-replicative chromatin maturation restricting TONSL activity has been shown to depend on SET8-catalyzed H4K20me1, which blocks the interaction between TONSL and chromatin23. H4K20me0 is read by the TONSL ARD domain, which is not present in plant TSK orthologs (SET8 orthologs are also absent in plants)55. Both TSK and TONSL contain the TPR domain that mediates the specific interaction with the H3.1 variant18. TSK binding to H3.1 is inhibited by methylation of K27 in plants18, but our in vitro results suggest that the TPR domain of TONSL is insensitive to mono-methylation at H3.1K27. This indicates that in contrast to plants, misregulation of TONSL activity in mammals may not occur as a result of genome-wide loss of H3K27 methylation due to PRC2 inhibition by H3.1K27M. However, and again in contrast to plant TSK, this study demonstrates that the TPR domain of TONSL displays enhanced binding to H3.1K27M compared to WT H3.1. Therefore, it is possible that H3.1K27M directly recruits TONSL to chromatin and forms stable complexes that are not inhibited by the post-replicative chromatin maturation step mediated by SET8 via H4K20me1. This hypothesis will need to be tested in cell culture systems, but if confirmed, would support a model where H3.1K27M directly induces misregulation of TONSL activity, which may lead to DNA damage and genomic instability as in plants. Interestingly, our results suggest that the K27M mutation also increases the affinity of TONSL for the H3.3 variant compared to the non-mutated H3.3 protein. Therefore, there is a possibility that H3.3K27M expression may also lead to the misregulation of TONSL, with consequences for genome maintenance.
Finally, our findings are in line with other studies that point to diverse effects of H3K27M on the regulation of DNA repair pathways and genomic stability29–33. These studies and our work suggest that H3.1K27M not only disrupts cellular identity via loss of H3K27me3, but also modulates the activity of DNA repair pathways to alter genomic integrity and induce tumorigenesis. Although this model underscores the incredible potency of H3K27M in disrupting key biological functions (i.e., epigenetic programming and genomic stability) required for cellular homeostasis, it also points to new therapeutic routes associated with the DNA damage response that could be applied to help manage or cure cancers characterized by this oncomutation.
Methods
Plant materials
Plants were grown at 22 °C under cool-white fluorescent lights (~100 μmol m−2 s−1) in long-day conditions (16 h light/8 h dark). The T-DNA insertion mutants atxr5/6 (At5g09790/At5g24330, SALK_130607/SAIL_240_H01)25, tsk/bru1-4 (At3g18730, SALK_034207)56, rad51 (At5g20850, GK_134A01)49, ku80-7 (At1g48050, SALK_112921)57, rad17-2 (At5g66130, SALK_009384)58 and mre11 (At5g54260, SALK_028450)59 are in the Col-0 ecotype background and were obtained from the Arabidopsis Biological Resource Center (Columbus, Ohio). Transgenic plants expressing H3.1 WT (HTR9), H3.1K27M (HTR9 K27M), H3.1::H3.3 WT (HTR5 driven by the HTR9 promoter), and H3.1::H3.3K27M (HTR5 K27M driven by the HTR9 promoter) were made by transforming Col-0 plants or DNA repair mutants (tsk, rad17, rad51, ku80 and mre11).
Cloning
To generate transgenic expression constructs (H3.1 WT, H3.1K27M, H3.1::H3.3 WT, and H3.1::H3.3K27M), the coding sequences of HTR9 (At5g10400) or HTR5 (At4G40040) and the HTR9 promoter (1027 bp upstream of the start codon) were cloned into MoClo system level 0 plasmids pICH41308 and pICH41295, respectively. The H3.1K27M and H3.3K27M mutations were engineered by site-directed mutagenesis (QuikChange II XL, Agilent Technologies, Santa Clara, CA). The terminator of HSP18.2 (At5g59720, 248 bp downstream of stop codon) was cloned into pICH41276. The level 1 clone was seamlessly assembled using Type-II restriction enzyme BsaI in the order of Promoter-CDS-terminator into destination vector pICH47802. The level 1 clones were later assembled into level 2 final clones combined with level 1 Ole::RFP selection cassette using BpiI, which allows for Agrobacterium transformation and RFP selection of transformants in planta.
To generate TSK/TONSL-TPR and TONSL-TPR + ARD expression constructs, the coding sequences of the TPR domains of A. thaliana TSK (aa 1-525) and of mouse TONSL (aa 1-465), and the joint domains TPR + ARD of TONSL (aa 1-693) were cloned into the pET32a vector as previously described18. As for the GST-fused peptides, the coding sequences of the N-terminal tails of A. thaliana H3.1 (aa 1-58) and H3.3 (aa 1-58) were fused with a C-terminal GST tag by cloning into pET28 as previously described18. The H3.1K27M and H3.3K27M mutations were introduced by site-directed mutagenesis using the QuikChange II XL kit (Agilent Technologies). Briefly, mutagenic primers containing the K27M substitution were designed and used for PCR amplification of the plasmid template. The PCR product was then treated with DpnI to digest the parental DNA template, followed by transformation into E. coli XL10-Gold ultracompetent cells (Agilent Technologies). The plasmids were sequenced to verify the introduction of the desired mutations.
For the histone methyltransferase assays, ATXR6 (residues 25-349) was cloned into pGEX-6P as previously described25.
Plant transformation
Plant transformations were done using the floral dip method60. Briefly, 400 ml of Agrobacterium (strain GV3101) liquid culture grown overnight at 28 °C was spun down at 3220 × g for 25 min and resuspended in 500 mL of transformation solution (5% sucrose and 0.02% Silwet L-77). Arabidopsis flowers were dipped into the bacterial solution for 30 s. T1 transgenic plants were selected with the seed-specific RFP reporter (OLE1-RFP) with an SFA Light Base (Nightsea, Lexington, MA). Transformations into mutants were either done with homozygous plants (rad17 and ku80) or heterozygous plants (tsk, rad51 and mre11), due to low seed set or sterility49,61,62. Transgenic tsk, rad51, and mre11 homozygous plants were identified in the T1 population.
Survival assay
Seeds were germinated and grown on ½ MS plates at 22 °C under cool-white fluorescent lights (~100 μmol m−2 s−1) in long-day conditions (16 h light/8 h dark) for 5 days, transferred to soil, and continued growing under the same conditions. The number of surviving plants was counted on days 0, 4, 8, 12, 16, and 20 after transferring to the soil. The survival rate was calculated as the number of live plants divided by the number of total seeds for each genotype.
Anthocyanin measurements
Anthocyanin content was determined using a modified version of a previously published protocol63. 200 μL of extraction buffer (50% methanol and 5% acetic acid) was added to 50 mg of ground leaf tissue, followed by centrifugation. The supernatant was carefully decanted into a new tube. This step was repeated to ensure the complete transfer of the supernatant. Absorbances were measured at 530 nm and 657 nm wavelengths. Anthocyanin concentration, expressed as Abs530 per gram fresh weight, was calculated using the formula [Abs530 − (0.25 × Abs657)] × 5.
Comet assay
DNA damage was assessed in three-week-old plants using a Comet Assay Kit (Trevigen). In brief, leaves were sectioned into small fragments with a razor blade in 500 μl of PBS buffer containing 20 mM EDTA and maintained on ice. The nuclei were then released into suspension, filtered through a 50 μm nylon mesh into a fresh tube, and subsequently mixed with an equal volume of low-melting-point agarose. The mixture was immediately spread onto CometSlides. Following a 1-h lysis step at 4 °C, the slides were immersed in 1× Tris-acetate electrophoresis buffer for 30 min to allow unwinding of the DNA before electrophoresis at 4 °C for 10 min. Post-electrophoresis, the slides were stained with SYBR Gold for visualization of the nuclei. Imaging was conducted using a Nikon Eclipse Ni-E microscope. Quantitative analysis of DNA damage was performed using the OpenComet plugin for ImageJ64.
MMS genotoxic assay
Seeds were germinated and grown on vertically-oriented ½ MS plates with or without 100 μg/ml methyl methanesulfonate (MMS) (Thermo Fisher Scientific, Bohemia, NY) under cool-white fluorescent lights (~100 μmol m−2 s−1) in long-day conditions (16 h light/8 h dark). Root length measurements were done 10 days (No MMS) or 20 days (100 μg/ml MMS) after germination, respectively. The relative root length was calculated by dividing the root length of individual seedlings grown in the presence of MMS by the average root length of plants of the same genotype grown on ½ MS plates.
Somatic recombination assay
H3.1 WT and H3.1K27M transgenic plants were generated by transforming a previously described inverted repeat GUS reporter line37. Experiments were performed at least three times in 4-week-old plants as previously described18.
RNA-seq
For each biological replicate, leaves from three plants cultivated in the same flat were combined for RNA extraction using the RNeasy Plant Mini Kit (Qiagen, Hilden, Germany). The integrity of RNA was confirmed with the Agilent 2100 Bioanalyzer Nano RNA Assay (Agilent, Santa Clara, CA). Sequencing libraries were prepared at the Yale Center for Genome Analysis (YCGA) with 200 ng of total RNA using oligo-dT beads for poly A selection. Sequencing (101 bp paired-end) was performed on an Illumina NovaSeq X Plus flow cell. Fastp (version 0.21.0 with default parameters) was used to filter and trim paired-end reads65 and reads with quality inferior to 20 were removed. STAR (version 2.7.2a) was used to align the data against the Arabidopsis TAIR1066 genome with a maximum of two mismatches (--outFilterMismatchNmax 2)67. Biological replicates were analyzed for consistency by principal component analysis (PCA) using FactoMineR68 The PCA was performed using all genes with an average TPM over ≥5 in all samples (Supplementary Fig. 7). For the analysis of the percentage of H3.1 gene transcripts (HTR1, HTR2, HTR3, HTR9, HTR13 and H3.1 transgene), the trimmed reads were mapped and quantified using Salmon (salmon v1.4.0, default parameters)69. For the expression analysis of DNA repair response genes, we utilized a curated list of genes previously assembled by the Britt laboratory. This comprehensive list, accessible via the Britt lab’s website (http://brittlab.ucdavis.edu/plant-dna-repair-genes), includes key genes involved in plant DNA repair mechanisms (see Supplementary Data 1 for the complete gene list). Paired-end fragments corresponding to TEs were determined with featureCounts (version 1.6.4)70. Transposable elements (TEs) were defined as previously described71. TEs were considered to be upregulated if they showed a ≥2-fold upregulation as compared to Col-0 in both biological replicates and had a value of TPM ≥ 1. The heatmap was generated with the R heatmap.2 function from the ggplot2 package72.
Nuclei DAPI staining and Immunostaining
Two-week-old leaves were fixed in 3.7% formaldehyde in cold Tris buffer (10 mM Tris-HCl pH 7.5, 10 mM NaEDTA, 100 mM NaCl) for 20 min under vacuum. The formaldehyde solution was removed, and leaves were washed twice for 10 min in Tris buffer. The leaves were chopped using razor blades in 150 μl LB01 buffer (15 mM Tris-HCl pH7.5, 2 mM NaEDTA, 0.5 mM spermine-4HCl, 80 mM KCl, 20 mM NaCl and 0.1% Triton X-100), then filtered through a 30 μm mesh (Sysmex Partec, Gorlitz, Germany). 10 μl of lysate was added to 10 μl of sorting buffer (100 mM Tris-HCl pH 7.5, 50 mM KCl, 2 mM MgCl2, 0.05% Tween-20, and 5% sucrose) and dried onto a coverslip for 30 min. Cold methanol was added to each coverslip for 3 min. Methanol was aspirated and TBS-Tx (20 mM Tris pH 7.5, 100 mM NaCl, 0.1% Triton X-100) was added for 5 min. TBS-Tx was then removed. For DAPI staining only, the coverslips were immediately mounted onto slides with Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA). For immunostaining experiments, the following steps were performed: the coverslips were blocked by adding 75 μl of Abdil-Tx (2% BSA in TBS-Tx) to each coverslip for 30 min, followed by a 1 h incubation with the appropriate primary antibody (H3K27me1; Active Motif #61015, H3K27me3, Millipore #07-449, H3K27M, Active Motif #61803) diluted in Abdil-Tx. The coverslips were then washed with TBS-Tx. 30 μl of secondary antibody was added on each coverslip and incubated for 30 min followed by washing with TBS-Tx. Finally, the coverslips were mounted onto slides with Vectashield mounting medium with DAPI. Images (Z-series optical sections of 0.3 μm steps) were taken on a Nikon Eclipse Ni-E microscope with a 100X CFI PlanApo Lamda objective (Nikon, Minato City, Tokyo, Japan) equipped with an Andor Clara camera. Images were deconvolved using the imageJ deconvolution plugin.
ChIP-seq
ChIP was performed as described previously73, with some modifications. Briefly, rosette leaves from 2-week-old plants were fixed for 15 min in 1% formaldehyde, followed by flash freezing. Approximately 0.1 g of ground tissue was added to 10 mL of extraction buffer 1 (0.4 M sucrose, 10 mM Tris–HCl, 10 mM MgCl2) and filtered through a 40 µm mesh. Samples were centrifuge at 3000 g for 20 min. The pellets were resuspended in 1 mL of extraction buffer 2 (0.25 M sucrose, 10 mM Tris–HCl, 10 mM MgCl2, 1% Triton X-100) and centrifuged at 12,000 g for 10 min. 400 µL of extraction buffer 3 (1.7 M sucrose, 10 mM Tris-HCl [pH8.0], 0.15% Triton X-100) was used to resuspend the pellets. The homogenate was added to a fresh tube containing 400 µL of extraction buffer 3, followed by centrifugation for 1 h at 16,000 g. The pellets were resuspended in nuclei lysis buffer (50 mM Tris–HCl pH 8.0, 10 mM EDTA, 1% SDS), and chromatin was sheared using a Bioruptor 200 sonicator (20 times on a 30-s ON, 30-s OFF cycle). The supernatants were centrifuged at 16,000 g for 5 min. ChIP dilution buffer (1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris–HCl pH 8.0, 167 mM NaCl) was added to the supernatant to obtain 10× volume. Antibodies (2 μL of H3K27me3 antibody [Millipore #07-449] or 2 μL of H3K27me1 antibody [ABclonal A22170]) were added to 750 μL of diluted sample and incubated at 4 °C overnight. An equal amount of Drosophila chromatin (Active Motif #53083) was added to each sample to allow for quantitative comparisons of samples displaying very different amounts of H3K27 methylation marks (ChIP-Rx)74. Immunoprecipitation was performed using protein A magnetic beads (New England BioLabs, Ipswich, MA). The beads were washed, resuspended in 200 µL of elution buffer (1% SDS and 0.1 M NaHCO3), and incubated at 65 °C for 15 min for elution. After uncrosslinking, samples were treated with proteinase K and purified using ChIP DNA Clean and Concentrator (Zymo Research, Irvine, CA).
ChIP sequencing libraries were prepared at the YCGA with a TruSeq Library Prep Kit (Illumina, San Diego, CA). They were validated using an Agilent Bioanalyzer 2100 High sensitivity DNA assay and quantified using the KAPA Library Quantification Kit for Illumina® Platforms kit. Sequencing was done on an Illumina NovaSeq 6000 using the S4 XP workflow. Fastp (version 0.21.0 with default parameters) was used to filter and trim paired-end reads65. The reads with quality scores <20 were removed. Duplicate reads were also removed using the Picard toolkit (https://broadinstitute.github.io/picard) (MarkDuplicates with REMOVE_DUPLICATES=true). To calculate the Rx scaling factor of each biological replicate, Drosophila-derived IP read counts were normalized to the number of input reads. Spike-in normalization was performed as previously described75,76. We used α = r/Nd_IP74 to compute the scaling factor α for each replicate, with Nd_IP corresponding to the number of reads (in millions) aligning to the D. melanogaster genome in the IP and with r = 100 * Nd_i / (Na_i + Nd_i), where Nd_i and Na_i are the number of input reads (in millions) aligning to the D. melanogaster or A. thaliana genome, respectively. We generated bedgraph files with a bin size of 10 bp using deepTools77 using Rx factors to scale each of the samples. We called the broad peaks using macs2 (2.2.9.1)78, with the parameters -g 1.2e8 --down-sample --broad on the wildtype data set. 8412 peaks were detected (wt_H3K27me3_peaks.broadPeak). Then using bedtools (v2.30.0) intersect79, we selected the genes overlapping with the H3K27me3 peaks (Supplementary Data 3). 7071 genes were kept for further analysis with deeptools (gene_withK27me3_peaks.bed). Using deeptools (version: 3.5.5) with scale-regions parameter and looking 1.5 kb upstream and downstream of the genes77, we computed the profile plot and made the heatmap with RRPM from H3K27me3 normalized data. In order to generate the chromosomal representation in Fig. 2C, featureCounts (version 1.6.4) was used to count the paired-end reads within 100-kb regions of the genome and scaled by adjusting the number of reads in each bin with Rx factors. Consistency between biological replicates was confirmed by Pearson correlation using deepTools2 (Supplementary Fig. 8)77.
Histone protein extraction
Histone protein extraction was performed as previously described80 with some modifications. For Western blot samples, ~80 mg of plant material was ground in 1 ml of cold Nuclear Isolation Buffer (NIB) (10 mM MES, pH5.5, 10 mM NaCl, 10 mM KCl, 10 mM MgCl2, 0.5 mM spermidine, 0.1 mM spermine, 0.15% v/v NP-40, 2.5 mM DTT, 0.4 mM PMSF, 1 μg/ml pepstatin A)80 and transferred to a 2-ml Dounce homogenizer. The plant homogenate was then processed with a loose pestle in the Dounce homogenizer for 50 strokes on ice. The lysate was filtered through two layers of Miracloth into new tubes and centrifuged. The supernatant was discarded, and the pellet was washed three times with NIB. The nuclear pellet was then resuspended in 1 ml of 0.4 N H2SO4. This mixture was transferred to a 2-ml Dounce homogenizer and homogenized with 40 strokes on ice to disrupt the nuclei. After centrifugation, the supernatant was transferred to a fresh tube and mixed with 264 μl of trichloroacetic acid dropwise to precipitate the proteins. The mixture was incubated on ice for 3 h to ensure complete precipitation. Following incubation, the histone pellet was centrifuged at 21,000 x g for 15 min at 4 °C. The supernatant was carefully removed, and the pellet was washed with 500 μl of ice-cold acetone to remove any remaining acid and impurities. The acetone wash step was repeated once more to ensure thorough cleaning. The pellet was then air-dried at room temperature until no visible moisture remained. Finally, the dried histone pellet was resuspended in 25 μl of histone storage buffer (10 mM Tris-HCl, pH 7.5, 0.1 mM EDTA) for storage at −80 °C.
For the mass spectrometry samples, 2.5 g of plant materials was ground into a very fine powder and transferred into 30 ml of fresh NIB buffer. The mixture was gently rocked for 10 min at 4 °C, and then transferred to a 40-ml loose Dounce homogenizer and homogenized with 50 strokes on ice. The lysate was filtered through two layers of Miracloth into new tubes and centrifuged at 900 x g for 10 min. The supernatant was discarded, and the pellet was washed three times with 10 ml fresh NIB (resuspend, rock for 3 min, centrifuge for 5 min). The washed pellet was resuspended in 1 ml of 0.4 N H2SO4, transferred to a 2-ml tight Dounce homogenizer, and homogenized with 50 strokes on ice. The mixture was incubated on a rotating platform for 1 h at 4 °C. After centrifugation, the supernatant was transferred to a fresh tube and mixed with 264 μl of trichloroacetic acid dropwise to precipitate the proteins. The mixture was incubated on ice for 3 h to ensure complete precipitation. Following incubation, the histone pellet was centrifuged at 21,000 x g for 10 min at 4 °C. The supernatant was carefully removed, and the pellet was washed with 500 μl of ice-cold acetone to remove any remaining acid and impurities. The acetone wash step was repeated. After a quick air-dry, the pellet was resuspended in 50 ml H2O and stored at −80 °C. 5% of the histone samples were run on the 15% SDS-PAGE gel and stained with Coomassie Blue to check for the quality of histones.
Western blotting
Total histone samples were subjected to Western blot analysis. Laemmli sample buffer (Bio-Rad, Hercules, CA) was added to each total histone sample, incubated at 95 °C for 3 min, and loaded onto a 4–20% gradient SDS-PAGE gel. Following electrophoresis, proteins were transferred onto membranes using a semi-dry blotter in a Tris-glycine transfer buffer containing 20% methanol. The membranes were then blocked with 5% non-fat dry milk in TBST (20 mM Tris-HCl, pH 7.6, 150 mM NaCl, 0.1% Tween-20) for 1 h at room temperature to prevent non-specific binding. The membranes were then probed with H3K27me1 (Active Motif: 61015) or H3K27me3 (Millipore: 07-449) for 1 h, followed by incubation with a secondary HRP-labeled antibody (Sigma) at a 1:10,000 dilution for 1 h at room temperature. Blots were visualized using the Bio-Rad Clarity Western ECL Substrate and imaged with a Bio-Rad ChemiDoc MP Imaging System. Relative quantification of Western blot bands was performed with Image J.
Nucleosome assembly
Recombinant nucleosome arrays for histone methyltransferase assays were assembled as described previously81. In short, histones were expressed in E. coli and purified from inclusion bodies. Histone octamers consisting of Arabidopsis H2A.13 (At3g20670), H2B (At3g45980), and Xenopus H4 along with either Xenopus H3 fused to a C-terminal Strep tag (H3-CT Strep) or Arabidopsis H3.1 (At1g09200) with K27A or K27M mutations were reconstituted by dialysis into refolding buffer (10 mM Tris pH 8, 1 mM EDTA, 5 mM b-mercaptoethanol, 2 M NaCl) and purified by size exclusion chromatography on a Superdex 200 gel filtration column (GE Healthcare). To form nucleosome arrays, histone octamers were assembled onto a plasmid containing 12 repeats of the 601 nucleosome positioning sequence connected via a 30-bp linker using gradient dialysis from 2 M to 0.4 M NaCl followed by step dialysis into TE.
Histone methyltransferase assay
Histone methyltransferase assays were performed as previously described82. Briefly, 0.5 μg of GST-ATXR6 and 0.5 μg of H3-CT-Strep nucleosomes were added to a total reaction volume of 25 μl. We either added no inhibitor or 0.5 μg of K27A or K27M nucleosomes. The reactions were incubated for 1 h at 30 °C. SDS-PAGE sample buffer was added to each tube followed by heating to 95 °C for 5 min. Samples were resolved by SDS-PAGE (15% gels) and transferred to a PVDF membrane. Coomassie stain solution (45% methanol, 10% acetic acid, 0.25% Coomassie Brilliant Blue R) was used to stain the membrane followed by destaining (45% methanol, 10% acetic acid). Membranes were air-dried, sprayed with EN3HANCE (PerkinElmer), and exposed to autoradiography film overnight at −80 °C. Films were developed and bands were quantified using the software ImageJ.
Flow cytometry
Rosette leaves from 2-week-old plants were finely chopped in 0.2–0.5 ml Galbraith buffer (45 mM MgCl2, 20 mM MOPS, 30 mM sodium citrate, 0.1% Triton X-100, 40 μg/ml RNase A) and filtered through a 30 μm mesh (Sysmex Partec). Isolated nuclei were stained by adding 20 μg/ml propidium iodide (Sigma) to each sample, followed by vortexing. The samples were analyzed using a BD FACSAria II sorter (Becton Dickinson, Franklin Lakes, NJ). FlowJo 10.9.0 (Tree Star, Ashland, Oregon) was used to generate profiles and for quantification (nuclei counts and rCV values). Each biological replicate represents one plant.
DNA-seq
Genomic DNA was extracted as previously described83. For each biological replicate, 5 plants were pooled to obtain ~40 mg of leaf tissue. DNA sequencing libraries were prepared at the YCGA. Genomic DNA was sonicated to an average fragment size of 350 bp using a Covaris E220 instrument (Covaris, Woburn, MA). The libraries were generated using the xGen Prism library prep kit for NGS (Integrated DNA Technologies, Coralville, IA). Paired-end 150 bp sequencing was performed on an Illumina NovaSeq 6000 using the S4 XP workflow (Illumina, San Diego, CA). Raw FASTQ data were filtered and trimmed using the fastp tool (--length_required 20, --qualified_quality_phred 20)65. The filtered sequences were then mapped to the genome (TAIR10) using BWA-MEM default parameters (10.48550/arXiv.1303.3997). Mapped reads were sorted, duplicates were removed and indexed using SAMtools84. Biological replicates were analyzed for consistency with deepTools2 (Supplementary Fig. 9)77. The program featurecount (version 2.0.6)70 was used to count the paired-end fragments present in each 50-kb region of the A. thaliana genome as previously described18. The log2 ratio was centered on the average ratio of any two compared libraries normalized to the first 5 Mbp of chromosome 1. Plot profiles were generated using R (version 4.3.2)85 and Gviz86.
Protein expression
Plant histone-GST fusion proteins and AtTSK-TPR were expressed in Rosetta (DE3) E. coli (Sigma, St. Louis, MO). The bacterial cultures were grown in Luria-Bertani (LB) medium, and expression of the fusion proteins was induced with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) at room temperature. Human histone-GST fusion proteins, mouse TONSL-TPR, and mouse TONSL-TPR + ARD were expressed in ArcticExpress (DE3) E. coli (Agilent, Santa Clara, CA). Human histone-GST fusion proteins, TONSL-TPR and TONSL-TPR + ARD were expressed at 16 °C for 24 h and induced with IPTG (1 mM) at OD600nm = 0.4.
To purify the histone-GST fusion proteins, harvested cell pellets were resuspended in PBS (1X Phosphate-Buffered Saline: 137 mM NaCl, 10 mM phosphate, 2.7 mM KCl, pH 7.4) supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF) prior to lysis by sonication and clarification by centrifugation. The clear lysate was then applied to a Glutathione Sepharose 4B affinity column. After thorough washing with PBS to remove unbound material, the fusion proteins were eluted with Elution Buffer (EB: 50 mM Tris-HCl, 50 mM NaCl, 30 mM reduced L-Glutathione, 10% glycerol, pH 8.0). The eluted proteins were then concentrated using Amicon® Ultra Centrifugal Filter Units (MilliporeSigma) with a 10 kDa molecular weight cutoff. The elution buffer was replaced with storage buffer (50 mM Tris-HCl, 50 mM NaCl, 10% glycerol, pH 8.0) by centrifuging at 4000 × g for 20 min. The concentrated and purified proteins were aliquoted and preserved at −80 °C for long-term storage.
ATXR6 expression in E.coli and purification was described in detail in a previous publication25. The purification of AtTSK-TPR, TONSL-TPR, and TONSL-TPR + ARD (containing an N-terminal Trx-His-S tag and a C-terminal His tag) was performed as previously described with minor modifications18. Briefly, the cell pellets were resuspended in NPI-10 buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, pH 8.0) containing 1 mM PMSF, and sonicated for 2.5 min (30 s pulse, 1 min break, 5 cycles). After centrifugation, the supernatant was transferred into a new 50 ml Falcon tube and added with washed agarose Ni-NTA His beads. The expressed proteins were trapped after passing through the Ni-NTA agarose column. The column was then washed with 7.5 ml NPI-20 (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole, pH 8.0) twice, and eluted with 10 ml NPI-250 buffer (50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazole, pH 8.0). The eluted proteins were further concentrated using Amicon Ultra Centrifugal Filter (50 kDa) to 1.5 ml for size exclusion chromatography (SEC). The concentrated proteins were injected into SEC and eluted using an SEC buffer (25 mM Hepes, 200 mM NaCl, pH 7.5). Peak fractions from the S200 column were pooled, concentrated to 1–2 mg ml−1, flash frozen under liquid nitrogen, and stored at −80 °C.
Histone binding assays
For the TSKTPR -histone binding assays, 2 μg of AtTSK were combined with either 2 μg of GST or GST-tagged histone tails (aa 1 to 58 of plant H3.1) in 400 μl of binding buffer (25 mM Tris-HCl, 250 mM NaCl, 0.05% NP-40, pH 8.0). The mixture was incubated overnight at 4 °C with rotation. Subsequently, 15 μl of prewashed Glutathione Sepharose 4B agarose beads (Cytiva, Marlborough, MA) were added to each sample and incubated for 30 min to pull-down the GST-tagged proteins. The beads were then washed four times for 5 min with 1 mL of binding buffer while rotating at 4 °C. The final wash was performed with binding buffer containing 150 mM NaCl. The proteins were eluted with 15 μl of 2× SDS loading buffer and denatured by boiling at 95 °C for 5 min. Samples were then resolved on a 10% SDS-PAGE gel. The lower section of the gel underwent Coomassie staining to assess the GST and GST-tagged proteins, while the upper section was analyzed by Western blot using an anti-His antibody (Sigma; H1029). The TONSLTPR +ARD and TONSLTPR binding assays were performed as described above with the following modifications. The binding buffer consisted of 50 mM Tris-HCl pH8.0, 300 mM NaCl, 5% glycerol, 0.25% NP-40, 0.2 mM EDTA, 1 mM DTT. The binding reaction was incubated for 2 h at 4 °C. The samples were resolved on a 4–20% gradient gel. Each pull-down experiment was repeated a minimum of three times.
Nucleosome binding assay
The nucleosome binding assays were performed as described for the histone binding assays with TONSLTPR + ARD. The recombinant human biotinylated nucleosomes were obtained from EpiCypher (Durham, NC).
LC-MS/MS analysis
The extracted histones were prepared for chemical derivatization and digestion as described previously87,88. In brief, the lysine residues from histones were derivatized with the propionylation reagent (1:2 reagent:sample ratio) containing acetonitrile and propionic anhydride (3:1), and the solution pH was adjusted to 8.0 using ammonium hydroxide. The propionylation was performed twice and the samples were dried on speed vac. The derivatized histones were then digested with trypsin at a 1:50 ratio (wt/wt) in 50 mM ammonium bicarbonate buffer at 37 °C overnight. The N-termini of histone peptides were derivatized with the propionylation reagent twice and dried on speed vac. The peptides were desalted with the self-packed C18 stage tip. The purified peptides were then dried and reconstituted in 0.1% formic acid. An LC-MS/MS system consisting of a Vanquish Neo UHPLC coupled to an Orbitrap Exploris 240 (Thermo Scientific) was used for peptide analysis. Histone peptide samples were maintained at 7 °C on sample tray in LC. Separation of peptides was carried out on an Easy-Spray™ PepMap™ Neo nano-column (2 µm, C18, 75 µm × 150 mm) at room temperature with a mobile phase. The chromatography conditions consisted of a linear gradient from 2 to 32% solvent B (0.1% formic acid in 100% acetonitrile) in solvent A (0.1% formic acid in water) over 48 min and then 42–98% solvent B over 12 min at a flow rate of 300 nL/min. The mass spectrometer was programmed for data-independent acquisition (DIA). One acquisition cycle consisted of a full MS scan, 35 DIA MS/MS scans of 24 m/z isolation width starting from 295 m/z to reach 1100 m/z. Typically, full MS scans were acquired in the Orbitrap mass analyzer across 290–1100 m/z at a resolution of 60,000 in positive profile mode with an auto maximum injection time and an AGC target of 300%. MS/MS data from HCD fragmentation was collected in the Orbitrap. These scans typically used an NCE of 30, an AGC target of 1000%, and a maximum injection time of 60 ms. Histone MS data were analyzed with EpiProfile89.
Split-luciferase complementation assays
The TPR domain (aa 1-524) of Arabidopsis TSK, fused with a nuclear localization signal (NLS), and the NLS alone, were cloned into the Gateway destination vector pGWB-cLUC (Addgene Plasmid #174051). Histones H3.1 and H3.1K27M (aa 1-136) were inserted into the Gateway destination vector pGWB-nLUC (Addgene Plasmid #174050). Agrobacterium tumefaciens GV3101 strains harboring these constructs were introduced into the leaves of 4-week-old Nicotiana benthamiana plants. For co-infiltration experiments, Agrobacterium cultures adjusted to cell densities of 0.1–0.5 at OD600 were mixed in equal volumes. To inhibit gene silencing, the tomato bushy stunt virus (TBSV) p19 silencing suppressor, encoded within the pDGB3alpha2_35S:P19:Tnos vector (Addgene Plasmid #68214), was also included in the infiltration mixture. The Agrobacterium inoculum was prepared by resuspension in infiltration buffer (10 mM MgCl2, 10 mM MES, pH 5.6, and 150 μM acetosyringone) and incubated at room temperature for 2 h. The inoculum was then gently infiltrated into the abaxial side of the leaves using a 1 ml needleless syringe. At 48–72 h post-infiltration, a 1 mM luciferin solution was applied to the leaves. Before imaging, detached leaves were kept in darkness for 10 min to reduce chlorophyll fluorescence interference. Luminescence was captured with a charge-coupled device camera. Image analysis and quantification were performed using ImageJ software.
RT-qPCR
RNA was isolated from two-week-old leaf tissue using TRIzol (Invitrogen, Carlsbad, CA). RNA samples were treated with RQ1 RNase-free DNase (Promega, Madison, WI) at 37 °C for 30 min. 750 ng of total RNA was used to produce cDNA with iScript cDNA Synthesis Kit (Bio‐Rad, Hercules, CA). Real-time PCR was done using KAPA SYBR FAST qPCR Master Mix (2X) Kit (Kapa Biosystems, Wilmington, MA) on a CFX96 Real-Time PCR Detection System (Bio‐Rad, Hercules, CA). Relative quantities were calculated using the Ct method with ACTIN7 (At5g09810) as the normalizer90. At least three biological replicates were performed for each experiment. Primers are listed in Supplementary Table 1.
Plant genotyping
Leaves from 2 or 3-week-old plants were homogenized in 500 μl DNA extraction buffer (200 mM Tris-HCl (pH 8.0), 250 mM NaCl, 25 mM EDTA, and 1% SDS) and 50 μl phenol:chloroform:isoamyl alcohol (25:24:1). Each sample was centrifuged for 10 min at 21,000 x g. 350 μl of the aqueous layer was transferred to a 1.5 ml tube containing 350 μl isopropanol. Samples were then mixed, incubated at room temperature for 15 min, and then spun down at 21,000 x g for 10 min. The supernatant was removed to secure the pellets. 400 μl 70% ethanol was used to wash the pellets. After centrifugation at 21,000 x g for 5 min, the ethanol was removed, and the pellets were dissolved in 50 μl water. Genotypes of individual T-DNA insertion T1 transgenic plants were determined by running two sets of genotyping PCR using GoTaq DNA polymerase (Promega, Madison, WI). WT primer sets amplify the original sequence of the given genes, while the mutant primer sets amplify the mutated sequence with T-DNA insert. Primers are listed in Supplementary Table 1.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
We thank Ling Xu from Yale University for help with protein purification. We also thank members of the Jacob laboratory for their comments and discussion. This project was made possible by a grant (R35GM128661) from the National Institutes of Health (NIH), a Yale Cancer Center Pilot Award to Y.J. (funded by NIH P30CA016359), and an equipment support grant to the Yale Center for Genomic Analysis (NIH 1S10OD030363−01A1). Work in the Voigt lab was supported by the Wellcome Trust ([104175/Z/14/Z], Sir Henry Dale Fellowship (to P.V.) and the UK Biotechnology and Biological Sciences Research Council (BBS/E/B/000C0421). Support for this work in the laboratory of J.C.v.W. came from NIH grant R01AG078926, and NIH grants AI165840 and CA196539 in the lab of B.A.G.
Author contributions
Y.J. supervised the work and conceptualized the study and the experiments with Y.C.H. and W.Y. All the experiments were performed by W.Y., Y.C.H., and C.L. The genomic analyses were done by A.P. and W.Y. Mass spectrometry analysis was performed by F.N.D., R.D., and B.A.G. The nucleosome arrays were generated by D.V. and P.V. Funding for the project was secured by Y.J., J.v.W., P.V, and B.A.G. The manuscript was written by Y.J. and C.L., with contributions from W.Y. and Y.C.H.
Peer review
Peer review information
Nature Communications thanks Cécile Raynaud and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
Sequencing data (RNA-seq, ChIP-seq and DNA-seq datasets) generated in this study are available from the NCBI’s Sequence Read Archive (SRA) under accession numbers PRJNA1121202. The mass spectrometry raw data has been deposited at the MassIVE database with the Proteome Exchange accession code PXD062467. Additional materials reported in this study are available upon request from the corresponding author. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Wenxin Yuan, Yi-Chun Huang.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-58892-2.
References
- 1.Louis, D. N. et al. cIMPACT-NOW update 2: diagnostic clarifications for diffuse midline glioma, H3 K27M-mutant and diffuse astrocytoma/anaplastic astrocytoma, IDH-mutant. Acta Neuropathol.135, 639–642 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Schwartzentruber, J. et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature482, 226–231 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Wu, G. et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat. Genet.44, 251–253 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castel, D. et al. Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol.130, 815–827 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bender, S. et al. Reduced H3K27me3 and DNA hypomethylation are major drivers of gene expression in K27M mutant pediatric high-grade gliomas. Cancer Cell24, 660–672 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Chan, K. M. et al. The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes Dev.27, 985–990 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis, P. W. et al. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science340, 857–861 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venneti, S. et al. Evaluation of histone 3 lysine 27 trimethylation (H3K27me3) and enhancer of Zest 2 (EZH2) in pediatric glial and glioneuronal tumors shows decreased H3K27me3 in H3F3A K27M mutant glioblastomas. Brain Pathol.23, 558–564 (2013). [DOI] [PMC free article] [PubMed]
- 9.Ebert, A. et al. Su(var) genes regulate the balance between euchromatin and heterochromatin in Drosophila. Genes Dev.18, 2973–2983 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrari, K. J. et al. Polycomb-dependent H3K27me1 and H3K27me2 regulate active transcription and enhancer fidelity. Mol. Cell53, 49–62 (2014). [DOI] [PubMed]
- 11.Shen, X. et al. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol. Cell32, 491–502 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasini, D. et al. Characterization of an antagonistic switch between histone H3 lysine 27 methylation and acetylation in the transcriptional regulation of Polycomb group target genes. Nucleic Acids Res.38, 4958–4969 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tie, F. et al. CBP-mediated acetylation of histone H3 lysine 27 antagonizes Drosophila Polycomb silencing. Development136, 3131–3141 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harutyunyan, A. S. et al. H3K27M in gliomas causes a one-step decrease in H3K27 methylation and reduced spreading within the constraints of H3K36 methylation. Cell Rep.33, 108390 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harutyunyan, A. S. et al. H3K27M induces defective chromatin spread of PRC2-mediated repressive H3K27me2/me3 and is essential for glioma tumorigenesis. Nat. Commun.10, 1262 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loyola, A. & Almouzni, G. Marking histone H3 variants: how, when and why? Trends Biochem. Sci.32, 425–433 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Loyola, A., Bonaldi, T., Roche, D., Imhof, A. & Almouzni, G. PTMs on H3 variants before chromatin assembly potentiates their final epigenetic state. Mol. Cell24, 309–316 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Davarinejad, H. et al. The histone H3.1 variant regulates TONSOKU-mediated DNA repair during replication. Science375, 1281–1286 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duro, E. et al. Identification of the MMS22L-TONSL complex that promotes homologous recombination. Mol. Cell40, 632–644 (2010). [DOI] [PubMed] [Google Scholar]
- 20.O’Connell, B. C. et al. A genome-wide camptothecin sensitivity screen identifies a mammalian MMS22L-NFKBIL2 complex required for genomic stability. Mol. Cell40, 645–657 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Donnell, L. et al. The MMS22L-TONSL complex mediates recovery from replication stress and homologous recombination. Mol. Cell40, 619–631 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piwko, W. et al. RNAi-based screening identifies the Mms22L-Nfkbil2 complex as a novel regulator of DNA replication in human cells. EMBO J.29, 4210–4222 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saredi, G. et al. H4K20me0 marks post-replicative chromatin and recruits the TONSL-MMS22L DNA repair complex. Nature534, 714–718 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacob, Y. et al. Selective methylation of histone H3 variant H3.1 regulates heterochromatin replication. Science343, 1249–1253 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacob, Y. et al. ATXR5 and ATXR6 are H3K27 monomethyltransferases required for chromatin structure and gene silencing. Nat. Struct. Mol. Biol.16, 763–768 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacob, Y. et al. Regulation of heterochromatic DNA replication by histone H3 lysine 27 methyltransferases. Nature466, 987–991 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stroud, H. et al. DNA methyltransferases are required to induce heterochromatic re-replication in Arabidopsis. PLoS Genet.8, e1002808 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Bockaj, I. et al. The H3.3K27M oncohistone affects replication stress outcome and provokes genomic instability in pediatric glioma. PLoS Genet.17, e1009868 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giacomini, G. et al., Aberrant DNA repair reveals a vulnerability in histone H3.3-mutant brain tumors. Nucleic Acids Res. 52, 2372–2388 (2024). [DOI] [PMC free article] [PubMed]
- 31.Grobner, S. N. et al. The landscape of genomic alterations across childhood cancers. Nature555, 321–327 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Mackay, A. et al., Integrated molecular meta-analysis of 1000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell32, 520–537.e525 (2017). [DOI] [PMC free article] [PubMed]
- 33.Zhang, Y. et al., Histone H3K27 methylation modulates the dynamics of FANCD2 on chromatin to facilitate NHEJ and genome stability. J. Cell Sci.131, jcs215525 (2018). [DOI] [PubMed]
- 34.Sanders, D. et al. Histone lysine-to-methionine mutations reduce histone methylation and cause developmental pleiotropy. Plant Physiol.173, 2243–2252 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okada, T., Endo, M., Singh, M. B. & Bhalla, P. L. Analysis of the histone H3 gene family in Arabidopsis and identification of the male-gamete-specific variant AtMGH3. Plant J.44, 557–568 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Li, Z. & Ahammed, G. J. Plant stress response and adaptation via anthocyanins: a review. Plant Stress10, 100230 (2023). [Google Scholar]
- 37.Lucht, J. M. et al. Pathogen stress increases somatic recombination frequency in Arabidopsis. Nat. Genet.30, 311–314 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Jacob, Y. & Michaels, S. D. H3K27me1 is E(z) in animals, but not in plants. Epigenetics4, 366–369 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sahu, V. & Lu, C. Oncohistones: hijacking the histone code. Annu. Rev. Cancer Biol.6, 293–312 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fransz, P., De Jong, J. H., Lysak, M., Castiglione, M. R. & Schubert, I. Interphase chromosomes in Arabidopsis are organized as well defined chromocenters from which euchromatin loops emanate. Proc. Natl. Acad. Sci. USA99, 14584–14589 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ingouff, M. et al. Zygotic resetting of the HISTONE 3 variant repertoire participates in epigenetic reprogramming in Arabidopsis. Curr. Biol.20, 2137–2143 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Lindroth, A. M. et al. Dual histone H3 methylation marks at lysines 9 and 27 required for interaction with CHROMOMETHYLASE3. EMBO J.23, 4286–4296 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown, Z. Z. et al. Strategy for “detoxification” of a cancer-derived histone mutant based on mapping its interaction with the methyltransferase PRC2. J. Am. Chem. Soc.136, 13498–13501 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Justin, N. et al. Structural basis of oncogenic histone H3K27M inhibition of human polycomb repressive complex 2. Nat. Commun.7, 11316 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stafford, J. M. et al. Multiple modes of PRC2 inhibition elicit global chromatin alterations in H3K27M pediatric glioma. Sci. Adv.4, eaau5935 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feng, W. et al. Large-scale heterochromatin remodeling linked to overreplication-associated DNA damage. Proc. Natl. Acad. Sci. USA114, 406–411 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Potok, M. E. et al. The role of ATXR6 expression in modulating genome stability and transposable element repression in Arabidopsis. Proc. Natl. Acad. Sci. USA119, e2115570119 (2022). [DOI] [PMC free article] [PubMed]
- 48.Raynaud, C. et al. Two cell-cycle regulated SET-domain proteins interact with proliferating cell nuclear antigen (PCNA) in Arabidopsis. Plant J.47, 395–407 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Li, W. et al. The Arabidopsis AtRAD51 gene is dispensable for vegetative development but required for meiosis. Proc. Natl. Acad. Sci. USA101, 10596–10601 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Kregten, M. et al. T-DNA integration in plants results from polymerase-theta-mediated DNA repair. Nat. Plants2, 16164 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Luedeman, M. E. et al. Poly(ADP) ribose polymerase promotes DNA polymerase theta-mediated end joining by activation of end resection. Nat. Commun.13, 4547 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Symington, L. S. & Gautier, J. Double-strand break end resection and repair pathway choice. Annu. Rev. Genet.45, 247–271 (2011). [DOI] [PubMed] [Google Scholar]
- 53.Syed, A. & Tainer, J. A. The MRE11-RAD50-NBS1 complex conducts the orchestration of damage signaling and outcomes to stress in DNA replication and repair. Annu. Rev. Biochem.87, 263–294 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bundock, P. & Hooykaas, P. Severe developmental defects, hypersensitivity to DNA-damaging agents, and lengthened telomeres in Arabidopsis MRE11 mutants. Plant Cell14, 2451–2462 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang, Y. C., Yuan, W. & Jacob, Y. The role of the TSK/TONSL-H3.1 pathway in maintaining genome stability in multicellular eukaryotes. Int. J. Mol. Sci.23, 9029 (2022). [DOI] [PMC free article] [PubMed]
- 56.Brzezinka, K., Altmann, S. & Baurle, I. BRUSHY1/TONSOKU/MGOUN3 is required for heat stress memory. Plant Cell Environ.42, 771–781 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Valuchova, S. et al. Protection of arabidopsis blunt-ended telomeres is mediated by a physical association with the Ku heterodimer. Plant Cell29, 1533–1545 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heitzeberg, F. et al. The Rad17 homologue of Arabidopsis is involved in the regulation of DNA damage repair and homologous recombination. Plant J.38, 954–968 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Samanic, I., Simunic, J., Riha, K. & Puizina, J. Evidence for distinct functions of MRE11 in Arabidopsis meiosis. PLoS One8, e78760 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J.16, 735–743 (1998). [DOI] [PubMed] [Google Scholar]
- 61.Takeda, S. et al. BRU1, a novel link between responses to DNA damage and epigenetic gene silencing in Arabidopsis. Genes Dev.18, 782–793 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Puizina, J., Siroky, J., Mokros, P., Schweizer, D. & Riha, K. Mre11 deficiency in Arabidopsis is associated with chromosomal instability in somatic cells and Spo11-dependent genome fragmentation during meiosis. Plant Cell16, 1968–1978 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakata, M. & Ohme-Takagi, M. Quantification of anthocyanin content. Bio-Protoc.4, e1098 (2014). [Google Scholar]
- 64.Gyori, B. M., Venkatachalam, G., Thiagarajan, P. S., Hsu, D. & Clement, M. V. OpenComet: an automated tool for comet assay image analysis. Redox Biol.2, 457–465 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics34, i884–i890 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lamesch, P. et al. The Arabidopsis Information Resource (TAIR): improved gene annotation and new tools. Nucleic Acids Res.40, D1202–D1210 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lê, S., Josse, J. & Husson, F. FactoMineR: an R package for multivariate analysis. J. Stat. Softw.25, 1–18 (2008). [Google Scholar]
- 69.Noda, M. et al. Salmon: scalable ab-initio light-matter simulator for optics and nanoscience. Comput. Phys. Commun.235, 356–365 (2019). [Google Scholar]
- 70.Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics30, 923–930 (2014). [DOI] [PubMed] [Google Scholar]
- 71.Panda, K. et al. Full-length autonomous transposable elements are preferentially targeted by expression-dependent forms of RNA-directed DNA methylation. Genome Biol.17, 170 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wickham, H. ggplot2 213 (Springer, 2009).
- 73.Villar, C. B. & Kohler, C. Plant chromatin immunoprecipitation. Methods Mol. Biol.655, 401–411 (2010). [DOI] [PubMed] [Google Scholar]
- 74.Orlando, D. A. et al. Quantitative ChIP-Seq normalization reveals global modulation of the epigenome. Cell Rep.9, 1163–1170 (2014). [DOI] [PubMed] [Google Scholar]
- 75.Dong, J. et al. H3.1K27me1 maintains transcriptional silencing and genome stability by preventing GCN5-mediated histone acetylation. Plant Cell33, 961–979 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nassrallah, A. et al. DET1-mediated degradation of a SAGA-like deubiquitination module controls H2Bub homeostasis. Elife7, e37892 (2018). [DOI] [PMC free article] [PubMed]
- 77.Ramirez, F. et al. deepTools2: a next-generation web server for deep-sequencing data analysis. Nucleic Acids Res.44, W160–W165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol.9, R137 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics26, 841–842 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scheid, R. et al. Histone acid extraction and high throughput mass spectrometry to profile histone modifications in Arabidopsis thaliana. Curr. Protoc.2, e527 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Voigt, P. et al. Asymmetrically modified nucleosomes. Cell151, 181–193 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jacob, Y. & Voigt, P. In vitro assays to measure histone methyltransferase activity using different chromatin substrates. Methods Mol. Biol.1675, 345–360 (2018). [DOI] [PubMed] [Google Scholar]
- 83.Clarke, J. D. Cetyltrimethyl ammonium bromide (CTAB) DNA miniprep for plant DNA isolation. Cold Spring Harb. Protoc.2009, pdb prot5177 (2009). [DOI] [PubMed]
- 84.Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics25, 2078–2079 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Team R. C. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2018).
- 86.Hahne, F. & Ivanek, R. Visualizing genomic data using Gviz and bioconductor. Methods Mol. Biol.1418, 335–351 (2016). [DOI] [PubMed] [Google Scholar]
- 87.Bhanu, N. V., Sidoli, S. & Garcia, B. A. A workflow for ultra-rapid analysis of histone post-translational modifications with direct-injection mass spectrometry. Bio Protoc.10, e3756 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sidoli, S. Bhanu, N. V. Karch, K. R. Wang, X. Garcia, B. A. Complete workflow for analysis of histone post-translational modifications using bottom-up mass spectrometry: from histone extraction to data analysis. J. Vis. Exp.2016, e54112 (2016). [DOI] [PMC free article] [PubMed]
- 89.Yuan, Z. F. et al. EpiProfile quantifies histone peptides with modifications by extracting retention time and intensity in high-resolution mass spectra. Mol. Cell Proteom.14, 1696–1707 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Sequencing data (RNA-seq, ChIP-seq and DNA-seq datasets) generated in this study are available from the NCBI’s Sequence Read Archive (SRA) under accession numbers PRJNA1121202. The mass spectrometry raw data has been deposited at the MassIVE database with the Proteome Exchange accession code PXD062467. Additional materials reported in this study are available upon request from the corresponding author. Source data are provided with this paper.