Abstract
Natriuretic peptides (NPs) have an important role in lipid metabolism in skeletal muscle and adipose tissue in animals. C-type natriuretic peptide (CNP) is an important NP, but the molecular mechanisms that underlie its activity are not completely understood. Treatment of intramuscular fat (IMF) and subcutaneous fat (SCF) adipocytes with CNP led to decreased differentiation, promoted proliferation and lipolysis, and increased the expression of natriuretic peptide receptor B (NPRB) mRNA. Silencing natriuretic peptide C (NPPC) had the opposite results in IMF and SCF adipocytes. Transcriptome analysis found 665 differentially expressed genes (DEGs) in IMF adipocytes and 991 in SCF adipocytes. Seven genes in IMF adipocytes (FABP4, APOA1, ACOX2, ADIPOQ, CD36, FABP5, and LPL) and eight genes in SCF adipocytes (ACOX3, ACSL1, APOA1, CPT1A, CPT2, FABP4, PDPK1 and PPARα) are related to fat metabolism. Fifteen genes were found to be enriched in the peroxisome proliferator-activated receptor (PPAR) pathway. Integrated analysis identified 113 intersection genes in IMF and SCF adipocytes, two of which (APOA1 and FABP4) were enriched in the PPAR pathway. In conclusion, CNP may regulated lipid metabolism through the NPRB-PPAR pathway in both IMF and SCF adipocytes, FABP4 and APOA1 may be the key genes that mediated CNP regulation of fat deposition.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-86433-w.
Keywords: C-type natriuretic peptide, Intramuscular adipocytes, Subcutaneous adipocytes, PPAR pathway
Subject terms: Molecular biology, Transcriptomics
Background and summary
Subcutaneous fat (SCF), abdominal fat (AbF), and intramuscular fat (IMF) are present in poultry. IMF content is associated with tenderness, flavor, and juiciness1. Excess SCF and abdominal fat are discarded during slaughtering and processing and have been reported to cause environmental pollution2. The contribution of SCF deposition is generally not considered in broiler production, and for that reason, high IMF and low SCF deposition are the main objectives of broiler breeding. Study of the differences in the molecular activity resulting in IMF or SCF deposition would have important theoretical and practical significance.
Hormones are bioactive substances that regulate growth and development, and they also regulate lipid metabolism. Thyroid hormones directly or indirectly stimulate hepatic lipid synthesis and degradation and help to maintain lipid metabolism and energy balance3. Growth hormone stimulates adipogenesis in 3T3-L1 cells4. Follicle stimulating hormone (FSH) has been shown to promote the differentiation of abdominal adipocytes5, and enhance IMF content in breast muscle6. Adiponectin7and estrogen8 were found to decrease fat deposition in chickens. Different hormones have different roles in the regulation of lipid metabolism.
Natriuretic peptides (NPs) are peptide hormones, including atrial natriuretic peptide (ANP)9, brain natriuretic peptide (BNP)10, and C-type natriuretic peptide (CNP)11in mammals, and BNP, CNP and VNP in poultry12. NPs act by binding to specific natriuretic peptide receptor A (NPRA) and natriuretic peptide receptor B (NPRB)13and have different effects on adipose tissue and skeletal muscle metabolism. ANP and BNP were reported to promote lipolysis in human fat cells14, control brown fat thermogenesis in mice15, and participate in mitochondrial oxidative metabolism and fat oxidation in human skeletal muscle myotubes16. CNP was shown to regulate food intake and energy expenditure in mice17, and overexpression in endothelial cells increased energy expenditure and improved insulin sensitivity and hepatic lipid metabolism in a high-fat diet-induced obesity model18. The results of previous studies show that NPs are involved in lipid metabolism in adipose tissue and skeletal muscle in mammals.
BNP stimulates lipolysis in the abdominal adipocytes of chickens by upregulating the expression of its receptor NPRA19, but the relationship between CNP and lipid metabolism is not clear. The evidence of studies in mammals suggests that CNP would be expected to regulate lipid metabolism in adipose tissue and skeletal muscle in chickens. This study aimed to clarify the effect of CNP on lipid metabolism in chickens and the molecular mechanisms underlying its activity in adipose tissue and skeletal muscle. Study of differences in the molecular pathways of CNP in lipid metabolism in adipose and skeletal muscle tissues would add to our understanding of the molecular mechanisms of NP regulation of lipid metabolism and provide important theoretical guidance for the breeding of high-quality broilers.
Methods
Preadipocyte culture
SCF preadipocytes were isolated from S3 strain chickens (Institute of Poultry Science, Jiangsu, China) at 7–10 days of age as previously described20. This strain has yellow feathers, green feet, good meat quality and excellent production performance. Cells were cultured in 96-well and 6-well culture dishes in Dulbecco’s Modified Eagle Medium (DMEM)/F12 (1:1) containing 10% fetal bovine serum and 1% penicillin/streptomycin at 37 °C in a humidified atmosphere of 5% CO2in air. When the cells reached 70% confluence, differentiation was induced with oleic acid (305 µmol/L). IMF preadipocytes were isolated from breast muscle tissue of female chickens of the same strain and age as previously described21. The method of cell culture was the same as for that of SCF adipocytes.
CNP treatment
Chicken CNP (Phoenix Pharmaceuticals, Burlingame, CA USA), was added to the culture media at concentrations of 10−6, 10−7 and 10−8 mol/L on day 0. The control group was (DMEM)/F12 (1:1) containing 10% fetal bovine serum and 1% penicillin/streptomycin. Based on phenotype data from the preliminary experiment, 10−7 mol/L was determined as the optimal concentration (the data is not shown). Cell proliferation was assayed with a Cell Counting Kit-8 (CCK-8; Dojindo, Kyushu, Japan) and 5-ethynyl-2’-deoxyuridine (EdU) staining (Ruibo, Guangzhou, China) in eight replicate wells, with three replicate wells for each treatment. Adipogenesis was monitored by morphologic examination of the cellular accumulation of lipid droplets by Oil Red O staining. On days 3 and 6, adipocytes were fixed with 10% formaldehyde, washed with phosphate buffered saline, and stained with Oil Red O (0.3% in 60% isopropanol) followed by extensive washes. Stained triglyceride droplets were visualized and photographed.
NPPC siRNA synthesis and transfection
The NPPC siRNA sequence was synthesized by GenePharma (Shanghai GenePharma, Shanghai, China). According to the mRNA sequence of chicken NPPC (NC_006108.3), three pairs of siRNA primers were designed with Oligo Designer version 3.0 (Shanghai GenePharma) (Table 1), and the NPPC−221 primer was validated in vitro.
Table 1.
NPPC siRNA primers.
| Primer name | Sense(5’−3’) | Antisense(5’−3’) |
|---|---|---|
| NPPC-Gallus-68 | GGCAAUGGCCAAACCUAUUTT | AAUAGGUUUGGCCAUUGCCTT |
| NPPC-Gallus-110 | GCUGUUAGAUGAGGAGCUGTT | CAGCUCCUCAUCUAACAGCTT |
| NPPC-Gallus-221 | GACCCGAAACACCAGAGAUTT | AUCUCUGGUGUUUCGGGUCTT |
Preadipocytes were plated on 12-well or 96-well plates in DMEM/F12 without antibiotics. Transfection was performed at 70–80% confluency (Qiagen, Düsseldorf, Germany). Adipocytes were cultured in 12-well plates and transfected with NPPC−221 siRNA (1,000 nmol per well) before evaluating gene expression by quantitative real-time polymerase chain reaction (qPCR) and cell differentiation by Oil Red O staining. After transfection with NPPC−221 siRNA (250 nmol per well), proliferation was monitored in each study group by CCK8 assays of cells growing in 96-well plates.
Lipolysis
Chicken CNP (10−7 mol/L) was added to the media, and the differentiated adipocytes were incubated for an additional 6 days. The glycerol concentration in the medium was determined following each treatment in three replicate wells using a commercially available assay kit (Jiancheng, Nanjing, China).
RNA extraction
Total RNA was isolated from adipocytes using extraction kits (Qiagen, Düsseldorf, Germany) following the manufacturer’s instructions. An ND-1000 spectrophotometer (Agilent Technologies, Palo Alto, CA, USA) was used to measure the concentration and purity of the RNA (A260:280 ≥ 1.8 and ≤ 2.0). RNA integrity (RIN ≥ 7) was evaluated with a 2100 Bioanalyzer Lab-on-Chip system (Agilent Technologies). RNA samples were stored at − 80 °C until used.
mRNA sequencing
Total RNA from SCF and IMF adipocytes treated with and without 10−7mol/L CNP for 6 days was used for mRNA sequencing in three replicate wells for each treatment. Data were analyzed as previously described19. Fragments per kilobase of exon per million mapped reads (FPKM) was used to quantify gene expression. Differentially expressed genes (DEGs) all had p-values ≤ 0.05 and fold changes ≥ 1.5. Significantly enriched pathways were analyzed by Kobas v3.0 (kobas.cbi.pku.edu.cn/help.do) as described by Mao et al.22 and Wu et al.23. Pathways with < 3 known chicken genes were discarded.
qPCR of genes
Gene expression was quantified by qPCR (Qiagen, Düsseldorf, Germany). The final concentration of each primer (Table 2) was 10 µmol/µL.
Table 2.
qPCR primers.
| Gene | Sequence (5’ to 3’) |
|---|---|
| CPT2 | F: TTATCCGGTTTGTGCCTTCGT |
| R: ATTCCCCTTTCTCAGTACCAGT | |
| PDPK1 | F: AAATCTGCCTGTAAAAGCTCT |
| R: AGCCTAATCTTTTGGTAGCATC | |
| PPARA | F: CCATTACGGAGTACATGCTT |
| R: AAAGGCACTTCTGAAAACGA | |
| LPL | F: GGTGACCTGCTTATGCTA |
| R: ATATTGCTGCCTCTTCTC | |
| ACOX2 | F: CATGCTTCGTGCCTTTCGACT |
| R: CCCTTCATTGTGCAGATCCG | |
| ADIPOQ | F: GCACGTACTTCTTTGCCTACCAC |
| R: GTTGCCCTCCCCGTACACC | |
| CD36 | F: ATTGCATATGATAATTGGCTTG |
| R: CATGTACGATATTGTGCCAT | |
| FABP5 | F: TGTGAAAACGGAAAGCACCT |
| R: CTGCTCCTGGATCAATGACC | |
| FABP4 | F: TGGCCGTGAAGATGTTGAAAG |
| R: CGCAGGTATTCCCGAAGGTTG | |
| APOA1 | F: GGACCAGTTCTCCGCCAAGT |
| R: TGCACCAGAGGCGTCATC | |
| ACOX3 | F: TTACTGTCGATCATTAGCCAT |
| R: CATAGCCACCTTGATAGAGC | |
| ACSL1 | F: CAAAGGAGAAGGTGAGGTGTG |
| R: CTTCAACGTACCGTTTGGTAG | |
| CPT1A | F: ATGTTTAATACCTCCCGCATC |
| R: CCTATCTCCTGCAGTAAGAGC | |
| NPPC | F: ACTTCTTTTTTGCCCTGGATTCTTC |
| R: CTACTGGGATTGAGCCATCTTGC |
Statistical analysis
Significant differences were assessed by Student’s t-test using SPSS, version 19.0 (IBM Corp; Armonk, USA). P < 0.05 was considered significant.
Data Availability
The datasets used and/or analyzed in this study are available from the corresponding author on reasonable request.
Data records
Effect of CNP on lipid metabolism in IMF adipocytes
IMF adipocyte cell morphology Adipocytes isolated from breast muscle were cultured for 2 days in T25 flasks and evaluated with an inverted microscope. Mature adipocytes began to cluster, a few spindle cells appeared, and some mature adipocytes began to dedifferentiate (Fig. 1A). On day 5, the number of prespindle adipocytes had increased (Fig. 1B); on day 7, the dedifferentiated cells proliferated abundantly (Fig. 1C) and were subcultured. The rate of cell proliferation was high (Fig. 1D). Lipid droplets were clearly visible after induced differentiation for 3 days (Fig. 1E), and large, round, red lipid droplets were visible 6 days after differentiation induced by oleic acid, confirming that the cells were IMF adipocytes (Fig. 1F).
Fig. 1.
Morphological changes of intramuscular preadipocytes (inverted microscope, 100×). (A–C) Cellular morphology of IMF preadipocytes 2, 5, and 7 days after isolation. (D) Morphology of IMF adipocytes 24 h after passage. (E) Differentiation of IMF adipocytes cultured with oleic acid for 3 days. (F) IMF adipocytes stained with Oil red O after induced differentiation for 3 days.
CNP induction enhanced IMF preadipocyte proliferation, inhibited adipocyte differentiation, and promoted lipolysis
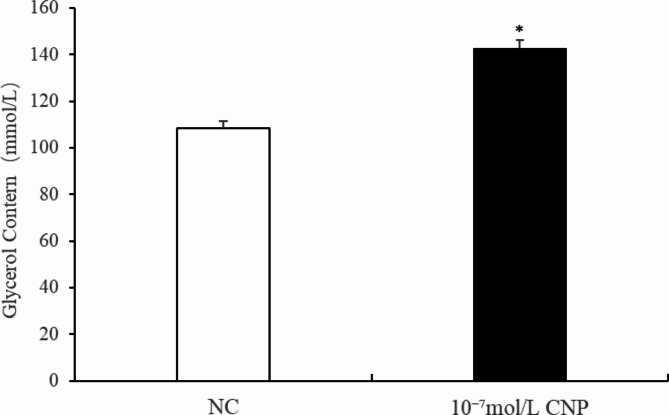
The number of preadipocytes increased significantly (p < 0.05) after 24 and 72 h of culture with 10−7 mol/L CNP (Fig. 2A). EdU staining also confirmed that the proliferation rate of CNP-induced cells was increased compared with control cells (Fig. 2B). Differentiated adipocytes were treated with exogenous 10−7 mol/L CNP for 3 and 6 d. As shown in Fig. 3A, adipocyte differentiation decreased significantly on treatment with 10−7 mol/L CNP (p < 0.05). Oil Red O staining showed that the lipid droplets were slightly smaller and fewer in number in cells treated with 10−7 mol/L CNP compared with controls (Fig. 3B) Lipolysis was evaluated by measuring glycerol levels in the culture medium. The effects of CNP treatment on the lipolysis of adipocytes are shown in Fig. 4. CNP treatment significantly enhanced glycerol levels compared with those in control cultures at 6 d (p < 0.05).
Fig. 2.
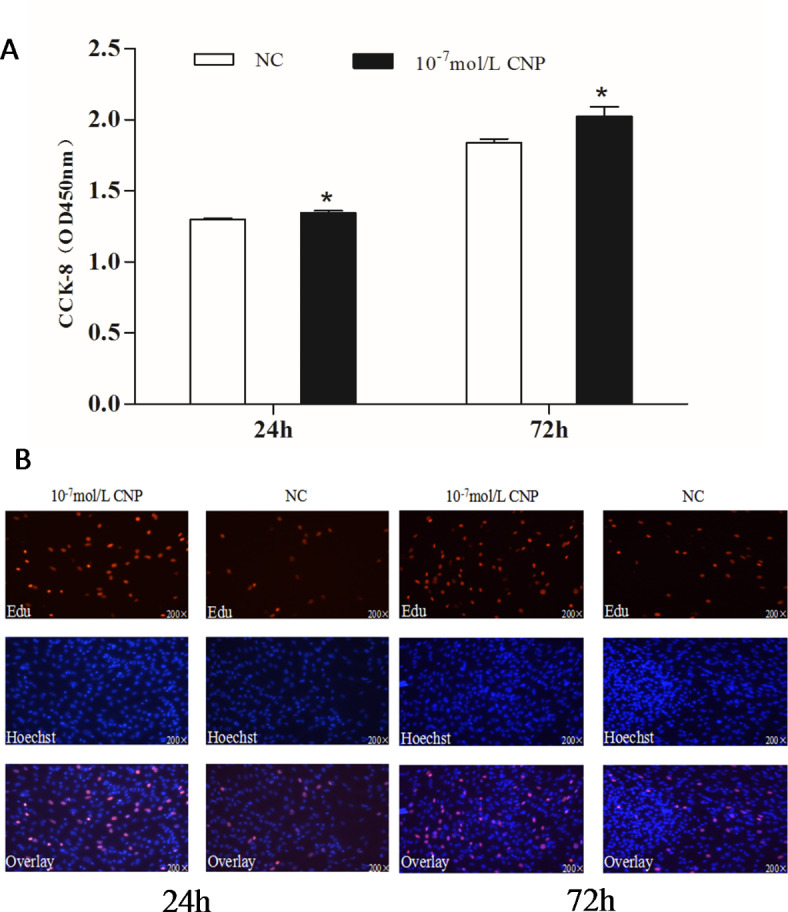
Effect of CNP on the proliferation of intramuscular preadipocyte. (A) The number of preadipocytes was significantly increased by CNP (Cell Counting Kit-8, n = 8, *p < 0.05 vs. control group). (B) EdU staining of intramuscular preadipocytes induced by CNP for 24 h and 72 h (n = 3). EdU, number of proliferative cells; Hoechst, number of cells before proliferation; Overlay, superimposition of the two images shows the location of proliferating cells.
Fig. 3.
Effect of CNP on differentiation of intramuscular adipocytes. (A) CNP 10−7 mol/L significantly decreased the differentiation of adipocytes (n = 3; **p < 0.01 vs. control). (B) Morphologic changes and lipid deposition induced by 10−7 mol/L CNP in adipocytes in vitro (inverted microscope, 400× magnification). Oil Red O staining shows that CNP 10−7 mol/L decreased both the size and number of the droplets in each accumulation compared with cells not treated with CNP.
Fig. 4.
Change of the glycerol content at 6 days after CNP treatment in intramuscular adipocytes (n = 3), (**p < 0.01 vs. control).
NPPC interference reduced cell proliferation, enhanced adipocyte differentiation and reduced lipolysis in IMF adipocytes
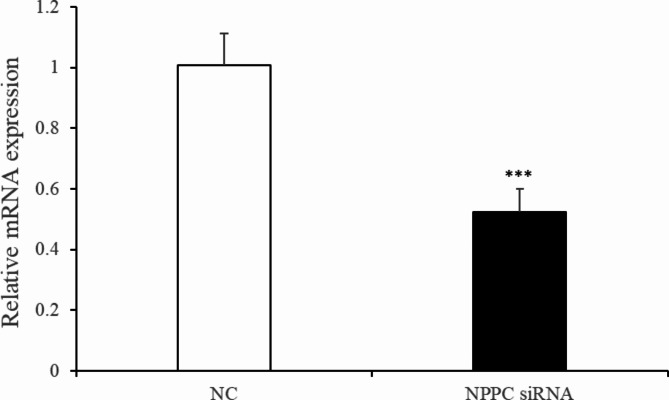
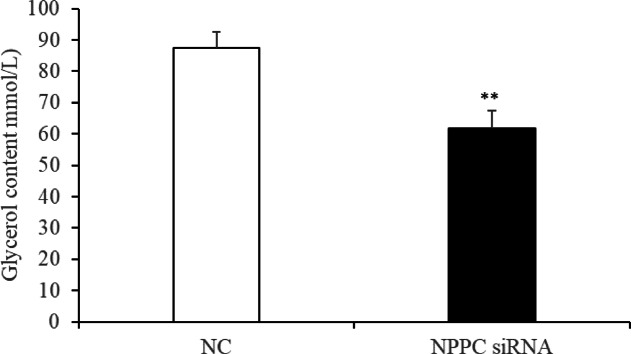
Preadipocytes were transfected with either the NPPC siRNA or the negative control vector. The expression of NPPC mRNA, compared with that of the control group, decreased significantly at 24 h after transfection with NPPC siRNA (Fig. 5, p < 0.01). The effects of NPPC interference on the proliferation of preadipocytes and their differentiation into adipocytes are shown in Figs. 6 and 7, respectively. The number of cells was significantly reduced at both 24 and 72 h after NPPC interference (Fig. 6, p < 0.05). Oil Red O staining showed that the lipid droplets were slightly larger, and their numbers were increased in groups treated with NPPC siRNA compared with those in control groups (Fig. 7B). NPPC interference significantly reduced glycerol levels compared with those in control cultures at 4 d (Fig. 8, p < 0.05).
Fig. 5.
Change of NPPC mRNA expression after 24 h transfection with NPPC siRNA in the intramuscular adipocytes.
Fig. 6.
Effect of NPPC interference on the proliferation of intramuscular preadipocytes. (A) Number of preadipocytes after transfection with NPPC siRNA (CCK8 kit, n = 8, *p < 0.05 vs. control group).); B. EdU staining of chicken preadipocytes after transfection with NPPC siRNA. EdU, number of proliferative cells; Hoechst, number of cells before proliferation; Overlay, superimposition of the two images shows the location of proliferating cells.
Fig. 7.
Effect of NPPC interference on the differentiation of intramuscular adipocytes. (A) Adipocyte differentiation increased significantly after transfection with NPPC siRNA (n = 3) (**p < 0.01 vs. control). (B) Morphologic changes and lipid deposition after transfection with NPPC siRNA in adipocytes in vitro (inverted microscope, 400× magnification). Lipid droplets (stained with Oil Red O) accumulated as larger clusters and in greater numbers in cells transfected with NPPC siRNA compared with the negative control vector.
Fig. 8.
Change of the glycerol content at 4 d after transfection with NPPC siRNA in intramuscular adipocytes. (n = 3), (**p < 0.01 vs. control).
Molecular mechanism of CNP regulation of IMF lipid deposition
CNP promoted NPRBmRNA expression in IMF adipocytes As shown in Fig. 9, treatment with exogenous 10−7 mol/L CNP for 6 d significantly increased the expression of NPRB mRNA (p < 0.05). The difference in NPRA expression in treated compared with control cells was not significant (p > 0.05).
Fig. 9.
Change of NPRB expression in intramuscular adipocytes at 6 d after CNP treatment ( n = 3), (*p < 0.05 and **p < 0.01 vs. control).
Key genes and pathways responding to CNP regulation in IMF adipocytes
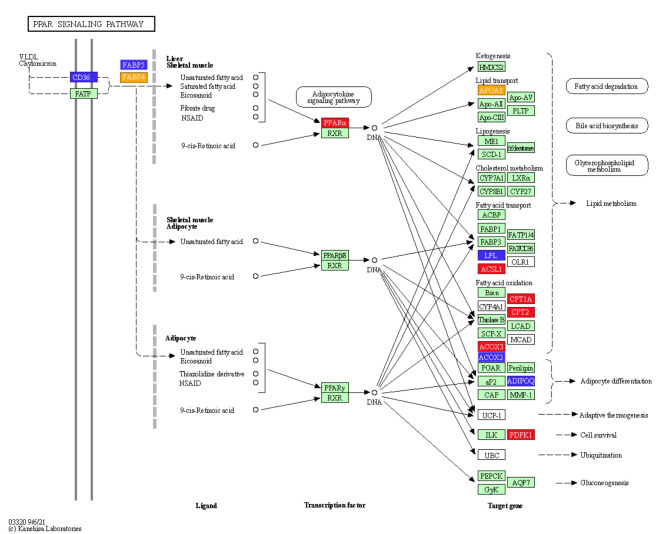
mRNA sequencing was used to identify key genes and pathways by which CNP influenced lipid metabolism in IMF adipocytes. DEGs were detected in total RNA isolated from cells treated with 10−7 mol/L CNP and control cells not exposed to CNP. The quality control data are shown in Table S1. A total of 665 DEGs (p < 0.05; log > 1.5 or < 0.67) were identified; 323 were upregulated and 342 were downregulated (Table S2). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of the DEGs found 11 pathways that were significantly enriched (Table 3, p ≤ 0.01). They were the PPAR signaling pathway, calcium signaling pathway, adrenergic signaling in cardiomyocytes, focal adhesion, cardiac muscle contraction, phosphatidylinositol signaling system, neuroactive ligand-receptor interaction, regulation of actin cytoskeleton, extracellular matrix-receptor interaction, AGE-RAGE signaling pathway in diabetic complications, and cell adhesion molecules. Seven genes (FABP4, APOA, ACOX2, ADIPOQ, CD36, FABP5and LPL) enriched in the PPAR pathway were reported to be closely related to lipid metabolism (Fig. 10; Table 3). qPCR verified seven of the DEGs identified by deep sequencing, and the RNA amplified from cells treated with 0 and 10−7 mol/L CNP validated the gene expression profile analysis. There was consistency between the qPCR assays and the deep sequencing analysis in terms of the direction of regulation and statistical significance (Fig. 11, p < 0.05). Therefore, it was hypothesized that CNP regulated lipid metabolism in IMF adipocytes via the NPRB-PPAR pathway and by promoting the expression of genes (e.g., FABP4, APOA, ACOX2, ADIPOQ, CD36, FABP5 and LPL) enriched in the PPAR pathway.
Table 3.
Significantly enriched pathways for differentially expressed genes in intramuscular adipocytes.
| Pathway | Enriched genes | P–value |
|---|---|---|
| Calcium signaling pathway | ADCY2,ADRA1D, ATP2B2,CACNA1H, CAMK1D, CAMK2A, CAMK4,CASQ2,CD38,CXCR4,HTR2B, HTR4,ip3ka, ITPR3,NCX1,PLCD4,TBXA2R, TNNC1,TNNC2 | 0.000 |
| Adrenergic signaling in cardiomyocytes | ACTC1,ADCY2,ADRA1D, ATP1B1,ATP2B2,BCL2,CAMK2A, CREB5,Myl2,MYL3,MYL4,NCX1,TNNC1,TNNT2 | 0.000 |
| Focal adhesion | ACTB, BCL2,CAV3,CHAD, fn1,IGF1,ITGA1,ITGA9,LAMA5,Myl2,MYL9,PDGFB, PGF, PPP1R12B, SHC4,THBS1, VTN | 0.000 |
| Cardiac muscle contraction | ACTC1,ATP1B1,CASQ2,Myl2,MYL3,MYL4,NCX1,TNNC1,TNNT2, UQCR11 | 0.000 |
| Phosphatidylinositol signaling system | DGKB, DGKG, DGKQ, INPP5J, ip3ka, IP6K3,ITPK1,ITPR3,MTMR1,PIK3C2A, PLCD4 | 0.000 |
| Neuroactive ligand-receptor interaction | ADM, ADRA1D, CHRM4,CHRNA1,CHRND, CHRNG, CNR1,CRHR2,EDN1,EDN2,EP4,GIPR, GRID1,GRM3,HTR2B, HTR4,PENK, S1PR3,SS2R, TBXA2R, VPAC2 | 0.001 |
| Regulation of actin cytoskeleton | ACTB, APC2,ARHGEF4,CHRM4,CXCR4,FGF7,Fgf9,fn1,IQGAP2,ITGA1,ITGA9,Myl2,MYL9,PDGFB, PPP1R12B | 0.001 |
| ECM-receptor interaction | CD36,CHAD, fn1,ITGA1,ITGA9,LAMA5,NPNT, THBS1,VTN | 0.006 |
| AGE-RAGE signaling pathway in diabetic complications | BCL2,CDKN1B, EDN1,F3,fn1,IL6,NOX1,PLCD4,SELE | 0.007 |
| Cell adhesion molecules (CAMs) | CADM3,CDH3,CDH4,CLDN1,ITGA9,NFASC, NRCAM, SELE | 0.010 |
| PPAR signaling pathway | ACOX2,ADIPOQ, APOA1,CD36,FABP4,FABP5,LPL | 0.010 |
Fig. 10.
Fig. 11.
Validation of differentially expressed genes by qPCR (n = 3), (**p < 0.01).
Effect of CNP on lipid metabolism in SCF adipocytes
CNP increased preadipocyte proliferation, decreased adipocyte differentiation and facilitated adipocyte lipolysis The number of preadipocytes was significantly increased by culture with 10−7 mol/L CNP for 24 and 48 h compared with controls (Fig. 12A, p < 0.05). EdU staining confirmed that the proliferation rate of CNP-induced cells was increased compared with control cells (Fig. 12B). Differentiated adipocytes were treated with exogenous 10−7 mol/L CNP for 3 and 6 d. Oil Red O staining showed that the lipid droplets were smaller and their numbers were decreased in study groups treated with 10−7 mol/L CNP compared with the control groups (Fig. 13A). The results showed that adipocyte differentiation was significantly decreased by treatment with CNP (Fig. 13B, p < 0.05). As shown in Fig. 14, CNP treatment significantly increased glycerol levels compared with the control group at 6 d. The results showed that CNP promoted lipolysis in SCF adipocytes.
Fig. 12.
Effect of CNP on the proliferation of subcutaneous preadipocytes.(A) Proliferation of preadipocytes induced for 24 h and 48 h by CNP (n = 8, * p < 0.05 vs. control group). (B) EdU staining of intramuscular preadipocytes induced for 24 h and 72 h by CNP (n = 3). EdU, number of proliferative cells; Hoechst, number of cells before proliferation; Overlay, superimposition of the two images shows the location of proliferating cells. *p < 0.05 vs. control group.
Fig. 13.
Effect of CNP on the differentiation of subcutaneous adipocytes. A, Morphologic changes and lipid deposition induced by 10−7mol/L CNP in adipocytes in vitro (inverted microscope, 200× magnification). Lipid droplets (stained with Oil Red O) were smaller and accumulated in fewer groups in cells exposed to 10−7mol/L CNP compared with untreated cells. B, Differentiation of adipocytes was significantly decreased by 10−7 mol/L CNP. (n = 3, **p < 0.01 vs. control).
Fig. 14.
Change of the glycerol content at 6 days after CNP treatment in subcutaneous adipocytes. (n = 3), (*p < 0.05 vs. control).
NPPC interference reduced cell proliferation, enhanced adipocyte differentiation, and reduced lipolysis. NPPC mRNA expression was significantly decreased compared with the control group at 24 h after transfection of preadipocytes with NPPC siRNA (Fig. 15, p < 0.05). As shown in Fig. 16, the number of cells was reduced significantly at 24 and 72 h after transfection with NPPC siRNA (p < 0.05). Lipid accumulation was increased at 3 d (Fig. 17A, p < 0.01). Staining with Oil Red O showed that the lipid droplets were slightly larger, and their number was increased in groups transfected with NPPC siRNA compared with the control groups (Fig. 17B). NPPC interference significantly reduced the glycerol level at 4 d compared with the control cultures (Fig. 18, p < 0.05).
Fig. 15.
Change of NPPC mRNA expression in subcutaneous adipocytes after transfection with NPPC siRNA for 24 h. (n = 3) (**p < 0.01).
Fig. 16.
Effect of NPPC interference on the proliferation of subcutaneous preadipocytes (n = 5) (**p < 0.01 vs. control). (A) The number of preadipocytes assayed by CCK8 kits after NPPC interference; (B) EdU staining assay of chicken preadipocytes transfected with NPPC siRNA. EdU, number of proliferative cells; Hoechst, number of cells before proliferation; Overlay, superimposition of the two images shows the location of proliferating cells.
Fig. 17.
Effect of NPPC interference on the differentiation of subcutaneous adipocytes (A) The differentiation of adipocytes was significantly increased after transfection with NPPC siRNA (n = 3) (**p < 0.01 vs. control). (B) Morphologic changes and lipid deposition after transfection with NPPC siRNA in adipocytes in vitro (inverted microscope, 400× magnification). Lipid droplets (stained with Oil Red O) accumulated in greater numbers and in larger groups in cells transfected with NPPC siRNA compared with the negative control vector.
Fig. 18.
Change of the glycerol content at 4 d after transfection with NPPC siRNA in subcutaneous adipocytes. (n = 3), (**p < 0.01 vs. control).
Molecular mechanism of CNP regulation of lipid deposition in SCF adipocytes
CNP enhanced NPRB expression qPCR showed that CNP treatment significantly increased the expression of NPRB mRNA at 6 d (Fig. 19, p < 0.05) and that differences in.
Fig. 19.
Change of NPRB expression in subcutaneous adipocytes at 6 d after CNP treatment ( n = 3), (*p < 0.05 and **p < 0.01 vs. control).
NPRA mRNA expression in treated and control cells were not significant.
Key genes and pathways responding to CNP regulation
Key DEGs and pathways were detected by mRNA sequencing of total RNA extracted from SCF adipocytes treated with 10−7 mol/L CNP and from control cells. The quality control data are shown in Table S1. A total of 991 DEGs (p < 0.05; log 1.5 > 1.5 or < 0.67) were identified, with 136 upregulated genes and 855 downregulated genes (Table S3). After KEGG pathway analysis of the DEGs, 12 pathways were significantly enriched (Table 4, p ≤ 0.01). They were PPAR signaling, ubiquitin mediated proteolysis, animal autophagy, vascular smooth muscle contraction, focal adhesion, regulation of actin cytoskeleton, apoptosis, endocytosis, transforming growth factor-beta signaling, progesterone-mediated oocyte maturation, protein processing in the endoplasmic reticulum, and NOD-like receptor signaling pathway. Eight genes (ACOX3, ACSL1, APOA1, CPT1A, CPT2, FABP4, PDPK1 and PPARα) enriched in PPAR pathway were reported to be closely related to lipid metabolism. qPCR verified the consistency of the eight DEGs identified by deep sequencing in terms of the direction of regulation and statistical significance (Fig. 20., p < 0.05). Taken together, the results support the hypothesis that CNP regulated lipid metabolism in SCF adipocytes via the NPRB-PPAR pathway and by increasing the expression of genes (e.g., ACOX3, ACSL1, APOA1, CPT1A, CPT2, FABP4, PDPK1 and PPARα) enriched in the PPAR pathway.
Table 4.
Significantly enriched pathways of differentially expressed genes in subcutaneous adipocytes.
| Pathway | Enriched genes | P-value |
|---|---|---|
| Ubiquitin mediated proteolysis | BIRC6,CUL1,CUL4A, CUL4B, CUL5,HERC1,HERC2,IAP3,ITCH, NEDD4,TRIP12,UBA6,UBE2L3,UBE3C, ANAPC4 | 0.007 |
| Autophagy - animal |
ATG13,ATG2B, CFLAR, EIF2AK4,MAPK1,MAPK9,Metazoa_SRP, PDPK1,PIK3C3 PIK3CA, PIK3CB, PRKAA1,PRKCQ, RB1CC1,RRAGD |
0.009 |
| Vascular smooth muscle contraction |
ADCY2,CALCRL, CALD1,cRhoA, GUCY1B3,MAPK1,MBSP, MRVI1,MYLK, PLA2G2E, PLA2G4A PRKCH, PRKCQ, ROCK1, ROCK2 |
0.009 |
| Focal adhesion | ARHGAP5,COL1A2,cRhoA, IAP3,ITGA2,ITGA4,ITGAV, ITGB1,ITGB8,MAPK1,MAPK9,MBSP, MYLK, PARVA, PDPK1,PIK3CA, PIK3CB, ROCK1,ROCK2, VTN, | 0.009 |
| Regulation of actin cytoskeleton | APC, ARHGEF4,BAIAP2,BDKRB1,cRhoA, DIAPH1,Fgf9,ITGA2,ITGA4,ITGAV, ITGB1,ITGB8,MAPK1,MBSP, MYLK, PIK3CA, PIK3CB, ROCK1,ROCK2, SCIN | 0.010 |
| Apoptosis | SPTAN1,PIK3CB, PIK3CA, PDPK1,MAPK9,MAPK1,IAP3,CTSZ, CTSO, CFLAR, CASP3,CASP2,ATM APAF1 | 0.031 |
| Endocytosis |
AP2B1,ARFGEF2,CAPZA1,cRhoA, DAB2,DNM3,EEA1,EHD4,IGF2R, IQSEC1,ITCH, KIF5B, NEDD4 RAB11FIP2,RABEP1,RBSN, STAM, TFRC, VPS29, VPS4B |
0.040 |
| TGF-beta signaling | THSD4,ROCK1,MAPK1,INHBA, DCN, CUL1,cRhoA, CHRD, BMPR-II, BMPR1A, AMH | 0.041 |
| PPAR signaling | ACOX3,ACSL1,APOA1,CPT1A, CPT2,FABP4,HMGCS1,PDPK1,PPARA | 0.043 |
| Progesterone-mediated oocyte maturation | ADCY2,ANAPC4,BUB1,HSP90AA1, MAPK1, MAPK9,PIK3CA, PIK3CB, RPS6KA3,RPS6KA6 | 0.045 |
| Protein processing in endoplasmic reticulum |
CUL1,DNAJC5,EIF2AK4,HSP90AA1,HSP90B1,HSPA4L, MAPK9,PDIA4,Sect. 62,SEL1L, TRAM1 UGGT2,WFS1,YOD1, ATXN3 |
0.048 |
| NOD-like receptor signaling | ANTXRL, cRhoA, ERBB2IP, HSP90AA1, IAP3, IFNAR1, MAPK1, MAPK9TANK, TBK1, TP53BP1, TRAF3, TRPM7 | 0.050 |
Fig. 20.
Validation of differentially expressed genes by qPCR (n = 3) (**p < 0.01 and ***p < 0.001).
Integrated analysis of DEGs from IMF adipocytes and SCF adipocytes
A total of 665 DEGs were identified in IMF adipocytes and 991 in SCF adipocytes. To further understand the effect of those DEGs on lipid deposition of IMF and SCF adipocytes, an integrated analysis was performed, and found 113 “intersection genes” that were common to both IMF and SCF adipocytes (Table S4). A total of 124 GO terms (gene number ≥ 2, p < 0.05) were significantly enriched based on 113 intersection genes, including integral component of membrane, cytoplasm, plasma membrane, extracellular space, signal transduction, cell adhesion, calcium ion binding, positive regulation of cell population proliferation, intracellular signal transduction, GTP binding, growth factor activity, positive regulation of gene expression, GTPase activity, cholesterol homeostasis, lipid binding, etc. The GO terms related to cell differentiation and lipid metabolism are shown in Fig. 21. KEGG pathway analysis of the intersection genes found that the significantly enriched pathways (Table 5) included PPAR signaling, tumor growth factor-beta signaling, calcium signaling, cytokine-cytokine receptor interaction, apelin signaling, adrenergic signaling in cardiomyocytes and cell adhesion molecules. It is noteworthy that the PPAR pathway was significantly enriched not only in intersection genes, but also in the DEGs in both IMF and SCF adipocytes. Two intersection genes, APOA1 and FABP4, were enriched in the PPAR pathway. The results suggest that CNP regulated lipid metabolism of IMF and SCF adipocytes through different genes enriched in the PPAR pathway and that APOA1 and FABP4 may be key genes (Fig. 22).
Fig. 21.
GO analysis of intersection genes in both IMF and SCF adipocytes.
Table 5.
Pathways significantly enriched in intersection genes.
| Pathway | Enriched genes | p-value |
|---|---|---|
| Cell adhesion molecules (CAMs) | CADM1,CADM3,CDH4,SELE | 0.003 |
| TGF-beta signaling pathway | AMH, INHBA, THSD4 | 0.009 |
| Calcium signaling pathway | ADCY2,ATP2B2,CAMK4,HTR2B | 0.011 |
| Cytokine-cytokine receptor interaction | AMH, CCR7, INHBA, TNFRSF11B | 0.016 |
| Apelin signaling pathway | ADCY2,MYL4,CAMK4 | 0.019 |
| Adrenergic signaling in cardiomyocytes | ADCY2,ATP2B2,MYL4 | 0.023 |
| PPAR signaling pathway | APOA1, FABP4 | 0.037 |
Fig. 22.
CNP regulated lipid metabolism through the NPRB-PPAR pathway in both IMF and SCF adipocytes. In IMF adipocytes, metabolism was regulated mainly by FABP4, FABP5, APOA1, ACOX2, ADIPOQ, CD36, and LPL enriched PPAR pathways, and by ACSL1, APOA1, CPT1A, CPT2, FABP4, PDPK1, ACOX3, and PPARα enriched PPAR pathways in SCF adipocytes.
Discussion
CNP and lipid metabolism in IMF adipocytes
Addition of 10−7 mol/L CNP to IMF adipocytes significantly decreased differentiation and increased cell proliferation and glycerin level. The findings clearly support the hypothesis that CNP played an important role in regulating lipid metabolism in the skeletal muscle of chickens. Addition of CNP to cultured IMF adipocytes significantly upregulated the expression of NPRB mRNA but had no effect on NPRA. Previous studies demonstrated that ANP stimulated lipolysis by binding to its receptor NPRAin both human and rat adipocytes24,25. CNP acts by binding to its receptor, NPRB26,27. The overexpression of CNP in adipocytes has been shown to decrease adipocyte hypertrophy via upregulation of NPRBin white adipose tissue28. mRNA sequencing identified seven DEGs (FABP4, FABP5, APOA1, ACOX2, ADIPOQ, CD36, and LPL) in adipocytes that are known to be linked to lipid metabolism and were found by KEGG analysis to be significantly enriched in the PPAR pathway. Fatty acid-binding protein 4 (FABP4) and fatty acid-binding protein 5 (FABP5) are cytoplasmic proteins involved in glucose and lipid metabolism29. FABP5interacts with numerous long-chain fatty acids of varying degrees of saturation, and participates in cis-bonded, polyunsaturated fatty-acid signaling30. High-density lipoprotein was significantly increased in obese (ob/ob) mice with a defect in the hepatic catabolism of apolipoprotein A1 (APOA1)31. CD36 was reported to promote fatty-acid uptake by muscle and fat cells32. Lipoprotein lipase (LPL) is the rate-limiting enzyme for the hydrolysis of the triglyceride core of circulating TG-rich lipoproteins, chylomicrons, and VLDLs33. Adiponectin (ADIPOQ) is a protein hormone secreted by adipocytes and regulates lipid and carbohydrate metabolism34, including promotion of fatty acid oxidation, decrease of plasma triglycerides, improvement of glucose metabolism and increased insulin sensitivity35. Of three Acyl-CoA oxidases (ACOXs), ACOX1, ACOX2, and ACOX3, ACOX2is a highly expressed regulatory gene in fatty acid metabolism pathways36. The results obtained here revealed that CNP bound with NPRB and upregulated its expression. It then changed the expression pattern of seven genes (FABP4, FABP5, APOA1, ACOX2, ADIPOQ, CD36, and LPL), which ultimately resulted in altered IMF adipocyte differentiation and lipolysis.
CNP and lipid metabolism in SCF adipocytes
Addition of 10−7 mol/L CNP to SCF adipocytes significantly decreased differentiation and increased proliferation and glycerin concentration. The results confirmed that CNP regulated lipid metabolism in not only IMF, but also in SCF adipocytes. More important, NPRB mRNA expression and the cGMP level were significantly increased, which is consistent with the trend in IMF adipocytes. Specifically, the PPAR pathway was also significantly enriched, but the enriched genes in SCF adipocytes were different from those in IMF adipocytes except for APOA1 and FABP4. Eight of the genes (ACSL1, APOA1, CPT1A, CPT2, FABP4, PDPK1, ACOX3 and PPARα) are known to be linked to lipid metabolism. Acyl-CoA synthetase long-chain family member 1 (ACSL1)influences lipolysis37and the β-oxidation rate in 3T3-L1 adipocytes38. The microRNA gga-miR-19b-3p was shown to accelerate adipocyte lipid deposition by downregulating ACSL1in chickens19. Peroxisome proliferator-activated receptor alpha (PPARα) is highly expressed in liver and muscle tissues, where it contributes to the β-oxidation of fatty acids and reduces lipid accumulation39. In chickens, PPARα mRNA expression was found to be negatively correlated with IMF from 0 to 8 w in thigh muscle tissue40. Carnitine palmitoyl transferase 2 (CPT2) is an enzyme required for mitochondrial long-chain fatty acid oxidation, and the loss of muscle CPT2led to accumulation of long-chain acylcarnitine and protected against diet-induced obesity and insulin resistance in mice41; Mitochondrial carnitine palmitoyl transferase 1a (CPT1A) in the liver mitochondrial outer membrane was found to catalyze the primary regulatory step in overall mitochondrial fatty acid oxidation42. ACOX3is involved in fatty acid metabolism43. The 113 intersection genes were included in six significantly enriched pathways. It is interesting that the PPAR pathway was also significantly enriched, including APOA1 and FABP4, suggesting that the PPAR pathway is the key pathway in CNP-regulated lipid metabolism in both IMF and SCF adipocytes, APOA1 and FABP4 may be the key genes.
In summary, our data showed that CNP regulated lipid metabolism through the NPRB-cGMP-PPAR pathway in both IMF and SCF adipocytes. In IMF adipocytes, metabolism was regulated mainly by FABP4, FABP5, APOA1, ACOX2, ADIPOQ, CD36, and LPL enriched PPAR pathways, and by ACSL1, APOA1, CPT1A, CPT2, FABP4, PDPK1, ACOX3, and PPARα enriched PPAR pathways in SCF adipocytes. APOA1 and FABP4 were key genes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank International Science Editing (http://www.international science editing.com) for editing this manuscript.
Abbreviations
- AbF
Abdominal Fat
- ANP
Atrial Natriuretic Peptide
- BNP
Brain Natriuretic Peptide
- CCK-8
Cell Counting Kit-8
- CNP
Intramuscular Fat
- DEGs
Differentially Expressed Genes
- DMEM
Dulbecco’s Modified Eagle Medium
- EdU
5-ethynyl-2’-deoxyuridine
- FPKM
Fragments per Kilobase of Exon per Million Mapped Reads
- FSH
Follicle Stimulating Hormone
- IMF
Subcutaneous Fat
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LPL
Lipoprotein Lipase
- NPPC
Natriuretic Peptide C
- NPRA
Natriuretic Peptide Receptor A
- NPRB
Natriuretic Peptide Receptor B
- NPS
Natriuretic Peptides
- PPAR
Peroxisome Proliferator Activated Receptor
- qPCR
Quantitative Real-Time Polymerase Chain Reaction
- SCF
C-Type Natriuretic Peptide
- VLDL
Very Low-Density Lipoprotein
Author contributions
Conceptualization, Huayun Huang; validation, Longzhou Liu ; data curation, Qianbao Wang and Zhenyang Huang; formal analysis, Zhong Liang and Chunmiao Li; writing the original draft, Huayun Huang; supervision, Zhenhua Zhao and Wei Han.
Funding
The research was supported by National Key Research and Development Program of China (2021YFD1200803), and the China Agriculture Research System of MOF and MARA (CARS-42-Z05).
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Declarations
Ethics approval and consent to participate
The study was conducted following the Guidelines for Experimental Animals formulated by the Ministry of Science and Technology (Beijing, China). All experimental procedures were approved by the Science Research Department (in charge of animal welfare) of the Institute of Poultry Science, Chinese Academy of Agricultural Sciences (Jiangsu, China). All methods were carried out following the relevant guidelines and regulations. Also, all methods were reported following the ARRIVE guidelines.
Competing interests
The authors declare no competing interests.
Footnotes
Huayun Huang and Longzhou Liu contributed equally to this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhenhua Zhao, Email: zzh0514@163.com.
Wei Han, Email: hanwei830@163.com.
References
- 1.Hocquette, J. F. et al. Intramuscular fat content in meat-producing animals: development, genetic and nutritional control, and identification of putative markers. J. Animal:An Int. J. Anim. Bioscience, 4(2): 303 – 19(2010). [DOI] [PubMed]
- 2.Ankre-Badu, G. A. et al. Mapping main, epistatic and sex-specific QTL for body composition in a chicken population divergently selected for low or high growth rate. J. BMC Genomics. 11, 107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendoza, A. & Hollenberg, A. N. New insights into thyroid hormone action. Pharmacol. Ther.173, 135–145 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawai, M. et al. Growth hormone stimulates adipogenesis of 3T3-L1 cells through activation of the Stat5A/5B-PPARgamma pathway. J. Mol. Endocrinol.38 (1–2), 19–34 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Cui, H. et al. FSH stimulates lipid biosynthesis in chicken adipose tissue by upregulating the expression of its receptor FSHR. J. J. Lipid Res.53 (5), 909–917 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui, X-Y. et al. Follicle-stimulating hormone increases the intramuscular fat content and expression of lipid biosynthesis genes in chicken breast muscle. J. J. Zhejiang University-SCIENCE B. 17, 303–310 (2016). [Google Scholar]
- 7.Yan, J., Yang, H., Gan, L. & Sunet, C. Adiponectin-impaired adipocyte differentiation negatively regulates fat deposition in chicken. J. J. Anim. Physiol. Anim. Nutr.98 (3), 530–537 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Cooke, P. S. & Naaz, A. Role of estrogens in adipocyte development and function. J. Experimental Biology Med. (Maywood NJ). 229 (11), 1127–1135 (2004). [DOI] [PubMed] [Google Scholar]
- 9.de Bold, A. J., Borenstein, H. B., Veress, A. T. & Sonnenberg, H. A. rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Reprinted from Life Sci. 28:89–94, J. Journal of the American Society of Nephrology: JASN, 12(2): 403-9; discussion – 8, 8–9(2001). (1981). [DOI] [PubMed]
- 10.Sudoh, T., Kangawa, K., Minamino, N. & Matsuo, H. A. new natriuretic peptide in porcine brain. J. Nat.332 (6159), 78–81 (1988). [DOI] [PubMed] [Google Scholar]
- 11.Kojima, M., Minamino, N., Kangawa, K. & Matsuoet, H. Cloning and sequence analysis of a cDNA encoding a precursor for rat C-type natriuretic peptide (CNP). J. FEBS Lett.276 (1-2), 209–213 (1990). [DOI] [PubMed] [Google Scholar]
- 12.Trajanovska, S. et al. Genomic analyses and cloning of novel chicken natriuretic peptide genes reveal new insights into natriuretic peptide evolution. Peptides28 (11), 2155–2163 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Maack, T. et al. Physiological role of silent receptors of atrial natriuretic factor. J. Sci. (New York NY). 238 (4827), 675–678 (1987). [DOI] [PubMed] [Google Scholar]
- 14.Lafontan, M. et al. Control of lipolysis by natriuretic peptides and cyclic GMP. J. Trends Endocrinol. Metabolism: TEM. 19 (4), 130–137 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Bordicchia, M. et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. J. Clin. Invest.122 (3), 1022–1036 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engeli, S. et al. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J. J. Clin. Invest.122 (12), 4675–4679 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inuzuka, M. et al. C-type natriuretic peptide as a new regulator of food intake and energy expenditure. J. Endocrinol.151 (8), 3633–3642 (2010). [DOI] [PubMed] [Google Scholar]
- 18.BAE, C. R. et al. Overexpression of C-type natriuretic peptide in endothelial cells protects against Insulin Resistance and inflammation during Diet-induced obesity. J. Sci. Rep.7 (1), 9807 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang, H. Y. et al. Integrated analysis of microRNA and mRNA expression profiles in abdominal adipose tissues in chickens. J. Sci. Rep.5, 16132 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramsay, T. G. & Rosebrough, R. W. Hormonal regulation of postnatal chicken preadipocyte differentiation in vitro. Comp. Biochem. Physiol. B Biochem. Mol. Biol.,136(2): 245 – 53(2003). [DOI] [PubMed]
- 21.Zhang, T. et al. Analysis of long noncoding RNA and mRNA using RNA sequencing during the differentiation of intramuscular preadipocytes in chicken. J. PloS One. 12 (2), e0172389 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao, X., Cai, T., Olyarchuk, J. G. & Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. J. Bioinf. (Oxford England). 21 (19), 3787–3793 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Wu, J., Mao, X., Cai, T., Luo, J. & Wei, L. KOBAS server: a web-based platform for automated annotation and pathway identification. J. Nucleic acids research, 34(Web Server issue): W720-4(2006). [DOI] [PMC free article] [PubMed]
- 24.Sengenes, C., Moro, C., Galitzky, J. & Lafontan, M. [Natriuretic peptides: a new lipolytic pathway in human fat cells]. J. Med. Sciences: M/S. 21 Spec No, 29–33 (2005). [PubMed] [Google Scholar]
- 25.Nishikimi, T. et al. Stimulatory and inhibitory regulation of lipolysis by the NPR-A/cGMP/PKG and NPR-C/G(i) pathways in rat cultured adipocytes. J. Regul. Pept.153 (1–3), 56–63 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Komatsu, Y. et al. C-type natriuretic peptide (CNP) in rats and humans. J. Endocrinol.129 (2), 1104–1106 (1991). [DOI] [PubMed] [Google Scholar]
- 27.Chen, H. H. & Burnett, J. C. Jr. C-type natriuretic peptide: the endothelial component of the natriuretic peptide system. J. J. Cardiovasc. Pharmacol.32 (Suppl 3), S22–S28 (1998). [PubMed] [Google Scholar]
- 28.Bae, C. R. et al. Adipocyte-specific expression of C-type natriuretic peptide suppresses lipid metabolism and adipocyte hypertrophy in adipose tissues in mice fed high-fat diet. J. Sci. Rep.8 (1), 2093 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makowski, L., Brittingham, K. C., Reynolds, J. M., Suttles, J. & Hotamisligil, G. S. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and IkappaB kinase activities. J. J. Biol. Chem.280 (13), 12888–12895 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armstrong, E. H., Goswami, D., Griffin, P. R., Noy, N. & Ortlund, E. A. Structural basis for ligand regulation of the fatty acid-binding protein 5, peroxisome proliferator-activated receptor β/δ (FABP5-PPARβ/δ) signaling pathway. J. J. Biol. Chem.289 (21), 14941–14954 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silver, D. L., Jiang, X. C., Arai, T., Bruce, C. & Tall, A. R. Receptors and lipid transfer proteins in HDL metabolism. J. Annals of the New York Academy of Sciences, 902: 103 – 11; discussion 11 – 2 (2000). [DOI] [PubMed]
- 32.Ibrahimi, A. & Abumrad, N. A. Role of CD36 in membrane transport of long-chain fatty acids. Curr. Opin. Clin. Nutr. Metab. Care. 5, 139–145 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Wang, H. & Eckel, R. H. Lipoprotein lipase: from gene to obesity. J. Am. J. Physiol. Endocrinol. Metabolism. 297 (2), E271–E288 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Scherer, P. E., Williams, S., Fogliano, M., Baldini, G. & Lodish, H. F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. J. Biol. Chem.270 (45), 26746–26749 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Yamauchi, T. et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. J. Nat. Med.8 (11), 1288–1295 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Ferdinandusse, S. et al. A novel case of ACOX2 deficiency leads to recognition of a third human peroxisomal acyl-CoA oxidase. J. Biochim. et Biophys. acta Mol. Basis Disease. 1864 (3), 952–958 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Hall, A. M., Smith, A. J. & Bernlohr, D. A. Characterization of the Acyl-CoA synthetase activity of purified murine fatty acid transport protein 1. J. J. Biol. Chem.278 (44), 43008–43013 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Ellis, J. M. et al. Adipose acyl-CoA synthetase-1 directs fatty acids toward beta-oxidation and is required for cold thermogenesis. J. Cell. Metabolism. 12 (1), 53–64 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barbier, O. et al. Pleiotropic actions of peroxisome proliferator-activated receptors in lipid metabolism and atherosclerosis. J. Arterioscler. Thromb. Vascular Biology. 22 (5), 717–726 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Fu, R. Q. et al. Expression profiles of key transcription factors involved in lipid metabolism in Beijing-You chickens. J. Gene. 537 (1), 120–125 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Pereyra, A. S., Rajan, A., Ferreira, C. R. & Ellis, J. M. Loss of Muscle Carnitine Palmitoyltransferase 2 prevents Diet-Induced obesity and insulin resistance despite long-chain acylcarnitine Accumulation. J. Cell. Rep.33 (6), 108374 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee, K., Kerner, J. & Hoppel, C. L. Mitochondrial carnitine palmitoyltransferase 1a (CPT1a) is part of an outer membrane fatty acid transfer complex. J. J. Biol. Chem.286 (29), 25655–25662 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu, H. et al. iTRAQ-based quantitative proteomics analysis of Sprague-Dawley rats liver reveals perfluorooctanoic acid-induced lipid metabolism and urea cycle dysfunction. J. Toxicol. Lett.357, 20–32 (2021). [DOI] [PubMed] [Google Scholar]
- 44.Kanehisa, M. & Goto, S. KEGG: Kyoto Encyclopedia of genes and genomes. Nucleic Acids Res.28, 27–30 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci.28, 1947–1951 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res.51, D587–D592 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed in this study are available from the corresponding author on reasonable request.
Data records
Effect of CNP on lipid metabolism in IMF adipocytes
IMF adipocyte cell morphology Adipocytes isolated from breast muscle were cultured for 2 days in T25 flasks and evaluated with an inverted microscope. Mature adipocytes began to cluster, a few spindle cells appeared, and some mature adipocytes began to dedifferentiate (Fig. 1A). On day 5, the number of prespindle adipocytes had increased (Fig. 1B); on day 7, the dedifferentiated cells proliferated abundantly (Fig. 1C) and were subcultured. The rate of cell proliferation was high (Fig. 1D). Lipid droplets were clearly visible after induced differentiation for 3 days (Fig. 1E), and large, round, red lipid droplets were visible 6 days after differentiation induced by oleic acid, confirming that the cells were IMF adipocytes (Fig. 1F).
Fig. 1.
Morphological changes of intramuscular preadipocytes (inverted microscope, 100×). (A–C) Cellular morphology of IMF preadipocytes 2, 5, and 7 days after isolation. (D) Morphology of IMF adipocytes 24 h after passage. (E) Differentiation of IMF adipocytes cultured with oleic acid for 3 days. (F) IMF adipocytes stained with Oil red O after induced differentiation for 3 days.
CNP induction enhanced IMF preadipocyte proliferation, inhibited adipocyte differentiation, and promoted lipolysis
The number of preadipocytes increased significantly (p < 0.05) after 24 and 72 h of culture with 10−7 mol/L CNP (Fig. 2A). EdU staining also confirmed that the proliferation rate of CNP-induced cells was increased compared with control cells (Fig. 2B). Differentiated adipocytes were treated with exogenous 10−7 mol/L CNP for 3 and 6 d. As shown in Fig. 3A, adipocyte differentiation decreased significantly on treatment with 10−7 mol/L CNP (p < 0.05). Oil Red O staining showed that the lipid droplets were slightly smaller and fewer in number in cells treated with 10−7 mol/L CNP compared with controls (Fig. 3B) Lipolysis was evaluated by measuring glycerol levels in the culture medium. The effects of CNP treatment on the lipolysis of adipocytes are shown in Fig. 4. CNP treatment significantly enhanced glycerol levels compared with those in control cultures at 6 d (p < 0.05).
Fig. 2.

Effect of CNP on the proliferation of intramuscular preadipocyte. (A) The number of preadipocytes was significantly increased by CNP (Cell Counting Kit-8, n = 8, *p < 0.05 vs. control group). (B) EdU staining of intramuscular preadipocytes induced by CNP for 24 h and 72 h (n = 3). EdU, number of proliferative cells; Hoechst, number of cells before proliferation; Overlay, superimposition of the two images shows the location of proliferating cells.
Fig. 3.
Effect of CNP on differentiation of intramuscular adipocytes. (A) CNP 10−7 mol/L significantly decreased the differentiation of adipocytes (n = 3; **p < 0.01 vs. control). (B) Morphologic changes and lipid deposition induced by 10−7 mol/L CNP in adipocytes in vitro (inverted microscope, 400× magnification). Oil Red O staining shows that CNP 10−7 mol/L decreased both the size and number of the droplets in each accumulation compared with cells not treated with CNP.
Fig. 4.
Change of the glycerol content at 6 days after CNP treatment in intramuscular adipocytes (n = 3), (**p < 0.01 vs. control).
NPPC interference reduced cell proliferation, enhanced adipocyte differentiation and reduced lipolysis in IMF adipocytes
Preadipocytes were transfected with either the NPPC siRNA or the negative control vector. The expression of NPPC mRNA, compared with that of the control group, decreased significantly at 24 h after transfection with NPPC siRNA (Fig. 5, p < 0.01). The effects of NPPC interference on the proliferation of preadipocytes and their differentiation into adipocytes are shown in Figs. 6 and 7, respectively. The number of cells was significantly reduced at both 24 and 72 h after NPPC interference (Fig. 6, p < 0.05). Oil Red O staining showed that the lipid droplets were slightly larger, and their numbers were increased in groups treated with NPPC siRNA compared with those in control groups (Fig. 7B). NPPC interference significantly reduced glycerol levels compared with those in control cultures at 4 d (Fig. 8, p < 0.05).
Fig. 5.
Change of NPPC mRNA expression after 24 h transfection with NPPC siRNA in the intramuscular adipocytes.
Fig. 6.
Effect of NPPC interference on the proliferation of intramuscular preadipocytes. (A) Number of preadipocytes after transfection with NPPC siRNA (CCK8 kit, n = 8, *p < 0.05 vs. control group).); B. EdU staining of chicken preadipocytes after transfection with NPPC siRNA. EdU, number of proliferative cells; Hoechst, number of cells before proliferation; Overlay, superimposition of the two images shows the location of proliferating cells.
Fig. 7.
Effect of NPPC interference on the differentiation of intramuscular adipocytes. (A) Adipocyte differentiation increased significantly after transfection with NPPC siRNA (n = 3) (**p < 0.01 vs. control). (B) Morphologic changes and lipid deposition after transfection with NPPC siRNA in adipocytes in vitro (inverted microscope, 400× magnification). Lipid droplets (stained with Oil Red O) accumulated as larger clusters and in greater numbers in cells transfected with NPPC siRNA compared with the negative control vector.
Fig. 8.
Change of the glycerol content at 4 d after transfection with NPPC siRNA in intramuscular adipocytes. (n = 3), (**p < 0.01 vs. control).
Molecular mechanism of CNP regulation of IMF lipid deposition
CNP promoted NPRBmRNA expression in IMF adipocytes As shown in Fig. 9, treatment with exogenous 10−7 mol/L CNP for 6 d significantly increased the expression of NPRB mRNA (p < 0.05). The difference in NPRA expression in treated compared with control cells was not significant (p > 0.05).
Fig. 9.
Change of NPRB expression in intramuscular adipocytes at 6 d after CNP treatment ( n = 3), (*p < 0.05 and **p < 0.01 vs. control).
Key genes and pathways responding to CNP regulation in IMF adipocytes
mRNA sequencing was used to identify key genes and pathways by which CNP influenced lipid metabolism in IMF adipocytes. DEGs were detected in total RNA isolated from cells treated with 10−7 mol/L CNP and control cells not exposed to CNP. The quality control data are shown in Table S1. A total of 665 DEGs (p < 0.05; log > 1.5 or < 0.67) were identified; 323 were upregulated and 342 were downregulated (Table S2). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of the DEGs found 11 pathways that were significantly enriched (Table 3, p ≤ 0.01). They were the PPAR signaling pathway, calcium signaling pathway, adrenergic signaling in cardiomyocytes, focal adhesion, cardiac muscle contraction, phosphatidylinositol signaling system, neuroactive ligand-receptor interaction, regulation of actin cytoskeleton, extracellular matrix-receptor interaction, AGE-RAGE signaling pathway in diabetic complications, and cell adhesion molecules. Seven genes (FABP4, APOA, ACOX2, ADIPOQ, CD36, FABP5and LPL) enriched in the PPAR pathway were reported to be closely related to lipid metabolism (Fig. 10; Table 3). qPCR verified seven of the DEGs identified by deep sequencing, and the RNA amplified from cells treated with 0 and 10−7 mol/L CNP validated the gene expression profile analysis. There was consistency between the qPCR assays and the deep sequencing analysis in terms of the direction of regulation and statistical significance (Fig. 11, p < 0.05). Therefore, it was hypothesized that CNP regulated lipid metabolism in IMF adipocytes via the NPRB-PPAR pathway and by promoting the expression of genes (e.g., FABP4, APOA, ACOX2, ADIPOQ, CD36, FABP5 and LPL) enriched in the PPAR pathway.
Table 3.
Significantly enriched pathways for differentially expressed genes in intramuscular adipocytes.
| Pathway | Enriched genes | P–value |
|---|---|---|
| Calcium signaling pathway | ADCY2,ADRA1D, ATP2B2,CACNA1H, CAMK1D, CAMK2A, CAMK4,CASQ2,CD38,CXCR4,HTR2B, HTR4,ip3ka, ITPR3,NCX1,PLCD4,TBXA2R, TNNC1,TNNC2 | 0.000 |
| Adrenergic signaling in cardiomyocytes | ACTC1,ADCY2,ADRA1D, ATP1B1,ATP2B2,BCL2,CAMK2A, CREB5,Myl2,MYL3,MYL4,NCX1,TNNC1,TNNT2 | 0.000 |
| Focal adhesion | ACTB, BCL2,CAV3,CHAD, fn1,IGF1,ITGA1,ITGA9,LAMA5,Myl2,MYL9,PDGFB, PGF, PPP1R12B, SHC4,THBS1, VTN | 0.000 |
| Cardiac muscle contraction | ACTC1,ATP1B1,CASQ2,Myl2,MYL3,MYL4,NCX1,TNNC1,TNNT2, UQCR11 | 0.000 |
| Phosphatidylinositol signaling system | DGKB, DGKG, DGKQ, INPP5J, ip3ka, IP6K3,ITPK1,ITPR3,MTMR1,PIK3C2A, PLCD4 | 0.000 |
| Neuroactive ligand-receptor interaction | ADM, ADRA1D, CHRM4,CHRNA1,CHRND, CHRNG, CNR1,CRHR2,EDN1,EDN2,EP4,GIPR, GRID1,GRM3,HTR2B, HTR4,PENK, S1PR3,SS2R, TBXA2R, VPAC2 | 0.001 |
| Regulation of actin cytoskeleton | ACTB, APC2,ARHGEF4,CHRM4,CXCR4,FGF7,Fgf9,fn1,IQGAP2,ITGA1,ITGA9,Myl2,MYL9,PDGFB, PPP1R12B | 0.001 |
| ECM-receptor interaction | CD36,CHAD, fn1,ITGA1,ITGA9,LAMA5,NPNT, THBS1,VTN | 0.006 |
| AGE-RAGE signaling pathway in diabetic complications | BCL2,CDKN1B, EDN1,F3,fn1,IL6,NOX1,PLCD4,SELE | 0.007 |
| Cell adhesion molecules (CAMs) | CADM3,CDH3,CDH4,CLDN1,ITGA9,NFASC, NRCAM, SELE | 0.010 |
| PPAR signaling pathway | ACOX2,ADIPOQ, APOA1,CD36,FABP4,FABP5,LPL | 0.010 |
Fig. 10.
Fig. 11.
Validation of differentially expressed genes by qPCR (n = 3), (**p < 0.01).
Effect of CNP on lipid metabolism in SCF adipocytes
CNP increased preadipocyte proliferation, decreased adipocyte differentiation and facilitated adipocyte lipolysis The number of preadipocytes was significantly increased by culture with 10−7 mol/L CNP for 24 and 48 h compared with controls (Fig. 12A, p < 0.05). EdU staining confirmed that the proliferation rate of CNP-induced cells was increased compared with control cells (Fig. 12B). Differentiated adipocytes were treated with exogenous 10−7 mol/L CNP for 3 and 6 d. Oil Red O staining showed that the lipid droplets were smaller and their numbers were decreased in study groups treated with 10−7 mol/L CNP compared with the control groups (Fig. 13A). The results showed that adipocyte differentiation was significantly decreased by treatment with CNP (Fig. 13B, p < 0.05). As shown in Fig. 14, CNP treatment significantly increased glycerol levels compared with the control group at 6 d. The results showed that CNP promoted lipolysis in SCF adipocytes.
Fig. 12.
Effect of CNP on the proliferation of subcutaneous preadipocytes.(A) Proliferation of preadipocytes induced for 24 h and 48 h by CNP (n = 8, * p < 0.05 vs. control group). (B) EdU staining of intramuscular preadipocytes induced for 24 h and 72 h by CNP (n = 3). EdU, number of proliferative cells; Hoechst, number of cells before proliferation; Overlay, superimposition of the two images shows the location of proliferating cells. *p < 0.05 vs. control group.
Fig. 13.
Effect of CNP on the differentiation of subcutaneous adipocytes. A, Morphologic changes and lipid deposition induced by 10−7mol/L CNP in adipocytes in vitro (inverted microscope, 200× magnification). Lipid droplets (stained with Oil Red O) were smaller and accumulated in fewer groups in cells exposed to 10−7mol/L CNP compared with untreated cells. B, Differentiation of adipocytes was significantly decreased by 10−7 mol/L CNP. (n = 3, **p < 0.01 vs. control).
Fig. 14.
Change of the glycerol content at 6 days after CNP treatment in subcutaneous adipocytes. (n = 3), (*p < 0.05 vs. control).
NPPC interference reduced cell proliferation, enhanced adipocyte differentiation, and reduced lipolysis. NPPC mRNA expression was significantly decreased compared with the control group at 24 h after transfection of preadipocytes with NPPC siRNA (Fig. 15, p < 0.05). As shown in Fig. 16, the number of cells was reduced significantly at 24 and 72 h after transfection with NPPC siRNA (p < 0.05). Lipid accumulation was increased at 3 d (Fig. 17A, p < 0.01). Staining with Oil Red O showed that the lipid droplets were slightly larger, and their number was increased in groups transfected with NPPC siRNA compared with the control groups (Fig. 17B). NPPC interference significantly reduced the glycerol level at 4 d compared with the control cultures (Fig. 18, p < 0.05).
Fig. 15.
Change of NPPC mRNA expression in subcutaneous adipocytes after transfection with NPPC siRNA for 24 h. (n = 3) (**p < 0.01).
Fig. 16.
Effect of NPPC interference on the proliferation of subcutaneous preadipocytes (n = 5) (**p < 0.01 vs. control). (A) The number of preadipocytes assayed by CCK8 kits after NPPC interference; (B) EdU staining assay of chicken preadipocytes transfected with NPPC siRNA. EdU, number of proliferative cells; Hoechst, number of cells before proliferation; Overlay, superimposition of the two images shows the location of proliferating cells.
Fig. 17.
Effect of NPPC interference on the differentiation of subcutaneous adipocytes (A) The differentiation of adipocytes was significantly increased after transfection with NPPC siRNA (n = 3) (**p < 0.01 vs. control). (B) Morphologic changes and lipid deposition after transfection with NPPC siRNA in adipocytes in vitro (inverted microscope, 400× magnification). Lipid droplets (stained with Oil Red O) accumulated in greater numbers and in larger groups in cells transfected with NPPC siRNA compared with the negative control vector.
Fig. 18.
Change of the glycerol content at 4 d after transfection with NPPC siRNA in subcutaneous adipocytes. (n = 3), (**p < 0.01 vs. control).
Molecular mechanism of CNP regulation of lipid deposition in SCF adipocytes
CNP enhanced NPRB expression qPCR showed that CNP treatment significantly increased the expression of NPRB mRNA at 6 d (Fig. 19, p < 0.05) and that differences in.
Fig. 19.
Change of NPRB expression in subcutaneous adipocytes at 6 d after CNP treatment ( n = 3), (*p < 0.05 and **p < 0.01 vs. control).
NPRA mRNA expression in treated and control cells were not significant.
Key genes and pathways responding to CNP regulation
Key DEGs and pathways were detected by mRNA sequencing of total RNA extracted from SCF adipocytes treated with 10−7 mol/L CNP and from control cells. The quality control data are shown in Table S1. A total of 991 DEGs (p < 0.05; log 1.5 > 1.5 or < 0.67) were identified, with 136 upregulated genes and 855 downregulated genes (Table S3). After KEGG pathway analysis of the DEGs, 12 pathways were significantly enriched (Table 4, p ≤ 0.01). They were PPAR signaling, ubiquitin mediated proteolysis, animal autophagy, vascular smooth muscle contraction, focal adhesion, regulation of actin cytoskeleton, apoptosis, endocytosis, transforming growth factor-beta signaling, progesterone-mediated oocyte maturation, protein processing in the endoplasmic reticulum, and NOD-like receptor signaling pathway. Eight genes (ACOX3, ACSL1, APOA1, CPT1A, CPT2, FABP4, PDPK1 and PPARα) enriched in PPAR pathway were reported to be closely related to lipid metabolism. qPCR verified the consistency of the eight DEGs identified by deep sequencing in terms of the direction of regulation and statistical significance (Fig. 20., p < 0.05). Taken together, the results support the hypothesis that CNP regulated lipid metabolism in SCF adipocytes via the NPRB-PPAR pathway and by increasing the expression of genes (e.g., ACOX3, ACSL1, APOA1, CPT1A, CPT2, FABP4, PDPK1 and PPARα) enriched in the PPAR pathway.
Table 4.
Significantly enriched pathways of differentially expressed genes in subcutaneous adipocytes.
| Pathway | Enriched genes | P-value |
|---|---|---|
| Ubiquitin mediated proteolysis | BIRC6,CUL1,CUL4A, CUL4B, CUL5,HERC1,HERC2,IAP3,ITCH, NEDD4,TRIP12,UBA6,UBE2L3,UBE3C, ANAPC4 | 0.007 |
| Autophagy - animal |
ATG13,ATG2B, CFLAR, EIF2AK4,MAPK1,MAPK9,Metazoa_SRP, PDPK1,PIK3C3 PIK3CA, PIK3CB, PRKAA1,PRKCQ, RB1CC1,RRAGD |
0.009 |
| Vascular smooth muscle contraction |
ADCY2,CALCRL, CALD1,cRhoA, GUCY1B3,MAPK1,MBSP, MRVI1,MYLK, PLA2G2E, PLA2G4A PRKCH, PRKCQ, ROCK1, ROCK2 |
0.009 |
| Focal adhesion | ARHGAP5,COL1A2,cRhoA, IAP3,ITGA2,ITGA4,ITGAV, ITGB1,ITGB8,MAPK1,MAPK9,MBSP, MYLK, PARVA, PDPK1,PIK3CA, PIK3CB, ROCK1,ROCK2, VTN, | 0.009 |
| Regulation of actin cytoskeleton | APC, ARHGEF4,BAIAP2,BDKRB1,cRhoA, DIAPH1,Fgf9,ITGA2,ITGA4,ITGAV, ITGB1,ITGB8,MAPK1,MBSP, MYLK, PIK3CA, PIK3CB, ROCK1,ROCK2, SCIN | 0.010 |
| Apoptosis | SPTAN1,PIK3CB, PIK3CA, PDPK1,MAPK9,MAPK1,IAP3,CTSZ, CTSO, CFLAR, CASP3,CASP2,ATM APAF1 | 0.031 |
| Endocytosis |
AP2B1,ARFGEF2,CAPZA1,cRhoA, DAB2,DNM3,EEA1,EHD4,IGF2R, IQSEC1,ITCH, KIF5B, NEDD4 RAB11FIP2,RABEP1,RBSN, STAM, TFRC, VPS29, VPS4B |
0.040 |
| TGF-beta signaling | THSD4,ROCK1,MAPK1,INHBA, DCN, CUL1,cRhoA, CHRD, BMPR-II, BMPR1A, AMH | 0.041 |
| PPAR signaling | ACOX3,ACSL1,APOA1,CPT1A, CPT2,FABP4,HMGCS1,PDPK1,PPARA | 0.043 |
| Progesterone-mediated oocyte maturation | ADCY2,ANAPC4,BUB1,HSP90AA1, MAPK1, MAPK9,PIK3CA, PIK3CB, RPS6KA3,RPS6KA6 | 0.045 |
| Protein processing in endoplasmic reticulum |
CUL1,DNAJC5,EIF2AK4,HSP90AA1,HSP90B1,HSPA4L, MAPK9,PDIA4,Sect. 62,SEL1L, TRAM1 UGGT2,WFS1,YOD1, ATXN3 |
0.048 |
| NOD-like receptor signaling | ANTXRL, cRhoA, ERBB2IP, HSP90AA1, IAP3, IFNAR1, MAPK1, MAPK9TANK, TBK1, TP53BP1, TRAF3, TRPM7 | 0.050 |
Fig. 20.
Validation of differentially expressed genes by qPCR (n = 3) (**p < 0.01 and ***p < 0.001).
Integrated analysis of DEGs from IMF adipocytes and SCF adipocytes
A total of 665 DEGs were identified in IMF adipocytes and 991 in SCF adipocytes. To further understand the effect of those DEGs on lipid deposition of IMF and SCF adipocytes, an integrated analysis was performed, and found 113 “intersection genes” that were common to both IMF and SCF adipocytes (Table S4). A total of 124 GO terms (gene number ≥ 2, p < 0.05) were significantly enriched based on 113 intersection genes, including integral component of membrane, cytoplasm, plasma membrane, extracellular space, signal transduction, cell adhesion, calcium ion binding, positive regulation of cell population proliferation, intracellular signal transduction, GTP binding, growth factor activity, positive regulation of gene expression, GTPase activity, cholesterol homeostasis, lipid binding, etc. The GO terms related to cell differentiation and lipid metabolism are shown in Fig. 21. KEGG pathway analysis of the intersection genes found that the significantly enriched pathways (Table 5) included PPAR signaling, tumor growth factor-beta signaling, calcium signaling, cytokine-cytokine receptor interaction, apelin signaling, adrenergic signaling in cardiomyocytes and cell adhesion molecules. It is noteworthy that the PPAR pathway was significantly enriched not only in intersection genes, but also in the DEGs in both IMF and SCF adipocytes. Two intersection genes, APOA1 and FABP4, were enriched in the PPAR pathway. The results suggest that CNP regulated lipid metabolism of IMF and SCF adipocytes through different genes enriched in the PPAR pathway and that APOA1 and FABP4 may be key genes (Fig. 22).
Fig. 21.
GO analysis of intersection genes in both IMF and SCF adipocytes.
Table 5.
Pathways significantly enriched in intersection genes.
| Pathway | Enriched genes | p-value |
|---|---|---|
| Cell adhesion molecules (CAMs) | CADM1,CADM3,CDH4,SELE | 0.003 |
| TGF-beta signaling pathway | AMH, INHBA, THSD4 | 0.009 |
| Calcium signaling pathway | ADCY2,ATP2B2,CAMK4,HTR2B | 0.011 |
| Cytokine-cytokine receptor interaction | AMH, CCR7, INHBA, TNFRSF11B | 0.016 |
| Apelin signaling pathway | ADCY2,MYL4,CAMK4 | 0.019 |
| Adrenergic signaling in cardiomyocytes | ADCY2,ATP2B2,MYL4 | 0.023 |
| PPAR signaling pathway | APOA1, FABP4 | 0.037 |
Fig. 22.
CNP regulated lipid metabolism through the NPRB-PPAR pathway in both IMF and SCF adipocytes. In IMF adipocytes, metabolism was regulated mainly by FABP4, FABP5, APOA1, ACOX2, ADIPOQ, CD36, and LPL enriched PPAR pathways, and by ACSL1, APOA1, CPT1A, CPT2, FABP4, PDPK1, ACOX3, and PPARα enriched PPAR pathways in SCF adipocytes.
All data generated or analysed during this study are included in this published article [and its supplementary information files].