Abstract
Parkinson’s disease is a progressive neurodegenerative condition with a considerable health and economic burden1. It is characterized by the loss of midbrain dopaminergic neurons and a diminished response to symptomatic medical or surgical therapy as the disease progresses2. Cell therapy aims to replenish lost dopaminergic neurons and their striatal projections by intrastriatal grafting. Here, we report the results of an open-label phase I clinical trial (NCT04802733) of an investigational cryopreserved, off-the-shelf dopaminergic neuron progenitor cell product (bemdaneprocel) derived from human embryonic stem (hES) cells and grafted bilaterally into the putamen of patients with Parkinson’s disease. Twelve patients were enrolled sequentially in two cohorts—a low-dose (0.9 million cells, n = 5) and a high-dose (2.7 million cells, n = 7) cohort—and all of the participants received one year of immunosuppression. The trial achieved its primary objectives of safety and tolerability one year after transplantation, with no adverse events related to the cell product. At 18 months after grafting, putaminal 18Fluoro-DOPA positron emission tomography uptake increased, indicating graft survival. Secondary and exploratory clinical outcomes showed improvement or stability, including improvement in the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part III OFF scores by an average of 23 points in the high-dose cohort. There were no graft-induced dyskinesias. These data demonstrate safety and support future definitive clinical studies.
Subject terms: Embryonic stem cells, Neuroscience, Parkinson's disease, Movement disorders
Bilateral grafts of cryopreserved human embryonic stem cell-derived dopaminergic neuron progenitor cells into the putamen of patients with Parkinson’s disease in a phase I clinical trial showed safety, improvements in off-drug motor function and graft survival at 18 months after transplant.
Main
Parkinson’s disease (PD) is a debilitating neurodegenerative disorder characterized by a marked, progressive degeneration of dopaminergic neurons in the substantia nigra and their striatal projections. Motor features include bradykinesia with rigidity and/or resting tremor2–4; non-motor manifestations are wide-ranging and include autonomic, psychiatric, sleep and other symptoms that contribute to a decline in quality of life4,5. The global burden of PD is projected to exceed 14 million people by 20401. Medical therapies for PD focus on augmenting striatal dopamine receptor stimulation or acting on other transmitter pathways, such as acetylcholine6,7 or adenosine8. Surgical therapies include deep brain stimulation, which can improve motor symptoms9, and magnetic-resonance-guided focused ultrasound ablation, which can be effective for tremor and other motor features10,11. Experimental approaches under investigation include the use of anti-synuclein antibodies12,13, GLP1 receptor agonists14,15 and gene therapies16,17 aimed at neuroprotection, enhancing dopamine function or potential disease modification. Levodopa remains the cornerstone therapy but is associated with the gradual development of complications such as dyskinesias, a narrowing therapeutic window, off-target effects and exacerbation of non-motor symptoms2,18. While these therapies have an important role in symptom alleviation in the early stages, their efficacy inevitably wanes due to ongoing attrition of dopaminergic neurons, often accompanied by unwanted side effects such as dyskinesias. By delivering new midbrain neurons to the striatum, cell therapy aims to replace the degenerated dopaminergic projections and to achieve a sustained clinical improvement in motor symptoms.
Open-label studies of fetal ventral midbrain tissue grafts in the striatum showed some promising results19–24 but were not replicated in double-blind, randomized, placebo-controlled trials25,26. The latter trials faced multiple challenges in tissue access, and used variable clinical design, surgical strategies and immunosuppression approaches. They also reported relatively high rates of graft-induced dyskinesia, possibly mediated by serotonergic neuron contaminants27. In other long-term follow-up studies, a subgroup of patients experienced sustained clinical benefit after grafting, including independence of dopaminergic (DA) medical therapy28, with reports demonstrating long-term graft survival and striatal DA innervation after brain autopsy at 14 years29 and 24 years after surgery30. The lessons learned from these studies suggest a potentially substantial clinical benefit for cell therapy in PD; however, these studies also highlighted the need for a scalable cell source of midbrain DA neurons, compatible with stringent quality-control assessments and lacking unwanted cellular contaminants. Over the following two decades, an intense effort ensued across the globe to identify a renewable source of human midbrain DA neurons from pluripotent stem cells (such as hES cells or human induced pluripotent stem (iPS) cells) that is suitable for clinical use.
We have previously established a protocol for the derivation of midbrain DA neurons from hES cells31. PS cells are exposed to a carefully determined sequence and combination of patterning factors to undergo directed differentiation into midbrain DA neurons through a floor-plate intermediate stage. The resulting floor-plate-derived DA neurons show transcriptional, biochemical and physiological features of authentic midbrain DA neurons, and exhibit robust cell survival and function when grafted in mouse, rat and rhesus monkey models of Parkinson’s disease31. Optogenetic studies in grafted rodents demonstrated that functional improvement is dependent on graft neuronal activity and dopamine release and that the transplanted DA neurons functionally integrate with host striatal neurons32. The protocol was subsequently adapted to GMP-compatible conditions and large-scale cell manufacturing33. Stringent release criteria confirmed midbrain DA neuron identity and the absence of remaining pluripotent stem cells as well as other concerning contaminants such as serotonergic neurons and choroid plexus cells. Cryopreservation was also developed, and four lots comprising around 10 billion cells in total were produced. Investigational New Device (IND)-enabling studies, including tumorigenicity, toxicity and biodistribution confirmed safety, as well as efficacy in reversing rotational behaviour in murine models of PD. Detailed preclinical data were published in 202133,34. The same cryopreserved cell product tested here, named bemdaneprocel (formerly MSK-DA01), was then used in the clinical trial.
We conducted a first-in-human, multisite, open-label phase I trial to assess the safety and tolerability of bemdaneprocel in people with PD (NCT04802733). The full eligibility and exclusion criteria are listed in Supplementary Table 1; patients with cognitive impairment (defined by a Montreal Cognitive Assessment score of <26) or with dyskinesia (AIMS rating scale > 2) were excluded. The study included two cohorts: low-dose cohort A (0.9 million cells per putamen) and high-dose cohort B (2.7 million cells per putamen) (Fig. 1). Enrolment occurred sequentially, first into the low-dose cohort, then into the high-dose cohort after a 30-day pause for evaluation by the data safety monitoring committee. There were two surgical sites (New York, n = 9) and Toronto (n = 3) and three screening and follow-up sites (New York, Irvine and Toronto), depending on patient location of residence. The cell doses for injection were selected on the basis of the desired number of surviving DA neurons, and the measured survival rate of DA neurons in our IND-enabling studies, which were performed with the same lot of cells nominated for clinical use. The high dose targets the estimated number of healthy DA neurons in the intact substantia nigra35 (300,000 DA neurons), and the lower dose targets the estimated minimal number of surviving grafted DA neurons required for clinical benefit36 (100,000 DA neurons). The patients underwent a series of clinical assessments and rating scales, as well as magnetic resonance imaging (MRI) and 18Fluoro-DOPA (18F-DOPA) positron emission tomography (PET) imaging at the baseline and at regular intervals after grafting (Fig. 1).
Fig. 1. The study design.
Summary of the study design. Patients were enrolled sequentially into a low-dose then a high-dose cohort. The diagram indicates the timeline of immunosuppression, monthly laboratory and clinical assessments, and imaging studies throughout the study. Ongoing follow-up is anticipated for a minimum of 5 years.
The primary objective was to assess the safety and tolerability at 1 year after transplantation. Secondary objectives included the feasibility of stereotactic transplantation; survival of transplanted cells by 18F-DOPA uptake on PET imaging; motor effects measured using MDS-UPDRS Part III OFF scores37 and good ON times (without dyskinesia and with non-troublesome dyskinesia) based on adjusted PD diaries8; and continued safety and tolerability on the basis of the incidence of serious adverse events (SAEs) and adverse events (Supplementary Table 2). Exploratory outcomes included OFF time, ON times with troublesome dyskinesias, the Unified Dyskinesia Rating Scale (UDysRS), use of antiparkinsonian medications, the Non-Motor Symptom Scale (NMSS) and the patient-reported outcome on the basis of the PDQ-39 questionnaire. All safety data from all of the participants were used to conduct the formal analysis of the primary end point at 1 year after transplantation. Secondary and exploratory end points were also assessed at 1 year and 18 months after transplantation. No tests of statistical significance were performed due to the small number of participants.
Vials containing cryopreserved cells were thawed under aseptic conditions and suspended in transplantation medium, and the live-cell concentration was adjusted to 100,000 ± 10,000 cells per µl (ref. 34). Cells were loaded into a modified cannula (Smart Flow, Clearpoint Neuro), and administered stereotactically during a single surgical session under general anaesthesia. Cells were delivered into the post-commissural putamen bilaterally through a single burr hole on each side. In total, nine cell deposits were made in each putamen (three passes of the cannula; three deposits per pass). Surgery was performed using a frameless MRI-guided approach with intraoperative imaging at one site (New York) and a frame-based stereotactic approach at the other site (Toronto).
To prevent graft rejection, a short-term immunosuppressive regimen similar to that used for solid organ transplantation38,39 was initiated perioperatively and continued for 1 year. The participants received basiliximab 20 mg intravenously intraoperatively and postoperatively on day 4; methylprednisolone 500 mg intravenously immediately before surgery then tapered to oral prednisone 5 mg daily and continued for 1 year; and tacrolimus taken orally beginning on the day after surgery (day 1) and then adjusted to a target trough blood level of 4 to 7 ng ml−1 for a period of 1 year.
Demographics
In total, 12 participants were sequentially enrolled into the low-dose (n = 5) or high-dose (n = 7) cohort (Extended Data Fig. 1). The participants had a median age of 67.0 and 75% were male, with a median time since diagnosis of 9 years (Supplementary Table 3). All of the participants remain engaged in the clinical follow-up.
Extended Data Fig. 1. Patient Disposition.
Diagram detailing the disposition of all participants. The 12 participants who passed screening were enrolled sequentially into the low-dose cohort (N = 5) or high-dose cohort (N = 7).
Safety and tolerability
Predefined safety criteria for the primary outcome were satisfied at 12 months of follow-up after transplantation (Table 1). There were two SAEs reported: one participant in the low-dose cohort was hospitalized overnight with COVID-19 approximately 2 months after the surgery and one participant in the high-dose cohort experienced a single seizure attributed to the surgical procedure within 24 h of surgery that prolonged hospitalization by 1 day. The participant received levetiracetam for 2 months with no recurrence of seizure during or after discontinuation of the anticonvulsant. There were no deaths, no SAEs related to transplanted cells or immunosuppression, and there were no tumours, abnormal tissue overgrowth or intracerebral haemorrhages detected with MRI, which showed the expected postoperative changes (Extended Data Fig. 2).
Table 1.
Summary of treatment-emergent SAEs at 12 months post transplantation
| Low dose (n = 5) | High dose (n = 7) | |||||
|---|---|---|---|---|---|---|
| Participants reporting, n (%) (total number of events) | ||||||
| 0 events | 1 event | ≥2 events | 0 events | 1 event | ≥2 events | |
| TESAE | 4 (80.0) | 1 (20.0) (1) | 0 | 6 (85.7) | 1 (14.3) (1) | 0 |
| Related to surgery | 5 (100) | 0 | 0 | 6 (85.7) | 1 (14.3) (1) | 0 |
| Related to transplanted cells | 5 (100) | 0 | 0 | 7 (100) | 0 | 0 |
| Related to immunosuppressive drugs | 5 (100) | 0 | 0 | 7 (100) | 0 | 0 |
| Tumour or abnormal tissue overgrowth related to presence of transplanted cells | 5 (100) | 0 | 0 | 7 (100) | 0 | 0 |
| Intracerebral haemorrhage that is deemed life threatening | 5 (100) | 0 | 0 | 7 (100) | 0 | 0 |
| Deaths | 0 | 0 | ||||
Summary of treatment-emergent SAE (TESAE) for all patients, according to dose group.
Extended Data Fig. 2. Representative Magnetic Resonance images.
Representative axial T1-weighted and sagittal Flair MRI images obtained at baseline, 6 months, 12 months and 18 months. No evidence of intracerebral haemorrhage, mass, lesion, and/or cellular overgrowth. Transplanted cells were indiscernible; needle tracks exhibit gliosis as expected and are best seen in the lower panels. MRI: Magnetic resonance imaging.
In addition to the two SAEs reported in the 12-month post-transplantation analysis, an SAE of gastrointestinal haemorrhage requiring hospitalization was reported approximately 15 months after surgery, 3 months after discontinuation of prednisone. This was assessed as unrelated to the study procedures and was subsequently resolved.
Through 18 months after transplantation, there were 78 cumulative treatment-emergent adverse events (TEAEs), described in Extended Data Tables 1–5. Most were mild or moderate in severity except for one fall that was deemed to be unrelated to treatment. No TEAEs were related to bemdaneprocel. MRI imaging by 18 months did not show evidence of tumours or changes in the putaminal volume. Notably, there were no reports of adverse events or clinical indications of graft-induced dyskinesias (GIDs) to date. Transplant feasibility was achieved, with intraoperative delivery of the total number of all intended cell deposits in all of the participants.
Extended Data Table 1.
Summary of treatment-emergent adverse and serious adverse events
Summary of TEAE and TESAE through 18 months post transplantation. Data in each cell are presented as: Participants reporting (%) [number of events] unless otherwise indicated. *One SAE of COVID. †One SAE of gastrointestinal haemorrhage and one SAE of seizure. 18F-DOPA, 18 F−fluorodopa; MRI, magnetic resonance imaging; Q1, quartile 1; Q3, quartile 3; SAE, serious adverse event; TEAE, treatment-emergent adverse event; TESAE, treatment-emergent serious adverse event.
Extended Data Table 5.
TEAEs by System Organ Class: Infections and infestations
TEAE (treatment-emergent adverse events) by organ class. Data in each cell are presented as: Participants reporting (%) [number of events] unless otherwise indicated.
Secondary and exploratory outcomes
The two main secondary clinical readouts were MDS-UPDRS Part III OFF and changes in the number of waking hours in the ON state. A brief description of rating scales is provided in Supplementary Note 1. At 18 months after transplantation, the MDS-UPDRS Part III showed improvement in the OFF state with a decrease in mean (s.d.) of 8.6 points (29.2) from the baseline to 34.6 (9.2) in the low-dose cohort, and a mean decrease of 23.0 points (7.9) from the baseline to 25.2 (8.7) in the high-dose cohort (Fig. 2a,b). The mean good ON times, based on adjusted PD diaries, increased by 0.2 h (3.8 h) from the baseline to 12.3 h (3.2 h) in the low-dose cohort and by 2.7 h (1.6 h) from the baseline to 13.6 h (1.9 h) in the high-dose cohort (Fig. 2c,d). Among the exploratory end points (Fig. 2e–h), MDS-UPDRS Part III ON scores also improved with a decrease in the mean (s.d.) of 7.6 points (15.3) from the baseline to 13.4 (7.3) in the low-dose cohort and of 8.4 points (5.6) from the baseline to 15.9 (8.9) in the high-dose cohort (Fig. 2e). The mean (s.d.) OFF times, based on adjusted PD diaries, decreased by 0.8 h (3.3 h) from the baseline to 2.5 h (3.1 h) in the low-dose cohort and decreased by 2.7 h (1.8 h) from the baseline to 2.3 h (1.9 h) in the high-dose cohort (Fig. 2f). MDS-UPDRS Part II scores increased by a mean (s.d.) of 2.4 points (2.6) from the baseline to 13.2 (3.6) in the low-dose cohort and decreased by 2.7 points (5.2) from the baseline to 10.0 (4.6) in the high-dose cohort (Fig. 2g). The mean (s.d.) PDQ-39 summary index scores increased (worsened) by 0.4 points (7.4) from the baseline to 25.4 (6.8) in the low-dose cohort and decreased (improved) by 4.2 points (8.2) from the baseline to 10.9 (8.7) in the high-dose cohort (Extended Data Table 6). Across the total study population, ON times with troublesome dyskinesias (Extended Data Table 7) and use of antiparkinsonian medications were similar to the baseline (Extended Data Table 8). UDysRS scores were similar to the baseline in both cohorts (Fig. 2h). The NMSS scores varied across domains, with total scores trending towards stability in the high-dose cohort (Extended Data Table 9).
Fig. 2. Clinical outcomes 18 months after transplantation.
a,b, Secondary end points: individual MDS-UPDRS Part III OFF scores in the low-dose (a) and high-dose (b) cohorts at the baseline and at different timepoints after transplantation. c,d, Individual PD diary good ON time at the baseline and at different timepoints after transplantation in the low-dose (c) and high-dose (d) cohorts. Bold lines are mean ± s.d.; exact values are given as mean (s.d.) beneath each data point. e–h, Exploratory end points: individual scores on MDS-UPDRS Part III ON (e), Adjusted PD diary OFF time (f), MDS-UPDRS Part II (g) and UDysRS objective subscore (h) at the baseline and at different timepoints after transplantation in the low- and high-dose cohorts. Bold lines are mean ± s.d. The baseline value (0 months) is defined as the last recorded value before surgery. The double dagger symbol (‡) indicates the exception that, for MDS-UPDRS Part III OFF at 18 months after transplantation, n = 6. The total possible score for the MDS-UPDRS Part III scale is 132, for the MDS-UPDRS Part II scale is 52 and for the UDysRS objective scale is 44. Good ON time is the sum of ON time without dyskinesia and ON time with non-troublesome dyskinesia.
Extended Data Table 6.
39-item Parkinson’s Disease Questionnaire
Data in each cell are presented as: Participants reporting (%) [number of events] unless otherwise indicated.
The PDQ-39 contains eight domains (mobility [10 items], activities of daily living [6 items], emotional well-being [6 items], stigma [4 items], social support [3 items], cognition [4 items], communication [3 items], and bodily discomfort [3 items]). Each items is scored from 0 (never) to 4 (always); domain scores are calculated as a percentage of the maximum score (range of 0 to 100); the Summary Index is the arithmetic mean of all domain scores; lower scores indicate increasing quality of life. PDQ − 39, 39-item Parkinson’s Disease Questionnaire; SD, standard deviation.
Extended Data Table 7.
Adjusted PD Diary: ON Times with troublesome dyskinesias
Data in each cell are presented as: Participants reporting (%) [number of events] unless otherwise indicated. PD, Parkinson’s disease; SD, standard deviation.
Extended Data Table 8.
Anti-parkinsonian medications
Mean Levodopa- Equivalent Daily Dose, in mg tabulated by cohort and at baseline, 12 months and 18 months post transplantation. Data presented as mean and standard deviation (SD).
Extended Data Table 9.
Parkinson’s Disease Non-Motor Symptoms Scale
The Parkinson’s Disease Non-Motor Symptoms Scale (PD NMSS) consists of 30 items across 9 domains (cardiovascular [2 items], sleep/fatigue [4 items], mood/cognition [6 items], perceptual problems/hallucinations [3 items], attention/memory [3 items], gastrointestinal tract [3 items], urinary [3 items], sexual function [2 items], miscellaneous [4 items]). Each item is scored based on severity (0–3) and frequency (1–4); severity and frequency scores are multiplied and summed to produce domain scores and a total score of 0–360 (lower scores indicate less severe and frequent symptoms). PD NMSS, Parkinson’s Disease Non-Motor Symptom Scale; SD, standard deviation.
Graft survival
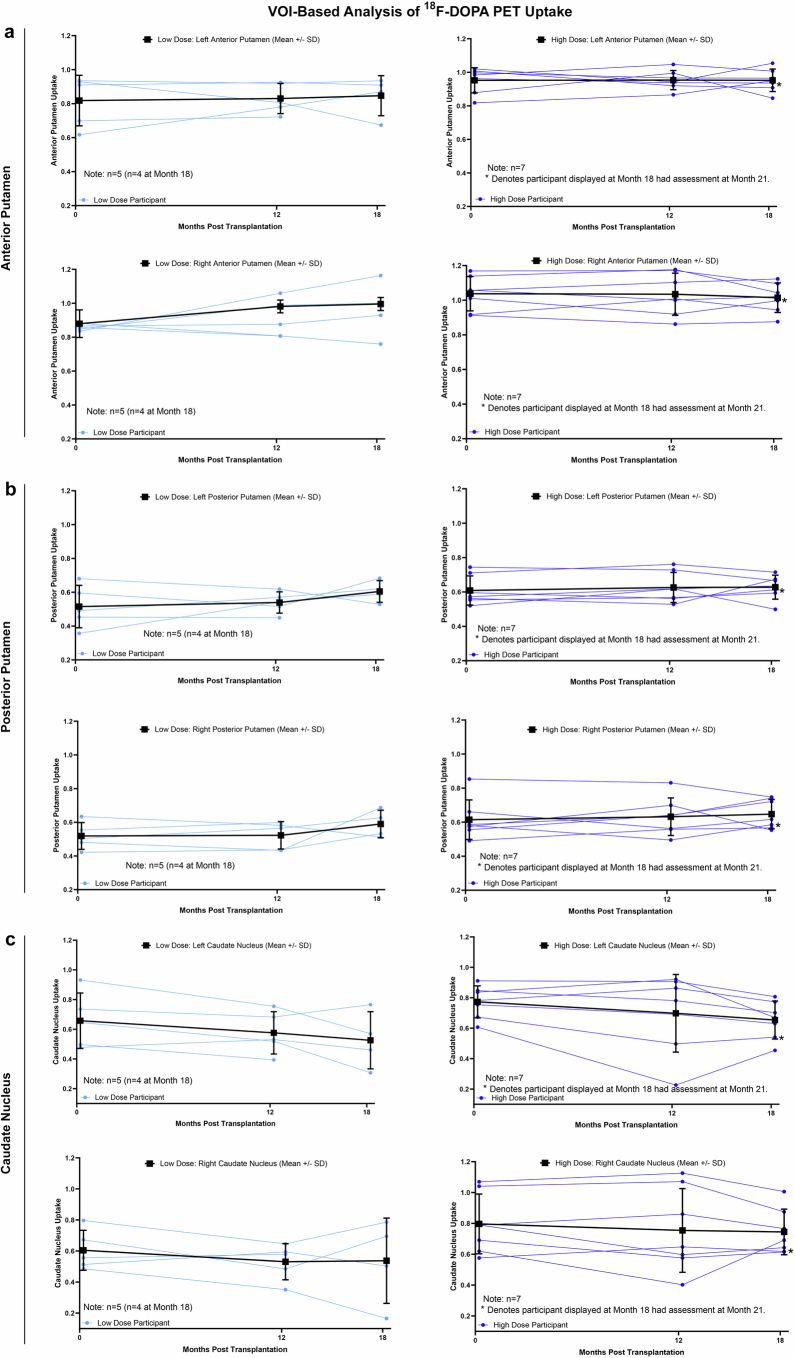
We performed serial 18F-DOPA PET imaging studies to obtain evidence of DA graft survival, a key secondary imaging-based end point. Quantitative analysis using the volume of interest (VOI) method revealed an increase in the mean uptake across the low- and high-dose cohorts bilaterally in the putamen, and stable to decreased uptake in the caudate. Uptake datapoints are shown for each individual patient in both cohorts (Extended Data Fig. 3). An image of the mean 18F-DOPA uptake signal is shown for the entire group (Fig. 3) and for the low- and high-dose cohorts (Extended Data Fig. 4) across multiple slices through the striatum. Individual patient data are also provided as source data. These data support DA neuron survival through the 18-month post-transplantation timepoint, 6 months after discontinuation of immunosuppression.
Extended Data Fig. 3. Quantification of 18F-DOPA PET uptake in the striatum.
Volume of interest (VOI)-based analysis of 18F-DOPA PET uptake signal in the anterior putamen (a), posterior putamen (b) and the caudate (c) at baseline, 12 months and 18 months, for individual patients in the low and high dose cohorts. Data is obtained via the VOI method as described in the Methods section. N = 12 for all timepoints with 2 exceptions: one patient missed the 18-month timepoint scan, and another patient had their 18-month timepoint scan performed at week 91 (at ~21 months) post grafting (data included). Bold lines in each panel represent the mean ± SD.
Fig. 3. PET images of 18F-DOPA uptake signal in the striatum.

Images of the 18F-DOPA PET uptake signal at multiple sections through the striatum, presented as the mean uptake of all patients (n = 12 at all timepoints except at 18 months, for which n = 11). Images of 18F-DOPA uptake were produced by dividing each original PET image by the occipital count and then subtracting 1. Formula: image of 18F-DOPA uptake = (PET image/occipital count − 1).
Extended Data Fig. 4. PET images of 18F-DOPA PET uptake signal in the two dose cohorts.
Images of 18F-DOPA PET uptake signal at multiple sections through the striatum, presented as mean uptake at baseline, 12 months, and 18 months in the low dose cohort (top) and high dose cohort (bottom). Images were produced by dividing each original PET image by occipital count and then subtracting 1. PET, positron emission tomography. N = 12 for all timepoints with 2 exceptions: one patient missed the 18-month timepoint scan, and another patient had their 18-month timepoint scan performed at week 91 (at ~21 months) post grafting (data included).
Discussion
In this first-in-human phase I trial, bilateral putaminal transplantation of bemdaneprocel, a hES cell-derived DA neuron progenitor cell product, was generally well tolerated and achieved predefined safety criteria at 12 months as a primary end point. Safety data through 18 months continued to indicate a favourable safety profile. The treatment process, including surgery and immunosuppression, was generally well tolerated. A high incidence of GID was reported in two randomized controlled trials25,26 of fetal ventral mesencephalic transplants (56% (n = 13 out of 23) and 15% (n = 5 out of 33) of grafted participants). This was a serious complication and was considered one of the major drawbacks of cell therapy at the time. The absence of GID in our study through 18 months is therefore particularly notable. This may relate to the lack of serotonergic neuron contaminants in bemdaneprocel, as preclinical studies confirmed the absence of serotonergic neurons in vitro and in vivo at 9 months after grafting34. Longer-term clinical and imaging follow-up is ongoing to monitor for the possibility of delayed onset GIDs or other adverse events.
Although this phase I study is not designed nor powered for determining efficacy, there is nonetheless a possibility of improvement in the key secondary outcomes, including the MDS-UPDRS OFF motor scores, reflecting improved motor function without the benefit of anti-Parkinsonian medication. This scale was selected as an outcome measure as it is the most widely used PD scale; it is well validated with high inter-rater reliability, and is sensitive to clinical changes40. Our data show a mean change of 8.6 points in the low-dose cohort and 23 points in the high-dose cohort, consistent with a moderate and large ‘clinically important difference’ in motor scores41, respectively. Coupled with the stabilization or possible improvement in other secondary and exploratory motor, non-motor and quality-of-life outcomes, the data suggest a promise for potential clinical benefit. However, these data need to be interpreted cautiously in view of the open-label study design, the small patient cohort, the relatively short observation period, the expected fluctuations in PD symptoms and the possibility of placebo effects, which can be substantial in studies of PD. Longer-term and larger studies are required to more rigorously evaluate the safety and efficacy of this cell therapy for PD. The clinical data also show a greater amplitude of changes in UPDRS motor scores and reduction in OFF times, and an earlier onset of improvement, in the high-dose group.
Establishing an optimal cell dose involves multiple considerations. While the grafted DA neuron progenitors are expected to become post-mitotic and mature without obvious in vivo expansion, as confirmed in preclinical studies, the rates of progressive graft maturation and functional integration within the host tissue are unknown42. Another unknown factor is the impact of the ongoing disease on the graft itself. Fetal grafting studies showed evidence of Lewy body formation within the graft; however, such disease transmission occurred only in later stages (more than a decade) after transplantation and affected only a minority of grafted DA neurons43,44. Nevertheless, the precise kinetics of hES cell-derived midbrain DA neuron maturation in grafts and their relative disease vulnerability remains unclear, which complicates the dose-finding process.
An encouraging finding in our study was the evidence of likely graft survival on the basis of 18F-DOPA PET, 6 months after cessation of immunosuppression. However, PET data should be interpreted cautiously owing to limitations in the resolution, signal-to-noise ratio and variability across and within patients as well as between the low- and high-dose cohorts. Furthermore, the correlation between 18F-DOPA PET uptake and clinical performance can be variable, and graft survival alone is not sufficient to predict long-term patient benefit. In past fetal allograft studies, the dosing, duration and type of immunosuppression was inconsistent.25,26,45. Data from one of the two randomized clinical trials suggested a potential decline in clinical function after discontinuation of immunosuppression25, yet autopsy reports in other studies demonstrated graft survival many years after surgery and discontinuation of immunosuppression. Our results suggest that the dosing and duration of immunosuppression were sufficient, well-tolerated and protected cell survival and function; longer-term follow-up data will be critical to confirm these findings. An alternative strategy to overcome graft rejection could lie in the use of an autologous iPS-cell-based DA-neuron-grafting approach. An initial single case report has been described to demonstrate feasibility46. However, consistently manufacturing and releasing an autologous product poses new challenges, and the possibility exists that the autologous nature of the cells may entail an increased risk of genetic susceptibility to develop disease. Thus, the feasibility of allografting without long-term immunosuppression, coupled with cryopreservation, offers an off-the-shelf approach33,47 that could facilitate the broader application of this therapy in the future.
Finally, our findings should be interpreted carefully within the context of a small and unblinded safety and tolerability study that achieved its primary outcome and was not designed to evaluate efficacy based on clinical outcomes. Despite this, the possible improvement in PD symptoms at 18 months after transplantation may translate in future trials to an important finding. There are currently several ES-cell- or iPS-cell-based grafting studies that are ongoing or close to initiation in the US, Asia and Europe, highlighting considerable enthusiasm for cell therapy for PD. These studies will probably differ in many aspects, including autologous versus allografts, cell dosing, patient selection, immunosuppression, surgical strategies and other criteria; they will therefore provide valuable insights into future study design and the value of cell grafting in PD. Taken together, our data support moving towards larger definitive clinical studies.
Methods
Cell preparation
In brief, master banks of undifferentiated WA09 (H9) cells (passage 28) were obtained from the WiCell Research Institute. The cells were manufactured under cGMP conditions to establish working cell banks that were further certified by Waisman Biomanufacturing. The cells passed a battery of tests including cell authentication by short tandem repeat (STR) analysis, karyotype and marker expression, as well as testing for adventitious viruses and mycoplasma. The full protocol is described in a previous publication34. cGMP manufacturing of MSK-DA01 cells, which were differentiated from hES cells (WA09 at passage 33), was performed at the Cell Therapy and Cell Engineering Facility (CTCEF) at MSKCC. WA09 cells were thawed and expanded on Geltrex coated flasks/dishes in Essential 8 basal medium with supplement for 10–14 days. Cells were split by Dispase (Stem cell Technologies) every 3–5 days at a ratio usually between 1:4 and 1:6. For MSK-DA01 differentiation, single cells WA09 were washed and plated on Geltrex at 400,000 cells per cm2 in Neurobasal medium with N2 and B27 (without vitamin A) containing 2 mM l-glutamine, 10 µM SB431542, 250 nM LDN193189 (LDN), 0.7 µM CHIR99021 and 500 ng ml−1 SHH with 10 µM Y-27632. The medium was replaced daily thereafter without adding Y-27632. On day 4, 7.5 µM CHIR99021 was added. The same medium was replaced on day 6. On day 7 SB, LDN and SHH were withdrawn from the medium. The same medium was changed on day 9. On day 10, Neurobasal medium (with B27) with 2 mM L-glutamine, 20 ng ml−1 BDNF, 20 ng ml−1 GDNF, 200 µM ascorbic acid, 500 µM dibutyryl-cAMP, 1 ng ml−1 TGFβ3 and 3 µM CHIR99021 was added. On day 11, cells were dissociated to single cells with Accutase for 30–40 min and replated on plates coated with poly-ornithine (15 µg ml−1, Sigma Aldrich), fibronectin (1 µg ml−1, Akron Biotech) and laminin (2 µg ml−1, Trevigen) at 800,000 cells per cm2 using the same medium as on day 10. On day 12, the medium was switched to include 10 µM DAPT and CHIR99021 was withdrawn. Complete medium changes were performed daily until collection at day 16. At day 16 of differentiation, MSK-DA01 cells were dissociated with Accutase for 30–40 min and filtered through a 40 µm cell strainer. The cell pellets were resuspended at a cell density of 8 million cells per ml of STEM-CELLBANKER and placed in a controlled-rate freezer (Thermo Fisher Scientific) for cryopreservation. Cryopreserved vials were stored in the secured GMP Facility freezer and monitored 24/7 by a Datatron system.
Study design and participants
This trial included two cohorts: a low-dose (cohort A; 0.9 million cells per putamen) and a high-dose (cohort B; 2.7 million cells per putamen) cohort (Extended Data Fig. 1). Enrolment occurred sequentially, first into the low-dose cohort, then into the high-dose cohort after a 30-day pause for evaluation by the data safety monitoring committee. The eligible participants (Supplementary Table 1) were aged at least 50 to 78 years (Canada) or at least 60 to 78 years (United States) with a confirmed diagnosis of PD for 3 to 20 years before screening and Hoehn–Yahr ON score of 0 to 3 (Canada) or ON score of 0 to 2 and OFF score of 3 to 4 (United States). The full inclusion and exclusion criteria are described in the protocol, which is provided in the Supplementary Information.
The participants attended screening, baseline and preoperative visits before surgery. After transplantation, the participants underwent a postoperative evaluation on day 10. A battery of questionnaires and clinical rating scales were administered during site visits throughout the study, with a total follow-up of 5 years after transplantation; the participants completed PD diaries at home before each visit, and adverse events were assessed throughout the trial.
Primary, secondary and exploratory outcomes
According to the protocol, the primary end point was the incidence of SAEs. Safety success criteria were defined as: two or fewer participants in either cohort developing two or more SAEs related to surgery, the presence of transplanted cells or immunosuppression; two or fewer participants in either cohort developing a tumour or abnormal tissue overgrowth related to the presence of transplanted cells; two or fewer participants in either cohort developing an intracerebral haemorrhage that was deemed to be life threatening; and one or zero deaths in either cohort. Secondary objectives (Supplementary Table 2) included the feasibility of stereotactic transplantation; survival of transplanted cells by 18F-DOPA uptake on PET imaging; motor effects measured by Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part III OFF scores and adjusted PD diary good ON times (the sum of ON time without dyskinesia and ON time with non-troublesome dyskinesia); and safety and tolerability by the incidence of SAEs and adverse events.
Neuroimaging
18F-DOPA PET studies and analysis were performed at a single imaging site by investigators blinded to clinical and dose information.
18F-DOPA PET acquisition
The participants underwent three-dimensional PET imaging at the baseline, and at 12 and 18 months after transplantation. Images were acquired on a high-resolution, high-sensitivity PET tomograph (GE Discovery IQ-5 ring PET/CT Scanner, General Electric Medical Systems; parameters: full-width-at-half-maximum = 5 mm, axial field of view = 26 cm, slice thickness = 3.3 mm, 79 slices). Acquisition began approximately 80 to 100 min after a 5.0 mCi intravenous injection of 18F-DOPA. The participants paused antiparkinsonian medication 12 h before scanning and were given 150 mg of carbidopa orally within 120 min before 18F-DOPA injection in accordance with FDA prescribing information. 18F-FDOPA PET image reconstruction used the VUEPointHD ViP (VPHD) method, a 3D iterative OSEM (ordered subset expectation maximization) algorithm with 4 iterations and 12 subsets after corrections for attenuation and scatter. The image resolution was approximately 5 mm in all directions.
18F-DOPA PET analyses
We created a priori group mean of striatal to occipital ratio (SOR-1) images computed for each timepoint, as well as a priori VOI-based analyses. The SOR-1 images (Fig. 3) were derived to provide an anatomical visualization of potential change in group mean18F-DOPA uptake signal over the striatum across the timepoints. The VOI-based analyses were performed to provide group × time quantification of 18F-DOPA uptake signal within prespecified regions within the striatum (that is, posterior putamen, surgical target region of implanted cells; caudate, control region, no implanted cells).
Mean image derivation in the standard brain space
18F-DOPA PET images for each participant were realigned across individual time frames and then registered together to the high-resolution T1 structural MRI scan (see below) using SPM12 software (https://www.fil.ion.ucl.ac.uk/spm). The registered PET images were converted into volumetric maps of specific 18F-DOPA uptake defined by (image/occipital counts − 1; SOR-1), with the occipital cortex used as a reference. The SOR-1 images of 18F-DOPA uptake before and after treatment were spatially normalized into the standard Montreal Neurological Institute anatomic space (https://mcin.ca/research/neuroimaging-methods/atlases). The group mean for the entire cohort was then produced from these images at each timepoint and displayed using the SOR-1 scale reported in the main text (Fig. 3).
MRI-guided VOI analysis of striatal 18F-DOPA uptake in the native brain space
18F-DOPA PET images for each participant were realigned across individual time frames and then registered together to the high-resolution T1 structural MRI scan (see below) in native space using SPM12. 18F-DOPA PET images were maintained in the native space without performing spatial normalization. Anatomical structures of the caudate nucleus and putamen were segmented in the MRI scan using an automated processing routine implemented with FSL software48 (https://fsl.fmrib.ox.ac.uk/fsl/docs/#/install/index). The regions of the anterior and posterior putamen were then separated using a semi-automated procedure involving realignment of the anterior commissure–posterior commissure planes on the MRI scan. 18F-DOPA counts were computed automatically in each striatal VOI, including the caudate nucleus, anterior and posterior putamen and occipital cortex (16 mm diameter sphere in each hemisphere). The 18F-DOPA uptake was then calculated (regional 18F-DOPA counts/occipital counts − 1) using FSL with the occipital cortex used as a reference. Individual datapoints for the anterior putamen, posterior putamen and caudate are shown for both the low- and high-dose cohorts (Extended Data Fig. 3). This method had been previously validated49,50 and used successfully in the PET imaging studies of fetal cell transplantation25,42,51,52.
MRI studies
MRI studies of the brain (1.5 Tesla and 3 Tesla) were performed using standardized acquisition protocols at screening, preoperatively for surgical planning, intraoperatively, postoperatively, and 1, 3, 6, 12 and 18 months after transplantation.
Demographics
Between 3 May 2021 and 30 March 2022, 17 participants were assessed for eligibility; five were excluded due to screening failure. The remaining 12 participants were sequentially enrolled into the low-dose (n = 5) or high-dose (n = 7) cohort (Extended Data Fig. 1). At the time of the last participant completing the 18-month visit, the median (min, max) follow-up time was 21.2 months (18.5, 24.7) and all of the participants were still engaged in clinical follow-up. The participants had a median age of 67.0 years (quartile 1 (Q1), Q3: 64.5, 70.0); 67% were white and 75% were male (Supplementary Table 3). The median (Q1, Q3) time since diagnosis was 9.0 (5.9, 11.5) years. At the baseline, all of the participants had a Hoehn–Yahr ON score of 2; 11 participants had a Hoehn–Yahr OFF score of 3 and one participant (low-dose cohort; Canada) had a score of 2.
TEAEs
TEAEs most frequently experienced by participants (Extended Data Table 6) were in the nervous system disorders (11 TEAEs in eight participants; Extended Data Table 7) and infections and infestations (10 TEAEs in seven participants; Extended Data Table 8) system/organ classes. Of the infections, one TEAE each of oral herpes and urinary tract infection was considered to be related to immunosuppression. No TEAEs were related to bemdaneprocel. Aside from two severe TEAEs (one fall deemed unrelated to treatment and the aforementioned SAE of gastrointestinal haemorrhage), all others were mild or moderate in severity (Extended Data Table 4).
Extended Data Table 4.
TEAEs by System Organ Class: Nervous System Disorders
TEAE (treatment-emergent adverse events) by organ class. Data in each cell are presented as: Participants reporting (%) [number of events] unless otherwise indicated.
Statistics
All safety data from all of the participants were used to conduct the formal analysis of the primary end point at 1 year after transplantation. Secondary and exploratory end points were also assessed at 1 year and 18 months after transplantation. Inferential statistical testing was not performed due to the small number of participants. Descriptive statistics (mean (standard deviation (s.d.)) or percentage (counts)) were tabulated by cohort.
Trial oversight
The trial was conducted in accordance with the protocol and all applicable local government laws, regulations and guidance, including policies with foundations in the World Medical Association Declaration of Helsinki, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. An institutional review board at Memorial Sloan Kettering Cancer Center and Weill Cornell Medicine reviewed and approved the clinical protocol (submitted with the Article) and the patient consent form. All of the participants signed the informed consent. (MSKCC: IRB no. 18-518 on 6/8/2021; WCM IRB no: 1810019690 on 14 January 2019). A data safety monitoring committee and the institutional review boards monitored trial progression and data handling. The Tri-institutional Human Embryonic Stem Cell Research Oversight committee (ESCRO) at MSKCC and WCM approved the study and the consent on 11 October 2019.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-025-08845-y.
Supplementary information
Supplementary Note 2: study protocol and statistical plan
Supplementary Tables 1–3
Source data
Acknowledgements
We thank the study participants, study investigators, study staff, the staff at the Cell Therapy and Cell Engineering Facility (Memorial Sloan Kettering Cancer Center) and the members of the Feinstein Institutes for Medical Research (Northwell Health) for their contributions to this research. This study was sponsored by BlueRock Therapeutics. The sponsor (BlueRock Therapeutics) collaborated with investigators on trial design and data collection, analysis and interpretation. The initial work to develop bemdaneprocel (MSK-DA01) was supported by NYSTEM grant C028503 and MSK Core Grant P30 CA008748. Under the direction of the authors, B. M. Hiller of Peloton Advantage, an OPEN Health company, provided medical writing support, which was funded by BlueRock Therapeutics.
Extended data figures and tables
Extended Data Table 2.
Summary of TEAEs related to immunosuppression regimen
Summary of TEAEs related to immunosuppression regimen. There were no TEAEs (treatment-emergent adverse events) after discontinuation of immunosuppression. Data in each cell are presented as: Participants reporting (%) [number of events] unless otherwise indicated.
Extended Data Table 3.
TEAEs by System Organ Class
TEAE (treatment-emergent adverse events) by organ class. Data in each cell are presented as: Participants reporting (%) [number of events] unless otherwise indicated.
Author contributions
V.T. served as a neurosurgeon investigator, performed surgery, contributed to preclinical data, clinical trial design and manuscript writing. H.S. served as principal investigator and neurologist on the trial. A.M.L., S.K.K., C.B. and K.K.H.Y. performed transplantation surgery as investigators on the trial. A.F. served as a neurologist investigator. Y.M., S.P. and D.E. performed the PET imaging studies, analysed the data and contributed to the PET screening of trial participants. M.T. and S.I. contributed to the preclinical data and to the study design. A.M.L. contributed to data analysis. N.A. contributed to medical monitoring. W.S. is the study statistician. L.S. contributed to the preclinical data, study design and manuscript preparation. C.H. was a neurologist investigator on the trial and contributed to the trial design. All of the authors contributed to and approved the manuscript.
Peer review
Peer review information
Nature thanks William Jagust, Hideyuki Okano, Mark Tuszynski and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
Data supporting the findings of this study, including de-identified participant-level data, are provided within the Article and its Supplementary Information. The study protocol and statistical analysis plan are also included in Supplementary Note 2. For further information on exploratory end points that are not described in this Article, investigators who provide a methodologically sound proposal may submit a request to medinfo@bluerocktx.com. Requests will be assessed and responded to within 30 days of receipt. Source data are provided with this paper.
Competing interests
V.T. is a scientific advisor and receives research support from BlueRock Therapeutics. H.S. has done consulting work for Novo Nordisk, BlueRock Therapeutics, Neurocrine and Neuroderm; and has also received clinical trial support from BlueRock Therapeutics, Prevail Therapeutics, Neuroderm, Sun Pharma, Meira GTX, Bukwang, Insightec, Biogen, Genentech, Cerevance, UCB and National Institutes of Health (NIH). A.M.L. has done consulting work for Medtronic, Abbott, Boston Scientific and Insightec; and is a scientific officer of Functional Neuromodulation. A.F. has stock ownership in Inbrain Pharma and has received payments as consultant and/or speaker from AbbVie, Abbott, Boston Scientific, CereGate, Dompé Farmaceutici, Inbrain Neuroelectronics, Ipsen, Medtronic, Iota, Syneos Health, Merz, Sunovion, Paladin Labs and UCB; and has received research support from AbbVie, BlueRock Therapeutics, Boston Scientific, Medtronic, Praxis and ES, and receives royalties from Springer. S.K.K. has received consultant fees, support and/or speaker honoraria from Novo Nordisk, Abbott, Boston Scientific and Medtronic. Y.M. has served as a consultant, study investigator and site principal investigator to acquire and/or analyse PET/SPECT and MRI brain images in multicentre clinical trials of stem cell and gene therapies for PD; and has received research support and paid travel from BlueRock Therapeutics and Aspen Neuroscience, research support from AskBio and MeiraGTx, and paid consulting services from Novo Nordisk. S.I., M.T., N.A. and W.S. are employees of BlueRock Therapeutics. A.L. is currently an employee of Dompé. L.S. is a scientific advisor and receives research support from BlueRock Therapeutics and is a scientific co-founder of DaCapo Brainscience. C.H. has received honoraria for consulting from Abbvie, AskBio and Certara; stock options for scientific advisory board participation from Axent Biosciences; honoraria for scientific advisory board participation from Bayer, Canary Health Technologies and ProJenX; honoraria for DSMB meetings from MeiraGTx; research support from BlueRock Therapeutics and Weston Brain Institute; and honoraria for presentations from the American Academy of Neurology, ASGCT and the Parkinson Study Group. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: L. Studer, C. Henchcliffe
Extended data
is available for this paper at 10.1038/s41586-025-08845-y.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-025-08845-y.
References
- 1.Dorsey, E. R. & Bloem, B. R. The Parkinson pandemic—a call to action. JAMA Neurol.75, 9–10 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Morris, H. R., Spillantini, M. G., Sue, C. M. & Williams-Gray, C. H. The pathogenesis of Parkinson’s disease. Lancet403, 293–304 (2024). [DOI] [PubMed] [Google Scholar]
- 3.Armstrong, M. J. & Okun, M. S. Diagnosis and treatment of Parkinson disease: a review. JAMA323, 548–560 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Poewe, W. et al. Parkinson disease. Nat. Rev. Dis. Primers3, 17013 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Heimrich, K. G., Schönenberg, A., Santos-García, D., Mir, P., Coppadis Study Group & Prell, T. The impact of nonmotor symptoms on health-related quality of life in Parkinson’s disease: a network analysis approach. J. Clin. Med.12, 2573 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung, K. A., Lobb, B. M., Nutt, J. G. & Horak, F. B. Effects of a central cholinesterase inhibitor on reducing falls in Parkinson disease. Neurology75, 1263–1269 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henderson, E. J. et al. Rivastigmine for gait stability in patients with Parkinson’s disease (ReSPonD): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol.15, 249–258 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Hauser, R. A. et al. A home diary to assess functional status in patients with Parkinson’s disease with motor fluctuations and dyskinesia. Clin. Neuropharmacol.23, 75–81 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Kremer, N. I. et al. STN-DBS electrode placement accuracy and motor improvement in Parkinson’s disease: systematic review and individual patient meta-analysis. J. Neurol. Neurosurg. Psychiatry94, 236–244 (2023). [DOI] [PubMed] [Google Scholar]
- 10.Krishna, V. et al. Trial of globus pallidus focused ultrasound ablation in Parkinson’s disease. N. Engl. J. Med.388, 683–693 (2023). [DOI] [PubMed] [Google Scholar]
- 11.Bond, A. E. et al. Safety and efficacy of focused ultrasound thalamotomy for patients with medication-refractory, tremor-dominant Parkinson disease: a randomized clinical trial. JAMA Neurol.74, 1412–1418 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang, A. E. et al. Trial of cinpanemab in early Parkinson’s disease. N. Engl. J. Med.387, 408–420 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Pagano, G. et al. Trial of prasinezumab in early-stage Parkinson’s disease. N. Engl. J. Med.387, 421–432 (2022). [DOI] [PubMed] [Google Scholar]
- 14.Standaert, D. G. GLP-1, Parkinson’s disease, and neuroprotection. N. Engl. J. Med.390, 1233–1234 (2024). [DOI] [PubMed] [Google Scholar]
- 15.Meissner, W. G. et al. Trial of lixisenatide in early Parkinson’s disease. N. Engl. J. Med.390, 1176–1185 (2024). [DOI] [PubMed] [Google Scholar]
- 16.Abeliovich, A., Hefti, F. & Sevigny, J. Gene therapy for Parkinson’s disease associated with GBA1 mutations. J. Parkinsons Dis.11, S183–S188 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Laar, A. D. et al. An update on gene therapy approaches for Parkinson’s disease: restoration of dopaminergic function. J. Parkinsons Dis.11, S173–S182 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Bie, R. M. A., Clarke, C. E., Espay, A. J., Fox, S. H. & Lang, A. E. Initiation of pharmacological therapy in Parkinson’s disease: when, why, and how. Lancet Neurol.19, 452–461 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Wenning, G. K. et al. Short- and long-term survival and function of unilateral intrastriatal dopaminergic grafts in Parkinson’s disease. Ann. Neurol.42, 95–107 (1997). [DOI] [PubMed] [Google Scholar]
- 20.Freed, C. R. et al. Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson’s disease. N. Engl. J. Med.327, 1549–1555 (1992). [DOI] [PubMed] [Google Scholar]
- 21.Brundin, P. et al. Bilateral caudate and putamen grafts of embryonic mesencephalic tissue treated with lazaroids in Parkinson’s disease. Brain123, 1380–1390 (2000). [DOI] [PubMed] [Google Scholar]
- 22.Hagell, P. et al. Sequential bilateral transplantation in Parkinson’s disease: effects of the second graft. Brain122, 1121–1132 (1999). [DOI] [PubMed] [Google Scholar]
- 23.Lindvall, O. et al. Evidence for long-term survival and function of dopaminergic grafts in progressive Parkinson’s disease. Ann. Neurol.35, 172–180 (1994). [DOI] [PubMed] [Google Scholar]
- 24.Peschanski, M. et al. Bilateral motor improvement and alteration of l-dopa effect in two patients with Parkinson’s disease following intrastriatal transplantation of foetal ventral mesencephalon. Brain117, 487–499 (1994). [DOI] [PubMed] [Google Scholar]
- 25.Olanow, C. W. et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann. Neurol.54, 403–414 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Freed, C. R. et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N. Engl. J. Med.344, 710–719 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Politis, M. et al. Serotonin neuron loss and nonmotor symptoms continue in Parkinson’s patients treated with dopamine grafts. Sci. Transl. Med.4, 128ra141 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Kefalopoulou, Z. et al. Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol71, 83–87 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hallett, P. J. et al. Long-term health of dopaminergic neuron transplants in Parkinson’s disease patients. Cell Rep.7, 1755–1761 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, W. et al. Extensive graft-derived dopaminergic innervation is maintained 24 years after transplantation in the degenerating parkinsonian brain. Proc. Natl Acad. Sci. USA113, 6544–6549 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kriks, S. et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature480, 547–551 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinbeck, J. A. et al. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson’s disease model. Nat. Biotechnol.33, 204–209 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim, T. W. et al. Biphasic activation of WNT signaling facilitates the derivation of midbrain dopamine neurons from hESCs for translational use. Cell Stem Cell28, 343–355 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piao, J. et al. Preclinical efficacy and safety of a human embryonic stem cell-derived midbrain dopamine progenitor product, MSK-DA01. Cell Stem Cell28, 217–229 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudow, G. et al. Morphometry of the human substantia nigra in ageing and Parkinson’s disease. Acta Neuropathol.115, 461–470 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hagell, P. & Brundin, P. Cell survival and clinical outcome following intrastriatal transplantation in Parkinson disease. J. Neuropathol. Exp. Neurol.60, 741–752 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Goetz, C. G. et al. Movement Disorder Society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov. Disord.22, 41–47 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Nelson, J. et al. Consensus recommendations for use of maintenance immunosuppression in solid organ transplantation: endorsed by the American College of Clinical Pharmacy, American Society of Transplantation, and International Society for Heart and Lung Transplantation: an executive summary. Pharmacotherapy42, 594–598 (2022). [DOI] [PubMed] [Google Scholar]
- 39.Neuberger, J. M. et al. Practical recommendations for long-term management of modifiable risks in kidney and liver transplant recipients: a guidance report and clinical checklist by the Consensus on Managing Modifiable Risk in Transplantation (COMMIT) Group. Transplantation101, S1–S56 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Martinez–Martin, P., Rodriguez-Blazquez, C. & Forjaz, M. J. in Neuroscience and Biobehavioral Psychology 8–16 (Elsevier, 2017).
- 41.Shulman, L. M. et al. The clinically important difference on the unified Parkinson’s disease rating scale. Arch. Neurol.67, 64–70 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Ma, Y. et al. Dopamine cell implantation in Parkinson’s disease: long-term clinical and 18F-FDOPA PET outcomes. J. Nucl. Med.51, 7–15 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li, J. Y. et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med.14, 501–503 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Kordower, J. H., Chu, Y., Hauser, R. A., Freeman, T. B. & Olanow, C. W. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med.14, 504–506 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Barker, R. A. Designing stem-cell-based dopamine cell replacement trials for Parkinson’s disease. Nat. Med.25, 1045–1053 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Schweitzer, J. S. et al. Personalized iPSC-derived dopamine progenitor cells for Parkinson’s disease. N. Engl. J. Med.382, 1926–1932 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirkeby, A. et al. Preclinical quality, safety, and efficacy of a human embryonic stem cell-derived product for the treatment of Parkinson’s disease, STEM-PD. Cell Stem Cell30, 1299–1314 (2023). [DOI] [PubMed] [Google Scholar]
- 48.Patenaude, B., Smith, S. M., Kennedy, D. N. & Jenkinson, M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage56, 907–922 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dhawan, V. et al. Comparative analysis of striatal FDOPA uptake in Parkinson’s disease: ratio method versus graphical approach. J. Nucl. Med.43, 1324–1330 (2002). [PubMed] [Google Scholar]
- 50.Jokinen, P. et al. Simple ratio analysis of 18F-fluorodopa uptake in striatal subregions separates patients with early Parkinson disease from healthy controls. J. Nucl. Med.50, 893–899 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Nakamura, T. et al. Blinded positron emission tomography study of dopamine cell implantation for Parkinson’s disease. Ann. Neurol.50, 181–187 (2001). [DOI] [PubMed] [Google Scholar]
- 52.Ma, Y. et al. Dyskinesia after fetal cell transplantation for parkinsonism: a PET study. Ann. Neurol.52, 628–634 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Note 2: study protocol and statistical plan
Supplementary Tables 1–3
Data Availability Statement
Data supporting the findings of this study, including de-identified participant-level data, are provided within the Article and its Supplementary Information. The study protocol and statistical analysis plan are also included in Supplementary Note 2. For further information on exploratory end points that are not described in this Article, investigators who provide a methodologically sound proposal may submit a request to medinfo@bluerocktx.com. Requests will be assessed and responded to within 30 days of receipt. Source data are provided with this paper.