Abstract
Severe COVID-19 is rare in children suggesting differences in immune response between children and adults. Limited information is available on how cellular immunity is modulated by COVID-19 vaccination and prior infection, and whether it is differentially modulated in children compared to adults. Here, we aimed to compare COVID-19 vaccine-induced functional T cell response between adults and children with and without previous SARS-CoV-2 infection. Adults (18–45 years; n = 45) and children (5–10 years; n = 51;), who received Pfizer-BioNTech COVID-19 vaccine or remained unvaccinated, and previously infected or not with SARS-CoV-2 were selected from two cross-sectional SARS-CoV-2 serosurveillance studies conducted in Bangladesh. Plasma nucleocapsid (N)-specific antibodies were measured by electrochemiluminescence immunoassay; IFN-γ, perforin and granzyme B secreting T cells were assessed using ELISpot assay. Vaccination in adults without previous infection, induced higher frequencies of IFN-γ and granzyme B secreting T lymphocytes compared to unvaccinated adults, while it increased only IFN-γ expression in vaccinated children. Previous infection increased IFN-γ response in unvaccinated adults only. Unvaccinated children showed higher granzyme B expression compared to adults irrespective of infection status. In vaccinated individuals, prior infection induced perforin expression in both adults and children. Children showed slightly different functional T cell response than adults in response to COVID-19 vaccination and infection. mRNA vaccination provided higher IFN-γ response in both adults and children, but induced cytotoxic T lymphocyte (CTL) response in adults only. Future studies may evaluate the impact of other types of COVID-19 vaccines on functional T cell immunity in children to confirm the findings.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-95870-6.
Keywords: Cytotoxic T lymphocyte, Perforin, Granzyme B, IFN-γ, Cellular immunity
Subject terms: Immunology, Diseases
Introduction
Cellular immunity is critical for the regulation of viral diseases. Both infection and vaccination induce CD4 and CD8 T cell responses. Although antibody responses are key to prevent infection1, T cell responses play critical roles in reducing disease severity and controlling infection2,3. Several studies have investigated the role of SARS-CoV-2-specific CD4 and CD8 T cells and cytotoxic T lymphocytes (CTL) in natural infections in various clinical spectra, and after COVID-19 vaccination, mostly focusing on phenotypic and antigen epitope specific changes4.
Assessing perforin and granzyme B secreting cells are proxy measures for evaluation of functional capacities of CTL and also natural killer (NK) cells, since both cells are the main source of these cytolytic molecules. The perforin-granzyme system of cytotoxicity is the main mechanism through which CTL and NK cells eradicate infected target cells5,6. During severe COVID-19 disease, there was exhaustion of circulating CD8+ T cells and NK cells, but the expression of the cytolytic proteins perforin and granzyme B was increased, indicating compensatory increase in cytotoxic potential of these cells as a host defense strategy7.
It is well recognized that children can be infected with SARS-CoV-2, but severe COVID-19 disease and deaths are less common in children than in adults and elderly3. Studies on natural SARS-CoV-2 infection have reported on both humoral and cellular immune responses showing differences between adults and children8–10. We have also described some differences in immuneprofile of SARS-CoV-2 infected and uninfected adults and children. Adults with prior infection displayed increased central and effector memory T cell phenotypes while children exhibited an ongoing innate inflammatory response11. Adaptive immunity is normally regarded immature in childhood. Accumulating evidence supports a role for innate immunity in the development of early life adaptive immunity, where epigenetic modifications confer immunological memory to innate immune cells; this enhanced response of the innate immune system to secondary infections is known as ‘trained immunity’11,12.
Vaccination of K18-hACE2 transgenic B-cell deficient (µMT) mice with mRNA vaccine BNT162b2 induced robust protective immunity, which was attributed to IFN-γ–driven cellular immunity13. Spike (S)-specific CD8+ T cell subpopulation expressing granzyme A, granzyme B and perforin was found to be induced in healthy donors at 4 weeks after the second dose of BNT162b2 vaccine14. However, reports evaluating the comparative effect of COVID-19 vaccination on T and NK cells responses between adults and children are scarce.
In this study, we aimed to evaluate and compare functional T cell response in adults and children aged 5–10 years following vaccination with two doses of Pfizer-BioNTech COVID-19 vaccine, with and without previous SARS-CoV-2 infection. Specifically, we determined SARS-CoV-2 peptide specific cytolytic granule-associated proteins granzyme B and perforin, and antiviral cytokine IFN-γ secreted by cytotoxic T lymphocytes.
Methods
Study design and participant selection
In this study, participants were selected from two cross-sectional SARS-CoV-2 serosurveillence studies conducted in Bangladesh. The first one was conducted (n = 3220) in urban Dhaka and Chattogram cities between October 2020 and February 202115. During this period, COVID-19 vaccine had not yet rolled out in Bangladesh. The second study (n = 7043) was carried out in 5 cities including Dhaka, between September 2021 to July 202216. During this time, COVID-19 vaccination had been introduced in Bangladesh but less than 50% of people were vaccinated at that time17.
Due to a lack of published studies on the assessment of functional cell response for sample size estimation, we assumed an effect size of 0.55 after vaccination with a significance level of 0.05 to achieve 80% power. Based on this assumption, the estimated sample size for the study was 50 adults and 50 children with vaccinated and unvaccinated ratio of 1:1 in both age groups. Both the vaccinated and unvaccinated groups were further divided into infected (with previous SARS-CoV-2 infection) and uninfected categories. Since, children were only given Pfizer-BioNTech Comirnaty (BNT162b2) vaccine, adults receiving the same vaccine were selected for the present study; adults receiving other vaccines (Moderna, Astrazeneca and Sinopharm) were not considered. Peripheral blood mononuclear cell (PBMC) samples from the 1st survey were used to evaluate the immune cell landscape among adults and children having previously been infected with SARS-CoV-2 in comparison to uninfected participants11. Thus, the above-mentioned categories of participants with adequate numbers of viable cells were selected by scrutinizing the remaining cryopreserved PBMCs samples from the 1st and 2nd surveys. All procedures were explained to participants and written informed consent was obtained from all participants and/or their legal guardians and assent was taken from 10 years old children.
Data and specimen collection
Data including socio-demographic information (age, sex, education, occupation, and family income), history of previous SARS-CoV-2 infection (based on anti-N-antibody titers), presence of COVID-like symptoms, and COVID-19 vaccination status was collected using a structured questionnaire. Information about the duration between vaccination and day of sample collection was recorded for both children and adults enrolled in the 2nd survey only. Height and weight of the participants were measured using a free-standing stadiometer (Seca 217, Hamburg, Germany) and digital weighing scale (Camry-EB9063, China), respectively to calculate body mass index (BMI). Blood was collected from all participants of the two survey studies for obtaining plasma, however, PBMC were separated from whole blood for only those who lived in Dhaka, and cryopreserved for the assessment of cellular immune response. Blood samples (8–10 mL) were collected in Li-heparin-coated tubes (S-Monovette Plasma, Sarstedt AG & Co. KG, Nümbrecht, Germany) from the participants at the time of enrollment. PBMC were separated from plasma through Ficoll-Paque density-gradient centrifugation, and were cryopreserved in fetal bovine serum (FBS) with 10% dimethyl sulfoxide (DMSO) and stored in liquid nitrogen. These cells were later utilized to assess functional T cell response by measuring SARS-CoV-2 peptide-specific interferon-gamma (IFN-γ), perforin, and granzyme B secreting cells.
Assessment of SARS-CoV-2-specific antibodies
Antibodies (IgG and IgM) specific for SARS-CoV-2 nucleocapsid (N) were measured in plasma samples for all participants15 using the Elecsys® Anti-SARS CoV-2 N immunoassay kits (Roche Diagnostics GmbH, Mannheim). Participants with a positive anti-N antibody titer were considered as having previous infection with or exposed to SARS-CoV-2.
Assessment of functional T cell response by ELISPOT assay
IFN-γ, perforin, and granzyme B secreting cells were measured in cryopreserved PBMC using human Granzyme B/Perforin Double-Color and IFN-γ ELISPOT kits (Cellular Technology Ltd. (CTL), OH, USA)) following manufacturer’s instructions. Cryopreserved PBMC were thawed, washed, counted and plated (in triplicate wells, 2 × 105 PBMC/well) on the 96 well ELISPOT plate pre-coated with capture antibodies. The two spike peptide pools were used as stimulating antigens in the ELISPOT assay, those were not specific for a particular variant of SARS-CoV-2. The peptide pools were made from the wild type SARS-CoV-2 virus without any mutation. These were overlapping peptide pool (PepMix 1™ and PepMix 2™) covering the spike glycoprotein and corresponding receptor-binding domain (RBD) of SARS-CoV-2 (JPT Peptide Technologies, Berlin, Germany). DMSO was used as negative control and phytohemagglutinin (PHA) as positive control. The plates were incubated at 37 °C with 5% CO2 (44 h for IFN-γ and 24 h for Granzyme B/Perforin). Spot forming units (SFU) were counted using the automated ImmunoSpot CTL Analyzer (CTL, OH, USA). The results are expressed as number of SFU per million PBMC (SFU/106 Cells).
Statistical analysis
The exposure variables in this study were vaccination with Pfizer-BioNTech COVID-19 mRNA vaccine and/or previous infection with SARS-CoV-2 in adults and children. The outcome variables were frequencies of IFN-γ, perforin and granzyme B secreting T cells. Demographic difference between adult and children was checked with descriptive statistics. Normality for all the outcome variables was assessed and non-normal association was examined by Skewness and Kurtosis. A log transformation (base 10) was applied to normalize the data. We used an independent t-test to compare the mean difference in IFN-γ and cytotoxic molecules between vaccinated and unvaccinated participants among uninfected individuals, as well as between vaccinated and unvaccinated participants among infected ones. To estimate the differential effects of vaccination, infection and differences between adults and children on the functional capacities of T cells, we also performed independent t-test. .
Since the numbers of samples belonging to different categories of participants were not sufficient, we conducted a power analysis to determine the impact of the revised sample size on the study’s power. With an expected effect size of 0.5 and a significance level of 0.05, the power for detecting differences between the vaccinated and unvaccinated groups was calculated to be approximately 0.772 (77.2%). This power estimate indicates that our study has 77.2% chance of detecting a moderate effect size, which is below the commonly desired threshold of 80% power and may limit the study’s ability to detect the effect size with high confidence.
All statistical analyses were conducted using STATA (Version 15), and graphical presentations were created with GraphPad Prism. Statistical significance was determined with a p-value threshold of < 0.10 for interactions and < 0.05 for all other comparisons.
Results
Participant characteristics
We selected a total of 96 participants, 45 adults and 51 children including previously infected (24 adults and 33 children) and uninfected participants (21 adults and 18 children) as well as vaccinated (22 adults and 19 children) and unvaccinated participants (23 adults and 32 children) (Fig. 1); We planned to collect 50 adults and 50 children for conducting this study. However, due to unavailability of adequate adults with optimum number of viable cells, we finally included 45 adults and 51 children in the analyses. The demographic characteristics, SARS-CoV-2 infection and COVID-19 vaccination status of study participants are given in Table 1. Among adults, higher proportion of participants belonged to non-slum areas (69%), were males (53%), engaged in any type of service (42%), and belonged to the 2nd quartile of income strata (38%, with monthly household income of BDT 20,000–40,000). Among children, majority were females (59%), enrolled from slum areas (55%) and belonged to lower income strata (31%). Higher proportion of adults (53%) and children (67%) have been previously infected with SARS-CoV-2, based on anti-N-antibody titers. None of the participants reported to have COVID-19-like symptoms during the previous 6 months’ time period or underwent RT-PCR confirmation of SARS-CoV-2 infection. Around 49% adults were immunized with Pfizer COVID-19 vaccine, while the proportion was lower in children (37%).
Fig. 1.

Flow Chart describing the selection of study participants.
Table 1.
Demographic characteristics of the study participants.
| Variables | Adult (n = 45) | Children (n = 51) |
|---|---|---|
| Age (yrs) | 29.0 ± 7.90 | 8.55 ± 2.06 |
| Sex | ||
| Male | 24(53.3%) | 21(41.2%) |
| Female | 21(46.7%) | 30(58.8%) |
| Locality | ||
| Slum | 14(31.1%) | 28(54.9%) |
| Non-slum | 31(68.9%) | 23(45.1%) |
| Household Income | ||
| BDT < 20,000 | 11(24.4%) | 16(31.4%) |
| BDT 20,000–40,000 | 17(37.8%) | 13(25.5%) |
| BDT 40,000–70,000 | 11(24.4%) | 14(27.5%) |
| > 70,000 | 6(13.3%) | 8(15.7%) |
| Occupation | ||
| Services | 19(42.2%) | – |
| Business | 8(17.8%) | – |
| Homemaker | 10(22.2%) | – |
| Unemployed | 4(8.90%) | – |
| Student | 4(9.30%) | 32(62.7%) |
| Out of school children | – | 19(37.3%) |
| Infection status (N-IgG) | ||
| Infected | 24(53.3%) | 33(64.7%) |
| Uninfected | 21(46.7%) | 18(35.3%) |
| Vaccination status | ||
| Vaccinated | 22(48.9%) | 19(37.2%) |
| Unvaccinated | 23(51.1%) | 32(62.7%) |
Data was presented as mean ± SD or number with percent in the parenthesis.
Cellular immune response
For describing the cellular immune response, we presented the results in three comparison groups. First, we selected uninfected participants who either received the mRNA vaccine or not and compared the immune response between them. Secondly, we selected unvaccinated participants, who were either previously infected with SARS-CoV-2 or not. The third group is a vaccinated group, who were either previously infected or not with SARS-CoV-2. The comparison of cellular immune response between any two groups was performed using independent t-test.
Effect of vaccination in uninfected participants
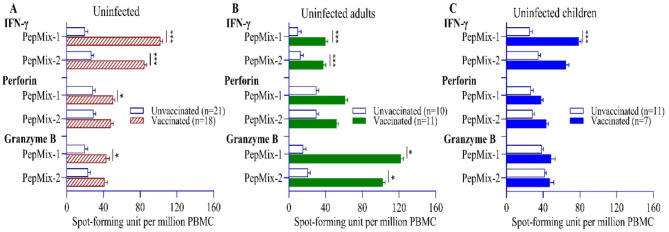
Among uninfected individuals, higher numbers of IFN-γ (reactive to both PepMix-1 and − 2 peptides), perforin (PepMix-1 only) and granzyme-B (PepMix-1 only) expressing T-cells were observed in vaccinated (n = 18) compared to unvaccinated participants (n = 21) (Fig. 2A). Among adults, vaccination generated higher number of IFN-γ and granzyme B secreting T-cells against both PepMix-1 and − 2 (Fig. 2B). In children, vaccination induced T-cell specific IFN-γ expression only, no effects were observed on the expression of cytotoxic granule molecules (Fig. 2C). We found no significant differences in cellular immune responses between adults (n = 11) and children (n = 7), within the vaccinated group (Supplementary Fig. 1A).
Fig. 2.
Comparison of IFN-γ and cytotoxic granule molecule secreting T cell responses between vaccinated and unvaccinated participants among uninfected individuals stratified by age. Plot (A) denotes all uninfected participant including both adults and children (red thatched and white bars), plot (B) denotes uninfected adult group (green and white bars), Plot (C) denotes uninfected children group (blue and white bars). Data were log-transformed and reported as geometric mean (back-transformed) with standard deviation. An independent t-test was conducted to calculate the p-value.
Effect of previous infection in unvaccinated participants
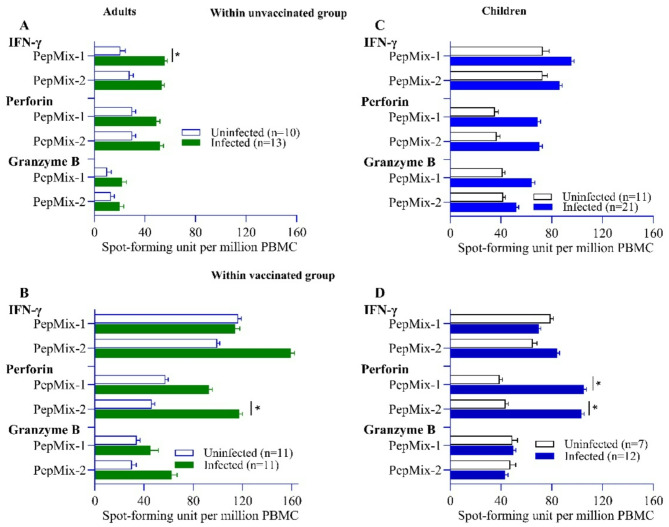
Previously infected adults displayed higher number of IFN-γ secreting T-cells compared to uninfected adults in response to PepMix-2 only (Fig. 3A). No changes were observed in cellular response in previously infected children compared to uninfected ones (Fig. 3C). In both uninfected and infected groups, children showed higher number of granzyme B secreting cells compared to adults (Table 2).
Fig. 3.
Comparison of cytotoxic granule molecules and IFN-γ secreting T cell responses between infected and uninfected participants among unvaccinated (plots A and C) and vaccinated (plots B and D) ones. Green with white bars denote adults (Left panel) and blue with white bars denote children (right panel). Data were log-transformed and reported as geometric mean (back-transformed) with standard deviation. An independent t-test was conducted to calculate the p-value.
Table 2.
Comparison of IFN-γ and cytotoxic granule molecule secreting T cell responses between unvaccinated adults and children with and without SARS-CoV-2 infection.
| Spot-forming units per | Infected (n = 34) | Uninfected (n = 20) | ||||
|---|---|---|---|---|---|---|
| million PBMC | Adults (n = 13) | Children (n = 21) | p-value | Adults (n = 10) |
Children (n = 10) | p-value |
| IFN-γ | ||||||
| PepMix-1 | 55.5 ± 2.29 | 95.5 ± 2.15 | 0.060 | 20.6 ± 3.96 | 72.8 ± 5.15 | 0.078 |
| PepMix-2 | 53.4 ± 2.09 | 86.1 ± 2.09 | 0.075 | 27.6 ± 3.04 | 72.6 ± 4.06 | 0.105 |
| Perforin | ||||||
| PepMix-1 | 49.2 ± 2.72 | 69.0 ± 2.39 | 0.310 | 30.1 ± 2.63 | 35.2 ± 2.72 | 0.728 |
| PepMix-2 | 52.0 ± 2.74 | 70.3 ± 2.32 | 0.352 | 30.0 ± 2.71 | 36.6 ± 2.67 | 0.656 |
| Granzyme B | ||||||
| PepMix-1 | 21.9 ± 3.51 | 64.4 ± 2.25 | 0.005 | 10.1 ± 3.24 | 41.2 ± 1.94 | 0.004 |
| PepMix-2 | 19.8 ± 3.40 | 52.0 ± 2.02 | 0.006 | 13.0 ± 2.83 | 41.7 ± 1.78 | 0.006 |
All the data were log-transformed and reported as geometric mean (back-transformed) with standard deviation. An independent t-test was conducted to calculate the p-value.
Effect of previous infection in vaccinated participants
Previous infection among vaccinated adults demonstrated increased number of perforin secreting T-cells (reactive to PepMix-2 only) compared to uninfected participants (mean 123.9 ± 2.52 vs. 42.7 ± 2.36, p = 0.029) (Fig. 3B). Similar increases in frequencies of perforin secreting cells (reactive to both PepMix 1 and 2) were noted among the vaccinated children with previous infection compared to uninfected ones (Fig. 3D). Prior infection did not have any effect on the expression of IFN-γ and granzyme B in either age group (Fig. 3). No differences were noted between adults and children among previously infected vaccinated group (Supplementary Fig. 1B).
Discussion
Several studies have been conducted to evaluate cellular immune response to COVID-19 vaccines in adults, but there have been relatively few reports in children. Moreover, nearly all of these studies have given attention to phenotypic changes and diversities of immune cells; limited information is available on how functional cellular immunity is modulated by COVID-19 vaccination. We therefore focused on this aspect of immunological response, and studied functional response of cytotoxic T lymphocytes to COVID-19 vaccination with or without past infection in adults and children.
Accumulating evidence indicates that besides antibodies, T cells play a major role in protection against severe COVID-19 disease18–20. All types of functional T cells (T-helper cells, CTL and NKT cells) secrete cytokines including IFN-γ to activate other immune cells and mediate immune response by enhancing antigen presentation, promoting the differentiation of T helper 1 (Th1) cells, or perform antiviral functions. CTL also exert their function by releasing cytotoxic molecules perforin and granzyme to eliminate the pathogen. Our findings of increased frequency of IFN- γ secreting T cells after vaccination of previously uninfected participants with BNT162b2 is supported by a number of studies21–23.. However, these studies applied interferon-gamma release assay (IGRA) using whole blood, which had the risk of getting contaminated with circulating plasma levels of IFN-γ; active secretion of the cytokine at cellular level was not assessed, as conducted in our study. Regarding cytotoxic molecules, one study reported no change in perforin and granzyme B expressing cells24, while another study showed increase in granzyme A, granzyme B and perforin expressing CD8+T cells in adults about one month after the second dose of BNT162b2 vaccine14. Our results are aligned with this study, demonstrating that immunization of adults with BNT162b2 vaccine increased the frequency of CTL secreting cytolytic granule molecule granzyme B. However, in children without prior infection, vaccination increased only IFN-γ secreting pan T-cells but not the cytotoxic granule molecule secreting CTL. Due to exposure to diverse infectious pathogens over the years, adults develop the capacity to rapidly respond with adaptive T cell immunity including cytolytic T cell response (IFN-γ and granzyme B secretion). We found that unvaccinated children showed elevated granzyme B expressing T cells compared to adults with or without prior infection (Table 2). It is possible that vaccination did not induce granzyme B expression due to pre-existing elevated frequencies of granzyme B-secreting CTL (Fig. 2).
Previous infection with SARS-CoV-2 in unvaccinated adults generated recall response of IFN-γ through memory CD8 T cells when stimulated with peptide antigens. One reason for the lack of increase in perforin and granzyme B expression may be that, many of the participants had asymptomatic infection (N-peptide positive) and it is possible that exposure to SARS-CoV-2 have occurred several weeks earlier, the changes in perforin and granzyme B expression were not detectable at this late stage. In contrast to adults, no recall response was noted in unvaccinated 5–10 years old children; prior infection did not show any effect on activated T-cell response of cytokine and cytotoxic molecules. Further investigations in larger sample size are needed to confirm the findings.
Past infection amplified post-vaccination secretion of perforin but not granzyme B or IFN-γ by antigen-stimulated T cells in both adults or children, suggesting that it was possible to bolster certain cytolytic functions of T cells in these age groups with two doses of mRNA vaccination. Aligning with our findings, one study in children demonstrated that vaccination with combination of SO-BERANA® 02/SOBERANA® Plus (recombinant RBD conjugated to tetanus toxoid) vaccines revealed similar level of cytotoxic granule proteins granzyme A, granzyme B, perforin, and granulin in both vaccinated and convalescent children25.Vaccination with adenovector vaccine ChAdOx1 nCoV-19, did not induce perforin secretion in adults, and perforin levels were similar to those found in patients with active COVID-19 disease26. Favresse et al. reported significant increase in IFN-γ secretion in adults who received a booster dose of Pfizer-BioNTech vaccine and were previously infected compared to those without any previous infection. After receiving the second booster dose, enhancement of IFN-γ secretion was observed only in participants without previous infection, suggesting that benefiting from a second booster vaccine dose was not likely if a threshold level of IFN-γ have already been achieved due to previous infection22. Our findings were somewhat different, we found that vaccination induced IFN-γ expression in both previously infected and uninfected adults, however, in children vaccination induced only IFN-γ expression without prior infection. With previous infection we did not observe any effect of vaccination on either cytokine or cytotoxic granule expression in children, which could be due to occurrence of infection several months ago.
Pre-existing elevated levels of cytolytic granule molecule in children may likely reflect an enhanced state of activation of the cytotoxic immune surveillance system due to repeated exposure to pathogens12. SARS-CoV-2 specific T cell responses have been shown to be influenced by age27,28. Growing evidence suggest that children exhibit low adaptive immune response, and they mount a stronger and faster innate immune response to the SAR-CoV-2 infection, particularly at the nasal mucosal barrier11,29–32. These data may indicate that heightened trained immunity among children plays a significant role in fighting SARS-CoV-2 infection. Even though two earlier studies have reported higher numbers of perforin expressing cells as well as mean levels of perforin per cell in children compared to adults, we did not find any difference between children and adults in our study33,34.
Strengths of our study includes functional evaluation of CTL cells that involves active release of cytotoxic molecules and cytokines upon stimulation with T cell stimulant (not just pre-existing levels of molecules circulating in serum). Another strength of the study was that, we had stored PBMC from participants who were not infected with SARS-CoV-2 (from the initial stage of onset of pandemic) and had not yet received COVID-19 vaccine at the time of sample collection. Finding such participants is next to impossible in the present time.
Limitations
This study has a number of limitations. First, we did not perform intracellular cytokine staining by flow cytometry to identify the phenotype of cells expressing perforin, granzyme B or IFN-γ. However, since PepMix peptides from SARS-CoV-2 are T cell stimulant, it is likely that T cells are secreting these molecules. Second, this was a community-based study, participants did not go for RT-PCR based testing unless acutely ill. As a confirmation for previous infection, we utilized the presence of N-specific antibody, though, it is quite possible that antibodies may have waned if an infection has occurred more than 5 to 8 months earlier, which would compromise the findings. Third, the study had a small sample size, thus we determined the power based on available sample size and found that there is a 77.2% chance of detecting a moderate effect size, which may limit the study’s ability to detect the effect size with high confidence.
Conclusion
In conclusion, there were small difference in functional T cell response of children compared to adults in response to COVID-19 vaccination and infection. The patterns of cellular immunity indicated that without previous infection, mRNA vaccination generated higher IFN-γ response (Th1) to SARS-CoV-2 peptides in both adults and children, but induced CTL response only in adults. Previous infection heightened post-vaccination perforin expression in both age groups. Children showed persistently increased frequencies of granzyme B-expressing T cells compared to adults, with or without prior SARS-CoV-2 infection probably reflecting an elevated steady-state level of immune surveillance. The impact of other types of COVID-19 vaccines (vector-, protein subunit- and whole inactivated virus-based vaccines) on functional T cell response may be evaluated in future studies comparing children with adults in various geographical settings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We warmly thank all of our participants for their consistently gracious welcome and sincere help during the study.
Author contributions
E.A., R.U.K.: Acquisition, Analysis, Interpretation, & Validation of data. M.T.T., M.J., M.S.H.: Data acquisition. M.A.H.: Statistical analysis, visualization. A.R.: Funding acquisition. P.S.: Methodology, Project Administration, Writing of MS. R.R.: Funding acquisition, Conceptualization, Writing of MS., E.A., R.U.K., M.T.T., M.J., M.A.H., M.S.H., M.V., M.Z.I., R.U.Z., A.R., P.S., R.R.: Reviewing and Editing of the MS.
Funding
This research was funded by The Foreign, Commonwealth & Development Office (FCDO; GR-02142), The United Nations Population Fund (UNFPA; GR-01967) and The United Nations Children’s Fund (UNICEF; GR-02141). icddr, b is also grateful to the Governments of Bangladesh, Canada, for providing core/unrestricted support for its operations and research. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data.
Data availability
All data that support the findings will be available upon reasonable request to corresponding author as per icddr, b policy (http://www.icddrb.org/policies).
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
“The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.” The study was approved by the institutional review board of icddr, b (PR-21069, dated 17 August 2021). All procedures were explained to participants and the written informed consent or assent was obtained from all participants and/or their legal guardians.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Evana Akhtar and Rakib Ullah Kuddusi contributed equally to this work.
References
- 1.Post, N. et al. Antibody response to SARS-CoV-2 infection in humans: A systematic review. PLoS One. 15(12), e0244126 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol.23(2), 186–193 (2022). [DOI] [PubMed] [Google Scholar]
- 3.Sette, A., Sidney, J. & Crotty, S. T cell responses to SARS-CoV-2. Annu. Rev. Immunol.41, 343–373 (2023). [DOI] [PubMed] [Google Scholar]
- 4.Shrotri, M. et al. T cell response to SARS-CoV-2 infection in humans: A systematic review. PLoS One16(1), e0245532 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, H. F., Schirra, C., Pattu, V., Krause, E. & Becherer, U. Lytic granule exocytosis at immune synapses: lessons from neuronal synapses. Front. Immunol.14, 1177670 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trapani, J. A. & Smyth, M. J. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol.2(10), 735–747 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Jiang, Y. et al. COVID-19 pneumonia: CD8(+) T and NK cells are decreased in number but compensatory increased in cytotoxic potential. Clin. Immunol.218, 108516 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang, C. K. et al. Aberrant hyperactivation of cytotoxic T-cell as a potential determinant of COVID-19 severity. Int. J. Infect. Dis.97, 313–321 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koch, C. M. et al. Age-related differences in the nasal mucosal immune response to SARS-CoV-2. Am. J. Respir Cell. Mol. Biol.66(2), 206–222 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mentor, G. et al. The effect of age and comorbidities: Children vs. Adults in their response to SARS-CoV-2 infection. Viruses16(5). (2024). [DOI] [PMC free article] [PubMed]
- 11.Akhtar, E. et al. Immune cell landscape in symptomatic and asymptomatic SARS-CoV-2 infected adults and children in urban Dhaka. Bangladesh Immunobiol.228(2), 152350 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Netea, M. G. et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol.20(6), 375–388 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang, X. et al. Vaccine-induced protection against SARS-CoV-2 requires IFN-gamma-driven cellular immune response. Nat. Commun.14(1), 3440 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nogimori, T. et al. Functional changes in cytotoxic CD8 + T-cell cross-reactivity against the SARS-CoV-2 Omicron variant after mRNA vaccination. Front. Immunol.13, 1081047 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raqib, R. et al. Seroprevalence of SARS-CoV-2 infection and associated factors among Bangladeshi slum and non-slum dwellers in pre-COVID-19 vaccination era: October 2020 to February 2021. PLoS One. 17(5), e0268093 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarker, P. et al. Serosurveillance among urban slum and non-slum populations immunized with COVID-19 vaccines in Bangladesh. Epidemiol. Infect.152, e14 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DHIS2. COVID-19 Vaccination Dashboard for Bangladesh 2024 [Available from: http://103.247.238.92/webportal/pages/covid19-vaccination-update.php.
- 18.Henry, B. M. et al. Anti-Endothelial cell antibodies are not frequently elevated in hospitalized patients with COVID-19. Acta Biomed.93(1), e2022026 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ledford, H. Killer’ immune cells still recognize omicron variant. Nature601(7893), 307 (2022). [DOI] [PubMed] [Google Scholar]
- 20.Wherry, E. J. & Barouch, D. H. T cell immunity to COVID-19 vaccines. Science377(6608), 821–822 (2022). [DOI] [PubMed] [Google Scholar]
- 21.Bonnet, B. et al. Decline of humoral and cellular immune responses against SARS-CoV-2 6 months after full BNT162b2 vaccination in hospital healthcare workers. Front. Immunol.13, 842912 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Favresse, J., Cabo, J. & Douxfils, J. Cellular immunity against SARS-CoV-2 is predominantly boosted in vaccinated individuals with no history of infection. J. Infect.87(2), e31–e2 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pighi, L., Henry, B. M., De Nitto, S., Salvagno, G. L. & Lippi, G. Cellular immunity against SARS-CoV-2 depends on the serological status. J. Infect.87(1), 57–58 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuapio, A. et al. NK cell frequencies, function and correlates to vaccine outcome in BNT162b2 mRNA anti-SARS-CoV-2 vaccinated healthy and immunocompromised individuals. Mol. Med.28(1), 20 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez-Nicado, R. et al. Comparative immune response after vaccination with SOBERANA((R)) 02 and SOBERANA((R)) plus heterologous scheme and natural infection in young children. Vaccines (Basel)11(11). (2023). [DOI] [PMC free article] [PubMed]
- 26.Familiar-Macedo, D. et al. Inflammatory and cytotoxic mediators in COVID-19 patients and in ChAdOx1 nCoV-19 (AZD1222) vaccine recipients. Cytokine171, 156350 (2023). [DOI] [PubMed] [Google Scholar]
- 27.Cohen, C. A. et al. SARS-CoV-2 specific T cell responses are lower in children and increase with age and time after infection. Nat. Commun.12(1), 4678 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paul, K. et al. Specific CD4 + T cell responses to ancestral SARS-CoV-2 in children increase with age and show cross-reactivity to beta variant. Front. Immunol.13, 867577 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia, R. et al. Immunological characterization and comparison of children with COVID-19 from their adult counterparts at single-cell resolution. Front. Immunol.15 (2024). [DOI] [PMC free article] [PubMed]
- 30.Pierce, C. A. et al. Natural mucosal barriers and COVID-19 in children. JCI Insight6(9). (2021). [DOI] [PMC free article] [PubMed]
- 31.Valentini, P., Sodero, G. & Buonsenso, D. The relationship between COVID-19 and innate immunity in children: A review. Child. (Basel)8(4). (2021). [DOI] [PMC free article] [PubMed]
- 32.Yoshida, M. et al. Local and systemic responses to SARS-CoV-2 infection in children and adults. Nature602(7896), 321–327 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahapatra, S. et al. High-resolution phenotyping identifies NK cell subsets that distinguish healthy children from adults. PLoS One12(8), e0181134 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rukavina, D. et al. Age-related decline of perforin expression in human cytotoxic T lymphocytes and natural killer cells. Blood92(7), 2410–2420 (1998). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data that support the findings will be available upon reasonable request to corresponding author as per icddr, b policy (http://www.icddrb.org/policies).