Abstract
Aim
Chronic periodontitis (CP), a prevalent inflammatory dental disease, has been linked to systemic conditions like Type 2 Diabetes Mellitus (T2DM). This systematic review and meta-analysis aimed to evaluate resistin levels in the Gingival Crevicular Fluid (GCF) of CP individuals with T2DM. The objective was to determine if resistin could be a potential biomarker for periodontal disease in T2DM individuals.
Methods
The review included data from seventeen clinical studies that investigated resistin levels in GCF of individuals diagnosed with CP and T2DM. Data were sourced from PubMed, Scopus, and EBSCOhost, selected for their extensive coverage of medical and dental research, ensuring thorough retrieval of relevant studies. From the initial seventeen studies, five complied with the strict inclusion criteria for meta-analysis.
Results
Using a comprehensive meta-analysis, the significance of GCF Resistin levels in individuals with CP and T2DM relative to the healthy groups was examined. In addition, a meta-analysis was carried out to look into the relationship between periodontal probing depth (PPD) and CP and T2DM. The results indicated that individuals with T2DM and CP had significantly higher GCF resistin levels than the other groups. Furthermore, the PPD in T2DM with CP was significantly greater than in the other groups.
Conclusion
The present review highlights the potential role of resistin as a biomarker to diagnose individuals with chronic periodontitis and T2DM.
Clinical significance
The significantly elevated levels of resistin suggest that resistin could serve as a potential biomarker for T2DM in individuals with CP. This could lead to improved methods of early diagnosis and treatment, which could enhance individual outcomes and quality of life.
Prospero registration
The registration number CRD42023467186.
Keywords: Biomarker, Chronic periodontitis, Gingival crevicular fluid, Periodontal pocket, Resistin, Diabetes mellitus
Graphical abstract
1. Introduction
Chronic Periodontitis(CP), the sixth complication of diabetes mellitus (DM) exhibits a two-way relationship with the latter.1 Studies have confirmed that altercations in the microflora coupled with disabled neutrophil functioning and compromised wound healing make DMa risk factor for periodontal disease.2, 3, 4 The results of a detailed meta-analysis further support this interrelationship.5 The continuous low-grade infectious state created due to chronic periodontitis,6 indirectly affects insulin sensitivity.7
There is a paradigm shift from clinical diagnosis to molecular diagnosis. With the presently available diagnostic tests and techniques, operator bias still exists and may vary between clinicians. Hence a more objective form of assessment is the need of the hour.8
Molecular biomarkers are known to be expressed during an infectious state. The evaluation of these molecular mediators can not only help clinicians to diagnose the disease process but it could also help in monitoring the response to treatment.
A hypothesis has been established that inflammation, lipids, and adipokines may foster the relationship between periodontitis and Type 2 Diabetes Mellitus (T2DM).9 People with diabetes are two to three times more likely to develop periodontitis compared to those without diabetes.10 It further indicated that Type 2 diabetes mellitus (T2DM) increases the risk of developing periodontitis by 34 %. This increased risk is closely linked to the level of blood sugar control. Conversely, systemically healthy individuals who have periodontitis often show higher levels of glycated haemoglobin (HbA1c) and fasting blood glucose, suggesting that severe periodontitis can elevate the risk of developing diabetes.11
Given how DM affects CP individuals, the relationship between CP and DM highlights the necessity for accurate diagnostic markers to forecast the course of oral disorders. Good diagnostic indicators are necessary to predict the course of oral disorders, and treating diabetes individuals' periodontitis can improve their glucose management. Insulin-like growth factor 1 (IGF-1) and insulin-like growth factor binding protein 2 (IGFBP-2) are two specific markers that have been established to identify the coexistence of CP and DM.12 According to a study, pancreatic diabetes, and pancreatic ductal adenocarcinoma (PDAC) may be distinguished from one another using biomarkers. IGF-1 may be able to tell if pancreatic diabetes is caused by PDAC or CP Furthermore, in the presence of DM, these markers might aid in differentiating between CP and PDAC.13 Additionally, studies are being done to understand better and identify the many phases of diabetes and its consequences through the use of novel biomarkers like proteins, metabolites, cytokines, and adipokines, including potential implications for coexisting conditions like CP.14
Due to its critical function in controlling the interaction between inflammation, lipids, and adipokines, resistin, an adipocytokine also known as Found in Inflammatory Zone 3 (FIZZ3) or Adipocyte-Specific Secretory Factor (ADSF), has attracted increased attention in the context of DM and CP.15,16 Resistin was unintentionally found in 2001 by Steppan et al. while researching adipocyte differentiation. When thiazolidinediones—a type of medication mostly used to improve insulin sensitivity in the treatment of diabetes—are introduced, resistin is noticeably downregulated in mature adipocytes.17
Resistin regulates diabetes, obesity, cardiovascular diseases (CVDs), atherosclerosis, and prediabetes in significant ways. Resistin may play therapeutic and diagnostic roles in inflammatory illnesses through serum. Increased resistin levels have been linked to the onset of insulin resistance, diabetes, and atherosclerosis, according to epidemiological and genetic research.18 The mechanisms underlying resistin's impact on insulin resistance in humans remain unclear. Similar to related rodent studies,19,20 in vitro studies have shown that recombinant human resistin could induce insulin resistance through 5′AMP-activated protein kinase (AMPK)-dependent and AMPK-independent suppressor of cytokine signaling-3 (SOCS-3) signaling pathways in Hepatoblastoma (HepG2) cells.21 Furthermore, given the location of resistin in human mononuclear cells, inflammation may be the mechanism that connects resistin with insulin resistance. Previous research has demonstrated the involvement of resistin in pro-inflammatory mechanisms. Resistin's expression has been linked to the production of pro-inflammatory cytokines like Tumor Necrosis Alpha (TNF-α) and interleukin-6 (IL-6) according to in vitro studies.22,23 Additionally, resistin may stimulate the expression of pro-inflammatory cytokines like (TNF-α) and Interleukin 6 (IL-6) via the nuclear factor-kappa B (NF-κB, p50/p65) signaling pathway.24
This discovery highlights the potential role of human resistin in inflammation and insulin resistance through a unique mechanism. Elevated resistin levels are linked to both pro-inflammatory effects and insulin resistance, and a comprehensive review has correlated resistin to chronic periodontitis (CP).25
However, a thorough investigation of the relationship between Type 2 Diabetes Mellitus (T2DM) and CP—particularly concerning resistin levels in gingival crevicular fluid—has yet to be conducted. Recognizing this gap, we designed a meta-analysis to evaluate resistin as a potential biomarker for T2DM individuals with CP. Additionally, our study examines the relationship between periodontal probing depth in CP individuals, both with and without T2DM.
2. Methods
2.1. Study design
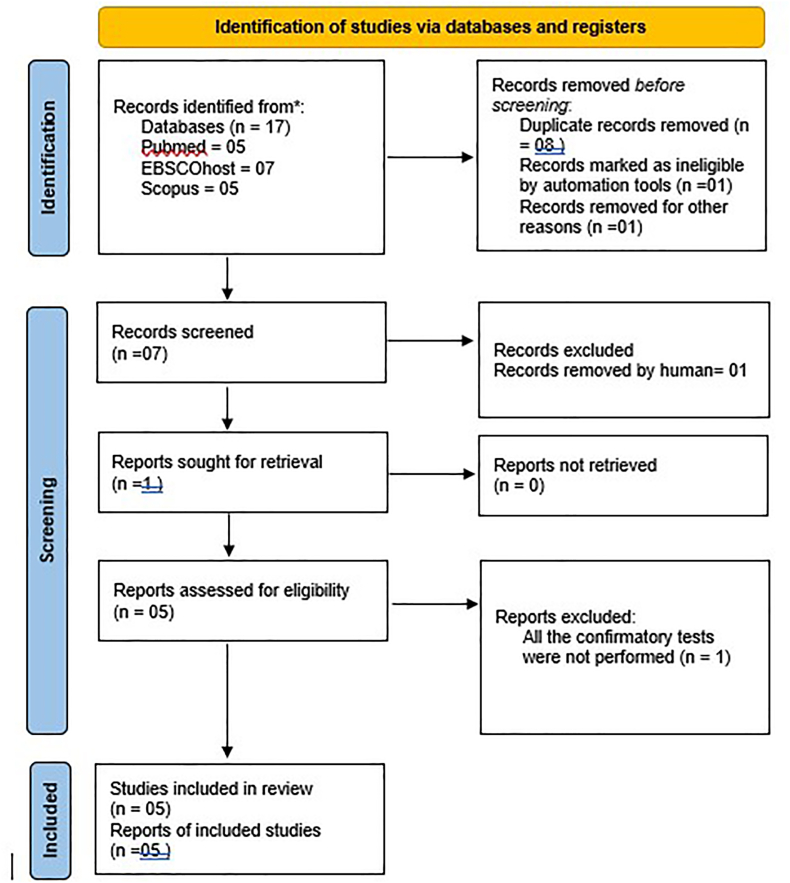
A systematic review and meta-analysis were undertaken to explore the correlation between resistin levels in GCF of teeth and the incidence of T2DM and CP. This review was meticulously structured in alignment with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.26 The study protocol was registered in PROSPERO with the registration number CRD42023467186, ensuring a structured and transparent approach (Fig. 1). Comprehensive assessments were carried out on full-text articles published in the period spanning from 1980 to 2023. This exhaustive research process was conducted over one year, from November 1, 2023 to October 30, 2024.
Fig. 1.
PRISMA flowchart.
2.2. Research question
The literature search was guided by the following question: “could the elevated levels of resistin in GCF help to distinguish the healthy individuals from T2DM individuals with CP?"
The search strategy was prepared based on Population/Problem, Exposure/Intervention, Comparison/Control, and Outcome (PECO). The interested population (P) was individuals who tested resistin biomarker level in GCF, exposure (E) was type 2 diabetes mellitus and CP, comparison (C) was healthy individuals and healthy oral condition, without any systemic disorders and outcome (O) was increased resistin levels in GCF (primary), periodontal probing depth (secondary), included study design was clinical studies (observational, cohort, prospective, and randomised control trials)
2.3. Inclusion criteria
(a) Clinical human observational studies and randomized clinical trials evaluating Resistin levels in human GCF to ensure the reliable and relevant evidence for clinical practice, (b) Clinical studies where the test individuals were diagnosed with CP and T2DM, (c) Studies that used a commercially available ELISA kit to analyze the levels of Resistin.
During the electronic and manual search processes, three investigators (A.G., A.K.D., and M.M.) reviewed the titles and abstracts of the publications. Publications not meeting the inclusion criteria were excluded at this stage. Subsequently, all remaining articles were retrieved and meticulously screened by a senior researcher (D.A.) to reach a consensus on their suitability for inclusion in the review.
2.4. Exclusion criteria
(a)Animal studies, (b) Studies not using gingival crevicular fluid as the test sample, (c) Studies that did not analyze Resistin, (d) narrative reviews, (e) Studies that did a qualitative analysis.
2.5. Information sources and search strategy
In conducting a comprehensive search, specific keywords were employed: “Resistin,” “gingival crevicular fluid,” “chronic periodontitis,” and “Diabetes Mellitus.” The devised search strategy was detailed as follows: (“resistin"[MeSH Terms] OR “resistin"[All Fields]) AND (“chronic periodontitis"[MeSH Terms] OR (“chronic"[All Fields] AND “periodontitis"[All Fields]) OR “chronic periodontitis"[All Fields]) AND (“gingival crevicular fluid"[MeSH Terms] OR (“gingival"[All Fields] AND “crevicular"[All Fields] AND “fluid"[All Fields]) OR “gingival crevicular fluid"[All Fields]) AND (“diabetes mellitus"[MeSH Terms] OR (“diabetes"[All Fields] AND “mellitus"[All Fields]) OR “diabetes mellitus"[All Fields]) [Table 1].
Table 1.
Search Strategy results.
| Data base | Search strategy | n |
|---|---|---|
| PubMed | (“resistin"[MeSH Terms] OR “resistin"[All Fields]) AND (“chronic periodontitis"[MeSH Terms] OR (“chronic"[All Fields] AND “periodontitis"[All Fields]) OR “chronic periodontitis"[All Fields]) AND (“gingival crevicular fluid"[MeSH Terms] OR (“gingival"[All Fields] AND “crevicular"[All Fields] AND “fluid"[All Fields]) OR “gingival crevicular fluid"[All Fields]) AND (“diabetes mellitus"[MeSH Terms] OR (“diabetes"[All Fields] AND “mellitus"[All Fields]) OR “diabetes mellitus"[All Fields]) | 05 |
| EBSCOhost | (“resistin"[MeSH Terms] OR “resistin"[All Fields]) AND (“chronic periodontitis"[MeSH Terms] OR (“chronic"[All Fields] AND “periodontitis"[All Fields]) OR “chronic periodontitis"[All Fields]) AND (“gingival crevicular fluid"[MeSH Terms] OR (“gingival"[All Fields] AND “crevicular"[All Fields] AND “fluid"[All Fields]) OR “gingival crevicular fluid"[All Fields]) AND (“diabetes mellitus"[MeSH Terms] OR (“diabetes"[All Fields] AND “mellitus"[All Fields]) OR “diabetes mellitus"[All Fields]) | 07 |
| Scopus | (“resistin"[MeSH Terms] OR “resistin"[All Fields]) AND (“chronic periodontitis"[MeSH Terms] OR (“chronic"[All Fields] AND “periodontitis"[All Fields]) OR “chronic periodontitis"[All Fields]) AND (“gingival crevicular fluid"[MeSH Terms] OR (“gingival"[All Fields] AND “crevicular"[All Fields] AND “fluid"[All Fields]) OR “gingival crevicular fluid"[All Fields]) AND (“diabetes mellitus"[MeSH Terms] OR (“diabetes"[All Fields] AND “mellitus"[All Fields]) OR “diabetes mellitus"[All Fields]) | 05 |
| Grey literature | (“resistin"[MeSH Terms] OR “resistin"[All Fields]) AND (“chronic periodontitis"[MeSH Terms] OR (“chronic"[All Fields] AND “periodontitis"[All Fields]) OR “chronic periodontitis"[All Fields]) AND (“gingival crevicular fluid"[MeSH Terms] OR (“gingival"[All Fields] AND “crevicular"[All Fields] AND “fluid"[All Fields]) OR “gingival crevicular fluid"[All Fields]) AND (“diabetes mellitus"[MeSH Terms] OR (“diabetes"[All Fields] AND “mellitus"[All Fields]) OR “diabetes mellitus"[All Fields]) | 00 |
The search was performed on November 21, 2023.
MeSH: Medical Subject Headings.
This strategy was tailored by (D.A., A.G., A.K.D. and M.M.) to ensure a thorough and focused retrieval of relevant literature. The search for relevant literature extended across multiple databases, including PubMed, Scopus, EBSCO, and clinical trial registry and was not constrained by language or publication year, covering all available literature up until May 30, 2024. A detailed summary of the search results from these databases is presented in [Table 1]. To further complete the research, a search for grey literature was conducted in OpenGrey and bibliography of included studies. Two investigators (A.G and A.K.D.) undertook a manual search through the references of the qualifying studies to identify additional pertinent studies. In instances where further information about a study or any other aspect was required, the authors of the relevant papers were contacted. For any arising uncertainties or questions, a senior investigator (D.A.) provided the necessary clarification.
2.6. Study selection and data collection process
The search strategy finalized in PubMed was meticulously applied to the three databases, with careful adherence to the pre-defined inclusion and exclusion criteria. Each study was thoroughly examined two to three times by three investigators individually (A.G., A.K.D., and M.M.) to ensure accuracy, completeness and duplicate removal. The data from these studies were then systematically gathered and entered into a Microsoft 365 Office-powered Excel sheet. After the careful compilation of data, a detailed table [Table 2, Table 3] was constructed to facilitate further analysis. In cases of any disagreements, a consensus was reached among the reviewers to resolve disputes.
Table 2.
Characteristics of the studies included.
| S.No. | Author year and journal | Number of groups | Age group | Control group | Method of GCF collection | Method of evaluation | Resistin levels in GCF of healthy controls (ng/ml) | Resistin levels in GCF of patients with CP (ng/ml) | Resistin levels in GCF of patients with CP and Type 2 DM | p | Inference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Hiroshima Y, et J Periodontal Res. 201227 | Gr P (n = 24): Patients with CP and no systemic disease Gr DM-P (n = 18) Type 2 DM + CP Gr H (n = 21) healthy controls with no systemic disease and oral disease |
29–78 years (mean of 56.4) | YES (healthy controls with no systemic and oral diseases) | Periopaper | ELISA | 0.78 + 0.61 | 2.55 + 2.04 | 1.84 + 1.97 | <0.05 | the resistin levels in GCF of patients with CP and Type 2 DM are significantly raised compared to healthy controls |
| 2. | Patel SP et al. Contemp Clin Dent. 201328 | Gr1 (n = 24): healthy controls with no systemic or oral disease Gr2 (n = 24): CP with uncontrolled diabetes Gr3 (n = 24): Type 2 DM controlled with CP Gr 4 (n = 24): CP with no systemic disease |
29–51 years | YES (healthy controls with no systemic and oral diseases) | Microcapillary method | ELISA | 4.75 + 1.81 | 9.25 + 3.3 | 7.58 + 2.96 | <0.05 | the resistin levels in GCF of patients with CP and Type 2 DM are significantly raised compared to healthy controls |
| 3. | Rode PA, J Indian Soc Periodontol. 201929 | Gr 1 (n = 20) Healthy with no systemic or oral disease Gr 2 (n = 20) CP with no systemic disease Gr 3 (n = 20) CP + Type 2 DM |
35–65 years | YES (healthy controls with no systemic and oral diseases) | Microcapillary method | ELISA | 0.15 + 0.15 | 1.55 + 0.75 | 2.43 + 0.62 | <0.0001 | the resistin levels in GCF of patients with CP and Type 2 DM are significantly raised compared to healthy controls |
| 4. | Govindaraj et al. Bioinformation. 202130 | Gr 1(n = 20): healthy with no systemic or oral disease Gr2 (n = 20): CP with no systemic disease Gr3 (n = 20): Type 2 DM with no CP Gr 4 (n = 20): Type 2 DM plus CP |
35–65 years | YES (healthy controls with no systemic and oral diseases) | Microcapillary method | ELISA | 0.001 + 0.001 | 0.001 + 0.001 | 0.0014 + 0.001 | <0.0001 | The resistin levels in GCF of patients with CP and Type 2 DM are significantly raised compared to healthy controls |
| 5. | Gokhale NH et al., J Periodontol. 201431 | Gr 1 (n = 15): Healthy controls with no systemic or oral diseases Gr 2 (n = 15): CP with no systemic disease Gr 3(n = 15): Type 2 DM with no CP Gr 4 (n = 15): Type 2 DM plus CP |
>35 years | YES (healthy controls with no systemic and oral diseases) | Microcapillary method | ELISA | 0.01 + 0.005 | 0.02 + 0.01 | 0.04 + 0.01 | 0.05 | the resistin levels in GCF of patients with CP and Type 2 DM are significantly raised compared to healthy controls |
| S. no. | Author Journal and year | PPD level for CP cases | CAL level for CP cases | Radiographic evidence of bone loss | Hb1Ac levels for Type 2 DM | RBS level for Type 2 DM | Method of collection GCF and volume | Number of sites | Time of collection | Storage temperature of GCF sample |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Hiroshima Y, et J Periodontal Res. 201227 | >5 mm | >3 mm | Yes | >5.8 % | >200 mg/dL | Paper strip placed for 10 s | – | Same day | Strips diluted and subjected to ELISA test |
| 2. | Patel SP et al. Contemp Clin Dent. 201328 | >5 mm | >3 mm | Yes | >7 % | >200 mg/dL | Micropipette with calibrated 1–5 μL. Sample collection time was 10 min |
1 site for periodontal group and multiple sites for healthy group | Subsequent day | −70 °C |
| 3. | Rode PA, J Indian Soc Periodontol. 201929 | >5 mm | >5 mm | Yes | 6.5–7 % | >200 mg/dL | Micropipette with calibrated 1–5 μL. Sample collection time was 10 min |
1 site for periodontal group and multiple sites for healthy group | Same day | −20 °C |
| 4. | Govindaraj et al. Bioinformation. 202130 | >5 mm | >4 mm | Yes | >8 % | >200 mg/dL | 1 μL was procured from each site. | 1 site for periodontal group and multiple sites for healthy group | Subsequent day after supragingival scaling | −20 °C |
| 5. | Gokhale NH et al., J Periodontol. 201431 | >5 mm | >3 mm | Yes | >6.5 % | >200 mg/dL | Micropipette with caliberated 1–5 μL. Sample collection time was 10 min. Volume collected = 4 μL |
1 site for periodontal group and multiple sites for healthy group | Subsequent day | −20 °C |
Table 3.
Comparison of Resistin Levels in T2DM + CP vs CP Only Patients.
| Study | T2DM + CP | CP Only |
|---|---|---|
| Gokhale et al., 2014 | 37.02 ± 10.94 (ng/ml) | 24.55 ± 7.91 (ng/ml) |
| Govindraj et al., 2021 | 1452.4 ± 137.8 (ng/ml) | 1160.2 ± 62.6 (ng/ml) |
| Hiroshima et al., 2012 | 1.84 ± 1.97 (ng/μL) | 2.55 ± 2.04 (ng/μL) |
| Patel et al., 2013 | 7.55 ± 2.96 (ng/μl) | 9.25 ± 3.3 (ng/μl) |
| Rode et al., 2019 | 2.434 ± 0.62 (ng/μl) | 1.55 ± 0.75 (ng/μl) |
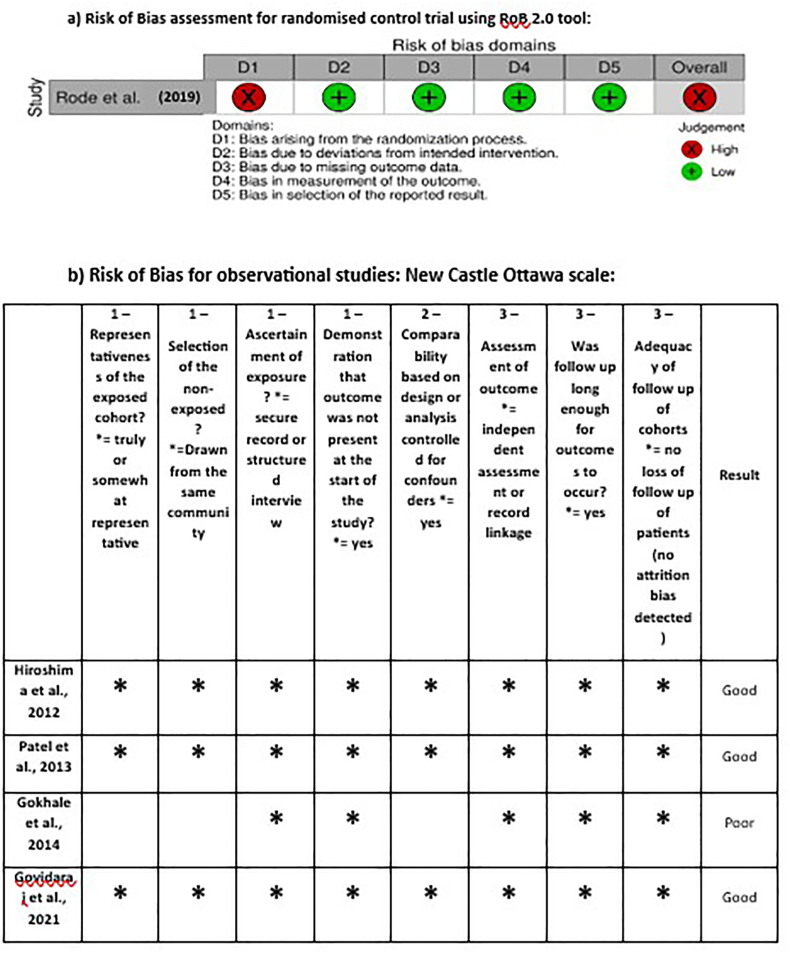
2.7. Risk of bias assessment
Two investigators (A.G., and A.K.D.) meticulously carried out the data collection and screening process, in strict adherence to the established inclusion criteria. The quality of the observational studies included in this systematic review was evaluated by two researchers using the Newcastle-Ottawa Scale (NOS), which operates on a scoring system ranging from 0 to 9 points. The NOS serves as an evaluative instrument designed to assess the risk of bias inherent in observational studies comprising four distinct domains pertaining to risk of bias evaluation. The risk of bias of the included randomised controlled trial by Rode et al. was assessed using the Cochrane RoB-2-tool.29 Each study was independently evaluated for each of the questions and assigned the closest answer by the two independent evaluators (A.G and A.K.D.). Following their assessments, all issues identified were collectively discussed to ensure a comprehensive evaluation. In instances of any discrepancies or disagreements, the final consensus was achieved by the intervention of a senior examiner (D.A.) who also acted as a mediator to resolve any differences effectively.
2.8. Summary measures and synthesis of results
Continuous data from eligible studies were only included in the meta-analysis. The continuous primary outcomes; GCF levels and periodontal probing depth (PPD) individual as well as the combined effect were assessed using the standardized mean difference (SMD) with 95 % CI which is equal mean difference divided by the pooled standard deviation. SMD greater than zero is considered as raised in the values whereas less than zero is regarded as a decline in the values. This effect size was considered small (0.2), medium (0.5), and large (0.8). Heterogeneity was examined by inspecting the forest plot and using the statistical test Q-test and I-square. The random-effect model using the DerSimonian and Lard approach was used to perform the meta-analysis pooled effect because of high heterogeneity. All analyses were conducted using the RevMan 5.3 (Review Manager v.5.3; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration 2014).
3. Results
In this systematic review, a comprehensive search across three databases (PubMed, EBSCOhost, Scopus) yielded 17 studies. After removing the duplicates, a pool of five studies remained for further scrutiny. These studies were then meticulously evaluated against the predefined eligibility and inclusion criteria, with the process and outcomes detailed by the updated PRISMA guidelines, as illustrated in Fig. 1.
The EndNote Basic Software (Thomson Reuters, New York, NY) was utilized for efficient reference management and duplicate elimination.
3.1. Risk of bias analysis
The Newcastle-Ottawa Scale (NOS) used to evaluate and categorise the quality of the observational studies (Fig. 2). After converting the results of the NOS to AHRQ standards, three out of four observational studies were designated as “Good quality studies,” and one was regarded as “poor quality study” in terms of quality scoring. The scores ranged from a minimum of 5 to a maximum of 8 stars. Most studies exhibited the lowest levels of comparability between cohorts, based on either design or analysis. Most of the studies did not address confounders like oral hygiene, previous dental history, habit of smoking etc.
Fig. 2.
Risk of Bias assessment of included studies a) Randomised control trial studies by RoB 2.0 tool, and b) observational studies by New- Castle Ottawa (NOS) tool.
RoB assessment for the study by Rode et al. presents a “high RoB” as illustrated in Fig. 2.29 Although the study design of the aforementioned study was a randomized controlled trial (RCT), the randomization of the included sample into the Healthy, chronic periodontitis, and chronic periodontitis with diabetes mellitus groups was not explained and would not have been possible. However, it is important to note the presence of heterogeneity among the included studies, among the six meta-analysis models it varies from 52 % to 97 %. This high level of heterogeneity underscores the need for a cautious interpretation of the pooled results and suggests the potential influence of varying non-randomized trials and adjustment of different potential confounders or populations on the outcomes.
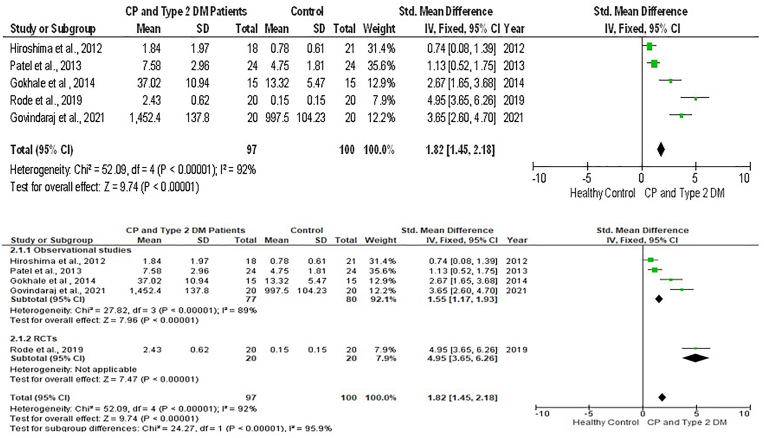
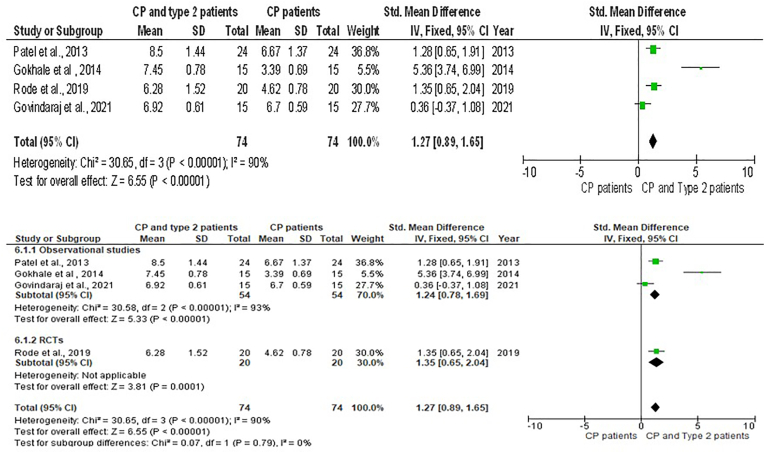
Six meta-analyses with sub-group analysis [Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8] were done to get a comprehensive understanding of the relationship between GCF resistin levels and CP with T2DM. In all the six meta-analyses a random effect model was applied due to the presence of high heterogeneity.
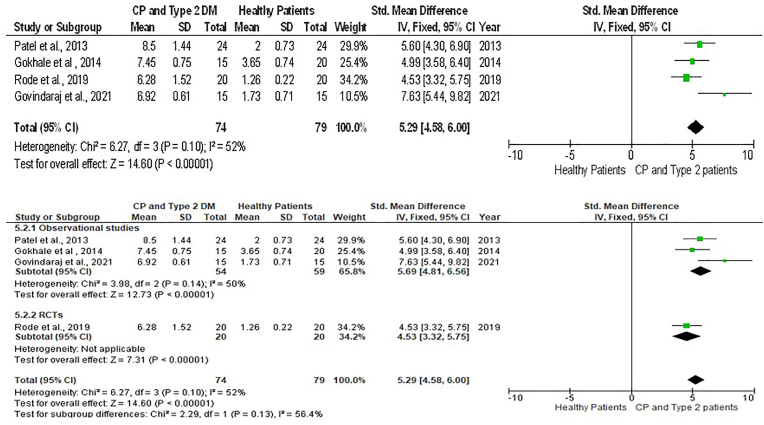
Fig. 3.
Forest plot showing the resistin levels in GCF of chronic periodontitis individuals with type 2 Diabetes Mellitus and healthy individuals and sub-group analysis.
Fig. 4.
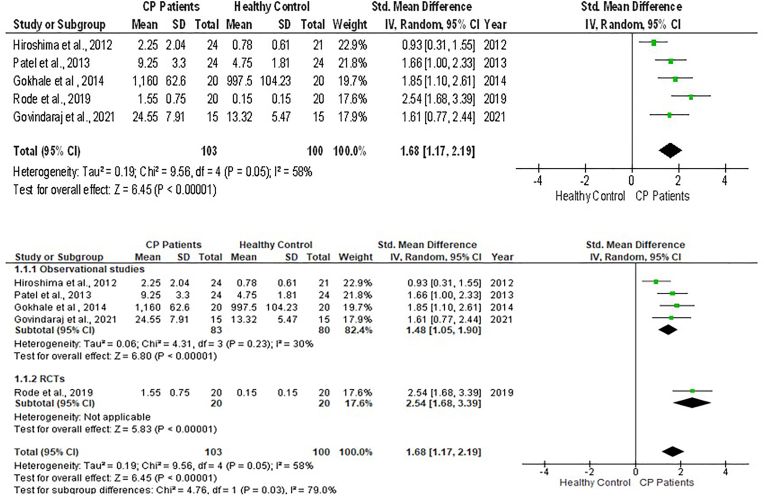
Forest plot showing the resistin levels in GCF of chronic periodontitis individuals and controls and sub-group analysis.
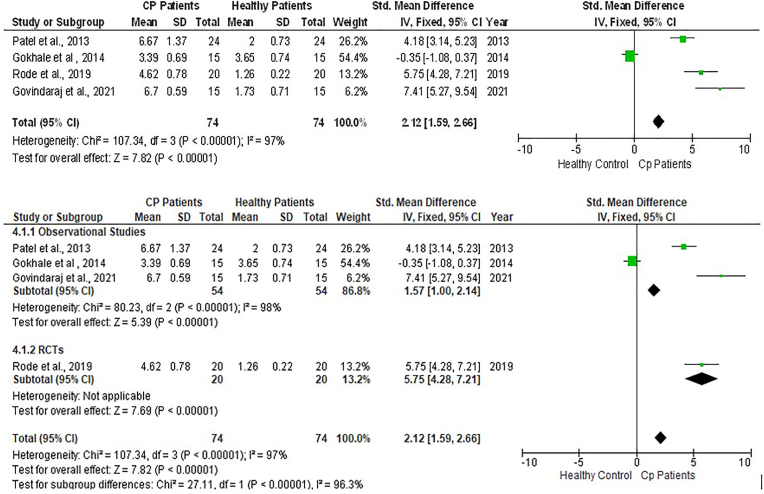
Fig. 5.
Forest plot showing the periodontal probing depth of chronic periodontitis individuals and controls and sub-group analysis.
Fig. 6.
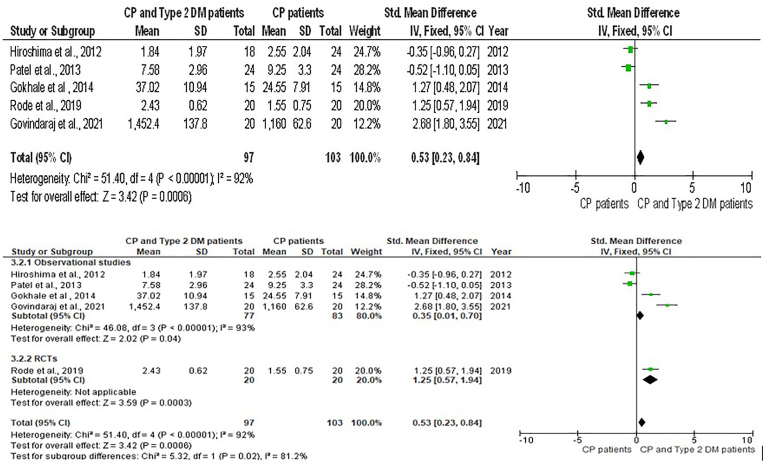
Forest plot showing the resistin levels in GCF chronic periodontitis individuals with type 2 Diabetes Mellitus and chronic periodontitis and sub-group analysis.
Fig. 7.
Forest plot showing the periodontal probing depth of chronic periodontitis individuals with type 2 Diabetes Mellitus and healthy individuals and sub-group analysis.
Fig. 8.
Forest plot showing the periodontal probing depth of chronic periodontitis individuals with type 2 Diabetes Mellitus and chronic periodontitis and sub-group analysis.
The forest plot comprising 5 studies reveals that, when the T2DM group was compared with the healthy control, the pooled GCF levels of resistin had a significantly large effect size in CP plus T2DM compared to healthy controls [SMD = 1.82(95 % CI: 1.45 to 2.18); p < 0.00001) and had a high value of heterogeneity (I2) of 92 %. Likewise, there was significant difference between them in sub-group analysis (p < 0.00001) (Fig. 3).
When the pooled GCF values of resistin were significantly raised with a large effect size in CP individuals compared to healthy controls [SMD = 1.68 (95 % CI: 1.17 to 2.19); p < 0.00001] showing a large Heterogeneity (I2) of 58 % among the studies (Fig. 4). When the pooled GCF levels of resistin groups with CP plus T2DM were compared to individuals with CP effect size was not statistically significant, albeit the effect size was medium [SMD = 0.53 (95 % CI: 0.23 to 0.84) p = 0.0006) for the CP with T2DM. Additionally, the heterogeneity among the studies was high (Fig. 5).
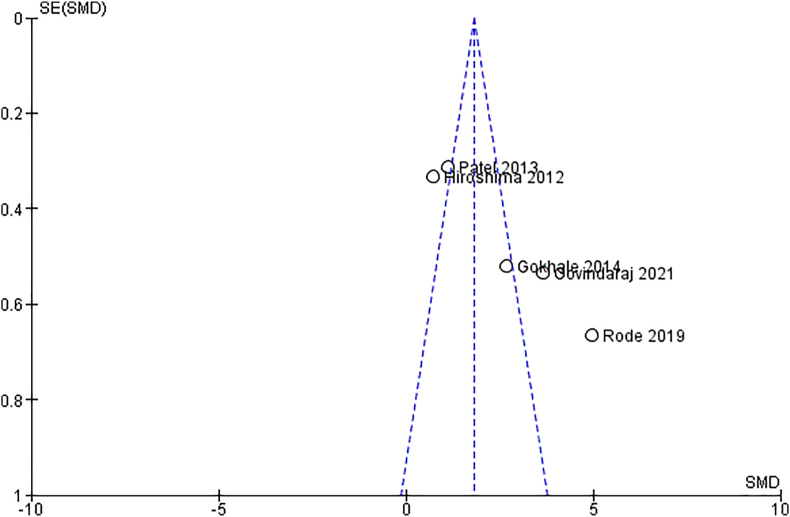
In addition to these meta-analyses were performed for relationship analysis between the Periodontal Probing depth (PPD) and experimental groups. The pooled effect size of PPD between the CP plus T2DM vs healthy group was revealed to be highly significant with quite a large effect size [SMD = 5.29 (95 % CI: 4.58 to 6.00); p < 0.00001; I2 = 52 %) (Fig. 6). This effect size value was higher when CP plus T2DM was compared with healthy controls. The analysis for PPD in the CP vs healthy group revealed the pooled effect size was large and highly significant [SMD = 2.12 (95 % CI: 1.59 to 2.66; p < 0.00001] data favoring the CP group and heterogeneity among the studies were 97 %. However, the analysis for PPD in T2DM and CP vs CP revealed that the pooled effect size of PPD was significantly having medium effect size [SMD = 1.27(95 % CI: 0.89 to 1.65); p < 0.00001; I2 = 90 %) affected in CP individuals with overlying systemic disease like T2DM compared to individuals with CP only. The publication bias assessed through funnel plot diagram (Fig. 9).
Fig. 9.
Funnel plot of studies showing the publication bias.
3.2. GCF sample collection methodology
In the systematic review, the collection of GCF was a pivotal part of each study's methodology. Among the included studies, the microcapillary technique was predominantly used for GCF collection. This method involves the use of microcapillary tubes, which are carefully inserted into the gingival crevice to collect the fluid. The technique is highly regarded for its precision and minimal invasiveness, allowing for accurate measurement of GCF volume and constituents. An exception to this common method was noted where the paper strip method was employed.27 This alternative approach involves placing absorbent paper strips into the gingival crevice to absorb the GCF, which is then quantified and analyzed. The choice of collection method can influence the volume and composition of GCF obtained, thereby potentially influencing the study outcomes.
3.3. Confirmatory tests and results
For the analysis of GCF samples in the included studies, a commercially available ELISA was uniformly utilized. ELISA is a widely recognized technique for detecting and quantifying substances, particularly proteins, in various sample types. Its high sensitivity and specificity make it an ideal choice for biomarker analysis. Each study adhered strictly to the manufacturer's instructions for the ELISA procedure, ensuring consistency and reliability in the assay results.
3.4. Characteristics of the studies
Patel SP and Raju PA explored the relationship between resistin levels in serum and GCF with periodontal inflammation and its association with single-nucleotide polymorphism (SNP) in the human resistin gene at −420.28 The methodology included precise measurement of resistin levels using a commercially available ELISA kit in both serum and GCF, providing a comprehensive view of resistin's presence and concentration in different bodily fluids in the context of periodontal health (Table 2, Table 3). The results of the study revealed a significant increase in resistin levels in the GCF in individuals with CP compared to healthy individuals. This difference pointed to the potential role of resistin as a marker for periodontal inflammation, indicating its increased presence in the context of periodontal disease.
The randomized control clinical trial conducted by Rode PA et al. offers significant insights into the genetic aspects of periodontal disease and diabetes.29 This study aimed to explore the influence of SNP on the expression of the resistin gene (RETN) at positions 420 and + 299 and its impact on resistin levels in serum and GCF in individuals with CP and T2DM. The investigation assessed the clinical parameters and resistin levels in serum and GCF in periodontally healthy individuals, who had CP, and those with CP and T2DM. The resistin levels were measured using enzyme-linked immunosorbent assays, while the RETN polymorphism at the specified positions was genotyped using polymerase chain reaction's restriction fragment length polymorphism technique. The results enrich our understanding of the genetic factors in periodontal and systemic health, suggesting the potential of genetic testing in the diagnosis and management of CP and T2DM, and paving the way for more personalized and effective treatment approaches.
Govindaraj et al., embarked on an investigation to estimate the levels of resistin in the GCF among various individual groups using a commercially available ELISA kit, focusing particularly on the comparison between diabetic and non-diabetic individuals with chronic periodontitis.30
The study's results revealed a strong positive correlation between GCF resistin levels and key clinical and biochemical parameters such as random blood sugar (RBS), Hemoglobin A1c (HbA1c), and other clinical indicators of periodontal health. Furthermore, the study highlighted the significant Resistin levels across the individual groups, with the highest levels observed in individuals with generalized CP and T2DM. This finding highlights the potential role of resistin as a biomarker for diabetes-related periodontitis, suggesting that elevated Resistin levels in GCF could be indicative of an underlying diabetic condition in individuals with CP.
Hiroshima undertook a detailed investigation into the levels of resistin in the GCF of individuals with and without DM and additionally explored the mechanism of resistin release in response to Porphyromonas Gingivalis lipopolysaccharide in human neutrophils.27 This research provides a unique insight into the role of resistance in the context of periodontal health and diabetes. The methodology involved the collection of GCF samples using periopaper strips, a standard technique for acquiring periodontal fluid. These samples were then analyzed using a commercially available ELISA kit, ensuring precise and reliable measurement of resistin levels. The study included subjects both with CP and DM, and healthy controls, allowing for a comparative analysis. A key finding from this study was the significantly higher mean levels of resistin in the GCF of individuals with CP and DM. This elevated level of resistin in individuals with periodontal and diabetic conditions suggests its potential role as an inflammatory marker specific to these diseases. Furthermore, the study interestingly observed that while resistin levels in plasma were not correlated with Hb1Ac levels, a marker for long-term blood glucose control, the GCF resistin levels were indeed correlated with periodontal inflammation. The study paves the way for future research to further elucidate these relationships and explore the potential of resistin as a biomarker for periodontal disease in diabetic individuals.
Gokhale NH conducted a clinical-biochemical study to analyze and compare the levels of resistin in the GCF of individuals with T2DM and CP to healthy individuals.31 The GCF was collected using the micropipette method and subjected to a commercially available ELISA kit. Results indicated a significant increase in resistin levels in GCF of individuals with T2DM and CP compared to the other groups. This led them to conclude that resistin could be considered a potential biomarker for individuals with T2DM and CP.
3.5. Publication bias
The funnel plot represents the logit transformed proportion (effect size) plotted against the standard error of studies and the vertical line in the funnel represents the weighted mean effect size (Fig. 9). The plot appears somewhat asymmetric, with more studies on the right side (positive SMD) than on the left. The asymmetry suggests that negative or small-effect studies may be underreported.
4. Discussions
Clinical studies and reviews have consistently established a strong association between periodontal disease and DM.10,32 Research indicates that DM poses a risk for the onset and advancement of periodontal diseases.33, 34, 35, 36 This link could propel researchers to look into particular biomarkers to help with the early detection of both illnesses. A detailed meta-analysis has further concluded the positive bidirectional relationship between DM and CP.37
Resistin which is an adipokine has garnered attention due to its role in inflammatory diseases although it was first implicated in insulin resistance.23 However, the research on the relationship between resistin and insulin resistance has yielded inconsistent results, possibly due to the confounding factor of obesity, which has been considered in some studies.38,39 A thorough meta-analysis conducted in 2016 concluded that obesity may not have as much of an impact on GCF proinflammatory biomarker levels as localized periodontal inflammation.40 Hence this systematic review and meta-analysis was conducted to investigate the effect of T2DM and CP on the GCF levels of resistin and whether this adipokine could be used as a disease-specific biomarker.
The studies revealed a clear rise in Resistin levels in GCF of individuals with CP and associated DM compared to the healthy controls. It has been recognized as a potential indicator of the local disease process, making it a valuable source for biomarker assessment. The role of GCF as a site-specific indicator of the disease was highlighted by Fatima et al. in their detailed review.41
The selected studies consistently revealed a significant increase in resistin levels in GCF among individuals with CP and associated DM compared to healthy controls, reinforcing the potential of resistin as a diagnostic biomarker. The inclusion of studies that conducted non-surgical periodontal therapy (NSPT) was noteworthy. These studies reported a marked decrease in resistin values post-treatment, accompanied by an improvement in blood Hb1Ac levels. This finding suggests a dual and reversible relationship between DM and CP, further emphasizing the clinical relevance of resistin as a biomarker in monitoring disease progression and treatment outcomes.29,42
A study by Hiroshima et al. and Patel et al. revealed a poor correlation between Hb1Ac levels and serum.27,28 Thus, as part of the inclusion criteria in this review, we included those studies that analyzed the levels of resistin in GCF. The comparison between serum and GCF resistin levels in the study by Patel et al. found no significant difference. There was no significant difference was observed in serum resistin levels among the test groups, however GCF levels of resistin were significantly elevated in DM and CP groups compared to healthy individuals and between the test groups. This suggests that GCF may serve as a site-specific indicator of both the local disease process in the periodontium and the systemic disease process of DM, highlighting the potential clinical utility of GCF resistin analysis. The methodological aspects of GCF collection and analysis were taken into account. In this review, only one study utilized the perio-paper method.27
Variations in the clinical settings, the volume of GCF collected, oral conditions at the time of sample selection, and potential operator bias could explain the observed heterogeneity among the studies. These factors should be considered when interpreting the results and designing future studies. However, these results must be interpreted with caution due to the limited number of included studies.
5. Conclusion
Implications for Clinical Practice: These findings can potentially improve clinical practice by enabling timely interventions and more precise disease management.
Implications for Clinical Research: Further research is warranted to validate its clinical utility and establish standardized protocols. Given that chronic periodontitis is a known complication of diabetes mellitus, resistin presents an exciting avenue for early detection and monitoring of both conditions.
Patient/parent/guardian consent
Not applicable since it is a review study.
Credit author statement
Dr. Dax Abraham: Concept, Design, Supervision, Writing, Critical review.
Dr. Alpa Gupta: Concept, Design, Supervision, Analysis and/or interpretation, Writing, Critical review.
Dr. Arun Kumar Duraismay: Concept, Design, Supervision, Analysis and/or interpretation, Writing, Critical review.
Dr. Mrinalini Mrinalini: Concept, Design, Supervision, Analysis and/or interpretation, Writing, Critical review.
Data sharing and data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Ethical statement and clinical trial registration
Not applicable since it is a review study.
PROSPERO registration
The study protocol was registered in PROSPERO with the registration number CRD42023467186.
Financial support
No financial support received for conducting this study at any stage.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank Dr. Rajeev Kumar Malhotra, Senior Scientist (All India Institute of Medical Sciences, New Delhi) for his detailed statistical analysis of the data provided.
References
- 1.Kudiyirickal M.G., Pappachan J.M. Periodontitis: an often-neglected complication of diabetes. World J Diabetes. 2024;15(3):318–325. doi: 10.4239/wjd.v15.i3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iacopino A.M. Periodontitis and diabetes interrelationships: role of inflammation. Ann Periodontol. 2001;6(1):125–137. doi: 10.1902/annals.2001.6.1.125. [DOI] [PubMed] [Google Scholar]
- 3.Brownlee M., Lilly Lecture Glycation and diabetic complications. Diabetes. 1993;43(6):836–841. doi: 10.2337/diab.43.6.836. 1994. [DOI] [PubMed] [Google Scholar]
- 4.Zambon J.J., Reynolds H., Fisher J.G., Shlossman M., Dunford R., Genco R.J. Microbiological and immunological studies of adult periodontitis in patients with noninsulin-dependent diabetes mellitus. J Periodontol. 1988;59(1):23–31. doi: 10.1902/jop.1988.59.1.23. [DOI] [PubMed] [Google Scholar]
- 5.Khader Y.S., Dauod A.S., El-Qaderi S.S., Alkafajei A., Batayha W.Q. Periodontal status of diabetics compared with nondiabetics: a meta-analysis. J Diabet Complicat. 2006;20(1):59–68. doi: 10.1016/j.jdiacomp.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 6.D'Aiuto F., Parkar M., Andreou G., et al. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. 2004;83(2):156–160. doi: 10.1177/154405910408300214. [DOI] [PubMed] [Google Scholar]
- 7.Yki-Järvinen H., Sammalkorpi K., Koivisto V.A., Nikkilä E.A. Severity, duration, and mechanisms of insulin resistance during acute infections. J Clin Endocrinol Metab. 1989;69(2):317–323. doi: 10.1210/jcem-69-2-317. [DOI] [PubMed] [Google Scholar]
- 8.Ghallab N.A. Diagnostic potential and future directions of biomarkers in gingival crevicular fluid and saliva of periodontal diseases: review of the current evidence. Arch Oral Biol. 2018;87:115–124. doi: 10.1016/j.archoralbio.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Grossi S.G. Treatment of periodontal disease and control of diabetes: an assessment of the evidence and need for future research. Ann Periodontol. 2001;6(1):138–145. doi: 10.1902/annals.2001.6.1.138. [DOI] [PubMed] [Google Scholar]
- 10.Preshaw P.M., Alba A.L., Herrera D., et al. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55(1):21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson T.C., Clarkson J.E., Worthington H.V., et al. Treatment of periodontitis for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev. 2022;4(4):CD004714. doi: 10.1002/14651858.CD004714.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Włodarczyk B., Borkowska A., Włodarczyk P., Małecka-Panas E., Gąsiorowska A. Insulin-like growth factor 1 and insulin-like growth factor binding protein 2 serum levels as potential biomarkers in differential diagnosis between chronic pancreatitis and pancreatic adenocarcinoma in reference to pancreatic diabetes. Przegląd Gastroenterol. 2021;16(1):36–42. doi: 10.5114/pg.2020.95091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen D.K., Korc M., Petersen G.M., et al. Diabetes, pancreatogenic diabetes, and pancreatic cancer. Diabetes. 2017;66(5):1103–1110. doi: 10.2337/db16-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaishya S., Sarwade R.D., Seshadri V. MicroRNA, proteins, and metabolites as novel biomarkers for prediabetes, diabetes, and related complications. Front Endocrinol. 2018;9:180. doi: 10.3389/fendo.2018.00180. Apr 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steppan C.M., Bailey S.T., Bhat S., et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 16.Patel L., Buckels A.C., Kinghorn I.J., et al. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300(2):472–476. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 17.Quinn C.E., Hamilton P.K., Lockhart C.J., McVeigh G.E. Thiazolidinediones: effects on insulin resistance and the cardiovascular system. Br J Pharmacol. 2008;153(4):636–645. doi: 10.1038/sj.bjp.0707452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kollari E., Zografou I., Sampanis C., et al. Serum adipokine levels in patients with type 1 diabetes are associated with degree of obesity but only resistin is independently associated with atherosclerosis markers. Hormones (Basel) 2022;21(1):91–101. doi: 10.1007/s42000-021-00328-9. [DOI] [PubMed] [Google Scholar]
- 19.Muse E.D., Obici S., Bhanot S., et al. Role of resistin in diet-induced hepatic insulin resistance. J Clin Investig. 2004;114(2):232–239. doi: 10.1172/JCI21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steppan C.M., Wang J., Whiteman E.L., Birnbaum M.J., Lazar M.A. Activation of SOCS-3 by resistin. Mol Cell Biol. 2005;25(4):1569–1575. doi: 10.1128/MCB.25.4.1569-1575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo Z., Zhang Y., Li F., et al. Resistin induces insulin resistance by both AMPK-dependent and AMPK-independent mechanisms in HepG2 cells. Endocrine. 2009;36(1):60–69. doi: 10.1007/s12020-009-9198-7. [DOI] [PubMed] [Google Scholar]
- 22.Kaser S., Kaser A., Sandhofer A., Ebenbichler C.F., Tilg H., Patsch J.R. Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem Biophys Res Commun. 2003;309(2):286–290. doi: 10.1016/j.bbrc.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Bokarewa M., Nagaev I., Dahlberg L., Smith U., Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174(9):5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 24.Silswal N., Singh A.K., Aruna B., Mukhopadhyay S., Ghosh S., Ehtesham N.Z. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem Biophys Res Commun. 2005;334(4):1092–1101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- 25.Akram Z., Rahim Z.H., Taiyeb-Ali T.B., et al. Resistin as potential biomarker for chronic periodontitis: a systematic review and meta-analysis. Arch Oral Biol. 2017;73:311–320. doi: 10.1016/j.archoralbio.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4. Mar 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiroshima Y., Bando M., Inagaki Y., et al. Resistin in gingival crevicular fluid and induction of resistin release by Porphyromonas gingivalis lipopolysaccharide in human neutrophils. J Periodontal Res. 2012;47(5):554–562. doi: 10.1111/j.1600-0765.2011.01466.x. [DOI] [PubMed] [Google Scholar]
- 28.Patel S.P., Raju P.A. Resistin in serum and gingival crevicular fluid as a marker of periodontal inflammation and its correlation with single-nucleotide polymorphism in human resistin gene at -420. Contemp Clin Dent. 2013;4(2):192–197. doi: 10.4103/0976-237X.114878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rode P.A., Kolte R.A., Kolte A.P., Purohit H.J., Ahuja C.R. Relevance of single-nucleotide polymorphism to the expression of resistin gene affecting serum and gingival crevicular fluid resistin levels in chronic periodontitis and type 2 diabetes mellitus: a randomized control clinical trial. J Indian Soc Periodontol. 2019;23(2):131–136. doi: 10.4103/jisp.jisp_361_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Govindaraj K., Sudhakar U., Bhuminathan S., Govindaraj J. Resistin levels in the gingival crevicular fluid among diabetic and non-diabetic chronic periodontitis patients. Bioinformation. 2021;17(10):899–902. doi: 10.6026/97320630017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gokhale N.H., Acharya A.B., Patil V.S., Trivedi D.J., Setty S., Thakur S.L. Resistin levels in gingival crevicular fluid of patients with chronic periodontitis and type 2 diabetes mellitus. J Periodontol. 2014;85(4):610–617. doi: 10.1902/jop.2013.130092. [DOI] [PubMed] [Google Scholar]
- 32.Llambés F., Arias-Herrera S., Caffesse R. Relationship between diabetes and periodontal infection. World J Diabetes. 2015;6(7):927–935. doi: 10.4239/wjd.v6.i7.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soskolne W.A., Klinger A. The relationship between periodontal diseases and diabetes: an overview. Ann Periodontol. 2001;6(1):91–98. doi: 10.1902/annals.2001.6.1.91. [DOI] [PubMed] [Google Scholar]
- 34.Novak M.J., Potter R.M., Blodgett J., Ebersole J.L. Periodontal disease in Hispanic Americans with type 2 diabetes. J Periodontol. 2008;79(4):629–636. doi: 10.1902/jop.2008.070442. [DOI] [PubMed] [Google Scholar]
- 35.Fernandes J.K., Wiegand R.E., Salinas C.F., et al. Periodontal disease status in gullah african americans with type 2 diabetes living in South Carolina. J Periodontol. 2009;80(7):1062–1068. doi: 10.1902/jop.2009.080486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matu N.K., Stephen L., Lalloo R. Prevalence and severity of periodontal disease: type 2 diabetics versus non-diabetics. SADJ. 2009;64(2):64–68. [PubMed] [Google Scholar]
- 37.Stöhr J., Barbaresko J., Neuenschwander M., Schlesinger S. Bidirectional association between periodontal disease and diabetes mellitus: a systematic review and meta-analysis of cohort studies. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-93062-6. Jul 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heilbronn L.K., Rood J., Janderova L., et al. Relationship between serum resistin concentrations and insulin resistance in nonobese, obese, and obese diabetic subjects. J Clin Endocrinol Metab. 2004;89(4):1844–1848. doi: 10.1210/jc.2003-031410. [DOI] [PubMed] [Google Scholar]
- 39.Azuma K., Katsukawa F., Oguchi S., et al. Correlation between serum resistin level and adiposity in obese individuals. Obes Res. 2003;11(8):997–1001. doi: 10.1038/oby.2003.137. [DOI] [PubMed] [Google Scholar]
- 40.Akram Z., Abduljabbar T., Abu Hassan M.I., Javed F., Vohra F. Cytokine profile in chronic periodontitis patients with and without obesity: a systematic review and meta-analysis. Dis Markers. 2016;2016 doi: 10.1155/2016/4801418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fatima T., Khurshid Z., Rehman A., Imran E., Srivastava K.C., Shrivastava D. Gingival crevicular fluid (GCF): a diagnostic tool for the detection of periodontal health and diseases. Molecules. 2021;26(5):1208. doi: 10.3390/molecules26051208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joshi A., Maddipati S., Chatterjee A., Lihala R., Gupta A. Gingival crevicular fluid resistin levels in chronic periodontitis with type 2 diabetes before and after non-surgical periodontal therapy: a clinico-biochemical study. Indian J Dent Res. 2019;30(1):47–51. doi: 10.4103/ijdr.IJDR_215_17. [DOI] [PubMed] [Google Scholar]