Abstract
A substantial body of evidence indicates a positive correlation between dyslipidemia and an elevated risk of chronic kidney disease, with renal interstitial fibrosis frequently serving as a common pathway in the advanced stages of chronic kidney disease progression. Hydrogen has anti-inflammatory and antioxidant properties, and magnesium hydride nanoparticle is a material with high hydrogen storage capacity. Magnesium hydride -fortified feed is capable of releasing hydrogen gas steadily and continuously within the digestive tract. A 12-week high-fat diet significantly elevated the serum urea and creatinine levels in mice. In contrast, dietary addition of magnesium hydride demonstrated a notable protective effect against pathological conditions. Additionally, magnesium hydride -fortified feed was found to reduce renal fibrosis and thereby improve renal function. In support of these findings, an in vitro study utilizing human kidney cortical proximal tubule epithelial cells (HK-2 cells) exposed to palmitic acid under conditions mimicking a high-fat diet confirmed the renoprotective effects of magnesium hydride. Furthermore, the primary target phosphatase and tensin homologue deleted on chromosome 10 and the molecular mechanisms underlying the effects of magnesium hydride, specifically its ability to inhibit the transforming growth factor-beta -Smad family member 2 and 3 (Smad2/3) axis through downregulating the expression of phosphatase and tensin homologue deleted on chromosome 10, were elucidated. Additionally, overexpression of Hes family BHLH transcription factor 1 can negate the beneficial effects of magnesium hydride, suggesting that Hes family BHLH transcription factor 1 may serve as an upstream regulatory target in the context of the effects of magnesium hydride. In conclusion, this study demonstrated that magnesium hydride functions as a safe and effective hydrogen source capable of inhibiting the activation of the transforming growth factor-beta/Smad2/3 and protein kinase B/mechanistic target of rapamycin pathways by increasing the expression of phosphatase and tensin homologue deleted on chromosome 10. This mechanism counteracts the progression of high-fat diet-induced chronic renal damage.
Keywords: chronic kidney disease, high-fat diet, magnesium hydride, phosphatase and tensin homolog deleted on chromosome ten, renal fibrosis
Introduction
Chronic kidney disease (CKD) represents a significant global public health challenge. According to data from the World Health Organization, approximately 10% of the global population is affected by CKD. This condition is especially critical in low- and middle-income countries, where access to diagnostic and therapeutic services is frequently constrained. Consequently, CKD has emerged as the 12th leading cause of mortality worldwide.1 The etiology of CKD is multifaceted and extensive, encompassing a variety of developmental, physiological, societal, cultural, and environmental factors. Notably, conditions such as obesity, dyslipidemia, hypertension, and other traditional metabolic syndromes play significant roles. Hyperlipidemia, characterized by either elevated triglyceride levels or reduced high-density lipoprotein cholesterol levels, has been demonstrated to be closely associated with an increased risk of renal insufficiency. Specifically, low high-density lipoprotein cholesterol has emerged as an independent risk factor for the progression of CKD. International guidelines characterize the hallmark lesions of this disease as glomerular sclerosis, tubular atrophy, and interstitial fibrosis, as observed in renal biopsy samples.2
Dyslipidemia, which is precipitated by various etiological factors, involves the accumulation of pathological lipids and metabolic byproducts in plasma, tissues, and organs. Upon exposure to a high-fat diet (HFD), nonadipose tissues, including metabolically significant organs such as the kidneys and liver, function as lipid reservoirs.3 The accumulation of adipose tissue exacerbates the risks associated with obesity and induces renal damage through multiple mechanisms. The “lipid nephrotoxicity” hypothesis suggests that lipid deposition in proximal tubule cells, the stroma, and mesangial cells disrupts the permeability of the glomerular basement membrane, leading to deleterious effects on renal structure and function.4 Fatty acids promote apoptosis in these tubule cells via the activation of peroxisome proliferator-activated receptor gamma.5 Lipid accumulation can impair mitochondrial function in renal tubular cells and hinder fatty acid oxidation within the epithelium, leading to ATP depletion, cell death, dedifferentiation, and intracellular fat storage. These processes exacerbate renal tubulointerstitial fibrosis through further lipid deposition.6 The intricate interplay of various cellular pathways, including transforming growth factor-beta (TGF-β)/Smad signaling, protein kinase B signaling, and the Notch pathway, serves as the primary driver of kidney fibrosis. Renal interstitial fibrosis constitutes a common pathway for the advanced progression of CKD, for which there are currently no dedicated therapeutic drugs specifically targeting CKD and renal fibrosis in clinical practice. phosphatase and tensin homologue deleted on chromosome 10 (PTEN), a potent tumor suppressor, serves as a major negative regulator of class I phosphatidylinositol 3-kinase (PI3K), serine/threonine-specific protein kinase (AKT), and the mechanistic target of rapamycin (mTOR)-defined signaling pathways, which are involved in the modulation of several critical cellular processes. The loss of PTEN can simultaneously induce unrestrained activation of the PI3K signaling pathway. Recent research has shown that PTEN plays a pivotal role in regulating essential organogenesis by promoting profibrotic signaling pathways. Importantly, the conjugation of PTEN with polyubiquitin chains (K27-polyUb) induces epithelial‒mesenchymal transition (EMT) in response to TGF-β, sonic hedgehog, connective tissue growth factor, interleukin-6, and hyperglycemia. This process is characterized by reduced expression of epithelial cell markers, such as E-cadherin, β-catenin, and cytokeratin, and increased expression of mesenchymal cell markers, including N-cadherin, vimentin, fibronectin (FN), and alpha-smooth muscle actin.7
The prevalence of CKD is increasing at an alarming rate, highlighting its multifaceted complexity and its critical role in exacerbating clinical and financial burdens. Currently, the comprehensive multimodal intervention strategies widely implemented in clinical practice include lifestyle modifications, nutritional adjustments, and the management of metabolic disorders. Additionally, the use of statins and dual renin‒angiotensin system blockers is prevalent. The maximum tolerated doses of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers are also recommended. Renal interstitial fibrosis represents a common pathway in the progression of CKD and has substantial implications for the potential deceleration of CKD through the inhibition of renal fibrosis.8 However, there are currently no pharmacological agents that specifically target renal cells or cause renal fibrosis in the clinic. Molecular hydrogen (H2), a colorless and odorless reductant, readily permeates cellular membranes, modulates mitochondrial electron transport, and attenuates oxidative stress, thereby reducing mitochondrial damage. Alternatively, it stabilizes the intracellular environment and influences the transcription of key inflammatory regulatory proteins, thereby exhibiting anti-inflammatory and antioxidant properties.9 Numerous studies have demonstrated its efficacy in mitigating organ fibrosis10,11 through these mechanisms. Magnesium hydride (MgH2), a reliable and efficient hydrogen storage medium, is orally absorbable and reacts with water to gradually release H2 within the body during digestion.12 Biocompatible, sustainable, consistent, predictable, and cost-effective, MgH2 has potential for the management of severe dyslipidemia. Given the potential of MgH2 as a therapeutic agent for chronic kidney injury induced by a HFD, we initiated this comprehensive study to investigate its protective effects and elucidate the underlying mechanisms.
Materials and Methods
Animals
In this study, 18 male C57BL/6 mice (Shanghai Jiesijie Laboratory Animal Co., Ltd., Shanghai, China; license No. SCXK 2018-004), aged 8 weeks and weighing between 21 and 24 g, were utilized. These mice were specific pathogen-free in grade and virus-free. The breeding conditions were maintained at 25°C with 50% relative humidity under a 12-hour light/dark cycle. The mice were provided with specialized feed and tap water and had ad libitum access to purified water and feed. All animal procedures were conducted in accordance with the guidelines approved by the Experimental Animal Ethics Committee of Naval Medical University (No. NMUMERC-2021-006).
Following a 1-week acclimatization period in the cages, the mice were subsequently assigned to experimental groups. Eighteen mice were randomly assigned to three groups, each consisting of six mice: the normal control group, which was fed a standard diet; the HFD group, which received a HFD (Wuxi Fanbo Biotechnology Co., Ltd., Wuxi, Jiangsu, China) with a fat energy supply ratio of 60%; and the MgH2 group, which was provided with the same HFD as the HFD group but supplemented with MgH2 (Center of Hydrogen Science, Shanghai, China) at a concentration of 0.5 g/kg (Figure 1A).
Figure 1.
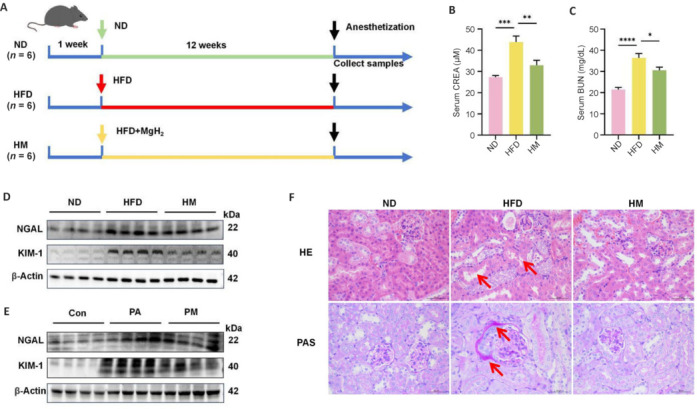
MgH2 improves HFD/PA-induced renal injury.
(A) Animal experiment chart. (B, C) Variations in CREA and BUN levels in the mice. These indicators increased under HFD conditions and decreased after MgH2 treatment. The data are expressed as the mean ± SD (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (one-way analysis of variance followed by Tukey’s post hoc test). (D, E) Western blotting analysis of the expression of KIM-1 and NGAL in mouse kidney (D) and HK-2 cells (E). The raw data for the quantitative results are shown in Additional Table 1. (F) HE staining and PAS staining of mouse kidney tissue (400× original magnification). A HFD induces changes in renal pathological structure (arrows), and MgH2 can improve this effect. BUN: Blood urea nitrogen; Con: control group; CREA: creatinine; H&E: hematoxylin and eosin; HFD: high-fat diet; HM: MgH2 group; KIM-1: kidney injury molecule-1; MgH2: magnesium hydride; ND: normal control; NGAL: neutrophil gelatinase-associated lipocalin; PA: palmitic acid; PAS: periodic acid-Schiff; PM: PA + MgH2 group.
Upon completion of the experimental period, all the mice were anesthetized with isoflurane, and blood samples were collected from the eyeballs. The collected blood was allowed to stand, followed by centrifugation to obtain the supernatant for further analysis. Following euthanasia of the mice, the kidneys, peritesticular fat, and various other organs were excised and weighed. The selected tissues were preserved in 10% neutral buffered formalin, while the remaining samples were stored at –80°C for subsequent analyses.
Serum biochemistry
Blood was collected from the eyeballs and centrifuged at 1000 × g for 15 minutes at 4°C. Serum samples were obtained, and the biochemical parameters of the serum, including urea nitrogen and creatinine, were assessed according to the manufacturer’s specifications (Mindray Global, Shenzhen, China).
Cell culture and intervention
Human kidney cortical proximal tubule epithelial cells (HK-2 cells, Wuhan Pricella Biotechnology Co., Ltd., Wuhan, China, Cat# CL-0109) were cultured in Dulbecco’s modified Eagle medium/nutrient mixture F-12 (DMEM/F-12; Servicebio, Wuhan, China, Cat# G4610-500ML) supplemented with 10% fetal bovine serum and maintained in a cell culture incubator (Esco Micro Pte. Ltd., Singapore) at 37°C and 5% CO2. Upon reaching approximately 70% confluency, the cells were incubated overnight in serum-free medium prior to drug intervention. The cells were divided into five groups: (1) Normal control group (Con): DMEM/F-12 medium; (2) palmitic acid (PA; MedchemExpress, Monmouth Junction, NJ, USA) group: DMEM/F-12 medium supplemented with 200 μM PA for constructing a cell model of renal tubular injury induced by high-fat conditions; (3) PA + MgH2 (PM) group: DMEM/F-12 medium supplemented with 200 μM PA and 500 μM MgH2; (4) control plasmid (Vector) group: DMEM/F-12 medium supplemented with 2 μg control plasmid (Genomeditech, Shanghai, China); and (5) Hes family BHLH transcription factor 1 (HES1) overexpression (HES1-OE) group: DMEM/F-12 medium supplemented with 2 μg HES1 overexpression plasmid (Genomeditech).
The day before transfection, 1 × 106 HK-2 cells were seeded into a 6-well plate. When the cells reached 70–80% confluence on the second day, the transfection system was configured according to the standard of the transfection instructions, and the mixture was homogeneous. Then, the mixture was added to the culture wells of 6-well plates after standing at room temperature for 30 minutes and then incubated in a cell culture incubator at 37°C. The medium was changed from 4–6 hours, and the cells were incubated for 18–48 hours. The transfection efficiency was detected under a fluorescence microscope (Leica, Wetzlar, Gemany) or by immunoblotting the proteins.
Renal histology and immunohistochemistry
Kidney tissue was fixed with 10% neutral fixative, embedded in paraffin, and sectioned. Following dewaxing and hydration, the sections were stained with hematoxylin‒eosin (Servicebio) and periodic acid‒Schiff (Servicebio). Immunohistochemical staining was conducted using specific antibodies against TGF-β (rabbit, 1:100; Proteintech, Wuhan, China; Cat# 21898-1-AP) and FN (rabbit, 1:100; CST, Danvers, CO, USA; Cat# 26836). This process included embedding, sectioning, dewaxing, antigen retrieval, blocking, incubation with primary antibodies at 4°C for 12 hours, and subsequent incubation with secondary antibodies (goat, 1:2000, Abcam, Cambridge, UK, Cat# ab205718) at 25°C for 1 hour. Following fixation with paraformaldehyde, the HK-2 cells were permeabilized with 0.5% Triton X-100 and subsequently blocked with 5% bovine serum albumin. This was followed by sequential incubation with primary antibodies, such as vimentin antibodies (rabbit, 1:1000, Proteintech, Cat# 60330-1-Ig), E-cadherin antibodies (rabbit, 1:50, Proteintech, Cat# 20874-1-AP), TGF-β antibodies (rabbit, 1:50, Proteintech, Cat# 21898-1-AP), Smad2 antibodies (rabbit, 1:50, Cloud-clone Corp, Wuhan, China, Cat# PAC124Hu01) and Alexa Fluor 488-tagged, a green fluorescent indicator, goat anti-rabbit antibody (1:500, Abcam, Cat# ab150077). The cells were then observed and imaged via an optical microscope.
Cell viability assay
Cell growth and proliferation were detected via a Cell Counting Kit-8 (CCK-8) detection kit (MedChemExpress). HK-2 cells were treated with PA, MgH2, and the PTEN inhibitor SF1670 (MedChemExpress) for 24 hours, with PA concentrations ranging from 0 to 1000 μM, MgH2 concentrations ranging from 0 to 1000 μM, and SF1670 concentrations ranging from 0 to 80 μM. Subsequently, Cell Counting Kit-8 reagent diluted at a ratio of 1:10 with complete DMEM/F-12 medium was added to each well, and the mixture was incubated for 1 hour. Finally, the absorbance of each well at 450 nm was measured via a microplate reader (BioTek, Winooski, VT, USA) to determine the cell survival rate.
Western blot analysis
To isolate proteins from mouse kidneys and HK-2 cells, the purified protein was first homogenized and then subjected to centrifugation. The resulting supernatant was subsequently quantified via a protein quantification kit (Thermo Fisher Scientific, Franklin, MA, USA). High-throughput polyacrylamide gel electrophoresis was employed for protein separation, after which the protein samples were transferred onto nitrocellulose membranes (GE, Boston, MA, USA). The membranes were then blocked with 5% bovine serum albumin at room temperature for 1 hour and incubated with the following primary antibodies overnight at 4°C: neutrophil gelatinase-associated lipocalin (NGAL) antibodies (rabbit, 1:1000, Cloud-clone Corp., Cat# PAB388Mu01 & PAB388Hu01), kidney injury molecule-1 (KIM-1) antibodies (rabbit, 1:1000, Cloud-clone Corp., Cat# PAA785Mu01 & PAA785Hu01), vimentin antibodies (rabbit, 1:1000, Proteintech, Cat# 60330-1-Ig), FN antibodies (rabbit, 1:1000, CST, Cat# 26836), E-cadherin antibodies (rabbit, 1:1000, Proteintech, Cat# 20874-1-AP), β-actin antibodies (rabbit, 1:1000, Proteintech, Cat# 66009-1-Ig), TGF-β antibodies (rabbit, 1:1000, Proteintech, Cat# 21898-1-AP), Smad2 antibodies (rabbit, 1:1000, Cloud-clone Corp., Cat# PAC124Mu01 & PAC124Hu01), and Smad3 antibodies (rabbit, 1:1000, Cloud-clone Corp., Cat# PAC123Mu01 & PAC123Hu01), phospho-Smad family member 2 (p-Smad2) antibodies (rabbit, 1:1000, CST, Cat# 18338), phospho-Smad family member 3 (p-Smad3) antibodies (rabbit, 1:1000, CST, Cat# 9520), PTEN antibodies (rabbit, 1:1000, CST, Cat# 9559), phospho-protein kinase B (p-AKT) antibodies (rabbit, 1:1000, CST, Cat# 4060), mTOR antibodies (rabbit, 1:1000, CST, Cat# 2983), phospho-mechanistic target of rapamycin (p-mTOR) antibodies (rabbit, 1:1000, CST, Cat# 5536), and HES1 antibodies (rabbit, 1:1000, CST, Cat# 11988). Then the membranes were washed with Tween 20/Tris-buffered saline three times and incubated with the anti-rabbit HRP-linked antibody (1:3000, CST, Cat# 14786) for 2 hours at room temperature. Band characterization was performed via dual-color thermal imaging technology (Tanon, Shanghai, China), and the results were further refined using ImageJ software.
Quantitative reverse transcription polymerase chain reaction
Total RNA was isolated from mouse kidney tissue and HK-2 cells with TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The RNA was subsequently reverse transcribed into complementary DNA with a commercially available kit (Yeasen, Shanghai, China). Quantitative reverse transcription polymerase chain reaction was then conducted with SYBR Green dye (Yeasen) to ensure precise quantification. The primer sequences for vimentin were 5′-CTG CTG GAA GGC GAG GAG AG-3’ and 5′-TCA ACC GTC TTA ATC AGG AGT GTT C-3’ (designed by Sangon, Shanghi, China). The gene expression data were normalized to the relative mRNA expression level of β-actin.
RNA transcriptome sequencing
RNA transcriptome sequencing was used to screen for differential genes in mice from the normal diet, HFD and MgH2 groups, and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis was performed on the differential genes to summarize the major enrichment pathways of the top 15 differential genes, which were all completed via the Gene Denovo Bioinformatics Analysis Platform (https://www.omicshare.com/tools/).
Fluorescent probes for lipid droplets
After being washed with PBS, HK-2 cells were stained with a Lipi-Blue fluorescent probe (DOJINDO, Kumamoto-ken, Japan, Cat#LD01 Lipi-Blue) for 15 minutes and then observed under a fluorescence microscope.
Statistical analysis
The statistical results are presented as the mean ± standard errors of the means (SEMs). GraphPad Software 8.0 (GraphPad Software, Boston, MA, USA; www.graphpad.com) was used for data analysis and graphic representation. Independent sampling t tests and one-way analysis of variance followed by Tukey post hoc tests were employed. Significance was declared at P < 0.05.
Results
Magnesium hydride ameliorates high-fat diet-induced dyslipidemia and renal injury
Following a 12-week dietary intervention involving three groups of mice, we evaluated kidney function influenced by a HFD through blood urea nitrogen and creatinine (Figure 1B and C), revealing abnormal elevations in both parameters in the HFD group, which were mitigated by MgH2 treatment. Additionally, we used western blotting to quantify the protein expression levels of NGAL and KIM-1 in vivo and in vitro. The results revealed an increase in their expression in the HFD/PA group, which was notably decreased by MgH2 (Figure 1D and E). Additionally, we evaluated the effects of a HFD on kidney damage and function through hematoxylin‒eosin (H&E) staining, periodic acid‒Schiff (PAS) staining, and assessment of glomerular injury. Hematoxylin‒eosin staining revealed significant thickening and adhesion of the glomerular basement membrane in the HFD group, along with intraglomerular matrix deposition induced by the HFD. In contrast, the administration of MgH2 resulted in a marked reduction in glomerular injury (Figure 1F).
Magnesium hydride mitigates lipid deposition and renal fibrosis in high-fat diet-fed mice
Lipid droplets, which are encapsulated in phospholipids and associated with dyslipidemia, represent critical markers of CKD that are precipitated by lipemic conditions.13 This renal pathology is further characterized by the intertubular fibrosis observed in HFD-fed mice. Renal fibrosis results from excessive extracellular matrix accumulation due to EMT stimulation.14 Western blot analysis corroborated these findings, revealing the upregulation of Vimentin and FN, along with the suppression of E-cadherin levels, thereby exacerbating fibrosis in the HFD and PA groups. Notably, these effects were significantly reversed by MgH2 treatment (Figure 2A and G). To elucidate the effects of MgH2 on antirenal fibrosis, a morphological analysis was conducted to assess FN expression in the kidneys of HFD-fed mice (Figure 2C). Furthermore, MgH2 significantly reduced the mRNA expression levels of the fibrotic marker vimentin (Figure 2B and H). The viability of HK-2 cells in the presence of PA (0–800 μM) and MgH2 (0–1000 μM) was assessed using a Cell Counting Kit-8 assay. MgH2 exhibits low toxicity toward HK-2 cells, with no inhibitory effect on cell growth even at a concentration of 1000 µM. In contrast, PA significantly inhibited cell viability at a concentration of 200 µM (Figure 2D and E). A pronounced accumulation of lipid droplets was observed in the PA-treated group (Figure 2F). Immunofluorescence staining revealed a marked improvement in fibrosis severity in the PM group subsequent to PA induction (Figure 2I and J).
Figure 2.
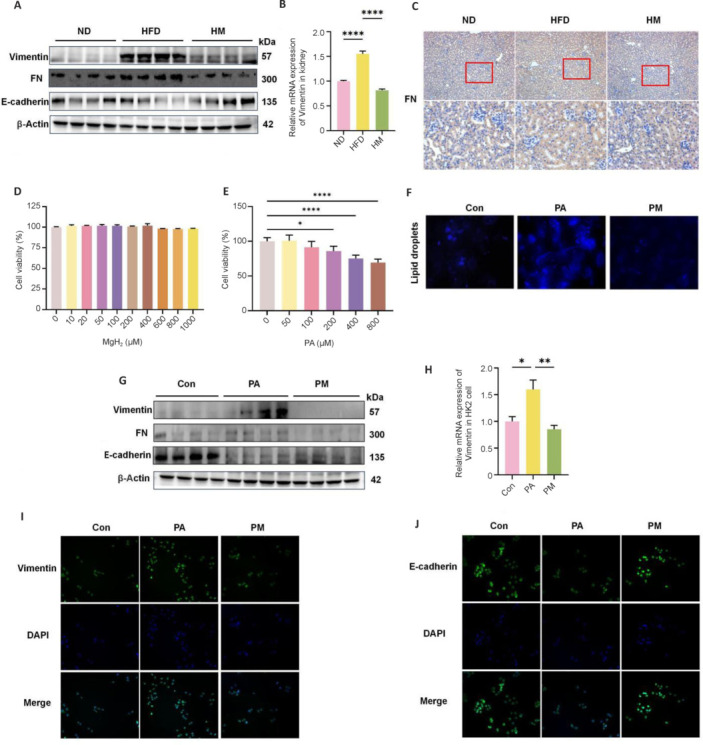
MgH2 ameliorates lipid accumulation and fibrosis in kidneys induced by HFD/PA
(A) Western blot analysis of fibrotic protein expression following MgH2 intervention in mouse kidneys. The raw data for the quantitative results are shown in Additional Table 1. (B) Variation in Vimentin mRNA expression in the mouse kidney. The data were normalized to those of the ND group. (C) FN immunohistochemistry in mouse kidney tissue (100× original magnification (upper), 400× original magnification (lower)), which increased in the HFD group and decreased after MgH2 treatment. (D, E) Effects of PA and MgH2 on HK-2 cell cytotoxicity. The data were normalized to those of the 0 μM group. (F) Fluorescence-based assessment of lipid droplets in HK-2 cells (400× original magnification). PA increased the lipid droplet content in HK-2 cells, and MgH2 treatment resulted in a reduction in lipid droplets. (G) Differential expression of fibrosis markers in HK2 cells as determined by Western blot analysis. The raw data for the quantitative results are shown in Additional Table 1. (H) Changes in Vimentin mRNA expression in HK-2 cells following MgH2 intervention. The data were normalized to those of the Con group. The data are expressed as the mean ± SD (n = 6). *P < 0.05, **P < 0.01, ****P < 0.0001 (one-way analysis of variance followed by Tukey’s post hoc test). (I, J) Immunofluorescence analysis of Vimentin and E-cadherin in response to MgH2 stimulation (400× original magnification). Vimentin was elevated in the PA group, whereas E-cadherin was decreased in the PA group. Both of these parameters partially returned to normal levels after MgH2 treatment. Con: Control group; DAPI: 4′,6-diamidino-2-phenylindole; FN: fibronectin; HFD: high-fat diet; HM: MgH2 group; MgH2: magnesium hydride; ND: normal control; PA: palmitic acid; PM: PA + MgH2 group.
Magnesium hydride inhibits the activation of the transforming growth factor-beta pathway
The results of the top 15 enriched KEGG pathways revealed that the PI3K/AKT signaling and TGF-β signaling pathways were important for the renoprotective effect of MgH2 on HFD-induced renal injury (Figure 3A). TGF-β is a pivotal mediator in the progression of renal fibrosis, and its dysregulation under pathological conditions exacerbates kidney injury.15 To examine whether MgH2 modulates TGF-β expression, we utilized immunohistochemical techniques to detect TGF-β in renal tissues (Figure 3F). Our findings indicated that TGF-β expression was elevated under HFD conditions and that this increase was mitigated by MgH2. Given that TGF-β promotes Smad2/3 phosphorylation and translocation,16 we also investigated downstream signaling molecules. Immunoblotting analyses conducted both in vivo and in vitro confirmed that MgH2 inhibited the phosphorylation of TGF-β and its downstream signaling pathway (Figure 3B and C). Furthermore, fluorescence analysis of cells revealed that MgH2 suppressed the PA-induced upregulation of TGF-β and Smad2 expression (Figure 3D and E).
Figure 3.
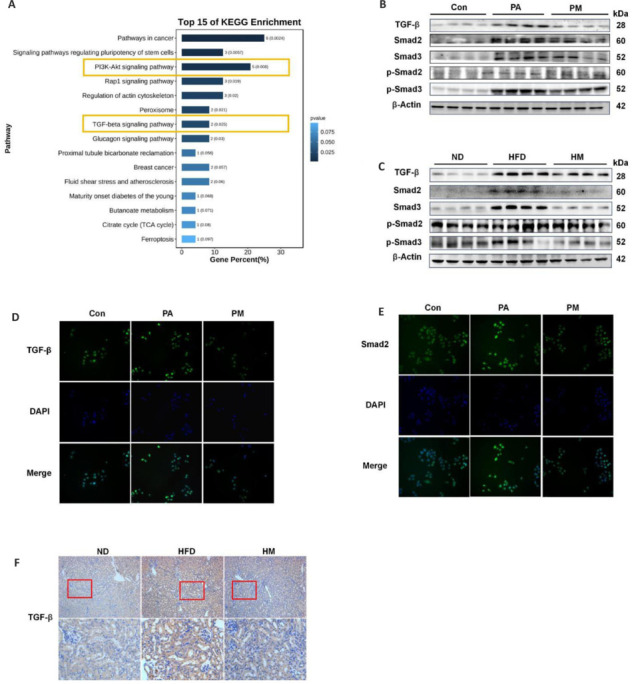
MgH2 inhibits the activation of the TGF-β pathway.
(A) KEGG enrichment of the top 15 signaling pathways. The raw data are shown in Additional Table 2 (1.1MB, pdf) . (B, C) Molecular immunoblotting results of the TGFβ-Smad2/3 pathway after MgH2 intervention in in vitro (B) and in vivo (C) experiments. The raw data for the quantitative results are shown in Additional Table 1. (D) Molecular immunofluorescence results of TGF-β in HK-2 cells after MgH2 intervention (400× original magnification). TGF-β increased after PA treatment but decreased after MgH2 treatment. (E) Molecular immunofluorescence results of Smad2 in HK-2 cells after MgH2 intervention (400× original magnification). Smad2 levels increased after PA treatment but decreased after MgH2 treatment. (F) TGF-β immunohistochemistry in mouse kidney tissue (100× original magnification (upper), 400× original magnification (lower)). Compared with that in the ND group, TGF-β was elevated in the HFD group and partially returned to normal levels after MgH2 treatment. Con: Control group; DAPI: 4’,6-diamidino-2-phenylindole; HFD: high-fat diet; HM: MgH2 group; KEGG: Kyoto Encyclopedia of Genes and Genomes; ND: normal control; PA: palmitic acid; PM: PA + MgH2 group; p-Smad2: phospho-Smad family member 2; p-Smad3: phospho-Smad family member 3; Smad2: Smad family member 2; Smad3: Smad family member 3; TGF-β: transforming growth factor-beta.
Magnesium hydride suppresses the activation of Smad family member 2 and 3 and protein kinase B/mechanistic target of rapamycin pathway through the modulation of phosphatase and tensin homologue deleted on chromosome 10
PTEN functions as a negative regulator of the PI3K/AKT/mTOR signaling pathway and is intricately associated with renal interstitial fibrosis.17 To further elucidate the regulatory effects of MgH2 on the AKT/mTOR pathway and to determine whether these effects are PTEN dependent, we conducted investigations at both the animal and cellular levels. Western blot analysis revealed that MgH2 inhibited the upregulation of the AKT/mTOR pathway under HFD/PA conditions and reversed the phosphorylation of associated signaling molecules (Figure 4A and B). To further investigate this phenomenon, we employed the PTEN inhibitor SF1670 at a working concentration of 5 μM for cytotoxicity analysis in HK-2 cells (Figure 4C). As anticipated, SF1670 treatment significantly reduced PTEN expression in HK-2 cells, thereby attenuating the inhibitory effect of MgH2 on the activation of the AKT/mTOR pathway (Figure 4D).
Figure 4.
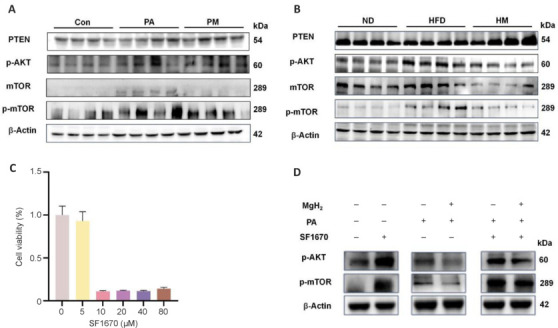
MgH2 suppresses AKT/mTOR pathway activation via modulation of PTEN in vitro.
(A, B) Western blotting results of the PTEN/AKT/mTOR pathway after MgH2 intervention in in vitro (A) and in vivo (B) experiments. The raw data for the quantitative results are shown in Additional Table 1. (C) Evaluation of HK-2 cell toxicity induced by SF1670 (a PTEN inhibitor). (D) Analysis of the effects of PTEN inhibition on the expression of proteins in the AKT/mTOR pathway. The raw data for the quantitative results are shown in Additional Table 1. Con: Control group; HFD: high-fat diet; HM: MgH2 group; MgH2: magnesium hydride; mTOR: mammalian target of rapamycin; ND: normal control; PA: palmitic acid; p-AKT: phospho-protein kinase B; PM: PA + MgH2 group; p-mTOR: phospho-mammalian target of rapamycin; PTEN: phosphatase and tensin homolog deleted on chromosome ten.
Counteracting magnesium hydride -mediated inhibition of Smad family member 2 and 3 and protein kinase B/mechanistic target of rapamycin R and the transforming growth factor-beta-Smad2/3 pathway via Hes family BHLH transcription factor 1
After identifying PTEN as a key target molecule for MgH2 action, we conducted an expression analysis of HES1, an upstream regulator of PTEN, to investigate additional potential upstream targets of PTEN. Therefore, we first assessed HES1 protein levels both in vitro and in vivo. Compared with that in the normal control or Con groups, HES1 expression was elevated in the HFD and PA groups but significantly decreased following MgH2 treatment (Figure 5A and B). HES1 was subsequently overexpressed using transfection plasmids, which resulted in increased transfection efficiency in both fluorescence and protein immunoblotting assays (Figure 5C). The western blotting results suggested that HES1 overexpression activated the phosphorylation of the AKT/mTOR and transforming growth factor-beta-Smad2/3 signaling pathways (Figure 5D), an effect that was reversed by MgH2. These findings imply that the inhibition of PTEN activates the AKT/mTOR and transforming growth factor-beta-Smad2/3 pathways, thereby diminishing the protective effect of MgH2 against renal injury.
Figure 5.
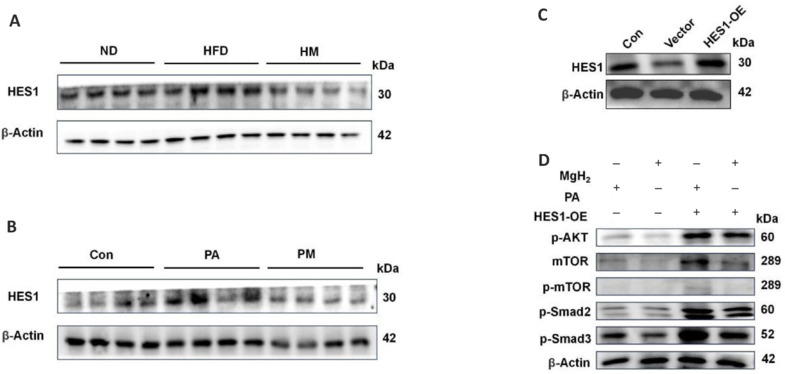
Mitigation of MgH2-induced inhibition of the AKT/mTOR and TGFβ-Smad2/3 pathways by HES1.
(A) In vivo protein expression levels of HES1. (B) In vitro protein expression levels of HES1. (C) HES1 overexpression was detected via western blot analysis. (D) Impact of HES1 overexpression on the protein expression levels of molecules in the TGFβ-Smad2/3 and AKT/mTOR pathways. The raw data for the quantitative results are shown in Additional Table 1. Con: Control group; HES1: Hes family BHLH transcription factor 1; HFD: high-fat diet; HM: MgH2 group; MgH2: magnesium hydride; mTOR: mammalian target of rapamycin; ND: normal control; OE: overexpression; PA: palmitic acid; p-AKT: phospho-protein kinase B; PM: PA + MgH2 group; p-mTOR: phospho-mammalian target of rapamycin; p-Smad2: phospho-Smad family member 2; p-Smad3: phospho-Smad family member 3; TGFβ: transforming growth factor-beta.
Discussion
MgH2, a solid hydrogen source with high storage capacity, is characterized by its efficiency and safety,18 in addition to its low cost, abundant reserves, and high energy storage capacity.19 In our study, we examined the potential applications of MgH2 in the medical field. MgH2 has demonstrated considerable promise in reducing oxidative stress, enhancing cellular function, reducing chronic inflammation, and effectively regulating blood lipid levels.18 The significant hydrolysis product of MgH2, H2, may be the key to exerting the aforementioned functions. H2 supplementation has been found to expedite fat and glucose metabolism, notably lowering total cholesterol and triglyceride levels while increasing the concentrations of vitamins C and E.20 Furthermore, it has been shown to improve insulin resistance and suppress the elevation of low-density lipoprotein levels.21 Molecular hydrogen has demonstrated substantial efficacy in both the prevention and treatment of various kidney diseases in animals and humans.22 Notably, MgH2 has antifibrotic effects on acute kidney injury by inhibiting the thioredoxin-interacting protein/NOD-like receptor thermal protein domain-associated protein 3/NF-κB pathway, thereby alleviating acetaminophen-induced kidney injury.23 However, its role in CKD remains understudied.
We investigated the potential ameliorative effects of MgH2 on CKD in HFD-fed mice. The mice were fed a HFD for 12 weeks, resulting in ectopic fat accumulation and renal injury. Notably, intervention with MgH2 had beneficial effects on renal function. Recent studies have highlighted the regulatory function of hydrogen in human lipid metabolism. Notably, hydrogen inhibits the expression of the coactivator peroxisome proliferator activated receptor 1 alpha gene, which in turn promotes fatty acid catabolism.24 Additionally, hydrogen-enriched water has been associated with increased expression of uncoupling protein 1 in brown adipose tissue.25 These pathways modulate a variety of metabolic processes, leading to a decrease in total cholesterol levels and an improvement in lipid profiles. MgH2 is recognized as a promising solid hydrogen carrier because of its high safety and efficiency. A substantial body of research has linked magnesium deficiency to lipid abnormalities and the development of metabolic syndrome.26 Magnesium deficiency has been shown to induce the expression of endothelial differentiation-related factor 1 and peroxisome proliferator-activated receptor gamma, which are implicated in adipogenesis.27,28 Our research demonstrated that MgH2 can alleviate renal tubulointerstitial injury and fibrosis induced by a HFD. However, whether magnesium is involved in the renoprotective effect of MgH2, in addition to the role of hydrogen, was not addressed in this study. This is also an important direction for research that we will pursue in the future.
Renal tissue, particularly tubular epithelial cells, predominantly relies on lipids for energy because of their extensive reabsorptive functions. These cells are notably susceptible to fibrotic progression following stimulation.29,30 The reduced self-regenerative capacity of tubular epithelial cells hinders their ability to undergo cellular differentiation, thereby promoting the accumulation of inflammatory cells within the kidneys and disrupting the balance between extracellular matrix production and degradation. The activation of myofibroblasts and the subsequent accumulation of extracellular matrix are critical processes in the development of kidney fibrosis, which is characterized by the deposition of proteins such as FN and collagen-1.31 A fundamental challenge in comprehending renal fibrosis lies in the elucidation of key molecular pathways responsible for the activation of profibrotic cells. The current consensus indicates that TGF-β1, along with its downstream Smad signaling cascade, is critically involved in this process.8 H2 has been shown to suppress TGF-β1-induced EMT, with hydrogen water counteracting the downregulation of Sirtuin 1 expression induced by TGF-β1.32 Concurrently, hydrogen water alleviated oxalate-induced kidney injury by diminishing oxidative stress, inflammation and fibrosis via the inhibition of the PI3K/AKT, NF-κB, and TGF-β signaling pathways.33 Consistent with these findings, our results indicated a reduction in TGF-β activity in kidney tissue and HK-2 cells following MgH2 intervention. Additionally, MgH2 has been shown to both activate Smad2 and Smad3 phosphorylation and suppress the expression of vimentin and FN, thereby substantiating the inhibition of the TGF-β pathway. In addition to the conventional TGF-β/Smad pathway, TGF-β also interacts with the PI3K/AKT signaling pathway to induce fibrosis. PI3K/AKT signaling is indispensable for TGF-β-induced EMT and cell migration, wherein activated Akt phosphorylates twist family bHLH transcription factor 1, leading to the release of TGF-β2 and subsequent signal amplification, thereby reinforcing Akt activity.34 In conjunction with the KEGG enrichment analysis of this study, MgH2 may inhibit the activation of the TGF-β pathway by regulating the PI3K/AKT pathway.
PTEN, in conjunction with PI3K and AKT, forms a critical signaling pathway.35,36 The effect of TGF-β on AKT is intercepted via the activation of PTEN, which serves as a therapeutic target.37 A previous study indicated that hydrogen molecules play regulatory roles in the PTEN/AKT/mTOR signaling pathway.38 However, the effect of MgH2 on PTEN remains largely unexplored. To this end, we explored the potential regulatory effect of MgH2 on PTEN as an upstream target of the TGF-β and AKT signaling pathways. Our findings indicate that SF1670 attenuates the inhibitory effects of MgH2 on TGF-β and AKT following PTEN suppression, confirming the regulatory effect of MgH2 on PTEN. These results suggest that MgH2 may activate PTEN to suppress TGF-β and AKT signaling, thereby mitigating renal injury. HES1 plays a crucial role in sustaining the self-renewal of cancer stem cells, promoting cancer metastasis and EMT, and promoting resistance to chemotherapy. In the context of kidney fibrosis, PTEN expression is frequently diminished through mechanisms that remain unclear, but it is subject to direct regulation by HES1. Correspondingly, our findings indicate that HES1 overexpression mitigates the beneficial effects of MgH2 in renal cells.
Several limitations of this research have been identified. First, this study represents the initial use of oral MgH2 in studies of chronic kidney injury; however, the concentration and distribution of H2 produced from its hydrolysis in vivo remain unclear. Second, the HFD employed in the present study, which induces abnormal lipid levels and accumulation, concurrently triggers inflammation and oxidative stress. Third, this study has not yet conducted further analysis on the effects of various hydrolysis products of MgH2. This suggests that the interplay between these factors may be influenced by MgH2, necessitating further investigations into the specific underlying mechanisms involved.
The results of the present study demonstrated that MgH2 has the potential to significantly mitigate HFD-induced renal injury. These findings indicate that MgH2 may attenuate renal damage by inhibiting the activation of the AKT-mTOR and transforming growth factor-beta-Smad pathways, likely through the upregulation of PTEN.
Additional files:
Additional Table 1: The raw data for the quantitative results of western blot analysis..
Additional Table 1.
The raw data for quantitative results of western blot analysis
| Figure1D | ND | HFD | HM | ND | HFD | HM | ||||||||||||||||||||
| ngal | 4964.36 | 7030.18 | 7676.18 | 7711.48 | 13375.48 | 12531.89 | 12857.13 | 10014.65 | 11246.6 | 11523.18 | 9158.48 | 5767.6 | 0.7251952 | 1.026970806 | 1.12133868 | 1.126495314 | 1.953894136 | 1.830662255 | 1.878173412 | 1.46294308 | 1.642906706 | 1.683309595 | 1.337873509 | 0.84253274 | ||
| kimi | 723.85 | 1008.94 | 3455.84 | 4611.77 | 13067.2 | 12561.67 | 17535.4 | 17849.25 | 11025.25 | 11328.18 | 10772.5 | 9583.25 | 0.295436921 | 0.411795437 | 1.410489368 | 1.882278274 | 5.333333333 | 5.127002979 | 7.157013999 | 7.285110812 | 4.499918371 | 4.623558222 | 4.396759316 | 3.911370964 | ||
| Figure1E | ND | HFD | HM | Con | PA | PM | ||||||||||||||||||||
| ngal | 5538.06 | 4018.89 | 4182.01 | 4460.94 | 8637.65 | 10298.31 | 12490.25 | 9855.06 | 8930.18 | 8664.89 | 7258.75 | 1089.34 | 1.217162732 | 0.883277381 | 0.919128127 | 0.980431761 | 1.898395046 | 2.263377271 | 2.745124973 | 2.165959154 | 1.962687707 | 1.904381892 | 1.595338436 | 0.2394167 | ||
| kimi | 7523.96 | 7820.86 | 7170.15 | 5702.38 | 41213.9 | 42510.25 | 41965.3 | 42177.69 | 34851.07 | 37979.47 | 34572.54 | 32208.9 | 1.066572162 | 1.108659743 | 1.016417204 | 0.80835089 | 5.842348768 | 6.026115138 | 5.948864794 | 5.978972512 | 4.940374628 | 5.383846463 | 4.900891118 | 4.56582918 | ||
| Figure2A | ND | HFD | HM | ND | HFD | HM | ||||||||||||||||||||
| Vim | 4939.58 | 5066.18 | 4558.36 | 4587.48 | 13178.6 | 14030.89 | 14965.6 | 13881.18 | 11850.6 | 12026.48 | 12748.01 | 13178.6 | 1.03167986 | 1.058121515 | 0.952058314 | 0.958140312 | 2.752480211 | 2.930489359 | 3.125712734 | 2.899220953 | 2.475114351 | 2.511848618 | 2.662547255 | 2.752480211 | ||
| FN | 23068.57 | 20313.81 | 29756.05 | 28293.71 | 38120.27 | 31397.42 | 35298.83 | 39541.2 | 18148.88 | 21951.12 | 19458.47 | 15070.23 | 0.909714416 | 0.801079816 | 1.173436743 | 1.115769025 | 1.503281701 | 1.23816455 | 1.39201756 | 1.559316406 | 0.715705298 | 0.865647516 | 0.767349284 | 0.59429802 | ||
| E-cad | 28887.4 | 22024.4 | 20740.69 | 27521.76 | 26598.47 | 20968.64 | 14292.98 | 11051.4 | 18165.93 | 24407.18 | 31750.88 | 36775.86 | 1.165116953 | 0.88831123 | 0.83653529 | 1.110036527 | 1.072797425 | 0.845729209 | 0.576479479 | 0.445736671 | 0.732687366 | 0.984416015 | 1.280609836 | 1.483282606 | ||
| Figure2G | ND | HFD | HM | Con | PA | PM | ||||||||||||||||||||
| Vim | 1093.63 | 2558.06 | 1478.23 | 1908.65 | 3564.48 | 7740.31 | 13008.84 | 13346.13 | 3506.28 | 2268.63 | 2393.7 | 2552.94 | 0.621506925 | 1.453738472 | 0.840074049 | 1.084680553 | 2.025684194 | 4.398796915 | 7.392888044 | 7.584569025 | 1.992609294 | 1.289256198 | 1.360333136 | 1.450828791 | ||
| FN | 3563.7 | 5581.82 | 5596.18 | 5198.94 | 11958.96 | 11233.72 | 9922.55 | 11942.25 | 6360.72 | 8078.43 | 7757.72 | 6191.48 | 0.71486171 | 1.119687232 | 1.122567781 | 1.042883278 | 2.398911971 | 2.253432187 | 1.990417559 | 2.395560022 | 1.275930963 | 1.620495631 | 1.556162691 | 1.241982203 | ||
| E-cad | 36977.28 | 37156.3 | 44872.88 | 39010.3 | 23147.83 | 19088.88 | 19763.52 | 23843.76 | 33343.66 | 39841.85 | 31439.95 | 23133.52 | 0.93603438 | 0.940566051 | 1.135901787 | 0.987497782 | 0.585958857 | 0.483211528 | 0.50028921 | 0.603575469 | 0.844053757 | 1.008547448 | 0.795863679 | 0.585596616 | ||
| Figure3B | ND | HFD | HM | ND | HFD | HM | ||||||||||||||||||||
| TGFb | 8247.86 | 13278.83 | 15356 | 10451.98 | 27148.37 | 40564.07 | 44639.95 | 44876.07 | 20377.59 | 14409 | 17113.18 | 13856.69 | 0.696982571 | 1.12212296 | 1.297653496 | 0.883240973 | 2.294163665 | 3.427852777 | 3.772283614 | 3.792236853 | 1.722001231 | 1.217627587 | 1.446143387 | 1.17095482 | ||
| smad2 | 6281.18 | 3256.48 | 4252.89 | 6243.89 | 12498.67 | 8531.77 | 9983.01 | 10867.6 | 8906.18 | 9496.18 | 5445.43 | 5980.48 | 1.25407648 | 0.650176396 | 0.849115823 | 1.246631301 | 2.495436858 | 1.70342071 | 1.993169762 | 2.169783633 | 1.778173984 | 1.895971138 | 1.087213818 | 1.194039863 | ||
| smad3 | 3138.06 | 3485.18 | 2709.48 | 3446.18 | 10520.89 | 11968.84 | 13514.13 | 11349.13 | 11167.01 | 10113.6 | 7273.38 | 6860.11 | 0.982262949 | 1.090917059 | 0.848110557 | 1.078709435 | 3.293206771 | 3.746438269 | 4.230138744 | 3.552459132 | 3.49545266 | 3.165718489 | 2.276684222 | 2.147324105 | ||
| p-smad2 | 9588.06 | 9459.84 | 8641.72 | 9229.87 | 10865.77 | 12139.31 | 10177.13 | 9546.18 | 11148.43 | 9808.96 | 9125.77 | 9059.18 | 1.038807416 | 1.024915566 | 0.936277289 | 0.999999729 | 1.177239447 | 1.315219685 | 1.102629533 | 1.034269975 | 1.207863922 | 1.062740574 | 0.988721133 | 0.981506516 | ||
| p-smad3 | 2256.87 | 3085.94 | 3055.7 | 3259.7 | 11880.72 | 13934.6 | 13827.65 | 12441.72 | 8901.36 | 10810.18 | 11772.89 | 12571.65 | 0.77434529 | 1.058804053 | 1.048428532 | 1.118422125 | 4.076344482 | 4.781042716 | 4.744347546 | 4.268826861 | 3.05410865 | 3.70903595 | 4.039347378 | 4.313406604 | ||
| Figure3C | ND | HFD | HM | Con | PA | PM | ||||||||||||||||||||
| TGFb | 4770.65 | 2514.82 | 2252.23 | 2867.65 | 9489.18 | 13014.77 | 11235.65 | 12159.6 | 7369.6 | 9690.89 | 9627.36 | 8471.18 | 1.538255672 | 0.810882402 | 0.726212481 | 0.924649446 | 3.059705691 | 4.196502316 | 3.622840146 | 3.920759995 | 2.376265079 | 3.124745372 | 3.104260662 | 2.731460217 | ||
| smad2 | 1590.216667 | 838.2733333 | 750.7433333 | 955.8833333 | 3163.06 | 4338.256667 | 3745.216667 | 4053.2 | 2456.533333 | 3230.296667 | 3209.12 | 2823.726667 | 1.538255672 | 0.810882402 | 0.726212481 | 0.924649446 | 3.059705691 | 4.196502316 | 3.622840146 | 3.920759995 | 2.376265079 | 3.124745372 | 3.104260662 | 2.731460217 | ||
| smad3 | 8533.25 | 10542.1 | 13480.35 | 11920.1 | 28297.47 | 33108.52 | 26719.64 | 25693.76 | 7598.28 | 9824.05 | 8017.52 | 8638.57 | 0.767451063 | 0.948120101 | 1.212376169 | 1.072052667 | 2.544976819 | 2.977666057 | 2.403072233 | 2.310808125 | 0.683363087 | 0.883541162 | 0.721068086 | 0.776923181 | ||
| p-smad2 | 24085.33 | 21628.69 | 18533.74 | 19991.98 | 20879.57 | 24374.57 | 32315.4 | 26209.69 | 26833.28 | 25201.28 | 25050.81 | 16066.62 | 1.143656426 | 1.027006494 | 0.880047351 | 0.94928973 | 0.991435634 | 1.157390562 | 1.53444918 | 1.244528532 | 1.274138785 | 1.196645669 | 1.189500822 | 0.762899791 | ||
| p-smad3 | 27177.05 | 28191.81 | 18961.05 | 5336.69 | 28540.28 | 31649.64 | 29149.81 | 25209.93 | 12264.23 | 18160.76 | 18174.93 | 14538.45 | 1.364539217 | 1.415489553 | 0.952020044 | 0.267951187 | 1.432985969 | 1.589104593 | 1.463590011 | 1.265771603 | 0.615777754 | 0.911838085 | 0.91254955 | 0.729964628 | ||
| Figure4A | ND | HFD | HM | ND | HFD | HM | ||||||||||||||||||||
| pten | 15990.91 | 15970.45 | 16364.08 | 12559.2 | 13761.33 | 16122.2 | 21192.01 | 23048.08 | 19498.28 | 24703.79 | 22473.28 | 20090.45 | 1.050571047 | 1.049226866 | 1.075087575 | 0.825114512 | 0.904092067 | 1.05919654 | 1.392272994 | 1.514213109 | 1.280998294 | 1.622989969 | 1.476449889 | 1.319902688 | ||
| p-akt | 5304.11 | 5211.06 | 6311.89 | 5893.48 | 7815.01 | 10559.72 | 12327.01 | 7003.43 | 7551.72 | 8605.31 | 12472.96 | 9430.53 | 0.933799989 | 0.917418336 | 1.111221828 | 1.037559847 | 1.375849342 | 1.859061448 | 2.170196659 | 1.232968935 | 1.32949657 | 1.514983359 | 2.195891471 | 1.660265117 | ||
| mtor | 2109.01 | 5085.47 | 10444.59 | 16487.42 | 39466.49 | 43420.44 | 28041.35 | 40250.37 | 23322.78 | 19195.12 | 17050 | 9260.52 | 0.24719917 | 0.596073021 | 1.224220833 | 1.932506976 | 4.625906737 | 5.089353168 | 3.286754659 | 4.717786095 | 2.733686353 | 2.249879199 | 1.99844754 | 1.085434804 | ||
| p-mtor | 3487.11 | 1700.53 | 4480.06 | 5271.89 | 6224.01 | 9478.31 | 6024.43 | 11270.65 | 7587.6 | 6862.72 | 6277.6 | 992.21 | 0.933656145 | 0.455308345 | 1.199513507 | 1.411522003 | 1.666447339 | 2.537769778 | 1.613010799 | 3.017659789 | 2.031541696 | 1.837458725 | 1.680795792 | 0.265659232 | ||
| Figure4B | ND | HFD | HM | Con | PA | PM | ||||||||||||||||||||
| pten | 11085.41 | 10364.18 | 10229.06 | 8110.77 | 9077.77 | 9496.6 | 9632.89 | 10076.48 | 9330.23 | 11408.77 | 12360.48 | 14886.18 | 1.1144078 | 1.041903099 | 1.028319588 | 0.815369513 | 0.912581284 | 0.954685944 | 0.968387074 | 1.012980838 | 0.937960895 | 1.146914934 | 1.242589613 | 1.496496305 | ||
| p-akt | 9409.16 | 9380.23 | 8888.65 | 8322.53 | 11423.65 | 9992.94 | 11552.06 | 8156.06 | 6249.94 | 5011.53 | 3647.23 | 4491.23 | 1.045445669 | 1.042231276 | 0.987612141 | 0.924710914 | 1.269274348 | 1.110309087 | 1.283541899 | 0.90621454 | 0.694426783 | 0.55682785 | 0.405241361 | 0.499017654 | ||
| mtor | 38997.64 | 27958.93 | 21612.12 | 28143.59 | 38857.78 | 44619 | 39391.2 | 17834.88 | 12085.42 | 15185.66 | 14826.88 | 29374.23 | 1.336539394 | 0.95821725 | 0.74069738 | 0.964545976 | 1.331746068 | 1.529196414 | 1.350027606 | 0.611242622 | 0.414195319 | 0.52044772 | 0.508151499 | 1.006722857 | ||
| p-mtor | 14370.98 | 12177.71 | 12836.42 | 9701.23 | 23785.12 | 22610.59 | 34352.12 | 34527.12 | 16611.59 | 10996.76 | 10944.71 | 4619.28 | 1.171077738 | 0.992350214 | 1.046027877 | 0.790544172 | 1.938227214 | 1.842515861 | 2.799322174 | 2.81358276 | 1.353662954 | 0.896115701 | 0.891874196 | 0.376420813 | ||
| Figure5A | ND | HFD | HM | ND | HFD | HM | ||||||||||||||||||||
| HESI | 31056.59 | 30746.59 | 34112.59 | 32013.83 | 31058.05 | 40329 | 38124.59 | 36038.37 | 25598.83 | 21309.42 | 15901.52 | 11745.69 | 0.971052516 | 0.961359685 | 1.06660507 | 1.000982728 | 0.971098166 | 1.260974786 | 1.192049064 | 1.126818813 | 0.80040366 | 0.666285832 | 0.497195958 | 0.367254803 | ||
| Figure5B | ND | HFD | HM | Con | PA | PM | ||||||||||||||||||||
| HESI | 20517.1 | 20460.64 | 25386.95 | 23655 | 37623.78 | 44901.95 | 27601.12 | 34633.3 | 20139.88 | 19285.95 | 14207.64 | 13312.2 | 0.911671658 | 0.909162873 | 1.128062094 | 1.051103375 | 1.671802247 | 1.995205716 | 1.226448125 | 1.538921096 | 0.894909991 | 0.856965848 | 0.63131255 | 0.591523921 | ||
Additional Table 2 (1.1MB, pdf) : The signaling pathways associated with KEGG enrichment.
The signaling pathway of KEGG enrichment
Funding Statement
Funding: This study was supported by the Medical Science and Technology Youth Cultivation Program (No. 21QNPY035), the Foundation of Naval Medical University (Nos. 2022MS003, 2022QN019), the Talents Training Program of Pudong Health Commission of Shanghai (No. PWRq2021–38), the National Natural Science Foundation of China (No. 82304921), and the Science and Technology Innovation Plan of Shanghai Science and Technology Commission (No. 23Y11920500).
Footnotes
Conflicts of interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Editor note: Xuejun Sun is an Editorial Board member of Medical Gas Research. He is blinded from reviewing or making decisions on the manuscript. The article was subject to the journal’s standard procedures, with peer review handled independently of this Editorial Board member and his research group.
Data availability statement:
All data relevant to the study are included in the article or uploaded as Additional files.
References
- 1.Tangri N, Svensson MK, Bodegård J, et al. Mortality, health care burden, and treatment of CKD: a multinational, observational study (OPTIMISE-CKD) Kidney360. 2024;5:352–362. doi: 10.34067/KID.0000000000000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389:1238–1252. doi: 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 3.Mladenović D, Vesković M, Šutulović N, et al. Adipose-derived extracellular vesicles - a novel cross-talk mechanism in insulin resistance, nonalcoholic fatty liver disease, and polycystic ovary syndrome. Endocrine. 2024;85:18–34. doi: 10.1007/s12020-024-03702-w. [DOI] [PubMed] [Google Scholar]
- 4.Ruan XZ, Varghese Z, Moorhead JF. An update on the lipid nephrotoxicity hypothesis. Nat Rev Nephrol. 2009;5:713–721. doi: 10.1038/nrneph.2009.184. [DOI] [PubMed] [Google Scholar]
- 5.Xu C, Hong Q, Zhuang K, et al. Regulation of pericyte metabolic reprogramming restricts the AKI to CKD transition. Metabolism. 2023;145:155592. doi: 10.1016/j.metabol.2023.155592. [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Ning X, Wei L, et al. Twist1 downregulation of PGC-1α decreases fatty acid oxidation in tubular epithelial cells, leading to kidney fibrosis. Theranostics. 2022;12:3758–3775. doi: 10.7150/thno.71722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fedorova O, Parfenyev S, Daks A, Shuvalov O, Barlev NA. The role of PTEN in epithelial–mesenchymal transition. Cancers (Basel) 2022;14:3786. doi: 10.3390/cancers14153786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang R, Fu P, Ma L. Kidney fibrosis: from mechanisms to therapeutic medicines. Signal Transduct Target Ther. 2023;8:129. doi: 10.1038/s41392-023-01379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saengsin K, Sittiwangkul R, Chattipakorn SC, Chattipakorn N. Hydrogen therapy as a potential therapeutic intervention in heart disease: from the past evidence to future application. Cell Mol Life Sci. 2023;80:174. doi: 10.1007/s00018-023-04818-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nie C, Zou R, Pan S, et al. Hydrogen gas inhalation ameliorates cardiac remodeling and fibrosis by regulating NLRP3 inflammasome in myocardial infarction rats. J Cell Mol Med. 2021;25:8997–9010. doi: 10.1111/jcmm.16863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizutani A, Endo A, Saito M, et al. Hydrogen-rich water reduced oxidative stress and renal fibrosis in rats with unilateral ureteral obstruction. Pediatr Res. 2022;91:1695–1702. doi: 10.1038/s41390-021-01648-7. [DOI] [PubMed] [Google Scholar]
- 12.Ma J, Dong S, Lu H, et al. The hydrogen storage nanomaterial MgH(2) improves irradiation-induced male fertility impairment by suppressing oxidative stress. Biomater Res. 2022;26:20. doi: 10.1186/s40824-022-00266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitrofanova A, Merscher S, Fornoni A. Kidney lipid dysmetabolism and lipid droplet accumulation in chronic kidney disease. Nat Rev Nephrol. 2023;19:629–645. doi: 10.1038/s41581-023-00741-w. [DOI] [PubMed] [Google Scholar]
- 14.Saraswati S, Martínez P, Serrano R, et al. Renal fibroblasts are involved in fibrogenic changes in kidney fibrosis associated with dysfunctional telomeres. Exp Mol Med. 2024;56:2216–2230. doi: 10.1038/s12276-024-01318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reiss AB, Jacob B, Zubair A, Srivastava A, Johnson M, De Leon J. Fibrosis in chronic kidney disease: pathophysiology and therapeutic targets. J Clin Med. 2024;13:1881. doi: 10.3390/jcm13071881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su Q, Wang JJ, Ren JY, et al. Parkin deficiency promotes liver cancer metastasis by TMEFF1 transcription activation via TGF-β/Smad2/3 pathway. Acta Pharmacol Sin. 2024;45:1520–1529. doi: 10.1038/s41401-024-01254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo L, Tan K, Luo Q, Bai X. Dihydromyricetin promotes autophagy and attenuates renal interstitial fibrosis by regulating miR-155-5p/PTEN signaling in diabetic nephropathy. Bosn J Basic Med Sci. 2020;20:372–380. doi: 10.17305/bjbms.2019.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen W, Zhang Y, Miao G, et al. The augment effects of magnesium hydride on the lipid lowering effect of atorvastatin: an in vivo and in vitro investigation. Med Gas Res. 2025;15:148–155. doi: 10.4103/mgr.MEDGASRES-D-23-00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larpruenrudee P, Bennett NS, Gu Y, Fitch R, Islam MS. Design optimization of a magnesium-based metal hydride hydrogen energy storage system. Sci Rep. 2022;12:13436. doi: 10.1038/s41598-022-17120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeBaron TW, Singh RB, Fatima G, et al. The Effects of 24-week, high-concentration hydrogen-rich water on body composition, blood lipid profiles and inflammation biomarkers in men and women with metabolic syndrome: a randomized controlled trial. Diabetes Metab Syndr Obes. 2020;13:889–896. doi: 10.2147/DMSO.S240122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Todorovic N, Fernández-Landa J, Santibañez A, et al. The effects of hydrogen-rich water on blood lipid profiles in clinical populations: a systematic review and meta-analysis. Pharmaceuticals (Basel) 2023;16:142. doi: 10.3390/ph16020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnsen HM, Hiorth M, Klaveness J. Molecular hydrogen therapy-a review on clinical studies and outcomes. Molecules. 2023;28:7785. doi: 10.3390/molecules28237785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Si Y, Liu L, Zhang Y, et al. Magnesium hydride protects against acetaminophen-induced acute kidney injury by inhibiting TXNIP/NLRP3/nf-κb pathway. Ren Fail. 2024;46:2330629. doi: 10.1080/0886022X.2024.2330629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamimura N, Ichimiya H, Iuchi K, Ohta S. Molecular hydrogen stimulates the gene expression of transcriptional coactivator PGC-1α to enhance fatty acid metabolism. NPJ Aging Mech Dis. 2016;2:16008. doi: 10.1038/npjamd.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostojic SM. Hydrogen-rich water as a dietary activator of brown adipose tissue and UCP1? Ann Nutr Metab. 2022;78:242–243. doi: 10.1159/000525175. [DOI] [PubMed] [Google Scholar]
- 26.Găman MA, Dobrică EC, Cozma MA, et al. Crosstalk of magnesium and serum lipids in dyslipidemia and associated disorders: a systematic review. Nutrients. 2021;13:1411. doi: 10.3390/nu13051411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Locatelli L, Fedele G, Castiglioni S, Maier JA. Magnesium deficiency induces lipid accumulation in vascular endothelial cells via oxidative stress-the potential contribution of EDF-1 and PPARγ. Int J Mol Sci. 2021;22:1050. doi: 10.3390/ijms22031050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu X, Chen Q, Li S, Xiong T. Peroxisome proliferator-activated receptors as biomarkers in cerebrovascular diseases: A narrative review. NeuroMarkers. 2025;2:100035. [Google Scholar]
- 29.Gu YY, Liu XS, Lan HY. Therapeutic potential for renal fibrosis by targeting Smad3-dependent noncoding RNAs. Mol Ther. 2024;32:313–324. doi: 10.1016/j.ymthe.2023.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao B, Li M, Li B, et al. The action mechanism by which C1q/tumor necrosis factor-related protein-6 alleviates cerebral ischemia/reperfusion injury in diabetic mice. Neural Regen Res. 2024;19:2019–2026. doi: 10.4103/1673-5374.390951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nørregaard R, Mutsaers HAM, Frøkiær J, Kwon TH. Obstructive nephropathy and molecular pathophysiology of renal interstitial fibrosis. Physiol Rev. 2023;103:2827–2872. doi: 10.1152/physrev.00027.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xing Z, Pan W, Zhang J, et al. Hydrogen rich water attenuates renal injury and fibrosis by regulation transforming growth factor-β induced Sirt1. Biol Pharm Bull. 2017;40:610–615. doi: 10.1248/bpb.b16-00832. [DOI] [PubMed] [Google Scholar]
- 33.Si Y, Liu L, Cheng J, et al. Oral hydrogen-rich water alleviates oxalate-induced kidney injury by suppressing oxidative stress, inflammation, and fibrosis. Front Med (Lausanne) 2021;8:713536. doi: 10.3389/fmed.2021.713536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo K. Signaling cross talk between TGF-β/Smad and other signaling pathways. Cold Spring Harb Perspect Biol. 2017;9:a022137. doi: 10.1101/cshperspect.a022137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashemi M, Etemad S, Rezaei S, et al. Progress in targeting PTEN/PI3K/Akt axis in glioblastoma therapy: Revisiting molecular interactions. Biomed Pharmacother. 2023;158:114204. doi: 10.1016/j.biopha.2022.114204. [DOI] [PubMed] [Google Scholar]
- 36.Fan B, Lu F, Du WJ, et al. PTEN inhibitor bisperoxovanadium protects against noise-induced hearing loss. Neural Regen Res. 2023;18:1601–1606. doi: 10.4103/1673-5374.358606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Hu Q, Li C, et al. PTEN-induced partial epithelial–mesenchymal transition drives diabetic kidney disease. J Clin Invest. 2019;129:1129–1151. doi: 10.1172/JCI121987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu H, Chen W, Liu W, et al. Molecular hydrogen regulates PTEN-AKT-mTOR signaling via ROS to alleviate peritoneal dialysis-related peritoneal fibrosis. FASEB J. 2020;34:4134–4146. doi: 10.1096/fj.201901981R. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The signaling pathway of KEGG enrichment
Data Availability Statement
All data relevant to the study are included in the article or uploaded as Additional files.


