Abstract
Background
Transthyretin (TTR) Cardiomyopathy (ATTR-CM) is characterized by the deposition of misfolded TTR monomers in the heart, leading to progressive heart failure. TTR-specific therapies offer a pharmacological approach to slow disease progression. However, there remains limited data on the efficacy, comparative effectiveness, and safety of these therapies. Therefore, we aim to perform a systematic review and meta-analysis of randomized controlled trials (RCTs) comparing TTR-specific therapies with placebo in patients with ATTR-CM.
Methods
We searched through Pubmed, Cochrane, and Embase databases. Our primary outcome was: (1) All Cause Mortality. We also performed a subgroup analysis comparing TTR stabilizers versus TTR knock-down therapies (RNA inhibitors and antisense oligonucleotides).
Results
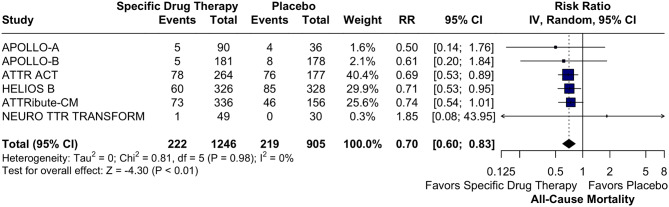
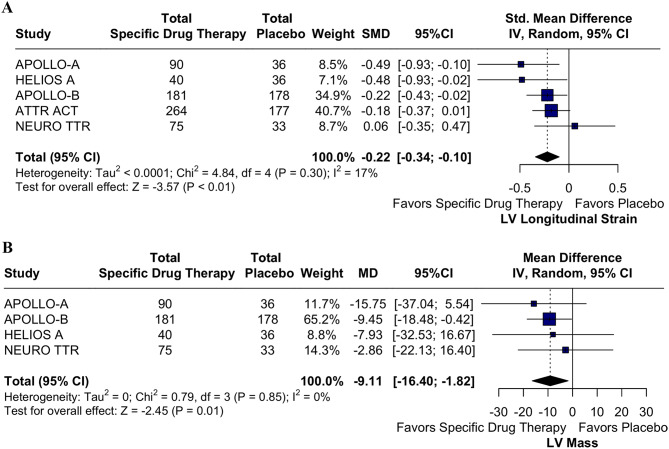
Nine RCTs were included, involving 2,713 patients, of whom 1,160 (59.34%) were assigned to the TTR-specific therapies group. In the pooled analysis, TTR-specific therapies were associated with a significant reduction in all-cause mortality (RR 0.70; 95% CI 0.60, 0.83; p < 0.01; I² = 0%), with both TTR stabilizers and knock-down therapies showing equally effective reductions (p = 0.97). Additionally, TTR-specific therapies improved LV longitudinal strain (SMD − 0.22; 95% CI -0.34, -0.10; p < 0.01; I² = 17%) and reduced LV mass (SMD − 9.11 g; 95% CI -16.4 g, -1.82 g; p = 0.01; I² = 0%).
Conclusion
This meta-analysis highlights the potential of TTR-targeting therapies as an effective option for managing ATTR-CM, with significant improvements in survival. No efficacy differences were found between TTR stabilizers and knock-down therapies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-025-04653-4.
Keywords: Transthyretin cardiac amyloidosis, Transthyretin gene RNA inhibitors, Transthyretin stabilizers, Transthyretin gene antisense oligonucleotides
Introduction
Transthyretin (TTR) amyloidosis (ATTR) is a disease characterized by the deposition of misfolded TTR monomers in vital organs. This condition arises either from destabilizing mutations in hereditary ATTR (hATTR) or from an age-associated mechanism in wild-type ATTR (wtATTR), frequently involving the heart and presenting as TTR amyloid cardiomyopathy (ATTR-CM) [1].
Recent research indicates that as many as 10–15% of older adults with heart failure (HF) with preserved ejection fraction may have undiagnosed wtATTR. The natural history of the disease, encompassing factors such as age of onset, primary phenotype, and clinical course, varies according to the specific mutation and familial traits [2]. Untreated patients with ATTR-CM typically experience progressive HF, with an approximate survival time of 3 to 5 years [3].
In recent years, novel TTR-targeting therapies have been developed. These therapies either reduce the production of TTR (RNA inhibitors and antisense oligonucleotides) or stabilize the circulating TTR molecule (TTR stabilizers). The TTR tetramer stabilizer tafamidis is currently the only approved agent for treating ATTR-CM [4]. Recent trials have demonstrated the efficacy of emerging therapies, including Vutrisiran, Patisiran, Eplontersen, Inotersen, and Acoramidis, in managing ATTR [5–12]. Therefore, we propose to conduct a systematic review and meta-analysis to compare the efficacy of ATTR-specific therapies with placebo and evaluate the comparative effectiveness of different therapeutic classes in patients with ATTR-CM.
Methods
This meta-analysis and systematic review was performed and reported according to the Cochrane Collaboration Handbook for Systematic Reviews of Intervention and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [13, 14]. The prospective meta-analysis protocol has been uploaded to the International Prospective Register of Systematic Reviews (CRD42024592297).
Eligibility criteria
There were no restrictions regarding publication date, status, or language. Studies were considered eligible for inclusion if they (1) were randomized controlled trials (RCTs); and (2) compared the following therapies for ATTR with placebo: Tafamidis, Acoramidis, Patisiran, Vutrisiran, Inotersen, and Eplontersen; 3) enrolled patients with ATTR-CM; and 4) presented data regarding any of the prespecified efficacy and safety endpoints. We excluded studies that (1) did not report any of the outcomes of interest, (2) had overlapping patient populations, and (3) abstracts presented in congresses.
Search strategy and data extraction
We systematically searched Medline via Pubmed, EMBASE, and Cochrane from the database inception to September 2024. The study selection process included reviewing titles and abstracts initially, followed by a thorough examination of the full texts of potentially suitable studies. The full search strategy is reported in the Supplemental Methods S3. Eight authors (J.F.; G.B.; R.P.; P.S.; C.F.; W.N.; V.A; A.C.), in pairs, independently and following a double-blinded model, extracted selected studies, reviewed the main reports and supplementary materials and extracted the relevant information from the included trials. Any discrepancies were resolved through consensus among the authors or addressed through deliberation with other review team members (A.P.; E.K.).
For the extraction of continuous outcomes, if the outcome was reported in the median or interquartile range (IQR) we used the Wan and Luo Eqs. [15, 16] to transform it into mean and standard deviation (SD) per Cochrane recommendation (Supplemental Methods S4). Moreover, if the data was reported in mean and 95% Confidence Interval (CI), we transformed it into mean and SD using the Cochrane Calculator [17].
Endpoints and subgroup analysis
This meta-analysis’s primary endpoints were (1) all-cause mortality and (2) cardiovascular (CV) mortality. We also included the secondary outcomes of (3) hospitalizations for heart failure (HF), (4) all-cause hospitalizations, (5) any adverse events (AE), (6) cardiac AE, (7) serious adverse events (SAE), (8) cardiac SAE, (9) left ventricular (LV) mass, and (10) LV longitudinal strain. A prespecified subgroup analysis for all-cause mortality was performed, comparing (1) TTR stabilizers versus TTR knock-down therapies (RNA inhibitors and antisense oligonucleotides). Additionally, we analyzed the primary outcomes, including only studies specifically designed for an ATTR-CM population. A full definition of each outcome can be found in Supplemental Methods S5.
Quality assessment
Eight review authors (J.F.; G.B.; R.P.; P.S.; C.F.; W.N.; V.A; A.C.) in pairs, independently assessed the risk of bias for each trial using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions [14], through Cochrane’s Risk of Bias 2 tool for randomized studies [18]. We resolved disagreements by discussion or by a third review author (E.K.; A.P.). We assessed the risk of bias according to the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other biases. We graded each trial as having a high, low, or unclear risk of bias for each domain. We also performed funnel plot analysis to appraise small study effects [19]. Finally, The GRADE [20] (Grading of Recommendations, Assessment, Development, and Evaluation) tool was used to assess the certainty of evidence for each outcome, with categorizations ranging from high to very low.
Statistical analysis
Endpoints were analyzed using a risk ratio (RR) for binary data and mean difference (MD) or standard mean difference (SMD) for continuous outcomes with 95% CI. If the outcome was reported in rates, we analyzed and reported our results in rate of mean (ROM) with 95% CI. Heterogeneity was examined with the Cochran Q test and I2 statistics; p-values inferior to 0.10, and I2 > 25% were considered significant for heterogeneity. The p-value was derived from the χ2 test based on the degree of freedom of the analysis. A significant interaction was confirmed if the p < 0.05 for our prespecified subgroup analysis. We analyzed the results using the random-effect model and the restricted maximum liked method (REML). R version 4.4.2 (R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analyses using the “meta” package [21].
Trial sequential analysis
We used the TSA 0.9.5.10 Beta software for trial sequential analysis (TSA) to confirm our meta-analysis results. The type of boundary value for the hypothesis test was set to a two-sided test with an alpha value of 5%. Once the cumulative studies in the Z-curve cross the conventional monitoring boundary or the futility area, the results are consistent and should be considered reliable evidence [22].
Post-hoc network meta-analysis
A post-hoc frequentist network meta-analysis was performed to estimate the head-to-head indirect effect size for the different specific therapies for ATTR-CM. We used the calculated pairwise comparison from the primary analysis to generate the indirect evidence. Consistency was tested using node splitting, and we used P-Scores, ranging from 0(worst) to 1(best), to rank each drug. The complete statistical methodology for the frequentist network meta-analysis can be found in Supplemental Methods S7.
Protocol deviations
We reported protocol deviations from CRD42024592297 in Supplemental MethodsS8.
Results
Study selection and baseline characteristics
The study selection is demonstrated in Fig. 1. The initial search identified 632 studies (PubMed [n = 81], Embase [n = 302], and Cochrane [n = 249]). After title and abstract screening and removing duplicates, 54 studies remained to be thoroughly reviewed according to the inclusion and exclusion criteria. Of these, 9 RCTs were included [4–12], comprising 2713 patients, of whom 1160 (59.34%) were treated with TTR-targeting therapies. A full description of the studies’ eligibility criteria can be found in Supplemental Methods S6. Study characteristics and drop-out rates are reported in Table 1 and Supplemental Tables S1 A and S1B. The included participants had a mean age of 68.34 years, were mostly male (86.86%), and 15.15% had a NYHA Class ≥ III. The mean follow-up was 26.26 months. 6 [5–10] studies included TTR knock-down therapies, while the remaining 3 [4, 11, 12] used TTR stabilizers. The distribution of countries included in the RCTs is detailed in Supplemental Table S2.
Fig. 1.
PRISMA Flow Diagram. PRISMA flow diagram of study screening and selection
Table 1.
Baseline characteristics of included studies
| Study | Follow-up, months | Intervention/Control | Number of patients | Age, mean | Female, n (%) | NYHA functional class, n (%) | ATTR amyloidosis stage, n (%) | NT-proBNP (pg/ml), mean | Amyloidosis type, n (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class I | Class II | Class > III | Stage 1 | Stage 2 | Stage 3 | ATTRm | ATTRwt | |||||||
| APOLLO-A 2018 [11] | 18 | Patisiran | 90 | 60 | 22 (24) | 34 (37.8) | 56 (62.2) | 0 | N/A | N/A | N/A | 756.4 | 90 (100) | 0 |
| Placebo | 36 | 62 | 6 (16,6) | 16 (44.4) | 20 (55.6) | 0 | N/A | N/A | N/A | 845.7 | 36 (100) | 0 | ||
| APOLLO-B 2023 [12] | 12 | Patisiran | 181 | 76* | 20 (11.05) | 10 (6) | 156 (86) | 15 (8) | 124 (69) | 46 (25) | 11 (6) | 2022 | 37 (20) | 144 (80) |
| Placebo | 178 | 76* | 18 (10.11) | 15 (8) | 150 (84) | 13 (7) | 120 (67) | 45 (25) | 13 (7) | 1955.2 | 28 (19) | 144 (80) | ||
| ATTR-ACT 2018 [17] | 30 | Tafamidis (20-80 mg) | 264 | 74.5 | 23 (8.7) | 24 (9.1) | 162 (61.4) | 78 (29.5) | N/A | N/A | N/A | 2995.9 | 63 (23.9) | 201 (76.1) |
| Placebo | 177 | 74.1 | 20 (11.3) | 13 (7.3) | 101 (57.1) | 63 (35.6) | N/A | N/A | N/A | 3161 | 43 (24.3) | 134 (75.7) | ||
| ATTRibute-CM 2024 [18] | 30 | Acoramidis | 421 | 77.4 | 37 (8.8) | 51 (12.1) | 293 (69.6) | 77 (18.3) | N/A | N/A | N/A | 2946 | 41 (9.7) | 380 (90.3) |
| Placebo | 211 | 77.1 | 25 (11.8) | 17 (8.1) | 162 (76.8) | 32 (15.2) | N/A | N/A | N/A | 2725 | 20 (9.5) | 191 (90.5) | ||
| HELIOS A 2022‡ [13] | 72 | Vutrisiran | 40 | 63.5 | 8 (20.0) | 4 (10) | 20 (50) | 0 | N/A | N/A | N/A | 824.8 | 40 (100) | 0 |
| Placebo | 36 | 62 | 6 (16.67) | 16 (44.4) | 20 (55.6) | 0 | N/A | N/A | N/A | 845.7 | 36 (100) | 0 | ||
| JUDGE 2019 [19] | 1 |
Acoramidis 400 mg/ 800 mg |
16/16 | 72.1 / 76.2 | 2 (12)/ 2 (12) | 0/0 | 10 (62) / 13 (81) | 6 (38) / 3 (19) | N/A | N/A | N/A | 3280.3 / 2630.4 | 6 (38) / 5 (31) | N/A |
| Placebo | 17 | 72.7 | 0 | 0 | 12 (71) | 5 (29) | N/A | N/A | N/A | 2611.3 | 3 (18) | N/A | ||
| NEURO TTR 2019 [15] | 15 | Inotersen | 112 | 59 | 35 (31) | N/A | N/A | N/A | 74 (66) | 38 (34) | 0 | N/A | 112 (100) | 0 |
| Placebo | 60 | 59.5 | 19 (32) | N/A | N/A | N/A | 42 (70) | 18 (30) | 0 | N/A | 60 (100) | 0 | ||
| NEURO TTR TRANSFORM 2023‡ [16] | 16.5 | Eplontersen | 144 | 53 | 44(31) | N/A | N/A | N/A | 115 (79.8) | 29 (20.1) | 0 | N/A | 144 (100) | 0 |
| Placebo | 60 | 59.5 | 19(32) | N/A | N/A | N/A | 42 (70) | 18 (30) | 0 | N/A | 60 (100) | 0 | ||
| HELIOS B 2024 [14] | 42 | Vutrisiran | 326 | 77* | 27 (8) | 49 (150 | 250 (77) | 27 (8) | 208 (64) | 100 (31) | 8 (6) | 2021 | 37 (11) | 289 (89) |
| Placebo | 328 | 76* | 22 (7) | 35 (11) | 258 (79) | 35 (11) | 229 (70) | 87 (27) | 12 (4) | 1801 | 39 (12) | 289 (88) | ||
Binary outcomes are reported in frequency and percentage. Continuous outcome reported in mean or median. 16 patients did not have heart failure; ‡ Control group was a historical placebo; * Reported in median; Abbreviations: ATTRm: hereditary type transthyretin amyloidosis; ATTRwt: wild type transthyretin amyloidosis; HF: heart failure; N/A: not available; NT-proBNP: N-terminal Pro B-type Natriuretic Peptide; NYHA: New York Heart Association
Risk of Bias assessment and small study effect
Six included trials [4, 5, 8, 9, 11, 12] were evaluated as having a low risk of bias in all domains. Two trials were assessed as having some concerns [6, 10], and one was evaluated as having a high risk of bias [7]. The individual RCT appraisal is reported in Supplemental Fig. S1A and S1B. Moreover, our funnel plot (Supplemental Fig. S2) shows a symmetrical distribution of studies with convergence toward the pooled treatment effect size as weights increased, suggesting no evidence of a small study effect. Our GRADE assessments are presented in Supplemental Table S3. The certainty of evidence was high for all-cause mortality, SAE, cardiac AE, and LV longitudinal strain. For other outcomes, the certainty ranged from moderate to low.
Pooled analysis
In those receiving TTR-specific therapies, there was a significant reduction in all-cause mortality (RR 0.70; 95% CI 0.60, 0.83; p < 0.01; I² = 0%; Fig. 2). The LV longitudinal strain endpoint demonstrated a statistically significant reduction in the TTR-specific therapy group (SMD − 0.22; 95% CI -0.34, -0.10; p < 0.01; I² = 17%; Fig. 3A). TTR-specific therapies consistently reduced LV mass compared to placebo (MD -9.11 g; 95% CI -16.4 g, -1.82 g; p = 0.01; I² = 0%; Fig. 3B).
Fig. 2.
Forest Plot for All Cause Mortality. TTR-specific therapies showed a significant reduction in all-cause mortality compared to placebo. Abbreviations: CI: Confidence Interval; RR: Risk Ratio; TTR: Transthyretin
Fig. 3.
A. Forest Plot for LV Longitudinal Strain. TTR-specific therapies significantly reduced LV longitudinal strain compared to placebo. Abbreviations: CI: Confidence Interval; SMD: Standardized Mean Difference; LV: Left Ventricle; TTR: Transthyretin. B. Forest Plots for LV Mass. TTR-specific therapies led to a consistent reduction in LV mass compared to placebo Abbreviations: CI: Confidence Interval; SMD: Standardized Mean Difference; LV: Left Ventricle; TTR: Transthyretin
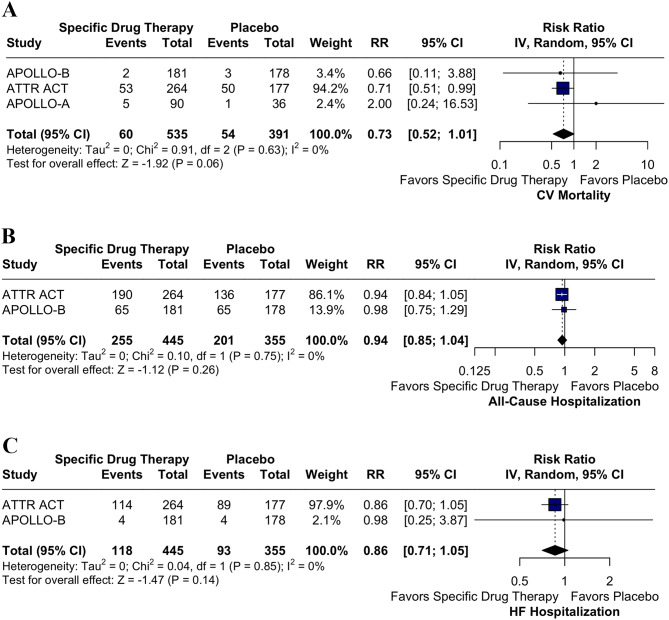
However, for the endpoints of CV mortality (RR 0.73; 95% CI 0.52, 1.01; p = 0.06; I² = 0%; Fig. 4A), all-cause hospitalization (RR 0.94; 95% CI 0.85, 1.04; p = 0.26; I² = 0%; Fig. 4B) and HF hospitalization (RR 0.86; 95% CI 0.71, 1.05; p = 0.14; I² = 0%; Fig. 4C) did not show statistically significant differences between the groups. The impact of treatment with TTR-specific therapies on N-terminal pro-B-type natriuretic peptide (NTpro-BNP), Kansas City Cardiomyopathy Questionnaire Overall Summary Score (KCCQ-OS), TTR levels, and the 6-minute walk test (6MWT) was evaluated, with baseline and post-intervention values provided in Supplemental Tables S4 to S7.
Fig. 4.
A. Forest Plot for CV Mortality. TTR-specific therapies did not show a statistically significant reduction in CV mortality compared to placebo. Abbreviations: CI: Confidence Interval; RR: Risk Ratio; CV: Cardiovascular; TTR: Transthyretin. B. Forest Plot for All Cause Hospitalization. There was no statistically significant difference in all-cause hospitalization between patients receiving TTR-specific therapies and placebo. Abbreviations: CI: Confidence Interval; RR: Risk Ratio; TTR: Transthyretin. C. Forest Plot for HF Hospitalization. TTR-specific therapies did not lead to a statistically significant difference in HF hospitalization compared to placebo. Abbreviations: CI: Confidence Interval; RR: Risk Ratio; HF: Heart Failure; TTR: Transthyretin
Subgroup analysis
Our subgroup analysis shows that both TTR Stabilizers (RR: 0.71; 95% CI 0.58, 0.86; p < 0.01; I² = 0%; Fig. 5) and knock-down therapies (RR: 0.70; 95%CI 0.53, 0.92; p = 0.01; I² = 0%; Fig. 5) therapies significantly reduced all-cause mortality. There was no evidence of difference in efficacy between these subgroups (p = 0.93; Fig. 5).
Fig. 5.
Forest Plot for Subgroup analysis of All Cause Mortality. TTR knock-down therapies and TTR stabilizers showed an equally effective reduction in all-cause mortality compared to placebo. Abbreviations: CI: Confidence Interval; RR: Risk Ratio; TTR: Transthyretin
Pooled analysis of trials specifically designed for ATTR-CM
In those receiving TTR-specific therapies, there was a significant reduction in all-cause mortality (RR 0.71; 95% CI 0.60, 0.83; p < 0.001; I² = 0%; Supplemental Fig. S3) and CV mortality (RR 0.71; 95% CI 0.51, 0.99; p = 0.041; I² = 0%; Supplemental Fig. S4).
Our subgroup analysis shows that both TTR Stabilizers (RR: 0.71; 95% CI 0.58, 0.86; p < 0.001; I² = 0%; Supplemental Fig. S5) and knock-down therapies (RR: 0.70; 95% CI 0.53, 0.93; p = 0.015; I² = 0%; Supplemental Fig. S5) therapies significantly reduced all-cause mortality. There was no evidence of a difference in efficacy between these subgroups (p = 0.98; Supplemental Fig. S5).
Adverse events
There were no significant increases in SAE (RR 0.91; 95% CI 0.86, 0.97; p < 0.01; I² = 0%; Supplemental Fig. S6), cardiac AE (RR 0.90; 95% CI 0.81, 0.99; p = 0.037; I² = 39%; Supplemental Fig. S7), or cardiac SAE (RR 0.98; 95% CI 0.82, 1.18; p = 0.861; I² = 0%; Supplemental Fig. S8) between the TTR-specific therapies group and the placebo group. Similarly, there was no significant difference in overall AE (RR 1.0; 95% CI 0.98, 1.01; p = 0.66; I² = 41%; Supplemental Fig. S9) between the two groups.
Trial sequential analysis
Our TSA results for the primary endpoint of All-cause mortality achieved the required information size, indicating a low risk of type 1 error (Supplemental Fig. S10).
Post-hoc network meta-analysis for the primary endpoints
In our post-hoc network meta-analysis, we observed no differences between the specific therapies for ATTR-CM in our indirect estimations for all-cause mortality and CV mortality endpoints. Patisiran (P-Score = 0.75; Supplemental Table S9) and Tafamidis (P-Score = 0.84; Supplemental Table S9), were ranked the best treatment for all-cause mortality and CV mortality endpoints. Detailed results for the post-hoc network meta-analysis can be found in Supplemental Results.
Discussion
In this updated systematic review and meta-analysis of 9 RCTs encompassing 2,713 patients, we compared TTR-specific therapies with placebo in the population with ATTR-CM. Our main findings were as follows: (1) TTR-specific therapies significantly reduced all-cause mortality; (2) no subgroup difference in all-cause mortality endpoint was observed between knock-down therapies and TTR stabilizers; (3) TTR-specific therapies significantly reduced LV mass and LV longitudinal strain; (4) no significant difference in CV mortality, all-cause hospitalization, or HF hospitalization between TTR-specific therapies and placebo; and (5) no significant differences in the incidence of AE between patients receiving TTR-specific therapies and in the placebo group. In our sensitivity analysis of only ATTR-CM designed trials, (6) TTR-specific therapies significantly reduced all-cause mortality; (7) no subgroup difference in all-cause mortality endpoint was observed between knock-down therapies and TTR stabilizers; (8) TTR-specific therapies slightly but significantly reduced CV mortality.
Therapeutic options for ATTR have advanced significantly. Historically, liver transplantation was considered the only method to address hATTR. It halts the production of mutant TTR but has limited efficacy due to the continued deposition of wtATTR in tissues. In contrast, recent FDA-approved therapies target the protein directly [23]. Currently, there are two major classes of TTR-targeting therapies for ATTR: TTR stabilizers, which bind to transthyretin, stabilizing the protein’s tetrameric structure and affecting rate-limiting steps in ATTR amyloidogenesis, and TTR knock-down therapies, such as RNA inhibitors and antisense oligonucleotides, target the gene encoding transthyretin, thereby reducing circulating levels of the implicated protein [24]. Approved through organ-specific trials, these therapies address polyneuropathy and cardiomyopathy, with some studies overlapping both. In our proposed systematic review and meta-analysis, we specifically evaluate TTR-targeting therapies for patients with cardiomyopathy, independent of other organ involvement. This approach enables us to compare different therapies for ATTR-CM, expand therapeutic options, and confirm the efficacy of these treatments.
The main result of our meta-analysis highlights the significant reduction in mortality rates with TTR-specific therapies in patients with ATTR-CM compared to placebo. Specifically, TTR-specific therapies reduced mortality by 30% (RR 0.70; 95% CI 0.60, 0.83; Fig. 2), underscoring their clinical efficacy in managing this condition. Our findings align with major RCTs included in the analysis, such as the ATTR-ACT trial, which evaluated tafamidis in ATTR-CM patients (RR 0.69 [0.53; 0.89]), and the HELIOS-B trial, which assessed vutrisiran in ATTR-CM patients (RR 0.71 [0.53; 0.95]). (RR 0.71 [0.53; 0.95]). Moreover, the ATTRibute-CM, APOLLO A, APOLLO B and NEURO TTR TRANSFORM RCTs did not yield statistically significant results individually. Additionally, some studies relied on historical placebo groups, which may introduce variability and affect comparability, such as HELIOS A, which referenced the placebo group from the APOLLO A trial, and NEURO TTR TRANSFORM, which used the placebo group from the NEURO TTR study. Although the historical placebo groups had similar endpoints and eligibility criteria, this may introduce bias in interpreting the results. However, our meta-analysis revealed 0% heterogeneity. These consistent results, confirmed by subgroup analysis (Fig. 5), trials explicitly designed for ATTR-CM (Supplemental Figs. S3–S5) populations, and funnel plot analysis (Supplemental Fig. S2), further validate the efficacy of both TTR stabilizers and knock-down therapies in reducing mortality among patients with ATTR-CM. Moreover, our findings align with observational phase 4 studies, such as Garcia-Pavia et al. [25], which reported survival rates at 30 and 42 months of 84.4% and 76.8%, respectively, in tafamidis-treated patients, compared to 70.0% and 59.3% in untreated patients.
In cardiac imaging, the term strain describes myocardial shortening and thickening, the fundamental features of myocardial fiber function [26, 27]. Our meta-analysis also demonstrated a significant improvement in LV longitudinal strain in patients on TTR therapy. In ATTR-CM, peak longitudinal strain from the apical 4-chamber view is independently associated with mortality, regardless of genotype or disease severity [26, 28]. Both mortality and LV longitudinal strain were improved in the TTR therapy group compared to placebo. In HELIOS-B, vutrisiran attenuated the decline in peak longitudinal strain compared to placebo (LS mean difference − 1.23; 95% CI: -1.73 to -0.73), aligning with the observed reduction in mortality. This suggests that TTR therapies may help to halt the progression of myocardial dysfunction.
Our analysis shows a significant reduction in LV mass with TTR-specific therapies compared to placebo (SMD − 9.11; 95% CI: -16.40 to -1.82]; I² = 0%), highlighting consistency across trials. This finding is clinically meaningful, as concentric LV hypertrophy with increased LV mass index is characteristic of ATTR-CM [29]. Like Dobner et al. [30], cohort studies observed similar LV mass reduction with tafamidis. However, the mechanisms—whether due to decreased amyloid deposition, clearance, or reverse remodeling—are yet to be fully understood. TTR-specific therapies also trended toward reducing CV mortality, with the strongest support from the ATTR-ACT study. Though reductions in all-cause and HF hospitalizations were observed, these outcomes lacked statistical significance. Given that fewer studies have been reported on these endpoints, further research with larger samples and longer follow-ups is essential to confirm these potential benefits and clarify the impact of TTR-targeting therapies on reducing these endpoints.
TTR-specific therapies consistently improved KCCQ-OS scores and 6MWT performance compared to placebo (Supplemental Tables S5 and S7) and showed reduced NT-proBNP levels (Supplemental Table S4), indicating slower ATTR-CM progression and enhanced functional capacity [31]. Safety analyses found no evidence of increased risk for overall AE, serious AE, cardiac AEs, or cardiac SAE associated with TTR-specific therapies, supporting a favorable safety profile. Continued monitoring of broader populations will be essential, as long-term effects remain to be fully understood.
An important aspect of our meta-analysis is that it addresses a contemporary cohort of patients with ATTR-CM, most of whom receive multidisciplinary care. It shows that both knock-down therapies and TTR stabilizers offer similar mortality reduction benefits, with consistent effect sizes across patient subgroups. Furthermore, our findings align with long-term extension studies, highlighting the sustained impact of these therapies with longer follow-ups, and emphasizing the benefits of early diagnosis and early initiation of treatment [32, 33]. However, the advantage of combining these two classes of medication or identifying optimal therapeutic strategies for severe cases, such as advanced HF, remains unclear and requires further investigation. Another significant issue is the lack of cost-effectiveness of available medications. The annual acquisition cost of disease-modifying therapies ranges from over $100,000 to nearly $600,000, several times higher than even liberal cost-effectiveness thresholds [34, 35].
Our meta-analysis has several important strengths, addressing key gaps in the existing literature. First, our TSA confirmed that the required information size was met, providing sufficient power to support the benefits of TTR-specific therapies in reducing mortality in patients with ATTR-CM. Second, our subgroup analysis also helps close a key gap in the literature by showing comparable efficacy between TTR knock-down therapies and TTR stabilizers, supporting the potential expansion of therapeutic options beyond tafamidis, currently the only FDA-approved ATTR-CM therapy. The specific treatment of cardiac amyloidosis has significant practical implications, as it represents a novel approach that effectively reduces amyloid deposition, improves cardiac function, modifies the natural course of the disease, and enhances patient outcomes. It is also important to acknowledge that more recent studies may benefit from earlier patient diagnoses. This leads to the inclusion of individuals with less severe disease compared to earlier cohorts. Current research indicates a shift towards a greater prevalence of wild-type forms, a reduced proportion of hereditary forms, lower NT-proBNP levels, and improved functional capacity in patients. These trends suggest that early diagnosis and treatment are increasingly influencing the current clinical landscape of the disease. These are important confounders that should be addressed in further studies.
This study has some limitations that warrant consideration. First, cross-trial comparisons are challenging due to differences in the enrolled cohorts. Additionally, some studies used historical placebo groups, which may introduce bias when interpreting the results. However, our sensitivity analyses validated our findings in most instances, thus confirming the results of the overall pooled analysis (Supplemental Figs. S2–S5). Second, we did not assess the effects in patients using a combination of two or more TTR-specific therapies. Third, a direct comparison between stabilizers and knock-down therapies was not possible. None of the included studies directly compared these two treatment classes, precluding the identification of subgroups that might benefit more from one over the other. Finally, due to study heterogeneity and the limited number of studies evaluating these endpoints, we could not assess the impact of TTR-specific therapies on NT-proBNP levels, quality of life, or the 6MWT. Future well-designed studies are needed to directly compare the efficacy of TTR targeting therapies, both as monotherapies and in combination, and to evaluate potential subpopulations that may benefit from one therapy over another.
Conclusion
Overall, this updated systematic review and meta-analysis provide strong evidence supporting the use of TTR-specific therapies to manage ATTR-CM. These therapies significantly improve all-cause mortality and have demonstrated a favorable safety profile in patients with ATTR-CM. No significant differences were found regarding efficacy between TTR stabilizers and TTR knock-down therapies. As the treatment landscape for ATTR-CM continues to evolve, TTR-specific therapies offer a novel and potentially disease-modifying option for patients with this challenging condition.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
N/A.
Abbreviations
- 6MWT
6-minute walk test
- AE
Adverse events
- ATTR
Transthyretin amyloidosis
- ATTR-CM
Transthyretin amyloid cardiomyopathy
- CI
Confidence interval
- CV
Cardiovascular
- FDA
US Food and Drug Administration
- HF
Heart failure
- hATTR
Hereditary transthyretin amyloidosis
- IQR
Interquartile range
- KCCQ-OS
Kansas City Cardiomyopathy Questionnaire Overall Summary Score
- LS
Least square
- LV
Left ventricular
- MD
Mean difference
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- RCT
Randomized controlled trial
- REML
Restricted maximum liked method
- ROM
Rate of mean
- RR
Risk ratio
- SAE
Serious adverse events
- SD
Standard deviation
- SMD
Standard mean difference
- TSA
Trial sequential analysis
- TTR
Transthyretin
- wtATTR
Wild-type transthyretin amyloidosis
Author contributions
Conceptualization and study design: A.P., L.P. Data collection and extraction: A.P., A.C., C.F., P.S., J.F., W.N., G.B., R.P., V.L. Statistical analysis: E.K., V.L. Results interpretation: L.P., F.F. Initial manuscript drafting: A.P., J.F., A.C., C.F., W.N., E.K., P.S. Critical manuscript revision: F.F., L.P. Figures and tables preparation: A.C., E.K., R.P., G.B. Supplementary Appendix organization: E.K., W.N., R.P., A.C., P.S. Overall project supervision: A.P., E.K.
Funding
Not applicable. This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Declarations
Ethics approval and consent to participate
Not applicable. This study did not involve human participants, animal subjects, or any data requiring ethical approval.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Alonzo Armani Prata, Pedro Gabriel Scardini, Ana Carolina Covre, Wilson Falco Neto, Julia Marques Fernandes, Gabriel Scarpioni Barbosa, Chris Fukunaga, Rafael Petri Pinheiro, Vanio L. J. Antunes are medical students.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/19/2025
The wrong Supplementary file was originally published with this article; it has now been replaced with the correct file.
References
- 1.Singh V, et al. State-of-the-art radionuclide imaging in cardiac transthyretin amyloidosis. J Nuclear Cardiology: Official Publication Am Soc Nuclear Cardiol Vol. 2019;26(1):158–73. 10.1007/s12350-018-01552-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruberg FL, et al. Transthyretin amyloid cardiomyopathy: JACC State-of-the-Art review. J Am Coll Cardiol Vol. 2019;73:2872–91. 10.1016/j.jacc.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonopoulos AS, et al. Prevalence and clinical outcomes of transthyretin amyloidosis: a systematic review and meta-analysis. Eur J Heart Fail Vol. 2022;24(9):1677–96. 10.1002/ejhf.2589. [DOI] [PubMed] [Google Scholar]
- 4.Maurer, Mathew S, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. New Engl J Med Vol. 2018;379(11):1007–16. 10.1056/NEJMoa1805689. [DOI] [PubMed] [Google Scholar]
- 5.Solomon SD, et al. Effects of Patisiran, an RNA interference therapeutic, on cardiac parameters in patients with hereditary Transthyretin-Mediated amyloidosis. Circulation Vol. 2019;139(4):431–43. 10.1161/CIRCULATIONAHA.118.035831. [DOI] [PubMed] [Google Scholar]
- 6.Maurer, Mathew S, et al. Patisiran treatment in patients with transthyretin cardiac amyloidosis. New Engl J Med Vol. 2023;389:1553–65. 10.1056/NEJMoa2300757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams D, et al. Efficacy and safety of Vutrisiran for patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy: a randomized clinical trial. Amyloid: Int J Experimental Clin Invest: Official J Int Soc Amyloidosis Vol. 2023;30(1):1–9. 10.1080/13506129.2022.2091985. [DOI] [PubMed] [Google Scholar]
- 8.Fontana M, et al. Vutrisiran in patients with transthyretin amyloidosis with cardiomyopathy. The new England journal of medicine, 10.1056/NEJMoa2409134. 30 Aug. 2024. 10.1056/NEJMoa2409134. [Google Scholar]
- 9.Benson MD, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. New Engl J Med Vol. 2018;379(1):22–31. 10.1056/NEJMoa1716793. [DOI] [PubMed] [Google Scholar]
- 10.Coelho T et al. Eplontersen for hereditary transthyretin amyloidosis with polyneuropathy. JAMA 330,15 (2023): 1448–58. 10.1001/jama.2023.18688 [DOI] [PMC free article] [PubMed]
- 11.Gillmore JD, et al. Efficacy and safety of acoramidis in transthyretin amyloid cardiomyopathy. New Engl J Med Vol. 2024;390(2):132–42. 10.1056/NEJMoa2305434. [DOI] [PubMed] [Google Scholar]
- 12.Judge DP, et al. Transthyretin stabilization by AG10 in symptomatic transthyretin amyloid cardiomyopathy. J Am Coll Cardiol Vol. 2019;74(3):285–95. 10.1016/j.jacc.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 29 Mar. 2021;372. 10.1136/bmj.n71. (Clinical research ed.). [DOI] [PMC free article] [PubMed]
- 14.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.5 (updated August 2024). Cochrane, 2024. Available from www.training.cochrane.org/handbook
- 15.Luo D, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res Vol. 2018;27(6):1785–805. 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 16.Wan X et al. Dec. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC medical research methodology vol. 14 135. 19 2014, 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed]
- 17.https://training.cochrane.org/resource/revman-calculator
- 18.Sterne JAC et al. Aug. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed.) vol. 366 l4898. 28 2019, 10.1136/bmj.l4898 [DOI] [PubMed]
- 19.Sterne JAC et al. Jul. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ (Clinical research ed.) vol. 343 d4002. 22 2011, 10.1136/bmj.d4002 [DOI] [PubMed]
- 20.Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from guidelinedevelopment.org/handbook. When referring to a specific chapter or subsection refer to it by the title and section number, not page numbers.
- 21.Balduzzi S, et al. How to perform a meta-analysis with R: a practical tutorial. Evidence-based Mental Health Vol. 2019;22(4):153–60. 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trial Sequential Analysis (TSA). [Computer program]. Version 0.9.5.10 beta. the Copenhagen trial unit, centre for clinical intervention research, the capital region. Copenhagen University Hospital– Rigshospitalet; 2021.
- 23.Brailovsky Y et al. Mar. TTR Amyloidosis: Current State of Affairs and Promise for the Future. JACC. Case reports vol. 10 101759. 15 2023, 10.1016/j.jaccas.2023.101759 [DOI] [PMC free article] [PubMed]
- 24.Tsoi MR, et al. Emerging therapies for transthyretin amyloidosis. Curr Oncol Rep Vol. 2023;25(6):549–58. 10.1007/s11912-023-01397-2. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Pavia P et al. Jun. Survival in a Real-World Cohort of Patients With Transthyretin Amyloid Cardiomyopathy Treated With Tafamidis: An Analysis From the Transthyretin Amyloidosis Outcomes Survey (THAOS). Journal of cardiac failure, S1071-9164(24)00222-7. 21 2024, 10.1016/j.cardfail.2024.06.003 [DOI] [PubMed]
- 26.Smiseth OA, et al. Myocardial Strain Imaging: Theory, Current Practice, and the Future. JACC. Cardiovascular imaging, S1936-878X(24)00301-2. 26 Aug. 2024. 10.1016/j.jcmg.2024.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Huntjens, Peter R, et al. Prognostic utility of echocardiographic atrial and ventricular strain imaging in patients with cardiac amyloidosis. JACC. Cardiovasc Imaging Vol. 2021;14:1508–19. 10.1016/j.jcmg.2021.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Chacko L, et al. Echocardiographic phenotype and prognosis in transthyretin cardiac amyloidosis. Eur Heart J Vol. 2020;41:1439–47. 10.1093/eurheartj/ehz905. [DOI] [PubMed] [Google Scholar]
- 29.Nakano T et al. May. Transthyretin Amyloid Cardiomyopathy: Impact of Transthyretin Amyloid Deposition in Myocardium on Cardiac Morphology and Function. Journal of personalized medicine vol. 12,5 792. 13 2022, 10.3390/jpm12050792 [DOI] [PMC free article] [PubMed]
- 30.Dobner S, et al. Impact of Tafamidis on myocardial function and CMR tissue characteristics in transthyretin amyloid cardiomyopathy. ESC Heart Fail Vol. 2024;11:2759–68. 10.1002/ehf2.14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ioannou A, et al. Prognostic value of a 6-Minute walk test in patients with transthyretin cardiac amyloidosis. J Am Coll Cardiol Vol. 2024;84(1):43–58. 10.1016/j.jacc.2024.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Judge DP et al. Nov. Long-Term Efficacy and Safety of Acoramidis in ATTR-CM: Initial Report From the Open-Label Extension of the ATTRibute-CM Trial. Circulation. 2024;18. 10.1161/CIRCULATIONAHA.124.072771. [DOI] [PMC free article] [PubMed]
- 33.Elliott P et al. Long-Term survival with Tafamidis in patients with transthyretin amyloid cardiomyopathy. Circ Heart Fail 15,1 (2022): e008193. 10.1161/CIRCHEARTFAILURE.120.008193 [DOI] [PMC free article] [PubMed]
- 34.Hellenbart EL et al. Disease-modifying therapies for amyloid transthyretin cardiomyopathy: current and emerging medications. Pharmacotherapy 45,2 (2025): 124–44. 10.1002/phar.4639 [DOI] [PMC free article] [PubMed]
- 35.Wasfy JH, Winn AN, Touchette DR et al. Disease Modifying Therapies for the Treatment of Transthyretin Amyloid Cardiomyopathy. 2024. Draft Evidence Report. Institute for Clinical and Economic Review. https://icer.org/assessment/transthyretin-amyloid‐cardiomyopathy‐2024
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].