Abstract
Major depressive disorder (MDD) is a multifactorial disorder involving genetic and environmental factors, with unclear pathogenesis. This study aims to explore the pathogenic pathway of MDD and its relationship with immune responses and to discover its potential targets by bioinformatics methods. We first applied gene set variation analysis (GSVA) and seven different immune infiltration algorithms to the GSE98793 dataset to determine the differences in signaling pathways, metabolic pathways, and immune cell infiltration between MDD patients and healthy controls. Differentially expressed genes between MDD patients and controls were obtained from five datasets (GSE98793, GSE32280, GSE38206, GSE39653, and GSE52790), and 113 machine learning methods were employed to construct MDD diagnostic models. Based on the constructed MDD diagnostic models, MDD patients were divided into high-risk and low-risk groups. GSVA and immune microenvironment analyses were conducted to investigate the differences between the two groups. Furthermore, potential drugs and therapeutic targets for the high-risk MDD group were explored to provide new insights and directions for the precise treatment of MDD. GSVA and immune infiltration results indicate that patients with MDD exhibit differences from normal individuals in various aspects, including biological processes, signaling pathways, metabolic processes, and immune cells. To investigate the functions and biological significance of differentially expressed genes in MDD patients, we performed GO and KEGG enrichment analyses on the differentially expressed genes from five databases (GSE98793, GSE32280, GSE38206, GSE39653, and GSE52790). By comparing the enrichment results across the five datasets, we found that the cell-killing signaling pathway was consistently present in the enriched signaling pathways of all datasets, suggesting that this pathway may play a crucial role in the pathogenesis of MDD. The random forest algorithm (AUC = 0.788) was selected as the optimal algorithm from 113 machine learning algorithms, leading to the development of a robust and predictive MDD algorithm, highlighting the important role of NPL in MDD. By dividing MDD into high and low-risk subgroups based on diagnostic model scores, enrichment pathways, and immunological results further demonstrated that high-risk MDD is associated with increased levels of reactive oxygen species, inflammation, and numbers of T cells and B cells. Through GSEA scoring, five upregulated pathways in the high-risk MDD group were identified, and multiple potential drugs such as Mibefradil, LY364947, ZLN005, STA- 5326, and vemurafenib were screened. Patients with MDD show differences in signaling pathways, metabolic pathways, and immune mechanisms. By constructing an MDD diagnostic model, we predicted the key genes of MDD and the characteristic pathways associated with a higher risk of MDD. This provides new insights for risk stratification identification and offers new perspectives for the clinical application of precision immunotherapy and drug development.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-97623-x.
Keywords: Major depression disorder (MDD), Machine learning, Gene set variation analysis (GSVA), Immune infiltration, Biomarker
Subject terms: Depression, Computational biology and bioinformatics, Machine learning
Introduction
Major depressive disorder (MDD) is a prevalent and debilitating mental health condition characterized by a constellation of symptoms, including persistent low mood, anhedonia, sleep disturbances, cognitive impairments, and social dysfunction1. In severe cases, MDD can lead to self-harm and suicidal behavior, further emphasizing the need for effective interventions and a deeper understanding of its pathogenesis2. The global burden of MDD is substantial and continues to rise, with the World Health Organization (WHO) projecting that depression will be one of the leading causes of disability worldwide by 20233,4. In China, the world’s most populous country, a national survey revealed that depression is the second leading cause of disability, underscoring the urgent need to address this growing public health concern5.
Despite the significant impact of MDD on individuals and society, its pathogenesis remains elusive, with multiple factors, including genetic, environmental, and pathological processes, contributing to its development. Several theories have been proposed to explain the underlying mechanisms of MDD, such as the hypothalamic-pituitary-adrenal (HPA) axis dysfunction hypothesis, the monoamine hypothesis, the inflammatory hypothesis, the genetic and epigenetic abnormality hypothesis, the structural and functional brain remodeling hypotheses, and the psychosocial hypotheses6. Animal model studies suggest that genetic factors account for approximately 40–50% of the risk of developing depression7. To date, nearly 200 genes have been associated with MDD, including mutations in the FKBP5 allele, which establishes a link between the HPA axis and the immune response8. Moreover, accumulating evidence points to the involvement of the immune system, particularly the inflammatory response, in the pathophysiology of MDD9,10.
Current treatments for MDD encompass a range of approaches, with medication and psychotherapy being the most highly recommended. Antidepressant medications, such as those targeting monoamine neurotransmitters (e.g., serotonin, norepinephrine, and dopamine), have demonstrated efficacy in treating MDD11,12. However, a significant proportion of patients exhibit inadequate response to these medications, leading to refractory depression13. Furthermore, treatment side effects can hinder patient adherence and even contribute to increased dysfunction, morbidity, and mortality14. Targeted immunotherapy may prove more suitable for MDD patients with elevated pro-inflammatory biomarkers, who may have a suboptimal response to traditional antidepressants15.
Given the limitations of current treatments and the complex nature of MDD pathogenesis, there is a pressing need to develop novel biomarkers for the diagnosis and treatment of MDD. In recent years, bioinformatics approaches have emerged as powerful tools for unraveling the mechanisms underlying various diseases. In this study, we employed gene set variation analysis (GSVA) to identify pathways and differentially expressed genes associated with MDD. Additionally, we assessed the immune microenvironment of MDD patients and explored potential biomarkers. By integrating multiple datasets and leveraging advanced bioinformatics techniques, such as machine learning algorithms, this study aims to provide a comprehensive analysis of the signaling and metabolic pathways, immune infiltration patterns, and key genes implicated in MDD pathogenesis. The identification of robust biomarkers and the construction of a machine learning-based diagnostic model for MDD could facilitate early detection, risk stratification, and personalized treatment strategies. Ultimately, this research endeavors to contribute to a deeper understanding of MDD pathogenesis and pave the way for the development of more effective and targeted interventions, thereby improving the lives of individuals affected by this debilitating disorder.
Data and methods
Data sources
Gene expression datasets were obtained from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/). We selected datasets based on the following criteria: (1) severe depression diagnosis; (2) case-control design; (3) whole blood samples from MDD patients and healthy controls; (4) MDD patients without comorbidities or prior medication. Five datasets met these criteria: GSE98793, GSE32280, GSE38206, GSE39653, and GSE52790 (see Supplementary Table 1)16–20.
Gene set variation Analysis(GSVA)in MDD patients
GSVA was performed on the GSE98793 dataset, which had the largest sample size, using Gene Ontology Biological Process (GOBP) and Kyoto Encyclopedia of Genes and Genomes (KEGG) gene sets. Differential analysis between MDD patients and healthy controls was conducted using the Limma package (FDR< 0.05, t > 2). Subsequently, Reactome and KEGG metabolic pathway gene sets were used to reassess metabolic pathway differences.
Immune infiltration in MDD patients
Seven immune infiltration methods (CIBERSORT, EPIC, ESTIMATE, MCPcounter, quanTIseq, TIMER, and xCell) were applied to the GSE98793 dataset to evaluate differences in immune cell types between MDD and control groups. The relative abundance of significantly different immune cells was calculated (FDR< 0.05).
Identification and analysis of differentially expressed genes (DEGs)
DEGs were identified across five datasets (GSE98793, GSE32280, GSE38206, GSE39653, GSE52790) using the “limma” package (FDR< 0.05, |log2 FC|> 0.2). Genes with log2 FC<− 0.2 and FDR< 0.05 were considered downregulated, while those with log2 FC > 0.2 and FDR< 0.05 were upregulated. Results were visualized using volcano plots.
GO and KEGG enrichment analysis
GO and KEGG enrichment analyses were performed on DEGs using the “clusterProfiler” package (FDR< 0.05) to explore potential signaling pathways.
MDD diagnostic model construction
Using 113 machine learning algorithms to calculate the AUC values for the five datasets, the average AUC values for each type of machine learning algorithm were obtained21. A higher AUC value indicates better diagnostic performance of the model. To construct an interpretable diagnostic model for MDD, we selected six genes identified by the random forest (RF) algorithm with the highest AUC value and employed logistic regression to establish a model based on these six genes. The model was applied to predict the risk of MDD in the training dataset, and residuals, Cook’s distances, and model coefficients were calculated and visualized to assess the model’s goodness of fit. Using the established model, we computed scores for MDD patients and healthy individuals and compared the differences between the two groups using the Wilcoxon rank-sum test. To evaluate the model’s performance, we conducted receiver operating characteristic (ROC) curve analysis on five datasets and the combined dataset using the “pROC” R package and calculated the area under the curve (AUC).
GSVA analysis of MDD diagnostic model subgroups
MDD patients were divided into high-risk and low-risk groups based on the Median value of MDD model scores. Similar to Method 2.2, we performed GSVA difference analyses using GOBP and KEGG for the MDD high and low-risk groups in the same way. After identifying the differences, we selected the Reactome and KEGG metabolic pathway genomes to again assess the differences in metabolic pathways in the MDD high and low-risk groups.
Immune infiltration analysis of MDD diagnostic model subgroups
Based on 7 different immune infiltration methods, the differences in immune cell types between the high-risk and low-risk groups of MDD were evaluated, and the relative abundance of immune cells with significant differences was calculated. A significance level of FDR<0.05 was used to indicate statistical significance.
MDD potential drug prediction
Using the R limma package to calculate differentially expressed genes between two groups of MDD patients. Conducting Gene Set Enrichment Analysis on these differentially expressed genes to obtain gene pathways specific to different high-risk groups of MDD. Enrichment of gene sets with FDR< 0.05 is considered significant. The L1000 FWD database (https://amp.pharm.mssm.edu/l1000fwd/) records genes that are upregulated or downregulated by over 16,000 drugs or small molecules in cancer cell lines. By comparing the enriched genes with those in the database, drug results can be obtained. Human cancer cell line drug sensitivity data can be downloaded from the Cancer Therapeutics Response Portal (CTRP v.2.0, https://portals.broadinstitute.org/ctrp) and PRISM Repurposing dataset (https://depmap.org/portal/prism/). The Area Under the Curve (AUC) values for drug sensitivity are calculated using the “pRRophetic” software package, where lower AUC values indicate higher sensitivity to potential drugs. Due to limitations in sample size and available metadata in the public datasets used, we were unable to account for potential confounding factors such as sex differences, age-related effects, and BMI. However, to address potential batch effects arising from different experimental or technical variations, we employed the ComBat method for batch effect correction. This approach helps to minimize technical variations while preserving biological variation.
Experimental validation
Peripheral blood samples (5 mL) were collected from 30 healthy controls and 30 patients diagnosed with Major Depressive Disorder (MDD) according to DSM- 5 criteria at Affiliated Hospital of North Sichuan Medical College. The study was approved by the Medical Ethics Committee of Affiliated Hospital of North Sichuan Medical College (Approval No. 2023ER050 - 1), and all participants provided written informed consent. Total RNA was extracted from whole blood using TRIzol reagent (Invitrogen, USA) following the manufacturer’s protocol. RNA concentration and purity were assessed using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, USA). Reverse transcription was performed using the PrimeScript RT kit (TaKaRa, Japan) with 1 µg of total RNA. Quantitative PCR (qPCR) was conducted on an ABI 7500 Real-Time PCR System (Applied Biosystems, USA) using SYBR Premix Ex Taq II (TaKaRa, Japan) and specific primers (see Supplementary Table 2). Relative expression levels were calculated using the 2^-ΔΔCt method, with GAPDH as the internal reference gene. All experiments were performed in triplicate.
Results
Aberrantly active signaling and metabolic pathways in MDD patients
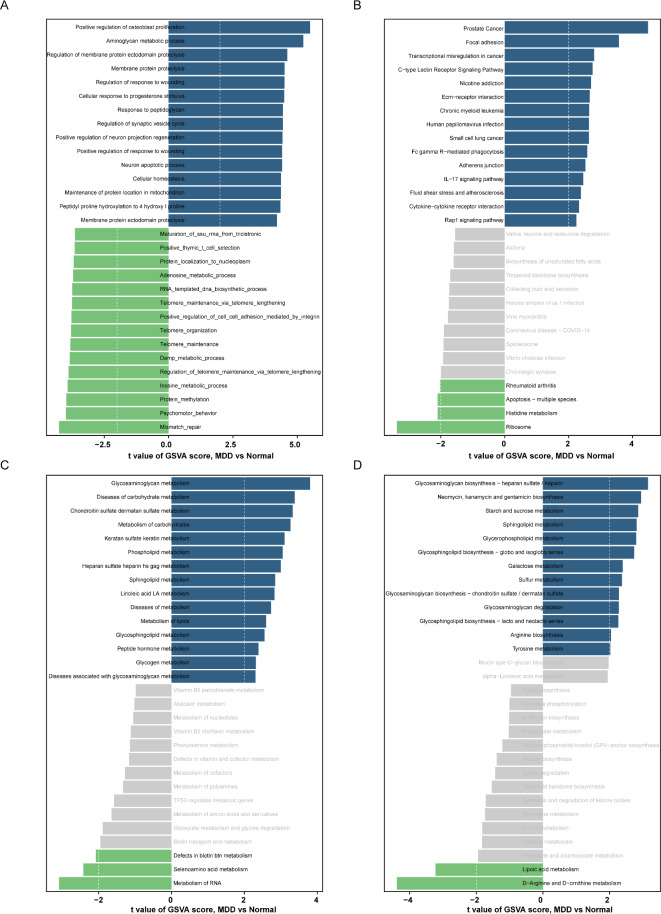
To investigate the differences in gene expression and metabolism between patients with MDD and healthy individuals, we performed Gene Set Variation Analysis (GSVA) on multiple gene sets, including GO, KEGG, and Reactome. The GSVA results based on the GO gene set revealed that several signaling pathways, such as those related to osteoblast proliferation, glycosaminoglycan metabolism, membrane protein proteolysis, wound response, and cellular response to progesterone and peptidoglycan, were abnormally active in MDD patients (Fig. 1A). Simultaneously, the GSVA results using the KEGG gene set showed that pathways involved in cell adhesion, transcriptional misregulation, receptor signaling, addiction, and cancer were aberrantly activated in MDD patients (Fig. 1B).
Fig. 1.
The gene set variation analysis (GSVA) for MDD groups and control groups based on the GSE98793 database. (A) GSVA scoring bar graph based on “GO” enrichment pathway; (B) GSVA scoring bar graph based on “KEGG enrichment pathway; (C) GSVA scoring bar graph based on “Reactome pathway; (D) GSVA scoring bar graph based on “KEGG metabolism pathway GSVA score bar graphs. Blue represents upregulated terms and green represents down-regulated terms.
To further elucidate the metabolic differences between MDD patients and healthy controls, we conducted GSVA analyses using Reactome and KEGG metabolic gene sets. The Reactome gene set results indicated that various metabolic processes, particularly those related to glycosaminoglycan, carbohydrate, and keratan sulfate metabolism, were significantly higher in MDD patients compared to healthy individuals (Fig. 1C). Similarly, the KEGG metabolic gene set results demonstrated that metabolic pathways involving glycosaminoglycan biosynthesis, antibiotic biosynthesis, starch and sucrose metabolism, sphingolipid metabolism, and glycerophospholipid metabolism were notably elevated in MDD patients (Fig. 1D). These results provide novel insights and directions for further understanding the pathogenesis of MDD, highlighting the potential involvement of these aberrant signaling and metabolic pathways in the development and progression of the disorder.
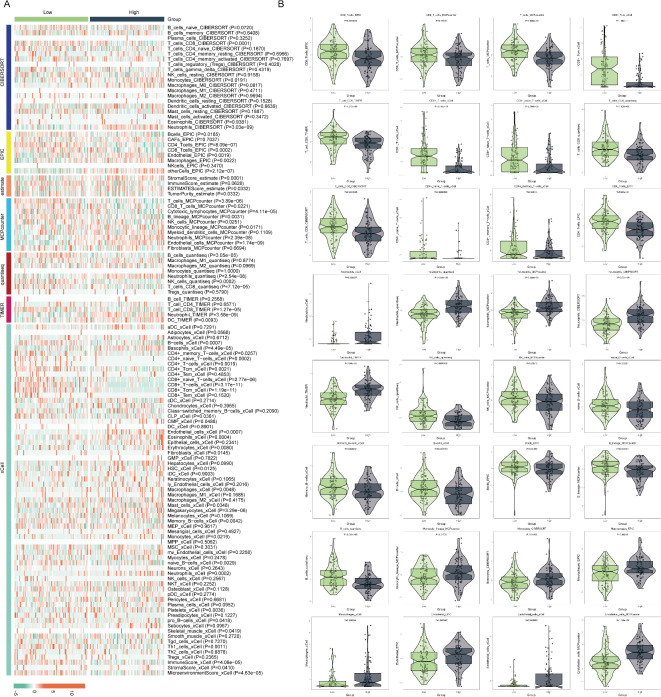
Immune cell infiltration analysis in patients with MDD
The immune system plays a crucial role in the pathogenesis of MDD. To investigate the differences in immune cell infiltration between MDD patients and healthy individuals, we employed seven distinct immune infiltration algorithms to assess and compare the immune profiles of the two groups. By generating a heatmap of the immune infiltration differences (Fig. 2A), we discovered significant variations in the composition of immune cells between MDD patients and healthy controls. Further analysis revealed that the peripheral blood of healthy individuals exhibited significantly higher levels of CD4 + T cells, CD8 + T cells, total T cells, and B cells compared to MDD patients (Fig. 2B), indicating a more active adaptive immune response in healthy subjects. Conversely, the peripheral blood of MDD patients displayed markedly elevated levels of macrophages, M1-type macrophages, neutrophils, and endothelial cells compared to healthy controls (Fig. 2B), suggesting that inflammatory processes and cytokine storms may be more prominent in MDD patients. Our findings underscore the substantial differences in immune cell infiltration between MDD patients and healthy individuals, with MDD patients exhibiting a relatively more active innate immune response and a comparatively weakened adaptive immune response.
Fig. 2.
Analysis of seven immune infiltration methods in the MDD and control groups based on the GSE98793 database. (A) Heat map with relative abundance differences of different immune cell types; (B) distribution of specific immune cell subpopulations violin plot.
Analysis of differentially expressed genes in patients with MDD
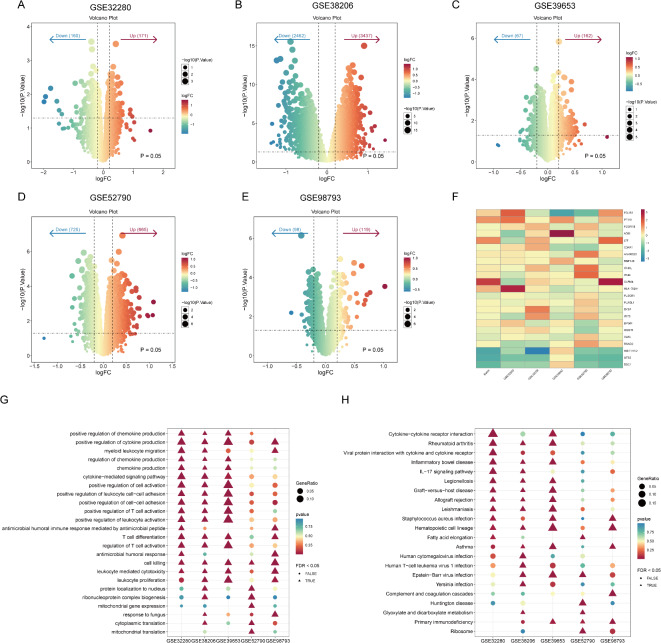
To comprehensively identify differentially expressed genes (DEGs) between patients with MDD and healthy individuals, we conducted an integrative analysis of five publicly available gene expression datasets, including GSE98793, GSE32280, GSE38206, GSE39653, and GSE52790. As a first step, we performed differential gene expression analysis on each dataset independently to identify genes that exhibited significant differential expression between MDD patients and normal controls. Our analysis revealed 217, 331, 5899, 229, and 1390 DEGs in the GSE98793, GSE32280, GSE38206, GSE39653, and GSE52790 datasets, respectively (Fig. 3A-E).
Fig. 3.
Differentially expressed gene analysis based on five Datasets GSE98793, GSE32280, GSE38206, GSE39653, and GSE52790. (A-E) Volcano plots of differentially expressed genes between MDD patients and healthy individuals in the five datasets; where genes located above the threshold line (P = 0.05) are significant, with red dots representing significantly up-regulated genes and blue dots representing significantly down-regulated genes; (F) heatmap of 23 hub genes, with the color scale indicating the level of expression, with red representing higher expression and blue representing lower expression; (G) GO-BP enrichment analysis of hub genes; (H) KEGG pathway analysis of hub genes.
To further refine our findings and pinpoint key genes that consistently showed differential expression across multiple datasets, we conducted an intersection analysis of the DEGs identified in the five datasets. Through this intersection analysis, we ultimately identified a set of 23 core genes, termed hub-genes, that displayed stable differential expression patterns between MDD patients and healthy individuals (Fig. 3F). These hub-genes primarily participate in the immune and inflammatory processes of MDD patients, including cell killing, regulation of chemokine production, positive regulation of cytokine production, positive regulation of cell activation, positive regulation of leukocyte cell-cell adhesion, and positive regulation of T cell activation (Fig. 3G). However, these biological processes seem less significant in the GSE52790 and GSE98793 datasets. KEGG pathway analysis revealed enrichment of these hub-genes in pathways such as Cytokine-cytokine receptor interaction, Viral protein interaction with cytokine and cytokine receptor, and the IL- 17 signaling pathway (Fig. 3H). Additionally, some genes were enriched in disease or infection pathways, such as Hematopoietic cell lineage, Legionellosis, Rheumatoid arthritis, Graft-versus-host disease, Leishmaniasis, and Epstein-Barr virus infection, suggesting a link between the pathogenesis of MDD and these diseases.
MDD diagnostic model construction and analysis
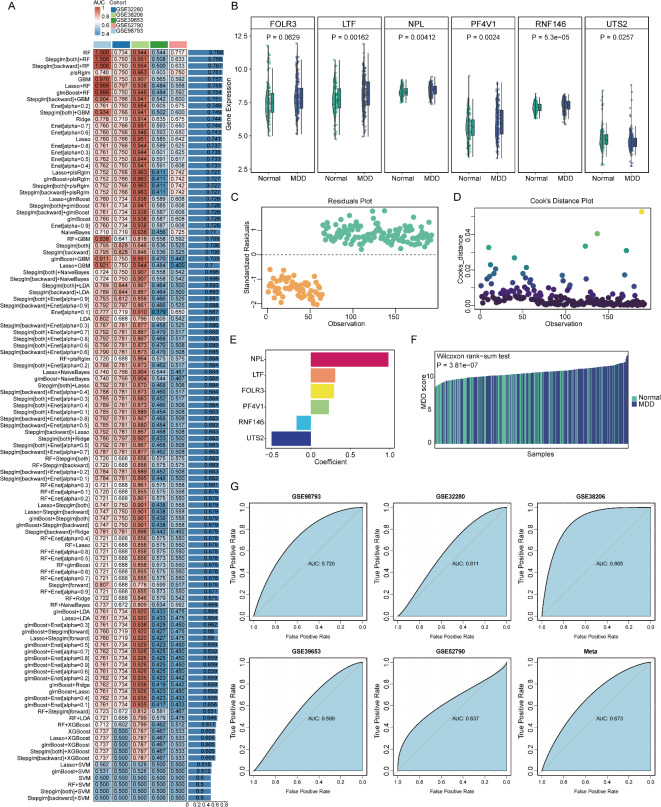
Using the core differentially expressed genes from five datasets, we employed 113 machine learning algorithms to identify feature genes for MDD. The closer the Area Under the Curve (AUC) value is to 1, the better the performance of the model22. The average AUC values indicated that the Random Forest (RF) algorithm performed the best, with an overall AUC value of 0.788. Based on the RF machine learning algorithm, we identified 6 key genes (Fig. 4A), including FOLR3, LTF, NPL, PF4 V1, and RNF146, which showed enhanced expression in MDD patients, while UTS2 exhibited decreased expression (Fig. 4B). Furthermore, we performed qPCR analysis on whole blood samples from MDD patients and healthy controls. As shown in Supplementary Fig. 1, FOLR3, LTF, NPL, PF4 V1, and RNF146 were significantly upregulated in MDD patient samples, with RNF146 showing particularly pronounced overexpression. Conversely, UTS2 was downregulated in MDD patient samples.(Figure S1 and Table S2).
Fig. 4.
Machine learning algorithm construction of MDD diagnostic model. (A) Identification of MDD feature genes using 113 machine learning algorithms; (B) Box plots of expression levels of 6 key genes; (C) Residual plot of MDD regression model; (D) Cook’s distance test plot of MDD regression model; (E) Bar plot of coefficients in MDD regression model; (F) Bar plot of MDD regression model scores for MDD patients and normal individuals; (G) ROC curves of the five datasets and the combined dataset (meta).
We then used these 6 genes to establish a logistic regression model for MDD diagnosis. The residuals were randomly distributed on both sides of the 0 line (Fig. 4C), with a large number of data points falling within the normal range of Cook’s Distance, indicating a good fit and robustness of the diagnostic model (Fig. 4D). The model formula indicates that the MDD score is determined by the expression level of LTF multiplied by 0.3084 plus the expression level of NPL multiplied by 0.9831 plus the expression level of FOLR3 multiplied by 0.2942 plus the expression level of PF4 V1 multiplied by 0.2283 minus the expression level of RNF146 multiplied by 0.1796 minus the expression level of UTS2 multiplied by 0.4960 (Fig. 4E). To further validate the diagnostic model, we combined the samples from the five datasets and found that the regression model scores of MDD patients were significantly higher than those of healthy controls (P = 3.81e- 07) (Fig. 4F). Moreover, the Receiver Operating Characteristic (ROC) curves of the five individual datasets and the combined dataset confirmed the accuracy of the MDD diagnostic model, with AUC values of 0.720, 0.61, 0.865, 0.599, 0.637, and 0.673, respectively. These results demonstrate the good predictive performance of the MDD diagnostic model (Fig. 4G).
Subgroup analysis of MDD diagnostic models
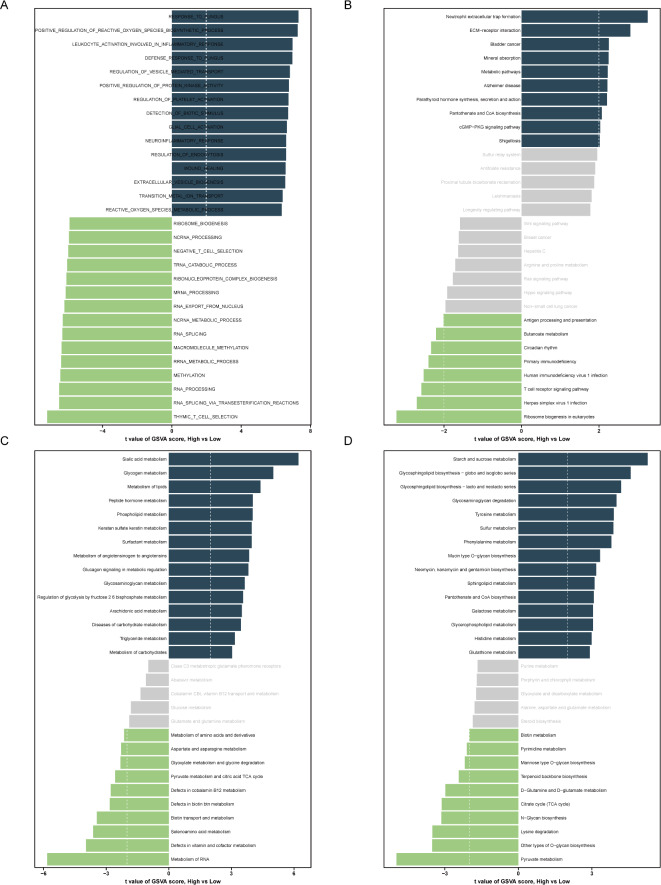
Based on the median MDD score, MDD patients were divided into two subgroups: a group with a high risk of MDD and a group with a low risk of MDD. We repeated GSVA across multiple gene sets to investigate gene expression and metabolic pathway differences between MDD subgroups. GSVA analysis with the GO genome showed that patients at high risk for MDD were associated with functional abnormalities related to immune and inflammatory responses, oxidative stress, changes in cell signaling, and neurological function (Fig. 5A). Also, the GSVA results of the KEGG genome showed that the high risk of MDD involved more complex pathway changes, including immune and inflammatory responses, cell signaling, metabolic processes, endocrine status, and neurodegenerative diseases (Fig. 5B). Accordingly, we further analyzed metabolic pathway differences between MDD subgroups with GSVA analyses based on the Reactome and KEGG metabolic genomes. The Reactome genomics results indicate that various metabolic pathways in the high-risk group of MDD patients are significantly enhanced, particularly in aspects of energy metabolism, inflammatory responses, and the synthesis and cellular signaling of neurotransmitters (Fig. 5C). The KEGG metabolic gene sets of the high-risk MDD group encompass the synthesis and breakdown processes of carbohydrates, lipids, amino acids, and nucleotides. Abnormalities in these metabolic pathways may be associated with the pathogenesis of MDD, especially in terms of energy metabolism, neurotransmitter synthesis, cellular signaling, and cellular antioxidant defense (Fig. 5D).
Fig. 5.
GSVA scores for high-risk and low-risk groups of MDD. (A) Bar plot of GSVA scores based on “GO” enriched pathways; (B) Bar plot of GSVA scores based on “KEGG enriched pathways; (C) Bar plot of GSVA scores based on “Reactome pathways; (D) Bar plot of GSVA scores based on “KEGG metabolic pathways. Blue indicates upregulated items, and green indicates downregulated items.
The above results again suggested the role of the immune system in the pathogenesis of MDD. The differences in immune cells in the MDD subgroups may reflect an imbalance between innate and adaptive immunity, by seven different immune infiltration algorithms reevaluated and compared (Fig. 6A). We found that CD8 T cells, T cells, CD8 + Tcm, CD8 + T-cells, CD8 + naive T-cells, CD4 T cells, CD4 + naive T-cells, CD4 + memory T-cells, naive B-cells, Memory B-cells B-cells, B-lineage, Neutrophils, and NK cells were significantly decreased in the MDD high-risk group, whereas neutrophils, monocyte lineage, monocytes, macrophages, and endothelial cells were significantly increased in the MDD high-risk group (FDR< 0.05) (Fig. 6B). This demonstrated that activation of the innate immune system and weakening of the adaptive immune system increased the risk of developing MDD. To further investigate the immunological aspects of MDD, we conducted a focused analysis on a curated set of immune-related genes. This analysis revealed significant differential expression in several key immune genes. Notably, we observed significant upregulation of genes involved in inflammatory pathways and innate immune responses, such as TLR4 (p = 3.35E- 09), TNFRSF1 A (p = 1.88E- 06), IL1R1 (p = 1.23E- 06), and IFNGR1 (p = 9.27E- 05) in the high depression group. We also found significant upregulation of IL6R (p = 0.003) and STAT3 (p = 8.70E- 04), suggesting activation of the JAK-STAT signaling pathway. Interestingly, CCL4 (p = 0.004) and CTLA4 (p = 0.046) were significantly downregulated in the high depression group, potentially indicating complex immune regulatory mechanisms. Additionally, NOS2 (p = 0.004) was upregulated, supporting the potential role of oxidative stress in MDD. These findings provide additional support for the involvement of immune dysregulation in MDD, particularly highlighting the activation of innate immune responses and inflammatory pathways (Figure S2).
Fig. 6.
Immune infiltration analysis for high-risk and low-risk groups of MDD. (A) Heatmap showing the relative abundance differences of immune cell types; (B) Violin plot of differential immune cell levels.
MDD potential drug prediction
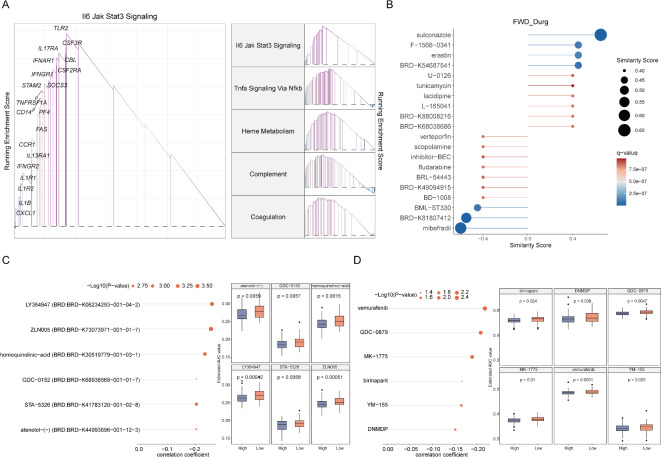
To investigate the molecular mechanisms of the MDD high-risk group, we employed the GSEA method to analyze the upregulated signaling pathways in the high-risk group. The results showed that the five most significantly upregulated pathways were the Il6-Jak-Stat3 signaling pathway, Tnfa-Nfkb signaling pathway, heme metabolism pathway, complement system-related pathway, and coagulation cascade pathway, respectively (Fig. 7A). These findings suggest that inflammatory responses, oxidative stress, immune dysfunction, and coagulation abnormalities may be important pathophysiological mechanisms in MDD high-risk individuals.
Fig. 7.
MDD potential drug prediction. (A) Enrichment results and top five upregulated signaling pathways in the high-score MDD group using GSEA algorithm; (B) Top ten drugs that promote and inhibit MDD patients obtained from the L100 FWD database; (C) Potential drug correlation and differential analysis for high and low-score MDD patients selected from the CTRP dataset; (D) Potential drug correlation and differential analysis for high and low-score MDD patients selected from the PRISM dataset.
By using the L1000 FWD database, we obtained drugs targeting these pathways (Fig. 7B). The Similarity Score of the drugs indicates the similarity between the gene expression patterns induced by the drugs and the patterns observed in MDD samples. Drugs with negative similarity scores, such as verteporfin, scopolamine, inhibitor-BEC, fludarabine, BRL- 54,443, BRD-K49094915, BD- 1008, BML-ST330, BRD-K81807412, and mibefradi, may have greater potential for MDD treatment. Additionally, we predicted potential drugs for MDD based on gene expression and drug sensitivity profiles from the CTRP and PRISM datasets (Fig. 7C-D). Differences in AUC values between the High and Low groups (Log2FC > 0.08) and negative correlation coefficients (r < − 0.30) between MDD and AUC were set as thresholds for compound selection. Drug response analysis and differential evaluation were performed on different MDD score groups. Six potential drugs generated from the CTRP database (LY364947, ZLN005, homoquinolinic-acid, GDC- 0152, STA- 5326, atenolol-(-)), and six potential drugs from the PRISM database (vemurafenib, GDC- 0879, MK- 1775, birinapant, YM- 155, DNMDP) have lower Estimated AUC values in the MDD-High group and are significantly negatively correlated with the risk score (P < 0.05). These findings contribute to understanding the biomarkers and potential therapeutic targets of MDD, providing valuable information for the development of new treatment strategies. We observed that some genes showed inconsistent expression directions across different cohorts. This variability could be attributed to the heterogeneous nature of MDD, differences in study populations, or technical variations in sample processing and data generation. To address this, we performed a meta-analysis using a random-effects model, which accounts for between-study heterogeneity. This approach allowed us to identify genes with consistent expression changes across the majority of studies, providing a more robust set of potential biomarkers. Future studies with larger, more homogeneous cohorts and standardized protocols may help to further elucidate these gene expression patterns.
Discussion
MDD is a severe neurological disorder with a high rate of recurrence and disability23. However, the intricate etiology of MDD, coupled with the absence of definitive diagnostic markers, often results in diagnostic uncertainty and challenges in treatment efficacy24. Despite numerous studies examining DEGs between MDD patients and healthy individuals, the fundamental causes of MDD have remained obscure. This situation underscores an urgent need for the identification of specific and sensitive biomarkers to facilitate precise MDD diagnosis. In our research, we commenced by revealing disparities in signaling and metabolic pathways, as well as in the profiles of immune cells, between individuals with MDD and those without. Subsequently, we advanced our research by analyzing data from five comprehensive datasets (GSE98793, GSE32280, GSE38206, GSE39653, GSE52790). Through this analysis, we not only identified a multitude of DGEs but also zeroed in on 23 pivotal hub-genes. These genes are crucially involved in immune and inflammatory responses, transcriptional regulation, and translational processes. Building upon these insights, we constructed a sophisticated MDD diagnostic model employing a diverse array of 113 machine-learning algorithms. This model not only enhances diagnostic accuracy but also, through its scoring mechanism, has guided the discovery of several potential therapeutic compounds. These collective findings represent a significant step forward in the quest to better understand, diagnose, and manage MDD. It’s important to note that some genes may be involved in multiple pathways, which could influence the interpretation of our pathway analysis results. This overlap highlights the complex interplay of molecular mechanisms in MDD and underscores the need for integrative approaches to understanding the disorder. However, it is important to acknowledge that our study is limited by the size and geographic diversity of the datasets used. Future research should aim to include larger, more diverse datasets to enhance the generalizability of these findings. They lay the groundwork for the development of dependable diagnostic tools and the formulation of targeted treatment strategies.
In recent years, the pivotal role of immune and inflammatory processes in the pathogenesis of MDD has gained widespread recognition. GSVA analysis reveals the differences between MDD patients, such as the positive regulation of the osteoblast proliferation process. Osteoblasts are large multinucleated cells that belong to the monocyte/macrophage lineage, which are derived from mesenchymal stem cells (MSC) and undergo three developmental stages: cell proliferation, extracellular matrix (ECM) secretion and matrix maturation, and matrix mineralization25. The development and function of osteoblasts are regulated by multiple factors, with the Wnt signaling pathway playing a key role in regulating gene expression, cell behavior, cell adhesion, lipid metabolism, and other processes26–28. Simultaneously, osteoblasts have been implicated in the pathogenesis of rheumatoid arthritis (RA)29. This association is particularly noteworthy as the signaling pathways involved in RA are significantly enriched in patients with MDD. The immune mechanisms link MDD with RA where pro-inflammatory cytokines commonly present in RA can impact monoaminergic neurotransmission, neurotrophic factors, and other factors, thereby increasing the risk of MDD30. Zhou et al.31suggested that Epstein-Barr virus infection, along with a decrease in T cells and B cells, could be potential factors contributing to the comorbidity of RA and depression. The cytokine IL17, produced by helper T cells, is involved in protecting epithelial cells and mucosa from bacterial and fungal invasion under physiological conditions26. This protective role of IL- 17 may explain the heightened expression observed in high-risk MDD groups in response to fungal. Furthermore, the results of the immune infiltration analysis fully demonstrated the immune cell dynamics in MDD. Previous studies have shown that MDD is associated with excessive activation of innate immune cells, such as a significant increase in the number of neutrophils and monocytes32. The immune infiltration results in our study confirmed the activation of innate immune cells, and it also revealed a reduction in adaptive immunity, evidenced by lower T and B cell counts. It’s worth noting that these results are based on computational inference from gene expression data using the LM22 signature matrix as a reference. While this approach provides valuable insights, future studies with direct immune cell quantification would be beneficial to validate these findings. There is growing evidence of changes in the immune system of MDD patients, such as T-cell dysfunction and imbalance in B-cell homeostasis33. Reduced T-cell and B-cell counts in MDD patients raise the risk of inflammation, malignancy, autoimmune diseases, and heightened susceptibility to MDD, thereby worsening symptoms34,35. In light of these findings, it is crucial to consider the immune system as a potential therapeutic target in MDD. The identification of specific pathways, such as glycosaminoglycan metabolism and IL- 17 signaling, aligns with emerging theories in MDD pathophysiology. For instance, the IL- 17 pathway has been implicated in neuroinflammation and synaptic plasticity, potentially contributing to MDD symptoms. Similarly, alterations in glycosaminoglycan metabolism may affect neurotransmitter function and neuroplasticity, further elucidating the complex molecular landscape of MDD.
The escalating size and complexity of biological data necessitate sophisticated analytical tools. Machine learning, with its ability to build predictive and informative models, is an ideal choice for this purpose. In our study, we compared 113 machine learning methods and identified the Random Forest (RF) algorithm as the most effective, with the highest average Area Under the Curve (AUC) value, indicating superior predictive performance. Based on this, we identified 5 key genes and found that all overlapped with the hub genes we obtained earlier, except for NPL. In the MDD diagnostic model, NPL (N-acetylneuraminate pyruvate lyase) had the highest coefficient, indicating its importance in diagnosing MDD. NPL is involved in the biosynthesis and activation of N-Acetylneuraminic acid (Neu5 Ac), a prevalent form of sialic acid in mammals36. Abnormality in Neu5 Ac has been linked to neurodevelopmental and metabolic disorders37. Notably, we observed a high enrichment of sialic acid metabolism in the high-risk MDD group, suggesting a potential role in MDD pathophysiology. Currently, there is a dearth of genetic research on NPL, and no association with MDD has been reported, indicating that NPL may represent a novel therapeutic target for MDD. Another gene of interest, UTS2, which had the second-highest coefficient, is predominantly expressed in cholinergic neurons in the brainstem and spinal cord38. UTS2 has been implicated in a variety of diseases, such as chronic non-bacterial osteomyelitis39, pleomorphic glioblastoma40, colon cancer41, and MDD42, all of which involve immune and inflammatory processes. The identification of these molecular biomarkers could revolutionize the predictive, diagnostic, and therapeutic landscape of MDD. It may serve as a valuable tool for personalized therapy in the future based on individual genetic profiles.
Based on the GSEA algorithm to identify the top five upregulated signaling pathways in the high-risk group of MDD, and conducted drug prediction. We obtained 10 negative similarity drugs from the L1000 FWD database. Notably, Mibefradil, with the highest similarity score, is a T-type Ca2+channel blocker that may exert therapeutic effects in MDD by inhibiting the cell cycle, promoting apoptosis, or alleviating pain43,44. Additional potential MDD drugs were predicted using the Connectivity Map (CTRP) and PRISM datasets. LY364947, a pyrazole-based small molecule inhibitor, inhibits the serine-threonine kinase activity of TGFβRI and reduces resistance to radiotherapy in glioblastoma-induced cells, breast cancer cell lines, and several non-small lung cancer cell lines45. ZLN005 inhibits TNF-α-induced pro-inflammatory cytokine expression46. Homoquinolinic acid is a potent stimulant for cortical neurons47. STA- 5326 suppresses inflammation by selectively inhibiting the expression of the gene encoding the p40 subunit present in IL- 12 and IL- 23 through selective inhibition of the c-Rel translocation48. Necrotic apoptosis is associated with a variety of immune disorders including inflammation, neurodegeneration, autoimmune diseases, and cancer, and vemurafenib is a potent inhibitor of necrotic apoptosis49. GDC- 0879 is a BRAF inhibitor that inhibits cellular pyroptosis50. WEE1 plays a key role in cell cycle regulation, and the antitumor effects of MK- 1775 as a WEE1 kinase inhibitor have been widely reported51. Our study explores dysregulated genes in MDD through diagnostic modeling and bioinformatics approaches, identifying potential targets for drug discovery. Among the predicted drugs, mibefradil and vemurafenib stand out due to their potential interactions with key pathways identified in our study. Mibefradil, as a T-type Ca2+ channel blocker, may modulate neurotransmission and neuroplasticity, processes often disrupted in MDD. Vemurafenib’s role in inhibiting necrotic apoptosis could potentially mitigate neuroinflammation in MDD. Future studies should prioritize these drugs for experimental validation, considering their mechanisms of action, safety profiles, and potential for repurposing in MDD treatment. These findings suggest that targeting inflammatory and neural-related genes could be a viable strategy for developing novel MDD treatments.
Limitations and future directions
This study has several limitations that should be considered. Firstly, the relatively small sample sizes in some cohorts may have limited our statistical power, potentially obscuring subtle effects. While we identified significant differences and patterns, larger cohorts are necessary to validate and extend these findings. Secondly, the heterogeneous nature of MDD, encompassing various subtypes and clinical presentations, may not be fully captured in our analysis. Thirdly, unmeasured confounding factors, such as lifestyle variables, medication use, or comorbid conditions, could have influenced our results. Lastly, the generalizability of our findings may be constrained by the limited diversity of our datasets, which may not fully represent the global MDD population. Future research should address these limitations by employing larger, more diverse cohorts, incorporating detailed clinical and demographic data, and utilizing advanced statistical methods to account for MDD heterogeneity and potential confounders. Such efforts will enhance our understanding of MDD’s molecular underpinnings and contribute to the development of more targeted therapeutic strategies.
Conclusion
In conclusion, this comprehensive study sheds light on the complex pathogenesis of major depressive disorder (MDD) by integrating multiple aspects, including signaling and metabolic pathway activities, immune infiltration patterns, and potential diagnostic biomarkers. We identified aberrantly active pathways related to osteoblast proliferation, glycosaminoglycan metabolism, membrane protein proteolysis, wound response, and cellular response to progesterone and peptidoglycan in MDD patients. Immune cell infiltration analysis revealed a more active innate immune response and a weakened adaptive immune response in MDD patients compared to healthy controls. Through differential gene expression and intersection analysis, we identified 23 core genes (hub-genes) that consistently showed differential expression patterns between MDD patients and healthy individuals, primarily participating in immune and inflammatory processes. Utilizing machine learning algorithms, we established a logistic regression model for MDD diagnosis based on six key genes, demonstrating good predictive performance. Subgroup analysis further highlighted the functional abnormalities and immune system imbalances associated with high-risk MDD patients. Lastly, we identified potential therapeutic targets and drugs for MDD treatment by analyzing upregulated signaling pathways and utilizing drug sensitivity profiles. Our findings provide novel insights into the underlying mechanisms of MDD and pave the way for the development of more effective diagnostic and treatment strategies, ultimately improving the lives of individuals affected by this disorder. Further research is necessary to validate these findings and translate them into clinical practice.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
not applicable.
Author contributions
Lei Tang: Investigation, Methodology, Data curation, Writing-Original draft preparation.Liling Wu & Mengqin Dai,: Investigation, Methodology, Data curation.Lu Liu & Nian Liu: Conceptualization, Funding acquisition, Writing-review & editing, Supervision, Project administration.
Funding
This study was also supported by the Natural Science Foundation of North Sichuan Medical College [grant number CBY24-QDA11], the Sichuan Science and Technology Program [grant number 2024 NSFSC2017, 2024ZYD0272], and the Opening Project of Functional and Molecular Imaging Key Laboratory of Sichuan Province [grant number SCU-HM- 202307001].
Data availability
The datasets extracted and analysed during the current study are available in the Gene Expression Omnibus (GEO) repository, accessible through the following accession numbers: GSE98793, GSE32280, GSE38206, GSE39653, and GSE52790 (https://www.ncbi.nlm.nih.gov/geo/). Additional data supporting the findings of this study are presented within the paper and are available upon reasonable request from the corresponding author, Lu Liu. All analysis codes used in this study can be obtained through a reasonable request to the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Ethics & inclusion statement
This study was conducted in accordance with the principles set out in the Global Code of Conduct for Research in Resource-Poor Settings. All data used in this research were obtained from publicly available databases. We ensured that our research objectives aligned with local and global health priorities. In our analysis and interpretation of the data, we were mindful of potential biases and strived to avoid generalizations that could perpetuate stereotypes. We have made efforts to ensure that our findings are accessible to the scientific community and, where applicable, to stakeholders in resource-poor settings who may benefit from this research. All authors contributed to and approved the final manuscript, and we have appropriately acknowledged the contributions of all collaborators and data sources. We are committed to the fair and equitable sharing of benefits that may arise from this research.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nian Liu, Email: liunian@nsmc.edu.cn.
Lu liu, Email: liuludoctor88@nsmc.edu.cn.
References
- 1.Dean, J. & Keshavan, M. The neurobiology of depression: an integrated view. Asian J. Psychiatry. 27, 101–111 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Su, Y., Ye, C., Xin, Q. & Si, T. Major depressive disorder with suicidal ideation or behavior in Chinese population: A scoping review of current evidence on disease assessment, burden, treatment and risk factors. J. Affect. Disord.340, 732–742 (2023). [DOI] [PubMed] [Google Scholar]
- 3.Barnett, R. & Depression Lancet (London England)393(10186), 2113 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Malhi, G. S., Mann, J. J. & Depression Lancet (London England)392(10161), 2299–2312 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Lu, J. et al. Prevalence of depressive disorders and treatment in China: a cross-sectional epidemiological study. Lancet Psychiatry. 8 (11), 981–990 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Cui, L. et al. Major depressive disorder: hypothesis, mechanism, prevention and treatment. Signal. Transduct. Target. Therapy. 9 (1), 30 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hao, Y., Ge, H., Sun, M. & Gao, Y. Selecting an appropriate animal model of depression. Int. J. Mol. Sci.20(19) (2019). [DOI] [PMC free article] [PubMed]
- 8.Ruiz, N. A. L. et al. Inflammatory process and immune system in major depressive disorder. Int. J. Neuropsychopharmacol.25 (1), 46–53 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Réus, G. Z., Manosso, L. M., Quevedo, J. & Carvalho, A. F. Major depressive disorder as a neuro-immune disorder: origin, mechanisms, and therapeutic opportunities. Neurosci. Biobehav. Rev.155, 105425 (2023). [DOI] [PubMed] [Google Scholar]
- 10.Patil, C. R., Gawli, S., Bhatt, S. & C. & Targeting inflammatory pathways for treatment of the major depressive disorder. Drug Discovery Today. 28 (9), 103697 (2023). [DOI] [PubMed] [Google Scholar]
- 11.Hassan, M., Amir, A., Shahzadi, S. & Kloczkowski, A. Therapeutic implications of MicroRNAs in depressive disorders: A review. Int. J. Mol. Sci.23(21) (2022). [DOI] [PMC free article] [PubMed]
- 12.Park, L. T. & Zarate, C. A. Jr. Depression in the primary care setting. N. Engl. J. Med.380 (6), 559–568 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rybak, Y. E. et al. Treatment-resistant major depressive disorder: Canadian expert consensus on definition and assessment. Depress. Anxiety. 38 (4), 456–467 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papakostas, G. I., Jackson, W. C., Rafeyan, R. & Trivedi, M. H. Inadequate response to antidepressant treatment in major depressive disorder. J. Clin. Psychiatry81(3) (2020). [DOI] [PubMed]
- 15.Drevets, W. C., Wittenberg, G. M., Bullmore, E. T. & Manji, H. K. Immune targets for therapeutic development in depression: towards precision medicine. Nat. Rev. Drug Discov.21 (3), 224–244 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leday, G. G. R. et al. Replicable and coupled changes in innate and adaptive immune gene expression in two Case-Control studies of blood microarrays in major depressive disorder. Biol. Psychiatry. 83 (1), 70–80 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yi, Z. et al. Blood-based gene expression profiles models for classification of subsyndromal symptomatic depression and major depressive disorder. PloS One. 7 (2), e31283 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belzeaux, R. et al. Responder and nonresponder patients exhibit different peripheral transcriptional signatures during major depressive episode. Translational Psychiatry. 2 (11), e185 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savitz, J. et al. Inflammation and neurological disease-related genes are differentially expressed in depressed patients with mood disorders and correlate with morphometric and functional imaging abnormalities. Brain. Behav. Immun.31, 161–171 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, Z. et al. Microarray profiling and co-expression network analysis of Circulating LncRNAs and mRNAs associated with major depressive disorder. PloS One9(3), e93388 (2014). [DOI] [PMC free article] [PubMed]
- 21.Liu, Z. et al. Machine learning-based integration develops an immune-derived LncRNA signature for improving outcomes in colorectal cancer. Nat. Commun.13 (1), 816 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Handelman, G. S. et al. eDoctor: machine learning and the future of medicine. J. Intern. Med.284 (6), 603–619 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Monroe, S. M. & Harkness, K. L. Major depression and its recurrences: life course matters. Ann. Rev. Clin. Psychol.18, 329–357 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Martin-Key, N. A. et al. The delta Study - Prevalence and characteristics of mood disorders in 924 individuals with low mood: results of the of the world health organization composite international diagnostic interview (CIDI). Brain Behav.11 (6), e02167 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amarasekara, D. S., Kim, S. & Rho, J. Regulation of osteoblast differentiation by cytokine networks. Int. J. Mol. Sci.22(6) (2021). [DOI] [PMC free article] [PubMed]
- 26.Berardi, S., Corrado, A., Maruotti, N., Cici, D. & Cantatore, F. P. Osteoblast role in the pathogenesis of rheumatoid arthritis. Mol. Biol. Rep.48 (3), 2843–2852 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ponzetti, M. & Rucci, N. Osteoblast differentiation and signaling: established concepts and emerging topics. Int. J. Mol. Sci.22(13) (2021). [DOI] [PMC free article] [PubMed]
- 28.Alekos, N. S., Moorer, M. C. & Riddle, R. C. Dual effects of lipid metabolism on osteoblast function. Front. Endocrinol.11, 578194 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komatsu, N. & Takayanagi, H. Mechanisms of joint destruction in rheumatoid arthritis - immune cell-fibroblast-bone interactions. Nat. Rev. Rheumatol.18 (7), 415–429 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Brock, J. et al. Immune mechanisms of depression in rheumatoid arthritis. Nat. Rev. Rheumatol.19 (12), 790–804 (2023). [DOI] [PubMed] [Google Scholar]
- 31.Zhou, T. T. et al. Potential diagnostic markers and therapeutic targets for rheumatoid arthritis with comorbid depression based on bioinformatics analysis. Front. Immunol.14, 1007624 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beurel, E., Toups, M. & Nemeroff, C. B. The bidirectional relationship of depression and inflammation: double trouble. Neuron107 (2), 234–256 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Debnath, M., Berk, M. & Maes, M. Translational evidence for the inflammatory response system (IRS)/Compensatory immune response system (CIRS) and neuroprogression theory of major depression. Prog. Neuro-psychopharmacol. Biol. Psychiatry. 111, 110343 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Wu, S., Yin, Y. & Du, L. The bidirectional relationship of depression and disturbances in B cell homeostasis: double trouble. Prog. Neuro-psychopharmacol. Biol. Psychiatry. 132, 110993 (2024). [DOI] [PubMed] [Google Scholar]
- 35.Sun, L., Su, Y., Jiao, A., Wang, X. & Zhang B. T cells in health and disease. Signal. Transduct. Target. Therapy. 8 (1), 235 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng, C., Hu, Z. X., He, M., Liu, L. & Voglmeir, J. Recombinant human N-acetylneuraminate lyase as a tool to study clinically relevant mutant variants. Carbohydr. Res.516, 108561 (2022). [DOI] [PubMed] [Google Scholar]
- 37.Liu, F., Simpson, A. B., D’Costa, E., Bunn, F. S. & van Leeuwen, S. S. Sialic acid, the secret gift for the brain. Crit. Rev. Food Sci. Nutr.63 (29), 9875–9894 (2023). [DOI] [PubMed] [Google Scholar]
- 38.Vaudry, H. et al. International union of basic and clinical pharmacology. XCII. Urotensin II, Urotensin II-related peptide, and their receptor: from structure to function. Pharmacol. Rev.67 (1), 214–258 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Fu, Z. et al. Transcriptome analysis based on machine learning reveals a role for autoinflammatory genes of chronic nonbacterial osteomyelitis (CNO). Sci. Rep.13 (1), 6514 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang, Y., Shen, L. & Sun, M. Prognostic significance and functional mechanism of UTS2 in glioblastoma multiforme. Curr. Cancer Drug Targets (2024). [DOI] [PubMed]
- 41.Hao, M. et al. Development of an immune-related gene prognostic risk model and identification of an immune infiltration signature in the tumor microenvironment of colon cancer. BMC Gastroenterol.23 (1), 58 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng, Z., Liu, J., He, S. & Gao, W. The Pyroptosis-Related signature predicts diagnosis and indicates immune characteristic in major depressive disorder. Front. Pharmacol.13, 848939 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li, P. et al. Mibefradil, a T-type Ca(2+) channel blocker also blocks Orai channels by action at the extracellular surface. Br. J. Pharmacol.176 (19), 3845–3856 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen, Y. L. et al. Chronic intrathecal infusion of Mibefradil, Ethosuximide and nickel attenuates nerve ligation-induced pain in rats. Br. J. Anaesth.115 (1), 105–111 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Tschernia, N. P. & Gulley, J. L. Tumor in the crossfire: inhibiting TGF-β to enhance cancer immunotherapy. BioDrugs: Clin. Immunotherapeutics Biopharmaceuticals Gene Therapy. 36 (2), 153–180 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu, Y., Du, M., Cao, Z. & He, H. PGC-1α attenuates TNF-α-induced inflammatory responses in OCCM-30 cells. J. Periodontal Res.57 (5), 1024–1033 (2022). [DOI] [PubMed] [Google Scholar]
- 47.Stone, T. W. Excitant activity of Methyl derivatives of quinolinic acid on rat cortical neurones. Br. J. Pharmacol.81 (1), 175–181 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keino, H. et al. Therapeutic effect of the potent IL-12/IL-23 inhibitor STA-5326 on experimental autoimmune uveoretinitis. Arthritis Res. Therapy. 10 (5), R122 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun, M. et al. Vemurafenib inhibits necroptosis in normal and pathological conditions as a RIPK1 antagonist. Cell Death Dis.14 (8), 555 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeng, R. et al. Predicting the prognosis of esophageal adenocarcinoma by a Pyroptosis-Related gene signature. Front. Pharmacol.12, 767187 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirai, H. et al. MK-1775, a small molecule Wee1 inhibitor, enhances anti-tumor efficacy of various DNA-damaging agents, including 5-fluorouracil. Cancer Biol. Ther.9 (7), 514–522 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets extracted and analysed during the current study are available in the Gene Expression Omnibus (GEO) repository, accessible through the following accession numbers: GSE98793, GSE32280, GSE38206, GSE39653, and GSE52790 (https://www.ncbi.nlm.nih.gov/geo/). Additional data supporting the findings of this study are presented within the paper and are available upon reasonable request from the corresponding author, Lu Liu. All analysis codes used in this study can be obtained through a reasonable request to the corresponding author.