Highlights
-
•
In this secondary analysis of the DASH (Dietary Approaches to Stop Hypertension)-Sodium trial, dietary sodium reduction independently lowered estimated 10-year ASCVD risk.
-
•
The combination of the DASH diet and sodium reduction resulted in the greatest relative decrease in ASCVD risk compared to either intervention alone.
-
•
The impact of sodium reduction on estimated ASCVD risk was greatest in women, Black adults, and participants with baseline stage 2 (≥140/90 mmHg) hypertension.
-
•
Our findings support dietary sodium reduction in addition to the DASH diet to mitigate cardiovascular disease risk.
Keywords: Diet, Sodium, Cardiovascular disease, Trial
Abstract
Background
The Dietary Approaches to Stop Hypertension (DASH) diet lowers estimated 10-year ASCVD (atherosclerotic cardiovascular disease) risk. The effects of dietary sodium reduction on ASCVD risk are uncertain. This study aims to evaluate the impact of sodium reduction, alone and combined with the DASH diet, on 10-year ASCVD risk scores.
Methods
The DASH-Sodium trial randomized adults with elevated blood pressure (average systolic blood pressure of 120 to 159 mm Hg and average diastolic blood pressure of 80 to 95 mm Hg) to the DASH diet or typical American diet. Within each arm, individuals consumed 3 different levels of sodium in random order: low, medium, and high. Each period lasted 30 days. Pooled cohort equation-estimated 10-year ASCVD risk scores were calculated at baseline and at the end of each feeding period. The primary outcomes of interest were the absolute and relative differences in 10-year ASCVD risk scores from baseline.
Results
Among the 412 participants (mean age 48 ± 10 years; 57 % female, 57 % Black), sodium reduction decreased ASCVD risk scores in both dietary arms. Compared to high sodium intake, low sodium intake changed ASCVD risk by -9.4 % (95 % CI -11.7, -7.0). When compared to a typical American diet, the DASH diet changed 10-year ASCVD by -5.3 % (95 % CI -9.3, -1.2). Compared to a high sodium-control diet, the combination of both low sodium intake with DASH changed ASCVD risk by -14.1 % (95 % CI -18.6, -9.3).
Conclusions
Sodium reduction and the DASH diet both independently reduced 10-year ASCVD risk scores. Moreover, the combined impact was additive. These findings support dietary sodium reduction in addition to the DASH diet for ASCVD prevention.
1. Introduction
Cardiovascular disease (CVD) is the leading cause of death globally [1]. Over 50 % of CVD cases are attributed to modifiable risk factors, including diet and physical activity [[2], [3], [4]]. In the United States, dietary habits are among the most suboptimal metrics of cardiovascular health (CVH) [5]. Strikingly, over 90 % of adults exceed the recommended amount of daily sodium intake [1].
The Dietary Approaches to Stop Hypertension (DASH) diet is recommended by national guidelines to promote CVH [1,6]. DASH eating patterns prioritize fruits, vegetables, whole grains, and low-fat dairy products while limiting saturated fat, cholesterol, and added sugars [7]. The DASH diet is associated with decreased incidence of CVD, [1] protective benefits against subclinical cardiac injury, [8] and lower 10-year atherosclerotic CVD (ASCVD) risk, [9] as measured by the Pooled Cohort Equation (PCE). Corresponding estimates of the impact of sodium reduction on ASCVD risk have not been performed.
The DASH-Sodium trial sought to evaluate the impact of sodium reduction, alone and in combination with the DASH diet, on blood pressure [10]. The study randomized adults with elevated blood pressure or hypertension to the DASH diet or a typical American diet; within each assigned diet, the study randomized each person to a sequence of 3 different sodium levels (low, medium, high). While the trial found that sodium reduction and the DASH diet independently improved blood pressure, [10] the effects of these dietary interventions on 10-year ASCVD risk were not assessed. In this secondary analysis of the DASH-Sodium trial, we evaluated the impact of dietary sodium reduction, alone and in combination with the DASH diet, on 10-year ASCVD risk scores. We hypothesized that sodium reduction alone would decrease risk and that the combined effect of sodium reduction with the DASH diet would be additive.
2. Methods
The DASH-Sodium trial was a multicenter, randomized trial conducted at 4 clinical centers (Baltimore, MD; Boston, MA; Durham, NC; and Baton Rouge, LA) from September 1997 through November 1999 [7,10]. Funded by the National Heart, Lung, and Blood Institute, the study design, protocol, and outcomes have been previously described [7,10]. In brief, The DASH-Sodium trial evaluated the impact of sodium intake with either the DASH diet or typical American diet (control) on blood pressure. The original study protocol was approved by the Institutional Review Boards at each center. All participants provided written, informed consent for study participation and subsequent analyses of their stored biospecimens. The current study was determined by the Institutional Review Board of Beth Israel Deaconess Medical Center to be exempt research (determination number: 2022D000994).
2.1. Participants
The DASH-Sodium trial studied adults aged 22 years and older with an average systolic blood pressure (SBP) of 120–159 mm Hg and an average diastolic blood pressure (DBP) of 80–95 mm Hg. Individuals with heart disease, renal insufficiency, poorly controlled dyslipidemia, insulin-dependent diabetes, excessive alcohol intake, or those on antihypertensive agents were excluded from the trial [10].
2.2. Dietary interventions
Following a parallel design, participants in the DASH-Sodium trial were randomized to either the DASH diet or American (control) diet over a 12-week period. The control diet was modeled to represent the macronutrient, mineral, and fiber consumption of a typical American diet. The DASH diet emphasized fruits, vegetables, whole grains, lean protein, legumes, and nuts while limiting sweets, sugary beverages, red meat, and processed foods. Relative to the control diet, the DASH diet contained more potassium, calcium, magnesium, and fiber [10].
Within each dietary pattern assignment, participants consumed each of 3 different levels of sodium: high (1.6 mg/kcal), intermediate (1.1 mg/kcal), and low (0.5 mg/kcal). Following a crossover design, each participant was randomly assigned 1 of 6 sequences to consume these sodium levels. Sodium intake was calculated based upon individualized participant energy requirements, taking into account body size and activity level. The high sodium level was representative of typical consumption patterns in the United States. The intermediate level reflected the upper limit of guideline recommendations, and the low level was intended to test the effects of sodium intake below guideline recommendations [10].
All participants initially consumed the high-sodium control diet for a 2-week run-in period. Individuals were then randomized to their respective dietary assignment and consumed each level of sodium for an average of 30 days. The study provided all cooked meals and snacks for participants. Each 30-day period was separated by a washout period (on average about 5 days) during which participants could resume their usual diets. Greater than 98 % of participants completed each intervention period [10].
2.3. Outcomes of interest
The primary outcomes of interest were the absolute and relative differences in 10-year ASCVD risk scores derived from the PCE following each 30-day feeding period [11]. Certain static covariates (race, sex) and covariates unlikely to change over the short term trial duration (age, current cigarette smoking, diagnosis of diabetes) were only determined at baseline, whereas dynamic covariates (total cholesterol, HDL, and SBP) were measured at baseline and at the end of each feeding period. Absolute and relative differences were also compared between select diets hypothesized to be healthiest (the DASH-low sodium diet) and the least healthy (the control diet with high sodium).
2.4. Measurements
Seated SBP and DBP were measured simultaneously by trained and certified observers using a random-zero sphygmomanometer. Baseline BP was averaged from readings collected over 5 visits, including 3 visits during the screening phase and 2 visits during the 2-week run-in period. BP measures from the end of each intervention period were averaged from 5 measurements taken at 5 different visits over the last 9 days of each period, with at least 2 of the readings taken within the last 4 days. Fasting blood samples were collected at the end of the run-in period and after each feeding period. Samples were sent for analysis to quantify total cholesterol, HDL cholesterol, and triglycerides, using enzymatic colorimetry, while LDL cholesterol was estimated via the Friedewald equation [12]. Body mass index was determined from height and weight measurements and obesity was defined as a body mass index ≥30 kg/m2.
2.5. Statistical analysis
Population characteristics were summarized overall and by dietary assignment using means and proportions. Ten-year ASCVD risk estimates were calculated at baseline and at the end of each 30-day intervention period. Risk scores were log-transformed due to data skew. The log-transformed values were analyzed to compare the absolute (difference in geometric means) and relative percentage-point difference (exponentiated difference in logarithmic means) from baseline of each respective dietary group (DASH versus control) and at the end of each intervention period across both diets as previously described [9,13].
For the diet effects (DASH versus control), absolute and relative log-transformed ASCVD calculations were compared at 4, 8, and 12-weeks, as participants remained on their assigned diets across all 3 feeding periods. For the sodium effect, absolute and log-transformed ASCVD calculations were estimated after 4 weeks, the duration of each sodium feeding period. Differences between sodium levels (low versus high, low versus medium, medium versus high) were compared within each dietary arm (DASH and control) and overall after observing there was no interaction between diet and sodium level. Comparisons were performed as previously described [9,13]. In brief, mean ASCVD risk values were compared via linear mixed effects models clustered by participant with an exchangeable covariance matrix. The fixed portion of the model included diet assignment, sodium level or visit, and the interaction of these (either diet-by-visit or diet-by-sodium level). The random effects portion of the model incorporated a unique participant identification number, establishing a random intercept.
In the primary analysis, we used the calculated 10-year ASCVD risk score. However, since the PCE is intended for individuals aged 40–79 with an SBP of 90–200 mm Hg, a DBP of 60–130 mm Hg, a total cholesterol of 130–320 mg/dL, and an HDL cholesterol of 20–100 mg/dL, we performed 2 sensitivity analyses to either exclude or impute participants who did not meet these criteria. In the imputed calculation, individual values that did not fall within the intended ranges were substituted for either the minimum or maximum accepted values. For example, those with HDL cholesterol levels below 20 mg/dL were given the level of 20 mg/dL, whereas those with HDL cholesterol levels >100 mg/dL were given the value of 100 mg/dL.
In a pre-specified stratified analysis, we also examined the effects in strata of the following CVD risk factors: age (<60 years and ≥60 years), sex (male or female), race (Black or non-Black), stage 2 hypertension status (≥140/90 mmHg), obesity (body mass index ≥30 kg/m2), and smoking status (current smoker, no or yes). Interaction terms (a three-way interaction term for comparisons of DASH versus control or DASH-low sodium versus control-high sodium) were used to determine the difference between strata.
Analyses were conducted using Stata software version 15.1 (StataCorp LLC, College Station, Texas). Statistical significance was defined as a two-tailed P-value <0.05.
3. Results
3.1. Baseline characteristics
Baseline characteristics overall and by diet assignment are shown below in Table 1. Of the 412 participants in the DASH-Sodium trial, 22 were missing covariates necessary for an ASCVD risk calculation and were excluded from our analysis. There were no meaningful differences in baseline characteristics between the 412 in the randomized trial and the 390 included in current analyses (Supplement Table 1).
Table 1.
Baseline characteristics overall and according to diet assignment.
| Variable | Overall (n=390) | Control (n=193) | DASH (n=197) |
|---|---|---|---|
| Age, years | 48.4 (10.0) | 49.2 (10.3) | 47.6 (9.6) |
| Female, % | 56.9 | 54.4 | 59.4 |
| Black, % | 57.2 | 57.0 | 57.4 |
| BMI ≥ 30 kg/m2, % | 38.7 | 39.9 | 37.6 |
| BMI, kg/m2 | 29.2 (4.8) | 29.5 (5.0) | 28.8 (4.7) |
| Hypertension, % | 40.0 | 39.9 | 40.1 |
| SBP, mm Hg | 134.7 (9.4) | 135.5 (9.4) | 134.0 (9.5) |
| DBP, mm Hg | 85.6 (4.4) | 85.7 (4.1) | 85.5 (4.8) |
| Total Cholesterol, mg/dL | 202.8 (35.4) | 202.6 (34.8) | 203.0 (36.0) |
| HDL cholesterol, mg/dL | 48.4 (13.0) | 48.1 (13.3) | 48.7 (12.7) |
| LDL cholesterol, mg/dL | 131.4 (30.1) | 131.6 (30.8) | 131.2 (29.5) |
| LDL cholesterol, mmol/L | 3.4 (0.8) | 3.4 (0.8) | 3.4 (0.8) |
| Current smoking, % | 10.5 | 10.9 | 10.2 |
Abbreviations: DASH = Dietary Approaches to Stop Hypertension; BMI = Body mass index; SBP = Systolic blood pressure; DBP = Diastolic blood pressure; HDL = High density lipoprotein; LDL = low density lipoprotein.
Values are mean (SD) or %, unless otherwise indicated.
3.2. Effects of the DASH diet
The mean 10-year ASCVD risk score at baseline was 3.13 % among those assigned the control diet and 2.63 % among those assigned the DASH diet. A greater reduction in estimated ASCVD risk was observed during follow-up among those assigned the DASH diet compared to those assigned the control diet (Fig. 1). Compared to the control diet, the DASH diet resulted in an absolute difference in 10-year ASCVD risk scores of −0.12 % [95 % CI −0.23, −0.02] and a relative difference of −5.33 % [95 % CI −9.32, −1.16]. Absolute and proportional intra- and inter-cohort differences are shown below in Table 2. Similar findings were observed in sensitivity analyses excluding or imputing covariates for the PCE (Supplement Tables 2 and 3).
Fig. 1.
Impact of Sodium Reduction on Estimated 10-year ASCVD risk in DASH and Control Diets. ASCVD risk represented on a log scale and generated using generalized estimating equations methods. ASCVD = Atherosclerotic Cardiovascular Disease. Ctrl = Control diet. DASH = Dietary Approaches to Stop Hypertension. Created in BioRender. Knauss, H. (2025) https://BioRender.com/g16w414.
Table 2.
Effects of DASH versus control diets, with and without sodium reduction, on 10-year ASCVD risk score.
| Diet |
Baseline Risk, % |
Follow-up Risk, % |
Change from Baseline |
Difference in Change from Baseline between Diets |
||
|---|---|---|---|---|---|---|
| Absolute, ppt | Relative, % | Absolute, ppt | Relative, % | |||
| Control | 3.13 (0.26) | 2.78 (0.23) | −0.35 | −11.32 | Reference | Reference |
| [−0.46, −0.24] | [−13.99, −8.56] | |||||
| DASH | 2.63 (0.22) | 2.21 (0.18) | −0.42 | −16.04 | −0.12 | −5.33 |
| [−0.52, −0.32] | [−18.55, −13.45] | [−0.23, −0.02] | [−9.32, −1.16] | |||
| Control-High | 3.13 (0.26) | 2.94 (0.24) | −0.19 | −6.05 | Reference | Reference |
| Sodium | [−0.31, −0.07] | [−9.57, −2.39] | ||||
| DASH-Low | 2.63 (0.22) | 2.12 (0.17) | −0.51 | −19.28 | −0.35 | −14.09 |
| Sodium | [−0.63, −0.39] | [−22.28, −16.17] | [−0.49, −0.21] | [−18.59, −9.34] | ||
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; DASH = Dietary Approaches to Stop Hypertension; PPT = percentage points.
Values followed by (SE) or [95 % CI]. There were 390 participants with 1560 ASCVD measurements. Baseline and follow-up risks calculated as geometric means. Absolute differences calculated as difference in geometric means modeled, using a mixed effects model, clustered by participants with exchangeable working matrix. Relative difference determined by exponentiated logarithmic means, using a mixed effects model, clustered by participants with an exchangeable working matrix.
3.3. Effects of sodium reduction
In the groups assigned to the control and DASH diets, low sodium intake resulted in the greatest ASCVD risk score reduction from baseline, relative to high and medium sodium intake (Fig. 1 and Supplement Table 4). There was no evidence that diet modified the effects of low sodium intake on risk (P-interaction =0.26). Thus, we report the overall results. Compared to the high sodium level, the low sodium level changed ASCVD risk by −0.34 percentage-points (95 % CI −0.45, −0.23) among adults assigned the control diet and by −0.17 percentage-points (95 % CI −0.25, −0.08) among adults assigned the DASH diet, whereas the medium sodium level changed ASCVD risk by −0.19 percentage-points (95 % CI −0.29, −0.09) in the control diet and by −0.09 percentage-points in the DASH diet (95 % CI −0.18, −0.01) (Table 3). Sensitivity analyses excluding or imputing PCE covariates demonstrated similar findings (Supplement Tables 5 and 6).
Table 3.
Effects of reducing sodium in control and DASH diets on 10-year ASCVD risk score.
| Sodium |
Overall (Control and DASH) |
Control |
DASH |
|||
|---|---|---|---|---|---|---|
| Level | Difference in Change from Baseline Between Sodium Levels* |
|||||
| Absolute, ppt | Relative, % | Absolute, ppt | Relative, % | Absolute, ppt | Relative, % | |
| LvH | −0.24 | −9.39 | −0.34 | −11.49 | −0.17 | −7.29 |
| [−0.31,−0.17] | [−11.70, −7.02] | [−0.45, −0.23] | [−14.49, −8.38] | [−0.25, −0.08] | [−10.76, −3.69] | |
| LvM | −0.14 | −5.49 | −0.15 | −4.99 | −0.07 | −3.27 |
| [−0.20, −0.07] | [−7.91, −3.02] | [−0.25, −0.04] | [−8.21, −1.65] | [−0.16, 0.01] | [−6.89, 0.49] | |
| MvH | −0.11 | −4.12 | −0.19 | −6.84 | −0.09 | −4.16 |
| [−0.17, −0.04] | [−6.57, −1.61] | [−0.29, −0.09] | [−10.00, −3.57] | [−0.18, −0.01] | [−7.74, −0.43] | |
Abbreviations: PPT = Percentage points; ASCVD, atherosclerotic cardiovascular disease; DASH = Dietary Approaches to Stop Hypertension; LvH = Low versus high sodium intake; LvM = Low versus medium sodium intake; MvH = Medium versus high sodium intake.
Values followed by (SE) or [95 % CI]. Overall baseline ASCVD risk was 2.87 % (standard error, 0.17). For the control diet, baseline ASCVD risk was 3.13 % (standard error, 0.27); for the DASH diet, baseline ASCVD risk was 2.63 % (standard error, 0.21). There were 390 participants with 1560 ASCVD measurements. There were 193 participants on the control diet with 772 ASCVD measurements. There were 197 participants on the DASH diet with 788 ASCVD measurements. Absolute differences calculated as difference in geometric means modeled, using a mixed effects model, clustered by participants with exchangeable working matrix. Relative difference determined by exponentiated logarithmic means, using a mixed effects model, clustered by participants with an exchangeable working matrix.*Sodium levels were compared within each person following a crossover design.
3.4. Combined effects of DASH diet and sodium reduction
Low sodium intake combined with the DASH diet resulted in significantly greater ASCVD risk score reduction than reducing sodium alone, in the control diet (Table 2). Relative to high sodium in combination with the control diet, low sodium intake plus the DASH diet resulted in an absolute difference in 10-year ASCVD risk of −0.35 % percentage-points (95 % CI −0.49, −0.21), or a relative difference of −14.09 % (95 % CI −18.59, −9.34). Similar findings were found with sensitivity analyses (Supplementary Tables 7 and 8).
3.5. Stratified analysis
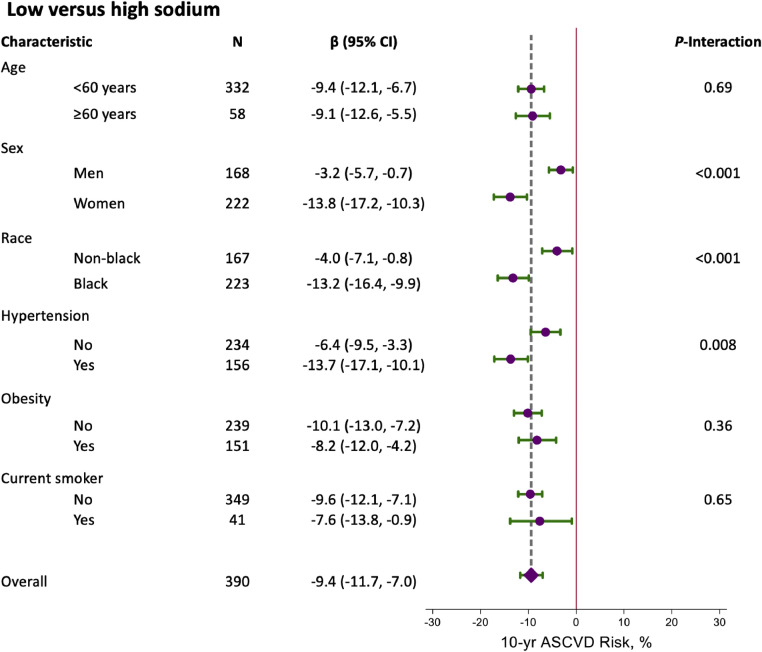
There was no evidence that effects on 10-year ASCVD risk score of the DASH diet versus control or the combined DASH-low sodium diet versus the control-high sodium diet differed by age, sex, race, hypertension, obesity, and smoking status (Supplement Figures 1 and 2). There was evidence that a low versus high sodium diet overall affected ASCVD risk more in women by −13.8 % (95 % CI: −17.2, −10.3) versus −3.2 % (95 % CI: −5.7, −0.7) in men (P-interaction <0.001). In addition, low versus high sodium changed ASCVD risk by −13.2 % (95 % CI: −16.4, −9.9) among Black versus −4.0 % (95 % CI: −7.1, −0.8) among non-Black adults (P-interaction <0.001) (Fig. 2). Effects were also greater among adults with stage 2 hypertension at baseline (−13.7 % versus −6.4 %; P-interaction = 0.008).
Fig. 2.
Relative reduction (95 % CI) in 10-year ASCVD risk score overall and stratified by age, sex, race, hypertension, obesity, and smoking status smoking status for low versus high sodium intake.
4. Discussion
In this population of 390 adults with elevated BP without prior CVD, our findings are three-fold: First, the DASH diet significantly reduced estimated ASCVD risk relative to the control, typical American diet. Second, effects from both DASH and sodium reduction were additive, such that the combination of the DASH diet and low sodium resulted in the greatest decrease in ASCVD risk. Third, compared to a typical high sodium intake level, reducing sodium to recommended levels or even lower levels beyond current recommendations decreased ASCVD risk scores with greater effects among women, Black adults, and participants with baseline stage II hypertension. These findings support prior work, suggesting a role for both sodium reduction and the DASH diet to improve cardiovascular health.
Observational evidence suggests that consumption of the DASH diet is associated with a lower risk of CVD events [[14], [15], [16]]. However, there are no long-term trials of the DASH diet and CVD events. Prior work has shown that the DASH diet lowers CVD risk factors (BP and LDL cholesterol), [7,10] subclinical cardiac injury, [13,17] and ASCVD risk [9]. The present study is consistent with this work, replicating the efficacy of the DASH diet for CVD risk reduction among a distinct study population. Our findings reaffirm the role for the DASH diet in CVD prevention, as recommended by current guidelines [18].
Lower sodium intake is associated with a lower risk of CVD in observational studies [[19], [20], [21]]. Moreover, multiple trials have shown that sodium reduction reduces BP [22]. Recently, the large SSaSS trial showed that reducing sodium through a potassium substitute prevented stroke and coronary heart disease; however, many have attributed this benefit to potassium [23]. There are virtually no trials directly examining the impact of sodium reduction alone on CVD risk or evaluating how different thresholds of reduction relate to CVD risk. The optimal amount of dietary sodium for prevention of CVD remains controversial. Current sodium consumption in the United States is estimated at 3500 mg/day [24]. AHA guidelines advise <1500 mg/day of sodium, though this cutoff has been scrutinized for insufficient support from long-term data [25,26]. Our study demonstrated that sodium reduction at both low and medium levels decreased estimated 10-year ASCVD risk relative to high intake. These findings support the need for ongoing public health policy prioritizing population-wide efforts at salt reduction, even at modest amounts.
Interestingly, the impact of salt reduction was most pronounced among Black adults and women. Race- and sex-based disparities in CVD prevention, presentation, and treatment are well-established. In particular, sodium intake among both Black adults and women is more strongly associated with BP and CVD risk compared with White adults and men, respectively [[27], [28], [29]]. Our findings reinforce the importance of sodium reduction among these groups.
Conversely, the effect of sodium reduction did not significantly differ across strata of age (<60 years and ≥60 years), obesity (body mass index ≥30 kg/m2), and smoking status. The absence of significant differences across these demographic groups may reflect that obesity, age, and smoking contribute to the risk of cardiovascular disease through mechanisms independent of sodium intake. For example, tobacco use is a known independent risk factor for cardiovascular disease and all cause mortality, whereas obesity may mediate disease through distinct metabolic pathways not impacted by sodium intake [1,18].
We found that the combined effects of the DASH diet and sodium reduction decreased 10-year ASCVD risk scores to a greater extent than either intervention alone. This is presumably driven by the additive benefit of each intervention on BP; [10,30] however, the effect of DASH on LDL cholesterol may also play a role [9]. Prior work suggests distinct mechanisms by which the DASH diet and sodium reduction impact CVD risk, with DASH reducing subclinical cardiac damage and systemic inflammation and lower sodium reducing cardiac strain [13]. Ultimately, our findings extend the prior literature and support that adopting a low sodium-DASH diet will result in larger reductions in CVD risk.
This study has several strengths. First, the DASH-Sodium trial was a multi-center randomized control trial with a rigorous design and high follow-up rates. Second, interventions were closely monitored with all meals and snacks provided, minimizing the opportunity to violate respective diet criteria. Third, dietary needs were tailored based upon individual energy requirements, allowing for the evaluation of sodium reduction on ASCVD risk scores irrespective of activity level or weight alterations.
Our study has clinical implications for cardiovascular health. The DASH diet has previously demonstrated a protective role in cardiac injury and mitigates estimated 10-year ASCVD risk. Our findings further expand on prior literature and demonstrates a role for sodium reduction, in addition to the DASH diet, for greater risk reduction. Importantly, benefits of sodium restriction were demonstrated at both low and medium levels of intake. While the greatest benefit was observed with the lowest sodium level, our findings suggest even moderate sodium reduction can be beneficial and may be a more feasible goal to maintain. Moreover, these effects were more pronounced in women and Black adults, reinforcing the importance of a low sodium-DASH diet among these groups.
Limitations of this study should be noted. First, the follow-up time was relatively short, with each sodium intervention lasting 30 days. Second, the study excluded individuals with known heart disease, renal insufficiency, diabetes mellitus (based on prevailing definitions), those with specific dietary needs, or persons on antihypertensive treatment [10]. A significant proportion of Americans meet at least one of these exclusion criteria; thus, our study should be replicated among patients with these conditions. Third, race was dichotomously characterized as Black versus White/other, in a similar fashion recommended by the PCE. The impact of the studied dietary interventions on non-Black minoritized groups cannot be determined from this study. Fourth, the PCE is an imperfect scoring system and does not account for all risk factors of ASCVD; thus, results may not be applied to patients with genetic or other CVD risk factors excluded from the PCE. Of note, the recent Predicting Risk of Cardiovascular Disease Events (PREVENT) calculator aims to address limitations of current risk calculators by incorporating cardiometabolic traits and social determinants of health [31]; however, our study did not collect all of the covariates (i.e. serum creatinine for estimated glomerular filtration rate) necessary for the PREVENT equations. Additionally, PREVENT has yet to be adopted by clinical practice guidelines for management of hypertension and dyslipidemia. Finally, while BP was assessed throughout the study, other PCE covariates, such as smoking or diabetes status, were only assessed at baseline. Moreover, we assumed that participants did not have diabetes, but it is possible they might have had undiagnosed or non-insulin dependent diabetes. While changes in these covariates over the relatively short study period were unlikely, they were not monitored.
In conclusion, in this population with elevated BP, the DASH diet and sodium reduction both significantly decreased estimated 10-year ASCVD risk. These effects were additive when combining both interventions versus either alone. Further, the impact of sodium reduction on 10-year ASCVD risk was most pronounced in women, Black adults, and participants with baseline stage II hypertension. Our findings reinforce the need for public efforts to promote adoption of DASH eating habits and sodium reduction to mitigate CVD risk.

Central illustration created in BioRender. Knauss, H. (2025) https://BioRender.com/g16w414.
Sources of funding
The measurement of cardiac biomarkers was supported by the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) R21HL144876. The original DASH trial was supported by grants (HL50981, HL50968, HL50972, HL50977, HL50982, HL02642, RR02635, and RR00722) from the NHLBI, the Office of Research on Minority Health, and the National Center for Research Resources of the NIH. One author was supported by NIH/ NHLBI K23HL135273 and R21HL144876.
CRediT authorship contribution statement
Hanna M. Knauss: Writing – review & editing, Writing – original draft, Visualization, Conceptualization. Lara C Kovell: Writing – review & editing, Validation, Methodology, Investigation, Conceptualization. Edgar R. Miller: Writing – review & editing, Methodology, Investigation, Conceptualization. Lawrence J. Appel: Writing – review & editing, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization. Kenneth J Mukamal: Writing – review & editing, Methodology, Investigation, Conceptualization. Timothy B Plante: Writing – review & editing, Methodology, Investigation, Conceptualization. Stephen P. Juraschek: Writing – review & editing, Writing – original draft, Validation, Supervision, Software, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Stephen Juraschek, MD, PhD reports financial support was provided by National Institutes of Health. Stephen Juraschek, MD, PhD reports financial support was provided by American Heart Association. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are indebted to the study participants for their sustained commitment during the DASH-Sodium Trial. Central illustration created in BioRender. Knauss, H. (2025) https://BioRender.com/g16w414.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2025.100980.
Appendix. Supplementary materials
References
- 1.Tsao C.W., Aday A.W., Almarzooq Z.I., Anderson C.A.M., Arora P., Avery C.L., Baker-Smith C.M., Beaton A.Z., Boehme A.K., Buxton A.E., Commodore-Mensah Y., Elkind M.S.V., Evenson K.R., Eze-Nliam C., Fugar S., Generoso G., Heard D.G., Hiremath S., Ho J.E., Kalani R., Kazi D.S., Ko D., Levine D.A., Liu J., Ma J., Magnani J.W., Michos E.D., Mussolino M.E., Navaneethan S.D., Parikh N.I., Poudel R., Rezk-Hanna M., Roth G.A., Shah N.S., St-Onge M.P., Thacker E.L., Virani S.S., Voeks J.H., Wang N.Y., Wong N.D., Wong S.S., Yaffe K., Martin S.S. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2023 update: a report from the American Heart Association. Circulation. 2023;147(8):e93–e621. doi: 10.1161/CIR.0000000000001123. Feb 21Epub 2023 Jan 25. Erratum in: Circulation. 2023 Feb 21;147(8):e622. Erratum in: Circulation. 2023 Jul 25;148(4):e4. PMID: 36695182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Cardiovascular Risk Consortium. C Magnussen, Ojeda F.M., Leong D.P., Alegre-Diaz J., Amouyel P., Aviles-Santa L., De Bacquer D., Ballantyne C.M., Bernabé-Ortiz A., Bobak M., Brenner H., Carrillo-Larco R.M., de Lemos J., Dobson A., Dörr M., Donfrancesco C., Drygas W., Dullaart R.P., Engström G., Ferrario M.M., Ferrières J., de Gaetano G., Goldbourt U., Gonzalez C., Grassi G., Hodge A.M., Hveem K., Iacoviello L., Ikram M.K., Irazola V., Jobe M., Jousilahti P., Kaleebu P., Kavousi M., Kee F., Khalili D., Koenig W., Kontsevaya A., Kuulasmaa K., Lackner K.J., Leistner D.M., Lind L., Linneberg A., Lorenz T., Lyngbakken M.N., Malekzadeh R., Malyutina S., Mathiesen E.B., Melander O., Metspalu A., Miranda J.J., Moitry M., Mugisha J., Nalini M., Nambi V., Ninomiya T., Oppermann K., d'Orsi E., Pająk A., Palmieri L., Panagiotakos D., Perianayagam A., Peters A., Poustchi H., Prentice A.M., Prescott E., Risérus U., Salomaa V., Sans S., Sakata S., Schöttker B., Schutte A.E., Sepanlou S.G., Sharma S.K., Shaw J.E., Simons L.A., Söderberg S., Tamosiunas A., Thorand B., Tunstall-Pedoe H., Twerenbold R., Vanuzzo D., Veronesi G., Waibel J., Wannamethee S.G., Watanabe M., Wild P.S., Yao Y., Zeng Y., Ziegler A., Blankenberg S. Global effect of modifiable risk factors on cardiovascular disease and mortality. N Engl J Med. 2023;389(14):1273–1285. doi: 10.1056/NEJMoa2206916. Oct 5Epub 2023 Aug 26. PMID: 37632466; PMCID: PMC10589462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yusuf S., Joseph P., Rangarajan S., Islam S., Mente A., Hystad P., Brauer M., Kutty V.R., Gupta R., Wielgosz A., AlHabib K.F., Dans A., Lopez-Jaramillo P., Avezum A., Lanas F., Oguz A., Kruger I.M., Diaz R., Yusoff K., Mony P., Chifamba J., Yeates K., Kelishadi R., Yusufali A., Khatib R., Rahman O., Zatonska K., Iqbal R., Wei L., Bo H., Rosengren A., Kaur M., Mohan V., Lear S.A., Teo K.K., Leong D., O'Donnell M., McKee M., Dagenais G. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;395(10226):795–808. doi: 10.1016/S0140-6736(19)32008-2. Mar 7Epub 2019 Sep 3. Erratum in: Lancet. 2020 Mar 7;395(10226):784. PMID: 31492503; PMCID: PMC8006904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian F., Chen L., Qian Z.M., Xia H., Zhang Z., Zhang J., Wang C., Vaughn M.G., Tabet M., Lin H. Ranking age-specific modifiable risk factors for cardiovascular disease and mortality: evidence from a population-based longitudinal study. EClinicalMedicine. 2023;64 doi: 10.1016/j.eclinm.2023.102230. Sep 27PMID: 37936651; PMCID: PMC10626167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lloyd-Jones D.M., Ning H., Labarthe D., Brewer L., Sharma G., Rosamond W., Foraker R.E., Black T., Grandner M.A., Allen N.B., Anderson C., Lavretsky H., Perak A.M. Status of cardiovascular health in US adults and children using the American Heart Association's new "life's essential 8" metrics: prevalence estimates from the National Health and Nutrition Examination Survey (NHANES), 2013 through 2018. Circulation. 2022;146(11):822–835. doi: 10.1161/CIRCULATIONAHA.122.060911. Sep 13Epub 2022 Jun 29. Erratum in: Circulation. 2022 Nov 15;146(20):e298. PMID: 35766033. [DOI] [PubMed] [Google Scholar]
- 6.Lichtenstein A.H., Appel L.J., Vadiveloo M., Hu F.B., Kris-Etherton P.M., Rebholz C.M., Sacks F.M., Thorndike A.N., Van Horn L., Wylie-Rosett J. 2021 Dietary guidance to improve cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2021;144(23):e472–e487. doi: 10.1161/CIR.0000000000001031. Dec 7Epub 2021 Nov 2. PMID: 34724806. [DOI] [PubMed] [Google Scholar]
- 7.Appel L.J., Moore T.J., Obarzanek E., Vollmer W.M., Svetkey L.P., Sacks F.M., Bray G.A., Vogt T.M., Cutler J.A., Windhauser M.M., Lin P.H., Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–1124. doi: 10.1056/NEJM199704173361601. Apr 17. PMID: 9099655. [DOI] [PubMed] [Google Scholar]
- 8.Belanger M.J., Kovell L.C., Turkson-Ocran R.A., Mukamal K.J., Liu X., Appel L.J., Miller E.R.3rd, Sacks F.M., Christenson R.H., Rebuck H., Chang A.R., Juraschek S.P. Effects of the dietary approaches to stop hypertension diet on change in cardiac biomarkers over time: results from the DASH-sodium trial. J Am Heart Assoc. 2023;12(2) doi: 10.1161/JAHA.122.026684. Jan 17Epub 2023 Jan 11. PMID: 36628985; PMCID: PMC9939071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong S.Y., Wee C.C., Kovell L.C., Plante T.B., Miller E.R.3rd, Appel L.J., Mukamal K.J., Juraschek S.P. Effects of diet on 10-year atherosclerotic cardiovascular disease risk (from the DASH Trial) Am J Cardiol. 2023;187:10–17. doi: 10.1016/j.amjcard.2022.10.019. Jan 15Epub 2022 Nov 29. PMID: 36459731; PMCID: PMC10122756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacks F.M., Svetkey L.P., Vollmer W.M., Appel L.J., Bray G.A., Harsha D., Obarzanek E., Conlin P.R., Miller E.R.3rd, Simons-Morton D.G., Karanja N., Lin P.H., DASH-Sodium Collaborative Research Group Effects on blood pressure of reduced dietary sodium and the Dietary approaches to stop hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. PMID: 11136953. [DOI] [PubMed] [Google Scholar]
- 11.Goff D.C., Jr, Lloyd-Jones D.M., Bennett G., Coady S., D'Agostino R.B., Sr, Gibbons R., Greenland P., Lackland D.T., Levy D., O'Donnell C.J., Robinson J.G., Schwartz J.S., Shero S.T., Smith S.C., Jr, Sorlie P., Stone N.J., Wilson P.W.F. ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;63(25 Pt B):2935–2959. doi: 10.1016/j.jacc.2013.11.005. 2014 Jul 1Epub 2013 Nov 12. Erratum in: J Am Coll Cardiol. 2014 Jul 1;63(25 Pt B):3026. PMID: 24239921; PMCID: PMC4700825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. JunPMID: 4337382. [PubMed] [Google Scholar]
- 13.Juraschek S.P., Kovell L.C., Appel L.J., Miller, Sacks F.M., Chang A.R., Christenson R.H., Rebuck H., Mukamal K.J. Effects of diet and sodium reduction on cardiac injury, strain, and inflammation: the DASH-sodium trial. J Am Coll Cardiol. 2021;77(21):2625–2634. doi: 10.1016/j.jacc.2021.03.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fung T.T., Chiuve S.E., McCullough M.L., Rexrode K.M., Logroscino G., Hu F.B. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168(7):713–720. doi: 10.1001/archinte.168.7.713. Apr 14Erratum in: Arch Intern Med. 2008 Jun 23;168(12):1276. PMID: 18413553. [DOI] [PubMed] [Google Scholar]
- 15.Sotos-Prieto M., Bhupathiraju S.N., Mattei J., Fung T.T., Li Y., Pan A., Willett W.C., Rimm E.B., Hu F.B. Association of changes in diet quality with total and cause-specific mortality. N Engl J Med. 2017;377(2):143–153. doi: 10.1056/NEJMoa1613502. Jul 13PMID: 28700845; PMCID: PMC5589446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiavaroli L., Viguiliouk E., Nishi S.K., Blanco Mejia S., Rahelić D., Kahleová H., Salas-Salvadó J., Kendall C.W., Sievenpiper J.L. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11(2):338. doi: 10.3390/nu11020338. Feb 5PMID: 30764511; PMCID: PMC6413235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juraschek S.P., Kovell L.C., Appel L.J., Miller E.R.3rd, Sacks F.M., Christenson R.H., Rebuck H., Chang A.R., Mukamal K.J. Associations between dietary patterns and subclinical cardiac injury: an observational analysis from the DASH trial. Ann Intern Med. 2020;172(12):786–794. doi: 10.7326/M20-0336. Jun 16Epub 2020 May 19. PMID: 32423348; PMCID: PMC7388686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J., Himmelfarb C.D., Khera A., Lloyd-Jones D., McEvoy J.W., Michos E.D., Miedema M.D., Muñoz D., Smith S.C., Jr, Virani S.S., Williams K.A., Sr, Yeboah J., Ziaeian B. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical Practice Guidelines. Circulation. 2019;140(11):e596–e646. doi: 10.1161/CIR.0000000000000678. Sep 10Epub 2019 Mar 17. Erratum in: Circulation. 2019 Sep 10;140(11):e649-e650. Erratum in: Circulation. 2020 Jan 28;141(4):e60. Erratum in: Circulation. 2020 Apr 21;141(16):e774. PMID: 30879355; PMCID: PMC7734661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook N.R., Cutler J.A., Obarzanek E., Buring J.E., Rexrode K.M., Kumanyika S.K., Appel L.J., Whelton P.K. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP) BMJ. 2007;334(7599):885–888. doi: 10.1136/bmj.39147.604896.55. Apr 28Epub 2007 Apr 20. PMID: 17449506; PMCID: PMC1857760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cook N.R., Appel L.J., Whelton P.K. Sodium intake and all-cause mortality over 20 years in the trials of hypertension prevention. J Am Coll Cardiol. 2016;68(15):1609–1617. doi: 10.1016/j.jacc.2016.07.745. Oct 11PMID: 27712772; PMCID: PMC5098805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma Y., He F.J., Sun Q., Yuan C., Kieneker L.M., Curhan G.C., MacGregor G.A., Bakker S.J.L., Campbell N.R.C., Wang M., Rimm E.B., Manson J.E., Willett W.C., Hofman A., Gansevoort R.T., Cook N.R., Hu F.B. 24-Hour urinary sodium and potassium excretion and cardiovascular risk. [DOI] [PMC free article] [PubMed]
- 22.Filippini T., Malavolti M., Whelton P.K., Naska A., Orsini N., Vinceti M. Blood pressure effects of sodium reduction: dose-response meta-analysis of experimental studies. Circulation. 2021;143(16):1542–1567. doi: 10.1161/CIRCULATIONAHA.120.050371. Apr 20Epub 2021 Feb 15. PMID: 33586450; PMCID: PMC8055199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin X., Paige E., Tian M., Li Q., Huang L., Yu J., Rodgers A., Elliott P., Wu Y., Neal B. The proportion of dietary salt replaced with potassium-enriched salt in the SSaSS: implications for scale-up. Hypertension. 2023;80(5):956–965. doi: 10.1161/HYPERTENSIONAHA.122.20115. MayEpub 2023 Jan 11. PMID: 36628969. [DOI] [PubMed] [Google Scholar]
- 24.Hu J.R., Sahni S., Mukamal K.J., Millar C.L., Wu Y., Appel L.J., Juraschek S.P. Dietary sodium intake and sodium density in the United States: estimates from NHANES 2005-2006 and 2015-2016. Am J Hypertens. 2020;33(9):825–830. doi: 10.1093/ajh/hpaa104. Sep 10PMID: 32619231; PMCID: PMC7481988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cook N.R., Appel L.J., Whelton P.K. Lower levels of sodium intake and reduced cardiovascular risk. Circulation. 2014;129(9):981–989. doi: 10.1161/CIRCULATIONAHA.113.006032. Mar 4Epub 2014 Jan 10. PMID: 24415713; PMCID: PMC4181831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.IOM (Institute of Medicine) National Academies Press; Washington, DC: 2013. Sodium intake in populations: assessment of evidence. [PubMed] [Google Scholar]
- 27.Yuan Y.E., Haas A.V., Rosner B., Adler G.K., Williams G.H. Elevated blood pressure and aldosterone dysregulation in young black women versus white women on controlled sodium diets. J Clin Endocrinol Metab. 2024;109(2):e773–e779. doi: 10.1210/clinem/dgad512. Jan 18PMID: 37650607; PMCID: PMC10795929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barris C.T., Faulkner J.L., Belin de Chantemèle E.J. Salt sensitivity of blood pressure in women. Hypertension. 2023;80(2):268–278. doi: 10.1161/HYPERTENSIONAHA.122.17952. FebEpub 2022 Aug 23. PMID: 35997024; PMCID: PMC9851945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howard G., Cushman M., Moy C.S., Oparil S., Muntner P., Lackland D.T., Manly J.J., Flaherty M.L., Judd S.E., Wadley V.G., Long D.L., Howard V.J. Association of clinical and social factors with excess hypertension risk in black compared with white US adults. JAMA. 2018;320(13):1338–1348. doi: 10.1001/jama.2018.13467. Oct 2PMID: 30285178; PMCID: PMC6233849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juraschek S.P., Miller E.R.3rd, Weaver C.M., Appel L.J. Effects of sodium reduction and the DASH diet in relation to baseline blood pressure. J Am Coll Cardiol. 2017;70(23):2841–2848. doi: 10.1016/j.jacc.2017.10.011. Dec 12Epub 2017 Nov 12. PMID: 29141784; PMCID: PMC5742671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan S.S., Coresh J., Pencina M.J., Ndumele C.E., Rangaswami J., Chow S.L., Palaniappan L.P., Sperling L.S., Virani S.S., Ho J.E., Neeland I.J., Tuttle K.R., Rajgopal Singh R., Elkind M.S.V., Lloyd-Jones D.M.; American Heart Association. Novel prediction equations for absolute risk assessment of total cardiovascular disease incorporating cardiovascular-kidney-metabolic health: a scientific statement from the American Heart Association. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.