Abstract
Congenital factor VII (FVII) deficiency is a rare autosomal recessive bleeding disorder characterized by prolonged prothrombin time (PT) and reduced FVII coagulant activity (FVII: C). Here, we present the case of a middle-aged male patient with gastrointestinal bleeding, who exhibited prolonged PT and decreased FVII: C levels. Gene sequencing analysis revealed compound heterozygous mutations in the F7 gene: c.64G > A (p.V22I) and c.506-1G > A. Based on the laboratory results and gene sequencing, the patient was diagnosed as FVII deficiency. After adding recombinant activated FVII (rFVIIa) for several days, the laboratory indicators returned to normal and the bleeding symptoms were relieved. In subsequent validation studies, we also identified the c.506-1G > A mutation in his older sister and daughter. Importantly, this represents the first documented case where both mutations coexist concurrently. Additionally, our literature review reveals that approximately 50% of mutation types associated with congenital FVII deficiency are located on exon 9; however, there is no significant correlation between the reduction in FVII: C levels and severity of clinical symptoms based on EAHAD database analysis.
Keywords: Congenital factor VII deficiency, Prothrombin time, EAHAD, Case report
Introduction
Factor VII (FVII) deficiency is a rare autosomal recessive disorder, with an occurrence probability of 1 in 500,000 [1]. FVII deficiency is characterized by a hemorrhagic disorder in which the FVII coagulant activity (FVII: C) falls below 70% [2]. This condition can be further classified into type I and type II based on the decrease in both FVII: C and FVII antigen level (FVII: Ag). In type I, both FVII: C and FVII: Ag decrease, while in type II only FVII: C declines while FVII: Ag remains normal. Although measuring FVII: Ag can help distinguish between type I and type II deficiencies, it does not provide information about propensity to bleed. Laboratory diagnostic criteria include a normal activated partial thromboplastin time (APTT) and prolonged prothrombin time (PT), while clinical heterogeneity is a characteristic feature of the disease, with manifestations ranging from asymptomatic to fatal severe bleeding. The regulation of FVII levels is influenced by genetic polymorphisms within the FVII gene [3]. FVII is a vitamin K-dependent serine protease that binds to tissue factors to activate exogenous clotting pathways. Insufficient levels of FVII result in abnormal clotting leading to bleeding episodes. Guglielmo et al. have reported common presentations of mild bleeding symptoms such as nosebleeds, bleeding gums, menstrual bleeding, and post-abrasion bleeding, whereas central nervous system (CNS) bleeds are less frequent [4]. Treatment for this disorder primarily involves supplementation with FVII through methods such as transfusion of fresh frozen plasma (FFP), plasma-derived FVII concentrates, recombinant activated FVII (rFVIIa), and prothrombin complex concentrates (PCCs). Here, we report on family with congenital FVII deficiency. The proband, a 54-year-old male patient, presented with severely low levels of FVII: C along with gastrointestinal bleeding. Following next generation sequencing (NGS) analysis, he received a diagnosis of congenital FVII deficiency. Figure 1 displays the progenitor pedigree of the family.
Fig. 1.
The pedigree of the family
Case presentation
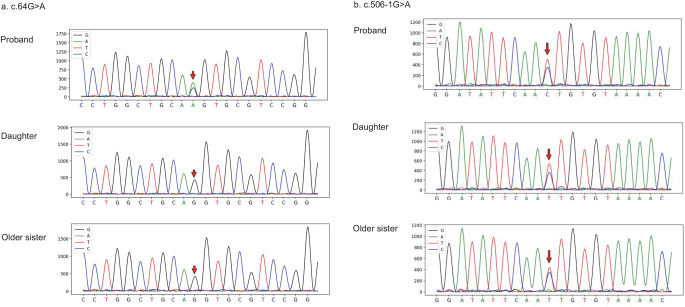
A 54-year-old male patient presented to the hematology department due to abnormal coagulation detected before undergoing a colonoscopy. He had experienced bloody stools for 10 days, with melena mixed with fresh blood, and an estimated blood loss of approximately 5 mL. Upon obtaining a detailed medical history, it was discovered that the patient had been experiencing nosebleeds for five to six years, without visiting a doctor. The patient has not taken any medications, such as anticoagulants or antiplatelet drugs, that could affect coagulation function. Furthermore, the patient’s older brother died from a cerebral hemorrhage, while the cause of hemorrhage of his parents remained unknown. Complete coagulation function tests revealed prolonged PT at 34.2 s (reference range 9–13 s), increased international normalized ratio (INR) at 3.35 (reference range 0.8–1.2), reduced FVII: C at 12% (reference range 55–170%), elevated FVIII: C at 182% (reference range 60–150%), and decreased FXIII: C at 64.5% (reference range 75.2-154.8%). To determine whether the FVII deficiency in the patient was congenital or acquired, NGS was performed on both the patient and his living relatives, excluding deceased family members such as older brother and parents but including daughter and older sister. Following peripheral blood analysis using NGS, heterozygous mutations were identified in chromosome13:113105905 c.64G > A (p.V22I) and chromosome13:113116765 c.506-1G > A. These mutations were found within the F7 gene (Fig. 1), which is associated with FVII deficiency. Additionally, a heterozygous variant of C.1851 A > C (p.R617S) was detected on chromosome1: 197,040,623 in the F13B gene, which is related to deficiency of the XIII-B subunit; meanwhile, the patient also exhibited reduced FXIII activity. All three variants are classified as of unknown significance according to the American College of Medical Genetics and Genomics (ACMG) classification. Furthermore, Sanger sequencing confirmed that the older sister and daughter also carried the heterozygous mutation of c.506-1G > A (Fig. 2); however, no clinical symptoms were observed in either individual. Subsequent treatment with rFVIIa (1 mg every 4 h after the first dose of 2 mg) resulted in both PT and FVII: C returning to normal levels. After the patient’s coagulation function normalized, a colonoscopy was performed, revealing multiple colonic polyps ranging in size from 0.3 to 0.6 cm. The polyps were subsequently removed. During the follow-up, the patient’s coagulation function remained normal, and the symptoms of gastrointestinal (GI) and nasal bleeding were controlled.
Fig. 2.
Gene sequencing and verification results. c.64G > A (p.V22I) and c.506-1G > A are marked with a red row
Discussion
Our analysis of the F7 gene, situated on chromosome 13 within the EAHAD database, inclusive of cases up to 2022, unveiled 271 mutation types across 1058 instances, primarily comprising missense mutations [5]. Figure 3 exhibits the mutation sites and respective frequencies across all cases within the database. The highest density of mutant gene variants was observed on exon 9, with c.1238G > A on exon 9 being the most prevalent mutant gene overall. After excluding unpublished literature, the incidence among 1046 patients from European and American populations (75.81%) was three times greater than that in the Asian population (24.19%).
Fig. 3.

The mutation sites and the number of mutations for all cases in the EAHAD database
In the literature review on FVII deficiency, it was found that the mutations c.64G > A [6–9] and c.506-1G > A [6, 10–16] have been reported in multiple studies. Importantly, this represents the initial case report in where two heterozygous mutations, c.64G > A (p.V22I) and c.506-1G > A, were simultaneously present, with site validation conducted among close relatives.
Within the EHAHD database, 700 patients were categorized according to the severity of clinical manifestations, adhering to the criteria outlined by Mariani et al. in 2005 (a. Severe: central nervous system (CNS) bleeding and/or GI bleeding and/or hemarthrosis; b. Moderate: three or more symptoms excluding CNS and/or GI bleeding and/or hemarthrosis; c. Mild: one or two symptoms excluding CNS and/or GI bleeding and/or hemarthrosis) [17]. This classification system offers a standardized framework for assessing the severity of FVII deficiency and its related symptoms, facilitating more precise diagnosis and treatment planning. Of the patients, 295 were asymptomatic, 151 had mild symptoms, 83 exhibited moderate symptoms, and 171 presented severe symptoms. Table 1 presents a comprehensive summary of the gene mutation locations and the severity of clinical symptoms for each individual case. The data elucidates patterns of symptom severity based on the mutation locations. Mutations in exons 3, 5 and introns 3, 5 and 7 predominantly result in severe symptoms. In contrast, mutations in introns 1 and 6 are typically linked to mild symptoms. Mutations in exons 1, 6, 8 and 9, along with intron 8, flanking (5’) and UTR (5’), were identified in asymptomatic patients. Only one mutation case has been reported for intron 4, and it resulted in a severe outcome. Likewise, exon 2 presents a single mutation case, characterized by mild symptoms. Interestingly, only one mutation site was identified spanning exons 1–9, and this particular case presented with asymptomatic symptoms. No mutation site was found on intron 2. The occurrence of asymptomatic (32.61%) and severe (36.96%) patients on exon 7 was comparable. The Chi-square test was employed to statistically analyze the relationship between mutation sites and the severity of clinical symptoms (Tabe 1). The results demonstrated a significant statistical correlation between mutation sites and the severity of clinical symptoms (P < 0.0001). This finding suggests that the various manifestations of mutation sites may, to some extent, determine the severity of clinical symptoms. Specifically, mutation sites may significantly impact the clinical phenotype of the disease, further validating the potential role of genetic mutations in its development. This patient carries both mutations and, based on the severity of clinical symptoms, is classified as having a severe form of the disease. In the EHAHD database, cases with either the c.64G > A or c.506-1G > A mutation are typically asymptomatic or exhibit mild to moderate symptoms. This patient harbors both mutations in a compound heterozygous state, leading to prolonged prothrombin time (PT) without an early onset of bleeding symptoms. Additionally, the patient has the F13B c.1851 A > C mutation and colon polyps, which may contribute to worsening GI bleeding symptoms.
Table 1.
The location of mutated genes and the clinical severity
| location | exon1 | exon2 | exon3 | exon4 | exon5 | exon6 | exon7 | exon8 | exon9 | intron1 | inton2 | intron3 | intron4 | intron5 | intron6 | intron7 | intron8 | flanking (5’) | UTR (5’) | exon1-9 | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| clinical severity | |||||||||||||||||||||
| asymptomatic | 7(41.17%) | 0 | 9(24.32%) | 0 | 7(20.59%) | 62(48.44%) | 15(32.61%) | 61(64.21%) | 295(44.10%) | 3(25.00%) | 0 | 3(27.27%) | 0 | 2(14.29%) | 3(23.08%) | 2(15.38%) | 34(54.84%) | 52(54.17%) | 3(50.00%) | 1(100%) | < 0.0001 |
| mild | 5(29.41%) | 1(100%) | 9(24.32%) | 1(100%) | 7(20.59%) | 31(24.22%) | 12(26.09%) | 20(21.05%) | 145(21.67%) | 7(58.33%) | 0 | 2(18.18%) | 0 | 2(14.29%) | 5(38.46%) | 3(23.08%) | 15(24.19%) | 22(22.92%) | 0 | 0 | |
| moderate | 1(5.88%) | 0 | 5(13.51%) | 0 | 2(5.88%) | 5(3.91%) | 2(4.35%) | 2(2.11%) | 106(15.84%) | 0 | 0 | 1(9.09%) | 0 | 1(7.14%) | 2(15.38%) | 0 | 3(4.83%) | 5(5.21%) | 2(33.33%) | 0 | |
| severe | 4(23.52%) | 0 | 14(37.84%) | 0 | 18(52.94%) | 30(23.44%) | 17(36.96%) | 12(12.63%) | 123(18.39%) | 2(16.67%) | 0 | 5(45.45%) | 4(100%) | 9(64.29%) | 3(23.08%) | 8(61.54%) | 10(16.13%) | 17(17.71%) | 1(16.67%) | 0 | |
| total | 17 | 1 | 37 | 1 | 34 | 128 | 46 | 95 | 669 | 12 | 0 | 11 | 4 | 14 | 13 | 13 | 62 | 96 | 6 | 1 |
UTR: untranslated region
Clinical severity was according to Mariani et al. 2005 (a. Severe: CNS bleeding and/or GI bleeding and/or hemarthrosis; b. Moderate: three or more symptoms excluding CNS and/or GI bleeding and/or hemarthrosis; c. Mild: one or two symptoms excluding CNS and/or GI bleeding and/or hemarthrosis)
Table 2 illustrates the correlation between FVII: C levels and the severity of clinical symptom. Among asymptomatic patients, the FVII: C proportion was below 1%, with the majority concentrated between 1% and 50%. Among those with FVII: C levels below 10%, 14.91% were asymptomatic, 29.24% were mild, 13.16% were moderate, and 42.69% were severe. Asymptomatic to mild bleeding predominates in individuals with FVII: C levels between 10% and 50%. Most patients exhibiting FVII: C levels greater than 50% were asymptomatic. Chi-square test analysis showed a significant association between FVII: C levels and clinical severity (P < 0.0001), indicating statistical significance.
Table 2.
The relationship between FVII: C and the severity of clinical symptom
| FVII: C | < 1% | 1–10% | 10–30% | 30–50% | > 50% | total | P |
|---|---|---|---|---|---|---|---|
| clinical severity | |||||||
| asymptomatic | 4 | 47 | 49 | 62 | 26 | 188 | < 0.0001 |
| mild | 41 | 59 | 17 | 23 | 5 | 145 | |
| moderate | 11 | 34 | 10 | 10 | 1 | 66 | |
| severe | 83 | 63 | 2 | 3 | 0 | 151 | |
| total | 139 | 203 | 78 | 98 | 32 | 550 |
FVII is a vitamin K-dependent glycoprotein that plays a critical role in the extrinsic pathway of the coagulation cascade. Following endothelial injury, tissue factor (TF) is exposed to plasma components, and binds to circulating FVII, forming the TF-FVIIa complex [18]. Sylvester et al. reported a case in which a patient with an FVII level of 21% undergoing cardiac surgery received intraoperative administration of 4-factor PCC at a dose of 25 units/kg as a source of FVII. One hour after administration, the FVII level increased from 27–58% [19]. Currently, no clear recommendation exists for the use of vitamin K to treat FVII deficiency. Given the patient’s FVII deficiency and potential bleeding risks, we will continue to assess the need for the use of PCC or rFVIIa as part of long-term care.
We believe that individuals who have experienced spontaneous bleeding events need to be monitored for clotting abnormalities, not only for their own well-being but also for the health of their relatives. Although NGS could not be performed after his older brother died from a brain hemorrhage, we suspect that FVII deficiency may have been the underlying cause of the hemorrhage.
The limitations of this study primarily stem from the small sample size, as the research is limited to a single case and its family members, which restricts the generalizability of the results. Additionally, the absence of screening in a larger population limits the applicability of the conclusions. We acknowledge the lack of in vitro functional validation studies to further confirm the pathogenicity of these mutations. Future research could enhance the reliability and generalizability of the results by incorporating a larger sample size and conducting functional validation studies. Furthermore, with respect to the literature review, there may be selection bias, as the reported cases predominantly focus on individuals with more prominent clinical symptoms.
Conclusion
Individuals experiencing spontaneous bleeding, including nosebleeds, gingival bleeding, easy bruising, increased menstruation, and other unprovoked bleeding, should be assessed for the potential absence of coagulation factors, which may indicate underlying coagulation abnormalities, following the exclusion relevant organic lesions. Diagnosing patients with congenital FVII deficiency requires, in addition to standard laboratory tests, NGS to analyze their genetic profile. Specifically for patients already diagnosed with the condition, NGS of their close relatives is essential to confirm the presence of mutation sites, enabling timely diagnosis and treatment of high-risk individuals when symptoms appear.
Acknowledgements
We are grateful to the patient and his family for their cooperation in regard to this report.
Author contributions
Y.J. contributed to literature writing, data collection and chart creation; X.Y. contributed to article revision and methodology. X.L. contributed to article review and revision. All authors approved the final version of the paper.
Funding
This work was supported by the Liaoning Provincial Central Government guides local science and technology development special projects [grant number 2023JH6/100200006].
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical standards and consent to publish declaration
The studies involving participant was reviewed and approved by the Ethics Committee of The First Hospital of China Medical University. The patient and his family provided their written informed consent to take part in this study. A copy of the written consent is available for review by the editorial office of this journal.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Palla R, Peyvandi F, Shapiro AD (2015) Rare bleeding disorders: diagnosis and treatment [J]. Blood 125(13):2052–2061 [DOI] [PubMed] [Google Scholar]
- 2.Sevenet PO, Kaczor DA, Depasse F, Factor VII, Deficiency (2017) From basics to clinical laboratory diagnosis and patient management [J]. Clin Appl Thromb Hemost 23(7):703–710 [DOI] [PubMed] [Google Scholar]
- 3.Bernardi F, Mariani G (2021) Biochemical, molecular and clinical aspects of coagulation factor VII and its role in hemostasis and thrombosis [J]. Haematologica 106(2):351–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mariani G, Bernardi F, Factor VII, Deficiency (2009) [J] Semin Thromb Hemost 35(4):400–406 [DOI] [PubMed] [Google Scholar]
- 5.Giansily-Blaizot M, Rallapalli PM, Perkins SJ et al (2020) The EAHAD blood coagulation factor VII variant database [J]. Hum Mutat 41(7):1209–1219 [DOI] [PubMed] [Google Scholar]
- 6.Kwon MJ, Yoo KY, Lee KO et al (2011) Recurrent mutations and genotype-phenotype correlations in hereditary factor VII deficiency in Korea [J]. Blood Coagul Fibrinolysis 22(2):102–105 [DOI] [PubMed] [Google Scholar]
- 7.S S, R M, K K, et al. Molecular characterization of Iranian patients with inherited coagulation factor VII deficiency. Balkan J Med Genet, (2017) 20(2): 19–26 [DOI] [PMC free article] [PubMed]
- 8.Wulff K, Herrmann FH (2000) Twenty two novel mutations of the factor VII gene in factor VII deficiency [J]. Hum Mutat 15(6):489–496 [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Wang S, Leng S et al (2021) Novel factor VII gene mutations in six families with hereditary coagulation factor VII deficiency [J]. J Clin Lab Anal 35(9):e23905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang M, Wang Z, Yu Z et al (2011) A novel missense mutation close to the charge-stabilizing system in a patient with congenital factor VII deficiency [J]. Blood Coagul Fibrinolysis 22(4):264–270 [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, Lee HJ, Bin JH et al (2009) A novel homozygous missense mutation in the factor VII gene of severe factor VII deficiency in a newborn baby [J]. Blood Coagul Fibrinolysis 20(2):161–164 [DOI] [PubMed] [Google Scholar]
- 12.Li R, Zhang M, Wang Z et al (2015) [Analysis of the gene mutation in an inherited FVII deficiency patient] [J]. Zhonghua Yi Xue Za Zhi 95(18):1401–1404 [PubMed] [Google Scholar]
- 13.Liu H, Wang HF, Cheng ZP et al (2015) Phenotypic and genotypic characterization of four factor VII deficiency patients from central China [J]. Blood Coagul Fibrinolysis 26(4):408–413 [DOI] [PubMed] [Google Scholar]
- 14.Peng W, Zhang S, Liu X et al (2016) [Mutation analysis and prenatal diagnosis for a family affected with congenital factor VII deficiency] [J]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 33(3):357–360 [DOI] [PubMed] [Google Scholar]
- 15.Quintavalle G, Riccardi F, Rivolta GF et al (2017) F7 gene variants modulate protein levels in a large cohort of patients with factor VII deficiency. Results from a genotype-phenotype study [J]. Thromb Haemost 117(8):1455–1464 [DOI] [PubMed] [Google Scholar]
- 16.Yu T, Wang X, Ding Q et al (2009) Using a minigene approach to characterize a novel splice site mutation in human F7 gene causing inherited factor VII deficiency in a Chinese pedigree [J]. Haemophilia 15(6):1262–1266 [DOI] [PubMed] [Google Scholar]
- 17.Mariani G, Herrmann FH, Dolce A et al (2005) Clinical phenotypes and factor VII genotype in congenital factor VII deficiency [J]. Thromb Haemost 93(3):481–487 [DOI] [PubMed] [Google Scholar]
- 18.Muller MP, Morrissey JH, Tajkhorshid E (2022) Molecular view into Preferential binding of the factor VII Gla domain to phosphatidic acid [J]. Biochemistry 61(16):1694–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sylvester KW, Couper G, Connors JM (2015) Prothrombin complex concentrate for factor VII replacement in a patient undergoing left ventricular assist device implantation with factor VII deficiency [J]. Am J Hematol 90(9):E185 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.