Abstract
Background
Preserved ratio impaired spirometry (PRISm) refers to a form of lung function deterioration, and previous studies have established the association with Chronic Obstructive Pulmonary Disease (COPD). Research has also shown the association between COPD and lipid metabolism disturbances. Despite these findings, the association between lipid metabolism markers and PRISm remains poorly understood.
Methods
This analysis was conducted on the 2007–2012 data from the National Health and Nutrition Examination Survey (NHANES), including a total of 9,431 participants. The Non-High-Density Lipoprotein Cholesterol to High-Density Lipoprotein Cholesterol Ratio (NHHR) was calculated based on lipid profiles, and PRISm patients were classified according to pulmonary function tests. To explore the association between NHHR and PRISm, multivariable logistic regression analysis was used.
Results
A strong linear association was observed between NHHR and PRISm. In Adjusted Model 2, the weighted multivariable logistic regression analysis revealed that each unit increase in NHHR increased the chance of developing PRISm by 8% (OR:1.08, 95%CI:1.01–1.16, P = 0.039).Participants within the highest NHHR tertile demonstrated a 1.36-fold increased likelihood of presenting with PRISm compared to those in the lowest NHHR tertile (OR:1.36, 95% CI: 1.01–1.83, P = 0.048). Additionally, weighted Restricted Cubic Spline affirmed a linear association between NHHR and PRISm (P for non-linearity = 0.637), while clear non-linear associations were found between NHHR and FEV1% predicted (P for non-linearity = 0.010) and FEV1/FVC (P for non-linearity = 0.023). Subgroup analysis and interaction tests revealed a significant interaction effect among different waist circumference categories (P for interaction = 0.020). Notably, in individuals without abdominal obesity, NHHR showed a strong positive association with PRISm (OR = 1.23, 95% CI: 1.07–1.42, P = 0.01).
Conclusion
These results indicate that NHHR is positively associated with PRISm and is significantly associated with the decline in lung function. This study offers distinctive perspectives that may contribute to the avoidance and management of early-stage pulmonary dysfunction.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-025-02571-0.
Keywords: NHHR, PRISm, NHANES, Lipid metabolism, Cross-sectional study
Background
Preserved ratio impaired spirometry (PRISm) is diagnosed when an individual has a normal FEV1/FVC but lower lung volumes as measured by spirometry (FEV1/FVC ≥ 0.7, FEV1 < 80% predicted) [1]. In 2023, this term was officially adopted by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) as a replacement for the "unclassified" category [2], offering a clear understanding of the diminished ventilatory efficiency seen in these patients. The concept's introduction is supported by clinical evidence showing that these patients may quickly progress to Chronic Obstructive Pulmonary Disease (COPD) [3]. Nevertheless, not all PRISm patients will acquire COPD [4, 5], and the condition’s reversibility highlights the importance of early intervention to manage lung function deterioration. Epidemiological data indicates that PRISm affects between 5 and 20% of the population [6–11]. Without timely intervention, the progression of these cases could significantly increase the prevalence of COPD, thus placing a greater strain on social and healthcare systems. Research also shows that PRISm is strongly association with a range of other health conditions, including asthma [12], chronic kidney disease [13], metabolic disorders [14], cardiovascular diseases [15], and diabetes [16].
The Association between lipid metabolism disorders and impaired lung function is multifaceted. Evidence suggests that abnormalities in triglyceride (TG) and high-density lipoprotein cholesterol (HDL-C) levels may play a role in the decline of lung function [17]. A reduction in HDL-C is strongly associated with an increase in the release of inflammatory mediators [18]. Moreover, lipid metabolism disorders lead to the buildup of lipid peroxidation products, which cause damage to airway tissues [19]. Even more importantly, obesity resulting from lipid metabolism disruptions limits chest expansion and increases airway resistance [20, 21]. However, most of the research so far has focused on severe or irreversible stages of lung dysfunction, with limited studies addressing early-stage lung impairment. This study primarily explores the association between early lung function decline and lipid metabolism disorders, with the goal of controlling disease progression at an early stage.
The non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) is a novel biomarker for assessing atherosclerotic lipid composition [22]. In recent years, it has proven to be a valuable predictive marker in diseases such as angina pectoris [23], diabetes [24], cancer [25], and obstructive sleep apnea [26]. Unlike the LDL-C/HDL-C ratio, which primarily reflects cholesterol transport [27], and the TG/HDL-C ratio, which is associated with insulin resistance and triglyceride-rich lipoproteins [28], NHHR provides a more integrated assessment of lipid metabolism. Notably, it offers enhanced sensitivity in evaluating high-density lipoprotein cholesterol metabolism. Population-based studies have confirmed its superior ability to predict cardiovascular risk, systemic inflammation, and metabolic dysfunction.
In the screening and assessment of PRISm, NHHR may prove to be a useful instrument. Research has shown that PRISm patients often exhibit abnormal lipid levels [29]. Dyslipidemia can intensify oxidative stress, promote endothelial dysfunction, and ultimately lead to lung tissue damage, all of which may accelerate PRISm progression. NHHR has been closely associated with systemic inflammation, a key contributor to airway and parenchymal remodeling in PRISm [15]. Furthermore, NHHR-related lipid imbalances may impair pulmonary microvascular function, reduce lung compliance and exacerbating ventilation-perfusion mismatch, thereby increasing cardiovascular risk in PRISm patients. Interestingly, a growing body of cohort studies has reported a higher incidence of cardiovascular disease in PRISm patients [30, 31]. As a result, understanding the association between PRISm and cardiovascular metabolism is crucial for assessing its prognosis and remains an important area of ongoing research. While these findings require further confirmation, some researchers suggest that lipid metabolism disorders could be an underlying mechanism contributing to the decline in lung function in PRISm patients [32].
This research evaluated the association between NHHR, lung function indices, and PRISm using data from a representative sample from the National Health and Nutrition Examination Survey (NHANES) datasets.
Materials and methods
Study cohort
This research employed data spanning three distinct periods (2007–2012) from the NHANES database for a cross-sectional analysis of United States (U.S.) adults. Health and demographic data were collected from the general U.S. population through a complex random sampling approach. All NHANES datasets used in this study were approved by the Ethical Review Board of the National Center for Health Statistics, with informed consent obtained from every participant [33]. Initially, 30,442 participants were screened, and after applying the exclusion criteria, 9,431 eligible individuals were included. The following are the exclusion criteria:1) age under 20 years (n = 12,729); 2) missing height data (n = 846); 3) missing pulmonary function data (n = 3,531); 4) pulmonary function quality control below grade B (n = 1,747); 5) FEV1/FVC < 0.7 (n = 1,512); 6) missing total cholesterol (TC) or HDL-C data (n = 486); 7) use of lipid-lowering medications for at least 180 days (n = 160). These factors could affect either lung function or lipid metabolism, potentially confounding the association between NHHR and lung function [34] (Fig. 1).
Fig. 1.
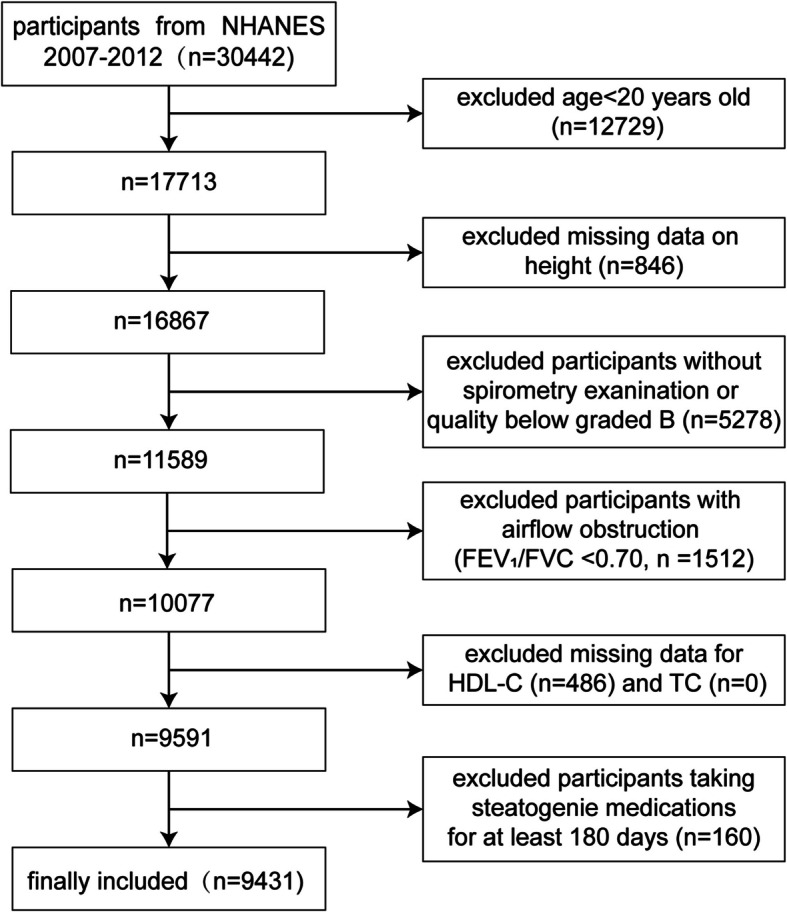
Flowchart of the study participants
Exposure definition
In this study, NHHR [22] was treated as the exposure variable, calculated based on the participants' lipid profiles. The formula used for calculation is: (TC-HDL)/HDL. The NHHR data were divided into three categories: Q1 (representing low levels), Q2 (representing moderate levels), and Q3 (representing high levels) for additional research.
Outcome definition
PRISm is characterized by a reduced predicted value of Forced Expiratory Volume in 1 s (FEV1%) while the ratio of Forced Expiratory Volume in 1 s to Forced Vital Capacity (FEV1/FVC) remains preserved [1], and was used as the outcome variable in this study. We collected pulmonary function data and used the NHANES III equations to derive predicted lung volume measurements for various racial groups. The crucial pulmonary function parameters include FEV1 and FVC [35]. All pulmonary function tests in this study were performed by well-trained technicians in accordance with the guidelines of the American Thoracic Society (ATS). The test quality was categorized from A to F (Supplementary Table 1). To enhance data accuracy and reliability, only tests meeting ATS data collection criteria with a quality control grade of B or higher were included.
Covariates
According to previous studies [36, 37], factors related to lipid metabolism and lung function were considered. These include five categories: (1) Demographic characteristics: age, sex, race, educational attainment, marital status, poverty-to-income ratio (PIR). (2) Body measurements: body mass index (BMI) and waist circumference (WC). (3) Inflammatory and biochemical markers: white blood cell count (WBC), lymphocyte count, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TB) and glycated hemoglobin (HBA1c). (4) Lifestyle factors: smoking status and alcohol habit. (5) Comorbidities: hypertension, diabetes, heart failure, coronary heart disease, cancer, arthritis, chronic respiratory diseases, and recent infection episodes. Further details on these covariates are provided in Supplementary Table 2.
Statistical analysis
This study is a large-scale cross-sectional analysis, designed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines. Following the recommendations from the Centers for Disease Control and Prevention, all statistical analyses were conducted using weights. The missing data for covariates was evaluated, revealing that the missing values were less than 5%. Consequently, we employed chained equations for multiple imputations to address the missing covariates and randomly selected one dataset from the five imputed datasets for analysis. Continuous variables were analyzed using weighted t-tests and are presented as means with standard deviations (SD), while categorical variables were assessed with chi-square tests and are shown as percentages (%). Additionally, a weighted multivariable logistic regression model was constructed to assess the association of NHHR with PRISm, results reported as odds ratios (OR) and 95% confidence intervals (CI). This study also established three models: an Unadjusted Model that does not adjust for any covariates, an Adjusted Model 1, which accounts for baseline demographic factors such as age, sex, race, marital status, education attainment, PIR, BMI, and WC, and an Adjusted Model 2, which incorporates a comprehensive set of covariates, including lifestyle factors (alcohol habit and smoking status), metabolic and cardiovascular conditions (hypertension, diabetes, heart failure, and coronary heart disease), comorbidities (cancer, arthritis, and chronic respiratory diseases), recent infection episodes, and laboratory parameters (WBC, lymphocyte count, ALT, AST, TB and HBA1c). To explore potential linear or nonlinear association among NHHR, PRISm, and lung function, weighted restricted cubic splines (RCS) analysis were employed. Furthermore, to investigate whether PRISm is influenced by other factors, this study conducted weighted subgroup analyses and interaction tests to determine if the association between NHHR and PRISm was consistent across populations. This study used R software (version 4.4.2) for all statistical analyses. P-value < 0.05 were considered significant.
Results
Baseline characteristics
Over the three cycles between 2007 and 2012, a total of 9,431 eligible participants were selected from the NHANES database. The overall prevalence of PRISm was found to be 8.62%. Within the study participants, 47.00% were male, and the median age was 43 years.
When participants were stratified by NHHR tertiles, those in the Q3 group exhibited a greater tendency to be male, around 44 years old, and more likely to have a partner. They also tended to have a lower socioeconomic status, characterized by lower income and education levels. Compared to the Q1 group, the prevalence of obesity in the Q3 group more than doubled. Significant variations were also observed in inflammatory and biochemical markers. In terms of lifestyle and comorbidities, individuals in the Q3 group were more likely to have a history of smoking and were at greater risk of conditions such as hypertension, diabetes, and arthritis. Moreover, PRISm was significantly more prevalent in the Q3 group than in the Q1 group (P < 0.05). Lung function parameters, including FVC and FEV1, also showed significant disparities, with participants in the Q3 group presenting higher FVC and FEV1 values than those in the Q1 group (P < 0.05). Further details are available in Table 1. The total weighted sample size in this study was 131,602,235 individuals, categorized into Q1, Q2, and Q3 groups based on NHHR levels, Supplementary Table 3 provides the weighted participant counts for each group.
Table 1.
Baseline study population characteristics (weighted)
| Characteristic | Total (n = 9431) | Q1 (n = 3144) | Q2 (n = 3144) | Q3 (n = 3143) | P-value |
|---|---|---|---|---|---|
| Sex (%) | < 0.001 | ||||
| Male | 4433 (47.00%) | 1027 (32.67%) | 1467 (46.66%) | 1939 (61.69%) | |
| Female | 4998 (53.00%) | 2117 (67.33%) | 1677 (53.34%) | 1204 (38.31%) | |
| Age (year) | 43 [31, 56] | 40 [28, 56] | 44 [31, 57] | 44 [34,55.25] | < 0.001 |
| Race (%) | < 0.001 | ||||
| Mexican American | 1663 (17.63%) | 426 (13.55%) | 544 (17.30%) | 693 (22.05%) | |
| Other Hispanic | 1083 (11.48%) | 298 (9.48%) | 385 (12.25%) | 400 (12.73%) | |
| Non-Hispanic White | 3934 (41.71%) | 1315 (41.83%) | 1270 (40.39%) | 1349 (42.92%) | |
| Non-Hispanic Black | 1907 (20.22%) | 782 (24.87%) | 660 (20.99%) | 465 (14.79%) | |
| Other Race | 844 (8.95%) | 323 (10.27%) | 285 (9.06%) | 236 (7.51%) | |
| Poverty-to-income ratio (%) | < 0.001 | ||||
| < 1 | 1959 (20.77%) | 664 (21.12%) | 607 (19.31%) | 688 (21.89%) | |
| > = 1, < = 3 | 3809 (40.39%) | 1166 (37.09%) | 1278 (40.65%) | 1365 (43.43%) | |
| > 3 | 3663 (38.84%) | 1314 (41.79%) | 1259 (40.04%) | 1090 (34.68%) | |
| Marital status (%) | < 0.001 | ||||
| Married/Living with Partner | 5693 (60.36%) | 1726 (54.90%) | 1896 (60.31%) | 2071 (65.89%) | |
| Without Partner | 3738 (39.64%) | 1418 (45.10%) | 1248 (39.69%) | 1072 (34.11%) | |
| Educational attainment (%) | < 0.001 | ||||
| Less than high school | 2229 (23.63%) | 593 (18.86%) | 738 (23.47%) | 898 (28.57%) | |
| High School Grad/GED or equivalent | 2054 (21.78%) | 600 (19.08%) | 699 (22.23%) | 755 (24.02%) | |
| Above high school | 5148 (54.59%) | 1951 (62.05%) | 1707 (54.29%) | 1490 (47.41%) | |
| Body mass index (%) | < 0.001 | ||||
| < = 24.9 kg/m2 | 2600 (27.57%) | 1460 (46.44%) | 735 (23.38%) | 405 (12.89%) | |
| > 24.9, < = 29.9 kg/m2 | 3169 (33.60%) | 921 (29.29%) | 1126 (35.81%) | 1122 (35.70%) | |
| > 29.9 kg/m2 | 3662 (38.83%) | 763 (24.27%) | 1283 (40.81%) | 1616 (51.42%) | |
| Waist circumference (%) | < 0.001 | ||||
| Male < 102 cm, female < 88 cm | 4238 (44.94%) | 1868 (59.41%) | 1298 (41.28%) | 1072 (34.11%) | |
| Male > = 102 cm, female > = 88 cm | 5193 (55.06%) | 1276 (40.59%) | 1846 (58.72%) | 2071 (65.89%) | |
| HBA1c (%) | < 0.001 | ||||
| < 5.7% | 6264 (66.42%) | 2366 (75.25%) | 2086 (66.35%) | 1812 (57.65%) | |
| > = 5.7% | 3167 (33.58%) | 778 (24.75%) | 1058 (33.65%) | 1331 (42.35%) | |
| ALT (U/L) | 26.551 ± 22.501 | 22.459 ± 22.401 | 25.583 ± 16.911 | 31.613 ± 26.233 | < 0.001 |
| AST (U/L) | 26.312 ± 19.589 | 25.433 ± 22.959 | 25.424 ± 12.321 | 28.078 ± 21.630 | < 0.001 |
| Bilirubin (total), umol/L | 12.754 ± 5.103 | 12.926 ± 5.238 | 12.639 ± 4.883 | 12.699 ± 5.178 | 0.582 |
| White blood cell (1000 cells/uL) | 7.115 ± 2.316 | 6.631 ± 1.960 | 7.077 ± 2.090 | 7.638 ± 2.715 | < 0.001 |
| Lymphocyte (1000 cells/uL) | 2.164 ± 0.778 | 2.016 ± 0.672 | 2.159 ± 0.819 | 2.318 ± 0.804 | < 0.001 |
| Smoking status (%) | < 0.001 | ||||
| Yes | 3898 (41.33%) | 1134 (36.07%) | 1272 (40.46%) | 1492 (47.47%) | |
| No | 5533 (58.67%) | 2010 (63.93%) | 1872 (59.54%) | 1651 (52.53%) | |
| Alcohol habit (%) | 0.104 | ||||
| > = 12 drinks/year | 7077 (75.04%) | 2329 (74.08%) | 2310 (73.47%) | 2438 (77.57%) | |
| < 12 drinks/year | 2354 (24.96%) | 815 (25.92%) | 834 (26.53%) | 705 (22.43%) | |
| Hypertension (%) | < 0.001 | ||||
| Yes | 3134 (33.23%) | 877 (27.89%) | 1075 (34.19%) | 1182 (37.61%) | |
| No | 6297 (66.77%) | 2267 (72.11%) | 2069 (65.81%) | 1961 (62.39%) | |
| Diabetes (%) | 0.015 | ||||
| Yes | 899 (9.53%) | 263 (8.37%) | 317 (10.08%) | 319 (10.15%) | |
| No | 8532 (90.47%) | 2881 (91.63%) | 2827 (89.92%) | 2824 (89.85%) | |
| Heart failure (%) | 0.225 | ||||
| Yes | 123 (1.30%) | 36 (1.15%) | 50 (1.59%) | 37 (1.18%) | |
| No | 9308 (98.70%) | 3108 (98.85%) | 3094 (98.41%) | 3106 (98.82%) | |
| Coronary heart disease (%) | 0.170 | ||||
| Yes | 160 (1.70%) | 49 (1.56%) | 50 (1.59%) | 61 (1.94%) | |
| No | 9271 (98.30%) | 3095 (98.44%) | 3094 (98.41%) | 3082 (98.06%) | |
| Arthritis (%) | 0.004 | ||||
| Yes | 1899 (20.14%) | 583 (18.54%) | 662 (21.06%) | 654 (20.81%) | |
| No | 7532 (79.86%) | 2561 (81.46%) | 2482 (78.94%) | 2489 (79.19%) | |
| Cancer (%) | 0.560 | ||||
| Yes | 541 (5.74%) | 194 (6.17%) | 172 (5.47%) | 175 (5.57%) | |
| No | 8890 (94.26%) | 2950 (93.83%) | 2972 (94.53%) | 2968 (94.43%) | |
| Chronic respiratory diseases (%) | 0.307 | ||||
| Yes | 1225 (12.99%) | 435 (13.84%) | 392 (12.47%) | 398 (12.66%) | |
| No | 8206 (87.01%) | 2709 (86.16%) | 2752 (87.53%) | 2745 (87.34%) | |
| Recent infection episodes (%) | 0.547 | ||||
| Yes | 362 (3.84%) | 114 (3.63%) | 115 (3.66%) | 133 (4.23%) | |
| No | 9069 (96.16%) | 3030 (96.37%) | 3029 (96.34%) | 3010 (95.77%) | |
| FEV1/FVC (%) | 80.50 ± 5.40 | 81.20 ± 5.80 | 80.20 ± 5.30 | 80.00 ± 4.90 | < 0.001 |
| FEV1 (%) | 98.00 ± 13.30 | 99.90 ± 13.50 | 97.80 ± 13.20 | 96.30 ± 13.00 | < 0.001 |
| FVC (ml) | 3949.39 ± 1054.35 | 3842.47 ± 1022.77 | 3928.47 ± 1067.32 | 4077.28 ± 1059.34 | < 0.001 |
| FEV1 (ml) | 3175.34 ± 859.67 | 3118.82 ± 853.88 | 3148.56 ± 866.42 | 3258.66 ± 852.59 | < 0.001 |
| PRISm (%) | < 0.001 | ||||
| Yes | 813 (8.62%) | 221 (7.03%) | 273 (8.68%) | 319 (10.15%) | |
| No | 8618 (91.38%) | 2923 (92.97%) | 2871 (91.32%) | 2824 (89.85%) | |
A stratified comparison of participant characteristics based on the presence of PRISm showed that Individuals with PRISm tended to be older and had lower income and education levels. Metabolically, they were more likely to be obese, especially with abdominal obesity, and had a higher prevalence of abnormal glycated hemoglobin levels. Their inflammatory markers were also generally elevated compared to the general population. In terms of lifestyle, they were less likely to consume alcohol but more likely to have a history of smoking. Additionally, they had a greater likelihood of comorbid conditions, including hypertension, diabetes, arthritis, coronary heart disease, heart failure, and chronic respiratory diseases. Moreover, PRISm patients had higher NHHR indices, with most of them falling into the Q3 group of NHHR levels, as shown in Supplementary Table 4.
Likelihood of PRISm occurring is associated with NHHR
This study demonstrated a significant association between NHHR and PRISm. In the weighted multivariable logistic regression analysis, Unadjusted Model revealed that each 1-unit increase in NHHR was associated with a 16% increased risk of developing PRISm (95% CI: 1.10, 1.22, P < 0.001). Upon Adjusted Model 1, the risk remained elevated by 11% (95% CI: 1.03, 1.19, P = 0.005). In Adjusted Model 2, each point increase in NHHR was associated with an 8% increase in the incidence of PRISm (95% CI: 1.01, 1.16, P = 0.039). Stratification by NHHR tertiles (Q1, Q2, Q3) showed that in Adjusted Model 2, participants in Q3 had 1.36 times higher odds of presenting with PRISm than those in Q1 (95% CI: 1.01, 1.83, P = 0.048). This association remained statistically significant in both Adjusted Model 1 and Unadjusted Model, with trend tests across all models consistently confirming significance (Table 2).
Table 2.
The association between NHHR and PRISm (weighted)
| Unadjusted Model | Adjusted Model 1 | Adjusted Model 2 | |||
|---|---|---|---|---|---|
| Outcome variables | OR (95%CI), P-value | ||||
| NHHR | 1.16 (1.10, 1.22), < 0.001 | 1.11(1.03,1.19), 0.005 | 1.08(1.01,1.16), 0.039 | ||
| NHHR Tertiles | |||||
| Q1 | Reference | Reference | Reference | ||
| Q2 | 1.28(1.00,1.63), 0.052 | 1.12(0.86,1.47), 0.084 | 1.08(0.83,1.45), 0.591 | ||
| Q3 | 1.76(1.38,2.26), < 0.001 | 1.45(1.08,1.95), 0.014 | 1.36(1.01,1.83), 0.048 | ||
| P for trend | < 0.001 | 0.011 | 0.039 | ||
The linear and nonlinear associations between NHHR, PRISm, and lung function parameters
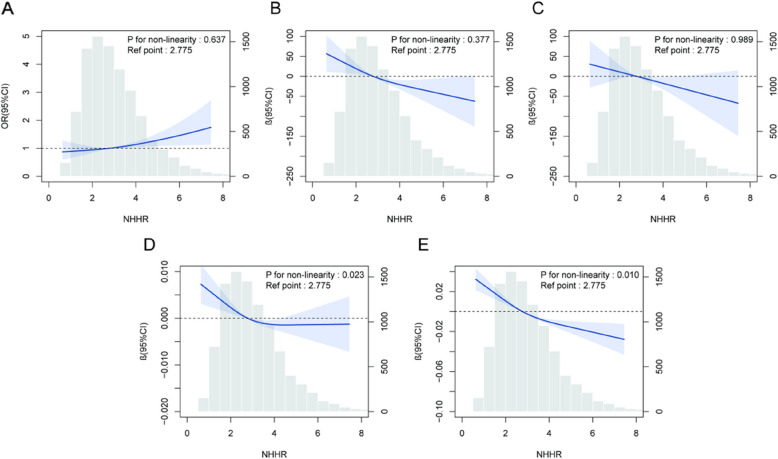
This study applied weighted RCS analysis to examine the likely linear association between NHHR and PRISm in Adjusted Model 2. The outcomes show a linear association between NHHR and PRISm (P for non-linearity = 0.637), indicating that each unit increases in NHHR, the risk of PRISm rises proportionally (Fig. 2A). This study also analyzed the association between NHHR and lung function parameters. NHHR showed a linear association with FVC and FEV1 (NHHR and FEV1: Fig. 2B; NHHR and FVC: Fig. 2C), while there was a clear nonlinear association between NHHR and FEV1% predicted value as well as FEV1/FVC (NHHR and FEV1/FVC: Fig. 2D; NHHR and FEV1%: Fig. 2E). More specifically, when the NHHR was at low to moderate levels, changes in FEV1/FVC and predicted FEV1% were relatively stable. However, when NHHR surpassed 2.775, the decline in lung function metrics accelerated significantly, indicating an accelerated risk. This result remained consistent after adjusting for all confounders, reinforcing that NHHR not only serves as a linear indicator of early PRISm risk but also reflects the complex, nonlinear dynamics of lung function decline (Fig. 2).
Fig. 2.
Exposure–response associations for NHHR and PRISm derived by restricted cubic spline modeling (weighted). A A linear association exists between NHHR and PRISm. B A linear association exists between FEV1 and PRISm. C A linear association exists between FVC and PRISm. D A non-linear association exists between NHHR and FEV1/FVC. E A non-linear association exists between NHHR and FEV1%
Subgroup analysis
We evaluated the association between PRISm and NHHR across different populations using weighted interaction tests and subgroup analysis. The subgroup analysis based on waist circumference revealed a significant interaction effect (P for interaction = 0.020). Specifically, among individuals without abdominal obesity, NHHR showed a significant positive association with PRISm (OR = 1.23, 95% CI: 1.07–1.42, P = 0.010). However, this association was not statistically significant in individuals with abdominal obesity. In contrast, stratified analyses based on demographic, lifestyle, and clinical factors—including sex, race, marital status, education attainment, PIR, BMI, smoking status and alcohol habit, as well as histories of hypertension, diabetes, heart failure, coronary heart disease, chronic respiratory diseases, recent infection episodes, arthritis, and cancer—showed no significant variation in the NHHR-PRISm association (P for interaction > 0.05). This suggests that the association between NHHR and PRISm is generally stable across different subgroups, with a significant interaction observed only in the waist circumference-based analysis (Table 3).
Table 3.
Subgroup analysis of association between NHHR and PRISm risk(weighted)
| NHHR | PRISm | ||
|---|---|---|---|
| Subgroup | OR (95%CI) | P-value | P for interaction |
| Sex (%) | 0.119 | ||
| Male | 1.16 (1.07, 1.27) | < 0.01 | |
| Female | 0.96 (0.86, 1.08) | 0.48 | |
| Race (%) | 0.612 | ||
| Mexican American | 1.01 (0.86, 1.19) | 0.87 | |
| Other Hispanic | 1.00 (0.82, 1.23) | 0.97 | |
| Non-Hispanic White | 1.09 (0.99, 1.20) | 0.06 | |
| Non-Hispanic Black | 1.04 (0.89, 1.22) | 0.58 | |
| Other Race | 1.13 (0.93, 1.38) | 0.19 | |
| Educational attainment (%) | 0.504 | ||
| Less Than high school | 1.04 (0.90, 1.19) | 0.60 | |
| High school grad/GED or equivalent | 1.06 (0.88, 1.27) | 0.53 | |
| Above high school | 1.11 (1.01, 1.22) | 0.03 | |
| Poverty-to-income ratio (%) | 0.065 | ||
| < 1 | 1.21 (1.08, 1.36) | < 0.01 | |
| > = 1, < = 3 | 1.01 (0.89, 1.15) | 0.82 | |
| > 3 | 1.08 (0.96, 1.22) | 0.19 | |
| Marital status (%) | 0.866 | ||
| Married/Living with partner | 1.08 (0.99, 1.19) | 0.07 | |
| Without partner | 1.05 (0.90, 1.22) | 0.52 | |
| Body mass index (%) | 0.284 | ||
| < = 24.9 kg/m2 | 1.17 (0.96, 1.44) | 0.12 | |
| > 24.9, < = 29.9 kg/m2 | 1.12 (0.95, 1.32) | 0.16 | |
| > 29.9 kg/m2 | 1.05 (0.96, 1.15) | 0.30 | |
| Waist circumference (%) | 0.020 | ||
| Male < 102 cm, female < 88 cm | 1.23 (1.07, 1.42) | 0.01 | |
| Male > = 102 cm, female > = 88 cm | 1.03 (0.94, 1.12) | 0.54 | |
| HBA1c (%) | 0.976 | ||
| < 5.7% | 1.07 (0.95, 1.20) | 0.26 | |
| > = 5.7% | 1.07 (0.95, 1.22) | 0.25 | |
| Alcohol habit (%) | 0.364 | ||
| yes | 1.10 (1.00, 1.21) | 0.05 | |
| no | 1.03 (0.89, 1.18) | 0.72 | |
| Smoking status (%) | 0.793 | ||
| Yes | 1.11 (0.99, 1.24) | 0.07 | |
| No | 1.04 (0.93, 1.16) | 0.49 | |
| Hypertension (%) | 0.316 | ||
| Yes | 1.04(0.92, 1.17) | 0.55 | |
| No | 1.11(0.99, 1.24) | 0.06 | |
| Diabetes (%) | 0.381 | ||
| Yes | 1.03 (0.88, 1.20) | 0.71 | |
| No | 1.09 (1.01, 1.18) | 0.03 | |
| Heart failure (%) | 0.823 | ||
| Yes | 0.92 (0.56, 1.53) | 0.75 | |
| No | 1.08 (1.01, 1.16) | 0.04 | |
| Coronary heart disease (%) | 0.103 | ||
| Yes | 0.78 (0.48, 1.27) | 0.26 | |
| No | 1.08 (1.01, 1.16) | 0.03 | |
| Arthritis (%) | 0.761 | ||
| Yes | 1.11 (0.98, 1.27) | 0.1 | |
| No | 1.08 (1.00, 1.17) | 0.05 | |
| Cancer (%) | 0.529 | ||
| Yes | 0.91 (0.68, 1.23) | 0.53 | |
| No | 1.09 (1.01, 1.17) | 0.03 | |
| Chronic respiratory diseases (%) | 0.562 | ||
| Yes | 1.12 (0.96, 1.31) | 0.13 | |
| No | 1.07 (0.98, 1.16) | 0.11 | |
| Recent infection episodes (%) | 0.098 | ||
| Yes | 1.02 (0.79, 1.33) | 0.85 | |
| No | 1.09 (1.01, 1.17) | 0.03 | |
Discussion
This large cross-sectional cohort analysis, which included 9,431 participants from the 2007–2012 NHANES database, found a strong independent association between NHHR and PRISm. In multivariable logistic regression analysis, each one-unit increase in NHHR was strongly associated with the incidence of PRISm. Furthermore, RCS analyses indicated a strong linear association between NHHR and PRISm in all subjects, whereas the association with lung function indices (the FEV1/FVC and FEV1% predicted) showed a significant non-linear trend. Specifically, Subgroup analyses and interaction tests further enhanced the reliability of these findings. Subgroup analysis and interaction testing confirmed a significant interaction effect across different WC categories. The association between NHHR and PRISm was stronger and positively associated with individuals without abdominal obesity.
PRISm, an unstable state of lung function, is primarily characterized by impaired lung capacity [15, 38]. However, the underlying mechanisms of PRISm remain unclear, and there are no established methods for early clinical prevention. Nuria and colleagues found [39] low eosinophil levels and high glycated hemoglobin were observed in the PRISm population, pointing to the possibility that metabolic abnormalities may be more prominent than typical inflammatory responses in this group. This finding challenges the conventional view that associates wheezing and asthma-related inflammation as the primary pathological mechanism. Additionally, our research revealed that NHHR has a significant impact on airflow limitation and reduced lung capacity, suggesting that NHHR may serve as a more sensitive indicator of lung function changes prior to the onset of PRISm. Current research indicates that COPD progresses through multiple stages [40], with the longitudinal decline in FEV1 serving as an early indicator of the transition from GOLD stage 0 (early COPD) to clinical COPD [41]. This pattern of lung function decline mirrors the characteristics seen in PRISm.
Unlike previous studies, this is the first study to establish an association between NHHR, an indicator reflecting both atherosclerotic and protective aspects of lipid metabolism, and PRISm. Previous research has primarily focused on the association between standard lipid indicators and cardiovascular and metabolic disorders; however, growing evidence suggests that lipid metabolism disorders may also affect lung function [42–45]. Specific lipid molecules can regulate lung tissue cells in a manner that is dependent on cell type [46], which may have varied impacts on lung function impairment. Moreover, lung function decline is often accompanied by lipid metabolism disturbance [47]. Similarly, early declines in lung function tend to show signs of lipid metabolism disruption, and these metabolic abnormalities are closely associated to the severity of chronic lung disorders and the possibility of severe exacerbations. In a large-scale cross-sectional investigation conducted by Lucia Cestelli [12], baseline risk factor analysis found that obesity was more prevalent in the PRISm group than in those with normal lung function, which is consistent with our findings. Obesity, as a marker of disruptions in lipid metabolism, shows a significant positive association with PRISm, prompting us to place more emphasis on the role of lipid metabolism in this group of lung function abnormalities. Furthermore, previous research [1] has shown that individuals with PRISm tend to have higher BMI and an increased prevalence of diabetes, which aligns with our findings. This suggests that PRISm, as a form of lung function decline, may overlap with metabolic dysfunction.
Moreover, this study revealed that NHHR showed a stronger association with PRISm in individuals without abdominal obesity, whereas the association was not significant in those with abdominal obesity. This suggests that NHHR fluctuations may serve as a better indicator of lipid metabolism-related lung function impairment in individuals without abdominal obesity. Abdominal obesity is commonly associated with metabolic complications such as diabetes and coronary artery disease [48, 49]. These individuals are not only affected by lipid metabolism disorders but also by other metabolic disturbances, which may weaken the predictive value of NHHR in this group. Conversely, among those without abdominal obesity, NHHR appears to be more sensitive to early lung function decline. This could be attributed to its role in promoting inflammation and oxidative stress, which may impair endothelial function and pulmonary microcirculation, ultimately reflecting underlying lipid metabolism disturbances. These findings underscore the importance of further evaluating this biomarker as a potential indicator of early lung function impairment, particularly in individuals without abdominal obesity. Future studies should explore whether monitoring NHHR levels could aid in identifying high-risk individuals and guiding early preventive interventions. To summarize, the affiliation associating lipid metabolism and lung function is intricate, while regulating lipid levels may provide potential therapeutic strategies for improving lung health.
HDL-C is an important component of NHHR and has a regulatory effect on early lung function damage. Studies indicate that the association between HDL-C and pulmonary function parameters mainly involves processes such as modulation of the inflammatory microenvironment, maintenance of oxidative stress balance, and protection of the vascular endothelium. HDL-C regulates lipid homeostasis in type II alveolar epithelial cells and modulates inflammatory factor production in lung stroma [50], demonstrating significant biological effects. Moreover, the antioxidant properties of HDL-C help reduce oxidative stress levels and protect lung parenchymal cells from damage caused by reactive oxygen species [51]. NHHR serves as a key marker of HDL-C metabolic status, a decrease in NHHR may reflect either a reduction in HDL-C or an increase in non-HDL-C, appears to diminish these protective effects, promoting systemic inflammation and oxidative stress, impairing alveolar repair, and contributing to the onset of PRISm. A large-scale study has shown a significant positive association between HDL-C and lung function parameters [52]. However, in Reed’s research [53], HDL-C in COPD patients increased in the later stages of the disease, which is consistent with our findings and is associated with complications in advanced COPD and medication use. This might also be associated with the heterogeneity of COPD, while PRISm, as an early form of lung function impairment, maintains relatively consistent characteristics in its association with lipid metabolism dysfunction. Additionally, pro-atherogenic lipoproteins also contribute to NHHR, and elevated levels are closely associated with endothelial dysfunction, potentially accelerating atherosclerosis through increased oxidative stress and inflammation [54]. Given that numerous large cohort studies [6, 14, 15, 29] have indicated that PRISm is associated with an increased risk of cardiovascular mortality, and some studies [12] have reported a higher prevalence of heart disease in PRISm patients with impaired lung function, these findings further support the association between NHHR and PRISm.
Strengths and limitations
The primary strength of this study lies in its use of a large-scale prospective cohort design, coupled with weighted analytical methods that appropriately account for a range of potential confounding factors. Utilizing nationally representative data and incorporating sample weights, this study provides an exhaustive analysis into the association among NHHR, PRISm, and lung function in U.S. adults. Notably, it is the first to discover a substantial linear association between NHHR and PRISm.
Still, this research has a few limitations. Because of the cross-sectional nature of the study, we cannot draw causal conclusions about the association between NHHR and PRISm. While we adjusted for multiple confounders, the potential impact of residual confounding factors, and unmeasured inflammatory markers—cannot be fully excluded. Future randomized controlled trials are needed to establish the causal association between lipid metabolism disturbances and lung function decline and to assess whether targeting NHHR through treatment could improve the course of PRISm.
Conclusion
In this study, elevated NHHR levels were found to be associated with a higher prevalence of PRISm and were strongly associated with impaired lung function. Therefore, monitoring NHHR levels provides a viable method for early identification of abnormal lipid metabolism and decreased lung function.
Supplementary Information
Acknowledgements
The authors express their gratitude to the NHANES database for uploading valuable datasets.
Abbreviations
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- ATS
American Thoracic Society
- BMI
Body mass index
- COPD
Chronic Obstructive Pulmonary Disease
- CI
Confidence intervals
- FVC
Forced Vital Capacity
- FEV1
Forced Expiratory Volume in 1 s
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- HBA1c
Glycated hemoglobin
- HDL-C
High-density lipoprotein cholesterol
- NHANES
National Health and Nutrition Examination Survey
- NHHR
Non-HDL-C and HDL-C ratio
- OR
Odds ratios
- PIR
Poverty-to-income ratio
- PRISm
Preserved Ratio Impaired Spirometry
- RCS
Regression cubic splines
- SD
Standard deviations
- TB
Total bilirubin
- TC
Total Cholesterol
- TG
Triglycerides
- U.S.
United States
- WBC
White blood cell
- WC
Waist circumference
Authors’ contributions
Qilei Zhu created the study subject and handled data analysis, Ran He, Yiqin Yan, Lihan Xiang, Yarong Li and Yi Yang advanced the article's conceptualization and writing, Dandan Hu, Liming Lou directed the article's writing direction. The submission of the manuscript was reviewed and approved by all authors.
Funding
Not applicable.
Data availability
In this study, publicly accessible datasets were evaluated. Visit https://www.cdc.gov/nchs/nhanes/index.htm to get this data.
Declarations
Ethics approval and consent to participate
The NHANES protocol was approved by the Ethics Review Board of the NCHS. Each participant provided written informed consent during the survey. This study is a secondary analysis based on the publicly available NHANES database, and therefore, does not require further ethical review by the hospital's ethics committee.
Consent for publication
The authors gave their approval for the publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wan ES, Castaldi PJ, Cho MH, Hokanson JE, Regan EA, Make BJ, et al. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res. 2014;15(1):89. 10.1186/s12931-014-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global strategy for prevention, diagnosis and management of COPD: 2023 report 2023. 10 December 2023. Available from: https://goldcopd.org/2023-gold-report-2/.
- 3.Martinez FJ, Agusti A, Celli BR, Han MK, Allinson JP, Bhatt SP, et al. Treatment Trials in Young Patients with Chronic Obstructive Pulmonary Disease and Pre-Chronic Obstructive Pulmonary Disease Patients: Time to Move Forward. Am J Respir Crit Care Med. 2022;205(3):275–87. 10.1164/rccm.202107-1663SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adibi A, Sadatsafavi M. Looking at the COPD spectrum through “PRISm.” Eur Respir J. 2020;55(1):1902217. 10.1183/13993003.02217-2019. [DOI] [PubMed] [Google Scholar]
- 5.Wan ES, Hokanson JE, Regan EA, Young KA, Make BJ, DeMeo DL, et al. Significant Spirometric Transitions and Preserved Ratio Impaired Spirometry Among Ever Smokers. Chest. 2022;161(3):651–61. 10.1016/j.chest.2021.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He D, Sun Y, Gao M, Wu Q, Cheng Z, Li J, et al. Different Risks of Mortality and Longitudinal Transition Trajectories in New Potential Subtypes of the Preserved Ratio Impaired Spirometry: Evidence From the English Longitudinal Study of Aging. Front Med. 2021;8:755855. 10.3389/fmed.2021.755855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guerra S, Sherrill DL, Venker C, Ceccato CM, Halonen M, Martinez FD. Morbidity and mortality associated with the restrictive spirometric pattern: a longitudinal study. Thorax. 2010;65(6):499–504. 10.1136/thx.2009.126052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan ES. The Clinical Spectrum of PRISm. Am J Respir Crit Care Med. 2022;206(5):524–5. 10.1164/rccm.202205-0965ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y, Ampon MR, Abramson MJ, James AL, Maguire GP, Wood-Baker R, et al. Prevalence and characteristics of adults with preserved ratio impaired spirometry (PRISm): Data from the BOLD Australia study. Chron Respir Dis. 2025;22:14799731241312688. 10.1177/14799731241312687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax. 2003;58(5):388–93. 10.1136/thorax.58.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez-Padilla R, Montes de Oca M, Thirion-Romero I, Wehrmeister FC, Lopez MV, Valdivia G, et al. Trajectories of spirometric patterns, obstructive and PRISm, in a population-based cohort in Latin America. Int J Chron Obstruc Pulmon Dis. 2023;18:1277–85. 10.2147/copd.S406208. [DOI] [PMC free article] [PubMed]
- 12.Cestelli L, Johannessen A, Gulsvik A, Stavem K, Nielsen R. Risk Factors, Morbidity, and Mortality in Association With Preserved Ratio Impaired Spirometry and Restrictive Spirometric Pattern: Clinical Relevance of Preserved Ratio Impaired Spirometry and Restrictive Spirometric Pattern. Chest. 2025;167(2):548–60. 10.1016/j.chest.2024.08.026. [DOI] [PubMed] [Google Scholar]
- 13.Patel I, Zhang J, Chai Y, Qiao Y, Gong H, Xu H, et al. Preserved ratio impaired spirometry, airflow obstruction, and their trajectories in relationship to chronic kidney disease: a prospective cohort study. Sci Rep. 2025;15(1):3439. 10.1038/s41598-025-86952-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wijnant SRA, De Roos E, Kavousi M, Stricker BH, Terzikhan N, Lahousse L, et al. Trajectory and mortality of preserved ratio impaired spirometry: the Rotterdam Study. Eur Respir J. 2020;55(1). 10.1183/13993003.01217-2019. [DOI] [PubMed]
- 15.Wan ES, Balte P, Schwartz JE, Bhatt SP, Cassano PA, Couper D, et al. Association Between Preserved Ratio Impaired Spirometry and Clinical Outcomes in US Adults. JAMA. 2021;326(22):2287–98. 10.1001/jama.2021.20939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein OL, Krishnan JA, Glick S, Smith LJ. Systematic review of the association between lung function and Type 2 diabetes mellitus. Diabetic medicine : a journal of the British Diabetic Association. 2010;27(9):977–87. 10.1111/j.1464-5491.2010.03073.x. [DOI] [PubMed] [Google Scholar]
- 17.Naveed B, Weiden MD, Kwon S, Gracely EJ, Comfort AL, Ferrier N, et al. Metabolic syndrome biomarkers predict lung function impairment: a nested case-control study. Am J Respir Crit Care Med. 2012;185(4):392–9. 10.1164/rccm.201109-1672OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rohatgi A, Westerterp M, von Eckardstein A, Remaley A, Rye KA. HDL in the 21st Century: A Multifunctional Roadmap for Future HDL Research. Circulation. 2021;143(23):2293–309. 10.1161/circulationaha.120.044221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye L, Mao S, Fang S, Zhang J, Tan Y, Gu W. Increased Serum Romo1 Was Correlated with Lung Function, Inflammation, and Oxidative Stress in Chronic Obstructive Pulmonary Disease. Inflammation. 2019;42(5):1555–60. 10.1007/s10753-019-01017-x. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Z, Li J, Si J, Ma B, Shi H, Lv J, et al. A large-scale genome-wide association analysis of lung function in the Chinese population identifies novel loci and highlights shared genetic aetiology with obesity. Eur Respir J. 2021;58(4). 10.1183/13993003.00199-2021. [DOI] [PMC free article] [PubMed]
- 21.Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12(9):755–67. 10.1080/17476348.2018.1506331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheng G, Liu D, Kuang M, Zhong Y, Zhang S, Zou Y. Utility of Non-High-Density Lipoprotein Cholesterol to High-Density Lipoprotein Cholesterol Ratio in Evaluating Incident Diabetes Risk. Diabetes, metabolic syndrome and obesity : targets and therapy. 2022;15:1677–86. 10.2147/dmso.S355980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui Y, Choi M. Association between the non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and angina pectoris in US adults: a cross-sectional retrospective study based on NHANES 2009–2018. Lipids Health Dis. 2024;23(1):347. 10.1186/s12944-024-02343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu B, Li M, Yu Z, Zheng T, Feng X, Gao A, et al. The non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) as a predictor of all-cause and cardiovascular mortality in US adults with diabetes or prediabetes: NHANES 1999–2018. BMC Med. 2024;22(1):317. 10.1186/s12916-024-03536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie W, Liu H, Lin Q, Lian L, Liang B. Association of non-high-density lipoprotein to high-density lipoprotein ratio (NHHR) with prognosis in cancer survivors: a population-based study in the United States. Front Nutr. 2024;11:1430835. 10.3389/fnut.2024.1430835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Q, Hu Z, Zhang H, Shi X, Li X, Zhu X. A cross-sectional study of the correlation of the ratio of non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol (NHHR) with obstructive sleep apnea (OSA) in adult populations: NHANES (2005–2008 and 2015–2020). Medicine. 2024;103(40):e39965. 10.1097/md.0000000000039965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li MY, Chen JH, Chen C, Kang YN. Association between egg consumption and cholesterol concentration: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2020;12(7). 10.3390/nu12071995. [DOI] [PMC free article] [PubMed]
- 28.Yin H, Huang W, Yang B. Association between METS-IR index and obstructive sleep apnea: evidence from NHANES. Sci Rep. 2025;15(1):6654. 10.1038/s41598-024-84040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Washio Y, Sakata S, Fukuyama S, Honda T, Kan OK, Shibata M, et al. Risks of Mortality and Airflow Limitation in Japanese Individuals with Preserved Ratio Impaired Spirometry. Am J Respir Crit Care Med. 2022;206(5):563–72. 10.1164/rccm.202110-2302OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2021;143(21):e984–1010. 10.1161/cir.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan ES, Fortis S, Regan EA, Hokanson J, Han MK, Casaburi R, et al. Longitudinal Phenotypes and Mortality in Preserved Ratio Impaired Spirometry in the COPDGene Study. Am J Respir Crit Care Med. 2018;198(11):1397–405. 10.1164/rccm.201804-0663OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Godfrey MS, Jankowich MD. The Vital Capacity Is Vital: Epidemiology and Clinical Significance of the Restrictive Spirometry Pattern. Chest. 2016;149(1):238–51. 10.1378/chest.15-1045. [DOI] [PubMed] [Google Scholar]
- 33.NHANES - NCHS Research Ethics Review Board Approval 2022. Available from: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx.
- 34.Duan S, Zhang Y, Wu SJ, Jiang LZ, Zhang J, Gan Y, et al. Atorvastatin attenuates inflammatory infiltration and vascular remodeling in lung of hypercholesterolemia rabbits. Exp Lung Res. 2010;36(10):573–92. 10.3109/01902141003739715. [DOI] [PubMed] [Google Scholar]
- 35.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–68. 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 36.Wu R, Gong H. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and chronic obstructive pulmonary disease: the mediating role of dietary inflammatory index. Front Nutr. 2024;11:1427586. 10.3389/fnut.2024.1427586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng Y, Liu W, Zhu X, Xu M, Lin B, Bai Y. Associations of dietary inflammation index and composite dietary antioxidant index with preserved ratio impaired spirometry in US adults and the mediating roles of triglyceride-glucose index: NHANES 2007–2012. Redox Biol. 2024;76:103334. 10.1016/j.redox.2024.103334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higbee DH, Granell R, Davey Smith G, Dodd JW. Prevalence, risk factors, and clinical implications of preserved ratio impaired spirometry: a UK Biobank cohort analysis. Lancet Respir Med. 2022;10(2):149–57. 10.1016/s2213-2600(21)00369-6. [DOI] [PubMed] [Google Scholar]
- 39.Olvera N, Agusti A, Vonk JM, Wang G, Hallberg J, Boezen HM, et al. Heterogeneity of reduced FEV(1) in early adulthood: A looking forward, looking backwards analysis. Respirology (Carlton, Vic). 2025. 10.1111/resp.14876. [DOI] [PMC free article] [PubMed]
- 40.Mirza S, Benzo R. Chronic Obstructive Pulmonary Disease Phenotypes: Implications for Care. Mayo Clin Proc. 2017;92(7):1104–12. 10.1016/j.mayocp.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zafari Z, Sin DD, Postma DS, Löfdahl CG, Vonk J, Bryan S, et al. Individualized prediction of lung-function decline in chronic obstructive pulmonary disease. CMAJ. 2016;188(14):1004–11. 10.1503/cmaj.151483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franssen FM, O’Donnell DE, Goossens GH, Blaak EE, Schols AM. Obesity and the lung: 5. Obesity and COPD Thorax. 2008;63(12):1110–7. 10.1136/thx.2007.086827. [DOI] [PubMed] [Google Scholar]
- 43.Hanson C, Rutten EP, Wouters EF, Rennard S. Influence of diet and obesity on COPD development and outcomes. Int J Chron Obstruct Pulmon Dis. 2014;9:723–33. 10.2147/copd.S50111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamonaca P, Prinzi G, Kisialiou A, Cardaci V, Fini M, Russo P. Metabolic disorder in Chronic Obstructive Pulmonary Disease (COPD) patients: towards a personalized approach using marine drug derivatives. Mar Drugs. 2017;15(3):81. 10.3390/md15030081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kilk K, Aug A, Ottas A, Soomets U, Altraja S, Altraja A. Phenotyping of chronic obstructive pulmonary disease based on the integration of metabolomes and clinical characteristics. Int J Mol Sci. 2018;19(3). 10.3390/ijms19030666. [DOI] [PMC free article] [PubMed]
- 46.Chen H, Li Z, Dong L, Wu Y, Shen H, Chen Z. Lipid metabolism in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2019;14:1009–18. 10.2147/copd.S196210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang P, Zhao Y, Wei H, Wu W, Guo Z, Ma S, et al. Causal Relationships Between Blood Lipid Levels and Chronic Obstructive Pulmonary Disease: A Mendelian Randomization Analysis. Int J Chron Obstruct Pulmon Dis. 2025;20:83–93. 10.2147/copd.S476833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wan H, Wang Y, Xiang Q, Fang S, Chen Y, Chen C, et al. Associations between abdominal obesity indices and diabetic complications: Chinese visceral adiposity index and neck circumference. Cardiovasc Diabetol. 2020;19(1):118. 10.1186/s12933-020-01095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross R, Després JP. Abdominal obesity, insulin resistance, and the metabolic syndrome: contribution of physical activity/exercise. Obesity (Silver Spring, Md). 2009;17(Suppl 3):S1-2. 10.1038/oby.2009.381. [DOI] [PubMed] [Google Scholar]
- 50.Kotlyarov S. High-Density Lipoproteins: A Role in Inflammation in COPD. Int J Mol Sci. 2022;23(15). 10.3390/ijms23158128. [DOI] [PMC free article] [PubMed]
- 51.Kim C, Lee JM, Park SW, Kim KS, Lee MW, Paik S, et al. Attenuation of Cigarette Smoke-Induced Emphysema in Mice by Apolipoprotein A-1 Overexpression. Am J Respir Cell Mol Biol. 2016;54(1):91–102. 10.1165/rcmb.2014-0305OC. [DOI] [PubMed] [Google Scholar]
- 52.Cirillo DJ, Agrawal Y, Cassano PA. Lipids and pulmonary function in the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2002;155(9):842–8. 10.1093/aje/155.9.842. [DOI] [PubMed] [Google Scholar]
- 53.Reed RM, Iacono A, DeFilippis A, Eberlein M, Girgis RE, Jones S. Advanced chronic obstructive pulmonary disease is associated with high levels of high-density lipoprotein cholesterol. J Heart Lung Transplant. 2011;30(6):674–8. 10.1016/j.healun.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 54.Jakubowski H, Witucki Ł. Homocysteine Metabolites, Endothelial Dysfunction, and Cardiovascular Disease. Int J Mol Sci. 2025;26(2). 10.3390/ijms26020746. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
In this study, publicly accessible datasets were evaluated. Visit https://www.cdc.gov/nchs/nhanes/index.htm to get this data.