Abstract
Background
Early National Institutes of Health Stroke Scale (NIHSS) assessment may provide practical benefits over 90‐day modified Rankin Scale (mRS), but it is unclear how it compares in adjudicating randomized clinical trial (RCT) results in acute ischemic stroke.
Methods and Results
We searched Ovid Medline (inception to April 1, 2023) and included RCTs of acute therapies for acute ischemic stroke with data for both 90‐day mRS and NIHSS within 7 days. Primary outcome was agreement between trial results (classified as positive, negative, or neutral) based on 24‐hour NIHSS and 90‐day mRS scores. We additionally assessed agreement for 2‐hour, 48‐hour, 72‐ to 96‐hour, and 5‐ to 7‐day NIHSS scores. We aimed to validate our findings using individual patient data from the ESCAPE (Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke) and ESCAPE‐NA1 (Safety and Efficacy of Nerinetide [NA‐1] in Subjects Undergoing Endovascular Thrombectomy for Stroke) RCTs. We included 116 trials (44 387 patients), contributing 165 NIHSS assessments. The 24‐hour NIHSS scores resulted in the same classification as 90‐day mRS scores in 61/73 (83.6%) trials (Cohen's kappa, 0.64 [95% CI: 0.45–0.83] and Gwet's agreement coefficient 1, 0.79 [95% CI: 0.67–0.90]). Agreement was not statistically different by timing of NIHSS assessments (range 75%–100%, P=0.33). Individual patient data showed higher agreement for assessments between 48 hours and 7 days, varying by NIHSS dichotomization cutoffs (NIHSS score, 0–2; 2 hours, 56.6%; 24 hours, 66.6%; 48 hours, 71.8%; 5–7 days: 76.5%, P<0.01; NIHSS score, 0–7; 2 hours, 72.8%; 24 hours, 80.5%; 48 hours, 83.1%; 5–7 days: 84.7%, P<0.01).
Conclusions
The 24‐hour NIHSS scores aligned with 90‐day mRS scores in 84% of RCT results, indicating intermediate‐to‐good agreement. However, individual patient data showed that early NIHSS risks misclassifying around 1/4 patients. These data contribute to a better understanding of the nuances of early NIHSS score as an outcome in acute ischemic stroke RCTs.
Keywords: acute ischemic stroke, modified Rankin Scale, National Institutes of Health Stroke Scale, randomized controlled trial
Subject Categories: Ischemic Stroke
Nonstandard Abbreviations and Acronyms
- mRS
modified Rankin Scale
- NIHSS
National Institutes of Health Stroke Scale
- SMD
standardized mean difference
Clinical Perspective.
What Is New?
In this analysis that included 44 387 patients with acute ischemic stroke from 116 randomized controlled trials, the agreement in classifying trials as positive versus neutral versus negative between 24‐hour National Institutes of Health Stroke Scale (NIHSS) score and 90‐day modified Rankin Scale score was 83.6% (Cohen's kappa: 0.64 and Gwet's agreement coefficient 1, 0.79); trial‐level agreement did not differ significantly across NIHSS assessment time points between 24 hours and 7 days, although individual patient data of 1420 participants indicated somewhat higher agreement for later NIHSS assessments.
What Are the Clinical Implications?
These data will help inform the discussion on early NIHSS score as an alternative outcome measure to 90‐day modified Rankin Scale score in randomized controlled trials on acute ischemic stroke interventions.
Individual patient data showed that NIHSS score within 7 days risks misclassifying up to one fourth of patients with respect to 90‐day modified Rankin Scale score, although agreement improves with later assessments; therefore, when seeking to prognosticate 90‐day functional recovery in clinical practice, it may be prudent to wait at least 1 week to assess the poststroke NIHSS score.
The most accepted outcome for assessing the efficacy of interventions in phase III randomized clinical trials (RCT) of acute ischemic stroke is the functional neurological outcome measured on the modified Rankin Scale (mRS) at 90‐day follow‐up. 1 The mRS combines motor and cognitive components mixing the concepts of impairment, disability, and handicap. 2 The National Institutes of Health Stroke Scale (NIHSS) is a purely impairment‐based scale that determines neurological deficits on examination. The NIHSS is less prone to psychosocial confounding factors, and if assessed well before the 90‐day period (eg, within the first week after stroke onset), it may reflect a purer assessment of a patient's posttreatment neurological status. A previous review of primary outcome measures in stroke trials showed that the NIHSS was in second place after the mRS as the most frequently chosen primary outcome measure in stroke trials. 3
Using the NIHSS assessed within 7 days follow‐up could be a reasonable early surrogate outcome measure to the mRS at 90 days and could additionally decrease the costs associated with conducting stroke trials by reducing the required follow‐up time. 4 Some modeling studies have also suggested that using the NIHSS might allow a reduction in the sample size of RCTs. 4 , 5 Despite these hypothetical advantages, there are a paucity of data examining whether clinical trial results observed when using the NIHSS (measured within 7 days) as an outcome align with those obtained using the 90‐day mRS score. We therefore aimed to determine the agreement between these 2 outcomes using published data from RCTs of acute ischemic stroke, and to further validate our findings using individual patient data from 2 RCTs that measured the NIHSS at multiple time points.
METHODS
Data Availability
The analysis for this study is based on published results from individual studies. All extracted data from the individual studies and the code used for performing the analyses can be made available upon reasonable request to the corresponding author. The ESCAPE‐NA1 (Safety and Efficacy of Nerinetide [NA‐1] in Subjects Undergoing Endovascular Thrombectomy for Stroke) individual patient data are not currently publicly available for distribution.
Ethical Approval
Approval from an institutional or regional review board was not required as all study level data used in this study were extracted from publications. For the ESCAPE (Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke and ESCAPE‐NA1 trials, the ethics board at each participating site approved the trial.
Registration and Reporting Standards
We conducted this systematic review according to a protocol that we registered (International Prospective Register of Systematic ReviewsCRD42023445515) and report the results consistent with Preferred Reporting Items for Systematic Reviews and Meta‐Analyses. 6
Search Strategy
We used a comprehensive electronic strategy (Table S1) to search Ovid Medline from inception to April 1, 2023, for articles meeting our eligibility criteria. We performed backward citation searching using the bibliographies of included studies.
Eligibility Criteria
We included RCTs published in English, German, Spanish, French, or Dutch (reflecting the language skills of the study team) in peer‐reviewed journals, reporting on adult patients with acute ischemic stroke, examining interventions initiated within 48 hours of stroke onset and completed within 7 days of stroke onset and with available data for both 90‐day mRS score and NIHSS score within 7 days. A detailed list of inclusion and exclusion criteria is provided in Table 1.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion criteria | |
| 1. | Randomized controlled trial |
| 2. | Reports on both mRS and NIHSS in the main paper or supplement |
| 3. | Minimum follow‐up period of 90‐d for the mRS |
| 4. | NIHSS assessed within 7 d (168 h) follow‐up |
| 5. | Includes acute‐phase stroke patients (within 48 h of stroke onset) |
| 6. | Studies in humans |
| 7. | Reports in English, German, Dutch, French, or Spanish |
| Exclusion criteria | |
| 1. | Nonacute phase interventions (ie, intervention started >48 h after onset or continued for more than 7 d after onset) |
| 2. | Primarily a secondary prevention study (main outcome is recurrent stroke/transient ischemic attack) |
| 3. | Studies not reporting on original data (meta‐analysis and reviews) |
mRS indicates modified Rankin Scale; and NIHSS, National Institutes of Health Stroke Scale.
Study Selection
Four reviewers (L.A.R., J.M.O., M.K., A.G.) independently used the systematic review management platform Covidence to screen titles and abstracts for eligibility and assess the full text of potentially eligible studies for final inclusion using rigorous application of the inclusion and exclusion criteria. All studies were assessed by at least 2 authors. In case of disagreement, the authors resolved discrepancies in consensus meetings whenever possible, with A.G. having the final vote for conflict resolution.
Data Extraction
Six reviewers (L.A.R., J.M.O., M.K., A.S., R.V.M., A.G.) independently extracted the characteristics and outcomes of each trial. For publications reporting on multiple trials or phases of trials, data were extracted for each trial or trial phase separately. We extracted aspects of study design as well as patients and treatment characteristics. We aimed to collect data on NIHSS outcomes at all available time points within 7 days post stroke. Thus, studies reporting NIHSS scores on >1follow‐up time‐point could contribute to multiple timepoints. Data on NIHSS scores were collected in all available formats, including median with interquartile range or mean±SD, dichotomized scores or change from baseline NIHSS score. Data on 90‐day mRS score were extracted as the full distribution across all scores of the mRS whenever possible. If this was not reported, we extracted data on the distribution by dichotomized scores or by summary statistics (median with interquartile range or mean±SD). If studies applied dichotomization for mRS, NIHSS, or both we recorded the definitions that were used. In case of disagreement, the authors resolved discrepancies in consensus meetings.
Risk of Bias
We used version 2 of the Cochrane risk‐of‐bias tool for randomized trials for assessing risk of bias in randomized trials in the following domains 7 : (1) randomization process, (2) deviations from intended interventions, (3) missing outcome data, (4) measurement of the outcome, and (5) selection of the reported results. In case of disagreement, the authors resolved discrepancies in consensus meetings.
Outcomes
The primary outcome was agreement between results of the trial assessed on the NIHSS at 24‐hour follow‐up and the results of the trial assessed on the mRS at 90‐day follow‐up. Secondary outcomes were agreement between trial results as assessed on the NIHSS at 2‐hour, 48‐hour, 72‐ to 96‐hour, and 5‐ to 7‐day follow‐up and the mRS score at 90‐day follow‐up.
We used the authors' specifications of favorable versus unfavorable outcomes for dichotomizing scores for each RCT for both the 90‐day mRS and early NIHSS scores. We classified trial results on the NIHSS and mRS as positive versus neutral versus negative. A result on the NIHSS was defined as positive if (1) both the point estimate and the lower boundary of the 95% CI of the odds ratio for favorable NIHSS were >1 (indicating statistically significant improvement on the dichotomized NIHSS) or (2) if the point estimate and the upper boundary of the 95% CI of the standardized mean differences (SMDs) were <0 (indicating improvement of continuous NIHSS scores, including scores reported as change in NIHSS score compared with baseline NIHSS score). Results were classified as negative if the point estimate and upper boundary of the 95% CI were <1 for dichotomized scores and >0 for continuous scores. All other results were classified as neutral. Results measured using mRS scores at 90‐day follow‐up were categorized into positive, negative, and neutral results in the same manner.
Statistical Analysis
We quantified the distribution of cohort‐level characteristics with descriptive analyses. We summarized the effect sizes observed for each intervention arm versus control in each trial, for each timing of the NIHSS until 7 days and for the 90‐day mRS. Effect sizes were grouped as either odds ratios (ORs) or SMDs depending on how results were presented in the included studies. To facilitate synthesis of studies reporting mean and median summary statistics for effect sizes, these were transformed into SMD's whenever possible. Unadjusted summary effect measures were calculated in STATA version 17 using the metan command. We separately calculated each summary effect measure (SMDs and ORs) for each outcome (NIHSS and mRS), including each available time point of NIHSS assessment (2‐hour, 24‐hour, 48‐hour, 72‐ to 96‐hour, and 5‐ to 7‐day follow‐up).
We determined the percentage of agreement across trials between NIHSS and 90‐day mRS scores in terms of a trichotomized classification of trial results as positive versus neutral versus negative results. We also examined additional measures of agreement including Cohen's kappa and Gwet's agreement coefficient 1 (AC1). 8 Cohen's kappa is a statistical measure that quantifies agreement for categorical ratings by accounting for the agreement occurring by chance. Gwet's AC1 is another reliability coefficient that adjusts for chance agreement; it has the advantage of providing more stable and unbiased estimates than Cohen's kappa, particularly in cases of high or low prevalence of certain categories. When there is unbalanced category prevalence, use of Cohen's kappa can result in misleadingly low kappa values, even in the setting of good agreement, also known as the kappa paradox. 9 Using the Gwet's AC1 in addition to Cohen's kappa can provide a more stable coefficient with varying prevalence or marginal probability, or at least provide contrast with the Cohen's kappa estimate to better understand how changing category definitions or prevalence affects agreement.
We classified the Cohen's kappa and Gwet's AC1 values according to the cutoffs proposed by Fleiss et al. 10 (with values <0.40 considered poor agreement, 0.40–0.74 considered intermediate to good, and 0.75–1.00 considered excellent) and by Landis and Koch 11 (with values of <0.20 considered poor agreement, 0.21–0.40 considered fair, 0.41–0.60 considered moderate, 0.61–0.80 considered substantial, and 0.81–1.00 considered almost perfect). 11 We did this for all NIHSS time points combined as well as for each individual NIHSS time point separately. To determine whether agreement differed between the different time points of NIHSS assessment we performed a chi‐square test and a chi‐square test for trend. We further assessed agreement between NIHSS and 90‐day mRS scores when dichotomizing trials as positive versus neutral/negative and determined the sensitivity and specificity for the dichotomized classification of trials for each NIHSS time point using the 90‐day mRS scoreas reference standard. Sensitivity here meant the proportion of trials for which the NIHSS analysis yielded a positive result, out of all trials for which the mRS scoreyielded a positive result. Specificity meant the proportion of trials for which the NIHSS analysis yielded a negative/neutral result, out of all trials for which the mRS scoreyielded a negative/neutral result.
Individual Patient Data Analysis
To corroborate our findings, we pooled individual patient data from the ESCAPE and ESCAPE‐NA1 trials. 12 , 13 These trials were selected because they were the only trials that assessed the NIHSS on 4 time points in a similar manner. The mRS was dichotomized at 0 to 2, indicating good functional outcome. NIHSS scores were dichotomized at 0 to 2 (indicating good outcome) in accordance with the trial protocols. 12 , 13 Additionally, we assessed NIHSS scoredichotomized at 0 to 7 because previous research indicated this may be a stronger predictor of 90‐day mRS score. 14 We assessed agreement measures (percentage agreement, sensitivity, specificity, Cohen's kappa, and Gwet's AC1) between 90‐day mRS and NIHSS scores assessed at 2 hours, 24 hours, 48 hours, and 5 to 7 days. For sensitivity, this meant examining the proportion of all patients who had a favorable outcome according to the NIHSS, out of all patients who had a favorable outcome according to the mRS. For specificity, this meant the proportion of patients who had an unfavorable outcome according to the NIHSS, out of all patients who had an unfavorable outcome according to the mRS. Furthermore, we calculated SMDs between the treatment and control arms for the NIHSS at each time point and for the 90‐day mRS scoreusing Cohen's d. They were derived by applying an ANOVA followed by a mean comparison of the estimates in the combined data of the trials. For the NIHSS, we derived the SMDs after ANCOVA including baseline NIHSS score as a covariate as recommended in recent trial simulations. 15
RESULTS
After screening 1401 references and excluding ineligible studies, we included 116 RCTs reporting on 90‐day mRS and NIHSS scores within 7 days, representing a total of 44 387 patients. 12 , 13 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127
Nine of 116 RCTs tested more than 1 intervention (Table 2). Thrombolytic/antithrombotic medication was the most frequently tested intervention type (37/116 [31.9%] trials, total of 115 245 patients). The median age of the study population was 68 (interquartile range, 66–72) years, and 25 218 (56.8%) were male. The median time from stroke onset to initiation of treatment was 6 (interquartile range, 5–11) hours and median duration of the intervention was 1 (1–24) hours.
Table 2.
Study Characteristics
| Characteristic | Median (IQR) or N (%) | Studies reporting characteristic | Number of patients |
|---|---|---|---|
| Age, y | 68 (66–72) | 116 | 44 387 |
| Male sex | 25 218 (56.8%) | 116 | 44 387 |
| Baseline NIHSS score | 13 (9–16) | 113 | 43 409 |
| Time from onset to start intervention in hours | 6 (5–11) | 116 | 44 387 |
| Duration of intervention in hours | 1 (1–24) | 116 | 44 387 |
| Number of study arms | |||
| 2 | 106 (91.4%) | 106 | 34 431 |
| 3 | 7 (6.0%) | 7 | 9192 |
| 4 | 2 (1.7%) | 2 | 136 |
| 6 | 1 (0.9%) | 1 | 628 |
| Intervention type | |||
| Endovascular therapy (including device) | 30 (25.9%) | 30 | 8809 |
| Cerebroprotectant | 27 (23.3%) | 27 | 14 598 |
| Thrombolytic/antithrombotic | 37 (32.8%) | 37 | 15 245 |
| Other | 22 (19.0%) | 22 | 5735 |
| Large vessel occlusion | 15 044 (69.0%) | 60 | 21 788 |
| Independent prestroke | 28 228 (90.9%) | 66 | 31 050 |
| Year of publication | 2019 (2014–2021) | 116 | 44 555 |
| Timing NIHSS assessment* | |||
| 2 h | 4 (3.4%) | 4 | 1832 |
| 24 h | 74 (63.8%) | 74 | 26 908 |
| 48 h | 25 (21.6%) | 25 | 8106 |
| 72–96 h | 14 (12.0%) | 14 | 2684 |
| 5–7 d | 53 (45.7%) | 53 | 24 815 |
| Format of reported NIHSS score* | |||
| Mean/median | 62 (53.4%) | 62 | 25 538 |
| Change from baseline | 24 (20.7%) | 24 | 6069 |
| Dichotomized | 57 (49.1%) | 57 | 27 036 |
| Format of reported mRS score* | |||
| Mean/median | 41 (35.3%) | 41 | 15 054 |
| Dichotomized | 110 (94.8%) | 110 | 43 808 |
| Full ordinal range* | 76 (65.5%) | 76 | 37 659 |
More than 100% because results may be presented in multiple ways in a single study.
IQR indicates interquartile range; mRS, modified Rankin Scale; and NIHSS, National Institutes of Health Stroke Scale.
In general, there were some concerns regarding overall bias in almost half (53/116, 45.7%) of included studies while 41/116 (35.3%) studies were deemed to be at low risk of overall bias. (Figure, Figure S1). When examining the individual domains, most studies were at low risk of bias arising from the randomization process 79/116 (68.1%), deviations from intended interventions 74/116 (63.8%), missing outcome data 96/116 (82.8%), outcomes measurement 89/116 (76.7%), and selection of the reported results 64/116 (55.2%).
Figure 1. Risk of bias assessment of included studies.
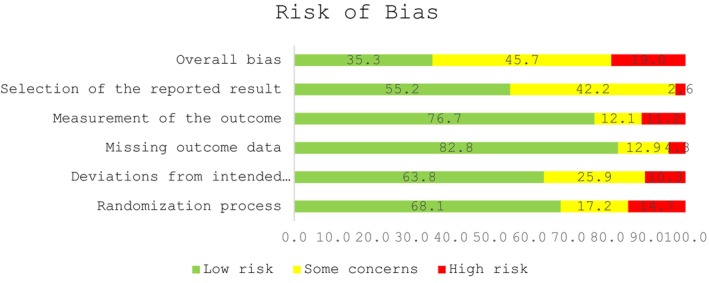
The 116 included RCTs contributed 165 NIHSS assessments performed across the 5 time points. Unadjusted summary effects for NIHSS and mRS outcomes are presented in Figures S2 through S16. When classifying trial results in a trichotomous manner as positive, negative, or neutral and combining results for all NIHSS time points, there was agreement between NIHSS and mRS scores in 138/165 (83.6%) assessments. NIHSS score was assessed at 24 hours in 73/116 included trials and there was agreement between results assessed on 24‐hour NIHSS and mRS scores in 61/73 studies (84%, Table 3; Table S2). For 24‐hour assessment Cohen's kappa was 0.64 (indicating intermediate to good 10 or substantial agreement 11 depending on the applied cutoffs) and Gwet's AC1 was 0.79 (indicating excellent 10 or substantial agreement 11 ). Besides assessment at 24 hours, NIHSS score was most frequently reported for 7‐day follow‐up (36/116 trials) with 86% of these trials showing agreement between results assessed on NIHSS and mRS. Agreement for the remaining time points ranged from 75% to 100%. We observed no significant differences for rates of agreement between NIHSS and mRS scores across the different time points (P=0.33). The effect sizes obtained with the NIHSS score in terms of SMDs were generally similar to those obtained with the 90‐day mRS score (Table S2).
Table 3.
Agreement Between NIHSS and mRS (Positive Versus Neutral Versus Negative)
| NIHSS timing | Agreement NIHSS/mRS scores | Agreement—good outcome* | Agreement—neutral outcome* | Agreement—poor outcome* | Cohen's kappa (95% CI) | Gwet's AC1 (95% CI) |
|---|---|---|---|---|---|---|
| 2 h | 3/3 (100%) | 0 | 3/3 (100%) | 0 | NA | NA |
| 24 h | 61/73 (83.6%) | 17/21 (81.0%) | 43/50 (86.0%) | 1/2 (50.0%) | 0.64 (0.45–0.83) | 0.79 (0.67–0.90) |
| 48 h | 18/24 (75.0%) | 3/6 (50.0%) | 15/17 (88.2%) | 0/1 (0.0%) | 0.35 (−0.08 to 0.79) | 0.69 (0.44–0.94) |
| 72–96 h | 12/12 (100%) | 2/2 (100%) | 10/10 (100%) | 0 | NA | NA |
| 5–7 d | 44/51 (86.2%) | 8/12 (66.7%) | 34/36 (94.4%) | 2/3 (66.7%) | 0.67 (0.43–0.90) | 0.83 (0.70–0.95) |
AC1 indicates agreement coefficient 1; mRS, modified Rankin Scale; and NIHSS, National Institutes of Health Stroke Scale.
Cases in which 90‐d mRS agreed with the NIHSS impression (ie, positive, neutral or negative), out of all NIHSS assessments with that impression at that time point.
When considering dichotomized classification of trial results as positive versus negative/neutral, agreement in trial classification was similar compared with the trichotomized results. The 24‐hour NIHSS score had a sensitivity of 81.0% (95% CI, 58.1%–94.6%) and specificity of 86.8% (95% CI, 74.2%–94.5%) for classifying the trials as positive versus negative/neutral with the 90‐day mRS score as the reference standard. The 7‐day NIHSS score had a corresponding sensitivity and specificity of 80.0% (95% CI, 44.4%–97.5%) and 90.2% (95% CI, 76.9%–97.5%), respectively (Table S3).
Agreement in Individual Patient Data
Among 1420 patients (315 in ESCAPE and 1105 in ESCAPE‐NA1), 90‐day mRS score and at least 1 early NIHSS measurement within 7 days post stroke were available for 1409 patients (99.2%).
When classifying trial results trichotomized as positive versus negative versus neutral, there was agreement between NIHSS and mRS in overall trial results (positive for ESCAPE, neutral for ESCAPE‐NA1) for all time points except 2‐hour NIHSS score. When dichotomizing NIHSS scores at 0 to 2 and combining results for all time points, there was agreement with 90‐day mRS outcomes in 3523/5195 (67.8%) assessments. Specifically, among 1564 NIHSS assessments classified as good outcome, 1438 (91.9%) were also classified as good outcome by the 90‐day mRS score; however, among 3631 poor NIHSS outcomes, only 2085 (57.4%) also had poor 90‐day mRS outcomes. Agreement measures increased for later versus earlier NIHSS assessments (2 hours: 56.6%; 24 hours: 66.6%; 48 hours: 71.8%; 5–7 days: 76.5%, P<0.01; Cohen's kappa and Gwet's AC1 rising from 0.21 and 0.19 [ie, poor/slight agreement] respectively at 2 hours to 0.54 and 0.53 [ie, intermediate to good/moderate] at 5–7 days, Table 4, Table S4). For all time points, sensitivity was low (2 hours 26.2% and 5–7 days 67.6%) and specificity was high (2 hours 97.6% and 5–7 days 89.7%). Agreement measures with 90‐day mRS scores were higher when dichotomizing NIHSS scores at 0 to 7 (percentage agreement 4169/5195 [80.3%], with good mRS outcomes in 2409/2860 [84.2%] good NIHSS outcomes and poor mRS outcomes in 1760/2335 [75.4%] bad NIHSS outcomes), with similar improvement over time (2 ‐hours: 72.8%; 24 hours: 80.5%; 48 hours: 83.1%; 5–7 days: 84.7%, P<0.01; Cohen's kappa and Gwet's AC1 rising from 0.47 and 0.46 [ie, intermediate to good/moderate] respectively at 2 hours to 0.67 and 0.61 [ie, intermediate to good/substantial] at 5–7 days, Table 4). Sensitivity increased (2 hours: 62.9%, 5–7 days: 86.2%) and specificity decreased (2 hours: 93.3%, 5–7 days: 72.1%). That being said, the effect sizes obtained with the NIHSS in terms of SMDs were similar at each time point to those obtained with the 90‐day mRS score (Table 5).
Table 4.
Agreement Between NIHSS (Dichotomized at 0–2 and Dichotomized at 0–7) and mRS Using Individual Patient Data
| NIHSS timing | Agreement—overall NIHSS/mRS scores | Agreement—good outcome* | Agreement—poor outcome* | Cohen's kappa (95% CI) | Gwet's AC1 (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|---|
| NIHSS score dichotomized at 0–2 d | |||||||
| 2 h | |||||||
| ESCAPE | 193/296 (65.2) | 24/28 (85.7) | 169/268 (63.1) | 0.19 (0.11–0.28) | 0.44 (0.33–0.55) | 19.5 (12.9–27.6) | 97.7 (94.2–99.4) |
| ESCAPE‐NA1 | 539/998 (54.0) | 171/181 (94.5) | 368/817 (45.0) | 0.20 (0.17–0.24) | 0.11 (0.05–0.18) | 27.6 (24.1–31.3) | 97.4 (95.2–98.7) |
| Combined | 732/1294 (56.6) | 195/209 (93.3) | 537/1085 (49.5) | 0.21 (0.18–0.24) | 0.19 (0.13–0.25) | 26.2 (23.1–29.6) | 97.6 (96.0–98.7) |
| 24 h | |||||||
| ESCAPE | 214/302 (70.9) | 47/54 (87.0) | 167/248 (67.3) | 0.35 (0.26–0.45) | 0.50 (0.39–0.60) | 36.7 (28.4–45.7) | 96.0 (92.0–98.4) |
| ESCAPE‐NA1 | 697/1066 (65.4) | 307/328 (93.6) | 390/738 (52.9) | 0.36 (0.32–0.41) | 0.31 (0.25–0.37) | 46.9 (43.0–50.8) | 94.9 (92.3–96.8) |
| Combined | 911/1368 (66.6) | 354/382 (92.7) | 557/986 (56.5) | 0.37 (0.33–0.41) | 0.35 (0.30–0.40) | 45.2 (41.7–48.8) | 95.2 (93.2–96.8) |
| 48 h | |||||||
| ESCAPE | 213/288 (74.0) | 56/63 (88.9) | 157/225 (69.8) | 0.44 (0.34–0.53) | 0.54 (0.44–0.64) | 45.2 (36.2–54.4) | 95.7 (91.4–98.3) |
| ESCAPE‐NA1 | 661/930 (71.1) | 306/328 (93.3) | 355/602 (59.0) | 0.45 (0.40–0.50) | 0.42 (0.36–0.48) | 55.3 (51.1–59.5) | 94.2 (91.3–96.3) |
| Combined | 874/1218 (71.8) | 362/391 (92.6) | 512/827 (61.9) | 0.46 (0.41–0.50) | 0.44 (0.39–0.49) | 53.5 (49.6–57.3) | 94.6 (92.4–96.4) |
| 5–7 d | |||||||
| ESCAPE | 219/285 (76.8) | 76/91 (83.5) | 143/194 (73.7) | 0.52 (0.42–0.62) | 0.56 (0.46–0.66) | 59.8 (50.8–68.4) | 90.5 (84.8–94.6) |
| ESCAPE‐NA1 | 787/1030 (76.4) | 451/491 (91.9) | 336/539 (62.3) | 0.53 (0.49–0.58) | 0.53 (0.48–0.59) | 69.0 (65.3–72.5) | 89.4 (85.8–92.3) |
| Combined | 1006/1315 (76.5) | 527/582 (90.6) | 479/733 (65.4) | 0.54 (0.50–0.58) | 0.53 (0.48–0.58) | 67.6 (64.1–70.8) | 89.7 (86.8–92.2) |
| NIHSS score dichotomized at 0–7 d | |||||||
| 2 h | |||||||
| ESCAPE | 222/296 (75.0) | 69/89 (77.5) | 153/207 (73.9) | 0.46 (0.36–0.57) | 0.54 (0.44–0.64) | 56.1 (46.9–65.0) | 88.4 (82.7–92.8) |
| ESCAPE‐NA1 | 720/998 (72.1) | 398/454 (87.7) | 322/544 (59.2) | 0.45 (0.40–0.51) | 0.45 (0.39–0.50) | 64.2 (60.3–68.0) | 85.2 (81.2–88.6) |
| Combined | 942/1294 (72.8) | 467/543 (86.0) | 475/751 (63.3) | 0.47 (0.42–0.51) | 0.46 (0.41–0.50) | 62.9 (59.3–66.3) | 86.2 (83.0–89.0) |
| 24 h | |||||||
| ESCAPE | 244/302 (80.8) | 101/132 (76.5) | 143/170 (84.1) | 0.61 (0.52–0.70) | 0.62 (0.53–0.71) | 78.9 (70.8–85.6) | 82.2 (75.7–87.6) |
| ESCAPE‐NA1 | 857/1066 (80.4) | 526/606 (86.8) | 331/460 (72.0) | 0.60 (0.55–0.64) | 0.62 (0.57–0.67) | 80.3 (77.1–83.3) | 80.5 (76.4–84.3) |
| Combined | 1101/1368 (80.5) | 627/738 (85.0) | 474/630 (75.2) | 0.61 (0.56–0.65) | 0.61 (0.57–0.66) | 80.1 (77.1–82.8) | 81.0 (77.6–84.1) |
| 48 h | |||||||
| ESCAPE | 237/288 (82.3) | 108/143 (75.5) | 129/145 (89.0) | 0.65 (0.56–0.73) | 0.65 (0.56–0.74) | 87.1 (79.9–92.4) | 78.7 (71.6–84.7) |
| ESCAPE‐NA1 | 775/930 (83.3) | 478/558 (85.7) | 297/372 (79.8) | 0.65 (0.60–0.70) | 0.68 (0.63–0.73) | 86.4 (83.3–89.2) | 78.8 (74.3–82.8) |
| Combined | 1012/1218 (83.1) | 586/701 (83.6) | 426/517 (82.4) | 0.66 (0.61–0.70) | 0.67 (0.63–0.71) | 86.6 (83.7–89.0) | 78.7 (75.1–82.1) |
| NIHSS score dichotomized at 5–7 d | |||||||
| ESCAPE | 240/285 (84.2) | 121/160 (75.6) | 119/125 (95.2) | 0.69 (0.61–0.77) | 0.68 (0.60–0.77) | 95.3 (90.0–98.3) | 75.3 (67.8–81.8) |
| ESCAPE‐NA1 | 876/1030 (84.9) | 608/718 (84.7) | 266/312 (85.3) | 0.66 (0.61–0.71) | 0.73 (0.69–0.77) | 93.0 (90.7–94.8) | 70.7 (65.9–75.3) |
| Combined | 1114/1315 (84.7) | 729/878 (83.0) | 385/437 (88.1) | 0.67 (0.63–0.71) | 0.71 (0.68–0.75) | 93.3 (91.4–95.0) | 72.1 (68.1–75.9) |
AC1 indicates agreement coefficient 1; ESCAPE, ESCAPE‐NA1, Safety and Efficacy of Nerinetide (NA‐1) in Subjects Undergoing Endovascular Thrombectomy for Stroke; mRS, modified Rankin Scale; and NIHSS, National Institutes of Health Stroke Scale.
Cases in which 90‐d mRS agreed with the NIHSS impression (ie, good or bad), out of all NIHSS assessments with that impression at that time point.
Table 5.
Standardized Mean Difference in NIHSS (Intervention Minus Control Arm) at Different Early Time Points Versus 90‐Day mRS Using Individual Patient Data
| Measure and timing | SMD (95% CI) |
|---|---|
| NIHSS score—2 h | |
| ESCAPE | −0.36 (−0.59 to −0.13) |
| ESCAPE‐NA1 | −0.03 (−0.15 to 0.09) |
| Combined | −0.10 (−0.21 to 0.01) |
| NIHSS score —24 h | |
| ESCAPE | −0.50 (−0.73 to −0.28) |
| ESCAPE‐NA1 | −0.02 (−0.14 to 0.10) |
| Combined | −0.12 (−0.22 to −0.01) |
| NIHSS score —48 h | |
| ESCAPE | −0.69 (−0.93 to −0.46) |
| ESCAPE‐NA1 | −0.02 (−0.15 to 0.11) |
| Combined | −0.17 (−0.28 to −0.06) |
| NIHSS—5 to 7 d | |
| ESCAPE | −0.74 (−0.98 to −0.50) |
| ESCAPE‐NA1 | −0.03 (−0.15 to 0.10) |
| Combined | −0.18 (−0.29 to −0.07) |
| mRS score—90 d | |
| ESCAPE | −0.52 (−0.75 to −0.30) |
| ESCAPE‐NA1 | −0.03 (−0.14 to 0.09) |
| Combined | −0.12 (−0.23 to −0.02) |
Negative Values Indicate Treatment Benefit. ESCAPE indicates Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke; ESCAPE‐NA1, Safety and Efficacy of Nerinetide (NA‐1) in Subjects Undergoing Endovascular Thrombectomy for Stroke; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; and SMD, standardized mean difference.
DISCUSSION
In this systematic review and analysis, which included 44 387 patients from 116 trials with information on 170 NIHSS assessments over 5 time points, we observed that trial results as measured on 24‐hour NIHSS score agreed with results as assessed on the 90‐day mRS in almost 9 out of 10 trials. This resulted in a Cohen's kappa of 0.64 and Gwet's AC1 of 0.79. Agreement between NIHSS within 7‐day follow‐up and 90‐day mRS scores was not significantly different between the various time points. Individual patient data did show improvement for agreement over time and suggested that at an individual level NIHSS within 7 days risks misclassifying 20% to 30% of patients with respect to favorable 90‐day mRS depending on the definitions that are used.
Using the NIHSS within the first 7 days after stroke onset, rather than 90‐day mRS, as the primary outcome in acute stroke trials has several advantages. First, it would drastically reduce the required follow‐up period, which in turn would result in lower operating costs for stroke trials. The use of the NIHSS within 7 days also minimizes interference from confounding factors, such as other medical conditions or socioeconomic barriers unrelated to the ischemic stroke or the tested intervention and thus allows for a better estimation of the direct treatment effect of the intervention on outcome. 128 , 129 Using early NIHSS score as a primary outcome may allow for a smaller sample size; For instance, a simulation study showed that using NIHSS at day 7, rather than 90‐day mRS, could reduce the required sample size by almost a quarter. 4
There are also important downsides to the NIHSS to consider. Compared with the mRS, the NIHSS may be more difficult to interpret, especially for patients. Furthermore, it shows a weaker association with quality of life and its statistical characteristics are less optimal. 130 , 131 Through historical precedent, 90‐day mRS has become the standard for stroke trials and it is favored as the primary outcome by key regulators. The 85% agreement between early NIHSS and 90‐day mRS scores in the current study might be insufficient to convince regulators of the role of early NIHSS as a primary outcome. Adoption of early NIHSS as a substitute primary outcome in stroke trials would be limited if regulatory approval cannot be obtained based on such trials.
A previous study showed that NIHSS increasingly predicts functional outcome at 90 days as the time since stroke onset progresses, accounting for one quarter of the variance at 1 to 3 hours after stroke onset, half at 24 hours, and three quarters at 90 days. 132 However, our trial‐level results indicated a consistently high level of agreement across all assessed time points within 1 week of follow‐up, whereas our individual patient data showed there was higher agreement for later assessments. These findings hold potential implications for the design of future stroke trials, as relying on the NIHSS measurement at 24 hours may be more feasible and practical when assessing outcome on a study level, considering that patients are often transferred or discharged from the participating hospital, which complicates later in‐person assessment. However, when the goal is to predict outcomes at an individual patient‐level, our results indicate it might be better to wait at least 1 week before assessing the NIHSS. Furthermore, predicting functional outcomes for individual patients is more accurate when combining several radiological and clinical factors, including early NIHSS score, rather than relying solely on early NIHSS score. Several prediction tools have been developed and demonstrated to accurately predict functional outcomes on an individual level, though many have not been externally validated. 133 , 134
Although early NIHSS assessment may be a more direct measure of postintervention impairment, it is vulnerable to interrater variabilities, and these factors may also affect how agreement from one time point to another plays out in practice, especially when comparing NIHSS assessments across different trials. In previous studies, most NIHSS items showed moderate to excellent interrater reliability but certain items, such as ataxia assessment, consistently showed poor interrater reliability. 135 , 136 This is a potential pitfall of introducing early NIHSS score as a primary outcome in stroke trials. However, problems with interrater variability are also well appreciated for the mRS and are harder to narrow down to specific components given the global/holistic nature of the assessment compared with the examination‐based NIHSS. 137 , 138
The interpretation of the presented kappa and Gwet's AC1 values varies depending on the definitions used for the cutoff thresholds. For instance, the kappa value for NIHSS assessed at 24 hours was classified as intermediate to good according to the criteria proposed by Fleiss et al. 10 However, using Landis and Koch's criteria it was categorized as substantial, whereas Altman's criteria would classify it as good. 11 , 139 These variations highlight that the specific terminology used to describe agreement depends on the chosen framework. Nevertheless, across all definitions, the agreement between NIHSS at 24 hours and 90‐day mRS consistently falls within the intermediate to good range.
By aggregating results from >100 trials, our findings further strengthen and corroborate the use of early NIHSS outcome assessments in stroke trials. Another study assessed the correlation between NIHSS at 5 to 7 days and 90‐day mRS in the MR CLEAN and IMS III (Interventional Management of Stroke III) trials and showed that the treatment effect on the mRS is largely mediated by NIHSS at 24 hours follow‐up. 140 Other studies also showed that NIHSS score within 1 week of follow‐up is the most important predictor of functional outcome on the 90‐day mRS. 141
This study has several limitations. First, there was considerable heterogeneity in definitions that were used for dichotomization of NIHSS and mRS scores in the included studies. This may have potentially affected agreement between the outcomes, although the extent to which this may have influenced our results is unknown. Second, we used 90‐day mRS as the reference standard although it may be affected by various factors that occur independent of acute ischemic stroke or the assessed intervention. 128 , 129 This could have led to disagreement between early NIHSS and 90‐day mRS scores resulting in a potential underestimation of the performance of early NIHSS score, whereas in fact early NIHSS score better reflects the neurological injury. There was considerable variability in the way early NIHSS was reported in the included studies, both in terms of timing and the method of presentation (dichotomized, means/median or change compared with baseline). It is unclear how this may have influenced our findings. Although there is consensus that reporting results of a shift analysis of the full ordinal scale is the desirable way to report 90‐day mRS outcomes, no clear agreement exists for assessing early NIHSS score. 142 Future studies should establish the optimal way of analyzing early NIHSS measurements, both in terms of timing and analytical methods, to standardize its use as an alternative to 90‐day mRS as the primary outcome in stroke trials. Ideally, such studies should look at individual patient data across a larger number of studies.
CONCLUSIONS
NIHSS assessed at 24‐hours aligned with 90‐day mRS scores in 84% of trials for identifying positive versus neutral versus negative RCT results, indicating intermediate to good agreement. However, individual patient data showed that NIHSS score within 7 days risks misclassifying around 1 in 4 patients with respect to 90‐day mRS score, with some improvement in agreements for assessments between 48 hours and 7 days. These data contribute to a better understanding of the nuances of considering early NIHSS score as an outcome measure in acute ischemic stroke RCTs.
Sources of Funding
This work was supported by the Heart and Stroke Foundation of Canada New Investigator Award.
Disclosures
Leon A. Rinkel is supported by the Niels Stensen Fellowship. Johanna M. Ospel received consulting fees from Nicolab. Mayank Goyal has received grants from NoNo, Medtronic, and Cerenovus via the University of Calgary; royalties or licenses from GE Healthcare and Microvention; consulting fees from Microvention, Medtronic, Stryker, and Mentice and has stock options in Circle Neurovascular. Aravind Ganesh reports consulting fees and honoraria from Atheneum, AlphaSights, MD Analytics, MyMedicalPanel, Creative Research Designs, CTC Communications Corp, Alexion, Biogen, and Servier Canada; research support from Alberta Innovates, Campus Alberta Neuroscience, Brain Canada, the Canadian Cardiovascular Society, Panmure House, the Government of Canada INOVAIT and New Frontiers in Research programs, the Heart and Stroke Foundation of Canada and the Canadian Institutes of Health Research; stock/stock options from SnapDx Inc and LetsGetProof; and a patent application for a system for patient monitoring and cuff‐based therapies – all outside the scope of the published work.
Supporting information
Tables S1–S4
Figures S1–S16
This article was sent to Fadar O. Otite, MD, SM, Assistant Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.124.040304
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1. Saver JL. Optimal end points for acute stroke therapy trials: best ways to measure treatment effects of drugs and devices. Stroke. 2011;42:2356–2362. doi: 10.1161/STROKEAHA.111.619122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wood P. International Classification of Impairments, Disabilities and Handicaps: A Manual of Classification Relating to the Consequences of Disease. Geneva: World Health Organization; 1980. [Google Scholar]
- 3. Quinn TJ, Dawson J, Walters MR, Lees KR. Functional outcome measures in contemporary stroke trials. Int J Stroke. 2009;4:200–205. doi: 10.1111/j.1747-4949.2009.00271.x [DOI] [PubMed] [Google Scholar]
- 4. Kerr DM, Fulton RL, Lees KR. Seven‐day NIHSS is a sensitive outcome measure for exploratory clinical trials in acute stroke: evidence from the virtual international stroke trials archive. Stroke. 2012;43:1401–1403. doi: 10.1161/strokeaha.111.644484 [DOI] [PubMed] [Google Scholar]
- 5. Young FB, Weir CJ, Lees KR. Comparison of the National Institutes of Health stroke scale with disability outcome measures in acute stroke trials. Stroke. 2005;36:2187–2192. doi: 10.1161/01.STR.0000181089.41324.70 [DOI] [PubMed] [Google Scholar]
- 6. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Higgins JPT, Savović J, Page MJ, Elbers RG, S JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane; 2022. [Google Scholar]
- 8. Gwet KL. Computing inter‐rater reliability and its variance in the presence of high agreement. Br J Math Stat Psychol. 2008;61:29–48. doi: 10.1348/000711006X126600 [DOI] [PubMed] [Google Scholar]
- 9. Feinstein AR, Cicchetti DV. High agreement but low kappa: I. The problems of two paradoxes. J Clin Epidemiol. 1990;43:543–549. doi: 10.1016/0895-4356(90)90158-L [DOI] [PubMed] [Google Scholar]
- 10. Fleiss JL, Levin B, Park MC. Statistical Methods for Rates and Proportions. 3rd ed. John Wiley & Sons, Inc; 2003. [Google Scholar]
- 11. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- 12. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 13. Hill MD, Goyal M, Menon BK, Nogueira RG, McTaggart RA, Demchuk AM, Poppe AY, Buck BH, Field TS, Dowlatshahi D, et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE‐NA1): a multicentre, double‐blind, randomised controlled trial. Lancet. 2020;395:878–887. doi: 10.1016/s0140-6736(20)30258-0 [DOI] [PubMed] [Google Scholar]
- 14. Mistry EA, Yeatts S, Havenon A, Mehta T, Arora N, Rosa FDLRL, Starosciak AK, Siegler JE, Mistry AM, Yaghi S, et al. Predicting 90‐day outcome after thrombectomy: baseline‐adjusted 24‐hour NIHSS is more powerful than NIHSS score change. Stroke. 2021;52:2547–2553. doi: 10.1161/STROKEAHA.120.032487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mistry EA, Yeatts SD, Khatri P, Mistry AM, Detry M, Viele K, Harrell FE Jr, Lewis RJ. National Institutes of Health stroke scale as an outcome in stroke research: value of ANCOVA over analyzing change from baseline. Stroke. 2022;53:e150–e155. doi: 10.1161/strokeaha.121.034859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group . Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1588. doi: 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- 17. Use of anti‐ICAM‐1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology. 2001;57:1428–1434. doi: 10.1212/wnl.57.8.1428 [DOI] [PubMed] [Google Scholar]
- 18. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of a randomized phase 2 trial. Stroke. 2005;36:880–890. doi: 10.1161/01.Str.0000157668.39374.56 [DOI] [PubMed] [Google Scholar]
- 19. Albers GW, Goldstein LB, Hall D, Lesko LM. Aptiganel hydrochloride in acute ischemic stroke: a randomized controlled trial. JAMA. 2001;286:2673–2682. doi: 10.1001/jama.286.21.2673 [DOI] [PubMed] [Google Scholar]
- 20. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega‐Gutierrez S, McTaggart RA, Torbey MT, Kim‐Tenser M, Leslie‐Mazwi T, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–718. doi: 10.1056/nejmoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alexandrov AV, Köhrmann M, Soinne L, Tsivgoulis G, Barreto AD, Demchuk AM, Sharma VK, Mikulik R, Muir KW, Brandt G, et al. Safety and efficacy of sonothrombolysis for acute ischaemic stroke: a multicentre, double‐blind, phase 3, randomised controlled trial. Lancet Neurol. 2019;18:338–347. doi: 10.1016/s1474-4422(19)30026-2 [DOI] [PubMed] [Google Scholar]
- 22. An JQ, Cheng YW, Guo YC, Wei M, Gong MJ, Tang YL, Yuan XY, Song WF, Mu CY, Zhang AF, et al. Safety and efficacy of remote ischemic postconditioning after thrombolysis in patients with stroke. Neurology. 2020;95:e3355–e3363. doi: 10.1212/WNL.0000000000010884 [DOI] [PubMed] [Google Scholar]
- 23. Anderson CS, Robinson T, Lindley RI, Arima H, Lavados PM, Lee TH, Broderick JP, Chen X, Chen G, Sharma VK, et al. Low‐dose versus standard‐dose intravenous Alteplase in acute ischemic stroke. N Engl J Med. 2016;374:2313–2323. doi: 10.1056/NEJMoa1515510 [DOI] [PubMed] [Google Scholar]
- 24. Aoki J, Kimura K, Morita N, Harada M, Metoki N, Tateishi Y, Todo K, Yamagami H, Hayashi K, Terasawa Y, et al. YAMATO study (tissue‐type plasminogen activator and edaravone combination therapy). Stroke. 2017;48:712–719. doi: 10.1161/STROKEAHA.116.015042 [DOI] [PubMed] [Google Scholar]
- 25. Bang OY, Chung JW, Kim SK, Kim SJ, Lee MJ, Hwang J, Seo WK, Ha YS, Sung SM, Kim EG, et al. Therapeutic‐induced hypertension in patients with noncardioembolic acute stroke. Neurology. 2019;93:e1955–e1963. doi: 10.1212/wnl.0000000000008520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barlinn K, Jakubicek S, Siepmann T, Chernyshev OY, Pallesen LP, Wienecke M, Hermann W, Graehlert X, Alexandrov AW, Vosko M, et al. Autotitrating bilevel positive airway pressure in large vessel steno‐occlusive stroke patients with suspected sleep apnea: a multicenter randomized controlled study. Front Neurol. 2021;12:667494. doi: 10.3389/fneur.2021.667494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barreto AD, Ford GA, Shen L, Pedroza C, Tyson J, Cai C, Rahbar MH, Grotta JC. Randomized, multicenter trial of ARTSS‐2 (Argatroban with recombinant tissue plasminogen activator for acute stroke). Stroke. 2017;48:1608–1616. doi: 10.1161/strokeaha.117.016720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 29. Blanco M, Nombela F, Castellanos M, Rodriguez‐Yáñez M, García‐Gil M, Leira R, Lizasoain I, Serena J, Vivancos J, Moro MA, et al. Statin treatment withdrawal in ischemic stroke. A controlled randomized study. Neurology. 2007;69:904–910. doi: 10.1212/01.wnl.0000269789.09277.47 [DOI] [PubMed] [Google Scholar]
- 30. Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, Guillemin F. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15:1138–1147. doi: 10.1016/s1474-4422(16)30177-6 [DOI] [PubMed] [Google Scholar]
- 31. Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Dowling RJ, Yan B, Bush SJ, Dewey HM, Thijs V, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378:1573–1582. doi: 10.1056/NEJMoa1716405 [DOI] [PubMed] [Google Scholar]
- 32. Campbell BCV, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, et al. Endovascular therapy for ischemic stroke with perfusion‐imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 33. Cao J, Lin H, Lin M, Ke K, Zhang Y, Zhang Y, Zheng W, Chen X, Wang W, Zhang M, et al. RECO flow restoration device versus solitaire FR with the intention for Thrombectomy study (REDIRECT): a prospective randomized controlled trial. J Neurosurg. 2021;134:1569–1577. doi: 10.3171/2020.3.Jns193356 [DOI] [PubMed] [Google Scholar]
- 34. Chamorro A, Amaro S, Castellanos M, Segura T, Arenillas J, Martí‐Fábregas J, Gállego J, Krupinski J, Gomis M, Cánovas D, et al. Safety and efficacy of uric acid in patients with acute stroke (URICO‐ICTUS): a randomised, double‐blind phase 2b/3 trial. Lancet Neurol. 2014;13:453–460. doi: 10.1016/s1474-4422(14)70054-7 [DOI] [PubMed] [Google Scholar]
- 35. Chen HS, Cui Y, Zhou ZH, Dai YJ, Li GH, Peng ZL, Zhang Y, Liu XD, Yuan ZM, Jiang CH, et al. Effect of argatroban plus intravenous alteplase vs intravenous alteplase alone on neurologic function in patients with acute ischemic stroke: the ARAIS randomized clinical trial. JAMA. 2023;329:640–650. doi: 10.1001/jama.2023.0550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, Boccardi E. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368:904–913. doi: 10.1056/NEJMoa1213701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue‐type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA. 1999;282:2019–2026. doi: 10.1001/jama.282.21.2019 [DOI] [PubMed] [Google Scholar]
- 38. Culp WC, Onteddu SS, Brown A, Nalleballe K, Sharma R, Skinner RD, Witt T, Roberson PK, Marsh JD. Dodecafluoropentane emulsion in acute ischemic stroke: a phase Ib/II randomized and controlled dose‐escalation trial. J Vasc Interv Radiol. 2019;30:1244–1250. doi: 10.1016/j.jvir.2019.04.020 [DOI] [PubMed] [Google Scholar]
- 39. Deng C, Campbell D, Diprose W, Eom C, Wang K, Robertson N, Short TG, Brew S, Caldwell J, McGuinness B, et al. A pilot randomised controlled trial of the management of systolic blood pressure during endovascular thrombectomy for acute ischaemic stroke. Anaesthesia. 2020;75:739–746. doi: 10.1111/anae.14940 [DOI] [PubMed] [Google Scholar]
- 40. Eggers J, König IR, Koch B, Händler G, Seidel G. Sonothrombolysis with transcranial color‐coded sonography and recombinant tissue‐type plasminogen activator in acute middle cerebral artery main stem occlusion: results from a randomized study. Stroke. 2008;39:1470–1475. doi: 10.1161/strokeaha.107.503870 [DOI] [PubMed] [Google Scholar]
- 41. Elkind MSV, Veltkamp R, Montaner J, Johnston SC, Singhal AB, Becker K, Lansberg MG, Tang W, Kasliwal R, Elkins J. Natalizumab in acute ischemic stroke (ACTION II). A randomized, placebo‐controlled trial. Neurology. 2020;95:e1091–e1104. doi: 10.1212/wnl.0000000000010038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. England TJ, Hedstrom A, O'Sullivan SE, Woodhouse L, Jackson B, Sprigg N, Bath PM. Remote ischemic conditioning after stroke trial 2: a phase IIb randomized controlled trial in hyperacute stroke. J Am Heart Assoc. 2019;8:e013572. doi: 10.1161/JAHA.119.013572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fischer U, Kaesmacher J, Strbian D, Eker O, Cognard C, Plattner PS, Bütikofer L, Mordasini P, Deppeler S, Pereira VM, et al. Thrombectomy alone versus intravenous alteplase plus thrombectomy in patients with stroke: an open‐label, blinded‐outcome, randomised non‐inferiority trial. Lancet. 2022;400:104–115. doi: 10.1016/s0140-6736(22)00537-2 [DOI] [PubMed] [Google Scholar]
- 44. Ginsberg MD, Palesch YY, Hill MD, Martin RH, Moy CS, Barsan WG, Waldman BD, Tamariz D, Ryckborst KJ. High‐dose albumin treatment for acute ischaemic stroke (ALIAS) part 2: a randomised, double‐blind, phase 3, placebo‐controlled trial. Lancet Neurol. 2013;12:1049–1058. doi: 10.1016/s1474-4422(13)70223-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gusev EI, Martynov MY, Nikonov AA, Shamalov NA, Semenov MP, Gerasimets EA, Yarovaya EB, Semenov AM, Archakov AI, Markin SS. Non‐immunogenic recombinant staphylokinase versus alteplase for patients with acute ischaemic stroke 4·5 h after symptom onset in Russia (FRIDA): a randomised, open label, multicentre, parallel‐group, non‐inferiority trial. Lancet Neurol. 2021;20:721–728. doi: 10.1016/s1474-4422(21)00210-6 [DOI] [PubMed] [Google Scholar]
- 46. Haley EC Jr, Thompson JL, Grotta JC, Lyden PD, Hemmen TG, Brown DL, Fanale C, Libman R, Kwiatkowski TG, Llinas RH, et al. Phase IIB/III trial of tenecteplase in acute ischemic stroke: results of a prematurely terminated randomized clinical trial. Stroke. 2010;41:707–711. doi: 10.1161/strokeaha.109.572040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Han B, Ma T, Liu Z, Wu Y, Tan W, Sun S, Li X, Shao C, Tang D, Sun J. Efficacy and safety of tirofiban in clinical patients with acute ischemic stroke. Front Neurol. 2021;12:785836. doi: 10.3389/fneur.2021.785836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. He YD, Guo ZN, Qin C, Jin H, Zhang P, Abuduxukuer R, Yang Y. Remote ischemic conditioning combined with intravenous thrombolysis for acute ischemic stroke. Ann Clin Transl Neurol. 2020;7:972–979. doi: 10.1002/acn3.51063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hemmen TM, Raman R, Guluma KZ, Meyer BC, Gomes JA, Cruz‐Flores S, Wijman CA, Rapp KS, Grotta JC, Lyden PD. Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS‐L): final results. Stroke. 2010;41:2265–2270. doi: 10.1161/strokeaha.110.592295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Herisson F, Godard S, Volteau C, Le Blanc E, Guillon B, Gaudron M. Early sitting in ischemic stroke patients (SEVEL): a randomized controlled trial. PLoS One. 2016;11:e0149466. doi: 10.1371/journal.pone.0149466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hess DC, Wechsler LR, Clark WM, Savitz SI, Ford GA, Chiu D, Yavagal DR, Uchino K, Liebeskind DS, Auchus AP, et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet Neurol. 2017;16:360–368. doi: 10.1016/s1474-4422(17)30046-7 [DOI] [PubMed] [Google Scholar]
- 52. Huo X, Ma G, Tong X, Zhang X, Pan Y, Nguyen TN, Yuan G, Han H, Chen W, Wei M, et al. Trial of endovascular therapy for acute ischemic stroke with large infarct. N Engl J Med. 2023;388:1272–1283. doi: 10.1056/NEJMoa2213379 [DOI] [PubMed] [Google Scholar]
- 53. Investigators TAiIS . Abciximab in acute ischemic stroke. A randomized, double‐blind, placebo‐controlled, dose‐escalation study. Stroke. 2000;31:601–609. doi: 10.1161/01.STR.31.3.601 [DOI] [PubMed] [Google Scholar]
- 54. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Román L, Serena J, Abilleira S, Ribó M, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 55. Jovin TG, Li C, Wu L, Wu C, Chen J, Jiang C, Shi Z, Gao Z, Song C, Chen W, et al. Trial of thrombectomy 6 to 24 hours after stroke due to basilar‐artery occlusion. N Engl J Med. 2022;387:1373–1384. doi: 10.1056/NEJMoa2207576 [DOI] [PubMed] [Google Scholar]
- 56. Kaste M, Murayama S, Ford GA, Dippel DW, Walters MR, Tatlisumak T. Safety, tolerability and pharmacokinetics of MCI‐186 in patients with acute ischemic stroke: new formulation and dosing regimen. Cerebrovasc Dis. 2013;36:196–204. doi: 10.1159/000353680 [DOI] [PubMed] [Google Scholar]
- 57. Khatri P, Kleindorfer DO, Devlin T, Sawyer RN Jr, Starr M, Mejilla J, Broderick J, Chatterjee A, Jauch EC, Levine SR, et al. Effect of alteplase vs aspirin on functional outcome for patients with acute ischemic stroke and minor nondisabling neurologic deficits: the PRISMS randomized clinical trial. JAMA. 2018;320:156–166. doi: 10.1001/jama.2018.8496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim JS, Lee KB, Park JH, Sung SM, Oh K, Kim EG, Chang DI, Hwang YH, Lee EJ, Kim WK, et al. Safety and efficacy of otaplimastat in patients with acute ischemic stroke requiring tPA (SAFE‐TPA): a multicenter, randomized, double‐blind, placebo‐controlled phase 2 study. Ann Neurol. 2020;87:233–245. doi: 10.1002/ana.25644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Koga M, Yamamoto H, Inoue M, Asakura K, Aoki J, Hamasaki T, Kanzawa T, Kondo R, Ohtaki M, Itabashi R, et al. Thrombolysis with alteplase at 0.6 mg/kg for stroke with unknown time of onset: a randomized controlled trial. Stroke. 2020;51:1530–1538. doi: 10.1161/strokeaha.119.028127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kohler E, Prentice DA, Bates TR, Hankey GJ, Claxton A, van Heerden J, Blacker D. Intravenous minocycline in acute stroke: a randomized, controlled pilot study and meta‐analysis. Stroke. 2013;44:2493–2499. doi: 10.1161/strokeaha.113.000780 [DOI] [PubMed] [Google Scholar]
- 61. Kvistad CE, Næss H, Helleberg BH, Idicula T, Hagberg G, Nordby LM, Jenssen KN, Tobro H, Rörholt DM, Kaur K, et al. Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR‐TEST 2, part A): a phase 3, randomised, open‐label, blinded endpoint, non‐inferiority trial. Lancet Neurol. 2022;21:511–519. doi: 10.1016/s1474-4422(22)00124-7 [DOI] [PubMed] [Google Scholar]
- 62. Langezaal LCM, van der Hoeven E, Mont'Alverne FJA, de Carvalho JJF, Lima FO, Dippel DWJ, van der Lugt A, Lo RTH, Boiten J, Lycklama À Nijeholt GJ, et al. Endovascular therapy for stroke due to basilar‐artery occlusion. N Engl J Med. 2021;384:1910–1920. doi: 10.1056/NEJMoa2030297 [DOI] [PubMed] [Google Scholar]
- 63. Lapergue B, Blanc R, Gory B, Labreuche J, Duhamel A, Marnat G, Saleme S, Costalat V, Bracard S, Desal H, et al. Effect of endovascular contact aspiration vs stent retriever on revascularization in patients with acute ischemic stroke and large vessel occlusion: the ASTER randomized clinical trial. JAMA. 2017;318:443–452. doi: 10.1001/jama.2017.9644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. LeCouffe NE, Kappelhof M, Treurniet KM, Rinkel LA, Bruggeman AE, Berkhemer OA, Wolff L, van Voorst H, Tolhuisen ML, Dippel DWJ, et al. A randomized trial of intravenous Alteplase before endovascular treatment for stroke. N Engl J Med. 2021;385:1833–1844. doi: 10.1056/NEJMoa2107727 [DOI] [PubMed] [Google Scholar]
- 65. Lewandowski CA, Frankel M, Tomsick TA, Broderick J, Frey J, Clark W, Starkman S, Grotta J, Spilker J, Khoury J, et al. Combined intravenous and intra‐arterial r‐TPA versus intra‐arterial therapy of acute ischemic stroke: Emergency Management of Stroke (EMS) Bridging Trial. Stroke. 1999;30:2598–2605. doi: 10.1161/01.str.30.12.2598 [DOI] [PubMed] [Google Scholar]
- 66. Li W, Qi Z, Ma Q, Ding J, Wu C, Song H, Yang Q, Duan J, Liu L, Kang H, et al. Normobaric hyperoxia combined with endovascular treatment for patients with acute ischemic stroke: a randomized controlled clinical trial. Neurology. 2022;99:e824–e834. doi: 10.1212/WNL.0000000000200775 [DOI] [PubMed] [Google Scholar]
- 67. Liu X, Dai Q, Ye R, Zi W, Liu Y, Wang H, Zhu W, Ma M, Yin Q, Li M, et al. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open‐label, randomised controlled trial. Lancet Neurol. 2020;19:115–122. doi: 10.1016/s1474-4422(19)30395-3 [DOI] [PubMed] [Google Scholar]
- 68. Logallo N, Novotny V, Assmus J, Kvistad CE, Alteheld L, Rønning OM, Thommessen B, Amthor KF, Ihle‐Hansen H, Kurz M, et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR‐TEST): a phase 3, randomised, open‐label, blinded endpoint trial. Lancet Neurol. 2017;16:781–788. doi: 10.1016/s1474-4422(17)30253-3 [DOI] [PubMed] [Google Scholar]
- 69. Lorenzano S, Vestri A, Lancia U, Bovi P, Cappellari M, Stanzione P, Samà D, Bruscoli M, Cavazzuti M, Zini A, et al. Thrombolysis in elderly stroke patients in Italy (TESPI) trial and updated meta‐analysis of randomized controlled trials. Int J Stroke. 2021;16:43–54. doi: 10.1177/1747493019884525 [DOI] [PubMed] [Google Scholar]
- 70. Lowhagen Henden P, Rentzos A, Karlsson JE, Rosengren L, Leiram B, Sundeman H, Dunker D, Schnabel K, Wikholm G, Hellstrom M, et al. General Anesthesia versus conscious sedation for endovascular treatment of acute ischemic stroke: the AnStroke trial (anesthesia during stroke). Stroke. 2017;48:1601–1607. doi: 10.1161/STROKEAHA.117.016554 [DOI] [PubMed] [Google Scholar]
- 71. Lyden P, Hemmen T, Grotta J, Rapp K, Ernstrom K, Rzesiewicz T, Parker S, Concha M, Hussain S, Agarwal S, et al. Results of the ICTuS 2 trial (Intravascular Cooling in the Treatment of Stroke 2). Stroke. 2016;47:2888–2895. doi: 10.1161/strokeaha.116.014200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ma H, Campbell BCV, Parsons MW, Churilov L, Levi CR, Hsu C, Kleinig TJ, Wijeratne T, Curtze S, Dewey HM, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019;380:1795–1803. doi: 10.1056/NEJMoa1813046 [DOI] [PubMed] [Google Scholar]
- 73. Martins SO, Mont'Alverne F, Rebello LC, Abud DG, Silva GS, Lima FO, Parente BSM, Nakiri GS, Faria MB, Frudit ME, et al. Thrombectomy for stroke in the public health care system of Brazil. N Engl J Med. 2020;382:2316–2326. doi: 10.1056/NEJMoa2000120 [DOI] [PubMed] [Google Scholar]
- 74. Mitchell PJ, Yan B, Churilov L, Dowling RJ, Bush SJ, Bivard A, Huo XC, Wang G, Zhang SY, Ton MD, et al. Endovascular thrombectomy versus standard bridging thrombolytic with endovascular thrombectomy within 4·5 h of stroke onset: an open‐label, blinded‐endpoint, randomised non‐inferiority trial. Lancet. 2022;400:116–125. doi: 10.1016/s0140-6736(22)00564-5 [DOI] [PubMed] [Google Scholar]
- 75. Mizuma A, Yamashita T, Kono S, Nakayama T, Baba Y, Itoh S, Asakura K, Niimi Y, Asahi T, Kanemaru K, et al. Phase II trial of intravenous low‐dose granulocyte colony‐stimulating factor in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2016;25:1451–1457. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.022 [DOI] [PubMed] [Google Scholar]
- 76. Mousavi SA, Saadatnia M, Khorvash F, Hoseini T, Sariaslani P. Evaluation of the neuroprotective effect of dextromethorphan in the acute phase of ischaemic stroke. Arch Med Sci. 2011;7:465–469. doi: 10.5114/aoms.2011.23413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Muir KW, Ford GA, Messow CM, Ford I, Murray A, Clifton A, Brown MM, Madigan J, Lenthall R, Robertson F, et al. Endovascular therapy for acute ischaemic stroke: the Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE) randomised, controlled trial. J Neurol Neurosurg Psychiatry. 2017;88:38–44. doi: 10.1136/jnnp-2016-314117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Muscari A, Puddu GM, Santoro N, Serafini C, Cenni A, Rossi V, Zoli M. The atorvastatin during ischemic stroke study: a pilot randomized controlled trial. Clin Neuropharmacol. 2011;34:141–147. doi: 10.1097/WNF.0b013e3182206c2f [DOI] [PubMed] [Google Scholar]
- 79. Nacu A, Kvistad CE, Naess H, Øygarden H, Logallo N, Assmus J, Waje‐Andreassen U, Kurz KD, Neckelmann G, Thomassen L. NOR‐SASS (Norwegian Sonothrombolysis in Acute Stroke Study): randomized controlled contrast‐enhanced Sonothrombolysis in an unselected acute ischemic stroke population. Stroke. 2017;48:335–341. doi: 10.1161/strokeaha.116.014644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 81. Padma Srivastava MV, Bhasin A, Bhatia R, Garg A, Gaikwad S, Prasad K, Singh MB, Tripathi M. Efficacy of minocycline in acute ischemic stroke: a single‐blinded, placebo‐controlled trial. Neurol India. 2012;60:23–28. doi: 10.4103/0028-3886.93584 [DOI] [PubMed] [Google Scholar]
- 82. Pancioli AM, Adeoye O, Schmit PA, Khoury J, Levine SR, Tomsick TA, Sucharew H, Brooks CE, Crocco TJ, Gutmann L, et al. Combined approach to lysis utilizing eptifibatide and recombinant tissue plasminogen activator in acute ischemic stroke‐enhanced regimen stroke trial. Stroke. 2013;44:2381–2387. doi: 10.1161/strokeaha.113.001059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pancioli AM, Broderick J, Brott T, Tomsick T, Khoury J, Bean J, del Zoppo G, Kleindorfer D, Woo D, Khatri P, et al. The combined approach to lysis utilizing eptifibatide and rt‐PA in acute ischemic stroke: the CLEAR stroke trial. Stroke. 2008;39:3268–3276. doi: 10.1161/strokeaha.108.517656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. de la Pérez Ossa N, Abilleira S, Jovin TG, García‐Tornel Á, Jimenez X, Urra X, Cardona P, Cocho D, Purroy F, Serena J, et al. Effect of direct transportation to thrombectomy‐capable center vs local stroke center on neurological outcomes in patients with suspected large‐vessel occlusion stroke in nonurban areas: The RACECAT randomized clinical trial. JAMA. 2022;327:1782–1794. doi: 10.1001/jama.2022.4404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pico F, Lapergue B, Ferrigno M, Rosso C, Meseguer E, Chadenat ML, Bourdain F, Obadia M, Hirel C, Duong DL, et al. Effect of in‐hospital remote ischemic perconditioning on brain infarction growth and clinical outcomes in patients with acute ischemic stroke: the RESCUE BRAIN randomized clinical trial. JAMA Neurol. 2020;77:725–734. doi: 10.1001/jamaneurol.2020.0326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Price CI, Shaw L, Islam S, Javanbakht M, Watkins A, McMeekin P, Snooks H, Flynn D, Francis R, Lakey R, et al. Effect of an enhanced paramedic acute stroke treatment assessment on thrombolysis delivery during emergency stroke care: a cluster randomized clinical trial. JAMA Neurol. 2020;77:840–848. doi: 10.1001/jamaneurol.2020.0611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pruvost‐Robieux E, Benzakoun J, Turc G, Marchi A, Mancusi RL, Lamy C, Domigo V, Oppenheim C, Calvet D, Baron JC, et al. Cathodal transcranial direct current stimulation in acute ischemic stroke: pilot randomized controlled trial. Stroke. 2021;52:1951–1960. doi: 10.1161/strokeaha.120.032056 [DOI] [PubMed] [Google Scholar]
- 88. Qiu T, Li C, Huang L, Xiao H, Deng X, Dai X, Fu S, Wang J, Gong Q, Luo Q, et al. Tirofiban combined with heparin's effect and safety in the treatment of mild to moderate acute ischemic stroke. Neurol Res. 2021;43:220–224. doi: 10.1080/01616412.2020.1839690 [DOI] [PubMed] [Google Scholar]
- 89. Qiu Z, Li F, Sang H, Luo W, Liu S, Liu W, Guo Z, Li H, Sun D, Huang W, et al. Effect of intravenous tirofiban vs placebo before endovascular thrombectomy on functional outcomes in large vessel occlusion stroke: the RESCUE BT randomized clinical trial. JAMA. 2022;328:543–553. doi: 10.1001/jama.2022.12584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ramakrishnan TCR, Kumaravelu S, Narayan SK, Buddha SS, Murali C, Majeed PHA, Meenakshi‐Sundaram S, Wadia RS, Sharma V, Basu I, et al. Efficacy and safety of intravenous tenecteplase bolus in acute ischemic stroke: results of two open‐label, multicenter trials. Am J Cardiovasc Drugs. 2018;18:387–395. doi: 10.1007/s40256-018-0284-1 [DOI] [PubMed] [Google Scholar]
- 91. Ren C, Xu G, Liu Y, Liu G, Wang J, Gao J. Effect of conscious sedation vs. general anesthesia on outcomes in patients undergoing mechanical thrombectomy for acute ischemic stroke: a prospective randomized clinical trial. Front Neurol. 2020;11:170. doi: 10.3389/fneur.2020.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Renú A, Millán M, San Román L, Blasco J, Martí‐Fàbregas J, Terceño M, Amaro S, Serena J, Urra X, Laredo C, et al. Effect of intra‐arterial alteplase vs placebo following successful thrombectomy on functional outcomes in patients with large vessel occlusion acute ischemic stroke: the CHOICE randomized clinical trial. JAMA. 2022;327:826–835. doi: 10.1001/jama.2022.1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Requena M, Olivé‐Gadea M, Muchada M, Hernández D, Rubiera M, Boned S, Piñana C, Deck M, García‐Tornel Á, Díaz‐Silva H, et al. Direct to angiography suite without stopping for computed tomography imaging for patients with acute stroke: a randomized clinical trial. JAMA Neurol. 2021;78:1099–1107. doi: 10.1001/jamaneurol.2021.2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ringleb P, Bendszus M, Bluhmki E, Donnan G, Eschenfelder C, Fatar M, Kessler C, Molina C, Leys D, Muddegowda G, et al. Extending the time window for intravenous thrombolysis in acute ischemic stroke using magnetic resonance imaging‐based patient selection. Int J Stroke. 2019;14:483–490. doi: 10.1177/1747493019840938 [DOI] [PubMed] [Google Scholar]
- 95. Roaldsen MB, Eltoft A, Wilsgaard T, Christensen H, Engelter ST, Indredavik B, Jatužis D, Karelis G, Kõrv J, Lundström E, et al. Safety and efficacy of tenecteplase in patients with wake‐up stroke assessed by non‐contrast CT (TWIST): a multicentre, open‐label, randomised controlled trial. Lancet Neurol. 2023;22:117–126. doi: 10.1016/s1474-4422(22)00484-7 [DOI] [PubMed] [Google Scholar]
- 96. Roffe C, Nevatte T, Sim J, Bishop J, Ives N, Ferdinand P, Gray R; Investigators ftSOS, Group tSOC . Effect of routine low‐dose oxygen supplementation on death and disability in adults with acute stroke: the stroke oxygen study randomized clinical trial. JAMA. 2017;318:1125–1135. doi: 10.1001/jama.2017.11463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rusyniak DE, Kirk MA, May JD, Kao LW, Brizendine EJ, Welch JL, Cordell WH, Alonso RJ. Hyperbaric oxygen therapy in acute ischemic stroke: results of the hyperbaric oxygen in acute ischemic stroke trial pilot study. Stroke. 2003;34:571–574. doi: 10.1161/01.str.0000050644.48393.d0 [DOI] [PubMed] [Google Scholar]
- 98. Sarraj A, Hassan AE, Abraham MG, Ortega‐Gutierrez S, Kasner SE, Hussain MS, Chen M, Blackburn S, Sitton CW, Churilov L, et al. Trial of endovascular thrombectomy for large ischemic strokes. N Engl J Med. 2023;388:1259–1271. doi: 10.1056/NEJMoa2214403 [DOI] [PubMed] [Google Scholar]
- 99. Saver JL, Goyal M, Bonafe A, Diener H‐C, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, et al. Stent‐retriever thrombectomy after intravenous t‐PA vs. t‐PA alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 100. Schonenberger S, Uhlmann L, Hacke W, Schieber S, Mundiyanapurath S, Purrucker JC, Nagel S, Klose C, Pfaff J, Bendszus M, et al. Effect of conscious sedation vs general anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: a randomized clinical trial. JAMA. 2016;316:1986–1996. doi: 10.1001/jama.2016.16623 [DOI] [PubMed] [Google Scholar]
- 101. Schwarz S, Al‐Shajlawi F, Sick C, Meairs S, Hennerici MG. Effects of prophylactic antibiotic therapy with mezlocillin plus sulbactam on the incidence and height of fever after severe acute ischemic stroke. Stroke. 2008;39:1220–1227. doi: 10.1161/STROKEAHA.107.499533 [DOI] [PubMed] [Google Scholar]
- 102. Shuaib A, Bornstein NM, Diener HC, Dillon W, Fisher M, Hammer MD, Molina CA, Rutledge JN, Saver JL, Schellinger PD, et al. Partial aortic occlusion for cerebral perfusion augmentation: safety and efficacy of NeuroFlo in Acute Ischemic Stroke trial. Stroke. 2011;42:1680–1690. doi: 10.1161/strokeaha.110.609933 [DOI] [PubMed] [Google Scholar]
- 103. Simonsen CZ, Yoo AJ, Sorensen LH, Juul N, Johnsen SP, Andersen G, Rasmussen M. Effect of general anesthesia and conscious sedation during endovascular therapy on infarct growth and clinical outcomes in acute ischemic stroke: a randomized clinical trial. JAMA Neurol. 2018;75:470–477. doi: 10.1001/jamaneurol.2017.4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Song H, Wang Y, Ma Q, Chen H, Liu B, Yang Y, Zhu J, Zhao S, Jin X, Li Y, et al. Efficacy and safety of recombinant human prourokinase in acute ischemic stroke: a phase IIa randomized clinical trial. Transl Stroke Res. 2022;13:995–1004. doi: 10.1007/s12975-022-01012-9 [DOI] [PubMed] [Google Scholar]
- 105. Sun J, Liang F, Wu Y, Zhao Y, Miao Z, Zhang L, Gelb AW, Chan MTV, Peng Y, Han R. Choice of anesthesia for endovascular treatment of acute ischemic stroke (CANVAS): results of the CANVAS pilot randomized controlled trial. J Neurosurg Anesthesiol. 2020;32:41–47. doi: 10.1097/ana.0000000000000567 [DOI] [PubMed] [Google Scholar]
- 106. Tao C, Nogueira RG, Zhu Y, Sun J, Han H, Yuan G, Wen C, Zhou P, Chen W, Zeng G, et al. Trial of endovascular treatment of acute basilar‐artery occlusion. N Engl J Med. 2022;387:1361–1372. doi: 10.1056/NEJMoa2206317 [DOI] [PubMed] [Google Scholar]
- 107. Teal P, Davis S, Hacke W, Kaste M, Lyden PD, Fierus M. A randomized, double‐blind, placebo‐controlled trial to evaluate the efficacy, safety, tolerability, and pharmacokinetic/pharmacodynamic effects of a targeted exposure of intravenous repinotan in patients with acute ischemic stroke: modified randomized exposure controlled trial (mRECT). Stroke. 2009;40:3518–3525. doi: 10.1161/strokeaha.109.551382 [DOI] [PubMed] [Google Scholar]
- 108. Tian D‐C, Shi K, Zhu Z, Yao J, Yang X, Su L, Zhang S, Zhang M, Gonzales RJ, Liu Q, et al. Fingolimod enhances the efficacy of delayed alteplase administration in acute ischemic stroke by promoting anterograde reperfusion and retrograde collateral flow. Ann Neurol. 2018;84:717–728. doi: 10.1002/ana.25352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Torgano G, Zecca B, Monzani V, Maestroni A, Rossi P, Cazzaniga M, Manganaro D, Boiti C, Zilioli E, Borutti G, et al. Effect of intravenous tirofiban and aspirin in reducing short‐term and long‐term neurologic deficit in patients with ischemic stroke: a double‐blind randomized trial. Cerebrovasc Dis. 2010;29:275–281. doi: 10.1159/000275503 [DOI] [PubMed] [Google Scholar]
- 110. Turk AS 3rd, Siddiqui A, Fifi JT, de Leacy RA, Fiorella DJ, Gu E, Levy EI, Snyder KV, Hanel RA, Aghaebrahim A, et al. Aspiration thrombectomy versus stent retriever thrombectomy as first‐line approach for large vessel occlusion (COMPASS): a multicentre, randomised, open label, blinded outcome, non‐inferiority trial. Lancet. 2019;393:998–1008. doi: 10.1016/s0140-6736(19)30297-1 [DOI] [PubMed] [Google Scholar]
- 111. van den Berg SA, Uniken Venema SM, Reinink H, Hofmeijer J, Schonewille WJ, Miedema I, Fransen PSS, OP DM, Raaijmakers TWM, van Dijk GW, et al. Prehospital transdermal glyceryl trinitrate in patients with presumed acute stroke (MR ASAP): an ambulance‐based, multicentre, randomised, open‐label, blinded endpoint, phase 3 trial. Lancet Neurol. 2022;21:971–981. doi: 10.1016/s1474-4422(22)00333-7 [DOI] [PubMed] [Google Scholar]
- 112. van der Steen W, van de Graaf RA, Chalos V, Lingsma HF, van Doormaal PJ, Coutinho JM, Emmer BJ, de Ridder I, van Zwam W, van der Worp HB, et al. Safety and efficacy of aspirin, unfractionated heparin, both, or neither during endovascular stroke treatment (MR CLEAN‐MED): an open‐label, multicentre, randomised controlled trial. Lancet. 2022;399:1059–1069. doi: 10.1016/s0140-6736(22)00014-9 [DOI] [PubMed] [Google Scholar]
- 113. Wan Y, Tian H, Wang H, Wang D, Jiang H, Fang Q. Selective intraarterial hypothermia combined with mechanical thrombectomy for acute cerebral infarction based on microcatheter technology: a single‐center, randomized, single‐blind controlled study. Front Neurol. 2023;14:1039816. doi: 10.3389/fneur.2023.1039816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wang Y, Li S, Pan Y, Li H, Parsons MW, Campbell BCV, Schwamm LH, Fisher M, Che F, Dai H, et al. Tenecteplase versus alteplase in acute ischaemic cerebrovascular events (TRACE‐2): a phase 3, multicentre, open‐label, randomised controlled, non‐inferiority trial. Lancet. 2023;401:645–654. doi: 10.1016/s0140-6736(22)02600-9 [DOI] [PubMed] [Google Scholar]
- 115. White PM, Gregson B, Cora EA, Dixit A, Subramanian G, Joshi Y, Simister R, Lakey R, Maiter A, Ford GA. Evaluation of stroke thrombectomy including patients where IV thrombolysis is contraindicated or has failed: a randomized trial of two novel thrombectomy devices. J Neurointerv Surg. 2021;13:311–318. doi: 10.1136/neurintsurg-2020-016038 [DOI] [PubMed] [Google Scholar]
- 116. Yang P, Song L, Zhang Y, Zhang X, Chen X, Li Y, Sun L, Wan Y, Billot L, Li Q, et al. Intensive blood pressure control after endovascular thrombectomy for acute ischaemic stroke (ENCHANTED2/MT): a multicentre, open‐label, blinded‐endpoint, randomised controlled trial. Lancet. 2022;400:1585–1596. doi: 10.1016/s0140-6736(22)01882-7 [DOI] [PubMed] [Google Scholar]
- 117. Yang P, Zhang Y, Zhang L, Zhang Y, Treurniet KM, Chen W, Peng Y, Han H, Wang J, Wang S, et al. Endovascular Thrombectomy with or without intravenous Alteplase in acute stroke. N Engl J Med. 2020;382:1981–1993. doi: 10.1056/NEJMoa2001123 [DOI] [PubMed] [Google Scholar]
- 118. Yoshimura S, Sakai N, Yamagami H, Uchida K, Beppu M, Toyoda K, Matsumaru Y, Matsumoto Y, Kimura K, Takeuchi M, et al. Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med. 2022;386:1303–1313. doi: 10.1056/NEJMoa2118191 [DOI] [PubMed] [Google Scholar]
- 119. Zhang X, Zhong W, Ma X, Zhang X, Chen H, Wang Z, Lou M. Ginkgolide with intravenous alteplase thrombolysis in acute ischemic stroke improving neurological function: a multicenter, cluster‐randomized trial (GIANT). Front Pharmacol. 2021;12:792136. doi: 10.3389/fphar.2021.792136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zhang Y, Hua W, Li Z, Peng Y, Han Z, Li T, Yin C, Wang S, Nan G, Zhao Z, et al. Efficacy and safety of a novel thrombectomy device in patients with acute ischemic stroke: a randomized controlled trial. Front Neurol. 2021;12:686253. doi: 10.3389/fneur.2021.686253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhang Y, Liu P, Li Z, Peng Y, Chen W, Zhang L, Chu J, Kuai D, Chen Z, Wu W, et al. Endovascular treatment of acute ischemic stroke with a fully radiopaque retriever: a randomized controlled trial. Front Neurol. 2022;13:962987. doi: 10.3389/fneur.2022.962987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Zhang Y, Wang J, Ma Z, Mu G, Liang D, Li Y, Qian X, Zhang L, Shen F, Zhang L, et al. Prospective pilot study of tirofiban in progressive stroke after intravenous thrombolysis. Front Neurol. 2022;13:982684. doi: 10.3389/fneur.2022.982684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zhang Y, Zhang QQ, Fu C, Wang L, Zhang GQ, Cao PW, Chen GF, Fu XM. Clinical efficacy of tirofiban combined with a solitaire stent in treating acute ischemic stroke. Braz J Med Biol Res. 2019;52:e8396. doi: 10.1590/1414-431x20198396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zhao J, Yuan F, Song C, Yin R, Chang M, Zhang W, Zhang B, Yu L, Jia Y, Ma Y, et al. Safety and efficacy of three enteral feeding strategies in patients with severe stroke in China (OPENS): a multicentre, prospective, randomised, open‐label, blinded‐endpoint trial. Lancet Neurol. 2022;21:319–328. doi: 10.1016/s1474-4422(22)00010-2 [DOI] [PubMed] [Google Scholar]
- 125. Zhu Z, Fu Y, Tian D, Sun N, Han W, Chang G, Dong Y, Xu X, Liu Q, Huang D, et al. Combination of the immune modulator fingolimod with alteplase in acute ischemic stroke: a pilot trial. Circulation. 2015;132:1104–1112. doi: 10.1161/circulationaha.115.016371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Zi W, Qiu Z, Li F, Sang H, Wu D, Luo W, Liu S, Yuan J, Song J, Shi Z, et al. Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional Independence in patients with acute ischemic stroke: the DEVT randomized clinical trial. JAMA. 2021;325:234–243. doi: 10.1001/jama.2020.23523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Zinkstok SM, Roos YB. Early administration of aspirin in patients treated with alteplase for acute ischaemic stroke: a randomised controlled trial. Lancet (London, England). 2012;380:731–737. doi: 10.1016/S0140-6736(12)60949-0 [DOI] [PubMed] [Google Scholar]
- 128. Ganesh A, Menon BK, Assis ZA, Demchuk AM, Al‐Ajlan FS, Almekhlafi MA, Rempel JL, Shuaib A, Baxter BW, Devlin T, et al. Discrepancy between post‐treatment infarct volume and 90‐day outcome in the ESCAPE randomized controlled trial. Int J Stroke. 2021;16:593–601. doi: 10.1177/1747493020929943 [DOI] [PubMed] [Google Scholar]
- 129. Ganesh A, Ospel JM, Menon BK, Demchuk AM, McTaggart RA, Nogueira RG, Poppe AY, Almekhlafi MA, Hanel RA, Thomalla G, et al. Assessment of discrepancies between follow‐up infarct volume and 90‐day outcomes among patients with ischemic stroke who received endovascular therapy. JAMA Netw Open. 2021;4:e2132376. doi: 10.1001/jamanetworkopen.2021.32376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Lees KR, Bath PM, Schellinger PD, Kerr DM, Fulton R, Hacke W, Matchar D, Sehra R, Toni D. Contemporary outcome measures in acute stroke research: choice of primary outcome measure. Stroke. 2012;43:1163–1170. doi: 10.1161/strokeaha.111.641423 [DOI] [PubMed] [Google Scholar]
- 131. Ali M, Fulton R, Quinn T, Brady M, Lees KR, Alexandrov A, Bath PM, Bluhmki E, Bornstein N, Claesson L, et al. How well do standard stroke outcome measures reflect quality of life? A retrospective analysis of clinical trial data. Stroke. 2013;44:3161–3165. doi: 10.1161/STROKEAHA.113.001126 [DOI] [PubMed] [Google Scholar]
- 132. Saver JL, Altman H. Relationship between neurologic deficit severity and final functional outcome shifts and strengthens during first hours after onset. Stroke. 2012;43:1537–1541. doi: 10.1161/STROKEAHA.111.636928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chalos V, Venema E, Mulder MJHL, Roozenbeek B, Steyerberg EW, Wermer MJH, Lycklama à Nijeholt GJ, van der Worp HB, Goyal M, Campbell BCV, et al. Development and validation of a postprocedural model to predict outcome after endovascular treatment for ischemic stroke. JAMA Neurol. 2023;80:940–948. doi: 10.1001/jamaneurol.2023.2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Jampathong N, Laopaiboon M, Rattanakanokchai S, Pattanittum P. Prognostic models for complete recovery in ischemic stroke: a systematic review and meta‐analysis. BMC Neurol. 2018;18:26. doi: 10.1186/s12883-018-1032-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Lyden P, Brott T, Tilley B, Welch KM, Mascha EJ, Levine S, Haley EC, Grotta J, Marler J. Improved reliability of the NIH stroke scale using video training. NINDS TPA Stroke Study Group. Stroke. 1994;25:2220–2226. doi: 10.1161/01.str.25.11.2220 [DOI] [PubMed] [Google Scholar]
- 136. Lyden P, Raman R, Liu L, Grotta J, Broderick J, Olson S, Shaw S, Spilker J, Meyer B, Emr M, et al. NIHSS training and certification using a new digital video disk is reliable. Stroke. 2005;36:2446–2449. doi: 10.1161/01.Str.0000185725.42768.92 [DOI] [PubMed] [Google Scholar]
- 137. Quinn TJ, Dawson J, Walters MR, Lees KR. Reliability of the modified Rankin Scale. Stroke. 2009;40:3393–3395. doi: 10.1161/STROKEAHA.109.557256 [DOI] [PubMed] [Google Scholar]
- 138. Wilson JT, Hareendran A, Hendry A, Potter J, Bone I, Muir KW. Reliability of the modified Rankin Scale across multiple raters: benefits of a structured interview. Stroke. 2005;36:777–781. doi: 10.1161/01.STR.0000157596.13234.95 [DOI] [PubMed] [Google Scholar]
- 139. Altman DG. Practical statistics for medical research. Douglas G. Altman, Chapman and Hall, London, 1991. No. of pages: 611. Price: £32.00. Stat Med. 1991;10:1635–1636. doi: 10.1002/sim.4780101015 [DOI] [Google Scholar]
- 140. Chalos V, van der Ende NAM, Lingsma HF, Mulder M, Venema E, Dijkland SA, Berkhemer OA, Yoo AJ, Broderick JP, Palesch YY, et al. National Institutes of Health stroke scale: an alternative primary outcome measure for trials of acute treatment for ischemic stroke. Stroke. 2020;51:282–290. doi: 10.1161/strokeaha.119.026791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Sajobi TT, Menon BK, Wang M, Lawal O, Shuaib A, Williams D, Poppe AY, Jovin TG, Casaubon LK, Devlin T, et al. Early trajectory of stroke severity predicts long‐term functional outcomes in ischemic stroke subjects. Stroke. 2017;48:105–110. doi: 10.1161/STROKEAHA.116.014456 [DOI] [PubMed] [Google Scholar]
- 142. Ganesh A, Luengo‐Fernandez R, Wharton RM, Rothwell PM. Ordinal vs dichotomous analyses of modified Rankin scale, 5‐year outcome, and cost of stroke. Neurology. 2018;91:e1951–e1960. doi: 10.1212/wnl.0000000000006554 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figures S1–S16
Data Availability Statement
The analysis for this study is based on published results from individual studies. All extracted data from the individual studies and the code used for performing the analyses can be made available upon reasonable request to the corresponding author. The ESCAPE‐NA1 (Safety and Efficacy of Nerinetide [NA‐1] in Subjects Undergoing Endovascular Thrombectomy for Stroke) individual patient data are not currently publicly available for distribution.


