Abstract
Background
Pulmonary embolism (PE) is a life-threatening cardiovascular condition with increasing global incidence. Obesity is a significant risk factor for PE, although its reported relationship with outcomes is inconsistent. This study aimed to investigate the impact of obesity on clinical outcomes in patients with high-risk PE.
Methods
We conducted a retrospective analysis of US adult patients hospitalized with high-risk PE from 2016 to 2019 using the National Inpatient Sample database. Patients were categorized into three groups based on BMI: non-obese, obese (30 to < 40 kg/m2), and severely obese (≥40 kg/m2). We compared baseline characteristics, in-hospital procedures, and outcomes among these groups. Multivariable logistic regression models assessed the relationship between obesity levels and in-hospital outcomes.
Results
Of 752,660 patients with PE, 29,610 (3.9 %) were classified as high-risk. The distribution among BMI categories was: non-obese (77.1 %), obese (8.8 %), and severely obese (14.1 %). Severely obese patients were younger (mean age 55.7 vs. 66.1 years for non-obese, p < 0.001) and more likely to be female (63.2 % vs. 51.4 % for non-obese, p < 0.001). After adjustment, obese and severely obese patients had lower odds of in-hospital mortality (obese: aOR 0.50, p < 0.001; severely obese: aOR 0.69, p < 0.001) and major adverse cardiovascular and cerebrovascular events (obese: aOR 0.50, p < 0.001; severely obese: aOR 0.72, p < 0.001).
Conclusion
Our study revealed an “obesity paradox” in high-risk PE patients, with obese and severely obese individuals showing lower mortality and fewer complications despite higher comorbidity rates. These findings emphasize the need for tailored risk assessment and treatment strategies in obese patients with high-risk PE.
Keywords: Pulmonary embolism, Obesity, Risk factors, Outcomes
1. Introduction
Pulmonary embolism (PE) is a potentially life-threatening cardiovascular condition with global estimates suggesting an increase in incidence in the past two decades, with rates from 39 to 115 cases per 100,000 population per year [1]. Despite advancements in diagnosis and management, PE is associated with significant mortality rates accounting for 0.46 % of all recorded deaths, and age-standardized mortality rates ranging from 0 to 24 deaths per 100,000 population-years across different countries [2].
Obesity, with a body mass index (BMI) at or above 30, has become a global health crisis with an estimated 890 million adults living with obesity in 2022 [3]. Obesity is strongly associated with other comorbidities and increased mortality risk [4,5]. Obesity is also a significant risk factor for the development of PE [[6], [7], [8], [9]]. Obese individuals exhibit a prothrombotic state characterized by increased levels of procoagulant factors and impaired fibrinolysis, which substantially elevates their risk of venous thromboembolism (VTE) and, consequently, PE [10].
Despite the increased risk of PE associated with obesity, Keller et al. [11] revealed a paradoxical relationship between obesity and mortality in patients with acute PE. This phenomenon, known as the “obesity paradox,” suggests that obese patients with PE may have actually lower in-hospital mortality rates compared to normal-weight patients [11]. However, there is lack of confirmatory data in the United States, particularly among patients with high-risk PE who are at greatest risk for adverse events. Therefore, the primary objective of our study was to evaluate the impact of obesity on clinical outcomes in large, nationwide US cohort of patients with high-risk PE.
2. Methods
2.1. Data source
The National Inpatient Sample (NIS), available since 1988, is one of the largest publicly available all payer inpatient healthcare databases in the United States. The dataset includes approximately 7 million hospital admissions each year, about a 20 percent stratified sample of all discharges from U.S. community hospitals, excluding rehabilitation and long term acute care facilities. The NIS is part of the Healthcare Cost and Utilization Project (HCUP) and is designed to provide estimates of inpatient utilization, access, costs, quality, and outcomes at local, regional, and national levels across the nation [12].
2.2. Study design and population
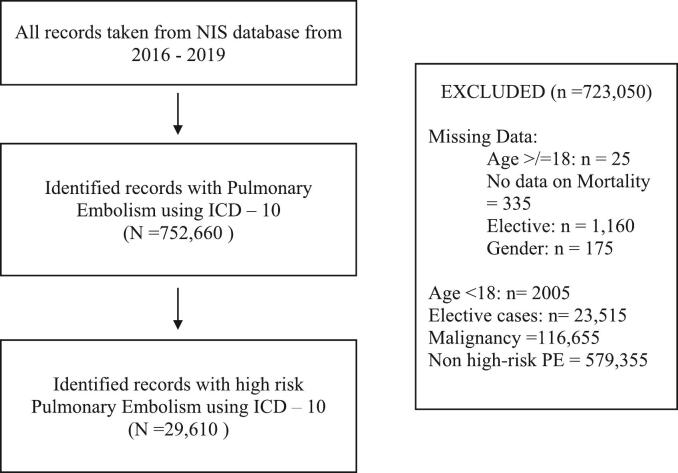
In this retrospective study, we analyzed adult patients (aged 18 years and older) who were hospitalized with a primary discharge diagnosis of acute PE from 2016 to 2019, categorized into three groups based on BMI: non-obese (BMI < 30 kg/m2), obese (BMI 30 to < 40 kg/m2), and severely obese (BMI ≥ 40 kg/m2) [13]. Patient selection was based on the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes, which were introduced in 2016 and offered more detailed information than the former ICD-9 system. Table S1 lists the ICD-10 codes related to patient and procedural characteristics. For each hospital discharge, we recorded patient demographics such as age, gender, race, admission day (weekday or weekend), expected primary payer, and median household income based on ZIP code. We excluded records with missing information on age, gender, elective status (planned/non-emergency admissions), admission type and day, and mortality from our analysis (refer to Fig. 1 for the study flow diagram). Patients with a diagnosis of malignancy were excluded from this analysis to minimize potential bias arising from the complex relationship between malignancy, body weight, and clinical outcomes. Each discharge record included data on up to 30 diagnoses.
Fig. 1.
Flow diagram.
In this study, a “high-risk PE” was defined as PE with cardiogenic shock, mechanical ventilation, mechanical circulatory support (MCS), or vasopressors [14,15]. Supplementary Table S1 contains a comprehensive list of ICD-10-CM codes used to identify PE. These codes also helped classify procedural details during hospitalization, such as systemic thrombolysis, catheter-directed thrombolysis, ultrasound-facilitated catheter-directed thrombolysis, catheter-directed embolectomy, surgical embolectomy or thrombectomy, and inferior vena cava (IVC) filter placement.
2.3. Outcomes
The primary outcome of interest was the difference in all-cause in-hospital mortality between patients with different BMI groups: non-obese (BMI < 30 kg/m2), obese (BMI 30 to < 40 kg/m2), and severely obese (BMI ≥ 40 kg/m2). Secondary outcomes, including in-hospital adverse events [major adverse cardiovascular and cerebrovascular events (MACCE), all-cause mortality, major bleeding, intracranial hemorrhage (ICH), non-ICH bleeding events, length of stay and cost] were also evaluated. The MACCE was defined as a composite of all-cause mortality, acute ischemic CVA and cardiac complications. Cardiac complications included coronary artery dissection, pericardial effusion (including tamponade), Dressler's syndrome, post MI angina, intracardiac thrombus and acute mechanical complications. Gastrointestinal, retroperitoneal, intracranial, intracerebral hemorrhage, as well as periprocedural hemorrhage, unspecified hemorrhage or requiring blood transfusion were defined as major bleeding. The treatments that the participants also received including invasive management procedures such as systemic thrombolysis, catheter directed thrombolysis, ultrasound-facilitated catheter directed thrombolysis, catheter directed embolectomy, surgical embolectomy/thrombectomy and inferior vena cava (IVC) filter placement.
2.4. Statistical analysis
Statistical analysis was performed on IBM SPSS version 29. Continuous variables were presented as median and interquartile range, due to skewed data, and categorical data were presented as frequencies and percentages. Pearson's chi square test was used to compare categorical variables, and continuous variables were compared using t-test or the Kruskal Wallis test, as appropriate. Sampling weights were used to calculate the estimated total discharges as specified by the Agency for Healthcare Research and Quality (AHRQ). Multivariable logistic regression models were used to assess the relationship between level of obesity and in-hospital outcomes. Odds ratios (ORs) with 95 % confidence intervals (CIs) were produced by these models, adjusted for baseline differences between groups. Adjustment was made for a range of covariates such as age, gender, weekend admission, hospital characteristics (bed size, region, and teaching status), and clinical factors. Factors included ventricular fibrillation (VF), ventricular tachycardia (VT), atrial fibrillation (AF), heart failure (HF), hypertension, valvular heart disease, dyslipidemia, chronic liver disease, chronic lung disease, chronic kidney disease, anemia, thrombocytopenia, coagulopathies, diabetes mellitus, systemic thrombolysis, catheter-directed thrombolysis, ultrasound-facilitated catheter-directed thrombolysis, catheter-directed embolectomy, surgical embolectomy/thrombectomy and inferior vena cava (IVC) filter placement.
3. Results
Of a total of 752,660 patients with a primary diagnosis of acute PE, 29,610 (3.9 %) were classified as having high-risk and were included in this analysis. Of these high-risk PE patients, 22,825 (77.1 %) were non-obese, 2,615 (8.8 %) were obese, and 4,170 (14.1 %) were severely obese. Table 1 showed the baseline characteristics of the patients. Mean age decreased progressively through the BMI groups with severely obese patients’ mean age of 55.7 years compared to 62.6 years for obese and 66.1 years for non-obese (p < 0.001). Female representation was also highest in the severely obese group (63.2 %) compared to 51.7 % and 51.4 % in the non-obese and obese groups, respectively (p < 0.001).
Table 1.
Baseline characteristics of patients with high-risk pulmonary embolism stratified by body mass index (BMI) category.
| Non-obese | Obese | Severely Obese | P-value | |
|---|---|---|---|---|
| NIS discharge weight (Number, %) | 22,825 (77.1 %) | 2,615 (8.8 %) |
4,170 (14.1 %) |
<0.001 |
| Mean Age | 66.1 | 62.6 | 55.7 | <0.001 |
| Age Group Categories, % | <0.001 | |||
| Under 50 Years | 15.3 | 19.7 | 32.7 | |
| 50 to 75 Years | 54.1 | 59.7 | 61.0 | |
| Over 75 Years | 30.6 | 20.7 | 6.2 | |
| Female, % | 51.7 | 51.4 | 63.2 | <0.001 |
| Weekend admission, % | 26.8 | 25.4 | 26.6 | 0.380 |
| Ethnicity,% | <0.001 | |||
| White | 65.8 | 64.9 | 64.5 | |
| Black | 22.1 | 24.6 | 25 | |
| Hispanic | 7.1 | 7.5 | 6.1 | |
| Asian | 1.6 | 0.6 | 0.8 | |
| Native | 0.4 | 0.4 | 0.1 | |
| Other | 3.1 | 2 | 3.5 | |
| Hospital Region,% | <0.001 | |||
| Northeast | 17.1 | 15.3 | 15.5 | |
| Midwest or North Central | 22.1 | 26 | 28.5 | |
| South | 41.1 | 39.4 | 38.7 | |
| West | 19.8 | 19.3 | 17.3 | |
| Hospital Bed Size,% | <0.001 | |||
| Small | 14.8 | 14.9 | 14.7 | |
| Medium | 29.3 | 31.4 | 26.5 | |
| Large | 55.9 | 53.7 | 58.8 | |
| Hospital Location/Teaching Status,% | <0.001 | |||
| Rural | 4.5 | 4.4 | 4.4 | |
| Urban non-teaching | 17.3 | 19.3 | 15.2 | |
| Teaching | 78.2 | 76.3 | 80.3 | |
| Median ZIP income | <0.001 | |||
| 1st Quartile | 31.4 | 29.8 | 34.7 | |
| 2nd Quartile | 26 | 26.7 | 27.2 | |
| 3rd Quartile | 23.9 | 24.6 | 24.4 | |
| 4th Quartile | 18.8 | 18.9 | 13.8 | |
| Primary Expected Payer,% | <0.001 | |||
| Medicare | 60.3 | 52.3 | 39.8 | |
| Medicaid | 11.1 | 11.1 | 17.3 | |
| Private Insurance | 21.4 | 29.5 | 37.1 | |
| Self-pay | 4.2 | 4.4 | 4.4 | |
| No charge | 0.2 | 0 | 0 | |
| Other | 2.9 | 2.7 | 1.4 | |
| Record Characteristics, % | ||||
| Ventricular Fibrillation | 3.6 | 3.6 | 3.2 | 0.440 |
| Ventricular Tachycardia | 6.8 | 6.3 | 5.9 | 0.060 |
| Cardiogenic Shock | 29.4 | 27.7 | 30 | 0.120 |
| Saddle PE | 18.5 | 22.2 | 23.6 | <0.001 |
| Acute cor pulmonale | 20.3 | 24.5 | 29.7 | <0.001 |
| Cardiac arrest | 36.6 | 25.8 | 27.8 | <0.001 |
| Comorbidities, % | ||||
| Heart Failure | 33.3 | 36.9 | 38 | <0.001 |
| Valvular Heart Disease | 8.9 | 7.3 | 6.7 | <0.001 |
| Hypertension | 61.9 | 71.3 | 66.7 | <0.001 |
| Diabetes Mellitus | 28.4 | 39.4 | 40 | <0.001 |
| Dyslipidemia | 31.4 | 36.9 | 31.4 | <0.001 |
| Atrial Fibrillation/Flutter | 21.4 | 22.6 | 18.3 | <0.001 |
| Smoking | 30.4 | 37.9 | 28.4 | <0.001 |
| Dementia | 9.8 | 3.8 | 1.6 | <0.001 |
| Chronic Kidney Disease | 18.9 | 18.2 | 18.9 | 0.620 |
| Chronic Lung Disease | 23.7 | 27.9 | 25.9 | <0.001 |
| Anemia | 38.9 | 39.4 | 36.5 | 0.008 |
| Thrombocytopenia | 14.8 | 14.9 | 12 | <0.001 |
| Coagulopathy | 13.2 | 12.2 | 12.8 | 0.333 |
| Chronic Liver Disease | 0.9 | 0.8 | 0.1 | <0.001 |
| Peripheral Vascular Disease | 4.5 | 3.3 | 2.2 | <0.001 |
| Previous Acute Myocardial Infarction | 4.9 | 5.2 | 4.8 | 0.781 |
| Previous PCI | 4.2 | 5.4 | 3.5 | <0.001 |
| Previous CABG | 6.8 | 8.4 | 3.6 | <0.001 |
| Previous CVA | 5.9 | 5.7 | 3.5 | <0.001 |
Severely obese patients had a higher prevalence of saddle PE (23.6 % vs 18.5 % in non-obese and 22.2 % in obese, p < 0.001) and acute cor pulmonale (29.7 % vs 20.3 % in non-obese and 24.5 % in obese, p < 0.001). Interestingly, cardiac arrest was more frequent in non-obese (36.6 %) than in obese (25.8 %) and severely obese (27.8 %) patients (p < 0.001).
The variations in comorbidities were quite distinct between BMI groups. Heart failure was present in 38 % of severely obese, 36.9 % of obese, and 33.3 % of non-obese (p < 0.001) patients. Obese patients had the highest prevalence of hypertension and dyslipidemia (71.3 % and 36.9 %, respectively) compared to non-obese (61.9 % and 31.4 %) and severely obese patients (p < 0.001 for both). Obese patients also had the highest smoking rates (37.9 %) versus non-obese and severely obese (30.4 %, 28.4 %, p < 0.001). A complete list of comorbidities and their prevalence is provided in Table 1.
3.1. In-Hospital procedures and outcomes
3.1.1. Crude rates
Table 2 showed the analysis of in-hospital management and clinical outcomes of patients with high-risk PE, stratified by BMI groups. In terms of management, severely obese patients were more likely to receive catheter-directed procedures (13.8 %) compared to obese (12 %) and non-obese patients (8.2 %, p < 0.001). Systemic thrombolysis was most common in obese patients (26.4 %), followed by severely obese (25.7 %) and non-obese patients (22.7 %, p < 0.001). Interestingly, catheter-directed embolectomy was most frequent in obese patients (5.7 %), followed by non-obese (4.7 %), and least common in severely obese patients (3.7 %, p < 0.001).
Table 2.
In-hospital management and clinical outcomes of patients with High-Risk Pulmonary Embolism Stratified by Body Mass Index (BMI) Category.
| Non-obese | Obese | Severely Obese | P-value | |
|---|---|---|---|---|
| NIS discharge weight | 22,825 | 2,615 | 4,170 | <0.001 |
| Management, % | ||||
| Systemic thrombolysis | 22.7 | 26.4 | 25.7 | <0.001 |
| Catheter-directed thrombolysis | 8.2 | 12 | 13.8 | <0.001 |
| Ultrasound-facilitated catheter-directed thrombolysis | 1.8 | 2.3 | 3.1 | <0.001 |
| Catheter-directed embolectomy | 4.7 | 5.7 | 3.7 | <0.001 |
| Surgical embolectomy / thrombectomy | 2.6 | 4 | 4 | <0.001 |
| IVC filter | 15.2 | 14.7 | 15.2 | 0.780 |
| Circulatory and Ventilatory support | ||||
| Use of vasopressors | 15.4 | 14.9 | 15.6 | 0.736 |
| Mechanical Ventilation | 67 | 60 | 64.7 | <0.001 |
| ECMO | 3.3 | 3.3 | 4.8 | <0.001 |
| Clinical outcomes, % | ||||
| All-cause mortality | 43.1 | 27.2 | 32.4 | <0.001 |
| MACCE | 46.3 | 30 | 36.6 | <0.001 |
| Major bleeding | 12.3 | 10.5 | 9.6 | <0.001 |
| ICH | 3.1 | 1.5 | 1.7 | <0.001 |
| Non-ICH | ||||
| Retroperitoneal | 1.7 | 2.1 | 2 | 0.190 |
| Gastrointestinal | 7.2 | 6.1 | 5.6 | <0.001 |
| Procedure related | 1 | 1 | 1 | 0.940 |
| Length of Stay, days, mean | 8.3 | 8.8 | 10.4 | <0.001 |
| Total charge, $, mean | 152,967.94 | 164,298.94 | 184,746.64 | <0.001 |
There are differences in mechanical ventilation use, with severely obese and obese patients having a lower rate compared to non-obese patients (64.7 % and 60 % vs. 67.0 %, respectively, p < 0.001). Conversely, severely obese patients are more likely to receive ECMO (4.8 % vs. 3.3 % in non-obese patients, p < 0.001).
For clinical outcomes, all-cause mortality was higher in non-obese (43.1 %) than in severely obese (32.4 %) and obese (27.2 %, p < 0.001) patients. Similarly, MACCE rates were also highest in non-obese (46.3 %), versus severely obese (36,6%) and obese patients (30 %), p < 0.001. In addition, major bleeding complications were also more common in non-obese patients (12.3 %) compared to obese (10.5 %) and severely obese patients (9.6 %, p < 0.001).
Severely obese patients also exhibited a longer hospital stay (mean 10.4 days vs. 8.3 days in non-obese patients, p < 0.001) and incurred higher total charges ($184,746.64 vs. $152,967.94 in non-obese patients, p < 0.001). A complete list of in-hospital procedures and outcomes is provided in Table 2.
Fig. 2 illustrates the disposition of patients with high-risk PE, stratified by Body Mass Index (BMI) Category. Obese and severely obese patients were more likely to have been discharged home (23.7 % and 21.6 % vs. 17.0 %, p < 0.001), to a short-term facility (7.5 % and 6.2 % vs. 5.1 %, p < 0.001), to an intermediate care facility (29.3 % and 26.7 % vs. 24.4 %, p < 0.001), and to have received home health services (11.9 % and 12.8 % vs. 9.8 %, p < 0.001) compared to non-obese patients.
Fig. 2.
Disposition of patients with high-risk pulmonary embolism stratified by body mass index (BMI) category. Legend:  Non-Obese.
Non-Obese.  Obese.
Obese.  Severely obese.
Severely obese.
3.1.2. Adjusted analysis
The multivariate analysis showed several significant findings (Table 3). Patients who were obese had higher odds of undergoing systemic thrombolysis (aOR 1.26, p < 0.001), catheter-directed thrombolysis (aOR 1.54, p < 0.001), catheter-directed embolectomy (aOR 1.31, p = 0.004), and surgical embolectomy/thrombectomy (aOR 1.76, p < 0.001). Similar trends were observed in severely obese patients with higher odds for catheter directed thrombolysis (aOR 1.80, p < 0.001), ultrasound-facilitated catheter directed thrombolysis (aOR 1.52, p < 0.001), and IVC filter placement (aOR 1.17, p = 0.002). Interestingly, severely obese patients had lower odds of catheter-directed embolectomy (aOR 0.77, p = 0.005) compared to non-obese patients.
Table 3.
Crude and adjusted odds ratios for in-hospital procedures and complications of patients with High-Risk Pulmonary Embolism stratified by Body Mass Index (BMI) category.
| Obese | Severely Obese | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome |
Crude OR |
P value | aOR (95 % CI) | P value |
Crude OR |
P value | aOR (95 % CI) | P value |
| In-Hospital Procedures | ||||||||
| Systemic thrombolysis | 1.49 (1.43––1.55) | <0.001 | 1.26 (1.15–––1.39) | <0.001 | 1.62 (1.57–1.68) | <0.001 | 1.09 (1.01––1.19) | 0.030 |
| Catheter-directed thrombolysis | 1.94 (1.87–2.01) | <0.001 | 1.54 (1.35–––1.75) | <0.001 | 2.14 (2.08–2.21) | <0.001 | 1.80 (1.62––2.01) | <0.001 |
| Ultrasound-facilitated catheter-directed thrombolysis | 1.92 (1.79 – 2.06) | <0.001 | 1.21 (0.92–––1.59) | 0.181 | 2.20 (2.07–2.33) | <0.001 | 1.52 (1.23––1.88) | <0.001 |
| Catheter-directed embolectomy | 1.68 (1.56–1.80) | <0.001 | 1.31 (1.09–––1.56) | 0.004 | 1.45 (1.35–1.55) | <0.001 | 0.77(0.65––0.92) | 0.005 |
| Surgical embolectomy/thrombectomy | 1.40 (1.19–1.63) | <0.001 | 1.76 (1.40–––2.20) | <0.001 | 1.48 (1.29–1.69) | <0.001 | 1.47 (1.22–––1.78) | <0.001 |
| IVC filter | 0.98 (0.95–1.02) | 0.302 | 1.02 (0.91–––1.15) | 0.686 | 0.94 (0.91–0.97) | <0.001 | 1.17 (1.06–1.29) | 0.002 |
| In-Hospital Complications | ||||||||
| MACCE | 0.56 (0.53–0.59) | <0.001 | 0.50 (0.46–––0.55) | <0.001 | 0.59 (0.57–0.62) | <0.001 | 0.72 (0.67––0.77) | <0.001 |
| Mortality | 0.46 (0.43–0.48) | <0.001 | 0.50 (0.45–––0.54) | <0.001 | 0.59 (0.56–0.62) | <0.001 | 0.69 (0.64–––0.74) | <0.001 |
| Major Bleeding | 0.81 (0.76–0.85) | <0.001 | 0.87 (0.76–––1.0) | 0.057 | 0.72 (0.68–0.75) | <0.001 | 0.77 (0.69–––0.87) | <0.001 |
| ICH | 0.75 (0.66–0.85) | <0.001 | 0.47 (0.34–––0.66) | <0.001 | 0.47 (0.41–0.54) | <0.001 | 0.47 (0.37–––0.61) | <0.001 |
Reference: non-obese; adjusted for age, gender, weekend admission, hospital bed size, region and location/teaching status, VF, VT, AF, HF, hypertension, valvular heart disease, dyslipidemia, smoking status, chronic liver disease, chronic lung disease, chronic kidney disease, anemia, thrombocytopenia, coagulopathies, diabetes mellitus, systemic thrombolysis, catheter directed thrombolysis, ultrasound facilitated catheter directed thrombolysis, catheter directed embolectomy, surgical embolectomy/thrombectomy and inferior vena cava (IVC) filter placement.
In terms of in-hospital complications, both obese and severely obese patients demonstrated significantly lower odds of MACCE (Obese: aOR 0.50, p < 0.001; severely obese: aOR 0.72, p < 0.001) and mortality (obese: aOR 0.50, p < 0.001; severely obese: aOR 0.69, p < 0.001) compared to non-obese patients. Finally, severely obese patients had lower odds of major bleeding (aOR 0.77, p < 0.001), while there was no statistical difference in major bleeding among obese patients (aOR 0.87, p = 0.057).
3.1.3. Sensitivity analysis
Table S2 presents a detailed analysis of in-hospital clinical outcomes among patients with high-risk PE, stratified by both age groups and BMI categories. All-cause mortality was highest in non-obese patients across all ages (40.8 % under 50, 45.0 % aged 50–75, 50.7 % over 75; all p < 0.001), while obese cohorts had lower rates (24.3 %, 29.0 %, 37.4 %, respectively), with severely obese (BMI ≥ 40) patients showing intermediate mortality in younger groups (38.1 % under 50) but the lowest mortality in older adults (30.2 % over 75). MACCE followed similar trends, with non-obese patients having the highest rates (37.2 % under 50, 41.6 % aged 50–75, 48.3 % over 75; all p < 0.001), while obese and severely obese groups demonstrated progressively lower rates. Major bleeding complications were significantly lower in obese patients under 50 (4.9 % vs. 12.3 % non-obese, p < 0.001), though differences diminished in older cohorts (50–75: 12.8 % obese vs. 12.4 % non-obese, p = 0.003; over 75: 9.8 % obese vs. 12.2 % non-obese, p = 0.081). Intracranial hemorrhage rates were consistently lower in obese and severely obese patients across ages (under 50: 1.0–1.5 % vs. 3.9 % non-obese; 50–75: 1.7–1.8 % vs. 3.1 %; both p < 0.001), though not significant in those over 75 (1.6 % vs. 2.6 %, p = 0.190).
Our sensitivity analysis excluding cardiac arrest cases revealed persistent associations between obesity and outcomes in high-risk PE patients (Table S3). After adjustment, obese patients showed significantly higher odds of receiving interventional therapies including systemic thrombolysis (aOR 1.37, p < 0.001), catheter-directed thrombolysis (aOR 1.47, p < 0.001), and surgical embolectomy/thrombectomy (aOR 1.85, p < 0.001) compared to non-obese patients. Similarly, severely obese patients had higher odds of receiving catheter-directed thrombolysis (aOR 1.84, p < 0.001) and ultrasound-facilitated catheter-directed thrombolysis (aOR 1.44, p = 0.002). In terms of in-hospital complications, both obese and severely obese patients demonstrating significantly lower odds of MACCE (obese: aOR 0.50, p < 0.001; severely obese: aOR 0.68, p < 0.001) and mortality (obese: aOR 0.49, p < 0.001; severely obese: aOR 0.62, p < 0.001).
4. Discussion
The study evaluated the impact of obesity on management and outcomes in high-risk PE patients, using data from 29,610 (4.1 %) patients categorized as having high-risk PE without known malignancy. The analysis revealed several key insights: First, severely obese patients were younger and included a higher proportion of females compared to non-obese and obese groups. Second, obese and severely obese patients had a higher prevalence of comorbidities including heart failure, hypertension and diabetes mellitus. Third, severely obese patients had longer length of stay and higher total charges. Fourth, after adjustment, obese and severely obese patients were more likely to receive systemic thrombolysis, catheter-directed thrombolysis, ultrasound-facilitated catheter-directed thrombolysis, and surgical embolectomy/thrombectomy. Finally, we observed an 'obesity paradox' in clinical outcomes, with obese and severely obese patients having significantly lower mortality, MACCE, and major bleeding complications than non-obese patients.
Our findings of younger age and a higher proportion of females among severely obese patients with PE are consistent with previous publications [11,16,17]. A study by Keller et al. reported that obese PE patients were younger (67.0 vs 73.0 years) and more frequently female (60.2 % vs 52.7 %) compared to non-obese patients [11]. Tamimi et al. [16] also noted that severely obese PE patients were more often female (60 % vs. 48 %) and younger (57 vs. 66 years) compared with their non-obese counterparts. These observations may be explained by the interplay of hormonal factors, especially in younger women, including use of oral contraceptives or hormone replacement therapy, which can enhance PE risk in conjunction with obesity [17,18]. Moreover, this increased risk in younger, female obese patients may be explained by the higher percentage of body fat at the same BMI in women compared to men and by the chronic inflammatory state induced by obesity, which may contribute to hypercoagulability [10,17].
Our study showed that obese and severely obese patients with high-risk PE had a higher prevalence of certain comorbidities like heart failure, hypertension and diabetes mellitus as seen in existing literature. For instance, in a study conducted by Keller et al. found that obese patients with PE were more likely than non-obese patients to have hypertension and diabetes [11]. The relationship of obesity with comorbidities in PE patients indicates the highly intricate relationship between obesity and cardiovascular health. Obesity induces a chronic low grade inflammatory state that may increase the inflammatory response in PE and lead to comorbidities [10]. Understanding these relationships is crucial for developing targeted interventions and improving outcomes in obese patients with high-risk PE.
While our findings align with previous studies on PE [11,16,19] regarding hospital stay and costs, our specific focus on high-risk PE provides novel insights. In a study by Samaranayake et al. of patients with massive PE receiving thrombolysis, the length of hospital stay was higher in the obese group compared to the non-obese group (median 11 versus 7 days respectively, p = 0.04) [19]. Tamimi et al. reported that severely obese patients with PE incurred higher total charges ($64,688 vs $55,993, adjusted difference 10,492, [95 % CI 8,019–12,964], p < 0.01) and have longer hospital stays (4.7 days vs 4.2 days, adjusted difference 0.67 [95 % CI 0.5–0.8], p < 0.01) compared to non-severely obese patients. These results indicate that obesity is associated with greater resource utilization in patients with PE.
Our study confirms and expands upon a previous study [11] regarding the higher likelihood of aggressive interventions in obese and severely obese patients with PE. While obese patients received more aggressive therapies, multivariable models adjusted for PE severity markers confirmed the persistence of the obesity paradox. This suggests clinicians may escalate care preemptively in obese patients due to perceived vulnerability, whereas the mortality benefit likely reflects obesity-associated physiological adaptations rather than differential PE severity at presentation. Importantly, we extend this understanding to the specific context of high-risk PE, a critical subset that has received limited attention in prior research. Keller et al [11] demonstrated that obese patients received systemic thrombolysis (6.4 % vs 4.3 %, p < 0.001) and surgical embolectomy (0.3 % vs. 0.1 %, p < 0.001) more often than the reference group (non-obesity and non-underweight). Several factors may contribute to this phenomenon: obese patients tend to be more closely monitored, and therefore deterioration is detected earlier and intervention is more prompt; and obese patients are often considered to be higher risk and are more often referred to specialists and more aggressive management strategies are used. may be more often referred to specialists, leading to more aggressive management strategies. This trend of more aggressive treatment in obese patients with PE is similar to other cardiovascular diseases. Oreopoulos et al. showed that overweight and mildly to moderately obese patients were more likely to undergo revascularization procedures than subjects with normal BMI despite lower risk coronary anatomy [20]. This persisted with different treatment strategies, including medical management, coronary artery bypass graft or percutaneous coronary intervention [20].
Our findings of an 'obesity paradox', where obese and severely obese patients demonstrate lower mortality compared to non-obese patients, has been observed in various cardiovascular conditions [11,17,21,22]. Keller et al. reported decreased all-cause in-hospital mortality rates in obese patients with PE, regardless of age, sex, comorbidities, or reperfusion treatment [11]. Similarly, Stein et al reported that among stable patients not treated with thrombolytic therapy, mortality was 3.8 % for obese patients and 8.4 % for non-obese patients (RR 0.45) [17]. Further supporting this trend, Alkhalfan et al. showed that obesity was associated with a significant lower risk of 30 day PE related mortality (adjusted HR 0.29, p = 0.036; 95 % CI 0.09–0.92) and a higher BMI was paradoxically associated with a significantly lower risk of PE related mortality (HR = 0.91per1kg/m2 increase, p = 0.049; 95 % CI 0.83– 0.999) [21]. Barba et al. also reported that obese patients with acute venous thromboembolism have less than half the mortality rate when compared with normal BMI patients [22]. However, the interpretation of this phenomenon requires careful consideration, especially in the context of high-risk PE. While previous studies have attributed this paradox to factors such as younger age at diagnosis and earlier presentation [11,[23], [24], [25], [26], [27]], our analysis controlled for age, suggesting that other mechanisms may be at play. Interestingly, despite having a higher prevalence of saddle embolism and cor pulmonale, obese and severely obese patients in our study still demonstrated better outcomes. This counterintuitive phenomenon may be explained by several factors. Hainer et al. found that the obesity paradox is influenced by body composition, with BMI potentially being a better indicator of lean body mass than adiposity in some patient populations, especially among the elderly [28]. Obese patients have greater right ventricular mass and volume, which may confer an advantage in coping with acute increases in right ventricular overload in PE [29]. Furthermore, patients with obesity have a greater left ventricular mass and thicker interventricular septum, which may make them more resistant to septal bowing and thus decrease the risk for the development of obstructive shock [30]. The persistent mortality benefit despite higher comorbidity burdens underscores the role of obesity-associated cardiopulmonary reserve. Greater right ventricular mass and septal thickness in obese patients may mitigate acute cor pulmonale, allowing better tolerance of hemodynamic stress during PE.
An observed “obesity paradox” implies that BMI should be taken into account when assessing risk and deciding on treatment approaches. But clinicians should be careful not to underestimate the severity of PE in obese patients, whose outcomes may be better. Furthermore, limitations should be acknowledged. Limitations include: (1) Retrospective design with potential administrative data inaccuracies; (2) Lack of long-term follow-up and absence of chronic treatment/medication data; (3) Inability to differentiate body composition profiles − BMI cannot distinguish between protective adiposity with preserved muscle mass and sarcopenic obesity, where low muscle mass may mediate poorer outcomes through mechanisms like systemic inflammation; and (4) possible selection bias toward less severe PE phenotypes in obese patients. The absence of CT-derived muscle mass measurements prevents assessment of whether preserved lean mass in obese individuals explains part of the observed paradox through metabolic advantages or greater cardiopulmonary reserve. Despite these limitations, the strengths of the research include a thorough analysis of a nationally representative sample of size that increases generalizability of the findings. By utilizing the NIS database, we were able to examine a broad patient population across different healthcare settings in real world clinical practices and outcomes.
5. Conclusion
In conclusion, our study showed that obesity and high-risk pulmonary embolism presents an intriguing “obesity paradox.” Obese and severely obese patients demonstrated paradoxically lower mortality and fewer complications despite higher rates of comorbidities and more aggressive interventions. This finding underscores the need for a tailored approach to risk assessment and treatment strategies in this patient population. Specifically, clinicians should consider: (1) modifying existing prognostic scores to account for this paradox, as standard calculators may overestimate mortality risk in obese patients; (2) establishing BMI-specific thresholds for biomarkers like NT-proBNP, which may present differently despite significant right ventricular dysfunction; (3) implementing obesity-adjusted comorbidity assessment, recognizing that traditional risk factors carry different weights in this population; and (4) developing individualized treatment protocols with careful consideration for earlier intervention in apparently stable obese patients, whose physiological reserve may mask clinical deterioration.
Grant Support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Ziv Shachar: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Investigation, Formal analysis, Data curation. Marlon V. Gatuz: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Investigation, Formal analysis, Data curation. Adam Folman: Writing – review & editing, Supervision. Maguli S. Barel: Writing – review & editing, Supervision. Rami Abu-Fanne: Writing – review & editing, Supervision. Dmitry Abramov: Writing – review & editing, Validation, Resources, Conceptualization. Mamas A. Mamas: Writing – review & editing, Validation, Resources, Conceptualization. Ariel Roguin: Writing – review & editing, Validation, Supervision, Conceptualization. Ofer Kobo: Writing – review & editing, Visualization, Validation, Supervision, Software, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2025.101682.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Jareño Esteban J.J., de Miguel Díez J., Fernández Bermejo L.A. Pulmonary embolism and comorbidity. Open Respir. Arch. 2022 Jun 8;4(3) doi: 10.1016/j.opresp.2022.100188. PMID: 37496583; PMCID: PMC10369663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barco S., Valerio L., Gallo A., Turatti G., Mahmoudpour S.H., Ageno W., Castellucci L.A., Cesarman-Maus G., Ddungu H., De Paula E.V., Dumantepe M., Goldhaber S.Z., Guillermo Esposito M.C., Klok F.A., Kucher N., McLintock C., Ní Áinle F, Simioni P., Spirk D., Spyropoulos A.C., Urano T., Zhai Z.G., Hunt B.J., Konstantinides S.V. Global reporting of pulmonary embolism-related deaths in the World Health Organization mortality database: Vital registration data from 123 countries. Res. Pract. Thromb. Haemost. 2021;5(5) doi: 10.1002/rth2.12520. PMID: 34263098; PMCID: PMC8268665, Jun 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization, Obesity and overweight [Internet], Geneva: World Health Organization; 2024 Mar 1 [cited 2024 Nov 22], Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 4.Koskinas K.C., Van Craenenbroeck E.M., Antoniades C., et al. Obesity and cardiovascular disease: an ESC clinical consensus statement. Eur Heart J. 2024;45(38):4063–4098. doi: 10.1093/eurheartj/ehae508. [DOI] [PubMed] [Google Scholar]
- 5.Csige I., Ujvárosy D., Szabó Z., et al. The impact of obesity on the cardiovascular system. J. Diabetes Res. 2018;2018 doi: 10.1155/2018/3407306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang G., De Staercke C., Hooper W.C. The effects of obesity on venous thromboembolism: A review. Open J. Prev. Med. 2012;2:499–509. doi: 10.4236/ojpm.2012.24069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edeer A.D., Comez S., Damar H.T., Savci A. Prevalence and risk factors of venous thromboembolism in postoperative patients: A retrospective study. Pak. J. Med. Sci. 2018;34:1539–1544. doi: 10.12669/pjms.346.16021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lentz S.R. Thrombosis in the setting of obesity or inflammatory bowel disease. Blood. 2016;128:2388–2394. doi: 10.1182/blood-2016-05-716720. [DOI] [PubMed] [Google Scholar]
- 9.El-Menyar A., Asim M., Al-Thani H. Obesity paradox in patients with deep venous thrombosis. Clin. Appl. Thromb. Hemost. 2018;24:986–992. doi: 10.1177/1076029617727858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hotoleanu C. Association between obesity and venous thromboembolism. Med. Pharm. Rep. 2020;93(2):162. doi: 10.15386/mpr-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keller K., Hobohm L., Münzel T., et al. Survival benefit of obese patients with pulmonary embolism. Mayo Clin. Proc. 2019;94(10):1960–1973. doi: 10.1016/j.mayocp.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 12.HCUP-US NIS Overview, Accessed November 1, 2024, https://hcup-us.ahrq.gov/nisoverview.jsp.
- 13.Centers for Disease Control and Prevention (CDC), BMI categories for adults, CDC [Internet], [cited 2025 Mar 8]. Available from: https://www.cdc.gov/bmi/adult-calculator/bmi-categories.html.
- 14.Sedhom R., Megaly M., Elbadawi A., et al. Sex differences in management and outcomes among patients with high-risk pulmonary embolism: a nationwide analysis. Mayo Clin. Proc. 2022;97(10):1872–1882. doi: 10.1016/j.mayocp.2022.03.022. [DOI] [PubMed] [Google Scholar]
- 15.Sedhom R., Beshai R., Moussa P., Megaly M., Mohsen A., Abramov D., Stoletniy L., Elgendy I.Y. Outcomes with malignancy-associated high-risk pulmonary embolism: a nationwide analysis. Mayo Clin. Proc. 2024 Jan;99(1):81–89. doi: 10.1016/j.mayocp.2023.03.019. Epub 2023 Aug 26 PMID: 37632484. [DOI] [PubMed] [Google Scholar]
- 16.Tamimi O., Tamimi F., Gotur D.B. Clinical impact of morbid obesity on pulmonary embolism hospitalizations. Chest. 2023;164(4):A5949. doi: 10.1016/j.chest.2023.07.3834. [DOI] [Google Scholar]
- 17.Stein P.D., Matta F., Goldman J. Obesity and pulmonary embolism: the mounting evidence of risk and the mortality paradox. Thromb. Res. 2011 Dec;128(6):518–523. doi: 10.1016/j.thromres.2011.10.019. Epub 2011 Nov 10 PMID: 22078437. [DOI] [PubMed] [Google Scholar]
- 18.Kabrhel C., Varraso R., Goldhaber S.Z., Rimm E.B., Camargo C.A. Prospective study of BMI and the risk of pulmonary embolism in women. Obesity. 2009;17:2040–2046. doi: 10.1038/oby.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samaranayake C.B., Keir G., McCabe C., et al. Thrombolysis for massive pulmonary embolisms in morbid obesity: a multisite case–control study. ERJ Open Res. 2021;7(1):00762–02020. doi: 10.1183/23120541.00762-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oreopoulos Antigone, McAlister Finlay A., Kalantar-Zadeh Kamyar, Padwal Raj, Ezekowitz Justin A., Sharma Arya M., Kovesdy Csaba P., Fonarow Gregg C., Norris Colleen M. The relationship between body mass index, treatment, and mortality in patients with established coronary artery disease: a report from APPROACH. Eur. Heart J. 2009;30(21):2584–2592. doi: 10.1093/eurheartj/ehp288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alkhalfan F., Bukhari S., Rosenzveig A., Moudgal R., Khan S.Z., Ghoweba M., Chaudhury P., Cameron S.J., Tefera L. The obesity mortality paradox in patients with pulmonary embolism: insights from a tertiary care center. J. Clin. Med. 2024 Apr 19;13(8):2375. doi: 10.3390/jcm13082375. PMID: 38673648; PMCID: PMC11051153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barba R., Zapatero A., Losa J.E., Valdés V., Todolí J.A., Di Micco P., Monreal M., Investigators Riete. Body mass index and mortality in patients with acute venous thromboembolism: findings from the RIETE registry. J. Thromb. Haemost. 2008 Apr;6(4):595–600. doi: 10.1111/j.1538-7836.2008.02907.x. Epub 2008 Jan 17. PMID: 18208535. [DOI] [PubMed] [Google Scholar]
- 23.Keller K., Munzel T., Ostad M.A. Sex-specific differences in mortality and obesity paradox of patients with myocardial infarction ages >70 y. Nutrition. 2018;46:124–130. doi: 10.1016/j.nut.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Lavie C.J., De Schutter A., Parto P., et al. Obesity and prevalence of cardiovascular diseases and prognosis: the obesity paradox updated. Prog. Cardiovasc. Dis. 2016;58(5):537–547. doi: 10.1016/j.pcad.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Angeras O., Albertsson P., Karason K., et al. Evidence for obesity paradox in patients with acute coronary syndromes: a report from the Swedish coronary angiography and angioplasty registry. Eur. Heart J. 2013;34(5):345–353. doi: 10.1093/eurheartj/ehs217. [DOI] [PubMed] [Google Scholar]
- 26.Bucholz E.M., Beckman A.L., Krumholz H.A., Krumholz H.M. Excess weight and life expectancy after acute myocardial infarction: the obesity paradox reexamined. Am. Heart J. 2016;172:173–181. doi: 10.1016/j.ahj.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z.J., Zhou Y.J., Galper B.Z., Gao F., Yeh R.W., Mauri L. Association of body mass index with mortality and cardiovascular events for patients with coronary artery disease: a systematic review and meta-analysis. Heart. 2015;101(20):1631–1638. doi: 10.1136/heartjnl-2014-307119. [DOI] [PubMed] [Google Scholar]
- 28.V. Hainer, I. Aldhoon-Hainerová, Obesity paradox does exist, Diabetes Care. 2013 Jul;36(Supplement_2):S276-S281. [DOI] [PMC free article] [PubMed]
- 29.Chahal H., McClelland R.L., Tandri H., Jain A., Turkbey E.B., Hundley W.G., Barr R.G., Kizer J., Lima J.A.C., Bluemke D.A., et al. Obesity and right ventricular structure and function: The MESA-right ventricle study. Chest. 2012;141:388–395. doi: 10.1378/chest.11-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chowdhury J.M., Zhao H., Moores L.K., Rali P. Obesity paradox in VTE outcomes: an evolving concept. Chest. 2020;158:1290–1291. doi: 10.1016/j.chest.2020.02.081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.