Abstract
Metastatic neuroblastoma is a highly aggressive pediatric malignancy with poor prognosis. Radiotherapy is commonly used as part of multimodal treatment, but its impact on survival outcomes remains controversial. This study investigates the association between radiotherapy and survival in patients with metastatic neuroblastoma using data from the Surveillance, Epidemiology, and End Results (SEER) database. A retrospective analysis was conducted on 4,850 patients diagnosed with metastatic neuroblastoma from the SEER database (2004–2015). After applying exclusion criteria, 981 patients were included, with 368 receiving radiotherapy. Propensity score matching (PSM) was used to balance baseline characteristics between the radiotherapy and non-radiotherapy groups, resulting in 234 patients in each group. Survival outcomes were analyzed using Kaplan–Meier curves and Cox proportional hazards models. Before PSM, no significant difference in overall survival (OS) and cancer-specific survival (CSS) was observed between patients who received radiotherapy and those who did not. After PSM, there was a trend toward improved OS and CSS in the radiotherapy group, though statistical significance was not reached. Cox regression analysis identified age ≥ 1 year and non-adrenal primary tumor site as significant independent predictors of poorer OS and CSS. Radiotherapy was not an independent predictor of survival in the multivariate analysis, but a trend toward a survival benefit was noted, particularly in patients with larger tumors and those who underwent surgery. Radiotherapy may improve survival in metastatic neuroblastoma patients with large tumors or surgical resection, though its independent benefit remains uncertain. Personalized strategies require integrating updated COG risk stratification with biomarkers and prospective trials assessing long-term outcomes to refine treatment approaches.
Keywords: Metastatic neuroblastoma, Radiotherapy, Survival analysis, SEER database, Propensity score matching, Cox regression analysis
Subject terms: Paediatric cancer, Cancer
Introduction
Neuroblastoma, an embryonal tumor originating primarily from the autonomic nervous system, includes neuroblastoma, ganglioneuroblastoma, and ganglioneuroma. Notably, neuroblastoma comprises 97% of these tumors, characterized by heterogeneity in site of occurrence, histopathological features, and biological properties1. It ranks among the most common extracranial solid tumors in children and is the most prevalent cancer type in infants under 12 months2,3. Predominantly affecting children, neuroblastoma accounts for approximately 15% of all childhood cancer deaths, with an incidence rate of 107 cases per million4. The onset is closely associated with age, with a median diagnosis age of 17.3 months, and 40% of patients are diagnosed before the age of one. The 5-year survival rate is estimated to range from 75 to 98% for children with low- and intermediate-risk neuroblastoma, while it falls below 50% for those with high-risk neuroblastoma5. Recurrence or progression at metastatic sites remains the leading cause of neuroblastoma-related mortality6.
Radiation therapy is a standard treatment for high-risk neuroblastoma, typically administered following consolidation therapy to prevent local tumor recurrence7,8. However, debate persists regarding whether the radiation field should encompass lymph nodes adjacent to the primary tumor9,10. Similarly, there is uncertainty about which metastatic lesions should be irradiated and how this affects the risk of local and overall recurrence11,12. Conflicting data exist on the efficacy of radiation therapy for patients with residual tumors at the primary site post-consolidation therapy. Although radiation therapy has demonstrated some efficacy in treating high-risk neuroblastoma, there is no level one evidence supporting its optimal use13,14. Additionally, there is a scarcity of clinical studies on metastatic neuroblastoma in children, particularly large-scale studies focused on survival prognosis. This gap in research has impeded the development of mature treatment strategies for metastatic neuroblastoma, highlighting the critical need for more foundational and clinical studies to provide patients with more precise and personalized treatment options.
This study aims to leverage the Surveillance, Epidemiology, and End Results (SEER) database and apply propensity score matching (PSM) analysis to evaluate the overall and specific effects of radiation therapy on the prognosis of metastatic neuroblastoma. The objective is to generate evidence that will inform clinical decision-making and provide valuable reference data for the design of future clinical trials.
Materials and methods
Patients and variables
Specific clinicopathological data and prognostic outcomes of neuroblastoma patients from 2004 to 2015 were retrieved from the SEER database. Ethical approval or local statements were not required for this study, as all data utilized were obtained through publicly accessible methods from the SEER database.
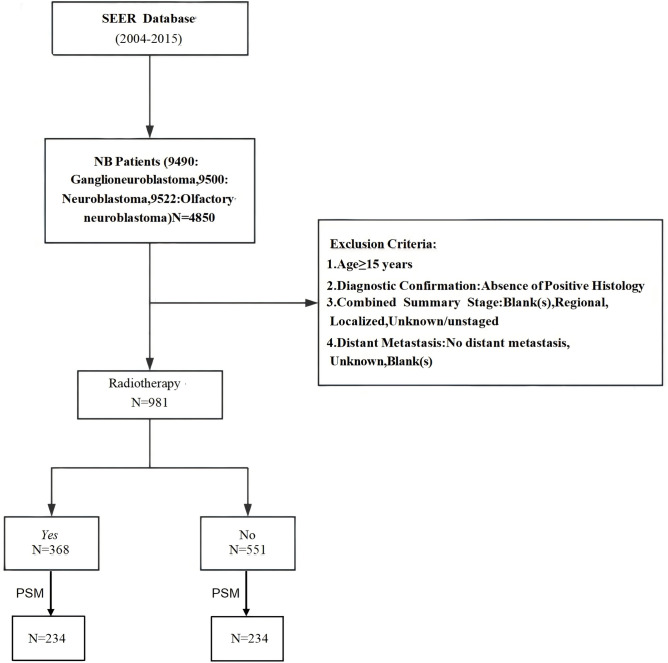
Data were obtained for patients diagnosed with neuroblastoma according to the International Classification of Diseases for Oncology (ICD-O-3) histology codes: 9490 (ganglioneuroblastoma), 9500 (neuroblastoma, not otherwise specified), 9504 (spongioneuroblastoma), and 9522 (olfactory neuroblastoma). The following variables were extracted: age at diagnosis, gender, race, year of diagnosis, primary site, tumor grade (defined as well-differentiated I–II, poorly differentiated III, and undifferentiated IV), tumor size, histology, chemotherapy, surgery, radiation therapy, metastasis, survival time, and cause of death. Exclusion criteria included: (1) age ≥ 15 years; (2) diagnosis not confirmed by positive histology; (3) summary stage categorized as blank, regional, localized, unknown, or unstaged; (4) absence of distant metastasis, or unknown/blank metastasis status. The study flowchart is shown in Fig. 1, and all included patients (n = 919) were divided into two groups based on whether they received radiation therapy.
Fig. 1.
Flow chart for screening patients.
Statistical analysis
Baseline characteristics of patients and treatments were summarized using descriptive statistics. Differences in clinical characteristics between the radiotherapy and non-radiotherapy groups were analyzed with chi-square and Fisher’s exact tests, as appropriate. To minimize selection bias and ensure comparability between the radiation therapy group and the non-radiation therapy group, PSM was applied. The matching variables included age, gender, race, primary tumor site, tumor grade, tumor size, histological type, chemotherapy status, and surgical intervention. A 1:1 nearest neighbor algorithm without replacement was used, resulting in 234 matched pairs of patients.
Kaplan–Meier survival curves were utilized to analyze survival outcomes, including overall survival (OS) and cancer-specific survival (CSS), with differences between groups assessed via the log-rank test. Cox proportional hazards models were applied to identify independent prognostic factors for survival, with results expressed as hazard ratios (HR) and 95% confidence intervals (CI). Both univariate and multivariate analyses were conducted, with variables showing significance (P < 0.05) in univariate analysis included in the multivariate model. Statistical analyses were performed using R software (version 4.3.0), with a P-value of less than 0.05 considered statistically significant.
Results
Baseline patient characteristics
Among the 919 patients diagnosed with metastatic neuroblastoma in the SEER database from 2004 to 2015, 368 received radiation therapy, while 551 did not. The demographic characteristics of patients with or without radiation therapy are summarized in Table 1. Overall, no significant associations were observed with gender, tumor grade, or histological type. However, statistically significant differences were noted for age (P < 0.001), race (P = 0.013), primary site (P = 0.049), tumor size (P < 0.001), chemotherapy (P < 0.001), and surgery (P < 0.001). After PSM, the radiation therapy group and the non-radiation therapy group each comprised 234 matched patients. No significant associations were observed between variables in the two groups after PSM (Table 1).
Table 1.
Characteristics of radiation therapy in patients afflicted with metastatic neuroblastoma.
| Variable | Group | Before PSM | P_value | After PSM | P_value | ||
|---|---|---|---|---|---|---|---|
| Radiotherapy N (%) | Non-radiotherapy N (%) | Radiotherapy N (%) | Non-radiotherapy N (%) | ||||
| Age (years) | < 1 | 37 (10.1) | 225 (40.8) | < 0.001 | 35 (15) | 32 (13.7) | 0.792 |
| ≥ 1 | 331 (89.9) | 326 (59.2) | 199 (85) | 202 (86.3) | |||
| Sex | Male | 212 (57.6) | 305 (55.4) | 0.544 | 129 (55.1) | 144 (61.5) | 0.189 |
| Female | 156 (42.4) | 246 (44.6) | 105 (44.9) | 90 (38.5) | |||
| Race | White | 258 (70.1) | 417 (75.7) | 0.013 | 171 (73.1) | 174 (74.4) | 0.601 |
| Black | 73 (19.8) | 70 (12.7) | 40 (17.1) | 33 (14.1) | |||
| Others | 37 (10.1) | 64 (11.6) | 23 (9.8) | 27 (11.5) | |||
| Primary site | Adrenal | 250 (67.9) | 338 (61.3) | 0.049 | 159 (67.9) | 167 (71.4) | 0.482 |
| Others | 118 (32.1) | 213 (38.7) | 75 (32.1) | 67 (28.6) | |||
| Grade | Grades I–II | 4 (1.1) | 6 (1.1) | 0.907 | 4 (1.7) | 1 (0.4) | 0.298 |
| Grades III–IV | 215 (58.4) | 312 (56.6) | 135 (57.7) | 145 (62) | |||
| Unknown | 149 (40.5) | 233 (42.3) | 95 (40.6) | 88 (37.6) | |||
| Tumor Size | < 5 cm | 61 (16.6) | 119 (21.6) | < 0.001 | 40 (17.1) | 39 (16.7) | 0.906 |
| ≥ 5 cm | 237 (64.4) | 273 (49.5) | 144 (61.5) | 141 (60.3) | |||
| Unknown | 70 (19.0) | 159 (28.9) | 50 (21.4) | 54 (23.1) | |||
| Histologic type | Ganglioneuroblastoma | 26 (7.1) | 36 (6.5) | 0.899 | 18 (7.7) | 18 (7.7) | 1.000 |
| Neuroblastoma | 341 (92.7) | 514 (93.3) | 216 (92.3) | 216 (92.3) | |||
| Olfactory neuroblastoma | 1 (0.3) | 1 (0.2) | 0 (0.0) | 0 (0.0) | |||
| Chemotherapy | Yes | 366 (99.5) | 501 (90.9) | < 0.001 | 232 (99.1) | 232 (99.1) | 1.000 |
| No | 2 (0.5) | 50 (9.1) | 2 (0.9) | 2 (0.9) | |||
| Surgery | Yes | 322 (87.5) | 327 (59.3) | < 0.001 | 200 (85.5) | 185 (79.1) | 0.090 |
| No | 46 (12.5) | 224 (40.7) | 34 (14.5) | 49 (20.9) | |||
Survival analysis for OS and CSS
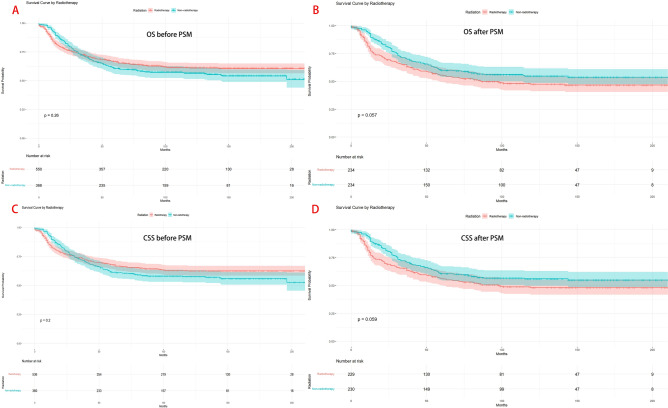
Before PSM, there were no significant differences in OS and CSS between patients who received radiation therapy and those who did not (P > 0.05), as shown in Fig. 2A and C.
Fig. 2.
Comparison of OS and CSS between the radiation therapy and the non-radiation therapy groups. (A) OS before PSM; (B) OS after PSM. (C) CSS before PSM; (D) CSS after PSM.
After 1:1 matching, analysis of OS and CSS between the 234 patients in the radiation therapy group and the 234 patients in the non-radiation therapy group also showed no significant statistical differences (P > 0.05), as seen in Fig. 2B and D.
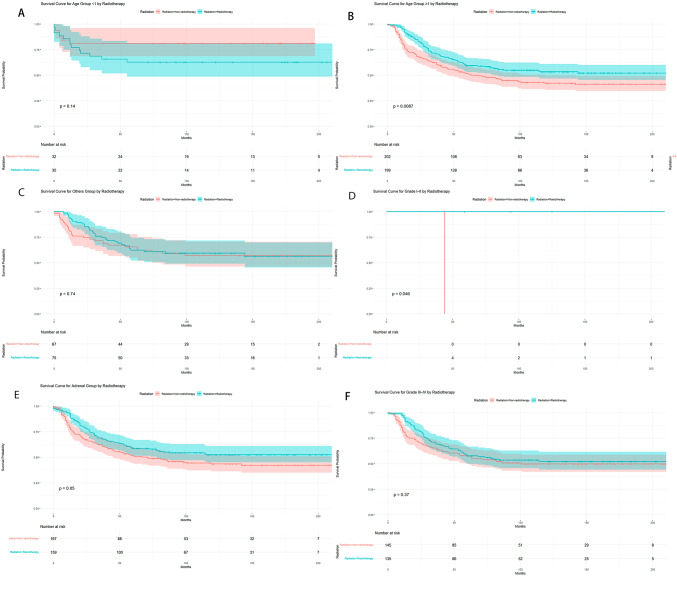
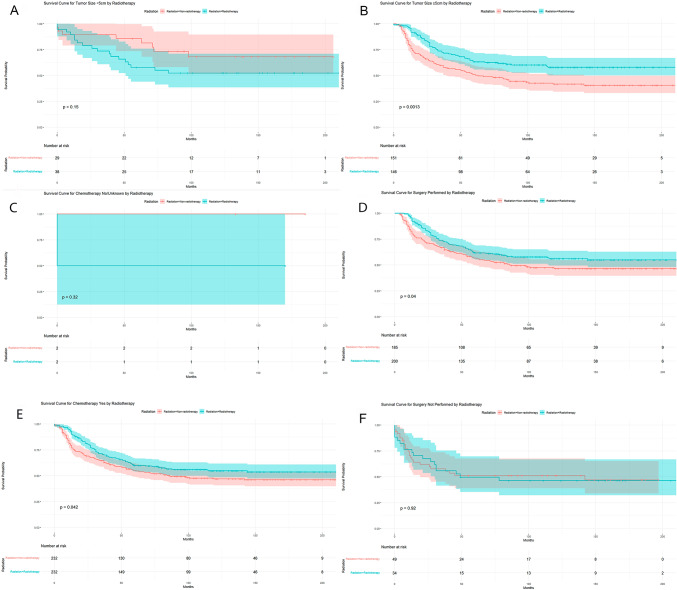
However, when stratifying tumor patients by age, primary site, tumor differentiation grade, tumor size, chemotherapy, and surgery, it was found that OS was significantly better in the radiation therapy group compared to the non-radiation therapy group for patients aged ≥ 1 year (P = 0.009, Fig. 3B), patients with tumor differentiation Grades I–II (P = 0.046, Fig. 3E), patients with tumor size ≥ 5 cm (P = 0.001, Fig. 4B), patients who received chemotherapy (P = 0.042, Fig. 4C), and patients who underwent surgery (P = 0.040, Fig. 4E). However, there were no differences in OS between the radiation therapy and non-radiation therapy groups for patients aged < 1 year, with tumors in the primary site, with Grades III–IV differentiation, with tumor size < 5 cm, without chemotherapy, and without surgery (Figs. 3A, C, D, F, 4A, D, and F).
Fig. 3.
Comparison of OS between radiotherapy and non-radiotherapy groups after stratifying tumor patients by age, primary site, tumor differentiation grade, tumor size, chemotherapy and surgery.
Fig. 4.
Comparison of OS between radiotherapy and non-radiotherapy groups after stratifying tumor patients tumor size, chemotherapy and surgery.
Prognostic factors in metastatic neuroblastoma
Cox regression analysis assessed the impact of various factors on OS and CSS in patients with metastatic neuroblastoma, as detailed in Tables 2 and 3. Multivariate analysis revealed that patients aged 1 year or older had a significantly higher risk of mortality compared to those under 1 year, with HRs for OS at 1.918 (95% CI 1.185–3.104, P = 0.008) and similar results for CSS (HR = 1.918, 95% CI 1.185–3.104, P = 0.008). Additionally, the primary tumor site was identified as a crucial factor influencing survival outcomes. After adjusting for other variables, patients with tumors located outside the adrenal gland demonstrated higher survival rates for both OS (HR = 0.680, 95% CI 0.501–0.922, P = 0.013) and CSS (HR = 0.673, 95% CI 0.493–0.920, P = 0.013).
Table 2.
Univariate and multivariate Cox regression model analysis of OS between the Radiotherapy group and the Non-radiotherapy group.
| Variable | Group | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P_value | Hazard ratio (95% CI) | P_value | ||
| Age(years) | < 1 | Reference | Reference | ||
| ≥ 1 | 1.833 (1.144–2.937) | 0.012 | 1.918 (1.185–3.104) | 0.008 | |
| Sex | Female | Reference | Reference | ||
| Male | 0.981 (0.748–1.286) | 0.888 | 0.974 (0.744–1.274) | 0.845 | |
| Race | White | Reference | Reference | ||
| Black | 1.074 (0.738–1.565) | 0.709 | 1.122 (0.784–1.606) | 0.529 | |
| Others | 1.471 (0.977–2.214) | 0.065 | 1.487 (0.984–2.246) | 0.060 | |
| Primary site | Adrenal | Reference | Reference | ||
| Others | 0.753 (0.556–1.019) | 0.066 | 0.680 (0.501–0.922) | 0.013 | |
| Grade | Grades I–II | Reference | Reference | ||
| Grades III–IV | 2.873 (0.402–20.555) | 0.293 | 3.402 (0.468–24.722) | 0.226 | |
| Unknown | 3.029 (0.422–21.750) | 0.271 | 3.372 (0.465–24.470) | 0.229 | |
| Tumor size | < 5 cm | Reference | Reference | ||
| ≥ 5 cm | 1.303 (0.857–1.982) | 0.216 | 1.262 (0.827–1.926) | 0.280 | |
| Unknown | 1.397 (0.870–2.245) | 0.167 | 1.302 (0.803–2.111) | 0.285 | |
| Histologic Type | Neuroblastoma | Reference | Reference | ||
| Ganglioneuroblastoma | 0.937 (0.563–1.560) | 0.802 | 0.950 (0.565–1.596) | 0.846 | |
| Olfactory neuroblastoma | NA | NA | NA | NA | |
| Chemotherapy | Yes | Reference | Reference | ||
| No | 0.502 (0.070–3.580) | 0.492 | 0.597 (0.081–4.377) | 0.612 | |
| Surgery | Yes | Reference | Reference | ||
| No | 1.361 (0.968–1.913) | 0.076 | 1.407 (0.969–2.042) | 0.073 | |
| Radiotherapy | Yes | Reference | Reference | ||
| No | 1.295 (0.990–1.694) | 0.059 | 1.275 (0.974–1.669) | 0.077 | |
Significant values are in bold.
Table 3.
Univariate and multivariate Cox regression model analysis of CSS between the Radiotherapy group and the Non-radiotherapy group.
| Variable | Group | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P_value | Hazard ratio (95% CI) | P_value | ||
| Age (years) | < 1 | Reference | Reference | ||
| ≥ 1 | 1.833 (1.144–2.937) | 0.012 | 1.918 (1.185–3.104) | 0.008 | |
| Sex | Female | Reference | Reference | ||
| Male | 0.981 (0.748–1.286) | 0.888 | 0.954 (0.726–1.256) | 0.739 | |
| Race | White | Reference | Reference | ||
| Black | 1.074 (0.738–1.565) | 0.709 | 1.090 (0.744–1.597) | 0.659 | |
| Others | 1.471 (0.977–2.214) | 0.065 | 1.469 (0.965–2.237) | 0.073 | |
| Primary site | Adrenal | Reference | Reference | ||
| Others | 0.753 (0.556–1.019) | 0.066 | 0.673 (0.493–0.920) | 0.013 | |
| Grade | Grades I–II | Reference | Reference | ||
| Grades III–IV | 2.873 (0.402–20.555) | 0.293 | 3.327 (0.458–24.185) | 0.235 | |
| Unknown | 3.029 (0.422–21.750) | 0.271 | 3.347 (0.461–24.302) | 0.232 | |
| Tumor size | < 5 cm | Reference | Reference | ||
| ≥ 5 cm | 1.303 (0.857–1.982) | 0.216 | 1.237 (0.809–1.891) | 0.327 | |
| Unknown | 1.397 (0.870–2.245) | 0.167 | 1.261 (0.775–2.052) | 0.350 | |
| Histologic type | Neuroblastoma | Reference | Reference | ||
| Ganglioneuroblastoma | 0.937 (0.563–1.560) | 0.802 | 0.982 (0.583–1.653) | 0.945 | |
| Olfactory neuroblastoma | NA (NA–NA) | NA | NA (NA–NA) | NA | |
| Chemotherapy | Yes | Reference | Reference | ||
| No | 0.502 (0.070–3.580) | 0.492 | 0.589 (0.080–4.320) | 0.603 | |
| Surgery | Yes | Reference | Reference | ||
| No | 1.361 (0.968–1.913) | 0.076 | 1.462 (1.001–2.137) | 0.049 | |
| Radiotherapy | Yes | Reference | Reference | ||
| No | 1.295 (0.990–1.694) | 0.059 | 1.278 (0.970–1.682) | 0.081 | |
Significant values are in bold.
In the multivariate model for OS (HR = 1.275, 95% CI 0.974–1.669, P = 0.077) or CSS (HR = 1.278, 95% CI 0.970–1.682, P = 0.081), radiation therapy itself did not reach statistical significance as an independent prognostic factor. However, the analysis suggests a trend toward survival benefit from radiation therapy, especially when considering the marginal P-values. These results highlight the complexity of treatment decisions for metastatic neuroblastoma, where multiple factors, including age and tumor location, play a crucial role in patient prognosis.
Discussion
This study provides critical insights into the role of radiotherapy in treating metastatic neuroblastoma, emphasizing its potential impact on survival outcomes. The major findings suggest a trend toward improved OS and CSS in patients who received radiotherapy after propensity score matching, though these results did not achieve statistical significance. Subgroup analyses indicated that radiotherapy may confer a survival benefit, particularly in patients with larger tumors and those who underwent surgery. However, in the multivariate Cox regression analysis, radiotherapy did not emerge as an independent predictor of survival, implying that its benefits may be context-dependent, influenced by factors such as tumor size and surgical intervention. These findings highlight the complexity of treating metastatic neuroblastoma and the necessity for individualized treatment strategies that account for the unique characteristics of each patient’s disease.
The findings of our study align with and build upon previous research investigating the impact of radiotherapy on survival outcomes in neuroblastoma. Consistent with our results, several studies have indicated that radiotherapy may confer a survival advantage in specific subgroups of neuroblastoma patients, particularly those with residual disease post-surgery or larger primary tumors15–17. For example, Tesson18 underscored the potential benefits of radiotherapy in improving local control, thereby potentially enhancing overall survival, especially in high-risk cases. Our study supports these findings, demonstrating a trend toward improved survival in patients receiving radiotherapy, although a statistically significant difference was not observed in the overall cohort after PSM.
However, our study differs from some earlier reports in its emphasis on the lack of independent prognostic significance of radiotherapy in multivariate analysis. While previous studies, such as those by De et al.19, have highlighted the potential of radiotherapy to significantly improve outcomes in certain patient populations, our findings suggest that its benefits may be more context-specific, influenced by factors like tumor size and surgical intervention. Moreover, unlike other studies that have demonstrated clear survival benefits with radiotherapy across broader patient populations20,21, our results indicate that these benefits may not be universally applicable, underscoring the need for personalized treatment approaches in metastatic neuroblastoma.
A key strength of this study is the use of PSM to minimize selection bias, providing a more accurate assessment of radiotherapy’s impact on survival outcomes in metastatic neuroblastoma22,23. PSM facilitated the creation of well-balanced groups with comparable baseline characteristics, addressing a significant limitation in many previous studies that lacked such rigorous matching procedures24. This methodological approach allowed us to more effectively isolate the effect of radiotherapy, reducing the influence of confounding variables such as age, tumor size, and extent of surgical intervention. Despite this robust design, our study found that radiotherapy was not an independent predictor of survival in the multivariate analysis, challenging the conventional wisdom that suggests a universal benefit of radiotherapy in this patient population25. These findings imply that while PSM is a powerful tool for observational studies, the complex nature of metastatic neuroblastoma treatment outcomes may require even more nuanced approaches, potentially including stratified or subgroup analyses, to fully understand the contexts in which radiotherapy is most beneficial26.
Despite the strengths of our study, several limitations must be acknowledged. First, as a retrospective analysis using data from the SEER database, the study is inherently subject to the biases and limitations associated with observational research, including potential inaccuracies in data reporting and coding27. Additionally, the SEER database lacks detailed information on treatment protocols, such as radiation dose and field, which are critical for accurately assessing the true impact of radiotherapy on survival outcomes1. Furthermore, the study population was limited to patients under 15 years of age, which may restrict the generalizability of the findings to older populations typically affected by neuroblastoma28. This study was based on data from 2004 to 2015 and utilized the older COG risk classification system, which categorized all patients with International Neuroblastoma Staging System (INSS) stage 4 as high-risk. However, the updated COG risk classification system29 has significantly refined risk stratification by integrating the International Neuroblastoma Risk Group Staging System (INRGSS) and chromosomal abnormalities, such as 1p/11q deletions. For instance, metastatic patients without MYCN amplification or segmental chromosomal aberrations (SCA) may now be reclassified as intermediate- or low-risk, potentially reducing the need for radiotherapy. This heterogeneity in risk grouping may partially explain the lack of overall survival benefit observed in our study. Future studies should incorporate molecular biomarkers to prospectively validate the role of radiotherapy in patients with true high-risk features such as MYCN amplification or SCA.
Future studies should aim to overcome these limitations by incorporating prospective designs with more detailed clinical data, including specifics of radiotherapy treatment and its timing relative to other modalities. Additionally, research exploring the molecular and genetic factors influencing the response to radiotherapy in metastatic neuroblastoma is needed30. Such studies could pave the way for personalized treatment approaches, optimizing the use of radiotherapy in conjunction with other therapeutic strategies31. Finally, examining the impact of radiotherapy on quality of life and long-term survivorship in this patient population would provide a more comprehensive understanding of its role in neuroblastoma treatment32.
Conclusion
This study highlights the nuanced role of radiotherapy in the treatment of metastatic neuroblastoma. While radiotherapy did not demonstrate an independent survival benefit in the overall cohort, subgroup analyses suggested potential clinical utility in patients with larger tumor burdens or those undergoing surgical intervention. The limitations of the older risk stratification system underscore the need for more precise definitions of radiotherapy indications. Future research should integrate the revised COG risk classification system with molecular biomarkers to better identify patient subgroups that may derive the greatest benefit from radiotherapy. Additionally, prospective studies incorporating detailed clinical and molecular data are essential to refine therapeutic strategies and improve outcomes for patients with this aggressive malignancy. Further exploration of the long-term effects of radiotherapy on survival and quality of life will also be critical to informing patient-centered treatment approaches.
Acknowledgements
The authors want to acknowledge all of the participants and investigators for contributing and sharing summary-level data on SEER.
Author contributions
SXL proposed the conception, designed the study and supervised the whole analysis. AA acquired the data and performed statistical analysis. FA drafted the article. KL contributed to the data acquisition and checked the statistical analysis.
Funding
Supported by Natural Science Foundation of Xinjiang Uygur Autonomous Region (Grant number: 2024D01A106).
Data availability
Publicly available datasets were analyzed in this study. The data can be found here: https://seer.cancer.gov/data/
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ailikamu Aierken and Falide Atabieke contributed equally to this work.
Contributor Information
Shui-Xue Li, Email: Lishuixue@sina.com.
Kai Li, Email: likai6265123@163.com.
References
- 1.Che, W. Q. et al. How to use the Surveillance, Epidemiology, and End Results (SEER) data: Research design and methodology. Mil. Med. Res.10(1), 50. 10.1186/s40779-023-00488-2 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takita, J. Molecular basis and clinical features of neuroblastoma. JMA J.4(4), 321–331. 10.31662/jmaj.2021-0077 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurney, J. G. et al. Infant cancer in the U.S.: Histology-specific incidence and trends, 1973 to 1992. J. Pediatr. Hematol. Oncol.19(5), 428–432. 10.1097/00043426-199709000-00004 (1997). [DOI] [PubMed] [Google Scholar]
- 4.Matthay, K. K. et al. Neuroblastoma. Nat. Rev. Dis. Primers2, 16078. 10.1038/nrdp.2016.78 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Mlakar, V. et al. 11q deletion in neuroblastoma: A review of biological and clinical implications. Mol. Cancer16(1), 114. 10.1186/s12943-017-0686-8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.London, W. B. et al. Historical time to disease progression and progression-free survival in patients with recurrent/refractory neuroblastoma treated in the modern era on Children’s Oncology Group early-phase trials. Cancer123(24), 4914–4923. 10.1002/cncr.30934 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haas-Kogan, D. A. et al. Impact of radiotherapy for high-risk neuroblastoma: A Children’s Cancer Group study. Int. J. Radiat. Oncol. Biol. Phys.56(1), 28–39. 10.1016/s0360-3016(02)04506-6 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Marcus, K. J. et al. Primary tumor control in patients with stage 3/4 unfavorable neuroblastoma treated with tandem double autologous stem cell transplants. J. Pediatr. Hematol. Oncol.25(12), 934–940. 10.1097/00043426-200312000-00005 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Simon, T., Häberle, B., Hero, B., von Schweinitz, D. & Berthold, F. Role of surgery in the treatment of patients with stage 4 neuroblastoma age 18 months or older at diagnosis. J. Clin. Oncol.31(6), 752–758. 10.1200/JCO.2012.45.9339 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Braunstein, S. E. et al. Role of the extent of prophylactic regional lymph node radiotherapy on survival in high-risk neuroblastoma: A report from the COG A3973 study. Pediatr. Blood Cancer66(7), e27736. 10.1002/pbc.27736 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazloom, A. et al. Radiation therapy to the primary and postinduction chemotherapy MIBG-avid sites in high-risk neuroblastoma. Int. J. Radiat. Oncol. Biol. Phys.90(4), 858–862. 10.1016/j.ijrobp.2014.07.019 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erratum to: Polishchuk, A. L., Li, R., Hill-Kayser, C. et al. Likelihood of bone recurrence in prior sites of metastasis in patients with high-risk neuroblastoma. Int. J. Radiat. Oncol. Biol. Phys. 89, 839–845 (2014). Int. J. Radiat. Oncol. Biol. Phys. 93(3), 729 (2015). 10.1016/j.ijrobp.2015.06.037 [DOI] [PubMed]
- 13.Simon, T. et al. Intensified external-beam radiation therapy improves the outcome of stage 4 neuroblastoma in children > 1 year with residual local disease. Strahlenther. Onkol.182(7), 389–394. 10.1007/s00066-006-1498-8 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Arumugam, S., Manning-Cork, N. J., Gains, J. E., Boterberg, T. & Gaze, M. N. The evidence for external beam radiotherapy in high-risk neuroblastoma of childhood: A systematic review. Clin. Oncol. (R Coll Radiol).31(3), 182–190. 10.1016/j.clon.2018.11.031 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Wawrzuta, D. et al. Revisiting the role of radiotherapy in the treatment of neuroblastoma 4S: 30 years of institutional experience and systematic review. Clin. Transl. Radiat. Oncol.47, 100791. 10.1016/j.ctro.2024.100791 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salemi, F. et al. Neuroblastoma: Essential genetic pathways and current therapeutic options. Eur. J. Pharmacol.926, 175030. 10.1016/j.ejphar.2022.175030 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Robbins, J. R. et al. Radiation therapy as part of local control of metastatic neuroblastoma: The St Jude Children’s Research Hospital experience. J. Pediatr. Surg.45(4), 678–686. 10.1016/j.jpedsurg.2009.11.003 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tesson, M. et al. An evaluation in vitro of the efficacy of nutlin-3 and topotecan in combination with 177Lu-DOTATATE for the treatment of neuroblastoma. Oncotarget9(49), 29082–29096. 10.18632/oncotarget.25607 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Ioris, M. A. et al. Local control in metastatic neuroblastoma in children over 1 year of age. BMC Cancer15, 79. 10.1186/s12885-015-1082-7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caussa, L., Hijal, T., Michon, J. & Helfre, S. Role of palliative radiotherapy in the management of metastatic pediatric neuroblastoma: A retrospective single-institution study. Int. J. Radiat. Oncol. Biol. Phys.79(1), 214–219. 10.1016/j.ijrobp.2009.10.031 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Sultan, I., Ghandour, K., Al-Jumaily, U., Hashem, S. & Rodriguez-Galindo, C. Local control of the primary tumour in metastatic neuroblastoma. Eur. J Cancer45(10), 1728–1732. 10.1016/j.ejca.2009.04.021 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Liang, J., Hu, Z., Zhan, C. & Wang, Q. Using propensity score matching to balance the baseline characteristics. J. Thorac. Oncol.16(6), e45–e46. 10.1016/j.jtho.2020.11.030 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Atabieke, F. et al. Association between frailty and hepatic fibrosis in NAFLD among middle-aged and older adults: Results from NHANES 2017–2020. Front. Public Health12, 1330221. 10.3389/fpubh.2024.1330221 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan, C. J. Reducing bias using propensity score matching. J. Nucl. Cardiol.25(2), 404–406. 10.1007/s12350-017-1012-y (2018). [DOI] [PubMed] [Google Scholar]
- 25.Canete, A. et al. Poor survival for infants with MYCN-amplified metastatic neuroblastoma despite intensified treatment: The International Society of Paediatric Oncology European Neuroblastoma Experience. J. Clin. Oncol.27(7), 1014–1019. 10.1200/JCO.2007.14.5839 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Baek, S., Park, S. H., Won, E., Park, Y. R. & Kim, H. J. Propensity score matching: A conceptual review for radiology researchers. Korean J Radiol.16(2), 286–296. 10.3348/kjr.2015.16.2.286 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aierken, A. et al. No bidirectional relationship between inflammatory bowel disease and diverticular disease: A genetic correlation and Mendelian randomization study. Front. Genet.15, 1334473. 10.3389/fgene.2024.1334473 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shahraki, K. & Suh, D. W. Neglected metastatic neuroblastoma. Ophthalmology131(7), 826. 10.1016/j.ophtha.2023.08.004 (2024). [DOI] [PubMed] [Google Scholar]
- 29.Irwin, M. S. et al. Revised neuroblastoma risk classification system: A report from the Children’s Oncology Group. J. Clin. Oncol.39(29), 3229–3241. 10.1200/JCO.21.00278 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moghimi, B. et al. Preclinical assessment of the efficacy and specificity of GD2-B7H3 SynNotch CAR-T in metastatic neuroblastoma. Nat. Commun.12(1), 511. 10.1038/s41467-020-20785-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shum, T. et al. Constitutive signaling from an engineered IL7 receptor promotes durable tumor elimination by tumor-redirected T cells. Cancer Discov.7(11), 1238–1247. 10.1158/2159-8290.CD-17-0538 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seong, B. K. et al. A metastatic mouse model identifies genes that regulate neuroblastoma metastasis. Cancer Res.77(3), 696–706. 10.1158/0008-5472.CAN-16-1502 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly available datasets were analyzed in this study. The data can be found here: https://seer.cancer.gov/data/