Abstract
The XX/XY sex chromosome system is highly conserved across mammals, with rare exceptions where males lack a Y chromosome. Among these is the genus Tokudaia, a group of spiny rats comprising three species with unique sex chromosome systems deviating from the typical XX/XY pattern. While Tokudaia osimensis and Tokudaia tokunoshimensis have completely lost the Y chromosome, they retain some Y-linked genes on the X chromosome. In contrast, Tokudaia muenninki retains large sex chromosomes where both the X and Y chromosomes have fused with an autosome pair, carrying multi-copied Y-linked genes, including Sry. In this study, we generated chromosome-level genome assemblies for male individuals of all three Tokudaia species. By investigating loci typically associated with rodent Y-linked genes, we characterized sequences derived from the Tokudaia Y-chromosomal most recent common ancestor (Tokudaia Y-MRCA) and traced their evolutionary trajectories. Our analyses revealed that an initial X-to-Y translocation of a sequence containing the boundary-associated segmental duplication in a common ancestor of Tokudaia marked the beginning of their unique sex chromosome evolution. The boundary-associated segmental duplication, uniquely multi-copied in Tokudaia, facilitated further rearrangements through nonallelic homologous recombination and duplications. These processes culminated in subsequent Y-to-X translocations and duplications, leading to the complete loss of the Y chromosome as a distinct entity while preserving Y-linked genes in a multicopy state on the X chromosome. These findings highlight Tokudaia's rapid sex chromosome evolution within 3 million years and provide insights into the mechanisms underlying Y chromosome loss, contributing to a broader understanding of sex chromosome evolution in rodents.
Keywords: Y chromosome, comparative genomics, spiny rat, chromosome evolution, chromosome-level genome assembly
Introduction
The Sry-dependent sex determination system, encoded on the Y chromosome, is conserved in most mammals (Sinclair et al. 1990; Koopman et al. 1991; Goodfellow and Lovell-Badge 1993). However, certain mammals, such as Tokudaia and Ellobius, have atypical sex chromosomes and distinct sex determination systems (Matthey 1953; Honda et al. 1977, 1978). The genus Tokudaia includes three species—Tokudaia muenninki (Okinawa spiny rat), Tokudaia osimensis (Amami spiny rat), and Tokudaia tokunoshimensis (Tokunoshima spiny rat)—which are endemic to the islands of Okinawa, Amami Ōshima, and Tokunoshima in southwestern Japan. Phylogenetic studies indicate that T. muenninki diverged approximately 2.5 to 2.7 million years ago (Mya) (Murata et al. 2012), with T. osimensis and T. tokunoshimensis diverging later, approximately 1.1 Mya (Murata et al. 2010). The karyotypes of T. osimensis (2n = 25) and T. tokunoshimensis (2n = 45) exhibit an XO/XO system, where males lack a Y chromosome and both sexes have only one X chromosome (Honda et al. 1977, 1978; Nakamura et al. 2007). Additionally, the male-determining factor Sry is absent in both species (Soullier et al. 1998; Sutou et al. 2001). As a result, T. osimensis and T. tokunoshimensis utilize a Sry-independent sex determination system, prompting investigations into novel mechanisms of sex determination (Kobayashi et al. 2007; Terao et al. 2022). Recent whole-genome analyses of T. osimensis revealed a 17-kbp male-specific duplication upstream of Sox9, including a region homologous to enh14 in mice. This duplication was found to promote Sox9 expression in gene-edited mice; however, no sex reversal occurred, suggesting that it is necessary but not sufficient for male determination, leaving the mechanism unresolved (Terao et al. 2022). Moreover, fluorescence in situ hybridization (FISH) analysis has revealed that some Y chromosome genes commonly found in mammals have been translocated to the distal region of the X chromosome in T. osimensis and T. tokunoshimensis (Sutou et al. 2001; Arakawa et al. 2002; Kuroiwa et al. 2010). In contrast, T. muenninki (2n = 44) has a typical XX/XY karyotype; however, its sex chromosomes have fused with autosomes, creating a large pseudoautosomal region (PAR) (Murata et al. 2010, 2012). RNA-seq analysis indicates that recombination suppression has initiated in the autosome-derived region physically adjacent to the ancestral sex chromosomes, leading to sequence divergence (Murata et al. 2015). Additionally, multiple Y-linked genes, including Sry, have undergone multicopy amplification in T. muenninki, suggesting that it may possess a unique sex determination mechanism (Murata et al. 2010, 2016). Consequently, Tokudaia represents an appealing model for studying chromosomal evolution, especially that of sex chromosomes. However, all three species are endangered (IUCN 2024), limiting the availability of research resources. Previous studies have primarily relied on bacterial artificial chromosome (BAC)-based FISH analyses (Kobayashi et al. 2008; Kuroiwa et al. 2010; Murata et al. 2016), partial genome analysis (Murata et al. 2016), and RNA-seq to identify genes (Murata et al. 2015). The only whole-genome analysis identifying a male-specific region in T. osimensis used short-read sequencing, resulting in fragmented X chromosome sequences and restricting comprehensive sex chromosome analysis (Terao et al. 2022). These genome data were later used in the analysis by Li et al. (2024), which confirmed the translocation of several additional Y-linked genes to the X chromosome beyond those already identified by FISH (Sutou et al. 2001; Arakawa et al. 2002; Kuroiwa et al. 2010). However, their study was limited to gene presence confirmation and did not conduct any genome sequence-based analysis. Moreover, the genomes of the other two Tokudaia species have not even been sequenced.
In this study, we constructed chromosome-level genome assemblies for male individuals of all three Tokudaia species using long-read sequencing and Hi-C technology, enabling a comprehensive comparative genomic analysis of their sex chromosomes. Our primary aim is to establish high-quality genome assemblies and compare their chromosomal structures to understand how the Y chromosome of their common ancestor (the Tokudaia Y-chromosomal most recent common ancestor, Tokudaia Y-MRCA) has evolved and contributed to shaping the current sex chromosome structures in each species. By investigating these structural changes in the Tokudaia lineage, we aim to provide insights into the diversity of sex chromosome evolution in mammals.
Results
Genome Construction of the Three Tokudaia Species
We constructed chromosome-level genome assemblies for all three Tokudaia species using PacBio CLR and Hi-C data, with detailed methodology and statistics provided in Scientific Data (Okuno et al. 2023). Unfortunately, the ancestral Y chromosome of T. muenninki was highly fragmented, prompting us to acquire additional PacBio HiFi data for reanalysis, which allowed us to achieve chromosome-level assembly for all chromosomes, including the Y chromosome. By reconstructing of the T. muenninki genome using HiFi reads, we successfully generated assembly with a N50 of 171.5 Mbp, totaling 3,674.6 Mbp (Table 1), which is approximately 1,014 Mbp longer than the previously assembled genome. This discrepancy is likely due to the inclusion of highly repetitive heterochromatic regions in the assembly. The estimated genome size is 3,362.6 Mbp, and when accounting for the half-coverage of the sex chromosomes, this result demonstrates good concordance. Notably, this assembly successfully resolved the highly fragmented Y chromosome from the previous assembly and reconstructed the neo-ancX chromosome as a single sequence, including the heterochromatic region. A summary of the genome assembly results for all three species is provided in Table 1. As shown in Table 1 and supplementary fig. S19, Supplementary Material online our assemblies exhibit high continuity and completeness, as evaluated by BUSCO gene assessment. However, independent experimental validation of the assembly has not been conducted.
Table 1.
Assembly statistics of the three Tokudaia species genomes
| T. osimensis | T. tokunoshimensis | T. muenninki | |
|---|---|---|---|
| 2n = 25, XO/XO | 2n = 45, XO/XO | 2n = 44, XX/XY | |
| Genome assembly statistics | |||
| #Scaffolds | 123 | 159 | 34 |
| #Chromosome-level scaffolds | 13 | 23 | 23 |
| #Unplaced scaffolds | 110 | 136 | 11 |
| Total scaffold length (bp) | 2,445,255,239 | 2,477,294,292 | 3,674,585,994 |
| Total Chr.-level scaffold length (bp) | 2,440,098,537 | 2,473,996,358 | 3,651,392,569 |
| Anchored to chromosome (%) | 99.79 | 99.87 | 99.37 |
| Longest scaffold (bp) | 269,377,760 | 179,766,896 | 346,715,556 |
| Contig N50 (bp) | 8,462,634 | 13,597,559 | 138,437,150 |
| Contig L50 | 88 | 57 | 11 |
| Scaffold N50 (bp) | 234,036,378 | 125,076,325 | 171,451,252 |
| Scaffold L50 | 5 | 9 | 8 |
| Gaps (bp) | 270,541 | 205,000 | 9,500 |
| BUSCO evaluation (v5.7.1, genome mode, glires_odb10) | |||
| Complete BUSCOs (%) | 98.72 | 98.74 | 98.80 |
| Single-copy BUSCOs (%) | 98.15 | 97.99 | 97.96 |
| Duplicated BUSCOs (%) | 0.57 | 0.75 | 0.84 |
| Fragmented BUSCOs (%) | 0.70 | 0.72 | 0.67 |
| Missing BUSCOs (%) | 0.57 | 0.54 | 0.52 |
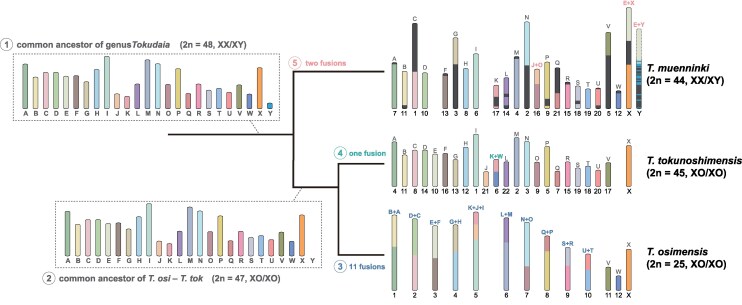
Subsequently, we analyzed the synteny relationships among the three species and predicted their ancestral karyotypes (Fig. 1, supplementary figs. S1 and S2, Supplementary Material online). Synteny in both autosomes and the X chromosome was highly conserved across species, with karyotype differences attributable to chromosomal fusions. The common ancestral karyotype likely comprised 23 autosomes and sex chromosomes (2n = 48), whereas the common ancestors of T. osimensis and T. tokunoshimensis likely lost the Y chromosome, resulting in a 2n = 47 karyotype. All chromosomal fusions appeared to have occurred in a species-specific manner: T. osimensis has 12 autosomes and one X chromosome resulting from 11 chromosomal fusions; T. tokunoshimensis has 22 autosomes and one X chromosome because of one fusion; and T. muenninki has 21 autosomes and sex chromosomes resulting from two fusions, one involving a sex chromosome and the other an autosome.
Fig. 1.
Evolutionary transitions from ancestral chromosomes of the three Tokudaia species. Evolutionary transitions from ancestral chromosomes to the current karyotypes of the three species, based on synteny analysis. The 25 ancestral chromosomes of the common ancestor (labeled A to Y, where A to W are autosomes, and X/Y are sex chromosomes) are shown, along with the fusion events between corresponding chromosomes. The common ancestor of the three species is estimated to have a karyotype of 2n = 48 (XX/XY) (1). After divergence, the common ancestor of T. osimensis and T. tokunoshimensis lost the Y chromosome, leading to a 2n = 47 (XO/XO) karyotype (2). Subsequent divergence led to 11 chromosomal fusions in T. osimensis, resulting in the current karyotype of 2n = 25 (XO/XO) (3), and one chromosomal fusion in T. tokunoshimensis, leading to the current 2n = 45 (XO/XO) karyotype (4). In contrast, T. muenninki experienced two chromosomal fusions, including a fusion between autosome E and a sex chromosome (5), resulting in 2n = 44 (XX/XY).
Genomic Overview of the Tokudaia Y-MRCA-Originated Locus in the Three Tokudaia Species
In contrast to other chromosomes—such as the X chromosome shown as an example in supplementary fig. S3a, Supplementary Material online—no chromosome-scale synteny, or even synteny at the megabase level, was detected in the Y chromosome due to its extensive structural rearrangements and differentiation. To identify regions derived from Tokudaia Y-MRCA, we searched for known rodent Y-linked genes across the whole genome sequences. The copy numbers and chromosomal locations of the identified Y-linked genes are summarized in Table 2. As shown in Fig. 2a, in T. osimensis and T. tokunoshimensis, these genes were detected at a single locus in the distal Xq region (Xq-region1). In contrast, in T. muenninki, they were distributed across three distinct regions: the heterochromatic region between the neo-sex and ancestral X chromosome segments (Xhet-region, previously classified as a heterochromatic region based on cytogenetic studies; Murata et al. 2010; Murata et al. 2012), the distal Xq region (Xq-region2), and around the ancestral Y region (ancY-het-region). Xq-region2 is distinct from Xq-region1 and originated from an independent translocation event. Before delving into the details of each locus, we first provide an overview of these identified Y-MRCA-derived regions. The structural differences among these loci are striking. Xq-region1 in T. osimensis and T. tokunoshimensis spans approximately 900 kb to 1 Mb, whereas Xq-region2 in T. muenninki consists of duplicated 150 kb segments totaling around 300 kb. In contrast, the Xhet-region and ancY-het-region in T. muenninki are much larger, spanning 80 to 100 Mb, though they are rich in repetitive sequences. Despite the relatively short divergence time of approximately 2.5 million years between T. muenninki and the other two species, extensive structural rearrangements and repeat insertions have independently occurred in each lineage. As a result, while small-scale synteny can be detected at certain loci, no large-scale synteny is observed (supplementary fig. S3b, Supplementary Material online and Fig. 2b). The highest degree of synteny at the gene order level is observed between Xq-region1 in T. osimensis/T. tokunoshimensis and the proximal part of ancY-het-region in T. muenninki (Fig. 2c-c), where three genes (Ddx3y, Uty, and Tsp1y) are retained in the same order within a region of approximately 350 kb. Beyond this, only scattered instances of two-gene synteny are detected (Fig. 2c-a, b, and d). Given these substantial differences in genomic structure and scale, direct comparisons between the former Y-derived loci of T. muenninki and those in T. osimensis/T. tokunoshimensis are challenging. Moreover, no closely related outgroup species with a fully assembled Y chromosome exists, making ancestral state reconstruction difficult.
Table 2.
Comparison of Y-linked gene numbers across Tokudaia Y-MRCA-derived regions and other rodent species reported to have atypical sex chromosomes
| T. osimensis | T. tokunoshimensis | T. muenninki | M. oregoni a | E. lutescens b | E. talpinus b | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (XO/XO) | (XO/XO) | (XX/XY) | (XMO/XPXM) | (XO/XO) | (XX/XX) | |||||||||||
| Xq-region1 | Xq-region1 | Xhet-region | Xq-region1 | Xq-region2 | ancY-het-region | Male XP | Female XM | X | X | |||||||
| Intact | Pseudo | Intact | Pseudo | Intact | Pseudo | Intact | Pseudo | Intact | Pseudo | Intact | Pseudo | Intact/pseudo | Intact/pseudo | |||
| Ddx3y | 1 | … | 1 | … | … | … | … | … | … | … | 1 | … | o | o | … | … |
| Uty | 1 | … | 1 | … | … | … | … | … | … | … | 1 | … | o | o | … | … |
| Eif2s3y | 1 | … | 1 | … | … | … | … | … | … | … | 11 | 2 | o | o | 1 | 1 |
| Uba1y | … | … | … | … | … | 53 | … | … | … | … | … | 8 | ? | ? | … | … |
| Usp9y | … | 1 | … | 1 | … | 1 | … | … | … | … | 7 | 24 | ? | ? | 1 | … |
| Sry | … | … | … | … | … | … | … | … | … | … | 5 | 58 | o | o | … | … |
| Rbmy | … | … | … | … | … | … | … | … | … | … | 5 | 40 | o | o | … | … |
| Tspy | 1 | … | 1 | … | … | … | … | … | 2 | … | 62 | 35 | … | … | … | … |
| Zfy | 1 | … | 1 | … | 1 | … | … | … | 2 | … | 15 | 78 | ? | ? | 1 | 1 |
| Kdm5d | 2 | … | … | 1 | … | … | … | … | … | … | … | … | o | o | … | … |
| BASD | 3 | … | 4 | … | 1 | … | 2 | … | … | 2 | 105 | … | … | … | … | … |
Fig. 2.
Tokudaia Y-MRCA-derived regions in the three species. a) Representation of chromosomal regions containing Tokudaia Y-chromosomal most recent common ancestor (Y-MRCA)-derived sequences in the three species. In T. muenninki, two regions were identified on the X chromosome (Xhet-region, Xq-region2) and one large region on the Y chromosome (ancY-het-region). In both T. osimensis and T. tokunoshimensis, a single region was identified on the X chromosome (Xq-region1). b) Dot-plot comparison of Y-MRCA-derived regions between T. osimensis (Xq-region1 and its surrounding regions) and T. muenninki (Y-MRCA-derived regions). In T. muenninki, the corresponding X chromosomal regions are: A) the 3′ terminal region of the Xhet-region and B) the region spanning Xq-region1 to Xq-region2, while the Y chromosomal regions correspond to blocks 0 to 10. The specific positions of these regions are indicated in a). Strong synteny, visualized as continuous diagonal lines, is observed between upstream and downstream regions of T. osimensis Xq-region1 and the corresponding Xq-region1 in T. muenninki. In contrast, in the Y-derived regions, synteny is fragmented, appearing as sparse and short diagonal lines, indicating disrupted structural conservation. c) Magnified views of the regions outlined by squares in b). The region labels (a to d) correspond to those indicated in b). The figure shows that the longest syntenic segment between T. osimensis Xq-region1 and T. muenninki is within block 0 of ancY, spanning approximately 400 kb, where three genes (Ddx3y, Uty, and Tspy1) are retained in the same order. Beyond this region, only scattered instances of two-gene synteny are observed.
Tokudaia Y-MRCA-Originated Locus in T. osimensis and T. tokunoshimensis Genomes
As mentioned above, regions likely derived from Tokudaia Y-MRCA in both T. osimensis and T. tokunoshimensis were located at the same distal X chromosome locus, with an inversion observed between the two species (Fig. 3a). This locus contains seven Y-linked genes (Zfy, Kdm5d, Eif2s3y, Uty, Tspy, Ddx3y, and Usp9y) and was the only region in both species that retained intact Y-linked genes. Otherwise, only a previously reported Rbmy processed pseudogene was found on an autosome (supplementary fig. S4, Supplementary Material online) (Kuroiwa et al. 2010).
Fig. 3.
Tokudaia Y-MRCA-derived Xq-region1 in T. osimensis and T. tokunoshimensis. a) Synteny analysis of Xq-region1 and surrounding areas in T. osimensis and T. tokunoshimensis, alongside corresponding regions in T. muenninki and mouse. Seven synteny blocks (SB1 to SB7) were identified among the four species, with alignment lines connecting homologous regions. Chromosome origins are distinguished by fill patterns and labels: X chromosome-derived regions are labeled SB1–SB4, Y chromosome-derived regions are SB5, SB6, and SB5′, and autosomal regions are also indicated. Boundary-associated segmental duplications (BASDs) are shown at the edges of synteny boundaries. SB1 to SB4 on the mouse X chromosome are considered ancestral, while SB5, SB6, and SB5′ (a duplicated portion of SB5, potentially originating from Tokudaia Y-MRCA with some autosomal contributions), were inserted into the Xq-region1 of T. osimensis and T. tokunoshimensis. In T. muenninki, SB7, containing repeated sequences similar to those downstream of SB4, was inserted in this region. Structural variations, including inversions, were observed between T. osimensis and T. tokunoshimensis, particularly in SB5, SB6, SB2, and SB3. b) A schematic diagram of the evolutionary events following the insertion of SB5 and SB6 (derived from Tokudaia Y-MRCA) in T. osimensis and T. tokunoshimensis, based on sequence duplication traces and the most parsimonious prediction of structural variation. Initially, the common ancestor underwent the insertion of SB5 and SB6 (in reverse orientation) between SB1 and SB2 via BASD-mediated NAHR. Subsequently, SB5 and BASD were duplicated and translocated, forming SB5′. After species divergence, each lineage experienced two inversion events, shaping the current genome structures of both species. c) Close-up view of the boundary regions where the insertions likely occurred, using the mouse genome as a reference. Diagonal alignment lines with the mouse genome indicate that the insertion was mediated by the BASD, located between SB1 and SB2 in the mouse genome. The alignments of regions flanking Mm-BASD001, along with their corresponding positions, further confirm the BASD-mediated insertion.
Next, we compared the genomes of both species with the X chromosomes of T. muenninki and mice, which lack the insertion of Y-derived sequences in this locus. We defined synteny blocks (SBs) and predicted the evolutionary events that occurred among the four species. Consequently, the region surrounding this locus was divided into seven SBs (Fig. 3a, supplementary fig. S5 and table S1, Supplementary Material online). SB1 to 4 were X chromosome-derived sequences common to all four species, with their order and orientation in mice considered ancestral (supplementary fig. S6, Supplementary Material online). Meanwhile, SB5, SB5′, and SB6 were found only in T. osimensis and T. tokunoshimensis, indicating an insertion between SB1 and SB4 in their common ancestor. Interestingly, SB5 contains not only Y-linked genes but also autosomal-derived Uggt2 and Dnajc3 genes (supplementary fig. S7, Supplementary Material online). As intact copies of these genes are present on autosomes, a genomic region containing parts of these genes is duplicated and translocated. SB5′ (99.2 kbp in T. osimensis, 61.3 kbp in T. tokunoshimensis) is a duplicated sequence of part of the Uggt2 and Dnajc3 regions within SB5, likely duplicated after insertion into their common ancestor. It is uncertain whether these sequences first integrated into the ancestral Y before translocating to the X or were directly inserted into the ancestral X. SB7, found only in T. muenninki, is a repeat-rich region homologous to repetitive sequences downstream of SB4 in T. osimensis and T. tokunoshimensis and is inserted within SB2 (supplementary fig. S5b, Supplementary Material online).
To understand how the genomic structure of the Xq-region1 in both species arose, we conducted a detailed analysis to infer the most plausible sequence of genomic rearrangements. Given the extensive structural variations observed in Tokudaia Y-MRCA-derived regions, resolving these recent events provides valuable insights into the dynamics of Y-derived sequences in this lineage. By estimating the most parsimonious insertion positions and arrangements of SB5 and SB6 to minimize the number of inversions in both species (supplementary fig. S8a, Supplementary Material online), we identified two possible scenarios, both suggesting that SB5 and SB6 were inserted between SB1 and SB2 (supplementary fig. S8b, Supplementary Material online). Among these, the scenario depicted in Fig. 3b (supplementary fig. S8b, Supplementary Material online, right) was found to be the most likely. This conclusion is supported by sequence analysis of T. osimensis, which revealed that the SB5′ likely originated from SB5 through an inverted duplication event mediated by a shared sequence at its boundaries (supplementary fig. S9, Supplementary Material online). Additionally, the arrangement of homologous sequences around SB5 and SB5′ suggests that an inversion occurred within SB5 after this duplication event. Further details on these rearrangement scenarios can be found in supplementary fig. S9, Supplementary Material online.
Chromosomal rearrangements often leave identifiable sequence features at their junctions, providing insights into their underlying mechanisms. Given that SB5 and SB6 were inserted at the boundary between SB1 and SB2—specifically between the genes Ids and Eola1—in both T. osimensis and T. tokunoshimensis, we examined this region to determine whether it harbored any characteristic sequences. Our analysis identified a 6.7 kbp sequence shared between the 3′ end of SB1 and the 5′ end of SB2, exhibiting over 98% sequence identity in T. osimensis. This sequence exceeds the commonly used criteria for segmental duplications (SDs; >1 kb length and >90% sequence identity) (Bailey and Eichler 2006). Based on these characteristics, we refer to this sequence as the boundary-associated segmental duplication (BASD; supplementary table S2, Supplementary Material online) throughout the manuscript. Although details of BASD are discussed in a later section, we note here that in common rodents, including Mus musculus and T. muenninki, BASD is typically present in up to two copies within the Xq-region1 locus. However, in T. osimensis and T. tokunoshimensis, the copy number has increased to three and four, respectively. Specifically, In M. musculus and T. muenninki, Mm-BASD001 and Tm-BASD001 are located at the SB1–SB2 boundary, and Mm-BASD002 and Tm-BASD002 are located at the SB2–SB3 boundary. In T. osimensis, To-BASD001 is found at the SB1–SB6 boundary, To-BASD002 at the SB5–SB2 boundary, and To-BASD003 at the SB2–SB5′ boundary. In T. tokunoshimensis, Tt-BASD001 is located at the SB1–SB3 boundary, Tt-BASD002 at the SB5′–SB5 boundary, Tt-BASD003 at the SB5–SB2 boundary, and Tt-BASD004 at the SB6–SB4 boundary (Fig. 3a). The BASD corresponding to Mm-BASD001 likely facilitated the insertion of SB5 and SB6 via nonallelic homologous recombination (NAHR; Fig. 3c). Interestingly, the BASD was also found at the SB2–SB5′ boundary in T. osimensis and at the SB5′–SB5 and SB6–SB4 boundaries in T. tokunoshimensis, indicating its role in further structural changes post-insertion (Fig. 3a).
Tokudaia Y-MRCA-Originated Locus in T. muenninki Genome
Before discussing individual loci, we provide an overview of the sex chromosomes of T. muenninki based on our genome assembly. As shown in Figs. 4a and 5a, each of the ancestral X (ancX) and ancestral Y (ancY) chromosomes fused with one of the two homologous copies of the same autosome (forming neo-sex chromosomes), resulting in large, extended sex chromosomes. In this study, the terms “neo-X”, “neo-Y”, and “neo” are used in both structural and sequence contexts. Structurally, “neo-X” and “neo-Y” refer to the autosomal segments that have fused with the ancestral X and Y chromosomes, respectively, while “neo” refers more generally to the autosome-derived portion of the sex chromosomes. In sequence-based analyses, we use “neo-X” or “neo-Y” when sequence differences allow us to distinguish their origin, and “neo” when such differences are not evident. The X chromosome comprises an autosomal and ancX region, separated by an approximately 80 Mbp heterochromatic region consisting of repetitive sequences. Meanwhile, the Y chromosome consists of autosomal and ancY regions that are positioned in close proximity, unlike the X chromosome, where the corresponding regions are separated by a large heterochromatic region. Additionally, part of the Tokudaia Y-MRCA-originated region is located on the proximal side of the autosomal region. The ancY region alternates between highly repetitive heterochromatic sequences and Y-linked gene regions, forming a mosaic structure. The assembled Y chromosome sequence extends from the differentiated neo-Y region to the distal end of ancY. Details of the Tokudaia Y-MRCA-originated locus in the T. muenninki genome are discussed below.
Fig. 4.
Tokudaia Y-MRCA-derived region in X chromosome of T. muenninki. a) Overview of the Tokudaia Y-chromosomal most recent common ancestor (Y-MRCA)-derived region identified in the X chromosome of T. muenninki. This region appears in two distinct locations: the Xhet-region and Xq-region2. b) Self-dot-plot alignment of the neo-ancX chromosome of T. muenninki. The central region of neo-ancX consists of a heterochromatic region composed of repetitive elements. c) Enlarged view of the Xhet-region (115.2 to 198.3 Mbp). Genes and BASD are shown as horizontal lines in the genome. Regions of Y-chromosome origin, X-chromosome origin, heterochromatin, and BASD are distinguished by their labels and positions. We identified 52 copies of partial Uba1y (pseudogene) within the heterochromatic region spanning approximately 80 Mbp. On the 3′ side of this region, intact Zfy, pseudogenes of Uba1y and Usp9y, and BASD, are located. The ancX region begins at Zfp300. At the bottom, a dot-plot alignment diagram shows how the highlighted 542 kbp basic repeat unit aligns within the region. The heterochromatic region is composed of irregular repeats of a basic unit that includes partial Uba1y. Genes labeled with “-ps” represent pseudogenes, while intact genes are indicated in bold. d) Enlarged view of the 3′ side of the Xhet-region (195.3 to 199.0 Mbp). This figure shows an enlargement of the area between the heterochromatic region and the start of the ancX region, as shown in b). Within the genomic region depicted by boxed outlines, Uba1y pseudogenes, intact Zfy, BASD, and Usp9y pseudogenes are aligned. Genes labeled with “-ps” represent pseudogenes, while intact genes are indicated in bold. e) Enlarged view of Xq-region2 (344.9 to 346.0 Mbp). Between the X-linked genes Ikbkg and Gab3, a 162 kbp T. muenninki-specific insertion of the Tokudaia Y-MRCA-originated sequence, containing intact Zfy, Tspy, and BASD, was identified (indicated by the lower arrow). This sequence, along with an approximately 300 kbp region containing it (indicated by the upper arrow), has undergone an inverted duplication. Genes labeled with “-ps” represent pseudogenes, while intact genes are indicated in bold.
Fig. 5.
Tokudaia Y-MRCA-derived region in the Y chromosome of T. muenninki. a) Overview of the Tokudaia Y-chromosomal most recent common ancestor (Y-MRCA)-derived region identified in the Y chromosome of T. muenninki. The Tokudaia Y-MRCA-derived region spans a broad ancY-het-region on the Y chromosome. In the ancY-het-region, the ancY sequence is divided into blocks 1 to 10 by the heterochromatic regions, whereas block 0 exists on the proximal side of the neo-Y region. Blocks 0 and 1 are separated by the neo-Y-region-derived sequences. b) Self-dot-plot alignment of the Y chromosome of T. muenninki. The Y chromosome includes the genomic region extending from the differentiated neo-Y region to the distal end of ancY, spanning upstream approximately 100 Mbp into the neo-sex chromosome, with minimal differences compared to the neo-X chromosome. The neo-Y and ancY regions are closely adjacent. The highly duplicated Tokudaia Y-MRCA-originated region is divided into ten blocks by heterochromatic regions composed of repetitive sequences, forming a mosaic structure between the two regions. Arrows at the top of the figure indicate the repeat units (three copies each) that constitute the palindromic structures. c) Enlarged view of Tokudaia Y-MRCA-derived region blocks in the ancY region. The enlarged view of blocks 0 to 10 displays the Y-linked genes and BASD present in each block. Identical genes are shown in matching labels, corresponding to those listed in supplementary table S3, Supplementary Material online. The 372 kbp region previously identified via bacterial artificial chromosome-based analysis is highlighted in the figure. Genes labeled with “-ps” represent pseudogenes, while intact genes are indicated in bold.
The X chromosome contains Y-linked genes in two regions: the central Xhet-region and the distal Xq-region2. The Xhet-region (115.2 to 198.3 Mbp) primarily consists of heterochromatic sequences, with a distinct 2.5 Mbp sequence on the 3′ side. Approximately 76% of this heterochromatic region is composed of irregular repeats of a 542 kbp basic unit, with 52 partial copies of Uba1y at the ends (Fig. 4b and supplementary fig. S10a to e, Supplementary Material online). The following 2.5 Mbp region contains intact Zfy, pseudogenes of Uba1y and Usp9y, and BASD (Fig. 4c). In Xq-region2, a T. muenninki-specific Tokudaia Y-MRCA-derived 162 kbp insertion, containing intact Zfy, Tspy, and BASD, was identified. This sequence, along with an approximately 300 kbp region containing it, underwent inverted duplication (Fig. 4d and supplementary fig. S10f and g, Supplementary Material online).
The ancY region of the Y chromosome exhibits an uneven distribution of Y-linked genes, which are organized into ten distinct blocks (blocks 1 to 10; Fig. 5, supplementary figs. S11a and S12 and supplementary table S3, Supplementary Material online). Notably, the longest inter-block region lies between blocks 7 and 8, where highly repetitive ∼120 kbp ancY-specific sequences are densely present (supplementary fig. S11b and c, Supplementary Material online). These sequences are also found between other inter-blocks, with a total of 481 copies spanning 36.4 Mbp—accounting for approximately 23% of the entire ancY region (supplementary fig. S11d to f, Supplementary Material online). Additionally, the regions spanning blocks 4–5, 5–6, and 6–7—including parts of the adjacent gene-containing regions—form relatively long palindromic structures totaling approximately 6 Mb (Fig. 5b and supplementary table S4, Supplementary Material online). While large palindromes are a well-known feature of human and mouse Y chromosomes (Shirleen et al. 2014; Rhie et al. 2023), neither these palindromes nor the abovementioned ∼120 kb repetitive sequences found in the heterochromatic clusters showed detectable sequence similarity to human or mouse Y chromosomes in BLASTN searches (>70% identity), suggesting that these elements are unique to T. muenninki.
Turning our attention to the gene content, in addition to the gene clusters located within blocks 1 to 10, we identified a distinct Y-linked gene cluster near the proximal end of the neo-Y chromosome, referred to as block 0. This block is separated from block 1 by neo-Y-derived sequences. Most blocks contain highly duplicated sequences with multiple intact or pseudogenized copies of Y-linked genes (supplementary table S3, Supplementary Material online). However, Kdm5d was detected only as a partial fragment (exons 11 to 17, 7 out of 26 exons) in the block 1, with a frameshift mutation in exon 14. No autosomal copy was identified. As our analysis includes only genes with at least 50% coverage, Kdm5d was considered absent in T. muenninki. Further details are provided in supplementary fig. S13, Supplementary Material online. Notably, blocks (1), 2, 3, and 4, as well as blocks 5, (6), 7, (8), and 9, form groups with similar Y-linked gene copy numbers and high genomic similarity. In contrast, block 0 contains only Ddx3y and Uty, including the 372 kbp sequence identified in a previous BAC-based study (Fig. 5c) (Murata et al. 2016). The Y-linked genes in block 0 are mostly single-copy and show little homology with other blocks, distinguishing them from the others.
We then look into the region surrounding block 0. This region (7.2 to 12.9 Mbp) contains repetitive sequences at both the 5′ (7.2 to 9.4 Mbp) and the 3′ (12.5 to 12.9 Mbp) ends (Fig. 6a). Notably, just downstream of the 3′ repetitive sequences lies a neo-Y-chromosome-derived 4.6 Mbp sequence, which continues into block 1. Alignment with the X chromosome (Fig. 6a, left) revealed that this 4.6 Mbp neo-Y sequence between blocks 0 and 1 underwent an inversion relative to the homologous neo-X sequence. Synteny with homologous regions in T. osimensis and T. tokunoshimensis suggests that the inversion occurred on the Y chromosome (supplementary fig. S14, Supplementary Material online). When the neo-Y region (7.2 to 17.5 Mbp) is inverted, the neo-Y-chromosome-derived region becomes continuous (Fig. 6a, right), aligning the repetitive sequences at the 5′ end of block 0 with those at the 5′ end of block 1 to form a continuous block 0–1 region. This suggests that the neo-Y region originally had the genomic structure shown in Fig. 6a (right) and later underwent inversion to form the current structure (Fig. 6a, left).
Fig. 6.
Comparative analysis of the block 0 surrounding region in the T. muenninki Y chromosome with the corresponding neo-X region. a) Comparative analysis of the T. muenninki Y chromosome blocks 0 to 1 surrounding region with the corresponding X chromosome region. The left panel shows a comparison of the current sequences. Upstream of block 0, a clear alignment between the neo-X and neo-Y regions is observed, while downstream, the neo-X region aligns with the Y chromosome region between block 0 and block 1 in an inverted orientation. Further downstream, the X chromosome forms heterochromatin, while the Y chromosome forms block 1. A self-dot-plot alignment of the corresponding Y chromosome region is shown at the bottom of the figure. In the right panel, the region from block 0 to just before block 1 (indicated by an arrow on the Y chromosome) is inverted. This operation connects the neo-X versus. neo-Y alignment directly up to the start of block 0, with the X chromosome forming heterochromatin and the Y chromosome displaying a continuous connection from block 0 to block 1. The dot-plot further confirms the continuity of repetitive sequences at the 5′ ends of both block 0 and block 1, forming a continuous repetitive sequence region. b) Distribution of heterozygous single nucleotide polymorphism (hetero-SNP) density across the entire X chromosome, excluding heterochromatin regions, along with a zoomed view of the 12.5 Mbp region surrounding the proximal end of the neo-sex chromosome. Data from the female individual are shown in the upper track, while data from the male individual are shown in the lower track. Below this expanded view, an alignment between neo-X versus. neo-Y (male) and neo-X versus. neo-X (female) are shown. Around the proximal end of the neo-sex chromosome (near 104.2 Mbp), the hetero-SNP density in the male sharply increases, while the hetero-SNP density in the female remains consistently low. Concurrently, the similarity between neo-X and neo-Y decreases, indicating areas of discontinuous differentiation. These areas were designated as Strata 0 (spanning from the distal end of the neo-sex chromosome to 104.2 Mbp) and Strata 1. While Strata 1 is further divided into two subregions (S1a and S1b) by block 0, no clear differences in hetero-SNP density were observed between them in the male. However, there was a slight tendency for higher hetero-SNP values in the male and lower neo-X versus. neo-Y identity near the heterochromatin. c) List of genes located in Strata 1 (neo-X and neo-Y regions). Tandem duplications of Tnp2 and Prm3 were observed on the neo-X side, whereas Bfar and Pla2g10 were deleted on the neo-Y side. Additionally, eight genes were pseudogenized on the neo-Y side, indicating degeneration within the Y side of the neo region.
In the male genome, heterozygous single nucleotide polymorphism (SNP) density and sequence identity between homologous chromosomes were examined across the X chromosome (Fig. 6b, blue line). A pronounced increase in heterozygous SNPs, along with a decrease in sequence identity, emerged near 104.2 Mbp, between Grin2a and Rpl39l, indicating the onset of sequence differentiation between the neo-X and neo-Y regions. In contrast, analysis of SNP density within a female individual (between two X chromosomes) (Fig. 6b, pink line) revealed uniformly low heterozygous SNP rates across the entire chromosome including neo-X region. These results suggest that recombination occurs between the neo-X and neo-Y regions from the distal end to 104.2 Mbp, defining this segment as the pseudoautosomal region (PAR, Strata 0), whereas the region beyond this point has undergone differentiation and no longer recombines. The differentiated region beyond 104.2 Mbp is referred to as Strata 1, which is further divided into two subregions the inversion described above into two subregions—Strata 1a (S1a) and Strata 1b (S1b)—by the inversion described earlier (Fig. 6b). However, no marked differences were observed between these two regions in terms of heterozygous SNP density or sequence divergence, suggesting that the inversion itself had limited impact on the overall degree of differentiation within Strata 1. Information on genes located in Strata 1 is presented in Fig. 6c. On the neo-Y side, eight genes have become pseudogenes with partial deletions, illustrating the ongoing degeneration of this region.
Analysis of BASD
In the previous sections, we examined the current state of Tokudaia Y-MRCA-originated genomic regions, identifying not only Y-linked genes but also BASDs as significant elements within these regions. To further characterize BASD, we conducted homology searches against known sequence databases. When we performed a nucleotide search of identified BASDs, as well as a translated search (ORF-based) against the nonredundant databases, we did not find any homologous sequences outside rodent genomes. Additionally, no significant matches to known repetitive elements were detected, suggesting that BASD is a rodent-specific sequence. Based on this result, we expanded our investigation into the genomes of nine rodent species to determine the distribution of BASDs beyond the Tokudaia species. The results showed that, except for Tokudaia species, 1 to 2 copies of BASD were found only in regions corresponding to the Xq-region1 of other rodent genomes, with no copies present in other chromosomal regions (supplementary fig. S15, Supplementary Material online). In T. osimensis and T. tokunoshimensis, BASD was also observed exclusively in Xq-region1, with three and four copies, respectively, as noted earlier (Fig. 3a). Conversely, in T. muenninki, the BASD was found not only at two locations within Xq-region1 but also in two partial sequences within Xq-region2 (Fig. 4d), one in the terminal ancX region of the Xhet-region (Fig. 4c), one in the Y chromosome block 0 region, and at 104 locations across blocks 1 to 10 (Fig. 5b, supplementary tables S3 and S5, and fig. S16, Supplementary Material online). This suggests that, while BASD is typically restricted to two sites within Xq-region1 across rodents, it has undergone duplication and translocation to regions harboring Y genes in Tokudaia species, particularly in T. muenninki, where it has extended beyond Xq-region1.
To further clarify how the BASD and its surrounding regions are distributed within the Tokudaia genus, we analyzed the evolutionary relationships based on BASD homology. Figure 7 and supplementary fig. S17, Supplementary Material online illustrate the phylogenetic tree of the identified BASDs, along with the alignment results for each BASD-surrounding region, against the two BASD-flanking sequences in T. muenninki, which are considered to retain the ancestral configuration. The tree shows a clade consisting first of mouse BASDs, followed by BASDs from T. muenninki's Xq-region1 (indicated by the vertical orange bar). Below this, BASDs found outside Xq-region1 in T. muenninki, as well as those found in Xq-region1 of T. osimensis and T. tokunoshimensis, form a large single clade, all of which are considered to be contained within the Tokudaia Y-MRCA-derived regions (indicated by the vertical blue bar). Additionally, a comparison with the ancestral-type Tm-BASD-flanking regions revealed that these BASDs aligned with much of the surrounding region of Tm-BASD002 (up to 1.8 kbp upstream [2α] and 10.8 kbp downstream [2γ]), suggesting that these sequences likely originated from a translocation of the ancestral Tm-BASD002-flanking region on the X chromosome to the Y chromosome (X-to-Y event), with no discrepancies. Furthermore, the upstream region of Tm-BASD001 corresponds to To-BASD001 and Tt-BASD001, while its downstream region aligns with To-BASD002, To-BASD003, and Tt-BASD003, confirming that the Tokudaia Y-MRCA-derived sequence was inserted between SB1 and SB2 in both T. osimensis and T. tokunoshimensis.
Fig. 7.
Phylogenetic relationships of the boundary-associated segmental duplications (BASDs) in Tokudaia and mouse genomes, with alignment to T. muenninki's ancestral BASDs (Tm-BASD001 and Tm-BASD002). a) Left: Phylogenetic tree showing the relationship among BASDs found in the genomes of the three Tokudaia species and mouse. Right: Regions around each BASD that align with the flanking sequences of the putative ancestral BASDs are distinguished based on their correspndence to Tm-BASD001 and Tm-BASD002. Among the BASDs identified in the ancY region, 93 were collapsed for clarity. The full tree with the expanded clade is available in supplementary fig. S17, Supplementary Material online. b) Based on the results shown in a), this panel illustrates the current configuration of sequences flanking each BASD, as well as the presumed ancestral state surrounding BASD001 prior to the insertion of Tokudaia Y-MRCA-derived sequences into the Xq-region1 in T. osimensis and T. tokunoshimensis. Dashed lines indicate Tokudaia Y-MRCA-derived segments. The close proximity of α, BASD, and γ supports a model in which these sequences formed a circular intermediate (eccDNA) prior to integration.
Further analysis of the downstream region of To-BASD001 revealed that it corresponds to the downstream region of Tm-BASD002 (2γ), while the upstream region of To-BASD002 corresponds to the upstream region of Tm-BASD002 (2α; Fig. 7b). This suggests that the inserted sequence between SB1 and SB2 in T. osimensis and T. tokunoshimensis has a structure of 2γ-[Y and autosome-derived sequence]-2α, with BASD sequences positioned at both insertion boundaries. Given that the original arrangement of these sequences was 2α-BASD-2γ, the question arises as to why they are now separated into BASD-2γ and 2α-BASD flanking the inserted sequence. One plausible explanation is that the 2α-BASD-2γ-[Y and autosome-derived sequences] initially formed an extrachromosomal circular DNA (eccDNA) (Zhao et al. 2022; Holt et al. 2023) intermediate, which subsequently integrated into the X chromosome via NAHR mediated by the BASD sequence.
Discussion
The Tokudaia genus represents a remarkable exception to the conserved XX/XY sex chromosome system prevalent in mammals. Through our chromosome-level genome assemblies and comparative analyses, we conducted a comprehensive exploration of the current Tokudaia Y-MRCA-derived regions in all three species and identified BASDs as key structural elements specifically present in these regions. Furthermore, by elucidating the evolutionary traces remaining in the genome mediated by BASDs, we shed light on the evolutionary trajectory of Tokudaia Y-MRCA-derived regions.
Based on these results, we propose the most plausible scenario for the evolution of Tokudaia Y-MRCA regions, which involves multiple translocation events, as summarized in Fig. 8. Initially, in the common ancestor of the genus Tokudaia, the BASD002 and its surrounding regions (α-β-γ, where β represents the BASD itself) from Xq-region1 were duplicated and translocated to Tokudaia Y-MRCA (Fig. 8-(1), X-to-Y event). After the divergence of T. muenninki, the SB6–SB5 region (Tokudaia Y-MRCA-originated region with an autosome-derived segment), including BASD, formed eccDNA in the common ancestor of T. osimensis and T. tokunoshimensis. This induced NAHR with BASD001, resulting in the insertion of Tokudaia Y-MRCA-originated sequences into the X chromosome and the replacement of the ancestral BASD001 with Y-derived BASD (Fig. 8-(2)). Subsequently, inversions and duplications formed SB5′, and after the divergence of T. osimensis and T. tokunoshimensis, further inversions led to their current genome structures (Fig. 8-(3) and (4)). The present hypothesis suggests two translocations: one from the X chromosome to the Y chromosome and the other from Y to X, a phenomenon similar to the formation of the cattle color-sidedness locus through two translocations via circular intermediates (Durkin et al. 2012). Additionally, it has been shown that the insertion sequence into the X chromosome contains not only Y-derived sequences but also fragments of autosomal origin. Given that 20% of eccDNA found in human germline cells is composed of multiple genomic segments (Henriksen et al. 2022), it is plausible that autosomal and Y-derived segments formed chimeric structures during eccDNA formation, subsequently co-integrated into the X chromosome.
Fig. 8.
Schematic diagram of Y chromosome-related evolutionary events inferred for the three Tokudaia species based on the results of previous analyses.
In T. muenninki (Fig. 8-(5)), autosomes fused with the X and Y chromosomes, already containing duplicated BASDs, forming sex chromosomes with large pseudoautosomal regions. The ancY-het-region contains Y-linked genes, such as Ddx3y and Uty, which are only found in block 0, suggesting that block 0 retains the ancestral state. Furthermore, as shown in the phylogenetic tree of BASDs (Fig. 7a), the BASD located in block 0 diverges earliest among all BASDs identified in the Tokudaia Y-MRCA-derived regions of T. muenninki. This strongly suggests that block 0 represents the initial site of BASD insertion during the earliest X-to-Y translocation event (Fig. 8-(1)).
Successive duplications of the ancestral Y regions likely formed blocks 1 to 10, while recombination suppression at the proximal end of the neo-sex chromosomes fostered the development of divergent regions. An inversion involving block 0 and the terminal neo-Y region may have contributed to the current neo-Y–ancY structure. The presence of multiple BASD-Zfy-Tspy sets in the Xq-region2 and ancY regions suggests that a portion of the Y chromosome translocated into Xq-region2.
The Xhet-region (the heterochromatic region between the neo-X chromosome and the ancestral X chromosome) likely serves as an intercalary heterochromatic block (IHB), preventing Xist-driven gene dosage compensation from affecting neo-X regions. IHBs are thought to form early after autosome-sex chromosome fusion. While IHBs have been reported in other rodents (Dobigny et al. 2004; Veyrunes et al. 2004; Deuve et al. 2006; Oliveira da Silva et al. 2022), specific IHB sequences were first identified in T. muenninki. Partial Uba1y copies were found within the repetitive elements comprising the Xhet-region, with Y-linked sequences, including BASD, located at the last 2.5 Mbp of the Xhet-region. These sequences may have been acquired through translocations between the X and Y chromosomes, with duplications forming the current IHBs spanning approximately 80 Mbp (Fig. 4c).
To further understand the integration of Tokudaia Y-MRCA-derived sequences into Xq-region1 and Xq-region2, we examined both the genes incorporated and the genomic regions where these sequences were inserted. Although T. osimensis and T. tokunoshimensis have lost their Y chromosomes, some genes originally encoded in Xq-region1, such as Zfy, Eif2s3y, Uty, Tspy, and Ddx3y, remained intact. These genes may have been preserved primarily due to their essential role in male development, as suggested by previous studies on Y-linked gene conservation across mammals (Bellott et al. 2014). The survival of dosage-sensitive genes on the Y chromosome has been linked to their function in transcriptional and translational regulation, highlighting their significance beyond male-specific roles (Bellott et al. 2014). The retention of Y-linked genes has also been observed in other rodents that have lost their Y chromosome. A comparative analysis of Y-linked genes across Ellobius species (Mulugeta et al. 2016), Microtus oregoni (Couger et al. 2021), and Tokudaia (this study) is summarized in Table 2. This comparison highlights that Eif2s3y is consistently retained in all examined species that have lost their Y chromosome, reinforcing its essential role in male fertility. Prior studies have shown that Eif2s3y is sufficient to drive spermatogonial differentiation, enabling the formation of haploid germ cells that are functional in assisted reproduction, even in the absence of other Y-linked genes (Yamauchi et al. 2014). Additionally, Zfy is retained in most species, although it has lost some exons in M. oregoni. This supports previous findings that highlight the importance of Zfy in spermatogenesis (Yamauchi et al. 2022). Interestingly, some of these genes, including Eif2s3y, are also present in the X chromosome (XM), which is shared by both sexes of M. oregoni (Table 2). Although their retention in females may be selectively neutral, current evidence strongly supports their essential role in male viability. After the divergence of the two Tokudaia species, duplication and inversion events were observed; however, the synteny of the seven Y-linked genes on SB5 was maintained, further supporting strong selective pressure on the X chromosome.
We next examined the genomic context of the insertion sites. The presence of BASD is a key factor in these regions. Structural rearrangements observed in both Xq-region1 and Xq-region2 across various species suggest that these regions are susceptible to structural variation and may have been receptive to the insertion of Tokudaia Y-MRCA-derived sequences (supplementary figs. S10f and S18, Supplementary Material online). Instability in the syntenic regions of Xq-region1 has also been observed in mice and humans (Warburton et al. 2004; Mueller et al. 2008; Swanepoel et al. 2020; Jackson et al. 2021), supporting the idea that structural flexibility facilitated rearrangements. While large segmental duplications are present in the Xq-region1 of humans, mice, and other species, they are spatially separated from BASD and exhibit lineage-specific variation in structure and gene content (supplementary fig. S18, Supplementary Material online), indicating that this region is structurally unstable and prone to recurrent rearrangements.
Despite these advances—namely, the identification of the initial X-to-Y translocation and the subsequent incorporation of Tokudaia Y-MRCA-derived regions into structurally dynamic areas of the X chromosome mediated by BASD—key questions remain. Chief among them is the functional role of BASD and its contribution to the structural stability of these chromosomes, as well as the precise molecular mechanisms driving the observed translocations. While our study focuses on elucidating how these chromosomal rearrangements have occurred, the ultimate evolutionary pressures that led to these changes remain an open question. Investigating the ecological, reproductive, and genetic factors influencing Y chromosome loss in Tokudaia will be crucial for future studies. Another major question is the novel sex determination system that arose in T. osimensis and T. tokunoshimensis. While we have characterized the genomic changes associated with this transition, the regulatory mechanisms governing male differentiation remain unclear. Investigating how these species compensate for the loss of the Y chromosome is essential for understanding the broader evolutionary forces driving sex chromosome transformation.
Previous studies on the creeping vole (M. oregoni) and Ellobius have provided key insights into the retention of Y-linked genes, but have primarily focused on gene-level analyses rather than large-scale chromosomal architecture. Structural analyses of genome reorganization following Y chromosome loss remain largely unexplored in these species. In contrast, our study leveraged high-quality, chromosome-level genome assemblies of three closely related Tokudaia species, enabling a detailed characterization of the structural rearrangements accompanying Y chromosome loss. Particularly, the ability to compare T. osimensis and T. tokunoshimensis, which diverged only ∼1 million years ago, has provided an unprecedented opportunity to track lineage-specific chromosomal changes within Y-derived regions. Further challenges include the difficulty of functional validation due to the endangered status of Tokudaia and the lack of an outgroup species with a typical XX/XY system, particularly one with a fully assembled Y chromosome genome. Addressing these gaps will require innovative approaches, such as structural modeling and comparative analyses with closely related rodent species.
In conclusion, this study provides a foundational genomic resource and a crucial step toward understanding the mechanisms and evolutionary implications of Y chromosome loss. By integrating detailed structural and comparative analyses, we establish Tokudaia as a key model for investigating rapid sex chromosome evolution and underscore the importance of further comparative studies to fully elucidate the genomic consequences of Y chromosome loss in mammals.
Materials and Methods
Genome Reconstruction of T. muenninki Using PacBio HiFi Reads
The whole genome assembly of T. muenninki was reconstructed by newly obtaining PacBio HiFi reads and assembling them together with previous data. The remaining DNA obtained during the analysis in a previous study was used for sequencing. Genomic DNA was sheared into fragment sizes ranging from 15 to 20 kb using the Megaruptor 3 system (Hologic, MA, USA). A HiFi library was prepared using a SMRTbell Prep Kit 3.0 (Pacific Biosciences, CA, USA), followed by size selection using the Blue Pippin system (Sage Science, MA, USA) to remove short fragments. The library was sequenced on the Revio system using a Revio polymerase kit (Pacific Biosciences, CA, USA) and a Revio sequencing plate (Pacific Biosciences, CA, USA) with 30-h movies per SMRT cell. A total of 201.5 Gb of HiFi reads were generated from two Revio SMRT cells and processed using the PacBio SMRT Link v13.1.0.221972 software.
In addition to the newly obtained HiFi reads, previously acquired PacBio CLR reads and Arima Hi-C reads from the same individual were used as input. Assembly was performed using Hifiasm v0.19.8 (Cheng et al. 2021), treating the PacBio CLR reads as ultra-long reads. Following this, Hi-C scaffolding was applied to achieve chromosome-level assembly. Examination of the input assembly contigs prior to Hi-C scaffolding revealed the presence of sex chromosome-derived contigs that were not included in the p_ctg output of the Hifiasm assembly. Consequently, these sex chromosome-derived contigs—identified in hap1_ctg and hap2_ctg based on Hi-C contact information—were reverted, and Hi-C scaffolding was then conducted using YaHS v1.2 (Zhou et al. 2023) with the Arima Hi-C reads. The Hi-C contact map was visualized using Juicebox v1.11.0828 (Robinson et al. 2018), and extensive manual curation was performed to fix mis-assemblies and mis-scaffoldings. Supplementary fig. S11, Supplementary Material online illustrates the Hi-C contact map constructed from the final sequences.
Chromosome-to-Chromosome Alignment and Prediction of Karyotype Evolution
For pairwise sequence alignments between the three Tokudaia species, the query sequences were first fragmented into 500 kbp segments using the “seqkit” sliding command (Shen et al. 2016) and aligned to the reference genome using minimap2 v2.23 with the -c option (Li 2018). Only primary alignments exceeding 100 kbp were visualized using a lyla-plot. Genomic alignments enabled the prediction of chromosome fusion events in each of the three species, as along with the karyotype of the common ancestors of T. osimensis and T. tokunoshimensis and the genus Tokudaia. The karyotypes of these species and their ancestors were visualized on a unified scale using the RIdeogram library in the R package (Hao et al. 2020).
Prediction of Y-Linked Genes
We identified Y-linked genes across the entire genome of T. osimensis by manually integrating homology- and RNA-seq-based predictions. For homology-based predictions, M. musculus protein sequences and previously reported T. osimensis proteins were splice-aligned to the genome using Spaln v2.3.3 (Iwata and Gotoh 2012) to predict gene structures. For RNA-seq-based predictions, RNA-seq reads from male and female brains (SRR8429976 to SRR8429982) were mapped to the genome using HISAT2 v. 2.2.1 (Kim et al. 2019), and the mapped reads were assembled into candidate transcripts using StringTie v2.2.0 (Kovaka et al. 2019). The open reading frames (ORFs) of the predicted transcripts were identified using TransDecoder (https://github.com/TransDecoder/TransDecoder). For T. tokunoshimensis, Y-linked gene structures were predicted by splice-aligning T. osimensis Y-linked genes using Spaln v2.3.3. To predict multicopy Y-linked genes from the T. muenninki genome, comprehensive gene structure prediction was conducted in two steps: (i) identification of homologous loci for the Y-linked genes and (ii) prediction of the exon–intron structure of the Y genes at each locus. First, the Y-linked genes of T. osimensis (or M. musculus when T. osimensis data were unavailable) were aligned to the genome using TBLASTN (Altschul et al. 1990). Regions extending 10 kbp upstream and downstream of the alignment segments, covering more than 50% of the query length, were extracted. For each locus, the exon–intron structure was predicted on the chromosome masked outside the homologous Y-gene region using Exonerate (Slater and Birney 2005). Finally, genes with the longest ORFs, covering at least 80% of the query length, were classified as intact, whereas others were considered pseudogenes.
Prediction of Genome Rearrangements in Y-Originated Loci of T. osimensis and T. tokunoshimensis Using Maximum Parsimony
We aimed to predict the insertion loci of the Y-derived sequence in the common ancestor of T. osimensis and T. tokunoshimensis, as well as the inversion events that occurred in their respective lineages. First, SBs were manually defined for the three Tokudaia species and mouse genomes, based on interspecies alignments using minimap2 v2-2.24 and MAFFT v7.490 (Katoh and Standley 2013). We then inferred the order and orientation of the SBs in the common ancestor of the two species using the maximum parsimony method, making the following three assumptions: (i) the order and orientation of the SBs before the insertion of the Y-derived sequence into the X chromosome of the common ancestor was SB1(+)–SB2(+)–SB3(+)–SB4(+); (ii) SB5 and SB6, originating from the Y chromosome, were inserted together at the same locus; and (iii) duplicated sequences, including SB5′, were excluded when calculating the edit distance. We considered three candidate insertion loci (between SB1 and SB2, between SB2 and SB3, and between SB3 and SB4) and eight possible combinations of the orientation and order of SB5 and SB6 as potential inserted sequence structures in the common ancestor of the two species. For these 24 combinations, we calculated the minimum number of inversions required to derive the current structure of T. osimensis and T. tokunoshimensis from the ancestral structure, calculated separately for each species using UniMoG (Hilker et al. 2012).
Homologous Search of BASDs and Phylogenetic Analysis
To identify BASDs, we aligned the genomic sequences of the mouse BASD (5,908 bp and 5,870 bp) as queries using BLASTN (Altschul et al. 1990). Alignments shorter than 500 bp were excluded, while split alignments within 3,000 bp of the genomic position were merged. Regions with alignments longer than 3,000 bp were considered potential BASDs. This process was repeated iteratively, using the candidate sequences as new queries. Sequences longer than 5,000 bp were designated as final BASDs for further analysis. Multiple sequence alignments of obtained BASDs were performed using MAFFT v7.4904, removing spurious sequences or poorly aligned regions with trimAI v1.4.16 (Capella-Gutiérrez et al. 2009). Phylogenetic analysis was performed using IQ-TREE v1.6.12 (-b 1000) (Nguyen et al. 2015) and visualized using iTOL (Letunic and Bork 2021).
Detection of the Basic Repeating Unit in Heterochromatin Regions of T. muenninki Sex Chromosomes
To investigate the repetitive units within the heterochromatic region between the neo-sex chromosome and ancestral X on the X chromosome of T. muenninki, a self-alignment of the X chromosome from 114 to 120 Mbp was performed using BLASTN. This region encompasses the terminal portion of the neo-sex chromosome and a portion of the adjacent heterochromatin. The alignment results were visualized using a dot-plot diagram. According to the dot-plot diagram, the repetitive unit (115,656,799 to 116,199,009 bp) of the heterochromatin was manually identified. Subsequently, the region from 114 to 199 Mbp of the X chromosome was aligned to the repetitive unit using BLASTN, and the alignment results were visualized in dot-plot diagrams. Similarly, to investigate the repetitive units within the heterochromatic region of the Y chromosome, a self-alignment of the Y chromosome from 104.9 to 107.0 Mbp, corresponding to the heterochromatic region between blocks 7 and 8, was performed using BLASTN. The entire region from 90 to 122 Mbp of the Y chromosome was aligned to a manually determined repetitive unit (104,993,405 to 105,113,388 bp) using BLASTN, and the alignment results were visualized in dot-plot diagrams.
Detection of Heterozygous SNPs on the Neo-Sex Chromosome of T. muenninki
Illumina reads were mapped to the T. muenninki genome, excluding the Y chromosome, using bwa-mem2 v2.2.1 (Vasimuddin et al. 2019). Reads with less than 90% identity or less than 80% alignment coverage were filtered out using the Samtools view command (Li et al. 2009). SNP calling was performed using Bcftools v1.13 (Danecek et al. 2021) with the “mpileup” command (parameter: -d 200), followed by the “call” command with the -m option. Heterozygous SNPs were selected based on the following criteria: single nucleotide substitutions, allele frequency (AF) between 0.25 and 0.75, and read depth between 21 (half of the mode) and 65 (1.5 times the mode).
Gene Prediction of the Neo-Sex Chromosome S1 Region in T. muenninki
We predicted gene structures within the differentiation regions (Strata 1) of the neo-sex chromosomes by combining homology- and RNA-seq-based approaches. For homology-based prediction, the protein sequences from M. musculus, Rattus rattus, and previously annotated T. muenninki sequences were splice-aligned to the sex chromosomes using Spaln v2.3.3 to predict gene structures. For the RNA-seq-based prediction, RNA-seq reads from the male brain, liver, and testis (DRR059293 to DRR059295), as well as from male and female fibroblasts (DRR059296 to DRR059302) of T. muenninki, were mapped to the sex chromosomes using HISAT2 v. 2.2.1. The mapped reads were assembled into candidate transcripts using StringTie v2.2.0, and ORFs of the predicted transcripts were identified using TransDecoder.
Supplementary Material
Acknowledgments
We thank the technical staff at the National Institute of Genetics and Itoh Laboratory for their contributions to library preparation, sequencing, and data management.
Contributor Information
Miki Okuno, Division of Microbiology, Department of Infectious Medicine, Kurume University School of Medicine, Kurume, Fukuoka 830-0011, Japan.
Kentaro Matsuoka, School of Life Science and Technology, Institute of Science Tokyo, Tokyo 152-8550, Japan.
Yuta Mochimaru, School of Life Science and Technology, Institute of Science Tokyo, Tokyo 152-8550, Japan.
Takahiro Yamabe, School of Life Science and Technology, Institute of Science Tokyo, Tokyo 152-8550, Japan.
Mayou Okano, School of Life Science and Technology, Institute of Science Tokyo, Tokyo 152-8550, Japan.
Takamichi Jogahara, Faculty of Law, Economics and Management, Okinawa University, Naha, Okinawa 902-8521, Japan.
Atsushi Toyoda, Comparative Genomics Laboratory, National Institute of Genetics, Mishima, Shizuoka 411-8540, Japan; Advanced Genomics Center, National Institute of Genetics, Mishima, Shizuoka 411-8540, Japan.
Asato Kuroiwa, Reproductive and Developmental Science, Biosystems Science Course, Graduate School of Life Science, Hokkaido University, Sapporo, Hokkaido 060-0810, Japan; Division of Reproductive and Developmental Biology, Department of Biological Sciences, Faculty of Science, Hokkaido University, Sapporo, Hokkaido 060-0810, Japan.
Takehiko Itoh, School of Life Science and Technology, Institute of Science Tokyo, Tokyo 152-8550, Japan.
Supplementary Material
Supplementary material is available at Molecular Biology and Evolution online.
Author Contributions
Mi.O., A.T., A.K., and T.I. conceived the study. T.J. collected samples. A.T. performed DNA extraction and sequencing. Mi.O., K.M., Y.M., T.Y., Ma.O., and T.I. contributed to the data analysis. Mi.O., A.K., and T.I. wrote the manuscript. All authors reviewed, edited, and approved the final version of the manuscript.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers 16H06279 (PAGS), 22H04925 (PAGS), 22H02598, and 23K18093.
Data Availability
The HiFi sequencing data for T. muenninki have been submitted to DDBJ under accession numbers DRR613121 and DRR613122. The genome assemblies for T. muenninki have been deposited in DDBJ under accession numbers BAAFZZ010000001 to BAAFZZ010000034.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990:215(3):403–410. 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Arakawa Y, Nishida-Umehara C, Matsuda Y, Sutou S, Suzuki H. X-chromosomal localization of mammalian Y-linked genes in two XO species of the Ryukyu spiny rat. Cytogenet Genome Res. 2002:99(1-4):303–309. 10.1159/000071608. [DOI] [PubMed] [Google Scholar]
- Bailey JA, Eichler EE. Primate segmental duplications: crucibles of evolution, diversity and disease. Nat Rev Genet. 2006:7(7):552–564. 10.1038/nrg1895. [DOI] [PubMed] [Google Scholar]
- Bellott DW, Hughes JF, Skaletsky H, Brown LG, Pyntikova T, Cho T-J, Koutseva N, Zaghlul S, Graves T, Rock S, et al. Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators. Nature. 2014:508(7497):494–499. 10.1038/nature13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009:25(15):1972–1973. 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Concepcion GT, Feng X, Zhang H, Li H. Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat Methods. 2021:18(2):170–175. 10.1038/s41592-020-01056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couger MB, Roy SW, Anderson N, Gozashti L, Pirro S, Millward LS, Kim M, Kilburn D, Liu KJ, Wilson TM, et al. Sex chromosome transformation and the origin of a male-specific X chromosome in the creeping vole. Science (1979). 2021:372:592–600. 10.1126/science.abg7019. [DOI] [PubMed] [Google Scholar]
- Danecek P, Bonfield JK, Liddle J, Marshall J, Ohan V, Pollard MO, Whitwham A, Keane T, McCarthy SA, Davies RM, et al. Twelve years of SAMtools and BCFtools. Gigascience. 2021:10(2):giab008. 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuve JL, Bennett NC, O’Brien PCM, Ferguson-Smith MA, Faulkes CG, Britton-Davidian J, Robinson TJ. Complex evolution of X and Y autosomal translocations in the giant mole-rat, Cryptomys mechowi (Bathyergidae). Chromosome Res. 2006:14(6):681–691. 10.1007/s10577-006-1080-3. [DOI] [PubMed] [Google Scholar]
- Dobigny G, Ozouf-Costaz C, Bonillo C, Volobouev V. Viability of X-autosome translocations in mammals: an epigenomic hypothesis from a rodent case-study. Chromosoma. 2004:113(1):34–41. 10.1007/s00412-004-0292-6. [DOI] [PubMed] [Google Scholar]
- Durkin K, Coppieters W, Drögemüller C, Ahariz N, Cambisano N, Druet T, Fasquelle C, Haile A, Horin P, Huang L, et al. Serial translocation by means of circular intermediates underlies colour sidedness in cattle. Nature. 2012:482(7383):81–84.. 10.1038/nature10757. [DOI] [PubMed] [Google Scholar]
- Goodfellow PN, Lovell-Badge R. SRY and sex determination in mammals. Annu Rev Genet. 1993:27(1):71–92. 10.1146/annurev.ge.27.120193.000443. [DOI] [PubMed] [Google Scholar]
- Hao Z, Lv D, Ge Y, Shi J, Weijers D, Yu G, Chen J. RIdeogram: drawing SVG graphics to visualize and map genome-wide data on the idiograms. PeerJ Comput Sci. 2020:6:e251. 10.7717/peerj-cs.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen RA, Jenjaroenpun P, Sjøstrøm IB, Jensen KR, Prada-Luengo I, Wongsurawat T, Nookaew I, Regenberg B. Circular DNA in the human germline and its association with recombination. Mol Cell. 2022:82(1):209–217.e7. 10.1016/j.molcel.2021.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilker R, Sickinger C, Pedersen CNS, Stoye J. UniMoG—a unifying framework for genomic distance calculation and sorting based on DCJ. Bioinformatics. 2012:28(19):2509–2511. 10.1093/bioinformatics/bts440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S, Arrey G, Regenberg B. Did circular DNA shape the evolution of mammalian genomes? Trends Biochem Sci. 2023:48(4):317–320. 10.1016/j.tibs.2022.09.010. [DOI] [PubMed] [Google Scholar]
- Honda T, Suzuki H, Itoh M. An unusual sex chromosome constitution found in the Amami spinous country-rat. Tokudaia osimensis osimensis. Jpn J Genet. 1977:52:247–249. 10.1266/JJG.52.247. [DOI] [Google Scholar]
- Honda T, Suzuki H, Itoh M, Hayashi K. Karyotypical differences of the Amami spinous country-rats. Tokudaia osimensis osimensis obtained from two neighbouring islands. Jpn J Genet. 1978:53:297–299. 10.1266/jjg.53.297. [DOI] [Google Scholar]
- IUCN . 2024. The IUCN red list of threatened species. Version 2024-1. [Internet]. Available from: https://www.iucnredlist.org.
- Iwata H, Gotoh O. Benchmarking spliced alignment programs including Spaln2, an extended version of Spaln that incorporates additional species-specific features. Nucleic Acids Res. 2012:40(20):e161. 10.1093/nar/gks708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EK, Bellott DW, Cho T-J, Skaletsky H, Hughes JF, Pyntikova T, Page DC. Large palindromes on the primate X chromosome are preserved by natural selection. Genome Res. 2021:31(8):1337–1352. 10.1101/gr.275188.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM. MAFFT Multiple Sequence Alignment Software Version 7: improvements in performance and usability. Mol Biol Evol. 2013:30(4):772–780. 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol. 2019:37(8):907–915. 10.1038/s41587-019-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Yamada F, Hashimoto T, Abe S, Matsuda Y, Kuroiwa A. Exceptional minute sex-specific region in the X0 mammal, Ryukyu spiny rat. Chromosome Res. 2007:15(2):175–187. 10.1007/s10577-006-1093-y. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Yamada F, Hashimoto T, Abe S, Matsuda Y, Kuroiwa A. Centromere repositioning in the X chromosome of XO/XO mammals, Ryukyu spiny rat. Chromosome Res. 2008:16(4):587–593. 10.1007/s10577-008-1199-5. [DOI] [PubMed] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991:351(6322):117–121. 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- Kovaka S, Zimin A V, Pertea GM, Razaghi R, Salzberg SL, Pertea M. Transcriptome assembly from long-read RNA-seq alignments with StringTie2. Genome Biol. 2019:20(1):278. 10.1186/s13059-019-1910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroiwa A, Ishiguchi Y, Yamada F, Shintaro A, Matsuda Y. The process of a Y-loss event in an XO/XO mammal, the Ryukyu spiny rat. Chromosoma. 2010:119(5):519–526. 10.1007/s00412-010-0275-8. [DOI] [PubMed] [Google Scholar]
- Letunic I, Bork P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021:49(W1):W293–W296. 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics. 2018:34(18):3094–3100. 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, Subgroup 1000 Genome Project Data Processing . The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009:25(16):2078–2079. 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Song S, Zhang J. Where are the formerly Y-linked genes in the Ryukyu spiny rat that has lost its Y chromosome? Genome Biol Evol. 2024:16(3):evae046. 10.1093/gbe/evae046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthey R. Les Chromosomes des Muridae. Révision critique et matériaux nouveaux pour servir à l’histoire de l’évolution chromosomique chez ces rongeurs. Revue suisse de zoologie. 1953:60(4):225–283. https://www.biodiversitylibrary.org/part/215411. [Google Scholar]
- Mueller JL, Mahadevaiah SK, Park PJ, Warburton PE, Page DC, Turner JMA. The mouse X chromosome is enriched for multicopy testis genes showing postmeiotic expression. Nat Genet. 2008:40(6):794–799. 10.1038/ng.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulugeta E, Wassenaar E, Sleddens-Linkels E, Van Ijcken WFJ, Heard E, Grootegoed JA, Just W, Gribnau J, Baarends WM. Genomes of Ellobius species provide insight into the evolutionary dynamics of mammalian sex chromosomes. Genome Res. 2016:26(9):1202–1210. 10.1101/gr.201665.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata C, Kuroki Y, Imoto I, Kuroiwa A. Ancestral Y-linked genes were maintained by translocation to the X and Y chromosomes fused to an autosomal pair in the Okinawa spiny rat Tokudaia muenninki. Chromosome Res. 2016:24(3):407–419. 10.1007/s10577-016-9531-y. [DOI] [PubMed] [Google Scholar]
- Murata C, Kuroki Y, Imoto I, Tsukahara M, Ikejiri N, Kuroiwa A. Initiation of recombination suppression and PAR formation during the early stages of neo-sex chromosome differentiation in the Okinawa spiny rat, Tokudaia muenninki. BMC Evol Biol. 2015:15(1):234. 10.1186/s12862-015-0514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata C, Yamada F, Kawauchi N, Matsuda Y, Kuroiwa A. Multiple copies of SRY on the large Y chromosome of the Okinawa spiny rat, Tokudaia muenninki. Chromosome Res. 2010:18(6):623–634. 10.1007/s10577-010-9142-y. [DOI] [PubMed] [Google Scholar]
- Murata C, Yamada F, Kawauchi N, Matsuda Y, Kuroiwa A. The Y chromosome of the Okinawa spiny rat, Tokudaia muenninki, was rescued through fusion with an autosome. Chromosome Res. 2012:20(1):111–125. 10.1007/s10577-011-9268-6. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Kuroiwa A, Nishida-Umehara C, Matsubara K, Yamada F, Matsuda Y. Comparative chromosome painting map between two Ryukyu spiny rat species, Tokudaia osimensis and Tokudaia tokunoshimensis (Muridae, Rodentia). Chromosome Res. 2007:15(6):799–806. 10.1007/s10577-007-1163-9. [DOI] [PubMed] [Google Scholar]
- Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015:32(1):268–274. 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno M, Mochimaru Y, Matsuoka K, Yamabe T, Matiz-Ceron L, Jogahara T, Toyoda A, Kuroiwa A, Itoh T. Chromosomal-level assembly of Tokudaia osimensis, Tokudaia tokunoshimensis, and Tokudaia muenninki genomes. Sci Data. 2023:10(1):927. 10.1038/s41597-023-02845-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira da Silva W, Rosa CC, Ferguson-Smith MA, O’Brien PCM, Saldanha J, Rossi RV, Pieczarka JC, Nagamachi CY. The emergence of a new sex-system (XX/XY1Y2) suggests a species complex in the “monotypic” rodent Oecomys auyantepui (Rodentia, Sigmodontinae). Sci Rep. 2022:12(1):8690. 10.1038/s41598-022-12706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhie A, Nurk S, Cechova M, Hoyt SJ, Taylor DJ, Altemose N, Hook PW, Koren S, Rautiainen M, Alexandrov IA. The complete sequence of a human Y chromosome. Nature. 2023:621(7978):344–354. 10.1038/s41586-023-06457-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JT, Turner D, Durand NC, Thorvaldsdóttir H, Mesirov JP, Aiden EL. Juicebox.js provides a cloud-based visualization system for Hi-C data. Cell Syst. 2018:6(2):256–258.e1. 10.1016/j.cels.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Le S, Li Y, Hu F. SeqKit: a cross-platform and ultrafast toolkit for FASTA/Q file manipulation. PLoS One. 2016:11(10):e0163962. 10.1371/journal.pone.0163962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf A-M, Lovell-Badge R, Goodfellow PN. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990:346(6281):240–244. 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- Slater GSC, Birney E. Automated generation of heuristics for biological sequence comparison. BMC Bioinformatics. 2005:6(1):31. 10.1186/1471-2105-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirleen SYQ, Alföldi J, Pyntikova T, Brown LG, Graves T, Minx PJ, Fulton RS, Kremitzki C, Koutseva N, Mueller JL. Sequencing the mouse Y chromosome reveals convergent gene acquisition and amplification on both sex chromosomes. Cell. 2014:159(4):800–813. 10.1016/j.cell.2014.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soullier S, Hanni C, Catzeflis F, Berta P, Laudet V. Male sex determination in the spiny rat Tokudaia osimensis (Rodentia: Muridae) is not Sry dependent. Mamm Genome. 1998:9(7):590–592. 10.1007/s003359900823. [DOI] [PubMed] [Google Scholar]
- Sutou S, Mitsui Y, Tsuchiya K. Sex determination without the Y chromosome in two Japanese rodents Tokudaia osimensis osimensis and Tokudaia osimensis spp. Mamm Genome. 2001:12(1):17–21. 10.1007/s003350010228. [DOI] [PubMed] [Google Scholar]
- Swanepoel CM, Gerlinger ER, Mueller JL. Large X-linked palindromes undergo arm-to-arm gene conversion across Mus lineages. Mol Biol Evol. 2020:37(7):1979–1985. 10.1093/molbev/msaa059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao M, Ogawa Y, Takada S, Kajitani R, Okuno M, Mochimaru Y, Matsuoka K, Itoh T, Toyoda A, Kono T, et al. Turnover of mammal sex chromosomes in the Sry-deficient Amami spiny rat is due to male-specific upregulation of Sox9. Proc Natl Acad Sci U S A. 2022:119(49):e2211574119. 10.1073/pnas.2211574119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasimuddin M, Misra S, Li H, Aluru S. Efficient architecture-aware acceleration of BWA-MEM for multicore systems. In: Proceedings of the 2019 IEEE International Parallel and Distributed Processing Symposium (IPDPS). Rio de Janeiro (Brazil): IEEE; 2019. p. 314–324. 10.1109/IPDPS.2019.00041. [DOI] [Google Scholar]
- Veyrunes F, Catalan J, Sicard B, Robinson TJ, Duplantier J-M, Granjon L, Dobigny G, Britton-Davidian J. Autosome and sex chromosome diversity among the African pygmy mice, subgenus Nannomys (Murinae; Mus). Chromosome Res. 2004:12(4):369–382. 10.1023/B:CHRO.0000034098.09885.e6. [DOI] [PubMed] [Google Scholar]
- Warburton PE, Giordano J, Cheung F, Gelfand Y, Benson G. Inverted repeat structure of the human genome: the X-chromosome contains a preponderance of large, highly homologous inverted repeats that contain testes genes. Genome Res. 2004:14(10a):1861–1869. 10.1101/gr.2542904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi Y, Matsumura T, Bakse J, Holmlund H, Blanchet G, Carrot E, Ikawa M, Ward MA. Loss of mouse Y chromosome gene Zfy1 and Zfy2 leads to spermatogenesis impairment, sperm defects, and infertility. Biol Reprod. 2022:106(6):1312–1326. 10.1093/biolre/ioac057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi Y, Riel JM, Stoytcheva Z, Ward MA. Two Y genes can replace the entire Y chromosome for assisted reproduction in the mouse. Science (1979). 2014:343:69–72. 10.1126/science.1242544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yu L, Zhang S, Su X, Zhou X. Extrachromosomal circular DNA: current status and future prospects. Elife. 2022:11:e81412. 10.7554/eLife.81412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, McCarthy SA, Durbin R. YaHS: yet another Hi-C scaffolding tool. Bioinformatics. 2023:39(1):btac808. 10.1093/bioinformatics/btac808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The HiFi sequencing data for T. muenninki have been submitted to DDBJ under accession numbers DRR613121 and DRR613122. The genome assemblies for T. muenninki have been deposited in DDBJ under accession numbers BAAFZZ010000001 to BAAFZZ010000034.