Abstract
The majority of non-NF2 schwannomatosis (non-NF2 SWN) patients experience debilitating pain. Yet, it is not known why only some schwannomas cause pain or whether mutations in SWN-related genes, (SMARCB1 or LZTR1) differentially influence pain signaling pathways. Here, we established cell lines from non-NF2 SWN tumors resected from patients with varying degrees of pain and bearing mutations in different SWN-related genes. Compared with conditioned medium (CM) collected from “nonpainful” SWN tumors, CM from “painful” SWN tumors contained elevated levels of specific inflammatory cytokines (IL-6, IL-8, VEGF), and was able to enhance sensory neuron responsiveness to noxious TRPV1 and TRPA1 agonists in vitro. In in vivo studies, injection of CM from painful non-NF2 SWN into the hind paws of healthy mice evoked both more acute pain behavior and greater enhancement of mechanical stimulus-evoked behavioral responses than did CM from nonpainful non-NF2 SWN. Furthermore, the behavioral effects of painful CM differed as a function of the SWN-related gene mutations identified in the tumors of origin. Painful SMARCB1 mutant CM, for example, sensitized mice to mechanical stimulation at low forces, compared to non-painful tumor CM and control media, but this effect waned over time. In contrast, CM from a painful tumor with no detectable germline mutation in NF2, SMARCB1 or LZTR1 caused the greatest increase in responsiveness to low mechanical forces and this effect lasted for 2 days post-injection. These experiments establish a paradigm for examining the mechanisms by which painful SWN tumors bearing different mutations produce their sensory effects and will thus facilitate better understanding and, potentially, treatment of the pain endured by non-NF2 SWN patients.
Subject terms: Molecular biology, Neuroscience
Introduction
Patients with non-NF2 schwannomatosis (non-NF2-SWN) develop multiple tumors along major peripheral nerves1. Non-NF2 schwannomatosis is a disease that manifests in many cases as a pain syndrome. Often patients experience pain prior to the detection of a palpable mass. Pain may or may not be directly related to the size or location of the tumor, and not all tumors are painful2. Surgical resection is the current standard of care for patients who harbor painful schwannomas, however tumor removal may not provide lasting pain relief due to tumor growth and is complicated by overall tumor burden3. Many patients have been trialed on up to 10 different medications for pain with little relief4. We do not understand the etiology of non-NF2 schwannomatosis related pain. Neuropathic, nociceptive, and inflammatory pain have all been described by patients5 and different symptoms of pain have been described even within a patient6.
To complicate matters, schwannomatosis as a disease entity has recently been divided into subgroups based on gene mutations found in patients with multiple schwannomas7. The most common form of heritable SWN is NF2-related schwannomatosis, in which patients harbor germline mutations in the NF2 tumor suppressor gene. The features of NF2-related SWN include development of bilateral vestibular schwannomas (acoustic neuromas) in addition to peripheral nerve schwannomas, neurological symptoms related to hearing and balance, and a decrease in life expectancy8. NF2-related SWN typically presents with hearing loss, tinnitus, and balance problems. Non-NF2 SWN is a previously stated, a disease that results in a multiple tumor phenotype along peripheral nerves where most, but not all schwannomas cause pain. Non-NF2 related SWN is the focus of our work, specifically examining painful phenotypes and underlying causes of pain.
Mutations in either of two genes on chromosome 22q11 (LZTR1 or SMARCB1) account for ~ 90% of familial non-NF2 schwannomatosis. In sporadic disease, these mutations account for ~ 45% of cases. Patients harbor mutations in either SMARCB1 or LZTR1 but not both7,8. Schwannomatosis is therefore classified as 1) SMARCB1 related, 2) LZTR1 related, or 3) Schwannomatosis Not Elsewhere Classified (NEC). The NEC group does not harbor a mutation in SMARCB1, LZTR1, or NF2 in the germline. Verification of mutation status in 2 separate tumors, if available, is needed to rule out mosaic NF2-SWN in these patients. It is currently unknown whether mutation status influences the painful phenotype of non-NF2 schwannomatosis, although it has been suggested that patients with mutations in LZTR1 express an increased incidence of pain9.
Due to the heterogeneity of the painful phenotype seen between and within non-NF2 schwannomatosis patients, we speculate that there is an alteration occurring in the schwannoma itself and that this alters the behavior of nociceptive neurons. We are particularly interested in how the tumors’ “secretome” affects sensory neurons. To test our hypothesis, we established immortalized (SV40 large T antigen-transfected) cell lines from resected tumors from non-NF2 SWN patients. Tumors were selected based on varying degrees of patient reported pain (no, mild, or severe pain) using the Visual Analog Scale (0–10). Cell lines demonstrated the same gene expression as the tumors from which they were derived, as confirmed by Illumina HT-12 microarray expression analysis10. In our previous study, we found that conditioned medium (CM) collected from painful SWN tumors, but not that from nonpainful SWN tumors, contained increased amounts of multiple cytokines, upregulated the expression of pain-associated genes in cultured dorsal root ganglion (DRG) neurons, and increased the responsiveness of these neurons to noxious agonists for transient receptor potential vanilloid 1 (TRPV1) and transient receptor potential ankyrin 1 (TRPA1) channels in vitro11.
Our prior in vitro studies showed convincing evidence that substances secreted by SWN tumors sensitize neurons and alter neuronal gene expression. However, they did not address whether this might translate into pain alterations in vivo. In this study, using well-established methods to quantify pain levels in healthy mice, we therefore assessed the effects of non- NF2 SWN CM on acute and mechanically-evoked pain behaviors. In addition, we examined the influence of non- NF2 SWN mutations on cytokine secretion in tumor CM and on the sensitivity of CM-treated sensory neurons to TRPA1 and TRPV1 agonists. This study expands our previously published in vitro findings and validates the notion of mechanistic heterogeneity in the pain experienced in non-NF2 schwannomatosis.
Results
Our lab collects tumor samples from patients with schwannomatosis that require surgery. We have a well- established, annotated tumor bank of schwannomas from non-NF2 schwanonnmatosis patients, with varying degrees of pain. Prior to surgery, patients rate their pain using a 0–10 scale using the Visual Analogue Scale (VAS) to measure pain intensity. Sometimes patients with SWN have tumors removed due to reasons that are not related to pain, such as increased growth rate. We consider those tumors to be in the “non-painful” group. Also, we include tumors where the patient indicated their pain to be less than 3 in the “non-painful” category12. Patients who undergo tumor removal specifically for pain reduction and report a pain score of greater than 7 as “painful”. We group the collected tumors this way to detect potential targets that are the most meaningful.
Painful non-NF2 SWN tumor CM contains elevated levels of cytokines and chemokines
In our prior study11, we published a qualitative description of elevated levels of cytokines in CM from painful (n = 4) and non-painful (n = 3) tumors using the Proteome Profiler Human Cytokine XL Array (R&D Systems). To validate and further explore these differences, we narrowed down our analysis to 9 candidates (CCL2, IL-6, IL-8, VEGF, GDF-15, CXCL1, CXCL5, CCL20 and GM-CSF) indicated by the cytokine array to be highly expressed and performed quantitative ELISAs on an expanded cohort of SWN tumor CMs (Painful CM n = 12 samples; Non-painful CM n = 8 samples) (Fig. 1). These assays revealed significantly higher levels of IL-6 (p = 0.03), IL-8 (p = 0.01), VEGF (p = 0.008) and GDF-15 (p = 0.01) in painful CM, compared with non-painful CM (Fig. 1). These findings both support and extend our observation of elevated cytokine release by painful SWN tumors. As many of these cytokines have been previously linked to pain, they also provide a plausible mechanism for nociceptor sensitization by painful non-NF2 SWN CM.
Fig. 1.
Painful Schwannoma CM contains elevated levels of secreted cytokines and chemokines. Painful CM (n = 12 samples, gray bars) and non-painful CM (n = 8 samples, black bars) were examined by ELISA to measure amounts of specific cytokines. CCL2, IL-6, IL-8, VEGF, GDF-15, CXCL1, CXCl5, CCl20 and GM-CSF were tested. Painful CM contains significantly higher levels of IL-6 (p = 0.03), IL-8 (p = 0.01), VEGF (p = 0.008) and GDF-15 (p = 0.01). Unpaired t-test with Welch’s correction was used to determine significance.
Conditioned media from painful schwannomas causes an increase in pain behaviors in mice
Our prior analysis of non-NF2 SWN CM effects was confined to in vitro assays11. To assess the effects of non-NF2 SWN CM in vivo, we therefore injected one hindpaw of healthy C57Bl6 mice with conditioned media from painful (n = 40 mice) or non-painful tumors (n = 30 mice), or with non-conditioned medium (Dulbecco’s modified eagle’s medium/10% FBS/2uM forskolin; n = 40 mice). We utilized media from 4 separate painful tumors, and 3 separate non-painful tumors that had been analyzed in our prior in vitro study11. To test for acute pain responses to CM, mice were video recorded for 10 min following injection. Mice that received injections of CM from non-painful tumors showed increased paw licking or flinching, compared to those injected with control media (p = 0.0002). Painful CM caused mice to exhibit an even greater acute behavioral response compared to control media (p < 0.0001) and non-painful tumor CM (p = 0.01) (Fig. 2a).
Fig. 2.
Conditioned media from painful SWN tumors cause increased pain behaviors upon intra- plantar paw injection. Ten microliters of CM were injected into the hindpaw of C57black6 mice. CM from 4 painful tumors n = 40 mice and CM from 3 non-painful tumors n = 30 mice were tested. Control media (non-conditioned Schwann cell media, DMEM 10% FBS, and 2% forskolin) was injected into an additional 40 mice.(a)Acute pain response was assessed by examining the number of times a mouse licked or flinched the affected paw. CM from painful tumors significantly increased the number of licks/flinches over a 10-min observation period compared to non-painful CM (p = 0.01) and control media (p < 0.0001). Brown-Forsythe ANOVA test with Dunnett’s T3 multiple comparisons was used to determine significance (b). Response to evoked mechanical pain was examined using Von Frey filaments. Increasing forces from 0.02 g to 1 g were tested. Each filament was tested 6 times. The percent response was calculated for each mouse at each force. Response was measured prior to injection and one hour after the final injection. Painful CM induced an increased response to light touch (black squares) compared to control media (white squares) at all forces less than 0.4 g (#) Painful media caused increased response compared to non-painful CM (gray squares) at 0.07 g (24.6 + /- SEM 1.76 vs 14.44 + /- SEM 2.0 p = 0.007*) and 0.16 g (30.16 + /- SEM 1.47 vs 21.6 + /- SEM 3.0 p = 0.02*). Two-way ANOVA with Dunnett’s multiple comparisons test was used to determine significance between groups at different forces.
Following the observation period, and to simulate our in vitro CM sensitization study, mice were injected once daily with CM for two additional days. One hour after the final injection, the mice were examined for mechanical sensitivity using the von Frey filament assay. Pre-injection, mice exhibited the expected pattern of increasing response frequency with increasing stimulus force. Following injection, painful CM treated mice exhibited hypersensitivity to lower forces (0.02 g, 0.04 g, 0.07 g, and 0.16 g), compared with those injected with control media or non-painful CM (Fig. 2b; Supplementary Fig. 1).
In vitro and in vivo response to painful and non-painful CM segregated by mutation group
Effect of SMARCB1, LZTR1, and NEC mutant tumor CM on cultured DRG neurons.
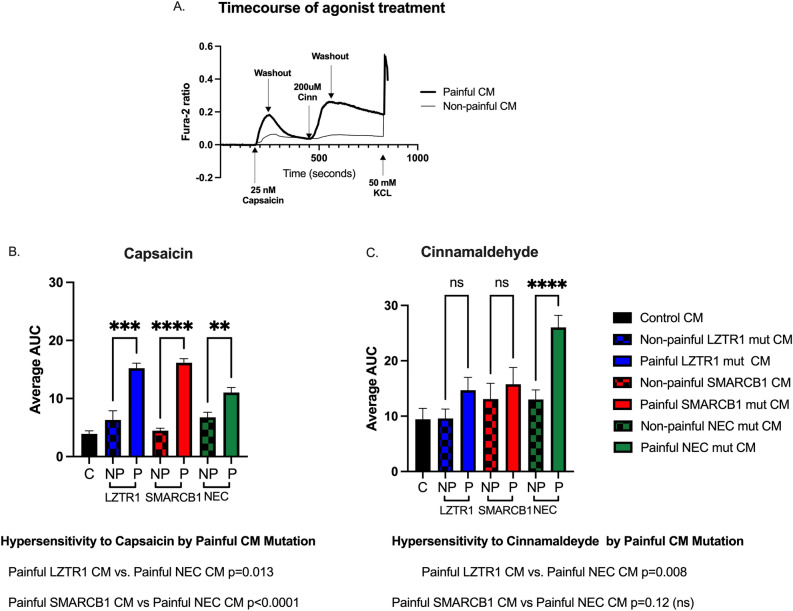
Our previous work demonstrated that cultured mouse dorsal root ganglion (DRG) neurons that were pretreated with CM from painful tumors, but not that from non-painful tumors, were hypersensitized to low doses of capsaicin (~ 25 nM), a TRPV1 agonist and/or cinnamaldehyde (~ 200uM), a TRPA1 agonist. We also noted that each painful tumor tested had a slightly different effect on the cultured neurons11. At the time of that publication, mutations were not considered as diagnostics criteria for SWN and were thus not examined. We subsequently collected additional painful (patient-reported pain score > 7) and non-painful (patient-reported pain score < 3) tumors from patients with known germline mutations in SMARCB1 and LZTR1. We also made use of confirmed SWN NEC tumors where sequencing of SMARCB1 and LZTR1 detected no mutations in either gene. One painful tumor and one non-painful tumor from each gene group were used to create cell lines and CM. We then repeated our sensory neuron sensitization experiments using these additional CMs from pain score- and mutation-matched groups. Mouse DRGs were dissociated and the resulting cells were plated and incubated with CM for 48 h. Following incubation, the neurons were subjected to fluorescent calcium imaging analysis during challenge with low-dose capsaicin and cinnamaldehyde as previously described11. Low-dose capsaicin caused a rapid influx of calcium in a subset of neurons that quickly returned to baseline levels upon washout, resulting in a bell-shaped curve, while low-dose cinnamaldehyde evoked a slower increase in calcium in a subset of neurons that very slowly declined upon washout but did not return to baseline levels, resulting in an overall larger area under the curve (Fig. 3a). All painful CMs sensitized DRG neurons to low dose capsaicin compared with their matched non-painful counterparts as demonstrated by either an increased magnitude of response (among responsive cells) or an increase in the percentage of cells responding to capsaicin (Fig. 3b, Table 1). However, differences were seen between painful CM groups based on mutation. Painful SMARCB1 mut CM (AUC = 16.2) and LZTR1 mut CM (AUC = 15.6) enhanced the response to low-dose capsaicin more than NEC mut CM (AUC = 11) (p = 0.0001; p = 0.003, respectively) (Fig. 3b). Conversely, painful NEC mut CM enhanced the response to low-dose cinnamaldehyde (AUC 21.3) more than non-painful NEC tumor CM (AUC = 11.8) (p = 0.01) or painful LZTR1 tumor CM (AUC = 9.59) (p = 0.007) (Fig. 3c). While pretreatment of DRG cells with painful SMARCB1 CM and non-painful SMARCB1 CM both increased the magnitude of response to cinnamaldehyde, compared to control CM, painful SMARCB1 boosted the percentage of cells sensitized to cinnamaldehyde (44% painful SMARCB1 CM vs 17% non-painful SMARCB1 CM p = 0.0001) (Fig. 3c, Table 1).
Fig. 3.
Conditioned media from painful SWN tumors sensitize DRG neurons to the TRPV1 agonist, capsaicin, and the TPRA1 agonist, cinnamaldehyde. (a). Representative calcium imaging trace DRG cells were pre-treated with painful schwannoma CM, non-painful schwannoma CM, or control media for 48 h before imaging. Fura-2 ratio measurements were recorded at 2 s intervals. The datapoints were corrected for differing baseline readings of Fura-2. Area under the curve was calculated for each treatment. Six coverslips of DRGs were tested per CM. Brown-Forsythe ANOVA test with Dunnett’s T3 multiple comparisons was employed to determine statistical significance p < 0.05 is considered significant (b) TRPV1 agonist, Capsaicin stimulus CM from painful tumors increased the responsiveness of the DRGs to capsaicin as calculated by area under the curve. Solid filled bars are painful CM. Checkered bars are non-painful CM. Comparisons between painful CMs of different mutations were also assessed. (c) TRPA1 agonist, Cinnamaldehyde stimulus CM from painful NEC tumor sensitized DRG neurons to low dose cinnamaldehyde (p = 0.0001). Additionally, comparisons between painful CMs of different mutations were also examined.
Table 1.
Number of cells responsive to TRP agonists.
| 25 nM Capsacin | 200uM Cinnamaldehyde | |
|---|---|---|
| Control CM | 56/230 (24%) | 22/147 (15%) |
| NP LZTR1 | 28/148 (19%) | 44/207 (21%) |
| P LZTR1 | 293/540 (54%)**** | 48/209 (24%) |
| NP SMARCB1 | 106/418 (25%) | 25/149 (17%) |
| P SMARCB1 | 395/597 (66%)**** | 46/104 (44%)**** |
| NP NEC | 61/226 (27%) | 86/316 (27%) |
| P NEC | 151/319 (47%)**** | 226/414 (54%)**** |
| **** p < 0.0001 | ||
Chi-square analysis p < 0.05 is significant.
Acute Behavioral Response to CM differs by mutation
The mouse behavior studies described in Fig. 2 were next repeated, considering mutation status, using CM from our expanded cohort of samples. The CM examined in Fig. 3 was injected into the foot pad of C57Bl6 mice (10/group). Mice were observed for 10 min and the number of times a mouse licked or flinched the injected paw was recorded. All painful SWN CMs caused an increase in licking and flinching compared to control media (Fig. 4). However, only LZTR1 painful CM caused an increased acute pain response compared to their non-painful LZTR1 CM counterpart (p = 0.006). Further comparison between tumor genotypes revealed that CM from painful tumors with LZTR1 mutations caused an increase in acute pain response compared to painful CM from SMARCB1 (p = 0.0002) and NEC tumors (p = 0.0001) (Fig. 4). In addition, non-painful LZTR1 CM also elicited more of an acute pain response than control media (p = 0.0001), non-painful SMARCB1 (p = 0.0007) and non-painful NEC CM (p = 0.0001). No significant differences in cumulative licks and flinches were noted between painful and non-painful tumor CM from SMARCB1 or NEC tumors.
Fig. 4.
Acute Response to CM differs by mutation. Painful CM from a SMARCB1 mut tumor, LZTR1 mut tumor, and NEC mut tumors were tested. All painful tumors had a pain score greater than 7. One non-painful tumor (pain score < 3) from each mutation group was used for comparison. Ten mice were injected per CM. All painful SWN CMs caused an increase in licking and flinching compared to control media. LZTR1 painful CM caused an increased acute pain response compared to non-painful LZTR1 CM compared to control media (p = 0.006, p = 0.0001, respectively). Brown-Forsythe ANOVA with Dunnett’s T3 multiple comparisons tests were used to determine significance.
CM-evoked behavioral hypersensitivity to mechanical stimuli varies by mutation
We next examined the responsiveness of CM-injected mice to punctate mechanical stimuli evoked by von Frey monofilaments (0.02 to 0.07 g). Mice were assayed one hour and again 24 and 48 h after the final CM injection. CM from painful SMARCB1mutant tumor sensitized the injected paw to evoked mechanical pain, compared to non-painful SMARCB1 CM at low forces (0.04 g p = 0.03; 0.07 g p = 0.01) (Fig. 5a). This effect subsided over the ensuing 24 h (Fig. 5b). Forty-eight hours post-injection, no differences between painful and non-painful CM were detected (Fig. 5c). An even more prominent sensitization to mechanical stimuli was produced by CM from painful NEC tumor. Injection of this CM elicited a greater degree of mechanical responsiveness than that from nonpainful NEC tumor at the lowest force tested (0.02 g) (NEC mut painful vs NEC mut non-painful p = 0.0016) as well as at 0.04 g (NEC mut painful vs NEC mut non-painful p < 0.0001) and 0.07 g (p < 0.0001) (Fig. 5a). Moreover, this effect was durable, in that hypersensitivity persisted 24 h post-injection (0.04 g p = 0.0017; 0.07 g p = 0.0002) (Fig. 5b). Forty-eight hours post-injection, mice treated with painful NEC CM still demonstrated an apparently increased response to light touch, but the difference from non-painful NEC CM was not statistically significant (Fig. 5c). No statistically significant differences in punctate mechanical sensitivity were observed between non-painful LZTR1-related CM and painful LZTR1-related CM at any time point post injection (Figs. 5 a,b,c).
Fig. 5.
Response to painful stimuli differs between SWN mutational groups. Evoked mechanical hypersensitivity to light touch (0.02 g, 0.04 g, and 0.07 g) was assayed using Von Frey (VF) filaments. Percent response was calculated as in Fig. 2b. Four separate rounds of VF were performed on each mouse over the course of the experiment. The first round was performed prior to any injections with the subsequent rounds being performed 1 h following the final injection, 24 h post final injection, and 48 h post final injection. 5a). No differences were seen between mutation groups of non-painful CM (checked bars). Painful NEC mut CM (Green Bars) and SMARCB1 mut CM (Red Bars) caused an increase in response to light touch at 0.04 g and 0.07 g compared to corresponding non-painful CM (checked bars), while painful LZTR1 (Blue bars) did not affect response to light touch at any force. Painful NEC mut CM gave a significant response to light touch at the lowest Von Frey filament force tested (0.02 g, p = 0.01). 5b). Twenty-four hours post-injection, painful NEC CM maintained a significant response to light touch compared to non-painful NEC CM at 0.04 g (p = 0.02) and 0.07 g (p = 0.004). 5c). Forty-eight hours post-injection, increased hypersensitivity to light touch was still demonstrated in the painful NEC CM treated mouse cohort. Two-way ANOVA with repeated measures, and Tukey’s multiple comparisons were used to determine significance.
Levels of specific cytokines and chemokines in the injection cohort CMs
Finally, we assayed the CM used in the in vivo experiments above for levels of CCL2, IL-6, IL-8, VEGF, GDF-15, CXCL1, CXCL5, CCL20 and GM-CSF, using ELISA assays (R&D systems). When comparing painful CMs by mutation, painful NEC CM (Fig. 6, Green bars) contained higher levels of IL-8, CCL2, and CCL20 than Painful SMARCB1 CM (Fig. 6, Red bars) and Painful LZTR1 CM (Fig. 6, Blue bars). Painful LZTR1 CM contained higher levels of GDF-15, CXCL1 and GM-CSF than Painful NEC and Painful SMARCB1 CM.
Fig. 6.

Cytokine levels in CM by mutation status ELISA was used to examine the levels of our candidate panel of cytokines/chemokines in painful CM from our injection cohort. CCL2, IL-6, IL-8, VEGF, GDF-15, and CXCL1, CXCL5, CCL20 and GM-CSF were tested. Painful NEC CM (Green Bars) contains higher levels of IL-8, CCL2, and CCL20 than Painful SMARCB1CM (red bars) and Painful LZTR1 CM (Blue bars). Painful LZTR1 contained higher levels of GDF-15, CXCL1 and GM-CSF than Painful NEC and Painful SMARCB1 CM.
Discussion
Patients with non-NF2 schwannomatosis describe their pain experience with great diversity. While the majority of patients experience chronic pain, symptoms can be localized to a tumor or may be diffuse. In some cases a particular tumor may not cause any pain. Our prior study focused on deciphering the molecular mechanisms of painful tumors (Pain score > 7) compared to “non-painful” tumors (pain score < 3)11. Those in vitro experiments revealed that CM collected from painful SWN tumors, but not that from non-painful SWN tumors, contained increased amounts of multiple cytokines, upregulated the expression of pain-associated genes in sensory neuron cultures, and increased neuronal responses to noxious TRPV1 and TRPA1 agonists11. In the present study, these and additional CM samples were injected in vivo to assess whether the secretome of SWN cells can induce painful behaviors in mice. We found that painful tumor-derived CMs increase acute pain behaviors when injected into the glabrous skin of C57Bl6 mice. An increase in licking and flinching occurred after a single injection of painful CM (Fig. 2a). Behavioral responsiveness to punctate mechanical stimuli also increased in mice injected with painful CM. While this increase between the grouped cohorts was statistically significant overall (Fig. 2b), variability was evident between painful tumors (Supplementary Fig. 1). This variability may reflect that seen with these CM samples in vitro11. CM samples that hypersensitized neurons to capsaicin in vitro also hypersensitized mice to punctate stimuli. Moreover, the samples that sensitized neurons to both cinnamaldehyde and capsaicin in vitro11, produced the largest enhancement of mechanically-evoked pain in vivo (painful Tumor 3; Supplementary Figs. 1 & 2).
In 2022, experts in the field of peripheral nerve sheath tumors established new criteria for diagnosis of schwannomatosis7. All tumors of Schwann cell origin were to be classified as schwannomatosis. Neurofibromatosis type 2 was renamed to NF2-related schwannomatosis7,13. The multiple tumor disorder we have been studying, formerly called SWN, was renamed and subdivided to reflect tumor mutation status. As a group, this disease is termed non-NF2 schwannomatosis (non-NF2-SWN). Subgroups of tumors are classified as SMARCB1-related SWN, LZTR1-related SWN, or SWN not elsewhere classified (NEC). Based on this reclassification, in the present study we repeated our in vitro and in vivo studies using an additional set of samples with known germline mutations in these genes. It is not known whether mutations in SMARCB1 or LZTR1 correlate with a patient’s experience with pain. However, based on our new data, we believe tumor mutation status may contribute to variability in cytokine secretion and neuronal sensitization in vitro and in vivo.
CM from painful tumors with mutations in SMARCB1 produced significant sensitization to punctate mechanical stimuli that was evident one hour after injection but subsided by 24 h. Painful SMARCB1 CM contains elevated levels of Il-6, VEGF, and GDF-15, compared to non-painful tumor CM. Previous studies demonstrated that IL-6 induced mechanical hypersensitivity in otherwise naive rats in vivo and increased DRG neuron sensitivity to capsaicin in vitro14. Another study demonstrated that IL-6 injection also increased hyperalgesia in the rat footpad. Intriguingly, this effect peaked 2–3 h after injection, was still demonstrated 6 h post injection, but subsided 24 h post injection15, consistent with the kinetic profile exhibited by painful SMARCB1 CM in the present study.
Injection of painful NEC mutant CM caused an amplified, long-lasting response to punctate mechanical stimuli, even increasing the response to the lowest force tested. Painful NEC CM also contains elevated levels of IL-6 VEGF, CXCL-1, and GDF-15, compared to non-painful CM. However, it additionally contains elevated levels of IL-8, CCL2, CXCL-5, and CCL20, compared to painful CM from SMARCB1 and LZTR1 mutant tumor cells. Interestingly, painful NEC CM also caused an increased response to the TRPA1 agonist cinnamaldehyde in our in vitro calcium imaging experiments. There is a growing consensus that TRPA1 is involved in mechanical hypersensitivity in different types of chronic pain under pathological conditions16–18. Among the cytokines elevated in painful NEC CM, CCL2 is a promising candidate for initiating and maintaining mechanical hypersensitivity. Previous studies showed that spinal administration of CCL2 in rats induced sustained painful mechanical hypersensitivity that lasted 4 days post-injection19. In addition, CCL2 acting on its receptor CCR2, sensitizes TRPA1, causing a sustained influx of calcium into cells20. Based on these previous findings it is possible that elevated CCL2 in painful NEC CM, activates or sensitizes TRPA1 and thereby induces an amplified and sustained mechanical hypersensitivity. IL-8 is another promising candidate mediator of lasting mechanical hypersensitivity. Injection of IL-8 into the footpad of the naïve rat is known to cause mechanical hyperalgesia21. IL-8 acts through the receptor CXCR1/CXCR2 during inflammation. Previous studies demonstrated that IL-8 is involved in long lasting mechanical hypersensitivity that persists even after an inflammatory response has resolved 22, again consistent with our in vivo and in vitro findings on painful NEC CM. Lastly, another study demonstrated that intraplantar injection of CXCL5 into the rat paw caused a dose-dependent reduction in mechanical pain thresholds compared to vehicle control, which returned to baseline levels by 24 h23. Based on these findings, targeting elevated cytokines may be an effective strategy to treat mechanically evoked pain induced by painful NEC SWN CM.
Painful LZTR1 mutant CM did not induce hypersensitivity to light touch compared to non-painful LZTR1 mutant CM. However, acute pain-related behaviors were greater in response to CM from nonpainful LZTR1 mut CM than that from control medium and were greater yet in response to painful LZTR1 CM. Painful LZTR1 mutant CM also evoked a larger acute pain response than either SMARCB1 or NEC CM (p = 0.0004 and p = 0.0003, respectively). This finding leads us to speculate that tumors with mutations in LZTR1 may have a different signature of secreted cytokines that increase acute pain. In fact, painful LZTR1 CM was the only sample that contained an elevated level of GM-CSF by ELISA and the highest level of CXCL-1 compared to the other painful CMs. The role of GM-CSF in pain and inflammation has been previously examined24. GM-CSF receptors are expressed in a subset of small/medium diameter neurons that also express TRPV1. In vivo, intraplantar injection of GM-CSF in mice caused favoring of the affected paw24.
Our study demonstrates definitive differences in levels of cytokines secreted by painful and non-painful tumors and significant effects on neuronal sensitization in vitro. The effects of CM in vivo may appear to be more subtle. This stems from several factors. Firstly, patient reported pain scores are noted to have inherent flaws. We have taken great efforts in selecting our cohort of tumors to limit the subjectivity of pain reporting that could influence our results. Our cohort of “non-painful” tumors are from patients who reported pain scores of less than 3. These tumors were not removed because they were causing pain to the patient but rather because of increased growth or neurological deficit. Painful tumors were surgically excised from patients who reported scores of 7 + specifically to relieve pain. Secondly, all tumors whether painful or non-painful secreted cytokines into conditioned media, therefore we expected to see some background response in our mouse experiments. Painful tumors demonstrated higher levels of cytokines in CM but the specific cytokine or combination of cytokines that cause a painful phenotype are still under investigation. Variability in cytokine levels from painful tumor to painful tumor may dilute the response to injected CM when the samples are pooled. Thirdly, our Von Frey method and analysis employed a conservative approach. We used a percent response to filament size and force method of testing. This method focused in on the lowest forces. Our analysis used a conservative approach (two-way ANOVA with Tukey’s multiple comparisons), which compared all groups (painful, non-painful, and control) at all forces measured resulting in significant but more modest p values (see supplementary table for statistics). Also, mouse models of evoked pain are not exactly representative of the human experience25. Humans living with chronic pain can vocalize their symptoms and express pain interference in daily tasks, in addition to providing descriptives of sharp, dull, burning pain. The emotional and cognitive influences of mood, and anxiety are confounding factors that are recognized in the human pain experience. We recognize the shortcomings in pain behavioral testing in mouse models. Punctate evoked stimuli may not capture the essence of the pain experienced by an animal25. Non-reflexive behavioral assays are being developed in mice such as facial grimacing, changes in weightbearing, and home cage analytics for spontaneous behaviors26 to better capture a comprehensive assessment of pain in experimental animals. These assays in addition to the traditional evoked pain response assay used in our current study will allow future rodent research that parallels the human experience.
In conclusion, our findings demonstrate that painful SWN CM sensitizes mice to painful stimuli and that both the magnitude and duration of the pain hypersensitivity, as well as the cytokine/chemokine content of these CMs differ as a function of the SWN-associated gene mutation. These data, derived using an expanded cohort of painful and non-painful CM, validate our previous in vitro results. Additional testing of painful CM in vivo may lead to a better understanding of the etiology of SWN-related pain and guide the rational development of personalized therapies for pain in patients suffering from these disorders.
Methods
Tumor cells and conditioned media
Schwannomatosis-related tumors were collected from surgical cases from January 2014 through March 2021, occurring at Johns Hopkins School of Medicine. Informed, written consent was obtained prior to surgery. The Institutional Review Board (IRB) of the Johns Hopkins School of Medicine approved consent forms and study design. All research was performed in accordance with relevant guidelines/regulations and approved by the IRB Study Number NA_00069904. The diagnosis of SWN was confirmed by Johns Hopkins Pathology. Self-reported pain scores and mutation status were collected prior to surgery. Patients were tested for germline mutations in LZTR1, SMARCB1, and NF2 by an outside lab and reported by the patient. Tumor tissue was not sequenced. Human Schwannomatosis cell lines were established from participant tumors as described in Ostrow et al. 2015. Cell cultures were maintained in D10 media (DMEM, 10% FBS, 5% penicillin/streptomycin) with mitogens (2uM Forskolin). Media for testing was collected from these cultures at 48 h intervals at 80% confluence Conditioned medium (CM) was centrifuged to remove cell debris and passed through a 0.22 micron filter. CM was frozen in aliquots and stored at -80C for future use.
ELISA
Protein levels in CM were normalized to SERPINE1. The internal controls of the Human Cytokine XL Array (R&D systems) and SERPINE1 consistently demonstrated the mean pixel density across all CMs tested. Therefore, SERPINE1 was used as the internal control protein to normalize CM input prior to cytokine ELISA. Levels of SERPINE1 were tested in undiluted CMs to determine concentration using the R&D Quantikine total SERPINE1 ELISA kit. CMs were then diluted to a final concentration of 2 ng of SERPINE1 prior to the ELISA assays. All ELISA experiments were performed according to a similar framework with reagent variation determined by their kit-specific instructions (R&D systems). In brief, CM samples and standards were pipetted into a microplate pre-coated with monoclonal specific antibodies, where target polypeptides were bound and further tagged with target-specific enzyme-linked polyclonal antibodies before proportional color analysis of added development substrate. Absorbance at 450 nM was measured. Each sample was performed in triplicate. The amount of protein in each sample (pg/ml) was interpolated from a standard curve (Graphpad Prism 10).
Mouse cohorts and experiments
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Johns Hopkins University School of Medicine (Approval ID MO23M18) and were in accordance with the guidelines provided by the National Institute of Health and the International Association for the Study of Pain. Animal experiments presented in this study are reported in accordance with ARRIVE guidelines.
Dissociated Mouse DRG cell cultures
Dorsal root ganglia (DRG) were harvested from 12 week old C57BL6J mice into Complete Saline Solution (CSS; NaCl 137 mM, KCl 5.3 mM, MgCl2-6H2O 1 mM, Sorbitol 25 mM, HEPES 10 mM, CaCl2-2H2O 3 mM). DRG were digested with TM Liberase (Trituration Moderate; 0.35U/mL in CSS, 50 mM EDTA) at 37 °C for 20 min in a rotating wheel hybridization oven, followed by TL Liberase (Trituration Low; 0.25U/mL in CSS, 50 mM EDTA, 30U/mL Papain) at 37 °C for 15 min by the same method. These were resuspended in complete DRG medium (DMEM/F12, 10%FBS, 1% glutamine, 5% penicillin/streptomycin) containing BSA (1.5 mg/mL) and Trypsin Inhibitor (1.5 mg/mL) before mechanical dissociation and passage through a 70um mesh filter. DRG were then spotted in 20uL TI/BSA/DMEM on poly-L-lysine/laminin coated coverslips and left to adhere for 1 h in an incubator. Wells containing coverslips were then flooded with complete DRG medium.
Calcium imaging
The day after establishment of primary mouse DRG cultures, culture medium was replaced with schwannomatosis cell CM and the cells were further incubated for 48 h at 37 °C. Three separate DRG cultures were prepared for each CM to be tested. Four coverslips containing at least 30 neurons/slip were treated with CM. A minimum of 100 DRG neurons were tested per condition. Cells were treated with CM for 48 h before loading with 2 μM fura-2 acetoxymethyl ester (Molecular Probes) in calcium imaging buffer (CIB, containing in mM: 130 NaCl, 3 KCl, 2.5 CaCl2, 0,6 MgCl2, 10 HEPES, 1.2 NaHCO3, 10 glucose, pH 7.45, 290 mOsm adjusted with mannitol). Coverslips with fura-2-loaded cells were mounted on an inverted fluorescence microscope (TE200, Nikon). Images were acquired with a cMOS camera (NEO, Andor) using an excitation filter wheel (Ludl) equipped with 340 and 380 nm filters. Data were acquired using NIS Elements imaging software (Nikon). Fluorescence changes are expressed as the ratio of fluorescence emission at 520 nm upon stimulation at 340 to that upon stimulation at 380 nm (F340/F380). Capsaicin experiments: Capsaicin was dissolved in CIB at a net concentration of 25 nM. During continual imaging at ~ 2 s intervals cells were perfused with 25 nM Capsaicin for 30 s with a recovery period of washing with unmodified CIB. Cinnamaldehyde experiments: Similarly, DRG cells were exposed to 200 uM cinnamaldehyde/CIB solution for 2 min followed by a 4 min washout with CIB. The datapoints were corrected for differing baseline readings of Fura-2. Perfusion with KCl at a concentration of 50 mM was used to identify viable neurons. Area under the curve was calculated for each treatment.
Conditioned media injections
Eight week old C57BL6J mice were placed in an immobilization cone and injected with 10uL of conditioned media in the right hind paw using a 20 g insulin syringe. Ten mice were injected per conditioned media. Injections were repeated for 48 h, 3 injections total. Mice were injected in groups of 20, (2 CMs; 10 mice per CM) to simplify handling and subsequent testing. CMs were deidentified and the experimenter was blinded to the code for the injections and subsequent behavioral assays to eliminate bias. All samples were the same in volume, color, and viscosity.
Post-injection observations for acute pain behaviors
Immediately following day 1 injections all animals were recorded for a 10 min observation period. Mice were placed into vertical plexiglass watch cylinders arranged in an arc of 5 opposite a mounted camera, in such a position to see all angles inside each cylinder. The mice were recorded from the placement of the first mouse in the group of five until 10 min after the placement of the last in the group. The observation period of each mouse was defined as 10 min, beginning when they were placed in the watch cylinder, and was used during the review and scoring of these recordings. The recorded 10 min observation periods were then scored for reactive behavior (licking, flinching). Each instance of deliberate licking of the underside of the injected paw was considered a Lick, while each instance of rapid recoil or wave of the injected paw was considered a Flinch. Particular attention was paid to distinguish reactions from grooming behavior.
Von frey assessment
Following the final injection, CM injected mice were placed into plexiglass cages in groups of 5 on top of a wire-mesh platform and given an acclimation period of twenty minutes, coinciding with the time intervals 1 h, 24 h, and 48 h following final injections. Following this period, wire filaments of seven increasing gauge and force (0.02 g, 0.04 g, 0.07 g, 0.16 g, 0.4 g, 0.6 g, 1 g) were applied to the hindpaws of each mouse to the point of bending, six times per filament size. The response for each filament introduction to each paw was recorded as positive or negative. A dose–response frequency curve was calculated for each mouse.
Data analysis and statistics
For ELISA: The level of specific cytokines were determined by interpolation of a standard curve using Graphpad Prism10. Data from Painful CM (n = 12 samples) were grouped and compared to Non-painful CM (n = 8 samples). Two-tailed unpaired t-tests with Welch’s correction were used to determine significance (Graphpad Prism 10). p value < 0.05 was considered significant.
For calcium imaging: The area under the curve of fura-2 ratio, with subtraction of the baseline obtained prior to each stimulus, was used to compare the effects of painful vs. non-painful CM. Brown-Forsythe and Welch ANOVA tests with Dunnett’s T3 multiple comparisons test were used to compare the effects of CM groups (Graphpad Prism10). In addition, the percentage of cells responding to capsaicin, or cinnamaldehyde stimulation was analyzed. An individual cell’s response to stimuli was considered to be positive if the fura-2 ratio was greater than 0.1 after baseline subtraction. Chi-squared analysis was performed to test significance between the percentage of cells responding to painful and non-painful CM. p value < 0.05 was considered significant.
For acute pain: Brown-Forsythe One-way ANOVA test and post-hoc Dunnett’s T3 multiple comparisons test were performed to determine significance between groups using Graphpad Prism 10.
For Von Frey analysis: Two-way ANOVA with repeated measures, and Tukey’s multiple comparison tests were performed to determine significance between groups using Graphpad Prism 10.
Supplementary Information
Acknowledgements
The Blaustein Pain Foundation, Pamela Mars Wright Foundation, and The Johns Hopkins Neurosurgery Pain Research Institute supported this work. We thank members of the Hoke and Caterina labs for helpful discussions.
Author contributions
R.R. conducted experiments, performed data analysis, and co-wrote the manuscript. K.L.O. conceived the project, designed the experiments, conducted experiments, analyzed the data, and co-wrote the manuscript. A.B. and M.J.C. facilitated experimental design, assisted in data analysis, and revised the manuscript. All authors reviewed and approved the manuscript.
Data availability
The authors will make materials, data and protocols available upon request. Please contact the corresponding author, Kimberly L Ostrow PhD; kostrow3@jhmi.edu.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-99820-0.
References
- 1.MacCollin, M. et al. DiagNECtic criteria for schwannomatosis. Neurology64(11), 1838–1845 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Gonzalvo, A. et al. Schwannomatosis, sporadic schwannomatosis, and familial schwannomatosis: A surgical series with long-term follow-up. Clin. Article. J. Neurosurg.114(3), 756–762 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Halvorsen, C. M. et al. The long-term outcome after resection of intraspinal nerve sheath tumors: Report of 131 consecutive cases. Neurosurgery77(4), 585–592 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Merker, V. L. et al. Clinical features of schwannomatosis: A retrospective analysis of 87 patients. Oncologist17(10), 1317–1322 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mansukhani, S. A. et al. Familial Schwannomatosis: A DiagNECtic Challenge. J. Clin. Diagn. Res.11(2), 01–03 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iorno, V. et al. Including cannabinoids in the treatment of painful schwannomatosis. Brain Behav8(7), e01011 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plotkin, S. R. et al. Updated diagNECtic criteria and nomenclature for neurofibromatosis type 2 and schwannomatosis: An international consensus recommendation. Genet. Med.24(9), 1967–1977 (2022). [DOI] [PubMed] [Google Scholar]
- 8.Evans, D. G. et al. ERN GENTURIS clinical practice guidelines for the diagNECis, treatment, management and surveillance of people with schwannomatosis. Eur. J. Hum. Genet.30(7), 812–817 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jordan, J. T. et al. Pain correlates with germline mutation in schwannomatosis. Medicine (Baltimore)97(5), e9717 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostrow, K. L. et al. Immortalized human schwann cell lines derived from tumors of schwannomatosis patients. PLoS ONE10(12), e0144620 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ostrow, K. L. et al. The secretomes of painful versus nonpainful human schwannomatosis tumor cells differentially influence sensory neuron gene expression and sensitivity. Sci. Rep.9(1), 13098 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serlin, R. C. et al. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain61(2), 277–284 (1995). [DOI] [PubMed] [Google Scholar]
- 13.Planet, M., Kalamarides, M. & Peyre, M. Schwannomatosis: a Realm Reborn: year one. Curr. Opin. Oncol.35(6), 550–557 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Fang, D. et al. Interleukin-6-mediated functional upregulation of TRPV1 receptors in dorsal root ganglion neurons through the activation of JAK/PI3K signaling pathway: roles in the development of bone cancer pain in a rat model. Pain156(6), 1124–1144 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Cunha, F. Q. et al. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br. J. Pharmacol.107(3), 660–664 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eid, S. R. et al. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory-and neuropathy-induced mechanical hypersensitivity. Mol. Pain4, 1744 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan, S. et al. IQGAP1 promotes chronic pain by regulating the trafficking and sensitization of TRPA1 channels. Brain146(6), 2595–2611 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrus, M. et al. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol. pain3, 1744 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dansereau, M. A. et al. Spinal CCL2 pronociceptive action is no longer effective in CCR2 receptor antagonist-treated rats. J. Neurochem.106(2), 757–769 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Jung, H. et al. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J. Neurochem.104(1), 254–263 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunha, F. Q. et al. Interleukin-8 as a mediator of sympathetic pain. Br J Pharmacol104(3), 765–767 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sachs, D. et al. Tumour necrosis factor-alpha, interleukin-1beta and interleukin-8 induce persistent mechanical nociceptor hypersensitivity. Pain96(1–2), 89–97 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Dawes, J. M. et al. CXCL5 mediates UVB irradiation-induced pain. Sci Transl Med3(90), 90ra60 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Achuthan, A. et al. Granulocyte macrophage colony-stimulating factor induces CCL17 production via IRF4 to mediate inflammation. J. Clin. Investig.126(9), 3453–3466 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pattison, L. A. et al. Digging deeper into pain: an ethological behavior assay correlating well-being in mice with human pain experience. Pain165(8), 1761–1773 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bohic, M. et al. Mapping the neuroethological signatures of pain, analgesia, and recovery in mice. Neuron111(18), 2811–2830 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors will make materials, data and protocols available upon request. Please contact the corresponding author, Kimberly L Ostrow PhD; kostrow3@jhmi.edu.