Abstract
BACKGROUND
Reproductive interference (i.e. sexual interaction between males of one species and females of another species that reduce the fitness of one or both the interacting individuals) is an important species interaction significantly affecting population dynamics and persistence. However, its exploitation in pest control remains overlooked. Here, we investigated the possible integration of reproductive interference into the sterile insect technique (SIT) to develop a heterospecific SIT (h‐SIT). Under this approach, contrary to the classic SIT, sterile heterospecific males from closely related, nonpest species are released to compete with the pest population for mates. To this end, we focused on the invasive pest species Drosophila suzukii and used D. melanogaster as the control species. First, we investigated the effect of irradiation on D. melanogaster sterility and longevity. Then, we tested the mating performance of irradiated males and their ability to reduce the D. suzukii fitness.
RESULTS
We found by microcosm experiments that: (i) irradiation induced high levels of D. melanogaster male sterility without reducing longevity; (ii) irradiated D. melanogaster males court D. suzukii females as much as D. suzukii males do, and they couple, mate with and inseminate heterospecific females; (iii) irradiated D. melanogaster males significantly reduce the offspring of D. suzukii females under two different species ratios.
CONCLUSION
Our results provide the first foundations for the development of a h‐SIT against D. suzukii, an approach which can be tested against other groups of pest species. © 2025 The Author(s). Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: reproductive interference, pest control, sterile insect technique, Drosophila suzukii, Drosophila melanogaster
Irradiated Drosophila melanogaster males successfully court and mate with D. suzukii females and significantly reduce numbers of D. suzukii offspring under different species ratios.

1. INTRODUCTION
Species interactions, such as predation, parasitism or competition, can significantly affect the population dynamics of the interacting species, affecting their abundance and persistence. 1 These interactions have been used for a long time to control pest species in agriculture and are the foundation of modern biological control approaches. 2
Along with the above species interactions, reproductive interference is now recognized as a major ecological process affecting population dynamics and species persistence. 3 , 4 , 5 , 6 , 7 Reproductive interference (or satyrization in animals) consists of any sexual interaction between species that reduces the fitness of one or both interacting individuals. 8 It occurs as a result of incomplete mating barriers between species and can occur at any stage of mate acquisition, from courtship to mating and hybridization. 3 Reproductive interference has been documented under laboratory and field conditions in a wide variety of sexually reproducing taxa. 5 , 6 Theoretical and empirical studies showed that reproductive interference, as competition, is density‐dependent and can result in population or species exclusion. 4 , 9
The first attempts to exploit reproductive interference for pest control date back to the first half of the 20th Century. During the 1930s–40s, in pioneering works, F. L. Vanderplank and colleagues applied reproductive interference to suppress tsetse fly, Glossina swynnertoni Austen (reviewed in 10 ). Laboratory experiments showed that offspring produced by cross‐mating between G. swynnertoni (the vector species) and Glossina morsitans Westwood (a nonvector species) had low fertility, as all hybrid males and some hybrid females were sterile. Then, a large field experiment was carried out in the Shinyanga area, Tanzania, by releasing fertile G. morsitans pupae over 7 months. After the mass release the density of G. swynnertoni was drastically reduced, demonstrating the potential of hybrid sterility and sexual interference to suppress tsetse flies. 9 , 10
Although the experience with tsetse flies showed the potential effectiveness of reproductive interference for controlling a pest species, it did not lead to common exploitation of this approach. A major concern about heterospecific sterility was that pre‐mating isolation mechanisms could constrain mating between released heterospecific males and wild females, leading to failure to control the target species in the field. 11 , 12 A further concern has been the risk of introducing non‐native pest species. The mass release of fertile individuals of a closely related species that is non‐native or a pest itself, would, at least potentially, lead to replacing one pest species with another. These concerns and the success in the 1950s of Knipling and his team in eradicating the screwworm Cochliomyia hominivorax (Coquerel) using ionizing radiation, 13 has led to the spread of the homospecific sterile insect technique (SIT) as the major approach exploiting sterility in pest control during the last decades. 14 , 15 , 16 , 17 , 18 It consists of mass rearing, sterilization by ionizing radiation, and massive release of conspecific individuals into the target population. The nonfertile mating between the released males and wild females leads to a progressive decline of the target pest population. 19 , 20
However, a renewed interest has recently been in exploiting reproductive interference to control pest species. 8 , 21 , 22 , 23 , 24 Mitchell et al. 8 reviewed the literature on reproductive interference in natural populations. They highlighted the effects of these processes on population decline in nature and proposed a framework for their use in pest control. 8
Interestingly, McInnis 21 tested the potential use of sterilized males of the oriental fruit fly Bactrocera dorsalis (Hendel) against wild carambola fruit fly B. carambolae, suggesting that the concern about using non‐native or potential pest species to control another pest can be overcome if the released heterospecific individuals are sterile, thus integrating reproductive interference and SIT. More recently, Honma et al. 22 proposed a framework for incorporating reproductive interference into a classic SIT program. They argued that the sterile males released in a SIT program to suppress the wild population of the same species also could lead to suppression of a closely related pest species through reproductive interference (an approach that they called sterile interference).
In this paper, we aimed to explore the potential use of reproductive interference to develop a heterospecific SIT (h‐SIT) approach against the spotted wing fly Drosophila suzukii Matsumura. This approach, contrary to the classic SIT, is based on using sterile heterospecific males from closely related species to compete with the pest population for mates. D. suzukii is an invasive pest that has spread in the last few decades from its native range in East Asia throughout North America, Europe and South America. 25 , 26 Unlike most Drosophilidae, D. suzukii can lay eggs in unripe and healthy fruits, causing severe economic losses for fruit industries worldwide. 27 , 28 We selected the fruit fly Drosophila melanogaster to induce reproductive interference. Previous studies showed that post‐mating isolation between D. melanogaster and D. suzukii is complete, whereas there is an incomplete pre‐mating isolation. 24 , 29 Wolf and colleagues recently assessed the potential for hybridization between gene drive‐modified D. suzukii individuals and nontarget Drosophila species in Europe. They found by male mating behavior tests that D. melanogaster males frequently showed interest in D. suzukii females but did not achieve copulation. 29 Accordingly, in our previous study, by investigating reproductive interference between nonirradiated D. melanogaster males and D. suzukii, we found that D. melanogaster males successfully courted D. suzukii females. Furthermore, they could inseminate D. suzukii, leading to egg deposition; however, no hybrids were produced as these eggs did not progress to larval development. Finally, the presence of nonirradiated D. melanogaster males under different species ratios also imposed fitness costs on D. suzukii females, resulting in reduced D. suzukii offspring production. 24 These results are a baseline for exploiting irradiated D. melanogaster males in an h‐SIT approach.
Here, we specifically aimed: (i) to assess the effect of irradiation doses on the sterility degree and longevity of D. melanogaster males. To this end, we irradiated virgin D. melanogaster males with two gamma‐ray doses (60 and 80 Gy) and assessed their fertility, through mating trials with D. melanogaster females, and their longevity; (ii) to analyze the mating performance of irradiated D. melanogaster males in courting and mating with D. suzukii females; (iii) to evaluate if irradiated D. melanogaster males can reduce D. suzukii fitness with whom they mated. To this end, we analyzed the effect of irradiated D. melanogaster males on the fertility of D. suzukii females using different species ratios.
2. MATERIAL AND METHODS
2.1. Laboratory colonies
Drosophila suzukii and D. melanogaster individuals from the laboratory rearing facilities of the Sapienza University of Rome were used in this study. 24 Both species were reared on an artificial diet consisting of agar (7 g), table sugar (16 g), precooked ground maize (72 g), mother yeast (18 g), soy flour (10 g) and methylparaben (2.5 g). 30 The colonies were maintained in entomological cages (30 × 30 × 30 cm), in a climate chamber at 25 ± 1 °C under a 14 h:10 h, light:dark cycle.
2.2. Effect of irradiation dose on sterility and longevity of D. melanogaster males
In order to evaluate the effect of irradiation doses on the sterility degree of D. melanogaster males, mating trials between irradiated males and fertile D. melanogaster females were carried out. Virgin D. melanogaster adults were obtained by checking the adult emergence from the pupae confined in rearing Falcon tubes (50 mL) every 30 min, where they completed their full larval development. As soon as new individuals emerged, we divided them into cages (15 × 15 × 15 cm) according to the gender. This procedure allows us to be sure to use only virgin males and females. After selection, D. melanogaster males were sterilized using gamma radiation. Males (48, 72 or 96 h old) were placed in 50‐mL Falcon tubes containing wet cotton to avoid dehydration and transported to the Calliope Facility at ENEA Casaccia Research Centre (Rome). The Calliope Facility is a pool‐type irradiation facility equipped with a 60Co (mean energy 1.25 MeV) radio‐isotopic source array in a high‐volume (7.0 × 6.0 × 3.9 m) shielded cell. The irradiation cell can provide different dose rates by placing the samples in specific positions and exposing them for varying periods. 31 We provided irradiation doses of 60 and 80 Gy to D. melanogaster males, according to Nelson et al. 32 and Henneberry et al. 33 The dose rate was 175.03 Gy h−1 (2.92 Gy min−1). After irradiation, five irradiated males were placed in Falcon tubes (50 mL) with five unirradiated D. melanogaster females. Each Falcon tube contained food substrate to allow females to lay eggs. We assessed two control treatments: ‘Home’, where we used unirradiated D. melanogaster males, which had not undergone transport stress but had always been kept in the climate chamber conditions; and ‘Trip’, where we used unirradiated D. melanogaster males previously transported to the Calliope facility without receiving any irradiation dose. In this way, we evaluated the impact that the stress caused by transport could have on their fertility. We performed 12 to 16 replicates for each irradiation dose (60 and 80 Gy) and the control treatment (0 Gy). The couples were left together for 6 days and then removed. New individuals that emerged were removed and counted, and oviposition substrates were checked daily until no newborn individuals were observed. We further evaluated the impact of the highest irradiation dose (80 Gy) on the sterility of D. melanogaster females, by carrying out mating trials between irradiated females with fertile D. melanogaster males as described above. This information can be useful in developing a h‐SIT approach because, in mass‐rearing conditions, sexing may not be completely accurate, and females could inadvertently be released into the field.
In order to evaluate the effect of irradiation doses on male longevity, we compared the average lifespan between irradiated and unirradiated D. melanogaster males. Virgin D. melanogaster males were selected and irradiated as described above. A cage (30 × 30 × 30 cm) with 20 males was set up for each dose treatment (60 and 80 Gy). Two more cages were set up as control, one ‘Home’ and one as ‘Trip’, as in the previous experiment. Mortality was recorded every morning until all flies had died.
2.3. Mating performance of irradiated D. melanogaster males
All the following assays were carried out using only the D. melanogaster males irradiated with an 80 Gy irradiation dose, which led to higher sterility without affecting male longevity (see Results section).
First, the mating performance of D. melanogaster males was investigated in no‐choice and choice tests. In no‐choice tests, we set up four experimental conditions by placing in 15‐mL Falcon tubes: (1) one unirradiated D. melanogaster male and one D. melanogaster female; (2) one irradiated D. melanogaster male at 80 Gy and one D. melanogaster female; (3) one irradiated D. melanogaster male at 80 Gy and one D. suzukii female; and (4) one D. suzukii male and one D. suzukii female. In this way, it has been possible to understand the average courtship time of D. melanogaster males with conspecific and heterospecific females, and investigate if the irradiated D. melanogaster males court D. suzukii females as much as D. suzukii males. In the choice test, we set up an experimental condition by placing in 15‐mL Falcon tubes one D. suzukii female, one D. suzukii male and one irradiated D. melanogaster male, and analyzing the courtship behavior of D. melanogaster males and D. suzukii males separately when they co‐occurred.
The experiments were carried out with virgin individuals selected as described above. After 5 min acclimatation, we recorded 10 min of the individual's behavior with a Tough TG‐6 camera (Olympus, Tokyo, Japan). The videos were used to analyze the courtship behavior elements of D. melanogaster and D. suzukii males 34 , 35 using BORIS software. 36 Twenty replicates were carried out.
Second, we investigated if irradiated D. melanogaster males were able to mate with D. suzukii females and impregnate them. We set up two experimental conditions in 50‐mL Falcon tubes with food substrate: a control condition where we placed one virgin D. suzukii female alone, to investigate if females lay unfertilized eggs; and an experimental condition where we placed one virgin D. suzukii female and one irradiated D. melanogaster male at 80 Gy as described previously. We left the individuals inside the tubes for 6 days, to allow the females to oviposit. After that time, we removed the individuals and, through a EZ4W stereomicroscope (Leica, Wetzlar, Germany) at 5× magnification, we checked daily the possible occurrence of eggs laid by D. suzukii females in food substrates. If present, eggs were photographed with a stereomicroscope digital camera, and monitored for eclosion and for eventual larval development. Forty replicates were carried out in both conditions.
2.4. Effect of irradiated D. melanogaster males on D. suzukii fitness
We evaluated whether the reproductive interactions between D. melanogaster males and D. suzukii individuals result in fitness costs for D. suzukii females. To this end, we set up three experimental setting by placing in entomological cages (15 × 15 × 15 cm): (1) five pairs of unirradiated virgin D. suzukii (selected as described above) without D. melanogaster males; (2) 40 irradiated D. melanogaster males and five pairs of virgin D. suzukii to test an 8:1 over‐flooding ratio (sterile: WT males, OFR); and (3) 60 irradiated D. melanogaster males with five pairs of virgin D. suzukii to test an OFR of 12:1. 20 Then, we compared the number of newborn individuals that emerged from cages with only D. suzukii pairs and from cages where D. suzukii pairs plus irradiated D. melanogaster males co‐occurred.
2.5. Data analysis
In order to evaluate the effect of the irradiation doses on the sterility degree of D. melanogaster adults, a GLM model (generalized linear model; package MASS) 37 was applied. We used the irradiation dose of D. melanogaster adults as a fixed effect (0, 60, 80 Gy) on the offspring produced by D. melanogaster females (the response variable), which was recorded as an event with a continuous distribution. We analyzed the offspring produced with a negative binomial distribution applied to the GLM model. The model family was selected comparing the aikake information criterion (AIC) and Bayesian information criterion (BIC) estimators and the likelihood ratio test. Tukey's multiple comparisons of means was performed as a post hoc test using the multcomp package in R. 38
In order to evaluate the effect of the irradiation doses on the longevity of D. melanogaster males, survival distributions of the four D. melanogaster groups (‘Home’ and ‘Trip’ controls, ‘60 Gy’, ‘80 Gy’) were computed using the Kaplan–Meier method. 39 To this end, the survival and survminer R packages were used. 40 The differences between survival distributions were estimated using the log‐rank test.
In order to analyze the courtship behavior data, we used a GLM model with a negative binomial distribution, according to model selection estimators, for comparing the courtship time among no‐choice conditions. As a post hoc test, we performed Tukey's honestly significant difference test. To compare the time spent by D. melanogaster and D. suzukii males in courting D. suzukii females in the choice test, we used the Wilcoxon–Mann–Whitney U‐test (dplyr package).
Regarding the effect of D. melanogaster on D. suzukii fitness, a GLMM analysis was performed using the D. suzukii offspring as a response variable with a continuous distribution. In the analysis, the number of D. melanogaster males (0, 40 or 60 individuals) in each replicate was considered a fixed effect (the explanatory variables). The age of the experimental individuals (48, 72 and 96 h old) was considered a random effect because they are a sampling of infinite possible combinations, and we were not interested in studying them as such but instead in identifying whether they constituted a source of significant variability. We applied a negative binomial distribution to the GLMM effect, comparing the models based on the optimal parsimony principle (AIC and BIC estimators) and the likelihood ratio test. We performed the Tukey multiple comparisons of means as a post hoc test.
All analyses were performed using R v3.6.2. 41
3. RESULTS
3.1. Effect of irradiation dose on sterility and longevity of D. melanogaster
In the mating trials aimed to assess the effect of irradiation on male sterility, the mean number of offspring produced by D. melanogaster females was 128.66 (± 10.67) (mean ± SE) in the ‘Home’ and 149.9 (± 18.07) in the ‘Trip’ control conditions. In the treatments at 60 and 80 Gy, the number of offspring produced by D. melanogaster females was 35.81 (± 4.08) and 29.84 (± 3.62), respectively (Fig. 1). The GLM model showed a significant effect of irradiation dose on the number of offspring produced by D. melanogaster females when the males were irradiated at 60 Gy and 80 Gy (Table 1). The Tukey multiple comparisons tests showed a significant reduction in D. melanogaster offspring produced when D. melanogaster males were irradiated at 60 Gy (z = −6.268, P = < 0.001) and 80 Gy (z = − 9.335, P = < 0.001) than in the ‘Home’ condition. There was a significant reduction also with irradiated males at 60 Gy (z = −6.784, P = < 0.001) and 80 Gy (z = − 9.702, P = < 0.001) than in the ‘Trip’ condition. A significant reduction in D. melanogaster offspring also was observed between the conditions with males irradiated at 60 and 80 Gy (z = −3.455, P = 0.003). There were no significant differences between the two control conditions (P > 0.05) (Fig. 1).
Figure 1.
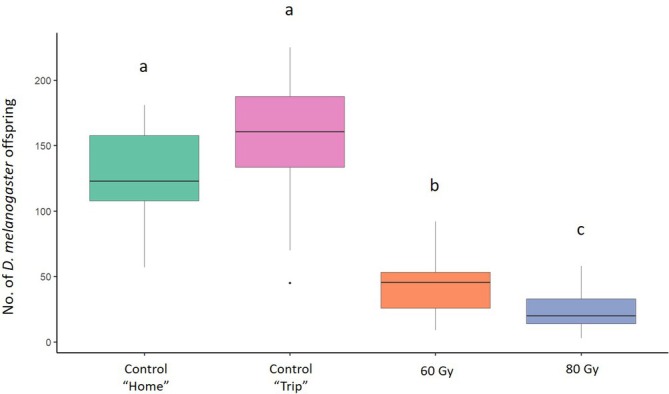
Effect of irradiation on sterility of Drosophila melanogaster males. Comparison between the offspring originated from unirradiated D. melanogaster females coupled with unirradiated D. melanogaster males (green column and pink column, respectively) and irradiated at 60 and 80 Gy (orange and blue column, respectively). Different letters indicate significant differences by Tukey multiple comparisons tests (P < 0.05). Boxplots show median values (middle line), interquartile range (box) and range values.
Table 1.
Effect of irradiation doses on sterility of Drosophila melanogaster males and females. Generalized linear model (GLM) values are shown. Values in boldface indicate significant differences (P < 0.05).
| Fixed effects | Estimate | ±SE | z‐value | P‐value |
|---|---|---|---|---|
| Males | ||||
| (Intercept) | 4.8572 | 0.1306 | 37.191 | <2e−16 |
| Control ‘Trip’ | 0.1527 | 0.1934 | 0.790 | 0.43 |
| IRR60 | −1.1004 | 0.1756 | −6.268 | 3.66e−10 |
| IRR80 | −1.6960 | 0.1817 | −9.335 | <2e−16 |
| Females | ||||
| (Intercept) | 4.2850 | 0.1428 | 30.009 | <2e−16 |
| Control ‘Trip’ | 0.1144 | 0.2016 | 0.568 | 0.57 |
| IRR80 | −1.9741 | 0.2109 | −9.358 | <2e−16 |
In the mating trials aimed to assess the effect of irradiation on female sterility, the mean number of offspring produced by D. melanogaster females was 72.6 (± 10.34) (mean ± SE) in the ‘Home’ and 81.4 (± 13.86) in the ‘Trip’ conditions. In the treatments at 80 Gy, the number of offspring produced by D. melanogaster females was 10.08 (± 1.39) (Fig. 2). The GLM model showed a significant effect of the irradiation dose on the number of offspring produced by irradiated D. melanogaster females at 80 Gy (Table 1). The Tukey multiple comparisons tests showed a significant reduction in D. melanogaster offspring produced when D. melanogaster females were irradiated at 80 Gy (z = − 9.358, P = < 1e‐04) than in the ‘Home’ condition. There was a significant reduction also with irradiated females at 80 Gy (z = − 9.917, P = < 1e−04) than in the ‘Trip’ condition. There were no significant differences between the two control conditions (P > 0.05) (Fig. 2).
Figure 2.
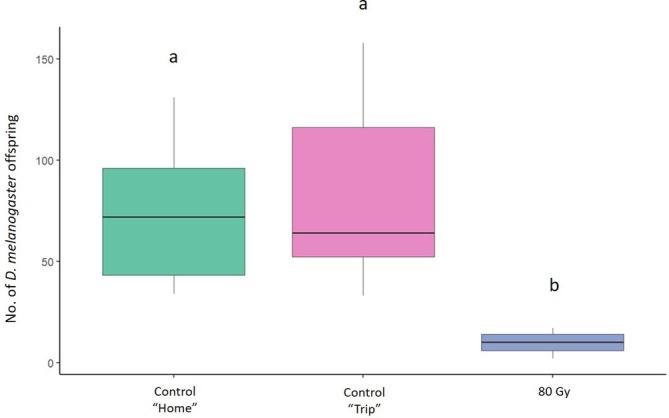
Effect of irradiation on sterility of Drosophila melanogaster females. Comparison between the offspring originated from unirradiated D. melanogaster females coupled with unirradiated D. melanogaster males (green and pink columns, respectively) and irradiated females at 80 Gy (blue column). Different letters indicate significant differences by Tukey multiple comparisons tests (P < 0.05). Boxplots show median values (middle line), interquartile range (box) and range values.
The Kaplan–Meier survival curves showed significant differences in the lifespan of D. melanogaster males among treatments (60 Gy, 80 Gy, ‘Trip’ and ‘Home’ cage control groups) (Mantel–Cox log‐rank; χ2 = 17.8, d.f. = 3, P = 5e−04) (Fig. 3). The pairwise comparisons test showed that the individuals irradiated at 60 and 80 Gy had higher survival probability than control individuals. Significant differences were indeed observed between the males irradiated at 60 Gy and those of the ‘Home’ (P = 0.0308) and the ‘Trip’ (P = 0.0151) groups, as well as between the males irradiated at 80 Gy and those of the two control groups (’Home’ P = 0.0053; ‘Trip’ P = 0.0028). No significant differences were observed between the two control groups (P = 0.7859) and between the two experimental conditions (P = 0.8541).
Figure 3.
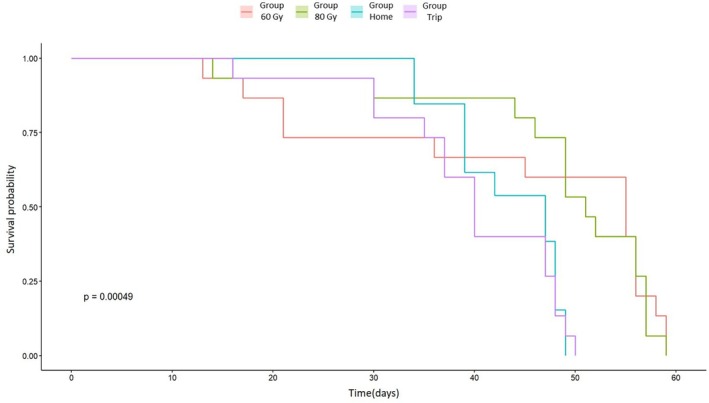
Effect of different treatments on the longevity of Drosophila melanogaster males. Kaplan–Meier survival curves for each condition are shown. The P‐value of the log‐rank test also is shown.
3.2. Mating performance of the irradiated D. melanogaster males
The mating performance of irradiated D. melanogaster males toward D. suzukii females was investigated by courtship and mating trials.
First, we investigated if irradiated D. melanogaster males were able to court D. suzukii females as much as D. suzukii males in no‐choice and choice tests. Under all experimental conditions the typical behavior elements during courtship were observed, including ‘orientation’ (i.e. the male approaches the female, quivering the abdominal and scissoring its wings), ‘tapping,’ (i.e. the male hits the female abdomen, or middle and hind legs by stretching his foreleg); ‘wing spreading’ and ‘wing scissoring’ (i.e. the male is oriented toward the female front, quivers with the abdomen and scissors his wings keeping them at 180° for seconds to expose the upper side and wing spot toward to female). 34 , 35
In the no‐choice tests, the average courtship time spent by unirradiated D. melanogaster males courting unirradiated D. melanogaster females was 23.37% (± 5.19)(mean ± SE); the average courtship time spent by irradiated D. melanogaster males at 80 Gy courting unirradiated D. melanogaster females was 20.84% (± 3.61); the average courtship time spent by irradiated D. melanogaster males courting D. suzukii females was 26.41% (± 5.90); the average courtship time spent by D. suzukii males courting D. suzukii females was 35.36% (± 8.17). The GLM model showed no significant differences in the average courtship time among conditions. The Tukey multiple comparisons tests showed no significant differences among the four conditions (P > 0.05) (Fig. 4).
Figure 4.
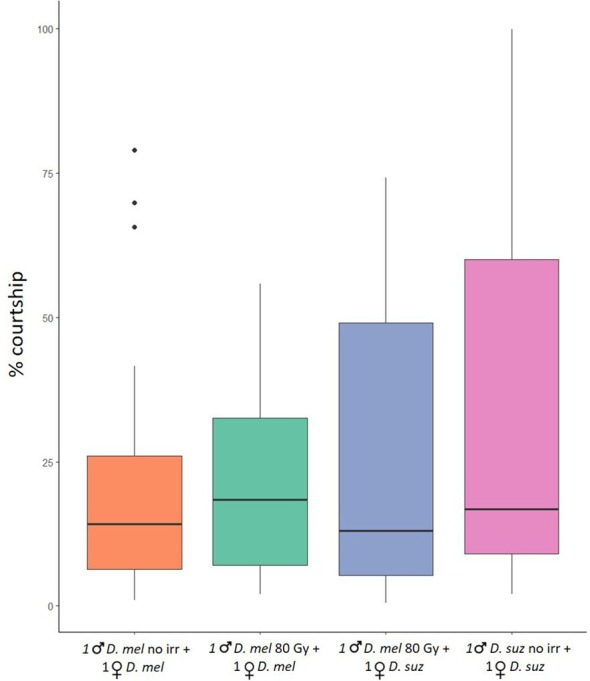
Mating performance of irradiated Drosophila melanogaster males in no‐choice tests. Time spent in conspecific and heterospecific courtship behavior by unirradiated and irradiated D. melanogaster males, and unirradiated D. suzukii males. Percentage of the total time spent courting D. melanogaster females by unirradiated D. melanogaster males (orange column – conspecific courtship); percentage of the total time spent courting D. melanogaster females by irradiated D. melanogaster males (green column – conspecific courtship); percentage of the total time spent courting D. suzukii females by irradiated D. melanogaster males (blue column – heterospecific courtship); percentage of the total time spent courting D. suzukii females by unirradiated D. suzukii males (pink column – conspecific courtship). Tukey multiple comparisons tests (P > 0.05). suz = suzukii; mel = melanogaster. Boxplots show median values (middle line), interquartile range (box) and range values.
In choice tests, where D. melanogaster males and D. suzukii males were placed together in 15‐mL falcon tubes with D. suzukii females, the average courtship time spent by D. melanogaster males was 19.43% (± 6.16) and the average courtship time spent by D. suzukii males was 9.52% (± 2.29). There were no significant differences between the courtship time of D. suzukii and D. melanogaster males (Wilcoxon–Mann–Whitney test W = 194.5, P = 0.9105) (Fig. 5).
Figure 5.
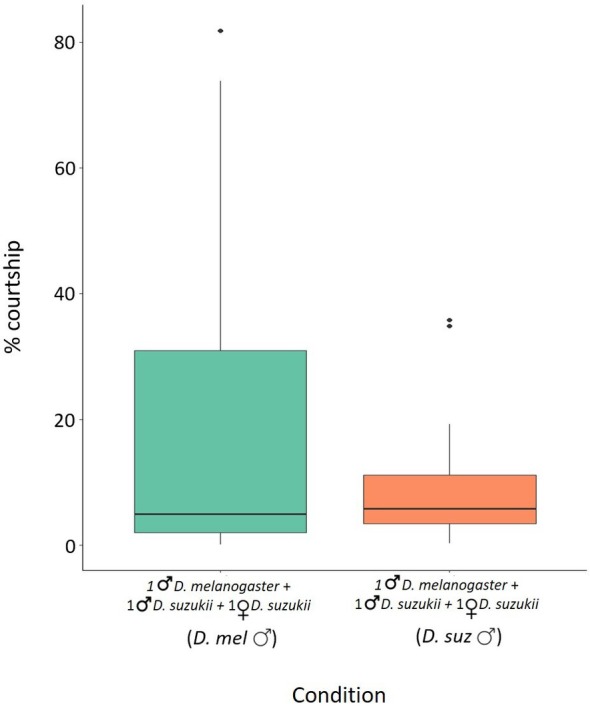
Mating performance of irradiated Drosophila melanogaster males in choice tests. Time spent in courting Drosophila suzukii females by D. suzukii and irradiated D. melanogaster males. Percentage of the total time spent courting D. suzukii females by irradiated D. melanogaster males and D. suzukii males when placed together (green and orange column, respectively) Wilcoxon–Mann–Whitney U‐test P > 0.05. Boxplots show median values (middle line), interquartile range (box) and range values.
Second, we investigated if irradiated D. melanogaster males could mate with and impregnate D. suzukii females. To this end, we analyzed the occurrence of oviposited eggs by virgin D. suzukii females confined alone and with irradiated D. melanogaster males. In monitoring the food substrates of each replicate daily, we found no eggs in 39 of 40 substrates in the condition with only virgin D. suzukii females. In the condition with virgin D. suzukii females paired with irradiated D. melanogaster males, eggs were found in 12 of 40 replicates (30%), but no subsequent larval development was observed.
3.3. Effect of irradiated D. melanogaster males on D. suzukii fitness
The mean number of the individuals originating from five pairs of D. suzukii was 37.95 (± 3.62) (± SE) in the control tests, whereas it was 16.11 (± 5.58) and 13.89 (± 3.69), when 40 and 60 irradiated D. melanogaster males were added, respectively (Fig. 6). The GLMM model showed a significant effect on the D. suzukii offspring (Table 2). The Tukey multiple comparisons tests showed a significant reduction of D. suzukii offspring when 40 (z = −2.758, P = 0.0160) and 60 (z = −3.218, P = 0.0038) irradiated D. melanogaster males were placed with D. suzukii couples (Fig. 6), whereas no significant differences were observed between treatments with 40 and 60 D. melanogaster irradiated males (z = −0.408, P = 0.9118).
Figure 6.
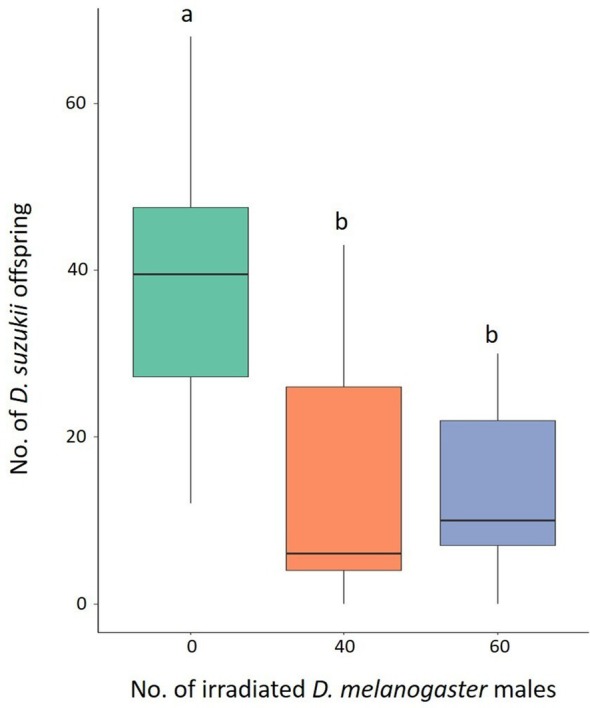
Effect of irradiated Drosophila melanogaster males on D. suzukii fitness. Offspring originated from five pairs of D. suzukii without irradiated D. melanogaster males (green column) and with 40 (orange column) or 60 (blue column) males. Different letters mean significant differences by Tukey multiple comparisons tests (P < 0.05). Eighteen replicates were carried out for the condition without D. melanogaster males. Nine replicates were carried out for each of the two conditions involving the presence of D. melanogaster males. Boxplots show median values (middle line), interquartile range (box) and range values.
Table 2.
Effect of irradiated Drosophila melanogaster males on D. suzukii fitness. Generalized linear mixed model (GLMM) model values are shown. Values in boldface indicate significant differences (P < 0.05).
| Fixed effects | Estimate | ±SE | z‐value | P‐value |
|---|---|---|---|---|
| (Intercept) | 3.6361 | 0.1756 | 20 711 | <2−‐16 |
| 40 D. melanogaster males | −0.8566 | 0.3105 | −2.758 | −0.00581 |
| 60 D. melanogaster males | −1.0050 | 0.3123 | −3.218 | −0.00129 |
4. DISCUSSION
4.1. Irradiation dose, D. melanogaster male sterility and longevity
Heterospecific SIT and SIT approaches can be developed under similar theoretical frameworks. Irradiation dose is an important factor affecting the sterility degree, male longevity, and performance. 42 It is, therefore, necessary to find a sufficiently high dose to induce sterility that also has a minimum impact on the biological quality of the irradiated males. 43
We found high levels of sterility in both D. melanogaster males and females (Figs 1 and 2). As observed also in other insect species, 44 , 45 , 46 we found that females showed higher sensitivity to irradiation than males. Although it affected fertility, irradiation did not reduce male longevity. Interestingly, irradiated males exhibited a longer lifespan compared to control individuals. This finding contrasts with some results reported in the literature on Diptera. For example, Lanouette et al. 47 observed no significant differences in the longevity of D. suzukii males exposed to increasing irradiation doses (30–120 Gy). By contrast, Chen et al. 48 reported that longevity in D. suzukii began to decrease at doses exceeding 90 Gy. Likewise, in Aedes aegypti, survival of irradiated males was significantly reduced compared to controls. 41 , 49 Despite the disparity between our findings and those of other studies, the phenomenon of increased longevity following irradiation has been documented in other species, including some Anopheles species 50 , 51 and the stink bug pest Bagrada hilaris, 52 where irradiated males at 80 Gy exhibited a longer average lifespan than control individuals. The reasons for such results could be multiple, ranging from molecular and cellular changes to biological, physical and human‐related factors. 53 , 54 Understanding the mechanisms underlying this result would certainly warrant further in‐depth analysis. However, within the context of this study, sterile males with stable or extended longevity are likely to remain in the environment longer, effectively competing with fertile males and contributing to population suppression over a prolonged period.
As observed in previous studies, 32 , 33 we also obtained residual fertility in our results. It will be interesting to test if higher irradiation doses lead to higher male sterility without reducing male performance. Notably, the purpose of male sterilization is different in SIT and h‐SIT approaches. In the former, male sterilization is the way to introduce sterility into the wild pest population. Residual fertility could hamper the SIT effectiveness as wild females could mate with released unsterilized males and produce fertile offspring. 20 , 55 , 56 Conversely, in the h‐SIT, the introduction of sterility in the target pest population is ensured by the reproductive postzygotic isolation mechanisms between the released heterospecific males and wild females of the target species. 10 , 21 Male sterilization avoids potential adverse environmental effects if non‐native or potentially pest species are used in the h‐SIT. In the case of h‐SIT using D. melanogaster, residual fertility would not reduce the released males' effectiveness because unirradiated males would induce sterility in D. suzukii females. 24 Furthermore, there are no concerns about the potential release of fertile D. melanogaster males. Indeed, D. melanogaster is not considered an agricultural pest as the female oviposits on rotten fruits. 57
4.2. Mating performance of the irradiated D. melanogaster males and effect on D. suzukii fitness
Heterospecific SIT is based on the sterility between heterospecifics. The unfertile mating between the released heterospecific males and wild females leads to a progressive decline of the target pest population. 19 , 20 Therefore, the potential use of h‐SIT as a control method strictly depends on the mating ability of the released irradiated males. 3 Our results supported that the irradiated D. melanogaster males at 80 Gy can be effective at different stages of mate acquisition, from courtship to mating. The analysis of the courtship behavior showed that D. melanogaster males courted D. suzukii females as much as D. suzukii males (Figs 4 and 5). Notably, irradiated D. melanogaster males exhibited comparable average courtship time toward D. suzukii females and conspecific females (Fig. 4). This behavior may be driven by reproductive interference, primarily through misdirected courtship by D. melanogaster males. Male insects, including D. melanogaster, often exhibit less selective mate choice owing to their lower reproductive investment compared to females. 58 This tendency can lead to heterospecific courtship behaviors. Additionally, the larger body size of D. suzukii females, often associated with higher fecundity, could make them appear more attractive to D. melanogaster males, as body size is commonly used as a proxy for reproductive potential. 59 , 60
The results of mating trials showed that in the control condition with virgin D. suzukii females alone, there were no eggs oviposited in all 40 replicates with only one exception; larval development and production of D. suzukii individuals were observed in that replicate, suggesting that we inadvertently selected a D. suzukii female that was likely to have mated before the experiment. Notably, we found that in the test conditions (one D. suzukii female placed with one irradiated D. melanogaster male), D. suzukii females oviposited eggs in 30% of the replicates (12 of 40), and no larval development was observed in any of the replicates. This result confirms that irradiated D. melanogaster males could couple, mate and impregnate D. suzukii females, but the postzygotic isolation between D. suzukii and D. melanogaster is complete. 24
The results of our study clearly show that irradiated D. melanogaster males can lead to reproductive interference in D. suzukii. Indeed, D. suzukii had significantly reduced offspring in the presence of irradiated D. melanogaster males, irrespective of the species ratio used (Fig. 6). The reproductive interference on D. suzukii by irradiated D. melanogaster males is likely to be the result of interference during courtship and mating, as described above. However, other factors could contribute to the offspring reduction of D. suzukii by D. melanogaster males. First, it has been shown that D. melanogaster, during courtship, produces cis‐vaccenyl acetate (cVA), which acts as a repellent to D. suzukii female for laying eggs. 61 , 62 Furthermore, D. melanogaster males release substances through seminal fluid that reduce female remating in homospecific matings. 63 , 64 This latter phenomenon deserves future studies. Indeed, if it also occurs in heterospecific matings between D. suzukii and D. melanogaster, it would be of great interest for h‐SIT, preventing D. suzukii females from remating with co‐specific wild males.
4.3. ‘The importance of being melanogaster’
The above arguments make us optimistic about using D. melanogaster in h‐SIT against D. suzukii and in testing this approach under well‐isolated confined‐field facilities.
However, let us highlight a further issue that makes h‐SIT particularly exciting to explore: D. melanogaster is a model species. The huge amount of data available on the genetics and biology of D. melanogaster can be exploited to address classical problems in developing SIT. 65 , 66 , 67 Despite considerable research, for most species where SIT is used, we lack a deep understanding of the effects of radiation, rearing conditions (diet, light, density), as well as the effects of environmental conditions on individual biology and male performance in the field. Furthermore, some authors have emphasized the need to build ‘a better male’ 68 to overcome the prevailing approach based on overflooding ratios (sterile: wild males) in the field, which can hamper the economic feasibility of SIT. In this context, D. melanogaster, whose biology has been dissected at the molecular, cellular and physiological levels, could significantly contribute to unveiling these critical issues, making D. melanogaster–suzukii a potential model system for the development of heterospecific SIT.
5. CONCLUSIONS
Reproductive interaction between heterospecific individuals can have multiple effects on pest management. When pre‐mating barriers between interfertile species are weak, hybridization can occur and significantly threaten pest control. It can favor pest outbreaks by improving population fitness and adaptation to environmental conditions or by leading to control failures as a consequence of the introgression of pesticide resistance alleles from one species to another. 69 , 70 However, reproductive interaction can be beneficial for pest management. 8 , 22 Here, we highlight the potential use of reproductive interference in pest management by h‐SIT. This approach would broaden our control toolbox by offering additional options to control important agricultural pests. In the last decades, the new sequencing technologies combined with classical reproductive incompatibility studies have improved our knowledge of pre‐ and postzygotic isolation in sibling pest species, allowing us to identify even more numerous potential systems for the development and application of the h‐SIT control approach. 71 , 72
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
DP and MC conceived the study. DP, MC and DC designed the study. FC, VM, JS and AV collected the data. FC and VM analyzed the data. DP wrote the first draft of the manuscript. All authors contributed critically to the drafts and approved the final manuscript.
ACKNOWLEDGEMENTS
We thank Elisa Michelangeli and Mark Eltenton for their technical help. This study was carried out within the Agritech National Research Center and received funding from the European Union Next‐Generation EU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR) – MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4 – D.D. 1032 17 June 2022, CN00000022). This manuscript reflects only the authors' views and opinions, neither the European Union nor the European Commission can be considered responsible for them. FC was funded by PhD resources within PON ‘RICERCA E INNOVAZIONE’ 2014–2020, AZIONE IV.5 ‘DOTTORATI SU TEMATICHE GREEN’, D.M. 1061 10 August 2021. Open access publishing facilitated by Universita degli Studi di Roma La Sapienza, as part of the Wiley ‐ CRUI‐CARE agreement.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Figshare at: 10.6084/m9.figshare.28805657.
REFERENCES
- 1. Cain ML, Bowman WD and Hacker SD, Ecology, 2nd edn. Oxford University Press, Incorporate, Oxford, p. 648 (2011). [Google Scholar]
- 2. Hoddle MS and Van Driesche RG, Biological control of insect pests, in Encyclopedia of Insects. Academic Press, Cambridge MA, pp. 91–101 (2009). [Google Scholar]
- 3. Gröning J and Hochkirch A, Reproductive interference between animal species. Q Rev Biol 83:257–282 (2008). [DOI] [PubMed] [Google Scholar]
- 4. Kishi S, Nishida T and Tsubaki Y, Reproductive interference determines persistence and exclusion in species interactions: sexual interference governs competition. J Anim Ecol 78:1043–1049 (2009). [DOI] [PubMed] [Google Scholar]
- 5. Kyogoku D, Reproductive interference: ecological and evolutionary consequences of interspecific promiscuity. Popul Ecol 57:253–260 (2015). [Google Scholar]
- 6. Shuker DM and Burdfield‐Steel ER, Reproductive interference in insects: reproductive interference in insects. Ecol Entomol 42:65–75 (2017). [Google Scholar]
- 7. Gómez‐Llano M, Germain RM, Kyogoku D, McPeek MA and Siepielski AM, When ecology fails: how reproductive interactions promote species coexistence. Trends Ecol Evol 36:610–622 (2021). 10.1016/j.tree.2021.03.003. [DOI] [PubMed] [Google Scholar]
- 8. Mitchell C, Leigh S, Alphey L, Haerty W and Chapman T, Reproductive interference and Satyrisation: mechanisms, outcomes and potential use for insect control. J Pest Sci 95:1023–1036 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ribeiro JMC, Can satyrs control pests and vectors? J Med Entomol 25:431–440 (1988). [DOI] [PubMed] [Google Scholar]
- 10. Klassen W, Curtis CF and Hendrichs J, History of the sterile insect technique, in Sterile insect technique. CRC Press, Boca Raton, pp. 1–44 (2021). [Google Scholar]
- 11. Davidson G, The potential use of sterile hybrid males for the eradication of member species of the Anopheles gambiae complex. Bull World Health Organ 40:221–228 (1969). [PMC free article] [PubMed] [Google Scholar]
- 12. Davidson G, Odetoyinbo JA, Colussa B and Coz J, Field attempt to assess the mating competitiveness of sterile males produced by crossing 2 member species of the Anopheles gambiae complex. Bull World Health Organ 42:55–67 (1970). [PMC free article] [PubMed] [Google Scholar]
- 13. Knipling EF, Screwworm eradication: concepts and research leading to the sterile‐male method, in Smithsonian Report for 1958. Smithsonian Inst., Washington DC, pp. 409–418 (1959). [Google Scholar]
- 14. Enkerlin WR, Impact of fruit fly control programmes using the sterile insect technique, in Sterile insect technique. CRC Press, Boca Raton, pp. 979–1006 (2021). [Google Scholar]
- 15. Feldmann U, Dyck VA, Mattioli RC, Jannin J and Vreysen MJB, Impact of tsetse fly eradication programmes using the sterile insect technique, in Sterile Insect Technique. CRC Press, Boca Raton, pp. 1051–1080 (2021). [Google Scholar]
- 16. Lees RS, Carvalho DO and Bouyer J, Potential impact of integrating the sterile insect technique into the fight against disease‐transmitting mosquitoes, in Sterile Insect Technique. CRC Press, Boca Raton: 10811118 (2021). [Google Scholar]
- 17. Simmons GS, Suckling DM, Carpenter JE, Addison MF, Dyck VA and Vreysen MJB, Improved quality management to enhance the efficacy of the sterile insect technique for lepidopteran pests. J Appl Entomol 134:261–273 (2010). [Google Scholar]
- 18. Vargas‐Terán M, Spradbery JP, Hofmann HC and Tweddle NE, Impact of screwworm eradication programmes using the sterile insect technique, in Sterile insect technique. CRC Press, Boca Raton, pp. 949–978 (2021). [Google Scholar]
- 19. Bourtzis K and Vreysen MJB, Sterile insect technique (SIT) and its applications. Insects 12:638 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dyck VA, Hendrichs J and Robinson AS, Sterile Insect Technique: Principles and Practice in Area‐Wide Integrated Pest Management, 2nd edn. CRC Press, Boca Raton: (2021). [Google Scholar]
- 21. McInnis DO, Rendon P, Jang E, Van Sauers‐Muller A, Sugayama R and Malavasi A, Interspecific mating of introduced, sterile Bactrocera dorsalis with Wild B. Carambolae (Diptera: Tephritidae) in Suriname: a potential case for cross‐species sterile insect technique. Ann Entomol Soc Am 92:758–765 (1999). 10.1093/aesa/92.5.758. [DOI] [Google Scholar]
- 22. Honma A, Kumano N and Noriyuki S, Killling two bugs with one stone: a perspective for targeting multiple pest species by incorporating reproductive interference into sterile insect technique: reproductive interference and SIT. Pest Manag Sci 75:571–577 (2019). [DOI] [PubMed] [Google Scholar]
- 23. Tsurui‐Sato K, Fujimoto S, Deki O, Suzuki T, Tatsuta H and Tsuji K, Reproductive interference in live‐bearing fish: the male guppy is a potential biological agent for eradicating invasive mosquitofish. Sci Rep 9:5439 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cerasti F, Mastrantonio V, Dallai R, Cristofaro M and Porretta D, Applying Satyrization to insect Pest control: the case of the spotted wing drosophila, Drosophila suzukii Matsumura. Insects 14:569 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fraimout A, Debat V, Fellous S, Hufbauer RA, Foucaud J, Pudlo P et al., Deciphering the routes of invasion of Drosophila suzukii by means of ABC random forest. Mol Biol Evol 34:980–996 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tait G, Mermer S, Stockton D, Lee J, Avosani S, Abrieux A et al., Drosophila suzukii (Diptera: Drosophilidae): a decade of research towards a sustainable integrated Pest management program. Brewer M, ed. by. J Econ Entomol 114:1950–1974 (2021). [DOI] [PubMed] [Google Scholar]
- 27. Bolda M, Goodhue RE and Zalom FG, Spotted wing drosophila: potential economic impact of a newly established pest. Agric Resour Econ 13:5–8 (2010). [Google Scholar]
- 28. De Ros G, Conci S, Pantezzi T and Savini G, The economic impact of invasive pest Drosophila suzukii on berry production in the province of Trento, Italy. J Berry Res 5:89–96 (2015). [Google Scholar]
- 29. Wolf S, Collatz J, Enkerli J, Widmer F and Romeis J, Assessing potential hybridization between a hypothetical gene drive‐modified Drosophila suzukii and nontarget drosophila species. Risk Anal 43:1921–1932 (2023). [DOI] [PubMed] [Google Scholar]
- 30. Silva‐Soares NF, Nogueira‐Alves A, Beldade P and Mirth CK, Adaptation to new nutritional environments: larval performance, foraging decisions, and adult oviposition choices in Drosophila suzukii . BMC Ecol 17:21 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baccaro S and Cemmi A, Gamma Irradiation CALLIOPE Facility at ENEA – Casaccia Research Centre. ENEA, Rome, p. 48 (2019). [Google Scholar]
- 32. Nelson FRS, Drosophila melanogaster: effects of gamma radiation on fecundity and longevity. J Econ Entomol 66:257–258 (1973). [DOI] [PubMed] [Google Scholar]
- 33. Henneberry TJ, Effects of gamma radiation on the fertility and longevity of Drosophila melanogaster . J Econ Entomol 56:279–281 (1963). [Google Scholar]
- 34. Lasbleiz C, Ferveur JF and Everaerts C, Courtship behaviour of Drosophila melanogaster revisited. Anim Behav 72:1001–1012 (2006). [Google Scholar]
- 35. Revadi S, Lebreton S, Witzgall P, Anfora G, Dekker T and Becher P, Sexual behavior of Drosophila suzukii . Insects 6:183–196 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Friard O and Gamba M, boris: a free, versatile open‐source event‐logging software for video/audio coding and live observations. Methods Ecol Evol 7:1325–1330 (2016). [Google Scholar]
- 37. Ripley B, Venables B, Bates DM, Hornik K, Gebhardt A, Firth D et al., Package ‘mass’. Cran r 538:113–120 (2013). [Google Scholar]
- 38. Hothorn T, Bretz F and Westfall P, Simultaneous inference in general parametric models. Biom J 50:346–363 (2008). [DOI] [PubMed] [Google Scholar]
- 39. Kaplan E and Meier P, Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481 (1958). [Google Scholar]
- 40. Kassambara A, Kosinski M and Biecek P, survminer: Drawing Survival Curves using ‘ggplot2. R package version 0.4.9 (2021).
- 41. R Core Team , A Language and Environment for Statistical Computing. R‐Foundation for Computer Statistics, Vienna, Austria: (2019). [Google Scholar]
- 42. Ernawan B, Anggraeni T, Yusmalinar S and Ahmad I, Investigation of developmental stage/age, gamma irradiation dose, and temperature in sterilization of male Aedes aegypti (Diptera: Culicidae) in a sterile insect technique program. J Med Entomol 59:320–327 (2022). [DOI] [PubMed] [Google Scholar]
- 43. Lance DR and McInnis DO, Biological basis of the sterile insect technique, in Sterile Insect Technique. CRC Press, Boca Raton, pp. 113–142 (2021). [Google Scholar]
- 44. Sassù F, Nikolouli K, Pereira R, Vreysen MJB, Stauffer C and Cáceres C, Irradiation dose response under hypoxia for the application of the sterile insect technique in Drosophila suzukii . PLoS One Dec 31;14(12):e0226582 (2019). 14:e0226582 (2019). 10.1371/journal.pone.0226582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arredondo J, Aguirre‐Medina JF, Meza JS, Cancino J and Díaz‐Fleischer F, Does the effect of irradiation dose vary between flies selected and non‐selected to resist desiccation? The case of Anastrepha ludens (Diptera: Tephritidae). J Econ Entomol 113:2679–2687 (2020). [DOI] [PubMed] [Google Scholar]
- 46. Bakri A, Mehta K and Lance DR, Sterilizing insects with ionizing radiation, in Sterile Insect Technique. Principles and Practice in Area‐Wide Integrated Pest Management, ed. by Dyck VA, Hendrichs J and Robinson AS. Springer, Dordrecht, the Netherlands, pp. 233–269 (2005). [Google Scholar]
- 47. Lanouette G, Brodeur J, Fournier F, Martel V, Vreysen M, Cáceres C et al., The sterile insect technique for the management of the spotted wing drosophila, Drosophila suzukii: establishing the optimum irradiation dose. Ling E, editor. PLoS One 12:e0180821 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen Y, Pan H, Li J, Pan D, Liu P and Hu H, Effects of irradiated sterile male and mating sequence on the fertility of Drosophila suzukii (Diptera: Drosophilidae). J Insect Sci 22:22 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yamada H, Maïga H, Kraupa C, Somda NSB, Mamai W, Wallner T et al., Radiation dose‐fractionation in adult Aedes aegypti mosquitoes. Parasite 30:5 (2023). 10.1051/parasite/2023005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Abdel‐Malek AA, Tantawy AO and Wakid AM, Studies on the eradication of anopheles pharoensis Theobald by the sterile‐male technique using Cobalt‐60. I. Biological effects of gamma radiation on the different developmental stages. J Econ Entomol 59:672–678 (1966). [DOI] [PubMed] [Google Scholar]
- 51. Helinski ME, Parker AG and Knols BG, Radiation‐induced sterility for pupal and adult stages of the malaria mosquito anopheles arabiensis . Malar J 5:41 (2006). 10.1186/1475-2875-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cristofaro M, Sforza RFH, Roselli G, Paolini A, Cemmi A, Musmeci S et al., Effects of gamma irradiation on the fecundity, fertility, and longevity of the invasive stink bug Pest Bagrada hilaris (Burmeister) (Hemiptera: Pentatomidae). Insects 13:787 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bakri A, Heather N, Hendrichs J and Ferris I, Fifty years of radiation biology in entomology: lessons learned from IDIDAS. Ann Entomol Soc Am 98:1–12 (2005). [Google Scholar]
- 54. Arthur V, Machi A and Mastrangelo T, Ionizing radiations in entomology, inEvolution of ionizing radiation research. IntechOpen, pp. 213–234 (2015). [Google Scholar]
- 55. Plá I, de García Oteyza J, Tur C, Martínez MÁ, Laurín MC, Alonso E et al., Sterile insect technique Programme against Mediterranean fruit Fly in the Valencian community (Spain). Insects 12:415 (2021). 10.3390/insects12050415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Oliva CF, Jacquet M, Gilles J, Lemperiere G, Maquart PO, Quilici S et al., The sterile insect technique for controlling populations of Aedes albopictus (Diptera: Culicidae) on Reunion Island: mating vigour of sterilized males. PLoS One 7:e49414 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Markow TA, The secret lives of drosophila flies. Elife 4:e06793 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Arnqvist G and Rowe L, Sexual conflict, Vol. 27. Princeton university press, New Jersey, USA: (2005). [Google Scholar]
- 59. Bonduriansky R, The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol Rev 76:305–339 (2001). [DOI] [PubMed] [Google Scholar]
- 60. Lev A and Pischedda A, Male size does not affect the strength of male mate choice for high‐quality females in Drosophila melanogaster . J Evol Biol 36:1255–1265 (2023). [DOI] [PubMed] [Google Scholar]
- 61. Dekker T, Revadi S, Mansourian S, Ramasamy S, Lebreton S, Becher PG et al., Loss of drosophila pheromone reverses its role in sexual communication in Drosophila suzukii . Proc R Soc Lond B Biol Sci 282:20143018 (2015). 10.1098/rspb.2014.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shaw B, Brain P, Wijnen H and Fountain MT, Reducing Drosophila suzukii emergence through inter‐species competition: Reducing D. Suzukii emergence through competition. Pest Manag Sci 74:1466–1471 (2018). [DOI] [PubMed] [Google Scholar]
- 63. Manning A, A sperm factor affecting the receptivity of drosophila melanogaster females. Nature 194:252–253 (1962). [Google Scholar]
- 64. Gromko MH, Newport MEA and Kortier MG, Sperm dependence of female receptivity to Remating in Drosophila melanogaster . Evolution 38:1273–1282 (1984). [DOI] [PubMed] [Google Scholar]
- 65. Das SR, Maselko M, Upadhyay A and Smanski MJ, Genetic engineering of sex chromosomes for batch cultivation of non‐transgenic, sex‐sorted males. PLoS Genet 16:e1009180 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liu J, Rayes D and Akbari OS, A fluorescent sex‐sorting technique for insects with the demonstration in Drosophila melanogaster . GEN Biotechnol 3:35–44 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lutrat C, Giesbrecht D, Marois E, Whyard S, Baldet T and Bouyer J, Sex sorting for Pest control: It's raining men! Trends in parasitology. 35:649–662 (2019). [DOI] [PubMed] [Google Scholar]
- 68. Pérez‐Staples D, Shelly TE and Yuval B, Female mating failure and the failure of ‘mating’ in sterile insect programs. Entomol Exp Appl 146:66–78 (2013). [Google Scholar]
- 69. Corrêa AS, Cordeiro EM and Omoto C, Agricultural insect hybridization and implications for pest management. Pest Manag Sci 75:2857–2864 (2019). [DOI] [PubMed] [Google Scholar]
- 70. Porretta D and Canestrelli D, The ecological importance of hybridization. Trends Ecol Evol 38:1097–1108 (2023). [DOI] [PubMed] [Google Scholar]
- 71. Ivey V and Hillier NK, Hybridization in heliothine moths: impacts on reproduction, pheromone communication, and pest management. Front Ecol Evol 11:1208079 (2023). [Google Scholar]
- 72. Saveer AM, Becher PG, Birgersson G, Hansson BS, Witzgall P and Bengtsson M, Mate recognition and reproductive isolation in the sibling species Spodoptera littoralis and Spodoptera litura . Front Ecol Evol 2:18 (2014). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in Figshare at: 10.6084/m9.figshare.28805657.


