Abstract
Background.
Severe injury induces systemic microvascular impairment that reduces microvascular blood flow (MBF), even after resuscitation to normal blood pressure. These changes are associated with organ dysfunction and death, but the underlying causes and potential therapeutic approaches to address them remain unclear. Two possible contributors are hyperadhesive VWF secretion from an activated endothelium and oxidative modification of hemostatic proteins. N-acetylcysteine has been shown to address both of these processes and increase MBF in other disease states with similar features.
Methods.
Anesthetized, male Sprague-Dawley rats were subjected to a standardized polytrauma and pressure-targeted catheter hemorrhage. They then received either no treatment (Control) or a single bolus of NAC, followed by autologous whole blood transfusion. Renal MBF was measured using contrast-enhanced ultrasound (CEUS) at prespecified time points. von Willebrand factor (VWF) multimer gels and other laboratory studies were performed. Histologic analysis of vascular thrombi was also performed on uninjured tissue from rats undergoing either this trauma protocol or a sham procedure.
Results.
NAC increased MBF at 3 hours after resuscitation. This was accompanied by a decrease in VWF multimer size that was not seen in the Control group. Histologic data showed an overall increase in systemic thrombus burden associated with trauma.
Conclusions.
NAC improves renal MBF, possibly by reducing VWF multimer size and reducing microthrombus burden. This is significant both mechanistically and therapeutically. It sheds light on the possible pathways involved in causing microvascular obstruction after trauma and identifies possible treatment approaches that could be developed further. Ultimately, targeting these pathways could move us closer to resuscitation strategies that optimize vital organ MBF.
Keywords: Microvascular blood flow, trauma, antioxidant, von Willebrand factor, rats
Introduction
Severe trauma elicits a systemic decrease in microvascular blood flow (MBF) that can persist even after resuscitation to normal blood pressures.1–4 These changes are associated with multiple organ dysfunction syndrome (MODS) and death, signifying that they represent an important barrier to effective resuscitation.1–5 In fact, parameters of microcirculatory flow might be better predictors of the development of MODS than traditional hemodynamic measures, like blood pressure, highlighting its significance.4 Using contrast-enhanced ultrasound (CEUS) with parametric image analysis in a rat model of trauma and resuscitation, we have previously shown that the decrease in MBF is due to microcirculatory obstruction, rather than changes in blood rheology or vascular tone.6 This reinforces what has been shown previously using other quantification modalities.1,3,4,7 However, the cause of these obstructions and possible therapeutic approaches to addressing them remain unknown.
There are several salient possible contributors to microvascular obstruction after trauma. Given the sudden, large-scale, widespread activation of the hemostatic system that is triggered to survive the initial trauma, it is likely that microvascular thrombi are involved. The alterations in hemostasis that are commonly observed after trauma are complex and interconnected.8–10 However, two of the characteristic changes that could contribute to microthrombus formation are diffuse endothelial activation with subsequent von Willebrand factor (VWF) release and oxidative stress.10 These mechanisms are consistent with the pathophysiology of a thrombotic microangiopathy (TMA). Although schistocytes and significant hemolysis have not been described after trauma, other features of TMA have.
Tissue injury and hemorrhagic shock induce systemic endothelial activation.11–14 Upon activation, the endothelium secretes its abundant stores of VWF multimer strings.13–15 The newly secreted VWF is naïve to its primary cleaving protease, ADAMTS13 (a disintegrin and metalloprotease with a thrombospondin type 1 motif, member 13), and is thus ultralarge.13,16 These larger VWF multimers are more prone to spontaneous platelet adhesion, activation, and subsequent thrombus formation.17,18
In parallel, severe trauma triggers a rapid surge in inflammation and subsequent oxidative stress in the blood.19–21 We have shown that proteins in the coagulation cascade are susceptible to oxidative modification in these settings, and this oxidation can alter their function.22,23 Additionally, neutrophil-mediated oxidation of VWF and ADAMTS13 render the VWF resistant to cleavage, leading to larger, more adhesive multimers anchored to the endothelium.24,25 In other disease states, this can lead to thrombotic angiopathies.26–28
N-acetylcysteince (NAC) is an agent that could address both of these pathologic pathways. In other disease states with similar features of endothelial activation, VWF release, oxidative burst, and diffuse thrombus formation (e.g., thrombotic thrombocytopenic purpura, myocardial infarction, ischemic stroke), NAC has been shown to improve MBF.29–32 However, the microcirculatory derangements caused by polytrauma are unique, and the effect of NAC on MBF in trauma remains unexplored. One study showed that NAC administration protected the function of several organs in a rat model of hemorrhagic shock followed by cold seawater immersion, though the mechanism was not clearly demonstrated and the role of tissue injury was not incorporated.33 However, the microcirculatory derangements caused by polytrauma are relatively unique, and the effect of NAC on MBF in trauma remains relatively unexplored. A well-described mechanism for NAC’s ability to improve blood flow in other disease states is to reduce the disulfide bonds in the VWF that is released from endothelial cells (ECs), thus breaking up the ultralarge, hyperadhesive multimers into smaller strings that are less likely to spontaneously trigger thrombus formation.29–31 We have also shown that NAC and other antioxidant agents can reverse the oxidative modifications of hemostatic proteins like fibrinogen, restoring normal function.23 This makes NAC an ideal early candidate to test in an animal model of trauma-induced microvascular obstruction, both to begin to elucidate the underlying mechanisms of this process and to identify possible therapeutic avenues for further exploration.
In this study, we tested the hypothesis that NAC would reverse impairment of renal MBF in a rat model of severe trauma, hemorrhagic shock, and resuscitation. In short, we show that NAC reduced VWF multimer size and improved MBF after resuscitation. We also present histologic data confirming the presence of vascular microthrombi after trauma. Taken together, these findings lend further support to the concept that diffuse microthrombus formation is responsible for post-traumatic microvascular obstruction, identify endothelial activation and oxidative stress as possible contributors, and provide early insight into a possible therapeutic strategy for further development.
Materials and Methods
Animal Protocol
All experimental protocols were approved by the University of Washington Institutional Animal Care and Use Committee. Animal preparation, trauma, hemorrhage, and resuscitation protocols were similar to our previous study, apart from the administration of NAC.6 Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 275–325 g were acclimated to the facility for at least 1 day with free access to food and water. Immediately before the procedure, animals were injected with 0.375 mg xylazine (20 mg/mL) and 13.125 mg ketamine (100 mg/mL) intramuscularly for analgesia and initial sedation. They were then placed on inhaled isoflurane (3% via induction chamber) to achieve deep anesthesia. Tracheostomy was performed, and animals were placed on a ventilator with inhaled isoflurane starting at 0.5% and titrated to maintain deep anesthesia. The right carotid artery was cannulated (BTPE-60 polyethylene tubing, Instech Laboratories Inc., Plymouth Meeting, PA) and connected to pressure transduction (MP150, Biopac Systems, Goleta, CA). The right internal jugular (IJ) vein was cannulated with a 4 F double-lumen catheter (Cook Medical, Bloomington, IN). The animals were connected to arterial waveform monitors to calculate heart rate (HR) and mean arterial pressure (MAP). A rectal temperature probe was inserted for continuous measurement. A warming lamp was modulated with a goal of maintaining baseline temperature. Animals were allowed to stabilize on the ventilator for a minimum of 20 minutes before baseline measurements.
After stabilization, baseline measurements were taken. Then, animals were subjected to a standardized polytrauma and hemorrhage protocol derived from a model published by Darlington et al that created TIC in rats.34 In summary, they underwent laparotomy, right and medial lobe liver crush, stretch injury of 10 cm of small bowel, left femur fracture, and left quadriceps crush, followed by a catheter hemorrhage at a rate of 1 mL/min. In our model, the hemorrhage portion was targeted to a MAP of 35–45 mmHg. Animals were held at this target pressure for 60 min, hemorrhaging additional volume as needed. All hemorrhaged blood was collected in syringes with 0.109 M (3.2%) sodium citrate at a standard 1:9 volume ratio. The syringes were then placed on a rocker for gentle agitation.
After 60 minutes of shock, animals were randomized to either the Control or the NAC group. Control animals did not receive any treatment before transfusion. NAC animals received an infusion of NAC (American Regent, Shirley, NY) at a dose of 150 mg/kg, diluted in sterile water to a volume of 3 mL/kg per package insert and infused over 5 min. This was followed immediately by shed blood transfusion.
All animals were resuscitated by autologous blood transfusion (1 mL/kg/min) with the shed, citrated blood that was collected during the hemorrhage portion. They were observed for three hours after completion of transfusion without any further intervention. Animals that died early were excluded from the study in order to generate complete timelines of the treatment effects.
At the end of the observation period, animals were euthanized with a 39 mg pentobarbital overdose (390 mg/mL).
Contrast-Enhanced Ultrasound Measurements
Renal MBF was measured with CEUS of the right kidney using image capture and analysis techniques described previously.6 In short, a linear array transducer was positioned in the mid-kidney short-axis plane. Contrast-specific amplitude modulation imaging was used with transmit frequency 2–8 MHz, mechanical index 0.19, and dynamic range 54 dB (Logiq E9™, General Electric Healthcare, Chicago, IL). A 1:10 dilution of microbubble ultrasound contrast agent (Definity™, Lantheus Medical Imaging Inc., North Billerica, MA) in 0.9% normal saline was continuously infused at a rate of 40 μL/min. Once steady state of contrast was reached, MBF was quantified using the continuous infusion destruction-replenishment method.35 To summarize, a 20-frame burst of high mechanical index ultrasound (MI=0.19) was used to destroy microbubbles in the frame, then a time-intensity curve of contrast signal intensity was generated as the microbubbles replenished the tissue in the frame. The time-intensity curve was then fit to the function y = A (1 - eβt), where A represents the microvascular blood volume or plateau of the curve and β represents the blood flux rate or rate constant of the refill, a measure of how quickly the signal intensity approaches its plateau. The total MBF was then calculated by multiplying A × β = Aβ, which has been shown to correlate well with MBF as measured by microsphere injection.36 At each timepoint, three ninety-second videos were collected with a frame rate of 2/sec, gain of 17, low and high mechanical indices of 14 and 100.
After experiment completion, DICOM video files were exported and converted to MOV format using Horos v3.3.6 conversion software (Nimble Co LLC, Annapolis, MD). The MOV files were then analyzed using Narnar software (Narnar, Lake Oswego, OR) on an iPad Pro (Apple Inc., Cupertino, CA). Each video was converted to grayscale, and a horseshoe-shaped region of interest was drawn over the renal parenchyma, excluding the renal pelvis and large vessels at the hilum. The time-intensity curves for these regions were fitted to the equation above, and the A and β parameters were recorded. The parameters were averaged across the three videos captured at each timepoint in order to minimize minor temporal variations.
VWF Multimer Gels
The VWF multimer size distribution was measured in plasma collected at baseline, end of the shock period, and end of the resuscitation period. Samples were electropheresed on 1.7% agarose gel for 18.5 hr. VWF was detected using polyclonal rabbit anti-human VWF antibody (Agilent Dako, Santa Clara, CA) at a final concentration of 0.5 μg/mL overnight at 4°C, followed by a secondary horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody (Fisher Scientific, Waltham, MA) at a dilution of 1:8,000 in 5% milk-1X tris-buffered saline (TBS) for 1 hr at room temperature. Images were captured using an ImageQuant 350 gel imager (GE Healthcare, Chicago, IL). Densitometry analysis was performed using ImageQuant TL software (GE Healthcare, Chicago, IL).
Laboratory Testing
Arterial blood was collected via the carotid catheter. For blood gases, blood was collected in heparinized capillary tubes and analyzed with an ABL90 Flex Plus machine (Radiometer, Copenhagen, Denmark). For rotational thromboelastometry (ROTEM), 900 μL of blood was collected in syringes with 0.109 M (3.2%) buffered sodium citrate (Haemonetics Corporation, Boston, MA) at a standard 1:9 volume ratio and analyzed with a ROTEM Delta (Tem Innovations GmbH, Munich, Germany). For complete blood counts (CBC), blood was collected in ethylenediaminetetraacetic acid (EDTA) microtainers (Becton Dickinson, Franklin Lakes, NJ) and analyzed with a VetScan HM5 Hematology Analyzer (Zoetis Inc., Parsippany-Troy Hills, NJ).
Histology
New histologic analysis of microvascular thrombus burden was performed in a group of rats from a previously published set of related experiments.6 In that study, rats were subjected to either the polytrauma-hemorrhage protocol described above or a sham procedure involving anesthesia and instrumentation only. Immediately after euthanasia, biopsies of kidney, small bowel, liver, lung, and heart were excised, placed in 10% formalin, and gently mixed. Tissue was trimmed, fixed in paraffin, sectioned, and stained for fibrin with phosphotungstic acid hematoxylin (PTAH) to visualize vascular thrombi. A previously described scoring system to quantify the thrombus burden in the tissue was used that ranged from 0 (no thrombi) to 3 (greater than 5 thrombi).37 The scorer was blinded to treatment group.
Statistical Analysis
Variables were first tested for normality using the Shapiro-Wilk test. If both sham and trauma groups were normally distributed (Shapiro-Wilk p value > 0.05), they were compared by parametric tests, and otherwise nonparametric tests were used. Single measurement, descriptive data were compared by either unpaired, two-tailed t-test (parametric) or Mann-Whitney test (nonparametric). As reported below, all variables with repeated measures were found to be normally distributed and therefore were analyzed using repeated measures analysis of variance (RM-ANOVA) with terms for time, treatment group, and the interaction between time and treatment group. The Geisser-Greenhouse correction was made to avoid the assumption of sphericity. However, because of the inflection point caused by resuscitation partway through the protocol, the results for these terms are difficult to interpret. Rather, differences between treatment groups at individual time points were identified as the comparisons of interest a priori. VWF multimer gel size distributions were analyzed by comparing the percentage of high molecular weight VWF multimers (HMW, >10 protomers) at each time point to that at baseline in each treatment group. All pairwise comparisons were made with the Sidak correction for multiple comparisons. A p value less than 0.05 was considered significant. Statistical analysis was completed using GraphPad Prism v10.2.3 (GraphPad Software LLC, Boston, MA).
Results
Descriptive Characteristics
Descriptive data are summarized in Table 1. There were no significant differences in body weight, hemorrhage volume, or contrast infusion volume between control and NAC groups.
Table 1.
Descriptive data for control and NAC groups. If both groups were normally distributed (Shapiro-Wilk p value > 0.05), variables are presented as mean ± standard deviation and were compared by unpaired, two-tailed t-test. Otherwise, they were presented as median (interquartile range) and were compared by Mann-Whitney test.
| Control (n=7) | NAC (n=6) | Comparison p value |
|||
|---|---|---|---|---|---|
| Shapiro-Wilk test p value |
Value | Shapiro-Wilk test p value |
Value | ||
| Weight (g) | 0.448 | 351 ± 25.6 | 0.701 | 340 ± 15.0 | 0.356 |
| Hemorrhage volume (mL) | 0.076 | 5.0086 ± 1.73 | 0.413 | 6.68 ± 1.81 | 0.418 |
| Total contrast infusion volume (mL) | 0.028 | 4.02 (4.00–4.24) | 0.355 | 4.06 (4.02–4.15) | 0.836 |
Vital Sign Data
MAPs were normally distributed in the control (Shapiro-Wilk test p=0.726) and NAC (p=0.510) groups. There were no significant differences between groups at any time point (p>0.050). In both groups, the MAP dropped during the shock period, consistent with the study design, and increased modestly after resuscitation (Figure 1A).
Figure 1.
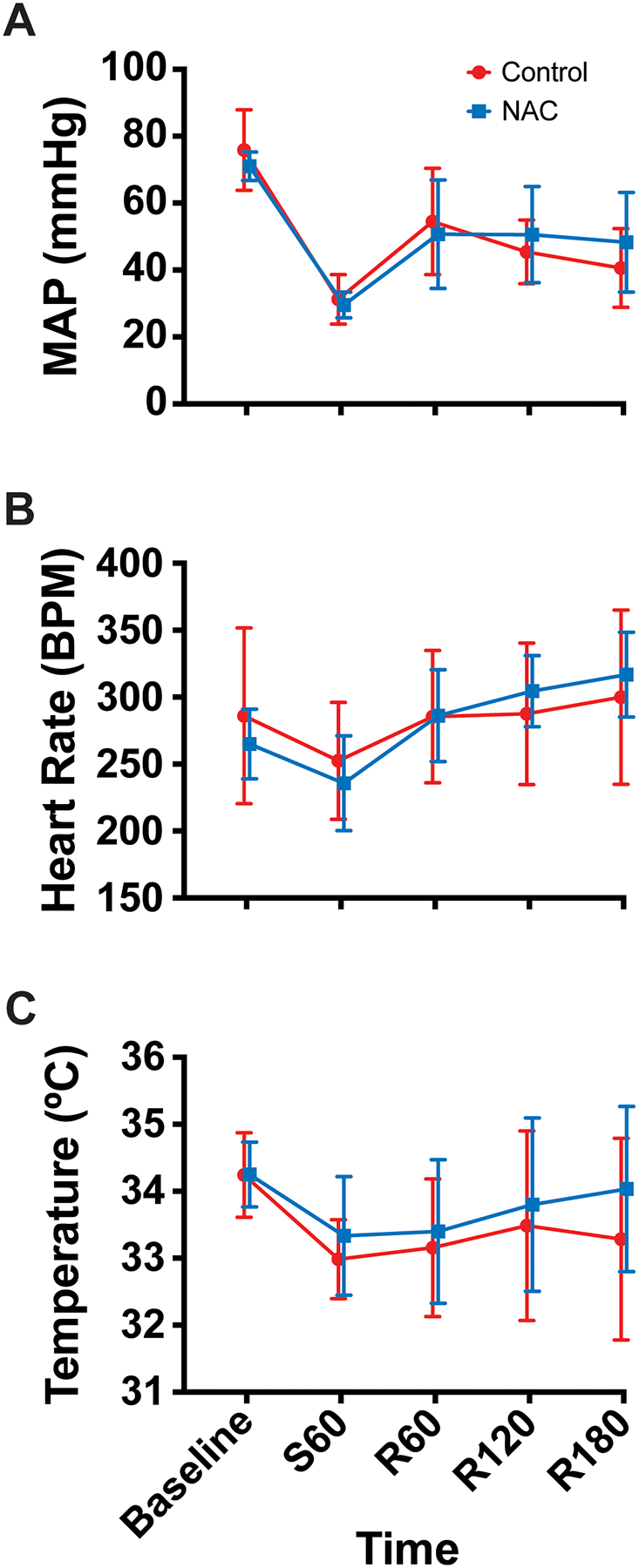
Vital sign data (mean, standard deviation) (Control n=7, NAC n=6). (A) Mean arterial pressure (MAP), (B) Heart rate (HR), and (C) Temperature over time. Time points are denoted with “S” for shock period or “R” for resuscitation period and the number of minutes elapsed in that period.
HRs were normally distributed in the control (p=0.120) and NAC (p=0.848) groups. There were no significant differences between groups at any time point (p>0.050). Both groups displayed a minor decrease in HR during the shock period, follow by a steady increase after resuscitation (Figure 1B).
Temperatures were normally distributed in the control (p=0.287) and NAC (p=0.562) groups. There were no significant differences between groups at any time point (p>0.050). In both groups, the temperature dropped during shock and modestly improved after resuscitation (Figure 1C).
Contrast-Enhanced Ultrasound Data
Total MBF (Aβ) values were normally distributed in the control (p=0.406) and NAC (p=0.548) groups. There were no differences in MBF between groups at baseline, during shock, or early after resuscitation. However, there was a gradual separation between groups, and at 180 min after resuscitation, the NAC group had significantly higher MBF than the control group (p=0.013) (Figure 2A).
Figure 2.
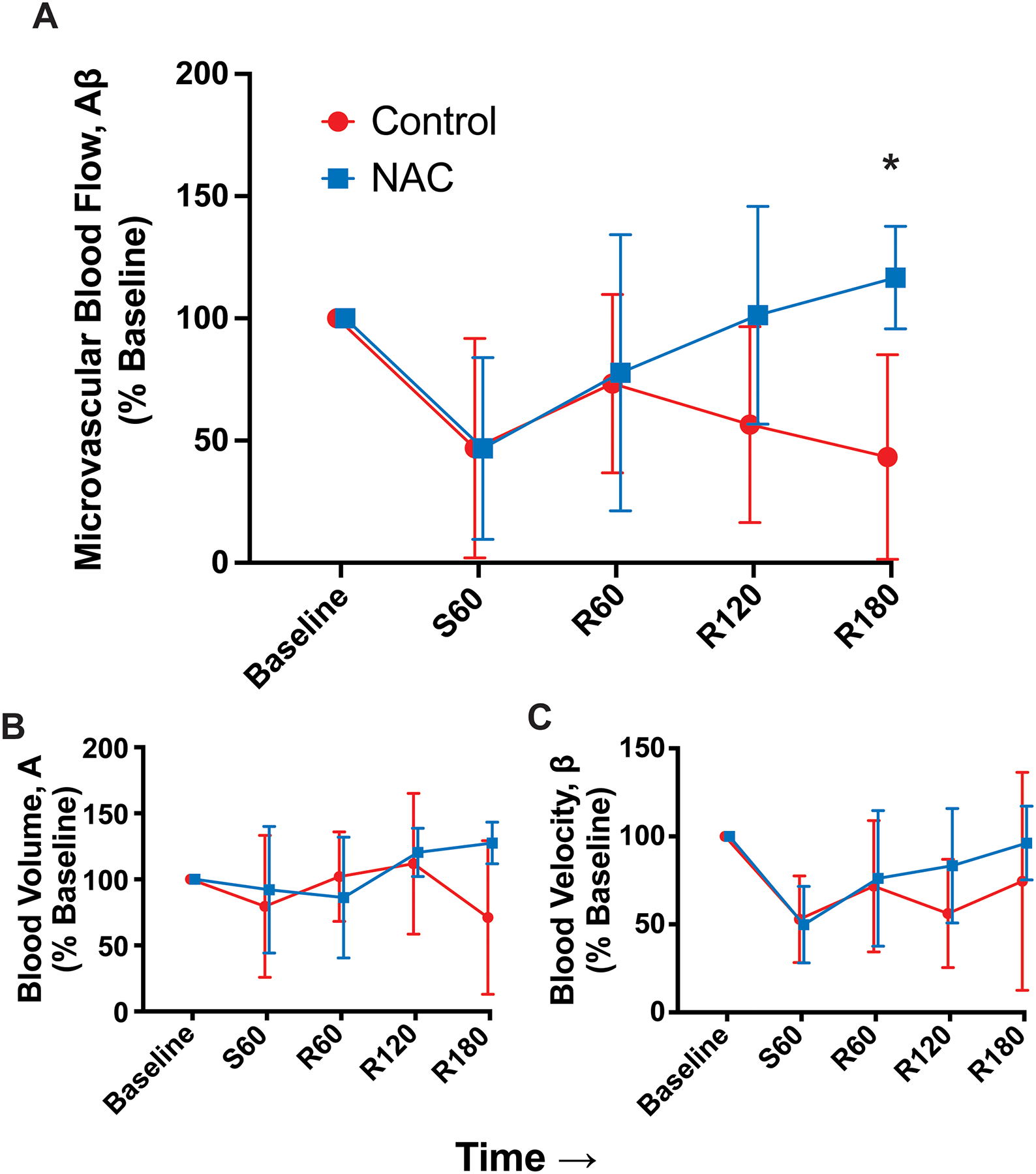
Contrast-enhanced ultrasound data, normalized to mean at baseline (mean, standard deviation) (Control n=7, NAC n=6). (A) Total blood flow, (B) blood volume, and (C) blood velocity over time. Asterisk represents time point that is significantly different between groups. Time points are denoted with “S” for shock period or “R” for resuscitation period and the number of minutes elapsed in that period.
This separation appears to have been driven by a combination of nonsignificant decreases in both blood volume (A), which is a marker of microvascular patency, and blood velocity (β). Microvascular blood volume (A) values were normally distributed in the control (p=0.541) and NAC (p=0.484) groups. There were no significant differences between groups at any time point (p>0.050), though the control group had a decrease in A values at the end of the resuscitation period that likely contributed to the differences in Aβ at that time point (Figure 2B).
Microvascular blood velocity (β) values were normally distributed in the trauma (p=0.473) and NAC (p=0.501) groups. There were no significant differences between groups at any time point (p>0.050) (Figure 2C). Toward the end of the resuscitation period, there were nonsignificant separations in β values with the NAC group rising slightly above the control group, which likely also contributed to the differences in Aβ at that time point.
Raw CEUS and all other repeated measures data are provided in Supplemental Digital Content (SDC Table 1). Representative example CEUS videos are also included in Supplemental Digital Content (SDC Videos).
VWF Multimer Gels
Plasma for VWF multimer gels was only available for three Control rats and four NAC rats. VWF HMW percentages were normally distributed in the Control (p=0.181) and NAC (p=0.834) groups. In the NAC group, the HMW percentage was significantly lower at 180 minutes after resuscitation than at baseline (p=0.005) (Figure 3). There were no significant differences between time points in the Control group (p>0.050).
Figure 3.
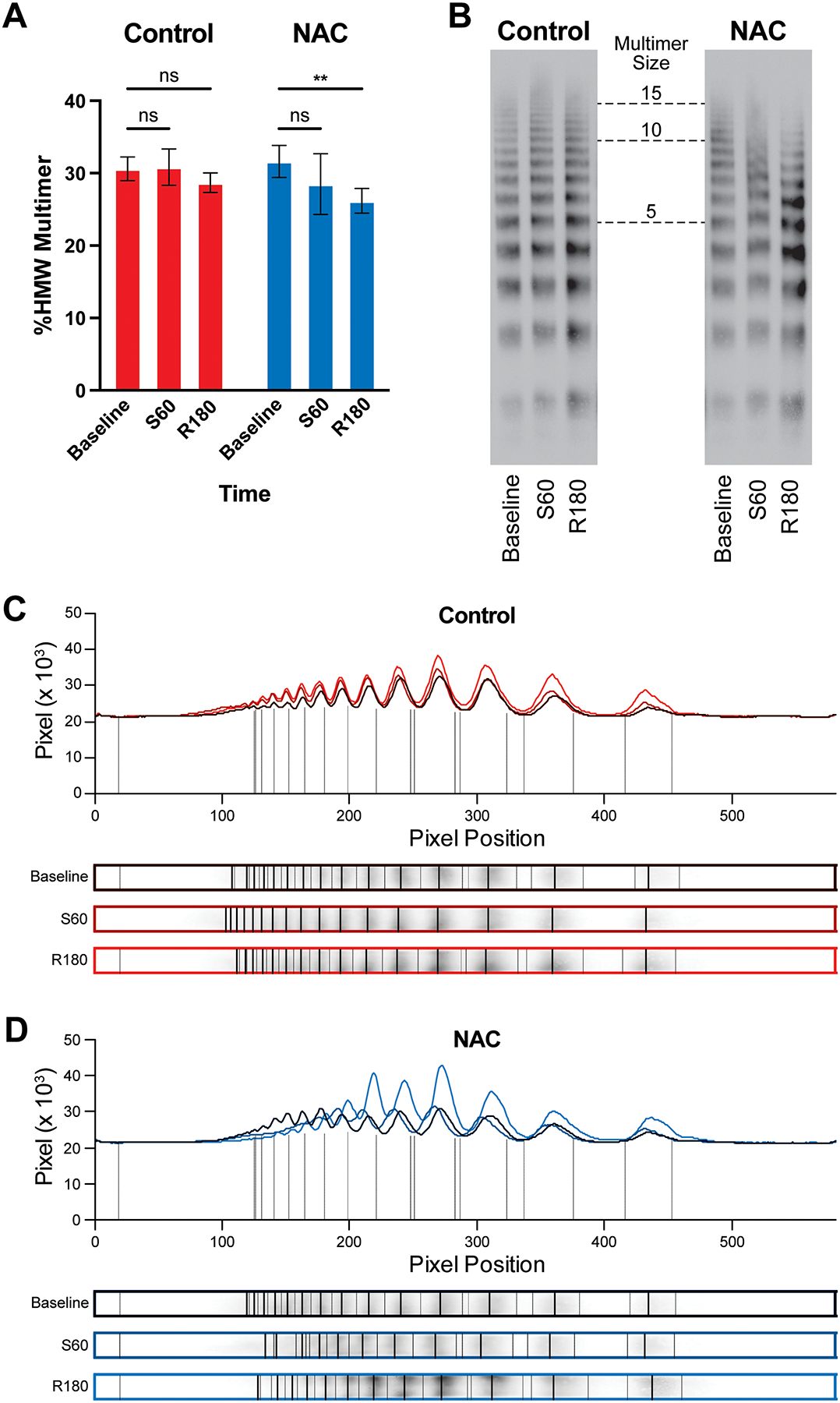
VWF multimer gel data. (A) Percentage of high molecular weight VWF multimers (HMW, >10 protomers) over time in Control and NAC groups (Control n=3, NAC n=4). Example gels (B) and densitometry analyses (C&D) demonstrating an upshift toward larger multimers from baseline to the end of the shock period, followed by a downshift at the end of the resuscitation period that was more pronounced in the NAC group. Time points are denoted with “S” for shock period or “R” for resuscitation period and the number of minutes elapsed in that period. Asterisks denote p<0.010.
Laboratory Data
Serum lactate concentrations were normally distributed in the control (p=0.527) and NAC (p=0.620) groups. There were no significant differences between groups at any time point (p>0.050). In both groups, lactate concentration rose dramatically during shock then improved modestly after resuscitation (Figure 4A).
Figure 4.
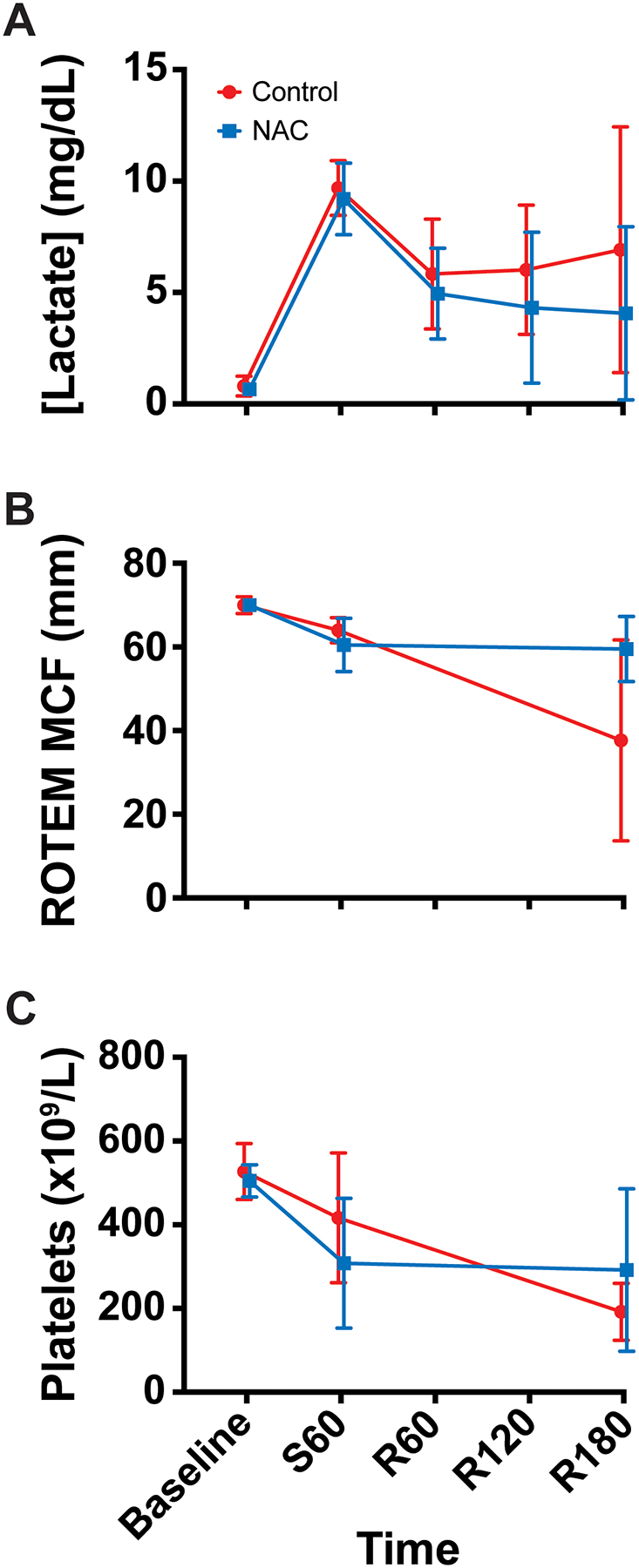
Laboratory data (mean, standard deviation). (A) Serum lactate concentration (Control n=7, NAC n=6), (B) rotational thromboelastometry maximum clot firmness (ROTEM MCF) (Control n=3, NAC n=2), and (C) platelet count (Control n=7, NAC n=6) over time. Time points are denoted with “S” for shock period or “R” for resuscitation period and the number of minutes elapsed in that period.
ROTEM data were not available for one trauma and one sham rat, due to equipment malfunction requiring service. ROTEM maximum clot firmness (MCF) values were normally distributed in the control (p=0.335) and NAC (p=0.165) groups. There were no significant differences between groups at any time point (p>0.050). In both groups, the MCF decreased from baseline after shock and remained low at the end of the protocol (Figure 4B).
Platelet counts were normally distributed in the control (p=0.631) and NAC (p=0.131) groups. There were no significant differences between groups at any time point (p>0.050). Both groups displayed a decrease in circulating platelet count during shock and after resuscitation (Figure 4C).
Histology
Compared to rats undergoing sham surgery, rats subjected to this polytrauma-hemorrhagic shock protocol exhibited an overall higher microthrombus burden across all organs (p=0.043) (Figure 5A). There were no significant differences between groups in any individual organ (p>0.050), though there were several trends that contributed to the overall combined difference, most notably in the intestine (Figure 5B–F). Representative images of intestine are included in Figure 5G&H.
Figure 5.

Histology data (Sham n=6, Trauma n=7). PTAH thrombus score for (A) all tissue combined, (B) kidney, (C) intestine, (D) liver, (E) lung, and (F) heart between rats subjected to sham procedure (green) and to trauma-shock-resuscitation protocol (red). Asterisk denotes p<0.050. (G) Example H&E (top) and PTAH (bottom) 200x images of intestine showing absent thrombi in a Sham rat (left) and high thrombus burden in the mucosal and submucosal vasculature of a Trauma rat (right) with arrows denoting thrombi. PTAH, phosphotungstic acid hematotoxylin; H&E, hematoxylin and eosin.
Discussion
This paper describes an improvement in renal MBF after administration of NAC in a rat model of polytrauma and hemorrhagic shock. This bears both mechanistic and early therapeutic significance.
The causes of post-traumatic microvascular obstruction are not well-described, but we present data implicating endothelial activation, VWF secretion, and microthrombus formation. Our histologic data confirm that trauma causes microvascular thrombi in uninjured tissue. Previous studies have shown an increase in plasma VWF concentration and multimer size after trauma, suggesting endothelial activation and VWF hyperadhesiveness as a possible cause of these thrombi. Our multimer gels confirm that the increase in MBF after NAC administration was accompanied by a decrease in VWF multimer size. This is consistent with the known effects of NAC in other disease states, and it further implicates this pathway as a culprit in microvascular thrombus formation. The reduction in VWF multimer size by NAC was less dramatic in our study than that seen in other animal models. This could be inherent to the pathophysiology of trauma or due to a difference in NAC dosing. We administered 150 mg/kg NAC, which is the recommended loading dose in the package insert for acetaminophen overdose, but it is significantly lower than the doses used in previous studies to reduce and solubilize large VWF multimers (400–800 mg/kg).29,31,38 The administration of higher doses must be counterbalanced with the importance of limiting crystalloid fluid volume in a trauma model. In early pilot experiments, rats were given higher doses of super-concentrated NAC to achieve the higher dose without excessive fluid volume. These rats died very soon after administration, presumably from caustic effects of the hyperosmolar solution. However, given the effect seen with the dose of NAC given, further testing with higher doses and alternative antioxidant and anti-inflammatory agents is warranted. Taken together, our data are consistent with VWF-mediated microthrombus formation after trauma that is susceptible to clearance with a reducing agent.
This study also represents an early step in advancing MBF-targeted resuscitation techniques. The fact that a reducing agent could improve MBF identifies this class of drug as a salient target for future therapeutic development. Given the pathways discussed above, it also identifies some other drug classes as potentially useful. For example, while antioxidant agents (e.g., NAC, vitamin C) could break down large VWF multimers by reducing disulfide bonds, anti-inflammatory agents could mitigate the oxidative burst that contributes to VWF hyperadhesiveness, and cleaving proteases (e.g., ADAMTS13, neutrophil elastase) could digest the large VWF multimers. High-density lipoprotein (HDL) also represents a promising agent that mitigates VWF adhesiveness by preventing shear-induced self-association.39 Each of these agents has inherent advantages and disadvantages, including varying impacts on bleeding time, which would need to be taken into account when developing an intervention. This line of investigation could eventually allow us to optimize resuscitation to the level of the microcirculation.
There are several limitations to this study. First, this study does not fully define the mechanism by which NAC improves MBF. In particular, the effects on ROTEM are especially ill-defined, because of the very low sample size caused by equipment servicing. Additionally, histology was not performed in the NAC group, tempering any direct conclusions around the effect of NAC on microvascular thrombosis. Second, the experimental timeline truncates at 3 hours after resuscitation, and it is possible there are further changes in MBF later in the clinical course. Detectable changes in renal function (creatinine, urine output, glomerular filtration rate) also would not be expected during this 3-hour time window. In humans, MODS tends to occur days after injury.40 Though microvascular obstruction would be expected to precede MODS if it is indeed the cause, it is unclear how dynamic this process is during this time. Third, there was no volume control given to the Control group, leaving the possibility that NAC’s effect was elicited simply by the fluid volume administered. It seems unlikely that the additional 1 mL of intravascular volume provided by the NAC infusion on top of the average of 10.3 mL of fluid already administered to each rat (6.2 mL shed blood transfusion + 4.1 mL contrast-saline infusion) would account for the differences in MBF observed. However, this limitation will bear consideration if future experiments do not reproduce similar effects from NAC. Fourth, the study excluded animals that did not survive the full protocol. This was designed as a partially mechanistic study, rather than a pragmatic trial of a novel therapy. This limits interpretations of overall clinical efficacy of NAC in these settings, which would need to be evaluated separately. Fifth, the study used only male rats. To maximize statistical power and avoid the variation introduced by the well-described differences in trauma response between sexes, only one sex was used. Males were chosen because the trauma population is predominantly male. However, this limits generalizability of our findings. Finally, this study only measured MBF in one organ. While the kidneys are sensitive to shock and are commonly involved in MODS after trauma, it is possible that different organs undergo different alterations in MBF with different timelines.
In conclusion, this paper demonstrates that NAC administration decreases VWF multimer size and improves MBF after severe injury, hemorrhagic shock, and resuscitation. The primary mechanism of microvascular obstruction after trauma appears to be microthrombus formation, and VWF release from an activated endothelium is a probable contributor. Our results support this mechanism and identify VWF hyperadhesiveness and oxidative stress as therapeutic targets. Further investigation focused on defining the specific mechanisms involved could enable development of novel therapeutic strategies to optimize vital organ MBF after trauma.
Supplementary Material
SDC Table 1. Repeated Measures Data. Raw longitudinal data for all variables with repeated measures. CEUS, contrast-enhanced ultrasound. ROTEM MCF, rotational thromboelastometry maximal clot firmness.
SDC Videos. Renal CEUS Videos. Representative example contrast-enhanced ultrasound videos of Control (left) and NAC (right) groups at Baseline (top), Shock60 (middle), and Resuscitation180 (bottom). Clips show tissue-subtracted contrast signal after destruction from high-mechanical index ultrasound, replenishing the microvasculature from contrast influx during continuous contrast infusion.
Acknowledgments
This work was supported by funding from the National Institutes of Health (K08HL146840) and the University of Washington Department of Emergency Medicine.
Footnotes
Conflicts of Interest
The authors have no conflicts to disclose.
References
- 1.Domizi R, Damiani E, Scorcella C, et al. Association between sublingual microcirculation, tissue perfusion and organ failure in major trauma: A subgroup analysis of a prospective observational study. PloS one. 2019;14(3):e0213085. doi: 10.1371/journal.pone.0213085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campos-Serra A, Mesquida J, Montmany-Vioque S, et al. Alterations in tissue oxygen saturation measured by near-infrared spectroscopy in trauma patients after initial resuscitation are associated with occult shock. Eur J Trauma Emerg Surg. Feb 2023;49(1):307–315. doi: 10.1007/s00068-022-02068-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tachon G, Harrois A, Tanaka S, et al. Microcirculatory alterations in traumatic hemorrhagic shock. Critical care medicine. Jun 2014;42(6):1433–41. doi: 10.1097/ccm.0000000000000223 [DOI] [PubMed] [Google Scholar]
- 4.Hutchings SD, Naumann DN, Hopkins P, et al. Microcirculatory Impairment Is Associated With Multiple Organ Dysfunction Following Traumatic Hemorrhagic Shock: The MICROSHOCK Study. Critical care medicine. Sep 2018;46(9):e889–e896. doi: 10.1097/ccm.0000000000003275 [DOI] [PubMed] [Google Scholar]
- 5.Ward KR. The microcirculation: linking trauma and coagulopathy. Transfusion. Jan 2013;53 Suppl 1:38s–47s. doi: 10.1111/trf.12034 [DOI] [PubMed] [Google Scholar]
- 6.St John A, Wang X, Ringgold K, et al. Assessment of abnormal skeletal muscle perfusion by contrast-enhanced ultrasound with parametric imaging in rats after severe injury, hemorrhagic shock, and whole blood resuscitation. Shock (Augusta, Ga). Nov 16 2023;doi: 10.1097/shk.0000000000002267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szczesny G, Veihelmann A, Nolte D, Messmer K. Changes in the local blood and lymph microcirculation in response to direct mechanical trauma applied to leg: in vivo study in an animal model. The Journal of trauma. Sep 2001;51(3):508–17. doi: 10.1097/00005373-200109000-00014 [DOI] [PubMed] [Google Scholar]
- 8.Teeter W, Neal MD, Brown JB, MacLeod JBA, Vesselinov R, Kozar RA. TRAUMA-INDUCED COAGULOPATHY: PREVALENCE AND ASSOCIATION WITH MORTALITY PERSIST 20 YEARS LATER. Shock (Augusta, Ga). Sep 1 2024;62(3):380–385. doi: 10.1097/shk.0000000000002416 [DOI] [PubMed] [Google Scholar]
- 9.John AE, White NJ. Platelets and Fibrinogen: Emerging Complexity in Trauma-Induced Coagulopathy. Seminars in thrombosis and hemostasis. Mar 2020;46(2):125–133. doi: 10.1055/s-0039-1701017 [DOI] [PubMed] [Google Scholar]
- 10.Moore EE, Moore HB, Kornblith LZ, et al. Trauma-induced coagulopathy. Nat Rev Dis Primers. Apr 29 2021;7(1):30. doi: 10.1038/s41572-021-00264-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kregel HR, Hatton GE, Isbell KD, et al. Shock-Induced Endothelial Dysfunction is Present in Patients With Occult Hypoperfusion After Trauma. Shock (Augusta, Ga). Jan 1 2022;57(1):106–112. doi: 10.1097/shk.0000000000001866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krocker JD, Lee KH, Henriksen HH, et al. Exploratory Investigation of the Plasma Proteome Associated with the Endotheliopathy of Trauma. International journal of molecular sciences. Jun 1 2022;23(11)doi: 10.3390/ijms23116213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dyer MR, Plautz WE, Ragni MV, et al. Traumatic injury results in prolonged circulation of ultralarge von Willebrand factor and a reduction in ADAMTS13 activity. Transfusion. May 22 2020;doi: 10.1111/trf.15856 [DOI] [PubMed] [Google Scholar]
- 14.Dujardin RWG, Kisters JEC, Wirtz MR, et al. Shock-Driven Endotheliopathy in Trauma Patients Is Associated with Leucocyte Derived Extracellular Vesicles. International journal of molecular sciences. Dec 15 2022;23(24)doi: 10.3390/ijms232415990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeineddin A, Dong JF, Wu F, Terse P, Kozar RA. Role of Von Willebrand Factor after Injury: It May Do More Than We Think. Shock (Augusta, Ga). Jun 1 2021;55(6):717–722. doi: 10.1097/shk.0000000000001690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong JF, Moake JL, Nolasco L, et al. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. Dec 1 2002;100(12):4033–9. doi: 10.1182/blood-2002-05-1401 [DOI] [PubMed] [Google Scholar]
- 17.Arya M, Anvari B, Romo GM, et al. Ultralarge multimers of von Willebrand factor form spontaneous high-strength bonds with the platelet glycoprotein Ib-IX complex: studies using optical tweezers. Blood. Jun 1 2002;99(11):3971–7. doi: 10.1182/blood-2001-11-0060 [DOI] [PubMed] [Google Scholar]
- 18.Li F, Li CQ, Moake JL, Lopez JA, McIntire LV. Shear stress-induced binding of large and unusually large von Willebrand factor to human platelet glycoprotein Ibalpha. Annals of biomedical engineering. Jul 2004;32(7):961–9. doi: 10.1023/b:abme.0000032458.88212.54 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. Mar 4 2010;464(7285):104–7. doi: 10.1038/nature08780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manson J, Thiemermann C, Brohi K. Trauma alarmins as activators of damage-induced inflammation. Br J Surg. Jan 2012;99 Suppl 1:12–20. doi: 10.1002/bjs.7717 [DOI] [PubMed] [Google Scholar]
- 21.Pugin J How tissue injury alarms the immune system and causes a systemic inflammatory response syndrome. Ann Intensive Care. Jul 12 2012;2(1):27. doi: 10.1186/2110-5820-2-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han CY, Wang X, Ringgold KM, et al. Novel melanocortin fusion protein inhibits fibrinogen oxidation and degradation during trauma-induced coagulopathy. Blood. Jun 26 2023;doi: 10.1182/blood.2022019164 [DOI] [PubMed] [Google Scholar]
- 23.Han CY, Pichon TJ, Wang X, et al. Leukocyte activation primes fibrinogen for proteolysis by mitochondrial oxidative stress. Redox Biol. May 2022;51:102263. doi: 10.1016/j.redox.2022.102263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Fu X, Wang Y, et al. Oxidative modification of von Willebrand factor by neutrophil oxidants inhibits its cleavage by ADAMTS13. Blood. Jan 21 2010;115(3):706–12. doi: 10.1182/blood-2009-03-213967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Chen J, Ling M, Lopez JA, Chung DW, Fu X. Hypochlorous acid generated by neutrophils inactivates ADAMTS13: an oxidative mechanism for regulating ADAMTS13 proteolytic activity during inflammation. The Journal of biological chemistry. Jan 16 2015;290(3):1422–31. doi: 10.1074/jbc.M114.599084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oggianu L, Lancellotti S, Pitocco D, et al. The oxidative modification of von Willebrand factor is associated with thrombotic angiopathies in diabetes mellitus. PloS one. 2013;8(1):e55396. doi: 10.1371/journal.pone.0055396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Hobbs WE, Le J, Lenting PJ, de Groot PG, López JA. The rate of hemolysis in sickle cell disease correlates with the quantity of active von Willebrand factor in the plasma. Blood. Mar 31 2011;117(13):3680–3. doi: 10.1182/blood-2010-08-302539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Chung DW. Inflammation, von Willebrand factor, and ADAMTS13. Blood. Jul 12 2018;132(2):141–147. doi: 10.1182/blood-2018-02-769000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Reheman A, Gushiken FC, et al. N-acetylcysteine reduces the size and activity of von Willebrand factor in human plasma and mice. The Journal of clinical investigation. Feb 2011;121(2):593–603. doi: 10.1172/jci41062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozawa K, Packwood W, Muller MA, et al. Removal of endothelial surface-associated von villebrand factor suppresses accelerate datherosclerosis after myocardial infarction. Journal of translational medicine. May 1 2024;22(1):412. doi: 10.1186/s12967-024-05231-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez de Lizarrondo S, Gakuba C, Herbig BA, et al. Potent Thrombolytic Effect of N-Acetylcysteine on Arterial Thrombi. Circulation. Aug 15 2017;136(7):646–660. doi: 10.1161/circulationaha.117.027290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bueche CZ, Garz C, Kropf S, et al. NAC changes the course of cerebral small vessel disease in SHRSP and reveals new insights for the meaning of stases - a randomized controlled study. Exp Transl Stroke Med. 2013;5:5. doi: 10.1186/2040-7378-5-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Zhu Y, Zhang Z, et al. N-Acetyl-L-Cysteine Protects Organ Function After Hemorrhagic Shock Combined With Seawater Immersion in Rats by Correcting Coagulopathy and Acidosis. Front Physiol. 2022;13:831514. doi: 10.3389/fphys.2022.831514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darlington DN, Craig T, Gonzales MD, Schwacha MG, Cap AP, Dubick MA. Acute coagulopathy of trauma in the rat. Shock (Augusta, Ga). May 2013;39(5):440–6. doi: 10.1097/SHK.0b013e31829040e3 [DOI] [PubMed] [Google Scholar]
- 35.Nguyen T, Davidson BP. Contrast Enhanced Ultrasound Perfusion Imaging in Skeletal Muscle. J Cardiovasc Imaging. Jul 2019;27(3):163–177. doi: 10.4250/jcvi.2019.27.e31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. Feb 10 1998;97(5):473–83. doi: 10.1161/01.cir.97.5.473 [DOI] [PubMed] [Google Scholar]
- 37.Berthelsen LO, Kristensen AT, Tranholm M. Purified thromboplastin causes haemostatic abnormalities but not overt DIC in an experimental rabbit model. Thrombosis research. Oct 2010;126(4):337–44. doi: 10.1016/j.thromres.2010.06.022 [DOI] [PubMed] [Google Scholar]
- 38.Tersteeg C, Roodt J, Van Rensburg WJ, et al. N-acetylcysteine in preclinical mouse and baboon models of thrombotic thrombocytopenic purpura. Blood. Feb 23 2017;129(8):1030–1038. doi: 10.1182/blood-2016-09-738856 [DOI] [PubMed] [Google Scholar]
- 39.Chung DW, Chen J, Ling M, et al. High-density lipoprotein modulates thrombosis by preventing von Willebrand factor self-association and subsequent platelet adhesion. Blood. Feb 4 2016;127(5):637–45. doi: 10.1182/blood-2014-09-599530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sauaia A, Moore EE, Johnson JL, et al. Temporal trends of postinjury multiple-organ failure: still resource intensive, morbid, and lethal. The journal of trauma and acute care surgery. Mar 2014;76(3):582–92, discussion 592–3. doi: 10.1097/ta.0000000000000147 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDC Table 1. Repeated Measures Data. Raw longitudinal data for all variables with repeated measures. CEUS, contrast-enhanced ultrasound. ROTEM MCF, rotational thromboelastometry maximal clot firmness.


