Abstract
Plants are vital to human space exploration, providing oxygen, food, and psychological benefits to astronauts while contributing to water regeneration by recycling organic waste. However, microgravity, or reduced gravity, in space presents a considerable environmental challenge to plant growth. Understanding plant biology under both gravity and microgravity conditions is critical for advancing space exploration. In recent years, substantial progress has been made in understanding how gravity affects plants and its implications for future space agriculture, although a more comprehensive review is still needed. This review provides an overview of technological platforms used to simulate and study microgravity effects, detailing their historical background and key characteristics. It also summarizes recent advances in understanding plant gravitropism, including critical steps such as gravity sensing, signal transduction, and curvature response. The impacts of microgravity on plants are examined at phenotypic, cellular, and molecular levels. Studies on plant biology in microgravity have greatly expanded our knowledge, laying the foundation for the future of space agriculture and exploration. Additionally, we discuss agricultural systems designed for space, focusing on bioregenerative life-support systems, selection and breeding of plants suited for space environments, and their potential applications. Finally, we highlight the challenges and future research directions in plant biology and space agricultural systems.
Key words: microgravity platforms, gravitropism, space agriculture
This review provides an overview of technological platforms used to study microgravity effects, the mechanisms underlying plant gravitropism, and the impacts of altered gravity on plants. It also discusses space agriculture systems, including bioregenerative life-support systems and the selection of space-adapted plants, highlighting their potential applications. Finally, the review outlines key challenges and future research directions in plant biology and space agriculture.
Introduction
Growing plants in space has been a key topic since the early days of human space exploration. Space agriculture research formally began with studies by Jack Myers and colleagues, who investigated the use of algae for oxygen production and carbon dioxide removal for the United States (US) Air Force and the National Aeronautics and Space Administration (NASA) in the 1950s and 1960s (Myers, 1954). After these initial efforts, scientists began sending crops into space, with the first successful seed germination occurring aboard the Soviet Kosmos 110 in 1966 (Stanković, 2001). To date, five plant species—namely Arabidopsis thaliana (Arabidopsis), wheat (Triticum aestivum L.), pea (Pisum sativum), Brassica rapa L., and rice (Oryza sativa L.)—have successfully completed seed-to-seed cycles in space (Merkys et al., 1984; Musgrave et al., 2000; Sychev et al., 2007; Jia et al., 2024). Plants are now recognized as critical components for sustaining long-term human space exploration. However, the unique microgravity environment poses challenges for plant growth; controlled space environments face limitations such as restricted space, water, air, and light. Research on plant biology and the development of related technologies have substantially deepened our understanding of plant–gravity interactions and advanced the field of space agriculture.
The advancement of space agricultural technology has been increasingly recognized as a critical research area for supporting long-term human presence in extraterrestrial environments. Although some comprehensive review articles have effectively showcased recent progress in this field—such as the work by De Micco et al., which emphasizes the pivotal role of plant and microbial science and technology in the development of bioregenerative life-support systems (BLSSs) in space (De Micco et al., 2023)—most existing publications adopt more focused approaches. These studies often concentrate on specific aspects, such as the effects of reduced gravity on particular biological systems, the physiological challenges faced by humans in space, or impacts on other organisms. In contrast to these narrower perspectives, this review seeks to offer a broader understanding of the field by tracing its historical development and highlighting milestones that have shaped current technologies. Additionally, we aim to provide insights into future directions for space agriculture, exploring emerging trends and innovations that could address the complex challenges of sustaining human life in extraterrestrial environments. By examining the evolution of space agricultural research, we hope to contribute a comprehensive perspective on the state-of-the-art and potential trajectories of this transformative domain.
Technological platforms for microgravity studies
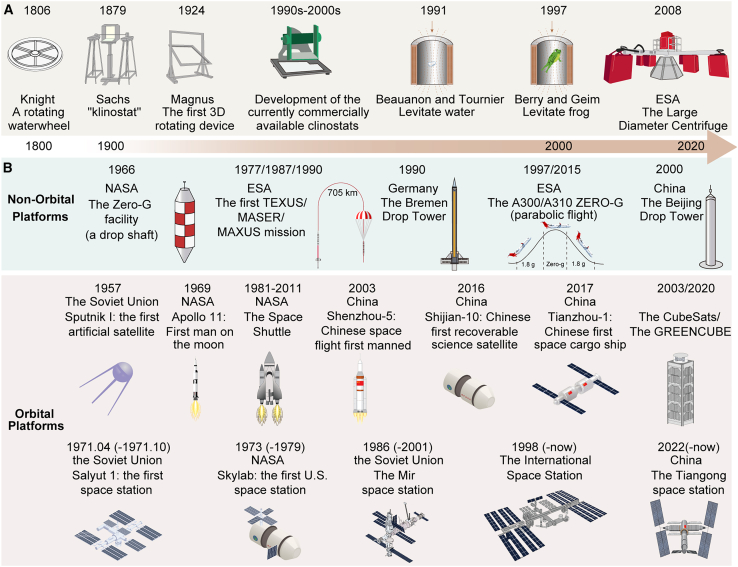
Understanding organismal responses to microgravity is critical for human space exploration. However, Earth’s constant gravity presents challenges for direct study. To address this, various platforms simulate or recreate microgravity, enabling research on the adaptations of plants and humans to these unique conditions (Figure 1).
Figure 1.
Chronological development of simulated and real microgravity research platforms.
(A) Ground-based microgravity research facilities and in-space partial gravity simulation platforms.
(B) Real microgravity platforms, including non-orbital and orbital research facilities.
Development of microgravity research platforms
Efforts to simulate microgravity began in the 18th century with ground-based facilities. Knight’s 1806 rotating waterwheel constituted the first major milestone, revealing plant gravitropism and inspiring the development of the clinostat and centrifuge, which are now widely used in microgravity research. The two-dimensional (2D) clinostat evolved into a three-dimensional (3D) version with two rotating axes, which was later commercialized (Hoson et al., 1997; Hemmersbach et al., 2006). Magnetic levitation, rooted in Earnshaw’s 1842 discovery, became another tool, enabling researchers to levitate organic materials and living organisms (Beaugnon and Tournier, 1991). Modern centrifuges, derived from Knight’s prototype, offer hypergravity and partial gravity environments for diverse biological experiments, with the European Space Agency’s (ESA) Large Diameter Centrifuge (LDC) providing 1–20 times the force of gravity (1–20g) (Loon et al., 2008; Frett et al., 2016).
The second milestone was the achievement of real microgravity. Non-orbital platforms such as drop towers, parabolic flights, and sounding rockets emerged between the 1960s and 2000s. Drop towers create near-vacuum conditions, enabling brief but high-quality microgravity experiments. Parabolic flights simulate microgravity during free-fall phases, whereas sounding rockets provide longer durations of microgravity. Platforms such as the Airbus Zero-G and ESA sounding rockets continue to support microgravity research.
The third milestone was the development of orbital platforms, beginning with early satellites and culminating in space stations. The Soviet Union’s 1971 space station and the subsequent International Space Station (ISS) represent advanced platforms for extended microgravity experiments. China’s Tiangong station, completed in 2022, is expected to make substantial contributions to space research (Shen et al., 2018).
Characteristics of microgravity research platforms
Microgravity research platforms fall into two categories: simulated microgravity using ground-based facilities and real microgravity via free-fall motion (Brungs et al., 2016; Böhmer and Schleiff, 2019). Ground-based simulated microgravity platforms include clinostats and magnetic levitators; real microgravity platforms include drop towers, parabolic flights, sounding rockets, and orbital platforms such as satellites and space stations (Table 1).
Table 1.
Comparison of specifications between ground-based simulated microgravity facilities and real microgravity platforms.
| Methods | Time | Operation | Use frequency | g-level | Hypergravity (acceleration stage) | |
|---|---|---|---|---|---|---|
| Ground-based simulated microgravity facilities | 2D clinostat | Hours to weeks | Manned | Unlimited | ≤10−3 | – |
| 3D clinostat (RPM) | 10–4 | – | ||||
| Magnetic levitator | Minutes to hours | <10−2 | – | |||
| Real microgravity platforms | Drop tower | 2.5–9.3 s | Automatic | 2–3 times/day | 10−3–10−6 | 30–65g |
| Parabolic flight | ∼20 s per parabola | Manned | 2 campaigns/year; 3 flights/campaign; 31 parabolas/flight | 10–2 | ∼2g | |
| Sounding rockets | 5–10 min | Automatic | 1 campaign (launch)/2 years | ≤10−4 | 10–50g | |
| Space shuttle and satellite | Weeks to months | Mainly automatic | Rarely | 10–6 | High launch and re-entry g | |
| International Space Station | Months to years | Automatic & manned | Rarely | 10–6 | – | |
| Tiangong Space Station | Months | Automatic & manned | – | 10–6 | – |
Ground-based simulated microgravity
Clinostats disrupt organisms’ gravity perception through rapid rotation, with variations such as random positioning machines (RPMs) enhancing the simulation. Magnetic levitators counteract gravity with magnetic forces, enabling gravity levels that range from 0g to 2g (Brungs et al., 2016).
Real microgravity
Drop towers provide high-quality microgravity in vacuum conditions, although experiment frequency is limited. Parabolic flights generate alternating microgravity and hypergravity phases. Sounding rockets offer longer microgravity durations during suborbital flights. Orbital platforms in low Earth orbit allow extended studies, supporting experiments on plant growth, development, and molecular biology (Morrow et al., 2016).
Centrifuges complement these platforms by generating partial gravity or 1g controls in space stations, as well as hypergravity conditions. They play a critical role in isolating variables and fine-tuning gravity levels for experiments (Kiss, 2015). This broad range of platforms has revolutionized microgravity research, driving discoveries essential for the advancement of space exploration.
Pros and cons of microgravity platforms for plant research
Research platforms provide a solid foundation for further study, making it essential to acquire a comprehensive understanding of their capabilities. Here, we summarize their principles, as well as their strengths and weaknesses (Table 2). Compared with real microgravity platforms, ground-based simulated microgravity facilities are more accessible, offer adjustable gravity, and have no operational time limits. Most importantly, they are cost-effective. However, the clinostat produces unnatural microgravity conditions with inevitable mechanical stress, whereas the magnetic levitator—despite effectively eliminating gravity—introduces a high-intensity magnetic field and uneven force distribution.
Table 2.
Principles, pros, and cons of microgravity research platforms.
| Methods | Principle | Pros | Cons | |
|---|---|---|---|---|
| Ground-based simulated microgravity facilities | Clinostat | Continuously changes sample orientation relative to the gravity vector to eliminate gravitational effects. |
|
|
| Magnetic levitator | Creates a counteracting magnetic force to balance gravity. Location within the magnetic field alters the magnitude and direction of the force. |
|
|
|
| Real microgravity platforms | Drop tower | Objects drop in a vacuum tube from the top of a tower, eliminating drag and friction to achieve high-quality microgravity. |
|
|
| Parabolic flight | Specially equipped aircraft execute parabolic maneuvers to follow a free-fall trajectory and achieve microgravity. |
|
|
|
| Sounding rockets | Rockets launched to suborbital altitudes follow a simple parabolic trajectory, providing a longer period of microgravity, before returning to Earth. |
|
|
|
| Orbital spaceflights |
|
|
|
To achieve real microgravity, platforms must be larger and more complex in design and structure, resulting in relatively high costs. Although non-orbital platforms are more accessible and affordable than orbital platforms, the duration of microgravity they provide is insufficient for plants to adequately perceive and respond to gravitational changes. Additionally, these platforms often introduce a hypergravity phase, making it difficult to isolate the effects of microgravity from other factors. The three non-orbital platforms are commonly used for testing aerospace devices, such as plant growth systems. In contrast, orbital platforms require considerable financial and human resources, making each flight highly valuable and necessitating thorough preparation. However, spaceflight is the only option for achieving long-term real microgravity with sufficient space and the possibility of manned operation. As a result, it is an ideal platform for studying the effects of microgravity at all levels, including molecular, cellular, tissue, and whole-plant levels.
Plant gravitropism
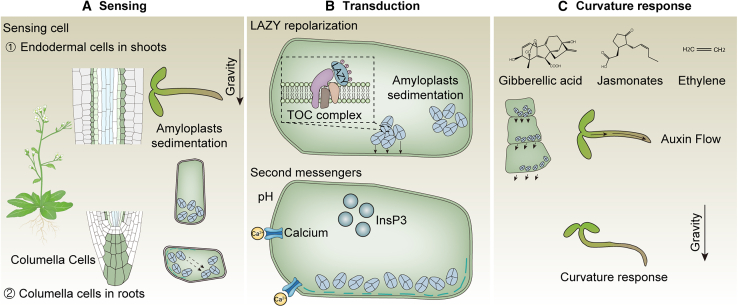
Plants have been continuously exposed to gravity throughout their evolution, making gravity a factor that influences nearly all biological, chemical, and physical processes in plants. The coordinated process of differential plant growth in response to gravity is known as gravitropism (Konings, 1995), a complex phenomenon involving several sequential stages: gravity sensing, signal transduction, and curvature response (Figure 2) (Su et al., 2017; Nakamura et al., 2019b).
Figure 2.
A model for plant gravitropism.
The process of plant gravitropism is artificially divided into three sequential stages.
(A) Gravity sensing (starch–statolith hypothesis): gravity stimulation induces the sedimentation of amyloplasts within sensing cells, including endodermal cells in shoots and columella cells in roots.
(B) Gravity transduction: the phosphorylated LAZY protein relocates to the lower side of the plasma membrane in response to amyloplast sedimentation, establishing a new polarity and initiating gravity signaling, which is transferred through a cascade of second messengers.
(C) Curvature response: the polar distribution of phytohormones—primarily auxin—drives differential growth rates that result in the plant’s curvature response.
Gravity sensing
In gravity sensing, the starch–statolith hypothesis is widely accepted by researchers today (Nakamura et al., 2019b; Takahashi et al., 2021). Plants perceive gravity using specialized gravity-sensing cells known as statocytes. Within statocytes, starch-filled amyloplasts, or statoliths, move freely and settle in response to gravistimulation (Sievers and Volkmann, 1977). Two well-characterized types of statocytes are columella cells in root caps and endodermal cells in shoots (Hashiguchi et al., 2013).
Columella cells are located in the inner part of the root cap. Root gravitropism is inhibited when the root cap is removed, either physically or through genetic manipulation. For instance, the Arabidopsis arf10-2 arf16-2 double mutant, which has severe developmental defects in the root cap—including the absence of columella cells containing amyloplasts—exhibits abnormal root gravitropism (Wang et al., 2005). Similarly, the Arabidopsis phosphoglucomutase (pgm) mutant, which impairs amyloplast sedimentation in columella cells, shows a reduced gravitropic response in roots (Kiss et al., 1997).
Endodermal cells in shoots function independently from root columella cells in gravity sensing. The Arabidopsis shoot gravitropic mutants sgr1 and sgr7, which lack a normal endodermis in hypocotyls and inflorescence stems, exhibit abnormal shoot gravitropism but retain normal root gravitropism with proper amyloplast sedimentation in the root cap (Fukaki et al., 1998), suggesting that the endodermis is essential for gravity sensing in shoots.
Starch accumulation plays a crucial role in the sedimentation of amyloplasts. In Arabidopsis, the starchless pgm mutant exhibits incomplete amyloplast sedimentation, resulting in a reduced gravitropic response in both roots and shoots. The partial gravitropic response in the pgm mutant suggests that starch accumulation, while important, is not absolutely essential for gravity sensing (Kiss et al., 1997; Fitzelle and Kiss, 2001; Morita, 2010). In contrast, the starch-excess mutant sex1, defective in a protein involved in starch mobilization, accumulates excess starch. As a result, plastids in the gravity-perceiving endodermal cells of the sex1 mutant are larger than those in the wild type, increasing hypocotyl sensitivity to gravity (Yu et al., 2001; Vitha et al., 2007). This indicates that an increase in the total mass of plastids leads to enhanced gravitropic sensitivity.
Intracellular components such as the cytoskeleton and vacuoles also influence amyloplast dynamics. In the agravitropic mutant shoot gravitropism 2 (sgr2), abnormal vacuolar membrane behavior restricts the movement of amyloplasts, preventing their sedimentation (Kato et al., 2002). Similarly, in the distorted1 (dis1) mutant, irregularly thick actin bundles surrounding amyloplasts in root columella cells hinder full amyloplast sedimentation, resulting in a reduced gravitropic response in roots (Zou et al., 2016). Additionally, Altered Response to Gravity 1 (ARG1) interacts with the cytoskeleton—likely actin—to contribute to gravity sensing. The arg1 mutant exhibits a reduced gravitropic response in both hypocotyls and roots (Boonsirichai et al., 2003).
Gravity transduction
The mechanism by which amyloplast sedimentation is converted into biochemical signals to trigger gravitropic responses remains unresolved. Two main models have been proposed: force-sensing and position sensor.
In the force-sensing model, amyloplast sedimentation exerts pressure on the endoplasmic reticulum (ER) or plasma membrane, deforming these membranes and activating mechanosensitive Ca2+-permeable channels (Perbal and Driss-Ecole, 2003; Hamilton et al., 2015). Another hypothesis within this model suggests that amyloplast tethering to the actin cytoskeleton generates tension, redirecting vesicle trafficking toward the sedimentation site. In contrast, the position sensor hypothesis posits that ligands on sedimented plastids interact directly with the plasma membrane to initiate signal transduction (Limbach et al., 2005).
A key discovery in gravitropism was the identification of LAZY family proteins as transducers of amyloplast sedimentation signals in statocytes. Initially described in mutants displaying abnormal stalk growth (Jenkins and Gerhardt, 1931; Jones and Adair, 1938), LAZY genes are critical for gravitropic responses in both shoots and roots across various species (Dong et al., 2013; Yoshihara et al., 2013; Taniguchi et al., 2017; Chen et al., 2020; Wang et al., 2024; Xu et al., 2024). In Arabidopsis, six LAZY genes are predominantly expressed in gravity-sensing cells (Nakamura et al., 2019a).
Recent findings revealed that LZY3 is phosphorylated by MPK kinase 5 (MKK5) and mitogen-activated protein kinase 3 (MPK3) during gravistimulation. Phosphorylation enhances LAZY’s affinity for TOC proteins on amyloplast surfaces; disruptions in TOC120 or TOC132 cause gravitropic defects due to reduced localization of LAZY protein on amyloplasts (Taniguchi et al., 2017). Upon amyloplast sedimentation, LAZY proteins may be dephosphorylated and relocate to the plasma membrane on the lower side of the cell, establishing new polarity (Taniguchi et al., 2017). This membrane relocation depends on basic and hydrophobic amino acids that are critical for membrane binding (Taniguchi et al., 2017; Chen et al., 2023).
LAZY proteins sediment with amyloplasts and translocate to the plasma membrane, transmitting positional information to RCC1-like domain (RLD) proteins. This process regulates auxin flow toward the site of sedimentation via PIN-FORMED-dependent transport (Kleine-Vehn et al., 2010; Taniguchi et al., 2017; Chen et al., 2023). The resulting polarized auxin distribution causes asymmetric growth, bending the organ toward gravity.
Second messengers, including calcium ions (Li et al., 2024), inositol triphosphate (InsP3), and protons, further transduce the gravity signal, facilitating auxin transport across the stimulated organ (Belyavskaya, 1996a; Perera et al., 1999; Fasano et al., 2001).
Differential distribution of phytohormones influences curvature response
The Cholodny–Went hypothesis posits that the bending growth characteristic of tropisms results from the polar distribution of auxin (Went, 1974). This hypothesis has been supported by numerous studies (Peer et al., 2011; Rosquete et al., 2013). Physiological analyses have identified a specific transport system responsible for auxin distribution, which affects differential growth. Differential auxin distribution plays an important role in determining the growth angle of primary and lateral roots (Rosquete et al., 2013; Roychoudhry et al., 2013). Mutants with defects in auxin synthesis, polar transport, or signal transduction often exhibit abnormal gravitropic responses (Stepanova et al., 2008; Rakusová et al., 2011; Baster et al., 2013).
In addition to auxin, other plant hormones—including jasmonic acid (JA), gibberellin (GA), ethylene, cytokinin, and brassinosteroid (BR)—function in concert with auxin to regulate root gravitropism. The polar distribution of JA appears to modulate gravitropism; plants with inhibited JA gradients or JA-deficient mutants exhibit delayed gravitropic responses (Gutjahr et al., 2005). GA signaling stabilizes the auxin transporter PIN-FORMED 2 (PIN2) on the lower side of roots during gravitropic responses (Willige et al., 2011; Löfke et al., 2013). Cytokinin redistributes rapidly (within minutes) toward the lower side of gravistimulated roots, and applying exogenous cytokinin to vertical roots induces root bending toward the application site (Aloni et al., 2004, 2006). Cytokinin regulates root cell elongation through both ethylene-dependent and -independent pathways (Street et al., 2016). BR regulates gravitropism by modulating polar auxin transport and influencing JA signaling pathways (Li et al., 2005; Retzer et al., 2019; Bai et al., 2024). In conclusion, plant gravitropism is a complex process regulated by a network of phytohormones.
Impacts of microgravity on plants
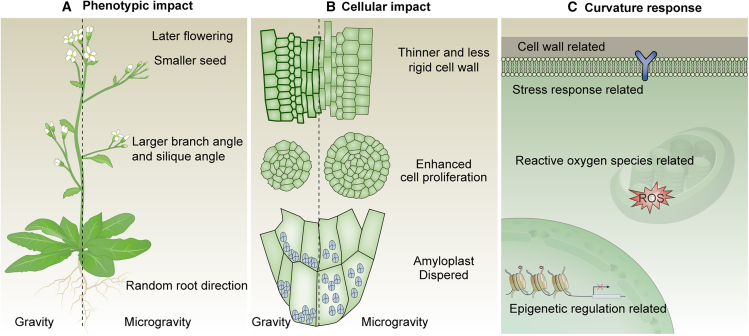
Here, we summarize the impacts of microgravity on plants at three levels: phenotypic, cellular, and signaling pathways (Figure 3).
Figure 3.
Impacts of microgravity on plants.
(A) At the phenotypic level, plants exhibit random root growth direction, larger branch and silique angles, delayed flowering, and smaller seed size.
(B) At the cellular level, plants in microgravity show dispersed amyloplasts, enhanced cell proliferation, and thinner, less rigid cell walls.
(C) Omics analyses reveal alterations in several signaling pathways under microgravity, including those related to the cell wall, stress responses, reactive oxygen species, and epigenetic regulation.
Impacts on phenotypes
In space agriculture, a major challenge comprises growing plants through their entire life cycle in space, often referred to as “from seed to seed.” Since the early days of human space exploration, seed-to-seed experiments have been conducted in space. Although plants evolved gravitropism on Earth and microgravity presents considerable environmental challenges, five plant species—Arabidopsis, wheat (T. aestivum L.), pea (P. sativum), B. rapa L., and rice (O. sativa L.)—have successfully completed their life cycles in space. These findings indicate that microgravity may have less severe effects on plant growth and development than initially anticipated (Merkys et al., 1984; Musgrave et al., 2000; Sychev et al., 2007; Jia et al., 2024).
Notable morphological changes occur in space-grown plants compared to Earth-grown plants. First, in the Arabidopsis seed-to-seed experiment conducted in the Advanced Astroculture (ADVASC) system aboard the ISS from 2001 to 2002, branches were larger, and silique angles were greater than those on Earth. In space-grown plants, branches grew nearly perpendicular to the inflorescence, even toward the root tray; siliques also emerged almost perpendicular to the stem (Link et al., 2014). Second, in the B. rapa L. seed-to-seed-to-seed experiment in the Svet greenhouse on the Mir space station in 1997, seeds produced in space were smaller than those grown on Earth, and starch was retained in mature space-grown seeds, consequently producing smaller second-generation space plants (Musgrave et al., 2000). Similarly, protein bodies in space-grown Arabidopsis seeds were 55% smaller compared to Earth-grown controls (Link et al., 2014). Furthermore, flowering time has been observed to change under microgravity conditions. In the Arabidopsis seed-to-seed experiment conducted in the Cell Biology Experiment Facility (CBEF) under continuous light on the Kibo module of the ISS in 2009, bolting occurred slightly earlier under microgravity than under 1g conditions in space. The number of flowers per plant significantly increased under microgravity, though flowering time was not significantly affected. However, in another Arabidopsis seed-to-seed experiment conducted in the Plant Culture Box (PCB) in the Chinese space lab TG-2, plants grown under long-day conditions (16 h light/8 h dark) in microgravity flowered later than those grown under the same photoperiod on Earth in either 1g-PCB or 1g-greenhouse conditions (Xie et al., 2021). These studies suggest that environmental factors (e.g., light, water, nutrition, air, and available growing volume) significantly influence plant growth and development, in addition to microgravity.
Impacts on root development
On Earth, plant roots orient themselves toward gravity—a phenomenon known as the positive gravitropic response—and away from blue or white light, exhibiting a negative phototropic response (Chen et al., 1999; Briggs, 2014). In the low-light environment of spaceflight, Arabidopsis roots displayed random growth directions (Paul et al., 2017). However, under blue or red light in space, Arabidopsis roots exhibited phototropic curvature in microgravity—a response that is either absent or reduced under higher gravity levels (Vandenbrink et al., 2016). These intriguing findings suggest a complex interplay between gravitropism and phototropism.
Additionally, roots grown along a vertical surface exhibit skewing and waving—phenomena typically attributed to gravity. Skewing refers to the slanted growth of roots away from the gravity vector, whereas waving describes the back-and-forth oscillation of root growth along its primary direction (Roux, 2012). Notably, several spaceflight experiments have shown that Arabidopsis roots also display skewing and waving in microgravity, consistent with their behavior on Earth (Paul et al., 2012).
Root length in microgravity has been examined in several experiments, with varying results. Soybean seedlings germinated during spaceflight showed increased primary root length, a higher number of lateral roots, and greater total fresh root weight (Levine et al., 2001). Similarly, sweet potato stem cuttings aboard the Space Shuttle produced more roots and exhibited greater total root length compared with ground-based controls (Mortley et al., 2008). However, rice roots grown in space showed no significant difference in length relative to those grown on Earth (Takakura et al., 1996). Intriguingly, in one experiment, Arabidopsis root length was shorter in space than on the ground (Paul et al., 2012), whereas another experiment revealed no significant change (Paul et al., 2017). The flavonoid-accumulating Arabidopsis mutant transparent testa 3 (tt3) exhibited enhanced correction of root growth direction under microgravity conditions (Villacampa et al., 2022). These findings suggest that root length does not consistently change under microgravity, varying across experiments or influenced by environmental factors such as light, water, flavonoids, and microgravity itself.
Impacts on cellular components
Cell wall
Plant cell walls, which provide mechanical strength, exhibit prominent changes under microgravity. Space-grown plants show thinner and less rigid cell walls due to altered biosynthesis and modification processes. Studies of O. sativa, Arabidopsis, and P. sativum have revealed reductions in cellulose and polysaccharides during spaceflight (Nedukha, 1996; Hoson et al., 2005).
Microgravity decreases cellulose crystallization, activates pectolytic enzymes, and alters calcium balance. Reduced polysaccharide content and molecular size lead to thinner, more extensible cell walls, stimulating plant growth (Hoson et al., 2014; Bulavin, 2016). During the STS-95 mission, rice roots and coleoptiles exhibited decreased cellulose and matrix polysaccharides but increased high-molecular-weight hemicellulose, resulting in thinner root cell walls and enhanced coleoptile extension (Hoson et al., 2002, 2003). Similar changes in Arabidopsis hypocotyls suggest increased malleability due to altered xyloglucan metabolism (Soga et al., 2002).
Microgravity also reduces lignin content across species such as pine, mung bean, and oat, due to decreased phenylalanine ammonia-lyase (PAL) and peroxidase activities. The STS-3 and STS-51F missions reported significant reductions in lignin content and shorter seedling growth under microgravity, supporting the hypothesis of suppressed lignin synthesis (Cowles et al., 1984, 1989). In contrast, hypergravity increases lignin content, which correlates with greater cell wall stiffness (Wakabayashi et al., 2009).
Proteomic and transcriptomic analyses of Arabidopsis revealed downregulation of genes encoding cell wall proteins, including cellulose synthases (CESA1, CESA3, CESA5, and CESA7) and cellulose-like proteins (CSLE1 and CSLG3), leading to improved cell wall malleability (Hoson et al., 2014; Olanrewaju et al., 2023a). Transcriptome profiling revealed reduced expression of genes related to cell wall organization, including xyloglucan endotransglucosylase (XTH) and pectin modification enzymes (Land et al., 2024). Glycome profiling also indicated altered xylans and xyloglucans in roots under spaceflight conditions compared with ground controls (Nakashima et al., 2023).
Cell membrane
The lipid bilayer of the cytoplasmic membrane acts as a boundary between the cell’s interior and the external environment; its structure and permeability are influenced by gravity. In Chlorella cells, microgravity increases membrane sinuosity, forming folds associated with enhanced metabolism and active transport. Lipid composition in cytoplasmic membranes from pea seedling roots and epicotyls under clinostatting differs significantly from ground controls, demonstrating substantial changes in lipid classes and fatty acid profiles (Polulakh Yu et al., 1989).
Microgravity decreases lipid peroxidation and increases plasma membrane microviscosity by 15%–20%. Although total phospholipid content remains stable, the proportion of unsaturated fatty acids (e.g., linoleic and linolenic acids) increases, while the fraction of saturated fatty acids (e.g., palmitic and stearic acids) decreases, thus maintaining membrane microviscosity. Sterol content, crucial for “lipid raft” formation, also increases under clinostatic conditions.
Transcriptional analyses reveal altered expression of genes linked to lipid signaling and membrane biosynthesis under microgravity. The plasma membrane, particularly its receptor-rich regions, serves as a hub for environmental signal perception. Proteomic studies of Arabidopsis aboard the ISS demonstrated significant changes in 149 membrane-associated proteins, with auxin metabolism and trafficking proteins depleted and stress- and defense-related proteins enriched—highlighting microgravity as a stress factor (Mazars et al., 2014). The predicted functions of these proteins suggest that those related to auxin metabolism and trafficking are reduced in the microsomal fraction under microgravity conditions, whereas proteins associated with stress responses, defense, and metabolism are more abundant, indicating that microgravity is perceived by plants as a stressful environment.
Cell cycle
Another significant effect of microgravity on plant cells is the “decoupling” of cell growth and proliferation (Manzano et al., 2008). Under Earth’s 1g environment, cell proliferation and growth are closely linked and interdependent processes. However, in microgravity, this relationship diverges—cell proliferation is enhanced, whereas cell growth is weakened (Boucheron-Dubuisson et al., 2016; Manzano et al., 2018). It has been suggested that this phenomenon is related to altered cell-cycle regulation, particularly due to shortening of the G2 phase (Matía et al., 2010). This shortening leads to a significant reduction in ribosome synthesis, causing nucleoli to become smaller and less active. Although the accelerated cell cycle may result in faster growth and increased cell length, it is generally detrimental to the plant.
Arabidopsis seedlings were taken aboard the space station for 10 days and germinated for 4 days. Results showed that seedlings grown in microgravity were larger than those in the control group; they displayed increased proliferation in the root meristem and decreased ribosome biogenesis, likely due to the accelerated cell cycle (Matía et al., 2007). In the Earth environment, the proliferation rate of root cortex cells was higher, and their nucleoli were more active compared to those of columellar cells. However, in microgravity, the central columella cells became longer with larger nucleoli, whereas cortex cells were shorter, more numerous, and densely packed, with smaller and less active nucleoli. Significant changes were observed in the cell cycle of root meristem cortical cells, resulting in enhanced cell proliferation and decreased activity per cell (Matía et al., 2005). This accelerated cell cycle can negatively affect overall plant development and morphogenesis. Additionally, diamagnetic levitation and mechanically simulated microgravity can cause similar uncoupling of cell proliferation and ribosome biogenesis in Arabidopsis seedlings, altering polar auxin transport (Manzano et al., 2013).
Amyloplasts and cytoskeleton
Statocytes are essential for plant gravity responses. The starch–statolith hypothesis posits that gravity-induced statolith displacement triggers second messengers, such as Ca2+, leading to auxin transport. In microgravity, amyloplast distribution shifts from the distal ends of cells (as observed under Earth’s gravity) to a dispersed, central localization (Perbal and Driss-Ecole, 1989; Kordyum and Brykov, 2021).
Studies examining the effects of microgravity revealed random amyloplast distribution in Picea glauca statocytes aboard the ISS, unlike their sedimentation on Earth (Rioux et al., 2015). B. napus roots in microgravity showed minimal statolith displacement; however, this environment triggered intracellular Ca2+ release, likely involved in gravity signal transduction (Bizet et al., 2018). Actin filaments, key components in gravity sensing, regulate root growth differently under microgravity. Amyloplasts may attach to actin via motor proteins, with dynein-driven movement becoming dominant in microgravity; these changes cause amyloplasts to shift toward the nucleus and potentially activate Ca2+ channels (Perbal et al., 1997; Nakashima et al., 2013). Ca2+, a crucial second messenger, shows rapid changes in microgravity, highlighting plants’ responsiveness to altered gravity (Belyavskaya, 1996b; Toyota et al., 2013). Experiments on Lepidium sativum roots exposed to microgravity after 1g acceleration showed statoliths moving toward the cell center via cytoskeletal elastic forces, and movement slowed over time (Perbal et al., 1997; Driss-Ecole et al., 2000). Additionally, microgravity disrupts microtubule organization in Arabidopsis root cells, affecting cellular structure (Yemets et al., 2024).
Omics studies reveal molecular responses to microgravity
Plants evolved under Earth’s gravity, whereas microgravity acts as a considerable stressor, impacting growth and development at the molecular level. Omics technologies have shown that microgravity alters stress responses, cell wall remodeling, photosynthesis, oxidative stress, and metabolism (Table 3). However, variations in experimental conditions during spaceflight—including plant species and data collection methods—have led to inconsistent results. Tools such as the ARABIDOMICS platform (Hauslage et al., 2020) and NASA’s GeneLab (Berrios et al., 2021) have helped standardize and share data; initiatives such as the International Standards for Space Omics Processing (ISSOP) promote global guidelines (Rutter et al., 2020).
Table 3.
Omics studies identifying molecular responses in plants under microgravity conditions.
| Biological process | Species and tissue | Treatment time | Gravity level | Omics technologies | Hardware platform | Reference |
|---|---|---|---|---|---|---|
| Lipid metabolism, response to stress factors, light signaling pathways | A. thaliana (wild type, and pin2 and pin3 mutants) | 5-day seedlings, 31 parabolas | Microgravity (μg), centrifuge 1g control (parabolic flight) | Microarray | Parabolic flight | Aubry-Hivet et al., 2014 |
| Auxin metabolism and signaling, calcium- mediated signaling, disease resistance, cell wall biochemistry | A. thaliana (WS) | 10-day seedlings, 20 parabolas, 40 parabolas, control | μg | Microarray | Parabolic flight | Paul et al., 2011 |
| Upregulation dominated by Ca2+- and ROS-related gene products, primary metabolism (glycolysis, gluconeogenesis, citrate cycle) | Callus cell cultures of A. thaliana (Col- 0) | 22 s microgravity | μg | Microarray analysis, mass spectrometry (MS) | Parabolic flight | Hausmann et al., 2014 |
| Calcium signaling, redox status, and stress response; gene ontology terms related to light and photosynthesis enriched under microgravity | A. thaliana (Ler) | Seed to seedling, 6 days in flight | μg, low-g (<0.1g) Moon gravity, centrifuge 1g control | RNA-seq | ISS European Modular Cultivation System (EMCS), with centrifuge control | Herranz et al., 2019 |
| Stress responses, carbohydrate metabolism, protein synthesis/degradation, intracellular trafficking/transport, signaling, cell wall biosynthesis | A. thaliana (Col-0) callus | 5 days in flight | μg, centrifuge 1g control | LC-ESI-MS/MS with iTRAQ labeling | Spacecraft Shenzhou 8 SIMBOX, with centrifuge control | Zhang and Zheng, 2015 |
| Upregulation of genes encoding plastid ribosomal proteins, mitochondrial electron transport chain components, and NADH dehydrogenase | A. thaliana (Col-0) callus | 5 days in flight | μg, centrifuge 1g control | Microarray | Spacecraft Shenzhou 8 SIMBOX, with centrifuge control | Fengler et al., 2015 |
| Alterations in cell wall development and oxygen-binding proteins | A. thaliana (Ler) | Seed to seedling, 96 h at 1g followed by 48 h at μg in flight | μg, centrifuge 1g control, ground 1g control | Microarray | ISS, EMCS, with centrifuge control | Correll et al., 2013 |
| Activation of proliferation-promoting pathways (cytokinin and auxin), and plastid/mitochondrial-encoded transcripts | A. thaliana (Col-0) | Seed to seedling, 6 days in flight | μg, Mars gravity level (0.3g), centrifuge 1g control | RNA-seq | ISS, EMCS, with centrifuge control | Villacampa et al., 2021 |
| Depletion of microsome-associated proteins, auxin metabolism, and trafficking; enrichment in stress response and defense metabolism | A. thaliana (Col-0) | Seed to seedling, 12-day treatment | μg, centrifuge 1g control, 1g on Earth | LC–MS/MS | ISS GENARA A hardware/EMCS, with centrifuge control | Mazars et al., 2014 |
| Downregulation of light perception, photosynthesis, and photosynthetic complex biosynthesis | A. thaliana (Ler) | Seed to seedling, 6 days (96 h at 1g and 48 h at μg or 1g) in flight | μg, centrifuge 1g control | RNA-seq | ISS, EMCS, with centrifuge control | Vandenbrink and Kiss, 2019 |
| Altered light sensing, cell wall architecture, and auxin-related gene expression | A. thaliana (leaves, hypocotyls, roots) | Seed to seedling, 12 days in flight | μg, ground 1g control | Microarray | ISS, Advanced Biological Research System (ABRS) | Paul et al., 2013 |
| Upregulation of heat-shock proteins; downregulation of peroxidase transcripts | A. thaliana (Col-0, Ler-0, WS-2) | Seed to seedling, 8 days in flight | μg, ground 1g control | RNA-seq | ISS, Biological Research in a Canister (BRIC) | Choi et al., 2019 |
| Downregulation of light signaling; upregulation of plant defense and cell wall metabolism | A. thaliana (WS, Col-0, Col-0/PhyD (phyD)/root tips) | 11 days in flight | μg, ground 1g control | RNA-seq | ISS, CARA Petri plates | Paul et al., 2017 |
| Changes in alternative splicing patterns | A. thaliana (Col-0 and WS) | Seed to seedling, 4 and 8 days in flight | μg, ground 1g control | RNA-seq | ISS, Vegetable Production System (VEGGIE) | Beisel et al., 2019 |
| HSPs and flavonoid biosynthesis significantly altered (e.g., ATHSP90.1, HSP235-M, ATHSP70) in young seedlings | A. thaliana (Col-0) | 7-day seedlings, 2 h and 16 h treatment | RPM, ground 1g control | Microarray | RPM | Kittang et al., 2013 |
| Repression of oxidative stress responses and cell wall remodeling | A. thaliana (Col-0 and vegetative actin mutant act2-3) | Seed to seedling, 14 days in flight | μg, ground 1g control | Microarray | Space shuttle Discovery, BRIC | Kwon et al., 2015 |
| Downregulation of UPR-related stress response genes (e.g., abscisic acid response, hypoxia, water deprivation, oxidative stress); upregulation of secondary metabolism genes associated with stress adaptation | A. thaliana (Col-0, atire1, bzip60, bzip28, bzip28 bzip60) | Seed to seedling, 14 days in flight | μg, ground 1g control | RNA-seq | ISS, BRIC | Angelos et al., 2021 |
| Differential abundance of cell wall-related proteins during spaceflight; upregulation of stress/defense, photosynthesis, translation, gene expression, and biogenesis pathways in leaves | A. thaliana (Adh::GFP, DR5r::GFP, and 35s::GFP lines) | Seed to seedling, 12 days in flight | μg, ground 1g control | iTRAQ-labeled MS/MS microarray | ISS, ABRS | Ferl et al., 2015 |
| Upregulation of ROS metabolism and cell wall organization; plastid-localized pathways activated | A. thaliana (Col-0) | Seed to seedling, 76 h in flight | μg, ground 1g control | iTRAQ-labeled LC–MS/MS RNA-seq | ISS, BRIC | Kruse et al., 2020 |
| Upregulation of xylan and pectin biosynthesis, metabolism, and transport; downregulation of hypoxia and heat-shock responses, DNA repair, and cell wall structure genes | A. thaliana (Ler) | Seed to seedling, 309 h in flight | μg, ground 1g control | Microarray, cell wall glycomics | ISS, BRIC | Johnson et al., 2017 |
| Carbohydrate and phosphate metabolism impacted by microgravity | A. thaliana (Ler, phyB-1) | 7-day seedlings, 24 h reorientation by 90°, in dark or unilateral blue/red light | – | Metabolomics, microarray | Millar and Kiss, 2013 | |
| Reduced DNA methylation levels in CHG, CHH, and CpG contexts; altered expression of genes linked to DNA methylation, hormone signaling, cell wall modification, and transposable elements (TEs) | A. thaliana (Col-0) | 6 days seedling, 60 h in flight | μg, ground 1g control | Whole-genome bisulfite sequencing | SJ-10 recoverable satellite | Xu et al., 2018 |
| Enrichment of abscisic acid signaling, protein phosphorylation, nitrate signaling; spaceflight-induced heritable differentially methylated regions (DMRs) retained through F3 generation | Offspring of A. thaliana (Col-0) grown for 11 days on satellite | Seed to seedling, 11 days in flight | μg, ground 1g control | RNA-seq, whole-genome bisulfite sequencing | SJ-10 recoverable satellite | Xu et al., 2021 |
| Altered DNA methylation and ROS signaling | A. thaliana (WS) | Seed to seedling, 11 days in flight, root and leaf collected separately | μg, ground 1g control | RNA-seq, whole-genome bisulfite sequencing | ISS, VEGGIE | Zhou et al., 2019 |
| Significant increases of methylation levels in met1-7 and elp2-5 mutants under spaceflight conditions | A. thaliana (Col-0, met1-7, elp2-5) | Seed to seedling, 11 days in flight | μg, ground 1g control | Whole-genome bisulfite sequencing, RNA-seq | ISS, VEGGIE | Paul et al., 2021 |
| Altered DNA methylation | A. thaliana (Col-0 and elp2-5) | Seed to seedling, 11 days in flight | μg, ground 1g control | Flap-enabled next-generation capture | ISS, VEGGIE | Zhou et al., 2024a |
| Changes in carbon metabolism, glycolysis, gluconeogenesis, and amino acid biosynthesis | A. thaliana | Seed to seedling, in flight | μg, onboard 1g control | Proteomics liquid chromatography (LC)–MS spectra | ISS, various hardware | Olanrewaju et al., 2023b |
| Reduced abundance of cellulose synthases, cellulose-like proteins, and tubulin cofactor B (TFCB) | A. thaliana (Col-0) | Seed to seedling, 10 days in flight | μg, ground 1g control | RNA-seq, TMT labeled LC–MS/MS proteomics analysis | ISS, BRIC, LED | Olanrewaju et al., 2023a |
| Regulation of auxin signaling, cell wall dynamics, and miRNA expression | A. thaliana Col-0 | Seed to seedling, 5–6 days in flight | μg, ground 1g control, onboard centrifuge 1g control | RNA-seq | ISS, EMCS | Land et al., 2024 |
| Upregulation of 20 of 32 ROS marker genes | Mizuna (Brassica rapa var. japonica) | Seed to seedling, 28 days in flight | μg, ground 1g control | RNA-seq | ISS, Lada | Sugimoto et al., 2014 |
| Upregulation of phosphatase 2C (accession CV735645) in Ceratopteris richardii spores after 1, 8, and 20 h under microgravity; HSP induction | Fern C. richardii | Spores, in flight with 1, 8, and 20 h of light | μg, ground 1g control | Microarray | NASA shuttle flight STS-93, BRIC flight canisters | Salmi and Roux, 2008 |
| Up- or downregulation of auxin-related and aquaporin genes | Etiolated Alaska pea (Pisum sativum L.) with/without 2,3,5-triiodobenzoic acid (TIBA) | Seed to seedling, 3 days in flight | μg, centrifuge 1g control | Microarray | ISS CBEF (includes centrifuge on space control) | Kamada et al., 2020 |
| Induction of HSPs and heat stress transcription factors HSFA2s and HSFB2s | Rice (E3 ubiquitin ligase SOIL-SURFACE ROOTING 1 (SOR1) mutant and wild type) | Seed to seedling, root, 6 h clinostat, and 4 h gravistimulation | – | RNA-seq | 3D clinostat | Kuya et al., 2023 |
Stress response components in microgravity
Heat-shock proteins
Heat-shock protein (HSP) genes, key regulators of stress responses, are significantly upregulated in microgravity. Studies of Arabidopsis and rice roots under simulated and actual microgravity have confirmed that HSPs and heat-shock transcription factors (HSFs) are induced and rapidly downregulated upon gravistimulation (Kittang et al., 2013; Choi et al., 2019; Kuya et al., 2023).
Unfolded protein response
The unfolded protein response (UPR) detects and resolves ER stress. Transcriptomic analyses of Arabidopsis UPR mutants during spaceflight revealed fewer differentially expressed genes (DEGs) compared with the wild type, highlighting the UPR’s partial role in transcriptional reprogramming under microgravity (Angelos et al., 2021).
Reactive oxygen species
Reactive oxygen species (ROS), crucial in stress signaling, show altered dynamics in space-grown Arabidopsis. Transcriptomic data have revealed the enrichment of ROS-scavenging genes (e.g., oxidases and peroxidases), whereas proteomic analyses show changes in protein oxidation states under microgravity (Sugimoto et al., 2014).
Alternative splicing
Alternative splicing (AS), which generates transcript isoforms in response to stress, was analyzed during the Advanced Plant Experiment-03 (APEX03). RNA sequencing (RNA-seq) of Arabidopsis roots from different ecotypes indicated that AS constitutes a key mechanism in plant adaptation to spaceflight (Beisel et al., 2019).
Epigenetics
DNA methylation, an epigenetic marker, is altered in space-grown plants. Arabidopsis seedlings aboard the SJ-10 satellite exhibited lower genome-wide methylation, affecting hormone signaling and cell wall genes. These changes were heritable up to the F3 generation (Xu et al., 2018, 2021). Other studies detected higher methylation levels within certain contexts in space-grown leaves, linked to ROS signaling and cell wall remodeling (Zhou et al., 2019; Paul et al., 2021). Methylation-regulating mutants showed increased methylation levels during spaceflight, suggesting epigenetic regulation of spaceflight-induced adaptations (Paul et al., 2021; Zhou et al., 2024b).
These findings highlight the complex molecular responses of plants to microgravity, driven by stress, signaling pathways, and epigenetic modifications.
Plant adaptations to space-specific environmental factors
Plants exhibit a remarkable capacity to adapt to the unique environmental conditions of space, particularly the microgravity environments encountered aboard the ISS. An understanding of these adaptive responses has profound implications for space agriculture, especially for sustainable food and pharmaceutical production during long-duration missions (Maffei et al., 2024). Although literature in this field remains limited, it is evident that several space-specific environmental factors significantly influence plant development.
Altered gravitropism
In microgravity, plants lose their ability to use gravity for orientation, resulting in reliance on alternative cues such as light (phototropism) and touch (thigmotropism). This shift is accompanied by cytoskeletal reorganization, in which actin filaments and microtubules adapt to the absence of gravitational force in ways that impact cell division, expansion, and polarity. The disruption of amyloplast sedimentation in gravity-sensing root cap cells further alters root growth patterns.
Photoreceptor modulation
In the absence of gravitational cues, light becomes the dominant environmental signal. Space-grown plants display altered expression and biosynthesis of key photoreceptors, including phototropins, phytochromes, and cryptochromes. Optimizing the red-to-blue light ratio under light-emitting diode (LED)-based lighting systems is critical for promoting efficient photosynthesis and morphogenesis.
Modified thermoperception
Microgravity influences thermosensory mechanisms, likely through changes in membrane fluidity and downstream signaling pathways. Plants exhibit adaptive plasticity in HSPs and ROS-scavenging systems to mitigate stress associated with temperature fluctuations.
Impaired gas exchange and water transport
The lack of buoyancy-driven convection in microgravity reduces gas-exchange efficiency, leading to localized accumulation of CO2 and ethylene, impaired transpiration, and altered stomatal dynamics and aquaporin activity. Consequently, space-based plant growth systems must tightly regulate airflow and humidity to support effective gas exchange and water transport.
Space agriculture
Space agriculture, discussed since the mid-20th century, aims to provide astronauts with food, oxygen, and clean water for long-term missions and planetary colonization (Wheeler, 2017). The field is still in its early stages, focusing on technologies such as hydroponics, optimized lighting, and resource-efficient cultivation systems.
Key technologies
Hydroponics
This soilless cultivation method offers precise nutrient control and high space efficiency, crucial for resource-limited environments. Recycled hydroponics has been successfully applied in space, such as in the cultivation of Glycine max within the Micro Ecological Life Support System Alternative (MELiSSA) (Paradiso et al., 2013). Innovations such as automated nutrient estimators and ion-detection algorithms further improve yield and nutrient management (Rajendran et al., 2024; Sangeetha and Periyathambi, 2024).
LED lighting
LED technology provides energy-efficient, long-lasting illumination for controlled agriculture. Studies have shown that adjustments of light quality and intensity optimize plant growth for species such as Cucumis sativus and Spinacia oleracea, both in space and on Earth (Graham et al., 2019; Zou et al., 2020).
Irrigation systems
Microgravity makes irrigation challenging. Systems such as capillary mats and porous tubes combined with cellulosic sponges have been designed to efficiently deliver water and nutrients (Paradiso et al., 2020). In a capillary mat system, pots are set on mats constantly moistened with nutrient solution (Nguyen et al., 2023). The Precursor of Food Production Unit (PFPU) project developed a modular cultivation system for edible tuberous plants in microgravity, finding cellulosic sponge to be the optimal cultivation substrate.
Environmental monitoring
Detection systems, such as nucleic acid amplification methods for microbial loads and chlorophyll fluorescence for assessing plant health, improve cultivation efficiency and reduce contamination risks (Amalfitano et al., 2020; Merrick et al., 2022). Sustainable soilless systems designed for multiple crop cycles further enhance spaceflight food production (Curry et al., 2024).
In situ resource utilization
Efficient use of Mars or Moon resources reduces transport costs (Ding et al., 2024). Studies using Martian and lunar soil simulants (MMS-1 and LHS-1) demonstrate their potential for intercropping and fertility enhancement through composting, enabling higher yields of nutritious crops such as potato (Solanum tuberosum) (Benavides-Mendoza et al., 2024; Caporale et al., 2024).
Space agriculture continues to evolve, integrating advanced technologies to create efficient, sustainable systems that will support long-term human space exploration.
Bioregenerative life-support system
A BLSS, also known as a controlled ecological life-support system (CELSS), is an artificial, highly closed ecosystem designed to sustain life in space. A BLSS typically incorporates three biological components: plants, animals, and microorganisms (Tang et al., 2021). These components interact through physical, biological, and chemical processes such as water and gas cycles, waste recycling, and nutrient cycling (Jin et al., 2021; Joris et al., 2024).
Currently, most research on closed life-support systems remains in the ground-based simulation stages (Liu et al., 2021a), with efforts primarily focused on fundamental biological processes and system construction (Yuan et al., 2019; Onoda et al., 2024).
In the 1950s and 1960s, Jack Myers and colleagues pioneered space agriculture by studying algae for oxygen production and carbon dioxide removal (Myers, 1954, 2002). In the 1960s, Russian scientists conducted research on microalgae, exploring its potential as a regenerator of the atmosphere within spacecraft cabins and in controlled-environment agriculture or regenerative man-made ecosystems (Golueke and Oswald, 1964). Their work demonstrated the feasibility of closed systems using gas-exchange equilibrium between humans and algae. NASA initiated space agriculture research in the 1960s (Armstrong, 1966) and later revived its bioregenerative efforts with the launch of the CELSS program in 1979. This program substantially expanded in the 1980s, incorporating crops such as wheat, soybean, lettuce, sweet potato, and potato (MacElroy and Bredt, 1984). In the early 1990s, Biosphere 2, an ambitious closed artificial ecosystem facility, was constructed in Arizona, USA (Nelson et al., 1992). This project aimed to enhance understanding of closed ecological systems and bioregenerative approaches for human life support. Around the same time, the ESA launched the MELiSSA project (Gòdia et al., 2002), which developed a life-support system using various microbial species and higher plants (Fulget et al., 1999). In the 1990s, Japanese scientists initiated research at the Closed Ecological Experiment Facility (CEEF), aiming to clarify life-support mechanisms within a completely closed system (Nitta et al., 2000).
Globally, advancements in BLSS research include NASA’s Advanced Plant Habitat, ESA’s MELiSSA project, and Russia’s BIOS-3, collectively driving progress in sustainable life-support systems for long-term space exploration.
Since the mid-1990s, China has made extensive progress in BLSS and artificial closed ecosystem development. The Lunar Palace 1 facility, built in 2013 at Beihang University, Beijing (Li et al., 2015), is a 500-m3 system integrating humans, plants, insects, and microbes. In 2014, a 105-day closed experiment achieved 100% oxygen and water recycling and 55% food regeneration (Fu et al., 2016). Research highlights include solid waste fermentation (Liu et al., 2020), yellow mealworm rearing (Li et al., 2015), and meal plan optimization (Fu et al., 2019). In 2016, a 180-day experiment in Shenzhen utilized a CELSS with six cabins and 25 plant species, examining atmospheric non-methane hydrocarbon (NMHC) trends (Dai et al., 2018) and advanced membrane bioreactor (MBR) water treatment methods (Li et al., 2018). From 2017 to 2018, the Lunar Palace 365 project conducted a 370-day experiment—the longest of its kind—with a closure degree of 98%. The experiment demonstrated water recycling that met national water quality standards in China using membrane biological activated carbon reactor technology; it revealed no lasting health impacts on crew members through analyses of salivary microbiota and cytokines (Yang et al., 2022; Zhao et al., 2022).
Plants in space agriculture
Plants are an essential component of long-term life-support systems in outer space and play a pivotal role in constructing BLSS systems (Ferl et al., 2002). First, higher plants absorb carbon dioxide and release oxygen through photosynthesis, contributing to the gas cycle in closed systems and providing a basic oxygen supply for humans. Second, as integral components of the ecosystem’s water cycle, plants purify wastewater and provide clean water for both humans and the system. Third, higher plants serve as fundamental food sources by supplying carbohydrates, amino acids, proteins, and trace elements, supporting the nutritional cycle in BLSSs. Additionally, plants recycle waste by absorbing nutrients from byproducts, promoting efficient waste management within the system. Finally, regular consumption of vegetables is essential for maintaining astronauts’ mental health during extended space missions. However, some plant species remain unsuitable for space agriculture.
Selection of suitable plants for space agriculture
Early space agriculture research primarily focused on algae (Sorokin and Myers, 1953) because they could effectively provide oxygen and remove carbon dioxide. However, algae cannot serve as the primary food source for astronauts due to their uneven nutritional profile and the challenge of using them to produce palatable food (Krauss, 1962). Consequently, modern research has shifted its focus toward higher plants, which offer balanced nutrition and greater versatility for food production.
There are three main considerations in selecting suitable plants for space agriculture: crop yield, developmental and cultivation conditions, and the quality of the crop as human food. Selection criteria vary depending on the planting approach. As early as 1962, the “Biologistics Symposium” at Wright-Patterson Air Force Base proposed a list of crops for dietary supplements on space missions (Pilgrim and Johnson, 1962). The suggested crops mainly included vegetables and perishable plants that could supplement stored food. It took an additional fifteen years to develop a more comprehensive list addressing broader human dietary needs, including staple crops that provide carbohydrates, proteins, and lipids (Hoff et al., 1982). Dueck et al. (2016) emphasized crop yield as a priority when using a Future Exploration Greenhouse (FEG) plant growth facility, and light-use efficiency is considered the most important factor in the yield category. After evaluation of key factors and underlying criteria, lettuce and cucumber ranked highest in suitability, followed by tomatoes and chives. El-Nakhel et al. (2019) found that red Salanova (Lactuca sativa L.) contained approximately 22% greater biomass at harvest relative to green Salanova, along with higher antioxidant capacity and more efficient light usage. This superior performance makes red Salanova an optimal candidate for space agriculture. Thus far, a wide variety of higher plants have been deemed suitable for space cultivation, and extensive research has been conducted to enhance their performance in space environments. Studies have demonstrated that tuber crops, such as potatoes and sweet potatoes (Ipomoea batatas [L.] Lam.), are among the most suitable crops for growing in space (Liu et al., 2021b). Several factors contribute to their suitability: (1) they have a high harvest index and tuber yield; (2) they are rich in high carbohydrates, providing a robust energy source for humans (Tibbitts, 1980; (3) they are easy to process into edible form; (4) they have high compressive strength and are resilient to mechanical stress; (5) their inedible parts decompose easily; (6) they can reproduce both asexually from tubers and sexually from seeds; and (7) reduced gravity does not impede the tuberization process (Wheeler, 2006).
Multiple studies have focused on the optimization of hydroponic conditions for growing tubers in controlled environments. For example, He et al. (2020) explored the impact of photon flux density (PFD) from white or white-plus-red LEDs on hydroponic sweet potato plantlets. They found that the number of newly developed leaves on the seedlings gradually increased, following a quadratic function, regardless of light quality. However, PFD significantly influenced leaf morphology, plant growth, and fluorescence characteristics. The results indicated that white LEDs with a red-to-blue ratio of 0.9 were optimal for promoting growth and conserving energy in hydroponic sweet potato production within a controlled environment. Additionally, the PFPU project aims to design a modular system for cultivating edible tuberous plants in microgravity (Paradiso et al., 2020). The study indicated that the proposed root module design—which includes a Kevlar bag as a container, cellulosic sponge as the cultivation substrate, and a porous tube-based water and nutrient distribution system—provided the necessary conditions for healthy plant growth and enabled completion of the tuber-to-tuber cycle.
An experiment conducted aboard a US Space Shuttle investigated the effects of microgravity on root growth, amyloplast distribution in root cells, and soluble sugar and starch concentrations in the stems of sweet potato (Mortley et al., 2008). The results indicated that the spaceflight environment did not adversely affect sweet potato metabolism. Furthermore, the use of vegetative cuttings to propagate sweet potato plants is a viable approach for space applications, particularly in short-duration spaceflight research. Cutting stems is easier and quicker than germinating seeds and enables the maintenance of genetic consistency from the initial planting.
Plant breeding in space
The unique conditions in space, such as radiation and microgravity, can induce changes in plants at the cellular, subcellular, and genetic levels (Ma et al., 2021; Mohanta et al., 2021). These factors contribute to genetic modification methods, producing plants suitable for both space cultivation and terrestrial agriculture. Space breeding offers several advantages over conventional breeding, including a wider spectrum of genetic variation, increased beneficial mutations, stable offspring traits, and increased yields (Song et al., 2023; Zong et al., 2025). Whereas conventional mutagenesis typically has a variation rate of 0.1%–0.5%, space radiation mutagenesis can achieve rates of 1%–5% (Salmi and Roux, 2008; Moeller et al., 2012). By 2020, China had developed approximately 200 plant varieties through space radiation (Yan et al., 2010).
In recent decades, modern breeding methods (e.g., genome selection, gene editing, and synthetic biology) have been widely applied in crop improvement, addressing yield, nutrition, and stress tolerance. These advancements have been driven by breakthroughs in molecular biology, genome sequencing, bioinformatics, and other omics technologies (Ewing et al., 2019). In 2021, Liu et al. introduced a “whole-body edible and elite plant” (WBEEP) strategy aimed at improving potatoes for space farming. Their goal was to reduce harmful solanine accumulation in potatoes by transforming solanine metabolism genes from tomatoes (Liu et al., 2021b). Approaches such as gene overexpression, gene silencing, gene knockout, and the reconstruction of biosynthetic pathways have been proposed to enhance the accumulation of beneficial nutrients, improve yield, and increase fertilizer-use efficiency in potatoes. Additionally, space-suitable plants such as sweet potato, which exhibit higher yield, nutritional value, and stress tolerance, can be utilized in terrestrial agriculture, particularly in controlled environments (Hill et al., 1992; Paradiso et al., 2020).
Concluding remarks and perspectives
Gravitropism and microgravity responses
In the past decade, substantial advances in the identification of gravitropism-related mutants—such as pgm, sgr, lazy, aux, pin, and pifs—have expanded our mechanistic understanding of plant gravitropism. We have also gained insights into amyloplast sedimentation and the role of statocytes in gravity sensing, as well as the involvement of calcium ions, InsP3, and protons in signal transduction and auxin distribution during curvature response. However, further research is needed to uncover the details of interactions among these pathways—for example, how amyloplast sedimentation is perceived within gravity-sensing cells.
Similarly, using both simulated and real microgravity platforms, plant responses to microgravity have been studied at morphological, cellular, and molecular levels in many species, including Arabidopsis, rice, wheat, and rapeseed. Especially with the development of omics biotechnologies, relevant pathways and biological processes altered by microgravity have been investigated at transcriptional, proteomic, and metabolic levels. Under stress, plants often produce more or new secondary metabolites, many of which possess high medicinal or nutritional value. For example, anthocyanins—which accumulate in large quantities under stress—exhibit various medicinal properties such as anticancer, antioxidant, and anti-inflammatory effects. Space microgravity, as a novel form of stress, is likely to induce the production of additional new and useful secondary metabolites.
Research platforms and biotechnology
With the development of simulated microgravity facilities—such as one-axial clinostats, 2D and 3D clinostats, RPMs, magnetic levitators, and centrifuges—scientists now have access to various precise gravity levels, including microgravity, reduced gravity, and hypergravity. Advanced real microgravity platforms, such as drop towers, parabolic flights, sounding rockets, and orbital spaceflights, provide opportunities for extended microgravity experiments. Additionally, the completion of the Chinese space station (Tiangong Space Station) in 2022 offers expanded opportunities for long-term space research. By combining simulated and real microgravity facilities, researchers can mitigate the limitations of each method and better isolate the effects of gravity in their studies.
Rapid advancements in cellular, molecular, and biotechnological research—including genomics, transcriptomics, proteomics, and metabolomics—have enabled the systematic analysis of plant responses to microgravity. Epigenetic alterations, pathways involved in microgravity adaptation, cellular changes, and metabolic responses in plants under microgravity conditions have been extensively documented through omics data. To support such studies, the ARABIDOMICS platform was designed to process large quantities of plant material for the extraction of RNA, phytohormones, and proteins, thus facilitating omics analysis in microgravity experiments. Additionally, platforms such as the GeneLab Data System and the Test of Arabidopsis Space Transcriptome (TOAST) were developed to collect, share, and analyze space-related omics data. New biotechnological methods are continually emerging. For instance, single-cell sequencing has been utilized to study the effects of spaceflight on humans, and Oxford Nanopore Technologies’ direct RNA-seq enables the analysis of RNA quantification and modification. The integration of multiple omics approaches has been, and will continue to be, instrumental in the systematic analysis of space biology, facilitating deeper insights into the biological impacts of space environments.
Research directions for plant adaptations to space-specific environmental factors
Single-cell and spatial transcriptomics will enable the identification of cell-type-specific responses to microgravity and spaceflight stress, thereby refining our understanding of developmental plasticity and metabolic reprogramming. For example, studies focused on root apical meristems in space environments may reveal how stem cell niches are maintained under altered gravitational cues. In parallel, advances in synthetic biology and bioengineering offer promising strategies to enhance plant adaptability, including compact growth habits, improved nutrient-use efficiency, and integrated biosensors for real-time environmental monitoring. Modular plant chassis systems could also be developed for specialized space applications, such as pharmaceuticals and biopolymers.
The field of epigenetics is poised to reveal how epigenetic memory shapes plant adaptation across multiple generations in space. Future studies will focus on chromatin remodeling, transgenerational stress adaptation, and DNA methylation and histone modification patterns. Another area of interest comprises plant–microbiome–environment interactions. Microgravity substantially alters root exudation, influencing the recruitment and behavior of microbial communities. Engineered beneficial microbes may be utilized in future systems to enhance nutrient cycling, augment plant immunity, and increase resilience to environmental stress.
The completion of seed-to-seed life cycles under spaceflight conditions will be essential for long-term sustainability. Expansion of these experiments to include a broader range of crop and medicinal species will be critical. Key research questions include the viability of pollen, fertilization processes, and the mechanisms that regulate seed dormancy and germination in microgravity.
To support these endeavors, integrative approaches combining multi-omics data with environmental sensor inputs will be crucial for modeling plant growth dynamics. Additionally, artificial intelligence–driven platforms for automated plant care and phenotypic monitoring will be indispensable in the management of space-based cultivation systems, including those designed for spacecraft, lunar habitats, or Martian greenhouses.
The future of space agriculture
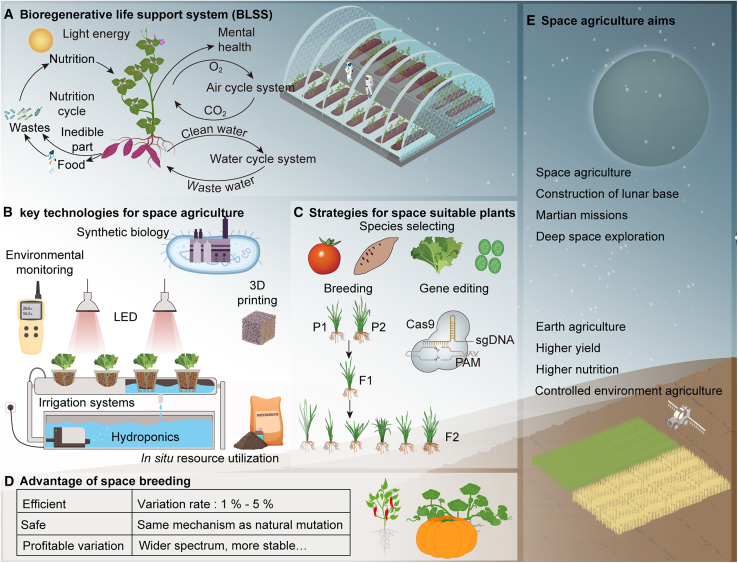
Decades of research have deepened our understanding of microgravity and its effects on biological systems, laying a strong foundation for the ambitious development of space agriculture (Figure 4) and proving essential in overcoming these challenges (Nguyen et al., 2023).
Figure 4.
Framework of space agriculture.
(A) The diverse and essential roles of plants in a bioregenerative life-support system (BLSS).
(B) Technologies designed to address and overcome the challenges of food production in space.
(C) Criteria and strategies for selecting suitable plants for space agriculture.
(D) Advantages of space breeding.
(E) Primary goals and objectives of space agriculture.
In recent decades, technological platforms have been transforming space agriculture from a theoretical possibility into a viable solution for long-term space exploration and colonization. Each system addresses the unique challenges of growing food in space—from resource scarcity to environmental control—ensuring that future missions can sustain human life far beyond Earth. Moreover, innovations in space farming are feeding back into agriculture on Earth, inspiring new methods for sustainable, high-efficiency food production in challenging environments. Several technological platforms have been developed to support space agriculture, addressing the unique challenges of growing plants in the harsh and resource-limited environment of space. These platforms integrate advancements in controlled environment agriculture, biotechnology, and engineering to create self-sustaining food production systems for space missions. As reviewed here, several key technologies have provided solid foundations that enable space agriculture. BLSSs integrate plants into life support, leveraging their natural processes to produce oxygen, absorb carbon dioxide, and recycle water while providing food. NASA’s CELSS and the ESA’s MELiSSA are examples of BLSSs, designed to create closed-loop ecosystems where plants play a central role in sustaining life (Gòdia et al., 2002). Additionally, hydroponics and aeroponics allow plants to grow without soil—an essential aspect of space farming (Wang et al., 2019). Given that natural sunlight is unavailable in spacecraft, artificial lighting is crucial for photosynthesis. Specialized LED systems have been developed to provide optimal wavelengths of light for plant growth (Sawatdee et al., 2023). Furthermore, plant growth in microgravity presents challenges regarding water and nutrient delivery, as fluids behave differently without gravity. Technologies such as capillary-based watering systems and engineered growth substrates (e.g., porous ceramic or fiber mats) help direct water and nutrients to plant roots (Arumugham et al., 2021). These systems have been tested on the ISS and are designed to replicate the functions of soil, ensuring that roots receive adequate moisture and oxygen.
However, many enabling technologies are still in the early stages of development. For example, larger-scale food production for extended missions or extraterrestrial colonies will require fully enclosed space greenhouses. Automated growth chambers and robotic plant care systems are under investigation to monitor environmental parameters such as light, temperature, humidity, and nutrient levels (Zabel et al., 2016; Nguyen et al., 2023). Precision agriculture technologies are also undergoing adaptation for space use, including sensors and imaging systems that monitor plant health in real time (Sarić et al., 2022). Biofeedback systems track factors such as plant respiration, photosynthesis rates, and root health, enabling adaptive control of environmental conditions. Remote monitoring and data collection are critical in space, where access to plants is limited and confirmation of consistent crop yields is essential (Tonnang et al., 2022). Plant biotechnology for space will benefit from genetic modification and synthetic biology approaches. Genome-editing tools such as CRISPR-mediated editing can create plants with enhanced resistance to radiation, increased stress tolerance, and improved nutrient efficiency (Ahmar et al., 2023). Similarly, synthetic biology enables the engineering of plants with metabolic pathways that support faster growth and more efficient water and nutrient use. Advanced 3D printing technology will be developed to produce necessary farming tools, components for hydroponic systems, and even custom plant scaffolds or containers on demand, using materials available in space. In situ resource utilization aims to harness local resources to support space farming (Santhoshkumar et al., 2024). Research into the use of Martian soil simulants and extraction of water from extraterrestrial environments is critical in the development of self-sufficient farming systems for Mars or the Moon (Kasiviswanathan et al., 2022; Nguyen et al., 2023; Maity and Saxena, 2024).
Moreover, space agriculture places humanity at a philosophical crossroads: it compels us to ask not just how we can grow food in space, but why we must. As we extend our reach into the cosmos, agriculture becomes a symbol of self-sufficiency, resilience, and the human drive to adapt and thrive in environments that seem hostile to life. It represents a transition from viewing space as an endless vacuum to considering it a place where life can flourish—an ultimate act of bioengineering and a bold statement of human potential. Therefore, space agriculture is not only about growing food in zero gravity but also about redesigning life itself to survive and prosper in the harshest environments, reflecting humanity’s aspirations and our evolving relationship with nature in a universe that challenges us to redefine what is possible.
Funding
This work was supported by grants from the National Key R&D Program of China (2021YFD2200505), the Science and Technology Commission of Shanghai Municipality (22JC1401300), the National Natural Science Foundation of China (32300207 and 32472220), and the Shanghai Municipal Afforestation & City Appearance and Environmental Sanitation Administration (G252407, G242407, G232405, G222413, and G222411).
Acknowledgments
No conflict of interest declared.
Author contributions
H.W., J.Y., L.Y., H.Z., and Z.J. conceived and revised the manuscript; H.N., W.Z., Z.Z., Y.D., W.Z., and M.Z. wrote the paper. All authors read and approved the manuscript.
Published: May 9, 2025
Contributor Information
Zehui Jiang, Email: jiangzh@icbr.ac.cn.
Huiqiong Zheng, Email: hqzheng@cemps.ac.cn.
Ling Yuan, Email: lyuan3@uky.edu.
Jun Yang, Email: jyang03@cemps.ac.cn.
Hongxia Wang, Email: hxwang@cemps.ac.cn.
References
- Ahmar S., Hensel G., Gruszka D. CRISPR/Cas9-mediated genome editing techniques and new breeding strategies in cereals – current status, improvements, and perspectives. Biotechnol. Adv. 2023;69 doi: 10.1016/j.biotechadv.2023.108248. [DOI] [PubMed] [Google Scholar]
- Aloni R., Aloni E., Langhans M., Ullrich C.I. Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann. Bot. 2006;97:883–893. doi: 10.1093/aob/mcl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni R., Langhans M., Aloni E., Ullrich C.I. Role of cytokinin in the regulation of root gravitropism. Planta. 2004;220:177–182. doi: 10.1007/s00425-004-1381-8. [DOI] [PubMed] [Google Scholar]
- Amalfitano S., Levantesi C., Copetti D., Stefani F., Locantore I., Guarnieri V., Lobascio C., Bersani F., Giacosa D., Detsis E., Rossetti S. Water and microbial monitoring technologies towards the near future space exploration. Water Res. 2020;177 doi: 10.1016/j.watres.2020.115787. [DOI] [PubMed] [Google Scholar]
- Angelos E., Ko D.K., Zemelis-Durfee S., Brandizzi F. Relevance of the Unfolded Protein Response to Spaceflight-Induced Transcriptional Reprogramming in Arabidopsis. Astrobiology. 2021;21:367–380. doi: 10.1089/ast.2020.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong R.C. Life support system for space flights of extended time periods. NASA CR-614. NASA Contract. Rep. NASA CR. 1966:1–567. [PubMed] [Google Scholar]
- Arumugham T., Kaleekkal N.J., Gopal S., Nambikkattu J., K R., Aboulella A.M., Ranil Wickramasinghe S., Banat F. Recent developments in porous ceramic membranes for wastewater treatment and desalination: A review. J. Environ. Manag. 2021;293 doi: 10.1016/j.jenvman.2021.112925. [DOI] [PubMed] [Google Scholar]
- Aubry-Hivet D., Nziengui H., Rapp K., Oliveira O., Paponov I.A., Li Y., Hauslage J., Vagt N., Braun M., Ditengou F.A., et al. Analysis of gene expression during parabolic flights reveals distinct early gravity responses in Arabidopsis roots. Plant Biol. 2014;16:129–141. doi: 10.1111/plb.12130. [DOI] [PubMed] [Google Scholar]
- Bai Q., Xuan S., Li W., Ali K., Zheng B., Ren H. Molecular mechanism of brassinosteroids involved in root gravity response based on transcriptome analysis. BMC Plant Biol. 2024;24 doi: 10.1186/s12870-024-05174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baster P., Robert S., Kleine-Vehn J., Vanneste S., Kania U., Grunewald W., De Rybel B., Beeckman T., Friml J. SCF(TIR1/AFB)-auxin signalling regulates PIN vacuolar trafficking and auxin fluxes during root gravitropism. EMBO J. 2013;32:260–274. doi: 10.1038/emboj.2012.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaugnon E., Tournier R. Levitation of water and organic substances in high static magnetic fields. J. Phys. III France. 1991;1:1423–1428. [Google Scholar]
- Beisel N.S., Noble J., Barbazuk W.B., Paul A.L., Ferl R.J. Spaceflight-induced alternative splicing during seedling development in Arabidopsis thaliana. NPJ Microgravity. 2019;5:9. doi: 10.1038/s41526-019-0070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyavskaya N.A. Calcium and Graviperception in Plants: Inhibitor Analysis. International Review of Cytology-a Survey of Cell Biology. 1996;168:123–185. [Google Scholar]
- Belyavskaya N.A. Free and membrane-bound calcium in microgravity and microgravity effects at the membrane level. Adv. Space Res. 1996;17:169–177. doi: 10.1016/0273-1177(95)00631-n. [DOI] [PubMed] [Google Scholar]
- Benavides-Mendoza A., Gonçalves R., Wamelink G.W.W., van der Putten P., Evers J.B. Intercropping on Mars: A promising system to optimise fresh food production in future martian colonies. PLoS One. 2024;19 doi: 10.1371/journal.pone.0302149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrios D.C., Galazka J., Grigorev K., Gebre S., Costes S.V. NASA GeneLab: interfaces for the exploration of space omics data. Nucleic Acids Res. 2021;49:D1515–D1522. doi: 10.1093/nar/gkaa887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizet F., Pereda-Loth V., Chauvet H., Gérard J., Eche B., Girousse C., Courtade M., Perbal G., Legué V. Both gravistimulation onset and removal trigger an increase of cytoplasmic free calcium in statocytes of roots grown in microgravity. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-29788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhmer M., Schleiff E. Microgravity research in plants: A range of platforms and options allow research on plants in zero or low gravity that can yield important insights into plant physiology. EMBO Rep. 2019;20 doi: 10.15252/embr.201948541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonsirichai K., Sedbrook J.C., Chen R., Gilroy S., Masson P.H. ALTERED RESPONSE TO GRAVITY is a peripheral membrane protein that modulates gravity-induced cytoplasmic alkalinization and lateral auxin transport in plant statocytes. Plant Cell. 2003;15:2612–2625. doi: 10.1105/tpc.015560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucheron-Dubuisson E., Manzano A.I., Le Disquet I., Matía I., Sáez-Vasquez J., van Loon J.J.W.A., Herranz R., Carnero-Diaz E., Medina F.J. Functional alterations of root meristematic cells of Arabidopsis thaliana induced by a simulated microgravity environment. J. Plant Physiol. 2016;207:30–41. doi: 10.1016/j.jplph.2016.09.011. [DOI] [PubMed] [Google Scholar]
- Briggs W.R. Phototropism: some history, some puzzles, and a look ahead. Plant Physiol. 2014;164:13–23. doi: 10.1104/pp.113.230573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brungs S., Egli M., Wuest S.L., M Christianen P.C., W A van Loon J.J., Ngo Anh T.J., Hemmersbach R. Facilities for Simulation of Microgravity in the ESA Ground-Based Facility Programme. Microgravity Sci. Technol. 2016;28:191–203. [Google Scholar]
- Bulavin I.V. Cytoskeleton orientation in the epidermal cells of roots formed de novo on leaf explants under clinorotation. Tsitol. Genet. 2016;50:58–64. [PubMed] [Google Scholar]
- Caporale A.G., Paradiso R., Palladino M., Arouna N., Izzo L., Ritieni A., De Pascale S., Adamo P. Assessment of Fertility Dynamics and Nutritional Quality of Potato Tubers in a Compost-Amended Mars Regolith Simulant. Plants. 2024;13 doi: 10.3390/plants13050747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Yu R., Li N., Deng Z., Zhang X., Zhao Y., Qu C., Yuan Y., Pan Z., Zhou Y., et al. Amyloplast sedimentation repolarizes LAZYs to achieve gravity sensing in plants. Cell. 2023;186:4788–4802.e15. doi: 10.1016/j.cell.2023.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Rosen E., Masson P.H. Gravitropism in higher plants. Plant Physiol. 1999;120:343–350. doi: 10.1104/pp.120.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Xu S., Tian L., Liu L., Huang M., Xu X., Song G., Wu P., Sato S., Jiang H., Wu G. LAZY3 plays a pivotal role in positive root gravitropism in Lotus japonicus. J. Exp. Bot. 2020;71:168–177. doi: 10.1093/jxb/erz429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W.G., Barker R.J., Kim S.H., Swanson S.J., Gilroy S. Variation in the transcriptome of different ecotypes of Arabidopsis thaliana reveals signatures of oxidative stress in plant responses to spaceflight. Am. J. Bot. 2019;106:123–136. doi: 10.1002/ajb2.1223. [DOI] [PubMed] [Google Scholar]
- Correll M.J., Pyle T.P., Millar K.D.L., Sun Y., Yao J., Edelmann R.E., Kiss J.Z. Transcriptome analyses of Arabidopsis thaliana seedlings grown in space: implications for gravity-responsive genes. Planta. 2013;238:519–533. doi: 10.1007/s00425-013-1909-x. [DOI] [PubMed] [Google Scholar]
- Cowles J.R., Lemay R., Jahns G.C., Scheld W.H., Peterson C. In: Plant Cell Wall Polymers. Lewis N.G., Paice M.G., editors. ACS; 1989. Lignification in young plant seedlings grown on earth and aboard the Space Shuttle; pp. 203–213. [Google Scholar]
- Cowles J.R., Scheld H.W., Lemay R., Peterson C. Growth and lignification in seedlings exposed to eight days of microgravity. Ann. Bot. 1984;54:33–48. doi: 10.1093/oxfordjournals.aob.a086865. [DOI] [PubMed] [Google Scholar]
- Curry A.B., Spern C.J., Khodadad C.L.M., Hummerick M.E., Spencer L.E., Torres J., Finn J.R., Gooden J.L., Monje O. Post-harvest cleaning, sanitization, and microbial monitoring of soilless nutrient delivery systems for sustainable space crop production. Front. Plant Sci. 2024;15 doi: 10.3389/fpls.2024.1308150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai K., Yu Q., Zhang Z., Wang Y., Wang X. Aromatic hydrocarbons in a controlled ecological life support system during a 4-person-180-day integrated experiment. Sci. Total Environ. 2018;610–611:905–911. doi: 10.1016/j.scitotenv.2017.08.164. [DOI] [PubMed] [Google Scholar]
- De Micco V., Amitrano C., Mastroleo F., Aronne G., Battistelli A., Carnero-Diaz E., De Pascale S., Detrell G., Dussap C.-G., Ganigué R., et al. Plant and microbial science and technology as cornerstones to Bioregenerative Life Support Systems in space. npj Microgravity. 2023;9 doi: 10.1038/s41526-023-00317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J., Xu Y., Tan J., Zhang H., Xiong X., Mei C., Li M., Xie G. How to make lunar soil suitable for cultivation? – A review. Sci. Total Environ. 2024;948 doi: 10.1016/j.scitotenv.2024.174603. [DOI] [PubMed] [Google Scholar]
- Dong Z., Jiang C., Chen X., Zhang T., Ding L., Song W., Luo H., Lai J., Chen H., Liu R., et al. Maize LAZY1 mediates shoot gravitropism and inflorescence development through regulating auxin transport, auxin signaling, and light response. Plant Physiol. 2013;163:1306–1322. doi: 10.1104/pp.113.227314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driss-Ecole D., Jeune B., Prouteau M., Julianus P., Perbal G. Lentil root statoliths reach a stable state in microgravity. Planta. 2000;211:396–405. doi: 10.1007/s004250000298. [DOI] [PubMed] [Google Scholar]
- Dueck T.A., Kempkes F., Meinen E., Stanghellini C. 46th International Conference on Environmental Systems. ICES; 2016. Choosing crops for cultivation in space. [Google Scholar]
- El-Nakhel C., Giordano M., Pannico A., Carillo P., Fusco G.M., De Pascale S., Rouphael Y. Cultivar-Specific Performance and Qualitative Descriptors for Butterhead Salanova Lettuce Produced in Closed Soilless Cultivation as a Candidate Salad Crop for Human Life Support in Space. Life. 2019;9 doi: 10.3390/life9030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing P.M., Runck B.C., Kono T.Y.J., Kantar M.B. The home field advantage of modern plant breeding. PLoS One. 2019;14 doi: 10.1371/journal.pone.0227079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano J.M., Swanson S.J., Blancaflor E.B., Dowd P.E., Kao T.H., Gilroy S. Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell. 2001;13:907–921. doi: 10.1105/tpc.13.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fengler S., Spirer I., Neef M., Ecke M., Nieselt K., Hampp R. A whole-genome microarray study of Arabidopsis thaliana semisolid callus cultures exposed to microgravity and nonmicrogravity related spaceflight conditions for 5 days on board of Shenzhou 8. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/547495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferl R., Wheeler R., Levine H.G., Paul A.L. Plants in space. Curr. Opin. Plant Biol. 2002;5:258–263. doi: 10.1016/s1369-5266(02)00254-6. [DOI] [PubMed] [Google Scholar]
- Ferl R.J., Koh J., Denison F., Paul A.L. Spaceflight induces specific alterations in the proteomes of Arabidopsis. Astrobiology. 2015;15:32–56. doi: 10.1089/ast.2014.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzelle K.J., Kiss J.Z. Restoration of gravitropic sensitivity in starch-deficient mutants of Arabidopsis by hypergravity. J. Exp. Bot. 2001;52:265–275. [PubMed] [Google Scholar]
- Frett T., Petrat G., W A van Loon J.J., Hemmersbach R., Anken R. Hypergravity Facilities in the ESA Ground-Based Facility Program – Current Research Activities and Future Tasks. Microgravity Sci. Technol. 2016;28:205–214. [Google Scholar]
- Fu Y., Guo R., Liu H. An optimized 4-day diet meal plan for ‘Lunar Palace 1. J. Sci. Food Agric. 2019;99:696–702. doi: 10.1002/jsfa.9234. [DOI] [PubMed] [Google Scholar]
- Fu Y., Li L., Xie B., Dong C., Wang M., Jia B., Shao L., Dong Y., Deng S., Liu H., et al. How to Establish a Bioregenerative Life Support System for Long-Term Crewed Missions to the Moon or Mars. Astrobiology. 2016;16:925–936. doi: 10.1089/ast.2016.1477. [DOI] [PubMed] [Google Scholar]
- Fukaki H., Wysocka-Diller J., Kato T., Fujisawa H., Benfey P.N., Tasaka M. Genetic evidence that the endodermis is essential for shoot gravitropism in Arabidopsis thaliana. Plant J. 1998;14:425–430. doi: 10.1046/j.1365-313x.1998.00137.x. [DOI] [PubMed] [Google Scholar]
- Fulget N., Poughon L., Richalet J., Lasseur C.h. MELISSA: global control strategy of the artificial ecosystem by using first principles models of the compartments. Adv. Space Res. 1999;24:397–405. doi: 10.1016/s0273-1177(99)00490-1. [DOI] [PubMed] [Google Scholar]
- Gòdia F., Albiol J., Montesinos J.L., Pérez J., Creus N., Cabello F., Mengual X., Montras A., Lasseur C. MELISSA: a loop of interconnected bioreactors to develop life support in space. J. Biotechnol. 2002;99:319–330. doi: 10.1016/s0168-1656(02)00222-5. [DOI] [PubMed] [Google Scholar]
- Golueke C.G., Oswald W.J. Role of Plants in Closed Systems. Annu. Rev. Plant Physiol. 1964;15:387–408. [Google Scholar]
- Graham T., Yorio N., Zhang P., Massa G., Wheeler R. Early seedling response of six candidate crop species to increasing levels of blue light. Life Sci. Space Res. 2019;21:40–48. doi: 10.1016/j.lssr.2019.03.001. [DOI] [PubMed] [Google Scholar]
- Gutjahr C., Riemann M., Müller A., Düchting P., Weiler E.W., Nick P. Cholodny-Went revisited: a role for jasmonate in gravitropism of rice coleoptiles. Planta. 2005;222:575–585. doi: 10.1007/s00425-005-0001-6. [DOI] [PubMed] [Google Scholar]
- Hamilton E.S., Schlegel A.M., Haswell E.S. United in diversity: mechanosensitive ion channels in plants. Annu. Rev. Plant Biol. 2015;66:113–137. doi: 10.1146/annurev-arplant-043014-114700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiguchi Y., Tasaka M., Morita M.T. Mechanism of higher plant gravity sensing. Am. J. Bot. 2013;100:91–100. doi: 10.3732/ajb.1200315. [DOI] [PubMed] [Google Scholar]
- Hauslage J., Görög M., Krause L., Schüler O., Schäfer M., Witten A., Kesseler L., Böhmer M., Hemmersbach R. ARABIDOMICS-A new experimental platform for molecular analyses of plants in drop towers, on parabolic flights, and sounding rockets. Rev. Sci. Instrum. 2020;91 doi: 10.1063/1.5120573. [DOI] [PubMed] [Google Scholar]
- Hausmann N., Fengler S., Hennig A., Franz-Wachtel M., Hampp R., Neef M. Cytosolic calcium, hydrogen peroxide and related gene expression and protein modulation in Arabidopsis thaliana cell cultures respond immediately to altered gravitation: parabolic flight data. Plant Biol. 2014;16:120–128. doi: 10.1111/plb.12051. [DOI] [PubMed] [Google Scholar]
- He D., Yan Z., Sun X., Yang P. Leaf development and energy yield of hydroponic sweet potato seedlings using single-node cutting as influenced by light intensity and LED spectrum. J. Plant Physiol. 2020;254 doi: 10.1016/j.jplph.2020.153274. [DOI] [PubMed] [Google Scholar]
- Hemmersbach R., Strauch S.M., Seibt D., Schuber M. Comparative studies on gravisensitive protists on ground (2D and 3D clinostats) and in microgravity. Microgravity Sci. Technol. 2006;18:257–259. [Google Scholar]
- Herranz R., Vandenbrink J.P., Villacampa A., Manzano A., Poehlman W.L., Feltus F.A., Kiss J.Z., Medina F.J. RNAseq Analysis of the Response of Arabidopsis thaliana to Fractional Gravity Under Blue-Light Stimulation During Spaceflight. Front. Plant Sci. 2019;10:1529. doi: 10.3389/fpls.2019.01529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W.A., Mortley D.G., Mackowiak C.L., Loretan P.A., Tibbitts T.W., Wheeler R.M., Bonsi C.K., Morris C.E. Growing root, tuber and nut crops hydroponically for CELSS. Adv. Space Res. 1992;12:125–131. doi: 10.1016/0273-1177(92)90018-s. [DOI] [PubMed] [Google Scholar]
- Hoff J.E., Howe J.M., Mitchell C.A. NASA; 1982. Nutritional and Cultural Aspects of Plant Species Selection for a Controlled Ecological Life Support System. [Google Scholar]
- Hoson T., Kamisaka S., Masuda Y., Yamashita M., Buchen B. Evaluation of the three-dimensional clinostat as a simulator of weightlessness. Planta. 1997;203:S187–S197. doi: 10.1007/pl00008108. [DOI] [PubMed] [Google Scholar]
- Hoson T., Saito Y., Soga K., Wakabayashi K. Signal perception, transduction, and response in gravity resistance. A nother graviresponse in plants. Adv. Space Res. 2005;36:1196–1202. [Google Scholar]
- Hoson T., Soga K., Mori R., Saiki M., Nakamura Y., Wakabayashi K., Kamisaka S. Stimulation of elongation growth and cell wall loosening in rice coleoptiles under microgravity conditions in space. Plant Cell Physiol. 2002;43:1067–1071. doi: 10.1093/pcp/pcf126. [DOI] [PubMed] [Google Scholar]
- Hoson T., Soga K., Wakabayashi K., Hashimoto T., Karahara I., Yano S., Tanigaki F., Shimazu T., Kasahara H., Masuda D., Kamisaka S. Growth stimulation in inflorescences of an Arabidopsis tubulin mutant under microgravity conditions in space. Plant Biol. 2014;16:91–96. doi: 10.1111/plb.12099. [DOI] [PubMed] [Google Scholar]
- Hoson T., Soga K., Wakabayashi K., Kamisaka S., Tanimoto E. Growth and cell wall changes in rice roots during spaceflight. Plant Soil. 2003;255:19–26. doi: 10.1023/a:1026105431505. [DOI] [PubMed] [Google Scholar]
- Jenkins M.T., Gerhardt F. A gene influencing the composition of the culm in maize. Agric. Res. Bull. 1931;138:124–151. [Google Scholar]
- Jia C., Zheng W., Liu F., Ding K., Yuan Y., Wang J., Xu D., Zhang T., Zheng H. Biological culture module for plant research from seed-to-seed on the Chinese Space Station. Life Sci. Space Res. 2024;42:47–52. doi: 10.1016/j.lssr.2024.04.005. [DOI] [PubMed] [Google Scholar]
- Jin X., Ai W., Li C., Zhang L., Yu Q., Tang Y., Dong W. Operation overview of a biological waste treatment system during the 4-crew 180-day integrated experiment in the controlled ecological life support system (CELSS) Life Sci. Space Res. 2021;29:15–21. doi: 10.1016/j.lssr.2021.02.004. [DOI] [PubMed] [Google Scholar]
- Johnson C.M., Subramanian A., Pattathil S., Correll M.J., Kiss J.Z. Comparative transcriptomics indicate changes in cell wall organization and stress response in seedlings during spaceflight. Am. J. Bot. 2017;104:1219–1231. doi: 10.3732/ajb.1700079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J.W., Adair C.R. A “LAZY” MUTATION IN RICE. J. Hered. 1938;29:315–318. [Google Scholar]
- Joris P., Lombard E., Paillet A., Navarro G., Guillouet S.E., Gorret N. Recycling potential of Cupriavidus necator for life support in space: Production of SCPs from volatile fatty acid and urea mixture. J. Biotechnol. 2024;396:18–27. doi: 10.1016/j.jbiotec.2024.10.001. [DOI] [PubMed] [Google Scholar]
- Kamada M., Oka M., Miyamoto K., Uheda E., Yamazaki C., Shimazu T., Sano H., Kasahara H., Suzuki T., Higashibata A., Ueda J. Microarray profile of gene expression in etiolated Pisum sativum seedlings grown under microgravity conditions in space: Relevance to the International Space Station experiment “Auxin Transport. Life Sci. Space Res. 2020;26:55–61. doi: 10.1016/j.lssr.2020.04.005. [DOI] [PubMed] [Google Scholar]
- Kasiviswanathan P., Swanner E.D., Halverson L.J., Vijayapalani P. Farming on Mars: Treatment of basaltic regolith soil and briny water simulants sustains plant growth. PLoS One. 2022;17 doi: 10.1371/journal.pone.0272209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Morita M.T., Fukaki H., Yamauchi Y., Uehara M., Niihama M., Tasaka M. SGR2, a phospholipase-like protein, and ZIG/SGR4, a SNARE, are involved in the shoot gravitropism of Arabidopsis. Plant Cell. 2002;14:33–46. doi: 10.1105/tpc.010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss J.Z. In: Plant Gravitropism: Methods and Protocols. Blancaflor E.B., editor. Springer New York; New York, NY: 2015. Conducting plant experiments in space; pp. 255–283. [Google Scholar]
- Kiss J.Z., Guisinger M.M., Miller A.J., Stackhouse K.S. Reduced gravitropism in hypocotyls of starch-deficient mutants of Arabidopsis. Plant Cell Physiol. 1997;38:518–525. doi: 10.1093/oxfordjournals.pcp.a029199. [DOI] [PubMed] [Google Scholar]
- Kittang A.-I., Winge P., van Loon J.j.w.a., Bones A.M., Iversen T.H. Adaptation response of Arabidopsis thaliana to random positioning. Adv. Space Res. 2013;52:1320–1331. [Google Scholar]
- Kleine-Vehn J., Ding Z., Jones A.R., Tasaka M., Morita M.T., Friml J. Gravity-induced PIN transcytosis for polarization of auxin fluxes in gravity-sensing root cells. Proc. Natl. Acad. Sci. USA. 2010;107:22344–22349. doi: 10.1073/pnas.1013145107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings A. Gravitropism of roots: an evaluation of progress during the last three decades. Bot. Acta. 1995;44:195–223. doi: 10.1111/j.1438-8677.1995.tb00781.x. [DOI] [PubMed] [Google Scholar]
- Kordyum E.l., Brykov V.o. Statoliths displacement in root statocytes in real and simulated microgravity. Kosm. nauka tehnol. 2021;27:78–84. [Google Scholar]
- Krauss R.W. Mass Culture of Algae for Food and Other Organic Compounds. Am. J. Bot. 1962;49:425–435. [Google Scholar]
- Kruse C.P.S., Meyers A.D., Basu P., Hutchinson S., Luesse D.R., Wyatt S.E. Spaceflight induces novel regulatory responses in Arabidopsis seedling as revealed by combined proteomic and transcriptomic analyses. BMC Plant Biol. 2020;20:237. doi: 10.1186/s12870-020-02392-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuya N., Nishijima R., Kitomi Y., Kawakatsu T., Uga Y. Transcriptome profiles of rice roots under simulated microgravity conditions and following gravistimulation. Front. Plant Sci. 2023;14 doi: 10.3389/fpls.2023.1193042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon T., Sparks J.A., Nakashima J., Allen S.N., Tang Y., Blancaflor E.B. Transcriptional response of Arabidopsis seedlings during spaceflight reveals peroxidase and cell wall remodeling genes associated with root hair development. Am. J. Bot. 2015;102:21–35. doi: 10.3732/ajb.1400458. [DOI] [PubMed] [Google Scholar]
- Land E.S., Sheppard J., Doherty C.J., Perera I.Y. Conserved plant transcriptional responses to microgravity from two consecutive spaceflight experiments. Front. Plant Sci. 2023;14 doi: 10.3389/fpls.2023.1308713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine L.H., Levine H.G., Stryjewski E.C., Prima V., Piastuch W.C. Effect of spaceflight on isoflavonoid accumulation in etiolated soybean seedlings. J. Gravitational Physiol. 2001;8:21–27. [PubMed] [Google Scholar]
- Li L., Xie B., Dong C., Hu D., Wang M., Liu G., Liu H. Rearing Tenebrio molitor L. (Coleptera: Tenebrionidae) in the “Lunar Palace 1” during a 105-day multi-crew closed integrative BLSS experiment. Life Sci. Space Res. 2015;7:9–14. doi: 10.1016/j.lssr.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Li L., Xu J., Xu Z.-H., Xue H.-W. Brassinosteroids Stimulate Plant Tropisms through Modulation of Polar Auxin Transport in Brassica and Arabidopsis. Plant Cell. 2005;17:2738–2753. doi: 10.1105/tpc.105.034397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Zhang L., Ai W., Dong W., Yu Q. A modified MBR system with post advanced purification for domestic water supply system in 180-day CELSS: Construction, pollutant removal and water allocation. J. Environ. Manag. 2018;222:37–43. doi: 10.1016/j.jenvman.2018.05.023. [DOI] [PubMed] [Google Scholar]
- Li X., Zhao R., Liu J., Li Z., Chen A., Xu S., Sheng X. Dynamic changes in calcium signals during root gravitropism. Plant Physiol. Biochem. 2024;208 doi: 10.1016/j.plaphy.2024.108481. [DOI] [PubMed] [Google Scholar]
- Limbach C., Hauslage J., Schäfer C., Braun M. How to activate a plant gravireceptor. Early mechanisms of gravity sensing studied in characean rhizoids during parabolic flights. Plant Physiol. 2005;139:1030–1040. doi: 10.1104/pp.105.068106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link B.M., Busse J.S., Stankovic B. Seed-to-seed-to-seed growth and development of Arabidopsis in microgravity. Astrobiology. 2014;14:866–875. doi: 10.1089/ast.2014.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Xie B., Liu H., Yao Z., Liu H. Effect of solid waste fermentation substrate on wheat (Triticum aestivum L.) growth in closed artificial ecosystem. Life Sci. Space Res. 2020;26:163–172. doi: 10.1016/j.lssr.2020.06.005. [DOI] [PubMed] [Google Scholar]
- Liu H., Yao Z., Fu Y., Feng J. Review of research into bioregenerative life support system(s) which can support humans living in space. Life Sci. Space Res. 2021;31:113–120. doi: 10.1016/j.lssr.2021.09.003. [DOI] [PubMed] [Google Scholar]
- Liu Y., Xie G., Yang Q., Ren M. Biotechnological development of plants for space agriculture. Nat. Commun. 2021;12 doi: 10.1038/s41467-021-26238-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfke C., Zwiewka M., Heilmann I., Van Montagu M.C.E., Teichmann T., Friml J. Asymmetric gibberellin signaling regulates vacuolar trafficking of PIN auxin transporters during root gravitropism. Proc. Natl. Acad. Sci. USA. 2013;110:3627–3632. doi: 10.1073/pnas.1300107110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loon J.J.W.A., Krause J., Cunha H., Goncalves J., Almeida H., Schiller P. ESA; 2008. (The Large Diameter Centrifuge, LDC, for Life and Physical Sciences and Technology). European Space Agency (Special Publication) ESA 663 SP. [Google Scholar]
- Ma L., Kong F., Sun K., Wang T., Guo T. From Classical Radiation to Modern Radiation: Past, Present, and Future of Radiation Mutation Breeding. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.768071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacElroy R.D., Bredt J. Current concepts and future directions of CELSS. Adv. Space Res. 1984;4:221–229. doi: 10.1016/0273-1177(84)90566-0. [DOI] [PubMed] [Google Scholar]
- Maffei M.E., Balestrini R., Costantino P., Lanfranco L., Morgante M., Battistelli A., Del Bianco M. The physiology of plants in the context of space exploration. Commun. Biol. 2024;7 doi: 10.1038/s42003-024-06989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity T., Saxena A. Challenges and innovations in food and water availability for a sustainable Mars colonization. Life Sci. Space Res. 2024;42:27–36. doi: 10.1016/j.lssr.2024.03.008. [DOI] [PubMed] [Google Scholar]
- Manzano A., Herranz R., den Toom L.A., Te Slaa S., Borst G., Visser M., Medina F.J., van Loon J.J.W.A. Novel, Moon and Mars, partial gravity simulation paradigms and their effects on the balance between cell growth and cell proliferation during early plant development. NPJ Microgravity. 2018;4:9. doi: 10.1038/s41526-018-0041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano A.I., Larkin O.J., Dijkstra C.E., Anthony P., Davey M.R., Eaves L., Hill R.J.A., Herranz R., Medina F.J. Meristematic cell proliferation and ribosome biogenesis are decoupled in diamagnetically levitated Arabidopsis seedlings. BMC Plant Biol. 2013;13:124. doi: 10.1186/1471-2229-13-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano A.I., Matía I., González-Camacho F., Carnero-Díaz E., van Loon J.J.W.A., Dijkstra C., Larkin O., Anthony P., Davey M.R., Marco R., Medina F.J. Germination of Arabidopsis Seed in Space and in Simulated Microgravity: Alterations in Root Cell Growth and Proliferation. Microgravity Sci. Technol. 2008;21:293–297. [Google Scholar]
- Matía I., González-Camacho F., Herranz R., Kiss J.Z., Gasset G., van Loon J.J.W.A., Marco R., Javier Medina F. Plant cell proliferation and growth are altered by microgravity conditions in spaceflight. J. Plant Physiol. 2010;167:184–193. doi: 10.1016/j.jplph.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Matía I., González-Camacho F., Marco R., Kiss J.Z., Gasset G., Loon J.v., Medina F.J. The “root” experiment of the “cervantes” spanish soyuz mission: Cell proliferation and nucleolar activity alterations in arabidopsis roots germinated in real or simulated microgravity. Microgravity Sci. Technol. 2007;19:128–132. [Google Scholar]
- Matía I., González-Camacho F., Marco R., Kiss J.Z., Gasset G., Medina F.-J. Nucleolar structure and proliferation activity of Arabidopsis root cells from seedlings germinated on the International Space Station. Adv. Space Res. 2005;36:1244–1253. [Google Scholar]
- Mazars C., Brière C., Grat S., Pichereaux C., Rossignol M., Pereda-Loth V., Eche B., Boucheron-Dubuisson E., Le Disquet I., Medina F.J., et al. Microgravity induces changes in microsome-associated proteins of Arabidopsis seedlings grown on board the international space station. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkys A.J., Laurinavicius R.S., Svegzdiene D.V. Plant growth, development and embryogenesis during Salyut-7 flight. Adv. Space Res. 1984;4:55–63. doi: 10.1016/0273-1177(84)90224-2. [DOI] [PubMed] [Google Scholar]
- Merrick T., Bennartz R., Jorge M.L.S.P., Pau S., Rausch J. Evaluation of Plant Stress Monitoring Capabilities Using a Portable Spectrometer and Blue-Red Grow Light. Sensors. 2022;22 doi: 10.3390/s22093411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar K.D.L., Kiss J.Z. Analyses of tropistic responses using metabolomics. Am. J. Bot. 2013;100:79–90. doi: 10.3732/ajb.1200316. [DOI] [PubMed] [Google Scholar]
- Moeller R., Reitz G., Nicholson The Protect Team W.L., Horneck G. Mutagenesis in bacterial spores exposed to space and simulated martian conditions: data from the EXPOSE-E spaceflight experiment PROTECT. Astrobiology. 2012;12:457–468. doi: 10.1089/ast.2011.0739. [DOI] [PubMed] [Google Scholar]
- Mohanta T.K., Mishra A.K., Mohanta Y.K., Al-Harrasi A. Space Breeding: The Next-Generation Crops. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.771985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita M.T. Directional Gravity Sensing in Gravitropism. Annu. Rev. Plant Biol. 2010;61:705–720. doi: 10.1146/annurev.arplant.043008.092042. [DOI] [PubMed] [Google Scholar]
- Morrow R.C., Richter R.C., Tellez G., Monje O., Wheeler R.M., Massa G.D., Dufour N.F., Onate B.G. 46th International Conference on Environmental Systems. 2016. A new plant habitat facility for the ISS. [Google Scholar]
- Mortley D.G., Bonsi C.K., Hill W.A., Morris C.E., Williams C.S., Davis C.F., Williams J.W., Levine L.H., Petersen B.V., Wheeler R.M. Influence of Microgravity Environment on Root Growth, Soluble Sugars, and Starch Concentration of Sweetpotato Stem Cuttings. J. Am. Soc. Hortic. Sci. 2008;133:327–332. [PMC free article] [PubMed] [Google Scholar]
- Musgrave M.E., Kuang A., Xiao Y., Stout S.C., Bingham G.E., Briarty L.G., Levenskikh M.A., Sychev V.N., Podolski I.G. Gravity independence of seed-to-seed cycling in Brassica rapa. Planta. 2000;210:400–406. doi: 10.1007/pl00008148. [DOI] [PubMed] [Google Scholar]
- Myers J. Basic remarks on the use of plants as biological gas exchangers in a closed system. J. Aviat. Med. 1954;25:407–411. [PubMed] [Google Scholar]
- Myers J. In one era and out the other. Photosynth. Res. 2002;73:21–28. doi: 10.1023/A:1020475502933. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Nishimura T., Morita M.T. Bridging the gap between amyloplasts and directional auxin transport in plant gravitropism. Curr. Opin. Plant Biol. 2019;52:54–60. doi: 10.1016/j.pbi.2019.07.005. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Nishimura T., Morita M.T. Gravity sensing and signal conversion in plant gravitropism. J. Exp. Bot. 2019;70:3495–3506. doi: 10.1093/jxb/erz158. [DOI] [PubMed] [Google Scholar]
- Nakashima J., Liao F., Sparks J.A., Tang Y., Blancaflor E.B., Palme K. The actin cytoskeleton is a suppressor of the endogenous skewing behaviour of Arabidopsis primary roots in microgravity. Plant Biol. 2014;16:142–150. doi: 10.1111/plb.12062. [DOI] [PubMed] [Google Scholar]
- Nakashima J., Pattathil S., Avci U., Chin S., Alan Sparks J., Hahn M.G., Gilroy S., Blancaflor E.B. Glycome profiling and immunohistochemistry uncover changes in cell walls of Arabidopsis thaliana roots during spaceflight. npj Microgravity. 2023;9 doi: 10.1038/s41526-023-00312-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedukha E.M. Possible mechanisms of plant cell wall changes at microgravity. Adv. Space Res. 1996;17:37–45. doi: 10.1016/0273-1177(95)00610-q. [DOI] [PubMed] [Google Scholar]
- Nelson M., Allen J.P., Dempster W.F. Biosphere 2: a prototype project for a permanent and evolving life system for Mars base. Adv. Space Res. 1992;12:211–217. doi: 10.1016/0273-1177(92)90026-t. [DOI] [PubMed] [Google Scholar]
- Nguyen M., Knowling M., Tran N.N., Burgess A., Fisk I., Watt M., Escribà-Gelonch M., This H., Culton J., Hessel V. Space farming: Horticulture systems on spacecraft and outlook to planetary space exploration. Plant Physiol. Biochem. 2023;194:708–721. doi: 10.1016/j.plaphy.2022.12.017. [DOI] [PubMed] [Google Scholar]
- Nitta K., Otsubo K., Ashida A. Integration test project of CEEF--a test bed for Closed Ecological Life Support Systems. Adv. Space Res. 2000;26:335–338. doi: 10.1016/s0273-1177(99)01073-x. [DOI] [PubMed] [Google Scholar]
- Olanrewaju G.O., Haveman N.J., Naldrett M.J., Paul A.-L., Ferl R.J., Wyatt S.E. Integrative transcriptomics and proteomics profiling of Arabidopsis thaliana elucidates novel mechanisms underlying spaceflight adaptation. Front. Plant Sci. 2023;14 doi: 10.3389/fpls.2023.1260429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanrewaju G.O., Kruse C.P.S., Wyatt S.E. Functional Meta-Analysis of the Proteomic Responses of Arabidopsis Seedlings to the Spaceflight Environment Reveals Multi-Dimensional Sources of Variability across Spaceflight Experiments. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms241914425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda Y., Nagahashi M., Yamashita M., Fukushima S., Aizawa T., Yamauchi S., Fujikawa Y., Tanaka T., Kadomura-Ishikawa Y., Ishida K., et al. Accumulated melanin in molds provides wavelength-dependent UV tolerance. Photochem. Photobiol. Sci. 2024;23:1791–1806. doi: 10.1007/s43630-024-00632-4. [DOI] [PubMed] [Google Scholar]
- Paradiso R., Ceriello A., Pannico A., Sorrentino S., Palladino M., Giordano M., Fortezza R., De Pascale S. Design of a Module for Cultivation of Tuberous Plants in Microgravity: The ESA Project “Precursor of Food Production Unit” (PFPU) Front. Plant Sci. 2020;11 doi: 10.3389/fpls.2020.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso R., De Micco V., Buonomo R., Aronne G., Barbieri G., De Pascale S., Legué V. Soilless cultivation of soybean for Bioregenerative Life-Support Systems: a literature review and the experience of the MELiSSA Project – Food characterisation Phase I. Plant Biol. 2014;16:69–78. doi: 10.1111/plb.12056. [DOI] [PubMed] [Google Scholar]
- Paul A.-L., Haveman N., Califar B., Ferl R.J. Epigenomic Regulators Elongator Complex Subunit 2 and Methyltransferase 1 Differentially Condition the Spaceflight Response in Arabidopsis. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.691790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A.-L., Manak M.S., Mayfield J.D., Reyes M.F., Gurley W.B., Ferl R.J. Parabolic Flight Induces Changes in Gene Expression Patterns inArabidopsis thaliana. Astrobiology. 2011;11:743–758. doi: 10.1089/ast.2011.0659. [DOI] [PubMed] [Google Scholar]
- Paul A.L., Amalfitano C.E., Ferl R.J. Plant growth strategies are remodeled by spaceflight. BMC Plant Biol. 2012;12:232. doi: 10.1186/1471-2229-12-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A.L., Sng N.J., Zupanska A.K., Krishnamurthy A., Schultz E.R., Ferl R.J. Genetic dissection of the Arabidopsis spaceflight transcriptome: Are some responses dispensable for the physiological adaptation of plants to spaceflight? PLoS One. 2017;12 doi: 10.1371/journal.pone.0180186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A.L., Wheeler R.M., Levine H.G., Ferl R.J. Fundamental Plant Biology Enabled by The Space Shuttle. Am. J. Bot. 2013;100:226–234. doi: 10.3732/ajb.1200338. [DOI] [PubMed] [Google Scholar]
- Peer W.A., Blakeslee J.J., Yang H., Murphy A.S. Seven things we think we know about auxin transport. Mol. Plant. 2011;4:487–504. doi: 10.1093/mp/ssr034. [DOI] [PubMed] [Google Scholar]
- Perbal G., Driss-Ecole D. Polarity of statocytes in lentil seedling roots grown in space (Spacelab D1 Mission) Physiol. Plantarum. 1989;75:518–524. doi: 10.1111/j.1399-3054.1989.tb05618.x. [DOI] [PubMed] [Google Scholar]
- Perbal G., Driss-Ecole D. Mechanotransduction in gravisensing cells. Trends Plant Sci. 2003;8:498–504. doi: 10.1016/j.tplants.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Perbal G., Driss-Ecole D., Tewinkel M., Volkmann D. Statocyte polarity and gravisensitivity in seedling roots grown in microgravity. Planta. 1997;203:S57–S62. doi: 10.1007/pl00008115. [DOI] [PubMed] [Google Scholar]
- Perera I.Y., Heilmann I., Boss W.F. Transient and sustained increases in inositol 1,4,5-trisphosphate precede the differential growth response in gravistimulated maize pulvini. Proc. Natl. Acad. Sci. USA. 1999;96:5838–5843. doi: 10.1073/pnas.96.10.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilgrim A.J., Johnson S.P. Armed Services Technical Information Agency; 1962. Investigation of selected higher plants as gas exchange mechanisms for closed ecological systems. Technical Documentary Report AMRL-TDR-62-127. [Google Scholar]
- Polulakh Yu A., Zhadko S.I., Klimchuk D.A., Baraboy V.A., Alpatov A.N., Sytnik K.M. Plant cell plasma membrane structure and properties under clinostatting. Adv. Space Res. 1989;9:71–74. doi: 10.1016/0273-1177(89)90057-4. [DOI] [PubMed] [Google Scholar]
- Rajendran S., Domalachenpa T., Arora H., Li P., Sharma A., Rajauria G. Hydroponics: Exploring innovative sustainable technologies and applications across crop production, with Emphasis on potato mini-tuber cultivation. Heliyon. 2024;10 doi: 10.1016/j.heliyon.2024.e26823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakusová H., Gallego-Bartolomé J., Vanstraelen M., Robert H.S., Alabadí D., Blázquez M.A., Benková E., Friml J. Polarization of PIN3-dependent auxin transport for hypocotyl gravitropic response in Arabidopsis thaliana. Plant J. 2011;67:817–826. doi: 10.1111/j.1365-313X.2011.04636.x. [DOI] [PubMed] [Google Scholar]
- Retzer K., Akhmanova M., Konstantinova N., Malínská K., Leitner J., Petrášek J., Luschnig C. Brassinosteroid signaling delimits root gravitropism via sorting of the Arabidopsis PIN2 auxin transporter. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-13543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux D., Lagacé M., Cohen L.Y., Beaulieu J. Variation in stem morphology and movement of amyloplasts in white spruce grown in the weightless environment of the International Space Station. Life Sci. Space Res. 2015;4:67–78. doi: 10.1016/j.lssr.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Rosquete M.R., von Wangenheim D., Marhavý P., Barbez E., Stelzer E.H.K., Benková E., Maizel A., Kleine-Vehn J. An auxin transport mechanism restricts positive orthogravitropism in lateral roots. Curr. Biol. 2013;23:817–822. doi: 10.1016/j.cub.2013.03.064. [DOI] [PubMed] [Google Scholar]
- Roux S.J. Root waving and skewing: unexpectedly in micro-g. BMC Plant Biol. 2012;12:231. doi: 10.1186/1471-2229-12-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychoudhry S., Del Bianco M., Kieffer M., Kepinski S. Auxin controls gravitropic setpoint angle in higher plant lateral branches. Curr. Biol. 2013;23:1497–1504. doi: 10.1016/j.cub.2013.06.034. [DOI] [PubMed] [Google Scholar]
- Rutter L., Barker R., Bezdan D., Cope H., Costes S.V., Degoricija L., Fisch K.M., Gabitto M.I., Gebre S., Giacomello S., et al. A New Era for Space Life Science: International Standards for Space Omics Processing. Patterns. 2020;1 doi: 10.1016/j.patter.2020.100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi M.L., Roux S.J. Gene expression changes induced by space flight in single-cells of the fern Ceratopteris richardii. Planta. 2008;229:151–159. doi: 10.1007/s00425-008-0817-y. [DOI] [PubMed] [Google Scholar]
- Sangeetha T., Periyathambi E. Automatic nutrient estimator: distributing nutrient solution in hydroponic plants based on plant growth. PeerJ Comput. Sci. 2024;10 doi: 10.7717/peerj-cs.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhoshkumar P., Negi A., Moses J.A. 3D printing for space food applications: Advancements, challenges, and prospects. Life Sci. Space Res. 2024;40:158–165. doi: 10.1016/j.lssr.2023.08.002. [DOI] [PubMed] [Google Scholar]
- Sarić R., Nguyen V.D., Burge T., Berkowitz O., Trtílek M., Whelan J., Lewsey M.G., Čustović E. Applications of hyperspectral imaging in plant phenotyping. Trends Plant Sci. 2022;27:301–315. doi: 10.1016/j.tplants.2021.12.003. [DOI] [PubMed] [Google Scholar]
- Sawatdee S., Jarunglumlert T., Pavasant P., Sakihama Y., Flood A.E., Prommuak C. Effect of mixed light emitting diode spectrum on antioxidants content and antioxidant activity of red lettuce grown in a closed soilless system. BMC Plant Biol. 2023;23 doi: 10.1186/s12870-023-04364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Guo S., Zhao P., Wang L., Wang X., Li J., Bian Q. Research on lettuce growth technology onboard Chinese Tiangong II Spacelab. Acta Astronaut. 2018;144:97–102. [Google Scholar]
- Sievers A., Volkmann D. Ultrastructure of gravity-perceiving cells in plant roots. Proc. R. Soc. Lond. B Biol. Sci. 1977;199:525–536. doi: 10.1098/rspb.1977.0160. [DOI] [PubMed] [Google Scholar]
- Soga K., Wakabayashi K., Kamisaka S., Hoson T. Stimulation of elongation growth and xyloglucan breakdown in Arabidopsis hypocotyls under microgravity conditions in space. Planta. 2002;215:1040–1046. doi: 10.1007/s00425-002-0838-x. [DOI] [PubMed] [Google Scholar]
- Song X., Li T., Gu H., Yin H. Space exposure enhanced pectin-degrading enzymes expression and activity in Aspergillus costaricaensis. World J. Microbiol. Biotechnol. 2023;39:295. doi: 10.1007/s11274-023-03740-y. [DOI] [PubMed] [Google Scholar]
- Sorokin C., Myers J. A high-temperature strain of Chlorella. Science. 1953;117:330–331. doi: 10.1126/science.117.3039.330. [DOI] [PubMed] [Google Scholar]
- Stanković B. 2001: a plant space odyssey. Trends Plant Sci. 2001;6:591–593. doi: 10.1016/s1360-1385(01)02158-6. [DOI] [PubMed] [Google Scholar]
- Stepanova A.N., Robertson-Hoyt J., Yun J., Benavente L.M., Xie D.Y., Dolezal K., Schlereth A., Jürgens G., Alonso J.M. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell. 2008;133:177–191. doi: 10.1016/j.cell.2008.01.047. [DOI] [PubMed] [Google Scholar]
- Street I.H., Mathews D.E., Yamburkenko M.V., Sorooshzadeh A., John R.T., Swarup R., Bennett M.J., Kieber J.J., Schaller G.E. Cytokinin acts through the auxin influx carrier AUX1 to regulate cell elongation in the root. Development. 2016;143:3982–3993. doi: 10.1242/dev.132035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S.H., Gibbs N.M., Jancewicz A.L., Masson P.H. Molecular Mechanisms of Root Gravitropism. Curr. Biol. 2017;27:R964–R972. doi: 10.1016/j.cub.2017.07.015. [DOI] [PubMed] [Google Scholar]
- Sugimoto M., Oono Y., Gusev O., Matsumoto T., Yazawa T., Levinskikh M.A., Sychev V.N., Bingham G.E., Wheeler R., Hummerick M. Genome-wide expression analysis of reactive oxygen species gene network in Mizuna plants grown in long-term spaceflight. BMC Plant Biol. 2014;14:4. doi: 10.1186/1471-2229-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sychev V.N., Levinskikh M.A., Gostimsky S.A., Bingham G.E., Podolsky I.G. Spaceflight effects on consecutive generations of peas grown onboard the Russian segment of the International Space Station. Acta Astronaut. 2007;60:426–432. [Google Scholar]
- Takahashi K., Takahashi H., Furuichi T., Toyota M., Furutani-Seiki M., Kobayashi T., Watanabe-Takano H., Shinohara M., Numaga-Tomita T., Sakaue-Sawano A., et al. Gravity sensing in plant and animal cells. NPJ Microgravity. 2021;7:2. doi: 10.1038/s41526-020-00130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura T., Goto E., Tanaka M. The effect of gravity on plant germination. Adv. Space Res. 1996;18:255–258. doi: 10.1016/0273-1177(95)00886-j. [DOI] [PubMed] [Google Scholar]
- Tang Y., Dong W., Ai W., Zhang L., Li J., Yu Q., Guo S., Li Y. Design and establishment of a large-scale controlled ecological life-support system integrated experimental platform. Life Sci. Space Res. 2021;31:121–130. doi: 10.1016/j.lssr.2021.08.001. [DOI] [PubMed] [Google Scholar]
- Taniguchi M., Furutani M., Nishimura T., Nakamura M., Fushita T., Iijima K., Baba K., Tanaka H., Toyota M., Tasaka M., Morita M.T. The Arabidopsis LAZY1 Family Plays a Key Role in Gravity Signaling within Statocytes and in Branch Angle Control of Roots and Shoots. Plant Cell. 2017;29:1984–1999. doi: 10.1105/tpc.16.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbitts T. Controlled Ecological Life Support System. Use of Higher plants. 1980 [Google Scholar]
- Tonnang H.E., Salifu D., Mudereri B.T., Tanui J., Espira A., Dubois T., Abdel-Rahman E.M. Advances in data-collection tools and analytics for crop pest and disease management. Curr. Opin. Insect Sci. 2022;54 doi: 10.1016/j.cois.2022.100964. [DOI] [PubMed] [Google Scholar]
- Toyota M., Furuichi T., Sokabe M., Tatsumi H. Analyses of a gravistimulation-specific Ca2+ signature in Arabidopsis using parabolic flights. Plant Physiol. 2013;163:543–554. doi: 10.1104/pp.113.223313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbrink J.P., Herranz R., Medina F.J., Edelmann R.E., Kiss J.Z. A novel blue-light phototropic response is revealed in roots of Arabidopsis thaliana in microgravity. Planta. 2016;244:1201–1215. doi: 10.1007/s00425-016-2581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbrink J.P., Kiss J.Z. Preparation of a Spaceflight Experiment to Study Tropisms in Arabidopsis Seedlings on the International Space Station. Methods Mol. Biol. 2019;1924:207–214. doi: 10.1007/978-1-4939-9015-3_17. [DOI] [PubMed] [Google Scholar]
- Villacampa A., Fañanás-Pueyo I., Medina F.J., Ciska M. Root growth direction in simulated microgravity is modulated by a light avoidance mechanism mediated by flavonols. Physiol. Plantarum. 2022;174 doi: 10.1111/ppl.13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villacampa A., Sora L., Herranz R., Medina F.J., Ciska M. Analysis of Graviresponse and Biological Effects of Vertical and Horizontal Clinorotation in Arabidopsis thaliana Root Tip. Plants. 2021;10 doi: 10.3390/plants10040734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitha S., Yang M., Sack F.D., Kiss J.Z. Gravitropism in the starch excess mutant of Arabidopsis thaliana. Am. J. Bot. 2007;94:590–598. doi: 10.3732/ajb.94.4.590. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K., Nakano S., Soga K., Hoson T. Cell wall-bound peroxidase activity and lignin formation in azuki bean epicotyls grown under hypergravity conditions. J. Plant Physiol. 2009;166:947–954. doi: 10.1016/j.jplph.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Wang J.W., Wang L.J., Mao Y.B., Cai W.J., Xue H.W., Chen X.Y. Control of root cap formation by MicroRNA-targeted auxin response factors in Arabidopsis. Plant Cell. 2005;17:2204–2216. doi: 10.1105/tpc.105.033076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Dong C., Gao W. Evaluation of the growth, photosynthetic characteristics, antioxidant capacity, biomass yield and quality of tomato using aeroponics, hydroponics and porous tube-vermiculite systems in bio-regenerative life support systems. Life Sci. Space Res. 2019;22:68–75. doi: 10.1016/j.lssr.2019.07.008. [DOI] [PubMed] [Google Scholar]
- Wang W., Huang L., Song Y., Gui S., Cao J., Zhang H., Du M., Chen J., Wang Z., Zhou J., et al. LAZY4 acts additively with the starch–statolith-dependent gravity-sensing pathway to regulate shoot gravitropism and tiller angle in rice. Plant Commun. 2024;5 doi: 10.1016/j.xplc.2024.100943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Went F.W. Reflections and Speculations. Annu. Rev. Plant Physiol. 1974;25:1–27. [Google Scholar]
- Wheeler R.M. Potato and Human Exploration of Space: Some Observations from NASA-Sponsored Controlled Environment Studies. Potato Res. 2006;49:67–90. [Google Scholar]
- Wheeler R.M. Agriculture for Space: People and Places Paving the Way. Open Agric. 2017;2:14–32. [Google Scholar]
- Willige B.C., Isono E., Richter R., Zourelidou M., Schwechheimer C. Gibberellin regulates PIN-FORMED abundance and is required for auxin transport-dependent growth and development in Arabidopsis thaliana. Plant Cell. 2011;23:2184–2195. doi: 10.1105/tpc.111.086355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Wang L., Zheng H. Molecular Basis to Integrate Microgravity Signals into the Photoperiodic Flowering Pathway in Arabidopsis thaliana under Spaceflight Condition. Int. J. Mol. Sci. 2021;23 doi: 10.3390/ijms23010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Chen H., Hu J., Cai W. Potential evidence for transgenerational epigenetic memory in Arabidopsis thaliana following spaceflight. Commun. Biol. 2021;4:835. doi: 10.1038/s42003-021-02342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Chen H., Jin J., Cai W. Single-base resolution methylome analysis shows epigenetic changes in Arabidopsis seedlings exposed to microgravity spaceflight conditions on board the SJ-10 recoverable satellite. NPJ Microgravity. 2018;4:12. doi: 10.1038/s41526-018-0046-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Song S., Jiang H., Wu G., Chen Y. Effects of LAZY family genes on shoot gravitropism in Lotus japonicus. Plant Sci. 2024;348 doi: 10.1016/j.plantsci.2024.112234. [DOI] [PubMed] [Google Scholar]
- Yan S., Gao W., Lu F., Zhao R. Research progress on mutation by spaceflight in medicinal plants breeding. Zhongguo Zhongyao Zazhi. 2010;35:385–388. [PubMed] [Google Scholar]
- Yang J., Fu Y., Liu H. Microbiomes of air dust collected during the ground-based closed bioregenerative life support experiment “Lunar Palace 365. Environ. Microbiome. 2022;17:4. doi: 10.1186/s40793-022-00399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemets A., Shadrina R., Blume R., Plokhovska S., Blume Y. Autophagy formation, microtubule disorientation, and alteration of ATG8 and tubulin gene expression under simulated microgravity in Arabidopsis thaliana. npj Microgravity. 2024;10:31. doi: 10.1038/s41526-024-00381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara T., Spalding E.P., Iino M. AtLAZY1 is a signaling component required for gravitropism of the Arabidopsis thaliana inflorescence. Plant J. 2013;74:267–279. doi: 10.1111/tpj.12118. [DOI] [PubMed] [Google Scholar]
- Yu T.S., Kofler H., Häusler R.E., Hille D., Flügge U.I., Zeeman S.C., Smith A.M., Kossmann J., Lloyd J., Ritte G., et al. The Arabidopsis sex1 mutant is defective in the R1 protein, a general regulator of starch degradation in plants, and not in the chloroplast hexose transporter. Plant Cell. 2001;13:1907–1918. doi: 10.1105/TPC.010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M., Custaud M.A., Xu Z., Wang J., Yuan M., Tafforin C., Treffel L., Arbeille P., Nicolas M., Gharib C., et al. Multi-System Adaptation to Confinement During the 180-Day Controlled Ecological Life Support System (CELSS) Experiment. Front. Physiol. 2019;10:575. doi: 10.3389/fphys.2019.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel P., Bamsey M., Schubert D., Tajmar M. Review and analysis of over 40 years of space plant growth systems. Life Sci. Space Res. 2016;10:1–16. doi: 10.1016/j.lssr.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zheng H.Q. Changes in Plastid and Mitochondria Protein Expression in Arabidopsis Thaliana Callus on Board Chinese Spacecraft SZ-8. Microgravity Sci. Technol. 2015;27:387–401. [Google Scholar]
- Zhao T., Liu G., Liu D., Yi Y., Xie B., Liu H. Water recycle system in an artificial closed ecosystem - Lunar Palace 1: Treatment performance and microbial evolution. Sci. Total Environ. 2022;806 doi: 10.1016/j.scitotenv.2021.151370. [DOI] [PubMed] [Google Scholar]
- Zhou M., Ferl R.J., Paul A.-L. Light has a principal role in the Arabidopsis transcriptomic response to the spaceflight environment. npj Microgravity. 2024;10 doi: 10.1038/s41526-024-00417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Riva A., Gauthier M.-P.L., Kladde M.P., Ferl R.J., Paul A.-L. Single-molecule long-read methylation profiling reveals regional DNA methylation regulated by Elongator Complex Subunit 2 in Arabidopsis roots experiencing spaceflight. Biol. Direct. 2024;19 doi: 10.1186/s13062-024-00476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Sng N.J., LeFrois C.E., Paul A.L., Ferl R.J. Epigenomics in an extraterrestrial environment: organ-specific alteration of DNA methylation and gene expression elicited by spaceflight in Arabidopsis thaliana. BMC Genom. 2019;20:205. doi: 10.1186/s12864-019-5554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong Y., Zhao Z., Zhou K., Duan X., Han B., He C., Huang H., Jiang H. Metabolome and transcriptome analysis of anthocyanin biosynthesis reveal key metabolites and candidate genes in red-stemmed alfalfa (Medicago sativa) BMC Genom. 2025;26:323. doi: 10.1186/s12864-025-11529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J.J., Zheng Z.Y., Xue S., Li H.H., Wang Y.R., Le J. The role of Arabidopsis Actin-Related Protein 3 in amyloplast sedimentation and polar auxin transport in root gravitropism. J. Exp. Bot. 2016;67:5325–5337. doi: 10.1093/jxb/erw294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou T., Huang C., Wu P., Ge L., Xu Y. Optimization of Artificial Light for Spinach Growth in Plant Factory Based on Orthogonal Test. Plants. 2020;9 doi: 10.3390/plants9040490. [DOI] [PMC free article] [PubMed] [Google Scholar]