Abstract
Background.
Adherence of kidney transplant recipients (KTRs) to prescribed regimens is vital for long-term graft function. This study aimed to identify adherence rates using objective and composite measures, risk factors for nonadherence, and the latter’s impact on posttransplant outcomes.
Methods.
A retrospective single-center cohort study was conducted among KTR transplanted from January 1, 2003, to December 31, 2017. Overall nonadherence was defined as 1 or more of the following in the first-year posttransplant: (1) at least 1 missed clinic visit, (2) >30% missed laboratory visits, and (3) >40% coefficient of variation of calcineurin inhibitor levels. Logistic and Cox proportional hazards models were fitted to identify adherence risk factors and outcomes, respectively.
Results.
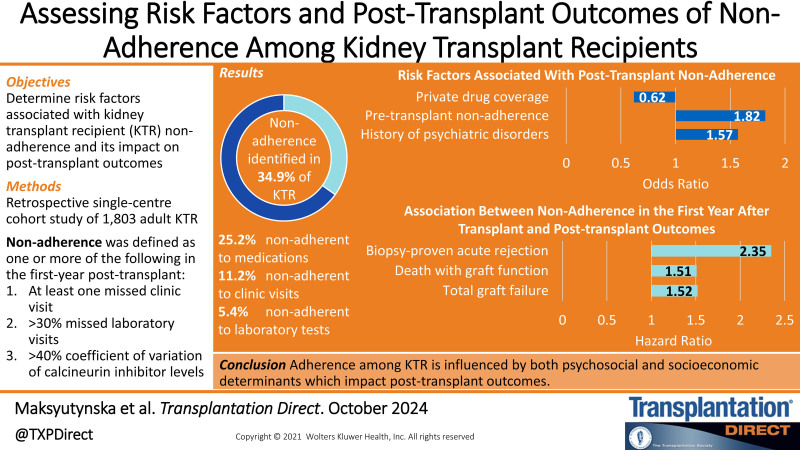
Among the included 1803 KTR, overall nonadherence was identified in 34.9%; 11.2% were nonadherent to clinic visits, 5.4% to laboratory tests, and 25.2% to medications. Recipient history of psychiatric disorders (odds artio [OR], 1.57; 95% confidence interval [CI], 1.22-2.02) or pretransplant nonadherence (OR, 1.82; 95% CI, 1.31–2.54), and private drug coverage (OR, 0.62; 95% CI, 0.48-0.80) were associated with posttransplant nonadherence. Any episode of nonadherence over the first year after transplant was associated with an increased risk of total graft failure (hazard ratio [HR], 1.52; 95% CI, 1.20-1.91), death with graft function (HR, 1.51; 95% CI, 1.11-2.05), and biopsy-proven acute rejection (HR, 2.35; 95% CI, 1.38-3.99).
Conclusions.
Adherence among KTR is influenced by both psychosocial and socioeconomic determinants which impact posttransplant outcomes. Our results emphasize feasible methods to monitor adherence and identify high-risk KTR.
Kidney transplantation is the gold standard treatment for kidney failure. As the field of transplantation is rapidly evolving, improvements to immunosuppression therapies, surgical techniques, biomarker identification, and organ allocation have optimized kidney transplant recipient (KTR) posttransplant outcomes.1-3 The adherence of transplant recipients is an important factor that complements the efficacy of treatments to improve patient outcomes.
Patient adherence is a broad term encompassing several domains within and beyond the field of transplantation. This includes adherence to medications (dosage and schedules), clinic visits, and scheduled laboratory tests, both pre- and posttransplantation. Among medications, immunosuppressants are of particular relevance to reduce the risk of allograft rejection.4 In solid organ transplantation, calcineurin inhibitors (CNIs) such as cyclosporine and tacrolimus are commonly used. These drugs have a narrow therapeutic index,5 such that changes in exposure over time can have a significant impact on patient health.6 However, because of varying pharmacokinetic parameters,7,8 regular monitoring and modulating drug levels is of great importance to ensure long-term graft function.
Patient adherence is most commonly measured in relation to medication dose and schedule compliance. This is achieved through objective and subjective measurements such as therapeutic drug monitoring to ascertain medication trough levels, self-reported questionnaires, in-person interviews, electronic pill bottle opening counts, intake diaries, and prescription fill dates.9-13 Additional measures of adherence include following laboratory testing schedules and attending clinical appointments with the nephrologist and care team.14
Because of differing nonadherence definitions and thresholds, the rates are highly variable among populations.15 Although there are various available methods, currently no standardized method to assess nonadherence in the transplant population exists.16,17 Moreover, risk factors predisposing KTR to nonadherence have yet to be systematically assessed or validated in larger study populations. Additionally, few studies have examined nonadherence as a composite factor made up of multiple discrete measures.
The diverse objectives of this study were to (1) study adherence rates in KTR defined using objective and composite criteria, (2) determine risk factors associated with KTR nonadherence in the first-year posttransplant, and (3) identify the subsequent impact of nonadherence on posttransplant outcomes beyond 1 y after transplant.
METHODS
Guidelines
This work complies with the Declaration of Helsinki and the Declaration of Istanbul. The study protocol was approved by the University Health Network Research Ethics Boards (19-5182.4).
Study Design and Population
A retrospective single-center cohort study was conducted among adult (>18 y of age) KTR who received a transplant at the University Health Network from January 1, 2003, to December 31, 2017. Patients were excluded from this study if they (1) had prior multiorgan transplants, (2) had prior kidney transplants, (3) received a transplant at another center, or (4) experienced primary graft nonfunction. Patient data were retrieved from our transplant center’s electronic health record, the Organ Transplant Tracking Record, and our research database, the Comprehensive Renal Transplant Research Information System.18
Definitions of Nonadherence
Overall nonadherence was defined as 1 or more of the following in the first-year posttransplant: (1) at least 1 missed clinic visit, (2) > 30% missed laboratory visits, and (3) > 40% coefficient of variation (CV) of CNI levels. To measure clinic visit nonadherence, only appointments marked as “no-show” were evaluated as missed; rescheduled or canceled appointments were not considered missed clinic visits.19 The following schedule is the expected frequency of patients’ clinic appointments within the first year following transplant: (1) weekly during the first month (2–4 visits depending on the date of discharge), (2) biweekly during the second and third months (4 visits), (3) monthly between the fourth and sixth months (3 visits), and (4) bimonthly between the seventh and twelfth months (3 visits).
Scheduled laboratory visit nonadherence was defined by the following standard institutional laboratory schedule (and the permissible time range to complete laboratory testing from the scheduled date) within the first year following transplant: (1) twice a week (±1 d) during the first month, (2) weekly (±3 d) during the second and third months, (3) biweekly (±1 wk) between the fourth and sixth months, and (4) monthly between the seventh and twelfth months (±2 wk).
CNI trough-level variability was used as an indicator of medication nonadherence. The variability was determined by the CV of CNI blood levels within our population using the following equation:
CNIi represents individual CNI trough levels for 1 patient, µ is the mean CNI trough level for the whole population, and N is the number of trough levels collected for 1 patient. These measurements were all performed over the first-year posttransplant. When applied to our population, the 75th percentile (third quartile) CV was 40% and was used as the threshold for nonadherence. The use of the 75th percentile as the cutoff has been previously used in the literature.20,21
Risk Factors and Outcomes
Recipient and donor risk factors were collected at baseline to assess their relationship with KTR nonadherence during the first year posttransplant. Recipient risk factors included age, sex, cause of end-stage renal disease (ESRD), history of smoking before transplant, history of diabetes mellitus, history of psychiatric disorders, history of pretransplant nonadherence, travel distance from recipient’s home to hospital (in kilometers), marital status, and drug coverage. Donor risk factors included living or deceased kidney donation. Pretransplant nonadherence was assessed using social work notes taken during detailed patient interviews at the transplant evaluation stage, with mention of any current or historic behavior of missed appointments or laboratory tests, missed or shortened dialysis sessions, inconsistent diabetes management, irregular medication intake, and other clinical domains.22 Similarly, social work notes were utilized to define a history of psychiatric disorders, which compiled information collected from patient self-reports, relatives, medical documentation, letters from psychiatrists, and referring physicians and centers.22 Thus, this variable was not based on a formal psychiatric assessment/diagnosis but summarizes any records of mental health concerns.
The outcomes of this study were the effect of nonadherence on posttransplant outcomes beyond 1-y posttransplant. The 1-y landmark allows the nonadherence exposure variable to accrue over the first year posttransplant. Posttransplant outcomes included death-censored graft failure (ie, return to chronic dialysis or need for preemptive retransplantation), death with graft function (ie, death prior graft loss), total graft failure (composite of the prior 2 outcomes), and biopsy-proven acute rejection after the origin of 1-y posttransplant.
Statistical Analysis
Descriptive statistics were used to assess baseline recipient and donor characteristics. Quantitative variables were expressed as a mean value with a SD or a median value with an interquartile range. Logistic regression models were fitted to evaluate the impact of risk factors on overall nonadherence and were reported as odds ratios (ORs) with 95% confidence intervals (CIs). The multivariable model was adjusted for all baseline risk factors. The Kaplan-Meier product limit method was used to graphically depict the time from cohort entry (ie, 1-y posttransplant) to the main study outcomes >10 y based on overall and domain-specific nonadherence groups. Differences in survival functions were evaluated using the log-rank test.
Cox proportional hazards models were fitted to study the effect of nonadherence on posttransplant outcomes and were reported as hazard ratios (HRs) with 95% CI. The model included overall nonadherence along with adjustment for recipient variables (age, sex, race, body mass index at transplant, history of diabetes mellitus, time on dialysis before transplant, peak panel-reactive antibody), donor variables (age, body mass index at donation, donor type), and transplant variables (cold ischemic time, type of induction, CNI at transplant discharge, delayed graft failure, transplant era). A two-tailed P value <0.05 was considered statistically significant, and all analyses were performed using Stata version 16 (StataCorp, College Station, TX).
RESULTS
Population Characteristics
Of the 2714 patients who were transplanted within the study period, 425 had a prior multiorgan transplant, 212 had a prior kidney transplant, 248 received a transplant from another center, and 26 experienced primary graft nonfunction transplants, resulting in 1803 patients being included in the study cohort (Figure 1). During 9011.5 person-years of follow-up, 327 total graft failures (136 death-censored graft failures and 191 deaths with graft function) were observed. Moreover, 55 biopsy-proven acute rejections were ascertained >8833.2 person-years of follow-up.
FIGURE 1.
Study flow diagram.
Among patients included in the study cohort, the mean recipient age was 51.7 (±13.4) y, and 60.7% were men (Table 1). The main cause of ESRD was glomerulonephritis in 34.4%. The majority of recipients had no prior history of smoking (55.6%), diabetes mellitus (67.4%), psychiatric disorders (81.9%), or pretransplant nonadherence (84.9%). Deceased donation was slightly more common (54.5%) when compared with living donation (45.5%), and drug coverage was almost equally distributed between private (50.5%) and public (49.5%) payers.
TABLE 1.
Baseline characteristics and adherence within the first-year posttransplant
| Baseline characteristics | No. of patients (N = 1803) | Total cohort | Adherence (n = 1173) | Nonadherence (n = 630) |
|---|---|---|---|---|
| Mean recipient age (y) | 1803 | 51.7 (±13.4) | 51.5 (±13.0) | 51.9 (±14.2) |
| Recipient sex | ||||
| Male | 1,095 | 60.7% | 726 (61.9%) | 369 (58.6%) |
| Female | 708 | 39.3% | 447 (38.1%) | 261 (41.4%) |
| Cause of end-stage renal disease | ||||
| Glomerulonephritis | 619 | 34.4% | 414 (35.4%) | 205 (32.6%) |
| Diabetes mellitus | 452 | 25.1% | 273 (23.3%) | 179 (28.5%) |
| Polycystic kidney disease | 238 | 13.2% | 165 (14.1%) | 73 (11.6%) |
| Other | 490 | 27.2% | 318 (27.2%) | 172 (27.3%) |
| Recipient history of smoking before transplant | ||||
| No | 993 | 55.6% | 647 (55.8%) | 346 (55.1%) |
| Yes | 794 | 44.4% | 512 (44.2%) | 282 (44.9%) |
| Recipient history of diabetes mellitus | ||||
| No | 1216 | 67.4% | 815 (69.5%) | 401 (63.7%) |
| Yes | 587 | 32.6% | 358 (30.5%) | 229 (36.4%) |
| Recipient history of psychiatric disorder | ||||
| No | 1477 | 81.9% | 994 (84.7%) | 483 (76.7%) |
| Yes | 326 | 18.1% | 179 (15.3%) | 147 (23.3%) |
| Recipient history of pretransplant nonadherence | ||||
| No | 1154 | 84.9% | 774 (88.1%) | 381 (79.4%) |
| Yes | 205 | 15.1% | 105 (12.0%) | 99 (20.6%) |
| Donor type | ||||
| Deceased | 982 | 54.5% | 621 (52.9%) | 361 (57.3%) |
| Living | 821 | 45.5% | 552 (47.1%) | 269 (42.7%) |
| Median travel distance from recipient’s home to hospital (km) | 1367 | 29.9 (11.2, 53.5) | 29.9 (11.4, 55.8) | 29.4 (10.3, 47.8) |
| Marital status | ||||
| Single | 256 | 18.7% | 169 (19.1%) | 87 (17.9%) |
| Married | 920 | 67.1% | 602 (68.0%) | 318 (65.4%) |
| Divorced/separated/widowed | 196 | 14.3% | 115 (13.0%) | 81 (16.7%) |
| Drug coverage | ||||
| Private | 695 | 50.5% | 490 (55.1%) | 205 (42.1%) |
| Public | 682 | 49.5% | 400 (44.9%) | 282 (57.9%) |
Assessing Risk Factors of Nonadherence
Overall nonadherence was identified in 630 (34.9%) patients; 202 (11.2%) patients were nonadherent to clinic visits, 98 (5.4%) to laboratory visits, and 454 (25.2%) to medication (Table S1, SDC, https://links.lww.com/TXD/A761). Univariable analysis showed that patients with diabetes mellitus in comparison with glomerulonephritis as their cause of ESRD were at a greater risk of overall nonadherence (OR, 1.32; 95% CI, 1.02-1.70; Table 2). Similarly, recipient history of diabetes mellitus (OR, 1.30; 95% CI, 1.06-1.59), psychiatric disorder (OR, 1.69; 95% CI, 1.32-2.16), and pretransplant nonadherence (OR, 1.92; 95% CI, 1.42-2.60) were risk factors associated with overall nonadherence. In a multivariable analysis, only recipient history of psychiatric disorders (OR, 1.57; 95% CI, 1.22-2.02) and pretransplant nonadherence (OR, 1.82; 95% CI, 1.31-2.54) were independent risk factors for overall nonadherence (Table 2). Furthermore, possessing private drug coverage showed a reduced risk for overall nonadherence (OR, 0.62; 95% CI, 0.48-0.80).
TABLE 2.
Logistic regression for the effect of recipient and donor factors on at least 1 episode of nonadherence within the first-year posttransplant
| Risk factors | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| Odd ratio (95% CI) | P | Odd ratio (95% CI) | P | |
| Recipient age (every 1 y increase) | 1.00 (0.99-1.01) | 0.59 | 1.00 (0.99-1.01) | 0.89 |
| Recipient sex (female vs male) | 1.15 (0.94-1.40) | 0.17 | 1.17 (0.95-1.44) | 0.15 |
| Cause of end-stage renal disease | ||||
| Diabetes mellitus vs glomerulonephritis | 1.32 (1.02-1.70) | 0.03 | 1.05 (0.68-1.60) | 0.84 |
| Polycystic kidney disease vs glomerulonephritis | 0.89 (0.65-1.23) | 0.50 | 0.97 (0.69-1.36) | 0.84 |
| Other vs glomerulonephritis | 1.09 (0.85-1.40) | 0.49 | 1.05 (0.81-1.36) | 0.71 |
| Recipient history of smoking before transplant (yes vs no) | 1.03 (0.85-1.26) | 0.75 | 0.98 (0.79-1.20) | 0.83 |
| Recipient history of diabetes mellitus (yes vs no) | 1.30 (1.06-1.59) | 0.01 | 1.20 (0.81-1.77) | 0.36 |
| Recipient history of psychiatric disorder (yes vs no) | 1.69 (1.32-2.16) | <0.001 | 1.57 (1.22-2.02) | 0.001 |
| Recipient history of nonadherence (yes vs no) | 1.92 (1.42-2.60) | <0.001 | 1.82 (1.31-2.54) | <0.001 |
| Donor type (living vs deceased) | 0.84 (0.69-1.02) | 0.08 | 0.95 (0.76-1.19) | 0.67 |
| Travel distance from recipient’s home to hospital (every 10-km increase) | 1.00 (0.99-1.00) | 0.30 | 1.00 (0.99-1.00) | 0.47 |
| Marital status | ||||
| Single vs married | 1.04 (0.78-1.39) | 0.80 | 0.87 (0.60-1.24) | 0.43 |
| Divorced/separated/widowed vs married | 1.29 (0.94-1.77) | 0.12 | 1.01 (0.73-1.41) | 0.94 |
| Drug coverage (private vs public) | 0.60 (0.48-0.75) | <0.001 | 0.62 (0.48-0.80) | <0.001 |
Exploring Nonadherence as a Risk Factor for Posttransplant Outcomes
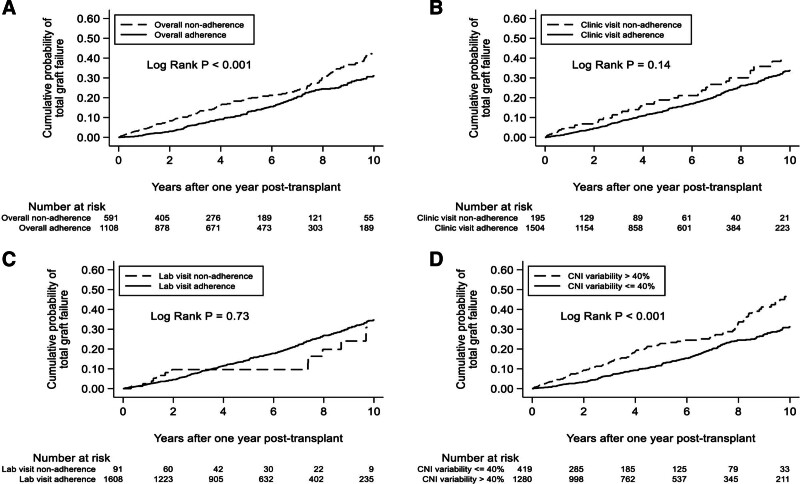
The cumulative probability of total graft failure was significantly greater in the overall nonadherent population in comparison with the adherent population (P < 0.001), driven primarily by medication nonadherence (P < 0.001; Figure 2). Nonadherence in any 1 of the 3 domains over the first year after transplant was associated with an increased risk of total graft failure (HR, 1.52; 95% CI, 1.20–1.91), death with graft function (HR, 1.51; 95% CI, 1.11-2.05), and biopsy-proven acute rejection (HR, 2.35; 95% CI, 1.38-3.99; Table 3). A trend toward an increased risk of death-censored graft failure was also observed (HR, 1.39; 95% CI, 0.96-2.01). CNI CV was consistently associated with an increased risk of graft, patient, and rejection outcomes, whereas biopsy-proven acute rejection was predicted by overall and domain-specific adherence measures (Table S2, SDC, https://links.lww.com/TXD/A761).
FIGURE 2.
Total graft failure over 10 y starting at 1-y posttransplant, categorized by nonadherence exposure: overall nonadherence (A), clinic visit nonadherence (B), laboratory visit nonadherence (C), and CNI variability (D).
TABLE 3.
Cox proportional hazards model for the effect of overall nonadherence on transplant outcomes starting 1-y posttransplant
| Outcome | Hazard ratio (95% CI) | P |
|---|---|---|
| Total graft failure after origin | 1.52 (1.20-1.91) | <0.001 |
| Death-censored graft failure after origin | 1.39 (0.96-2.01) | 0.08 |
| Death with graft function after origin | 1.51 (1.11-2.05) | 0.01 |
| Biopsy-proven acute rejection after origin | 2.35 (1.38-3.99) | 0.002 |
DISCUSSION
Patient adherence is a topic that spans many medical fields and is especially pertinent to the transplant population because of its significant impact on graft and patient outcomes.23 To investigate this further, our study adopted a novel approach to measure adherence in KTR, using a composite of readily accessible data from the hospital’s electronic record. Specifically, we used a 3-variable measure of nonadherence composed of clinic visit attendance, laboratory visit attendance, and CNI trough level variability with a priori thresholds. This was done to capture >1 dimension of adherence, unlike other approaches that rely on single, self-reported measures such as pill counts or patient-administered surveys. Rather, we conducted analyses using objective criteria and systematic thresholds to eliminate recall bias. Through this approach, we found overall nonadherence in our population to be 34.9% and align closely with the 30% observed in the heart transplant population.16 However, because of varying methodologies in measuring nonadherence, this percentage is highly variable among studies.15 To compare nonadherence rates among populations, and subsequently share strategies to improve them, a standardized and validated threshold must be developed. Our proposed adherence metric addresses several challenges associated with studying this outcome. It could be used for patient- and program-level monitoring, and incorporated into quality dashboards. Furthermore, local targets can also be set which may guide decision-making among healthcare providers.
The individual thresholds used for nonadherence in our analysis align with those used in the literature. For medication nonadherence in the literature, CV values ranging from 24.6% to 43% were associated with poor kidney graft function.12,24,25 This range corresponds to our study’s medication nonadherence threshold of CV >40%. The use of a higher cutoff considers the influence of pharmacokinetics which makes this measure variable among patients. In other transplant studies, nonadherence thresholds for clinic appointments ranged between 12% and 20%.19,26,27 This range is marginally higher than the cutoff value used in our analysis because we emphasized the importance of clinic visits within the first-year posttransplant. In this period, patients are most sensitive to complications of inadequate and overimmunosuppression, warranting timely dose adjustments.28 Clinic appointments are used to monitor and intervene in patient and graft health.29 Prior studies have employed a cutoff of 20% missed laboratory appointments, with a respective grace period, to define laboratory nonadherence.30 Their grace period allowed for 3 d of variance from the scheduled laboratory date in the first month and 7 d from the second to sixth months in the first-year posttransplant.30 However, in our analysis, the acceptable time range was shorter, growing to 1 wk starting at only the fourth month posttransplant, which accounts for our use of a greater nonadherence cutoff. Although not directly assessed in our study, an association between individual measures of nonadherence has been previously established, supporting the use of a composite overall nonadherence measure.19,30
Overall nonadherence was influenced by both psychosocial and socioeconomic determinants. In accordance with our findings, there is consensus that history of psychiatric disorders, history of nonadherence, and no supplemental private health coverage increase the risk for posttransplant nonadherence.15,31 This may be attributed to the high cost associated with medications required for comorbid conditions or difficulty incorporating additional medications into an existing schedule. More in-depth consideration of these factors during the evaluation process may help identify patients at greater risk for poor graft function after transplant.
In our analysis, significant increases in the risks for total graft failure and death with graft function were driven by medication nonadherence, potentially because of their narrow therapeutic window. As expected, the risk of total graft failure increased >10 y for both adherent and nonadherent KTR but was significantly higher in the nonadherent population. Additionally, the risk for biopsy-proven acute rejection starting 1 y after transplant increased 2-fold for nonadherent individuals. The negative effects of nonadherence on graft survival correspond to previous study findings.19 This finding highlights the importance and responsibility of KTR in managing their health with their care team.
Education is the most commonly used intervention to address adherence; however, it is not considered the most effective.32,33 At our center, mandatory pharmacist-led educational sessions are held to inform patients of their medications. More personalized interventions have also been explored in the literature and have been found to have positive impacts on adherence. These interventions include motivational interviewing and behavioral contracts that allow for reflection and intention-making, emphasizing the responsibility of the patient.34,35 Specifically, incorporating quality improvements at a personal level, through linking them to daily routines, environmental cues, and supportive people, was found to improve medication adherence in KTR.36 Furthermore, the use of health information technology, such as mobile apps to set reminders for medication, has been shown to improve adherence rates.9,37 Therefore, multidisciplinary approaches, combining education with additional interventions are recommended.9,38 However, further investigation of such integrative targeted interventions is required to ascertain their role in improving graft and patient outcomes and address limitations to existing studies with low methodological quality, small sample sizes, and heterogeneity in measurements.39,40 Furthermore, future research also warrants the consideration of the scalability, cost, and pragmatism of these interventions.41,42
Despite our study’s large and diverse population, it is limited to a single, adult cohort of KTR transplanted at a single center. Additional investigation is needed to determine whether our results are generalizable to other populations such as adolescents and young adults. Moreover, measuring nonadherence as a binary, compared with a continuous, variable may be identified as a loss of information.43 The binary approach allows for more distinct identification of patients that would require additional interventions and is easier to adapt to a clinical setting. As previously mentioned, the nonadherence thresholds are well-defined, within a range of those used in the literature, and have been validated in previous studies. The data collected were predominantly related to the transplant recipient, with donor data limited to deceased versus living. The tendency to adhere is unlikely related to other donor characteristics, so confounding by the latter is probably small. Furthermore, there may have been some variability in posttransplant follow-up practices among transplant physicians. However, the expected clinic and laboratory test schedules used in our study reflect those applicable to the majority of our population. Finally, observational studies are always at risk of residual confounding. The consistency of the overall and domain-specific results, as well as the efforts to account for numerous potential confounders in multivariable models, should help to mitigate the risk of significant bias.
In conclusion, our study offers a novel, feasible, and objective approach to study adherence in KTR. It further emphasizes that suboptimal adherence, as measured by the domains that were captured in this analysis, is strongly associated with adverse graft and patient outcomes. These trends can be readily measured and observed because transplant centers can capture adherence using the electronic health records of KTR and use them to flag high-risk patients for appropriate interventions. Whether the use of such a monitoring strategy will improve outcomes would best be evaluated in a clinical trial.
ACKNOWLEDGMENTS
The authors would like to acknowledge students from the Multi-Organ Transplant Student Research Training Program for their support with this article.
Supplementary Material
Footnotes
S.J.K. is a member of the Data and Safety Monitoring Board for a phase 2 clinical trial for Eledon Pharmaceuticals and acknowledges his position as a co-chair of Health Canada’s Organ Donation and Transplantation Collaborative Data System Working Group, a chair of the Trillium Gift of Life Network’s Transplant Performance Monitoring and Evaluation Committee, and a chair of The Transplantation Society’s Global Data Harmonization Committee. The other authors declare no funding or conflicts of interest.
K.M., B.B., X.W., and O.S. contributed to data collection and article preparation; Y.L. conducted formal analysis of the data; O.F. and S.J.K. contributed to study conception and design, and article preparation.
Access to clinical data collected as part of this research may be made available upon request from the corresponding author and approval of data sharing from the research ethics board at the institution.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
Contributor Information
Kateryna Maksyutynska, Email: k.maksyutynska@mail.utoronto.ca.
Benedict Batoy, Email: benedict.batoy@outlook.com.
Xinyu Wei, Email: xinyu.wei@mail.utoronto.ca.
Oswa Shafei, Email: Oswa.Shafei@wchospital.ca.
Yanhong Li, Email: yanhong.li@uhn.ca.
Olusegun Famure, Email: segun.famure@uhn.ca.
REFERENCES
- 1.Abramowicz D, Oberbauer R, Heemann U, et al. Recent advances in kidney transplantation: a viewpoint from the Descartes advisory board. Nephrol Dial Transplant. 2018;33:1699–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gokoel SRM, Gombert-Handoko KB, Zwart TC, et al. Medication non-adherence after kidney transplantation: a critical appraisal and systematic review. Transplant Rev (Orlando). 2020;34:100511. [DOI] [PubMed] [Google Scholar]
- 3.Wang JH, Skeans MA, Israni AK. Current status of kidney transplant outcomes: dying to survive. Adv Chronic Kidney Dis. 2016;23:281–286. [DOI] [PubMed] [Google Scholar]
- 4.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–2729. [DOI] [PubMed] [Google Scholar]
- 5.Johnston A. Equivalence and interchangeability of narrow therapeutic index drugs in organ transplantation. Eur J Hosp Pharm. 2013;20:302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uber PA, Ross HJ, Zuckermann AO, et al. Generic drug immunosuppression in thoracic transplantation: an ISHLT educational advisory. J Heart Lung Transplant. 2009;28:655–660. [DOI] [PubMed] [Google Scholar]
- 7.Borra LCP, Roodnat JI, Kal JA, et al. High within-patient variability in the clearance of tacrolimus is a risk factor for poor long-term outcome after kidney transplantation. Nephrol Dial Transplant. 2010;25:2757–2763. [DOI] [PubMed] [Google Scholar]
- 8.Monchaud C, Marquet P. Pharmacokinetic optimization of immunosuppressive therapy in thoracic transplantation: Part I. Clin Pharmacokinet. 2009;48:419–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myaskovsky L, Jesse MT, Kuntz K, et al. Report from the American Society of Transplantation Psychosocial Community of Practice Adherence Task Force: real-world options for promoting adherence in adult recipients. Clin Transplant. 2018;32:e13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leino AD, King EC, Jiang W, et al. Assessment of tacrolimus intrapatient variability in stable adherent transplant recipients: establishing baseline values. Am J Transplant. 2019;19:1410–1420. [DOI] [PubMed] [Google Scholar]
- 11.Reese PP, Bloom RD, Trofe-Clark J, et al. Automated reminders and physician notification to promote immunosuppression adherence among kidney transplant recipients: a randomized trial. Am J Kidney Dis. 2017;69:400–409. [DOI] [PubMed] [Google Scholar]
- 12.Kreuzer M, Prüfe J, Oldhafer M, et al. Transitional care and adherence of adolescents and young adults after kidney transplantation in Germany and Austria. Medicine (Baltimore). 2015;94:e2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patzer RE, Serper M, Reese PP, et al. Medication understanding, non-adherence, and clinical outcomes among adult kidney transplant recipients. Clin Transplant. 2016;30:1294–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dabbs AD, Song MK, Myers BA, et al. A randomized controlled trial of a mobile health intervention to promote self-management after lung transplantation. Am J Transplant. 2016;16:2172–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prendergast MB, Gaston RS. Optimizing medication adherence: an ongoing opportunity to improve outcomes after kidney transplantation. Clin J Am Soc Nephrol. 2010;5:1305–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sánchez R, Baillès E, Bastidas A, et al. A new quantitative approach to assessing noncompliance with medical recommendations in heart transplant recipients. Transplant Proc. 2016;48:2178–2180. [DOI] [PubMed] [Google Scholar]
- 17.Lavsa SM, Holzworth A, Ansani NT. Selection of a validated scale for measuring medication adherence. J Am Pharm Assoc (2003). 2011;51:90–94. [DOI] [PubMed] [Google Scholar]
- 18.Famure O, Phan NAT, Kim SJ. Health information management for research and quality assurance: the comprehensive renal transplant research information system. Healthc Manage Forum. 2014;27:30–36. [DOI] [PubMed] [Google Scholar]
- 19.Taber DJ, Fleming JN, Fominaya CE, et al. The impact of health care appointment non-adherence on graft outcomes in kidney transplantation. Am J Nephrol. 2017;45:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belaiche S, Décaudin B, Dharancy S, et al. Factors associated with the variability of calcineurin inhibitor blood levels in kidney recipients grafted for more than 1 year. Fundam Clin Pharmacol. 2018;32:88–97. [DOI] [PubMed] [Google Scholar]
- 21.Rayar M, Tron C, Jézéquel C, et al. High intrapatient variability of tacrolimus exposure in the early period after liver transplantation is associated with poorer outcomes. Transplantation. 2018;102:e108–e114. [DOI] [PubMed] [Google Scholar]
- 22.Gumabay FM, Novak M, Bansal A, et al. Pre-transplant history of mental health concerns, non-adherence, and post-transplant outcomes in kidney transplant recipients. J Psychosom Res. 2018;105:115–124. [DOI] [PubMed] [Google Scholar]
- 23.Butler JA, Roderick P, Mullee M, et al. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation. 2004;77:769–776. [DOI] [PubMed] [Google Scholar]
- 24.O’Regan JA, Canney M, Connaughton DM, et al. Tacrolimus trough-level variability predicts long-term allograft survival following kidney transplantation. J Nephrol. 2016;29:269–276. [DOI] [PubMed] [Google Scholar]
- 25.Goodall DL, Willicombe M, McLean AG, et al. High intrapatient variability of tacrolimus levels and outpatient clinic nonattendance are associated with inferior outcomes in renal transplant patients. Transplant Direct. 2017;3:e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fredericks EM, Magee JC, Eder SJ, et al. Quality improvement targeting adherence during the transition from a pediatric to adult liver transplant clinic. J Clin Psychol Med Settings. 2015;22:150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell T, Gooding H, Mews C, et al. Transition to adult care for pediatric liver transplant recipients: the Western Australian experience. Pediatr Transplant. 2017;21:e12820. [DOI] [PubMed] [Google Scholar]
- 28.Stallone G, Infante B, Grandaliano G. Management and prevention of post-transplant malignancies in kidney transplant recipients. Clin Kidney J. 2015;8:637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romano G, Simonella R, Falleti E, et al. Physical training effects in renal transplant recipients. Clin Transplant. 2010;24:510–514. [DOI] [PubMed] [Google Scholar]
- 30.Ryan JL, Dandridge LM, Fischer RT. Adherence to laboratory testing in pediatric liver transplant recipients. Pediatr Transplant. 2020;00:e13899. [DOI] [PubMed] [Google Scholar]
- 31.Dobbels F, Vanhaecke J, Dupont L, et al. Pretransplant predictors of posttransplant adherence and clinical outcome: an evidence base for pretransplant psychosocial screening. Transplantation. 2009;87:1497–1504. [DOI] [PubMed] [Google Scholar]
- 32.Berben L, Dobbels F, Kugler C, et al. Interventions used by health care professionals to enhance medication adherence in transplant patients: a survey of current clinical practice. Prog Transplant. 2011;21:322–331. [DOI] [PubMed] [Google Scholar]
- 33.Berben L, Bogert L, Leventhal ME, et al. Which interventions are used by health care professionals to enhance medication adherence in cardiovascular patients? A survey of current clinical practice. Eur J Cardiovasc Nurs. 2011;10:14–21. [DOI] [PubMed] [Google Scholar]
- 34.Chisholm-Burns MA, Spivey CA, Graff Zivin J, et al. Improving outcomes of renal transplant recipients with behavioral adherence contracts: a randomized controlled trial. Am J Transplant. 2013;13:2364–2373. [DOI] [PubMed] [Google Scholar]
- 35.Zomahoun HTV, Guénette L, Grégoire JP, et al. Effectiveness of motivational interviewing interventions on medication adherence in adults with chronic diseases: a systematic review and meta-analysis. Int J Epidemiol. 2017;46:589–602. [DOI] [PubMed] [Google Scholar]
- 36.Russell CL, Hathaway D, Remy LM, et al. Improving medication adherence and outcomes in adult kidney transplant patients using a personal systems approach: System CHANGETM results of the MAGIC randomized clinical trial. Am J Transplant. 2020;20:125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pérez-Jover V, Sala-González M, Guilabert M, et al. Mobile apps for increasing treatment adherence: systematic review. J Med Internet Res. 2019;21:e12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gandolfini I, Palmisano A, Fiaccadori E, et al. Detecting, preventing and treating non-adherence to immunosuppression after kidney transplantation. Clin Kidney J. 2022;15:1253–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mellon L, Doyle F, Hickey A, et al. Interventions for increasing immunosuppressant medication adherence in solid organ transplant recipients. Cochrane Database Syst Rev. 2022;9:CD012854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corr M, Walker A, Maxwell AP, et al. Non-adherence to immunosuppressive medications in kidney transplant recipients- a systematic scoping review. Transplant Rev (Orlando). 2025;39:100900. [DOI] [PubMed] [Google Scholar]
- 41.Foster BJ. Multicomponent interventions improve adherence-where do we go from here? Am J Transplant. 2020;20:5–6. [DOI] [PubMed] [Google Scholar]
- 42.Whittington M, Goggin K, Noel-MacDonnell J, et al. Cost-effectiveness analysis: personal systems approach in improving medication adherence in adult kidney transplant patients. J Healthc Qual. 2022;44:240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332:1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.