Visual Abstract
Key Words: cardiotoxicity, macrophage, NF-κB, SLAMF7, TRAF6
Highlights
-
•
SLAMF7 is a macrophage-associated membrane protein that plays a crucial role in regulating inflammation during DIC.
-
•
SLAMF7 interacts with TRAF6, inhibiting NF-κB signaling and proinflammatory macrophage activation, thereby reducing cardiac inflammation.
-
•
SLAMF7 deficiency exacerbates inflammation and cardiac dysfunction in DIC models, underscoring its protective role.
-
•
Recombinant SLAMF7 administration reduces inflammation and improves cardiac function in mice treated with doxorubicin.
Summary
Doxorubicin-induced cardiotoxicity (DIC) poses a significant challenge in cancer treatment. This study investigated the role of SLAMF7 in DIC, particularly in macrophage-mediated inflammation. Using SLAMF7 knockout mice, we found that SLAMF7 deficiency exacerbates DIC and amplifies inflammatory responses. Mechanistically, SLAMF7 interacts with TNF receptor-associated factor 6 to attenuate nuclear factor κB signaling, reducing oxidative stress and proinflammatory cytokines. Notably, administering recombinant SLAMF7 protein effectively mitigated DIC. These findings underscore the critical role of SLAMF7 in protecting against DIC, positioning it as a promising therapeutic target.
Doxorubicin (Dox), a potent anthracycline antibiotic, is a key chemotherapeutic agent used to treat various malignancies. However, its clinical application is significantly limited by dose-dependent cardiotoxicity, particularly in patients receiving high doses for anthracycline-sensitive breast cancer, where the risk of heart failure is markedly increased.1,2 Doxorubicin-induced cardiotoxicity (DIC) typically manifests as an atrophied and dysfunctional left ventricle, with cardiomyocyte atrophy being a primary feature of its pathogenesis.3 Despite extensive research, the intricate mechanisms driving DIC remain largely unknown, limiting treatment options for symptomatic management, with dexrazoxane being the sole U.S. Food and Drug Administration–approved medication for preventing DIC.4,5 Therefore, understanding the pathogenesis of DIC is essential for improving clinical outcomes and developing more targeted therapeutic interventions.
Recent clinical evidence highlights the pivotal role of inflammation in the development of cardiomyopathy, with studies showing that reducing cardiac inflammation can significantly improve cardiac function.6,7 Macrophages, the primary inflammatory cells infiltrating myocardial tissues, exhibit remarkable phenotypic plasticity in response to various cardiovascular insults.8,9 These cells play a dual role in cardiac pathology, both facilitating tissue regeneration and repair through secreting growth factors and exacerbating tissue damage via the production of reactive oxygen species (ROS) and proinflammatory cytokines.10 Macrophages undergo a biphasic response during cardiac injury and repair. Initially, the proinflammatory M1 macrophages dominate, secreting cytokines and chemokines that recruit additional monocytes and neutrophils to the site of injury.11, 12, 13 This phase is crucial for pathogen clearance and debris removal but can also exacerbate collateral tissue damage. Subsequently, a reparative phase occurs, characterized by the emergence of M2-like macrophages, which secrete anti-inflammatory mediators, such as interleukin (IL)-10 and transforming growth factor (TGF)-β, to promote healing and resolve inflammation. These M2 macrophages also up-regulate the expression of programmed death ligand 1 (PDL1) and PDL2, further contributing to the resolution of inflammation.14,15 However, the complex interplay between these macrophage phenotypes and the molecular events governing their transitions remains incompletely understood.
The signaling lymphocytic activation molecule (SLAM) family, consisting of 9 single-pass transmembrane receptors, plays critical roles in immune regulation. These receptors interact with SH2 domain-containing proteins via cytoplasmic immunoreceptor tyrosine-based switch motifs.16 Predominantly expressed on hematopoietic cells, SLAM receptors are pivotal in modulating immune functions and have been implicated in numerous immune-related disorders.17,18 The functional outcome of SLAM receptors engagement, whether activating or inhibiting immune cells, is largely dependent on their interaction with SLAM-associated protein (SAP) family adaptors.19 Typically, the presence of SAP proteins facilitates activating signals through the recruitment of Src family kinases, whereas the absence of SAP adaptors leads to phosphatase recruitment and inhibitory signaling. The activity of SLAM receptors is, therefore, finely tuned by the availability and abundance of SAP adaptors. Among SLAM family members, SLAMF7 is notable for its role in immune regulation. As a self-ligand, SLAMF7 engages in homotypic interactions and regulates the phagocytosis of hematopoietic tumor cells.20, 21, 22 SLAMF7 plays a complex role in immune regulation within macrophages. It helps bolter protective immunity against pathogens by dampening inflammatory responses and promoting M2 macrophage polarization.23,24 On the other hand, SLAMF7 can also trigger a potent inflammatory cytokine response, leading to aberrant macrophage activation and contributing to pathological inflammation, as observed in conditions such as Crohn’s disease and rheumatoid arthritis.25 Despite these insights, the role of SLAMF7 in the phenotypic modulation of cardiac macrophages remains unexplored, and its potential contribution to DIC pathogenesis is yet to be elucidated.
In this study, we investigated the role of SLAMF7 in DIC using a chronic DIC mouse model. Our findings reveal that Dox treatment down-regulates SLAMF7 expression in macrophages, thereby altering the phenotypic plasticity of cardiac macrophages throughout the progression of DIC. Furthermore, we propose that SLAMF7 activation may offer a novel therapeutic approach for mitigating DIC.
Methods
The data supporting the findings of this study are available from the corresponding author upon request. Detailed methodologies are provided in the Supplemental Appendix.
Mice
Wild-type (WT) (C57BL/6J background), SLAMF7 knockout (KO), SLAMF7flox/flox, and LysM-Cre mice were obtained from Cyagen Biosciences Inc. Male mice, aged 8 to 10 weeks and weighing 25 to 27 g, were utilized in all experiments. Global KO mice were generated using the CRISPR/Cas9 system. SLAMF7flox/flox mice were crossed with LysM-Cre mice to produce macrophage-specific conditional SLAMF7-deficient (SLAMF7flox/flox/LysMCre) mice. Age-matched WT, SLAMF7 KO, and macrophage-specific SLAMF7 KO mice were employed for in vivo studies, with at least 6 mice per group. All mice were housed under standard conditions, with room temperature maintained at 22 to 24 °C, with relative humidity at 40% to 70%, under a 12-h light/dark cycle, and provided ad libitum access to food and water. All animal protocols were approved by the Animal Care and Use Committee of Zhongshan Hospital, Fudan University (number 2023-004), and all procedures were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Investigators were blinded to the treatment/genotype groups during the experiment and quantification. Mice were randomly assigned to each group using a random number generator.
RNA Extraction and RT-qPCR
Total RNA was extracted from cardiac macrophages or heart tissues using TRIzol reagent (15596018, Invitrogen) in accordance with the manufacturer’s instructions. The concentration and purity of the extracted RNA were measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). Subsequently, 1 μg of total RNA was reverse transcribed into complementary DNA utilizing the Reverse Transcription Reagent Kit (RR036A, Takara Bio Inc, Kusatsu, Japan). The resulting cDNA was amplified via polymerase chain reaction using SYBR Green Universal PCR mix (RR820A, Takara Bio Inc, Kusatsu, Japan) and a CFX96 Real-Time System (Bio-Rad). The thermocycler conditions were as follows: initial denaturation at 95 °C for 10 second, followed by 39 cycles of 60 °C for 30 second for annealing and extension. Gapdh served as the internal reference gene. Relative expression levels or fold changes were calculated using the 2−ΔΔCt method. Primer sequences are provided in Supplemental Table 1.
Statistical analysis
Continuous variables are expressed as mean ± SEM. The Shapiro-Wilk test was used to assess data normality, and the Brown-Forsythe test was used to evaluate the homogeneity of variance. For normally distributed data, comparisons between 2 groups were made using the unpaired two-tailed Student’s t-test. For comparisons among multiple groups, a 1-way analysis of variance followed by Tukey post hoc tests was employed. The 2-way analysis of variance was utilized to analyze differences across several independent variables among multiple groups, with pairwise comparisons conducted using the Tukey test. In cases where the data were not normally distributed, the Mann-Whitney U test was applied for comparisons between 2 groups, and the Kruskal-Wallis test, followed by the false discovery rate method of Benjamini and Hochberg was used for multiple group comparisons. Survival rates were illustrated with Kaplan-Meier survival curves, and differences were analyzed using the log-rank test. Simple linear regressions were conducted for each pair of continuous covariates to calculate the Pearson correlation coefficient (r). A 2-sided P < 0.05 was considered statistically significant. Data analysis was performed using IBM SPSS Statistics (version 23.0), GraphPad Prism version 9.4.0, and R 4.1.0 (R Core Team).
Results
SLAMF7 expression is reduced in Dox-treated mouse hearts
To explore the potential involvement of SLAMFs in DIC, we assessed their expression profiles in both chronic and acute mouse models of DIC. Chronic cardiotoxicity was induced and sustained using a 4-week regimen of low-dose Dox, designed to simulate clinical chemotherapy scenarios (Supplemental Figure 1A). In the chronic DIC model, we observed a significant decrease in the mRNA expression of SLAMF6 and SLAMF7 in cardiac tissues, while SLAMF1 expression was notably elevated compared with control tissues (Figure 1A). These expression trends were consistent in the acute DIC model, which also exhibited reduced levels of SLAMF6 and SLAMF7 (Supplemental Figures 1B and 1C). Notably, SLAMF7 consistently exhibited the most significant down-regulation in response to Dox exposure. Western blot analysis corroborated these findings, revealing a significant reduction in SLAMF7 protein levels in cardiac tissues subjected to Dox treatment (Figure 1B and 1C). Immunofluorescence staining further confirmed the significant decrease in SLAMF7 levels in DIC cardiac tissue compared to saline-treated control tissue (Figure 1D, Supplemental Figure 1D). Additionally, enzyme-linked immunosorbent assays showed a significant drop in serum SLAMF7 concentrations in Dox-treated mice relative to control mice (Figure 1E). Given that Dox exposure causes cardiac injury, characterized by elevated plasma levels of cardiac troponin T (cTnT) and creatine kinase-MB (CK-MB), we investigated the correlation between SLAMF7 levels and these cardiac injury biomarkers. Our results demonstrated a pronounced inverse correlation between SLAMF7 levels and the concentrations of cTnT and CK-MB (Figures 1F and 1G). These findings suggest that SLAMF7 plays a crucial role in the pathogenesis of DIC.
Figure 1.
Down-Regulation of SLAMF7 Expression in Doxorubicin-Induced Cardiotoxicity Hearts
(A) Real-time qPCR analysis of SLAMs mRNA expression in a chronic doxorubicin-induced cardiotoxicity mouse model (n = 6 mice per group). (B and C) Western blot analysis (B) and quantification (C) of SLAMF7 levels in mouse cardiac tissue 4 weeks postsaline or doxorubicin (Dox) treatment (n = 8 mice per group). (D) Immunofluorescence images of SLAMF7 (red) and 4′,6-diamidino-2-phenylindole (DAPI) (blue) staining in cardiac tissue treated with saline or Dox. Negative control for SLAMF7 was included at the right of the image. Scale bar, 20 μm. (E) Serum SLAMF7 levels in Dox-treated mice and control groups (n = 8 mice per group). (F and G) Correlation analysis of serum SLAMF7 levels with cardiac troponin T (cTnT) and creatine kinase-MB (CK-MB) concentrations in a cohort of mice exposed to Dox for 4 weeks. Data are presented as means ± SEM. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. For statistical analyses, data in (A and E) were analyzed using a 2-tailed Student’s t-test; data in (C) were evaluated using the Mann-Whitney U test.
Loss of SLAMF7 exacerbates DIC
Next, we utilized wild-type (WT) and SLAMF7 knockout (KO) mice in a chronic DIC model to investigate the role of SLAMF7 in DIC (Supplemental Figures 2A and 2B). Although SLAMF7 KO mice exhibited an unchanged baseline phenotype in the absence of stress, Dox treatment led to markedly lower survival rates in these mice, with only 40% surviving compared with approximately 60% of WT mice (Supplemental Figure 2C). Echocardiographic evaluation revealed a significant decline in cardiac systolic function in SLAMF7 KO mice, evidenced by reduced left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) 4 weeks after the final Dox administration (Figure 2A). Consistent with impaired heart function, SLAMF7 KO mice exhibited significantly higher levels of cardiac injury markers, including cTnT and CK-MB, compared with WT control mice (Figure 2B). Furthermore, we assessed cardiac atrophy, another aspect of cardiotoxicity. Dox-treated SLAMF7 KO mice demonstrated substantial reductions in heart weight, cardiac dimensions, and cardiomyocyte cross-sectional area relative to the control group (Figures 2C and 2D). Masson’s trichrome and dihydroethidium staining further confirmed extensive interstitial fibrosis and elevated ROS levels in the cardiac tissue of SLAMF7 KO mice following Dox treatment, underscoring an exacerbation of cardiotoxic effects compared to WT mice (Figures 2E and 2F). Collectively, these findings indicate the detrimental impact of SLAMF7 deficiency in DIC.
Figure 2.
SLAMF7 Deficiency Aggravates Doxorubicin-Induced Cardiotoxicity
(A) M-mode echocardiographic analysis showing left ventricular ejection fraction (LVEF) and fractional shortening (LVFS) in wild-type (WT) and SLAMF7 knockout (KO) mice after saline or Dox treatment (n = 8 mice per group). (B) Plasma levels of cTnT and CK-MB in WT and SLAMF7 KO mice after saline or Dox treatment (n = 6 mice per group). (C) Left: Representative hematoxylin and eosin (H and E) staining showing gross heart sizes from WT and SLAMF7 KO mice after saline or Dox treatment. Scale bar, 1,000 μm. Right: Heart weight to tibia length (HW/TL) ratio in WT and SLAMF7 KO mice after Dox treatment (n = 6 mice per group). (D) Left: Representative wheat germ agglutinin staining (WGA) showing cross-sectional area of cardiomyocytes in WT and SLAMF7 KO mice after saline or Dox treatment. Scale bar, 50 μm. Right: Quantification of myocyte cross-sectional area (n = 6 mice per group). (E) Left: Representative Masson’s trichrome staining for myocardial fibrosis in WT and SLAMF7 KO mice after saline or Dox treatment. Scale bar, 50 μm. Right: Quantification of myocardial fibrosis (n = 6 mice per group). (F) Left: Representative dihydroethidium (DHE) staining of reactive oxygen species (ROS) levels in the cardiac tissue of WT and SLAMF7 KO mice after saline or Dox treatment. Scale bar, 50 μm. Right: Quantification of ROS levels (n = 6 mice per group). Data are presented as means ± SEM. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. For statistical analyses, data in (A to F) were analyzed using a 2-way analysis of variance followed by Tukey’s post hoc analysis. Abbreviations as in Figure 1.
Macrophage-specific SLAMF7 deficiency aggravates DIC
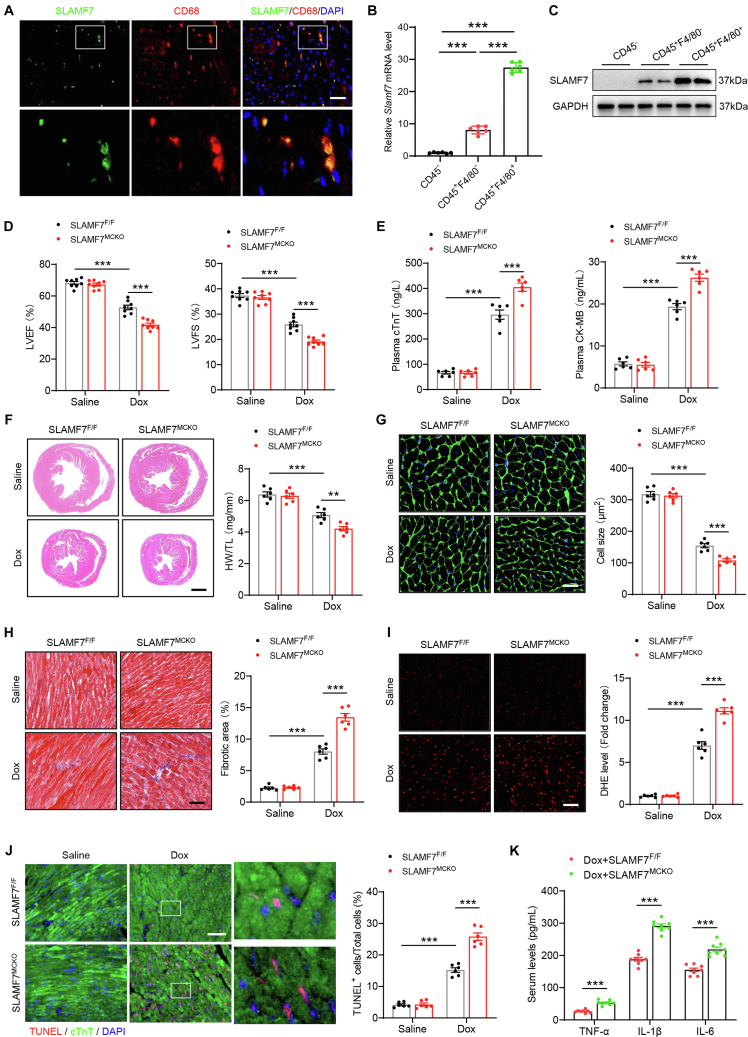
To identify the cellular origin of the down-regulated SLAMF7 observed in DIC cardiac tissues, we performed immunofluorescence staining. The analysis demonstrated colocalization of SLAMF7 with CD68, confirming its expression in macrophages (Figure 3A). To further delineate SLAMF7 expression within distinct cardiac cell populations, we performed enzymatic digestion on DIC mouse hearts and subsequently sorted the cells into CD45−, CD45+F4/80−, and CD45+F4/80+ subsets using fluorescence-activated cell sorting (Supplemental Figure 3A). SLAMF7 mRNA was most abundantly expressed in CD45+F4/80+ macrophages, with comparatively lower levels in CD45+F4/80− cells, and minimal expression in CD45− cells (Figure 3B). This mRNA expression profile was corroborated at the protein level, with robust SLAMF7 expression in CD45+F4/80+ macrophages, moderate expression in CD45+F4/80− cells, and negligible or undetectable levels in CD45− cells (Figure 3C). These observations highlight the predominant expression of SLAMF7 in cardiac macrophages.
Figure 3.
Macrophage-Specific Knockout of SLAMF7 Exacerbates Doxorubicin-Induced Cardiotoxicity in Mice
(A) Immunofluorescence costaining for SLAMF7 (green) and CD68 (red) on cardiac sections from mice 4 weeks post-Dox treatment. Nuclei were counterstained with DAPI (blue). (B) Quantitative analysis of Slamf7 mRNA expression across 3 FACS-sorted cell populations (CD45−, CD45+F4/80−, and CD45+F4/80+) from the cardiac tissue of mice 4 weeks post-Dox treatment. Data are presented as the fold change relative to the CD45− group (n = 4 samples, with hearts from 4-5 mice pooled per sample). (C) Measurement of SLAMF7 protein levels in the 3 FACS-sorted cell populations. Cardiac tissues from 3 mice were used for sorting. (D) M-mode echocardiographic analysis showing LVEF and LVFS in SLAMFF/F and SLAMF7MCKO mice after saline or Dox treatment (n = 8 mice per group). (E) Plasma levels of cardiac injury markers, cTnT and CK-MB, in SLAMFF/F and SLAMF7MCKO mice post-saline or Dox treatment (n = 6 mice per group). (F) Left: Representative hematoxylin and eosin (H and E) staining showing gross heart sizes in SLAMFF/F and SLAMF7MCKO mice after saline or Dox treatment (scale bar, 1,000 μm). Right: Heart weight to tibia length (HW/TL) ratio in SLAMFF/F and SLAMF7MCKO mice postsaline or Dox treatment (n = 8 mice per group). (G) Left: Representative WGA staining showing cardiomyocyte cross-sectional area in SLAMFF/F and SLAMF7MCKO mice after saline or Dox treatment. Scale bar, 50 μm. Right: Quantification of myocyte cross-sectional area (n = 6 mice per group). (H) Left: Representative Masson’s trichrome staining for myocardial fibrosis in SLAMFF/F and SLAMF7MCKO mice after saline or Dox treatment. Scale bar, 50 μm. Right: Quantification of fibrotic area (n = 6 mice per group). (I) Left: Representative DHE staining showing ROS levels in the hearts of SLAMFF/F and SLAMF7MCKO mice after saline or Dox treatment. Scale bar, 50 μm. Right: Quantification of ROS levels (n = 6 mice per group). (J) Left: Representative terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining showing the number of apoptotic cardiomyocytes in SLAMFF/F and SLAMF7MCKO mice after saline or Dox treatment. Scale bar, 50 μm. Right: Quantification of TUNEL+ cardiomyocytes (n = 6 mice per group). (K) Serum protein levels of inflammatory cytokines TNF-α (tumor necrosis factor-alpha), interleukin (IL)-1β (interleukin-1 beta), and IL-6 (interleukin-6) in SLAMFF/F and SLAMF7MCKO mice after saline or Dox treatment (n = 8 mice per group). Data are presented as means ± SEM. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. For statistical analyses, data in (B and K) were analyzed using a one-way analysis of variance followed by Tukey’s post hoc analysis; data in (D to J) were analyzed using a 2-way analysis of variance followed by Tukey’s post hoc analysis. Abbreviations as in Figures 1 and 2.
We then generated macrophage-specific SLAMF7-deficient (SLAMF7MCKO) mice by crossing SLAMF7flox/flox (SLAMF7F/F) with LysM-Cre mice to investigate the importance of cardiac macrophage-derived SLAMF7 in DIC (Supplemental Figures 3B and 3C). Echocardiographic data revealed that the absence of SLAMF7 specifically in macrophages markedly worsened systolic dysfunction induced by Dox, as indicated by reduced LVEF and LVFS (Figure 3D). Additionally, Dox challenge led to elevated levels of plasma cardiac injury markers, including cTnT and CK-MB, with these effects further exacerbated in SLAMF7MCKO mice compared with SLAMF7F/F control mice (Figure 3E). Moreover, SLAMF7MCKO mice exhibited exacerbated Dox-induced cardiac atrophy, as indicated by a decreased heart weight to tibia length (HW/TL) ratio and cardiomyocyte cross-sectional area compared to SLAMF7F/F mice (Figures 3F and 3G). Furthermore, SLAMF7MCKO mice subjected to Dox treatment showed increased levels of fibrosis, ROS accumulation, and a higher number of TUNEL+ cardiomyocytes than their SLAMF7F/F counterparts (Figures 3H to 3J). Serum analysis in the macrophage-specific SLAMF7-deficient DIC mouse model identified significantly elevated levels of proinflammatory cytokines, including TNF-α, IL-1β, and IL-6, in Dox-treated SLAMF7MCKO mice compared to SLAMF7F/F mice (Figure 3K). These findings suggest that cardiac macrophage-derived SLAMF7 plays a crucial protective role in DIC.
SLAMF7 deletion enhances proinflammatory macrophage polarization in DIC mice
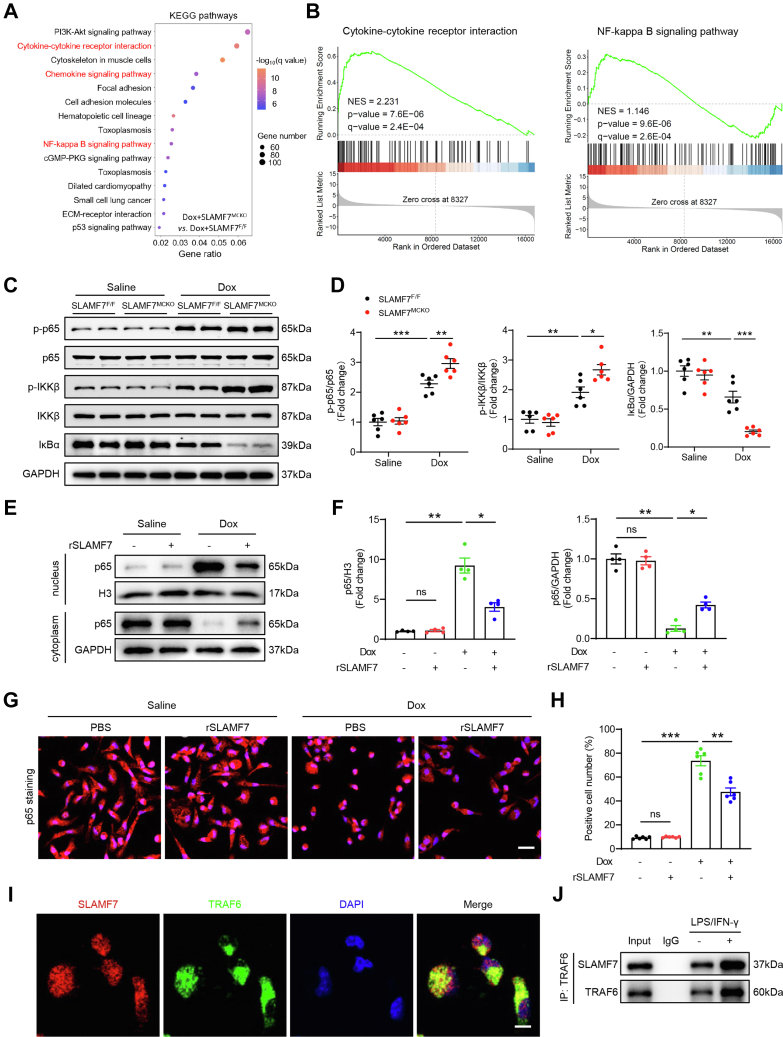
Using systemic and macrophage-specific SLAMF7 KO models, we demonstrated that SLAMF7 deletion exacerbated cardiac dysfunction and atrophy in DIC. We further conducted bulk RNA sequencing (RNA-seq) on cardiac tissue from SLAMF7MCKO and SLAMF7F/F mice 1 day post–final Dox administration, focusing on early post-treatment alterations (Supplemental Figure 4A). The RNA-seq results confirmed successful SLAMF7 deletion in macrophage-specific KO cardiac tissues, indicated by a significant reduction in Slamf7 transcripts compared with SLAMF7F/F control tissue (Supplemental Figure 4B). Compared with SLAMF7F/F tissue, SLAMF7 conditional deletion markedly intensified cardiac inflammatory signaling following Dox administration. Gene ontology analysis of differentially expressed genes in SLAMF7MCKO tissues revealed notable enrichment in pathways related to leukocyte migration, regulation of immune effector process, and chemokine receptor binding (Figure 4A). Additionally, gene set enrichment analysis demonstrated a marked positive association between SLAMF7 deficiency in macrophages and amplified immune response pathways compared with SLAMF7F/F tissues (Figure 4B).
Figure 4.
Effects of SLAMF7 Deletion on Macrophage Polarization in Doxorubicin-Induced Cardiotoxicity
(A) Gene ontology (GO) analysis of differentially expressed genes between SLAMF7MCKO and SLAMF7F/F cardiac tissue post-Dox treatment, with top enriched categories highlighted. (B) Gene set enrichment analysis of 1-day postfinal Dox treatment RNA-seq data for SLAMF7MCKO and SLAMF7F/F mice. (C) Representative flow cytometry plots for the sorting of macrophages from cardiac tissues of the indicated groups. (D) Quantification of M2 macrophages based on flow cytometry analysis (n = 6 mice per group). (E and F) Immunostaining images (E) for iNOS (M1 marker) and CD68 (macrophage marker) and ratios (F) of M1 macrophages (iNOS+CD68+) in cardiac tissues of mice. Scale bar: 20 μm. (G and H) Immunostaining images (G) for Arg1 (M2 marker) and CD68 (macrophage marker) and ratios (H) of M2 macrophages (Arg1+CD68+) in cardiac tissues of mice. Scale bar: 20 μm. (I) Quantification of mRNA levels for M1 marker genes encoding IL-1β, IL-6, TNF-α, iNOS, and MMP9 in macrophages (n = 6 samples per group). (J) Quantification of mRNA levels for M2 marker genes encoding VEGF, ARG1, TGFB, MRC1, and CD36 in macrophages (n = 6 samples per group). Data are presented as means ± SEM. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. For statistical analyses, data in (D, F, and H to J) were analyzed using a 2-tailed Student’s t-test; data in (J) were evaluated using the Mann-Whitney U test. BP = biological process; CC = cellular component; MF = molecular function; NES = normalized enrichment score.
Given these RNA-seq findings and the established role of macrophages in cardiac inflammation, we investigated the impact of SLAMF7 on inflammatory responses and macrophage polarization in DIC mice.26, 27, 28 Flow cytometry analysis revealed that the overall number of cardiac macrophages was similar between SLAMF7F/F and SLAMF7MCKO tissues following Dox treatment (Figure 4C, Supplemental Figure 4C). However, SLAMF7 deficiency in macrophages was associated with a reduced proportion of M2 macrophages in Dox-treated cardiac tissues (Figure 4D). To further validate these findings, we analyzed the cardiac immune cell profile using immunofluorescence staining. An enhanced transition from the M2 (Arg1+CD68+) to M1 (iNOS+CD68+) phenotype was confirmed in cardiac macrophages in SLAMF7MCKO mice after four weeks of Dox injection (Figures 4E to 4H). Furthermore, SLAMF7 deficiency correlated with an increased inflammatory transcriptional signature, as evidenced by up-regulated mRNA levels of key proinflammatory genes, such as Il1b, Il6, tumor necrosis factor-α (Tnfa), inducible nitric oxide synthase iNOS (Nos2), and matrix metallopeptidase 9 (Mmp9). In contrast, major anti-inflammatory genes, such as vascular endothelial growth factor A (Vegfa), Arg1, transforming growth factor beta 1 (tgfb1), Mrc1, and Cd36, were down-regulated (Figures 4I and 4J). Collectively, these findings underscore the pivotal role of SLAMF7 in modulating macrophage-mediated inflammatory responses and phenotype polarization in the context of DIC.
SLAMF7 attenuates inflammatory responses by inhibiting the TRAF6/NF-κB signaling pathway
To further elucidate the mechanisms by which SLAMF7 modulates macrophage phenotype during DIC, we performed Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis on RNA-seq data from cardiac tissues of SLAMF7MCKO and SLAMF7F/F mice 1 day post–Dox treatment. The results revealed significant dysregulation of genes involved in immune responses and nuclear factor-κB (NF-κB) signaling pathway in the cardiac tissues of Dox-treated SLAMF7MCKO mice (Figure 5A). Gene set enrichment analysis further supported these findings, demonstrating that SLAMF7 deletion in macrophages was associated with heightened NF-κB activity and enhanced immune responses in DIC models (Figure 5B, Supplemental Figure 5A). These results imply that SLAMF7 modulates the inflammatory response and cytokine production through its effects on the NF-κB signaling pathway. Subsequent experiments validated the differences in NF-κB signaling between SLAMF7MCKO and SLAMF7F/F mice following Dox treatment. Specifically, SLAMF7 deletion in macrophages resulted in elevated levels of phosphorylated NF-κB p65 (p-p65) (Figures 5C and 5D). Correspondingly, phosphorylated IκB kinase β (p-IKKβ) was markedly increased, while levels of the inhibitor of NF-κBα (IκBα) were significantly reduced in the cardiac tissues of SLAMF7MCKO mice compared to SLAMF7F/F littermates (Figures 5C and 5D). In vitro, Dox-stimulated bone marrow-derived macrophages treated with recombinant SLAMF7 (rSLAMF7) further corroborated these findings, showing significant inhibition of the NF-κB signaling pathway (Supplemental Figures 5B and 5C). Under basal conditions, IκBα binds NF-κB in the cytoplasm, preventing its nuclear translocation. Upon stimulation, IκBα is phosphorylated and degraded, releasing NF-κB to translocate to the nucleus and activate transcription.29,30 Western blotting of nuclear and cytoplasmic extracts and immunofluorescence assays confirmed that rSLAMF7 treatment reduced the nuclear translocation of NF-κB p65 (Figures 5E to 5H). These findings strongly suggest that SLAMF7 contributes to the repression of NF-κB signaling.
Figure 5.
SLAMF7 Facilitates Macrophage M2 Polarization Through TRAF6/NF-κB Signaling
(A) SLAMF7MCKO mice and their SLAMF7F/F littermates were subjected to Dox stimulation, and their hearts were collected for RNA-seq analysis. Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of differentially expressed genes. (B) Gene set enrichment analysis of RNA-seq data. (C) Representative immunoblotting images of nuclear factor κB (NF-κB) p-p65, NF-κB p65, p-IKKα/β, IKKα/β, and IκBα in SLAMF7F/F and SLAMF7MCKO cardiac tissues after saline or Dox treatment. (D) Quantification of panel C (n = 6 mice per group). E. Dox-treated bone marrow-derived macrophages (BMDMs) were stimulated with PBS or SLAMF7 recombinant proteins (rSLAMF7) for 24 hours; their cytoplasmic and nuclear fractions were isolated for western blotting assay. (F) Quantification of panel E (n = 4 samples per group). (G) Representative immunofluorescence images of NF-κB p65 (red) in BMDMs with or without rSLAMF7 treatment. Scale bars, 20 μm. (H) Quantification of panel G (n = 6 samples per group). (I) Confocal images showing the colocalization of SLAMF7 (red) and TRAF6 (green) in BMDMs Scale bar, 5 μm. (J) Coimmunoprecipitation assays confirming the interaction between SLAMF7 and TRAF6 in BMDMs Scale bar, 5 μm. Data are presented as means ± SEM. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. For statistical analyses, data in (D) were analyzed using a two-way analysis of variance followed by Tukey’s post hoc analysis; data in (F and H) were analyzed using a 1-way analysis of variance followed by Tukey’s post hoc analysis.
Next, we explored the potential intermediary signaling molecules that link SLAMF7 to the NF-κB pathway. Within the signaling cascade downstream of SLAM receptors, primary human monocytes express SAPs, including SAP and EAT-2.31 Typically, SLAM family receptors are activated in the presence of SAP family adaptors but exhibit inhibitory effects in their absence. In our study, SLAMF7 predominantly exerted inhibitory effects on macrophages in DIC cardiac tissue via the NF-κB pathway. Previous research has documented the interaction between SLAMF7 and SHIP1 in EAT-2–deficient natural killer (NK) cells.32 Importantly, SHIP1 is known to interact with TNF receptor-associated factor 6 (TRAF6), a key regulator of inflammation caused by its role in activating the NF-κB signaling pathway.23,33 This suggests a plausible mechanistic link whereby SLAMF7 might mitigate inflammatory responses. Subsequent subcellular localization studies indicated that SLAMF7 colocalized with TRAF6 (Figure 5I). Furthermore, coimmunoprecipitation of endogenous SLAMF7 and TRAF6 proteins indicated that they formed a stable complex in cultured macrophages, which was further augmented in response to lipopolysaccharide (LPS)/INF-γ stimulation (Figure 5J). Collectively, these results suggest that SLAMF7 mitigates inflammatory responses in DIC by inhibiting the TRAF6/NF-κB signaling pathway.
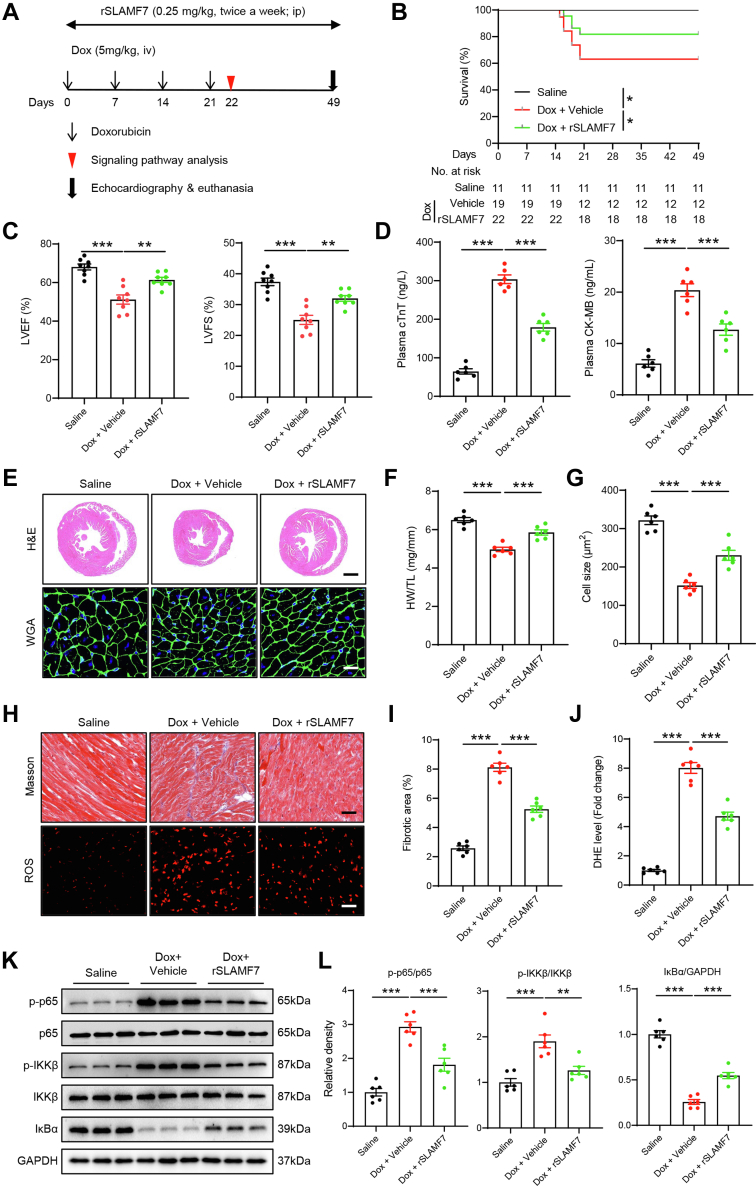
SLAMF7 activation mitigates DIC by suppressing inflammatory responses and cardiac injury
To further evaluate the therapeutic potential of SLAMF7 in vivo, we established a chronic DIC model with the administration of rSLAMF7 protein (Figure 6A).34 Treatment with rSLAMF7 led to a significant improvement in survival rates among DIC mice, alongside a marked reduction in cardiac contractile dysfunction. This was evidenced by increased LVEF and LVFS, coupled with lower levels of cardiac injury markers, including plasma cTnT and CK-MB, following Dox stimulation (Figures 6B to 6D). In alignment with enhanced cardiac function, rSLAMF7 treatment resulted in a noticeable increase in overall heart size and cardiomyocyte cross-sectional area in DIC models compared with control subjects (Figures 6E to 6G). Masson’s trichrome staining and dihydroethidium staining further revealed reduced collagen deposition and lower ROS levels in the cardiac tissues of rSLAMF7-treated mice (Figures 6H to 6J). Additionally, Dox treatment-induced cardiomyocyte apoptosis, as indicated by the number of TUNEL+ cells, was significantly mitigated by rSLAMF7 administration (Supplemental Figures 6A and 6B). Moreover, rSLAMF7-treated mice exhibited a substantial attenuation of inflammatory responses, characterized by reduced levels of cardiac proinflammatory cytokines, decreased infiltration of M1 macrophages, and suppression of NF-κB hyperactivation induced by Dox treatment (Figures 6K and 6L, Supplemental Figures 6B and 6C). In summary, these results suggest that SLAMF7 activation alleviates cardiac inflammation and exerts a protective effect against DIC.
Figure 6.
SLAMF7 Protects Against Doxorubicin-Induced Cardiotoxicity by Inhibiting Inflammation and Cardiac Injury
(A) Schematic diagram illustrating the experimental strategy for SLAMF7 activation. (B) Survival analysis. (C) M-mode echocardiograms illustrating LVEF and LVFS in saline-treated WT and Dox-treated mice with or without rSLAMF7 (n = 8 mice per group). (D) Plasma levels of cTnT and CK-MB in saline-treated WT and Dox-treated mice with or without rSLAMF7 (n = 6 mice per group). (E) Representative H and E (scale bar, 1,000 μm) and WGA (scale bar, 50 μm) staining of saline-treated WT and Dox-treated mice with or without rSLAMF7. (F) HW/TL ratio (n = 6 mice per group). G. Quantification of myocyte cross-sectional area (n = 6 mice per group). (H) Representative Masson’s trichrome (scale bar, 50 μm) staining of myocardial fibrosis and DHE (scale bar, 50 μm) staining indicating ROS levels of saline-treated WT and Dox-treated mice with or without rSLAMF7. (I) Quantification of fibrotic area (n = 6 mice per group). (J) Quantification of ROS levels (n = 6 mice per group). (K) Representative immunoblotting images of NF-κB signaling in saline-treated WT and Dox-treated mice with or without rSLAMF7. (L) Quantification of K (n = 6 mice per group). Data are presented as means ± SEM. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Statistical analyses for (C, D, F, G, I, J, and L) were analyzed using a one-way analysis of variance followed by Tukey’s post hoc analysis. Abbreviations as in Figures 2 and 3.
TRAF6 inhibition mediates the beneficial effects of SLAMF7 activation in DIC
To investigate whether the TRAF6/NF-κB axis serves as a downstream mediator of the cardioprotective effects elicited by SLAMF7 activation against DIC in vivo, we utilized the selective TRAF6 antagonist C25-140.35 If SLAMF7 and TRAF6 function within the same signaling cascade, concurrent inhibition should yield similar protective effects as those observed with TRAF6 inhibition alone. To test this, DIC mice were pretreated with C25-140 before the administration of rSLAMF7. As anticipated, C25-140 treatment alone significantly improved survival rates, enhanced LVEF and LVFS, decreased plasma cTnT and CK-MB levels, enlarged cardiomyocyte cross-sectional area, and attenuated fibrosis and ROS levels (Figures 7A to 7G, Supplemental Figures 7A to 7D). Notably, when TRAF6 was inhibited, subsequent rSLAMF7 treatment did not confer any additional cardioprotective benefits beyond those achieved with TRAF6 inhibition alone (Figures 7A to 7G, Supplemental Figures 7A to 7D). Additionally, C25-140 administration also attenuated cardiomyocyte apoptosis, reduced cardiac proinflammatory cytokine levels, and decreased M1 macrophages infiltration (Figures 7H to 7J). This suppression of cardiac inflammation was further substantiated by decreased NF-κB pathway activation, characterized by lower phosphorylated p65 and IKKβ levels and increased IκBα expression in C25-140–treated mice, irrespective of rSLAMF7 treatment (Figures 7K and 7L). Together, these findings suggest that the TRAF6/NF-κB axis plays a pivotal role in mediating the cardioprotective effects of SLAMF7 by modulating macrophage polarization and mitigating myocardial injury in the DIC mouse model.
Figure 7.
TRAF6 Inhibition Underlies the Beneficial Effects of SLAMF7 Activation in Doxorubicin-Induced Cardiotoxicity Mice
(A) M-mode echocardiograms illustrating LVEF and LVFS in WT and doxorubicin-induced cardiotoxicity mice treated with vehicle or TRAF6 inhibitor C25-140, with or without rSLAMF7 (n = 8 mice per group). (B) Plasma cTnT and CK-MB levels in WT and DIC mice treated with vehicle or C25-140, with or without rSLAMF7 (n = 6 mice per group). (C) Representative hematoxylin and eosin (H and E) staining showing gross heart sizes in WT and DIC mice treated with vehicle or C25-140, with or without rSLAMF7. Scale bar, 1,000 μm. (D) HW/TL ratio in WT and DIC mice treated with vehicle or C25-140, with or without rSLAMF7 (n = 6 mice per group). (E) Quantification of fibrotic area in WT and doxorubicin-induced cardiotoxicity mice treated with vehicle or C25-140, with or without rSLAMF7 (n = 6 mice per group). (F) Representative dihydroethidium (DHE) staining indicating reactive oxygen species (ROS) levels in WT and DIC mice treated with vehicle or C25-140, with or without rSLAMF7. Scale bar, 50 μm. (G) Quantification of ROS levels (n = 6 mice per group). (H) Quantification of TUNEL+ cardiomyocytes (n = 6 mice per group). (I) Serum levels of inflammatory cytokines TNF-α, IL-1β, and IL-6 in WT and doxorubicin-induced cardiotoxicity mice treated with vehicle or C25-140, with or without rSLAMF7 (n = 8 mice per group). (J) Quantification of M1 macrophages based on flow cytometry analysis (n = 6 mice per group). (K) Representative immunoblotting images of NF-κB signaling in WT and doxorubicin-induced cardiotoxicity mice treated with vehicle or C25-140, with or without rSLAMF7. (L) Quantification of K (n = 6 mice per group). Data are presented as mean ± SEM. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. For statistical analyses, data in (A, B, D, E, G to J, and L) were analyzed using a 1-way analysis of variance followed by Tukey’s post hoc analysis. Abbreviations as in Figures 2 and 3.
Discussion
In this study, we demonstrate that SLAMF7 expression in macrophages is notably down-regulated in response to Dox treatment. Our data indicate that SLAMF7 interacts with TRAF6, thereby suppressing NF-κB signaling and reducing proinflammatory macrophage polarization in the context of DIC (Supplemental Figure 8). Utilizing both global and macrophage-specific SLAMF7 KO mice, along with rSLAMF7 agonist, we provide compelling evidence that SLAMF7 exerts a protective role against DIC by mitigating inflammatory responses and preventing cardiac injury. In summary, our study elucidates the inhibitory role of SLAMF7 in Dox-induced inflammation and clarifies the underlying signaling mechanism, offering valuable insights into potential therapeutic targets for combating DIC.
SLAMF7, originally identified as a surface marker on NK cells, has garnered significant attention for its multifaceted roles in oncology and immunology.23,25,36 In multiple myeloma (MM), SLAMF7 has emerged as a critical therapeutic target, with clinical trials demonstrating the efficacy of elotuzumab, a monoclonal antibody targeting SLAMF7, particularly in combination therapies for relapsed or refractory MM.37 Moreover, SLAMF7-directed chimeric antigen receptor cells have shown substantial promise in preclinical MM models by effectively targeting and eradicating SLAMF7-expressing tumor cells.38 In addition, SLAMF7 plays an essential role in the phagocytosis of hematopoietic tumor cells through interactions with the Mac-1 integrin on phagocytes, thereby enhancing tumor cell clearance and immune surveillance.36 Beyond hematological malignancies, SLAMF7 is implicated in various immune responses, including those against HIV infection and polymicrobial sepsis,23,39 underscoring its broad regulatory functions. Given its multifaceted roles, SLAMF7 represents a valuable target for therapeutic interventions in both oncology and inflammatory diseases. In this context, our study presents novel evidence implicating SLAMF7 in the pathogenesis of DIC. We observed significant reduction in SLAMF7 expression in macrophages following Dox treatment. Both global and macrophage-specific SLAMF7 deletions significantly exacerbated cardiac toxicity in Dox-treated mice, primarily by amplifying inflammatory responses and intensifying cardiac injury. Further mechanistic analysis revealed that SLAMF7 attenuated the secretion of proinflammatory cytokines in vivo and in vitro by inhibiting the NF-κB signaling pathway. The interaction between SLAMF7 and TRAF6 appears to be a critical mechanism through which SLAMF7 suppresses NF-κB activation, thereby reducing inflammation and promoting a shift toward an anti-inflammatory M2 macrophage phenotype. Importantly, activation of SLAMF7 signaling with rSLAMF7 effectively mitigated Dox-induced cardiac toxicity and significantly improved survival in treated mice. In summary, our findings unveil a novel protective role for SLAMF7 in DIC by suppressing macrophage-mediated inflammatory responses and fostering a balanced immune environment.
Recent advances in understanding the role of inflammation and macrophage involvement in DIC have greatly expanded our knowledge of the disease's pathogenesis. Inflammatory responses are crucial in DIC, characterized by marked immune cell infiltration and the production of proinflammatory cytokines within myocardial tissue.40,41 Dox treatment skews the balance by increasing M1 macrophage populations while reducing M2 macrophages.10,13,42 The dynamic interplay between proinflammatory M1 macrophages derived from circulating monocytes and reparative M2 macrophages is influenced by the cytokine milieu and signaling pathways. Research indicates that M1 macrophages dominate the early phase of cardiac injury, followed by a subsequent increase in reparative M2 macrophages, whose proliferation is dependent on scavenger receptor A1.26 Immunomodulatory strategies that target the M1/M2 macrophage balance could be pivotal in resolving cardiac injury.43,44 Modulating macrophage polarization, through cytokines like IL-22, which governs macrophage differentiation,44 or natural compounds like glabridin, which influences gut microbiota and macrophage polarization, suggests that dietary interventions may hold promise for managing DIC.45 These findings underscore the protective role of M2 macrophages in alleviating DIC and highlight potential therapeutic avenues. We found that SLAMF7 interacts with TRAF6 to inhibit NF-κB signaling, thereby limiting proinflammatory macrophage polarization and promoting a shift toward an anti-inflammatory M2 phenotype. This regulation by SLAMF7 is crucial in mitigating Dox-induced cardiac injury and inflammation.
The biological activity of SLAMF7 is primarily facilitated by its ability to recruit SH2 domain-containing molecules to its immunoreceptor tyrosine-based switch motifs, which serve as binding sites for SAP family adaptors.16,46 Without SAP adaptors, SLAMF7 impairs NK cell function by engaging inhibitory effectors like SHP-1, SHP-2, and SHIP1.47 This dual functionality allows SLAMF7 to either activate or inhibit immune cell responses, contingent upon the cellular context and the availability of specific effector proteins.23,25,36,48 Factors, such as receptor density, cellular compartmentalization, and potentially unidentified binding partners, further influence these roles, highlighting the complex regulatory capacity of SLAMF7 in immune regulation. Our study shows that SLAMF7 mediates an inhibitory effect on immune response, likely through its interaction with inhibitory effectors. Previous reports suggest that the SLAMF7-SHIP1 interaction can impede the autoubiquitination of TRAF6, thereby diminishing TLR-triggered inflammatory responses by suppressing NF-κB and MAPK activation in macrophages.23 Consistent with this mechanism, our RNA-seq results indicated that SLAMF7 deficiency leads to enhanced activation of NF-κB and related inflammatory pathways. These findings led us to explore the role of TRAF6 in SLAMF7-mediated attenuation of DIC. TRAF6, a critical adaptor molecule within multiple signaling pathways, interacts with a range of substrates and plays a significant role in cardiovascular diseases, largely influenced by specific downstream signaling factors.49,50 Among these, the NF-κΒ pathway is a principal downstream target of TRAF6 and is critically involved in the pathological processes of cardiac conditions.23,30 The NF-κB pathway is integral to macrophage-mediated inflammatory responses, as well as macrophage proliferation, differentiation, and survival.51 Notably, SLAMF7 deficiency led to significant activation of canonical NF-κB signaling, as shown by increased levels of phosphorylated IKKα/β and p65, along with reduced IκBα levels. These results indicate that SLAMF7 suppresses proinflammatory macrophage polarization by inhibiting TRAF6/NF-κB signaling activation. Furthermore, we identified a direct interaction between SLAMF7 and TRAF6 in murine macrophages. The inhibition of TRAF6 activation underlies the beneficial effects observed in DIC models upon SLAMF7 activation. Collectively, our experiments underscore the critical role of TRAF6 in mediating the cardioprotective effects associated with SLAMF7 regulation.
Study limitations
First, we primarily focused on the inhibition of the TRAF6/NF-κB signaling-mediated inflammatory response as the key mechanism by which SLAMF7 influences DIC. However, other mechanisms, including phagocytosis and apoptosis, may be crucial in the context of SLAMF7 regulation. Additionally, we did not include a macrophage overexpression model in our study, which could provide additional insights into the direct effects of SLAMF7 on macrophage function. Last, because our study only included male mice, future research should incorporate female mice to explore potential sex differences in the impact of SLAMF7 deficiency on DIC.
Conclusions
Our study provides compelling evidence that SLAMF7 serves as a crucial negative regulator of DIC. This study sheds light on the regulatory mechanisms of SLAMF7 in DIC and elucidates the interaction between SLAMF7 and TRAF6 in transmitting downstream signals, offering promising insights for the development of therapeutic strategies targeting DIC.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: This study provides a comprehensive understanding of the protective role of macrophage-derived SLAMF7 in DIC. SLAMF7 mitigates DIC by inhibiting TRAF6-mediated NF-κB signaling, thereby reducing oxidative stress and proinflammatory cytokine production. The absence of SLAMF7 exacerbates DIC and inflammatory responses, highlighting its critical function in maintaining cardiac health under chemotherapeutic stress.
TRANSLATIONAL OUTLOOK: Future studies should explore the therapeutic potential of targeting SLAMF7, either through recombinant SLAMF7 administration or SLAMF7-TRAF6 pathway modulation, in preventing and treating DIC. Additionally, this study suggests that enhancing SLAMF7 activity could be a viable strategy for managing other inflammatory cardiovascular conditions, such as myocarditis and heart failure induced by cancer therapies.
Funding Support and Author Disclosures
This work was supported by the National Natural Science Foundation of China (T2288101, 82227803, 82071933, 82300292, and 82302213), National Key Research and Development Program of China (2021YFC2500500), and Natural Science Foundation of Shanghai (21ZR1413500, 20Y11912000, and 20JC1418400). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgements
The authors extend their gratitude to Cheng Wang (Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences) for their invaluable assistance with mouse colony management and for conducting animal experiments.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For an expanded Methods section as well as supplemental figures and a table, please see the online version of this paper.
Contributor Information
Xianhong Shu, Email: shu.xianhong@zs-hospital.sh.cn.
Junbo Ge, Email: jbge@zs-hospital.sh.cn.
Appendix
References
- 1.Wenningmann N., Knapp M., Ande A., et al. Insights into doxorubicin-induced cardiotoxicity: molecular mechanisms, preventive strategies, and early monitoring. Mol Pharmacol. 2019;96(2):219–232. doi: 10.1124/mol.119.115725. [DOI] [PubMed] [Google Scholar]
- 2.Mehta L.S., Watson K.E., Barac A., et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the american heart association. Circulation. 2018;137(8):e30–e66. doi: 10.1161/CIR.0000000000000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang S., Liu X., Bawa-Khalfe T., et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18(11):1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 4.Lipshultz S.E., Franco V.I., Miller T.L., et al. Cardiovascular disease in adult survivors of childhood cancer. Annu Rev Med. 2015;66:161–176. doi: 10.1146/annurev-med-070213-054849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dresdale A.R., Barr L.H., Bonow R.O., et al. Prospective randomized study of the role of N-acetyl cysteine in reversing doxorubicin-induced cardiomyopathy. Am J Clin Oncol. 1982;5(6):657–663. doi: 10.1097/00000421-198212000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Epelman S., Lavine K.J., Beaudin A.E., et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40(1):91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farbehi N., Patrick R., Dorison A., et al. Single-cell expression profiling reveals dynamic flux of cardiac stromal, vascular and immune cells in health and injury. eLife. 2019;8 doi: 10.7554/eLife.43882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilgendorf I., Frantz S., Frangogiannis N.G. Repair of the infarcted heart: cellular effectors, molecular mechanisms and therapeutic opportunities. Circ Res. 2024;134(12):1718–1751. doi: 10.1161/CIRCRESAHA.124.323658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halade G.V., Lee D.H. Inflammation and resolution signaling in cardiac repair and heart failure. EBioMedicine. 2022;79 doi: 10.1016/j.ebiom.2022.103992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vannella K.M., Wynn T.A. Mechanisms of organ injury and repair by macrophages. Annu Rev Physiol. 2017;79:593–617. doi: 10.1146/annurev-physiol-022516-034356. [DOI] [PubMed] [Google Scholar]
- 11.Wynn T.A., Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe S., Alexander M., Misharin A.V., et al. The role of macrophages in the resolution of inflammation. J Clin Invest. 2019;129(7):2619–2628. doi: 10.1172/JCI124615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavine K.J., Pinto A.R., Epelman S., et al. The macrophage in cardiac homeostasis and disease: JACC macrophage in CVD series (Part 4) J Am Coll Cardiol. 2018;72(18):2213–2230. doi: 10.1016/j.jacc.2018.08.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Amerongen M.J., Harmsen M.C., van Rooijen N., et al. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol. 2007;170(3):818–829. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao Y., Qian N., Xu J., et al. The roles of macrophages in heart regeneration and repair after injury. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.744615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannons J.L., Tangye S.G., Schwartzberg P.L. SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- 17.Lonial S., Dimopoulos M., Palumbo A., et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373(7):621–631. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 18.Wu N., Veillette A. SLAM family receptors in normal immunity and immune pathologies. Curr Opin Immunol. 2016;38:45–51. doi: 10.1016/j.coi.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Engel P., Eck M.J., Terhorst C. The SAP and SLAM families in immune responses and X-linked lymphoproliferative disease. Nat Rev Immunol. 2003;3(10):813–821. doi: 10.1038/nri1202. [DOI] [PubMed] [Google Scholar]
- 20.Ma C.S., Nichols K.E., Tangye S.G. Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules. Annu Rev Immunol. 2007;25:337–379. doi: 10.1146/annurev.immunol.25.022106.141651. [DOI] [PubMed] [Google Scholar]
- 21.Veillette A., Dong Z., Latour S. Consequence of the SLAM-SAP signaling pathway in innate-like and conventional lymphocytes. Immunity. 2007;27(5):698–710. doi: 10.1016/j.immuni.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Kim J.R., Horton N.C., Mathew S.O., et al. CS1 (SLAMF7) inhibits production of proinflammatory cytokines by activated monocytes. Inflamm Res. 2013;62(8):765–772. doi: 10.1007/s00011-013-0632-1. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y., Wang Q., Li M., et al. SLAMF7 regulates the inflammatory response in macrophages during polymicrobial sepsis. J Clin Invest. 2023;133(6) doi: 10.1172/JCI150224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu S., Chen Y., Lao J., et al. Signaling lymphocytic activation molecule family-7 alleviates corneal inflammation by promoting M2 polarization. J Infect Dis. 2021;223(5):854–865. doi: 10.1093/infdis/jiaa445. [DOI] [PubMed] [Google Scholar]
- 25.Simmons D.P., Nguyen H.N., Gomez-Rivas E., et al. SLAMF7 engagement superactivates macrophages in acute and chronic inflammation. Sci Immunol. 2022;7(68) doi: 10.1126/sciimmunol.abf2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H., Xu A., Sun X., et al. Self-maintenance of cardiac resident reparative macrophages attenuates doxorubicin-induced cardiomyopathy through the SR-A1-c-Myc axis. Circ Res. 2020;127(5):610–627. doi: 10.1161/CIRCRESAHA.119.316428. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y., Wu M., Zhong C., et al. M2-like macrophages transplantation protects against the doxorubicin-induced heart failure via mitochondrial transfer. Biomater Res. 2022;26(1):14. doi: 10.1186/s40824-022-00260-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gambardella J., Santulli G., Fiordelisi A., et al. Infiltrating macrophages amplify doxorubicin-induced cardiac damage: role of catecholamines. Cell Mol Life Sci. 2023;80(11):323. doi: 10.1007/s00018-023-04922-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frati G., Schirone L., Chimenti I., et al. An overview of the inflammatory signalling mechanisms in the myocardium underlying the development of diabetic cardiomyopathy. Cardiovascular research. 2017;113(4):378–388. doi: 10.1093/cvr/cvx011. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto M., Gohda J., Akiyama T., et al. TNF receptor-associated factor 6 (TRAF6) plays crucial roles in multiple biological systems through polyubiquitination-mediated NF-κB activation. Proc Jpn Acad Ser B Phys Biol Sci. 2021;97(4):145–160. doi: 10.2183/pjab.97.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dragovich M.A., Mor A. The SLAM family receptors: Potential therapeutic targets for inflammatory and autoimmune diseases. Autoimmun Rev. 2018;17(7):674–682. doi: 10.1016/j.autrev.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cruz-Munoz M.E., Dong Z., Shi X., et al. Influence of CRACC, a SLAM family receptor coupled to the adaptor EAT-2, on natural killer cell function. Nat Immunol. 2009;10(3):297–305. doi: 10.1038/ni.1693. [DOI] [PubMed] [Google Scholar]
- 33.Li M., Xia P., Du Y., et al. T-cell immunoglobulin and ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand engagement suppresses interferon-γ production of natural killer cells via β-arrestin 2-mediated negative signaling. J Biol Chem. 2014;289(25):17647–17657. doi: 10.1074/jbc.M114.572420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumaresan P.R., Lai W.C., Chuang S.S., et al. CS1, a novel member of the CD2 family, is homophilic and regulates NK cell function. Mol Immunol. 2002;39(1-2):1–8. doi: 10.1016/s0161-5890(02)00094-9. [DOI] [PubMed] [Google Scholar]
- 35.Brenke J.K., Popowicz G.M., Schorpp K., et al. Targeting TRAF6 E3 ligase activity with a small-molecule inhibitor combats autoimmunity. J Biol Chem. 2018;293(34):13191–13203. doi: 10.1074/jbc.RA118.002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen J., Zhong M.C., Guo H., et al. SLAMF7 is critical for phagocytosis of haematopoietic tumour cells via Mac-1 integrin. Nature. 2017;544(7651):493–497. doi: 10.1038/nature22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mai E.K., Goldschmid H., Miah K., et al. Elotuzumab, lenalidomide, bortezomib, dexamethasone, and autologous haematopoietic stem-cell transplantation for newly diagnosed multiple myeloma (GMMG-HD6): results from a randomised, phase 3 trial. The Lancet Haematology. 2024;11(2):e101–e113. doi: 10.1016/S2352-3026(23)00366-6. [DOI] [PubMed] [Google Scholar]
- 38.O'Neal J., Ritchey J.K., Cooper M.L., et al. CS1 CAR-T targeting the distal domain of CS1 (SLAMF7) shows efficacy in high tumor burden myeloma model despite fratricide of CD8+CS1 expressing CAR-T cells. Leukemia. 2022;36(6):1625–1634. doi: 10.1038/s41375-022-01559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Connell P., Pepelyayeva Y., Blake M.K., et al. SLAMF7 is a critical negative regulator of IFN-α-mediated CXCL10 production in chronic HIV infection. J Immunol. 2019;202(1):228–238. doi: 10.4049/jimmunol.1800847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaczmarek A., Krysko O., Heyndrickx L., et al. TNF/TNF-R1 pathway is involved in doxorubicin-induced acute sterile inflammation. Cell Death Dis. 2013;4(12) doi: 10.1038/cddis.2013.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krysko D.V., Kaczmarek A., Krysko O., et al. TLR-2 and TLR-9 are sensors of apoptosis in a mouse model of doxorubicin-induced acute inflammation. Cell Death Differ. 2011;18(8):1316–1325. doi: 10.1038/cdd.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams J.W., Giannarelli C., Rahman A., et al. Macrophage biology, classification, and phenotype in cardiovascular disease: JACC macrophage in CVD series (Part 1) J Am Coll Cardiol. 2018;72(18):2166–2180. doi: 10.1016/j.jacc.2018.08.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mittal M., Siddiqui M.R., Tran K., et al. Reactive oxygen species in inflammation and tissue injury. Antioxidants & redox signaling. 2014;20(7):1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye J., Wang Y., Xu Y., et al. Interleukin-22 deficiency alleviates doxorubicin-induced oxidative stress and cardiac injury via the p38 MAPK/macrophage/Fizz3 axis in mice. Redox Biol. 2020;36 doi: 10.1016/j.redox.2020.101636. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Huang K., Liu Y., Tang H., et al. Glabridin prevents doxorubicin-induced cardiotoxicity through gut microbiota modulation and colonic macrophage polarization in mice. Front Pharmacol. 2019;10:107. doi: 10.3389/fphar.2019.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sidorenko S.P., Clark E.A. The dual-function CD150 receptor subfamily: the viral attraction. Nat Immunol. 2003;4(1):19–24. doi: 10.1038/ni0103-19. [DOI] [PubMed] [Google Scholar]
- 47.Wilson T.J., Garner L.I., Metcalfe C., et al. Fine specificity and molecular competition in SLAM family receptor signalling. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0092184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan F., Wei J., Cheng Y., et al. SLAMF7 Promotes foam cell formation of macrophage by suppressing NR4A1 expression during carotid atherosclerosis. Inflammation. 2024;47(2):530–542. doi: 10.1007/s10753-023-01926-y. [DOI] [PubMed] [Google Scholar]
- 49.Abdullah M., Berthiaume J.M., Willis M.S. Tumor necrosis factor receptor-associated factor 6 as a nuclear factor kappa B-modulating therapeutic target in cardiovascular diseases: at the heart of it all. Transl Res. 2018;195:48–61. doi: 10.1016/j.trsl.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walsh M.C., Lee J., Choi Y. Tumor necrosis factor receptor- associated factor 6 (TRAF6) regulation of development, function, and homeostasis of the immune system. Immunol Rev. 2015;266(1):72–92. doi: 10.1111/imr.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oeckinghaus A., Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1(4) doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.