Abstract
Introduction
Reduced corneal sensation in individuals with type 2 diabetes mellitus (T2DM) leads to a dissociation between dry eye disease (DED) signs and symptoms, thereby affecting diagnostic accuracy. This study aimed to investigate the correlation between ocular surface signs and diabetic peripheral neuropathy (DPN) symptoms in patients with T2DM-associated DED.
Methods
The Michigan Neuropathy Screening Instrument Questionnaire (MNSIQ) was used to categorize patients with T2DM into MNSIQ-DPN and non-DPN groups. Ocular irritation symptoms were evaluated using the Ocular Surface Disease Index (OSDI) questionnaire. Ocular surface lesions were assessed via Cochet–Bonnet esthesiometry, corneal fluorescein staining (CFS), the Schirmer I tear test (SIT), tear meniscus height (TMH), noninvasive keratography break-up time (NIKf-BUT), and the meibomian gland loss (MGL) grade detected by OCULUS. Corneal nerve fiber parameters were evaluated using in vivo confocal microscopy (IVCM).
Results
A total of 116 patients with T2DM, comprising 76 non-DPN patients and 40 MNSIQ-DPN patients, along with 51 age-matched participants without diabetes, were enrolled. Although OSDI scores were equivalent between MNSIQ-DPN patients and non-DPN patients, MNSIQ-DPN patients presented significantly more severe CFS (p < 0.001), meibomian gland dysfunction (MGD) (p < 0.001), corneal nerve fiber loss (p < 0.001), sensory dysfunction (p = 0.02), and corneal microneuromas (p < 0.001). The MNSIQ score was significantly positively correlated with CFS (p < 0.001); MGD (p < 0.01); corneal nerve fiber loss, including corneal nerve fiber density and length and branch density, in the paracentral (all p < 0.001) and inferior-whorl areas (p < 0.01, p < 0.05 and p < 0.01, respectively); and corneal microneuromas, characterized by increased microneuroma numbers (p < 0.001) and areas (p < 0.001) in these regions.
Conclusion
MNSIQ scores were significantly and robustly correlated with the presence of corneal epithelial defects, MGD, and nerve fiber loss in patients with T2DM. These findings suggest that DPN is a critical factor in diabetic ocular surface complications, highlighting the importance of the MNSIQ for assessing these conditions.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40123-025-01150-x.
Keywords: Diabetes, Dry eye disease, Diabetic peripheral neuropathy, Microneuroma, Meibomian gland dysfunction, In vivo confocal microscopy
Key Summary Points
| Why carry out this study? |
| Diabetes-associated dry eye disease (DED) is the most common ocular surface condition in diabetes mellitus. Delayed diagnosis and intervention, often due to corneal hypoesthesia, can lead to diabetic neurotrophic keratopathy (DNK). |
| This study used the Michigan Neuropathy Screening Instrument Questionnaire (MNSIQ) to analyze the correlation between peripheral neuropathy symptoms and dry eye signs in patients with type 2 diabetes, highlighting the importance of MNSIQ as an indicator for DED. |
| What was learned from the study? |
| The MNSIQ reliably evaluated corneal epithelial integrity, lacrimal functional unit (LFU) function, and corneal nerve density and function in patients with type 2 diabetes-related DED. |
| Peripheral corneal neuropathy is crucial in diabetic ocular surface complications, highlighting the importance of including the MNSIQ score in routine ophthalmic evaluations. |
Introduction
Diabetes represents one of the most rapidly escalating health challenges of the twenty-first century, with the global prevalence of diabetes among adults increasing more than threefold over the past two decades [1]. In 2021, approximately 10.5% of adults worldwide had diabetes, totaling 536.6 million individuals [2]. By 2022, this number had increased to an estimated 828 million adults, representing an increase of 630 million since 1990 [3]. Prolonged hyperglycemia in diabetic patients leads to numerous complications affecting almost every organ system, including the vision system [4]. Diabetes-associated dry eye disease (DED) is the most common clinical condition affecting the ocular surface of patients with diabetes mellitus. A lack of timely intervention can lead to diabetic neurotrophic keratopathy (DNK) [5], which is characterized by an irregular, fragile cornea; superficial punctate keratopathy; delayed and incomplete wound healing; and persistent corneal epithelial erosion [4]. Corneal nerve damage, as evidenced by decreased sensitivity, often leads to the underdiagnosis and misdiagnosis of early-stage diabetes-associated DED. Since DNK typically remains undetected until significant symptoms manifest, irreversible damage may have already occurred at the time of diagnosis [6]. Consequently, early diagnosis, accurate assessment, and timely intervention are crucial for preventing the progression of DNK.
Diabetic peripheral neuropathy (DPN) is the most common complication of diabetes and affects more than 50% of patients with diabetes; DPN has various subtypes, with distal symmetric polyneuropathy (DSPN) being the most common form [7]. The primary clinical manifestation of DSPN is sensory neuropathy. Early symptoms include progressive symmetric numbness, tingling, and burning sensations in the limbs. In advanced stages, patients may experience proximal numbness; in severe cases, muscle weakness may also be observed [8, 9]. Diabetes-associated DED, a recognized ocular manifestation of DSPN, is identified as a leading cause of corneal morbidity in diabetic patients. The lacrimal functional unit (LFU), which includes the conjunctiva, cornea, and both the main and accessory lacrimal glands, operates under precise neural regulation [10]. DPN disrupts the tear film and impairs the function of the lacrimal and meibomian glands, resulting in tear instability, increased evaporation, inflammation, and epithelial defects [4]. Despite its importance, the prevalence of diabetes-associated DED disease in clinical settings is frequently underestimated because of the challenges associated with accurate assessment.
In vivo confocal microscopy (IVCM) is a noninvasive imaging technique in which high-quality images of the corneal C-fibers in the subbasal nerve plexus are acquired. Studies have shown that IVCM is comparable to measurement of intraepidermal nerve fiber density (IENFD) in biopsy samples in terms of diagnostic performance for clinical-level DPN. Considering the known relationship between damage to these fibers and DPN, the potential for their use as a surrogate biomarker for DPN has been identified [11, 12]. The major ocular surface complications in diabetic patients include DED, corneal nerve degeneration, and recurrent epithelial defects. These conditions complicate the accurate assessment of clinical lesion severity compared with individuals without diabetes. Given the established association between DNK and DPN, further investigation into the interaction between DPN and ocular surface lesions can assist physicians in managing these patients more effectively. Currently, the overlap and interaction between DPN and ocular surface lesions have been specifically addressed in only a few studies.
Our objective was to investigate the correlation between signs and symptoms of ocular surface lesions and symptoms of DPN in patients with type 2 diabetes mellitus (T2DM)-associated DED.
Methods
Study Design and Study Population
This prospective, observational, cross-sectional study was conducted at Qingdao Eye Hospital. The study protocol was approved by the local Committee of Qingdao Eye Hospital Research Ethics (approval number: 2019-33), and the procedures were performed in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all participants or their legal guardians.
Consecutive patients who were diagnosed with T2DM and visited the eye clinic of Qingdao Eye Hospital from June 2019 to March 2022 were enrolled in the study. The exclusion criteria were a history of ocular surgery; the use of ocular topical treatments within 1 month prior to the study; or a diagnosis of systemic diseases such as Sjögren’s syndrome, rheumatoid arthritis, systemic lupus erythematosus, or ankylosing spondylitis, which are considered independent risk factors for ocular surface disease. Patients from whom informed consent was not obtained were also excluded. The subjects without diabetes were volunteers who were free of any health problems, were not on any long-term medications, and had no corneal pathology or history of ocular or corneal surgery.
The Michigan Neuropathy Screening Instrument Questionnaire (MNSIQ) is an effective screening tool for evaluating DPN, consisting of 15 yes-or-no questions pertaining to foot sensation, such as pain, numbness, and temperature sensitivity [13, 14]. These questions were specifically chosen to reflect the most commonly reported symptoms of DPN. Additionally, two questions address non-neuropathic and primarily vascular symptoms. In the MNSIQ, the score is calculated by summing the responses indicating abnormal sensations, with a higher score signifying greater severity of neuropathy [15]. A score of ≥ 4, which has been validated as both specific and sensitive for the diagnosis of DPN, was used to define the presence of DPN (MNSIQ-defined DPN). The MNSIQ is a reliable and valid instrument in screening for diabetic neuropathy; the intraclass correlation coefficient (ICC) values were validated in both the European Portuguese version [16] and the Brazilian Portuguese version [17], which were 0.91 (95% CI 0.87–0.94) and 0.90 (95% CI 0.82–0.95), respectively. These high ICC values indicate the questionnaire's excellent stability. In addition, the internal consistency was verified by Cronbach's α coefficient (0.73, 95% CI 0.66–0.81) in this study. To prevent bias from contaminating the results of subjective tests, MNSIQ evaluation was prioritized before Ocular Surface Disease Index (OSDI) assessments, ocular examinations, and glycated hemoglobin (HbA1c) detection, and two assessors were randomly assigned to ensure impartiality.
In addition, to prevent bias from contaminating the data, we implemented the following masking procedures. First, prior to patient inclusion, a unified operation manual for ocular examinations was developed, and all investigators were trained to ensure consistency in measurement tools and methods. Second, we consecutively enrolled dry eye patients with type 2 diabetes from our clinics and concurrently recruited age- and sex-matched nondiabetic controls within the same time frame to minimize confounding factors. Third, we implemented a blinding procedure for outcome assessors by ensuring they had no access to participants' diabetes diagnosis information, thereby maintaining the objectivity of outcome evaluations. Additionally, during the initial analysis stage, data were anonymized, group labels were concealed, and steps were taken to reduce subjective biases among analysts.
The disease duration for all patients with T2DM was documented. HbA1c levels were measured in all participants. The MNSIQ was utilized to assess DPN symptoms, whereas the 12-item OSDI questionnaire [18] was administered to evaluate the severity of ocular irritation symptoms in patients with DED. All participants underwent comprehensive ophthalmologic examinations and specific tests for the assessment of corneal epithelial integrity, tear film quantity and quality, meibomian gland dysfunction (MGD), and corneal nerve sensitivity and morphology.
The OSDI questionnaire comprises 12 modules categorized into three subscales: ocular symptoms, vision functionality, and environmental triggers. For each module, patients indicated the frequency and/or severity of their symptoms using a five-point Likert scale. The total score ranges from 0 to 100, and with the cutoff value set at 12, a positive diagnosis of DED is indicated if the score is 13 or higher [19].
Clinical Ocular Surface Assessments
Corneal sensitivity was assessed using a Cochet–Bonnet esthesiometer (CBE, Luneau Ophtalmologie, Paris, France), which features a 0.12-mm retractable nylon monofilament. The standard operating procedure referred to published literature [20], and all corneal sensitivity measurements in this study were performed by two trained technicians with inspection service experience of more than 5 years. Prior to data collection, both technicians underwent a standardized training protocol to ensure operational consistency in CBE procedures. This training encompassed a theoretical session on the principles of corneal esthesiometry and CBE calibration, followed by repeated practical testing until inter-observer variability in measurements was reduced to less than 10% (as assessed by an intraclass correlation coefficient [ICC] > 0.90). To prevent bias from affecting the results, investigators performing CBE measurements were blinded to the clinical diagnosis and previous measurement results of participants. Additionally, all subject testing was conducted in a quiet room with controlled illumination to avoid environmental distractions.
Ocular surface integrity was assessed using fluorescein dye under slit-lamp examination with cobalt blue illumination. Corneal punctate staining was quantified using the National Eye Institute (NEI) scale, where the cornea is divided into five zones, each rated from 0 (absent) to 3 (severe) on the basis of the extent, size, and coalescence of punctate lesions, yielding a maximum possible score of 15 [21].
Tear film quantity was assessed using the Schirmer I tear test (SIT) and tear meniscus height (TMH). Schirmer strips (Clement Clarke, Essex, UK) were placed over the inferior temporal half of the lower lid margin in both eyes without prior anesthesia. The wet length (mm) after 5 min was recorded [22]. Tear film instability and TMH were evaluated with the Keratograph 5M (Oculus, Arlington, WA, USA), which generates illuminated patterns of concentric rings on the ocular surface and monitors their stability to detect tear film break-up and TMH. Additionally, the examiner everted each eyelid and utilized the infrared photography system of the keratograph to capture images of the meibomian glands. Meibomian gland dropout was graded using the Pult scale to assess the severity of MGD [23]. If lid eversion or image quality was insufficient to evaluate the dropout area, the result was recorded as “loss.”
IVCM
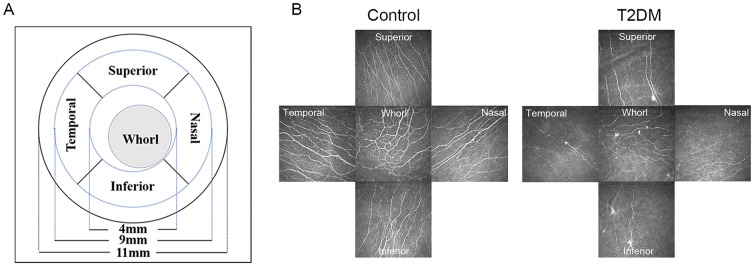
An experienced technician who was blinded to the study details followed a published protocol to perform laser scanning IVCM on a Heidelberg Retina Tomograph with the Rostock Cornea Module (Heidelberg Engineering, Heidelberg, Germany) [24]. The “Section” mode was used to capture images of the corneal upper, inferior, nasal, and temporal regions in both the paracentral and inferior-whorl areas (Fig. 1). The scanning depth extended from the superficial corneal epithelium to the endothelium. Images were acquired at a rate of 10 frames per second, with a field of view measuring 400 × 400 μm. Three focused, nonoverlapping images of the subbasal nerve plexus (SNP) from five areas were used for analysis. These 15 micrographs selected for each eye were analyzed using an automated software program (ACCMetrics; University of Manchester, Manchester, UK) [25] for the following four nerve parameters: (1) corneal nerve fiber density (CNFD) (the number of fibers/mm2, each frame area = 0.16 mm2), (2) corneal nerve branch density (CNBD) (the number of branch points on the main fibers/mm2), (3) corneal nerve fiber length (CNFL) (total length of fibers in mm/mm2), and (4) corneal nerve fiber width (CNFW) (average nerve fiber width in mm/mm2) [26].
Fig. 1.
Representative in vitro confocal microscopy (IVCM) images of A the five zones and their corresponding diameters and B comparative images of the four peripheral and whorl corneal zones from the right eye of a nondiabetic individual and a patient with type 2 diabetes mellitus (T2DM)
Microneuromas are defined as irregular enlargements of subbasal nerve terminals, hyperreflective areas, and bead-like alterations [27]. For quantification, we manually counted these structures in corneal nerve images from five regions following previously published methods [27, 28] and measured their area using ImageJ software. To avoid double-counting, microneuromas appearing in multiple frames were counted once.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism 9.5.0 software. Data are presented as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used for comparisons among the three groups if the conditions for a normal distribution and chi-square were satisfied; the Kruskal‒Wallis test was used if these two conditions were not satisfied. Multiple comparisons were made using post hoc tests with Bonferroni correction. Independent-sample t tests were applied to test two groups of quantitative data, and the χ2 test was used to test qualitative data. Correlation analyses were performed via the Spearman statistical method. A p value less than 0.05 was considered statistically significant.
Results
Demographic and Clinical Characteristics of the Study Populations
A total of 116 patients diagnosed with T2DM (60.83 ± 9.02 years) were included in the diabetic group, whereas 51 age- and sex-matched healthy participants (57.29 ± 7.99 years) were included in the control group. Within the diabetic group, 76 patients with T2DM did not have DPN (non-DPN group), whereas 40 patients had DPN, as determined by the MNSIQ (MNSIQ-DPN group).
The demographic data are presented in Table 1. There were no significant differences in age (p = 0.08) or sex (p = 0.24) among the groups. The duration of diabetes was significantly longer in the MNSIQ-DPN group (16.15 ± 9.61 years) than in the non-DPN group (11.75 ± 7.25 years, p = 0.01). HbA1c levels were significantly higher in the MNSIQ-DPN group (8.34 ± 1.81%) than in the non-DPN group (7.31 ± 1.30%, p < 0.001). Patients with T2DM in both the non-DPN group (31.58 ± 20.56, p < 0.001) and the MNSIQ-DPN group (37.51 ± 21.87, p < 0.001) had significantly higher OSDI scores than did those in the control group (9.84 ± 8.32). However, there was no significant difference in OSDI scores between the non-DPN and MNSIQ-DPN groups (p = 0.71). Corneal fluorescein staining (CFS) was significantly greater in the MNSIQ-DPN group (2.31 ± 2.83) than in both the non-DPN group (1.02 ± 2.48, p < 0.001) and the no diabetes group (0.16 ± 0.68, p < 0.001). There was no significant difference in CFS between the non-DPN group and the no diabetes group (p > 0.05) (Fig. 2A). Corneal sensitivity was significantly lower in both the non-DPN group (5.64 ± 0.86, p < 0.01) and the MNSIQ-DPN group (5.49 ± 0.81, p < 0.001) than in the no diabetes group (5.94 ± 0.28), with a more pronounced reduction in the MNSIQ-DPN group than in the non-DPN group (p = 0.02). Meibomian gland loss (MGL) grade was significantly higher in both the non-DPN group (3.47 ± 0.92, p < 0.001) and the MNSIQ-DPN group (4.00 ± 1.09, p < 0.001) than in the no diabetes group (2.55 ± 0.74), with a more significant increase in the MNSIQ-DPN group than in the non-DPN group (p = 0.01) (Fig. 2B). Additionally, the noninvasive keratography break-up time (NIKf-BUT) (p = 0.80) and SIT (p = 0.42) and TMH (p = 0.62) did not differ significantly among the groups.
Table 1.
Demographic and clinical characteristics of patients with T2DM
| Control group (n = 51) | Non-DPN group (n = 76) | MNSIQ-DPN group (n = 40) | p value | |
|---|---|---|---|---|
| Age (years) | 57.29 ± 7.99 | 60.37 ± 9.09 | 61.70 ± 8.95 | 0.08 |
| Male (%) | 20 (39.2%) | 38 (50.0%) | 14 (35.0%) | 0.24 |
| Diabetes duration (y) | N/A | 11.75 ± 7.25 | 16.15 ± 9.61# | < 0.05 |
| HbA1c (%) | 5.49 ± 0.45 | 7.31 ± 1.30* | 8.34 ± 1.81*# | < 0.001 |
| HbA1c level (mmol/mol) | 6.15 ± 0.72 | 9.06 ± 2.08* | 10.70 ± 2.89*# | < 0.001 |
| OSDI score (%) | 9.84 ± 8.32 | 31.58 ± 20.56* | 37.51 ± 21.87* | < 0.001 |
| MNSIQ score | N/A | 1.63 ± 0.73 | 5.65 ± 1.73# | < 0.001 |
| CFS | 0.16 ± 0.68 | 1.02 ± 2.48 | 2.31 ± 2.83*# | < 0.001 |
| Corneal sensitivity (mm) | 5.94 ± 0.28 | 5.64 ± 0.86* | 5.49 ± 0.81*# | < 0.001 |
| MGL grade | 2.55 ± 0.74 | 3.47 ± 0.92* | 4.00 ± 1.09*# | < 0.001 |
| NIKf-BUT (s) | 7.13 ± 6.02 | 6.67 ± 4.97 | 6.19 ± 4.48 | 0.80 |
| Schirmer I (mm) | 7.53 ± 3.79 | 6.83 ± 4.74 | 5.87 ± 3.65 | 0.42 |
| TMH (mm) | 0.24 ± 0.09 | 0.24 ± 0.11 | 0.24 ± 0.12 | 0.62 |
Data are presented as the mean ± standard deviation (SD) or n (%), and p values for comparison between the three groups. Symbols represent statistically significant differences
T2DM type 2 diabetes mellitus, DPN diabetic peripheral neuropathy, MNSIQ Michigan Neuropathy Screening Instrument Questionnaire, HbA1c glycated hemoglobin, OSDI Ocular Surface Disease Index, CFS corneal fluorescein staining, MGL meibomian gland loss, NIKf-BUT noninvasive keratography break-up time, TMH tear meniscus height, N/A not applicable
*Significant difference compared with the control group
#Significant difference compared with the non-diabetic peripheral nephropathy (DPN) group
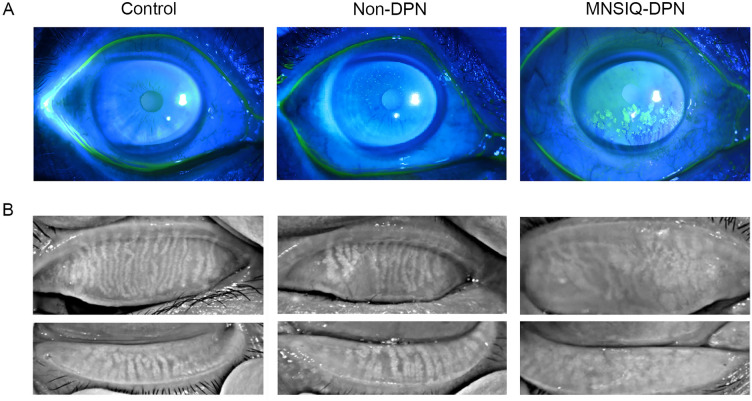
Fig. 2.
A Representative slit-lamp images with fluorescein staining revealed varying degrees of corneal involvement: control group (corneal fluorescein staining [CFS] = 0), the nondiabetic nephropathy (DPN) group (CFS = 2), and the Michigan Neuropathy Screening Instrument Questionnaire (MNSIQ)-DPN group (CFS = 5). B Infrared imaging of the upper and lower eyelids was performed using the Keratograph 5M system. Meibomian gland loss (MGL) grades were scored as follows: 1 for participants without diabetes, 3 for the non-DPN group, and 6 for the MNSIQ-DPN group
IVCM
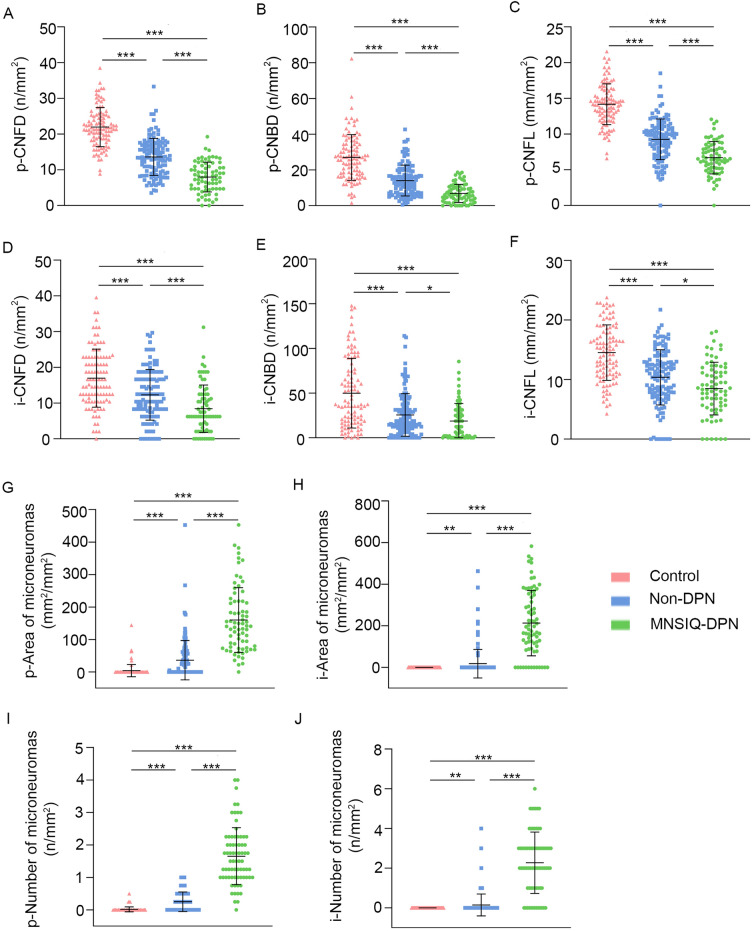
The findings and statistical comparisons are presented in Table 2 and Fig. 3. Representative images of the nerve morphology for each group are shown in Fig. 4. In the corneal paracentral (p-) area, p-CNFD, p-CNBD, and p-CNFL were significantly lower in both the non-DPN (p-CNFD: 10.63 ± 5.74 n/mm2, p < 0.001; p-CNBD: 10.16 ± 7.91 n/mm2, p < 0.001; p-CNFL: 7.85 ± 3.13 mm/mm2, p < 0.001) and MNSIQ-DPN (p-CNFD: 13.24 ± 6.46 n/mm2, p < 0.001; p-CNBD: 12.95 ± 7.85 n/mm2, p < 0.001; p-CNFL: 9.14 ± 3.20 mm/mm2, p < 0.001) groups compared to no diabetes group (p-CNFD: 21.96 ± 5.47 n/mm2; p-CNBD: 27.02 ± 12.78 n/mm2; p-CNFL: 14.17 ± 2.85 mm/mm2). However, p-CNFD (p < 0.001), p-CNBD (p < 0.001), and p-CNFL (p < 0.001) were greater in the non-DPN group than in the MNSIQ-DPN group. In the inferior-whorl (i-) area, i-CNFD, i-CNBD, and i-CNFL were significantly decreased in both the non-DPN (i-CNFD: 10.11 ± 7.29 n/mm2, p < 0.001; i-CNBD: 20.14 ± 22.39 n/mm2, p < 0.001; i-CNFL: 9.03 ± 4.82 mm/mm2, p < 0.001) and MNSIQ-DPN (i-CNFD: 10.14 ± 6.68 n/mm2, p < 0.001; i-CNBD: 25.23 ± 24.57 n/mm2, p < 0.001; i-CNFL: 10.15 ± 4.89 mm/mm2, p < 0.001) groups compared to no diabetes group (i-CNFD: 16.98 ± 8.12 n/mm2; i-CNBD: 49.93 ± 38.95 n/mm2; i-CNFL:14.54 ± 4.66 mm/mm2). In addition, a statistically significant difference was observed between the non-DPN and MNSIQ-DPN groups for i-CNFD (p < 0.01) and i-CNFL (p = 0.01). However, no statistically significant difference was found for i-CNBD between the two groups (p = 0.13). There was no significant difference in p-CNFW between the non-DPN and no diabetes groups (p = 0.37), the MNSIQ-DPN and no diabetes groups (p = 0.13), or the non-DPN and MNSIQ-DPN groups (p = 0.54) (Table 2). The level of i-CNFW was significantly lower in both the non-DPN group (0.0215 ± 0.0048 mm/mm2, p = 0.01) and the MNSIQ-DPN group (0.0207 ± 0.0064 mm/mm2, p < 0.01) than in the no diabetes group (0.0227 ± 0.0013 mm/mm2), and there was no significant difference between the MNSIQ-DPN and non-DPN groups (p = 0.47).
Table 2.
Comparison of corneal nerve fiber parameters between control subjects and patients with T2DM
| Control group (102 eyes) | Non-DPN group (123 eyes) | MNSIQ-DPN group (73 eyes) | p value | |
|---|---|---|---|---|
| p-CNFD (n/mm2) | 21.96 ± 5.47 | 13.58 ± 5.19* | 7.97 ± 4.16*# | < 0.001 |
| p-CNBD (n/mm2) | 27.02 ± 12.78 | 14.09 ± 8.64* | 6.89 ± 5.08*# | < 0.001 |
| p-CNFL (mm/mm2) | 14.17 ± 2.85 | 9.25 ± 2.86* | 6.69 ± 2.26*# | < 0.001 |
| p-CNFW (mm/mm2) | 0.0210 ± 0.0008 | 0.0214 ± 0.0013 | 0.0216 ± 0.0029 | 0.12 |
| i-CNFD (n/mm2) | 16.98 ± 8.12 | 12.40 ± 7.02* | 8.45 ± 6.63*# | < 0.001 |
| i-CNBD (n/mm2) | 49.93 ± 38.95 | 25.81 ± 23.84* | 18.83 ± 19.59* | < 0.001 |
| i-CNFL (mm/mm2) | 14.54 ± 4.66 | 10.46 ± 4.55* | 8.49 ± 4.43*# | < 0.001 |
| i-CNFW (mm/mm2) | 0.0227 ± 0.0013 | 0.0215 ± 0.0048* | 0.0207 ± 0.0064* | < 0.05 |
| p-Number of microneuromas (n/mm2) | 0.29 ± 0.62 | 0.49 ± 0.53* | 2.07 ± 1.25*# | < 0.001 |
| p-Area of microneuromas (mm2/mm2) | 19.49 ± 45.78 | 31.72 ± 36.57* | 231.60 ± 198.10*# | < 0.001 |
| i-Number of microneuromas (n/mm2) | 0.23 ± 0.51 | 0.79 ± 0.78* | 2.60 ± 1.84*# | < 0.001 |
| i-Area of microneuromas (mm2/mm2) | 15.35 ± 35.64 | 69.55 ± 95.41* | 299.80 ± 419.90*# | < 0.001 |
T2DM type 2 diabetes mellitus, DPN diabetic peripheral neuropathy, MNSIQ Michigan Neuropathy Screening Instrument Questionnaire, CNFD corneal nerve fiber density, CNBD corneal nerve branch density, CNFL corneal nerve fiber length, CNFW corneal nerve fiber width
*Significant difference compared with control group
#Significant difference compared with non-DPN group
Fig. 3.
Boxplots illustrating corneal confocal microscopy parameters of nerve fibers from the paracentral (A–C) and inferior-whorl areas (D–F), as well as microneuromas from the paracentral (G and H) and inferior-whorl areas (I and J). Significant differences, determined using post hoc tests with Bonferroni correction, are indicated (*p < 0.05; **p < 0.01; ***p < 0.001). DPN diabetic peripheral neuropathy, MNSIQ Michigan Neuropathy Screening Instrument Questionnaire, CNFD corneal nerve fiber density, CNBD corneal nerve branch density, CNFL corneal nerve fiber length
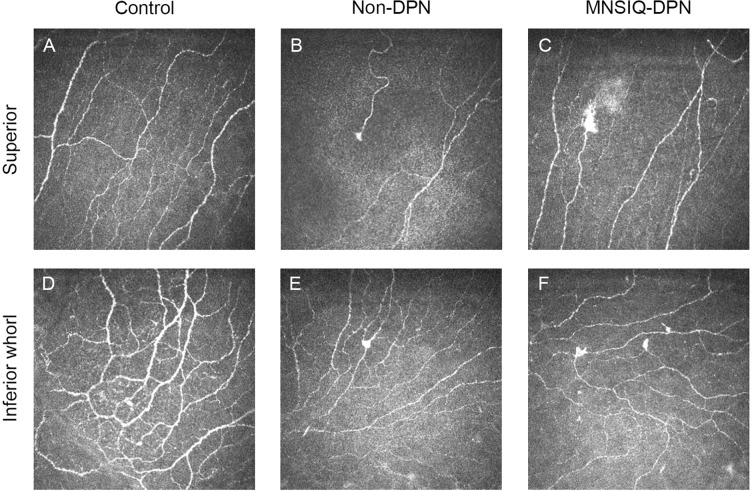
Fig. 4.
Corneal microneuroma formation in different groups. Representative in vitro confocal microscopy (IVCM) images of the superior cornea (first row) and the inferior-whorl cornea (second row). Images are representative images from participants without diabetes (A, D), participants with nondiabetic peripheral nephropathy (DPN) (B, E), and participants with Michigan Neuropathy Screening Instrument Questionnaire (MNSIQ)-DPN (C, F). In both the superior and inferior-whorl corneas, microneuromas were more evident in the MNSIQ-DPN group than in the non-DPN and control groups
The presence of corneal microneuromas in each group is shown in Fig. 3, and related data are shown in Table 2. Both the paracentral and inferior-whorl areas had a greater prevalence of corneal microneuromas in participants with MNSIQ-DPN and non-DPN than in those without diabetes. Specifically, in the paracentral area, participants with MNSIQ-DPN presented significantly greater numbers and areas of microneuromas (2.07 ± 1.25/mm2, p < 0.001; 231.60 ± 198.10 mm2/mm2, p < 0.001) than did those without diabetes (0.29 ± 0.62/mm2; 19.49 ± 45.78 mm2/mm2). Similarly, participants with non-DPN also presented significantly increased numbers of microneuromas (0.49 ± 0.53/mm2, p < 0.01; 31.72 ± 36.57 mm2/mm2, p < 0.01). Moreover, both the number and area of microneuromas were markedly greater in participants with MNSIQ-DPN than in those with non-DPN (p < 0.001 for both metrics). In the inferior-whorl area, participants with MNSIQ-DPN had a significantly greater number (2.60 ± 1.84/mm2, p < 0.001) and area (299.80 ± 419.90 mm2/mm2, p < 0.001) of microneuromas than did those without diabetes (0.23 ± 0.51/mm2; 15.35 ± 35.64 mm2/mm2). Similarly, participants with non-DPN also presented a significantly greater number (0.79 ± 0.78/mm2, p < 0.01) and area (69.55 ± 95.41 mm2/mm2, p < 0.01) of microneuromas than did those without diabetes. Moreover, participants with MNSIQ-DPN had markedly greater numbers and areas of microneuromas than did those with non-DPN (p < 0.001 for both metrics) (Fig. 4).
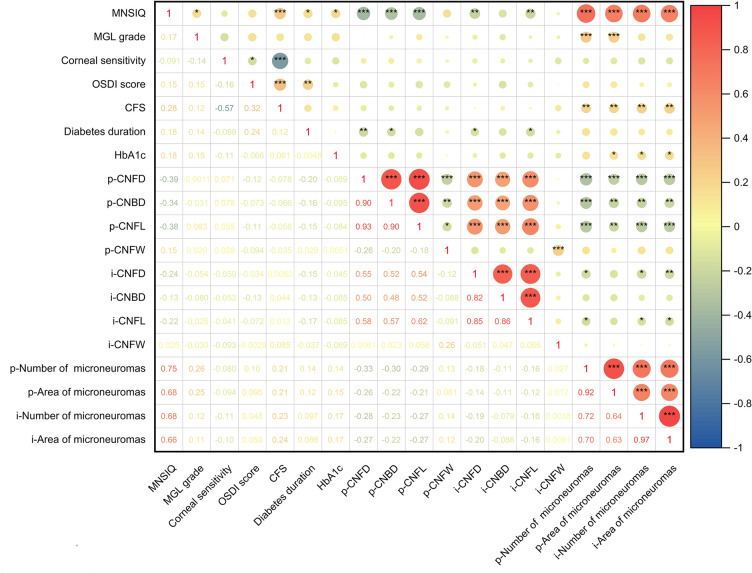
Associations Between DPN and Ocular Surface Changes in Diabetic Dry Eye Patients
The correlations between DPN and ocular surface parameter changes in patients were analyzed and are presented in Table 3 and Fig. 5. Spearman correlation analysis revealed that the MNSIQ score was significantly positively correlated with CFS (p < 0.001) and MGL grade (p < 0.01). However, no significant correlations were observed between MNSIQ scores and OSDI scores, NIKf-BUT, or SIT. Additionally, there was no significant correlation between MNSIQ scores and corneal sensitivity. In contrast, highly significant correlations were found between MNSIQ scores and corneal nerve fiber parameters, including the CNFD, CNBD, and CNFL, in both the paracentral (all p < 0.001) and inferior-whorl regions (p < 0.01, p < 0.05, and p < 0.01, respectively). DPN was significantly positively correlated with corneal microneuromas in the paracentral and inferior-whorl regions. Specifically, the MNSIQ score was significantly correlated with both the quantity and area of corneal microneuromas in these regions (p < 0.001 for both). Additionally, the distribution characteristics of corneal microneuromas in the paracentral and inferior-whorl areas of the cornea in patients with T2DM are presented in Table S1 and Figs. S1 and S2 in the Supplementary Material.
Table 3.
The correlation between MNSIQ scores and ocular surface parameters in patients with T2DM
| MNSIQ | r | p value |
|---|---|---|
| CFS | 0.2831 | < 0.001 |
| Corneal sensitivity (mm) | −0.0507 | 0.47 |
| MGL grade | 0.1875 | < 0.01 |
| p-CNFD (number/mm2) | −0.3910 | < 0.001 |
| p-CNBD (number/mm2) | −0.3496 | < 0.001 |
| p-CNFL (mm/mm2) | −0.3686 | < 0.001 |
| i-CNFD (number/mm2) | −0.2187 | < 0.01 |
| i-CNBD (number/mm2) | −0.1496 | < 0.05 |
| i-CNFL (mm/mm2) | −0.1873 | < 0.01 |
| p-Number of microneuromas (n/mm2) | 0.7478 | < 0.001 |
| p-Area of microneuromas (mm2/mm2) | 0.6512 | < 0.001 |
| i-Number of microneuromas (n/mm2) | 0.6911 | < 0.001 |
| i-Area of microneuromas (mm2/mm2) | 0.6743 | < 0.001 |
T2DM type 2 diabetes mellitus, MNSIQ Michigan Neuropathy Screening Instrument Questionnaire, CFS corneal fluorescein staining, CNFD corneal nerve fiber density, CNBD corneal nerve branch density, CNFL corneal nerve fiber length, MGL meibomian gland loss
Fig. 5.
Heatmap of the correlation analysis of the Michigan Neuropathy Screening Instrument Questionnaire (MNSIQ) score, ocular surface parameters, corneal nerve fiber parameters, and number and area of corneal microneuromas in type 2 diabetes mellitus (T2DM) patients. Significant differences, determined using Spearman correlation analysis, are indicated (*p < 0.05; **p < 0.01; ***p < 0.001). DPN diabetic peripheral neuropathy, CNFD corneal nerve fiber density, CNBD corneal nerve branch density, CNFL corneal nerve fiber length, CNFW corneal nerve fiber width, MGL meibomian gland loss, OSDI Ocular Surface Disease Index, CFS corneal fluorescein staining, HbA1c glycated hemoglobin
Discussion
In patients with diabetes-associated dry eye, a critical clinical challenge is the accurate evaluation and management of those exhibiting mild symptoms but with severe signs. Significant discrepancies between signs and symptoms are often observed, which may be linked to diminished corneal sensitivity [4]. The key finding of this study was the robust correlation between MNSIQ scores, which are considered the main standard for diagnosing DPN symptoms, and ocular surface abnormalities in patients with T2DM-associated DED. Specifically, compared with non-DPN patients with equivalent OSDI scores, patients with T2DM with MNSIQ-confirmed DPN presented higher CFS, MGD, and corneal nerve fiber loss; reduced corneal sensitivity; and greater numbers and areas of corneal microneuromas.
DPN is characterized by symmetric and distal axonal degeneration of sensory nerves. IVCM detection of corneal nerve fiber changes has become an ideal method for evaluating DPN and a Food and Drug Administration (FDA)-approved endpoint in clinical trials of peripheral and central neurodegenerative conditions because of its advantages of early diagnosis, accurate prediction, and reliable repeatability [12]. Our study revealed that participants with MNSIQ-DPN presented a significantly greater decrease in corneal nerve fiber loss and sensitivity than did all the other groups, including those without DPN. Moreover, the MNSIQ score was significantly negatively correlated with CNFD in patients with MNSIQ-DPN. When investigating the relationship between corneal nerve fiber loss and DPN in patients with diabetes using the neuropathy disability score (NDS), electrophysiological studies, and skin biopsies [29, 30], a progressive reduction in CNFD, CNFL, and CNBD was observed as the severity of DPN increased. In a large multicenter cohort study, an abnormally rapid annual loss of CNFL exceeding 6% was noted in 17% of diabetic patients. Such rapid CNFL loss may serve as a critical indicator for identifying patients at highest risk for the development and progression of DSPN [12]. Corneal sensitivity is correlated with the severity of DPN and serves as a potential marker [4, 31]. Our findings align with published conclusions, suggesting that the MNSIQ score can serve as a valuable clinical marker for evaluating diabetic corneal neuropathy.
It is important to highlight that our study did not observe a significant correlation between MNSIQ scores and corneal sensitivity. Notably, despite a marked reduction in corneal sensitivity in the MNSIQ-DPN group compared to the non-DPN group, this difference did not translate into a statistically significant relationship with MNSIQ scores. We posit that this phenomenon can be attributed to three principal factors. First, it is well documented that the cornea contains three established classes of nociceptors: mechanosensory, polymodal, and cold-sensory neurons. However, the Cochet–Bonnet method detects only mechanosensory neurons, which are sensitive to mechanical stimulation [4]. This suggests that corneal mechanical touch sensation has a limited scope and thus cannot fully represent the comprehensive sensory function of the cornea. Second, the patients with diabetes in our study demonstrated less pronounced reductions in corneal sensitivity and nerve fiber density than those with type 1 diabetes reported in the literature, which may introduce potential biases in data analysis [31]. Third, confounding factors such as smoking history, sleep disorders, and hyperlipidemia influence corneal sensitivity. We have not collected or analyzed data for these confounding factors due to limitations in sample size. Future research should employ strategies such as expanding the sample size, conducting stratified analyses of confounding factors, and integrating multicenter clinical studies to enhance the reliability of the findings.
Notably, in our study, corneal microneuromas were predominantly observed in participants with diabetes, particularly those diagnosed with MNSIQ-DPN. Recent studies have demonstrated that corneal microneuromas are promising biomarkers for various ocular surface diseases, including neuropathic corneal pain [32], DED [33], DNK [34], Sjögren’s syndrome [35], and postrefractive surgery complications [36]. In patients with T2DM with MNSIQ-DPN, who presented significantly higher HbA1c levels, both the density and area of corneal microneuromas were markedly increased and were correlated with higher MNSIQ scores. The presence of corneal microneuromas may reflect the impact of diabetes on peripheral nerve endings, with a greater number potentially indicating more severe clinical manifestations. The findings from a recent cross-sectional study corroborates our findings, showing that corneal microneuromas were more frequent and abundant in patients with T2DM with DPN with both painful and nonpainful DSPN [27]. Additionally, patients with T2DM with MNSIQ-DPN presented decreased corneal sensitivity, which was also positively correlated with lower OSDI scores, reflecting the influence of diabetic neuropathy on corneal sensitivity.
The maintenance and regeneration of the corneal epithelium depend on the balance of the proliferation, migration, differentiation, and apoptosis of limbal stem cells (LSCs) [37]. Corneal sensory nerves regulate epithelial renewal by releasing neuropeptides and growth factors that stimulate LSC activity [38]. In diabetes, chronic hyperglycemia causes nerve loss, inflammation, and oxidative stress, reducing key stem cell markers in the diabetic corneal limbus [39]. A recent study also revealed that sympathetic overactivation impairs LSC function and corneal regeneration via the NE-Adrb2-Shh signaling pathway [40]. In our study, the observed increases in MNSIQ and OSDI scores, together with reduced corneal sensitivity, were significantly correlated with elevated corneal fluorescein uptake in participants with MNSIQ-DPN associated with T2DM. These findings indicate a progressive deterioration of the corneal epithelial barrier function as DPN severity increases. The presence of corneal microneuromas, in conjunction with markedly reduced corneal nerve density and epithelial defects, may serve as an objective biomarker for the diagnosis of neurotrophic keratopathy (NK) [34, 41]. Our study revealed a strong positive correlation between the number and area of corneal microneuromas, suggesting that innervation-dependent dysfunction of corneal epithelial renewal plays a critical role in the pathogenesis of this condition. Cutaneous Schwann cells (SCs) and associated nerves degenerate interdependently in DPN, with SC defects contributing significantly to DPN pathogenesis [42]. Through single-cell messenger RNA (mRNA) analysis, Borschel demonstrated that terminally differentiated SCs release trophic factors that support LSCs, which are crucial for tissue regeneration [43]. These findings highlight SCs as potential therapeutic targets to improve diabetic LSC function and promote epithelial repair.
MGD is the most prevalent form of DED and is characterized by a reduction in tear film lipids. This lipid reduction leads to accelerated tear evaporation, compromised tear film stability, and impaired ocular surface hydration [44]. Approximately 80% of mixed dry eye (MDE) cases are attributable to MGD [45]. Patients with T2DM predominantly exhibit MGD accompanied by mildly reduced tear secretion. Notably, individuals with MNSIQ-DPN scores indicative of mild neuropathy exhibit significantly more severe MGD than do those without DPN. Although tear secretion levels in these patients do not differ significantly from those in nondiabetic patients, tear secretion levels are markedly lower than normal physiological levels reported in the literature [10]. In vivo studies have demonstrated that the pathophysiological mechanisms underlying MGD are closely associated with disruptions in lipid homeostasis, lipid accumulation, and abnormalities in lipid metabolism [46, 47]. Jende et al. reported that in patients with DPN, T2DM-related nerve lesions are associated with alterations in lipid metabolism [48]. Spearman correlation coefficient analysis revealed a significant association between the MGD grade and several parameters, including MNSIQ scores, the number of microneuromas, and the area of microneuromas in the corneal peripheral region. These findings suggest that asymptomatic MGD may serve as an early indicator of DED syndrome in patients with T2DM and that the MNSIQ could be a valuable tool for early and reliable diagnosis.
The HbA1c level and diabetes duration were strongly associated with the DPN and corneal nerve degeneration [12, 18]. Our results demonstrated a significant positive correlation between HbA1c levels and both the number of corneal microneuromas and the MNSIQ score. Additionally, diabetes duration exhibited a significant positive correlation with OSDI and MNSIQ scores, while showing a significant negative correlation with corneal nerve density in the peripheral and inferior-whorl regions. The findings were consistent with the results of previous studies [49].
The present study has several limitations that should be acknowledged. First, the relatively small sample size and the exclusive focus on patients with T2DM may restrict the generalizability of the findings, particularly given the lower prevalence of type 1 diabetes in China. Second, the study does not include objective measures for peripheral neuropathy, such as clinical evaluations of temperature and light touch sensitivity, or formal nerve conduction studies, which could have strengthened the diagnostic accuracy. Third, several potential confounding factors, including smoking history, sleep disorders, and hyperlipidemia, known to influence DPN, corneal nerve damage, and dry eye conditions, were not adequately controlled for in the analysis [50]. Due to the limited sample size, we were unable to collect or analyze data for these confounding factors. Additionally, our study explored the association between ocular surface-specific indicators but not between ocular surface indicators and systemic complications such as chronic kidney disease (CKD) due to limitations in cross-departmental collaboration. In future research, we aim to collaborate with the internal medicine department to integrate multidisciplinary data, thereby facilitating a more comprehensive investigation of the role of systemic factors.
Due to environmental constraints, we lack more accurate examinations of large and small fiber function in the clinical evaluation of peripheral nerve dysfunction, such as nerve conduction studies, quantitative sensory assessment, and skin biopsy.
Conclusions
This study revealed that the OSDI score is not a reliable indicator of the severity of ocular surface damage in DED patients with T2DM. The MNSIQ score, which is strongly correlated with multiple ocular surface parameters such as CFS, MGL grade, corneal nerve fiber density, and the numbers and areas of microneuromas in the peripheral and inferior-whorl regions, comprehensively reflects compromised corneal epithelial integrity, LFU function, and corneal nerve density and function. Consequently, peripheral corneal neuropathy plays a pivotal role in diabetic ocular surface complications, underscoring the importance of incorporating the MNSIQ into routine ophthalmic evaluations for diabetic patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of the study.
Medical Writing/Editorial Assistance
Thanks to American Journal Experts LLC for the language polishing assistance provided in the manuscript. This assistance was funded by the authors.
Author Contributions
Yangyang Zhang and Yanling Dong made significant contributions to the design and conception of the project, data analysis, and writing of the article. Yanling Liu collected the data and analyzed it; Dapeng Sun and Qianqian Kong detected the corneal nerve fiber by confocal microscopy; Dongfang Li and Rui Wang collected the data. Jia Yin contributed to the revision of the statistical methods during the article revision process; Lixin Xie provided important guidance in the process of article revision. Yangyang Zhang and Yanling Dong contributed to the interpretation of the results and critical revision of the manuscript. All the authors have read and approved the final manuscript. Yangyang Zhang and Yanling Dong are the study guarantors.
Funding
This study was supported by the National Natural Science Foundation of China (82101094 to Y.Z.), the Taishan Scholar Programme (202211342 to Y.Z.), and the Projects of Medical and Health Technology Development Programme in Shandong Province (202207020274 to Y.D.). Funding for editorial support in the drafting of this manuscript and for the journal’s Rapid Service Fee was provided by the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
Yanling Liu, Dapeng Sun, Qianqian Kong, Dongfang Li, Rui Wang, Jia Yin, Lixin Xie, Yanling Dong, and Yangyang Zhang declare that they have no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Ethical Approval
The study protocol was approved by the local Committee of Qingdao Eye Hospital Research Ethics (approval number: 2019-33), and the procedures were performed in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all participants or their legal guardians.
Contributor Information
Yanling Dong, Email: yanling1235@126.com.
Yangyang Zhang, Email: zhangyangyang@sdfmu.edu.cn.
References
- 1.Neeland IJ, Patel KV. Diabetes. In: Biomarkers in cardiovascular disease. 2019. p. 41–51.
- 2.Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Research and Clinical Practice; 2022. p. 183. [DOI] [PMC free article] [PubMed]
- 3.Zhou B, Rayner AW, Gregg EW, et al. Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: a pooled analysis of 1108 population-representative studies with 141 million participants. Lancet. 2024;404(10467):2077–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu FX, Lee PSY, Yang L, et al. The impact of sensory neuropathy and inflammation on epithelial wound healing in diabetic corneas. Prog Retin Eye Res. 2022;89:101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Q, Yang L, Wang Q, et al. Mechanistic investigations of diabetic ocular surface diseases. Front Endocrinol (Lausanne). 2022;13:1079541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Priyadarsini S, Whelchel A, Nicholas S, et al. Diabetic keratopathy: insights and challenges. Surv Ophthalmol. 2020;65(5):513–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callaghan BC, Price RS, Feldman EL. Distal symmetric polyneuropathy: a review. Jama. 2015;314(20):2172-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmichael J, Fadavi H, Ishibashi F, Shore AC, Tavakoli M. Advances in screening, early diagnosis and accurate staging of diabetic neuropathy. Front Endocrinol (Lausanne). 2021;12:671257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badian RA, Ekman L, Pripp AH, et al. Comparison of novel wide-field in vivo corneal confocal microscopy with skin biopsy for assessing peripheral neuropathy in type 2 diabetes. Diabetes. 2023;72(7):908–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438–510. [DOI] [PubMed] [Google Scholar]
- 11.Perkins BA, Lovblom LE, Lewis EJH, et al. Corneal confocal microscopy predicts the development of diabetic neuropathy: a longitudinal diagnostic multinational consortium study. Diabetes Care. 2021;44(9):2107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis EJH, Lovblom LE, Ferdousi M, et al. Rapid corneal nerve fiber loss: a marker of diabetic neuropathy onset and progression. Diabetes Care. 2020;43(8):1829–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17(11):1281–9. [DOI] [PubMed] [Google Scholar]
- 14.Mizokami-Stout KR, Li Z, Foster NC, et al. The contemporary prevalence of diabetic neuropathy in type 1 diabetes: findings from the T1D exchange. Diabetes Care. 2020;43(4):806–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herman WH, Pop-Busui R, Braffett BH, et al. Use of the Michigan neuropathy screening instrument as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the diabetes control and complications trial/epidemiology of diabetes interventions and complications. Diabet Med. 2012;29(7):937–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sartor CD, Oliveira MD, Campos V, Ferreira JSSP, Sacco ICN. Cross-cultural adaptation and measurement properties of the Brazilian Version of the Michigan Neuropathy Screening Instrument. Braz J Phys Ther. 2018;22(3):222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbosa M, Saavedra A, Severo M, Maier C, Carvalho D. Validation and reliability of the Portuguese version of the Michigan neuropathy screening instrument. Pain Pract. 2016;17(4):514–21. [DOI] [PubMed] [Google Scholar]
- 18.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118(5):615–21. [DOI] [PubMed] [Google Scholar]
- 19.Okumura Y, Inomata T, Iwata N, et al. A review of dry eye questionnaires: measuring patient-reported outcomes and health-related quality of life. Diagnostics (Basel). 2020;10(8):559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chao C, Stapleton F, Badarudin E, Golebiowski B. Ocular surface sensitivity repeatability with Cochet–Bonnet esthesiometer. Optom Vis Sci. 2015;92(2):183–9. [DOI] [PubMed] [Google Scholar]
- 21.Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640–50. [DOI] [PubMed] [Google Scholar]
- 22.Yawata N, Selva KJ, Liu YC, et al. Dynamic change in natural killer cell type in the human ocular mucosa in situ as means of immune evasion by adenovirus infection. Mucosal Immunol. 2016;9(1):159–70. [DOI] [PubMed] [Google Scholar]
- 23.Oydanich M, Maguire MG, Pistilli M, et al. Effects of omega-3 supplementation on exploratory outcomes in the dry eye assessment and management study. Ophthalmology. 2020;127(1):136–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tavakoli M, Malik RA. Corneal confocal microscopy: a novel non-invasive technique to quantify small fibre pathology in peripheral neuropathies. J Vis Exp. 2011(47):2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Graham J, Dabbah MA, et al. Small nerve fiber quantification in the diagnosis of diabetic sensorimotor polyneuropathy: comparing corneal confocal microscopy with intraepidermal nerve fiber density. Diabetes Care. 2015;38(6):1138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X, Graham J, Petropoulos IN, et al. Corneal nerve fractal dimension: A novel corneal nerve metric for the diagnosis of diabetic sensorimotor polyneuropathy. Invest Ophthalmol Vis Sci. 2018;59(2):1113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sierra-Silvestre E, Andrade RJ, Colorado LH, Edwards K, Coppieters MW. Occurrence of corneal sub-epithelial microneuromas and axonal swelling in people with diabetes with and without (painful) diabetic neuropathy. Diabetologia. 2023;66(9):1719–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moein H-R, Akhlaq A, Dieckmann G, et al. Visualization of microneuromas by using in vivo confocal microscopy: an objective biomarker for the diagnosis of neuropathic corneal pain? Ocul Surf. 2020;18(4):651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azmi S, Ferdousi M, Petropoulos IN, et al. Corneal confocal microscopy identifies small-fiber neuropathy in subjects with impaired glucose tolerance who develop type 2 diabetes. Diabetes Care. 2015;38(8):1502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46(5):683–8. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg ME, Tervo TM, Immonen IJ, Müller LJ, Grönhagen-Riska C, Vesaluoma MH. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci. 2000;41(10):2915–21. [PubMed] [Google Scholar]
- 32.Aggarwal S, Kheirkhah A, Cavalcanti BM, et al. Autologous serum tears for treatment of photoallodynia in patients with corneal neuropathy: efficacy and evaluation with in vivo confocal microscopy. Ocul Surf. 2015;13(3):250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guerrero-Moreno A, Liang H, Moreau N, et al. Corneal nerve abnormalities in painful dry eye disease patients. Biomedicines. 2021;9(10):1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yavuz Saricay L, Bayraktutar BN, Kenyon BM, Hamrah P. Concurrent ocular pain in patients with neurotrophic keratopathy. Ocul Surf. 2021;22:143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luzu J, Labbé A, Réaux-Le Goazigo A, et al. In vivo confocal microscopic study of corneal innervation in Sjögren’s Syndrome with or without small fiber neuropathy. Ocul Surf. 2022;25:155–62. [DOI] [PubMed] [Google Scholar]
- 36.Toh CJL, Liu C, Lee IXY, et al. Clinical associations of corneal neuromas with ocular surface diseases. Neural Regen Res. 2024;19(1):140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonnet C, González S, Roberts JS, et al. Human limbal epithelial stem cell regulation, bioengineering and function. Prog Retin Eye Res. 2021;85:100956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peterson DC, Hamel RN. Corneal Reflex. Treasure Island (FL): StatPearls. Disclosure: Renee Hamel declares no relevant financial relationships with ineligible companies: StatPearls Publishing Copyright © 2025, StatPearls Publishing LLC.; 2025
- 39.Zhu L, Titone R, Robertson DM. The impact of hyperglycemia on the corneal epithelium: molecular mechanisms and insight. Ocul Surf. 2019;17(4):644–54. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z, Yang L, Li Y, et al. Interference of sympathetic overactivation restores limbal stem/progenitor cells function and accelerates corneal epithelial wound healing in diabetic mice. Biomed Pharmacother. 2023;161:114523. [DOI] [PubMed] [Google Scholar]
- 41.Dieckmann G, Goyal S, Hamrah P. Neuropathic corneal pain. Ophthalmology. 2017;124(11):S34–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu X, Buhl CS, Sjogaard MB, et al. Structural changes in Schwann cells and nerve fibres in type 1 diabetes: relationship with diabetic polyneuropathy. Diabetologia. 2023;66(12):2332–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mirmoeini K, Tajdaran K, Zhang J, et al. Schwann cells are key regulators of corneal epithelial renewal. Investig Opthalmol Visual Sci. 2023;64(4):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glasgow BJ. Tear lipocalin and lipocalin-interacting membrane receptor. Front Physiol. 2021;12:684211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geerling G, Baudouin C, Aragona P, et al. Emerging strategies for the diagnosis and treatment of meibomian gland dysfunction: proceedings of the OCEAN group meeting. Ocul Surf. 2017;15(2):179–92. [DOI] [PubMed] [Google Scholar]
- 46.Guo Y, Zhang H, Zhao Z, et al. Hyperglycemia induces meibomian gland dysfunction. Investig Opthalmol Visual Sci. 2022;63(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H, Zhou Q, Wan L, et al. Lipidomic analysis of meibomian glands from type-1 diabetes mouse model and preliminary studies of potential mechanism. Exp Eye Res. 2021;210:108710. [DOI] [PubMed] [Google Scholar]
- 48.Jende JME, Groener JB, Oikonomou D, et al. Diabetic neuropathy differs between type 1 and type 2 diabetes: Insights from magnetic resonance neurography. Ann Neurol. 2018;83(3):588–98. [DOI] [PubMed] [Google Scholar]
- 49.Tummanapalli SS, Wang LL, Dhanapalaratnam R, et al. Moderate-severe peripheral neuropathy in diabetes associated with an increased risk of dry eye disease. Optom Vis Sci. 2024;101(9):563–70. [DOI] [PubMed] [Google Scholar]
- 50.Yu K, Bunya V, Maguire M, Asbell P, Ying G-S. Systemic conditions associated with severity of dry eye signs and symptoms in the dry eye assessment and management study. Ophthalmology. 2021;128(10):1384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.