Abstract
Ageing is a gradual biological process marked by a decline in physiological function, increasing susceptibility to disease, and mortality. Transposable elements (TEs) are repetitive DNA sequences capable of moving within the genome and thus potentially inducing mutations and disrupting normal cellular functions. Their mobile nature contributes to genomic variation, as transposition events can alter gene expression, chromosome structure, and the epigenetic landscape. To mitigate TE-induced damage, cells rely on epigenetic mechanisms, such as DNA methylation, histone modifications, and small RNAs, to repress TE activity. However, these silencing mechanisms become less effective with age, leading to increased TE activation. This review explores the dual role of TEs as both a cause and consequence of ageing, suggesting a complex relationship between TEs and the ageing process.
Keywords: senescence, transposable elements, chromatin, age-related diseases, long-lived organisms
Significance.
Transposable elements (TEs) are highly mutagenic repetitive DNA sequences that have long been understudied due to technical challenges in detecting and analyzing them. However, the emergence of long-read sequencing techniques has greatly advanced TE research, providing deeper insights into their genomic impact. Growing evidence now links TE activity to ageing and age-related diseases, suggesting that TEs can influence lifespan. In this review, we highlight recent discoveries showing that TE activation can be both a cause and a consequence of ageing, emphasizing the importance of studying these genomic elements.
Introduction
Ageing is of particular interest in biology, especially in understanding the underlying mechanisms that help elucidating patterns of longevity. Ageing is a gradual biological process characterized by a decline in physiological function and an increased susceptibility to disease and mortality over time (Kirkwood and Austad 2000; López-Otín et al. 2013). As organisms age, the accumulation of senescent cells (cells that have lost the ability to divide) contributes to progressive dysfunction at the organismal level, ultimately impairing survival and fertility.
The evolutionary theory of ageing suggests that the strength of natural selection, a measure of how effectively selection influences survival or reproduction, diminishes with age (Haldane 1941; Medawar 1946, 1952; Williams 1957). This decline forms the basis for hypotheses such as mutation accumulation and antagonistic pleiotropy. The mutation accumulation hypothesis posits that deleterious mutations expressed later in life persist in populations because natural selection is less effective at removing them when reproductive success is no longer significantly impacted (Medawar 1946, 1952). In contrast, the antagonistic pleiotropy hypothesis suggests that some genes have dual effects, offering benefits during early life, such as enhanced reproductive fitness, but causing negative effects in later life, contributing to ageing (Williams 1957). This trade-off arises because natural selection favours traits that enhance early-life fitness, even at the cost of late-life detriments.
Modern theories of ageing, which seek to explain its underlying mechanisms, are divided into two main categories: the error/damage and the programmed perspective (Jin 2010). The error/damage model proposes that ageing results primarily from the accumulation of cellular and molecular damage over time. This theory emphasises that environmental factors, lifestyle choices, and metabolic processes contribute to this damage (Weismann 1882; Davidovic et al. 2010; Jin 2010). In contrast, the programmed model views ageing as an inherent and essential part of the life cycle, driven by genetic and hormonal mechanisms rather than simply being a consequence of accumulated damage over time (Davidovic et al. 2010; Jin 2010).
Advances in whole-genome sequencing techniques have enabled the study of the genetic mechanisms involved in ageing. Among various genomic components, transposable element (TE) effects have been repeatedly linked to ageing due to their capacity to generate mutations with potential to disrupt normal cellular functions (Wood and Helfand 2013; Copley and Shorter 2023; Yushkova and Moskalev 2023). TEs are repetitive DNA sequences capable of moving (transpose) within the genome, which are commonly classified into two main classes based on their mechanism of transposition (Bourque et al. 2018). Class I elements, or retrotransposons, transpose via an RNA intermediate through a “copy and paste” mechanism. In contrast, Class II elements, or DNA transposons, move using a DNA intermediate and typically follow a “cut and paste” mechanism (Bourque et al. 2018). To capture the diversity within these broad categories, TEs are further classified in subclasses, orders, and superfamilies based on mechanistic and enzymatic criteria (Wicker et al. 2007). TEs are present in virtually all eukaryotic and prokaryotic genomes (Hua-Van et al. 2011; Kissinger and DeBarry 2011), and they typically represent a considerable fraction of the genomes, although their abundance is highly variable from one species to another (Guio and González 2019; Mérel et al. 2020). Due to their mobile and repetitive nature, TEs are a source of genomic variation. The DNA breaks and insertions associated with transposition events lead to obvious alterations to the genome (Bourque et al. 2018). The consequences of TE expression and mobilization can also have widespread effects, altering gene expression and structure, chromosome dynamics, as well as the epigenetic landscape of the genome (Casacuberta and González 2013; Bourque et al. 2018; Bourgeois and Boissinot 2019). The idea that TEs can contribute to ageing processes through mutations (associated with the error/damage theory of ageing) was first proposed by Shmookler Reis and Goldstein (1983) and Vijg et al. (1985). Building up on the same idea, the transposon ageing model, introduced a few years later by Murray (1990), postulates that an exponential increase of TE copy number with time could eventually kill the cell or the organism by inactivating essential genes. Indeed, the activation of TEs has been demonstrated to affect lifespan associated with DNA damage in several organisms like fruit flies, and mice (Wood et al. 2016; Simon et al. 2019). Similarly, TE activation has been recently associated with neurodegenerative, autoimmune, and cancer diseases, which can in turn affect organismal lifespan (Gorbunova et al. 2021).
To mitigate detrimental TE-related effects, TE activity (expression and/or transposition) is normally repressed by epigenetic mechanisms that can involve DNA methylation, histone modifications, and/or the production of small RNAs (reviewed in Slotkin and Martienssen 2007). Ageing disrupts these TE silencing mechanisms, increasing their activity. Examples of that have been documented in several organisms, where TE expression and sometimes TE transposition increased with age in different somatic tissues (Li et al. 2013; De Cecco et al. 2013a; Chen et al. 2016; Yang et al. 2022).
In this review, we explore current literature demonstrating that TE activity can be associated with both the causes and consequences of ageing, leading to a more complex hypothesis regarding the role of TEs in ageing processes.
TEs as a Source of Genomic Variation
TEs were first described by Barbara McClintock in the late 1940s in maize (Zea mays). Originally referred to as “controlling elements”, TEs were thought to move in response to environmental changes, thereby influencing gene expression (McClintock 1950, 1953). However, these ideas were initially rejected, and for many years, TEs were classified as “selfish” or “junk” genetic elements thought to cause only harmful effects (Strobel et al. 1979; Doolittle and Sapienza 1980; Orgel and Crick 1980; Hickey 1982). Due to the emergence of sequencing techniques, it is now understood that TEs are a significant component of genomes across various species (Bourque et al. 2018; Guio and González 2019; Wells and Feschotte 2020). Over the last decades, the importance of TEs has grown as their roles in influencing recombination rates, chromosomal rearrangements, mutagenesis, and gene regulation have been confirmed (Biémont 2010; Bourque et al. 2018). Additionally, TEs have been implicated in genome evolution and recent adaptation (Biémont and Vieira 2006; González et al. 2010; Arkhipova 2018; Xia et al. 2024). Therefore, the scientific community now acknowledges that while TEs can have detrimental effects, they have also played a significant role in shaping the structure, function, and evolution of genomes.
However, this same mobility that underlies their evolutionary impact also poses a risk to genome integrity. The ability of TEs to move within the genome is central to their mutagenic potential. Over time, the accumulation of transposition events within cells can lead to genomic instability at the organismal level, a phenomenon linked to both the transposon and the error/damage theories of ageing (Weismann 1882; Murray 1990; Davidovic et al. 2010; Jin 2010).
In addition to the effects directly caused by transposition events, TEs can alter both gene structure and expression. The impact of TEs on gene structure is determined by the specific location of their insertion. When inserted into introns, TEs can be incorporated as new exons (exonization), provide alternative splicing sites, or introduce alternative polyadenylation (poly(A)) signals (Schmitz and Brosius 2011; Chénais et al. 2012; Casacuberta and González 2013; Warren et al. 2015; Oliveira et al. 2023; Xia et al. 2024). TEs can also alter gene structure when inserted in other non-coding regions such as 5′ untranslated (5′ UTR) regions. There, they can, for example, introduce alternative transcription start sites, hence modulating gene transcript lengths (Faulkner et al. 2009; Batut et al. 2013; Coronado-Zamora and González 2023; Oliveira et al. 2023).
Furthermore, TEs can serve as a source of regulatory material for the modulation of gene expression (Bourque et al. 2018). They can harbour regulatory sequences, such as promoters, enhancers, or repressive elements, that fine-tune nearby gene expression, contribute to heterochromatin formation, and act as insulators to prevent the spread of heterochromatin (Chuong et al. 2017; Trizzino et al. 2017; Villanueva-Cañas et al. 2019). Additionally, TEs influence gene expression through post-transcriptional mechanisms, affecting mRNA decay, localization, and translation efficiency (Elbarbary et al. 2016). They also help define topological domains within the nucleus, facilitating chromatin loop formation (Mamillapalli et al. 2013).
These diverse effects highlight the role of TEs in shaping genomic evolution and in influencing gene regulation.
Regulatory Mechanisms of TEs
There are multiple epigenetic mechanisms regulating TE activity, including transcriptional and post-transcriptional repression (Slotkin and Martienssen 2007; Gebert and Rosenkranz 2015).
One of the mechanisms for silencing TEs involves RNA interference pathways, which depend on the production of small non-coding RNA molecules that complementary target and regulate TE transcripts (Slotkin and Martienssen 2007).
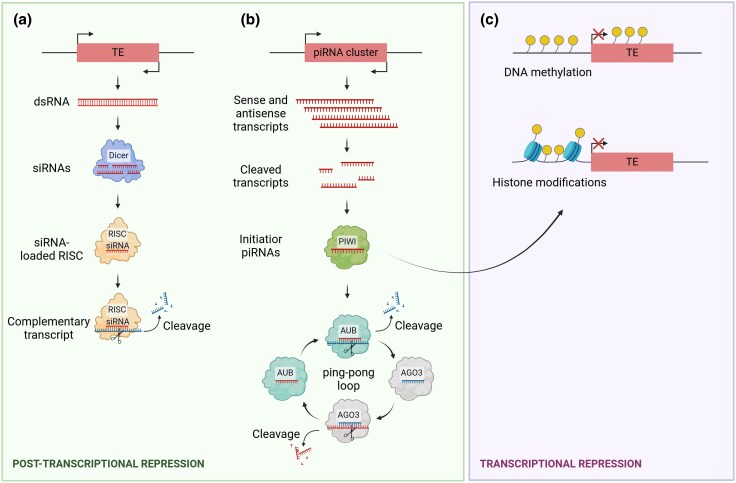
First, the small-interfering RNA (siRNA) pathway involves the cleavage of double-stranded RNA by Dicer proteins into short siRNAs. These siRNAs are then loaded into Argonaute (AGO) proteins to form the RNA-induced silencing complex (RISC), guiding the degrading complex to a complementary transcript for cleavage (Slotkin and Martienssen 2007; Gebert and Rosenkranz 2015; Fig. 1).
Fig. 1.
Schematic representation of the main regulatory mechanisms of TE activity. a) The small-interfering RNA (siRNA) pathway: double-stranded RNA (dsRNA) is cleaved by Dicer into siRNAs, which are loaded into Argonaute (AGO) proteins to form the RNA-induced silencing complex (RISC). This complex guides the cleavage of complementary TE transcripts. b) The Piwi-interacting RNA (piRNA) pathway: piRNAs, transcribed from piRNA clusters, bind to PIWI proteins, guiding them to TE loci where they induce repressive chromatin modifications, silencing TEs at the transcriptional level. Additionally, piRNAs can target TE transcripts for post-transcriptional cleavage via the Argonaute protein Aubergine (AUB), triggering the “ping-pong loop” to amplify piRNA production. c) Transcriptional regulation through chromatin modification: TEs can be silenced at the DNA level by heterochromatinization, mediated by DNA methylation and histone modifications such as deacetylation and methylation.
Second, the Piwi-interacting RNA pathway (piRNA) was first described as the main defence mechanism against TE transcripts in germ cells. But recently, this pathway has also been identified in the somatic cells of arthropods and molluscs (Martens et al. 2005; Ghildiyal et al. 2008; Lee et al. 2011; Perrat et al. 2013; Jones et al. 2016). The mechanisms of the piRNA pathway are well understood in mice and Drosophila, where extensive research has been conducted. In Drosophila, initiator piRNAs are transcribed from piRNA clusters, which consist of loci enriched in TE sequences and processed into 23- to 30-nucleotide fragments. These piRNAs bind to PIWI proteins and guide them to complementary TE loci in the genome, where they induce repressive chromatin modifications leading to transcriptional silencing of TEs (Hyun 2017; Fig. 1). Additionally, piRNAs can target TE transcripts for post-transcriptional cleavage, further inhibiting TE expression. In that case, piRNAs bind to the Argonaute protein Aubergine (AUB) and guide the cleavage of complementary TE transcripts. This cleavage releases responder piRNAs that can interact with Argonaute3 proteins (Hyun 2017; Fig. 1). These responder piRNAs target the original piRNA precursor, leading to further processing and amplification of piRNAs, which can subsequently silence additional TE transcripts. This process, known as the “ping-pong loop”, allows the amplification of piRNAs derived from TE transcripts (Hyun 2017; Fig. 1).
Finally, transcriptional regulation of TEs can also be achieved by suppressing their expression at the DNA level by inducing heterochromatisation through DNA methylation and/or histone modifications (Fig. 1). While not conserved across all eukaryotes, the methylation of cytosine residues in TE promoters by DNA methyltransferases is commonly associated with their transcriptional repression (Slotkin and Martienssen 2007). Similarly, modifications to the amino (N)-terminal tails of histones, such as deacetylation and methylation, create a repressive chromatin state around TEs. Indeed, nucleosomes associated with TEs are usually enriched in H3K9 methylation, a mark indicative of transcriptionally repressive and inactive chromatin (Martens et al. 2005).
Impact of Ageing on TE Expression
There is substantial evidence in the literature indicating that TEs can be activated with age leading to increased TE transcription in fruit flies (Table 1; Li et al. 2013; Chen et al. 2016; Jones et al. 2016; Wood et al. 2016; Brown et al. 2020a; Fabian et al. 2021; Giordani et al. 2022; Yang et al. 2022; Schneider et al. 2023), fish (Teefy et al. 2023; Xu et al. 2023), mice (De Cecco et al. 2013a; Van Meter et al. 2014), and humans (Colombo et al. 2018; Pehrsson et al. 2019; Senapati et al. 2023). Although it is generally agreed that this activation is due to an age-associated weakening of TE silencing processes, the precise molecular mechanisms underlying this phenomenon remain unclear.
Table 1.
Summary table of the impact of ageing on TE expression in different organisms
| Organism | Tissue | Mechanism | Impact on TE expression | References |
|---|---|---|---|---|
| D. melanogaster | Head | Loss of H3K9me2 | Increase | Brown et al. (2020a) |
| D. melanogaster | Head | Ago2 impairment in young flies | Increase | Li et al. (2013) |
| D. melanogaster | Fat body | LAM depletion (H3K9me3 decrease and H3K4me3 increase) | Increase | Chen et al. (2016) |
| D. melanogaster | Fat body, head | Loss of heterochromatin | Increase | Wood et al. (2016) |
| D. melanogaster | Fat body | piRNA pathway impairment | Increase | Jones et al. (2016) |
| D. melanogaster | Whole body, brain | ND | Increase | Yang et al. (2022) |
| M. musculus | Liver, muscle | Chromatin decondensation | Increase | De Cecco et al. (2013a) |
| M. musculus | Senescent cells | Chromatin decondensation | Increase | De Cecco et al. (2013b) |
| M. musculus | Cell lines | Loss of SIRT6 protein | Increase | Van Meter et al. (2014) |
| H. sapiens | Senescent cells | Hypomethylation | Increase | Colombo et al. (2018) |
| H. sapiens | Mammary luminal epithelial cells | Loss of methylation | Increase in breast cancer tissue | Senapati et al. (2023) |
ND, not determined.
In Drosophila melanogaster, differences in TE expression with age have been associated with changes in heterochromatin structure (Li et al. 2013; Chen et al. 2016; Wood et al. 2016; Brown et al. 2020a). For example, in older flies, a general loss of H3K9me2, a repressive histone modification typical of heterochromatin, has been observed at repetitive elements (Brown et al. 2020a). TE control in the soma of D. melanogaster is mediated by the siRNA pathway. Genetic manipulations of Ago2 gene, a key component of this pathway, in young flies resulted in elevated R2 and mdg4 TE transcripts in heads, together with an age-dependent expression acceleration of these two TE families, which are both classified as Class I retroelements (Li et al. 2013). In D. melanogaster fat bodies, an overall increased expression of total retrotransposons was found in old flies compared to young ones (Chen et al. 2016). Specifically, around 16% of the annotated retrotransposons had a significant upregulation (Chen et al. 2016). Since ageing has been associated with a gradual reduction of lamin-B (LAM), a protein involved in heterochromatin maintenance, the authors used LAM mutant flies to demonstrate that its depletion in young fat bodies leads to a similar upregulation of retrotransposons as observed in old fat bodies (Chen et al. 2016). They found that upon LAM depletion, there was a decrease in H3K9me3 (repressive histone mark) and an increase in H3K4me3 (activating histone mark) in some de-repressed TE families. This suggested that the age-related reduction of LAM could contribute to the loss of heterochromatin and the activation of retrotransposons in the fat bodies (Chen et al. 2016).
Building on this finding, another study also observed increased TE expression with age in both D. melanogaster fat bodies and heads (Wood et al. 2016). The researchers then genetically enhanced the activity of genes known to influence heterochromatin structure, such as Sir2, Su(var)3-9, and Dicer-2 (Wood et al. 2016). This intervention prevented the age-related loss of TE silencing observed in wild-type lines, further suggesting a common link between heterochromatin factors and the proper maintenance of TE silencing (Wood et al. 2016). Although genetic studies have demonstrated that components of the siRNA/piRNA pathway, such as Ago2 and Dicer-2, are crucial for TE control, their transcript levels have not been found significantly reduced in aged flies (Li et al. 2013; Chen et al. 2016). This suggests that factors beyond simple gene expression changes may be involved in age-associated TE reactivation and need further investigation. Additionally, a fully functional piRNA pathway was described in the adult fly fat body, whose suppression shortened lifespan (Jones et al. 2016). In this work, authors suggested that the presence of this piRNA pathway in normal somatic tissues may offer an additional cellular defence against TE reactivation and possible somatic genomic damage (Jones et al. 2016). However, further studies are needed to better understand the contribution of somatic small RNAs in the control of TE activity during ageing in wild-type populations of Drosophila.
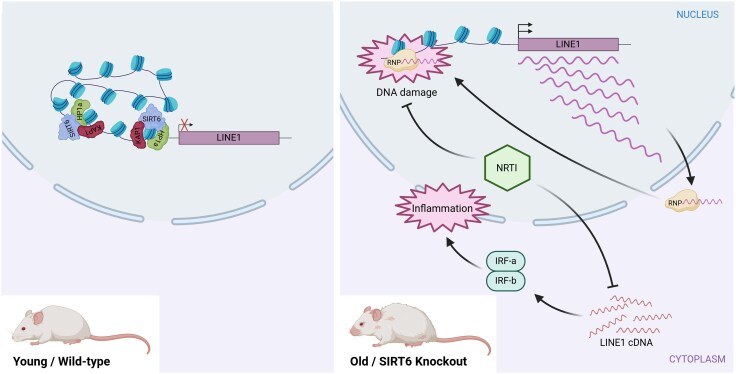
In mammals, including humans, numerous studies have documented an age-associated increase in TE transcripts (De Cecco et al. 2013a, 2013b; Van Meter et al. 2014; Colombo et al. 2018; LaRocca et al. 2020; Stow et al. 2021; Liu et al. 2023; Senapati et al. 2023; Pabis et al. 2024; Tsai et al. 2024). The mechanisms proposed to explain the increase in TE expression are primarily related to changes in chromatin conformation (Jintaridth and Mutirangura 2010; De Cecco et al. 2013b; Van Meter et al. 2014; Colombo et al. 2018; Senapati et al. 2023). Early evidence of this phenomenon was observed in mice, where various families of retrotransposons showed elevated transcription levels during ageing, both in vitro and in vivo (De Cecco et al. 2013a). Indeed, this increase in transcription was associated with a relatively more open chromatin in senescent cells (De Cecco et al. 2013b). In the same species, a specific mechanism was described to be responsible for the expression of LINE1 retroelements with age and stress involving the protein Sirtuin 6 (SIRT6) (Fig. 2; Van Meter et al. 2014). This protein represses LINE1 activity by binding to the 5′UTR of LINE1 loci and facilitates heterochromatin formation at these sites through its interaction with the Heterochromatin Protein 1a (HP1a) via the KRAB-associated protein 1 (Fig. 2; Van Meter et al. 2014). However, during ageing, SIRT6 is depleted from LINE1 loci, impeding the formation of heterochromatin and thus allowing LINE1 activation (Fig. 2; Van Meter et al. 2014).
Fig. 2.
Age-related activation of LINE1 elements due to SIRT6 depletion and its impact on genome stability and inflammation. In young/wild-type mouse cells, LINE1 elements are kept in a repressed state due to the regulatory role of SIRT6. SIRT6 ensures that the 5′ UTR region of LINE1 is packaged into transcriptionally inactive heterochromatin with the coordination of other factors such as KAP1 and HP1a. However, in old and senescent cells or in SIRT6 knockout mice, LINE1 elements become increasingly active. This activation is partly due to the inability to maintain the LINE1 5′ UTR in a stable heterochromatic state. As cells age, SIRT6 is depleted from the LINE1 5′ UTR, as it relocates to sites of DNA damage to facilitate DNA repair. The reduced presence of SIRT6 at the LINE1 5′ UTR weakens the silencing mechanisms, leading to the activation of LINE1 elements. The LINE1 transcripts can assemble into active ribonucleoprotein (RNP) complexes, which, upon transport to the nucleus, enhance LINE1 transposition and lead to DNA damage. Moreover, the cytoplasmic accumulation of LINE1 cDNA triggers a type I interferon (IRF) response, leading to pathological inflammation. Notably, inhibiting LINE1 replication with nucleoside reverse-transcriptase inhibitors (NRTIs) in aged mice significantly improved both mice health and lifespan.
In humans, artificially induced senescent cell lines exhibit a higher expression of TEs compared to non-senescent cell lines (Colombo et al. 2018). While epigenetic modifications do not always directly result in changes in TE expression, they can be strong indicators of potential shifts in their expression. Supporting this, a comprehensive epigenetic analysis of human TEs across various normal tissues and developmental stages revealed age-dependent variations in TE epigenetic profiles (Pehrsson et al. 2019). Similarly, DNA methylation profiling using whole-genome bisulfite sequencing in human mammary luminal epithelial cells (key cells implicated in breast cancer) from younger and older women showed that several TE subfamilies consistently exhibited methylation loss with age (Senapati et al. 2023).
Recent research suggests that transcriptional defects, such as increased intron retention and transcriptional readthrough may drive elevated TE expression during ageing and cellular senescence in mammals (Pabis et al. 2024). These defects become more frequent with age, potentially contributing to the rise in TE expression (Pabis et al. 2024). These findings indicate that transcriptional defects, rather than the sole expression of autonomous insertions, may be also responsible for age-related changes in transposon expression (Pabis et al. 2024).
Overall, the literature consistently reports an increased TE expression with ageing (Table 1), a trend that has been proposed as a reliable marker of biological age (LaRocca et al. 2021).
Impact of Ageing on TE Transposition
As previously mentioned, ageing can trigger the somatic expression of TEs, often as a result of disruptions in TE silencing mechanisms. However, evidence is limited regarding whether this age-induced TE expression leads to new transposition events. While there is some evidence of specific TE families increasing their copy number with age, this phenomenon has been observed in only a few species (Table 2).
Table 2.
Summary table of the impact of ageing on TE transposition in different organisms
| Organism | Tissue | Impact on TE transposition | TE family(ies) | Method | References |
|---|---|---|---|---|---|
| Drosophila melanogaster | Whole body | Increase | 412, copia (Class I) | Southern blot | Driver and McKechnie (1992) |
| D. melanogaster | Heads | Increase | mdg4 (Class I) | mdg4-TRAP system | Li et al. (2013) |
| D. melanogaster | Whole body | Increase | mdg4 (Class I) | mdg4-TRAP system | Wood et al. (2016) |
| D. melanogaster | Glial and neuronal cells | Increase | mdg4 (Class I) | CLEVR | Chang and Dubnau (2019) |
| D. melanogaster | Whole body/brain | Increase | ND | TIDAL software | Yang et al. (2022) |
| D. melanogaster | Whole body/heads/abdomens | Increase | ND | DeviaTE software | Fabian et al. (2021) |
| D. melanogaster | Brains | No differences | ND | TEMP software | Treiber and Waddell (2017) |
| D. melanogaster | Thoraces | No differences | ND | Retrofind software | Schneider et al. (2023) |
| M. musculus | Liver and muscle | Increase | LINE1 and MusD (Class I) | qPCR assay of genomic DNA | De Cecco et al. (2013a) |
| H. sapiens | Brain | Increase | LINE1 and Alu (Class I) | TLDR software | Ramirez et al. (2025) |
ND, not determined.
Some of the earliest evidence of age-related transposition was documented in D. melanogaster (Driver and McKechnie 1992). In this study, the increase in transposition with ageing was examined for the 412 and copia Class I retroelements (Driver and McKechnie 1992). Southern blot analysis of genomic DNA from old flies showed an increase in band intensity compared to young flies, indicating that new insertions of these two TE types occur with age (Driver and McKechnie 1992). In the same species, the use of the mdg4-TRAP reporter system was successfully used to reveal de novo transposition during ageing (Li et al. 2013; Wood et al. 2016). This system is designed so that green fluorescent protein (GFP) is expressed when an endogenous mdg4 retrotransposon inserts into an engineered site containing sequences from the ovo locus, a known hotspot for mdg4 insertion (Dej et al. 1998). Additionally, the use of the mdg4-TRAP system in flies subjected to a dietary restriction regimen (known to extend lifespan) demonstrated that nutritional shortage suppressed the age-related increase in mdg4 transposition events compared to flies on a high-calorie diet (Wood et al. 2016). Similarly, the development of a novel reporter system named Cellular Labelling of Endogenous retroVirus Replication (CLEVR), which is more sensitive and more versatile, also revealed an age-dependent increase of mdg4 retrotransposition in glial and neuronal cells (Chang and Dubnau 2019).
Subsequently, rather than focusing on specific transposition events within particular TE families, some studies expanded their analysis to nearly all described TE families in Drosophila using bioinformatic approaches on sequencing data (BOX 1) (Fabian et al. 2021; Yang et al. 2022). Using the software TIDAL to analyze whole-genome sequencing data from three different wild-type strains, researchers observed a higher number of uniquely detected TE insertions in aged flies compared to young fly genomes (Yang et al. 2022). Moreover, analysis of TE copy number using the software DeviaTE in populations selected for longevity revealed that TE families were generally more abundant in long-lived populations (Fabian et al. 2021). However, additional RNA-seq analysis showed a tendency toward a reduced TE expression in these populations, suggesting that controlling TE activity at the transcriptional level may be more critical for lifespan extension than regulating genomic insertions (Fabian et al. 2021).
Nevertheless, not all studies align with these findings. For example, the software TEMP did not detect a significant increase in new transposition events with age in genomic DNA from single adult fly neuronal cells (Treiber and Waddell 2017). Likewise, analysis of single-nucleus genome sequencing data using the software Retrofind revealed no significant increase in the number of TE insertions in nuclei from old flies compared with young ones (Schneider et al. 2023). Indeed, 76% of nuclei examined from indirect flight muscle and approximately 50% from thoraxes had no new insertions (Schneider et al. 2023).
In mice, age-related increase in TE transposition was reported for two specific Class I TE families, LINE1 and MusD. Quantitative PCR analysis of genomic DNA from skeletal muscle and liver tissues in 36-month-old mice revealed a significant increase in the copy number of these two TE families (De Cecco et al. 2013a). Moreover, authors also observed that LINE1 and MusD increase in naturally occurring age-associated cancers such as lymphoma and hepatocellular carcinoma (De Cecco et al. 2013a).
In humans, advances in long-read sequencing techniques have enabled the detection of novel transposition events in 18 frontal cortex brain samples from individuals between 67- and 92-year old (Ramirez et al. 2025). Using the software TLDR, which identifies retrotransposon insertions from long-read sequencing data, researchers found novel insertions of the Alu and LINE1 families in the ageing human brain (Ramirez et al. 2025). However, the authors noted that the number of Alu and LINE1 insertions identified per sample is likely underestimated due to the relatively low coverage of their long-read sequencing (Ramirez et al. 2025).
These findings highlight the complexity of TE regulation during ageing and indicate that further investigation is needed to fully understand the link between ageing and new transposition events, or whether such insertions are tissue-specific, as experimental and bioinformatic approaches can significantly influence the detection and interpretation of TE activity in ageing cells.
Impact of TE Activation in Longevity
TE activation (both expression and transposition) can impact cellular function in multiple ways. As already mentioned, TEs can modify the genome through their mobilization and influence host gene expression both in cis and trans, at transcriptional and post-transcriptional levels (Elbarbary et al. 2016; Chuong et al. 2017; Bourque et al. 2018; Bourgeois and Boissinot 2019). These alterations are a clear source of cellular damage, which can subsequently impact the organism's lifespan. Hence, the transposon ageing model suggests that the exponential increase in TE copy number over time within the genome could ultimately lead to cell or organism death as a result of an accumulation of somatic mutations (Murray 1990).
The effect of increased TE transposition on age-related phenotypes was first shown in Drosophila species (Driver and McKechnie 1992; Woodruff and Nikitin 1995). In these studies, artificial somatic transposition of P-element Class II insertions in D. melanogaster flies induced a reduction of lifespan in both males and females (Driver and McKechnie 1992; Woodruff and Nikitin 1995). Similar findings were observed with the somatic mobilization of the mariner element in D. simulans (Class II) (Nikitin and Woodruff 1995). However, this was not the case in D. melanogaster, likely because the tested strains contained only two potentially mobile copies of the mariner element (Nikitin and Woodruff 1995). Consequently, the somatic genomic damage caused by mariner elements would be less than that of P-elements, which had 17 putatively active copies in the tested D. melanogaster strains (Nikitin and Woodruff 1995). More recently, in the same species, no increase in TE transposition with age was observed. However, reduced expression of 412 and Roo Class I elements led to an extension in lifespan, suggesting that TE expression, rather than an increase in copy number, plays a key role in longevity (Schneider et al. 2023).
TE expression has also been associated with age-related phenotypes in Ago2 mutant flies. As expected, these mutants showed elevated TE expression in somatic tissues, along with progressive, age-related memory impairment and shorter lifespans compared to wild-type flies (Li et al. 2013). This finding aligned with previous works showing that mutations in Dicer-2 and loquacious genes, similarly result in reduced lifespans (Lim et al. 2011; Liu et al. 2012; Wood et al. 2016).
To investigate the contribution of TE activity to age-related effects, pharmacological inhibition of TE transposition using the reverse transcriptase inhibitor lamivudine (3TC) resulted in a lifespan extension in Dicer-2 mutants. These mutants exhibited increased double-strand breaks, likely caused by TE transposition, which was mitigated by 3TC treatment (Wood et al. 2016). Similarly, the loss of function of Hinfp, a zinc-finger transcription factor that interacts with Histone 1, a key chromatin assembly factor, leads to the derepression of numerous TEs (Nirala et al. 2021). This derepression accelerates ageing and promotes cancer-related phenotypes, such as abnormal cell proliferation and tissue transformation (Nirala et al. 2021). Rather than modifying genes involved in TE silencing, directly activating mdg4 insertions with a UAS-Gal4 system increased mortality in middle-aged flies, despite the retrotransposon being active early in life (Rigal et al. 2022). Authors suggested that young flies might tolerate TE activation, but as they age, TE activity could become detrimental, ultimately affecting their survival (Rigal et al. 2022). This may be due to the combined effects of age-related metabolic and physiological changes with TE activity, which together exacerbate cellular damage. Notably, this period coincides with the natural expression of endogenous TEs during normal ageing (Rigal et al. 2022).
In mice, as mentioned in a previous section, SIRT6 deficiency led to the derepression of LINE1 elements, resulting in the cytoplasmic accumulation of LINE1 cDNA. This triggered a type I interferon (IFN-I) response, causing pathological inflammation, which is a hallmark of ageing (Fig. 2; Simon et al. 2019). As a consequence, mice exhibited an acute, degenerative ageing-like phenotype, characterized by reduced lifespan and growth retardation. Notably, inhibiting LINE1 replication with nucleoside reverse-transcriptase inhibitors significantly improved the health and extended the lifespan of SIRT6 knockout mice, further validating these findings (Fig. 2; Mostoslavsky et al. 2006; Simon et al. 2019).
Similarly, in humans, LINE1 derepression during cellular senescence in fibroblasts leads to its upregulation, which in turn activates the IFN-I response. Again, this activation appears to depend on the accumulation of LINE1 cDNA in the cytoplasm, ultimately causing inflammation (De Cecco et al. 2019). Furthermore, LINE1 elements, which contain lamin-associated domains, are anchored to the nuclear lamina through SIRT7-mediated deacetylation, a mechanism that contributes to the regulation of their expression (Vazquez et al. 2019). Lamin proteins (structural proteins of the nuclear lamina that help maintain nuclear integrity and organize chromatin) are often lost or reduced in ageing and cancer cells. This loss likely compromises the ability of SIRT7 to tether LINE1 elements to the nuclear periphery, leading to their activation. As a result, LINE1 activation may contribute to phenotypes such as increased DNA damage and changes in gene expression (Vazquez et al. 2019).
Also in humans, a recent study has shown that chronological age (the time an organism has been alive) is not directly correlated with the expression of LTR, LINE, or SINE TE families in blood samples from multiple cancer-free cohorts (Tsai et al. 2024). Instead, the expression of these retrotransposons was found to be correlated with biological age (the physiological and functional state of an organism), with LTR and LINE expression associated with inflammatory responses, and SINE expression linked with the upregulation of the DNA repair pathways, both key hallmarks of ageing (Tsai et al. 2024). Therefore, SINE might contribute to ageing through genome instability, whereas LTR and LINE expression could be associated with inflammation (Tsai et al. 2024).
In models of premature ageing such as Hutchinson–Gilford progeria syndrome and Werner syndrome, senescent human muscle precursor cells exhibited a derepression of HERV-K insertions (Liu et al. 2023). This derepression led to the production of retrovirus-like particles, contributing to cellular senescence and accelerating ageing effects in younger cells, while also activating the innate immune response (Liu et al. 2023). In addition, neutralizing these HERV-K particles with antibodies has shown potential in mitigating these age-related processes (Liu et al. 2023). Likewise, early activation of LINE1 elements in progeroid cells, with signs of early-onset ageing, has been associated with heterochromatin loss and the emergence of senescent phenotypes (Della Valle et al. 2022). Targeting LINE1 RNA with antisense oligonucleotides helped restore heterochromatin epigenetic marks and lowered the expression of senescence-associated genes in both human cells and in a mouse model of Hutchinson–Gilford progeria syndrome, ultimately extending lifespan (Della Valle et al. 2022).
While TEs are primarily associated with promoting ageing or age-related phenotypes, there are also examples in the literature highlighting their beneficial effects on lifespan.
The evolution of telomeres is an example of antagonistic evolution of TEs. DNA polymerase is unable to fully replicate the ends of chromosomes, leading to their gradual shortening as the organism ages. Telomeres, which are chromosomal extensions, serve to protect against this loss of chromosomal length (Aubert and Lansdorp 2008). These structures are primarily composed of repeated sequences that, in Drosophila, are derived from TEs (Mason and Biessmann 1995). Notably, the elongation of telomeres in Drosophila is facilitated by specific retrotransposons: HeT-A, TART, and TAHRE (Pardue and DeBaryshe 2008). Consequently, the maintenance of telomeres and the control of TE activity within an organism must be carefully coordinated. Telomeric TEs are targeted by piRNAs in the same manner as other TEs, and disruptions in the piRNA pathway can lead to increased transcript levels of these TEs (Savitsky et al. 2006). Unlike other insertions, telomeric TEs are consistently transcribed in both sense and antisense orientations, which allows telomere maintenance despite the regulation exerted by the piRNA pathway (Danilevskaya et al. 1999).
Another example of the beneficial impact of TEs on ageing was observed in natural populations of D. melanogaster. The Hoppel insertion within the first intron of the longevity gene Indy enhances both fecundity and lifespan (Zhu et al. 2014). Moreover, the expression levels of Indy were found to be positively correlated with the presence of the Hoppel insertion (Zhu et al. 2014). However, homozygosity for the Indy insertion variant results in reduced fitness, thereby favouring the retention of the insertion allele in the heterozygous state (Zhu et al. 2014).
In summary, while there are rare instances where TE activity may positively influence the evolution of longevity, the overall evidence suggests that TE activity predominantly contributes to reduce lifespan. While the most evident consequence of TE expression with age is its potential to cause genomic instability through TE transposition, emerging evidence suggests that TE expression alone may also have an important role in reducing longevity, likely due to the activation of inflammatory responses. Although further research is necessary, targeting retrotransposon activity and the associated inflammation could potentially become a therapy for age-related diseases in the future (Simon et al. 2019).
The Role of TE Activity in Age-Related Diseases
Increased TE activity has been observed in various age-related diseases, often due to epigenetic control failures that occur naturally with ageing, as previously discussed. These disruptions can be exacerbated by genetic predispositions and environmental factors, influencing disease through different mechanisms (Gorbunova et al. 2021; Chénais 2022; Copley and Shorter 2023).
Elevated TE activity is known to cause DNA damage, primarily through double-strand breaks, which activate cell death pathways and contribute to neurodegeneration in diseases such as amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD), ataxia, and tauopathies like Alzheimer's disease (Frost et al. 2014; Takahashi et al. 2022; Liu et al. 2023). Comprehensive genome analyses have also shown that increased retrotransposition contributes to genomic instability across multiple cancer types (Rodriguez-Martin et al. 2020). This instability arises through mechanisms such as deletions, translocations, duplications, ultimately promoting cancer development and driving metastasis (Rodriguez-Martin et al. 2020).
The inflammatory response triggered by TE expression is also a common feature in many diseases including cancer, ALS/FTD, Alzheimer, multiple sclerosis, and autoimmune disorders (Zhao et al. 2018; Tossberg et al. 2020; Mosaddeghi et al. 2023; Ochoa et al. 2023; Zhen et al. 2023). This occurs because double-stranded RNA generated during retrotransposition are typically recognized by cells as foreign, often triggering the innate immune response, which in some cases leads to apoptosis.
The potential role of insertions as cis-regulatory sequences has also been linked with cancer and neurodegenerative diseases. In cancer, several studies have demonstrated that epigenetic changes in TE insertions can confer promoter-like functions to TEs, potentially driving oncogene expression (Lock et al. 2014; Deniz et al. 2020; Senapati et al. 2023; Lanciano and Cristofari 2024). Supporting this, a large tumour dataset analysis identified 1,068 cases of cryptic promoter activation involving TEs across 33 tumour types (Shah et al. 2023). In neurodegenerative diseases, SINE-VNTR-Alu (SVA) elements have been shown to regulate the expression of their neighbouring genes in human neuronal cells. For example, the genetic deletion of SVA elements in Alzheimer and Parkinson disease-associated risk loci resulted in epigenomic changes in the surrounding sequences through a significant reduction of H3K4me3 histone mark (van Bree et al. 2022). Moreover, the deletion of the SVA element at the BCKDK locus was associated with increased expression of the gene encoding the RNA-binding protein FUS (van Bree et al. 2022), which its abnormal aggregation has been previously linked to neurodegenerative diseases such as ALS and FTD (Deng et al. 2014).
Finally, TEs have been shown to contribute to cancer development by facilitating the tissue-specific transfer of circulating cell-free tumour-derived DNA (ctDNA; Cinar et al. 2024). These molecules facilitate tumour progression by transferring oncogenes between tumour cells, altering the tumour microenvironment, and modifying cellular responses to treatment (García-Olmo et al. 2010; Trejo-Becerril et al. 2012; Dvořáková et al. 2013; Cinar et al. 2024).
Overall, increased TE activity is implicated in a variety of age-related diseases, including neurodegenerative disorders and cancer, with mechanisms such as DNA damage, inflammatory responses, and dysregulated gene expression playing central roles in disease progression.
Transposable Elements and Sex Gap in Longevity
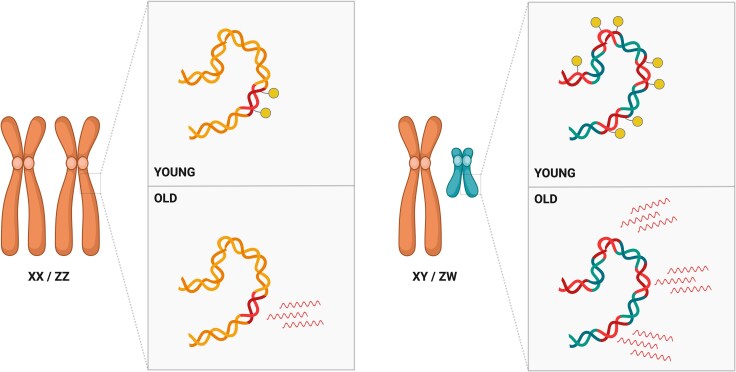
The age-related activation of TEs has been linked to the phenomenon of the sex gap in longevity. This refers to the fact that, in many animal species, males and females exhibit distinct mortality patterns leading to variations in longevity (Marais et al. 2018; Lemaître et al. 2020). Between males and females, the amount of TE sequences can differ substantially due to the presence of a repeat-rich Y or W chromosome in the heterogametic sex. One plausible explanation for the sex gap in longevity is the “toxic Y effect”, which suggests that the higher abundance of TEs on the Y (or W) chromosome compared to other chromosomes may have harmful consequences for the heterogametic sex (Fig. 3; Brown et al. 2020a; Nguyen and Bachtrog 2021; Peona et al. 2021; Warmuth et al. 2022; Teoli et al. 2024). This hypothesis proposes that, due to the TE-rich Y chromosome, more TEs may become active in older males than in females, potentially contributing to sex differences in ageing and the shorter lifespan observed in males (Fig. 3; Brown et al. 2020a; Nguyen and Bachtrog 2021; Peona et al. 2021; Warmuth et al. 2022; Teoli et al. 2024).
Fig. 3.
Schematic representation of the toxic Y effect. The heterogametic sex (XY or ZW chromosomes, right) harbours a higher abundance of TE sequences (red) compared to the homogametic sex (XX or ZZ chromosomes, left). These TEs are typically silenced by epigenetic mechanisms (yellow lollipops). However, in aged individuals, these silencing mechanisms may weaken, leading to the activation of TEs. Because the Y or W chromosomes are TE-rich, more TEs may become active in one sex than the other, potentially contributing to sex differences in ageing and reduced lifespan.
In D. melanogaster, it has been shown that ageing males experience a more rapid loss of H3K9me2 compared to females, leading to a greater TE activation during ageing in males (Brown et al. 2020b). This aligns with the observation that the Y chromosome, and TEs in general, impact chromatin structure genome-wide, supporting the “heterochromatin sink” model (Gatti and Pimpinelli 1992; Brown et al. 2020b). This model refers to the idea that the TE activation during ageing drains (hence “sink”) proteins and other factors that are usually involved in the maintenance of the heterochromatin. When these factors are depleted or mislocalized, the heterochromatin weakens further, creating a feedback loop where more TEs become activated, further straining the heterochromatin (Gatti and Pimpinelli 1992). Furthermore, putatively Y-linked TEs seem to lose heterochromatin more readily as individuals age, indicating that male-specific TEs are particularly susceptible to derepression in older flies (Brown et al. 2020a). Longevity assays on flies with abnormal sex chromosome configurations (XO, XXY, and XYY) also suggest that additional Y chromosomes could decrease lifespan (Brown et al. 2020a).
Recent transcriptomic data from human individuals with different karyotypes (XX, XY, XXY, and XYY) suggested that the presence and number of Y chromosomes may be associated with increased expression of certain TE subfamilies. This elevated TE expression could potentially contribute to a reduced lifespan in individuals carrying one or more Y chromosomes (Teoli et al. 2024).
However, using CRISPR/Cas9 to generate D. melanogaster Y chromosomes of varying sizes indicated that the size of the Y chromosome, and thus the amount of heterochromatin and TEs, did not contribute to sex-specific longevity differences (Delanoue et al. 2023). Similarly, in D. pseudoobscura, while larger Y chromosomes were associated with slightly increased TE expression, particularly in older males, these differences did not accelerate ageing (Nguyen and Bachtrog 2021).
As a result, the role of the Y chromosome in reducing male longevity through increased TE activity remains controversial, highlighting the need to investigate the contribution of TEs to sex differences in longevity across other species.
TEs and Their Association With Longevity in Long-Lived Organisms
Studying long-lived species could provide valuable insights into the mechanisms of ageing and cellular senescence, potentially clarifying the role of TEs in longevity. The concept of “negligible senescence” was first introduced to describe organisms with exceptionally slow ageing rates (characterized by gradual changes in physical appearance, reproductive capacity, and susceptibility to disease) such as certain conifers and fish species (Finch 1998). The mechanisms that contribute to slow senescence are diverse and species-specific, though some examples involving TEs have been reported (Sulak et al. 2016; Zhao et al. 2021; Jové et al. 2023; Sahm et al. 2024).
As expected given their mutagenic capacity, most of the studies of long-lived species show a consistent pattern of overall reduction in both TE abundance and TE expression (Sturm et al. 2017; Elsner et al. 2018; Teefy et al. 2020; Ricci et al. 2023). In Hydra, a long-lived freshwater cnidarian, mortality does not increase with age, indicating an absence of senescence (Schaible et al. 2015). One proposed mechanism behind Hydra's ability to evade ageing is the continued activity of the piRNA pathway in somatic cells (Sturm et al. 2017; Teefy et al. 2020). Indeed, the somatic activity of the piRNA pathway in Hydra represses TE expression in its somatic cell lineages, potentially contributing to the organism's exceptional longevity (Teefy et al. 2020).
The naked mole rat (Heterocephalus glaber) is a well-known organism studied for its resistance to cancer and its remarkable longevity compared to closely related species. A comparative genomic study between H. glaber and short-lived cancer-prone rodents (guinea pigs, rats, and mice) revealed a distinct TE landscape in H. glaber, reflecting a recent TE dynamic markedly different from the other rodents (Ricci et al. 2023). Notably, it has been observed that H. glaber did not accumulate non-LTR retrotransposons in recent evolutionary times, unlike the other species. Furthermore, an analysis of open reading frames with intact protein domains showed that naked mole rats possess only LINE elements lacking functional domains (Ricci et al. 2023).
Additionally, the comparison of the TE content and dynamic in long-lived bats from the genera Myotis, Rhinolophus, Pteropus, and Rousettus against the short-lived Molossus molossus (one of the bats with the shortest lifespan in the order Chiroptera) revealed a higher overall TE content in M. molossus (40.6%) compared to the long-lived bats (ranging from 28.84% to 34.29%; Ricci et al. 2023). Similar to the observations of the naked mole rat, long-lived bats showed a reduction in non-LTR retrotransposons. In contrast, the short-lived M. molossus had a high proportion of SINE elements, which rely on the transcriptional machinery of LINE elements for transposition (Ricci et al. 2023). This may impose a double burden of TE expression, potentially triggering inflammatory responses. The study suggests that both TE content and evolutionary TE dynamics, not just expression levels, may be linked to longevity (Ricci et al. 2023).
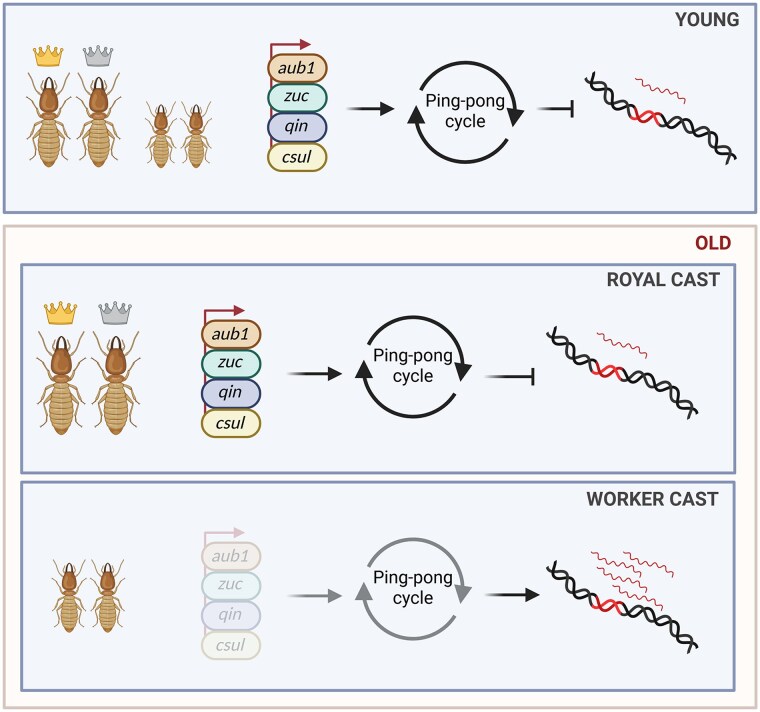
Social insects like honey bees and termites present valuable new models for ageing research due to the striking lifespan differences between colony members. The reproductive individuals, such as queens and kings, live for several decades, while sterile workers typically survive only a few weeks (Elsner et al. 2018). A transcriptomic analysis of old and young queens, kings, and termite workers (Macrotermes bellicosus) reported a 14.9% increase in TE expression only in old workers (Fig. 4; Elsner et al. 2018). This increase in TE expression appears to be linked to the downregulation of piRNA-pathway-related genes in workers compared to queens and kings, suggesting a reduction in the ping-pong cycle response in older workers (Fig. 4; Elsner et al. 2018).
Fig. 4.
Schematic representation of the mechanism underlying the difference in longevity between royal and worker castes in termites. In termites, the queen and king greatly outlive the workers. Analysis of TE expression in young and old samples of either cast revealed an increase in TE transcripts only in old workers, likely due to a piRNA pathway downregulation. Indeed, the downregulation of piRNA pathway effectors, such as aub1, zuc, qin and csul, in older workers results in decreased TE control, which subsequently leads to an increase in TE expression.
Although TEs are typically associated with negative effects on ageing, they have, in some cases, been found to play a beneficial role in the ageing process (Sulak et al. 2016; Zhao et al. 2021; Sahm et al. 2024). For example, blind mole rats (Spalax galili), small rodents known for their remarkably long lifespan (over 21 years) and their resistance to both spontaneous and induced tumour formation, provide a compelling example of how TE activation can function as a tumour suppressor (Zhao et al. 2021). In this species, the activation of retrotransposons triggers the innate immune system, initiating a coordinated cell death process that helps prevent tumour formation. Specifically, retrotransposons contribute to the formation of cytoplasmic RNA–DNA hybrids, which activate the cyclic GMP–AMP synthase (stimulator of IFN genes) IFN pathway, leading to the elimination of premalignant cells (Zhao et al. 2021). This protective mechanism is associated with reduced DNA methylation levels, allowing for the increased retrotransposon expression and, in turn, enhance tumour suppression (Zhao et al. 2021).
Another relevant example can be found in African elephants (Loxodonta africana), the largest living land mammal, where it has been observed that their genome contains 20 copies of the master tumour suppressor gene TP53, known as TP53 retrogenes (TP53RTG) (Sulak et al. 2016). TP53 was retroduplicated and increased in copy number through repeated segmental duplications (Sulak et al. 2016). Indeed, each of the TP53RTG retrogenes was found to be flanked by nearly identical clusters of TEs (Sulak et al. 2016). This expansion of the TP53RTG family might have enhanced the DNA damage response, resulting in increased resistance to cancer and extended longevity (Sulak et al. 2016; Vazquez and Lynch 2021).
Recently, a chromosome-level genome assembly of the Greenland shark (Somniosus microcephalus), the longest-lived vertebrate known with an estimated lifespan of around 400 years, revealed a TE content of 70.6%. This represents the highest proportion of TE content reported among all known shark species (Sahm et al. 2024). The expansion of TEs might be related to the expansion of DNA repair genes. Particularly, retrotransposon activity may have facilitated the expansion of DNA repair-related retrogenes, which in turn enabled the organism to tolerate higher levels of TE activity (Sahm et al. 2024).
Overall, these studies likely represent a glimpse of the vast potential for discovery regarding the relationship between TEs and extreme longevity, with examples of both negative and positive associations.
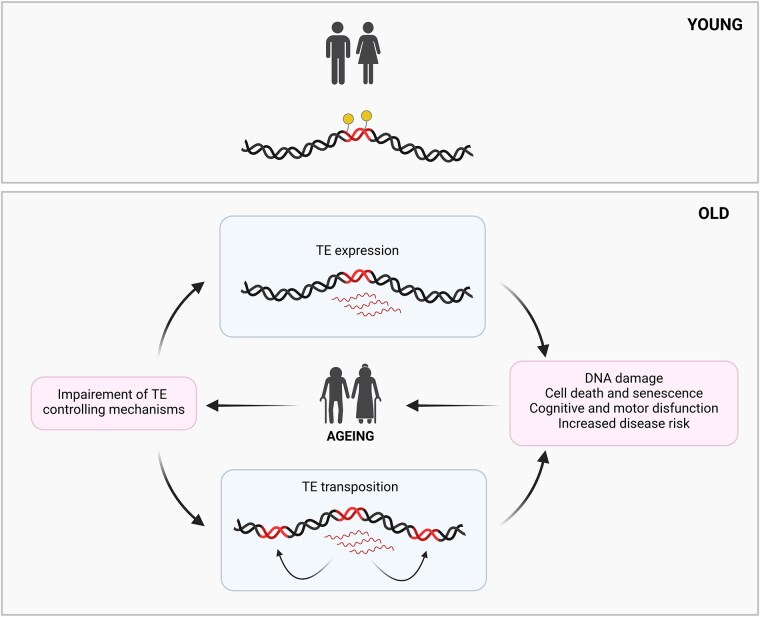
Conclusions and Perspectives
Based on the analysis of existing literature, we conclude that TE activity plays a feedforward role in ageing, acting both as a cause and a consequence (Fig. 5). TEs interact with various cellular pathways and epigenetic mechanisms that influence ageing, highlighting their complex involvement within a broader network of ageing-related processes.
Fig. 5.
TE activity as a cause and consequence of ageing. TEs are typically repressed by epigenetic mechanisms (represented as yellow lollipops). With ageing, the breakdown of these regulatory mechanisms leads to their deregulation, resulting in increased expression and occasional transposition. This elevated TE activity can contribute to DNA damage, cell death, and cellular senescence, as well as cognitive and motor dysfunction, ultimately raising disease risk and potentially accelerating age-related decline.
As ageing progresses, the breakdown of mechanisms that regulate TEs leads to their deregulation, resulting in increased expression and sometimes transposition. This activity contributes to genomic instability and can significantly impact cellular health, for example, through the production of TE-derived transcripts that may activate inflammatory pathways. Together, these effects drive age-related decline and disease (Fig. 5). Yet, intriguingly, some organisms with a high TE abundance do not experience reduced lifespans, suggesting that the relationship between TE abundance and ageing is far from straightforward.
Future research should aim to unravel the mechanisms that drive TE activation during ageing and investigate whether TE expression alone can have significant effects on the ageing process in different species. Advanced sequencing and bioinformatics techniques will be crucial for obtaining a more detailed assessment of TE activity at the copy-specific level, potentially revealing how individual elements contribute to age-related phenomena. Additionally, exploring the interactions between TEs and epigenetic regulators in the context of ageing holds great promise for developing novel therapeutic interventions, where targeted modulation of TE activity could help alleviate age-related dysfunctions.
BOX 1: Tools to Study TE Activity
Annotating TEs and analyzing their expression or transposition rates is challenging due to their repetitive nature, requiring specialized bioinformatic tools and pipelines (Teissandier et al. 2019; Lanciano and Cristofari 2020; Loreto et al. 2024). Here, we present a selection of some of the most recent and/or widely used tools that can aid in studying the dynamics of TEs.
A key question in the study of TEs and ageing is whether transposition increases with age, which can be addressed by comparing sequencing data from young and old samples. Nevertheless, inferring TE abundance or identifying low-frequency polymorphic TEs from sequencing reads, rather than from complete genome assemblies, presents unique challenges, especially when analyzing samples with nearly identical genome assemblies. This issue frequently arises in age-related comparisons of the same organism or tissue type. Indeed, a commonly employed experimental approach, pooled sequencing, involves combining tissues from multiple individuals and sequencing the DNA without tracking the origin of each read. In pooled sequencing data, methods for detecting structural variations in nearly identical genomes are generally limited to identifying variations shared by the majority within the pool. Detecting TE transpositions and estimating their frequencies in pooled sequencing datasets introduces additional computational challenges. Key obstacles include confidently identifying rare TE transposition events, recognizing reads that support the same transposition event, and mitigating biases due to non-uniform sequencing depth across the genome.
In studies of TE transposition and ageing, software such as TEMP (Zhuang et al. 2014) and TIDAL (Rahman et al. 2015) have been used to identify polymorphic TEs in short-read pool-sequencing data and estimate their frequencies, helping to determine whether ageing increases transposition rates (Treiber and Waddell 2017; Yang et al. 2022). Similarly, DeviaTE can be applied to both pool and individual sequencing data, and latest updates support the use of either long-read or short-read sequencing (Weilguny and Kofler 2019).
Recently, tools like TrEMOLO (Mohamed et al. 2023) and GraffiTE (Groza et al. 2024) have been developed to infer insertion frequencies of polymorphic TEs in pooled data from long-reads or genome assemblies, with the latter employing a pangenomic approach.
It is also essential in age-related studies to precisely measure TE expression, including copy-specific information (Lanciano and Cristofari 2020). TEs within the same family, by definition, share over 80% sequence identity, which complicates the investigation of TE regulation and expression in a copy-specific manner (Wicker et al. 2007; Lanciano and Cristofari 2020). Most genome-wide studies of TE expression analyze data at the family level. This process typically involves mapping short reads to either TE consensus sequences or specific TE copy sequences, followed by read counts for each family. Tools such as TEcount from the TEtools package (Lerat et al. 2017) and TEtranscripts (Jin et al. 2015) are commonly used for these analyses. Recently, numerous methods have emerged that leverage short-read sequencing datasets to address the multi-mapping issue and enable copy-level analyses (Lanciano and Cristofari 2020). These approaches utilize various algorithms to statistically reassign multi-mapped reads to specific locations, such as the expectation-maximization algorithm employed in TEtranscripts (Jin et al. 2015), SQuIRE (Yang et al. 2019), and Telescope (Bendall et al. 2019). TE expression pipelines that support the use of long-read sequencing data have been also recently developed (Kelsey et al. 2024; Rebollo et al. 2024).
A wide array of tools are available for analyzing TE transposition and expression, with new ones emerging as sequencing technologies continue to evolve (TE Hub Consortium et al. 2021). An extensive list of these tools, along with various other resources, can be found on the community-driven platform TEhub (https://tehub.org/).
Acknowledgments
We thank Tomás Carrasco-Valenzuela, Matthieu Boulesteix, Marie Fablet, and Rita Rebollo for their insightful comments on the manuscript. Figures were created with BioRender.com.
Contributor Information
Miriam Merenciano, Laboratoire de Biométrie et Biologie Evolutive, CNRS, UMR5558, Université Claude Bernard Lyon 1, Villeurbanne 69100, France.
Anaïs Larue, Laboratoire de Biométrie et Biologie Evolutive, CNRS, UMR5558, Université Claude Bernard Lyon 1, Villeurbanne 69100, France; INRAE, BF2I, UMR203, INSA Lyon, Villeurbanne 69621, France.
Chloé Garambois, Laboratoire de Biométrie et Biologie Evolutive, CNRS, UMR5558, Université Claude Bernard Lyon 1, Villeurbanne 69100, France.
William Vilas Boas Nunes, Laboratoire de Biométrie et Biologie Evolutive, CNRS, UMR5558, Université Claude Bernard Lyon 1, Villeurbanne 69100, France.
Cristina Vieira, Laboratoire de Biométrie et Biologie Evolutive, CNRS, UMR5558, Université Claude Bernard Lyon 1, Villeurbanne 69100, France.
Funding
This work was supported by the Agence Nationale de la Recherche (project LongevitY, grant Projet-ANR-20-CE02-0015), the HORIZON EUROPE Marie Sklodowska Curie Actions (project GeEpiAdaptation, grant 101065313), by the INSA Lyon and by the Université Claude Bernard Lyon 1.
Data Availability
No new data were generated or analyzed in support of this research.
Literature Cited
- Arkhipova IR. Neutral theory, transposable elements, and eukaryotic genome evolution. Mol Biol Evol. 2018:35(6):1332–1337. 10.1093/molbev/msy083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008:88(2):557–579. 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- Batut P, Dobin A, Plessy C, Carninci P, Gingeras TR. High-fidelity promoter profiling reveals widespread alternative promoter usage and transposon-driven developmental gene expression. Genome Res. 2013:23(1):169–180. 10.1101/gr.139618.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall ML, de Mulder M, Iñiguez LP, Lecanda-Sánchez A, Pérez-Losada M, Ostrowski MA, Jones RB, Mulder LCF, Reyes-Terán G, Crandall KA, et al. Telescope: characterization of the retrotranscriptome by accurate estimation of transposable element expression. PLoS Comput Biol. 2019:15(9):e1006453. 10.1371/journal.pcbi.1006453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biémont C. A brief history of the status of transposable elements: from junk DNA to major players in evolution. Genetics. 2010:186(4):1085–1093. 10.1534/genetics.110.124180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biémont C, Vieira C. Junk DNA as an evolutionary force. Nature. 2006:443(7111):521–524. 10.1038/443521a. [DOI] [PubMed] [Google Scholar]
- Bourgeois Y, Boissinot S. On the population dynamics of junk: a review on the population genomics of transposable elements. Genes (Basel). 2019:10(6):419. 10.3390/genes10060419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque G, Burns KH, Gehring M, Gorbunova V, Seluanov A, Hammell M, Imbeault M, Izsvák Z, Levin HL, Macfarlan TS, et al. Ten things you should know about transposable elements. Genome Biol. 2018:19(1):199. 10.1186/s13059-018-1577-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EJ, Nguyen AH, Bachtrog D. The Y chromosome may contribute to sex-specific ageing in Drosophila. Nat Ecol Evol. 2020a:4(6):853–862. 10.1038/s41559-020-1179-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EJ, Nguyen AH, Bachtrog D. The Drosophila Y chromosome affects heterochromatin integrity genome-wide. Mol Biol Evol. 2020b:37(10):2808–2824. 10.1093/molbev/msaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casacuberta E, González J. The impact of transposable elements in environmental adaptation. Mol Ecol. 2013:22(6):1503–1517. 10.1111/mec.12170. [DOI] [PubMed] [Google Scholar]
- Chang Y-H, Dubnau J. The gypsy endogenous retrovirus drives non-cell-autonomous propagation in a Drosophila TDP-43 model of neurodegeneration. Curr Biol. 2019:29(19):3135–3152.e4. 10.1016/j.cub.2019.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zheng X, Xiao D, Zheng Y. Age-associated de-repression of retrotransposons in the Drosophila fat body, its potential cause and consequence. Aging Cell. 2016:15(3):542–552. 10.1111/acel.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chénais B. Transposable elements and human diseases: mechanisms and implication in the response to environmental pollutants. Int J Mol Sci. 2022:23(5):2551. 10.3390/ijms23052551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chénais B, Caruso A, Hiard S, Casse N. The impact of transposable elements on eukaryotic genomes: from genome size increase to genetic adaptation to stressful environments. Gene. 2012:509(1):7–15. 10.1016/j.gene.2012.07.042. [DOI] [PubMed] [Google Scholar]
- Chuong EB, Elde NC, Feschotte C. Regulatory activities of transposable elements: from conflicts to benefits. Nat Rev Genet. 2017:18(2):71–86. 10.1038/nrg.2016.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinar M, Martinez-Medina L, Puvvula PK, Arakelyan A, Vardarajan BN, Anthony N, Nagaraju GP, Park D, Feng L, Sheff F, et al. Transposon DNA sequences facilitate the tissue-specific gene transfer of circulating tumor DNA between human cells. Nucleic Acids Res. 2024:52(13):7539–7555. 10.1093/nar/gkae427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo AR, Triche T, Ramsingh G. Transposable element expression in acute myeloid leukemia transcriptome and prognosis. Sci Rep. 2018:8(1):16449. 10.1038/s41598-018-34189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copley KE, Shorter J. Repetitive elements in aging and neurodegeneration. Trends Genet. 2023:39(5):381–400. 10.1016/j.tig.2023.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado-Zamora M, González J. Transposons contribute to the functional diversification of the head, gut, and ovary transcriptomes across Drosophila natural strains. Genome Res. 2023:33(9):1541–1553. 10.1101/gr.277565.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilevskaya ON, Traverse KL, Hogan NC, DeBaryshe PG, Pardue ML. The two Drosophila telomeric transposable elements have very different patterns of transcription. Mol Cell Biol. 1999:19(1):873–881. 10.1128/MCB.19.1.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovic M, Sevo G, Svorcan P, Milosevic DP, Despotovic N, Erceg P. Old age as a privilege of the “selfish ones.”. Aging Dis. 2010:1:139. [PMC free article] [PubMed] [Google Scholar]
- De Cecco M, Criscione SW, Peckham EJ, Hillenmeyer S, Hamm EA, Manivannan J, Peterson AL, Kreiling JA, Neretti N, Sedivy JM. Genomes of replicatively senescent cells undergo global epigenetic changes leading to gene silencing and activation of transposable elements. Aging Cell. 2013b:12(2):247–256. 10.1111/acel.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cecco M, Criscione SW, Peterson AL, Neretti N, Sedivy JM, Kreiling JA. Transposable elements become active and mobile in the genomes of aging mammalian somatic tissues. Aging (Albany, NY). 2013a:5(12):867–883. 10.18632/aging.100621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cecco M, Ito T, Petrashen AP, Elias AE, Skvir NJ, Criscione SW, Caligiana A, Brocculi G, Adney EM, Boeke JD, et al. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature. 2019:566(7742):73–78. 10.1038/s41586-018-0784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dej KJ, Gerasimova T, Corces VG, Boeke JD. A hotspot for the Drosophila gypsy retroelement in the ovo locus. Nucleic Acids Res. 1998:26(17):4019–4025. 10.1093/nar/26.17.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delanoue R, Clot C, Leray C, Pihl T, Hudry B. Y chromosome toxicity does not contribute to sex-specific differences in longevity. Nat Ecol Evol. 2023:7:1245–1256. 10.1038/s41559-023-02089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Valle F, Reddy P, Yamamoto M, Liu P, Saera-Vila A, Bensaddek D, Zhang H, Prieto Martinez J, Abassi L, Celii M, et al. LINE-1 RNA causes heterochromatin erosion and is a target for amelioration of senescent phenotypes in progeroid syndromes. Sci Transl Med. 2022:14(657):eabl6057. 10.1126/scitranslmed.abl6057. [DOI] [PubMed] [Google Scholar]
- Deng H, Gao K, Jankovic J. The role of FUS gene variants in neurodegenerative diseases. Nat Rev Neurol. 2014:10(6):337–348. 10.1038/nrneurol.2014.78. [DOI] [PubMed] [Google Scholar]
- Deniz Ö, Ahmed M, Todd CD, Rio-Machin A, Dawson MA, Branco MR. Endogenous retroviruses are a source of enhancers with oncogenic potential in acute myeloid leukaemia. Nat Commun. 2020:11(1):3506. 10.1038/s41467-020-17206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle WF, Sapienza C. Selfish genes, the phenotype paradigm and genome evolution. Nature. 1980:284(5757):601–603. 10.1038/284601a0. [DOI] [PubMed] [Google Scholar]
- Driver CJ, McKechnie SW. Transposable elements as a factor in the aging of Drosophila melanogaster. Ann N Y Acad Sci. 1992:673(1):83–91. 10.1111/j.1749-6632.1992.tb27439.x. [DOI] [PubMed] [Google Scholar]
- Dvořáková M, Karafiát V, Pajer P, Kluzáková E, Jarkovská K, Peková S, Krutílková L, Dvořák M. DNA released by leukemic cells contributes to the disruption of the bone marrow microenvironment. Oncogene. 2013:32(44):5201–5209. 10.1038/onc.2012.553. [DOI] [PubMed] [Google Scholar]
- Elbarbary RA, Lucas BA, Maquat LE. Retrotransposons as regulators of gene expression. Science. 2016:351(6274):aac7247. 10.1126/science.aac7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TE Hub Consortium, Elliott TA, Heitkam T, Hubley R, Quesneville H, Suh A, Wheeler TJ. TE hub: a community-oriented space for sharing and connecting tools, data, resources, and methods for transposable element annotation. Mob DNA. 2021:12(1):16. 10.1186/s13100-021-00244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner D, Meusemann K, Korb J. Longevity and transposon defense, the case of termite reproductives. Proc Natl Acad Sci U S A. 2018:115(21):5504–5509. 10.1073/pnas.1804046115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian DK, Dönertaş HM, Fuentealba M, Partridge L, Thornton JM. Transposable element landscape in Drosophila populations selected for longevity. Genome Biol Evol. 2021:13(4): evab031. 10.1093/gbe/evab031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner GJ, Kimura Y, Daub CO, Wani S, Plessy C, Irvine KM, Schroder K, Cloonan N, Steptoe AL, Lassmann T, et al. The regulated retrotransposon transcriptome of mammalian cells. Nat Genet. 2009:41(5):563–571. 10.1038/ng.368. [DOI] [PubMed] [Google Scholar]
- Finch CE. Variations in senescence and longevity include the possibility of negligible senescence. J Gerontol A Biol Sci Med Sci. 1998:53A(4):B235–B239. 10.1093/gerona/53A.4.B235. [DOI] [PubMed] [Google Scholar]
- Frost B, Hemberg M, Lewis J, Feany MB. Tau promotes neurodegeneration through global chromatin relaxation. Nat Neurosci. 2014:17(3):357–366. 10.1038/nn.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Olmo DC, Domínguez C, García-Arranz M, Anker P, Stroun M, García-Verdugo JM, García-Olmo D. Cell-free nucleic acids circulating in the plasma of colorectal cancer patients induce the oncogenic transformation of susceptible cultured cells. Cancer Res. 2010:70(2):560–567. 10.1158/0008-5472.CAN-09-3513. [DOI] [PubMed] [Google Scholar]
- Gatti M, Pimpinelli S. Functional elements in Drosophila melanogaster heterochromatin. Annu Rev Genet. 1992:26(1):239–275. 10.1146/annurev.ge.26.120192.001323. [DOI] [PubMed] [Google Scholar]
- Gebert D, Rosenkranz D. RNA-based regulation of transposon expression. Wiley Interdiscip Rev RNA. 2015:6(6):687–708. 10.1002/wrna.1310. [DOI] [PubMed] [Google Scholar]
- Ghildiyal M, Seitz H, Horwich MD, Li C, Du T, Lee S, Xu J, Kittler ELW, Zapp ML, Weng Z, et al. Endogenous siRNAs derived from transposons and mRNAs in Drosophila somatic cells. Science. 2008:320(5879):1077–1081. 10.1126/science.1157396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordani G, Cavaliere V, Gargiulo G, Lattanzi G, Andrenacci D. Retrotransposons down- and up-regulation in aging somatic tissues. Cells. 2022:11(1):79. 10.3390/cells11010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González J, Karasov TL, Messer PW, Petrov DA. Genome-wide patterns of adaptation to temperate environments associated with transposable elements in Drosophila. PLoS Genet. 2010:6(4):e1000905. 10.1371/journal.pgen.1000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbunova V, Seluanov A, Mita P, McKerrow W, Fenyö D, Boeke JD, Linker SB, Gage FH, Kreiling JA, Petrashen AP, et al. The role of retrotransposable elements in ageing and age-associated diseases. Nature. 2021:596(7870):43–53. 10.1038/s41586-021-03542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groza C, Chen X, Wheeler TJ, Bourque G, Goubert C. A unified framework to analyze transposable element insertion polymorphisms using graph genomes. Nat Commun. 2024:15(1):8915. 10.1038/s41467-024-53294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guio L, González J. New insights on the evolution of genome content: population dynamics of transposable elements in flies and humans. In: Anisimova M, editors. Evolutionary genomics: statistical and computational methods. New York, NY: Springer; 2019. p. 505–530. 10.1007/978-1-4939-9074-0_16. [DOI] [PubMed] [Google Scholar]
- Haldane JBS. New Paths in genetics. London, UK: Allen & Unwin; 1941. [Google Scholar]
- Hickey DA. Selfish DNA: a sexually-transmitted nuclear parasite. Genetics. 1982:101(3-4):519–531. 10.1093/genetics/101.3-4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua-Van A, Le Rouzic A, Boutin TS, Filée J, Capy P. The struggle for life of the genome's selfish architects. Biol Direct. 2011:6(1):19. 10.1186/1745-6150-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun S. Small RNA pathways that protect the somatic genome. Int J Mol Sci. 2017:18(5):912. 10.3390/ijms18050912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K. Modern biological theories of aging. Aging Dis. 2010:1. [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Tam OH, Paniagua E, Hammell M. TEtranscripts: a package for including transposable elements in differential expression analysis of RNA-seq datasets. Bioinformatics. 2015:31(22):3593–3599. 10.1093/bioinformatics/btv422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jintaridth P, Mutirangura A. Distinctive patterns of age-dependent hypomethylation in interspersed repetitive sequences. Physiol Genomics. 2010:41(2):194–200. 10.1152/physiolgenomics.00146.2009. [DOI] [PubMed] [Google Scholar]
- Jones BC, Wood JG, Chang C, Tam AD, Franklin MJ, Siegel ER, Helfand SL. A somatic piRNA pathway in the Drosophila fat body ensures metabolic homeostasis and normal lifespan. Nat Commun. 2016:7(1):13856. 10.1038/ncomms13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jové M, Mota-Martorell N, Fernàndez-Bernal A, Portero-Otin M, Barja G, Pamplona R. Phenotypic molecular features of long-lived animal species. Free Radic Biol Med. 2023:208:728–747. 10.1016/j.freeradbiomed.2023.09.023. [DOI] [PubMed] [Google Scholar]
- Kelsey MMG, Kalekar RA, Sedivy JM. TE-Seq: a transposable element annotation and RNA-Seq pipeline. Preprint. bioRxiv. 2024. 10.1101/2024.10.11.617912. [DOI] [Google Scholar]
- Kirkwood TBL, Austad SN. Why do we age? Nature. 2000:408(6809):233–238. 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- Kissinger JC, DeBarry J. Genome cartography: charting the apicomplexan genome. Trends Parasitol. 2011:27(8):345–354. 10.1016/j.pt.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciano S, Cristofari G. Measuring and interpreting transposable element expression. Nat Rev Genet. 2020:21(12):721–736. 10.1038/s41576-020-0251-y. [DOI] [PubMed] [Google Scholar]
- Lanciano S, Cristofari G. Cancer immunotherapy: how to exploit transposable elements? Clin Chem. 2024:70(1):17–20. 10.1093/clinchem/hvad091. [DOI] [PubMed] [Google Scholar]
- LaRocca D, Lee J, West MD, Labat I, Sternberg H. No time to age: uncoupling aging from chronological time. Genes (Basel). 2021:12(5):611. 10.3390/genes12050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca TJ, Cavalier AN, Wahl D. Repetitive elements as a transcriptomic marker of aging: evidence in multiple datasets and models. Aging Cell. 2020:19(7):e13167. 10.1111/acel.13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Bruck DJ, Dreves AJ, Ioriatti C, Vogt H, Baufeld P. In focus: spotted wing Drosophila, Drosophila suzukii, across perspectives. Pest Manag Sci. 2011:67(11):1349–1351. 10.1002/ps.2271. [DOI] [PubMed] [Google Scholar]
- Lemaître J-F, Ronget V, Tidière M, Allainé D, Berger V, Cohas A, Colchero F, Conde DA, Garratt M, Liker A, et al. Sex differences in adult lifespan and aging rates of mortality across wild mammals. Proc Natl Acad Sci U S A. 2020:117(15):8546–8553. 10.1073/pnas.1911999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerat E, Fablet M, Modolo L, Lopez-Maestre H, Vieira C. TEtools facilitates big data expression analysis of transposable elements and reveals an antagonism between their activity and that of piRNA genes. Nucleic Acids Res. 2017:45(4):e17. 10.1093/nar/gkw953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Prazak L, Chatterjee N, Grüninger S, Krug L, Theodorou D, Dubnau J. Activation of transposable elements during aging and neuronal decline in Drosophila. Nat Neurosci. 2013:16(5):529–531. 10.1038/nn.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D-H, Oh C-T, Lee L, Hong J-S, Noh S-H, Hwang S, Kim S, Han S-J, Lee YS. The endogenous siRNA pathway in Drosophila impacts stress resistance and lifespan by regulating metabolic homeostasis. FEBS Lett. 2011:585(19):3079–3085. 10.1016/j.febslet.2011.08.034. [DOI] [PubMed] [Google Scholar]
- Liu N, Landreh M, Cao K, Abe M, Hendriks G-J, Kennerdell JR, Zhu Y, Wang L-S, Bonini NM. The microRNA miR-34 modulates ageing and neurodegeneration in Drosophila. Nature. 2012:482(7386):519–523. 10.1038/nature10810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Liu Z, Wu Z, Ren J, Fan Y, Sun L, Cao G, Niu Y, Zhang B, Ji Q, et al. Resurrection of endogenous retroviruses during aging reinforces senescence. Cell. 2023:186(2):287–304.e26. 10.1016/j.cell.2022.12.017. [DOI] [PubMed] [Google Scholar]
- Lock FE, Rebollo R, Miceli-Royer K, Gagnier L, Kuah S, Babaian A, Sistiaga-Poveda M, Lai CB, Nemirovsky O, Serrano I, et al. Distinct isoform of FABP7 revealed by screening for retroelement-activated genes in diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A. 2014:111(34):E3534–E3543. 10.1073/pnas.1405507111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013:153(6):1194–1217. 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto ELS, de Melo ES, Wallau GL, Gomes TMFF. The good, the bad and the ugly of transposable elements annotation tools. Genet Mol Biol. 2024:46(3 suppl 1):e20230138. 10.1590/1678-4685-GMB-2023-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamillapalli A, Pathak RU, Garapati HS, Mishra RK. Transposable element ‘roo’ attaches to nuclear matrix of the Drosophila melanogaster. J Insect Sci. 2013:13(111):111. 10.1673/031.013.11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais GAB, Gaillard J-M, Vieira C, Plotton I, Sanlaville D, Gueyffier F, Lemaitre J-F. Sex gap in aging and longevity: can sex chromosomes play a role? Biol Sex Differ. 2018:9(1):33. 10.1186/s13293-018-0181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens JH, O'Sullivan RJ, Braunschweig U, Opravil S, Radolf M, Steinlein P, Jenuwein T. The profile of repeat-associated histone lysine methylation states in the mouse epigenome. EMBO J. 2005:24(4):800–812. 10.1038/sj.emboj.7600545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JM, Biessmann H. The unusual telomeres of Drosophila. Trends Genet. 1995:11(2):58–62. 10.1016/S0168-9525(00)88998-2. [DOI] [PubMed] [Google Scholar]
- McClintock B. The origin and behavior of mutable loci in maize. Proc Natl Acad Sci U S A. 1950:36(6):344–355. 10.1073/pnas.36.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock B. Induction of instability at selected loci in maize. Genetics. 1953:38(6):579–599. 10.1093/genetics/38.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medawar PB. Old age and natural death. Mod. Q. 1946:1:30–56. 10.4324/9780429299759-2. [DOI] [Google Scholar]
- Medawar PB. An unsolved problem of biology. London, UK: H. K. Lewis; 1952. [Google Scholar]
- Mérel V, Boulesteix M, Fablet M, Vieira C. Transposable elements in Drosophila. Mob DNA. 2020:11(1):23. 10.1186/s13100-020-00213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M, Sabot F, Varoqui M, Mugat B, Audouin K, Pélisson A, Fiston-Lavier A-S, Chambeyron S. TrEMOLO: accurate transposable element allele frequency estimation using long-read sequencing data combining assembly and mapping-based approaches. Genome Biol. 2023:24(1):63. 10.1186/s13059-023-02911-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosaddeghi P, Farahmandnejad M, Zarshenas MM. The role of transposable elements in aging and cancer. Biogerontology. 2023:24(4):479–491. 10.1007/s10522-023-10028-z. [DOI] [PubMed] [Google Scholar]
- Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006:124(2):315–329. 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Murray V. Are transposons a cause of ageing? Mutat Res. 1990:237(2):59–63. 10.1016/0921-8734(90)90011-F. [DOI] [PubMed] [Google Scholar]
- Nguyen AH, Bachtrog D. Toxic Y chromosome: increased repeat expression and age-associated heterochromatin loss in male Drosophila with a young Y chromosome. PLOS Genet. 2021:17(4):e1009438. 10.1371/journal.pgen.1009438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitin AG, Woodruff RC. Somatic movement of the manner transposable element and lifespan of Drosophila species. Mutat. Res. DNA Aging Gen. Instability Aging. 1995:338:43–49. 10.1016/0921-8734(95)00010-4. [DOI] [PubMed] [Google Scholar]
- Nirala NK, Li Q, Ghule PN, Chen H-J, Li R, Zhu LJ, Wang R, Rice NP, Mao J, Stein JL, et al. Hinfp is a guardian of the somatic genome by repressing transposable elements. Proc Natl Acad Sci U S A. 2021:118(41):e2100839118. 10.1073/pnas.2100839118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa E, Ramirez P, Gonzalez E, De Mange J, Ray WJ, Bieniek KF, Frost B. Pathogenic tau-induced transposable element–derived dsRNA drives neuroinflammation. Sci Adv. 2023:9(1):eabq5423. 10.1126/sciadv.abq5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira DS, Fablet M, Larue A, Vallier A, Carareto CMA, Rebollo R, Vieira C. ChimeraTE: a pipeline to detect chimeric transcripts derived from genes and transposable elements. Nucleic Acids Res. 2023:51(18):9764–9784. 10.1093/nar/gkad671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgel LE, Crick FHC. Selfish DNA: the ultimate parasite. Nature. 1980:284(5757):604–607. 10.1038/284604a0. [DOI] [PubMed] [Google Scholar]
- Pabis K, Barardo D, Sirbu O, Selvarajoo K, Gruber J, Kennedy BK. A concerted increase in readthrough and intron retention drives transposon expression during aging and senescence. eLife. 2024:12:RP87811. 10.7554/eLife.87811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue M-L, DeBaryshe PG. Drosophila telomeres: a variation on the telomerase theme. Fly (Austin). 2008:2(3):101–110. 10.4161/fly.6393. [DOI] [PubMed] [Google Scholar]
- Pehrsson EC, Choudhary MNK, Sundaram V, Wang T. The epigenomic landscape of transposable elements across normal human development and anatomy. Nat Commun. 2019:10(1):5640. 10.1038/s41467-019-13555-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peona V, Palacios-Gimenez OM, Blommaert J, Liu J, Haryoko T, Jønsson KA, Irestedt M, Zhou Q, Jern P, Suh A. The avian W chromosome is a refugium for endogenous retroviruses with likely effects on female-biased mutational load and genetic incompatibilities. Philos. Trans. R. Soc. B Biol. Sci. 2021:376(1833):20200186. 10.1098/rstb.2020.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrat PN, DasGupta S, Wang J, Theurkauf W, Weng Z, Rosbash M, Waddell S. Transposition-driven genomic heterogeneity in the Drosophila brain. Science. 2013:340(6128):91–95. 10.1126/science.1231965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman R, Chirn G, Kanodia A, Sytnikova YA, Brembs B, Bergman CM, Lau NC. Unique transposon landscapes are pervasive across Drosophila melanogaster genomes. Nucleic Acids Res. 2015:43(22):10655–10672. 10.1093/nar/gkv1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez P, Sun W, Dehkordi SK, Zare H, Fongang B, Bieniek KF, Frost B. Nanopore long-read sequencing unveils genomic disruptions in Alzheimer's disease. Preprint. bioRxiv. 2025:2024.02.01.578450, 10.1101/2024.02.01.578450 . [DOI] [Google Scholar]
- Rebollo R., Gerenton P., Cumunel E., Mary A., Sabot F., Burlet N., Gillet B., Hughes S., Oliveira S., Goubert D., et al. Identification and quantification of transposable element transcripts using long-read RNA-Seq in Drosophila germline tissues. Peer Community J. 2024:4:e89. 10.24072/pcjournal.457 . [DOI] [Google Scholar]
- Ricci M, Peona V, Boattini A, Taccioli C. Comparative analysis of bats and rodents' genomes suggests a relation between non-LTR retrotransposons, cancer incidence, and ageing. Sci Rep. 2023:13(1):9039. 10.1038/s41598-023-36006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigal J, Martin Anduaga A, Bitman E, Rivellese E, Kadener S, Marr MT. Artificially stimulating retrotransposon activity increases mortality and accelerates a subset of aging phenotypes in Drosophila. eLife. 2022:11:e80169. 10.7554/eLife.80169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Martin B, Alvarez EG, Baez-Ortega A, Zamora J, Supek F, Demeulemeester J, Santamarina M, Ju YS, Temes J, Garcia-Souto D, et al. Pan-cancer analysis of whole genomes identifies driver rearrangements promoted by LINE-1 retrotransposition. Nat Genet. 2020:52(3):306–319. 10.1038/s41588-019-0562-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm A, Cherkasov A, Liu H, Voronov D, Siniuk K, Schwarz R, Ohlenschläger O, Förste S, Bens M, Groth M, et al. The Greenland shark (Somniosus microcephalus) genome provides insights into extreme longevity. Preprint. BioRxiv. 2024:09.09.611499. 10.1101/2024.09.09.611499 . [DOI] [Google Scholar]
- Savitsky M, Kwon D, Georgiev P, Kalmykova A, Gvozdev V. Telomere elongation is under the control of the RNAi-based mechanism in the Drosophila germline. Genes Dev. 2006:20(3):345–354. 10.1101/gad.370206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible R, Scheuerlein A, Dańko MJ, Gampe J, Martínez DE, Vaupel JW. Constant mortality and fertility over age in Hydra. Proc. Natl. Acad. Sci. 2015:112(51):15701–15706. 10.1073/pnas.1521002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J, Brosius J. Exonization of transposed elements: a challenge and opportunity for evolution. Biochimie. 2011:93:1928–1934. 10.1016/j.biochi.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Schneider BK, Sun S, Lee M, Li W, Skvir N, Neretti N, Vijg J, Secombe J. Expression of retrotransposons contributes to aging in Drosophila. Genetics. 2023:224(2):iyad073. 10.1093/genetics/iyad073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senapati P, Miyano M, Sayaman RW, Basam M, Leung A, LaBarge MA, Schones DE. Loss of epigenetic suppression of retrotransposons with oncogenic potential in aging mammary luminal epithelial cells. Genome Res. 2023:33(8):1229–1241. 10.1101/gr.277511.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NM, Jang HJ, Liang Y, Maeng JH, Tzeng S-C, Wu A, Basri NL, Qu X, Fan C, Li A, et al. Pan-cancer analysis identifies tumor-specific antigens derived from transposable elements. Nat Genet. 2023:55(4):631–639. 10.1038/s41588-023-01349-3. [DOI] [PubMed] [Google Scholar]
- Shmookler Reis RJ, Goldstein S. Mitochondrial DNA in mortal and immortal human cells. Genome number, integrity, and methylation. J Biol Chem. 1983:258(15):9078–9085. 10.1016/S0021-9258(17)44633-3. [DOI] [PubMed] [Google Scholar]
- Simon M, Meter MV, Ablaeva J, Ke Z, Gonzalez RS, Taguchi T, Cecco MD, Leonova KI, Kogan V, Helfand SL, et al. LINE1 derepression in aged wild-type and SIRT6-deficient mice drives inflammation. Cell Metab. 2019:29(4):871–885.e5. 10.1016/j.cmet.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin RK, Martienssen R. Transposable elements and the epigenetic regulation of the genome. Nat Rev Genet. 2007:8(4):272–285. 10.1038/nrg2072. [DOI] [PubMed] [Google Scholar]
- Stow EC, Kaul T, deHaro DL, Dem MR, Beletsky AG, Morales ME, Du Q, LaRosa AJ, Yang H, Smither E, et al. Organ-, sex- and age-dependent patterns of endogenous L1 mRNA expression at a single locus resolution. Nucleic Acids Res. 2021:49(10):5813–5831. 10.1093/nar/gkab369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel E, Dunsmuir P, Rubin GM. Polymorphisms in the chromosomal locations of elements of the 412, copia and 297 dispersed repeated gene families in Drosophila. Cell. 1979:17(2):429–439. 10.1016/0092-8674(79)90169-7. [DOI] [PubMed] [Google Scholar]
- Sturm Á, Perczel A, Ivics Z, Vellai T. The Piwi-piRNA pathway: road to immortality. Aging Cell. 2017:16(5):906–911. 10.1111/acel.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulak M, Fong L, Mika K, Chigurupati S, Yon L, Mongan NP, Emes RD, Lynch VJ. TP53 copy number expansion is associated with the evolution of increased body size and an enhanced DNA damage response in elephants. eLife. 2016:5:e11994. 10.7554/eLife.11994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi F, Zhang C, Hohjoh H, Raveney B, Yamamura T, Hayashi N, Oki S. Immune-mediated neurodegenerative trait provoked by multimodal derepression of long-interspersed nuclear element-1. iScience. 2022:25(5):104278. 10.1016/j.isci.2022.104278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teefy BB, Adler A, Xu A, Hsu K, Singh PP, Benayoun BA. Dynamic regulation of gonadal transposon control across the lifespan of the naturally short-lived African turquoise killifish. Genome Res. 2023:33(1):141–153. 10.1101/gr.277301.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teefy BB, Siebert S, Cazet JF, Lin H, Juliano CE. PIWI–piRNA pathway-mediated transposable element repression in Hydra somatic stem cells. RNA. 2020:26(5):550–563. 10.1261/rna.072835.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teissandier A, Servant N, Barillot E, Bourc’his D. Tools and best practices for retrotransposon analysis using high-throughput sequencing data. Mob DNA. 2019:10(1):52. 10.1186/s13100-019-0192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoli J, Merenciano M, Fablet M, Necsulea A, Siqueira-de-Oliveira D, Brandulas-Cammarata A, Labalme A, Lejeune H, Lemaitre J-F, Gueyffier F, et al. Transposable element expression with variation in sex chromosome number: insights into a toxic Y effect on human longevity. Preprint. BioRxiv. 2024:08.03.550779. 10.1101/2023.08.03.550779 . [DOI] [Google Scholar]
- Tossberg JT, Heinrich RM, Farley VM, Crooke PS, Aune TM. Adenosine-to-inosine RNA editing of Alu double-stranded (ds)RNAs is markedly decreased in multiple sclerosis and unedited Alu dsRNAs are potent activators of proinflammatory transcriptional responses. J. Immunol. 2020:205(10):2606–2617. 10.4049/jimmunol.2000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiber CD, Waddell S. Resolving the prevalence of somatic transposition in Drosophila. eLife. 2017:6:e28297. 10.7554/eLife.28297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo-Becerril C, Pérez-Cárdenas E, Taja-Chayeb L, Anker P, Herrera-Goepfert R, Medina-Velázquez LA, Hidalgo-Miranda A, Pérez-Montiel D, Chávez-Blanco A, Cruz-Velázquez J, et al. Cancer progression mediated by horizontal gene transfer in an in vivo model. PLoS One. 2012:7(12):e52754. 10.1371/journal.pone.0052754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trizzino M, Park Y, Holsbach-Beltrame M, Aracena K, Mika K, Caliskan M, Perry GH, Lynch VJ, Brown CD. Transposable elements are the primary source of novelty in primate gene regulation. Genome Res. 2017:27(10):1623–1633. 10.1101/gr.218149.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y-T, Seymen N, Thompson IR, Zou X, Mumtaz W, Gerlevik S, Mufti GJ, Karimi MM. Expression of most retrotransposons in human blood correlates with biological aging. eLife. 2024:13:RP96575. 10.7554/eLife.96575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bree EJ, Guimarães RLFP, Lundberg M, Blujdea ER, Rosenkrantz JL, White FTG, Poppinga J, Ferrer-Raventós P, Schneider A-FE, Clayton I, et al. A hidden layer of structural variation in transposable elements reveals potential genetic modifiers in human disease-risk loci. Genome Res. 2022:32(4):656–670. 10.1101/gr.275515.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meter M, Kashyap M, Rezazadeh S, Geneva AJ, Morello TD, Seluanov A, Gorbunova V. SIRT6 represses LINE1 retrotransposons by ribosylating KAP1 but this repression fails with stress and age. Nat Commun. 2014:5(1):5011. 10.1038/ncomms6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez BN, Thackray JK, Simonet NG, Chahar S, Kane-Goldsmith N, Newkirk SJ, Lee S, Xing J, Verzi MP, An W, et al. SIRT7 mediates L1 elements transcriptional repression and their association with the nuclear lamina. Nucleic Acids Res. 2019:47(15):7870–7885. 10.1093/nar/gkz519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez JM, Lynch VJ. Pervasive duplication of tumor suppressors in Afrotherians during the evolution of large bodies and reduced cancer risk. eLife. 2021:10:e65041. 10.7554/eLife.65041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijg J, Mullaart E, Lohman PHM, Knook DL. UV-induced unscheduled DNA synthesis in fibroblasts of aging inbred rats. Mutat. Res. Repair Rep. 1985:146:197–204. 10.1016/0167-8817(85)90011-2. [DOI] [PubMed] [Google Scholar]
- Villanueva-Cañas JL, Horvath V, Aguilera L, González J. Diverse families of transposable elements affect the transcriptional regulation of stress-response genes in Drosophila melanogaster. Nucleic Acids Res. 2019:47(13):6842–6857. 10.1093/nar/gkz490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmuth VM, Weissensteiner MH, Wolf JBW. Accumulation and ineffective silencing of transposable elements on an avian W chromosome. Genome Res. 2022:32(4):671–681. 10.1101/gr.275465.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren IA, Naville M, Chalopin D, Levin P, Berger CS, Galiana D, Volff J-N. Evolutionary impact of transposable elements on genomic diversity and lineage-specific innovation in vertebrates. Chromosome Res. 2015:23(3):505–531. 10.1007/s10577-015-9493-5. [DOI] [PubMed] [Google Scholar]
- Weilguny L, Kofler R. DeviaTE: assembly-free analysis and visualization of mobile genetic element composition. Mol Ecol Resour. 2019:19(5):1346–1354. 10.1111/1755-0998.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weismann A. Ueber die Dauer des Lebens: ein Vortrag. Jena, Germany: G. Fischer; 1882. [Google Scholar]
- Wells JN, Feschotte C. A field guide to eukaryotic transposable elements. Annu Rev Genet. 2020:54(1):539–561. 10.1146/annurev-genet-040620-022145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker T, Sabot F, Hua-Van A, Bennetzen JL, Capy P, Chalhoub B, Flavell A, Leroy P, Morgante M, Panaud O, et al. A unified classification system for eukaryotic transposable elements. Nat Rev Genet. 2007:8(12):973–982. 10.1038/nrg2165. [DOI] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957:11(4):398–411. 10.1111/j.1558-5646.1957.tb02911.x. [DOI] [Google Scholar]
- Wood JG, Helfand SL. Chromatin structure and transposable elements in organismal aging. Front Genet. 2013:4:274. 10.3389/fgene.2013.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Jones BC, Jiang N, Chang C, Hosier S, Wickremesinghe P, Garcia M, Hartnett DA, Burhenn L, Neretti N, et al. Chromatin-modifying genetic interventions suppress age-associated transposable element activation and extend life span in Drosophila. Proc Natl Acad Sci U S A. 2016:113(40):11277–11282. 10.1073/pnas.1604621113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff RC, Nikitin AG. P DNA element movement in somatic cells reduces lifespan in Drosophila melanogaster: evidence in support of the somatic mutation theory of aging. Mutat Res. 1995:338(1-6):35–42. 10.1016/0921-8734(95)00009-u. [DOI] [PubMed] [Google Scholar]
- Xia B, Zhang W, Zhao G, Zhang X, Bai J, Brosh R, Wudzinska A, Huang E, Ashe H, Ellis G, et al. On the genetic basis of tail-loss evolution in humans and apes. Nature. 2024:626(8001):1042–1048. 10.1038/s41586-024-07095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Tang Y, Feng W, Yang Y, Cui Z. Comparative analysis of transposable elements reveals the diversity of transposable elements in decapoda and their effects on genomic evolution. Mar. Biotechnol. 2023:25(6):1136–1146. 10.1007/s10126-023-10265-w. [DOI] [PubMed] [Google Scholar]
- Yang N, Srivastav SP, Rahman R, Ma Q, Dayama G, Li S, Chinen M, Lei EP, Rosbash M, Lau NC. Transposable element landscapes in aging Drosophila. PLOS Genet. 2022:18(3):e1010024. 10.1371/journal.pgen.1010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WR, Ardeljan D, Pacyna CN, Payer LM, Burns KH. SQuIRE reveals locus-specific regulation of interspersed repeat expression. Nucleic Acids Res. 2019:47(5):e27. 10.1093/nar/gky1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkova E, Moskalev A. Transposable elements and their role in aging. Ageing Res Rev. 2023:86:101881. 10.1016/j.arr.2023.101881. [DOI] [PubMed] [Google Scholar]
- Zhao K, Du J, Peng Y, Li P, Wang S, Wang Y, Hou J, Kang J, Zheng W, Hua S, et al. LINE1 contributes to autoimmunity through both RIG-I- and MDA5-mediated RNA sensing pathways. J Autoimmun. 2018:90:105–115. 10.1016/j.jaut.2018.02.007. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Oreskovic E, Zhang Q, Lu Q, Gilman A, Lin YS, He J, Zheng Z, Lu JY, Lee J, et al. Transposon-triggered innate immune response confers cancer resistance to the blind mole rat. Nat Immunol. 2021:22(10):1219–1230. 10.1038/s41590-021-01027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Z, Chen Y, Wang H, Tang H, Zhang H, Liu H, Jiang Y, Mao Z. Nuclear cGAS restricts L1 retrotransposition by promoting TRIM41-mediated ORF2p ubiquitination and degradation. Nat Commun. 2023:14(1):8217. 10.1038/s41467-023-43001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C-T, Chang C, Reenan RA, Helfand SL. Indy gene variation in natural populations confers fitness advantage and life span extension through transposon insertion. Aging (Albany NY). 2014:6(1):58–69. 10.18632/aging.100634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J, Wang J, Theurkauf W, Weng Z. TEMP: a computational method for analyzing transposable element polymorphism in populations. Nucleic Acids Res. 2014:42(11):6826–6838. 10.1093/nar/gku323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.