Abstract
Carbapenems are potent antibiotics that reach sewage systems and then the environment, causing negative impacts. Thus, research on degrading processes to limit the carbapenem discharge in sewage systems is needed. Herein, fundamental aspects of high-frequency ultrasound alone and hybridized with the (photo)Fenton process to deal with a representative carbapenem antibiotic (meropenem, MERO) in water were considered. Initially, the action of ultrasound alone (at 578 kHz) on MERO in distilled water was tested for degradation, resulting in a partial removal (∼53 % after 120 min) and a moderate pseudo-first-order-kinetics (k: 6.3 × 10−3 min−1). Then, to enhance the MERO elimination ferrous ions were added to the ultrasound system, forming the sono-Fenton process. The increase in the ferrous ions concentration from 0 to 5 mg L−1 augmented the rate of MERO degradation (k changed from 6.3 to 15.7 × 10−3 min−1) and diminished the electric energy consumption from 1.22 to 0.49 kWh L−1. Afterward, the MERO treatment by the hybridized sono-photo-Fenton process (i.e., ultrasound combined with Fe2+ and UVA light) was evaluated, showing that the degradation efficiency was higher than by the sono-Fenton or photolysis (indeed, a synergistic index of 1.11 was obtained). Moreover, the sono-photo-Fenton process decreased the antimicrobial activity (against Staphylococcus aureus) after 30 min of treatment, indicating that the by-products did not have antimicrobial activity. The structures of primary by-products, at 50 % of MERO degradation, were elucidated through Fukui indices and LC-MS, finding that the pyrroline ring, β-lactam core, and thioether group on MERO were susceptible to the attacks of generated hydroxyl radicals (HO•) and the primary transformations occurred on such moieties of the antibiotic. Finally, the treatment of MERO in synthetic hospital wastewater by the action of the sono-photo-Fenton process was assessed, degrading 36 % of MERO at 60 min of treatment. The results from this research indicated that the hybridized processes could be an alternative to be used in niche applications for treating carbapenem antibiotics even in complex matrices, transforming them into less problematic compounds.
Keywords: Antibiotics degradation, Advanced oxidation processes, Processes combination, Transformation products, Water treatment
1. Introduction
Pharmaceuticals, such as antibiotics, are considered emerging concern pollutants worldwide due to their continuous entry, negative environmental effects, and persistence in the aquatic ecosystem [[1], [2], [3], [4]]. The presence of antibiotics in environmental water is mainly due to the inability of conventional methods in municipal wastewater treatment plants to eliminate them [1,[5], [6], [7]]. Antibiotics are considered pollutants of great concern because of their negative effects on public health, such as the widespread outbreak of antibiotic-resistant bacteria (ARB) and antibiotic-resistant genes (ARG) [[8], [9], [10]].
Carbapenem antibiotics (such as meropenem) are the last-line therapeutic option for patients when the regular antibiotics do not work to treat the bacterial infection. After consumption, those antibiotics reach the sewage system and wastewater treatment plants. However, the inability of the facilities to completely remove carbapenem antibiotics results in their discharge into the aquatic environment. For instance, some researchers have reported, concentrations at 169 ng L−1 and 206 ng L−1 of meropenem for wastewater treatment plants inlet and outlet, respectively [11,12].
According to the World Health Organization (WHO), carbapenem-resistant bacteria are a critical group of microorganisms [13]. For instance, in Latin American countries, such as Peru, the presence of carbapenem-resistant microorganisms in hospitals is widely reported [[14], [15], [16], [17]]. Carbapenem-resistant bacteria have also been found and isolated from waters worldwide [[18], [19], [20], [21], [22]]. One of the driving forces associated with the proliferation of resistant bacteria is the continuous input of antibiotics into the aquatic environment [23,24]. Therefore, it is necessary to eliminate antibiotics through the application of effective treatment methods, such as advanced oxidation processes (AOPs).
AOPs are useful in the degradation of pharmaceuticals in water [[25], [26], [27]]. There are well-known AOPs (e.g., activated ozone or UV/H2O2) that have been developed at full scale for the removal of organic compounds (as antibiotics) from wastewater [28,29]. AOPs based on activated ozone or UV/H2O2 provide high energy efficiency and produce HO• homogeneously, which makes the radical species react in a non-selective way with the target pollutants and matrix components in the aqueous matrix. However, other AOPs could still be solutions for specific applications, such as degradation of otherwise recalcitrant pollutants or decentralized water treatment applications [28].
The high-frequency ultrasound process (US) is an alternative to these well-known AOPs for eliminating antibiotics in aqueous samples [30,31]. In fact, previous works have shown the ability of sonochemical processes to selectively eliminate pharmaceuticals from complex aqueous matrices [[32], [33], [34]]. It is important to mention that at the moment, the treatment with the US for the removal of these compounds is only at the research stage (technology readiness level-TRL: 2–4). However, the US selectivity toward the degradation of hydrophobic organic pollutants makes this AOP very useful for niche applications [32]. Moreover, recent developments in ultrasound equipment to be operated in continuous mode can favor future applications at large scales [35]. Despite the use of high-frequency US for water treatment is still at low TRL levels, the study of fundamental aspects such as pollutant transformations, determination of the degradation efficiency, and hybridization with other processes could contribute to the progress of further larger US applications.
The US involves the acoustic cavitation phenomenon (i.e., formation, growth, and collapse of microbubbles), and the degradation of a pharmaceutical can take place via pyrolysis (for volatile compounds) or by attacks of sono-generated hydroxyl radicals (for non-volatile substances) coming from the water and oxygen molecules cleavage (Eqs. 1–4). In addition, US is also able to generate hydrogen peroxide as a by-product of hydroxyl radical combination (Eq. 5). To take advantage of the sono-generated H2O2, iron ions, and UV light can be added to the ultrasound process, producing a hybrid sono-(photo)-Fenton process. This hybrid process may enhance the degradation efficiency by producing the extra HO• through the Fenton reaction [30,36]. Hence, the hybrid processes involving ultrasound seem to be an alternative to dealing with antibiotics, such as meropenem (MERO), in aqueous samples.
| H2O + ))) → H• + HO• | (1) |
| O2 + ))) → 2O• | (2) |
| H2O + O• → 2HO• | (3) |
| O2 + H• → O• + HO• | (4) |
| 2HO• → H2O2 | (5) |
We should mention that some AOPs such as TiO2-based and g-C3N4-based heterogeneous photocatalysis [[37], [38], [39], [40]], electrochemical oxidation [41], catalytic ozonation [42], or photo-Fenton [43,44] can degrade MERO. Those works show the efficacy of diverse individual AOPs for degrading MERO in aqueous samples individually, but they do not consider the feasibility of hybrid processes with ultrasound. Meanwhile, our research team did an initial exploration of the ability of the sono-photo-Fenton process to degrade MERO in water [36]. It must be remarked that such a work mainly had a methodological focus, only showing the effects of operative parameters but not understanding the fundamental aspects of the sonochemical and sono-photo-catalytical processes.
Therefore, this work aimed to study the essential features of treating MERO in water by ultrasound alone and hybridized with the (photo)-Fenton process. Herein, the adjustment of the MERO degradation to the pseudo-first-order kinetics was shown initially. Then, it was evaluated the influence of the hydrophilic nature of MERO on the sonodegradation, by comparison with another organic pollutant. Furthermore, the role of iron concentration and ultrasonic power on both the degradation kinetics and electric energy consumption was established. Also, the elucidation of the primary transformations of MERO under the sono-photo-Fenton action and their link with the antimicrobial activity (AA) decrease were assessed. It can be mentioned that the rationalization of the by-products generation and AA diminution was supported with theoretical analyses based on the chemical structure such as Fukui indexes and probability of being active, respectively. Finally, the matrix influence on the carbapenem degradation was evaluated by treating it through the sono-photo-Fenton process in simulated hospital wastewater (which could be a potential niche application of ultrasound hybridized with the photo-Fenton process).
2. Experimental
2.1. Reagents
Meropenem trihydrate (MERO) was acquired from Matrix Scientific. Ammonium heptamolybdate tetrahydrate and sodium acetate trihydrate were obtained from J.T. Baker. Ammonium chloride, calcium chloride, monopotassium phosphate, methylene blue (MEBL), nutrient agar, potassium chloride, sodium chloride, sodium sulfate, and urea were purchased from Merck S.A. Iron sulfate heptahydrate and catalase (2000–5000 units mg−1) were acquired from Sigma-Aldrich. Acetonitrile (HPLC grade, Supelco), ultrapure water (Milli-Q system), and potassium iodide (Fisher Chemical, USA) were used experimentally. For the AA tests, Staphylococcus aureus ATCC 6538 was used as the probe microorganism.
2.2. Ultrasound reactor
A Meinhardt Ultrasound was used to perform the degradation experiments in a 500 mL glass batch reactor at 578 kHz, containing 300 mL of the solutions of MERO to be treated at an initial pH of 6.4 ± 0.2. The calorimetric method was employed to determine the actual acoustic power in the sonochemical system following the procedure described in the reference [45]. The temperature of the ultrasonic reactor was controlled at 19 ± 2 °C using a cooled water bath. For the hybridized sono-photo-Fenton process the addition of ferrous ions and UVA light were added to the ultrasonic system. The UVA light source was a cylindrical black lamp (Philips, 4 W of power, maximum emission at 365 nm; which was sleeved with a quartz jacket submerged into the sonochemical reactor). For the Fenton-based systems, the solutions of MERO to be treated also had an initial pH of 6.4 ± 0.2, and the pH was not controlled and it naturally evolved toward acidic values during the treatment (see the detailed explanation in Section 3.2 below). All experiments were carried out at least by duplicate, calculating the average values and the coefficients of variation (which were lower than 5 %). The figures and tables present the average values.
2.3. Analyses
The evolution of MERO was measured at 300 nm using an HPLC Agilent 1100 equipped with a Tecknokroma C-18 column (5 µm, 4.6x150 mm) and a diode array detector (DAD) as previously reported [36]. Then, the accomplishment of the MERO concentration evolution to pseudo-first-order kinetics (PFO) was evaluated by assessing the fitting to the mathematical expression, Ln C/Co = −kt, where C/Co represents the normalized concentration, t means the treatment time in min, and k is the rate constant in min−1 (as illustrated in Section 3.1, Fig. 1A). Besides, the electric energy consumed to degrade 50 % of MERO (EEC50) was calculated according to the mathematical expression, EEC50 = Pt50/V; where P is the electric power of the system in kW, t50 means the time needed to degrade 50 % of MERO in hours, and V represents the volume of the treated sample in L.
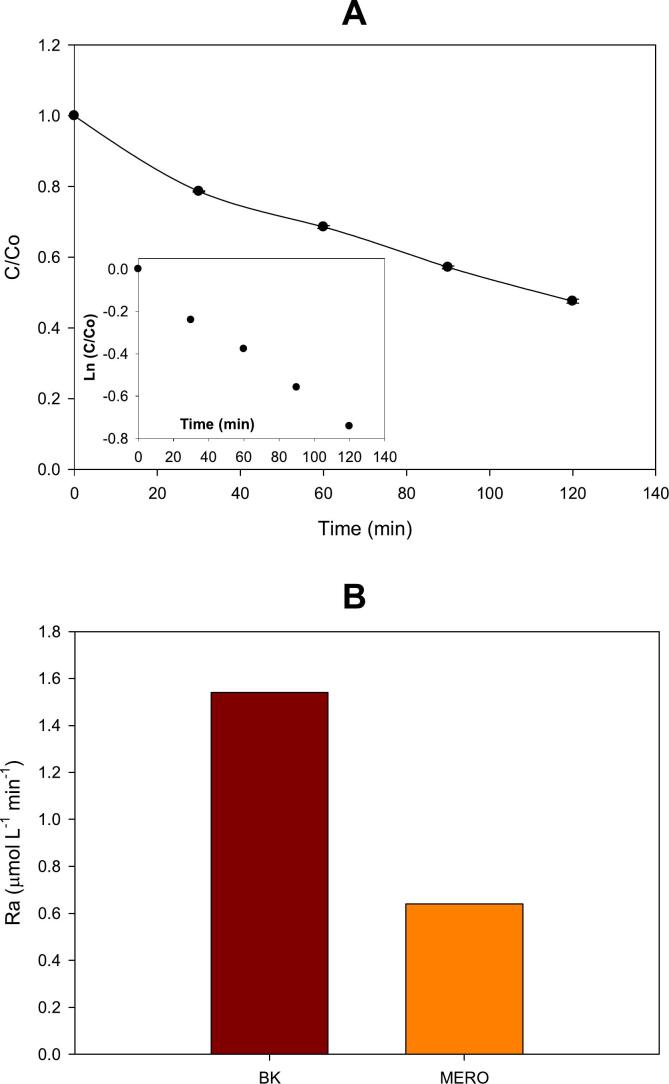
Fig. 1.
Sonolysis of meropenem. A. Meropenem degradation by ultrasound alone (inset: example of the adjustment of the antibiotic degradation to the PFO kinetics by plotting Ln (C/Co) vs. time). B. Rate of H2O2 accumulation (Ra) during sonolysis in the absence (BK) and presence of meropenem (MERO). Experimental conditions: [MERO]: 20 mg L−1; frequency: 578 kHz; and acoustic power: 23.8 W.
The accumulation of the sonogenerated hydrogen peroxide was estimated by the iodometric/spectrophotometric method, which uses potassium iodide and ammonium, as detailed previously [46]. The H2O2 accumulation has a linear relationship with the sonication time; thus, the rate of H2O2 accumulation (Ra) was calculated as the slope of the plot of hydrogen peroxide concentration vs. time [47]. On the other hand, the sonodegradation of MEBL (the other probe compound) was followed by measuring the absorbance at 665 nm using a UV5 (Mettler-Toledo) spectrophotometer.
Antimicrobial activity (AA) against a meropenem-sensitive Staphylococcus aureus was determined by analyzing the inhibition zone by the diffusion test on inoculated agars [33]. The structure of products of primary transformations, at 50 % of MERO degradation by sono-photo-Fenton, were elucidated using UPLC-Qtof-MS (Xevo G2-XS, Waters), equipped with an ESI system, operated in positive mode, with collision energies for fragmentation from 10 to 20 eV.
The theoretical analyses for explaining the generation of the by-products and AA diminution were performed using two free online softwares. To identify the moieties on MERO more susceptible to radical attacks, the f0 (Fukui index) was determined [39]. Such a calculation was performed using the freeware Rowan available online, which employs the xTB family of semiempirical methods developed by Stefan Grimme and co-workers at the University of Bonn [48]. More specific details about the calculations are provided on the Rowan Scientific platform at https://www.rowansci.com (accessed 2025-01-03) [48].
Regarding the AA diminution, this was connected to changes in the biological activities of the transformation products concerning MERO. Predictions about activities such as antibiotic carbapenem-like, muramoyltetrapeptide carboxypeptidase inhibitor, β-lactamase inhibitor, and cell wall synthesis inhibitor were made using the free online PASS software [49]. The procedure involved the input of the chemical structures in the SMILES format into the free online software; thereby, the PASS software generated the probabilities of being active (Pa) for MERO and its by-products.
3. Results and discussion
3.1. MER degradation US and pseudo-first-order kinetics
In the beginning, the carbapenem antibiotic was treated by high-frequency ultrasound alone (i.e., sonolysis). MERO evolution over time is shown in Fig. 1A. It can be noted that the MERO treatment was carried out at an initial concentration of 20 mg L−1 (this is much higher than those found at wastewater treatment plants; which are at ng L−1 levels). This high concentration allowed us to evaluate the fundamental aspects of MERO treatment by the ultrasound process (e.g., kinetics and the role of the pharmaceutical nature) using accessible analytical instruments such as a classical HPLC-DAD (see Section 2.3).
In addition to the pollutant evolution under the ultrasound action, the initial accumulation rate for H2O2 (Ra) in the MERO presence in distilled water was determined and compared with that in the antibiotic absence (BK, in Fig. 1B). From Fig. 1A was observed that after 120 min of sonication, ∼53 % of MERO was degraded. At the same time, the Ra was lower when the antibiotic was present in the distilled water; thus, denoting the interaction between the pollutant and the sonogenerated HO•. Since MERO is not a volatile substance, its degradation is mainly due to the action of radicals outside of the cavitation bubble [31]. The MERO degradation followed a PFO kinetics (see inset of Fig. 1A), exhibiting a k value of 6.3 × 10−3 min−1. This is consistent with several previous studies that have also evidenced that the sonodegradation of non-volatile organic pollutants (even β-lactam antibiotics) fits wells to PFO kinetics [30,50].
Besides the determination of the k value for the antibiotic, its elimination was compared with the degradation of MEBL at the same molar concentration, as another model pollutant, (which is an organic compound that also has nitrogen and oxygen moieties in its structure, Table 1) to verify the role of the pharmaceutical nature on the sonochemical process. We must mention that MEBL is used for the treatment of pediatric and adult patients with acquired methemoglobinemia, and historically, it has been utilized to treat malaria [51].
Table 1.
Structure, Log Kow, and k values for the treatment of MERO and MEBL.
| Pharmaceutical | Structure | Log Kow* |
k × 10−3 (min−1) |
|
|---|---|---|---|---|
| Sonolysis | Sono-Fenton** | |||
| MERO | 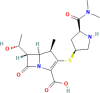 |
−0.60 | 6.3 | 11.8 |
| MEBL | 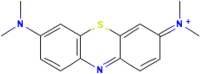 |
0.75 | 10.7 | 11.9 |
Values of Log Kow were taken from the PubChem database [51].
For the sono-Fenton system, 1.0 mg L−1 of ferrous ions was added to the ultrasound reactor.
From Table 1, which compares the k values for MERO and MEBL, it can be noted that MEBL had a higher sonodegradation rate constant than the carbapenem antibiotic. These results are associated with the hydrophobic nature of the target pollutants [32]. In fact, it has been shown that the most hydrophobic compounds are closer to the cavitation bubbles, and consequently, they can react with a higher number of radicals. As the Log Kow is a hydrophobicity indicator, this parameter was used herein. Table 1 contains the Log Kow values for the two pharmaceuticals. It can be noted that MEBL possesses a higher Log Kow value, thus explaining its faster degradation compared with MERO. On the other hand, as MERO has a relatively high hydrophilic character, the sonolysis alone is not completely useful for its degradation, and strategies to enhance the degradation of MERO are required.
3.2. MERO treatment by the sono-Fenton process
As evidenced in Fig. 1A, the sonochemical system was able to induce some degradation of MERO. However, the degradation level was limited (∼ 53 % after 120 min of treatment). Thus, a degradation improvement strategy involving the Fenton reaction (Eqs. 6–7) was considered. Due to the sonolytic system intrinsically accumulates H2O2 (at a rate of 0.645 µmol L−1 min−1, as shown in Fig. 1B), the addition of ferrous ions was done to generate the sono-Fenton systems. Table 1 presents the k values for the degradation MERO by the sono-Fenton system, and also its comparison with that for MEBL treatment.
| Fe2+ + H2O2 → Fe3+ + HO• + HO− | (6) |
| Fe3+ + H2O2 → Fe2+ + HOO• + H+ | (7) |
In the case of MERO, the iron presence in the sonochemical system significantly augmented the rate of degradation (k increased from 6.3 to 11.8 × 10−3 min−1, Table 1). On the contrary, the enhancement of the MEBL degradation by the iron presence was low (k only increased from 10.7 to 11.9 × 10−3 min−1, Table 1). These results can be explained considering the location of the pharmaceuticals and Fenton reaction regarding the cavitation bubble. The Fenton reaction occurs at the solution bulk [52,53] and MERO is far away from the cavitation bubbles due to its hydrophilic nature. As a consequence, the carbapenem antibiotic has more chance than MEBL to react with the radicals formed from the reaction between the added iron ions and the sonogenerated H2O2. Therefore, MERO degradation is improved more strongly than MEBL by the sono-Fenton process action.
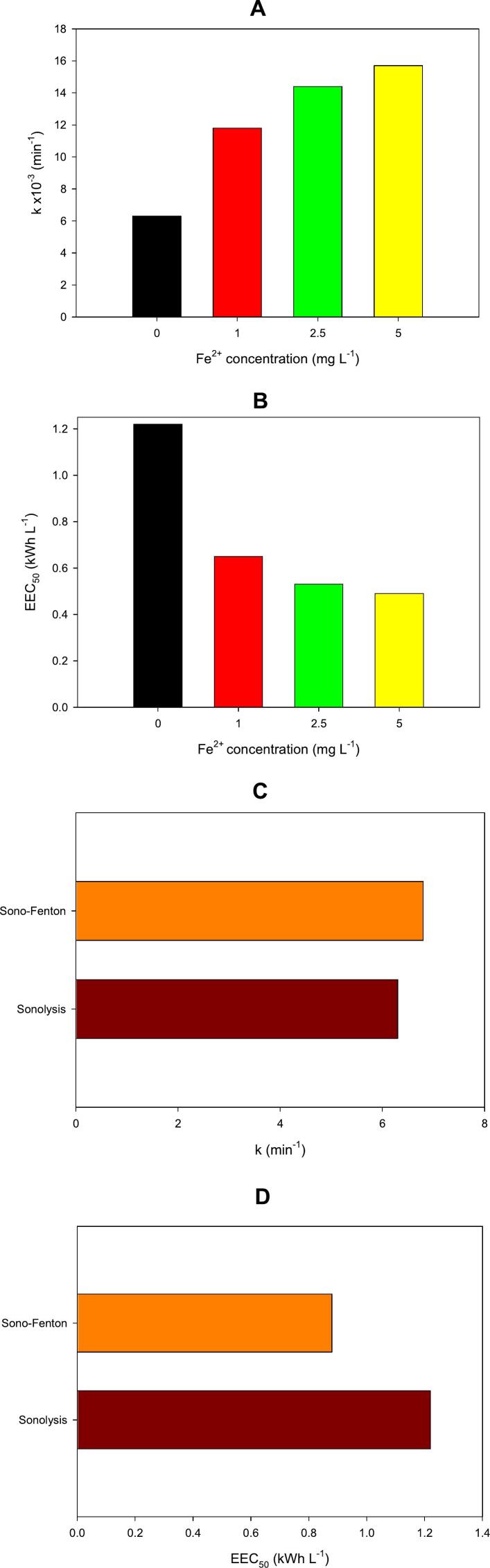
Considering the positive role of the iron addition on the MERO sonodegradation, the effect of ferrous ions concentration was established (Fig. 2A). Three concentrations of iron (i.e., 1.0, 2.5, and 5.0 mg L−1) were considered. It can be noted that the MERO elimination is increased as the iron concentration is augmented from 0 to 5.0 mg L−1. Indeed, the k values for the MERO degradation increased from 6.3 to 15.7 × 10−3 min−1; which is related to the ability of the iron to produce extra degrading hydroxyl radicals in the solution bulk by the Fenton process (Eqs. 6–7). However, it is important to take into account that higher iron concentrations could induce a detrimental effect [54] because an excess of ferrous ions induces a self-scavenger step and some iron precipitation as ferric hydroxides (Eqs. 8–9).
| Fe2+ + HO• → Fe3++HO− | (8) |
| Fe3+ + 3HO− → Fe(HO)3(s) | (9) |
Fig. 2.
Treatment of MERO by sono-Fenton. A. Effect of the Fe2+ concentration on the k values for the MERO degradation. B. Electric energy consumption (EEC50) by the sono-Fenton process at the different Fe2+ concentrations. C. Comparison of the k values for sonolysis (0 mg L−1 of Fe2+ and 23.8 W of acoustic power) and sono-Fenton (5 mg L−1 of Fe2+ and 8.1 W of acoustic power). D. Comparison of EEC50 for sonolysis and sono-Fenton.
The performance of the Fenton-based processes is strongly dependent on the solution pH. It is widely recognized that the best degrading results of such systems occur at a pH close to 3.0 [55]. Thus, it should be considered the pH change during the sono-Fenton hybrid process. Table 2 summarizes the pH change at 60 min of each process action. From Table 2, we can observe that the initial pH was ∼6.4 and after all treatments of MERO, the pH values diminished. The change was more noteworthy in the case of the Fenton-based systems.
Table 2.
Changes in the pH of the solution under the different treatments of MERO.
| Process* | pH value after 60 min of the process action |
|---|---|
| Sonolysis | 5.6 |
| Sono-Fenton (Fe2+: 1 mg L−1) |
4.0 |
| Sono-Fenton (Fe2+: 2.5 mg L−1) |
3.9 |
| Sono-Fenton (Fe2+: 5 mg L−1) |
3.8 |
| Sono-photo-Fenton (Fe2+: 5 mg L−1) |
3.8 |
Initial pH ∼ 6.4.
As seen in Table 2, the pH evolves toward acidic values. During the sonolysis, the pH decrease can be associated with the intrinsic formation of nitrous and nitric acids since N2 (coming from the dissolved air) is broken throughout the bubble collapse to produce such acids [56,57]. In the case of the hybrid systems involving the Fenton process, in addition to the intrinsic formation of nitric and nitrous acids, the secondary step of the Fenton reaction (Eq. 7) and the release of short-chain acidic structures from the target pollutant degradation [33] explain the higher pH decrease informed in Table 2. Thereby, the acidic solution generated in situ during the Fenton-based processes allowed these systems to achieve a good degradation of MERO in conditions where iron is expected to precipitate.
On the other hand, a critical aspect of the AOPs based on ultrasound is the high electric energy consumption [29]. Thus, to better support the superiority of the sono-Fenton process for degrading MERO, the EEC50 for this system was calculated (as described in Section 2.3), and it was compared with that for sonolysis (Fig. 2B). The results in Fig. 2B indicated that the electric energy consumption of the sono-Fenton systems (at 5.0 mg L−1 of iron) was less than a half of the required by the initial sonolytic process for degrading MERO. This is a direct consequence of the faster degradation of the carbapenem antibiotic by sono-Fenton.
The effect of ultrasound power on the degradation performance and the associated electric energy consumption were also tested (Fig. 2C and 2D). The results show the treatment of MERO by the sono-Fenton system (5.0 mg L−1 of iron) at 8.1 W of acoustic power compared to the sonolysis at 23.8 W. It can be noted that even at a lower ultrasound power the sono-Fenton process had a slightly higher k-value and a lower EEC50 than the sonolytic system (Fig. 2C and 2D). These last findings provide more arguments about the superiority of the sono-Fenton to deal with MERO regarding simple sonolysis.
It is relevant to consider that for the best case of the sono-Fenton system, the EEC50 was 0.49 kWh L−1 (equal to 490 kWh m−3), it belongs to the typical range for the electric energy consumption of ultrasound-based process (>100 kWh m−3); which is higher than other AOPs such those based on ozone or UVC/H2O2 [29]. Therefore, it is necessary to continue developing research on strategies for decreasing the EEC50 values through the optimization of electric energy use in ultrasound reactors, the enhancement of the treatable volume with the same input of electric energy to the reactor (e.g., the process operation in continuous mode) and/or the coupling with/addition of other low-energy-intensive subsystems.
3.3. Degradation of MERO by sono-photo-Fenton
In the previous section, the enhancement of the MERO degradation kinetics by the formation of a sono-Fenton process was shown. Despite the antibiotic degradation being faster, some hydrogen peroxide is still accumulated (see Ra value for sono-Fenton in Table 3). Therefore, as a strategy to consume part of the remnant H2O2 and get more elimination of MERO, UVA light was also added to the reaction system to form a sono-photo-Fenton process. Table 3 compares the k values for MERO degradation by sono-Fenton, direct photolysis, and sono-photo-Fenton (Fe2+: 5.0 mg L−1) processes.
Table 3.
Comparison of the values of k and Ra for the MERO treatment by sono-Fenton, photolysis, and sono-photo-Fenton.
| System |
k × 10−3 (min−1) |
Ra for H2O2 (µmol L−1 min−1) |
|---|---|---|
| Sono-Fenton | 15.5 | 0.306 |
| Photolysis | 1.3 | − |
| Sono-photo-Fenton | 18.6 | 0.288 |
| Synergy for sono-photo-Fenton* | 1.11 | |
−Not applicable.
The synergy was calculated as ksono-photo-Fenton/(ksono-Fenton + kphotolysis).
The degradation of MERO was enhanced by the UVA light addition to the sono-Fenton system, obtaining a k of 18.6 × 10−3 min−1 and synergy of 1.11. The synergy and the increment in the pseudo-first-order kinetic constant are associated with the extra formation of radicals by the iron photo-regeneration, as represented by Eq. 10 [58]. Besides, it should be mentioned that the used UVA also contributed to the MERO degradation via photolysis (although to a minor extent, k: 1.3 × 10−3 min−1). MERO has a small absorption tailing between 300 and 350 nm, while the UVA lamp starts its emission at 340 nm (with the maximum emission peak at 365 nm), which explains the contribution of photolysis to the sono-photo-Fenton system. This is consistent with previous work that has reported the photolytic degradation of MERO with light having components in the UVA region of the electromagnetic spectrum [59]. At this point, it is also relevant to mention that during the MERO treatment by sono-photo-Fenton, the experimental pH decreased from ∼6.4 up to 3.8 (see Table 2), which limits the iron precipitation, thus favoring the degradation of the target antibiotic under such a system.
| Fe3+ + H2O + UVA → Fe2+ + HO• + HO− | (10) |
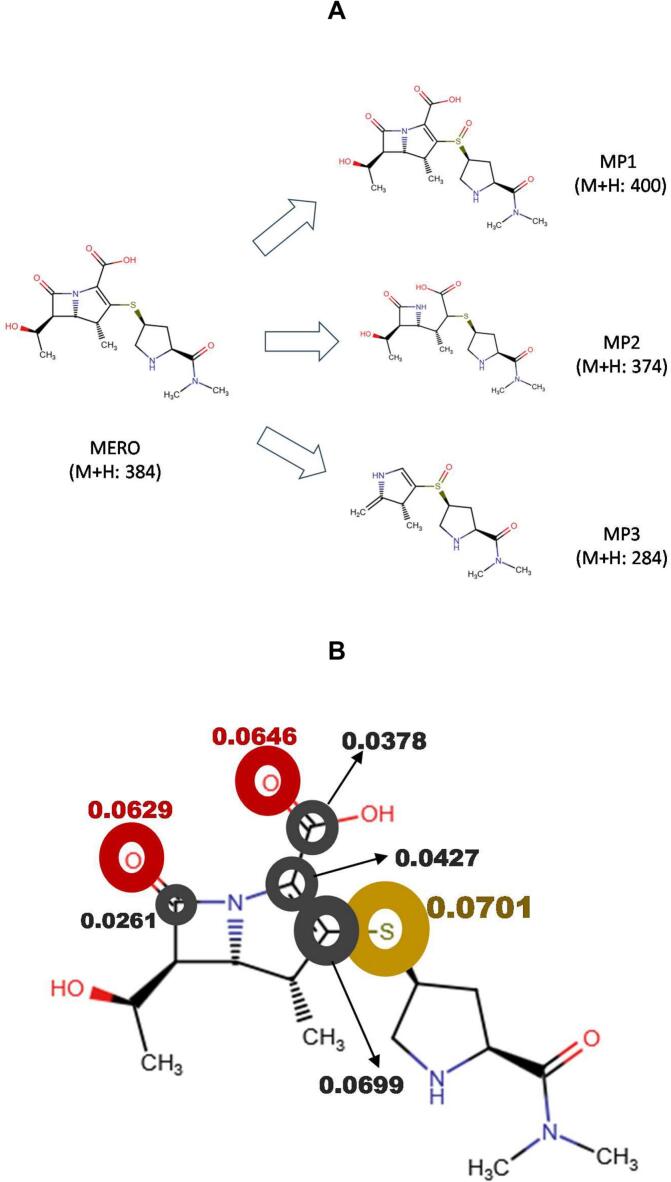
Since the sono-photo-Fenton process exhibited the highest degradation rate constant (k: 18.6 × 10−3 min−1, Table 3), the primary transformations induced by this process were determined. Fig. 3A presents the proposed chemical structure for the initial by-products of MERO degradation. The action of sono-photo-Fenton led to the formation of products presenting the oxidation on the thioether group (MP1, M + H: 400), the opening of the pyrroline ring (MP2, M + H: 374), and the cleavage of the β-lactam core (MP3, M + H: 284) regarding the parent carbapenem structure.
Fig. 3.
Primary transformations of MERO under the sono-photo-Fenton process. A. Proposed structures of the initial by-products. B. Fukui function for radical attacks on the carbapenem antibiotic (f0, which was determined using the free online software from the Rowan platform [48]); higher f0 values denote more reactive moieties on MERO.
To better understand the primary transformations induced on MERO, theoretical analysis of the Fukui function for radical interactions (f0) was calculated using the free online software from the Rowan platform [48]. This tool delivers information about the moieties of the target antibiotic more reactive to radical species [39]. According to this theoretical analysis (Fig. 3B), the highest f0 values were on the sulfur (0.0701) of the thioether, and the double bonds on the rings-type pyrroline (0.0699–0.0378) and β-lactam (0.0629–0.0261); thus, supporting the involvement of such atoms in the primary transformations, as observed in Fig. 3A. The literature has also reported that the thioether moiety of carbapenem antibiotics is very reactive toward HO•, which produces sulfoxide-type structures (as observed for MP1) [30,60]. In turn, the β-lactam ring on MERO is very strained. Hence, it is a site reactive toward radical species and characteristically experiences opening paths [39,60,61], generating transformation products such as MP3. Meanwhile, carbon–carbon double bonds and carboxylic acid are also attacked by HO• through the addition of the radical to pi-systems and hydrogen abstraction routes, respectively [55]. The combination of these two routes leads to the formation of MP2.
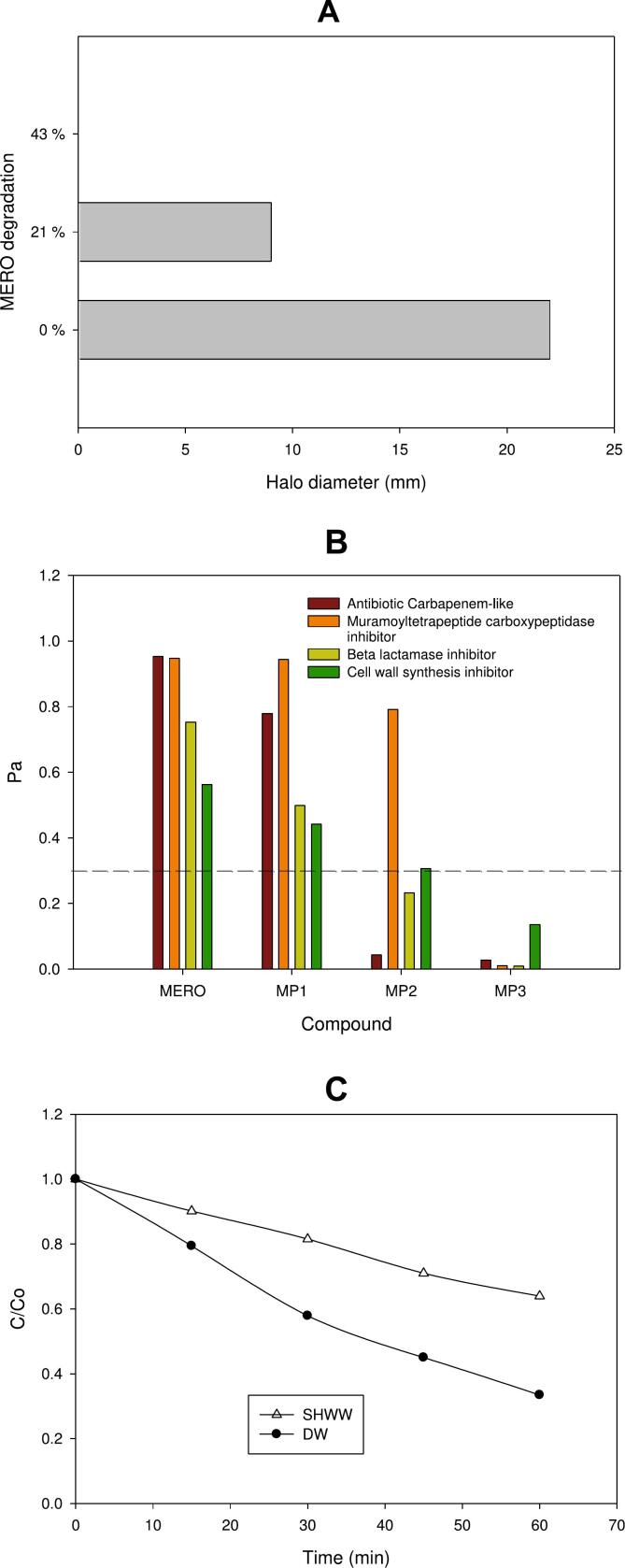
On the other side, to go beyond the MERO degradation, the change in the antimicrobial activity (AA) was tested using sensitive S. aureus as the probe microorganism (Fig. 4A). The AA was measured as the inhibition halo for the bacteria growth as a function of the MERO degradation. From Fig. 4A can be noted that the sono-photo-Fenton process completely decreased AA when 43 % of MERO was degraded. This result suggests that in the resultant solution, the antibiotic is below the effective concentration to induce a bactericidal action, and/or the primary intermediates have lower AA than MERO.
Fig. 4.
Treatment extent. A. Experimental antimicrobial activity (AA) against S. aureus, measured as the inhibition halo diameter (in mm). B. Predictions of biological activity for MERO and its primary transformation products (MP1-3); Pa value > 0.3 suggests that the compound is active (this threshold is represented by the dashed line in Fig. 4B). C. Comparison of the treatment of MERO in distilled water (DW) and synthetic hospital wastewater (SHWW) by sono-photo-Fenton. Experimental conditions: [MERO]: 20 mg L−1; [Fe2+]: 5 mg L−1; UVA light: 4 W; frequency: 578 kHz; and acoustic power: 23.8 W; temperature: 19 ± 2 °C.
To unravel the link between the antimicrobial activity decrease and the MERO degradation, predictions of the biological activity based on the chemical structure of primary transformation products were made by means of the PASS software [49]. Biological activities such as antibiotic carbapenem-like, muramoyltetrapeptide carboxypeptidase inhibitor, β lactamase inhibitor, and cell wall synthesis inhibitor (which are associated with the antimicrobial action mechanism) were taken into account (Fig. 4B). A comparison of probability to be active (Pa) for MERO and its by-products was considered.
As shown, in Fig. 4B, for most of the biological activities, the three primary degradation products had smaller Pa values than those for MERO. This is explained by the strong modifications to the carbapenem core (especially for MP2 and MP3 byproducts) that consistently support the decrease of AA presented in Fig. 4A. Besides, the connected AA elimination with the degradation of the carbapenem antibiotic by the sono-photo-Fenton system represents a good environmental effect due to this could limit the proliferation of antibiotic-resistant strains [32].
Finally, the treatment of MERO in a complex matrix was assessed. We considered synthetic hospital wastewater (SHWW, whose composition was taken from reference [62], and it has as main components urea, chloride, sulfate, and phosphate anions). Such a kind of matrix was selected because MERO is mainly used as a last-resort pharmaceutical in intra-hospital contexts [63,64], and approximately 70 % of the intravenously administered dose is released as unchanged meropenem in the urine [64] that ends up in the hospital sewage system [63].
Fig. 4C depicts the evolution of MERO in SHWW treated by sono-photo-Fenton. It can be seen that the degradation in SHWW is lower than in distilled water (DW). As the complex matrix has other substances (urea, chloride, sulfate, and phosphate anions) such compounds can scavenge the radical species formed during the sono-photo-Fenton process. In fact, these substances have a high reaction rate constant with HO•, 104–109 M−1 s−1 [65,66]. So, the MERO elimination in SHWW is slow but even it is similar to that obtained by sonolysis in DW (Fig. 1). Additionally, the trend line in Fig. 4C for the MERO degradation in SHWW suggests that if the treatment time is extended, complete elimination of MERO in this complex matrix could be achieved. Indeed, the k value for the MERO degradation in the complex matrix was 7.4 × 10−3 min−1. Thus, using this kinetic rate constant, the time required for degrading 99 % of the antibiotic is projected/estimated to be 10.4 h. Nevertheless, it must be taken into account that the antibiotic concentration utilized in the SHWW is very high (i.e., 20 mg L−1), and in actual hospital wastewater, the MERO concentration can be much lower (in the range of ng L−1 to µg L−1). Therefore, under more realistic conditions and significantly lower initial concentration of the target antibiotic, the treatment time is expected to be shorter than 10.4 h.
4. Conclusions
The ultrasound alone had a limited degrading action toward MERO, due to the hydrophilic character of this carbapenem antibiotic. Hence, the addition of ferrous ions to the sonochemical system improved the degradation of the target antibiotic. This improvement is due to the generation of extra radicals in the solution bulk through the Fenton reaction, which at the same time decreased the electric energy consumption. The iron concentration and ultrasound power played a key role in the degradation enhancement. The combination of ultrasound, iron, and UVA led to a faster and synergistic MERO degradation; where such a process diminished the antimicrobial activity, and transformed the parent compound into by-products with strong modifications on the antibiotic core, which links to the AA decreasing. The theoretical calculations of Fukui indices and biological activity provided support to better explain the attacks of radicals to MERO and the decrease of antimicrobial activity, respectively. Despite the kinetics of the treatment in SHWW being slower than in DW, the sono-photo-Fenton system induced degradation of the carbapenem in the complex matrix, demonstrating the good potentiality to deal with this class of antibiotics in aqueous samples.
CRediT authorship contribution statement
Efraím A. Serna-Galvis: Writing – original draft, Methodology, Investigation, Conceptualization. Kevin P. Celis-Llamoca: Methodology, Investigation, Formal analysis, Data curation. Ingrit E. Collantes-Díaz: Methodology, Formal analysis. Ricardo A. Torres-Palma: Writing – review & editing, Supervision, Conceptualization. Jessica I. Nieto-Juárez: Writing – review & editing, Supervision, Project administration, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors from UNI-Peru thank the financial support provided by the Faculty of Chemical and Textile Engineering at Universidad Nacional de Ingeniería (Project N° P-IQ-2022-000981) and Prociencia/Concytec through grant E041-01 (Project N° 32-2018). E. A. Serna-Galvis acknowledges the financial support provided through the project CODI-UdeA 2022-53586.
Footnotes
This article is part of a special issue entitled: ‘Selected Papers from EES 2024’ published in Ultrasonics Sonochemistry.
References
- 1.Nieto-Juárez J.I., Torres-Palma R.A., Botero-Coy A.M., Hernández F. Pharmaceuticals and environmental risk assessment in municipal wastewater treatment plants and rivers from Peru. Environ. Int. 2021;155 doi: 10.1016/j.envint.2021.106674. [DOI] [PubMed] [Google Scholar]
- 2.Rodríguez-Rodríguez C.E., Ramírez-Morales D., Gutiérrez-Quirós J.A., Rodríguez-Saravia S., Villegas-Solano D. Occurrence of pharmaceuticals in Latin America: case study on hazard assessment and prioritization in Costa Rica. Environ. Monit. Assess. 2024;196:739. doi: 10.1007/s10661-024-12872-z. [DOI] [PubMed] [Google Scholar]
- 3.Nassri I., Khattabi Rifi S., Sayerh F., Souabi S. Occurrence, pollution sources, and mitigation prospects of Antibiotics, anti-inflammatories, and endocrine disruptors in the aquatic environment. Environ. Nanotechnol. Monit. Manag. 2023;20 doi: 10.1016/j.enmm.2023.100878. [DOI] [Google Scholar]
- 4.Kovalakova P., Cizmas L., McDonald T.J., Marsalek B., Feng M., Sharma V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere. 2020;251 doi: 10.1016/j.chemosphere.2020.126351. [DOI] [PubMed] [Google Scholar]
- 5.Mishra S., Singh A.K., Cheng L., Hussain A., Maiti A. Occurrence of antibiotics in wastewater: Potential ecological risk and removal through anaerobic–aerobic systems. Environ. Res. 2023;226 doi: 10.1016/j.envres.2023.115678. [DOI] [PubMed] [Google Scholar]
- 6.Zhu L., Lin X., Di Z., Cheng F., Xu J. Occurrence, risks, and removal methods of antibiotics in urban wastewater treatment systems: a review. Water. 2024;16:3428. doi: 10.3390/w16233428. [DOI] [Google Scholar]
- 7.Wang K., Zhuang T., Su Z., Chi M., Wang H. Antibiotic residues in wastewaters from sewage treatment plants and pharmaceutical industries: Occurrence, removal and environmental impacts. Sci. Total Environ. 2021;788 doi: 10.1016/j.scitotenv.2021.147811. [DOI] [PubMed] [Google Scholar]
- 8.Cedeño-Muñoz J.S., Aransiola S.A., Reddy K.V., Ranjit P., Victor-Ekwebelem M.O., Oyedele O.J., Pérez-Almeida I.B., Maddela N.R., Rodríguez-Díaz J.M. Antibiotic resistant bacteria and antibiotic resistance genes as contaminants of emerging concern: Occurrences, impacts, mitigations and future guidelines. Sci. Total Environ. 2024;952 doi: 10.1016/j.scitotenv.2024.175906. [DOI] [PubMed] [Google Scholar]
- 9.Thibodeau A.J., Barret M., Mouchet F., Nguyen V.X., Pinelli E. The potential contribution of aquatic wildlife to antibiotic resistance dissemination in freshwater ecosystems: A review. Environ. Pollut. 2024;350 doi: 10.1016/j.envpol.2024.123894. [DOI] [PubMed] [Google Scholar]
- 10.Luo Y., Liu C., Wang Y., Yang Y., Mishra S. Occurrence, distribution and their correlation with different parameters of antibiotics and antibiotic resistance genes in lakes of China: A review. Mar. Pollut. Bull. 2023;193 doi: 10.1016/j.marpolbul.2023.115189. [DOI] [PubMed] [Google Scholar]
- 11.Guzman-Tordecilla M., Pacheco-Bustos C., Coronado-Posada N., Pedrosa-Gomes M., Martinez-Burgos W.J., Mejía-Marchena R., Zorman-Marques R. Exploring the ecotoxicological impact of meropenem on Lemna minor: Growth, photosynthetic activity, and oxidative stress. Environ. Res. 2024;258 doi: 10.1016/j.envres.2024.119409. [DOI] [PubMed] [Google Scholar]
- 12.Hrenovic J., Ivankovic T., Ivekovic D., Repec S., Stipanicev D., Ganjto M. The fate of carbapenem-resistant bacteria in a wastewater treatment plant. Water Res. 2017;126:232–239. doi: 10.1016/j.watres.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization (WHO), Guidelines for the prevention and control of carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in health care facilities, (2017). https://www.who.int/publications/i/item/guidelines-for-the-prevention-and-control-of-carbapenem-resistant-enterobacteriaceae-acinetobacter-baumannii-and-pseudomonas-aeruginosa-in-health-care-facilities. [PubMed]
- 14.Krapp F., Cuicapuza D., Salvatierra G., Buteau J.P., Amaro C., Astocondor L., Hinostroza N., Jacobs J., García C., Tsukayama P. Emerging carbapenem-resistant Klebsiella pneumoniae in a tertiary care hospital in Lima, Peru. Microbiol. Spectr. 2025;13 doi: 10.1128/spectrum.01825-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viñes J., Lopera C., Vergara A., Roca I., Vila J., Casals-Pascual C., Martínez J.A., García-Vidal C., Soriano A., Pitart C. Emergence of carbapenem-resistant Pseudomonas aeruginosa ST179 producing both IMP-16 and KPC-2: a case study of introduction from Peru to Spain. Microbiol. Spectr. 2024;12 doi: 10.1128/spectrum.00614-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rondon C., Garcia C., Krapp F., Machaca I., Olivera M., Fernández V., Villegas M., Vilcapoma P., Casapia M., Concha-Velasco F., Díaz J.C., Sarmiento F., Guillermo R., Farnham A., Sutter S.T., Kuenzli E. Antibiotic point prevalence survey and antimicrobial resistance in hospitalized patients across Peruvian reference hospitals. J. Infect. Public Health. 2023;16:52–60. doi: 10.1016/j.jiph.2023.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tickler I.A., La Torre J.C.G.D., Alvarado L., Obradovich A.E., Tenover F.C. Mechanisms of carbapenemase-mediated resistance among high-risk Pseudomonas aeruginosa lineages in Peru. J. Glob. Antimicrob. Resist. 2022;31:135–140. doi: 10.1016/j.jgar.2022.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Maguire M., Serna C., Montero Serra N., Kovarova A., O’Connor L., Cahill N., Hooban B., DeLappe N., Brennan W., Devane G., Cormican M., Morris D., Coughlan S.C., Miliotis G., Gonzalez-Zorn B., Burke L.P. Spatiotemporal and genomic analysis of carbapenem resistance elements in Enterobacterales from hospital inpatients and natural water ecosystems of an Irish city. Microbiol. Spectr. 2025;13 doi: 10.1128/spectrum.00904-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hubeny J., Korzeniewska E., Buta-Hubeny M., Zieliński W., Rolbiecki D., Harnisz M. Characterization of carbapenem resistance in environmental samples and Acinetobacter spp. isolates from wastewater and river water in Poland. Sci. Total Environ. 2022;822 doi: 10.1016/j.scitotenv.2022.153437. [DOI] [PubMed] [Google Scholar]
- 20.Ju X., Xiong P., Yan Z., Chen G., Cai C., Zhang R. Emergence of carbapenem-resistant Citrobacter spp. across human, animal, and water environments in China. Int. J. Antimicrob. Agents. 2025 doi: 10.1016/j.ijantimicag.2025.107463. [DOI] [PubMed] [Google Scholar]
- 21.Stefaniak K., Kiedrzyński M., Korzeniewska E., Kiedrzyńska E., Harnisz M. Preliminary insights on carbapenem resistance in Enterobacteriaceae in high-income and low-/middle-income countries. Sci. Total Environ. 2024;957 doi: 10.1016/j.scitotenv.2024.177593. [DOI] [PubMed] [Google Scholar]
- 22.Kempf M., Rolain J.-M. Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: clinical impact and therapeutic options. Int. J. Antimicrob. Agents. 2012;39:105–114. doi: 10.1016/j.ijantimicag.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Kusi J., Ojewole C.O., Ojewole A.E., Nwi-Mozu I. Antimicrobial resistance development pathways in surface waters and public health implications. Antibiotics. 2022;11:821. doi: 10.3390/antibiotics11060821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel M., Kumar R., Kishor K., Mlsna T., Pittman C.U., Mohan D. Pharmaceuticals of emerging concern in aquatic systems: chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019;119:3510–3673. doi: 10.1021/acs.chemrev.8b00299. [DOI] [PubMed] [Google Scholar]
- 25.Skalska-Tuomi K., Kaijanen L., Monteagudo J.M., Mänttäri M. Efficient removal of pharmaceuticals from wastewater: Comparative study of three advanced oxidation processes. J. Environ. Manage. 2025;375 doi: 10.1016/j.jenvman.2025.124276. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed Y., Maya A.A.S., Akhtar P., AlMohamadi H., Mohammad A.W., Ashekuzzaman S.M., Olbert A.I., Uddin M.G. Advancements and challenges in Fenton-based advanced oxidation processes for antibiotic removal in wastewater: From the laboratory to practical applications. J. Environ. Chem. Eng. 2025;13 doi: 10.1016/j.jece.2024.115068. [DOI] [Google Scholar]
- 27.Iqbal J., Shah N.S., Ali Khan J., Naushad M., Boczkaj G., Jamil F., Khan S., Li L., Murtaza B., Han C. Pharmaceuticals wastewater treatment via different advanced oxidation processes: Reaction mechanism, operational factors, toxicities, and cost evaluation – A review. Sep. Purif. Technol. 2024;347 doi: 10.1016/j.seppur.2024.127458. [DOI] [Google Scholar]
- 28.Hübner U., Spahr S., Lutze H., Wieland A., Rüting S., Gernjak W., Wenk J. Advanced oxidation processes for water and wastewater treatment – Guidance for systematic future research. Heliyon. 2024;10 doi: 10.1016/j.heliyon.2024.e30402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miklos D.B., Remy C., Jekel M., Linden K.G., Hübner U. Evaluation of advanced oxidation processes for water and wastewater treatment- A critical review. Water Res. 2018;139:118–131. doi: 10.1016/j.watres.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 30.Celis-Llamoca K., Serna-Galvis E.A., Torres-Palma R.A., Nieto-Juárez J.I. Sono-photo-Fenton action is improved by the addition of Passiflora edulis f. flavicarpa Degener (yellow passion fruit) Environ. Sci. Pollut. Res. 2024;31:64974–64986. doi: 10.1007/s11356-024-35522-w. [DOI] [PubMed] [Google Scholar]
- 31.Serna-Galvis E.A., Botero-Coy A.M., Martínez-Pachón D., Moncayo-Lasso A., Ibáñez M., Hernández F., Torres-Palma R.A. Degradation of seventeen contaminants of emerging concern in municipal wastewater effluents by sonochemical advanced oxidation processes. Water Res. 2019;154:349–360. doi: 10.1016/j.watres.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 32.Serna-Galvis E.A., Guateque-Londoño J.F., Silva-Agredo J., Porras J., Ávila-Torres Y., Torres-Palma R.A. Superior selectivity of high-frequency ultrasound toward chorine containing-pharmaceuticals elimination in urine: A comparative study with other oxidation processes through the elucidation of the degradation pathways. Ultrason. Sonochem. 2021;80 doi: 10.1016/j.ultsonch.2021.105814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serna-Galvis E.A., Silva-Agredo J., Giraldo-Aguirre A.L., Flórez-Acosta O.A., Torres-Palma R.A. High frequency ultrasound as a selective advanced oxidation process to remove penicillinic antibiotics and eliminate its antimicrobial activity from water. Ultrason. Sonochem. 2016;31:276–283. doi: 10.1016/j.ultsonch.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Guateque-Londoño J.F., Serna-Galvis E.A., Ávila-Torres Y., Torres-Palma R.A. Degradation of Losartan in Fresh Urine by Sonochemical and Photochemical Advanced Oxidation Processes. Water. 2020;12:3398. doi: 10.3390/w12123398. [DOI] [Google Scholar]
- 35.Meinhardt-Ultrasonics, Further Ultrasonics, Transducers. (2025). https://www.meinhardt-ultrasonics.com/english/transducers/further-ultrasonic/ (accessed April 19, 2025).
- 36.Celis-Llamoca K., Serna-Galvis E.A., Torres-Palma R.A., Nieto-Juárez J.I. High-frequency ultrasound processes as alternative methods for degrading meropenem antibiotic in water. MethodsX. 2022;9 doi: 10.1016/j.mex.2022.101835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmadmoazzam M., Akbari H., Adibzadeh A., Pourfadakari S., Akbari H. Visible-light-driven TiO2@Fe2O3/Chitosan nanocomposite with promoted photodegradation of meropenem and imipenem antibiotics by peroxymonosulfate. Environ. Technol. (United Kingdom) 2024;45:3456–3467. doi: 10.1080/09593330.2023.2218042. [DOI] [PubMed] [Google Scholar]
- 38.Cabrera-Reina A., Martínez-Piernas A.B., Bertakis Y., Xekoukoulotakis N.P., Agüera A., Sánchez Pérez J.A. TiO2 photocatalysis under natural solar radiation for the degradation of the carbapenem antibiotics imipenem and meropenem in aqueous solutions at pilot plant scale. Water Res. 2019;166 doi: 10.1016/j.watres.2019.115037. [DOI] [PubMed] [Google Scholar]
- 39.Wang J., Gao B., Dou M., Huang X., Ma Z. A porous g-C3N4 nanosheets containing nitrogen defects for enhanced photocatalytic removal meropenem: Mechanism, degradation pathway and DFT calculation. Environ. Res. 2020;184 doi: 10.1016/j.envres.2020.109339. [DOI] [PubMed] [Google Scholar]
- 40.Dou M., Wang J., Gao B., Ma Z., Huang X. A novel in-situ chemical oxidation channel – Selective pH-dependence of refractory β-lactam antibiotics in the synergistic mechanism of persulfate and g-C3N4 under visible light. Chem. Eng. J. 2020;394 doi: 10.1016/j.cej.2020.124899. [DOI] [Google Scholar]
- 41.Ahmadi A., Vogler B., Deng Y., Wu T. Removal of meropenem from environmental matrices by electrochemical oxidation using Co/Bi/TiO2 nanotube electrodes. Environ. Sci. Water Res. Technol. 2020;6:2197–2208. doi: 10.1039/D0EW00184H. [DOI] [Google Scholar]
- 42.Agudelo E.A., Cardona G. S.A. Advanced oxidation technology (ozone-catalyzed by powder activated carbon - portland cement) for the degradation of the meropenem antibiotic. Ozone Sci. Eng. 2021;43:88–105. doi: 10.1080/01919512.2020.1796582. [DOI] [Google Scholar]
- 43.Kordestani B., Jalilzadeh Yengejeh R., Takdastan A., Neisi A.K. A new study on photocatalytic degradation of meropenem and ceftriaxone antibiotics based on sulfate radicals: Influential factors, biodegradability, mineralization approach. Microchem. J. 2019;146:286–292. doi: 10.1016/j.microc.2019.01.013. [DOI] [Google Scholar]
- 44.Kordestani B., Takdastan A., Jalilzadeh Yengejeh R., Neisi A.K. Photo-Fenton oxidative of pharmaceutical wastewater containing meropenem and ceftriaxone antibiotics: influential factors, feasibility, and biodegradability studies. Toxin Rev. 2020;39:292–302. doi: 10.1080/15569543.2018.1520261. [DOI] [Google Scholar]
- 45.Kimura T., Sakamoto T., Leveque J.M., Sohmiya H., Fujita M., Ikeda S., Ando T. Standardization of ultrasonic power for sonochemical reaction. Ultrason. Sonochem. 1996;3 doi: 10.1016/S1350-4177(96)00021-1. [DOI] [Google Scholar]
- 46.Serna-Galvis E.A., Silva-Agredo J., Giraldo-Aguirre A.L., Torres-Palma R.A. Sonochemical degradation of the pharmaceutical fluoxetine: Effect of parameters, organic and inorganic additives and combination with a biological system. Sci. Total Environ. 2015;524–525:354–360. doi: 10.1016/j.scitotenv.2015.04.053. [DOI] [PubMed] [Google Scholar]
- 47.Camargo-Perea A.L., Serna-Galvis E.A., Lee J., Torres-Palma R.A. Understanding the effects of mineral water matrix on degradation of several pharmaceuticals by ultrasound: Influence of chemical structure and concentration of the pollutants. Ultrason. Sonochem. 2021;73 doi: 10.1016/j.ultsonch.2021.105500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.ROWAN, Molecular design and simulation tools for scientists, Rowan Sci. Corp. (2025). https://www.rowansci.com/ (accessed January 3, 2025).
- 49.W2D Team - PharmaExpert, PASS online, (2024). http://www.pharmaexpert.ru/passonline/index.php (accessed August 5, 2024).
- 50.Kim D.K., He Y., Jeon J., O’Shea K.E. Irradiation of ultrasound to 5-methylbenzotriazole in aqueous phase: Degradation kinetics and mechanisms. Ultrason. Sonochem. 2016;31:227–236. doi: 10.1016/j.ultsonch.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 51.PubChem, Methylen blue, Natl. Libr. Medicne-USA. (2025). https://pubchem.ncbi.nlm.nih.gov/compound/6099 (accessed January 2, 2025).
- 52.Montoya-Rodríguez D.M., Serna-Galvis E.A., Ferraro F., Torres-Palma R.A. Degradation of the emerging concern pollutant ampicillin in aqueous media by sonochemical advanced oxidation processes - Parameters effect, removal of antimicrobial activity and pollutant treatment in hydrolyzed urine. J. Environ. Manage. 2020;261 doi: 10.1016/j.jenvman.2020.110224. [DOI] [PubMed] [Google Scholar]
- 53.Montoya-Rodríguez D.M., Ávila-Torres Y., Serna-Galvis E.A., Torres-Palma R.A. Data on treatment of nafcillin and ampicillin antibiotics in water by sonochemistry. Data Br. 2020;29 doi: 10.1016/j.dib.2020.105361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kavitha V., Palanivelu K. The role of ferrous ion in Fenton and photo-Fenton processes for the degradation of phenol. Chemosphere. 2004;55:1235–1243. doi: 10.1016/j.chemosphere.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 55.Pignatello J.J., Oliveros E., MacKay A. Advanced oxidation processes for organic contaminant destruction based on the fenton reaction and related chemistry. Environ. Sci. Technol. 2006;36:1–84. doi: 10.1080/10643380500326564. [DOI] [Google Scholar]
- 56.Adewuyi Y.G. Sonochemistry: environmental science and engineering applications. Ind. Eng. Chem. Res. 2001;40:4681–4715. doi: 10.1021/ie010096l. [DOI] [Google Scholar]
- 57.Torres R., Pétrier C., Combet E., Moulet F., Pulgarin C. Bisphenol a mineralization by integrated ultrasound-UV-iron (II) treatment. Environ. Sci. Technol. 2007;41:297–302. doi: 10.1021/es061440e. [DOI] [PubMed] [Google Scholar]
- 58.Machado F., Teixeira A.C.S.C., Ruotolo L.A.M. Critical review of Fenton and photo ‑ Fenton wastewater treatment processes over the last two decades. Springer Berlin Heidelberg. 2023 doi: 10.1007/s13762-023-05015-3. [DOI] [Google Scholar]
- 59.Reina A.C., Martínez-Piernas A.B., Bertakis Y., Brebou C., Xekoukoulotakis N.P., Agüera A., Sánchez Pérez J.A. Photochemical degradation of the carbapenem antibiotics imipenem and meropenem in aqueous solutions under solar radiation. Water Res. 2018;128:61–70. doi: 10.1016/j.watres.2017.10.047. [DOI] [PubMed] [Google Scholar]
- 60.Szabó L., Tóth T., Engelhardt T., Rácz G., Mohácsi-farkas C., Takács E., Wojnárovits L. Change in hydrophilicity of penicillins during advanced oxidation by radiolytically generated •OH compromises the elimination of selective pressure on bacterial strains. Sci. Total Environ. 2016;551–552:393–403. doi: 10.1016/j.scitotenv.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 61.Trovó A.G., Pupo Nogueira R.F., Agüera A., Fernandez-Alba A.R., Malato S. Degradation of the antibiotic amoxicillin by photo-Fenton process – Chemical and toxicological assessment. Water Res. 2011;45:1394–1402. doi: 10.1016/j.watres.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 62.Serna-Galvis E.A., Cáceres-Peña A.C., Torres-Palma R.A. Elimination of representative fluoroquinolones, penicillins, and cephalosporins by solar photo-Fenton: degradation routes, primary transformations, degradation improvement by citric acid addition, and antimicrobial activity evolution. Environ. Sci. Pollut. Res. 2020;27:41381–41393. doi: 10.1007/s11356-020-10069-8. [DOI] [PubMed] [Google Scholar]
- 63.Sharma E., Chen Y., Kelso C., Sivakumar M., Jiang G. Soil & Environmental Health Navigating the environmental impacts and analytical methods of last-resort antibiotics : Colistin and carbapenems. Soil Environ. Heal. 2024;2 doi: 10.1016/j.seh.2024.100058. [DOI] [Google Scholar]
- 64.DrugBank, Meropenem excretion, DrugBank Online. (2025). https://go.drugbank.com/drugs/DB00760 (accessed January 5, 2025).
- 65.Lian L., Yao B., Hou S., Fang J., Yan S., Song W. Kinetic study of hydroxyl and sulfate radical-mediated oxidation of pharmaceuticals in wastewater effluents. Environ. Sci. Technol. 2017;51:2954–2962. doi: 10.1021/acs.est.6b05536. [DOI] [PubMed] [Google Scholar]
- 66.Buxton G.V., Greenstock C.L., Helman W.P., Ross A.B. Critical review of rate constants for reactions of hydrated electron, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O in Aqueous Solution) J. Phys. Chem. Ref. Data. 1988;513 doi: 10.1063/1.555805. [DOI] [Google Scholar]