Abstract
Pepstatins are potent inhibitors of aspartic proteases, featuring two statine residues crucial for target binding. However, the biosynthesis of pepstatins, especially their statine substructure, remains elusive. Here, we discover and characterize an unconventional gene cluster responsible for pepstatin biosynthesis, comprising discrete nonribosomal peptide synthetase and polyketide synthase genes, highlighting its trans-acting and iterative nature. Central to this pathway is PepI, an F420H2-dependent oxidoreductase. The biochemical characterization of PepI reveals its role in the tandem reduction of β-keto pepstatin intermediates. PepI first catalyzes the formation of the central statine, then produces the C-terminal statine moiety. The post-assembly-line formation of statine by PepI contrasts with the previously hypothesized biosynthesis involving polyketide synthase ketoreductase domains. Structural studies, site-directed mutagenesis, and deuterium-labeled enzyme assays probe the mechanism of F420H2-dependent oxidoreductases and identify critical residues. Our findings uncover a unique statine biosynthetic pathway employing the only known iterative F420H2-dependent oxidoreductase to date.
Subject terms: Biosynthesis, Oxidoreductases, X-ray crystallography, Enzyme mechanisms, Proteases
Pepstatins are potent inhibitors of aspartic proteases, featuring two statine residues crucial for target binding, however, their biosynthesis is elusive. Here, the authors discover and characterize an unconventional gene cluster responsible for pepstatin biosynthesis, and characterize the role of PepI, an F420H2-dependent oxidoreductase catalysing the tandem reduction of β-keto pepstatin intermediates.
Introduction
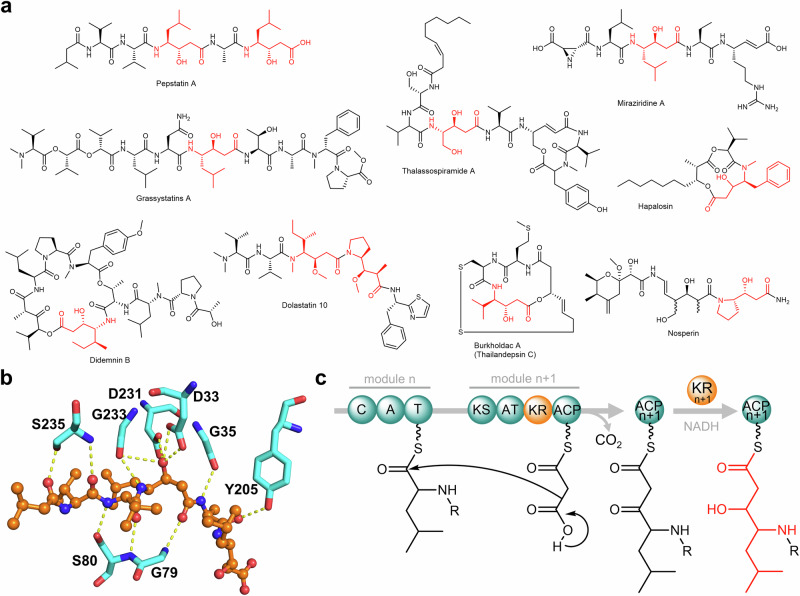
Pepstatins are highly potent inhibitors, effective in the pico- to nanomolar range, against various aspartic proteases (APs) such as pepsin and cathepsin D1–5. APs are endopeptidases discovered from all three domains of life and viruses. They are widely considered promising therapeutic targets owing to their vital roles in underlying physiological processes and the pathogenesis of various diseases. Notable examples include HIV-1 protease for HIV/AIDS, renin for hypertension, β- and γ-secretases for Alzheimer’s disease, plasmepsin for malaria, and the secreted APs for fungal infections6. Pepstatins are linear N-acyl pentapeptides featuring two (3S,4S)−4-amino-3-hydroxy-6-methylheptanoic acid residues, which are nonproteogenic γ-amino acids known as statines (Sta; Fig. 1a). Crystal structures of different APs in complex with pepstatins or structural analogs revealed that the central Sta residue forms hydrogen bonding with the two catalytic Asp residues7, vital to AP inhibition (Fig. 1b). Sta mimics the tetrahedral transition state during catalysis forming a non-cleavable structural analog of the scissile bond present in AP substrates. Insights into the inhibitory mechanism of pepstatin on APs have been instrumental in the development of peptide isostere classes of AP inhibitors8.
Fig. 1. Representative statine-containing natural products, pepstatin binding mode, and the proposed pathway for statine biosynthesis.
a Pepstatin A and representative known compounds with Sta/Sta-like residues (highlighted in red). b Interactions between pepstatin A and cathepsin D (PDB ID:1LYB). Only hydrogen bonds between pepstatin A (orange) and residues in the protein binding pocket (cyan) are shown. c The prior hypothesis on statine biosynthesis involving a modular PKS KR domain26.
Since the discovery of pepstatins from actinomycetes9,10, numerous studies have focused on their AP-inhibitory activity and mechanism. However, the pepstatin biosynthetic pathway has remained unclear. Pepstatins contain a Val-Val-Sta-Ala-Sta pentapeptide decorated with a variable N-terminal acyl chain2,10,11. The Sta residues in the pepstatin-like ahpatinins may be replaced by 4-amino-3-hydroxy-5-phenylpentanoic acid but retain the 3-OH-4-NH2 feature12,13. A feeding study using 14C-labeled compounds suggested the Sta residues in pepstatin are derived from l-leucine and malonate14. The Sta or Sta-like residues with the 3-OH-4-NH2 framework are also found in other natural products (representative examples in Fig. 1a)15–25. The biosynthesis of these substructures has been hypothesized to involve a collaborative process between nonribosomal peptide synthetase (NRPS) and polyketide synthase (PKS) modules26. In this proposed pathway, an NRPS module activates an α-amino acid such as leucine and loads it to the cognate peptidyl carrier protein (PCP) domain. The acyltransferase (AT) domain then attaches the malonyl-CoA extending unit to the acyl carrier protein (ACP) domain. A Claisen condensation reaction leads to the extension of the peptidyl thioester, forming a β-keto ester. In the didemnin biosynthetic gene cluster (BGC)27, isoleucine is activated, and the ketoreductase (KR) domain within the PKS module reduces the β-keto group to form the 3-OH-4-NH2 framework (Fig. 1c)26. A similar NRPS-PKS pair with a KR domain in the PKS module has also been observed in the BGCs of burkholdac23, hapalosin28, thalassospiramide29, and nosperin21. In contrast, the β-ketone functionality is retained in andrimid biosynthesis. Notably, the corresponding NRPS-PKS pair in the andrimid biosynthetic pathway lacks a KR domain, implying that the KR domain in PKS modules is responsible for 3-OH generation in Sta/Sta-like residue biosynthesis30. However, this pathway has not been experimentally verified and has only been rationalized based on the analysis of BGCs of natural products with the 3-OH-4-NH2 frameworks26.
In this work, we uncovered a unique post-assembly-line pathway for Sta biosynthesis that relies on a discrete oxidoreductase utilizing F420H2, a deazaflavin-based redox cofactor widely found in bacteria and archaea31–33. This pathway diverges from the conventional hypothesis, which attributes this function to a modular KR domain utilizing NADPH (Fig. 1c). Through gene knockout experiments and activation of the candidate pepstatin BGC, we characterized a noncanonical NRPS-PKS pathway responsible for pepstatin biosynthesis. Our findings suggest the involvement of in-trans and iterative NRPS and PKS mechanisms, indicating deviations from the traditional colinearity principle34,35. Deletion of pepI, encoding an F420H2-dependent oxidoreductase, resulted in an accumulation of the β-keto intermediates of pepstatins and related spontaneously decarboxylated products. Biochemical characterization of PepI in vitro unambiguously demonstrated the sequential reduction of β-ketones, starting with the central residue and then followed by the C-terminal moiety, ultimately leading to the formation of pepstatins. We determined the specific structural changes in the substrates and products of PepI through comprehensive structure elucidation and enzymatic characterization. The crystal structures of PepI and PepI in complex with the cofactor F420 allowed us to identify key residues His62, Tyr122, and Gln229. The roles of these residues are further rationalized by PepI-substrate modeling and site-directed mutagenesis studies. Moreover, biochemical assays utilizing a deuterium-labeled cofactor provide valuable insights into the catalytic mechanism of F420-dependent oxidoreductases. Notably, PepI-mediated Sta biosynthesis represents the only known iterative F420H2-dependent oxidoreductase, distinct from previously hypothesized PKS KR-dependent biosynthetic pathways for 3-OH-4-NH2 frameworks.
Results
Identification of the pepstatin BGC defying the colinearity rule
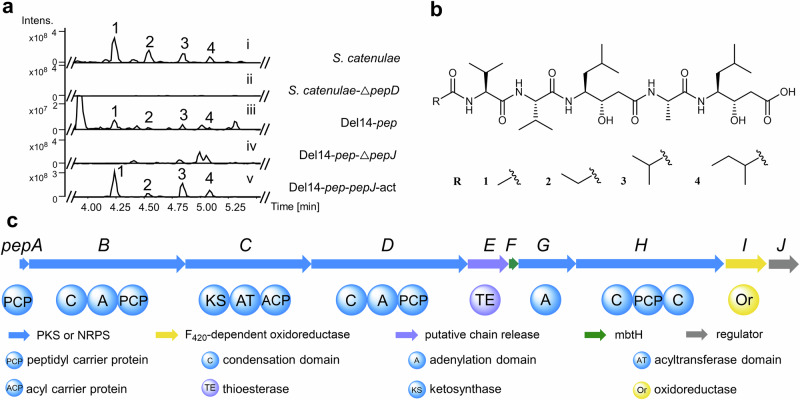
We cultivated the known pepstatin producer Streptomyces catenulae DSM4025836 and analyzed its fermentation broth using ultra-performance liquid chromatography-high-resolution mass spectrometry (UPLC-HRMS). Structural elucidation of the four purified pepstatin congeners (1-4) through nuclear magnetic resonance (NMR) spectroscopy revealed variations at the N-terminal acyl group, confirming congener 4 is a new derivative (Fig. 2a,b; Supplementary Figs. 1 and 26−42; and Supplementary Table 8)10,11.
Fig. 2. Identification of pepstatins and the pep biosynthetic gene cluster.
a UPLC-HRMS analysis (base peak chromatogram (BPC)) of pepstatin congeners (1-4) produced by Streptomyces catenulae DSM40258 (i); Knockingout pepD abolished pepstatin 1-4 production (ii); Pepstatin congeners (1-4) produced by heterologous expression of BGC pep in Del14 (Del14-pep) (iii); The production of 1-4 significantly decreased by pepJ deletion in Del14-pep-ΔpepJ (iv); The production of 1-4 increased in Del14-pep-pepJ-act by promoter exchange of pepJ (v). b The chemical structures of pepstatins isolated from Streptomyces catenulae DSM40258. c The schematic representation of the pepstatin BGC from S. catenulae DSM40258.
With pepstatin production verified, we performed complete genome sequencing of S. catenulae DSM40258 to decipher the BGC of pepstatin. Surprisingly, the absence of a straightforward candidate for an NRPS-PKS hybrid BGC suggested a noncanonical biosynthetic pathway. According to the colinearity principle, we expected to find five NRPS modules and two PKS modules in one BGC (Supplementary Fig. 2). However, none of the NRPS-related BGCs encoded more than four adenylation (A) domains, indicating an unconventional biosynthesis mechanism. Furthermore, we found no consecutive NRPS and PKS modules that matched the previously reported biosynthesis of statine/statine-like residues in the DSM40258 genome. Despite these unexpected challenges, we identified one NRPS-PKS hybrid BGC (designated as “pep”) with only three A domains that caught our attention. This BGC included one putative leucine-activating NRPS gene (pepB) adjacent to a PKS gene (pepC) with a truncated AT domain (Fig. 2c, Supplementary Table 5). To confirm the involvement of the pep BGC in pepstatin biosynthesis, we performed gene deletion of pepD, which encodes the potential alanine assembly module. Deleting the NRPS gene pepD completely abolished pepstatin production in S. catenulae DSM40258 (Fig. 2a; Supplementary Figs. 3 and 7a), demonstrating that pepD is essential for pepstatin biosynthesis.
To validate the biosynthetic pathway, the 18.3 kb pep BGC, comprising ten genes (pepA-J), was cloned and subsequently heterologously expressed in Streptomyces albus Del1437. The successful heterologous reconstitution of pepstatin production confirmed the BGC and its boundaries (Fig. 2a and Supplementary Fig. 4). Deletion of the LuxR-like transcriptional regulator gene pepJ significantly reduced pepstatin production, suggesting that pepJ functions as a positive regulator. Conversely, overexpression of pepJ under the kasOp promoter led to a more than 25-fold increase in pepstatin yield (Fig. 2a and Supplementary Figs. 5–7), enabling the identification of biosynthetic intermediates in subsequent studies.
A highly dissociated and nonlinear NRPS-PKS pathway for pepstatin biosynthesis
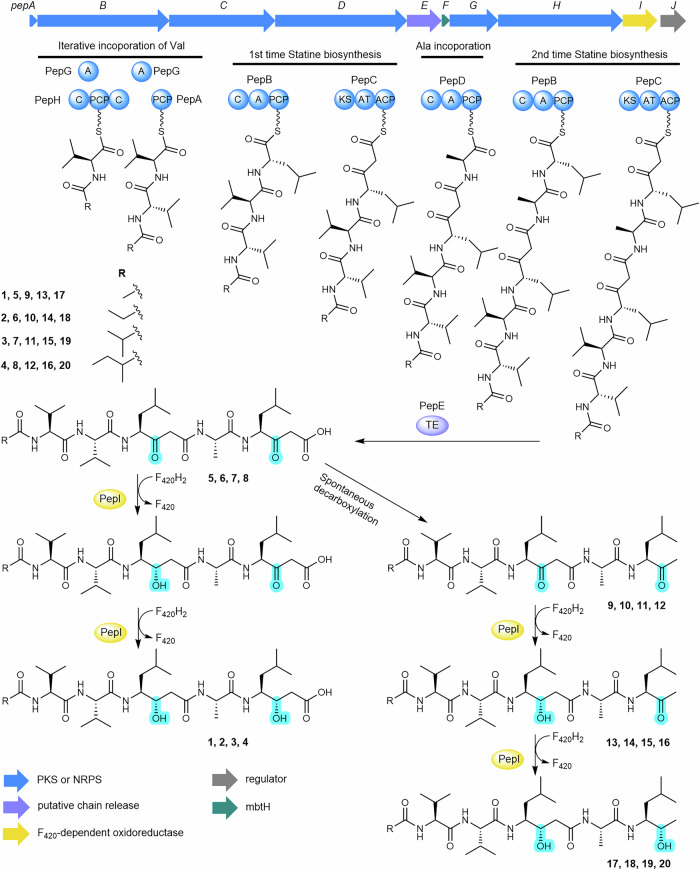
The streamlined pep BGC presents an interesting puzzle, as it contains fewer NRPS-PKS modules than the number of building blocks required for pepstatin biosynthesis, suggesting the involvement of noncanonical mechanisms. The pep BGC possesses only three A domains to assemble the pentapeptide backbone of pepstatins (Fig. 2c), which deviates from the colinearity principle. However, based on the chemical structure, pepstatin assembly hypothetically requires only three types of amino acids: a single l-alanine residue, and two occurrences each of l-valine and l-leucine (the precursor of statine), suggesting iterative use of A domains in its biosynthesis.
The A domain from the single-module NRPS gene pepD is predicted to activate alanine, while PepG, the stand-alone A domain protein, is predicted to activate threonine (Supplementary Table 5). PepH features an unusual C-PCP-C domain arrangement without any cognate A domains. PepG is likely promiscuous in substrate activation, capable of activating threonine and similar amino acids, including valine. However, the C domains in PepH might serve as gatekeepers, processing only proteins loaded with valine38. We propose that pepstatin biosynthesis initiates with the activation and in-trans loading of valine by PepG onto the PCP domain of PepH. The N-terminal C domain of PepH then acylates the Val-S-PepH species using various short-chain fatty acyl CoAs. A plausible subsequent step involves PepG reactivating valine and loading it onto the stand-alone PCP protein PepA. The C-terminal C domain of PepH may then facilitate chain extension by attacking the Val-S-PepA upon the thioester of the N-acyl-Val-S-PepH intermediate (Fig. 3).
Fig. 3. Proposed pepstatin biosynthesis pathway.
Fatty acids are likely activated to fatty acyl-CoA by acyl-CoA synthase and transferred to the carrier protein PepA, whereas PepG, PepH, PepB, PepC and PepD build up the peptide chain. The pentapeptide chain is subsequently released by PepE. Oxidation status changes are highlighted in turquoise to exhibit the two ketoreduction reactions catalyzed by PepI.
The A domain of the single-module NRPS gene pepB is predicted to activate leucine, while pepC, the sole PKS gene, encodes a KS-AT-ACP module. These findings suggest that PepB and PepC may collaborate to assemble the Sta residue backbone in pepstatins, similar to previously proposed Sta biosynthesis (Fig. 1c). However, the absence of a KR domain in PepB implies a remarkable new feature in Sta biosynthesis. We propose that PepB extends the nascent N-acyl-Val-Val chain with leucine, and PepC subsequently installs a malonyl-CoA to form the β-ketoacyl species. PepD then appears to activate and incorporate alanine into the growing NRP-PK chain. Following this, PepB and PepC may collaborate again to extend the lipopeptide backbone with a second β-ketoacyl building block. This second extension involving PepB and PepC following PepD, an unrelated NRPS module, is highly unusual and partially similar to the pass-back mechanism observed in thalassospiramide biosynthesis39. Notably, in pepstatin biosynthesis, this extension likely involves inter-protein interactions (Fig. 3), representing an in-trans mechanism distinct from intramodule interactions observed in other systems39. Overall, while the proposed pathway outlines a plausible biosynthetic logic, additional experimental data are needed to validate these hypotheses and clarify the unique enzymatic features of the pepstatin biosynthetic machinery.
The mechanism underlying the double reduction of the β-keto group during pepstatin biosynthesis remains unresolved due to the absence of a KR domain. The timing and nature of these unidentified reductions are particularly intriguing, given the presence of two Sta residues. We assumed it is likely that pepstatin biosynthesis, unlike other Sta-containing natural products, has evolved a unique β-keto reduction mechanism that may involve enzymes working either in tandem or sequentially. A prime candidate for this reduction process is PepI, a putative F420-dependent oxidoreductase, which shares low sequence identity (26.1%) with the TIM-barrel type F420-dependent methylenetetrahydromethanopterin reductase (Mer)40.
PepI is an F420H2-dependent ketone reductase
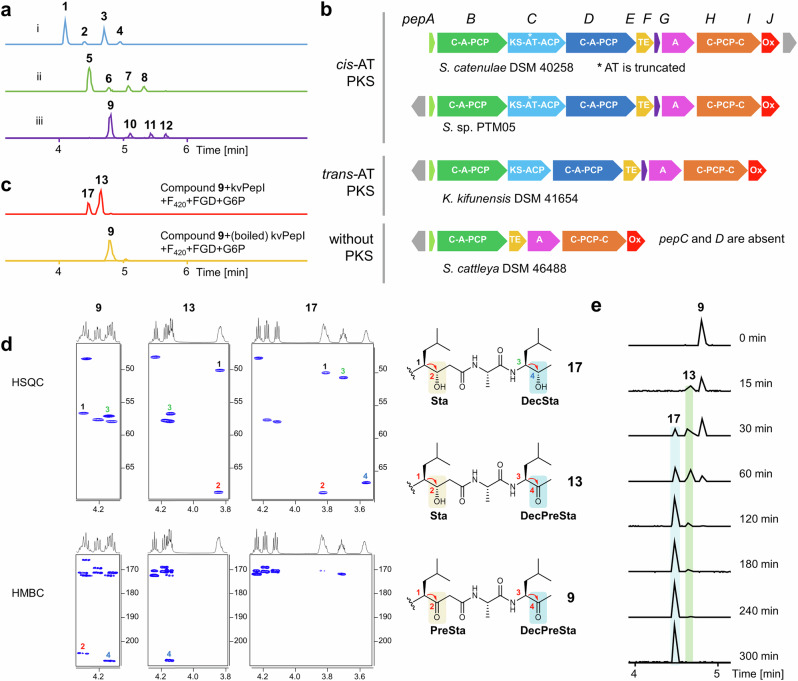
Deletion of the pepI gene in the heterologous expression system of the pep BGC abolished the production of 1-4, while a series of new peaks emerged, corresponding to unreduced β-keto intermediates 5-8 as identified by MS/MS analysis (Figs. 3 and 4a and Supplementary Figs. 8 and 9). However, the β-ketoacid compounds 5-8 decomposed rapidly through spontaneous decarboxylation into 9-12 (Fig. 4a), which lack the terminal carboxyl group, as confirmed by NMR analysis (Fig. 4d and Supplementary Figs. 10 and 43−62, Supplementary Table 9). Unlike the KR domain typically responsible for reduction during chain extension, the accumulation of the free β-keto intermediates (5-12) suggested that PepI functions as a tailoring enzyme, likely reducing the ketone groups at the β-position of the two β-diketone moieties after the intermediates are released from PepC (Fig. 3).
Fig. 4. PepI catalyzes tandem ketone reductions.
a UPLC-HRMS analysis of pepstatins and unreduced β-keto intermediates in the pepI deletion mutant. (i) EICs ([M + H]+, blue) of 1 (644.42), 2 (658.44), 3 (672.45), 4 (686.47) from the improved heterologous expression of pep (pepJ_act); (ii) EICs ([M + H]+, green) of 5 (640.39), 6 (654.40), 7 (668.42), 8 (682.44) and (iii) EICs ([M + H]+, purple) of 9 (596.40), 10 (610.42), 11 (624.44), 12 (638.45) from the pepI deletion mutant based on Del14-pep- pepJ-act. b Representative pep-like actinobacterial pathways classified into three types mainly distinguished by containing cis-AT PKS, trans-AT PKS, or without PKS. c In vitro characterization of kvPepI. EICs ([M + H]+, red) of 13 (598.42) and 17 (600.43) produced by kvPepI reaction with 9 and EIC ([M + H]+, orange) of 9 from the control reaction using boiled kvPepI and 9 were shown. d HSQC and HMBC slices of 9, 13, and 17, showing the structural changes at the Sta residue, decarboxylated Sta residue (DecSta), the precursor of Sta (PreSta), and the precursor of DecSta (DecPreSta). e Time-course of kvPepI using 9 as the substrate, supplemented with F420, FGD, and G6P. BPCs of the reactions were shown.
To investigate this distinct mechanism of statine formation, we aimed to characterize PepI in vitro. Recombinant PepI was successfully purified from E. coli BL21 (DE3). However, the recombinant protein exhibited unsatisfactory solubility (Supplementary Fig. 11b), precluding its use in downstream structural biology studies. To address this challenge, we searched public databases for homologs of PepI and identified several pep-like BGCs harboring pepI gene homologs. These BGCs are classified into three distinct subtypes, primarily based on variations in their PKS genes (Fig. 4b, Supplementary Fig. 11a, and Supplementary Table 6). The pep BGC represents the first subtype, characterized by a cis-AT PKS gene, while the second subtype features a trans-AT PKS gene that lacks the AT domain. Notably, all PKS modules in these BGCs are devoid of KR domains. We therefore hypothesize that PepI analogs serve as substitutes for KR domains to generate the 3-OH-4-NH2 moiety. The third subtype, however, lacks homologs of PepC and PepD, raising questions about its ability to produce Sta/Sta-like residues containing products (Fig. 4b). Among these, the homolog kvPepI from Kitasatospora viridis DSM44826, with 53.3% sequence identity to PepI, was more amenable to study (Supplementary Fig. 11b). Searching pepI homologous genes in genomes of producers of didemnin27, burkholdac23, hapalosin28, thalassospiramide29, and nosperin21 did not lead to any hit, further corroborating the unique Sta formation in pepstatin pathway.
We reasoned that 5-8 are likely the authentic intermediates for 1-4, but only the decarboxylated products 9-12 were stable enough to be isolated in sufficient quantities for in vitro assays. We primarily used the most abundant congener, 9, as the substrate in all PepI and kvPepI assays. To provide and regenerate the reduced cofactor F420H2, we supplemented the reaction mixture with F420, the F420-dependent glucose-6-phosphate dehydrogenase (FGD)41, and its substrate glucose-6-phosphate (G6P). After overnight incubation with PepI or kvPepI, UPLC-HRMS analysis revealed the complete consumption of 9 and the appearance of a new single-charged ion with a 4 Da increase, indicating the reduction of two ketones in 9 (Fig. 4c, Supplementary Figs. 10 and 12−14). Subsequently, we scaled up the kvPepI enzymatic assay using 9, which allowed us to purify new product 17 for NMR analysis, confirming the presence of the middle β-hydroxyl group and C-terminal secondary alcohol (Fig. 4d, Supplementary Figs. 68−72 and Supplementary Table 10).
We observed the same 4 Da mass shift using 10, 11, or 12 as substrates for PepI or kvPepI, indicating consistent dual ketone reduction supported by MS/MS analysis (Supplementary Figs. 10 and 12−14). Since the authentic substrates 5-8 were too unstable for isolation due to the rapid spontaneous β-keto decarboxylation, we used the fermentation broth of the mutant Del14-ΔpepI for in vitro assays. As anticipated, the incubation with PepI resulted in the production of 1-4, as detected by UPLC-HRMS (Supplementary Fig. 15). These results confirmed that PepI is the enzyme responsible for the dual ketone reduction in pepstatin biosynthesis. Removing F420, FGD, or G6P abolished PepI or kvPepI activity, underscoring the essential role of F420H2 in these transformations (Supplementary Figs. 13 and 14).
PepI catalyzes the tandem reduction of two ketones
With the role of PepI in reducing ketones during pepstatin biosynthesis confirmed, we focused on the timing of the two reduction steps. During our investigation, in addition to product 17, we identified a previously uncharacterized intermediate, 13, which was only 2 Da heavier than 9 (Fig. 4c). This led us to hypothesize that 13 could be an intermediate of 9 with only one of the ketones reduced. MS/MS and NMR analysis unambiguously determined the structure of purified 13. The HSQC and HMBC correlations of 13 clearly indicated the presence of a β-hydroxyl group at the middle residue (Sta3), with the methyl ketone at the terminal position remaining unchanged (Fig. 4d, Supplementary Figs. 10, 16, 58−62 and Supplementary Table 10). These findings are also corroborated by MS/MS analysis of the corresponding intermediates with an additional 2 Da when using 10, 11, or 12 as substrates (Supplementary Figs. 10 and 16).
To further examine the order of the two ketone reductions, we conducted time-course experiments using kvPepI. The time course-assay of kvPepI showed that 13 appeared first and reached the maximum concentration within three hours. Compound 17 emerged later than 13 and became apparent after one hour of incubation. The amount of 17 kept increasing for 5 h until 9 and 13 were nearly consumed completely (Fig. 4e). These results indicate that the reduction of the middle β-ketoamide moiety occurs before the reduction of the terminal ketone, establishing the sequential nature of PepI’s catalytic activity.
Structure of kvPepI
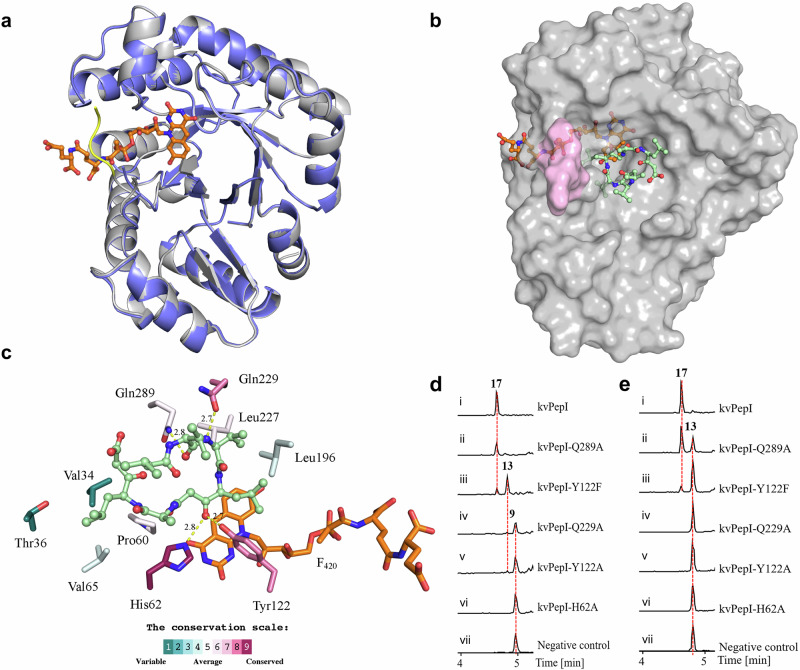
To better understand the mechanism underlying the reduction reaction, we determined the crystal structures of kvPepI and its complex with the cofactor F420, both to a resolution of 1.65 Å (Supplemtary Table 7). We found that kvPepI is structurally similar to other members of Class I F420-dependent enzymes (Fig. 5a, Supplementary Fig. 19a, b), including F420-dependent alcohol dehydrogenase (Adf, PDB ID: 1RHC; Supplementary Fig. 19c, C RMSD over entire length of the protein: 2.9 Å)42. Upon F420 binding, the kvPepI structure remains virtually unchanged, except for the stabilization of the residues 187–190 (Fig. 5a and Supplementary Fig. 20) that are part of a larger flexible loop (residues 181–201). This loop forms a lid over the lactyloligoglutamyl tail of F420, likely anchoring the F420 for catalysis. A number of hydrogen bonds and hydrophobic interactions further stabilize the cofactor (Supplementary Fig. 21a). Interestingly, the C5 of F420 was found to be positioned within 3.8 Å of a water molecule, held in place by His62 and Tyr122 (HOH570; Supplementary Fig. 21b). Overlay of Adf-acetone adduct structure with kvPepI-F420 revealed that the keto group of the acetone adduct occupies a position similar to that of the ordered water (HOH570, Fig. 5b, Supplementary Fig. 21a), suggesting that the highly conserved His62 acts as a general acid to protonate the keto group of 9. The His imidazole moiety likely acts as a proton relay through substrate protonation via the His62-Nɛ2 position and interaction with the carboxyl group of the Glu126 sidechain via the His62-Nδ1 (Supplementary Fig. 21b). Accordingly, the H62A mutation abolished the PepI activity (Fig. 5d, e). To rule out the possibility of H62A mutation causing a loss of activity due to allosteric effects, we also solved the structures of kvPepIH62A and kvPepIH62A in complex with F420, which are virtually identical to kvPepI apo and kvPepI-F420 complex structures, respectively (Supplementary Fig. 21c and Supplementary Table 7). Taken together, these findings strongly support the role of His62 as the proton-donating residue.
Fig. 5. Structural analysis, molecular modeling, and biochemical assays of kvPepI and mutants.
a Superposition of the kvPepI structure with (gray) and without (slate) F420 bound. Changes in the overall structure of the protein are minimal (Cα RMSD of 0.15 Å over all non-hydrogen atoms), except for the stabilization of a loop (residues 187 – 190; yellow line) that serves as a lid over the bound cofactor. b Hypothetical binding pose of U-shaped 9 in kvPepI – F420 complex structure. The missing loop (pink) was modeled using AlphaFold68,69. The orientation and conformation of 9 were modeled based on experimental data regarding the position of hydride transfer, as well as loss-of-activity-conferring amino acid mutations (details see experimental section). F420 (orange) and 9 (pale green) are shown as sticks (atom color: carbon black, nitrogen blue, oxygen red). c Schematic 3D representation of kvPepI – F420 interactions with modeled pose of 9. Hydrogen bonds are depicted with dotted yellow lines with distances given in Å, while all other residues form hydrophobic interactions with the substrate. The residues are colored according to the conservation score calculated using the ConSurf server74,75. Reactions using 9 (d) or 13 (e) as the substrate with kvPepI (i), Q289A (ii), Y122F (iii), Q229A (iv), Y122A (v), H62A (vi), and boiled kvPepI as negative control (vii). BPCs of reactions were shown.
Substrate mode of binding and mutational analysis of kvPepI
Despite repeated attempts, we could not determine the structure of either kvPepI or kvPepI-F420 in complex with 9. Therefore, we decided to model 9 in the kvPepI-F420 crystal structure using the gathered experimental data as well as the above-mentioned ordered water molecule to guide the position of the β-keto group of the oxidized statin precursor residue PreSta3 residue in 9 (Fig. 5b, c). For 9 two different binding modes were considered: linear and U-shaped conformation (Supplementary Fig. 22). However, only in the U-shaped conformation are two relatively well-conserved residues, Gln229 and Gln289, located close to the terminal end of the U-shaped 9 in the binding pocket (Fig. 5c and Supplementary Fig. 23c), forming hydrogen interactions with the amide bond of Val1 of 9. We reasoned that if these residues are critical for binding of 9, mutations to Ala should impair activity. Both Gln289A and Gln229A mutations were found to either result in complete loss of activity or impaired turnover of 9 and/or 13 (Fig. 5d, e). These data strongly support the binding of 9 in a U-shaped conformation. Moreover, a U-shaped conformation of 9 also nicely places the hydrophobic sidechains of 9 into hydrophobic pocket of the enzyme (Fig. 5b and Supplementary Fig. 23a) comprising residues that show varying degree of conservation (Fig. 5c, Supplementary Fig. 23b). Compound 9 is held in place primarily by hydrophic interactions such that it positions the carbonyl group (point of hydride attack) towards the F420 and His62 to ensure the correct enantioselective outcome of the reaction (Fig. 5c and Supplementary Fig. 25; vide infra).
The ketone is optimally placed for proton/hydride transfer, with the carbonyl carbon placed 2.9 Å and 3.8 Å from the Nɛ of His62 and C5 of F420, respectively. The side chain of Tyr122 packs against the Leu side chain and the β-keto group of PreSta3 of 9 (2.7 Å; Fig. 5c and Supplementary Fig. 23c). We thus wondered whether Tyr122 played a role in ketone reduction and produced kvPepIY122A and kvPepIY122F. When we tested the effect of kvPepIY122A on 9, we found ketone reduction to be significantly impaired, with only trace amounts of 13 observed (Fig. 5d). In contrast, kvPepIY122F resulted in an accumulation of 13 (Fig. 5d). Moreover, we tested the activities of kvPepIY122A and kvPepIY122F in comparison to kvPepI using purified 13 and observed only a tiny amount of 17 with kvPepIY122F but no formation of 17 was observed with kvPepIY122A (Fig. 5e). This implies that Tyr122 is crucial and acts at two points during catalysis: a) binding of 9, with hydrophobic interaction between phenyl ring and Sta helping orient the substrate for catalysis (Fig. 5c and Supplementary Fig. 23a, c), and b) during the second round of reduction by forming hydrogen bonding with 13. This likely involves the reduced ketone as kvPepIY122F and kvPepIY122A compared to kvPepI, either resulting in complete or significant loss of activity upon incubation with 13 (Fig. 5e). A comparison of the structure of kvPepIY122A-F420 complex to the corresponding structure of the wild-type holoenzyme revealed no major structural perturbations caused by the mutation (Supplementary Fig. 23d and Supplementary Table 7).
Whether the kvPeI employs either processive43 or distributive catalysis44 is not clear. The accumulation of 13 during time-course experiments (Fig. 4e) hints towards the latter. The conformational changes required to position the second keto reduction site close to His62 could be facilitated by the flexible loop located above the Sta (Fig. 5b; magenta). The first ketone reduction may also allow the formation of intra- and intermolecular hydrogen bonding, assisting reorientation of 13. Therefore, we envision a scenario where all the aforementioned factors likely contribute to the conformational change(s) needed to position the second keto group close enough to His62 for hydride transfer. Despite persistent efforts, we encountered challenges in obtaining the structure of kvPepI or variants in complex with F420, 13 or 17. Therefore, further studies are needed to gain a deeper understanding of the binding mode and molecular mechanism of the second keto reduction.
Hydride transfer from F420H2 to the keto carbon
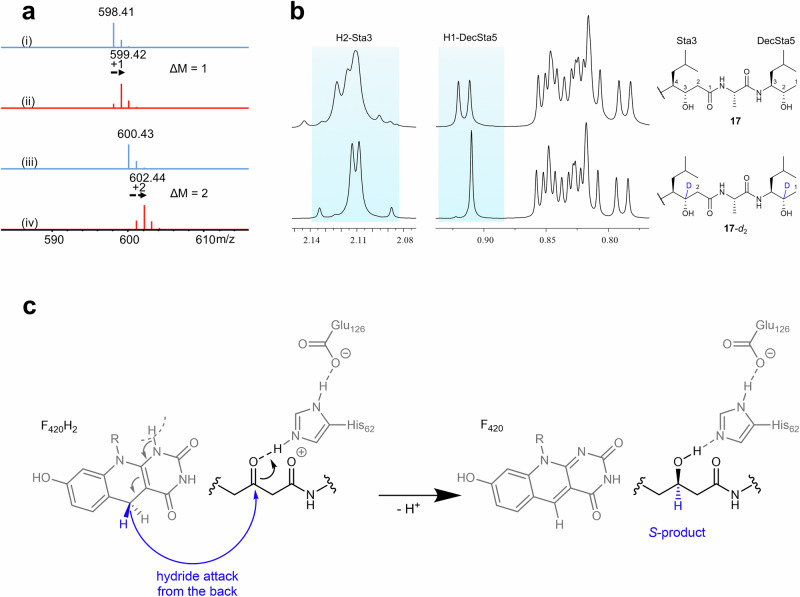
Since we observe only the S-enantiomer of 9, the specific orientation of substrate towards the F420 and His62 must be decisive in the enantioselective outcome of the reaction. To shed light on how and where the hydride is transferred from the C5 atom of F420H2, we performed kvPepI assays with deuterium-labeled reduced F420-5-d1. This labeled cofactor was synthesized through a coupled reaction using d-Glucose-1-d1 and hexokinase. Intermediates 13 and 17 were successfully produced and the main mass isotope ion increased by 1 and 2 Da, respectively (Fig. 6a). The mass shift indicates the incorporation of one deuterium in 13 and two deuteriums in 17. To determine the exact position of incorporation, we purified the labeled 13 and 17 from a scaled-up reaction. The NMR analysis unambiguously confirmed that the deuterium is attached to the carbon atom bearing the hydroxyl group (reduced from the ketone) (Fig. 6b, Supplementary Figs. 73−74 and Supplementary Table 10). These findings demonstrate that a direct hydride shift takes place from the cofactor to the carbonyl carbon. Furthermore, the stereo-configuration (S) of the carbon attached to the hydroxyl group in the middle Sta residue provides insight into the orientation of the pepstatin peptide backbone within the active site. In order to achieve the observed S-configuration while enabling protonation from His62 (vide supra), the peptide chain needs to be oriented as shown in Figs. 5c and 6c.
Fig. 6. Deuterium-labeled assays and mechanistic considerations for PepI.
a MS analysis of 13 and 17 in the kvPepI and 9 reactions without (i: 13, iii: 17, blue) or with (ii: 13-d1, iv: 17-d2, red) F420-5-d1 provided by the coupled hexokinase assay using d-Glucose-1-d1. b The 2 Da increase in the molecular mass indicated 17-d2 was the deuterated derivative of 17. The position of deuteration was deduced by comparing the 1H-NMR spectra.The methyl group H1-DecSta5 in 17-d2 showed a singlet whereas in 17 it was a doublet, together with the disappearance of the H2-DecSta5 signal in 17-d2, indicating the H2-DecSta5 was deuterated. Similarly, the H3-Sta3 was deuterated, as evidenced by the disappearance of H3-Sta3 signal and splitting pattern change of H2-Sta3. c Proposed reaction mechanism derived from the experimental observations indicates the orientation of the pepstatin peptide backbone in relation to His62 and F420 to achieve the observed S-product. R represents the lactyloligoglutamate tail of F420.
Discussion
Pepstatins have gained significant attention due to their potent inhibitory effects against various APs, which are promising therapeutic targets for treating a number of important diseases7. Despite this interest, the biosynthesis pathway of pepstatins has remained unknown. In this study, we identified the NRPS-PKS hybrid BGC responsible for pepstatin biosynthesis and revealed its distinctive disconnected and iterative utilization pattern. Most notably, we uncovered a unique F420H2-dependent post-assembly modification mechanism, which plays a crucial role in constructing the essential 3-OH-4-NH2 framework. This mechanism differs significantly from the previously hypothesized PKS KR-dependent pathway observed in the biosynthesis of other natural products (Fig. 1c)26.
The concise pepstatin assembly line stands out due to its highly disconnected nature, featuring unusual trans-acting and iterative enzymes. The potential iterative use of the discrete A domain PepG and its in-trans cooperation with PepH and PepA warrant further in-depth investigation into the biosynthetic mechanism. Furthermore, the stand-alone NRPS/PKS modules (PepB, PepC, and PepD) involved in the assembly of the Sta-Ala-Sta chain may operate through mechanisms distinct from those proposed for thalassospiramide biosynthesis, which involves a cross-modular pass-back chain extension strategy within a single multimodular megaenzyme39.
F420-dependent oxidoreductases have been reported in several natural product biosyntheses, typically mediating the hydrogenation of different double bonds in alkene, ketone, and imine moieties45–53. Notably, PepI stands out as the only biochemically characterized F420-dependent oxidoreductase capable of performing two rounds of reductions, underscoring the iterative fashion of pepstatin biosynthesis. Deletion of pepI led to the accumulation of β-diketone intermediates and the loss of pepstatin production. Through comprehensive structural and biochemical characterization of PepI/kvPepI, we confirmed that PepI/kvPepI sequentially reduces the middle β-ketone followed by the C-terminal β-ketone or methyl ketone.
Site-directed mutagenesis and kvPepI-9 modeling studies support the role of His62 as a potential catalytic residue, as corroborated by the abolishment of enzymatic activity regardless of whether 9 or 13 were used as the substrate. Two additional residues, Tyr122 and Gln229, were also found to be critical for PepI activity and, based on the modeling studies, are likely to be involved in substrate binding. Interestingly, mutating Tyr122 to Phe had a much greater effect on the second ketone reduction compared to the first step reduction, suggesting the vital role of the hydroxyl group of Tyr122 in positioning the substrate for the second reduction. In addition, the loss of kvPepI activity in the Q229A mutant supports the crucial role of Gln229 in stabilizing the substrate binding by forming hydrogen bonding in the modeling prediction. Interestingly, structural tolerance of the C-terminal ketone reduction indicates that the diketone is not a prerequisite for PepI activity. Further studies are currently underway to understand how 13 is oriented within the active site during the second reduction step.
In conclusion, this study unveils the long-sought-for, highly dissociated, and iterative biosynthetic pathway of pepstatins. The discovery of the KR domain-independent 3-OH-4-NH2 framework biosynthesis and the characterization of PepI as a tandem F420H2-dependent oxidoreductase expand the NRPS/PKS biosynthetic repertoire. Moreover, the data presented here on the PepI mechanism will likely contribute to a better understanding of underinvestigated F420-dependent oxidoreductases.
Methods
Bacterial strains, plasmids, and DNA manipulation
Bacterial strains and plasmids used in this study are listed in Supplementary Tables 1 and 2, respectively. All primers used in this study (listed in Supplementary Table 3) were synthesized by Sigma-Aldrich (Steinheim, Germany). In-silico analyses, including primers design, BGC annotation, and short-read mapping, were conducted using Geneious Prime® 2022.2.2 (Biomatters Ltd., New Zealand). E. coli DH10B was employed for general subcloning purposes. Plasmid DNA isolation kits were obtained from Qiagen. Q5® High-Fidelity DNA polymerase and HiFi DNA Assembly Mater Mix were procured from New England Biolabs (Ipswich, MA). Restriction enzymes and other standard molecular biology reagents were purchased from Thermo Scientific. All kits and enzymes were used in accordance with the manufacturers’ protocols. Red/ET recombineering–based plasmid modifications were performed using E. coli GB08-red, as described in previous studies54,55. Genomic DNA of Streptomyces strains was manually extracted following a cetyltrimethylammonium bromide-based protocol56.
Strain cultivation
E. coli was cultivated in LB medium (10 g/L tryptone, 5 g/L NaCl, 5 g/L yeast extract, pH 7.6) at 37 °C with shaking at 180 rpm. All Streptomyces strains were cultivated at 30 °C with shaking at 200 rpm. For Streptomyces catenulae DSM40258 and heterologous expression mutants, cells were grown in tryptone soya broth (TSB) medium (30 g/L TSB) for genomic DNA isolation, conjugation, or as starter cultures prior to fermentation. Conjugations were performed on MS agar plates (d-mannitol 20 g/L, soya bean meal 20 g/L, agar 20 g/L). Streptomyces catenulae medium (30 g/L glucose, 20 g/L corn steep liquor, 0.5 g/L dipotassium hydrogen phosphate, 0.2 g/L ammonium sulfate, 0.5 g/L magnesium sulfate, 5 g/L calcium carbonate, pH 7.0)57 was used for fermentation. Fermentation cultures were inoculated from 3 to 4 days TSB starting cultures (10% (v/v) inoculation volume) and cultivated for 4 days (24 h for Del14-pep-ΔpepI).
Genome sequencing and analysis
The genome of Streptomyces catenulae was sequenced using Illumina HiSeq and MinION Nanopore sequencing technologies at the genome analytics facility of the Helmholtz Centre for Infection Research (Braunschweig, Germany). BGCs were predicted by antiSMASH (http://antismash.secondarymetabolites.org/)58.
Construction of gene pepD deletion plasmid
The pepD gene in the chromosome of Streptomyces catenulae was deleted using the CRISPR-Cas9 gene editing tool pQS9, following previously described methods59. Spacer inserts, which included a gene-specific 20-nt guide sequence, were generated by annealing two 34-nt synthesized oligonucleotides, sgpepD-f and sgpepD-r. The annealed oligonucleotides were cloned into pQS9 at NcoI-XbaI to afford psgQS9-ΔpepD and verified by Sanger sequencing. The flanking region of gene pepD, 2 kb on each side, served as homologous recombination repair templates following Cas9-mediated gene editing. The 2.0 kb upstream homologous arm (UHA) and 2.0 kb downstream homologous arm (DHA) were amplified from genomic DNA of Streptomyces catenulae using primer pairs pepD-L-f/pepD-L-r and pepD-R-f/ pepD-R-r, respectively. These amplified fragments were cloned into psgQS9-ΔpepD at StuI using the Gibson assembly cloning kit, resulting in the plasmid pQS9-ΔpepD, which was verified by SalI digestion.
Heterologous expression of the pep BGC
The potential pepstatin BGC pep was divided into three fragments, each ~6 kb in size, with 20 bp homologous arms flanking both ends. These fragments were amplified using the primer pairs pep-1-f/pep-1-r, pep-2-f/pep-2-r, and pep-3-f/pep-3-r, respectively. Concurrently, the backbone plasmid was amplified using the primer pair p15A-f/p15A-r. The amplified plasmid backbone and the three pep gene cluster fragments were assembled via Gibson assembly, resulting in the formation of the plasmid p15A-pep.
Subsequently, a 4.3 kb phi31 integrase-apramycin resistance gene cassette was excised from the pR6K-phiC31-oriT plasmid through AseI digestion. This cassette was then introduced into the p15A-pep plasmid via Red/ET recombineering, yielding the recombinant plasmid p15A-int-pep. Plasmid p15A-int-pep was verified by NcoI digestion.
Pathway engineering of the pep BGC for heterologous expression
The gene pepJ in p15A-int-pep was replaced by the chloramphenicol resistance gene (cml), which was amplified using the primers pepJ-ko-f and pepJ-ko-r, via Red/ET recombination2,54. This process yielded the plasmid p15A-int-pep-ΔpepJ.
To enhance pepstatin production in the heterologous expression mutant, the upstream non-coding region of gene pepJ in plasmid p15A-int-pep was substituted with a cml-kasO cassette using Red/ET recombination, resulting in the plasmid p15A-int-pep-kasop-pepJ55.
For pepI inactivation, both pepI and the upstream non-coding region of pepJ in plasmid p15A-int-pep were replaced with cml-kasO cassette via Red/ET recombination, producing the plasmid p15A-int-pep-ΔpepI55.
Conjugation
In this study, we employed an adapted biparental or triparental conjugation protocol56 to introduce exogenous DNA into S. catenulae or S. albus Del14, respectively. The donor strain used for biparental conjugation was E. coli ET12567/pUZ8002. For triparental conjugation, the donor strain was E. coli DH10B, with E. coli HB101/pRK2013 serving as the helper strain.
After conjugation, the plates were allowed to dry and then further incubated at 30 °C until the exconjugants became visible, typically within 3−5 days. Visible exconjugants were inoculated to TSB media for genome DNA isolation. Each exconjugant was verified by PCR amplification with relevant primers (Supplementary Table 3).
Gene cloning and protein purification
The codon-optimized pepI gene was synthesized by GenScript and subsequently amplified using the primers pepI-f/pepI-r. The amplified pepI was cloned into the plasmid pCold I, resulting in the plasmid pCold-pepI. Additionally, the plasmids pET28b-sfpepI, pET28b-svpepI, and pET28b-kvpepI were ordered from GenScript.
For expressing the kvPepI site-directed mutants, H62A, Y122F, Y122A, and Q229A, plasmid pET28b was digested with NdeI/HindIII for cloning. The kvpepI gene was split into two parts, and the specific mutations were introduced at the junction through primers listed in Supplementary Table 3. The resulting mutant fragments were assembled into the plasmid pET28b via Gibson assembly. For the Q289A mutant, the pET28b plasmid was amplified using the primer pair pET-28b-f/pET-28b-r to serve as the backbone. The mutation was introduced through primers (listed in Supplementary Table 3) at the junction and cloned to plasmid pET28b by Gibson assembly.
All expression constructs were transformed into BL21 (DE3) cells. For protein purification, cells were grown overnight in LB containing relevant selection pressure at 37 °C. This overnight culture was diluted 1:100 into fresh LB medium supplemented with the appropriate antibiotics and incubated at 37 °C with shaking for 3−4 h. Until optical density (OD600) reached 0.6, the culture was rapidly cooled to 16 °C in ice water for 30 min. Subsequently, isopropyl β-d-1-thiogalactopyranoside (IPTG) was added to a final concentration of 0.1 mM to induce protein expression. The culture was then incubated with shaking at 16 °C for 16 h. Following this incubation period, the cells were harvested by centrifugation.
For protein purification, cell pellets were resuspended in lysis buffer (20 mM Tris pH 8.0, 200 mM NaCl, 10% glycerol (w/v), 20 mM imidazole, 1 mM TCEP) supplemented with cOmplete EDTA-free protease inhibitor tablets (Roche). The cell suspension was lysed by two passages through a cell disrupter (30 kpsi, Microfluidics Corp.), and the cell debris was removed by centrifugation at 40,000 × g for 30 min at 4 °C. The supernatant was then loaded onto a 5 mL HisTrap HP column (GE Healthcare) pre-equilibrated with the lysis buffer. The column was washed with 30 column volumes of lysis buffer, and the bound protein was eluted using an elution buffer (lysis buffer with 250 mM imidazole).
Fractions containing the target protein were subjected to size-exclusion chromatography using a HiLoad 16/600 Superdex 200 pg column (GE Healthcare) pre-equilibrated with gel filtration buffer (50 mM Tris pH 8.0, 200 mM NaCl, 1 mM TCEP). Protein purity was assessed by SDS-PAGE, and the fractions containing the highest purity protein were pooled and concentrated to approximately 5 mg/mL. The protein concentration was determined using a Nanodrop UV-Vis spectrophotometer at 280 nm, with theoretical extinction coefficients calculated using the ExPASy ProtParam tool.
Enzyme assay conditions
The crude extract containing compounds 5-8 previously stored at −80 °C, was dissolved in pre-cooled methanol to a concentration of 1 mg/mL and stored on ice. Purified compounds 9-12 were separately dissolved in methanol to create 1 mM stock solutions. The F420 and FGD enzyme used in the activity assays were prepared as previously described60,61.
A 50 µL reaction mixture was prepared in 1.5 mL Eppendorf tubes, containing 0.6 µM PepI, kvPepI, or other mutants, 20 mM Tris-HCl (pH 7.5), 10 µM F420, 2.5 mM glucose 6-phosphate, 0.45 µM F420-dependent glucose-6-phosphate dehydrogenase (FGD) and 10 µM substrate (or 2 µg crude extract). In the time-course experiments of kvPepI, substrate concentration was reduced to 1 µM. Unless otherwise noted, the reaction mixture was incubated at 30 °C for 5 h, then quenched with 0.2 M HCl, and stored at −80 °C until ready for analysis.
Before LC-MS analysis, an equal volume of methanol was added to the reaction mixture, and it was centrifuged at 8000 × g for 15 min at 15 °C to remove all the particles.
Sample preparation and UPLC-ESI-MS analysis
For small-scale fermentation (50 mL), Streptomyces cultures were harvested by centrifugation at 8000 × g for 15 min at 15 °C. The supernatant was supplemented with 2% (v/v) XAD16N resin and stirred for 2 h, followed by extraction with 50 mL methanol. The pelleted cells were resuspended in 50 mL methanol and agitated for 2 h. All fractions were evaporated to dryness under vacuum and then dissolved in 1 mL methanol to produce the crude extracts. The crude extract (10 μL) was diluted fivefold to 50 μL and centrifuged at 21,500 g for 15 min at 15 °C before UPLC-MS analysis.
Reverse phase UPLC-MS analysis was carried out using the following system and method. LC: Ultimate 3000 RS; HRMS: Bruker Maxis II (4Generation) Q-TOF using the Apollo II ESI source.; MS: Bruker amaZon speed ion trap mass spectrometer; Column: ACQUITY UPLC BEH C18 Column, 130 Å, 1.7 µm, 2.1 mm × 50 mm (Waters); Eluents: A: distilled water supplemented with 0.1% formic acid and B: distilled acetonitrile supplemented with 0.1% formic acid; Flow rate: 0.6 mL/min; column temperature: 45 °C. The gradient was as follows: (1) a 0.5 min isocratic step at 5% B, changed from 5% to 95 % B in 9 min, maintained at 95 % B for 1 min, decreased to 5% B in 0.5 min, and sustained 5% B for 2 min. (2) a 0.5 min isocratic step at 5% B, followed by a linear increase to 95% B in 18 min, maintained at 95 % B for 2 min, then equilibrated to the starting conditions (5% B) for 2 min. The UV-Vis spectra were recorded by a diode array detection (DAD) in the range from 200 to 600 nm. The mass detection was performed in the positive ESI mode. All HPLC-MS data were analyzed by Compass Data Analysis version 4.4 (Bruker Daltonics).
Compounds purification
To purify compounds 1-4, the cells and XAD16N resin from a 3-liter S. catenulae culture were harvested by centrifugation. The combined cells and resin were extracted with methanol. The extracts were concentrated using a rotary evaporator and then partitioned between methanol and hexane. The methanol phase was dried and further purified on a Sephadex LH-20 column (GE Healthcare) with methanol as the mobile phase. Fractions were pooled based on HPLC-MS analysis and were further purified by HPLC.
Semi-preparative purification of pepstatins was performed on an HCT HPLC-MS system using a Waters XSelect Peptide CSH C18 column (5 μm, 10 × 250 mm). The HPLC conditions were as follows: solvent A, H2O ( + 0.1% formic acid), and solvent B, ACN ( + 0.1% formic acid), at a flow rate of 5 mL/min and a column thermostatic at 45 °C. The gradient starts with a 2-minute isocratic step at 25 %B, followed by a ramp to 53%B in 16 min, maintained at 95%B for 3 min before returning to the initial condition in 2 min and re-equilibration for 2 min.
To purify compounds 9-12, a 3-liter culture of S. albus Del14-pep-ΔpepI was grown in liquid medium for 2 days, followed by the addition of activated carbon (1%, w/v). After centrifugation, the supernatant was removed, and the cells with activated carbon were extracted with methanol. The extracts were dried, and partitioned between ethyl acetate and deionized water (1:1, v/v). The organic phase containing compounds 9-12 was concentrated by rotary evaporation, and subsequently fractionated using flash chromatography on a Biotage Isolera One system with a 25 g Snapfit column. Elution was performed with ethyl acetate and methanol in the following gradient: 5 CV (column volume) of ethyl acetate, followed by a 10 CV linear increase from 0 to 20% methanol, and then a 10 CV linear increase from 20 to 100% methanol. Fractions were collected and further purified using a Dionex Ultimate 3000 SDLC low-pressure gradient system with a Waters XSelect Peptide CSH C18 column (5 μm, 10 × 250 mm). The gradient conditions were as follows: 0−2 min, 20%B; 2−12 min, 20−50%B; 12−15 min, 50-56%B; 15−17 min, 56−95%B; 17−19 min, 95%B; 19−-21 min, 95-20%B; 21−23 min, 20%B, with H2O ( + 0.1% formic acid) as eluent A and ACN ( + 0.1% formic acid) as eluent B. The separation was carried out with a column temperature of 45 °C at a flow rate of 5 mL/min.
To purify compounds 13 and 17, an in vitro ketoreduction reaction of substrate 9 was performed using the enzyme kvPepI, scaled up proportionally to 600 µL according to the method described in enzyme assay conditions. The reactions were quenched and extracted with ethyl acetate. The extracts were then concentrated and purified by HPLC, using the same separation conditions as those for intermediates 9-12.
Statine residue conformation verification
For l-statine (AAT Bioquest): Prepare two 1.5 ml Eppendorf tubes and add 50 µl of 10 mM l-statine solution to each. Adjust pH to approximately 9 by adding 20 µl of 1 M NaHCO3 to each tube. Then, add 20 µl of 1% Marfey’s reagent62 in acetone (d-FDLA and l-FDLA, respectively) to each tube, and incubate at 40 °C with shaking at 700 rpm for 1 to 2 h. Subsequently, neutralize the reaction by adding 10 µl of 2 N HCl, then dilute with 300 µl of acetonitrile. Centrifuge the mixture at 8000 × g for 15 min at 15 °C before UPLC-MS measurement. An aliquot (1 µL) of the analyte was injected, and the separation was achieved on a Waters Acquity BEH C18, 100 × 2.1 mm, 1.7 µm column using H2O ( + 0.1 % FA) and ACN ( + 0.1 % FA) as eluents. The flow rate is 550 µL/min at a column temperature of 45 °C.
For compounds 9, 13, 17, and 1, samples (approximately 100 µg each) were placed in 1.4 ml glass vials and heated in 100 µl of 6 M HCl at 110 °C for 45 min. The solvent was evaporated using a nitrogen flow at room temperature, and the residues were dissolved in 110 µL of H2O. Derivatization and analysis were performed in the same way as described above.
NMR conditions
NMR data were recorded in methanol-d4 or DMSO-d6 on a 500 MHz Avance III (UltraShield) spectrometer or a 700 MHz Avance III (Ascend) spectrometer, each equipped with a Helium-cooled CryoProbe (TCI). All observed chemical shift values (δ) are given in ppm and coupling constant values (J) in Hz. Chemical shifts were calibrated internally to the residual signal of either methanol-d4 (δH 3.31, δC 49.1) or DMSO-d6 (δH 2.50, δC 39.5). Multiplicities are described using the following abbreviations: s = singlet, d = doublet, t = triplet, q = quartet, dd = doublet of doublets, m = multiplet, br = broad signal.
Compound characterization
Acetyl-pepstatin (1): the molecular formula of 1 was determined to be C31H57N5O9 by High-resolution ESI-MS, m/z 644.4230 [M + H]+ (calcd. for C31H58N5O9, 644.4229). Analysis of the 1D and 2D NMR spectra (Supplementary Figs. 26−29, Supplementary Table 8), as well as the MS/MS fragmentation pattern (Supplementary Fig. 1), revealed that 1 has a peptide sequence of Ac-Val-Val-Sta-Ala-Sta. Hence, 1 was determined to be acetyl-pepstatin.
Propionyl-pepstatin (2) had the molecular formula C32H59N5O9, m/z 658.4383 [M + H]+ (calcd. for C31H60N5O9, 658.4386). The 1D NMR spectra of 2 were almost identical to that of 1, except for an additional CH2 group. Further interpretation of the 2D NMR data revealed a propionylated N-terminus. Thus, 2 was identified as propionyl-pepstatin (Supplementary Figs. 30−33, Supplementary Table 8).
Isobutyryl-pepstatin (Pepsinostreptin A) (3) had the molecular formula C33H61N5O9, m/z 672.4518 [M + H]+ (calcd. for C33H62N5O9, 672.4542). The 1D and 2D NMR spectra of 3 were similar to that of 1, the only difference was the N-terminal isobutyl group instead of the singlet methyl group. The structure of 3 was determined as isobutyryl-pepstatin (Supplementary Figs. 34−38, Supplementary Table 8).
2-methylbutanoyl-pepstatin (4) had the molecular formula C34H63N5O9, m/z 686.4660 [M + H]+ (calcd. for C34H64N5O9, 686.4699). The NMR data (Supplementary Figs. 39−42, Supplementary Table 8) and MS/MS fragmentation data (Supplementary Fig. 1) showed that 4 has the same amino acid sequence as 1-3. Thorough examination of the NMR data revealed that the fatty acid chain of 4 consisted of one methine group (δH-2 2.36, δC-2 43.4), one methylene group (δH-3 1.38/1.61, δC-3 28.3), and two methyl groups (δH-4 0.87, δC-4 12.4; δH-5 1.11, δC-5 18.2), indicating a 2-methylbutyl moiety. Therefore, the structure of 4 was characterized as 2-methylbutanoyl-pepstatin.
The molecular formula of compound 9 was determined to be C30H53N5O7 by ESI-HRMS, m/z 596.4017 [M + H]+ (calcd. for C30H54N5O7, 596.4018). 1D and 2D NMR data (Supplementary Figs. 43−47, Supplementary Table 9) in combination with mass spectrometric analysis (Supplementary Fig. 10) showed the presence of an oxidized statin residue (PreSta), with the hydroxy group converted into ketone group (δC-3 204.9). In addition, a decarboxylated and oxidized statin residue (DecPreSta) was identified. Finally, two valine, one alanine, and one acetyl group were identified. HMBC correlations together with MS/MS analysis determined the amino acid sequence, hence the planar structure of 9 was characterized.
Similarly, careful analysis of the NMR spectra (Supplementary Figs. 48−62, Supplementary Table 9) and MS/MS data (Supplementary Fig. 10) of compounds 10-12 showed they are the decarboxylated and oxidized intermedium of 2-3, respectively. Hence, the structures of compounds 10-12 were determined.
The molecular formula of 13 was determined to be C30H55N5O7 by High-resolution ESI-MS, m/z 598.4179 [M + H]+ (calcd. for C30H56N5O7, 598.4174). The NMR data (Supplementary Figs. 63−67, Supplementary Table 10) and MS/MS fragmentation data (Supplementary Fig. 16) indicated that the structure of 13 was similar to that of 9. The only difference is the PreSta residue in 9 was replaced by Sta residue in 13, indicated by the disappearance of one ketone signal (δC-3 204.9) and the appearance of one methine group (δH-3 3.84, δC-3 68.7).
Compound 17 had the molecular formula C30H57N5O7, m/z 600.4332 [M + H]+ (calcd. for C30H58N5O7, 600.4331). Compare the 1D and 2D NMR spectra (Supplementary Figs. 68−72, Supplementary Table 10) of 17 and 13 revealed that the ketone signal (δC-2 207.9) in 13 was replaced by the methine group (δH-2 3.56, δC-2 67.2) in 17. The structure of 17 was determined.
Crystallization and structure determination of kvPepI
Crystals of kvPepI, kvPepI – F420, kvPepIH62A, kvPepIH62A – F420, and kvPepIY122A – F420 were obtained at 18 °C in 30% (w/v) PEG 4000, 0.2 M Sodium acetate and 0.1 M Tris-Cl pH 8.5. For complex crystallization, the protein was incubated with excess F420 (1.5 mM) on ice overnight. Crystals were cryoprotected in mother liquor supplemented with 30% glycerol. The diffraction data was collected from a single crystal at 100 K at Petra III (Beamline: P11, DESY)63,64, processed using Xia265 or XDS66, and the structure was determined using PHASER molecular replacement67 using AlphaFold model generated using Colab notebook68,69. The structure was manually rebuilt in COOT70, refined using PHENIX Refine67, and validated using MolProbity71. The images presented were created using PyMOL (Schrödinger) and LigPlot+72.
Modeling of PepI: compound 9 complex
A model of the complex between the biosynthetic precursor of pepstatin and substrate of PepI (compound 9) and the kvPepI protein was generated based on the solved crystal structure (vide supra, PDB ID 9GM0) after the unresolved flexible loop Gly191-Thr194 was modeled by AlphaFold68,69 using the molecular operating environment (MOE, chemical computing group)73 in a stepwise manner:
The PDB file was loaded in MOE and the QuickPrep protein preparation procedure was applied with the following parameters: Tether strength for receptor = 30; Tether strength for ligand = 10; co-factor (F420) atoms were fixed; all other parameters were default.
The structure of compound 9 was inserted into the active site by first building the β-keto amide motif (precursor of the statine motif, “PreSta3”) in the orientation where the carbonyl oxygen is occupying the position of His62-coordinated water (H2O570; ordered water molecule from the main text) and the α-carbon, which is attacked by the hydride (or deuteride) in close proximity to the reactive center of co-factor F420 in the only possible orientation, which would lead to generation of the observed enantiomer (S-product; see Supplementary Fig. 25).
Growing of the peptidic structure of compound 9 into the pocket and orienting the hydrophobic side chains into hydrophobic pockets. The Steric constraints of the active site required to model a compound 9 in a loop/bent conformation with the loop occurring between Val1 and PreSta3 to remove clashes with the protein resulting in a U-shaped conformation.
Removal of all clashing water or solvent molecules as well as further finetuning of the complex structure.
A final QuickPrep step for localized energy minimization with the following parameters: Tether strength for receptor = 30; Tether strength for ligand = 10; co-factor (F420) atoms were fixed; all other parameters were default.
This yielded the proposed model for the interaction between PepI and the pepstatin precursor 9. Note that this model is an information-driven hypothesis, which shows a plausible orientation for enabling the generation of the S-product at residue 4 observed in the experimental studies highlighting that this enzyme-substrate complex is in principle possible and that the active site can accommodate compound 9 in this orientation. In general, coordinates for substrate atoms more distant to the β-keto statine precursor motif are characterized by ambiguities inherent to modeling efforts on highly flexible peptidic substrates.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Source data
Acknowledgements
This work is supported by the Helmholtz International Lab (InterLabs-0007). Research in Chengzhang Fu and Rolf Müller’s laboratory is funded by the Bundesministerium für Bildung und Forschung (BMBF). The authors acknowledge DESY (Hamburg, Germany), a member of the Helmholtz Association HGF, for the provision of experimental facilities. Parts of this research were carried out at PETRAIII (beamline P11), and we would like to thank Johanna Hakanpää, Guillaume Pompidor and Helena Taberma for assistance in using the photon beamline. Beamtime was allocated for proposal (Xh-20010236). Ghader Bashiri is supported by a Health Research Council of New Zealand grant (Hercus Health Research Fellowship 17/058).
Author contributions
C.F. conceived the study and analyzed the gene cluster. J.M. performed heterologous expression of BGC and in vitro biochemical studies. A.S. and H.Z. performed the protein crystallographic studies. H.Z. isolated the compounds and determined the chemical structures. G.B. purified F420 and FGD. M.E. performed the modeling. C.F., J.M., H.Z., A.S., L.H., M.E., and R.M. analyzed the data. C.F. and A.S. wrote the paper with input from all authors. J.M., H.Z., and A.S. contributed equally to the manuscript. All authors read and approved the final manuscript.
Peer review
Peer review information
Nature Communications thanks Andrea Mattevi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The pepstatin biosynthetic gene cluster sequence in this study has been deposited in GenBank under accession number PP947771. The protein crystal structure data generated in this study have been deposited in the Protein Data Bank under PDB IDs 9G64, 9GKH, 9GM0, 9GNC, and 9GND. All data that support the findings of this study are available in the main text, supplementary information and from corresponding author(s) upon request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jingjun Mo, Asfandyar Sikandar, Haowen Zhao.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-59785-0.
References
- 1.Kunimoto, S., Aoyagi, T., Morishima, H., Takeuchi, T. & Umezawa, H. Mechanism of inhibition of pepsin by pepstatin. J. Antibiot.25, 251–255 (1972). [DOI] [PubMed] [Google Scholar]
- 2.Aoyagi, T., Morishima, H., Nishizawa, R., Kunimoto, S. & Takeuchi, T. Biological activity of pepstatins, pepstanone A and partial peptides on pepsin, cathepsin D and renin. J. Antibiot.25, 689–694 (1972). [DOI] [PubMed] [Google Scholar]
- 3.Marks, N., Grynbaum, A. & Lajtha, A. Pentapeptide (pepstatin) inhibition of brain acid proteinase. Science181, 949–951 (1973). [DOI] [PubMed] [Google Scholar]
- 4.Matúz, K., Mótyán, J., Li, M., Wlodawer, A. & Tőzsér, J. Inhibition of XMRV and HIV-1 proteases by pepstatin A and acetyl-pepstatin. FEBS J.279, 3276–3286 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seelmeier, S., Schmidt, H., Turk, V., Helm, K. & von der Human immunodeficiency virus has an aspartic-type protease that can be inhibited by pepstatin A. Proc. Natl. Acad. Sci. USA85, 6612–6616 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosh, A. K. Aspartic Acid Proteases as Therapeutic Targets (Wiley-VCH Verlag GmbH & Co. KGaA, 2010).
- 7.Madala, P. K., Tyndall, J. D. A., Nall, T. & Fairlie, D. P. Update 1 of: Proteases universally recognize beta strands in their active sites. Chem. Rev.110, PR1–PR31 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Wlodawer, A. & Vondrasek, J. Inhibitors of HIV-1 protease: a major success of structure-assisted drug design. Annu. Rev. Biophys. Biomol. Struct.27, 249–284 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Umezawa, H., Aoyagi, T., Morishima, H., Matsuzaki, M. & Hamada, M. Pepstatin, a new pepsin inhibitor produced by Actinomycetes. J. Antibiot.23, 259–262 (1970). [DOI] [PubMed] [Google Scholar]
- 10.Morishima, H., Takita, T., Aoyagi, T., Takeuchi, T. & Umezawa, H. The structure of pepstatin. J. Antibiot.23, 263–265 (1970). [DOI] [PubMed] [Google Scholar]
- 11.Aoyagi, T., Yagisawa, Y., Kumagai, M., Hamada, M. & Morishima, H. Letter: new pepstatins, pepstatins Bu, Pr and Ac produced by Streptomyces. J. Antibiot.26, 539–541 (1973). [DOI] [PubMed] [Google Scholar]
- 12.Omura, S. et al. Ahpatinins, new acid protease inhibitors containing 4-amino-3-hydroxy-5-phenylpentanoic acid. J. Antibiot.39, 1079–1085 (1986). [DOI] [PubMed] [Google Scholar]
- 13.Sun, Y., Takada, K., Nogi, Y., Okada, S. & Matsunaga, S. Lower homologues of ahpatinin, aspartic protease inhibitors, from a marine Streptomyces sp. J. Nat. Products77, 1749–1752 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Morishima, H. et al. Biosynthetic studies on pepstatin. J. Antibiot.27, 267–273 (1974). [PubMed] [Google Scholar]
- 15.Kwan, J. C., Eksioglu, E. A., Liu, C., Paul, V. J. & Luesch, H. Grassystatins A-C from marine cyanobacteria, potent cathepsin E inhibitors that reduce antigen presentation. J. Med. Chem.52, 5732–5747 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, J., Currano, J. N., Carroll, P. J. & Joullié, M. M. Didemnins, tamandarins and related natural products. Nat. Prod. Rep.29, 404–424 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Stratmann, K., Burgoyne, D. L., Moore, R. E., Patterson, G. M. L. & Smith, C. D. Hapalosin, a cyanobacterial cyclic depsipeptide with multidrug-resistance reversing activity. J. Org. Chem.59, 7219–7226 (1994). [Google Scholar]
- 18.Luesch, H., Moore, R. E., Paul, V. J., Mooberry, S. L. & Corbett, T. H. Isolation of dolastatin 10 from the marine cyanobacterium Symploca species VP642 and total stereochemistry and biological evaluation of its analogue symplostatin 1. J. Nat. Prod.64, 907–910 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Nakao, Y., Fujita, M., Warabi, K., Matsunaga, S. & Fusetani, N. Miraziridine A, a novel cysteine protease inhibitor from the marine sponge Theonella aff. mirabilis1. J. Am. Chem. Soc.122, 10462–10463 (2000). [Google Scholar]
- 20.Oh, D.-C., Strangman, W. K., Kauffman, C. A., Jensen, P. R. & Fenical, W. Thalassospiramides A and B, immunosuppressive peptides from the marine bacterium Thalassospira sp. Org. Lett.9, 1525–1528 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Kampa, A. et al. Metagenomic natural product discovery in lichen provides evidence for a family of biosynthetic pathways in diverse symbioses. Proc. Natl. Acad. Sci. USA110, E3129–E3137 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan, P. et al. Phormidepistatin from the cyanobacterium UIC 10484: assessing the phylogenetic distribution of the statine pharmacophore. J. Nat. Prod.84, 2256–2264 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biggins, J. B., Gleber, C. D. & Brady, S. F. Acyldepsipeptide HDAC inhibitor production induced in Burkholderia thailandensis. Org. Lett.13, 1536–1539 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang, C. et al. Thailandepsins: bacterial products with potent histone deacetylase inhibitory activities and broad-spectrum antiproliferative activities. J. Nat. Prod.74, 2031–2038 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang, C., Flemming, C. J. & Cheng, Y.-Q. Discovery and activity profiling of thailandepsins A through F, potent histone deacetylase inhibitors, from Burkholderia thailandensis E264. MedChemComm3, 976–981 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh, C. T., O’Brien, R. V. & Khosla, C. Nonproteinogenic amino acid building blocks for nonribosomal peptide and hybrid polyketide scaffolds. Angew. Chem.52, 7098–7124 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu, Y. et al. Bacterial biosynthesis and maturation of the didemnin anti-cancer agents. J. Am. Chem. Soc.134, 8625–8632 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Micallef, M. L., D’Agostino, P. M., Sharma, D., Viswanathan, R. & Moffitt, M. C. Genome mining for natural product biosynthetic gene clusters in the Subsection V cyanobacteria. BMC Genom.16, 669 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross, A. C. et al. Biosynthetic multitasking facilitates thalassospiramide structural diversity in marine bacteria. J. Am. Chem. Soc.135, 1155–1162 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin, M., Fischbach, M. A. & Clardy, J. A biosynthetic gene cluster for the acetyl-CoA carboxylase inhibitor andrimid. J. Am. Chem. Soc.128, 10660–10661 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tzeng, S. F., Wolfe, R. S. & Bryant, M. P. Factor 420-dependent pyridine nucleotide-linked hydrogenase system of Methanobacterium ruminantium. J. Bacteriol.121, 184–191 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheeseman, P., Toms-Wood, A. & Wolfe, R. S. Isolation and properties of a fluorescent compound, factor 420, from Methanobacterium strain M.o.H. J. Bacteriol.112, 527–531 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grinter, R. & Greening, C. Cofactor F420: an expanded view of its distribution, biosynthesis and roles in bacteria and archaea. FEMS Microbiol. Rev.45; 10.1093/femsre/fuab021 (2021). [DOI] [PMC free article] [PubMed]
- 34.Challis, G. L. & Naismith, J. H. Structural aspects of non-ribosomal peptide biosynthesis. Curr. Opin. Struct. Biol.14, 748–756 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keating, T. A. & Walsh, C. T. Initiation, elongation, and termination strategies in polyketide and polypeptide antibiotic biosynthesis. Curr. Opin. Chem. Biol.3, 598–606 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Kakinuma, A., Kanamaru, T., Sugino, H., Asano, T. & Yoneda, M. Pepsinostrepins, novel protease inhibitors from streptomyces. US Patent Application US3907764A, filed 09 Apr. 1973, and published 23 Sep. 1975. Priority claimed from JP3537572A (07 Apr. 1972), and JP6841572A (07 Jul. 1972).
- 37.Myronovskyi, M. et al. Generation of a cluster-free Streptomyces albus chassis strains for improved heterologous expression of secondary metabolite clusters. Metab. Eng.49, 316–324 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Bloudoff, K. & Schmeing, T. M. Structural and functional aspects of the nonribosomal peptide synthetase condensation domain superfamily: discovery, dissection and diversity. Biochim. et. Biophys. Acta Proteins Proteom.1865, 1587–1604 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Zhang, J. J., Tang, X., Huan, T., Ross, A. C. & Moore, B. S. Pass-back chain extension expands multimodular assembly line biosynthesis. Nat. Chem. Biol.16, 42–49 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shima, S. et al. Structure of coenzyme F420 dependent methylenetetrahydromethanopterin reductase from two methanogenic archaea. J. Mol. Biol.300, 935–950 (2000). [DOI] [PubMed] [Google Scholar]
- 41.Oyugi, M. A., Bashiri, G., Baker, E. N. & Johnson-Winters, K. Investigating the reaction mechanism of F420-dependent glucose-6-phosphate dehydrogenase from Mycobacterium tuberculosis: kinetic analysis of the wild-type and mutant enzymes. Biochemistry55, 5566–5577 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Mascotti, M. L., Juri Ayub, M. & Fraaije, M. W. On the diversity of F420 -dependent oxidoreductases: a sequence- and structure-based classification. Proteins89, 1497–1507 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Dongen, S. F. M., Elemans, J. A. A. W., Rowan, A. E. & Nolte, R. J. M. Processive catalysis. Angew. Chem.53, 11420–11428 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Venkataraman, G. G., Miska, E. A. & Jordan, D. J. Processive and distributive non-equilibrium networks discriminate in alternate limits. J. Stat. Mech.2022, 83206 (2022). [Google Scholar]
- 45.Wang, P., Bashiri, G., Gao, X., Sawaya, M. R. & Tang, Y. Uncovering the enzymes that catalyze the final steps in oxytetracycline biosynthesis. J. Am. Chem. Soc.135, 7138–7141 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Ichikawa, H., Bashiri, G. & Kelly, W. L. Biosynthesis of the thiopeptins and identification of an F420H2-dependent dehydropiperidine reductase. J. Am. Chem. Soc.140, 10749–10756 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu, J. et al. Biosynthesis of the central piperidine nitrogen heterocycle in series a thiopeptides. Chin. J. Chem.37, 35–41 (2019). [Google Scholar]
- 48.Cheng, B., Guo, H., Wang, H., Zhao, Q. & Liu, W. Dissection of the enzymatic process for forming a central imidazopiperidine heterocycle in the biosynthesis of a series c thiopeptide antibiotic. J. Am. Chem. Soc.143, 13790–13797 (2021). [DOI] [PubMed] [Google Scholar]
- 49.Xu, M. et al. Functional genome mining reveals a Class V lanthipeptide containing a d-amino acid introduced by an F420 H2 -dependent reductase. Angew. Chem.59, 18029–18035 (2020). [DOI] [PubMed] [Google Scholar]
- 50.Steiningerova, L. et al. Different reaction specificities of F420H2-dependent reductases facilitate pyrrolobenzodiazepines and lincomycin to fit their biological targets. J. Am. Chem. Soc.142, 3440–3448 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Shi, J. et al. Discovery and biosynthesis of guanipiperazine from a NRPS-like pathway. Chem. Sci.12, 2925–2930 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu, H. J. et al. Redox modifications in the biosynthesis of alchivemycin A enable the formation of its key pharmacophore. J. Am. Chem. Soc.143, 4751–4757 (2021). [DOI] [PubMed] [Google Scholar]
- 53.Barra, L. et al. β-NAD as a building block in natural product biosynthesis. Nature600, 754–758 (2021). [DOI] [PubMed] [Google Scholar]
- 54.Zhang, Y., Buchholz, F., Muyrers, J. P. & Stewart, A. F. A new logic for DNA engineering using recombination in Escherichia coli. Nat. Genet.20, 123–128 (1998). [DOI] [PubMed] [Google Scholar]
- 55.Zhang, Y., Muyrers, J. P., Testa, G. & Stewart, A. F. DNA cloning by homologous recombination in Escherichia coli. Nat. Biotechnol.18, 1314–1317 (2000). [DOI] [PubMed] [Google Scholar]
- 56.Kieser, T. Practical streptomyces genetics (John Innes Foundation, 2000).
- 57.Kakinuma, A., Kanamaru, T., Sugino, H., Asano, T. & Yoneda, M. Pepsinostrepins, novel protease inhibitors from Streptomyces. U. S. Pat. No.3, 764 (1975). [Google Scholar]
- 58.Weber, T. et al. antiSMASH 3.0-a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res.43, W237–W243 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang, Q. et al. Dual-function chromogenic screening-based CRISPR/Cas9 genome editing system for actinomycetes. Appl. Microbiol. Biotechnol.104, 225–239 (2020). [DOI] [PubMed] [Google Scholar]
- 60.Bashiri, G., Squire, C. J., Moreland, N. J. & Baker, E. N. Crystal structures of F420-dependent glucose-6-phosphate dehydrogenase FGD1 involved in the activation of the anti-tuberculosis drug candidate PA-824 reveal the basis of coenzyme and substrate binding. J. Biol. Chem.283, 17531–17541 (2008). [DOI] [PubMed] [Google Scholar]
- 61.Bashiri, G., Rehan, A. M., Greenwood, D. R., Dickson, J. M. J. & Baker, E. N. Metabolic engineering of cofactor F420 production in Mycobacterium smegmatis. PloS ONE5, e15803 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marfey, P. Determination of d-amino acids. II. Use of a bifunctional reagent, 1,5-difluoro-2,4-dinitrobenzene. Carlsberg Res. Commun.49, 591–596 (1984). [Google Scholar]
- 63.Cianci, M. et al. P13, the EMBL macromolecular crystallography beamline at the low-emittance PETRA III ring for high- and low-energy phasing with variable beam focusing. J. Synchrotron Radiat.24, 323–332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meents, A. et al. Development of an in-vacuum x-ray microscope with cryogenic sample cooling for beamline P11 at PETRA III. Proceedings of SPIE8851, 88510K (2013).
- 65.Winter, G., Lobley, C. M. C. & Prince, S. M. Decision making in xia2. Acta Crystallogr. Sect. D., Biol. Crystallogr.69, 1260–1273 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr.66, 125–132 (2010). [DOI] [PMC free article] [PubMed]
- 67.Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. Sect. D. Struct. Biol.75, 861–877 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature596, 583–589 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat. Methods19, 679–682 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. Sect. D. Biol. Crystallogr.66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci.27, 293–315 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wallace, A. C., Laskowski, R. A. & Thornton, J. M. LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng.8, 127–134 (1995). [DOI] [PubMed] [Google Scholar]
- 73.Molecular Operating Environment (MOE), 2024.06; Chemical Computing Group ULC, 910-1010 Sherbrooke St. W., Montreal, QC H3A 2R7, Canada (2024).
- 74.Yariv, B. et al. Using evolutionary data to make sense of macromolecules with a “face-lifted” ConSurf. Protein Sci.32, e4582 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Landau, M. et al. ConSurf 2005: the projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Res.33, W299–W302 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The pepstatin biosynthetic gene cluster sequence in this study has been deposited in GenBank under accession number PP947771. The protein crystal structure data generated in this study have been deposited in the Protein Data Bank under PDB IDs 9G64, 9GKH, 9GM0, 9GNC, and 9GND. All data that support the findings of this study are available in the main text, supplementary information and from corresponding author(s) upon request. Source data are provided with this paper.