Abstract
The impact of DJ tube retention time on renal function has received scant attention from researchers. Nevertheless, there is a plethora of clinical evidence indicating that protracted stent retention can result in renal insufficiency, or even renal atrophy, which can consequently lead to loss of renal function or nephrectomy.A comprehensive review of the medical records of all patients who underwent DJ tube placement between 1 January 2010 and 1 September 2024 in our hospital was performed. Cases with a duration of DJ tube placement exceeding two years were selected for further analysis, as supported by previous studies. The final study population comprised 74 cases with indwelling DJ tubes for a minimum of two years. Renal size/glomerular width (PW) was measured on the basis of CT coronal scanning, and the mean value of PW and the rate of change of PW were calculated before the first placement of the DJ tube and at the last follow-up, respectively. Furthermore, the study recorded eGFR, serum creatinine (Scr), blood urea nitrogen (BUN), and blood uric acid (UA) at two time points: before and after DJ tube placement. The mean duration of indwelling DJ tubes was 67.94 ± 48.26 months in the unilateral DJ tube indwelling group (including isolated kidney cases) and 50.22 ± 29.65 months in the bilateral group. During the mean retention time of 67.94 ± 48.26 months, the mean PW change rates of unilateral DJ tube stented kidneys and healthy kidneys/unilateral kidneys were − 39.01 ± 26.1% and 16.52 ± 25.4%, respectively, which were statistically significant (P < 0.01). The mean rate of change in PW in the left and right sides of the bilateral DJ tube retention group was − 18.31 ± 36.3% over a mean retention time of 50.22 ± 29.65 months, which was statistically significant (P < 0.01). Furthermore, a statistically significant decrease of -37.81 ± 51.2% in eGFR was observed before and after bilateral DJ tube placement (P < 0.01). No statistically significant difference (P > 0.05) was observed in eGFR in the unilateral DJ tube placement group (including isolated kidney cases) and in Scr, BUN, and UA values in the unilateral and bilateral DJ tube placement groups before and after DJ tube placement. In the unilateral DJ tube-placement group, the duration of DJ tube placement exhibited a negative correlation with the rate of change in mean PW percentage (Pearson correlation coefficient r = -0.470, P = 0.002) and a positive correlation with the rate of change in eGFR (Pearson correlation coefficient r = 0.653, P < 0.01). Conversely, in the bilateral DJ tube retention group, DJ tube retention duration exhibited no significant correlation with the change in mean percentage of PW.However, it demonstrated a negative correlation with the rate of change in eGFR (Pearson correlation coefficient r = -0.443, P = 0.03). In patients with unilateral or bilateral indwelling DJ tubes, renal size may decrease over time despite the presence of an indwelling DJ tube, especially in patients with bilateral indwelling DJ tubes.
Keywords: Ureteral stents, Renal function, DJ tube, Renal parenchymal
Subject terms: Medical research, Nephrology, Urology
Introduction
Ureteral stenting is a common urological procedure, especially double J-tube ureteral stenting, which has been routinely used to relieve benign and malignant ureteral obstruction of various causes since its first use in 19711,2. Although percutaneous nephrostomy is a preferred approach for ureteral obstruction and patients prefer this procedure. However, double J-tube placement is more widely used in developing countries because of its longevity, low infection rate, and lack of need for an external drainage bag.In patients with ureteral obstruction due to benign disease, the ureteral stricture is fixed despite improvement in the primary disease or symptoms. This condition usually requires continuous, long-term indwelling double J-tube stenting. In contrast, patients with malignant tumours usually have a poorer prognosis and a shorter median overall survival. This makes long-term double J-tube stenting usually not feasible. However, long-term indwelling double J-tubes are the preferred option for early-stage malignancies in the gynaecology and pelvis, as well as for ureteral obstruction due to mid- to late-stage malignancies3-6.
Although previous research evidence suggests that double J-tubes can safeguard and enhance the renal function of obstructed kidneys by effectively draining urine, and their efficacy in treating ureteral obstruction is extremely high7–9. However, to the best of our knowledge, there are clinical cases of patients with decreased renal function or even renal atrophy due to prolonged indwelling double J-tubes. This is a complex issue that is not only related to the duration of retention, but also to the cause of obstruction, CKD stage, stent type, and condition. Clinical judgement of whether the obstruction has improved or resolved usually involves measurement of laboratory values reflecting renal function, imaging to determine whether the hydronephrosis has resolved and renal function imaging to directly observe unilateral renal function. Although renal function imaging accurately reflects the function of one side of the kidney, the high cost is prohibitive for most patients. Therefore, it is difficult to accurately and timely assess a patient’s renal function through laboratory tests and conventional CT examinations alone. Only a few researchers have focused on the actual renal function of kidneys with DJ tubes. This is because even if the renal function of the stented kidney deteriorates, the serum creatinine or estimated glomerular filtration rate remains essentially normal due to the compensatory effect of the contralateral kidney. We urge that attention should be paid to the effect of DJ tube retention time on renal function and suggest that renal function in stented kidneys should be assessed using an appropriate and accurate method, and renal parenchymal width might be a reliable method (PW)10–11. This study focused on the effect of retention time on renal function and renal parenchyma, as well as the effect of chronic kidney disease, hypertension and diabetes, and the cause of obstruction (benign or malignant disease) on renal function, and the potential causative mechanisms were summarised and discussed.
Materials and methods
Study population and design
Research population
The study protocol and information used in this manuscript were approved by the patients, the Institutional Review Board of the First Affiliated Hospital of Xiamen University, and the Ethics Committee of the First Affiliated Hospital of Xiamen University, and all methods were performed in accordance with the guidelines and regulations of the journal.This was a single-centre retrospective study of patients with indwelling DJ tubes between 1 January 2010 and 1 September 2024. The inclusion criteria were as follows: firstly, continuous placement of DJ tubes for a minimum of 24 months, as no significant change in the size of the patient’s kidneys was observed within one year of indwelling DJ tubes; and secondly, abdominal CT scans were performed before the initial placement of the DJ tubes and at the final follow-up. The exclusion criteria were as follows: firstly, those for whom information related to examinations, laboratory tests and CT scans was lacking; and secondly, those who were left in place for less than 24 months. The following data were obtained from the medical records: age, sex, duration of DJ tube retention in months, number of DJ tube changes, lateral position of the DJ tube (left or right), and potential comorbidities (diabetes mellitus, hypertension, chronic kidney disease, and the presence of hydronephrosis at the first visit). Furthermore, the following data were obtained from the medical records: hypertension, chronic kidney disease, presence of hydronephrosis at the initial visit, positive urine culture, history of treatment for pelvic disease (history of surgery, radiotherapy and chemotherapy) and likelihood of ureteral obstruction (classified as benign and malignant). Benign conditions included renal and ureteral stones, benign tumours of the uterus and bladder, and congenital renal disease. Malignant diseases included malignant neoplastic diseases of the kidneys, ureters, uterus, rectum and ovaries. Prior to and following the initial stent placement, a comprehensive set of biological markers was meticulously monitored, including blood urea nitrogen (BUN) and uric acid (UA), estimated glomerular filtration rate (EGFR), serum creatinine (Scr), complete blood count, urine leukocyte values, and urine cultures (bacterial values). Furthermore, the percentage change in EGFR and PW between the two time points (at the first indwelling DJ tube and the final follow-up) was calculated.The formula for calculating EGFR is referenced to the Chronic Kidney Disease Epidemiology Collaboration (CKD - EPI) equation.
Research methods and design
The determination of renal parenchymal width was achieved through a comprehensive review of coronal CT scans in all cases. While renal dynamic imaging is capable of accurately determining renal function on a single side, it is not as widely utilised and is comparatively expensive. The decision to utilise computed tomography stemmed from its established role as the preferred modality for disease review, complemented by its wider accessibility and cost-effectiveness. The renal parenchyma width was estimated on three occasions by the same investigator: firstly, before the first stent placement; and finally, at the final follow-up. The mean PW and the rate of change before and after were then calculated. The estimation process was conducted as outlined below: horizontal lines were delineated at the upper, middle and lower points of the CT coronal section of the kidney, a plumb line was drawn along the longest horizontal line of the renal parenchyma, and the renal parenchymal width (PW) was measured at the longest point of the upper, lower and middle lines, which were noted as points a, b and c, respectively (see Fig. 1). The parenchymal width was measured from the renal peritoneum to the renal collecting system, and the PW was measured at the three points. The mean PW was calculated, and the rate of change of the mean PW before and after placement was calculated. To minimise inter-observer variation, all the above measurements were performed by the same investigator. Concurrently, a correlation analysis was conducted between the retention time and the rate of change of mean PW, as well as the rate of change of eGFR, in both the unilateral DJ tube and bilateral DJ tube groups.
Fig. 1.

illustrates the methodology employed in the present study. Horizontal lines were drawn at the upper, middle, and lower points of the CT coronal section of the kidney. The longest renal parenchymal plumb line was then constructed along the horizontal line, and the parenchymal width (PW) was measured at the longest point of the upper, lower, and middle points, which were subsequently designated as points (a), (b), and (c), respectively.
Statistical analysis
All data were analysed using the statistical software package SPSS 27.0 (SPSS Inc., Chicago, IL, USA). The statistical analyses included the description of clinical data, including the calculation of medians, means, and percentages. Additionally, descriptive statistics were employed to delineate the baseline characteristics of the study population. The mean PW, BUN, UA, EGFR, and Scr before stent implantation and at the time of the last stent replacement were compared using a paired t-test or Wilcoxon signed-rank test, based on the results of the Shapiro-Wilk test. To identify parameters associated with the rate of change in mean PW, a Person’s rank correlation analysis or Mann-Whitney U-test were employed.All quantitative data conformed to normal distribution. A p-value of less than 0.05 was deemed statistically significant for all tests.
Results
General information
Table 1 provides a concise overview of the fundamental demographic characteristics of the study population. The total number of eligible cases was 74, but ultimately 4 cases were excluded due to missing data, and only 70 cases could be divided into unilateral and bilateral DJ tube retention groups (referred to as unilateral and bilateral groups). Within the unilateral group, there were 32 (68.1%) females and 15 (31.9%) males, while in the bilateral group, there were 12 (52.2%) females and 11 (47.8%) males. The mean age of the study population in the unilateral and bilateral groups was (51.68 ± 13.99) and (62.7 ± 11.20) years, respectively; the mean number of DJ tube changes was (9.60 ± 7.61) and 8.26 ± 9.50), respectively, and the mean duration of DJ tube placement was (67.94 ± 48.26) days. In the unilateral group, 57% of cases underwent DJ tube placement for malignancy, 57% had a history of pelvic surgery, 43% had received pelvic radiotherapy and 55% had received chemotherapy. Conversely, in the bilateral group, 95.7% of cases were attributed to malignancy, 78.3% had a history of pelvic surgery, 39.1% had a history of pelvic radiotherapy, and 78.3% had a history of chemotherapy. The high number of cases of malignant etiology may be due to the fact that patients with malignant tumours usually undergo regular pelvic or abdominal CT scans, whereas patients with benign cases usually undergo radiological or ultrasound examinations only. For instance, the reduced mean retention time observed in the bilateral group could be attributed to the occurrence of accelerated renal failure in patients with bilateral DJ tube retention, as evidenced by the prevalence of chronic kidney disease. The reduced incidence of hydronephrosis in the bilateral group may be attributable to patient adaptation to the procedure. The higher incidence of radiotherapy and chemotherapy in the bilateral group may be due to the limited range of treatment modalities available.
Table 1.
General information on the unilateral and bilateral groups, noting that the isolated kidney group is included in the unilateral group.
| n | Mean ± SD | Mean ± SD |
|---|---|---|
| Unilateral group(42 + 5) | Bilateral group(23) | |
| Age(years) | 51.68 ± 13.99 | 62.7 ± 11.20 |
| Retention time(moths) | 67.94 ± 48.26 | 50.22 ± 29.65 |
| Number of stent changes | 9.60 ± 7.61 | 8.26 ± 9.50 |
| Gender (female/male) | 32/47(48.5%) | 12/23(52.2%) |
| Right side | 25/47(53.2%) | - |
| Diabetes/Hypertension | 11/47(23.4%) | 8/23(34.8%) |
| Chronic kidney disease | 2/47(4.3%) | 11/23(47.8%) |
| First diagnosis of hydronephrosis | 45/47(95.7%) | 10/23(43.5%) |
| First diagnosis of positive urine culture | 29/47(61.7%) | 16/23(69.6%) |
| Malignant obstructive factors | 28/47(59.6%) | 22/23(95.7%) |
| History of pelvic surgery | 25/47(53.2%) | 18/23(78.3%) |
| History of pelvic radiation | 18/47(38.3%) | 9/23(39.1%) |
| History of chemotherapy | 27/47(57.4%) | 18/23(78.3%) |
Changes in PW and eGFR
As illustrated in Table 2, the data demonstrate a shift in mean PW and eGFR in both the unilateral and bilateral groups prior to the initial stent placement, as well as at the subsequent follow-up. The mean PW change of stented kidneys in the unilateral group was − 39.01 ± 26.1%, whereas that of the contralateral kidneys was 16.52 ± 25.1%, with a statistically significant difference (P < 0.01); and the change in their eGFR was 29.1 ± 1.03%, but the difference was not statistically significant (P = 0.355). In the unilateral group, over a mean period of (67.94 ± 48.26) months, the mean PW of stented kidneys decreased by 39.01%, whereas that of healthy kidneys increased by 16.52%. This indicated that the mean PW of healthy kidneys showed a different degree of increase. Conversely, the difference in eGFR before and after stent implantation was not statistically significant (P = 0.355). This finding suggests that the stented kidney may have undergone a reduction or loss in renal function, while the healthy kidney may have assumed a compensatory role. Consequently, eGFR alone is deemed unreliable as a measure of renal function in such cases. The mean PW change rate in the isolated kidney group was − 30.01 ± 16.1%, which was not statistically significant (P > 0.05); its eGFR change rate was 47.2 ± 78.2%, which was not statistically significant (P > 0.05). It seems paradoxical that the mean PW change in the isolated kidney group was lower than before stent implantation, while eGFR was higher than before placement, but neither was statistically significant.This may be due to the fact that there were fewer patients with isolated kidneys in the clinic with stenting or a shorter survival time, which resulted in a small number of cases that could not be evaluated statistically in detail.The mean rate of change in PW in the bilateral group was − 18.31 ± 36.4%, which was statistically significant (P < 0.01). The rate of change in their eGFR was − 37.81 ± 50.3%, which was statistically significant (P < 0.01). In the bilateral group, mean PW and mean rate of change in eGFR decreased compared to pre-treatment and were statistically significant. This indicates that in the bilateral group, over a mean period of 50.22 ± 29.65 months, there was a mean decrease of 18.31% in renal ‘PW’ (mean PW from left and right kidney) of the indwelling DJ tubes and a decrease of -29.21% in eGFR. Notably, the eGFR in the bilateral group decreased by 29.21% after stent retention, which may be due to lack of compensation from the contralateral kidney. In conclusion, prolonged unilateral retention of DJ tubes may result in uric acid, urea nitrogen and eGFR within the normal range, leading to a decline in renal function and parenchymal atrophy. Conversely, the bilateral group of patients with DJ tube placement may also experience renal atrophy, but their eGFR will instead be in the abnormally low range (despite normal UA and BUN), suggesting that eGFR can be used to some extent as a method of assessment during bilateral DJ tube placement. CT scans of representative patients in the unilateral, isolated kidney and bilateral groups showed changes in PW (Fig. 2).
Table 2.
Changes in mean renal parenchymal width and glomerular filtration rate before and after indwelling ureteral stents. Mean length of stay in unilateral, isolated kidney and bilateral groups: 67.29 ± 4.47 months, 73.40 ± 60.35 months, 50.22 ± 29.65 months; all using paired t-test; PW renal parenchymal width, eGFR estimated glomerular filtration rate.
| Before placement (Mean ± SD) | After placement (Mean ± SD) | Percentage change (%) | P value | |
|---|---|---|---|---|
| Unilateral group | ||||
| PW(stented kidney) | 1.53 ± 0.61 | 0.89 ± 0.45 | -39.01 ± 26.1 | <0.01 |
| PW(healthy kidney) | 2.16 ± 0.44 | 2.47 ± 0.52 | 16.52 ± 25.1 | <0.01 |
| eGFR | 89.70 ± 38.45 | 96.87 ± 42.84 | 29.79 ± 1.03 | 0.355 |
| Isolated kidney group | ||||
| PW | 2.30 ± 0.73 | 1.55 ± 0.28 | -30.1 ± 16.2 | 0.43 |
| eGFR | 47.49 ± 22.03 | 71.83 ± 53.42 | 47.63 ± 78.2 | 0.277 |
| Bilateral group | ||||
| PW | 2.09 ± 0.45 | 1.61 ± 0.48 | -18.31 ± 36.4 | <0.001 |
| eGFR | 82.34 ± 48.60 | 40.79 ± 29.92 | -37.81 ± 50.36 | <0.01 |
Fig. 2.
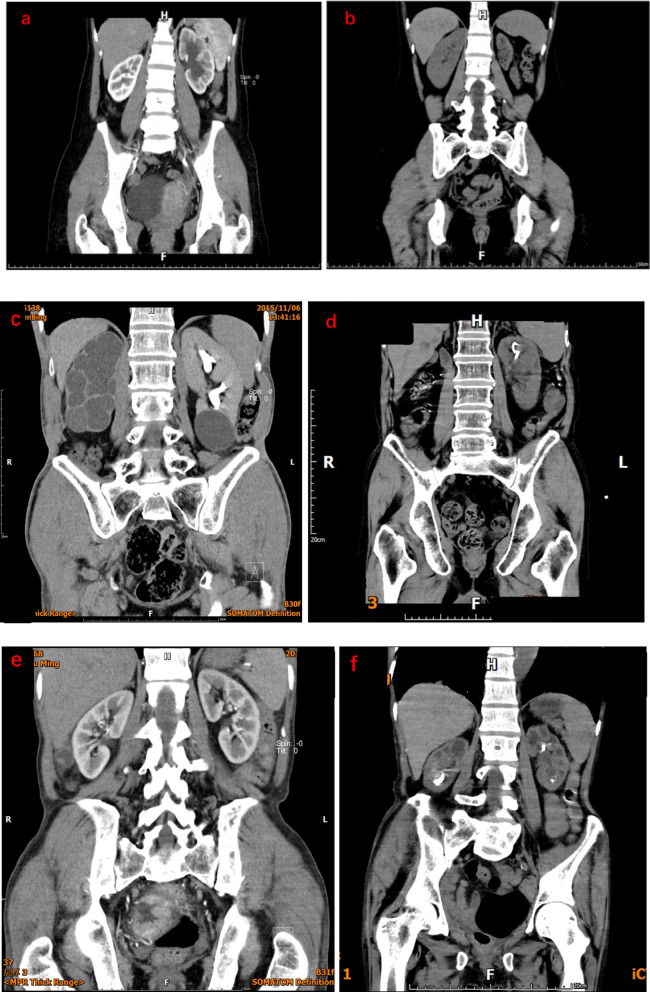
This image illustrates a typical case in the unilateral group: a 38-year-old female patient who had been fitted with a stent for a uterine malignancy, with left stent retention (a). The patient presented with left stent retention 81 months after the indwelling ureteral stent had been fitted. The left kidney exhibited a substantial reduction in size (b). As shown in the figure, a typical case in the isolated kidney group: male, 72 years old, malignant tumour of the lower ureter and bladder with left ureteral stent retention (c), after 91 months of retention the patient’s left kidney has significantly decreased in size (d). This is a typical case of bilateral stent retention (a), female, 45 years old, indwelling stenting for renal and ureteral stones, after 74 months of ureteral stent retention, the patient’s bilateral kidneys were observed to be significantly reduced in size.
Furthermore, Pearson’s rank correlation coefficient and the Mann-Whitney U test were utilised to assess the relationship between continuous and categorical variables, as well as the rate of change in mean PW in unilateral and bilateral groups, respectively.The results are presented in Tables 3, 4 and 5(isolated kidney group vs. unilateral group combined). The findings of the correlation analysis indicated that in the unilateral DJ tube indwelling group, the duration of DJ tube indwelling exhibited a negative correlation with the rate of change in mean PW percentage (Pearson correlation coefficient r = -0.470, P = 0.002) and a positive correlation with the rate of change in eGFR (Pearson correlation coefficient r = 0.653, P < 0.01). In summary, the longer the DJ tube remained in situ, the lower the stented renal PW. The scatter plot of the mean PW rate of change versus DJ tube retention time demonstrated a linear relationship, with an R2 value of 0.221 (Fig. 3). Conversely, within the bilateral group, no substantial correlation was identified between the rate of change in mean PW and DJ tube retention time, which may be attributable to the limited data set. However, a negative correlation was observed between the duration of DJ tube retention and the rate of change in eGFR (Pearson correlation coefficient r = -0.443, P = 0.03).The Mann-Whitney U test revealed that there was no statistically significant difference between the unilateral and bilateral groups for the various parameters.
Table 3.
Analysis of continuous and categorical variables in unilateral groups. Person test was taken for continuous variables and Mann-Whitney U-test for categorical variables and P < 0.05 was considered statistically significant.
| Unilateral group | Mean PW change rate | P vale |
|---|---|---|
| Continuous variable | Person’s correlation coefficient | |
| Age(years) | -0.07 | 0.64 |
| Retention time(moths) | -0.47 | <0.01 |
| Number of stent changes | -0.54 | <0.01 |
| Categorical variables | U vale/% | |
| Gender | 157 | 0.942 |
| Female | 76.20% | |
| Male | 23.80% | |
| Lateralisation | 199 | 0.794 |
| Left | 47.80% | |
| Right | 52.40% | |
| Diabetes/Hypertension | 110 | 0.249 |
| Yes | 21.40% | |
| No | 78.60% | |
| CKD | 18 | 0.905 |
| Yes | 2.40% | |
| No | 97.60% | |
| First diagnosis of hydronephrosis | 28 | 0.523 |
| Yes | 95.20% | |
| No | 4.80% | |
| First diagnosis urine(+) | 214 | 0.909 |
| Yes | 45.20% | |
| No | 54.80% | |
| Malignant obstructive factors | 177 | 0.363 |
| Yes | 59.50% | |
| No | 40.50% | |
| History of pelvic surgery | 201 | 0.701 |
| Yes | 57.10% | |
| No | 42.90% | |
| History of pelvic radiation | 201 | 0.703 |
| Yes | 42.90% | |
| No | 57.10% | |
| History of chemotherapy | 212 | 0.871 |
| Yes | 54.80% | |
| No | 45.20% | |
| Stent type | 168 | 0.301 |
| DJ tube | 61.90% | |
| Tumour stent tube | 38.10% | |
| Obstruction location | 170 | 0.989 |
| Upper | 73.80% | |
| Lower | 26.20% |
Table 4.
Analysis of continuous and categorical variables in the bilateral group. Person test was taken for continuous variables and Mann-Whitney U-test for categorical variables and P < 0.05 was considered statistically significant.
| Bilateral group | Mean PW change rate | P vale |
|---|---|---|
| Continuous variable | Person’s correlation coefficient | |
| Age(years) | 0.05 | 0.816 |
| Retention time(moths) | -0.21 | 0.336 |
| Number of stent changes | 0.286 | 0.186 |
| Categorical variables | U vale/% | |
| Gender | 59 | 0.949 |
| Female | 52.20% | |
| Male | 47.80% | |
| Diabetes/Hypertension | 59 | 0.949 |
| Yes | 34.80% | |
| No | 65.20% | |
| CKD | 59 | 0.667 |
| Yes | 47.80% | |
| No | 52.20% | |
| First diagnosis of hydronephrosis | 54 | 0.495 |
| Yes | 43.50% | |
| No | 56.50% | |
| First diagnosis urine(+) | 50 | 0.688 |
| Yes | 69.60% | |
| No | 30.40% | |
| Malignant obstructive factors | 7 | 0.546 |
| Yes | 95.70% | |
| No | 4.30% | |
| History of pelvic surgery | 33 | 0.371 |
| Yes | 78.30% | |
| No | 21.70% | |
| History of pelvic radiation | 39 | 0.655 |
| Yes | 78.30% | |
| No | 21.70% | |
| History of chemotherapy | 58 | 0.753 |
| Yes | 39.10% | |
| No | 60.90% | |
| Stent type | 42 | 0.823 |
| DJ tube | 78.30% | |
| Tumour stent tube | 21.70% | |
| Obstruction location | 22 | 0.465 |
| Upper | 87.00% | |
| Lower | 13.00% |
Table 5.
Changes in infection indicators before and after patients with indwelling stents in the bilateral group and unilateral group. There were a statistically significant difference in urine cultures before and after the indwelling stent.
| Paired difference (Mean ± SD) | 95% confidence interval of difference | P value | |
|---|---|---|---|
| Unilateral group | |||
| Blood WBC | -0.07 ± 3.89 | (-1.28, 1.15) | 0.91 |
| Urine WBC | -102.01 ± 1830.76 | (-672.51, 468.50) | 0.72 |
| Urine culture | -163.3 3 ± 381.83 | (-282.32, -44.35) | <0.01 |
| Bilateral group | |||
| Blood WBC | -3.94 ± 9.93 | (-8.24, 0.35) | 0.07 |
| Urine WBC | -1235 0.77 ± 2950.34 | (-2511.59, 40.05) | 0.06 |
| Urine culture | -52.04 ± 82.89 | (-87.88, -16.20) | <0.01 |
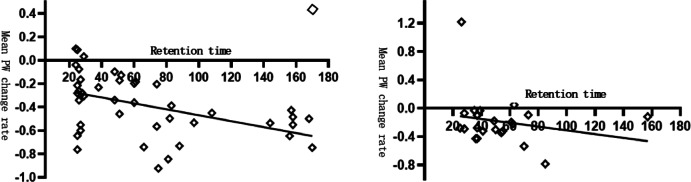
Fig. 3.
Depicts a scatterplot of the change in mean PW versus ureteral retention time (in months) for the unilateral and bilateral groups. There is a linear relationship between these variables, with an r² value of 0.221 for the unilateral group and no significant correlation between mean PW and retention time for the bilateral group (averaged over the left and right sides). It should be noted that the number of cases in the isolated kidney group was insufficient for analysis.
Discussion
It is widely accepted that in cases of ureteral obstruction, ureteral stenting protects renal function in the obstructed kidney unless it fails due to the alleviation of symptoms or the development of hydronephrosis, for example12–14. Consequently, clinicians may become somewhat indifferent to actual renal function with prolonged indwelling stents, particularly when laboratory tests are normal. However, our observations revealed a considerable number of patients with renal atrophy despite prolonged indwelling ureteral stenting, prompting us to question the efficacy of ureteral stents in preserving renal function in obstructed kidneys. It is noteworthy that even when clinicians detect abnormalities such as mild renal atrophy in patients by CT, they are unable to identify whether it is due to hydronephrosis or atrophy of the renal parenchyma itself. Therefore, the treatment modality is usually not changed (e.g., to percutaneous nephrolithotomy).To the best of our knowledge, this is the inaugural study in China to report the evolution of PW in patients with long-term indwelling ureteral stents. The findings of the study indicated that renal atrophy commenced when the duration of indwelling ureteral stenting exceeded two years, either unilaterally or bilaterally, particularly in cases where the stent had been in place for more than five years. Furthermore, it is our contention that eGFR during long-term unilateral indwelling stenting does not accurately reflect renal function, whereas eGFR during bilateral indwelling stenting can be employed as a reliable renal function assessment tool. It is noteworthy that PW may serve as a valuable assessment tool in both scenarios. A correlation analysis of the Person data revealed that the duration of the indwelling stent was a significant factor in both cases. Indeed, it has been identified in numerous clinical workups. However, given the lack of understanding regarding the mechanism by which renal atrophy occurs, there are currently no superior alternatives. Our preliminary view is that the mechanisms of impact on renal function and parenchyma are different for benign and malignant disease obstruction, and that malignant obstruction may also affect the kidneys through radiotherapy, chemotherapy and systemic disease.Moreover, there are no corresponding guidelines or prospective trials that systematically explore techniques other than ureteral stenting. As a consequence, the issue is typically either disregarded or silenced for the time being. The following section will examine the potential mechanisms by which renal atrophy may occur.
The precise mechanism by which prolonged indwelling ureteral stents result in reduced renal parenchyma or even renal atrophy remains uncertain. The following are some of the hypotheses that have been put forth: The initial hypothesis posits that chronic inflammatory stimulation results in damage to the renal pelvis and renal parenchyma. Urinary tract infection is one of the most common complications in patients with indwelling ureteral stents. The introduction of bacteria into the bladder can occur at the time of stent insertion or as a result of movement during the indwelling process15–16 In their study, Farsi et al. observed a 30% incidence of bacteriuria17. Paick et al. (2020) reported a 21% incidence of bacteriuria, while Kehinde et al. (2018) reported a 17% incidence18–19 Similarly, Lifshitz et al. (2017) reported a bacteriuria rate of 13% in 65 patients with ureteral stents20–21 Furthermore, Yeniyol et al. (2017) reported bacteriuria in 16% of patients, while Akay et al.(2017) reported bacteriuria in 24% of patients with ureteral stents at the time of ureteral stent removal22–24. However, it should be noted that none of the subjects included in the studies had undergone long-term stent placement, and the incidence of bacteriuria in one study was 60.9%, which is higher than the incidence reported in other studies22,23. This may be attributed to the fact that the subjects were observed for a longer duration than in previous studies, despite the regular replacement of their ureteral stents. These findings are consistent with those of another study, which demonstrated that the duration of ureteral stenting was significantly associated with the occurrence of bacteriuria25. Nevertheless, in patients presenting with severe ureteral stenosis or ureteral obstruction resulting from malignancy, long-term indwelling ureteral stents are typically indicated. In such cases, patients with ureteral stents that have been maintained in place despite regular replacement may be at an elevated risk of urinary tract infection in comparison to other patient groups. Furthermore, impaired renal function, particularly in CKD stages 4 and 5, may intensify bacterial aggregation and fixation, and even manifest as symptoms of severe urinary tract infections. It is noteworthy that one study concluded that the incidence of bacteriuria was significantly higher in women than in men with ureteral stents. The reason for this discrepancy is unclear. One potential explanation is the closer anatomical proximity of the female urethra to the genital tract. In the bilateral group, 52.2% of patients had a stent with stones after retention of the stent, compared to 53.7% in the unilateral group and 40% in the isolated kidney group.
Bacterial colonisation typically results in acute and chronic inflammatory cell infiltration, which initially damages the medullary tissue and the nearby cortex, subsequently leading to progressive atrophy and scar formation. This ultimately culminates in cortical thinning. Furthermore, the biofilm that forms on the surface of the ureteral stent prolongs the duration of urinary tract infections, thereby placing the patient at constant risk of infection or other complications. The formation of a biofilm will result in the development of a crust, and the two processes are inextricably linked. In our study, at the time of the last stent replacement, the stents were found to be full of stones in 53.7% of cases in the unilateral group and 52.2% in the bilateral group. It is evident that the wear and irritation between the stent and the renal pelvis, ureter, and bladder can result in the development of chronic inflammation. In conclusion, the incidence of bacteriuria with indwelling ureteral stents is not as low as previously assumed, particularly in patients with long-term indwelling ureteral stents. The results of our study also demonstrated that the positive urine culture results of patients before and after indwelling stenting exhibited some discrepancy, which was statistically significant (P < 0.01). Consequently, to prevent urinary tract infection, it may be advisable to minimise the duration of ureteral stenting wherever feasible.
The second hypothesis posits that, despite the numerous advantages of ureteral stents, prolonged use of these devices may still result in discomfort. This may manifest as irritation of the bladder mucosa, particularly the tricorneal membrane, at the distal end of the stent. Alternatively, smooth muscle spasms or ureteral reflux may lead to renal injury. A partial or complete ureteral obstruction results in ureteral dysfunction, as evidenced by the cessation of peristaltic activity and the transport of urine from the affected kidney to the bladder27–29. In vitro experiments have now demonstrated spontaneous contractile activity in isolated ureteral tissue specimens, with smooth muscle in the ureteral wall playing an important role. Given that the mechanism of peristaltic wave propagation in the ureter is currently unknown, it can be inferred that the propagation of peristaltic contractions is largely controlled by the smooth muscle cells of the ureteral wall. One study has concluded that the use of indwelling ureteral stents may result in the elimination of normal ureteral function. The researchers observed ureteral smooth muscle activity in 24 pigs at 48 h, 1, 2, 4 and 7 weeks after unilateral stent placement30–31.
A significant reduction in peristalsis was observed at 48 h following stent placement, with a persistent decline noted at one week post-placement. Furthermore, a greater absence of Gli1 expression in smooth muscle cells was observed in specimens with increasing ureteral inflammation. These findings are consistent with the results of a previous study which suggested that the propagation of ureteral smooth muscle contractions involves the action of calcium and calcium ion channels. Furthermore, the study proposed that three proteins (Gli, EPO, and α1-AR) play a role in the regulation of peristaltic activity of human ureteral tissue32–34. The results of animal experiments have demonstrated that tissues treated with the Gli protein inhibitor GANT61 exhibit a reduction in contractility in comparison to control tissues. In contrast, EPO demonstrated a reduction in contractility within five minutes of administration, indicating that its modulation of ureteral contractility occurs through a tachyphylactic mechanism. The authors propose that tamsulosin exerts its relaxation effect on ureteral smooth muscle by reducing the contractile activity of ureteral SMC, while not completely eliminating ureteral contraction. This hypothesis is supported by the in vitro results. The interstitial cells of Cajal (ICC), which are marked by Kit expression, are located throughout the upper urinary tract and exhibit morphological and molecular characteristics that are similar to those of intestinal pacemaker cells. The neutralisation of Kit activity in ureteral explants through the use of a neutralising antibody resulted in the observation of unidirectional peristaltic perturbation, yet no evidence of smooth muscle cell differentiation. This suggests that the function of Kit is necessary for the occurrence of ureteral peristalsis. It is well established that the expression of hyperpolarization-activated cyclic nucleotide-gated channel 3 (HCN3) channels is significantly correlated with spontaneous membrane polarization in cardiac pacemaker cells. Furthermore, such channels have been identified in the ureter. Inhibition of HCN ion channel activity has been demonstrated to result in the loss of proximal-distal ureteral contraction, indicating that Hcn3 function is essential for ureteral peristalsis35–37. In conclusion, the defective development of HCN3 and urothelial pacemaker cells, as indicated by the presence of c-KIT, would result in abnormal ureteral peristalsis and non-obstructive hydronephrosis.
Furthermore, previous studies have demonstrated that the sympathetic system plays a role in regulating ureteral peristalsis. For instance, stimulation of α-receptors in the human ureter has been shown to increase ureteral contraction, whereas α-blockers have been observed to decrease peak systolic pressures, thereby inducing ureteral dilatation. A study demonstrated that α-blockers had no notable impact on renal pelvic pressure in stented ureters in pigs. However, they did exhibit a detrimental effect on ureteral peristalsis, and the precise mechanism remains unclear. Alpha-blockers are frequently employed to alleviate low back pain in patients with ureteral obstruction or in patients with indwelling ureteral stents. It is plausible that this may also contribute to the attenuation of ureteral peristalsis. It is evident that the patient’s underlying malignancy or external compression can influence ureteral peristalsis, which may subsequently result in ureteral obstruction dysfunction. Despite the absence of peristalsis-related indexes in our study, the findings of previous animal experiments and in vitro experiments have provided some support for our hypothesis.
A third hypothesis, namely vesicoureteral reflux, may be another potential cause. The vesicoureteral bladder junction represents a crucial anatomical structure that serves to safeguard the upper urinary tract from the intermittent high pressure exerted by the bladder. It is equipped with an opening that enables the passage of urine from the ureter into the bladder, while simultaneously preventing retrograde flow of urine into the kidneys during the process of urination. Conversely, the presence of an indwelling ureteral stent prevents the closure of the ureterobladder junction and reduces or stops ureteral peristalsis38–39 In particular, irritation of the bladder mucosa by the presence of a foreign body within the bladder can result in spasm of the distal ureter and a rapid increase in intravesical pressure. This leads to the creation of an unfavourable pressure gradient that can encourage retrograde ascension of urine. This phenomenon typically occurs during the voiding phase, when elevated bladder pressure results in incomplete closure of the ureterovesical junction. The gap between the stent and the ureter both facilitates retrograde urine flow.
Furthermore, in an analysis of voiding cystourethrography in patients with stents, researchers observed reflux during voiding in 80% of cases, which may have contributed to the dystocia experienced by these patients during urination. Furthermore, another study reached the conclusion that vesicoureteral reflux along the stent occurred in 51.4% of patients, even after the immediate insertion of a ureteral stent. A study was conducted on 20 female dogs to evaluate the anatomical, fluid dynamic, functional and pathological changes associated with unilateral endoureteral stent placement. The glomerular filtration rate was assessed both prior to and following the placement of a unilateral stent, with the assessments conducted a few weeks apart. Additionally, weekly cystometry and cystography were conducted to ascertain the occurrence of reflux and to quantify the intravesical pressure responsible for this reflux40–42. The results demonstrated that in ureters with indwelling stents, the luminal volume of the ureter was enlarged three-fold (P < 0.002). There was no significant difference in GFR before and after stenting. Vesicoureteral reflux occurred at a mean intravesical pressure of 13.7 cm water column. Additionally, cystography showed ineffective ureteral peristalsis. The results demonstrated that indwelling ureteral stents caused vesicoureteral reflux and significant luminal dilatation. In a porcine model, Julia et al. graded the severity of vesicoureteral reflux according to the ureteral stent and found that indwelling stents tended to cause low-grade vesicoureteral reflux, mainly affecting the distal ureter43–44.
One study revealed that the prevalence of vesicoureteral reflux was approximately 33% at one week post-stenting and 83% at six weeks. These data indicate that the timing of stent placement has a substantial impact on the incidence of vesicoureteral reflux, with an average 40-fold increase observed at six weeks45–46 Furthermore, the removal of the stent has been demonstrated to reduce the risk of vesicoureteral reflux by 93%, thereby confirming that stent placement is the primary cause of this phenomenon. Another study also identified the time of stent placement as a risk factor for the development of vesicoureteral reflux. The results of this study demonstrated that the incidence of vesicoureteral reflux increased from 27% on the day of placement to 62–76% after 9 weeks of stent placement47–48 Furthermore, the 12-week follow-up demonstrated that vesicoureteral reflux remained present in 26% of the ureters six weeks following stent removal. This is likely attributable to stent-induced smooth muscle dilatation and ureteral lumen dilatation that does not dissipate immediately following stent removal. Furthermore, the persistence of vesicoureteral reflux has been documented in experimental settings up to three weeks following stent removal.
One study identified the characteristics of reflux after indwelling ureteral stents. Firstly, it was observed that vesicoureteral reflux did not occur at low bladder volumes. Secondly, reflux occurred progressively from a bladder volume of 120 ml, and thirdly, reflux usually occurred at a mean bladder volume of 240 ml. Secondly, the prevalence of vesicoureteral reflux was markedly elevated in patients who had been treated with long-term double J stent tubes. Thirdly, vesicoureteral reflux is more prevalent in older patients, potentially due to an elevated collagen to elastin ratio, which can result in diminished bladder capacity and compliance, consequently increasing the likelihood of reflux49–51.
In conclusion, ureteral stents traverse the ureterobladder junction en route from the ureter to the bladder, thereby compromising their anti-reflux functionality. This, in conjunction with a reduction in ureteral smooth muscle tone and diminished ureteral peristalsis, both of which are linked to the presence of the stent, results in vesicoureteral reflux. The urine within the bladder has a lower pH and similar bacterial levels to those of the external environment. In contrast, the urine within the renal pelvis has a higher pH. Consequently, in the event of vesicoureteral reflux during micturation, there is an increased risk of upper urinary tract-related complications, such as pyelonephritis or the formation of stones. This may result in parenchymal injury and a decline in renal function. Although vesicoureteral reflux associated with ureteral stents does not necessitate immediate treatment or surgery for symptomatic relief, recurrent vesicoureteral reflux may have a detrimental impact on patients who require long-term placement of ureteral stents. Therefore, the prevention of vesicoureteral reflux is of paramount importance for the quality of life of patients.
Another potentially important cause is the effects of radiotherapy, chemotherapy and systemic disease in patients with malignant obstruction. Firstly, pelvic radiotherapy has been shown to cause dose-related tissue damage, including collagen deposition and endarteritis52–53This ultimately results in ureteral stenosis. In a retrospective analysis of a database of Canadian adults with cervical cancer from 1994 to 2014, Welk et al. observed an incidence of ureteral stenosis after radiotherapy of 16%, which was higher than that in the group that had undergone surgery combined with radiotherapy (11%) and surgery alone (5%)54. Gillette et al. demonstrated a significant correlation between the incidence of ureteral stenosis and radiation exposure in animal studies. The extent of ureteral damage was found to increase in severity with rising radiation doses55. Van Kampen et al. demonstrated that the incidence of ureteral stenosis following ureteral radiotherapy in dogs exhibited a positive correlation with the area receiving radiotherapy. The incidence of ureteral strictures in patients with cervical cancer was found to be proportional to the total amount and single dose of radiotherapy administered following radiotherapy56–58. In the present study, pelvic radiotherapy was not found to be statistically associated with a statistically significant rate of change in mean PW. This may be due to the small study population and the lack of investigation of specific information about radiotherapy, such as specific information about the dose and location. Further research is required in this area. However, previous retrospective studies and animal studies have confirmed that radiotherapy to the pelvis can indeed cause damage to the ureter, which in turn can cause obstructive nephropathy.
It is important to note that certain drugs used in chemotherapy have the potential to cause renal damage while fighting cancer. For instance, cisplatin, methotrexate and mitomycin have been observed to induce renal dysfunction through direct toxic effects, while their respective metabolites have been demonstrated to exert direct toxicity on renal cells, resulting in the destruction of their normal structure and function. This, in turn, has been shown to affect tubular reabsorption and excretory function. Secondly, haemodynamic changes will also affect the vascular system of the kidney, resulting in a reduction of renal blood flow, which in turn causes renal ischemia and hypoxia, impairing the normal function of the kidneys. Thirdly, metabolic abnormalities can also affect the body’s metabolic balance, leading to the deposition of uric acid, calcium, phosphorus and other metabolites in the kidneys, forming crystals or stones. This can result in the obstruction of renal tubules, affecting the normal excretion of urine and triggering kidney damage.
It is evident that the compression of the tumour itself exerts an influence on renal function. The growth of the tumour in proximity to the kidney or ureter may result in the compression of the ureter, consequently leading to urinary tract obstruction. This, in turn, increases the pressure in the renal pelvis, hinders urine discharge, and ultimately results in hydronephrosis. This, in turn, can damage renal function in the long term. Furthermore, certain cancers may trigger paraneoplastic syndrome, which in turn can lead to an abnormal immune response in the body, resulting in the production of autoantibodies or cytokines. These, in turn, have the potential to damage renal blood vessels or glomeruli, thereby triggering renal lesions, such as membranous nephropathy and microscopic lesion nephropathy, amongst others.The metabolism in the body of cancer patients is usually in a disturbed state, and hyperuricaemia and hypercalcaemia may occur. The deposition of uric acid crystals or calcium salts within the renal tubules can result in their obstruction, thereby compromising the kidneys’ functionality and potentially culminating in renal failure.
Limitations
Firstly, as our study was conducted in a single centre, it is necessary to generalise these results with caution. However, our findings are in general agreement with those of KIM and LANNES et al. Therefore, prospective and randomised controlled experimental studies are needed to confirm these results. Thirdly, the relatively limited number of participants in the study is mainly due to the fact that cases of continuous indwelling ureteral stenting for more than two years are very rare in clinical practice. This is due to the fact that a significant number of patients may unfortunately pass away from the underlying disease before reaching the required time cut-off point. Fourth, the composition of the study population may not be representative of all patients with ureteral stents. This is because the majority of cases of ureteral obstruction in the clinical species are caused by malignant disease. Therefore, it is advisable to exercise caution when applying the conclusions drawn from this study to patients with ureteral obstruction due to benign etiology. Fifth, errors may occur in the measurement of renal parenchyma because patients may undergo a series of inflammatory changes in the kidneys during stenting, leading to hydronephrosis and oedema, which increase the width of the renal parenchyma. Sixth, we did not collect ultrasound data related to ureteral peristalsis in patients, which prevented us from delving into the mechanism. In addition, patients with indwelling ureteral stents due to pelvic tumours were not excluded from the study, which may introduce a degree of bias given that external obstruction was still present at the time of stent implantation.
Conclusion
In cases of ureteral obstruction, the size of the kidney diminishes over time despite the placement of a ureteral stent. This phenomenon is particularly evident in cases where the stent has been in situ for a period exceeding five years. The underlying pathophysiology is thought to be multifactorial, involving chronic ureteric obstruction, impaired ureteric peristalsis due to vesicoureteral reflux, irritation from the stent itself, and recurrent urinary tract infections.
Author contributions
TW and YD conceived and designed the study; ZY and QH collected and summarised the data; QH and ZY analysed and interpreted the data. All authors were involved in drafting the manuscript or reviewing important intellectual content, and all authors have read and ultimately approved the version to be published. Each author participated fully in the work and assumes public responsibility for the content of appropriate sections; each author agrees to take responsibility for all aspects of the work to ensure that issues related to the accuracy or completeness of any part of the work are properly investigated and resolved.
Funding
This work was supported in part by grants from Fujian Provincial Department of Science and Technology ( #2022J011353 and 2020CXB046 ).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical statement
The protocol of this study was approved by the Institutional Review Board of the First Hospital of Xiamen University.Informed consent was obtained from the patients for the case data and information used in this manuscript.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qianhao Huang, Zhiyong Zhang and Yifan Huang contributed equally to this work.
Contributor Information
Tao Wang, Email: taowang@xmu.edu.cn.
Yuedong Chen, Email: chenyuedong8@126.com.
References
- 1.Geavlete, P. et al. Ureteral stent complications - experience on 50,000 procedures. J. Med. Life. 14 (6), 769–775 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomer, N., Garden, E., Small, A. & Palese, M. Ureteral stent encrustation: epidemiology, pathophysiology, management and current technology. J. Urol.205 (1), 68–77 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Liatsikos, E. N. et al. Ureteral metal stents: 10-year experience with malignant ureteral obstruction treatment. J. Urol.182 (6), 2613–2617 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Haifler, M. et al. Tandem ureteral stents for malignant ureteral obstruction. J. Endourol. 34 (2), 222–226 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Kang, Q., Jiang, F., Yu, Y. & Yang, B. Application of metallic ureteral stents in gynecological malignancies: a literature review. Minim. Invasive Ther. Allied Technol.29 (1), 1–9 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Liu, L. et al. Can preoperative ureteral stents reduce the incidence of ureteral stricture after radiotherapy in patients with cervical cancer? BMC Urol.22 (1), 106 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yossepowitch, O. et al. Predicting the success of retrograde stenting for managing ureteral obstruction. J. Urol.166 (5), 1746–1749 (2001). [PubMed] [Google Scholar]
- 8.Wenzler, D. L. et al. Success of ureteral stents for intrinsic ureteral obstruction. J. Endourol. 22 (2), 295–299 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Rosevear, H. M. et al. Retrograde ureteral stents for extrinsic ureteral obstruction: nine years’ experience at university of Michigan. Urology70 (5), 846–850 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Piras, D. et al. Kidney size in relation to ageing, gender, renal function, birthweight and chronic kidney disease risk factors in a general population. Nephrol. Dial Transpl.35 (4), 640–647 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan, H. et al. Reduced kidney size and renal function of high-grade vesicoureteral reflux and intrarenal reflux in contrast-enhanced voiding urosonography. Front. Pediatr.12, 1478436 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar, A. et al. Ureteral obstruction in cancer patients: a qualitative study. Psychooncology25 (5), 605–609 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Peng, Y. L. et al. Ureteral stents cannot decrease the incidence of ureteroileal anastomotic stricture and leakage: A systematic review and meta-analysis. Int. J. Surg.93, 106058 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Roux, S. et al. Management of long ureteral stenosis: alternatives to indwelling ureteral stents. Prog Urol.31 (10), 598–604 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Liaw, A. & Knudsen, B. Urinary tract infections associated with ureteral stents: A review. Arch. Esp. Urol.69 (8), 479–484 (2016). [PubMed] [Google Scholar]
- 16.Scotland, K. B., Lo, J., Grgic, T. & Lange, D. Ureteral stent-associated infection and sepsis: pathogenesis and prevention: a review. Biofouling35 (1), 117–127 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Farsi, H. M., Mosli, H. A., Al-Zemaity, M. F., Bahnassy, A. A. & Alvarez, M. Bacteriuria and colonization of double-pigtail ureteral stents: long-term experience with 237 patients. J. Endourol. 9 (6), 469–472 (1995). [DOI] [PubMed] [Google Scholar]
- 18.Paick, S. H., Park, H. K., Oh, S. J. & Kim, H. H. Characteristics of bacterial colonization and urinary tract infection after indwelling of double-J ureteral stent. Urology62 (2), 214–217 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Kehinde, E. O. et al. Bacteriology of urinary tract infection associated with indwelling J ureteral stents. J. Endourol. 18 (9), 891–896 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Lifshitz, D. A. et al. Predictive value of urinary cultures in assessment of microbial colonization of ureteral stents. J. Endourol. 13 (10), 735–738 (1999). [DOI] [PubMed] [Google Scholar]
- 21.Nevo, A., Golomb, D., Lifshitz, D. & Yahav, D. Predicting the risk of sepsis and causative organisms following urinary stones removal using urinary versus stone and stent cultures. Eur. J. Clin. Microbiol. Infect. Dis.38 (7), 1313–1318 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Gorman, S. P., Jones, D. S., Bonner, M. C., Akay, M. & Keane, P. F. Mechanical performance of polyurethane ureteral stents in vitro and ex vivo. Biomaterials18 (20), 1379–1383 (1997). [DOI] [PubMed] [Google Scholar]
- 23.Akay, A. F., Aflay, U., Gedik, A., Sahin, H. & Bircan, M. K. Risk factors for lower urinary tract infection and bacterial stent colonization in patients with a double J ureteral stent. Int. Urol. Nephrol.39 (1), 95–98 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Rahman, M. A., Alam, M. M., Shahjamal, S., Islam, M. R. & Haque, M. E. Predictive value of urine cultures in evaluation of bacterial colonization of ureteral stents. Mymensingh Med. J.21 (2), 300–305 (2012). [PubMed] [Google Scholar]
- 25.Shabeena, K. S., Bhargava, R., Manzoor, M. A. P. & Mujeeburahiman, M. Characteristics of bacterial colonization after indwelling double-J ureteral stents for different time duration. Urol. Ann.10 (1), 71–75 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabih, A. & Leslie, S. W. Complicated urinary tract infections. In: StatPearls. edn. Treasure Island (FL): StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC.; (2024).
- 27.Liatsikos, E. N., Kagadis, G. C., Barbalias, G. A. & Siablis, D. Ureteral metal stents: a Tale or a tool? J. Endourol. 19 (8), 934–939 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Johnson, L. J., Davenport, D. & Venkatesh, R. Effects of Alpha-Blockade on ureteral peristalsis and intrapelvic pressure in an in vivo stented Porcine model. J. Endourol. 30 (4), 417–421 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Scotland, K. B. et al. Indwelling stents cause obstruction and induce ureteral injury and fibrosis in a Porcine model. BJU Int.131 (3), 367–375 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Janssen, C. et al. A role for the Hedgehog effector Gli1 in mediating Stent-induced ureteral smooth muscle dysfunction and aperistalsis. Urology104, 242e241–242e248 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Vogt, B. & Chokri, I. Characterization of Sonic Hedgehog/Gli1 signal expression in human ureter either Un-Stented or fitted with Double-Pigtail stent or a thread. Res. Rep. Urol.13, 529–533 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ricci, C. et al. [Combined radiologic-urologic procedure for the placement of ureteral stent in a case of bilateral iatrogenic ureteral lesion]. Urologia79 (1), 36–43 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Scotland, K. B., Bidnur, S., Wang, L., Chew, B. H. & Lange, D. Mediators of human ureteral smooth muscle contraction-a role for erythropoietin, Tamsulosin and Gli effectors. Transl Androl. Urol.10 (7), 2953–2961 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.34.Hurtado, R., Bub, G. & Herzlinger, D. The pelvis-kidney junction contains HCN3, a hyperpolarization-activated cation channel that triggers ureter peristalsis. Kidney Int.77 (6), 500–508 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cain, J. E., Islam, E., Haxho, F., Blake, J. & Rosenblum, N. D. GLI3 repressor controls functional development of the mouse ureter. J. Clin. Invest.121 (3), 1199–1206 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He, F. et al. The role of HCN channels in peristaltic dysfunction in human ureteral tuberculosis. Int. Urol. Nephrol.50 (4), 639–645 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iskander, S. M., Feeney, M. M., Yee, K. & Rosenblum, N. D. Protein kinase 2β is expressed in neural Crest-Derived urinary pacemaker cells and required for pyeloureteric contraction. J. Am. Soc. Nephrol.29 (4), 1198–1209 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosli, H. A., Farsi, H. M., al-Zimaity, M. F., Saleh, T. R. & al-Zamzami, M. M. Vesicoureteral reflux in patients with double Pigtail stents. J. Urol.146 (4), 966–969 (1991). [DOI] [PubMed] [Google Scholar]
- 39.Hübner, W. A., Plas, E. G., Trigo-Rocha, F. & Tanagho, E. A. Drainage and reflux characteristics of antireflux ureteral double-J stents. J. Endourol. 7 (6), 497–499 (1993). [DOI] [PubMed] [Google Scholar]
- 40.Austin, J. C. & Cooper, C. S. Vesicoureteral reflux: surgical approaches. Urol. Clin. North. Am.31 (3), 543–557 (2004). x. [DOI] [PubMed] [Google Scholar]
- 41.Yossepowitch, O. et al. Assessment of vesicoureteral reflux in patients with self-retaining ureteral stents: implications for upper urinary tract instillation. J. Urol.173 (3), 890–893 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Elmore, J. M., Kirsch, A. J., Perez-Brayfield, M. R., Scherz, H. C. & Koyle, M. A. Salvage extravesical ureteral reimplantation after failed endoscopic surgery for vesicoureteral reflux. J. Urol.176 (3), 1158–1160 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Çilesiz, N. C. et al. Four-Point injection technique of polyacrylate/polyalcohol copolymer in vesicoureteral reflux after kidneytransplant. Exp. Clin. Transpl.21 (5), 434–440 (2023). [DOI] [PubMed] [Google Scholar]
- 44.Lu, Y., Tay, J. Y. J., Lim, K. S. & Ng, L. G. Novel anti-reflux ureteral skirt: proof of concept in a Yorkshire-Landrace pig model. World J. Urol.42 (1), 437 (2024). [DOI] [PubMed] [Google Scholar]
- 45.Uvin, P., Van Baelen, A., Verhaegen, J. & Bogaert, G. Ureteral stents do not cause bacterial infections in children after ureteral reimplantation. Urology78 (1), 154–158 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Chung, J. M., Park, C. S. & Lee, S. D. Postoperative ureteral obstruction after endoscopic treatment for vesicoureteral reflux. Korean J. Urol.56 (7), 533–539 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chew, B. H. & Lange, D. Advances in ureteral stent development. Curr. Opin. Urol.26 (3), 277–282 (2016). [DOI] [PubMed] [Google Scholar]
- 48.de la Cruz, J. E. et al. Assessment of the grades of vesicoureteral reflux in stented ureters: an experimental study. Urol. Int.105 (7–8), 554–559 (2021). [DOI] [PubMed] [Google Scholar]
- 49.Kim, K. W. et al. CFD study on vesicoureteral reflux in the urinary tract with double J stent. Comput. Biol. Med.145, 105456 (2022). [DOI] [PubMed] [Google Scholar]
- 50.Lee, J., Sung, J., Jo, J. K. & So, H. 3D-Printing-Assisted extraluminal Anti-Reflux diodes for preventing vesicoureteral reflux through Double-J stents. Int. J. Bioprint. 8 (2), 549 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adhoni, M. Z. U., Al Homsi, A., Ali, Z. & Almushatat, A. Antireflux ureteral stents prevent Stent-Related symptoms: A Meta-Analysis of randomized controlled trials. Cureus15 (11), e49375 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.52.Zeng, G. H., Li, X., Wu, K. J. & Chen, W. Z. [Endoscopic management of bilateral ureteral obstruction after radiotherapy]. Ai Zheng. 23 (1), 108–109 (2004). [PubMed] [Google Scholar]
- 53.Dagoglu, N., Mahadevan, A., Nedea, E., Poylin, V. & Nagle, D. Stereotactic body radiotherapy (SBRT) reirradiation for pelvic recurrence from colorectal cancer. J. Surg. Oncol.111 (4), 478–482 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Welk, B., Wallis, C., D’Souza, D., McGee, J. & Nam, R. K. A Population-Based assessment of urologic procedures and operations after surgery or pelvic radiation for cervical Cancer. Int. J. Gynecol. Cancer. 28 (5), 989–995 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Gillette, S. L., Gillette, E. L., Powers, B. E., Park, R. D. & Withrow, S. J. Ureteral injury following experimental intraoperative radiation. Int. J. Radiat. Oncol. Biol. Phys.17 (4), 791–798 (1989). [DOI] [PubMed] [Google Scholar]
- 56.Heo, J. E., Jeon, D. Y., Lee, J., Han, H. H. & Jang, W. S. Prediction of stent failure for malignant ureteral obstruction in Non-Urological Cancer. Yonsei Med. J.64 (11), 665–669 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prontera, P. P. et al. Early diagnosis and management of arterio-ureteral fistulas: A literature review. Arch. Ital. Urol. Androl.95 (1), 10928 (2023). [DOI] [PubMed] [Google Scholar]
- 58.Versteegden, L. R. et al. Tubular collagen scaffolds with radial elasticity for Hollow organ regeneration. Acta Biomater.52, 1–8 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.