Abstract
The European Union (EU) actively promotes the adoption of organic farming, in which crop N requirements are satisfied via organic fertilizers, such as slurry. Maize (Zea mays L.) is a key crop for both feed and food production with high N uptake. In this short-term study, we tested fertigation with microfiltered slurry liquid faction for maize fertilization as viable strategy to enhance nitrogen use efficiency (NUE) under organic farming while reducing N losses, via ammonia (NH3), nitrous oxide (N2O), and nitrate leaching (NO3-). We compared three strategies (i) slurry application through surface broadcast of the liquid fraction before sowing as reference fertilization (“Ante” treatment, or “A”), (ii) slurry application through both pre-sowing broadcast of the liquid fraction and fertigation as side-dressing with the microfiltered liquid fraction (“Ante + Post” treatment, or “A + P”), and (iii) slurry microfiltered liquid fraction application as side dressing via fertigation (“Post” treatment, or “P”). Compared to “A”, cumulative N losses were reduced by 38% under “A + P” and 58% under “P”. Furthermore, NH3 volatilization decreased by 43% and 71% under “A + P” and “P”, respectively. These treatments also reduced N2O emissions by 30% and 37%. Nitrate leaching was reduced by 56% in the “P” treatment. Overall, the “P” strategy was the most effective in reducing N losses, while “A + P” tended to increase grain production (12.6 Mg ha-1) and NUE (38.1 kg grain kg-1 N supply) compared to “P” (11.0 Mg ha-1 and 35.5 kg grain kg-1 N supply). These results were primarily attributed to the improved synchronization between N supply and maize N requirements, emphasizing the risk associated with slurry application before sowing. Although conducted over a short experimental period, our study suggests that drip fertigation with slurries can overcome the potential yield losses of organic systems for crops with high N demand such as maize, while reducing N losses, fulfilling the environmental principles of organic farming and current requirements from EU policies.
Keywords: Drip fertigation, Cattle slurry, Organic maize, Nitrogen Use Efficiency
Subject terms: Agroecology, Environmental sciences
Introduction
The “Farm to Fork” strategy of the European Union (EU) aims to expand the extent of organic farming in the upcoming years, with a target of at least 25% of European agricultural land being dedicated to organic farming by 20301. However, it is crucial to align EU policies with the United Nations Sustainable Development Goals (SDGs), which include the need to safeguard food security by increasing agricultural production (SDG 2)2. Since crop yields are generally lower in organic compared to conventional farms due to soil nutrient deficiencies and problems with pests, weeds, and diseases3–6, it is paramount to identify techniques that enhance crop yields in organic systems to ensure a successful transition to more sustainable food systems.
Maize is one of the most important crops worldwide, and almost all countries within the EU grow maize for feed and food production, with 73 million tonnes produced in 2021, 6 million tonnes more than in 20207. However, the share of organic maize production is still confined across maize producing regions in the EU8,9, among other reasons, due to the high nutrient requirements of this crop10. Indeed, limited availability of nutrients, particularly nitrogen (N), has been identified as the main factor contributing to the lower yields of high N-demanding crops such as maize under organic farming4,6. Within the portfolio of organic fertilizers, according to the EU Reg. 848/2018, livestock slurries and manures are valuable N sources. Nevertheless, their adoption is limited to that from non-industrial farms and to digestates, limiting their use. The adoption of a solid–liquid separation process of raw slurries or downstream anaerobic digestion could maximize the options for their utilization under organic farming, sustaining the production of high N-demanding crops.
However, the application of manure and slurries to agricultural soils fuels the so-called “N cascade”, triggering the release of N losses in the form of ammonia (NH3) volatilization, nitrous oxide (N2O) emissions, and nitrate (NO3-) leaching11. Around 60% and 32% of the global anthropogenic NH3 and N2O emissions, respectively, are linked to livestock production systems12. Reducing these N losses via improved manure application is a prerequisite to fulfil the environmental principles of organic farming13. Ammonia volatilization causes ecosystem eutrophication and acidification, as well as human health issues as a source of PM2.514. While there are well-known strategies to reduce NH3 losses from manure distribution, such as surface application followed by immediate incorporation into the soil, direct injection, and trail hose distribution15,16; to our knowledge, the potential of drip fertigation with cattle slurry in reducing NH3 losses has been investigated only by Bacenetti et al.17. These authors found remarkable reductions in NH3 volatilisation with both pivot (-70%, on average) and, even more, drip fertigation (-83%, on average). Although broadcast distribution of slurries (with incorporation) remains the reference application method for most farming systems, the use of slurries for drip fertigation is now possible due to the recent development of appropriate microfiltration techniques18,19. A better understanding of the impact of this management option on NH3 volatilization is crucial to assess the overall efficiency of drip fertigation with microfiltered slurry.
Nitrous oxide emissions from N fertilization contribute to global warming and ozone depletion20. Drip irrigation allows to split N-fertilization in a few applications, which enhances yields by improving NUE, while eventually decreasing N2O emissions due to partial soil wetting, lowering soil moisture, and due to better temporal/spatial fertilizer distribution20–22; however, whether N2O reduction through drip fertigation can be achieved also with organic fertilizers, remains poorly investigated. Lombardi et al.23 adopted dairy farm slurry with subsurface drip fertigation (SDI) in comparison to chemical fertigation with dissolved urea. The authors found that fertigation with slurry increased N2O emissions, while no differences in maize yields were detected. On the other hand, Bacenetti et al.17 reported substantial reductions when adopting pivot (-22%, on average) or drip (-20%, on average) fertigation. Further research should therefore test the potential of drip fertigation with slurries to sustain maize yields in organic farming, avoiding side effects of higher GHG losses (including N2O) and other Nr loss pathways. Moreover, while Lombardi et al.23 adopted subsurface drip irrigation, this system is very uncommon in Italy. Drip irrigation plants with temporary driplines that are surface positioned and removed when needed are more common, and this could be useful for organic farming systems that often rely on ploughing to abate weeds.
Losses of reactive N through hydrological pathways primarily occur through NO3- leaching. The rate and frequency of N applications have been identified as major factors driving these losses24, particularly when cattle or pig slurries are used25,26. To mitigate NO3- leaching, strategies such as splitting N fertilization and reducing water supply have shown effectiveness with synthetic fertilizers27. Drip fertigation, which integrates these approaches, seems then a valuable tool to mitigate NO3- leaching, as suggested by several studies, mainly with mineral fertilizers28–30. However, while promising, the empirical evidence with animal slurries is scarce17,18,31.
To gain a more comprehensive perspective on the whole N loss chain after slurries application to land, the present study quantified the three main reactive N loss pathways (i.e., NH3, N2O and NO3-). The aim was to explore manure application strategies via fertigation that improve the efficiency of N use and reduce N losses in organic maize production. This was tested in a field experiment using split fertigation with microfiltered slurry applied from maize seed-dressing (P), a combination of fertigation from maize seed-dressing with pre-sowing application of liquid-separated slurry (A + P), and a single application at seeding as the reference N-fertilization strategy under organic farming (A). We tested the following hypotheses: (i) split N application through either pre-sowing slurry broadcast and side-dressing fertigation (A + P) or side-dressing fertigation only (P) can improve maize yields and N use efficiency compared to the full application before sowing (A); (ii) combining pre-sowing slurry broadcast and side-dressing fertigation (A + P) is a win–win strategy to abate NH3 volatilization (compared to full application before sowing; A), while keeping N2O emissions under control (as with side-dressing fertigation only; P); (iii) fertigation can abate NO3- leaching losses, through frequent and limited N supply.
Materials and methods
Site and soil characteristics
The field experiment was conducted in a commercial organic farm located in Piadena (45° 07′ 33.3″ N 10° 24′ 23.2″ E), Cremona Province, Po Valley (Lombardy, northern Italy). The farm had been under organic management for five years. The crop sequence on farm is a three-year crop rotation, with maize, sunflower and forage pea. Maize is irrigated with a surface drip irrigation system (drip lines), while sunflower and forage pea are rainfed. The local climate is typically humid subtropical according to the Köppen classification32, with a long-term average rainfall of 860 mm and mean annual air temperature of 14 °C. Meteorological data were recorded by a weather station near the field experiment (Meteosense Agrometeo™, Netsens LTD, Calenzano, Italy).
The soil is deep to moderately deep and is classified as fine, silty, mixed, mesic Oxyaquic Hapludalf33, with a silty-loam texture (sand 260 g kg-1, silt 520 g kg-1 and clay 220 g kg-1). Drainage is poor to locally good. Before establishing the trial, soil was sampled down to 0.3 m and analysed for the main physical and chemical properties: pH (in CaCl2) 7.48; bulk density of 1.29 g cm-3; electric conductivity of 167 µS cm-1; 7% of total carbonates; organic carbon (Walkley–Black) 19.7 g kg-1; total nitrogen (Kjeldahl) 1.59 g kg-1; available P (Olsen) 37 mg kg-1; exchangeable K (extraction in BaCl2) 353 mg kg-1 and cation exchange capacity (extraction in BaCl2) 14.7 cmol(+) kg-1.
Agronomic management and experiment description
The experiment encompassed three application strategies for organic maize fertilization: (i) the conventional approach of applying the slurry liquid fraction before maize sowing (“Ante” treatment, “A”); (ii) a hybrid method where 50% of the slurry liquid fraction was applied before sowing, and the remaining 50% was delivered through fertigation by injecting microfiltered liquid fraction into water and distributing it via drip lines (“Ante + Post” treatment, “A + P”); (iii) 100% applied with fertigation (“Post” treatment, “P”). In addition, a control treatment without any N input was included to calculate the contribution of organic matter mineralisation to N supply and to derive N2O emission factors. Therefore, the experimental field was divided into four areas of 0.5 ha each. The number of pseudo-replicates—within each of the four sectors—was four (Fig. 1).
Fig. 1.
Schematic representation of the experimental field depicting the placement of sampling devices for N2O emissions, NO3--leaching and NH3 volatilization. Roman letters stand for pseudo-replicates within each treatment (i.e., “Ante” [A], “Ante + Post” [A + P], “Post” [P], and control).
Pre-sowing fertilization occurred on March 29, 2022, while liquid fraction of dairy slurry was surface applied with a splash plate as base dressing before seedbed preparation to “A” (88 m3 ha-1, 265 kg N ha-1) and “A + P” (49 m3 ha-1, 147 kg N ha-1). After 24 h, slurry was buried at 0.3 m depth by ploughing, followed by rotary harrowing. For the sake of homogeneity, the same tillage operations were carried out in “P” and “control”, although no slurry liquid fraction was applied at this time for these treatments. Secondary rotary harrowing occurred on April 18, just before maize (Planta hybrid SNH 9503, FAO 500) seeding at a rate of 7 plants m-2 with rows spaced 0.7 m apart. Harvest for grain took place on August 14, 119 days after planting.
Slurry analysis, fertigation and irrigation events
Microfiltered slurry liquid fraction was obtained from a nearby organic dairy farm. Raw liquid slurry was first screw-pressed to eliminate most of the coarse solids (i.e., larger than 1.2 mm); downstream this separation, a microfilter (SEPCOM Micro-filter MFT, Saveco™ part of WAMGROUP, Ponte Motta/Cavezzo, Modena, Italy) with a 50 µm mesh was used to eliminate fine suspended solids to avoid clogging of emitters in the irrigation drip lines. Microfiltration was carried out in conjunction with the fertigation events.
The cumulative water supplied to maize from seeding to harvest was 2531 m3 ha-1. Specifically, out of six water application events, only two (the first and the last) were exclusively water supplies, while the other four were fertigations (at 1:10 slurry:water ratio). The treatment “P” had all four fertigation events, whereas “A + P” plots were only fertigated in the first and third events. In Table 1 are reported dry matter content, total and ammoniacal N concentrations for slurry liquid fraction after surface broadcast and microfiltered and diluted slurry liquid fraction at the time of the four fertigation events.
Table 1.
Chemical analysis and rate of N applications for the slurries supplied at pre-sowing (Broadcasting) and with fertigation events.
| Treatment | Dry matter content (%) | Total N on a fresh matter basis (g kg-1) | TAN (% Total-N) | Application rate (m3 ha-1) | Total-N applied (kg ha-1) | Applied TAN (kg ha-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | A + P | P | A | A + P | P | A | A + P | P | ||||
| Broadcasta | 3.85 | 3.01 | 54.57 | 88 | 49 | 0 | 265 | 147 | 0 | 145 | 80 | 0 |
| Fertigation Ib | 0.25 | 0.16 | 76.25 | 0 | 160 | 160 | 0 | 26 | 26 | 0 | 20 | 20 |
| Fertigation IIb | 1.03 | 0.68 | 59.32 | 0 | 0 | 94 | 0 | 0 | 64 | 0 | 0 | 38 |
| Fertigation IIIb | 0.97 | 0.66 | 44.82 | 0 | 148 | 148 | 0 | 97 | 97 | 0 | 44 | 44 |
| Fertigation IVb | 1.23 | 0.93 | 51.72 | 0 | 0 | 96 | 0 | 0 | 89 | 0 | 0 | 46 |
| Sum | 265 | 270 | 276 | 145 | 144 | 148 | ||||||
aData refers to liquid-separated and undiluted slurry.
bData refers to microfiltered and diluted (1:10) slurry liquid fraction.
| 1 |
Irrigation requirements (mm) were calculated as:with ET0 as the reference evapotranspiration in the Penman–Monteith Eq. (34), as provided by the field meteo-station; Kc as the tabulated crop coefficient for maize under irrigated conditions34; and effective precipitation as 80% of cumulative precipitation28.
Drip lines where spaced 1.4 m apart, one between two maize rows with emitters every 0.5 m and a nominal flow of 1 L h-1. Each drip line was opened or closed by means of a manual tap to select the fertigation treatments at the time of microfiltered slurry injection, and to apply the same amount of water used during fertigation to the unfertilized areas. Before surface broadcast fertilization and before each N fertigation event, multiple aliquots of liquid-separated and microfiltered slurry were sampled to evaluate the total Kjeldahl nitrogen (TKN), total ammonium nitrogen (TAN) and dry matter content (DM) (Table 1). At the time of fertigation, a slurry tanker was filled with microfiltered slurry, then transported to the experimental field and connected with a venturi pipe to an engine, pumping water from an irrigation channel. The microfiltered and diluted (1:10 ratio with water) slurry liquid fraction was then passed through a self-cleaning screen with 100-micron filters before being injected into the drip lines (Fig. 1). The correct dilution was assessed with a litre counter placed on the venturi system. However, a certain degree of seasonal variability was observed regarding the composition of the microfiltered and diluted fraction. The one used for the first fertigation had a lower DM and N concentration, probably because it derived from liquid manure accumulated during winter and spring, when rainfall is higher, and open lagoons collect more water. In contrast, higher DM percentages and N concentrations are observed in fertigation cycles 2, 3, and 4, as they originate from liquid manure accumulated during late spring and summer, when lagoons collect less precipitation.
Fertigation events took place around maize tasseling-flowering, on June 21, July 1, July 12, and July 21, 2022.
Maize yield and nitrogen use efficiency
Maize grain yield, biomass production and N uptake were determined by harvesting three rows from an area of 5 m2 for each replicate. Plants and ears were counted; ears were separated from plants and shelled to obtain grain yield and dry matter content after drying at 65 °C until constant weight. Similarly, plants were shredded, and subsamples were taken to determine dry matter. Once dry, these components were 2 mm grinded and analysed for total N content by the Kjeldahl method.
The harvest index was then obtained from the ratio of dry grain yield over the whole dry biomass production (grain + other plant components).
Nitrogen Use Efficiency (NUE) was defined according to López-Bellido and López-Bellido35:
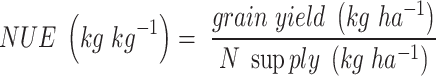 |
2 |
where N supply is the sum of N from fertilizers and from soil, accounting for nitrate and ammonium-N at the beginning of the experiment before maize planting, and for N from organic matter mineralisation, estimated using the control plots without fertilization36.
Soil moisture, temperature, NO3--N and NH4+-N content
Soil gravimetric water content (GWC), temperature, nitrate and ammonium N content were monitored concurrently with samplings for N2O emissions. Soil water content was gravimetrically measured for the 0–30 cm soil layer. Before maize sowing and until the drip lines were positioned, three random samples were taken from each pseudo-replicate to get an averaged value. When the drip lines were positioned until the end of the monitoring period, samplings were doubled for each sampling point: three were taken around the emitter (“wet” point), and three between maize rows without the irrigation line (“dry” point). The mean GWC for every pseudo-replicate was then derived from the average between “wet” and “dry” points. Water filled pore space (WFPS) was calculated as:
| 3 |
where total soil porosity (Φ) was calculated as follows:
| 4 |
with BD as the soil dry bulk density for the 0–30 cm soil layer, determined with the cylinder method, and 2.65, the soil particle density37.
Thirty cm-deep soil core samples were collected for mineral N content (NO3--N and NH4+-N) determination, following the same pattern for GWC: for each “wet” and “dry” point, three samples were made and then thoroughly homogenized. “Wet” and “dry” samples were kept separate and stored frozen at -20 °C until analysis. At that time, samples were defrosted, and 5 g were shaken with 20 mL of K2SO4 (0.05 M) for 2 h. After that, the filtered solution was analysed for both NO3- and NH4+-N. Nitrates were measured by dual wavelength spectroscopy method38, while NH4+-N was determined with the Berthelot colorimetric reaction39. A thermocouple sensor was then used to monitor soil temperature at a depth of 0–10 cm.
Nitrous oxide emissions
The N2O emissions were measured using the static chamber method21. Before irrigation started, a total of 13 measurements were made and an additional 14 were taken since irrigation and fertigation started, for a total of 27 measurements from March 17 to August 12, 2022. For each pseudo-replicate, at the beginning of the monitoring period, two steel collar frames (0.45 m diameter and 0.20 m hight) were inserted 5 cm into the soil on two interrow spaces for each pseudo replicate: one for the “wet” sampling point (with the drip line), and the second for the “dry” sampling point, between maize rows without drip line. To avoid preferential water flows during irrigation and fertigation events, chamber bases were carefully removed from “wet” points and put back into position once irrigation terminated.
At sampling time, the steel rings were hermetically closed with a 0.031 m3 PVC chamber, equipped with a 12 V fan inside. Samplings occurred at 0, 15, and 30 min after chamber closure from a three ways port: 30 mL of the air from the headspace volume of the chamber were sucked by a plastic syringe from a sampling port and then injected into a 12.5 mL pre-evacuated glass vial (Labco Ltd., Lampeter, United Kingdom). To standardize the monitoring protocol, measurements were taken in the late morning hours, generally between 9:00 and 11:00 a.m. The samples were analysed for N2O concentration with a fully automated gas chromatograph (Agilent 7890A, USA) equipped with an electron capture detector (ECD), and a Gerstel Maestro MPS2 autosampler.
Direct N2O fluxes were calculated using the linear or nonlinear increase in concentration in the chamber headspace over time, selected according to the emission pattern40. Cumulative emissions were estimated by linear interpolation. The emission factor for N2O (EF) as % of the total N applied, was calculated as:
| 5 |
where N2O emissions and N applied are expressed as kg N ha-1. Yield-scaled N2O-N emissions were finally calculated as the ratio of cumulative N2O emissions to maize grain yield.
Ammonia volatilization
Ammonia volatilization was monitored with the semi-static chamber method41. Briefly, a PVC cylinder of 0.20 m diameter and 0.30 m height was equipped with two polyfoam discs inside (each 3 cm high): one in the middle and one at the top end. Each of them was soaked in a 3% (weight/volume) oxalic acid in acetone solution as trapping medium for NH3 volatilisation. The foam in the middle captured NH3 volatilised from soil surface, the outer one only prevented from atmospheric contamination, thus were discarded. New foams were deployed inside the chamber before each sampling occasion.
After surface slurry liquid fraction application on March 29, eight chambers were promptly deployed in the field to capture volatilization from “A” and “A + P” (four in each plot), The foams were changed 1.5, 3, 6, 9, 24 and 96 h after slurry liquid fraction broadcasting. At 24 h, slurry liquid fraction incorporation started with mouldboard ploughing, thus chambers were temporarily removed and then brought back after harrowing. Measurements occurred 1.5, 3, 6, 24, 48 and 96 h since fertigation started.
To measure the trapped NH3, foams were rinsed with 500 mL of deionized water; then an aliquot of this leachate was analysed with an ion-selective electrode for NH3 (HANNA, HI 4101). This concentration, starting from the foam sampling surface, was then reported to hectare and to hours of sampling to derive a flux42. Given the unreliability of this monitoring method to derive absolute values of NH3-N losses43, correction indices derived from a previous calibration of the same static chambers44 were adopted. Given that measurement of NH3 emissions using semi-static systems can lead to a large bias, we consider these data on NH3 emissions as “potential NH3 emissions” (hereafter referred to as NH3). Cumulative volatilisation was calculated as the sum of NH3 emitted during each time interval. Yield-scaled NH3-N emissions were derived from the ratio of cumulative NH3 losses to maize grain yield.
Soil water and nitrogen leaching
Soil N leaching was calculated by coupling N concentration monitoring in the soil solution with drainage estimates obtained with the Soil Water Atmosphere Plant model (SWAP, v. 4.0.145). Nitrogen concentration in the soil solution was measured by means of ceramic suction cups inserted at 0.45 m depth, with a 45° angle to reduce the risk of preferential water flows. For each pseudo-replicate, two suction cups were deployed, following the same “wet” – “dry” pattern for N2O measures. Measurements started on April 15 and terminated on August 12, for a total of nine samplings. Samplings were done by applying a -60 kPa vacuum to the cups with a portable pump 24 h before each rain, irrigation, or fertigation event. Water samples were stored at -20 °C until analysed for NO3--N content with the same method for soil samples.
Water drainage at 45 cm depth was simulated with the SWAP model, which had been previously used for the same purpose under similar pedo-climatic conditions26,46. Further details on model implementation are reported in Supplementary materials. Nitrate leaching was then calculated by the trapezoidal rule26 as follows:
| 6 |
where c1 and c2 are the NO3--N concentration (mg N L-1) in suction cups of two consecutive sampling events, and v (mm) is the cumulative water flux at 45 cm depth between these occasions. Yield-scaled NO3-N leaching losses were calculated as the ratio of cumulative NO-3 leaching to maize grain yield.
Statistical analyses
The effect of slurry application strategies on maize yield and N-use efficiency, N-loss pathways (N2O emissions, NH3 volatilization, and NO3- leaching), and yield-scaled N-losses was evaluated with linear mixed-effect models, where (pseudo)replicates within sectors were considered as a random factor. Linear mixed effect model was used to account for the lack of independence among the individual units of observation21,47,48. Requirements for normality and homoscedasticity were checked with Shapiro–Wilk and Levene’s tests. Significant differences between the means of various treatments were further separated through post hoc Tukey’s HSD tests (p-value < 0.05). Principal Component Analysis (PCA) was adopted to understand the main relationships between soil variables, N-loss pathways, maize yield parameters and treatments (for further details on analysed parameters with PCA, see Fig. 7).
Fig. 7.
Biplot of Principal Component Analysis for main variables (Nitrogen Use Efficiency [NUE]; Grain yield [Grain]; maize vegetative biomass production [Biomass]; mean soil NO3--N content at 0.3 m depth [soil_NO3]; mean soil NH4+-N content at 0.3 m depth [soil_NH4]; mean soil Water Filled Pore Space content at 0.3 m depth [WFPS]; cumulative N2O emissions [N2O]; cumulative NH3 volatilisation [NH3] and cumulative NO3--N leaching losses [NO3_leaching]) as arrows, and clustered treatments (“Ante” [A], blue; “Ante + Post” [A + P], orange and “Post” [P], grey).
R 4.0.5 software49 with “nlme”50, “multcomp”51 and “factoextra”52 packages, were used for the linear mixed effect models, Tukey’s HSD tests, and Principal Component Analysis, respectively.
Results
Weather and soil conditions
From March 29 to August 12, the average air temperature was 19.1 °C, with an average wind speed of 0.83 m s-1. The cumulative rainfall was 170 mm. Soil temperature at a depth of 10 cm closely followed the air temperature for most of the monitoring period (Fig. 2), except for a lag between May 14 and June 21 when the soil temperature averaged 3.9 °C higher, reaching a peak of 28.9 °C on May 28, which was the highest temperature observed throughout the monitoring period.
Fig. 2.
Weather conditions during the field experiment and slurry application events.
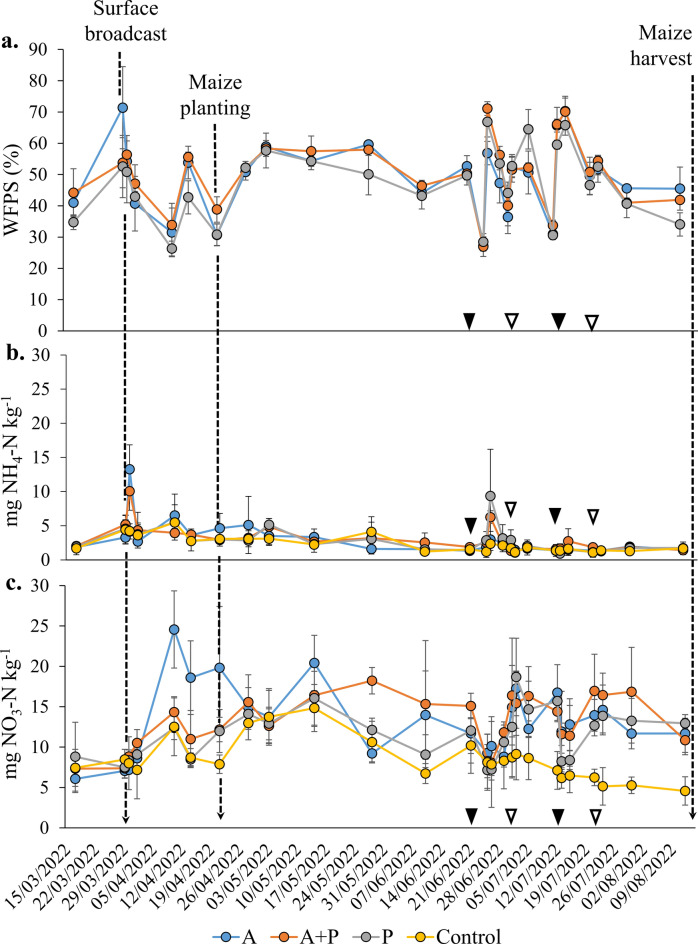
Soil water filled pore space (WFPS), averaged between the “wet” and “dry” positions, ranged from 26.3% to 71.4%, exceeding 60% only after irrigation and fertigation events, reaching the maximum on March 29 after slurry liquid fraction broadcast (Fig. 3a). Generally, WFPS quickly returned to the 30–40% range after these events, indicating fast soil drying. The longest period of relatively stable WFPS levels occurred from April 28 to June 21, with average values of 53.4%, 53.8%, and 48.3% for treatments “A”, “A + P”, and “P”, respectively, with no remarkable deviations from these averages. Extended periods with high soil moisture were rare.
Fig. 3.
Development of soil Water Filled Pore Space (a), ammoniacal (b) and nitric-N (c) content through the monitoring period at 0.3 m depth for the three treatments: Ante, “A”; Ante + Post, “A + P” and Post, “P”. Filled triangles represent fertigations on both “A + P” and “P”, while empty ones represent fertigations only for “P” treatment.
Maize yield and N use efficiency
Both grain (p-value = 0.049) and biomass yield (stalks, husks, and cobs; p-value = 0.018) were significantly higher in “A + P” than in “A” and “P”, respectively (Table 2). Conversely, grain yield in “P” and biomass in “A” showed intermediate values. The Harvest Index was 0.40 for both “A + P” and “P”, while numerically lower (0.38) for “A” (Table 2). No significant differences were detected between treatments in cumulative N uptake, while “A + P” was only numerically higher. Lastly, the “A + P” treatment showed higher NUE than “A”, suggesting a more effective conversion of absorbed N into grain, but only numerically higher than “P” (Table 2).
Table 2.
Linear mixed-effects models of yield components (grain and biomass), Harvest Index, total N uptake and Nitrogen Use Efficiency as affected by slurry application strategy.
| Thesis | Grain yield (Mg ha-1) | Biomass (Mg ha-1) | Harvest Index | N-uptake (kg N ha-1) | NUE (kg kg-1) |
|---|---|---|---|---|---|
| A | 10.50 ± 0.64 b | 17.24 ± 0.90 ab | 0.38 ± 0.02 | 236.67 ± 19.13 | 30.83 ± 1.99 b |
| A + P | 12.56 ± 0.72 a | 18.57 ± 0.53 a | 0.40 ± 0.01 | 288.54 ± 25.23 | 38.13 ± 2.03 a |
| P | 11.03 ± 0.74 ab | 16.47 ± 0.67 b | 0.40 ± 0.01 | 283.64 ± 13.64 | 35.50 ± 2.44 ab |
| p-value | 0.0496 | 0.0183 | 0.0757 | 0.0599 | 0.0345 |
Within columns, means followed by the same letter are not significantly different between treatments according to the Tukey’s HSD test (p = 0.05).
Soil NO3--N and NH4+-N content
On March 30, immediately after slurry liquid fraction distribution, NH4+-N levels in the 0–30 cm soil layer peaked at 13.28 and 10.07 mg kg-1 in the “A” and “A + P” treatments, respectively (Fig. 3b). Then, NH4+-N content rapidly decreased to background levels within two days (2.70, 4.35 and 3.62 mg kg-1 for “A”, “A + P” and control, respectively). In the “A” treatment, spikes of NH4+-N were observed until April 28, after which they gradually declined. The second major peak of NH4+-N occurred on June 26, following the first fertigation event, in both the “A + P” and “P” treatments. Following fertigation events did not cause noticeable increases in NH4+-N levels under any of the treatments. This was probably due to the higher proportion of ammoniacal N in microfiltered liquid slurry at the first fertigation event.
In contrast, NO3--N showed smoother but more prolonged peaks (Fig. 3c). Particularly in the “A” treatment, higher concentrations (24.56 mg kg-1) were observed 10 days after slurry liquid fraction broadcasting, following the decline in NH4+-N levels, and remained consistently higher than both the “A + P” and “P” treatments for at least the next 10 days. Other peaks in NO3--N occurred after precipitation events or irrigations. The day before fertigation began, NO3--N reached a minimum level, coinciding with low WFPS and the high N demand of maize at the silking stage. During the fertigation period, NO3--N generally followed the WFPS levels in all treatments, with declines observed after soil drying due to the high temperatures.
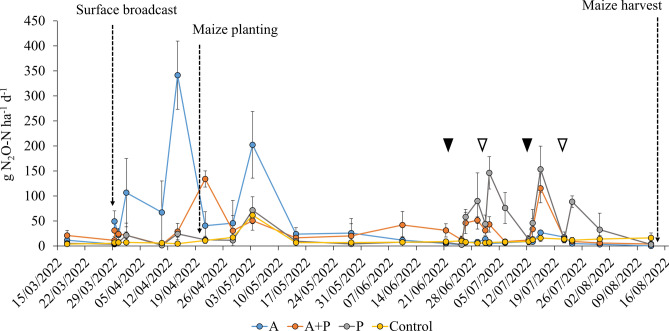
Nitrous oxide emissions
The most relevant peaks in N2O emissions were observed in the “A” treatment following surface slurry liquid fraction application and rain events, with the main occurrences on April 1, April 14, and May 3. Afterwards, the emissions declined to control levels throughout the experiment (Fig. 4). In the “A + P” treatment, a peak of 141 g N2O-N was reached on April 21, with a lag phase of nearly one month after surface slurry liquid fraction application. Unlike the “A” treatment, both the “A + P” and “P” treatments exhibited spikes in N2O emissions following fertigation events in the later part of the trial. Cumulative N2O-N emissions were significantly higher in the “A” treatment compared to the “A + P” and “P” treatments, with no significant differences between the latter two (Table 3).
Fig. 4.
Development of N2O-N emissions from soil surface, through the monitoring period for the three treatments: Ante, “A”; Ante + Post, “A + P” and Post, “P”; and control. Filled triangles represent fertigations on both “A + P” and “P”, while empty ones represent fertigations only for “P” treatment.
Table 3.
Cumulative N2O-N emissions, NH3-N volatilizations and NO3--N leaching through the monitoring campaign, N2O emission factor (EF), and total cumulative N losses. Within columns, means followed by the same letter are not significantly different between treatments according to the Tukey’s HSD test (p = 0.05).
| Thesis | Cumulative N2O-N losses (kg ha-1) |
Cumulative NH3-N losses (kg ha-1) |
Cumulative NO3-N losses (kg ha-1) |
N2O EF (%) |
Cumulative N losses (kg ha-1) |
|---|---|---|---|---|---|
| A | 6.45 ± 0.41 a | 14.04 ± 1.93 a | 39.67 ± 10.25 a | 1.76 ± 0.14 a | 60.16 ± 9.87 a |
| A + P | 4.48 ± 0.55 b | 8.06 ± 1.71 b | 24.82 ± 5.29 ab | 1.00 ± 0.23 b | 37.36 ± 5.94 b |
| P | 4.06 ± 0.22 b | 4.09 ± 1.00 c | 17.28 ± 2.81 b | 0.83 ± 0.11 b | 25.42 ± 2.15 b |
| p-value | < 0.0001 | < 0.0001 | 0.0378 | < 0.0001 | < 0.0001 |
Same trend was found for yield scaled N2O-N losses, where “A” almost doubled both “A + P” and “P”, with 0.61 against 0.36 and 0.37 kg N Mg grain-1, respectively (Table 4). That was the least impacting N-loss pathway among the three for both “A” and “A + P”, accounting for 11–12% respectively, but not for “P” that shared the same weight for NH3 volatilisations, with 16% over cumulative N losses.
Table 4.
Yield-scaled N2O-N, NH3-N and NO3--N losses. Within columns, means followed by the same letter are not significantly different between treatments according to the Tukey’s HSD test (p = 0.05).
| Thesis | Yield-scaled N2O-N losses (kg N Mg grain-1) |
Yield-scaled NH3-N losses (kg N Mg grain-1) |
Yield-scaled NO3-N losses (kg N Mg grain-1) |
Yield-scaled cumulative N-losses (kg N Mg grain-1) |
|---|---|---|---|---|
| A | 0.61 ± 0.02 a | 1.35 ± 0.27 a | 3.75 ± 0.77 a | 5.71 ± 0.70 a |
| A + P | 0.36 ± 0.06 b | 0.61 ± 0.11 b | 1.96 ± 0.32 b | 2.93 ± 0.31 b |
| P | 0.37 ± 0.04 b | 0.32 ± 0.06 b | 1.57 ± 0.26 b | 2.26 ± 0.19 b |
| p-value | < 0.0001 | < 0.0001 | 0.0123 | 0.0016 |
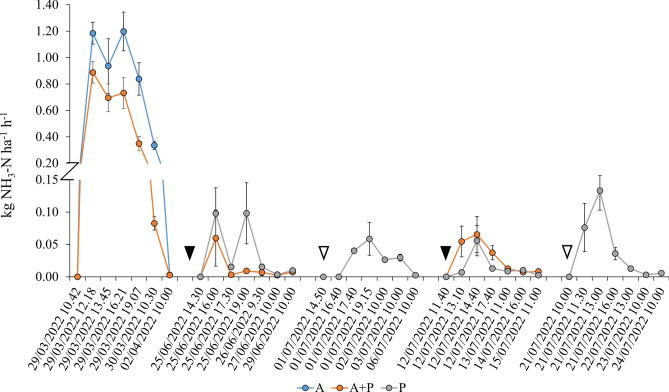
Ammonia volatilization
Ammonia volatilization fluxes following slurry liquid fraction surface broadcast were one order of magnitude higher compared to those after fertigation events (Fig. 5). On March 29, although the N rate applied to “A + P” was half the dose of the “A” treatment, the NH3 volatilisation in the first three hours after slurry liquid fraction distribution was only 25% lower, with two peaks observed at 1.5 and 5.5 h after application. Cumulatively, both the “A + P” and “P” treatments showed a significant reduction of 43% and 71%, respectively, compared to the “A” treatment, with “P” being significantly lower than “A + P” (Table 3). This pattern was numerically confirmed likewise for yield-scaled losses, since “A” outweighed both “A + P” and “P”, with 1.35, against 0.61 and 0.32 kg N Mg grain-1, respectively, however no significant differences between “A + P” and “P” were detected (Table 4). Ammonia volatilization accounted for 23%, 22%, and 16% of the total cumulative N-losses for the “A”, “A + P”, and “P” treatments, respectively. Interestingly this last one shared the same weight of N2O losses.
Fig. 5.
Development of NH3-N volatilisation after surface broadcast of liquid-separated slurry on “A” treatment (first event), and after fertigation events on Ante + Post, “A + P” and Post, “P” treatments. Filled triangles represent fertigations on both “A + P” and “P”, while empty ones represent fertigations only for “P” treatment.
Nitrate leaching
From surface slurry liquid fraction application until April 30, NO3--N concentrations in soil solution under “A” and “A + P” were 149-207% and 69-103% higher than that under “P”, respectively (Fig. 6a). These higher values gradually declined until June 29, when all three treatments reached similar levels, except for “P”, which increased to 29 mg NO3--N L-1 on the final date, more than six times higher than “A” and “A + P”. The average concentrations over the monitoring period were 42, 27, and 18 mg L-1 for “A”, “A + P”, and “P”, respectively.
Fig. 6.
Development of soil drainage-water NO3--N concentration (a), and NO3--N leaching losses (b) through the monitoring period at 0.45 m depth for the three treatments: Ante, “A”; Ante + Post, “A + P” and Post, “P”. Filled triangles represent fertigations on both “A + P” and “P”, while empty ones represent fertigations only for “P” treatment. The dashed line indicates a long period without measurements.
Cumulative N leaching losses were associated with precipitation events, with higher amounts leached on June 29 (Fig. 6b) after an estimated 85 mm of drainage water in the preceding 47 days (data not shown). Cumulative NO3--N leaching losses ranged between 17.28 kg N ha-1 for “P” and 39.67 kg N ha-1 for “A”, while “A + P” levelled to 24.82 kg N ha-1 without statistically significant differences between “A + P” and “A” or “P” (Table 3). Yield-scaled N-losses cleared similarities between “A” and “A + P” that resulted significantly lower, but not different from “P” (Table 4). Approximately 56% and 60% of the total losses occurred during the first half of May for “A” and “A + P”, respectively, while they accounted for 40% for “P”. Nitrate leaching was therefore the most impacting N escape route quantitatively, accounting for around 66% of cumulative N losses for both “A” and “A + P”, and 68% for “P”.
Relationships between variables
Principal Component Analysis showed that the two first principal dimensions explained around 78% of the variance (Fig. 7). Major contrasts were detected between the treatments: “A” was linked to high N losses including all major N-loss pathways, “A + P” was associated to high grain yield and NUE, while “P” was negatively related to N losses. The three N loss pathways were strongly correlated with each other and, in turn, strongly negatively related to NUE.
Discussion
Maize yield and N-use efficiency
Our results showed that yield and NUE tended to increase when N fertilization to maize was split between basal dressing application before sowing and fertigation events during the cropping cycle (i.e., “A + P”). This observation aligns with a common pattern observed in various crops and cultivation techniques, often ascribed to an improved synchronization between plant N demand and soil N availability53,54, and agrees with other observations of N-uptake rates by maize. For example, Subedi and Ma55 found substantial reductions in maize yields and grain filling when N application was restricted until V8, hence the limitation before seeding may have magnified those yield penalties. These authors also showed that 15N applied 3 weeks after silking was directly relocated to the grain, thus reinforcing the idea that N applied after tillering with fertigation may have stimulated grain instead of biomass production. Moreover, under our experimental conditions, soil N mineralization may have initially sustained maize N-requirements in “P” only during the first phase of the cropping cycle, as indicated by the soil NO3- concentration, decreasing after 15 of May. The same pattern occurred in “A + P”, however in this treatment half of the N dose was applied at broadcast, which prolonged N mineralization through May and June until the beginning of fertigations. Finally, full N application before sowing under “A” led to high N losses, thereby decreasing N availability for the maize crop.
We could have obtained different results on maize yields with lower levels of soil phosphorous (P) and potassium (K) fertility, since cattle slurries are valuable sources of those elements in case of soil scarcity56. Recent studies focusing on the dynamics of nutrients allocation after solid–liquid separation of livestock effluents with microfiltration units pointed out that, together with an increase in the removal of total solids, a larger share of P can be removed, while ammoniacal N and K can be recovered to a similar or higher extent within the liquid fraction19,57,58. Such a higher N/P ratio of the microfiltered liquid fraction could represent a more balanced fertilizer sustaining crop development through fertigation57. This could be of major interest for farms where soil P and K content is high, as in our case (37 mg P kg soil-1 and 353 mg K kg soil-1), and excessive P inputs would only lead to luxury consumption.
Nitrous oxide emissions
After slurry liquid fraction surface broadcasting, N2O emissions may have been initially produced by nitrification and later by denitrification. This is indicated by the shift in predominant mineral N form in the soil, with a peak of NH4+-N right after slurry application and increasing NO3--N values in the following days, and by the suitable WFPS conditions of 35–60% for coupled nitrification and denitrification59,60. The highest N2O peak was recorded two weeks after slurry liquid fraction broadcast application in “A”, following a 40-mm rain event and high soil NO3--N concentration. The combination of a dry–wet cycle with slurry may have increased C and N availability and microbial respiration, creating anaerobic microsites and boosting denitrification, even at WFPS values lower than 55%61,62. This is because slurries can be a hotspot of microbial activity triggering oxygen consumption rates. Water restricts gaseous diffusion within the soil, and therefore oxygen availability can be strongly limited in hotspots with slurry. Consequently, denitrification takes place over a wider range of soil moisture conditions with slurries than with mineral fertilisers or solid manure63.
Microfiltered slurry liquid fraction application through drip fertigation reduced N2O emissions under both “A + P” and “P”. This mitigation capacity of drip fertigation had been extensively observed with synthetic fertilizers22,64 and promising studies are emerging also with organic fertilizers: in Bacenetti et al.17, compared to digestate surface broadcast, N2O emissions were decreased by 22% (from 8.25 to 6.47 kg ha-1) and 20% (from 8.25 to 6.64 kg ha-1) through pivot and drip fertigation with digestate. Several factors might lead to this observation. Firstly, the dilution of N application both in terms of timing and rates allows for a higher synchrony between N supply and crop requirements, reducing the availability of soil mineral N for N2O-producing processes65. Second, due to the partial soil wetting associated with drip fertigation, the low N2O emissions from the dry areas between the drippers may compensate for the higher emitting surfaces surrounding the wet bulbs22,66.
The N2O emissions were similar when the entire N dose was applied through fertigation (“P”), and when split between pre-sowing and fertigation (“A + P”). Although it could be expected that full application with fertigation would have further reduced N2O emissions, this was not observed. This lack of difference may be attributed to the higher plant N uptake in “A + P”, reducing soil mineral N availability.
The calculated mean EF of 1.33% across all treatments, as well as the yield-scaled emissions, appear relatively high if compared to some studies in the literature. For instance, in the meta-analysis conducted by Cayuela et al.67 on Mediterranean systems, the EF for drip irrigation was found to be 0.51%. However, it is worth noting that the same meta-analysis indicated that organic-liquid fertilizers, such as pig and cattle slurries, had the highest EFs among different fertilizer types, with an EF of 0.85% ± 0.30 (N = 30). Furthermore, to our knowledge, there is no literature available reporting EFs specifically from fertigation with slurries, making it premature to establish a final reference value for this practice.
Ammonia volatilisation
In relation to applied TAN, the NH3 emission factors were lower than the reference values of 44% for broadcast cattle slurry application68. In detail, 9.68% and 5.60% of applied TAN were lost from “A” and “A + P”, respectively. The low NH3 volatilization may have been promoted by a combination of factors, such as high soil surface roughness (as a results of a previous soil tillage with a ripper) that eased slurry infiltration, low wind speed (only 0.3 m s-1 on average at the time of slurry application; data not shown), relatively low soil and air temperatures (averagely 13 and 15 °C, respectively), and moderate soil moisture content (17% GWC on average), which together have been reported to reduce NH3 volatilisation rates69,70. Furthermore, these factors may have also facilitated nitrification phenomena that, in turn, allow reduction in NH3 volatilisation by further lowering soil pH and NH4+ availability in the soil70. The NH3 volatilization rates from the “A + P” treatment after surface broadcast were proportionally higher compared to those from the “A” treatment in relation to the TAN application rate. This can be attributed to an increase in the surface-to-volume ratio, resulting in a greater exchange of NH3 with the air, particularly at lower rates of slurry liquid fraction application71,72.
We found that N application through fertigation at 50% or 100% reduced NH3 volatilisation by 43% and 71%, respectively. Immediate water addition after slurry application can reduce NH3 volatilization by improving infiltration into the soil, where NH4+ can be immobilised on cation exchange sites, thus reducing the potential for volatilisation73. Furthermore, slurry viscosity, a crucial determinant of the infiltration rates of this organic fertilizer and therefore of the associated NH3 losses69, may have been synergistically affected by the microfiltration treatment prior to application and by the delivery system through drip fertigation. This is because microfiltration reduces the fibrous components in the slurry, and because the viscosity is further decreased through dilution with irrigation water in the drip lines74, in turn increasing infiltration rates and reducing slurry exposure to air which consequently lowers NH3 volatilisation58. Microfiltration and water dilution have the additional technical benefit of increasing the lifespan of the dripline nozzles19.
The reductions we observed for NH3 volatilisation are in line with the findings of Bacenetti et al.17 and of Bortolini31: both observed major abatements in NH3 volatilisation from the adoption of fertigation with pivot (−70%)17, drip lines (-83%)17 or drop tubes (−89%)31 to supply organic fertilizers (microfiltered and liquid separated slurry).
Nitrate leaching
The concentrations of NO3--N in the soil solution during the latter half of April were notably high, especially under “A”, being – on average – higher than 80 mg NO3--N L-1, falling within the range reported by Perego et al.26 for the same region (0 – 101 mg L-1 at 0.5 m depth), but considerably lower than the maximum concentration of 300 mg L-1 in the topsoil reported by Mantovi et al.25 for an area in the Po valley with intensive pig slurry applications.
A significant finding in our study was that around 40% of NO3--N leaching losses in the treatment with full fertigation (“P”) occurred prior to any exogenous N supply. This highlights the substantial contribution of NO3- originating from the soil “reservoir”, which is often replenished after maize cultivation in the Po Plain leading to undesirable N surpluses75. Similar conclusions were drawn from studies conducted by Arbat et al.28 and Berenguer et al.76, underscoring the importance of determining soil NO3- concentration at sowing as a means of understanding and managing N dynamics more efficiently in agricultural systems.
Microfiltered slurry liquid-fraction application via fertigation was successful in reducing NO3- leaching losses compared to broadcast application. In addition to the increases in NUE obtained with this application method (at least for “A + P”), it is possible that the reductions are due to the spatial pattern of soil water and mineral N availability created by fertigation: due to the low mobility of NH4+ in the soil, this ion accumulates in the wet areas under the emitters receiving a higher amount of water, whereas NO3- from nitrification accumulates in the drier areas further away from the emitter66. Consequently, the potential for NO3- leaching decreases. Relevant decreases in NO3- leaching losses after fertigation with slurries have been recently reported also by Bortolini31 (-85%, on average) and by Bacenetti et al.17 (−40 — 45%, on average) further supporting the benefit to reduce leaching losses of these techniques.
Conclusion and implications
Although caution should be exercised drawing conclusions from a one-year experiment, our study indicates that drip fertigation can reduce all major N-loss pathways (NH3, N2O, NO3- leaching) while increasing maize yields, compared to slurry liquid fraction surface broadcast application. Indeed, the yield-scaled N losses of drip fertigated treatments were on average 55% lower than those of surface broadcast slurry liquid fraction application. Furthermore, drip fertigation averted the pollution swapping associated with “traditional” methods used to mitigate NH3 volatilisation after slurry application, such as low-trajectory (trailing hose and trailing shoe) and injection techniques. This is because these traditional methods have been often reported to stimulate N2O emissions by promoting the formation of hotspots with high N content and anaerobic conditions. Hence, to achieve even better results, research should explore if the benefits of drip fertigation can be further promoted by coupling this practice with other management options that enhance N retention in the slurry, like acidification or addition of biological nitrification inhibitors, on a research timespan longer than only one year. Moreover, a holistic approach considering all N escape routes such as N2 and NOx is becoming increasingly important, not only during field fertilization but also at stages such as storage and treatment processes like anaerobic digestion and microfiltration.
Overall, our promising results show that adopting drip fertigation with slurries can be a valuable option to overcome the potential yield losses of organic systems for crops with high N requirements such as maize, while reducing N losses, fulfilling the environmental principles of organic farming and current requirements from European Green Deal policies.
Supplementary Information
Acknowledgements
Financial support for this work was provided through the partners of the Joint Call of the Cofund ERA-Nets SusCrop (Grant N° 771134), FACCE ERA-GAS (Grant N° 696356), ICT-AGRI-FOOD (Grant N° 862665) and SusAn (Grant N° 696231). This work is also part of the project NODES which has received funding from the MUR – M4C2 1.5 of PNRR funded by the European Union—NextGenerationEU (Grant agreement no. ECS00000036). Additionally, this work was supported by the Foundation Romeo and Enrica Invernizzi (Milan, Italy). We would like to thank colleagues, technicians and students from the Agronomy group of Department of Sustainable Crop Production (Università Cattolica del Sacro Cuore of Piacenza), for their assistance during the whole experimental process. The authors acknowledge the farmers Mauro Begatti, Eros Rubes and Martino Boldini for the great support during the farm trial.
Author contributions
F.C. wrote the original draft of the manuscript and made editing, cured the dataset, made formal analysis and provided visualization of the graphs and tables. D.A., V.T., A.P., and A.F. conceived the experiment, reviewed and edited the manuscript. F.C., F.A., A.F., M.L., D.P. and P.G. led the experiment and collected data. All the authors reviewed the manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-01487-0.
References
- 1.European Commission. Communication from the commission to the European parliament, the European council, the council, the European economic and social committee and the committee of the regions. The European Green Deal. COM/2019/640 final. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2019%3A640%3AFIN (2019).
- 2.Colglazier, W. Sustainable development agenda: 2030. Science (80).349, 1048–1050 (2015). [DOI] [PubMed]
- 3.De Ponti, T., Rijk, B. & Van Ittersum, M. K. The crop yield gap between organic and conventional agriculture. Agric. Syst.108, 1–9 (2012). [Google Scholar]
- 4.Knapp, S. & van der Heijden, M. G. A. A global meta-analysis of yield stability in organic and conservation agriculture. Nat. Commun.9, 1–9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponisio, L. C. et al. Diversification practices reduce organic to conventional yield gap. Proc. R. Soc. B Biol. Sci.282, (2015). [DOI] [PMC free article] [PubMed]
- 6.Seufert, V., Ramankutty, N. & Foley, J. A. Comparing the yields of organic and conventional agriculture. Nature485, 229–232 (2012). [DOI] [PubMed] [Google Scholar]
- 7.EUROSTAT. Agricultural production - crops. https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agricultural_production_-_crops#Cereals (2022).
- 8.Meissle, M. et al. Pests, pesticide use and alternative options in European maize production: current status and future prospects. J. Appl. Entomol.134, 357–375 (2010). [Google Scholar]
- 9.European Commission. Organic farming in the EU – A decade of organic growth, January 2023. (2023).
- 10.Farneselli, M. et al. Nine-year results on maize and processing tomato cultivation in an organic and in a conventional low input cropping system. Ital. J. Agron.8, 2 (2013). [Google Scholar]
- 11.Galloway, J. N. et al. The nitrogen cascade. Bioscience53, 341–356 (2003). [Google Scholar]
- 12.van der Weerden, T. J. et al. Influence of key factors on ammonia and nitrous oxide emission factors for excreta deposited by livestock and land-applied manure. Sci. Total Environ.889, 164066 (2023). [DOI] [PubMed] [Google Scholar]
- 13.Oenema, O., Oudendag, D. & Velthof, G. L. Nutrient losses from manure management in the European Union. Livest. Sci.112, 261–272 (2007). [Google Scholar]
- 14.Wyer, K. E., Kelleghan, D. B., Blanes-Vidal, V., Schauberger, G. & Curran, T. P. Ammonia emissions from agriculture and their contribution to fine particulate matter: A review of implications for human health. J. Environ. Manage.323, 116285 (2022). [DOI] [PubMed] [Google Scholar]
- 15.Webb, J., Pain, B., Bittman, S. & Morgan, J. The impacts of manure application methods on emissions of ammonia, nitrous oxide and on crop response-A review. Agric. Ecosyst. Environ.137, 39–46 (2010). [Google Scholar]
- 16.Maris, S. C. et al. Strong potential of slurry application timing and method to reduce N losses in a permanent grassland. Agric. Ecosyst. Environ.10.1016/j.agee.2021.107329 (2021). [Google Scholar]
- 17.Bacenetti, J. et al. Reducing the environmental impact of maize by fertigation with digestate using pivot and drip systems. Biosyst. Eng.236, 27–38 (2023). [Google Scholar]
- 18.Gamble, J. D., Feyereisen, G. W., Papiernik, S. K., Wente, C. D. & Baker, J. M. Summer Fertigation of Dairy Slurry Reduces Soil Nitrate Concentrations and Subsurface Drainage Nitrate Losses Compared to Fall Injection. Front. Sustain. Food Syst.10.3389/fsufs.2018.00015 (2018). [Google Scholar]
- 19.Finzi, A. et al. Performance and sizing of filtration equipment to replace mineral fertilizer with digestate in drip and sprinkler fertigation. J. Clean. Prod.317, 128431 (2021). [Google Scholar]
- 20.Guo, C., Liu, X. & He, X. A global meta-analysis of crop yield and agricultural greenhouse gas emissions under nitrogen fertilizer application. Sci. Total Environ.831, 154982 (2022). [DOI] [PubMed] [Google Scholar]
- 21.Ardenti, F. et al. Matching crop row and dripline distance in subsurface drip irrigation increases yield and mitigates N2O emissions. F. Crop. Res.289, 108732 (2022). [Google Scholar]
- 22.Kuang, W., Gao, X., Tenuta, M. & Zeng, F. A global meta-analysis of nitrous oxide emission from drip-irrigated cropping system. Glob. Chang. Biol.27, 3244–3256 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Lombardi, B. et al. Is Dairy Effluent an Alternative for Maize Crop Fertigation in Semiarid Regions? An Approach to Agronomic and Environmental Effects. Animals12, 2025 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren, K. Y., Duan, Y. H., Xu, M. G. & Zhang, X. B. Effect of manure application on nitrogen use efficiency of crops in China: A meta-analysis. Sci. Agric. Sin.52, 2983–2996 (2019). [Google Scholar]
- 25.Mantovi, P., Fumagalli, L., Beretta, G. P. & Guermandi, M. Nitrate leaching through the unsaturated zone following pig slurry applications. J. Hydrol.316, 195–212 (2006). [Google Scholar]
- 26.Perego, A. et al. Nitrate leaching under maize cropping systems in Po Valley (Italy). Agric. Ecosyst. Environ.147, 57–65 (2012). [Google Scholar]
- 27.Wang, Z.-H. & Li, S. X. Nitrate N loss by leaching and surface runoff in agricultural land: A global issue (a review). In Advances in Agronomy (ed. Wang, Z.) (Elsevier Inc., 2019). [Google Scholar]
- 28.Arbat, G. et al. Soil water and nitrate distribution under drip irrigated corn receiving pig slurry. Agric. Water Manag.120, 11–22 (2013). [Google Scholar]
- 29.Zheng, J. et al. Drip fertigation sustains crop productivity while mitigating reactive nitrogen losses in Chinese agricultural systems: Evidence from a meta-analysis. Sci. Total Environ.886, 163804 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Zhu, Y., Zhang, H., Li, R., Zhu, W. & Kang, Y. Nitrogen fertigation affects crop yield, nitrogen loss and gaseous emissions: a meta-analysis. Nutr. Cycl. Agroecosystems127, 359–373 (2023). [Google Scholar]
- 31.Bortolini, L. A low environmental impact system for fertirrigation of maize with cattle slurry. Contemp. Eng. Sci.9, 201–213 (2016). [Google Scholar]
- 32.Rubel, F., Brugger, K., Haslinger, K. & Auer, I. The climate of the European Alps: Shift of very high resolution Köppen-Geiger climate zones 1800–2100. Meteorol. Zeitschrift26, 115–125 (2017). [Google Scholar]
- 33.Soil Survey Staff. Keys to Soil Taxonomy. (2014).
- 34.Allen, R. G., Pereira, L. S., Raes, D. & Smith, M. Crop evapotranspiration guidelines for computing crop requirements. FAO Irrig. Drain. Report modeling and application. J. Hydrol.285, 19–40 (1998). [Google Scholar]
- 35.López-Bellido, R. J. & López-Bellido, L. Efficiency of nitrogen in wheat under Mediterranean conditions: Effect of tillage, crop rotation and N fertilization. F. Crop. Res.71, 31–46 (2001). [Google Scholar]
- 36.Martínez, E. et al. The effects of dairy cattle manure and mineral N fertilizer on irrigated maize and soil N and organic C. Eur. J. Agron.83, 78–85 (2017). [Google Scholar]
- 37.Danielson, R. E. & Sutherland, P. L. Porosity. in Methods of Soil Analysis, Part 1: Physical and Mineralogical Methods (ed. Klute, A.) 443–461 (John Wiley & Sons, Ltd, 2018). 10.2136/sssabookser5.1.2ed.c18.
- 38.Maris, S. C., Teira-Esmatges, M. R., Arbonés, A. & Rufat, J. Effect of irrigation, nitrogen application, and a nitrification inhibitor on nitrous oxide, carbon dioxide and methane emissions from an olive (Olea europaea L.) orchard. Sci. Total Environ.538, 966–978 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Rhine, E. D., Mulvaney, R. L., Pratt, E. J. & Sims, G. K. Improving the Berthelot Reaction for Determining Ammonium in Soil Extracts and Water. Soil Sci. Soc. Am. J.62, 473 (1998). [Google Scholar]
- 40.Cocco, E. et al. How shallow water table conditions affect N2O emissions and associated microbial abundances under different nitrogen fertilisations. Agric. Ecosyst. Environ.261, 1–11 (2018). [Google Scholar]
- 41.Maris, S. C. et al. The interaction between types of cover crop residue and digestate application methods affects ammonia volatilization during maize cropping season. J. Environ. Qual. jeq2.20205 (2021) 10.1002/jeq2.20205. [DOI] [PubMed]
- 42.Bosch-Serra, À. D., Yagüe, M. R. & Teira-Esmatges, M. R. Ammonia emissions from different fertilizing strategies in Mediterranean rainfed winter cereals. Atmos. Environ.84, 204–212 (2014). [Google Scholar]
- 43.Alexander, J. R., Spackman, J. A., Wilson, M. L., Fernández, F. G. & Venterea, R. T. Capture efficiency of four chamber designs for measuring ammonia emissions. Agrosystems, Geosci. Environ.4, 1–11 (2021).
- 44.Capra, F. et al. Towards efficient N cycling in intensive maize: role of cover crops and application methods of digestate liquid fraction. GCB Bioenergy15, 867–885 (2023). [Google Scholar]
- 45.Kroes, J. G. et al. SWAP version 4; Theory description and user manual.www.wur.eu/environmental-research (2017).
- 46.Bonfante, A. et al. SWAP, CropSyst and MACRO comparison in two contrasting soils cropped with maize in Northern Italy. Agric. Water Manag.97, 1051–1062 (2010). [Google Scholar]
- 47.Bertora, C. et al. Dissolved organic carbon cycling, methane emissions and related microbial populations in temperate rice paddies with contrasting straw and water management. Agric. Ecosyst. Environ.265, 292–306 (2018). [Google Scholar]
- 48.Zhao, Y., De Maio, M., Vidotto, F. & Sacco, D. Influence of wet-dry cycles on the temporal infiltration dynamic in temperate rice paddies. Soil Tillage Res.154, 14–21 (2015). [Google Scholar]
- 49.R Core Team. R: A language and environment for statistical computing. (2020).
- 50.Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D. & R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–152. , https://CRAN.R-project.org/package=nlme. https://bugs.r-project.org (2021).
- 51.Hothorn, T., Bretz, F. & Westfall, P. Simultaneous Inference in General Parametric Models. Biometrical J.50, 346–363 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Kassambara, A. & Mundt, F. Package ‘factoextra’ Type Package Title Extract and Visualize the Results of Multivariate Data Analyses. (2020).
- 53.Abalos, D., Jeffery, S., Drury, C. F. & Wagner-Riddle, C. Improving fertilizer management in the U.S. and Canada for N2O mitigation: Understanding potential positive and negative side-effects on corn yields. Agric. Ecosyst. Environ.221, 214–221 (2016).
- 54.Cassman, K. G., Dobermann, A. R. & Walters, D. T. Agroecosystems, Nitrogen-use Efficiency, and Nitrogen Management. AMBIO A J. Hum. Environ.31, 132–140 (2002). [DOI] [PubMed] [Google Scholar]
- 55.Subedi, K. D. & Ma, B. L. Effects of N-deficiency and timing of N supply on the recovery and distribution of labeled 15N in contrasting maize hybrids. Plant Soil273, 189–202 (2005). [Google Scholar]
- 56.Edmeades, D. C. The long-term effects of manures and fertilisers on soil productivity and quality: a review. Nutr. Cycl. Agroecosystems66, 165–180 (2003). [Google Scholar]
- 57.Zielińska, M. & Bułkowska, K. Use of Membrane Techniques for Removal and Recovery of Nutrients from Liquid Fraction of Anaerobic Digestate. Membranes (Basel).15, 45 (2025). [DOI] [PMC free article] [PubMed]
- 58.Romio, C., Ward, A. J. & Møller, H. B. Characterization and valorization of biogas digestate and derived organic fertilizer products from separation processes. Front. Sustain. Food Syst.8, 1415508 (2024). [Google Scholar]
- 59.Bateman, E. J. & Baggs, E. M. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils41, 379–388 (2005). [Google Scholar]
- 60.Kool, D. M., Dolfing, J., Wrage, N. & Van Groenigen, J. W. Nitrifier denitrification as a distinct and significant source of nitrous oxide from soil. Soil Biol. Biochem.43, 174–178 (2011). [Google Scholar]
- 61.Aguilera, E., Lassaletta, L., Sanz-Cobena, A., Garnier, J. & Vallejo, A. The potential of organic fertilizers and water management to reduce N2O emissions in Mediterranean climate cropping systems review. Agric. Ecosyst. Environ.164, 32–52 (2013). [Google Scholar]
- 62.Congreves, K. A., Wagner-Riddle, C., Si, B. C. & Clough, T. J. Nitrous oxide emissions and biogeochemical responses to soil freezing-thawing and drying-wetting. Soil Biol. Biochem.117, 5–15 (2018). [Google Scholar]
- 63.Petersen, S. O. et al. Higher N2O emissions from organic compared to synthetic N fertilisers on sandy soils in a cool temperate climate. Agric. Ecosyst. Environ.358, 108718 (2023). [Google Scholar]
- 64.Abalos, D., Sanchez-Martin, L., Garcia-Torres, L., van Groenigen, J. W. & Vallejo, A. Management of irrigation frequency and nitrogen fertilization to mitigate GHG and NO emissions from drip-fertigated crops. Sci. Total Environ.490, 880–888 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Ma, Z., Ren, B., Zhao, B., Liu, P. & Zhang, J. Increasing grain yield, nitrogen use efficiency of summer maize and reducing greenhouse gas emissions by applying urea ammonium nitrate solution. Agron. J.114, 948–960 (2022). [Google Scholar]
- 66.Guardia, G. et al. Effect of inhibitors and fertigation strategies on GHG emissions, NO fluxes and yield in irrigated maize. F. Crop. Res.204, 135–145 (2017). [Google Scholar]
- 67.Cayuela, M. L. et al. Direct nitrous oxide emissions in Mediterranean climate cropping systems: Emission factors based on a meta-analysis of available measurement data. Agric. Ecosyst. Environ.238, 25–35 (2017). [Google Scholar]
- 68.van der Weerden, T. J. et al. Ammonia and nitrous oxide emission factors for excreta deposited by livestock and land-applied manure. J. Environ. Qual.50, 1005–1023 (2021). [DOI] [PubMed] [Google Scholar]
- 69.Sommer, S. G. & Hutchings, N. J. Ammonia emission from field applied manure and its reduction - Invited paper. Eur. J. Agron.15, 1–15 (2001). [Google Scholar]
- 70.Sommer, S. G. et al. Processes controlling ammonia emission from livestock slurry in the field. Eur. J. Agron.19, 465–486 (2003). [Google Scholar]
- 71.Huijsmans, J. F. M., Hol, J. M. G. & Hendriks, M. M. W. B. Effect of application technique, manure characteristics, weather and field conditions on ammonia volatilization from manure applied to grassland. NJAS Wageningen J. Life Sci.49, 323–342 (2001). [Google Scholar]
- 72.Misselbrook, T. H., Nicholson, F. A. & Chambers, B. J. Predicting ammonia losses following the application of livestock manure to land. Bioresour. Technol.96, 159–168 (2005). [DOI] [PubMed] [Google Scholar]
- 73.Sanz-Cobena, A., Misselbrook, T., Camp, V. & Vallejo, A. Effect of water addition and the urease inhibitor NBPT on the abatement of ammonia emission from surface applied urea. Atmos. Environ.45, 1517–1524 (2011). [Google Scholar]
- 74.Sun, G. et al. Effect of Moistube Fertigation on Infiltration and Distribution of Water-Fertilizer in Mixing Waste Biomass Soil. Sustainability11, 6757 (2019). [Google Scholar]
- 75.Zavattaro, L., Monaco, S., Sacco, D. & Grignani, C. Options to reduce N loss from maize in intensive cropping systems in Northern Italy. Agric. Ecosyst. Environ.147, 24–35 (2012). [Google Scholar]
- 76.Berenguer, P., Santiveri, F., Boixadera, J. & Lloveras, J. Nitrogen fertilisation of irrigated maize under Mediterranean conditions. Eur. J. Agron.30, 163–171 (2009). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.