Abstract
Background
Stoma outlet obstruction (SOO) is a significant complication following colorectal surgery with diverting ileostomy, but its prevalence and associated risk factors are not fully understood. This meta-analysis aimed to quantify the prevalence of SOO and identify key risk factors influencing its occurrence.
Methods
A systematic review and meta-analysis of 19 studies comprising 3287 patients were conducted. Pooled prevalence and odds ratios (ORs) for risk factors were calculated using a random-effects model. Subgroup and sensitivity analyses were performed to explore heterogeneity, and publication bias was assessed using funnel plots and Egger’s regression test.
Results
The pooled prevalence of SOO was 14% (95% CI = 11–18%, I2 = 84.9%). Subgroup analysis revealed higher prevalence in studies focusing on benign conditions (20%) and smaller sample sizes (< 100 patients, 16%). Key risk factors included high-output syndrome (OR = 4.23, 95% CI = 2.28–7.85), increased rectus abdominis thickness (OR = 3.51, 95% CI = 2.27–5.41), and laparoscopic surgery (OR = 4.04, 95% CI = 1.62–10.04). While publication bias was detected, but the trim-and-fill method indicated that the adjusted prevalence remained basically consistent with the overall pooled estimate.
Conclusions
SOO occurs in approximately 14% of patients undergoing colorectal surgery with diverting ileostomy. Key modifiable factors included high-output syndrome, rectus abdominis thickness, and laparoscopic surgery.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00384-025-04862-5.
Keywords: Stoma outlet obstruction, Colorectal surgery, Diverting ileostomy, Risk factors, Meta-analysis
Introduction
Colorectal surgery is pivotal in managing various colorectal diseases, including malignancies, inflammatory bowel diseases, and diverticular conditions. A critical aspect of these surgical interventions is the prevention of anastomotic leakage, a complication associated with significant morbidity and mortality. To mitigate this risk, surgeons often employ diverting ileostomy, which serves as a temporary fecal diversion to protect the distal anastomosis during the healing process [1, 2]. Despite its protective intent, diverting ileostomy is not without complications. One notable issue is stoma outlet obstruction (SOO), characterized by a blockage at the stoma site leading to symptoms such as abdominal pain, nausea, vomiting, and cessation of stoma output [3, 4]. SOO can result in prolonged hospital stays, additional surgical interventions, and a diminished quality of life for patients. Understanding the prevalence and identifying risk factors associated with SOO are essential for improving patient outcomes and guiding surgical practices [5].
The current literature presents varying incidence rates of SOO, reflecting differences in study populations, surgical techniques, and definitions of the condition. For instance, a retrospective cohort study reported an incidence of 5.6% in patients with sporadic rectal cancer and 27.3% in patients with ulcerative colitis undergoing stoma surgery [6]. Another study found an incidence of 18.4% among patients who underwent colorectal surgery with diverting ileostomy [7]. These variations underscore the need for a comprehensive analysis to ascertain the true prevalence of SOO. Increasing evidence indicated that several risk factors were associated with SOO, which including underlying ulcerative colitis, loop ileostomy construction, surgical site infections, and increased rectus abdominis thickness [7–9]. Currently, there is a lack of available evidence to systematically explore the risk factors of stoma outlet obstruction after colorectal surgery with diverting ileostomy.
Given the clinical significance of SOO and the variability in reported data, this meta-analysis aims to systematically evaluate the prevalence of SOO in patients undergoing colorectal surgery with diverting ileostomy. Furthermore, it seeks to identify and quantify the risk factors associated with SOO to provide evidence-based insights that can inform clinical practice and enhance patient care.
Materials and methods
This meta-analysis was conducted in accordance with the Guidelines for Meta-Analyses and Systematic Reviews of Observational Studies (MOOSE) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards [10, 11]. The processes of search strategy, data extraction, quality assessment, and statistical analysis were independently performed by two researchers (CK and LHP), with a third reviewer (GJX) consulted to resolve any discrepancies.
Literature search strategy
A comprehensive literature search was performed across multiple databases, including PubMed, EMBASE, and the Cochrane Library from the inception to October 2024. The search strategy incorporated a combination of Medical Subject Headings (MeSH) terms and keywords related to “stoma outlet obstruction,” “colorectal surgery,” “diverting ileostomy,” “prevalence,” “risk factors,” and their variants using appropriate Boolean operators. SOO was defined as the inability to pass stool or gas through the stoma, often accompanied by abdominal distension and other symptoms indicative of bowel obstruction. A diagnosis of SOO requires radiological confirmation, typically through cross-sectional imaging such as CT scans. The detailed search strategies are showed in supplementary 1.
Inclusion and exclusion criteria
We included studies that reported the prevalence of SOO following colorectal surgery with diverting loop ileostomy without language limitation, with a clear definition of SOO. Studies were excluded if they did not provide sufficient data on SOO prevalence or case reports, reviews, editorials, and conference abstracts.
Data extraction and quality assessment
Two independent reviewers (CK and LHP) screened titles and abstracts for eligibility. Full-text articles of potentially relevant studies were retrieved and assessed against the inclusion criteria. Discrepancies were resolved through discussion or consultation with a third reviewer (GJX). Data extracted included study characteristics (author, year of publication, country, study design), patient demographics (sample size, age, gender distribution), and clinical details (underlying diseases and type of ileostomy constructed), and outcomes (incidence/prevalence of SOO, identified risk factors, and their corresponding effect sizes). The quality of included studies was assessed using the Newcastle–Ottawa Scale (NOS) for observational studies [12]. Studies were rated as low, moderate, or high quality based on selection, comparability, and outcome assessment domains. Studies scoring 7 or higher were considered high quality.
Statistical analysis
The primary outcome was the prevalence of SOO in patients undergoing colorectal surgery with diverting ileostomy, while the secondary one was to identify risk factors associated with the development of SOO. Pooled prevalence rates of SOO were calculated using the DerSimonian-Laird random-effects model to account for potential heterogeneity among studies. Heterogeneity was assessed using the I2 statistic, with values > 50% indicating substantial heterogeneity. To explore potential sources of heterogeneity, subgroup analyses were conducted based on underlying disease (benign colorectal lesions vs. colorectal cancer), sample size (less than 100 vs. no less than 100), NOS scores (less than 7 points vs. no less than 7 points), study center (single-center vs. multi-center), and study region (Japan vs. others). We also explore the robustness of the overall pooled estimate using sensitivity analyses. Sensitivity analyses were performed by excluding studies with a high risk of bias (NOS score < 7) and assessing the impact of individual studies on the overall results by sequentially omitting each study. For identified risk factors, odds ratios (ORs) with 95% confidence intervals (CIs) were computed with a random-effects model. Multivariate or adjusted ORs reported in at least two studies were extracted for meta-analyses. Publication bias was evaluated using funnel plots and Egger or Begg’s regression test, with a p-value < 0.05 indicating significant publication bias [13]. If bias was detected, the trim-and-fill method was applied to adjust the pooled effect size [14]. All statistical analyses were performed using Stata version 12.0 (StataCorp, College Station, TX).
Results
Study selection
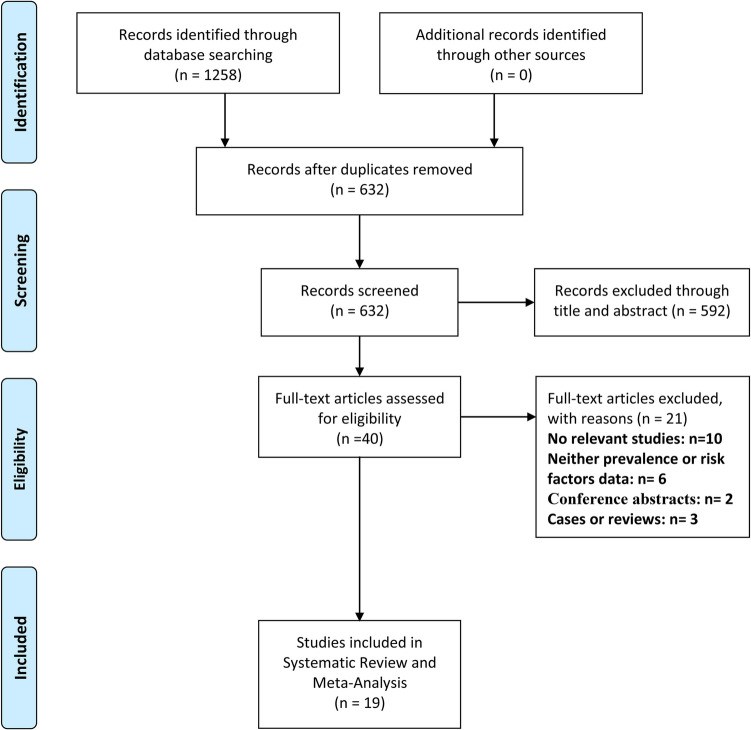
A total of 1258 items were identified through database searches. After the removal of duplicates, 632 studies remained for screening. Title and abstract review excluded 592 studies, leaving 40 full-text articles for detailed assessment. Among these, 21 studies were excluded for the following reasons: no relevant data (n = 10), absence of prevalence or risk factor analysis (n = 6), conference abstracts only (n = 2), and unsuitable study designs such as case reports or reviews (n = 3). Ultimately, a total of 19 studies met the inclusion criteria and were included in the meta-analysis [3, 4, 7–9, 15–28] (Fig. 1). Of all the included studies, three studies also reported other types of ostomies including end ileostomies and loop colostomies [4, 16, 24]. However, we merely extracted data involving diverting loop ileostomy in these studies for further investigation.
Fig. 1.
PRISMA flow diagram of study selection process
Characteristics of included studies
These included studies, conducted between 2005 and 2024, involved a total of 3287 patients undergoing colorectal surgery with diverting ileostomy. Most studies were conducted in Japan (n = 17), with one study from China and another from Italy (Table 1). The majority of studies (n = 18) were single-center, while one study was multi-center. Study populations included patients with colorectal cancer, inflammatory bowel disease, familial adenomatous polyposis, and other conditions. Diagnostic methods for SOO predominantly included clinical symptoms and imaging techniques such as CT or X-ray. The NOS quality scores ranged from 6 to 8, with 63% of studies rated as high quality (NOS ≥ 7).
Table 1.
Baseline characteristics of included studies
| Author (year) | Country | Enrollment period | Disease diagnosis | Surgical approach | Sample size | SOO diagnosis methods | Study center | NOS score |
|---|---|---|---|---|---|---|---|---|
| Takaaki Fujii (2005) [15] | Japan | 2008–2012 | IBD, FAP, and rectal cancer | Loop diverting ileostomy | 61 | X-ray and CT | Single-center | 6 |
| Gaku Ohira (2018) [16] | Japan | 2010–2015 | IBD, FAP, and CRC | Total colectomy or proctocolectomy with ileostomy | 88 | CT | Single-center | 7 |
| Koichi (2019) [17] | Japan | 2007–2017 | Rectal cancer | Laparoscopic rectal cancer surgery with loop ileostomy | 230 | CT | Single-center | 7 |
| Tomoaki (2020 [4] | Japan | 2008–2020 | UC | RPC and IPAA with diverting ileostomies | 90 | Clinical symptoms and CT | Single-center | 7 |
| Yutaro Hara (2020) [7] | Japan | 2015–2018 | IBD, colon perforation, and CRC | Colorectal surgery and diverting ileostomy | 103 | Clinical symptoms and CT | Single-center | 7 |
| Hiroya Enomoto (2021) [19] | Japan | 2014–2020 | Rectal cancer | Anterior rectal resection and diverting ileostomy | 100 | Clinical symptoms and CT | Single-center | 7 |
| Ryo Maemoto (2021) [20] | Japan | 2011–2019 | IBD, FAP, and rectal cancer | Laparoscopic colorectal surgery with diverting ileostomy | 155 | Clinical symptoms and CT | Single-center | 7 |
| Ryota Mori (2021) [22] | Japan | 2010–2020 | UC with cancer or dysplasia | IPAA with diverting ileostomy | 68 | CT | Single-center | 6 |
| Shigemasa Sasaki (2021) [21] | Japan | 2013–2015 | Rectal cancer | Laparoscopic rectal cancer surgery with loop ileostomy | 261 | Clinical symptoms and CT | Single-center | 8 |
| Tomoki Abe (2021) [18] | Japan | 2014–2020 | Rectal cancer | Rectal cancer surgery with loop ileostomy | 125 | Clinical symptoms and CT | Single-center | 7 |
| Kiyomitsu Kuwahara (2022) [9] | Japan | 2014–2021 | IBD and CRC | Laparoscopic colorectal surgery with diverting ileostomy | 63 | Clinical symptoms and CT | Single-center | 6 |
| Koichiro Kumano (2023) [24] | Japan | 2019–2022 | Rectal tumors | Diverting stoma construction following rectal resection | 27 | X-ray | Single-center | 6 |
| P Caprino (2023) [23] | Italy | 2010–2021 | UC | IPAA with diverting ileostomy | 75 | Clinical symptoms and CT | Single-center | 6 |
| Xiaowei Wang (2023) [25] | China | 2022 | Rectal cancer | Rectal cancer surgery with loop ileostomy | 38 | Clinical symptoms and CT | Single-center | 6 |
| Keisuke Ihara (2024) [3] | Japan | 2006–2021 | UC | Proctocolectomy and diverting ileostomy | 68 | Clinical symptoms and CT | Single-center | 7 |
| Masaya Kawai (2024) [27] | Japan | 2005–2017 | Rectal cancer | Rectal cancer surgery with loop ileostomy | 400 | Clinical symptoms and CT | Single-center | 6 |
| Takayuki Ogino (2024) [28] | Japan | 2010–2023 | UC and FAP | RPC and IPAA with diverting ileostomies | 106 | Clinical symptoms and CT | Multi-center | 7 |
| Yoshiko Matsumoto (2024) [8] | Japan | 2016–2021 | No available | Loop ileostomy | 188 | Clinical symptoms and CT | Single-center | 7 |
| Yuta Imaizumi (2024) [26] | Japan | 2018–2022 | Rectal cancer | Rectal cancer surgery with loop ileostomy | 92 | Clinical symptoms and CT | Single-center | 7 |
CRC, colorectal cancer; CT, computed tomography; FAP, familial adenomatous polyposis; IBD, inflammatory bowel disease; IPAA, ileal pouch-anal anastomosis; NOS, Newcastle–Ottawa Scale; RPC, restorative proctocolectomy; SOO, stoma outlet obstruction; UC, ulcerative colitis
Prevalence of stoma outlet obstruction
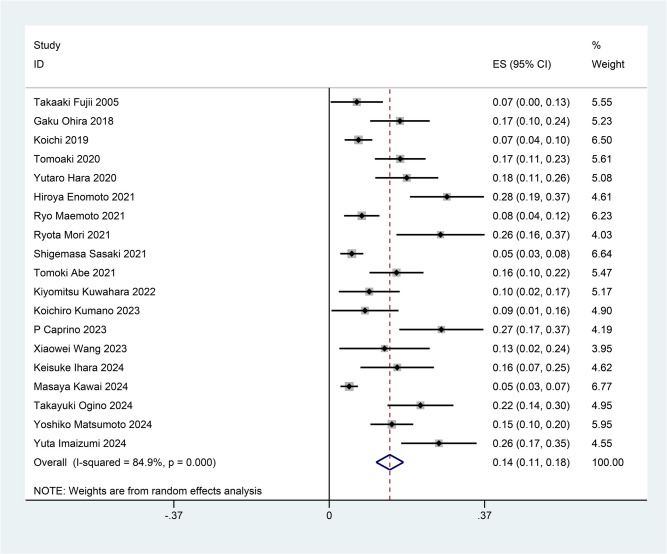
The pooled prevalence of SOO was 14% (95% CI = 11–18%), with substantial heterogeneity observed among studies (I2 = 84.9%, p < 0.001) (Fig. 2). Individual study estimates varied from 5.4% (Sasaki et al., 2021) [21] to 28% (Hara et al., 2020) [7].
Fig. 2.
Forest plot of the prevalence of stoma outlet obstruction after colorectal surgery with diverting ileostomy
We also conducted subgroup analyses to explore sources of heterogeneity (Table 2). Studies focusing on benign colorectal conditions (e.g., IBD and FAP) reported a higher prevalence (20%, 95% CI = 17–24%, I2 = 9.4%) compared to CRC-related cases (12%, 95% CI = 8–15%, I2 = 86.3%). Smaller studies (< 100 patients) reported a higher prevalence (16%, 95% CI = 10–22%, I2 = 74.6%) than larger studies (≥ 100 patients, 13%, 95% CI = 10–17%, I2 = 87.3%). Studies conducted in Japan reported a prevalence of 14% (95% CI = 11–17%, I2 = 85.1%), while studies outside Japan had a prevalence of 20% (95% CI = 7–33%, I2 = 69.2%). Multi-center studies reported a higher prevalence (22%, 95% CI = 14–30%) compared to single-center studies (14%, 95% CI = 11–17%).
Table 2.
Subgroup analysis of the prevalence of stoma outlet obstruction after colorectal surgery with diverting ileostomy
| Outcomes | Number of studies | OR (95% CI) | Heterogeneity, I2 (%) |
|---|---|---|---|
| Pooled results | 19 | 0.14 (0.11–0.18) | 84.9 |
| Subgroup analyses based on disease diagnosis | |||
| Benign colorectal lesions (IBD,FAP, and others) | 9 | 0.20 (0.17–0.24) | 9.4 |
| CRC | 12 | 0.12 (0.08–0.15) | 86.3 |
| Subgroup analyses based on sample size | |||
| Less than 100 | 8 | 0.16 (0.10–0.22) | 74.6 |
| No less than 100 | 11 | 0.13 (0.10–0.17) | 87.3 |
| Subgroup analyses based on quality of NOS scores | |||
| Less than 7 points | 7 | 0.13 (0.07–0.19) | 82.6 |
| No less than 7 points | 12 | 0.15 (0.11–0.20) | 84.9 |
| Subgroup analyses based on study center | |||
| Single-center | 18 | 0.14 (0.11–0.17) | 84.5 |
| Multi-center | 1 | 0.22 (0.14–0.30) | NA |
| Subgroup analyses based on study region | |||
| Japan | 17 | 0.14 (0.11–0.17) | 85.1 |
| Others | 2 | 0.20 (0.07–0.33) | 69.2 |
NOS, Newcastle–Ottawa Scale; IBD, inflammatory bowel disease; FAP, familial adenomatous polyposis; CRC, colorectal cancer; NA, no available
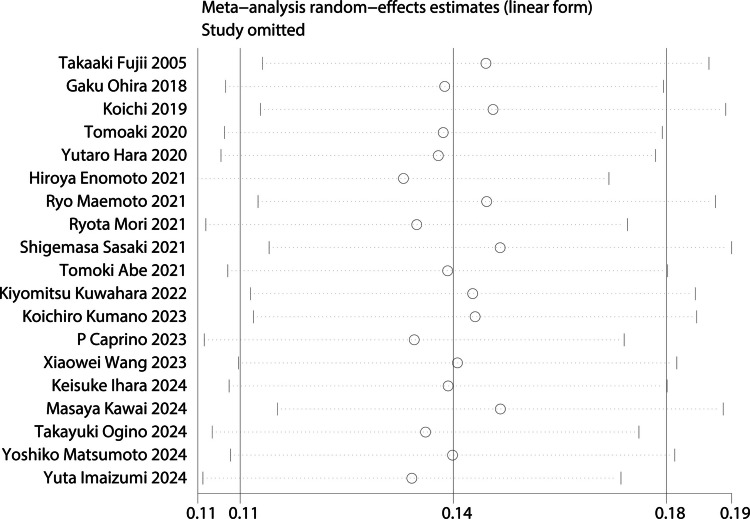
Subsequently, sensitivity analyses were performed to assess the robustness of findings by excluding studies with NOS scores < 7 and sequentially omitting individual studies. After removing these lower-quality studies, the pooled prevalence was recalculated based on the remaining high-quality studies (n = 12). The revised prevalence estimate was 13% (95% CI = 10–16%), with a slight reduction in heterogeneity (I2 = 82.6%). To evaluate the influence of individual studies on the pooled prevalence estimates, a leave-one-out sensitivity analysis was performed. Each study was sequentially removed from the meta-analysis, and the pooled prevalence was recalculated. None of the individual omissions led to substantial changes in the overall prevalence, which ranged from 13 to 15% (Fig. 3).
Fig. 3.
Sensitivity analysis for the pooled prevalence of stoma outlet obstruction after colorectal surgery with diverting ileostomy
Risk factors for SOO
This meta-analysis identified several key risk factors for SOO after colorectal surgery with diverting ileostomy. The pooled adjusted ORs and heterogeneity provided insights into their significance and consistency across studies (Table 3).
Table 3.
Risk factors of stoma outlet obstruction after colorectal surgery with diverting ileostomy
| Risk factors | Number of trials | Pooled adjusted OR (95% CI) | I2 (%) |
|---|---|---|---|
| Age < 60 | 4 | 1.37 (0.62–3.02) | 63.8 |
| Gender (female) | 3 | 0.40 (0.08–2.03) | 58.7 |
| BMI | 2 | 1.01 (0.85–1.21) | 0 |
| Total proctocolectomy | 2 | 3.95 (0.59–26.33) | 65 |
| Laparoscopic surgery | 3 | 4.04 (1.62–10.04) | 0 |
| Thickness of subcutaneous fat at stomal site | 4 | 1.27 (0.65–2.48) | 57.7 |
| Stoma site(Left side) | 2 | 3.13 (0.86–11.38) | 11.9 |
| Rectus abdominis thickness | 5 | 3.51 (2.27–5.41) | 0 |
| High-output syndrome | 5 | 4.23 (2.28–7.85) | 0 |
OR, odds ratio; CI, confidence interval
HOS is defined as a condition in which stoma output exceeds 1500 mL per day, often leading to electrolyte imbalances, dehydration, malnutrition, and renal dysfunction. HOS emerged as the most significant risk factor for SOO, with a pooled OR of 4.23 (five studies; 95% CI = 2.28–7.85, I2 = 0%). The strong and consistent association across five studies highlights the critical role of excessive stoma output in causing mechanical obstruction and functional stoma failure [3, 18, 22, 26, 28]. Effective postoperative management of stoma output is essential to reduce this risk.
Increased rectus abdominis muscle thickness was significantly associated with SOO, with a pooled OR of 3.51 (five studies; 95% CI: 2.27–5.41, I2 = 0%) [4, 18, 19, 21, 26]. The mechanical impact of thicker abdominal musculature, which may compress or narrow the stoma lumen, emphasizes the importance of preoperative anatomical evaluation and stoma site selection.
Laparoscopic surgery was associated with a fourfold increased risk of SOO (three studies; pooled OR = 4.04, 95% CI = 1.62–10.04, I2 = 0%) [3, 16, 28], which underscoring the need for precise surgical technique and training in laparoscopic cases.
Total proctocolectomy showed an elevated, but non-significant, risk of SOO (two studies; pooled OR = 3.95, 95% CI = 0.59–26.33, I2 = 65%) [16, 20]. The high heterogeneity suggests variability in patient populations and surgical techniques. This factor may warrant further investigation in larger, standardized studies. Left-sided stomas demonstrated a pooled OR of 3.13 (two studies; 95% CI = 0.86–11.38, I2 = 11.9%), although the result was not statistically significant [8, 16]. This trend may reflect anatomical differences affecting stoma functionality, but more research is needed to clarify its impact. Several factors, including subcutaneous fat thickness (pooled OR = 1.27, 95% CI = 0.65–2.48, I2 = 57.7%), age < 60 years (pooled OR = 1.37, 95% CI = 0.62–3.02, I2 = 63.8%), female gender (pooled OR = 0.40, 95% CI = 0.08–2.03, I2 = 58.7%), and BMI (pooled OR = 1.01, 95% CI = 0.85–1.21, I2 = 0%), were not significantly associated with SOO. The lack of significant findings suggests limited contributions of these factors to SOO risk.
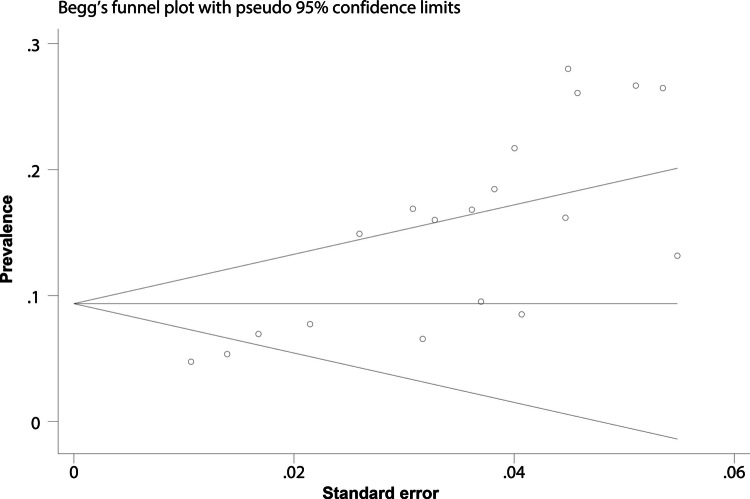
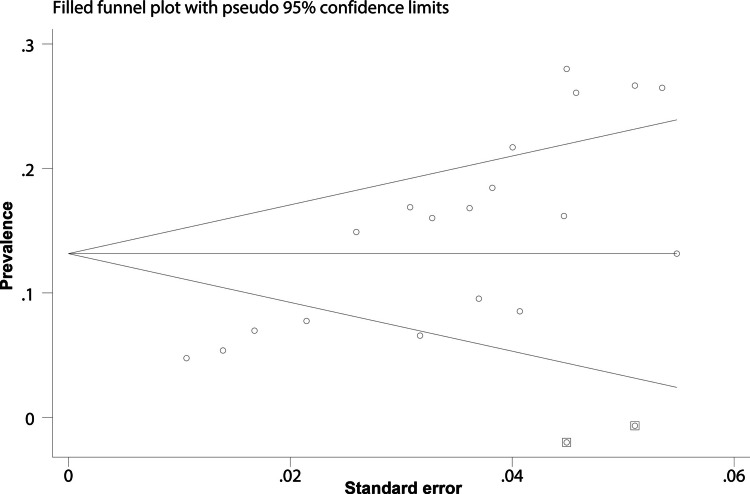
Publication bias
Publication bias was assessed using funnel plots and Egger’s regression test. Visual inspection of the funnel plot indicated asymmetry (Fig. 4), suggesting potential bias. Egger’s regression test confirmed this (p = 0.003). To address bias, the trim-and-fill method was applied and two “missing” studies were identified. After adding these missing studies, the adjusted pooled prevalence was 13.2% (95% CI = 10–16.3%), which was basically consistent with the primary analysis (Fig. 5).
Fig. 4.
Funnel plot for assessing publication bias
Fig. 5.
Funnel plot based on the trim-and-fill method was applied and two “missing” studies were identified
Discussion
Summary of key findings
This meta-analysis provides a comprehensive evaluation of the prevalence and associated risk factors of SOO following colorectal surgery with diverting ileostomy. The pooled prevalence of SOO was estimated at 14%. Notably, HOS, increased rectus abdominis muscle thickness, and laparoscopic surgical approach were identified as significant risk factors for SOO.
Interpretation of results
The overall prevalence of SOO at 14% underscores its clinical significance as a postoperative complication requiring proactive management. Substantial heterogeneity (I2 = 84.9%) among studies suggests that patient selection, surgical approaches, and healthcare practices significantly influence SOO rates. Higher SOO prevalence in patients with benign colorectal conditions may be attributable to more complex surgical procedures and higher physiological demands in this population. For example, IBD and FAP often require extensive resections and reconstructive procedures [29, 30], which may predispose patients to mechanical or functional complications at the stoma site.
The strong association between HOS and SOO (pooled OR = 4.23) emphasizes the role of excessive stoma output in the pathophysiology of SOO. HOS is thought to contribute to SOO through several mechanisms, including bowel distension, impaired motility, and fluid shifts that interfere with normal bowel function. The excessive fluid output can also lead to electrolyte imbalances and dehydration, further complicating postoperative recovery and promoting the development of SOO [31]. Also, HOS can occur as a secondary effect following the resolution of SOO, particularly as bowel function begins to return. However, when present in the perioperative phase, high-output stoma may contribute to the development of SOO due to its effects on fluid balance, electrolyte disturbances, and bowel edema. Proper management of HOS through electrolytes and stoma output control may mitigate its contribution to SOO. This finding underscores the importance of meticulous postoperative monitoring and interventions aimed at controlling stoma output through dietary modifications, pharmacological agents, or fluid management strategies. Increased rectus abdominis muscle thickness, identified as a significant risk factor (pooled OR = 3.51), highlights the anatomical challenges that may arise during stoma formation. Thicker abdominal musculature can compress the stoma lumen, particularly when combined with suboptimal stoma site selection [4, 5]. Preoperative imaging and assessment of abdominal wall thickness may help guide surgical planning to mitigate this risk. Laparoscopic surgery was associated with a fourfold increased risk of SOO. While tactile feedback may be reduced during laparoscopic surgery, the impact on stoma formation is minimized as it is performed extracorporeally. However, the learning curve and the complexity of laparoscopic procedures, particularly for less experienced surgeons, may still contribute to the risk of SOO. Surgeons with less experience or lower procedural volume may have a higher risk of complications, highlighting the importance of surgical training in minimizing this complication [32–34]. These findings suggest that surgeons performing laparoscopic procedures should receive specialized training in stoma formation techniques to minimize complications.
Clinical and public health implications
The findings of this study have important implications for clinical practice and public health. The diagnosis of SOO is often challenging due to its overlap with other postoperative conditions, such as paralytic ileus and small bowel obstruction. Cross-sectional imaging, including CT scans, is crucial to differentiate SOO from these conditions. Identifying HOS as a significant risk factor for SOO underscores the need for vigilant postoperative monitoring and interventions targeting stoma output. Clinicians should consider using fluid thickening agents, dietary modifications, and antidiarrheal medications to manage excessive output and reduce the risk of obstruction. Preoperative assessment of rectus abdominis muscle thickness using imaging modalities such as ultrasound or CT can guide stoma site selection, particularly in patients with a thicker abdominal wall. Surgeons may consider alternative sites or techniques, such as lateralizing the stoma or minimizing tension during creation, to optimize outcomes. The association between laparoscopic surgery and increased SOO risk highlights the importance of skill development and precision in minimally invasive procedures. Institutions should prioritize training programs that focus on stoma formation techniques, emphasizing the importance of achieving optimal stoma angulation and minimizing tension. Furthermore, the higher prevalence of SOO in smaller studies and multi-center studies may reflect variability in surgical expertise or institutional protocols, suggesting the need for standardization of care practices.
Future studies should aim to address the limitations identified in this analysis and provide more granular insights into the mechanisms underlying SOO. Prospective cohort studies with standardized definitions of SOO and consistent diagnostic criteria are essential to validate the findings and enhance comparability across studies. Randomized controlled trials evaluating preventive strategies, such as tailored stoma site selection or enhanced postoperative management protocols, could inform evidence-based guidelines. Research should also focus on elucidating the pathophysiological mechanisms linking HOS to SOO, potentially uncovering novel therapeutic targets. For example, understanding the role of inflammatory mediators, bowel wall remodeling, and mucosal integrity in stoma dysfunction could inform targeted interventions. Additionally, studies exploring the impact of patient-specific factors, such as genetic predisposition or comorbid conditions, on SOO risk may enable personalized surgical and postoperative care. Finally, expanding research beyond Japan to include diverse healthcare settings and populations will improve the generalizability of the findings and identify potential regional or systemic factors influencing SOO outcomes.
Diverting ileostomy is commonly used to mitigate the impact of AL, yet it carries complications such as dehydration, electrolyte imbalances, and SOO. Given these risks, selective use of ileostomy based on AL risk prediction models has gained attention. McKenna et al. and Sassun et al. validated AL risk scores for left- and right-sided colectomies, respectively, demonstrating moderate predictive ability [35, 36]. These models help identify patients at low risk of AL, allowing for more judicious ileostomy use. However, they do not incorporate stoma-related complications like SOO, which can significantly impact patient recovery. Our meta-analysis underscores that SOO is a relevant morbidity associated with ileostomy, warranting consideration alongside AL risk. Future studies should aim to integrate both AL and stoma-related complications into predictive frameworks to optimize ileostomy decision-making.
Strengths and limitations
This meta-analysis has several strengths, including a comprehensive literature search, robust methodology, and subgroup analyses that explored potential sources of heterogeneity. The inclusion of studies with high-quality NOS scores (≥ 7) enhances the credibility of the findings, while sensitivity analyses demonstrated the stability of the pooled prevalence estimates. The analysis also addressed potential publication bias using the trim-and-fill method, which confirmed that the adjusted pooled prevalence remained consistent with the primary estimate.
However, certain limitations should be acknowledged. Firstly, a key limitation of this meta-analysis is the inclusion of studies with various underlying pathologies, such as rectal cancer, colorectal cancer, and inflammatory bowel disease. While this broad approach provides a comprehensive view of SOO, it introduces heterogeneity due to differences in surgical techniques, anatomical variations, and postoperative outcomes. Focusing on a single pathology could have reduced this heterogeneity but would limit the generalizability of the findings. We deliberately included multiple pathologies to enhance the applicability of our results to a wider range of patients, reflecting real-world clinical practice. Despite the heterogeneity, sensitivity analyses showed that it had minimal impact on the pooled estimates, suggesting the robustness of the findings. However, future studies focusing on specific pathologies could provide more precise risk stratification for SOO and lead to more tailored clinical interventions. Secondly, the retrospective nature of most included studies precludes causal inferences between risk factors and SOO. Thirdly, the presence of publication bias, as indicated by funnel plot asymmetry and Egger’s test, suggests that smaller studies with negative findings may be underrepresented in the analysis. Finally, most studies were conducted in Japan, potentially limiting the applicability of the findings to other healthcare settings.
Conclusions
SOO is a prevalent complication following colorectal surgery with diverting ileostomy, with significant risk factors including HOS, increased rectus abdominis muscle thickness, and laparoscopic surgical approach. These findings highlight the importance of individualized patient assessment, meticulous surgical planning, and vigilant postoperative management to mitigate the risk of SOO. Future research should focus on validating these risk factors and developing targeted interventions to improve patient outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to express our gratitude to the authors of the included studies for generously providing the essential information required to conduct our systematic review with meta-analysis.
Author contributions
GJX, LHP, and CK were instrumental in the conception and design of the study. GJX, LHP, and CK were responsible for organizing the database. Statistical analyses were carried out by GJX, LHP, and CK, who also drafted the manuscript and assisted in interpreting the data and further enriched the study with their additional suggestions. The manuscript was thoroughly revised and finalized by CK. All authors have read and agreed to the published version of the manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Institutional review board statement
Not applicable.
Consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Babakhanlou R, Larkin K, Hita AG, Stroh J, Yeung SC (2022) Stoma-related complications and emergencies. Int J Emerg Med 15:17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burch J (2021) An overview of stoma-related complications and their management. Br J Community Nurs 26:390–394 [DOI] [PubMed] [Google Scholar]
- 3.Ihara K, Nakamura T, Takayanagi M, Fujita J, Maeda Y, Nishi Y, Shibuya N, Hachiya H, Ishizuka M, Tominaga K, Kojima K, Irisawa A (2024) Risk factors for stoma outlet obstruction after proctocolectomy for ulcerative colitis. J Anus Rectum Colon 8:18–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitahara T, Sato Y, Oshiro T, Matsunaga R, Nagashima M, Okazumi S (2020) Risk factors for postoperative stoma outlet obstruction in ulcerative colitis. World J Gastrointest Surg 12:507–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsujinaka S, Suzuki H, Miura T, Sato Y, Shibata C (2022) Obstructive and secretory complications of diverting ileostomy. World J Gastroenterol 28:6732–6742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okada S, Hata K, Emoto S, Murono K, Kaneko M, Sasaki K, Otani K, Nishikawa T, Tanaka T, Kawai K, Nozawa H (2018) Elevated risk of stoma outlet obstruction following colorectal surgery in patients undergoing ileal pouch-anal anastomosis: a retrospective cohort study. Surg Today 48:1060–1067 [DOI] [PubMed] [Google Scholar]
- 7.Hara Y, Miura T, Sakamoto Y, Morohashi H, Nagase H, Hakamada K (2020) Organ/space infection is a common cause of high output stoma and outlet obstruction in diverting ileostomy. BMC Surg 20:83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumoto Y, Aisu N, Kajitani R, Nagano H, Yoshimatsu G, Hasegawa S (2024) Complications associated with loop ileostomy: analysis of risk factors. Tech Coloproctol 28:60 [DOI] [PubMed] [Google Scholar]
- 9.Kuwahara K, Mokuno Y, Matsubara H, Uji M, Kobayashi I, Iyomasa S (2022) Risk factors for stoma outlet obstruction: preventing this complication after construction of diverting ileostomy during laparoscopic colorectal surgery. Jma j 5:207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama 283:2008–2012 [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605 [DOI] [PubMed] [Google Scholar]
- 13.Sedgwick P (2015) What is publication bias in a meta-analysis? BMJ 351:h4419 [DOI] [PubMed] [Google Scholar]
- 14.Jennions MD, Møller AP (2002) Publication bias in ecology and evolution: an empirical assessment using the ‘trim and fill’ method. Biol Rev Camb Philos Soc 77:211–222 [DOI] [PubMed] [Google Scholar]
- 15.Fujii T, Morita H, Sutoh T, Yajima R, Tsutsumi S, Asao T, Kuwano H (2015) Outlet obstruction of temporary loop diverting ileostomy. Hepatogastroenterology 62:602–605 [PubMed] [Google Scholar]
- 16.Ohira G, Miyauchi H, Hayano K, Kagaya A, Imanishi S, Tochigi T, Maruyama T, Matsubara H (2018) Incidence and risk factor of outlet obstruction after construction of ileostomy. J Anus Rectum Colon 2:25–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamura K, Matsuda K, Yokoyama S, Iwamoto H, Mizumoto Y, Murakami D, Nakamura Y, Yamaue H (2019) Defunctioning loop ileostomy for rectal anastomoses: predictors of stoma outlet obstruction. Int J Colorectal Dis 34:1141–1145 [DOI] [PubMed] [Google Scholar]
- 18.Abe T, Nishimura J, Yasui M, Matsuda C, Haraguchi N, Nakai N, Wada H, Takahashi H, Omori T, Miyata H, Ohue M (2021) Risk factors for outlet obstruction in patients with diverting ileostomy following rectal surgery. J Anus Rectum Colon 5:254–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enomoto H, Suwa K, Takeuchi N, Hannya Y, Tsukazaki Y, Ushigome T, Okamoto T, Eto K (2021) Risk of outlet obstruction associated with defunctioning loop ileostomy in rectal cancer surgery. Cancer Diagn Progn 1:465–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maemoto R, Tsujinaka S, Miyakura Y, Fukuda R, Kakizawa N, Takenami T, Machida E, Kikuchi N, Kanemitsu R, Tamaki S, Ishikawa H, Rikiyama T (2021) Risk factors and management of stoma-related obstruction after laparoscopic colorectal surgery with diverting ileostomy. Asian J Surg 44:1037–1042 [DOI] [PubMed] [Google Scholar]
- 21.Sasaki S, Nagasaki T, Oba K, Akiyoshi T, Mukai T, Yamaguchi T, Fukunaga Y, Fujimoto Y (2021) Risk factors for outlet obstruction after laparoscopic surgery and diverting ileostomy for rectal cancer. Surg Today 51:366–373 [DOI] [PubMed] [Google Scholar]
- 22.Mori R, Ogino T, Sekido Y, Hata T, Takahashi H, Miyoshi N, Uemura M, Doki Y, Eguchi H, Mizushima T (2022) Long distance between the superior mesenteric artery root and bottom of the external anal sphincter is a risk factor for stoma outlet obstruction after total proctocolectomy and ileal-pouch anal anastomosis for ulcerative colitis. Ann Gastroenterol Surg 6:249–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caprino P, Giambusso M, Sacchetti F, Potenza AE, Pastena D, Panunzi S, Piergentili I, Sofo L (2023) Risk factors and outcomes of restorative proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis. Retrospective study of 75 single center cases. Eur Rev Med Pharmacol Sci 27:1945–1953 [DOI] [PubMed] [Google Scholar]
- 24.Kumano K, Kitaguchi D, Owada Y, Kinoshita E, Moue S, Furuya K, Ohara Y, Enomoto T, Oda T (2023) A comparative study of stoma-related complications from diverting loop ileostomy or colostomy after colorectal surgery. Langenbecks Arch Surg 408:139 [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Wang Y, Lin B, Liu Y, Gu J, Ling L, Xu D, Ding K (2023) Transatmospheric ileal stoma manometry can be applied for the early detection of stoma outlet obstruction. Front Oncol 13:1187858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imaizumi Y, Takano Y, Okamoto A, Nakano T, Takada N, Sugano H, Takeda Y, Ohkuma M, Kosuge M, Eto K (2024) High-output stoma is a risk factor for stoma outlet obstruction in defunctioning loop ileostomies after rectal cancer surgery. Surg Today 54:106–112 [DOI] [PubMed] [Google Scholar]
- 27.Kawai M, Sakamoto K, Honjo K, Okazawa Y, Takahashi R, Kawano S, Munakata S, Sugimoto K, Ishiyama S, Takahashi M, Kojima Y, Tomiki Y (2024) Benefits and risks of diverting stoma creation during rectal cancer surgery. Ann Coloproctol 40:467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogino T, Sekido Y, Mizushima T, Fujii M, Mori R, Takeda M, Hata T, Hamabe A, Miyoshi N, Uemura M, Doki Y, Eguchi H (2024) Temporary loop end ileostomy reduces the risk of stoma outlet obstruction: a comparative clinical study in patients undergoing restorative proctocolectomy and ileal pouch-anal anastomosis. Surg Today. 10.1007/s00595-024-02944-5 [DOI] [PMC free article] [PubMed]
- 29.Coste M, Cao S, Kayal M, Wang YHW, Hahn SJ, Khaitov S, Sylla PA, Dubinsky MC, Plietz MC, Greenstein AJ (2024) A review of early small bowel obstructions in staged IPAA procedures. Surg Endosc 39(1):624–631 [DOI] [PubMed]
- 30.Vasen HFA, Ghorbanoghli Z, de Ruijter B, Trinidad RA, Langers AMJ, Peeters KCMJ, Bonsing BA, Hardwick JCH (2019) Optimizing the timing of colorectal surgery in patients with familial adenomatous polyposis in clinical practice. Scand J Gastroenterol 54:733–739 [DOI] [PubMed] [Google Scholar]
- 31.Hayden DM, Pinzon MC, Francescatti AB, Edquist SC, Malczewski MR, Jolley JM, Brand MI, Saclarides TJ (2013) Hospital readmission for fluid and electrolyte abnormalities following ileostomy construction: preventable or unpredictable? J Gastrointest Surg 17:298–303 [DOI] [PubMed] [Google Scholar]
- 32.Benlice C, Delaney CP, Liska D, Hrabe J, Steele S, Gorgun E (2018) Individual surgeon practice is the most important factor influencing diverting loop ileostomy creation for patients undergoing sigmoid colectomy for diverticulitis. Am J Surg 215:442–445 [DOI] [PubMed] [Google Scholar]
- 33.Tanaka M, Kanemitsu Y, Ueno H, Kobayashi H, Konishi T, Ishida F, Yamaguchi T, Hinoi T, Inoue Y, Tomita N, Ishida H, Sugihara K (2017) Prognostic impact of hospital volume on familial adenomatous polyposis: a nationwide multicenter study. Int J Colorectal Dis 32:1489–1498 [DOI] [PubMed] [Google Scholar]
- 34.Tyler R, Foss H, Phelan L, Radley S, Geh I, Karandikar S (2023) Impact of surgeon volume on 18-month unclosed ileostomy rate after restorative rectal cancer resection. Colorectal Dis 25:253–260 [DOI] [PubMed] [Google Scholar]
- 35.Sassun R, Larson DW, Bews KA, Kelley SR, Mathis KL, Habermann EB, McKenna NP (2024) When is diversion indicated after right-sided colon resections? J Surg Res 303:361–370 [DOI] [PubMed] [Google Scholar]
- 36.McKenna NP, Bews KA, Cima RR, Crowson CS, Habermann EB (2022) Validation of a left-sided colectomy anastomotic leak risk score and assessment of diversion practices. Am J Surg 224:971–978 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.