Abstract
Neurodegenerative diseases, including Alzheimer's and Parkinson's, are characterized by progressive neuronal loss, leading to cognitive and motor impairments. Early diagnosis remains a challenge due to the slow progression of symptoms and the limitations of current diagnostic methods. Nanobiosensors, leveraging the high sensitivity and specificity of nanotechnology, offer a promising, noninvasive, and cost-effective approach for detecting disease biomarkers at ultra-low concentrations. This review highlights recent advancements in nanobiosensor technology, including the integration of gold nanoparticles, quantum dots, and carbon nanotubes, which have significantly enhanced biomarker detection precision. Furthermore, it examines the advantages of nanobiosensors over traditional diagnostic techniques, such as improved sensitivity, rapid detection, and minimal invasiveness. The potential of these innovative sensors to revolutionize early disease detection and improve patient outcomes is discussed, along with existing challenges in clinical translation, including stability, reproducibility, and regulatory considerations. Addressing these limitations will be crucial for integrating nanobiosensors into routine clinical practice and advancing personalized medicine for neurodegenerative disorders.
Keywords: Neurodegenerative diseases, nanobiosensors, Alzheimer's disease, Parkinson's disease, biomarkers, nanotechnology, biosensing, electrochemical sensors
Introduction
Neurodegenerative diseases, which progressively damage the central nervous system (CNS) and lead to a gradual decline in nerve cell function, are challenging to diagnose early due to their slow progression.1,2 Subtle first symptoms may resemble other illnesses. The absence of precise diagnostic tools exacerbates the problem. Early warning indicators may be misinterpreted by both patients and clinicians. Undiagnosed or misdiagnosed patients waste critical therapy time. Earlier treatment slows disease development. Research seeks biomarkers. 3 This trend necessitates early detection. Furthermore, healthcare systems face significant stress. 4 An integrated approach could improve results. Ongoing research aims to develop nanobiosensors for early diagnosis of neurodegenerative diseases, including Alzheimer's and Parkinson's (PD). 5
Nanobiosensors employ nanotechnology for the detection of biological indicators. 6 Their sensitivity is remarkable. Furthermore, specificity is achieved. Their potential is under investigation. 7 Nanotechnology-based nanobiosensors hold promise for revolutionizing healthcare by enabling early and precise diagnosis, which is essential for improving therapeutic outcomes and potentially halting disease progression. 8 This innovative research focuses on enhancing patient outcomes by diagnosing CNS disorders through the detection of specific biomarkers. 9 Neurological disorders correlate with specific biomarkers. Diagnosing CNS disorders poses notable challenges due to the limitations of traditional methods, which are often invasive, costly, and imprecise.10,11 Nanobiosensors provide a promising, noninvasive, and cost-effective method for early detection, potentially enhancing patient outcomes. 12 While still under development, research suggests that these sensors could offer improved biocompatibility and lower risks compared to traditional options. 13 Ongoing studies aim to clarify their capabilities, limitations, and ethical implications. Specifically, nanobiosensors are being explored for better detection of neurodegenerative diseases such as Alzheimer’s and PD, focusing on identifying genetic mutations and environmental risk factors, including toxin exposure.14–18 Nanobiosensors, known for their high sensitivity and specificity, have become valuable in early biomarker detection, although early diagnosis remains challenging. 19 It is common for biomarkers to be found in small amounts in body fluids because they are complicated, have a wide range of protein changes, and are biologically variable in human samples. 20 Conventional detection methods face challenges in accurately identifying specific proteins. Techniques like affinity capture and advanced liquid chromatography-mass spectrometry (LC-MS) enhance protein enrichment and separation, addressing these limitations. 21 Using a panel of biomarkers improves test sensitivity and specificity by providing complementary disease insights. 22 Advanced detection technologies, including biosensors, further enhance biomarker assays in physiological fluids by minimizing matrix interference. 23 Nanobiosensors, leveraging nanomaterials’ unique properties such as increased surface area and reactivity, offer significant advantages in biomarker detection. 24 Increase biomarker-sensor surface interactions. This improves detection limits. Thus, facilitating earlier diagnosis. Nanobiosensors use biorecognition elements as their underlying concept. 25 Nanobiosensors utilize elements like antibodies, aptamers, or enzymes to selectively bind specific biomarkers, with transducers converting these interactions into detectable signals—whether electrical, optical, or mechanical. 26 Researchers are developing electrochemical, optical, and piezoelectric nanobiosensors, each with unique advantages: electrochemical sensors offer high sensitivity and compact design, 27 optical sensors provide real-time monitoring, and piezoelectric sensors ensure precise measurements. 28 The integration of nanotechnology has improved traditional biosensors, enabling simultaneous detection of multiple biomarkers and enhancing diagnostic accuracy.29,30 Nanobiosensors offer promising advances for diagnosing neurodegenerative diseases, despite challenges in achieving stability, reproducibility, and handling complex biological samples that may affect detection performance.31,32 With enhanced sensitivity and specificity, nanobiosensors show promise in facilitating early diagnosis and improving patient outcomes, while ongoing research seeks to resolve current limitations and advance clinical adoption. 33 This review highlights the role of nanobiosensor technology in diagnosing neurodegenerative diseases, particularly Alzheimer's and PD—two age-related disorders of critical neurological importance. Alzheimer's disease primarily impairs memory and cognitive function due to amyloid plaque buildup, causing neuron degeneration and symptoms such as language confusion, mood changes, and behavioral issues.34,35 In contrast, PD involves dopamine depletion, leading to motor-related symptoms like tremors, rigidity, and bradykinesia.36,37 Early diagnosis in both diseases can greatly improve patient quality of life. Nanobiosensors, with their high sensitivity and specificity, show promise for early detection. 38 Traditional diagnostic methods often lack the sensitivity needed, resulting in potential false positives or negatives. For instance, enzyme-linked immunosorbent assays (ELISA) detect cancer biomarkers, such as prostate-specific antigen, at levels of 10–100 ng/mL, whereas nanobiosensors using materials like quantum dots, carbon nanotubes, and gold nanoparticles achieve detection limits down to 10 pg/mL, significantly improving sensitivity.39,40 Constructing nanobiosensors requires careful selection of nanomaterials and biological components to ensure high sensitivity and specificity, along with functionalization to maintain sensor bioactivity. 41 Table 1 illustrates a nanobiosensor application for neurodegenerative disease detection, with added technologies like photonic crystals, microfluidics, and wearable devices enhancing sensitivity, specificity, and user convenience.42–44 These devices are essential for diagnostics, environmental monitoring, and drug discovery applications. 45 Optical microscopy, integrated with nanoparticle tracking and surface-sensitive imaging, allows for biomolecule size analysis on lipid bilayers, while wide-field extinction microscopy provides data on nanoparticle size, shape, and orientation. 43
Table 1.
Nanobiosensor for neurodegenerative disease detection.
| Sensor type | Detection technique | Detection limit | References |
|---|---|---|---|
| Polysaccharide | Optical-based detection | 70 nM | 46 |
| Chemical | Microcantilever sensing | 6 ng | 47 |
| Aptamer | Electrochemical detection | 10 pM | 48 |
| Peptide | Surface plasmon resonance technique | — | 49 |
| Antibody | Conjugation-based method | 13057.0 pg/mL | 50 |
| Antibody | Graphical analysis | Derived from gut | 51 |
| Indirect | Ultrasonication-based detection | Cerebrospinal fluid | 52 |
| Antibody | Enzyme-linked immunosorbent assay | Plasma | 53 |
| Aptamer | Colorimetric assay | 1 nM | 54 |
| Aptamer | Surface plasmon resonance detection | 0.64 fM | 55 |
| Antibody | Voltammetric analysis | 10 aM | 56 |
Incorporating carbon nanotubes and silver nanoparticles into electrodes enhances electrochemical catalysis, improving selectivity and sensitivity with micromolar detection limits. 57 Emerging technologies such as photonic crystals, microfluidics, and wearable systems can significantly improve the sensitivity, specificity, and ease of use of nanobiosensor-based diagnostic tools for neurodegenerative conditions, including Alzheimer’s and PD.
Parkinson's disease
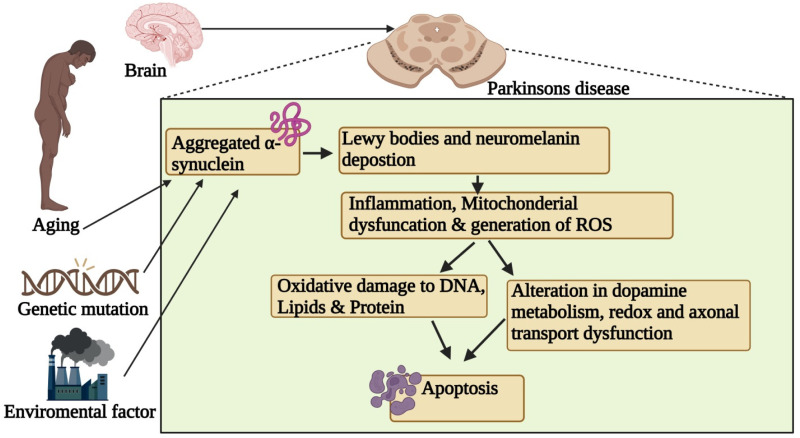
PD is characterized by a progressive decline in motor functions due to the loss of dopamine-releasing neurones, as depicted in Figure 1. This neurodegenerative disorder (NDD) impacts neurones, leading to memory loss and cognitive deficits, as well as issues in coordination, balance, tremors, ambulation difficulties, and rigidity. The onset of PD symptoms is gradual, intensifying over time, typically affecting individuals aged 50 and above, regardless of gender. PD is primarily linked to the degeneration or destruction of neurones in the brain responsible for regulating bodily movements. The resulting decrease in dopamine release causes movement disorders. Additionally, patients with PD experience a loss of nerve endings that produce norepinephrine, contributing to further neurological impairments. Currently, no specific treatments exist for PD, and available therapies, procedures, and drugs aim only to mitigate its symptoms.58–61
Figure 1.
Parkinson's disease pathophysiology.
Therapeutic options for PD encompass proteins such as human glial cell line-derived neurotrophic factor (hGDNF), which has demonstrated potential efficacy.
Ansorena et al. 62 devised a rapid and efficient method for producing high concentrations of pure hGDNF using mammalian cell-derived technology. Moreover, nanoparticle (NP)-mediated biomarker identification has shown potential for diagnosing PD (Table 1). PD is characterized by the degeneration of dopaminergic neurones in the substantia nigra and a decrease in dopamine levels in the striatum, leading to motor symptoms including tremors, rigidity, and cognitive decline. A significant feature of PD is α-synuclein amyloid accumulation, a disease hallmark. Adam et al. 63 designed a biosensor for detecting α-synuclein aggregation, a critical biomarker, facilitating early diagnosis and management of PD. Additionally, ZnO nanocomposites on aluminum microelectrodes serve as effective substrates for antibody binding to the α-synuclein antigen. Aghili et al. 64 developed an electrochemical nanobiosensor targeting circulating biomarkers like miR-195 for early PD detection.
Gold nanowires (GNWs) and exfoliated graphene oxide (EGO) were utilized to modify a screen-printed carbon electrode. A thiolated single-stranded probe selectively hybridized to miRNA-195, while doxorubicin acted as an electrochemical indicator for differential pulse voltammetry. This miR-195-based nanobiosensor shows promise as a diagnostic instrument for PD.
Aging, genetic mutations, and environmental factors contribute to α-synuclein aggregation, forming Lewy bodies that trigger inflammation, mitochondrial dysfunction, and ROS generation, ultimately leading to oxidative stress, DNA damage, altered dopamine metabolism, axonal dysfunction, and dopaminergic neuron apoptosis.
Alzheimer's disease
The degenerative neurological condition Alzheimer's causes cognitive decline and memory loss.
It affects critical functions such as communication, cognitive reasoning, and the ability to perform daily tasks. Early symptoms include trouble recalling recent conversations, problem-solving difficulties, and disorientation related to time and place. 65 As Alzheimer’s disease advances, patients experience worsening memory impairment and a decline in language abilities, which makes it challenging to express thoughts clearly. 66 Behavioral symptoms, such as mood fluctuations, agitation, and aggression, may also emerge. 67 Alzheimer's disease is caused by genetic, environmental, and lifestyle factors that lead to amyloid plaque buildup and tau tangles, resulting in neuronal damage and cell death. 68 The hippocampus, essential for memory, is notably impacted. Aging is the primary risk factor, with most patients over age 65, although early-onset cases can affect younger individuals. A family history of Alzheimer's and certain health conditions, such as cardiovascular disease and diabetes, further elevate risk. 69
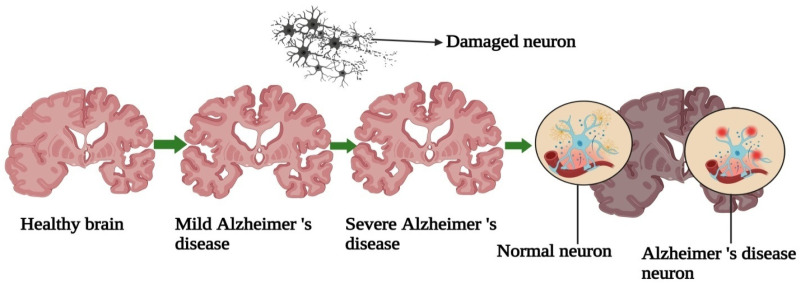
Diagnosis involves a thorough assessment, including physical, neurological, and mental exams, as well as brain imaging to exclude other conditions. Emerging cerebrospinal fluid (CSF) biomarkers improve diagnostic accuracy. 70 While Alzheimer's lacks a cure, cholinesterase inhibitors and memantine provide temporary cognitive improvements, aiding symptom management and quality of life. Nonpharmacological approaches, like cognitive therapy and social engagement, are also crucial. The exact cause of the cause of Alzheimer's is unknown, however genetic, environmental, and lifestyle factors may contribute. Familial predisposition is a crucial genetic factor that raises the risk. 34 Researchers are also examining environmental influences, though the interaction between these factors is complex. Understanding these interactions may enable earlier detection and intervention. Oxidative stress, caused by an imbalance between reactive oxygen species (ROS) and antioxidant defenses, contributes significantly to Alzheimer's disease progression. 71 The brain's high oxygen demand and lipid content make it particularly susceptible to ROS damage, impairing proteins, lipids, and DNA, leading to neuronal dysfunction and cell death.72,73 This oxidative stress accelerates synaptic dysfunction—a precursor to neuronal death and cognitive decline—by interfering with essential synaptic processes, thereby creating a cycle that worsens disease progression.74–76 Understanding this relationship may uncover new therapeutic targets, with antioxidants being explored as potential treatments. Although antioxidants have shown promise in reducing oxidative damage, clinical trial results have been inconsistent, suggesting that addressing multiple disease pathways may be more effective. 77 The global Alzheimer's population is over 50 million and estimated to reach 152 million by 2050. The societal and economic impact of Alzheimer's is immense, with global costs reaching an estimated $1 trillion annually. Despite extensive research, a cure remains elusive. Figure 2 compares the brain and neuronal structures of healthy individuals with those affected by Alzheimer's. 78
Figure 2.
Structures and neurons of healthy and Alzheimer's brains.
While amyloid-beta is a well-known biomarker, other potential biomarkers, such as the tau protein, may also be detected using advanced technologies like nanobiosensors. Acetylcholine-generating subcortical nuclei have a structure with similar properties. The buildup of hyperphosphorylated tau protein forms neurofibrillary tangles, a key feature of Alzheimer's disease.79,80 Tau levels in CSF and neuroimaging techniques offer insights into disease mechanisms and support early diagnosis, with neuroinflammatory markers also emerging as useful diagnostic indicators. 81 Neuroinflammation, marked by increased inflammatory cytokines and activated microglia, is essential to Alzheimer's disease progression and may serve as a valuable biomarker for enhancing diagnostic accuracy and understanding of the disease. 82
Role of nanobiosensors in Alzheimer's biomarker detection
Nanobiosensors integrate nanotechnology with biosensing mechanisms to detect biological markers with high sensitivity and specificity. Their application in Alzheimer's diagnosis enhances the detection of amyloid-beta and tau proteins in biological fluids like blood, saliva, and CSF, allowing for noninvasive, rapid, and cost-effective screening.3,83
Electrochemical nanobiosensors: These sensors utilize nanomaterials such as gold nanoparticles, carbon nanotubes, and graphene to improve electron transfer and enhance detection sensitivity. They can detect amyloid-beta peptides and phosphorylated tau proteins at ultra-low concentrations, significantly outperforming conventional immunoassays. 84
Optical nanobiosensors: Leveraging techniques like surface plasmon resonance (SPR), fluorescence resonance energy transfer (FRET), and quantum dot-based detection, optical nanobiosensors enable real-time monitoring of biomarker interactions. These sensors have shown promise in detecting amyloid-beta aggregates with high specificity. 85
Piezoelectric nanobiosensors: Utilizing nanoscale materials such as zinc oxide nanowires and quartz crystal microbalances, piezoelectric biosensors detect biomarker interactions by measuring frequency shifts upon binding of amyloid-beta or tau proteins, providing label-free and highly sensitive analysis. 86
Magnetic nanobiosensors: These sensors use magnetic nanoparticles (MNPs) conjugated with antibodies or aptamers to selectively capture and detect biomarkers in complex biological samples. They enhance sensitivity and enable efficient biomarker separation, reducing background noise in detection assays. 87
Surface-enhanced Raman spectroscopy (SERS)-based nanobiosensors: These sensors exploit the unique optical properties of nanomaterials, such as gold and silver nanoparticles, to amplify Raman signals from biomarker molecules. This technique allows for ultra-sensitive detection of amyloid-beta and tau proteins at very low concentrations. 85
Multiple sclerosis
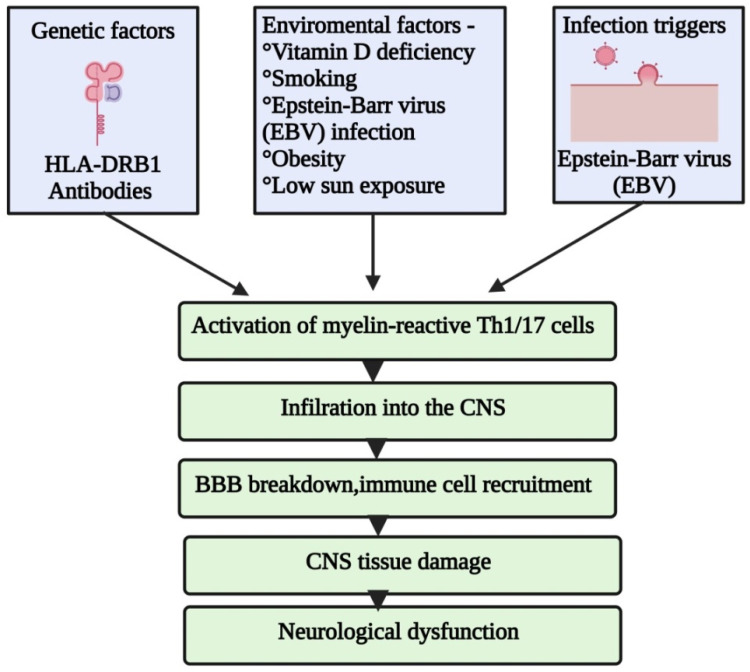
Multiple sclerosis (MS) involves inflammatory lesions in the brain and spinal cord's white matter, leading to axonal loss, neuronal damage, and demyelination (Figure 3). 87 Peripheral macrophages cross a compromised blood–brain barrier (BBB), contributing to axonal injury and demyelination, while leukocyte infiltration occurs due to increased adhesion molecule synthesis. 85 Advances in nanoscience provide novel strategies for diagnosing neurological diseases and developing therapies. Neuroimaging techniques like CT, MRI, and magnetic resonance spectroscopy have proven effective in monitoring neurodegenerative illnesses.88–91 Nanotechnology improves imaging techniques and supports the creation of novel diagnostic methods for CNS diseases.92–94 The heterogeneity of MS complicates the identification of a universal diagnostic marker. However, a synthetic glycoprotein antigen probe, CSF114(Glc), has shown promise in detecting autoantibodies associated with MS. 95 Additionally, a gold SPR biosensor was developed to differentiate MS patients from healthy individuals, although it exhibited low sensitivity but high specificity. 96 Nanoparticle-based biomarker detection is also being explored for MS diagnosis.
Figure 3.
Pathophysiology of multiple sclerosis: potential factors that cause MS.
Nanobiosensors in MS diagnosis
Recent advancements in nanobiosensor technology have significantly improved the detection of MS biomarkers. These sensors offer enhanced sensitivity, specificity, and real-time monitoring capabilities. Notable developments include:
Electrochemical biosensors: These devices have been engineered to detect specific MS-related biomarkers such as neurofilament light chain (NfL), myelin basic protein (MBP), and chitinase-3-like protein 1 (CHI3L1). Research has demonstrated that graphene-based electrochemical sensors can detect NfL at picomolar concentrations, improving diagnostic accuracy. 97
SPR sensors: SPR-based nanobiosensors have shown potential in differentiating MS patients from healthy individuals. Gold nanoparticle-enhanced SPR sensors exhibit high specificity in detecting autoantibodies linked to MS, facilitating early-stage diagnosis with minimal invasiveness. 98
Quantum dot-based biosensors: These nanobiosensors leverage fluorescent quantum dots for highly sensitive biomarker detection. Recent studies indicate that quantum dots conjugated with antibodies against MBP can identify early demyelination events, providing a novel approach for MS diagnosis. 99
Point-of-care (POC) nanobiosensors: Wearable and portable biosensors are emerging as viable tools for real-time MS monitoring. These devices, integrated with microfluidic chips, enable rapid biomarker detection in CSF and blood, allowing continuous disease tracking. 99
Magnetic nanoparticle-based biosensors: Functionalized MNPs are used for the highly selective capture and detection of MS-related biomarkers. MNP-based biosensors enhance signal amplification, improving the detection of MS-specific autoantibodies. 97
Graphene-based field-effect transistor (FET) Biosensors: These highly sensitive biosensors utilize graphene's unique electrical properties to detect MS biomarkers at ultra-low concentrations, making them valuable for early-stage diagnosis. 100
Nanopore biosensors: Nanopore-based biosensors offer real-time, label-free detection of MS-associated protein biomarkers. These sensors provide high specificity by analyzing single-molecule interactions within a confined nanopore environment. 101
Optical fiber biosensors: Using fiber optic technology, these biosensors detect MS biomarkers with high precision by measuring changes in refractive index, enabling noninvasive and real-time diagnostics. 102
Aptamer-based biosensors: Aptamer-functionalized nanobiosensors provide a highly selective approach for MS biomarker detection. These biosensors utilize synthetic DNA or RNA aptamers that bind to specific proteins associated with MS, improving diagnostic accuracy. 103
Nanocantilever biosensors: These biosensors detect molecular interactions by measuring mechanical deflections in nanocantilever structures, offering high sensitivity for detecting MS-associated proteins in biofluids. 104
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig's disease, is a fast-progressing NDD that primarily affects motor neurons, leading to muscle weakness, paralysis, and eventual respiratory failure. ALS affects about 1–3 people per 100,000 worldwide, with a typical survival of 2 to 4 years after diagnosis, and currently has no effective treatments. 101 Recent studies in biomarker research indicate that the Bcl-2-SODox complex, particularly in sporadic and familial ALS cases, might act as a biomarker due to mutant SOD1 protein's interaction with mitochondrial Bcl-2. 102 While elevated cystatin C levels in CSF have been associated with ALS progression, they do not predict disease onset. 103 A three-protein CSF biomarker panel, including VGF and cystatin C, showed 91% sensitivity and 97% specificity in predicting ALS, outperforming individual proteins. 104 A panel of 14 biomarkers associated with iron homeostasis, growth factors, and cytokines distinguished ALS patients from healthy controls with 89.2% accuracy, 87.5% sensitivity, and 91.2% specificity. 103
Nanobiosensor applications in ALS diagnosis
Nanobiosensors integrate biological recognition elements with nanomaterial-based transducers, offering high sensitivity, specificity, and real-time monitoring capabilities. The following nanobiosensor platforms have shown significant potential for ALS biomarker detection:
Electrochemical nanobiosensors
Electrochemical biosensors utilize conductive nanomaterials such as carbon nanotubes (CNTs), graphene, and gold nanoparticles (AuNPs) to enhance signal transduction. These sensors detect biomarkers through redox reactions, generating measurable electrical signals. Some notable applications in ALS diagnosis include. 84
Graphene-based electrochemical sensors for detecting neurofilament proteins in blood samples with high sensitivity. 105
Gold nanoparticle-functionalized electrodes for the rapid quantification of TDP-43 aggregates in CSF. 106
Screen-printed carbon electrodes (SPCEs) modified with nanomaterials to enhance signal amplification and facilitate portable ALS diagnostics. 107
Enzyme-based electrochemical biosensors for detecting oxidative stress markers in ALS patients. 108
Nanozymes in electrochemical sensors to mimic enzymatic activity and enhance biomarker detection. 109
Optical nanobiosensors
Optical biosensors rely on fluorescence, SPR, and colorimetric assays to detect ALS-related biomarkers with high specificity. Key innovations include: 85
Quantum dot (QD)-based biosensors for the multiplexed detection of ALS biomarkers such as TDP-43 and SOD1. 110
SERS biosensors for real-time monitoring of neurofilament levels in biofluids. 111
Localized LSPR sensors functionalized with aptamers to detect ALS-related protein aggregates at ultra-low concentrations. 112
FRET sensors for detecting misfolded protein aggregates in ALS. 113
Biosensors integrated with microfluidic platforms for high-throughput ALS biomarker screening. 114
Nanoplasmonic and piezoelectric biosensors
Nanoplasmonic biosensors utilize metal nanoparticles to enhance light-matter interactions, improving the sensitivity of ALS biomarker detection. 112
Piezoelectric biosensors employ nanomaterials such as zinc oxide (ZnO) nanowires to measure the mechanical changes induced by biomarker binding, allowing label-free detection of ALS-associated proteins. 115
Magnetoplasmonic biosensors that combine magnetic and plasmonic properties for improved biomarker specificity. 116
Molecularly imprinted polymer (MIP) nanobiosensors designed to selectively capture ALS biomarkers. 117
Advantages of nanobiosensors in ALS detection. 118
The application of nanobiosensors for ALS diagnostics provides several key advantages:
Early detection: The ultra-high sensitivity of nanobiosensors enables the detection of ALS biomarkers at pre-symptomatic stages, allowing for timely intervention.
Noninvasive sampling: Many nanobiosensors are designed for use with blood, saliva, or urine, reducing the need for invasive lumbar punctures.
POC testing: Portable and cost-effective biosensor platforms facilitate ALS screening outside of traditional laboratory settings.
Rapid and real-time analysis: Unlike conventional assays, nanobiosensors provide immediate readouts, improving diagnostic turnaround time.
Multiplexing capabilities: Advanced biosensors can simultaneously detect multiple ALS biomarkers, enhancing diagnostic accuracy and specificity.
Importance of early diagnosis
Neurodegenerative disorders are typically detected late, after significant neuron loss and reduced neuroplasticity, when symptoms manifest.115–117 This delay contributes to the inefficacy of conventional symptomatic treatments.118–120 Preventative treatments and early (preclinical) diagnosis could extend quality of life during the preclinical phase, yet no effective early diagnostic methods exist.121,122 Neurological disorders, resulting from brain or nervous system dysfunction, rank as the second leading cause of global disability, with approximately 80% of cases occurring in low- and middle-income countries. No single test can conclusively diagnose most neurological diseases. Multiple diagnostic methods, including EEG, EMG, MRI, enzymatic assays, and genetic testing like PCR, are employed. 123 However, traditional diagnostic techniques face challenges such as automation, cost, and accuracy. Advanced biosensors and nanobiosensors are emerging as promising tools for early disease detection and improving diagnosis.124,125
Role of nanobiosensors in early diagnosis
Nanobiosensors offer a highly sensitive and specific approach to detecting neurodegenerative disease biomarkers in their early stages. These sensors integrate nanotechnology with biosensing elements, allowing for real-time, noninvasive, and cost-effective diagnostics. Compared to conventional methods, nanobiosensors can detect lower concentrations of biomarkers in biological fluids such as CSF, plasma, and serum, significantly improving early disease identification.
Examples of nanobiosensor technologies
Electrochemical nanobiosensors—provide high sensitivity for detecting neurotransmitters like dopamine and biomarkers like α-synuclein, aiding in early PD diagnosis. 3
Optical nanobiosensors—utilize quantum dots and plasmonic nanoparticles to enhance fluorescence-based detection, enabling precise identification of amyloid-beta plaques associated with Alzheimer's disease. 85
Piezoelectric nanobiosensors—detect molecular interactions at ultra-low concentrations, facilitating the early detection of multiple NDDs. 86
Magnetoplasmonic nanobiosensors—combine magnetic and plasmonic properties to improve the specificity of biomarker detection for neurodegenerative diseases. 116
Microfluidic nanobiosensors—integrate lab-on-a-chip technology to enable high-throughput screening of biomarkers, enhancing early detection efficiency. 118
SERS nanobiosensors—Utilize nanostructured substrates to amplify Raman signals, allowing ultra-sensitive detection of neurodegenerative disease biomarkers. 95
FET-based nanobiosensors—use semiconductor nanomaterials for real-time, label-free detection of biomolecules with high specificity. 126
Importance of nano-transducers
Regardless of the condition's contagiousness, healing and therapy hinge on a prompt and precise diagnosis. Immunofluorescence, ELISA, and microscopic techniques are therapeutically relevant; nonetheless, they possess some drawbacks, such as elevated costs, imprecise results, low specificity, awkward application, and restricted sensitivity. 127 To address the previously mentioned disadvantages, the clinical demand for high-throughput, biocompatible, efficient, and rapid diagnostic techniques remains unmet. Over recent decades, various sensing methods have been used to detect biomarkers linked to both communicable and noncommunicable diseases. 128 These technologies employ high-conductivity electrodes that may track or identify electroactive proteins relevant to a medical condition, hence providing a robust signal. 118 All the aforementioned properties are incorporated in a sensing apparatus known as a biosensor. Consequently, employing biosensors for the detection of biological markers may represent a feasible approach.
Recent advancements in biosensor technology have initiated transformative shifts across various sectors, including food processing, agriculture, healthcare, and biological research. 95 Biosensors are now categorized based on their biological component, which can be a nucleic acid, enzyme, or antibody, and their transducing element, which may be calorimetric, optical, acoustic, or electrochemical.126,129 The large surface area of nanoparticles enhances their role as transducers, indicating that incorporating nanomaterials into electrical systems may advance transduction mechanisms in nanoelectromechanical systems (NEMS). 130 Modern biosensors offer distinct advantages, including increased observability, faster response times, higher sensitivity and accuracy, and improved outcomes compared to earlier glucose- and chemical-based biosensors. 131 Biosensors employ tissue-specific macromolecules, organic organelles, microbes, enzymes, and immunosensors (antibodies) as detection methods. The transduction methods rely on the physiochemical differences produced by detection and sensing mechanisms. Consequently, many biosensor transducer mechanisms encompass calorimetric, optical, piezoelectric, and electrochemical methods. 132 Acoustic and ultrasonic represent two prominent biosensor methodologies utilizing piezoelectric transducers; electrochemical, amperometric, and conductometric constitute three principal mechanisms of electrochemical transducer biosensors; and optical transducer biosensor mechanisms encompass fluorescence, absorbance, and chemiluminescence (CL).124,133,134
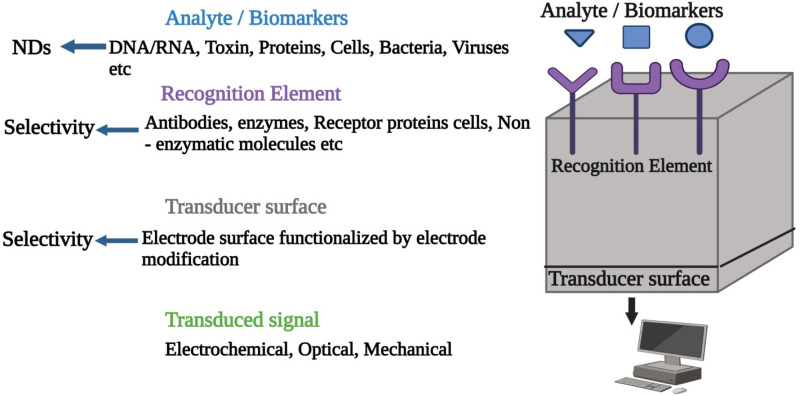
Biosensors operate based on cell signaling, and as previously mentioned, the essential components of an effective biosensor are electronic parameters, a biotransducer, and a biorecognition element, which may encompass a monitor/display, amplifier, and microprocessor. 135 The biorecognition element, or bioreceptor, is tailored to detect a specific analyte, allowing for evaluation of its properties. 136 The transducer then converts this interaction into a measurable signal, which is processed and amplified by electrical components, with the output signal's strength reflecting the analyte concentration. 137 In an amperometric sensor, the bioreceptor includes a specialized biomaterial placed near the transducer or an inactivated enzyme. The analyte undergoes a chemical interaction with the biomaterial. This leads to the creation of a novel analyte that yields a measurable electrical response. Occasionally, an analyte is conveyed to the system, which is subsequently discharged, cooled, or heated using hydrogen ions or electrons. The transducer may subsequently regulate the corresponding mechanism and transform it into electrical signals that could be computed and modified.133,137,138
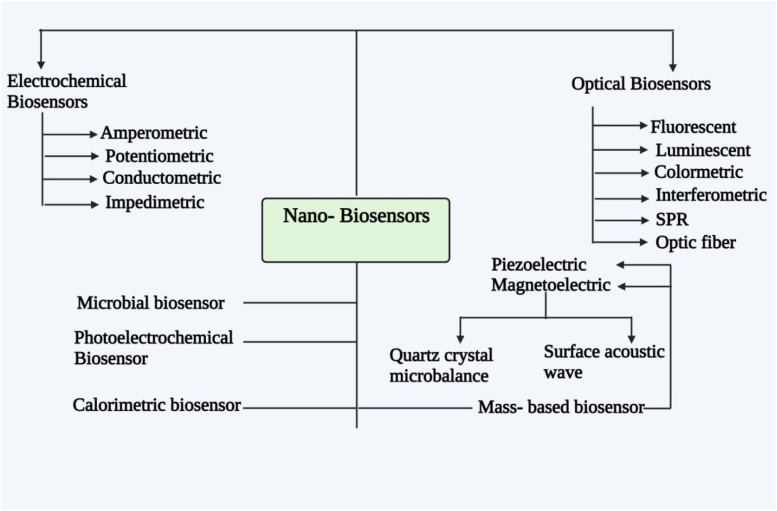
A biosensor's functionality depends on its core components, including the specific bioreceptor (e.g. antibody, enzyme, DNA) and the transduction mechanism used to detect targets. The transducer generates a small electrical signal, typically against a high baseline, which is then refined by extracting a baseline signal from an uncoated transducer for accuracy. Due to the biosensor's relatively slow reaction rate, issues like electrical noise are minimized, resulting in an analog signal output that can be digitized and processed by a microprocessor. This processed data is then either stored or analyzed as needed. Biosensors can operate in vivo or ex vivo, sourcing data directly from environmental or biological parameters. 139 Consequently, Figure 4 illustrates various types of biosensors, including mass-based, calorimetric, photoelectrochemical, microbiological, optical, and electrochemical biosensors, along with their subcategories. Electrochemical biosensors utilize electrochemical transducers and are categorized into impedimetric, conductometric, potentiometric, and amperometric types. Conversely, optical biosensors use an optical transducer paired with a biorecognition element and are classified into types like optical fiber, SPR, interferometric, colorimetric, luminescent, and fluorescent sensors. Microbial biosensors incorporate microbial components as transducers to produce measurable signals corresponding to analyte concentration
Figure 4.
Several of the most prevalent biosensor types and subtypes.
Photoelectrochemical biosensors capitalize on the photon-to-electricity conversion process that transpires simultaneously with photon absorption and charge separation. Calorimetric biosensors measure heat changes during chemical reactions, while mass-based biosensors detect binding events through changes in surface acoustic wave oscillation due to mass increases on the sensor surface. 139
Biomarkers
Biomarkers facilitate more accurate and rapid disease diagnosis, stratify patient populations to identify individuals likely to respond to pharmacological therapies, confirm that medication is effectively targeting the CNS or peripheral nervous system (PNS), and offer prognostic insights regarding disease progression. They also determine optimal drug responses. Biomarkers will enable preclinical drug development and the identification of potential therapeutic targets. Moreover, biomarkers may function as an essential connection between the human patient population and preclinical disease models, with shared biomarkers providing crucial mechanistic links and identifying potential therapy targets within the model system. Due to the intrinsic heterogeneity of the patient population with neurodegenerative diseases, which is characterized by numerous and sporadic genetic variants, patient stratification through biomarkers is essential. This approach significantly reduces the number of patients required for clinical studies and enhances clinical trial design. 133
Biomarkers exist in various forms, including imaging, biochemical, and genetic markers, and are measured by many methods. Currently, genetic mutations linked to a specific NDD are the most clinically relevant biomarkers, but emerging technologies are uncovering additional biomarkers for epigenetic modifications, microRNAs, and messenger RNA. Recent findings indicate that long noncoding RNAs function as biomarkers for certain neurological disorders and play a role in neurodegenerative mechanisms. A substantial amount of research is exploring the potential of extracellular RNAs as biomarkers for human diseases, such as brain injuries and NDDs. Genetic markers for neurodegenerative diseases span noncoding RNA, RNA, and DNA, with RNA-based biomarkers conserved across neurodegenerative diseases. This suggests common molecular pathways and interconnected functions among RNAs and genes across CNS cell types. 133 The recent failure of Alzheimer's disease-modifying drugs may indicate that patients in clinical trials were already in compromised states. Validated biomarkers are essential for the diagnosis and detection of preclinical stages of Alzheimer's, facilitating the development of therapeutic interventions. The integration of biomarkers from biological fluids, including CSF, with neuropsychological evaluations and molecular imaging may enhance diagnostic specificity and sensitivity, facilitating the identification of patients in early stages when pharmacological interventions are most viable. Biomarkers such as brain hypoperfusion or hypometabolism assessed via 18F-fluorodeoxyglucose (FDG)-PET, brain atrophy through MRI, increased CSF tau and/or phosphotau concentrations, decreased CSF amyloid-1–42 peptide (A42) levels, and affirmative amyloid or tau PET imaging have all been identified as indicators of progression to Alzheimer's disease, with clinical potential.
Although biochemical biomarkers are still developing, they have potential as pharmacodynamic tools in clinical trials, facilitating pharmaceutical development and serving as diagnostic tools. Thus, there is an urgent need to expand biomarkers for PD and to accelerate the identification of biomarkers that can detect the transition from pre-motor to motor symptoms. Nonmotor symptoms, such as mood disturbances, olfactory deficits, gastrointestinal dysfunction, and sleep disturbances, often precede motor symptoms in PD but are not exclusive to the disease. Therefore, biomarkers predicting the course from nonmotor to motor symptoms would be highly valuable in research assessing pharmacological interventions aimed at halting disease progression. Additional biomarkers are required for monitoring pharmaceutical efficacy in disease progression and in clinical trials, possibly combining biochemical, imaging, and genetic markers. 134
Many studies on nanoparticles for PD diagnostics have emphasized dopamine nanobiosensors. 140 Shin et al. 141 engineered silver–molybdenum disulfide (Ag/MoS₂) nanoparticles to improve electrochemical signaling for dopamine detection, showing promise for diagnostic applications in PD. Additionally, Vazquez-Guardado et al. 138 developed an enzyme-free dopamine biosensor that combines a nanostructured plasmonic substrate with a plasma-separating microfluidic chip and oxygen-deficient cerium oxide (CeO₂) nanoparticles, enabling label-free detection of antigens and biomarkers in unprocessed bodily fluids.
Optical biosensors
Specificity and sensitivity are key metrics in evaluating biosensors, which typically consist of a biological recognition element to capture the target analyte and a sensing component that converts biological interactions into measurable signals. The specificity is determined by the combination of biological sensing elements and recognition mechanisms, whereas sensitivity largely depends on the receptor, which can include a microbe, enzyme, nucleic acid (such as an aptamer), or antibody.
Recent advances have focused on improving the immobilization process, substrate, and procedural methodologies to enhance sensitivity. Nanomaterials with unique optical properties, including quantum dots, carbon nanotubes, graphene, and metallic nanoparticles (e.g. silver and gold) Have been increasingly employed to amplify the detection of targets. Quantum dots are favored for fluorescence detection due to their broad absorption spectrum and resistance to photobleaching. Metallic nanoparticles play a crucial role in amplifying signals for SERS-based detection, making optical biosensors with nanomaterials central to modern analytical diagnostics, particularly in cancer diagnosis. They offer short detection times, ease of use, and high sensitivity. Optical detection techniques for exosomal cancer biomarkers include fluorescence, electrochemiluminescence (ECL), CL, immunochromatographic assay (ICA), colorimetric analysis, SPR, and SERS. 138 Shawky et al. 140 developed a simplified optical biosensor utilizing gold nanoparticles for detecting nucleic acid transcripts, specifically tyrosyl DNA phosphodiesterase 2 (TDP2) and topoisomerase 1 (TOP1), which are linked to neurological and malignant conditions. This gold aggregating gold (GAG) assay offers rapid RNA quantification, serving as an alternative to traditional methods like real-time PCR. Haes et al. 142 A nanoscale optical biosensor utilizing localized SPR was developed to detect antibodies against amyloid-derived diffusible ligands (ADDLs), presenting an innovative diagnostic approach for Alzheimer's disease by analyzing human brain extracts and CSF from Alzheimer's patients and healthy controls.
Electrochemical biosensors
The fundamental principle of the electrochemical sensor, illustrated in Figure 5, involves the application of a variable or constant voltage to the electrode, leading to alterations in the detection material on the electrode surface, which generates an electrical signal. 142 Electrochemical sensors are utilized to ascertain the electrochemical and electrical properties of target substances or molecules for quantitative or qualitative detection and analysis. Chemically modified electrodes are currently a prominent subject of investigation in the field of electrochemistry. Most current research centers on electrochemical biosensors, classified into affinity and catalytic types. Catalytic biosensors leverage enzymes’ transfer and catalytic properties; however, maintaining enzyme activity in neutral conditions imposes strict operational and material requirements and adds significant cost. 141
Figure 5.
Basic concept of a biosensor system and the major components used to detect NDs.
Consequently, developing more efficient methods for building electrochemical enzyme-free sensors, stabilizing immobilized media, and identifying effective electron transfer media are among the critical challenges that require advancement in this field. Electrochemical sensors offer high sensitivity compared to other sensing methods, including calorimetric, magnetic, optical, piezoelectric, and acoustic techniques, while also being cost-effective, portable, and compatible with microfabrication, making them popular in therapeutic settings. Neurobiological signs exist in minimal amounts in biological fluids, requiring the implementation of highly sensitive detection technologies. 143
Since 1962, when Lyons and Clark introduced the glucose enzyme electrode, Numerous techniques have been established to alter electrode surfaces in biosensors, enhancing accuracy, sensitivity, and selectivity.143,144 Enzyme-based electrochemical sensors, building on this innovation, offer high selectivity due to enzyme specificity, allowing precise detection of individual enzymes in complex samples. 145 Nonetheless, alterations in environmental conditions, like the pH and temperature, substantially impact the activity of enzymes and other physiologically active compounds, significantly limiting the applicability of enzyme biosensors. The production process for the enzyme sensor is intricate, and inconsistent thickness may lead to reduced repeatability and oxygen interference. 146 Furthermore, enzyme-based biosensors may incur high costs attributed to the significant expense of enzymes. 147
The utilization and advancement of nonenzyme biosensors represent a novel research domain. Various enzyme-free methods are available for detecting biomarkers linked to neurodegenerative diseases, including colorimetric sensors, surface-enhanced Raman scattering biosensors, fluorescence imaging sensors, and electrochemical techniques. 148 Khalilzadeh et al. 149 developed an electrochemical method utilizing microRNA (miR) to identify miR-146a, a recognised biomarker for neurological disorders. The gold substrate was functionalized with capture microRNA (C-miR) to detect target microRNA (T-miR) miR-146a in a bioassay, using an optimized C-miR concentration for capturing T-miR on the electrode surface. Results from unprocessed human blood samples show that this biosensor effectively detects miR-146a, a potential biomarker for neurodegenerative diseases. Liu et al. describe a similar ratiometric electrochemical biosensor for detecting trinucleotide repeat sequences d(CAG)n, employing Exo III-assisted amplification on a graphene-modified electrode. This biosensor, with dual signals from ferrocene- and methylene blue-labeled DNAs, offers a reliable method for d(CAG)n repeat analysis and potential clinical application in neurodegenerative disease diagnostics (Figure 5).
Nanobiosensor
A sensor is a device that detects and measures an analyte within a sample. 150 In a biosensor, a biological reaction is sensed and converted into an electrical signal, involving a transducer and a detection system (receptor). Biosensors convert biological reactions from enzymes, tissues, or cells into electrical signals via a transducer, with their efficacy relying on stable biological elements and sensor interactions. Nanobiosensors leverage nanotechnology to enhance detection accuracy and sensitivity, making them essential tools in medical diagnostics, environmental monitoring, and food safety. Their compact size supports minimally invasive applications, enabling regular health monitoring.151–153 In environmental settings, nanobiosensors detect low-concentration pollutants and toxins, allowing for timely interventions, while in food safety, they expedite the identification of contaminants like pathogens and pesticides.154,155
Nanobiosensors comprise three core elements—a bioreceptor, transducer, and electronic system—that detect target molecules and transform these interactions into quantifiable signals. Known for their compactness, portability, and ability to provide real-time data, these sensors have advanced with new nanomaterials, enhancing their utility in personal healthcare and disease monitoring.155,156 Nanobiosensors also show promise as an alternative to traditional diagnostics like neuroimaging, offering enhanced sensitivity and specificity. 157 Nanoscale components, including nanoparticles and nanostructures, enhance nanobiosensor sensitivity through enzyme immobilization, improving both testing efficiency and cost-effectiveness.158,159 The integration of nanotechnology with electrochemical methods has also increased the speed and reliability of these sensors. 160 This advancement holds significant potential for transformative applications in disease management, notably for early cancer detection. 161 Nanobiosensors have demonstrated their utility in diagnosing chronic conditions like kidney disease and tuberculosis. 162 Some nanobiosensors operate without labels, allowing the detection of difficult-to-tag molecules, expanding their application in healthcare. 163 They significantly improve molecular diagnostics, offering more sensitive and rapid biomarker detection. 164 Nanomaterials, including nanoparticles and carbon nanotubes, are increasingly utilized in biosensor platforms to improve performance and expand application scope. 165 A notable advancement is multiplexed nanobiosensors, enabling simultaneous detection of multiple analytes for a thorough patient assessment. 166 Additionally, microfluidics and lab-on-a-chip technologies have further enhanced these capabilities 167 (Table 2).
Table 2.
PD: a selection of NP-based assays for the identification of particular indicators.
| Marker of disorder | NP | Diagnosis modality | Experiment type | BBB crossing | Ref. |
|---|---|---|---|---|---|
| α-Synclein | Immuno/magnetic particle | Immunoassay | In vitro | Not applicable | 168 |
| Gold nanorod | Surface plasmon | In vitro | Not applicable | 169 | |
| DA Receptor | Immuno-targeted far-red QDs | Fluorescence | In vivo (acute rat brain slide) | Intraventricular injection | 170 |
| DA | PEGylated PFPBA NPs (100 nm) | Near-infrared fluorescence | In vivo (mouse) | Yes | 171 |
| PEGylated PFPBA NPs (120 nm) | Fluorescence quenching | In vivo (zebrafish) | Yes | 172 |
Nanobiosensors for neurodegenerative disease diagnosis
Nanobiosensors significantly enhance clinical diagnostics by integrating biological detectors with sensor-transducer systems, offering high sensitivity and specificity in disease detection, including PD. 25 Recent advancements in nanofabrication, as demonstrated by Kalinke et al., 173 have improved diagnostic accuracy for biomarkers like dopamine and ascorbic acid. Carbon paste electrodes modified with carbon nanotubes and silver nanoparticles 174 and zinc oxide nanowire arrays 173 have effectively lowered detection limits for biomarkers, such as dopamine and uric acid, critical in Parkinson's diagnosis. Additionally, electrochemical and immunosensors facilitate the precise detection of alpha-synuclein, a key protein linked to the disease.175,176 Innovations in molecular fingerprint polymers and graphene-based biosensors have demonstrated superior analytical performance, enabling real-time, in vivo detection with exceptional sensitivity.162,177,178 These advancements indicate that nanobiosensors may transform the early diagnosis and management of neurodegenerative diseases through accurate, real-time monitoring of disease progression. 178
Future prospective
Nanobiosensor technology shows significant potential for advancing neurodegenerative disease diagnostics. With ongoing research, sensor miniaturization and performance improvements are making these devices increasingly suitable for POC and routine screening applications. Wearable nanobiosensors, for instance, enable real-time biomarker monitoring, potentially allowing early detection of diseases like Alzheimer's and PD, even before symptoms appear. Furthermore, integrating nanobiosensors with artificial intelligence and machine learning can enhance diagnostic accuracy through complex data analysis and predictive capabilities. Multiplexed biosensors, which detect multiple biomarkers simultaneously, will facilitate differential diagnosis based on distinct biomarker profiles. Furthermore, innovations in nanomaterial synthesis will enhance the sensor stability, biocompatibility, and sensitivity, supporting their reliability in clinical applications. These advancements could drive widespread adoption of nanobiosensors for early intervention and better management of neurodegenerative diseases. Future research should also focus on addressing current challenges, such as regulatory approval, cost reduction, and large-scale manufacturing, to ensure that these technologies can be effectively integrated into healthcare systems worldwide.
Conclusion
The early diagnosis of neurodegenerative diseases is crucial for improving patient outcomes and slowing disease progression. Traditional diagnostic methods often fail to provide early detection due to their invasive nature and limited sensitivity. Nanobiosensors represent a groundbreaking advancement, integrating the specificity of biological recognition elements with the unparalleled sensitivity of nanomaterials. By enabling ultra-sensitive and real-time biomarker detection, these innovative tools offer a noninvasive and highly accurate diagnostic approach.
Nanotechnology-driven biosensing systems have revolutionized early disease detection by significantly enhancing biomarker specificity, stability, and signal amplification. The integration of quantum dots, carbon nanotubes, and gold nanorods has led to superior diagnostic accuracy, reducing the risks of false positives and negatives. Furthermore, the real-time monitoring capabilities of electrochemical, optical, and piezoelectric biosensors make them indispensable in the field of neurodegenerative diagnostics.
Despite their immense potential, challenges such as reproducibility, biocompatibility, and large-scale clinical translation remain. Future advancements in sensor miniaturization, multiplexed detection, and artificial intelligence-driven analysis will further refine nanobiosensor applications, making them an essential component of personalized medicine. As these sensors continue to evolve, they have the potential to revolutionize neurodegenerative disease management by facilitating early intervention, enhancing patient monitoring, and ultimately transforming the landscape of neurological healthcare.
Acknowledgments
The authors are thankful to Kampala International University and Galgotias University for providing the necessary facilities to conduct this research work.
Abbreviations
- CNS
central nervous system
- LC-MS
liquid chromatography-mass spectrometry
- ELISA
enzyme-linked immunosorbent assays
- PD
Parkinson's disease
- hGDNF
human glial cell line-derived neurotrophic factor
- GNWs
Gold nanowires
- EGO
exfoliated graphene oxide
- MS
Multiple sclerosis
- BBB
blood–brain barrier
- ALS
Amyotrophic lateral sclerosis
- NEMS
nanoelectromechanical systems
- CL
chemiluminescence
- PNS
peripheral nervous system
- NDD
neurodegenerative disorder
- FDG
fluorodeoxyglucose
- Ag/MoS2
silver-molybdenum disulfide
- SERS
surface-enhanced Raman scattering
- ECL
electrochemiluminescence
- ICA
immunochromatographic assay
- SPR
surface plasmon resonance
- TDP2
tyrosyl DNA phosphodiesterase 2
- TOP1
topoisomerase 1
- GAG
gold aggregating gold
- ADDLs
amyloid-derived diffusible ligands
- miR
microRNA
Footnotes
ORCID iD: Sarad Pawar Naik Bukke https://orcid.org/0000-0002-5693-2953
Consent to publication: All authors read and agreed to the final copy of the findings as contained in the manuscript.
Author contributions: Shikha Yadav and Ananda Kumar Chettupalli conceived and designed the study. Ajaypal Singh, Shatrudhan Prajapati, Shikha Yadav, Ananda Kumar Chettupalli, Buyinza Nicholas and Sarad Pawar Naik Bukke, carried out the study, analyzed and interpreted the data. Buyinza Nicholas, Ananda Kumar Chettupalli, Sarad Pawar Naik Bukke, drafted the manuscript and revised the manuscript. All authors read and make the final corrections.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Data availability statement: The datasets/information used for this study are available on within the manuscript.
Declarations: I hereby declare that this submission is entirely my own work, in my own words, and that all sources used in researching it are fully acknowledged and all quotations properly identified.
References
- 1.Lamptey RNL, Chaulagain B, Trivedi R, et al. A review of the common neurodegenerative disorders: current therapeutic approaches and the potential role of nanotherapeutics. Int J Mol Sci [Internet] 2022. [cited 2024 Dec 1]; 23: 1851. https://www.mdpi.com/1422-0067/23/3/1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakraborty S. Study of Neurodegenerative Disease Progression and Early Detection using Deep Learning Algorithms . Graduate School, Inje University, 2020. [Google Scholar]
- 3.Porsteinsson AP, Isaacson RS, Knox S, et al. Diagnosis of early Alzheimer’s disease: clinical practice in 2021. J Prev Alzheimers Dis 2021; 8: 371–386. [DOI] [PubMed] [Google Scholar]
- 4.Deuschl G, Beghi E, Fazekas F, et al. The burden of neurological diseases in Europe: an analysis for the global burden of disease study 2017. Lancet Public Health [Internet] 2020. [cited 2024 Dec 1]; 5: e551–e567. https://linkinghub.elsevier.com/retrieve/pii/S2468266720301900 [DOI] [PubMed] [Google Scholar]
- 5.Choi HK, Choi J-H, Yoon J. An updated review on electrochemical nanobiosensors for neurotransmitter detection. Biosensors [Internet] 2023. [cited 2024 Dec 1]; 13: 892. https://www.mdpi.com/2079-6374/13/9/892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdel-Karim R. Nanotechnology-Enabled biosensors: a review of fundamentals, materials, applications, challenges, and future scope. Biomed Mater Devices [Internet] 2024. [cited 2024 Dec 1]; 2: 759–777. https://link.springer.com/10.1007/s44174-023-00147-z [Google Scholar]
- 7.Trevethan R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front Public Health 2017; 5: 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Setyati R, Astuti A, Utami T, et al. The importance of early detection in disease management. J World Future Med Health Nurs 2024; 2: 51–63. [Google Scholar]
- 9.Valenzuela-Amaro HM, Aguayo-Acosta A, Meléndez-Sánchez ER, et al. Emerging applications of nanobiosensors in pathogen detection in water and food. Biosensors [Internet] 2023. [cited 2024 Dec 1]; 13: 922. https://www.mdpi.com/2079-6374/13/10/922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khazaei M, Hosseini MS, Haghighi AM, et al. Nanosensors and their applications in early diagnosis of cancer. Sens Bio-Sens Res [Internet] 2023. [cited 2024 Dec 1]; 41: 100569. https://linkinghub.elsevier.com/retrieve/pii/S2214180423000211 [Google Scholar]
- 11.Erkmen C, Uslu B, Tiğ GA. Nanobiosensors: construction and diagnosis of disease. In: Azad UP, Chandra P. (eds) Handb nanobioelectrochemistry [internet]. Singapore: Springer Nature Singapore, 2023. [cited 2024 Dec 1], pp.639–660. https://link.springer.com/10.1007/978-981-19-9437-1_29 [Google Scholar]
- 12.Thakur M, Wang B, Verma ML. Development and applications of nanobiosensors for sustainable agricultural and food industries: recent developments, challenges and perspectives. Environ Technol Innov [Internet] 2022. [cited 2024 Dec 1]; 26: 102371. https://linkinghub.elsevier.com/retrieve/pii/S2352186422000530 [Google Scholar]
- 13.Motas JG, Gorji NE, Nedelcu D, et al. XPS, SEM, DSC and nanoindentation characterization of silver nanoparticle-coated biopolymer pellets. Appl Sci [Internet] 2021. [cited 2024 Dec 1]; 11: 7706. https://www.mdpi.com/2076-3417/11/16/7706 [Google Scholar]
- 14.Fazlali P, Mahdian A, Soheilifar MS, et al. Nanobiosensors for early detection of neurodegenerative disease. J Compos Compd [Internet] 2022. [cited 2024 Dec 1]; 4: 31–40. https://jourcc.com/index.php/jourcc/article/view/jcc414 [Google Scholar]
- 15.Wang H, Yang F, Zhang S, et al. Genetic and environmental factors in Alzheimer’s and Parkinson’s diseases and promising therapeutic intervention via fecal microbiota transplantation. Npj Park Dis [Internet] 2021. [cited 2024 Dec 1]; 7: 70. https://www.nature.com/articles/s41531-021-00213-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García J-C, Bustos R-H. The genetic diagnosis of neurodegenerative diseases and therapeutic perspectives. Brain Sci [Internet] 2018. [cited 2024 Dec 1]; 8: 222. https://www.mdpi.com/2076-3425/8/12/222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nabi M, Tabassum N. Role of environmental toxicants on neurodegenerative disorders. Front Toxicol 2022; 4: 837579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar S, Singh H, Feder-Kubis J, et al. Recent advances in nanobiosensors for sustainable healthcare applications: a systematic literature review. Environ Res 2023; 238: 117177. [DOI] [PubMed] [Google Scholar]
- 19.Tolosa E, Garrido A, Scholz SW, et al. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol 2021; 20: 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zanganeh S, Abbasgholinejad E, Doroudian M, et al. The current landscape of glioblastoma biomarkers in body fluids. Cancers [Internet] 2023. [cited 2024 Dec 1]; 15: 3804. https://www.mdpi.com/2072-6694/15/15/3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo J, Kufer R, Li D, et al. Technical advancement and practical considerations of LC-MS/MS-based methods for host cell protein identification and quantitation to support process development. mAbs [Internet] 2023. [cited 2024 Dec 1]; 15: 2213365. https://www.tandfonline.com/doi/full/10.1080/19420862.2023.2213365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodaghi A, Fattahi N, Ramazani A. Biomarkers: promising and valuable tools towards diagnosis, prognosis and treatment of Covid-19 and other diseases. Heliyon [Internet] 2023. [cited 2024 Dec 1]; 9: e13323. https://linkinghub.elsevier.com/retrieve/pii/S2405844023005303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh SU, Chatterjee S, Lone SA, et al. Advanced wearable biosensors for the detection of body fluids and exhaled breath by graphene. Microchim Acta [Internet] 2022. [cited 2024 Dec 1]; 189: 236. https://link.springer.com/10.1007/s00604-022-05317-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang X, Zhu Y, Kianfar E. Nano biosensors: properties, applications and electrochemical techniques. J Mater Res Technol [Internet] 2021. [cited 2024 Dec 1]; 12: 1649–1672. https://linkinghub.elsevier.com/retrieve/pii/S2238785421002751 [Google Scholar]
- 25.Banigo A, Azeez T, Ejeta K, et al. Nanobiosensors: applications in biomedical technology. IOP Conf Ser Mater Sci Eng [Internet] 2020. [cited 2024 Dec 1]; 805: 012028. https://iopscience.iop.org/article/10.1088/1757-899X/805/1/012028 [Google Scholar]
- 26.Antiochia R. Nanobiosensors as new diagnostic tools for SARS, MERS and COVID-19: from past to perspectives. Microchim Acta [Internet] 2020. [cited 2024 Dec 1]; 187: 639. http://link.springer.com/10.1007/s00604-020-04615-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang W, Wang R, Luo F, et al. Miniaturized electrochemical sensors and their point-of-care applications. Chin Chem Lett [Internet] 2020. [cited 2024 Dec 1]; 31: 589–600. https://linkinghub.elsevier.com/retrieve/pii/S1001841719305509 [Google Scholar]
- 28.Momin RT. Piezoelectric Sensors for Real-time Monitoring and Quality Control in Additive Manufacturing [Internet]. arXiv; 2023 [cited 2024 Dec 1]. https://arxiv.org/abs/2310.14321
- 29.Malik S, Singh J, Goyat R, et al. Nanomaterials-based biosensor and their applications: a review. Heliyon [Internet] 2023. [cited 2024 Dec 1]; 9: e19929. https://linkinghub.elsevier.com/retrieve/pii/S2405844023071372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rohilla D, Chaudhary S; Department of Chemistry and Centre of Advanced Studies in Chemistry, Panjab University, Chandigarh 160014, India, Umar A, Department of Chemistry, College of Science and Arts, Najran University, Najran-11001, Kingdom of Saudi Arabia, Promising Centre for Sensors and Electronic Devices (PCSED), Najran University, Najran-11001, Kingdom of Saudi Arabia: an overview of advanced nanomaterials for sensor applications. Eng Sci [Internet] 2021; 16: 47–70. [cited 2024 Dec 1]. http://www.espublisher.com/journals/articledetails/552 [Google Scholar]
- 31.Yang T, Duncan TV. Challenges and potential solutions for nanosensors intended for use with foods. Nat Nanotechnol 2021; 16: 251–265. [DOI] [PubMed] [Google Scholar]
- 32.Lo PK. Nanometre-Scale biosensors revolutionizing applications in biomedical and environmental research. Biosensors [Internet] 2023. [cited 2024 Dec 1]; 13: 969. https://www.mdpi.com/2079-6374/13/11/969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krajcovicova L, Klobusiakova P, Rektorova I. Gray matter changes in Parkinson’s and Alzheimer’s disease and relation to cognition. Curr Neurol Neurosci Rep [Internet] 2019. [cited 2024 Dec 1]; 19: 85. http://link.springer.com/10.1007/s11910-019-1006-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.2024 Alzheimer’s disease facts and figures. Alzheimers Dement J Alzheimers Assoc 2024; 20: 3708–3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anawal L, Chandakavate S, Lalitha S, et al. A comprehensive review on Alzheimer’s disease. World J Pharm Pharm Sci 2021; 10: 1170–1185. [Google Scholar]
- 36.Olson M, Lockhart TE, Lieberman A. Motor learning deficits in Parkinson’s disease (PD) and their effect on training response in gait and balance: a narrative review. Front Neurol 2019; 10: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jankovic J, Tan EK. Parkinson’s disease: etiopathogenesis and treatment. J Neurol Neurosurg Psychiatry [Internet] 2020. [cited 2024 Dec 1]; 91: 795–808. https://jnnp.bmj.com/lookup/doi/10.1136/jnnp-2019-322338 [DOI] [PubMed] [Google Scholar]
- 38.Rather SA, Mustafa RA, Ashraf MV, et al. Implications of nano-biosensors in the early detection of neuroparasitic diseases. In: Gautam A, Chaudhary V. (eds) Theranostic Appl Nanotechnol Neurol Disord [Internet]. Singapore: Springer Nature Singapore, 2023. [cited 2024 Dec 1], pp.43–83. https://link.springer.com/10.1007/978-981-99-9510-3_3 [Google Scholar]
- 39.Garg S, Sachdeva A, Peeters M, et al. Point-of-care prostate specific antigen testing: examining translational progress toward clinical implementation. ACS Sens 2023; 8: 3643–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Darwish MA, Abd-Elaziem W, Elsheikh A, et al. Advancements in nanomaterials for nanosensors: a comprehensive review. Nanoscale Adv 2024; 6: 4015–4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu Y, Liu T, Wang H, et al. Applications of nanomaterial technology in biosensing. J Sci Adv Mater Devices [Internet] 2024. [cited 2024 Dec 1]; 9: 100694. https://linkinghub.elsevier.com/retrieve/pii/S246821792400025X [Google Scholar]
- 42.Malmir N, Fasihi K. A highly-sensitive label-free biosensor based on two dimensional photonic crystals with negative refraction. J Mod Opt [Internet] 2017. [cited 2024 Dec 1]; 64: 2195–2200. https://www.tandfonline.com/doi/full/10.1080/09500340.2017.1346828 [Google Scholar]
- 43.Malmir K, Okell W, Trichet AAP, et al. Characterization of nanoparticle size distributions using a microfluidic device with integrated optical microcavities. Lab Chip [Internet] 2022. [cited 2024 Dec 1]; 22: 3499–3507. https://xlink.rsc.org/?DOI=D2LC00180B [DOI] [PubMed] [Google Scholar]
- 44.Safarkhani M, Aldhaher A, Heidari G, et al. Nanomaterial-assisted wearable glucose biosensors for noninvasive real-time monitoring: pioneering point-of-care and beyond. Nano Mater Sci [Internet] 2024. [cited 2024 Dec 1]; 6: 263–283. https://linkinghub.elsevier.com/retrieve/pii/S2589965123000776 [Google Scholar]
- 45.Rabiee N, Akhavan O, Fatahi Y, et al. CaZnO-based nanoghosts for the detection of ssDNA, pCRISPR and recombinant SARS-CoV-2 spike antigen and targeted delivery of doxorubicin. Chemosphere 2022; 306: 135578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giordano C, Albani D, Gloria A, et al. Nanocomposites for neurodegenerative diseases: hydrogel-nanoparticle combinations for a challenging drug delivery. Int J Artif Organs 2011; 34: 1115–1127. [DOI] [PubMed] [Google Scholar]
- 47.Khatri A, Punjabi N, Ghosh D, et al. Detection and differentiation of α-Synuclein monomer and fibril by chitosan film coated nanogold array on optical sensor platform. Sens Actuators B Chem [Internet] 2018; 255: 115–127. [Google Scholar]
- 48.Hijaz BA, Volpicelli-Daley LA. Initiation and propagation of α-synuclein aggregation in the nervous system. Mol Neurodegener [Internet] 2020. [cited 2024 Dec 1]; 15: 19. https://molecularneurodegeneration.biomedcentral.com/articles/10.1186/s13024-020-00368-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taghdisi SM, Danesh NM, Nameghi MA, et al. A novel electrochemical aptasensor based on nontarget-induced high accumulation of methylene blue on the surface of electrode for sensing of α-synuclein oligomer. Biosens Bioelectron [Internet] 2019. [cited 2024 Dec 1]; 123: 14–18. https://linkinghub.elsevier.com/retrieve/pii/S0956566318307796 [DOI] [PubMed] [Google Scholar]
- 50.Mollenhauer B, Bowman FD, Drake D, et al. Antibody-based methods for the measurement of α-synuclein concentration in human cerebrospinal fluid – method comparison and round robin study. J Neurochem [Internet] 2019. [cited 2024 Dec 1]; 149: 126–138. https://onlinelibrary.wiley.com/doi/10.1111/jnc.14569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao H, Zhao Z, He Z, et al. Detection of Parkinson’s disease through the peptoid recognizing α-synuclein in serum. ACS Chem Neurosci [Internet] 2019. [cited 2024 Dec 1]; 10: 1204–1208. https://pubs.acs.org/doi/10.1021/acschemneuro.8b00540 [DOI] [PubMed] [Google Scholar]
- 52.Ng ASL, Tan YJ, Lu Z, et al. Plasma alpha-synuclein detected by single molecule array is increased in PD. Ann Clin Transl Neurol 2019; 6: 615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Altay MF, Kumar ST, Burtscher J, et al. Development and validation of an expanded antibody toolset that captures alpha-synuclein pathological diversity in Lewy body diseases. NPJ Park Dis 2023; 9: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kakuda K, Ikenaka K, Araki K, et al. Ultrasonication-based rapid amplification of α-synuclein aggregates in cerebrospinal fluid. Sci Rep [Internet] 2019. [cited 2024 Dec 1]; 9: 6001. https://www.nature.com/articles/s41598-019-42399-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Youssef P, Kim WS, Halliday GM, et al. Comparison of different platform immunoassays for the measurement of plasma alpha-synuclein in Parkinson’s disease patients. J Park Dis [Internet] 2021. [cited 2024 Dec 1]; 11: 1761–1772. https://journals.sagepub.com/doi/full/10.3233/JPD-212694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun K, Xia N, Zhao L, et al. Aptasensors for the selective detection of alpha-synuclein oligomer by colorimetry, surface plasmon resonance and electrochemical impedance spectroscopy. Sens Actuators B Chem [Internet] 2017. [cited 2024 Dec 1]; 245: 87–94. https://linkinghub.elsevier.com/retrieve/pii/S0925400517301788 [Google Scholar]
- 57.Akhavan O, Ghaderi E, Rahighi R. Toward single-DNA electrochemical biosensing by graphene nanowalls. ACS Nano [Internet] 2012. [cited 2024 Dec 1]; 6: 2904–2916. https://pubs.acs.org/doi/10.1021/nn300261t [DOI] [PubMed] [Google Scholar]
- 58.Jang SJ, Lee C-S, Kim TH. α-Synuclein oligomer detection with aptamer switch on reduced graphene oxide electrode. Nanomater Basel Switz 2020; 10: 832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adam H, Gopinath SCB, Krishnan H, et al. Cyclic and differential pulse voltammetric measurements on fibrils formation of alpha synuclein in Parkinson’s disease by a gold interdigitated tetraelectrodes. Process Biochem [Internet] 2024. [cited 2024 Dec 1]; 136: 212–220. https://linkinghub.elsevier.com/retrieve/pii/S1359511323003793 [Google Scholar]
- 60.Benabid AL, Chabardes S, Mitrofanis J, et al. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol 2009; 8: 67–81. [DOI] [PubMed] [Google Scholar]
- 61.Cacciatore I, Baldassarre L, Fornasari E, et al. Recent advances in the treatment of neurodegenerative diseases based on GSH delivery systems. Oxid Med Cell Longev [Internet] 2012. [cited 2024 Dec 1]; 2012: 1–12. http://www.hindawi.com/journals/omcl/2012/240146/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carradori D, Eyer J, Saulnier P, et al. The therapeutic contribution of nanomedicine to treat neurodegenerative diseases via neural stem cell differentiation. Biomaterials 2017; 123: 77–91. [DOI] [PubMed] [Google Scholar]
- 63.Ansorena E, Casales E, Aranda A, et al. A simple and efficient method for the production of human glycosylated glial cell line-derived neurotrophic factor using a Semliki Forest virus expression system. Int J Pharm 2013; 440: 19–26. [DOI] [PubMed] [Google Scholar]
- 64.Adam H, Gopinath SCB, Hashim U. Integration of aluminium interdigitated electrodes with zinc oxide as nanocomposite for selectively detect alpha-synuclein for Parkinson’s disease diagnosis. J Phys Conf Ser [Internet] 2021. [cited 2024 Dec 1]; 2129: 012094. https://iopscience.iop.org/article/10.1088/1742-6596/2129/1/012094 [Google Scholar]
- 65.Alzheimer Soc. Risk factors [Internet]. 2018. https://alzheimer.ca/sites/default/files/documents/Risk-factors_Alzheimer-Society-Canada-2023.pdf
- 66.Yang S-Y, Chiu M-J, Lin C-H, et al. Development of an ultra-high sensitive immunoassay with plasma biomarker for differentiating Parkinson disease dementia from Parkinson disease using antibody functionalized magnetic nanoparticles. J Nanobiotechnology [Internet] 2016. [cited 2024 Dec 1]; 14: 41. http://jnanobiotechnology.biomedcentral.com/articles/10.1186/s12951-016-0198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar J, Eraña H, López-Martínez E, et al. Detection of amyloid fibrils in Parkinson’s disease using plasmonic chirality. Proc Natl Acad Sci U S A 2018; 115: 3225–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khan J, Yadav S, Alam M. Potential neuroprotective strategies using smart drug delivery systems for Alzheimer’s disease. Infect Disord - Drug Targets [Internet] 2024. [cited 2024 Dec 1]; 24: e231023222565. https://www.eurekaselect.com/222565/article [DOI] [PubMed] [Google Scholar]
- 69.Feng P, Chen Y, Zhang L, et al. Near-Infrared fluorescent nanoprobes for revealing the role of dopamine in drug addiction. ACS Appl Mater Interfaces 2018; 10: 4359–4368. [DOI] [PubMed] [Google Scholar]
- 70.Qian C-G, Zhu S, Feng P-J, et al. Conjugated polymer nanoparticles for fluorescence imaging and sensing of neurotransmitter dopamine in living cells and the brains of zebrafish Larvae. ACS Appl Mater Interfaces 2015; 7: 18581–9. [DOI] [PubMed] [Google Scholar]
- 71.Bouwman FH, Frisoni GB, Johnson SC, et al. Clinical application of CSF biomarkers for Alzheimer’s disease: from rationale to ratios. Alzheimers Dement Diagn Assess Dis Monit [Internet] 2022. [cited 2024 Dec 1]; 14: e12314. https://alz-journals.onlinelibrary.wiley.com/doi/10.1002/dad2.12314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bai R, Guo J, Ye X-Y, et al. Oxidative stress: the core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res Rev [Internet] 2022. [cited 2024 Dec 1]; 77: 101619. https://linkinghub.elsevier.com/retrieve/pii/S1568163722000617 [DOI] [PubMed] [Google Scholar]
- 73.Singh A, Kukreti R, Saso L, et al. Oxidative stress: a key modulator in neurodegenerative diseases. Molecules [Internet] 2019. [cited 2024 Dec 1]; 24: 1583. https://www.mdpi.com/1420-3049/24/8/1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tauffenberger A, Magistretti PJ. Reactive oxygen Species: beyond their reactive behavior. Neurochem Res 2021; 46: 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Subramanian J, Savage JC, Tremblay M-È. Synaptic loss in Alzheimer’s disease: mechanistic insights provided by two-photon in vivo imaging of transgenic mouse models. Front Cell Neurosci [Internet]. 2020. [cited 2024 Dec 1]; 14: 592607. https://www.frontiersin.org/articles/10.3389/fncel.2020.592607/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davinelli S, Medoro A, Savino R, et al. Sleep and oxidative stress: current perspectives on the role of NRF2. Cell Mol Neurobiol [Internet] 2024. [cited 2024 Dec 1]; 44: 52. https://link.springer.com/10.1007/s10571-024-01487-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ansari MA, Rao MS, Al-Jarallah A. Insights into early pathogenesis of sporadic Alzheimer’s disease: role of oxidative stress and loss of synaptic proteins. Front Neurosci 2023; 17: 1273626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chaudhary P, Janmeda P, Docea AO, et al. Oxidative stress, free radicals and antioxidants: potential crosstalk in the pathophysiology of human diseases. Front Chem [Internet] 2023. [cited 2024 Dec 1]; 11: 1158198. https://www.frontiersin.org/articles/10.3389/fchem.2023.1158198/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Subramanian A, Tamilanban T, Alsayari A, et al. Trilateral association of autophagy, mTOR and Alzheimer’s disease: potential pathway in the development for Alzheimer’s disease therapy. Front Pharmacol 2022; 13: 1094351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sinsky J, Pichlerova K, Hanes J. Tau protein interaction partners and their roles in Alzheimer’s disease and other tauopathies. Int J Mol Sci [Internet] 2021. [cited 2024 Dec 1]; 22: 9207. https://www.mdpi.com/1422-0067/22/17/9207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rawat P, Sehar U, Bisht J, et al. Phosphorylated tau in Alzheimer's disease and other tauopathies. Int J Mol Sci 2022; 23: 12841. Published 2022 Oct 25.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cáceres C, Heusser B, Garnham A, et al. The Major hypotheses of Alzheimer’s disease: related nanotechnology-based approaches for its diagnosis and treatment. Cells 2023; 12: 2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deuschl G, Beghi E, Fazekas F, et al. The burden of neurological diseases in Europe: an analysis for the global burden of disease study 2017. Lancet Public Health 2020; 5: e551–e567. [DOI] [PubMed] [Google Scholar]
- 84.Negahdary M, Angnes L. Electrochemical aptamer-based nanobiosensors for diagnosing Alzheimer’s disease: a review. Mater Sci Eng C Mater Biol Appl 2022; 135: 112689. [DOI] [PubMed] [Google Scholar]
- 85.Gao F, Li F, Wang J, et al. SERS-based optical nanobiosensors for the detection of Alzheimer’s disease. Biosensors 2023; 13: 880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jang J, Kim K, Yoon J, et al. Piezoelectric materials for ultrasound-driven dissociation of Alzheimer’s β-amyloid aggregate structure. Biomaterials 2020; 255: 120165. [DOI] [PubMed] [Google Scholar]
- 87.Kaushik A, Jayant RD, Tiwari S, et al. Nano-biosensors to detect beta-amyloid for Alzheimer’s disease management. Biosens Bioelectron 2016; 80: 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cai Y, Liu J, Wang B, et al. Microglia in the neuroinflammatory pathogenesis of Alzheimer’s disease and related therapeutic targets. Front Immunol [Internet] 2022. [cited 2024 Dec 1]; 13: 856376. https://www.frontiersin.org/articles/10.3389/fimmu.2022.856376/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Petry KG, Boiziau C, Dousset V, et al. Magnetic resonance imaging of human brain macrophage infiltration. Neurother J Am Soc Exp Neurother 2007; 4: 434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blezer ELA, Deddens LH, Kooij G, et al. In vivo MR imaging of intercellular adhesion molecule-1 expression in an animal model of multiple sclerosis. Contrast Media Mol Imaging 2015; 10: 111–121. [DOI] [PubMed] [Google Scholar]
- 91.Silverman DHS, Alavi A. PET Imaging in the assessment of normal and impaired cognitive function. Radiol Clin North Am 2005; 43: 67–77, x. [DOI] [PubMed] [Google Scholar]
- 92.Tucker KA, Robertson KR, Lin W, et al. Neuroimaging in human immunodeficiency virus infection. J Neuroimmunol 2004; 157: 153–162. [DOI] [PubMed] [Google Scholar]
- 93.Silva GA. Neuroscience nanotechnology: progress, opportunities and challenges. Nat Rev Neurosci 2006; 7: 65–74. [DOI] [PubMed] [Google Scholar]
- 94.Burn DJ, O’Brien JT. Use of functional imaging in Parkinsonism and dementia. Mov Disord Off J Mov Disord Soc 2003; 18: S88–S95. [DOI] [PubMed] [Google Scholar]
- 95.Wearne SL, Rodriguez A, Ehlenberger DB, et al. New techniques for imaging, digitization and analysis of three-dimensional neural morphology on multiple scales. Neuroscience 2005; 136: 661–680. [DOI] [PubMed] [Google Scholar]
- 96.Bocti C, Rockel C, Roy P, et al. Topographical patterns of lobar atrophy in frontotemporal dementia and Alzheimer’s disease. Dement Geriatr Cogn Disord 2006; 21: 364–372. [DOI] [PubMed] [Google Scholar]
- 97.Kharati M, Foroutanparsa S, Rabiee M, et al. Early diagnosis of multiple sclerosis based on optical and electrochemical biosensors: comprehensive perspective. Curr Anal Chem [Internet] 2020. [cited 2025 Mar 18]; 16: 557–569. https://www.eurekaselect.com/164960/article [Google Scholar]
- 98.Masson J-F. Surface plasmon resonance clinical biosensors for medical diagnostics. ACS Sens 2017; 2: 16–30. 10.1021/acssensors.6b00763 [DOI] [PubMed] [Google Scholar]
- 99.Can Demirdöğen B. A literature review of biosensors for multiple sclerosis: towards personalized medicine and point-of-care testing. Mult Scler Relat Disord 2021; 48: 102675. [DOI] [PubMed] [Google Scholar]
- 100.Badillo-Ramírez I, Carreón YJP, Rodríguez-Almazán C, et al. Graphene-Based biosensors for molecular chronic inflammatory disease biomarker detection. Biosensors 2022; 12: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen X, Zhou S, Wang Y, et al. Nanopore single-molecule analysis of biomarkers: providing possible clues to disease diagnosis. Trends Anal Chem TRAC 2023; 162: 117060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kharati M, Foroutanparsa S, Rabiee M, et al. Early diagnosis of multiple sclerosis based on optical and electrochemical biosensors: comprehensive perspective. Curr Anal Chem [Internet] 2020. [cited 2025 Mar 30]; 16: 557–569. https://www.eurekaselect.com/164960/article [Google Scholar]
- 103.Real-Fernández F, Passalacqua I, Peroni E, et al. Glycopeptide-based antibody detection in multiple sclerosis by surface plasmon resonance. Sensors 2012; 12: 5596–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang GY, Rayner SL, Chung R, et al. Advances in nanotechnology-based strategies for the treatments of amyotrophic lateral sclerosis. Mater Today Bio 2020; 6: 100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Turco A, Primiceri E, Chiriacò MS, et al. Advancing amyotrophic lateral sclerosis disease diagnosis: a lab-on-chip electrochemical immunosensor for ultra-sensitive TDP-43 protein detection and monitoring in serum patients’. Talanta 2024; 273: 125866. [DOI] [PubMed] [Google Scholar]
- 106.Tapia-Arellano A, Cabrera P, Cortés-Adasme E, et al. Tau- and α-synuclein-targeted gold nanoparticles: applications, opportunities, and future outlooks in the diagnosis and therapy of neurodegenerative diseases. J Nanobiotechnology [Internet] 2024. [cited 2025 Mar 30]; 22: 248. https://jnanobiotechnology.biomedcentral.com/articles/10.1186/s12951-024-02526-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Da Silva IS, Cardoso AR, Sales MGF. Electrochemical biosensor-based detection assays for early diagnosis of neurodegenerative disorders. In: Smart Diagn Neurodegener Disord [Internet]. Elsevier, 2024. [cited 2025 Mar 30], pp.155–177. https://linkinghub.elsevier.com/retrieve/pii/B9780323955393000107 [Google Scholar]
- 108.Song Z, Zhou Y, Han X, et al. Recent advances in enzymeless-based electrochemical sensors to diagnose neurodegenerative diseases. J Mater Chem B [Internet] 2021. [cited 2025 Mar 30]; 9: 1175–1188. https://xlink.rsc.org/?DOI=D0TB02745F [DOI] [PubMed] [Google Scholar]
- 109.Chen Y, Li B, Li K, et al. Superoxide dismutase nanozymes: current status and future perspectives on brain disease treatment and diagnosis. Chem Commun [Internet] 2024. [cited 2025 Mar 30]; 60: 4140–4147. https://xlink.rsc.org/?DOI=D3CC06288K [DOI] [PubMed] [Google Scholar]
- 110.Parihar A, Choudhary NK, Khan R. POCT Devices for neurodegenerative disorders: from lab to clinics. In: Smart Diagn Neurodegener Disord [Internet]. Elsevier, 2024. [cited 2025 Mar 30], pp.279–310. https://linkinghub.elsevier.com/retrieve/pii/B9780323955393000156 [Google Scholar]
- 111.Hoffmann C, Stumpf A. Comparison of A1 and A2A receptor dynamics using FRET based receptor sensors. SpringerPlus [Internet] 2015. [cited 2025 Mar 30]; 4: L6. https://springerplus.springeropen.com/articles/10.1186/2193-1801-4-S1-L6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Amrollahi P, Zheng W, Monk C, et al. Nanoplasmonic sensor approaches for sensitive detection of disease-associated exosomes. ACS Appl Bio Mater 2021; 4: 6589–6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Parihar A, Khan R, Srivastava AK. (eds). Smart diagnostics for neurodegenerative disorders: neuro-sensors. London: Academic Press, Elsevier, 2023. [Google Scholar]
- 114.Mir M, Palma-Florez S, Lagunas A, et al. Biosensors integration in blood-brain barrier-on-a-chip: emerging platform for monitoring neurodegenerative diseases. ACS Sens 2022; 7: 1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Woelfle T, Bourguignon L, Lorscheider J, et al. Wearable sensor technologies to assess motor functions in people with multiple sclerosis: systematic scoping review and perspective. J Med Internet Res 2023; 25: e44428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mitchell RM, Freeman WM, Randazzo WT, et al. A CSF biomarker panel for identification of patients with amyotrophic lateral sclerosis. Neurology 2009; 72: 14–19. [DOI] [PubMed] [Google Scholar]
- 117.Bezard E, Gross CE. Compensatory mechanisms in experimental and human parkinsonism: towards a dynamic approach. Prog Neurobiol 1998; 55: 93–116. [DOI] [PubMed] [Google Scholar]
- 118.Blesa J, Trigo-Damas I, Dileone M, et al. Compensatory mechanisms in Parkinson’s disease: circuits adaptations and role in disease modification. Exp Neurol 2017; 298: 148–161. [DOI] [PubMed] [Google Scholar]
- 119.Golpich M, Amini E, Mohamed Z, et al. Mitochondrial dysfunction and biogenesis in neurodegenerative diseases: pathogenesis and treatment. CNS Neurosci Ther 2017; 23: 5–22. doi: 10.1111/cns.12655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miller DB, O’Callaghan JP. Biomarkers of Parkinson’s disease: present and future. Metabolism 2015; 64: S40–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Akhtar RS, Stern MB. New concepts in the early and preclinical detection of Parkinson’s disease: therapeutic implications. Expert Rev Neurother 2012; 12: 1429–1438. [DOI] [PubMed] [Google Scholar]
- 122.Postuma RB, Berg D. Advances in markers of prodromal Parkinson disease. Nat Rev Neurol 2016; 12: 622–634. [DOI] [PubMed] [Google Scholar]
- 123.Elsworth JD. Parkinson’s disease treatment: past, present, and future. J Neural Transm Vienna Austria 1996 2020; 127: 785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jamebozorgi K, Taghizadeh E, Rostami D, et al. Cellular and molecular aspects of Parkinson treatment: future therapeutic perspectives. Mol Neurobiol 2019; 56: 4799–4811. [DOI] [PubMed] [Google Scholar]
- 125.Mehta SH, Adler CH. Advances in biomarker research in Parkinson’s disease. Curr Neurol Neurosci Rep 2016; 16: 279–310. [DOI] [PubMed] [Google Scholar]
- 126.Alimohammadzadeh E, Hedley J. Advances in graphene field effect transistors (FETs) for amine neurotransmitter sensing. Appl Sci [Internet] 2024. [cited 2025 Mar 30]; 14: 10109. https://www.mdpi.com/2076-3417/14/22/10109 [Google Scholar]
- 127.George Kerry R, Ukhurebor KE, Kumari S, et al. A comprehensive review on the applications of nano-biosensor-based approaches for non-communicable and communicable disease detection. Biomater Sci [Internet] 2021. [cited 2024 Dec 1]; 9: 3576–3602. https://xlink.rsc.org/?DOI=D0BM02164D [DOI] [PubMed] [Google Scholar]
- 128.Ukhurebor KE, Onyancha RB, Aigbe UO, et al. A methodical review on the applications and potentialities of using nanobiosensors for disease diagnosis. Desimone MF, editor. BioMed Res Int [Internet] 2022. [cited 2024 Dec 1]; 2022: 1–20. https://www.hindawi.com/journals/bmri/2022/1682502/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shokati A, Naser Moghadasi A, Nikbakht M, et al. A focus on allogeneic mesenchymal stromal cells as a versatile therapeutic tool for treating multiple sclerosis. Stem Cell Res Ther 2021; 12: 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Singh S, Dhanjal DS, Sonali B, et al. An insight in bacteriophage based biosensors with focus on their detection methods and recent advancements. Environ Technol Innov [Internet] 2020. [cited 2024 Dec 1]; 20: 101081. https://linkinghub.elsevier.com/retrieve/pii/S235218642031381X [Google Scholar]
- 131.Morais AL, Rijo P, Batanero Hernán MB, et al. Biomolecules and electrochemical tools in chronic non-communicable disease surveillance: a systematic review. Biosensors 2020; 10: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yoon J, Shin M, Lee T, et al. Highly sensitive biosensors based on biomolecules and functional nanomaterials depending on the types of nanomaterials: a perspective review. Mater Basel Switz 2020; 13: 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mohankumar P, Ajayan J, Mohanraj T, et al. Recent developments in biosensors for healthcare and biomedical applications: a review. Measurement [Internet] 2021. [cited 2024 Dec 1]; 167: 108293. https://linkinghub.elsevier.com/retrieve/pii/S0263224120308332 [Google Scholar]
- 134.Rodrigues D, Barbosa AI, Rebelo R, et al. Skin-Integrated wearable systems and implantable biosensors: a comprehensive review. Biosensors 2020; 10: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Carpenter AC, Paulsen IT, Williams TC. Blueprints for biosensors: design, limitations, and applications. Genes (Basel) 2018; 9: 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.El-Said WA, Abdelshakour M, Choi J-H, et al. Application of conducting polymer nanostructures to electrochemical biosensors. Mol Basel Switz 2020; 25: 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.De Corcuera JIR, Cavalieri RP, Powers JR. Prototype instruments for laboratory and on-line measurement of lipoxygenase activity. Food Sci Technol Int [Internet] 2003. [cited 2024 Dec 2]; 9: 5–9. https://journals.sagepub.com/doi/10.1177/1082013203009001001 [Google Scholar]
- 138.Ukhurebor KE. The role of biosensor in climate smart organic Agriculture toward agricultural and environmental sustainability. In: Agrometeorology [Internet]. 2020; 5–22. https://api.semanticscholar.org/CorpusID:225605185 . [Google Scholar]
- 139.Jeromin A, Bowser R. Biomarkers in neurodegenerative diseases. Adv Neurobiol 2017; 15: 491–528. [DOI] [PubMed] [Google Scholar]
- 140.Rosenthal LS, Drake D, Alcalay RN, et al. The NINDS Parkinson’s disease biomarkers program. Mov Disord Off J Mov Disord Soc 2016; 31: 915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shin J-W, Yoon J, Shin M, et al. Electrochemical dopamine biosensor composed of silver encapsulated MoS2 hybrid nanoparticle. Biotechnol Bioprocess Eng [Internet] 2019. [cited 2024 Dec 2]; 24: 135–144. https://link.springer.com/10.1007/s12257-018-0350-1 [Google Scholar]
- 142.Sim TM, Tarini D, Dheen ST, et al. Nanoparticle-Based technology approaches to the management of neurological disorders. Int J Mol Sci 2020; 21: 6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vázquez-Guardado A, Barkam S, Peppler M, et al. Enzyme-Free plasmonic biosensor for direct detection of neurotransmitter dopamine from whole blood. Nano Lett 2019; 19: 449–454. [DOI] [PubMed] [Google Scholar]
- 144.George Kerry R, Ukhurebor KE, Kumari S, et al. A comprehensive review on the applications of nano-biosensor-based approaches for non-communicable and communicable disease detection. Biomater Sci [Internet] 2021. [cited 2024 Dec 2]; 9: 3576–3602. https://xlink.rsc.org/?DOI=D0BM02164D [DOI] [PubMed] [Google Scholar]
- 145.Shawky SM, Awad AM, Abugable AA, et al. Gold nanoparticles - an optical biosensor for RNA quantification for cancer and neurologic disorders diagnosis. Int J Nanomedicine 2018; 13: 8137–8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Haes AJ, Chang L, Klein WL, et al. Detection of a biomarker for Alzheimer’s disease from synthetic and clinical samples using a nanoscale optical biosensor. J Am Chem Soc 2005; 127: 2264–2271. [DOI] [PubMed] [Google Scholar]
- 147.Viswanathan S, Radecka H, Radecki J. Electrochemical biosensors for food analysis. Monatshefte Für Chem - Chem Mon [Internet] 2009. [cited 2024 Dec 2]; 140: 891–899. http://link.springer.com/10.1007/s00706-009-0143-5 [Google Scholar]
- 148.Zhang H-L, Lai G-S, Han D-Y, et al. An amperometric hydrogen peroxide biosensor based on immobilization of horseradish peroxidase on an electrode modified with magnetic dextran microspheres. Anal Bioanal Chem [Internet] 2008; ; 390: 971–977. [DOI] [PubMed] [Google Scholar]
- 149.Tavakolian-Ardakani Z, Hosu O, Cristea C, et al. Latest trends in electrochemical sensors for neurotransmitters: a review. Sensors [Internet] 2019. [cited 2024 Dec 2]; 19: 2037. https://www.mdpi.com/1424-8220/19/9/2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Clark LC, Lyons C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann N Y Acad Sci 1962; 102: 29–45. [DOI] [PubMed] [Google Scholar]
- 151.Campàs M, Prieto-Simón B, Marty J-L. A review of the use of genetically engineered enzymes in electrochemical biosensors. Semin Cell Dev Biol 2009; 20: 3–9. [DOI] [PubMed] [Google Scholar]
- 152.Adeel M, Rahman MM, Caligiuri I, et al. Recent advances of electrochemical and optical enzyme-free glucose sensors operating at physiological conditions. Biosens Bioelectron 2020; 165: 112331. [DOI] [PubMed] [Google Scholar]
- 153.Shadlaghani A, Farzaneh M, Kinser D, et al. Direct electrochemical detection of glutamate, acetylcholine, choline, and adenosine using non-enzymatic electrodes. Sensors 2019; 19: 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Khalilzadeh B, Rashidi M, Soleimanian A, et al. Development of a reliable microRNA based electrochemical genosensor for monitoring of miR-146a, as key regulatory agent of neurodegenerative disease. Int J Biol Macromol 2019; 134: 695–703. [DOI] [PubMed] [Google Scholar]
- 155.Shi K, Hu K, Wang S, et al. Structural studies of electrochemically activated glassy carbon electrode: effects of chloride anion on the redox responses of copper deposition. Electrochimica Acta [Internet] 2007. [cited 2024 Dec 2]; 52: 5907–5913. https://linkinghub.elsevier.com/retrieve/pii/S0013468607004021 [Google Scholar]
- 156.Borj M, Taghizadehborojeni S, Shokati A, et al. Urinary tract infection among diabetic patients with regard to the risk factors, causative organisms and their antimicrobial susceptibility profiles. 2020.
- 157.Khan MZH, Hasan MR, Hossain SI, et al. Ultrasensitive detection of pathogenic viruses with electrochemical biosensor: state of the art. Biosens Bioelectron 2020; 166: 112431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Majdinasab M, Mishra RK, Tang X, et al. Detection of antibiotics in food: new achievements in the development of biosensors. TrAC Trends Anal Chem [Internet] 2020. [cited 2024 Dec 2]; 127: 115883. https://linkinghub.elsevier.com/retrieve/pii/S0165993620301126 [Google Scholar]
- 159.Sode K, Yamazaki T, Lee I, et al. Biocapacitor: a novel principle for biosensors. Biosens Bioelectron [Internet] 2016. [cited 2024 Dec 2]; 76: 20–28. https://linkinghub.elsevier.com/retrieve/pii/S0956566315303122 [DOI] [PubMed] [Google Scholar]
- 160.Zhou Q, Tang D. Recent advances in photoelectrochemical biosensors for analysis of mycotoxins in food. TrAC Trends Anal Chem [Internet] 2020. [cited 2024 Dec 2]; 124: 115814. https://linkinghub.elsevier.com/retrieve/pii/S0165993619306909 [Google Scholar]
- 161.Usha SP, Manoharan H, Deshmukh R, et al. Attomolar analyte sensing techniques (AttoSens): a review on a decade of progress on chemical and biosensing nanoplatforms. Chem Soc Rev [Internet] 2021. [cited 2024 Dec 2]; 50: 13012–13089. https://xlink.rsc.org/?DOI=D1CS00137J [DOI] [PubMed] [Google Scholar]
- 162.Kulkarni MB, Ayachit NH, Aminabhavi TM. Recent advancements in nanobiosensors: current trends, challenges, applications, and future scope. Biosensors [Internet] 2022. [cited 2024 Dec 2]; 12: 892. https://www.mdpi.com/2079-6374/12/10/892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bhatia D, Paul S, Acharjee T, et al. Biosensors and their widespread impact on human health. Sens Int [Internet] 2024. [cited 2024 Dec 2]; 5: 100257. https://linkinghub.elsevier.com/retrieve/pii/S2666351123000311 [Google Scholar]
- 164.Javaid M, Haleem A, Singh RP, et al. Exploring the potential of nanosensors: a brief overview. Sens Int [Internet] 2021. [cited 2024 Dec 2]; 2: 100130. https://linkinghub.elsevier.com/retrieve/pii/S2666351121000516 [Google Scholar]
- 165.Tulchinsky TH. Ethical issues in public health. In: Case stud public health [internet]. Elsevier, 2018. [cited 2024 Dec 2], pp.277–316. https://linkinghub.elsevier.com/retrieve/pii/B9780128045718000275 [Google Scholar]
- 166.Guruprasath N, Sankarganesh P, Adeyeye SAO, et al. Review on emerging applications of nanobiosensor in food safety. J Food Sci [Internet] 2024. [cited 2024 Dec 2]; 89: 3950–3972. https://ift.onlinelibrary.wiley.com/doi/10.1111/1750-3841.17149 [DOI] [PubMed] [Google Scholar]
- 167.Kumari M, Gupta V, Kumar N, et al. Microfluidics-based nanobiosensors for healthcare monitoring. Mol Biotechnol [Internet] 2024. [cited 2024 Dec 2]; 66: 378–401. https://link.springer.com/10.1007/s12033-023-00760-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Aghili Z, Nasirizadeh N, Divsalar A, et al. A highly sensitive miR-195 nanobiosensor for early detection of Parkinson’s disease. Artif Cells Nanomedicine Biotechnol [Internet] 2018. [cited 2024 Dec 1]; 46: 32–40. https://www.tandfonline.com/doi/full/10.1080/21691401.2017.1411930 [DOI] [PubMed] [Google Scholar]
- 169.2020 Alzheimer’s disease facts and figures. Alzheimers Dement [Internet] 2020. [cited 2024 Dec 1]; 16: 391–460. https://alz-journals.onlinelibrary.wiley.com/doi/10.1002/alz.12068 [Google Scholar]
- 170.Banovic S, Zunic LJ, Sinanovic O. Communication difficulties as a result of dementia. Mater Socio-Medica 2018; 30: 221–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fassero M, Mannion C, Manning L, et al. The effects of Alzheimer’s disease on behavior. 2021; 20: 76–93. [Google Scholar]
- 172.Suresh S, Singh S A, Rushendran R, et al. Alzheimer’s disease: the role of extrinsic factors in its development, an investigation of the environmental enigma. Front Neurol [Internet] 2023. [cited 2024 Dec 1]; 14: 1303111. https://www.frontiersin.org/articles/10.3389/fneur.2023.1303111/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dhahi T, Yousif Dafhalla AK, Tayfour OE, et al. Advances in nano sensors for monitoring and optimal performance enhancement in photovoltaic cells. iScience [Internet] 2024. [cited 2024 Dec 2]; 27: 109347. https://linkinghub.elsevier.com/retrieve/pii/S2589004224005686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Barbosa AI, Rebelo R, Reis RL, et al. Current nanotechnology advances in diagnostic biosensors. Med DEVICES Sens [Internet] 2021. [cited 2024 Dec 2]; 4: e10156. https://onlinelibrary.wiley.com/doi/10.1002/mds3.10156 [Google Scholar]
- 175.Attaallah R, Antonacci A, Arduini F, et al. Nanobiosensors for bioclinical applications: pros and cons. In: Patra JK, Fraceto LF, Das G, Campos EVR. (eds) Green nanoparticles [internet]. Cham: Springer International Publishing, 2020. [cited 2024 Dec 2], pp.117–149. http://link.springer.com/10.1007/978-3-030-39246-8_5 [Google Scholar]
- 176.Cavalcante FTT, De A. Falcão IR, Da S. Souza JE, et al. Designing of nanomaterials-based enzymatic biosensors: synthesis, properties, and applications. Electrochem [Internet] 2021. [cited 2024 Dec 2]; 2: 149–184. https://www.mdpi.com/2673-3293/2/1/12 [Google Scholar]
- 177.Wahab MRA, Palaniyandi T, Ravi M, et al. Biomarkers and biosensors for early cancer diagnosis, monitoring and prognosis. Pathol - Res Pract [Internet] 2023. [cited 2024 Dec 2]; 250: 154812. https://linkinghub.elsevier.com/retrieve/pii/S0344033823005125 [DOI] [PubMed] [Google Scholar]
- 178.Kalinke C, De Oliveira PR, Banks CE, et al. 3D-printed Immunosensor for the diagnosis of Parkinson’s disease. Sens Actuators B Chem [Internet] 2023. [cited 2024 Dec 2]; 381: 133353. https://linkinghub.elsevier.com/retrieve/pii/S0925400523000680 [Google Scholar]
- 179.Sharifianjazi F, Moradi M, Parvin N, et al. Magnetic CoFe2O4 nanoparticles doped with metal ions: a review. Ceram Int [Internet] 2020. [cited 2024 Dec 1]; 46: 18391–18412. https://linkinghub.elsevier.com/retrieve/pii/S0272884220311469 [Google Scholar]
- 180.Lolli F, Mulinacci B, Carotenuto A, et al. An N-glucosylated peptide detecting disease-specific autoantibodies, biomarkers of multiple sclerosis. Proc Natl Acad Sci U S A 2005; 102: 10273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Julien J-P. ALS: astrocytes move in as deadly neighbors. Nat Neurosci 2007; 10: 535–537. [DOI] [PubMed] [Google Scholar]
- 182.Wilson ME, Boumaza I, Lacomis D, et al. Cystatin C: a candidate biomarker for amyotrophic lateral sclerosis. PloS One 2010; 5: e15133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pasinetti GM, Ungar LH, Lange DJ, et al. Identification of potential CSF biomarkers in ALS. Neurology 2006; 66: 1218–1222. [DOI] [PubMed] [Google Scholar]
- 184.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 2011; 7: 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ma Z-L, Wang Z-L, Zhang F-Y, et al. Biomarkers of Parkinson’s disease: from basic research to clinical practice. Aging Dis 2024; 15: 1813–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tang J, Jiang S, Liu Y, et al. Electrochemical determination of dopamine and uric acid using a glassy carbon electrode modified with a composite consisting of a Co(II)-based metalorganic framework (ZIF-67) and graphene oxide. Microchim Acta [Internet] 2018. [cited 2024 Dec 2]; 185: 486. http://link.springer.com/10.1007/s00604-018-3025-x [DOI] [PubMed] [Google Scholar]
- 187.Zhang J, Shao S, Zhou D, et al. ZnO nanowire arrays decorated 3D N-doped reduced graphene oxide nanotube framework for enhanced photocatalytic CO2 reduction performance. J CO2 Util [Internet] 2021. [cited 2024 Dec 2]; 50: 101584. https://linkinghub.elsevier.com/retrieve/pii/S2212982021001517 [Google Scholar]
- 188.Sun K, Xia N, Zhao L, et al. Aptasensors for the selective detection of alpha-synuclein oligomer by colorimetry, surface plasmon resonance and electrochemical impedance spectroscopy. Sens Actuators B Chem [Internet] 2017. [cited 2024 Dec 2]; 245: 87–94. https://linkinghub.elsevier.com/retrieve/pii/S0925400517301788 [Google Scholar]
- 189.Mandala SHS, Liu T-J, Chen C-M, et al. Enhanced plasmonic biosensor utilizing paired antibody and label-free Fe3O4 nanoparticles for highly sensitive and selective detection of Parkinson’s α-synuclein in Serum. Biosensors 2021; 11: 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Atar N, Yola ML. A novel QCM immunosensor development based on gold nanoparticles functionalized sulfur-doped graphene quantum dot and h-ZnS-CdS NC for interleukin-6 detection. Anal Chim Acta [Internet] 2021. [cited 2024 Dec 2]; 1148: 338202. https://linkinghub.elsevier.com/retrieve/pii/S0003267021000143 [DOI] [PubMed] [Google Scholar]
- 191.Venton BJ, Cao Q. Fundamentals of fast-scan cyclic voltammetry for dopamine detection. Analyst [Internet] 2020. [cited 2024 Dec 2]; 145: 1158–1168. https://xlink.rsc.org/?DOI=C9AN01586H [DOI] [PMC free article] [PubMed] [Google Scholar]