ABSTRACT
Osteoarthritis (OA) is a common musculoskeletal disorder impacting millions in the United States, presenting with joint pain, stiffness, and reduced mobility. Its complex origins and lack of clear early‐stage symptoms make early detection challenging. Traditional diagnostic methods, including imaging, are often used when significant cartilage loss has already occurred. However, serum biomarkers offer potential for earlier and less invasive detection. For our review, articles published from 1980 to 2024 that analyzed OA serum biomarkers were retrieved from PubMed, Embase, and Web of Science. The analysis included biomarker frequency, percent changes from baseline levels, and logistic regression to assess correlations with OA. Several biomarkers exhibited altered levels in OA, classified into inflammatory, collagenous, mechanical stress, and other categories. Inflammatory markers such as IL‐6 and MPO showed significant elevation, while TNF‐α showed minimal correlation with OA. Collagenous markers, especially COMP, were consistently elevated in patients, correlating with disease severity. Additionally, PIIANP showed a strong negative correlation with OA progression. Obesity‐related markers, including resistin, were also associated with OA, and logistic regression confirmed IL‐6, COMP, and resistin as strongly correlated with OA, with PIIANP demonstrating a significant inverse relationship. This review highlights the critical role of serum biomarkers in OA detection and progression. Markers like IL‐6, COMP, and PIIANP offer significant potential for early diagnosis. Integrating these biomarkers into clinical practice may facilitate earlier intervention, potentially slowing OA progression. Future research should focus on validating these findings across larger, diverse populations and refining therapeutic strategies targeting these biomarker pathways.
Keywords: biomarkers, detection, early‐stage, osteoarthritis, serum
A systematic review of biomarkers associated with osteoarthritis progression. Meta‐analysis of dysregulation among the biomarkers distinguishes it from baseline patients as well as other pathologies, suggesting advancements in early diagnosis of the disease.

1. Introduction
Osteoarthritis (OA) is the most prevalent musculoskeletal disorder of synovial joints, affecting over 32.5 million adults in the United States (“OA” 2023) [1]. According to the Centers for Disease Control and Prevention (CDC), 13.9% of adults 25 years and older and 33.6% of adults 65 and older are affected by OA [2]. OA presents with pain, stiffness, deformity, and reduced function. This progressive condition is related to age, sex, and chronic mechanical stress from high‐impact sports, repetitive motion, or obesity. Due to the complex etiopathogenesis and vague presentation, it is difficult to diagnose it at an early stage, and many patients experience extensive cartilage loss well before a diagnosis is made. This deterioration often leads to a significant impact on joint mobility that progresses to a decrease in overall quality of life. Furthermore, no cost‐effective and minimally invasive treatments are available for advanced OA. The only viable long‐term solution at this stage is joint replacement [3]. Considering all these reasons, early detection of OA has paramount importance in negating the consequences of the disease.
Biomolecules, which are produced by normal and abnormal cells, are constantly circulating throughout the bloodstream. These molecules can be detected by serum assay and can serve as markers for various disease states. Recent studies in progressive diseases like cancer and multiple sclerosis have successfully demonstrated that changes in serum biomarker concentrations can be detected before the manifestation of clinical symptoms [4, 5]. In OA, serum biomarkers result from either a local inflammatory response [6] or leakage of proteins out of the joint space [7], allowing for changes in serum concentrations to be detected. The first changes involve damage to the extracellular matrix of articular cartilage, forcing a local response to remodel the matrix. As the damage progresses, chondrocytes shift from producing molecules that aid in tissue repair to molecules that replace cartilage with bone [8].
Current detection and diagnosis of OA includes a combination of patient history, physical exam, and imaging techniques. X‐rays are commonly used to visualize the joint space narrowing, bone spurs, subchondral cysts and sclerosis, and other structural abnormalities associated with the disease. The Kellgren–Lawrence scale is often used to classify the severity of OA based on these findings and ranges from grade 0 to 4 [9]. Blood tests are not currently used for the diagnosis of OA but could be an affordable and minimally invasive alternative that requires further investigation. Early detection of OA through serum biomarkers analysis could avoid the need for imaging techniques and reduce the cost incurred for its treatment.
Current research on OA patients has focused mostly on biomarkers involved in the inflammatory processes and only identified these molecules after OA symptoms have already started [10]. This review will expand on the current knowledge of the biomarkers not only related to inflammation, but also on the markers related to mechanical stress, obesity, aging, along with the effects of the sex hormones. We reviewed articles that highlight levels of serum biomarkers at different stages of OA to better understand the molecular pathogenesis and progression of the disease.
2. Methods
This systematic review was performed following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) guidelines.
2.1. Data and Literature Sources
We searched PubMed, Embase, Cochrane, and Web of Science for articles related to OA that were published from January 1980 through December 2023. The following keywords were utilized to collect comparison articles: “Early‐stage osteoarthritis,” “Serum biomarkers,” “inflammatory,” “hormonal,” “mechanical stress,” and “age‐related.” Furthermore, the bibliographies of the selected articles were searched to identify the additional relevant articles. Exactly 120 potential articles were identified to be screened before analysis.
2.2. Inclusion and Exclusion Criteria
The titles and abstracts of all the articles were thoroughly reviewed. The articles covering serum biomarkers associated with OA, along with the analysis of the dysregulated biomarkers, were included for the final review. Studies analyzing upregulated or downregulated biomarkers were included in this review. Articles in English or including an English translation were included. Studies where full texts were not available or did not provide adequate information pertaining to this analysis were excluded from this review (Figure 1).
FIGURE 1.
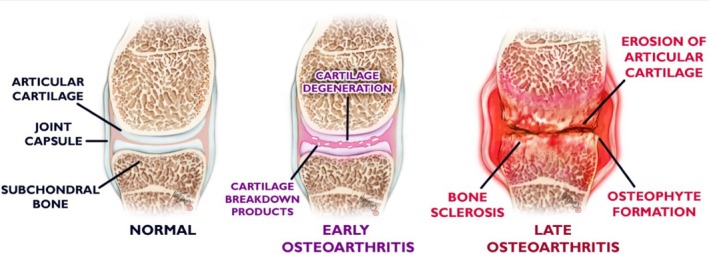
Stages and pathology of OA.
2.3. Assessment of Study Quality
Utilizing the inclusion and exclusion criteria, four reviewers (A.L., J.B., R.M., A.S.) independently assessed all potential articles and collectively decided on the final inclusion of the articles for the review, and differences were resolved with thorough discussion with the senior author before final entry. Of the 120 screened, 41 articles were included for the review (see Figure 2).
FIGURE 2.
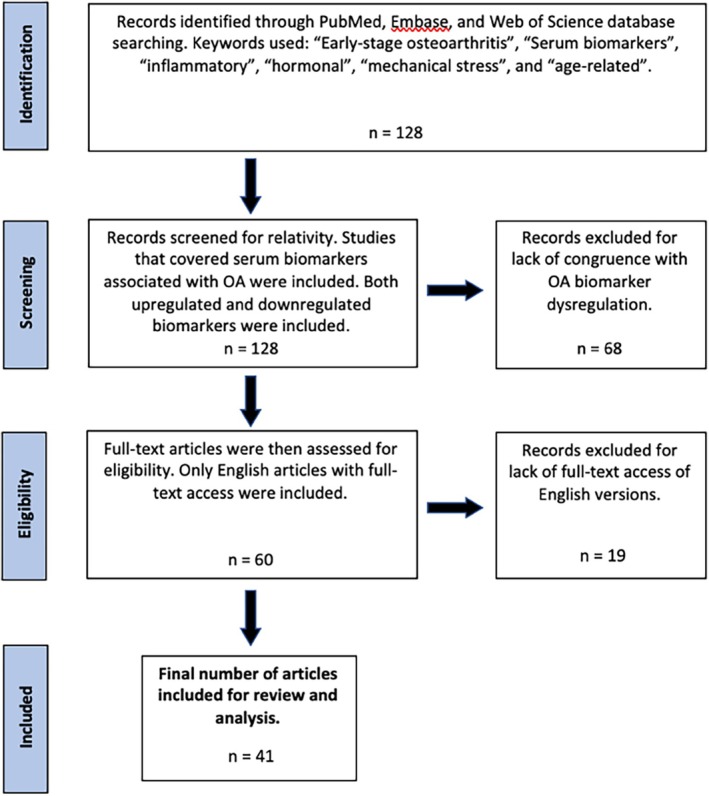
Flowchart for study exclusion and inclusion criteria. Exactly 128 articles were initially identified for potential inclusion, and 41 articles were included for final review and analysis.
2.4. Types of Dysregulation Measurements
Reviewers analyzed the role of biomarkers from the selected studies in two ways. First, we measured the frequency of each biomarker to identify biomarkers commonly researched in OA. Second, the biomarkers were analyzed by the percent change from baseline serum concentrations. Percent change was used due to the large range of both baseline and abnormal concentrations for each biomarker.
2.5. Statistical Methods
Statistical analysis was conducted using R Studio, Version 2023.12.1 + 402, Posit Software, PBC. To assess heterogeneity among studies that did not include general population‐adjusted data, we conducted Cochran's Q test to evaluate data variability and effect sizes. Based on this, four studies were not included in the final data analysis. Outcome measures were presented as Mean ± Standard Deviation (SD). Before conducting significance tests, the normality of the data was assessed using the Kolmogorov–Smirnov test to determine whether the distribution of serum biomarker concentrations followed a normal distribution. The average percent difference was calculated to quantify the changes in serum biomarker levels between different groups. For significance testing, Mann–Whitney U tests were used to compare serum biomarker concentrations between baseline (healthy individuals) and OA patients. A p value of ≤ 0.05 was considered statistically significant. To further explore the strength of association between biomarker concentrations and the presence of OA, logistic regression was performed. This allowed for the evaluation of how individual biomarker levels correlate with the likelihood of having OA, adjusting for confounders where applicable. Finally, to assess for publication bias we used funnel plot analysis, which demonstrated a symmetrical distribution of studies, suggesting no significant publication bias. Additionally, Egger's test was non‐significant (p = 0.23), indicating that small‐study effects were unlikely to influence the data analysis results.
3. Results
In osteoarthritis (OA), several serum biomarkers were found in altered concentrations when compared with a healthy population. To better understand the multi‐factorial pathogenesis of OA, we grouped the biomarkers analyzed in our studies into the following categories: inflammatory, collagenous, and mechanical stress‐related, for improved understanding of their range of biochemical changes occurring in the joint.
3.1. Inflammatory
Interleukins (ILs) are chemical messengers that have been linked to OA. These cytokines are produced by various cell types in the human body and serve as signaling molecules to promote activation and proliferation during inflammatory processes (Solimando et al. 2022). Though ILs have been tied to many systemic conditions, as they are a part of several normal regulatory processes, not all ILs are dysregulated in OA. Among them, IL‐6, IL‐1, IL‐1b, and IL‐8 are mentioned in the reviewed papers that are related to OA.
IL‐6 concentrations were found to be elevated in many different inflammatory conditions, but only at intermittent levels. When infused at higher levels, IL‐6 was shown to increase hepatic CRP production while simultaneously decreasing the production of other interleukins [11]. Eight studies examined the correlation between serum IL‐6 concentrations and OA in our review (Figure 3). Livshits et al. examined IL‐6 concentrations in a longitudinal study of OA progression, which compared serum concentrations with Kellgren/Lawrence grades. IL‐6 was found to be significantly higher in affected individuals, with concentrations increasing as OA progressed. This is concurrent with findings from other studies in this review. Interestingly, Ishibashi et al. showed upregulation of IL‐6 in OA, but without any significant correlation. Overall, our collective analysis displayed a significant difference in IL‐6 serum concentrations between baseline and OA patients (W = 475,686, p value = 7.058e−06, Figure 4).
FIGURE 3.
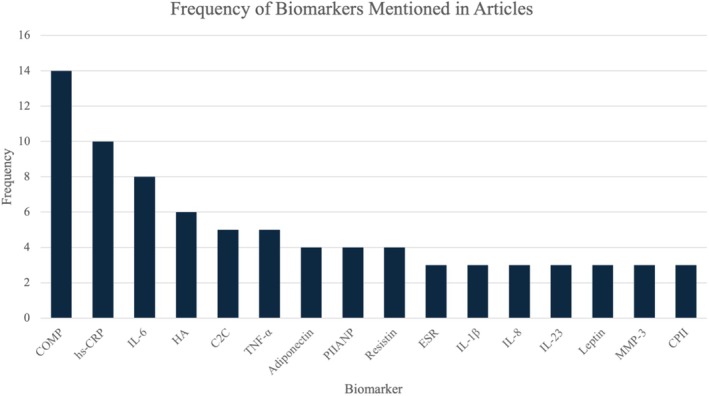
Number of times biomarkers are mentioned in the articles reviewed. Included biomarkers mentioned in ≥ 3 articles; n = 41 articles. C2C, cartilage type II collagen; COMP, cartilage oligomeric matrix protein; CPII, rate of type II procollagen synthesis; ESR, erythrocyte sedimentation rate; HA, hyaluronic acid; hs‐CRP, high‐sensitivity C‐reactive protein; PIIANP, N‐propeptide of type IIA procollagen.
FIGURE 4.
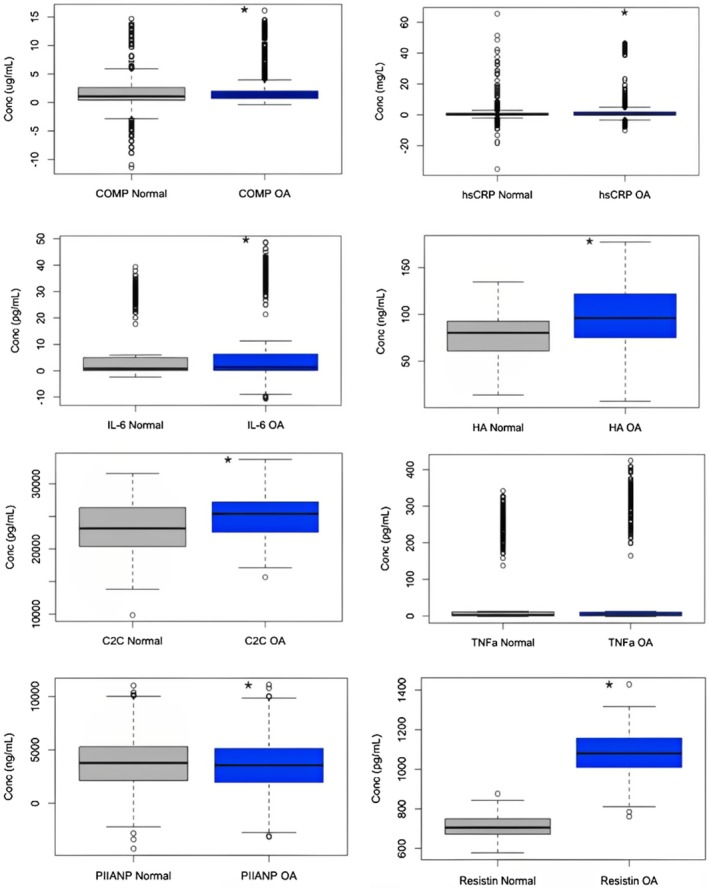
Concentrations of each biomarker under normal conditions compared with diagnostic OA. Kolgorov–Smirnov test was used to determine nonparametric data, and then Mann–Whitney U test was used to determine significant differences. COMP, hsCRP, IL‐6, HA, C2C, and Resistin serum levels in clinically diagnosed OA patients were all significantly greater than baseline levels. PIIANP serum concentrations in OA were significantly lower.
In addition to IL‐6, IL‐1, IL‐1b, and IL‐8 have also been studied in OA. IL‐1 and, more specifically, IL‐1b, were significantly up‐regulated in OA. While IL‐1 is expressed constitutively in the body and regulates body temperature, IL‐1b is primarily expressed under disease conditions, offering a unique relationship with OA, which is not considered a systemic disease. Liu et al., who examined IL‐1 and IL‐6 levels through OA progression in post‐menopausal women, noted that, while IL‐1 levels do increase significantly with OA severity, this relationship is not as stark as with IL‐6. However, IL‐1/1b is also believed to contribute to cartilage destruction similar to IL‐6 [12]. IL‐8, which functions mainly as a chemotactic factor for inflammatory leukocytes, was analyzed by Zhao et al. along with IL‐6 levels in knee osteoarthritic (KOA) patients. IL‐8 was found to be significantly unregulated in KOA patients, but still less prominent when compared with IL‐6 levels [13]. This finding aligns with IL‐8's chemotactic function, and it is hypothesized that, since OA is only a minor inflammatory condition, IL‐8 levels are only moderately significantly unregulated.
Other notable interleukins associated with OA progression are IL‐2, IL‐4, IL‐15, IL‐17A, IL‐17F, IL‐21, IL‐22, IL‐23, and IL‐38. All of them were shown to be up‐regulated in OA, but less significantly than IL‐6, IL‐1/1b, and IL‐8. Additionally, for most of these minor interleukin OA biomarkers, significant up‐regulation only occurs in later, more severe stages of OA [14, 15, 16].
Several additional inflammatory chemical messengers are linked to OA. Two of the non‐specific markers of local inflammation, C‐reactive protein (CRP) and erythrocyte sedimentation rate (ESR), are some of the most common markers of systemic inflammatory diseases [17]. When the levels of IL‐6 are increased, the liver responds by producing more CRP that can bind Fc receptors and activate complement. Therefore, CRP concentrations are an effective measurement for inflammation, while the high‐sensitivity CRP (hsCRP) immunoassay has been adopted to detect smaller changes in CRP [18]. The hsCRP marker was studied in 10 out of the 41 articles found in this review (Figure 3). In patients with knee OA, hsCRP, along with ESR, was shown to be upregulated and is likely associated with swelling and tenderness of the joint [17]. Our analysis conforms with this result, showcasing significant hsCRP upregulation in diagnostic OA when compared with the control group (W = 539,031, p value = 0.0002968, Figure 4). Since CRP is a fairly non‐specific biomarker in terms of OA, another study investigated matrix metalloproteinase degradation products (CRPM) which could hold more diagnostic value. Saberi Hosnijeh et al. investigated the role of CRPM in OA and discovered an association between CRPM and OA incidence and progression [19]. Increased levels of CRPM contained predictive value of OA progression at a 5‐year follow‐up, providing a potential early diagnostic biomarker.
Produced by macrophages in response to acute inflammatory conditions, tumor necrosis factor α (TNF‐α) acts as a master regulator of the pro‐inflammatory cytokines. This cytokine was mentioned in five articles (Figure 3) and initiates the production of prostaglandin E2, the synthesis of MMPs, and the activation of neutrophils, which contribute to the disease's pathogenesis, especially in the elderly population [11, 12]. Our analysis found no significant upregulation of TNF‐α in diagnostic OA (W = 233,346, p value = 0.07334, Figure 4). By looking at post‐menopausal women with OA, one research group investigated levels of TNF‐α in serum compared with a control of post‐menopausal women without OA. The study found that increased concentrations of TNF‐α were present when OA was more severe [12]. This could indicate that another biomarker, like CCL3, is involved in the progression of the disease. However, this study was conducted in post‐menopausal women who have decreased estrogen, compressing the data into a specific demographic. When looking at both men and women with OA, the serum levels of TNF‐α were not found to be significantly altered [11, 13]. This complex relationship demonstrates a marker that might need more investigation because, in theory, the pro‐inflammatory regulator should be upregulated in OA.
3.2. Collagenous
One of the most heavily researched collagenous proteins associated with OA is collagen oligomeric matrix protein (COMP). It was analyzed in 14 of the 41 studies analyzed (Figure 3). This protein is a 524 kDa glycoprotein that consists of 5 arms with a central assembly domain, a flexible stand, and a peripheral globular domain. Also known as thrombospondin, COMP is a calcium‐binding protein that binds to type II collagen fibers and participates in the matrix assembly in the articular cartilage [18, 20, 21]. Though the exact function of COMP remains unknown, the protein is a catalyst of collagen formation and acts as an important degradation product of articular cartilage [22]. In 1998, Petersson et al. isolated the non‐collagenous protein from the ECM of cartilage, noting that it was not unique to the tissue. The study also concluded that a protein's concentration in serum reflects the release from all cartilage or bone structures and the elimination of the protein at various metabolic points during the process. With that, Petersson et al. found an increase in COMP concentrations in individuals with OA compared with a healthy control [23]. From there, research has concentrated on learning more about the association of COMP with the etiology of OA. Serum concentrations of COMP were elevated in OA patients, with high levels correlated with destructive joint changes [21, 24, 25].
In OA, osteophytes are created as a response to damaged cartilage, attempting to create new bone material. One study completed by Golightly et al. discovered that increased serum levels of COMP were associated with the generation of osteophytes and joint space narrowing [26]. This was confirmed by a study by Kumm et al. who found a significant positive correlation between osteophyte progression and COMP levels [27]. These elevated levels could indicate synovial inflammation which triggers the release of protein fragments [26]. Osteoarthritic pain occurs as the disease progresses and the joint breaks down over time. When investigating COMP concentrations with regard to pain, a positive correlation was discovered [22, 24]. The progressive nature of the disease can be categorized by the Kellgren–Lawrence (KL) score, with increasing grades as the disease worsens. As suspected, COMP levels were increased in severe OA (KL grades 3 and 4) when compared with mild OA (KL grades 1 and 2) [20, 22, 24]. Our collective analysis also showed a significant difference between COMP serum concentration between baseline and OA patients (W = 211,442, p value = 1.137e−05, Figure 4). Due to this, COMP has been one of the most promising biomarkers for OA incidence and progression [19]. Although statistical significance was found in these reports, the ranges of COMP between normal and OA individuals overlap substantially, limiting the ability of COMP to be a single biomarker for diagnosis [24].
Matrix metalloproteinases (MMPs) are a family of protease enzymes that participate in the remodeling of the ECM. MMPs have been split into classes based on structure and substrate binding, including collagenases, gelatinases, stromelysins, and matrilysins. Chondrocytes and macrophages communicate through paracrine interactions to secrete the inflammatory cytokines, MMPs, growth factors, as well as tissue inhibitors of metalloproteinases (TIMPs) when OA damage occurs. In a study investigating synovial cells, levels of proinflammatory cytokines and MMPs were decreased upon the removal of macrophages [28]. In the same study, synovial fibroblast production of MMPs was shown to be regulated by the proinflammatory cytokines, TNF‐α and IL‐1β.
One of the collagenases, MMP‐1, was upregulated in intermediate cases of OA. Additionally, researchers found that the overexpression of serum amyloid A‐activating factor 1 was linked to the expression of the MMP‐1 promoter [29]. Another study investigated MMP‐3 in relation to OA because it has been previously studied as a biomarker for RA [30]. Playing a key role in the development of OA, this molecule is produced in response to mechanical stress as well as exposure to inflammatory cytokines and was found to be upregulated in early and intermediate cases of OA [29, 30]. In another study, MMP‐3 was found to be upregulated only in those with radiographic knee OA [31]. Weak positive correlations between MMP‐3 and serum Il‐6 or adiponectin concentrations were also found [30]. As part of the matrilysins class, MMP‐7 is another protein thought to play a role in OA. One study by Ling et al. discovered that elevated levels of MMP‐7 were present in patients who later developed OA. Their theory involved a shift from constitutively expressed MMP‐2 levels to MMP‐7, making the chondrocyte ECM vulnerable to degradation [15]. Finally, MMP‐9 was also found to be upregulated in pre‐OA individuals at the beginning of a longitudinal study when compared with healthy individuals who did not develop OA. These levels increased through the different stages of OA, leading to another biomarker that could detect the disease's progression and aid in early diagnosis [25].
Another collagenous protein that has varying serum concentrations in OA is N terminal propeptide of Type IIA procollagen (PIIANP). This protein was studied in four of the articles included in this review and is a product of type II collagen synthesis (Figure 3). In many synovial fluid studies, PIIANP is found to be upregulated, but concentration varies in serum. Van Spil et al. showed that serum PIIANP is consistently upregulated in OA in both obese and healthy BMI patients [32]. Another study, Kumm et al., supported this finding by showcasing a positive correlation between OA osteophyte formation and PIIANP concentration in women [27]. However, in men, this study showed a negative correlation between PIIANP and joint space narrowing (JSN) related to OA. Many more studies supported this negative correlation between sPIIANP and JSN [22, 33, 34]. In our analysis, we found a significant decrease in serum PIIANP levels in those with diagnostic OA (W = 789,275, p value = 0.03596, Figure 4). Overall, significantly decreased serum PIIANP levels are suggestive of OA progression, but this correlation may be diminished by patient obesity.
One collagenous biomarker known to be universally downregulated in OA is hyaluronic acid (HA), which was analyzed in six articles (Figure 3). HA is produced by synovial fibroblasts found in joints and helps with the support and fluidity of the joint, especially weight‐bearing joints. In OA, covalent crosslinking of HA is stimulated by the inflammation induced by TNF‐α and IL‐1β. Research with an equine OA model demonstrated an increased level of covalent cross‐linked HA, which impairs HA and decreases the ability to lubricate the articular cartilage, bind to aggrecan, and activate MMPs [35]. Thus, it is well understood that synovial fluid HA levels decrease in OA [36]. In fact, HA injections into the affected joint capsule provide temporary relief of OA symptoms and are a clinically accepted therapeutic option [37]. However, more recent studies focused on serum HA levels actually showed upregulation in OA progression [36]. While not as significant a correlation as synovial fluid HA, sHA appears to be increased in early‐stage OA, levels out in the mild stage, and then increases again in late‐stage OA [22]. Our analysis confirms the presence of elevated serum HA in diagnostic OA (W = 6111, p value = 1.242e−05, Figure 4). One theory explaining this difference between synovial fluid and serum HA levels in OA is that, while the affected joint has decreased expression of HA due to synovial damage, the damage releases molecules into serum that act as messengers, promoting HA upregulation in other, healthy joints. It is this non‐osteoarthritic joint HA upregulation that is thought to contribute to increased sHA in OA patients [26]. Regardless, the unique association of sHA in each stage of OA could allow for stage classification in future OA patients.
3.3. Obesity/Mechanical Stress Related
A variety of cytokines related to mechanical stress have been linked to the development of OA. Of these cytokines, an area of particular interest is with adipokines, which are specifically secreted by adipocytes. Various adipokines include leptin, adiponectin, resistin, and others [32]. Resistin was studied in 4 of the articles included in this review and has even been used to initiate OA in healthy animal joints. Additionally, previous in vitro studies have established resistin in the pathogenic release of oxidative products, immune cell chemotaxis, proteoglycan synthesis inhibition, and chondrocyte apoptosis [13]. In a 2012 study by Van Spil et al., plasma leptin was positively associated with the presence of knee OA on radiographs. However, this association disappeared when there was an adjustment for BMI. There were no significant associations seen with plasma adiponectin compared with the presence, incidence, or progression of knee OA, but plasma resistin was shown to have a positive association with the presence of knee OA [32]. In another study by Zhao et al., the plasma concentrations of resistin were also evaluated, comparing subjects without OA, with pre‐X‐ray defined knee OA, and X‐ray defined knee OA. They found plasma resistin levels were significantly elevated when the pre‐X‐ray defined group was compared with the control group. Our analysis showed that serum resistin was moderately upregulated in OA compared with baseline patients (W = 15,579, p value < 2.2e−16, Figure 4). There was also a significant positive correlation with resistin and the KL grade [13].
3.4. Strength of Association
In addition to measuring significant differences, the strength of association was measured for each biomarker regarding OA progression. The results indicate that PIIANP has a strong negative correlation with OA, suggesting that higher levels of this biomarker are linked to a lower likelihood of OA progression, although the wide confidence interval suggests some variability. Resistin shows a slight negative correlation with OA, but with more certainty around its estimate. In contrast, IL‐6, HA, CRP, and COMP are positively associated with OA, with IL‐6, HA, and CRP showing modest but reliable positive correlations, indicating that higher levels of these markers increase the likelihood of OA. COMP demonstrates a larger positive estimate, implying a stronger association with OA, though there is more variability in its confidence interval. TNF‐α and C2C, however, show near‐zero estimates, indicating little to no correlation with OA in this model. Overall, the analysis highlights PIIANP as a potential protective biomarker, while IL‐6, HA, CRP, and COMP are linked to OA progression (Table 1, Figure 5).
TABLE 1.
Logistic regression model results showing the estimated effect, standard deviation, and significance (p value) for each biomarker in relation to osteoarthritis progression.
| Estimate | Standard deviation | p | |
|---|---|---|---|
| COMP | 1.58E+00 | 2.59E−01 | 1.19E−09 |
| C2C | 1.16E−03 | 1.92E−04 | 1.67E−09 |
| CRP | 3.54E−01 | 8.03E−02 | 1.04E−05 |
| TNFa | −7.61E−03 | 8.96E−03 | 3.95E−01 |
| IL6 | 4.22E−01 | 9.41E−02 | 7.31E−06 |
| HA | 4.18E−01 | 5.39E−02 | 8.88E−15 |
| Resa | −1.69E−01 | 2.10E−02 | 9.69E−16 |
| PIIANP | −1.31E+00 | 3.51E−01 | 1.90E−04 |
FIGURE 5.
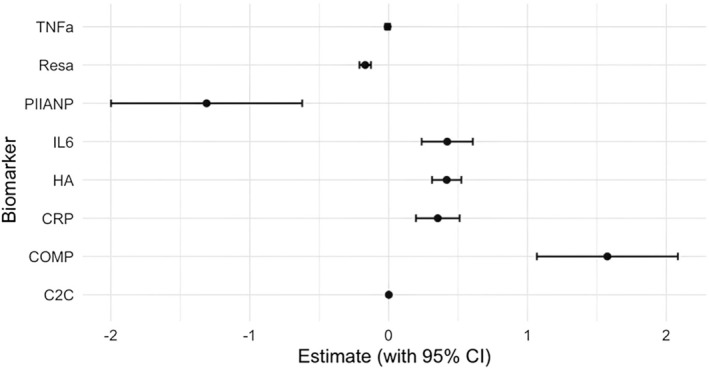
Forest plot of the biomarker predictors based upon logistic regression analysis. C2C, cartilage type II collagen; COMP, cartilage oligomeric matrix protein; HA, hyaluronic acid; hs‐CRP, high‐sensitivity C‐reactive protein; PIIANP, N‐propeptide of type IIA procollagen; Resa, resistin.
4. Discussion
OA is a multifaceted disease marked by the degradation of joint cartilage, inflammation, and subchondral bone remodeling. Our comprehensive review and analysis of 41 studies identified several key biomarkers that are dysregulated in OA. These biomarkers span various categories, including inflammatory mediators, collagenous markers, and obesity/mechanical stress markers. This detailed understanding of biomarkers can significantly contribute to early diagnosis and the development of new treatments [1, 7].
4.1. Inflammatory Markers
Inflammatory cytokines, such as IL‐1β, TNF‐α, and IL‐6, are consistently elevated in OA patients. These cytokines are pivotal in promoting joint inflammation and cartilage breakdown, correlating with disease severity and progression [11, 38, 39]. Our analysis confirmed that IL‐6, in particular, showed significant upregulation across multiple studies, reinforcing its potential as a key marker for OA progression [8]. Logistic regression results further support this finding, with IL‐6 showing a positive correlation with OA, suggesting it could be used to track disease progression (Figure 5). In contrast, TNF‐α displayed a weak or negligible correlation with OA based on logistic regression, which aligns with mixed findings in the literature regarding its role in OA pathology (Figure 6). Finally, C‐reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were upregulated in OA, reflecting the inflammatory state associated with the disease [17].
FIGURE 6.
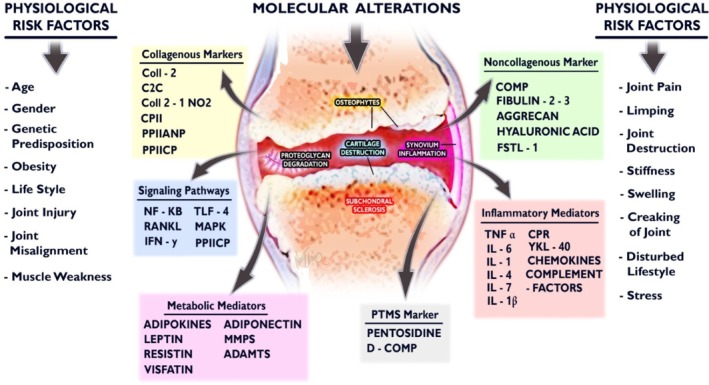
OA risk factors and correlating biomarkers detected in synovial fluid.
4.2. Collagenous Markers
One of the most heavily researched collagen‐related proteins associated with OA is collagen oligomeric matrix protein (COMP). COMP is a non‐collagenous glycoprotein involved in matrix assembly in articular cartilage. Elevated serum levels of COMP have been consistently associated with OA severity and progression [20, 21, 24]. Our analysis revealed that COMP levels are higher in OA patients compared with healthy controls, indicating its potential as a biomarker for disease monitoring [23, 26, 27]. Matrix metalloproteinases (MMPs), specifically MMP‐1, MMP‐3, and MMP‐7, play significant roles in the degradation of the extracellular matrix in OA. MMP‐1 and MMP‐3 were upregulated in OA and linked to cartilage breakdown [29, 30]. MMP‐7 has also been implicated in the progression of OA, suggesting its utility in early diagnosis and monitoring disease progression [15].
4.3. Obesity/Mechanical Stress Markers
Adipokines, such as leptin, adiponectin, and resistin, are cytokines secreted by adipose tissue and have been linked to OA. Plasma leptin levels were positively associated with knee OA presence and progression, although these associations diminish when adjusted for BMI [32]. Resistin levels were also elevated in OA patients, indicating its role in OA pathogenesis through oxidative stress, immune cell chemotaxis, and chondrocyte apoptosis [13].
4.4. Implications for Early Diagnosis
The consistent dysregulation of these biomarkers underscores their potential utility in early OA diagnosis. Detecting changes in biomarkers like aggrecan fragments, MMPs, and inflammatory cytokines before significant joint damage occurs could enable earlier interventions, potentially slowing disease progression and improving patient outcomes. Analyzing the significance of dysregulation and the strength of association between these biomarkers can help develop a unique biomarker signature that distinguishes OA from other conditions where some of these markers may also be dysregulated. This could lead to more accurate diagnoses and better‐targeted treatments [7, 10].
Implementing biomarker screening in clinical practice could revolutionize OA diagnosis by allowing clinicians to take a more proactive approach to diagnosis. This would facilitate the development of personalized treatment plans and early lifestyle interventions to mitigate risk factors, such as obesity and joint overuse, thereby delaying or preventing the onset of severe OA [5, 26].
4.5. Therapeutic Development
Understanding the molecular pathways associated with these dysregulated biomarkers offers avenues for developing targeted therapies. For instance, inhibiting key inflammatory cytokines like IL‐1β and TNF‐α with specific antagonists could mitigate the inflammatory processes driving cartilage degradation and reduce the production of MMPs. Similarly, direct MMP inhibitors could potentially reduce cartilage breakdown by targeting the enzymatic activity responsible for collagen and aggrecan degradation [30]. Additionally, the modulation of HA levels through intra‐articular injections has shown promise in providing symptomatic relief and improving joint function [37]. Further treatment to decrease the level of HA crosslinks or prevent them from developing would allow for reduced joint friction and improved symptoms.
Emerging therapeutic strategies might also focus on enhancing the repair capabilities of cartilage. For instance, treatments aimed at increasing the production of lubricin or promoting the synthesis of high‐molecular‐weight hyaluronic acid could improve joint lubrication and reduce wear [40]. Gene therapy and biologics targeting specific pathways involved in cartilage metabolism and inflammation hold potential for more effective long‐term management of OA [41].
5. Conclusion
Our review highlights the significant dysregulation of various biomarkers in OA, emphasizing their potential roles in early diagnosis and therapeutic development. By identifying and analyzing these biomarkers, we can enhance our understanding of OA pathogenesis, paving the way for more effective management of this debilitating condition.
While our review provides a comprehensive overview of biomarker dysregulation in OA, further studies are necessary to validate these findings in larger and more diverse populations. Longitudinal studies are essential to establish the temporal relationship between biomarker levels and disease progression.
Future research should also explore the integration of multi‐biomarker panels to increase diagnostic accuracy and predictive power. The development of standardized protocols for biomarker assessment will be crucial for translating these findings into clinical practice. Furthermore, investigating the role of novel biomarkers and their potential interactions with known markers could provide deeper insights into the complex molecular mechanisms underlying OA. Advances in proteomics and metabolomics may reveal new biomarkers or molecular patterns associated with OA, offering further opportunities for early diagnosis and personalized treatment approaches [42]. Additionally, exploring the genetic and epigenetic factors that contribute to OA susceptibility and progression could identify new targets for intervention and help stratify patients based on their risk profiles [43]. Early biomarker‐based diagnosis, coupled with targeted therapies, holds the promise of improving patient outcomes and reducing the overall burden of OA.
Author Contributions
Austin Lawrence: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, visualization, writing – original draft, writing – reviewing and editing. Joseph Boesel: conceptualization, data curation, formal analysis, investigation, methodology, resources, software, visualization, writing – original draft. Rogelio Martinez Aguilar: data curation, investigation, writing – original draft. Drew Gryczewski: writing – original draft. Ahmed Suparno Bahar Moni: conceptualization, supervision, writing – reviewing, and editing.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding: The authors received no specific funding for this work.
References
- 1. Ahmed U., Anwar A., Savage R. S., et al., “Biomarkers of Early Stage OA, Rheumatoid Arthritis and Musculoskeletal Health,” Scientific Reports 5 (2015): 9259, 10.1038/srep09259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neogi T., “The Epidemiology and Impact of Pain in OA,” OA Cartilage 21, no. 9 (2013): 1145–1153, 10.1016/j.joca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sinusas K., “OA: Diagnosis and Treatment,” American Family Physician 85, no. 1 (2012): 49–56. [PubMed] [Google Scholar]
- 4. Lynskey S. J., Macaluso M. J., Gill S. D., McGee S. L., and Page R. S., “Biomarkers of Osteoarthritis—A Narrative Review on Causal Links With Metabolic Syndrome,” Life (Basel) 13, no. 3 (2023): 730, 10.3390/life13030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saxby H., Mikropoulos C., and Boussios S., “An Update on the Prognostic and Predictive Serum Biomarkers in Metastatic Prostate Cancer,” Diagnostics (Basel) 10, no. 8 (2020): 549, 10.3390/diagnostics10080549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sturmer T., Brenner H., Koenig W., and Gunther K. P., “Severity and Extent of OA and Low Grade Systemic Inflammation as Assessed by High Sensitivity C Reactive Protein,” Annals of the Rheumatic Diseases 63, no. 2 (2004): 200–205, 10.1136/ard.2003.007674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bay‐Jensen A. C., Thudium C. S., and Mobasheri A., “Development and Use of Biochemical Markers in OA: Current Update,” Current Opinion in Rheumatology 30, no. 1 (2018): 121–128, 10.1097/BOR.0000000000000467. [DOI] [PubMed] [Google Scholar]
- 8. Braaten J. A., Banovetz M. T., DePhillipo N. N., et al., “Biomarkers for OA Diseases,” Life (Basel) 12, no. 11 (2022): 1779, 10.3390/life12111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kellgren J. H. and Lawrence J. S., “Radiological Assessment of Osteo‐Arthrosis,” Annals of the Rheumatic Diseases 16, no. 4 (1957): 494–502, 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fissolo N., Benkert P., Sastre‐Garriga J., et al., “Serum Biomarker Levels Predict Disability Progression in Patients With Primary Progressive Multiple Sclerosis,” Journal of Neurology, Neurosurgery, and Psychiatry 95, no. 5 (2024): 410–418, 10.1136/jnnp-2023-332251. [DOI] [PubMed] [Google Scholar]
- 11. Livshits G., Zhai G., Hart D. J., et al., “Interleukin‐6 Is a Significant Predictor of Radiographic Knee OA: The Chingford Study,” Arthritis and Rheumatism 60, no. 7 (2009): 2037–2045, 10.1002/art.24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu Y. P., Li J., Xin S. B., and X J., “Study the Relevance Between Inflammatory Factors and Estradiol and Their Association With Knee OA in Postmenopausal Women,” European Review for Medical and Pharmacological Sciences 22, no. 2 (2018): 472–478, 10.26355/eurrev_201801_14197. [DOI] [PubMed] [Google Scholar]
- 13. Zhao X. Y., Yang Z. B., Zhang Z. J., et al., “CCL3 Serves as a Potential Plasma Biomarker in Knee Degeneration (OA),” OA Cartilage 23, no. 8 (2015): 1405–1411, 10.1016/j.joca.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 14. Jiang L., Zhou X., Huang C., et al., “The Elevated Expression of IL‐38 Serves as an Anti‐Inflammatory Factor in Osteoarthritis and Its Protective Effect in Osteoarthritic Chondrocytes,” International Immunopharmacology 94 (2021): 107489, 10.1016/j.intimp.2021.107489. [DOI] [PubMed] [Google Scholar]
- 15. Ling S. M., Patel D. D., Garnero P., et al., “Serum Protein Signatures Detect Early Radiographic OA,” OA Cartilage 17, no. 1 (2009): 43–48, 10.1016/j.joca.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhu J., Ruan G., Cen H., et al., “Association of Serum Levels of Inflammatory Markers and Adipokines With Joint Symptoms and Structures in Participants With Knee Osteoarthritis,” Rheumatology (Oxford) 61, no. 3 (2022): 1044–1052, 10.1093/rheumatology/keab479. [DOI] [PubMed] [Google Scholar]
- 17. Hanada M., Takahashi M., Furuhashi H., Koyama H., and Matsuyama Y., “Elevated Erythrocyte Sedimentation Rate and High‐Sensitivity C‐Reactive Protein in OA of the Knee: Relationship With Clinical Findings and Radiographic Severity,” Annals of Clinical Biochemistry 53, no. Pt 5 (2016): 548–553, 10.1177/0004563215610142. [DOI] [PubMed] [Google Scholar]
- 18. Kondhalkar A., Ambad R., Bhatt N., and Jha R. K., “Determination of Clinical Utility of Novel Biochemical Markers in OA,” Journal of Pharmaceutical Research International 33 (2021): 240–245, 10.9734/jpri/2021/v33i39A32166. [DOI] [Google Scholar]
- 19. Saberi Hosnijeh F., Siebuhr A. S., Uitterlinden A. G., et al., “Association Between Biomarkers of Tissue Inflammation and Progression of OA: Evidence From the Rotterdam Study Cohort,” Arthritis Research & Therapy 18 (2016): 81, 10.1186/s13075-016-0976-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vilim V., Olejarova M., Machacek S., Gatterova J., Kraus V. B., and Pavelka K., “Serum Levels of Cartilage Oligomeric Matrix Protein (COMP) Correlate With Radiographic Progression of Knee OA,” OA Cartilage 10, no. 9 (2002): 707–713, 10.1053/joca.2002.0819. [DOI] [PubMed] [Google Scholar]
- 21. Wislowska M. and Jablonska B., “Serum Cartilage Oligomeric Matrix Protein (COMP) in Rheumatoid Arthritis and Knee OA,” Clinical Rheumatology 24, no. 3 (2005): 278–284, 10.1007/s10067-004-1000-x. [DOI] [PubMed] [Google Scholar]
- 22. Papaneophytou C., Alabajos‐Cea A., Viosca‐Herrero E., et al., “Associations Between Serum Biomarkers of Cartilage Metabolism and Serum Hyaluronic Acid, With Risk Factors, Pain Categories, and Disease Severity in Knee OA: A Pilot Study,” BMC Musculoskeletal Disorders 23, no. 1 (2022): 195, 10.1186/s12891-022-05133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petersson I. F., Boegard T., Dahlstrom J., Svensson B., Heinegard D., and Saxne T., “Bone Scan and Serum Markers of Bone and Cartilage in Patients With Knee Pain and OA,” OA Cartilage 6, no. 1 (1998): 33–39, 10.1053/joca.1997.0090. [DOI] [PubMed] [Google Scholar]
- 24. Clark A. G., Jordan J. M., Vilim V., et al., “Serum Cartilage Oligomeric Matrix Protein Reflects OA Presence and Severity: The Johnston County OA Project,” Arthritis and Rheumatism 42, no. 11 (1999): 2356–2364, . [DOI] [PubMed] [Google Scholar]
- 25. Pavelka K., Forejtova S., Olejarova M., et al., “Hyaluronic Acid Levels May Have Predictive Value for the Progression of Knee OA,” OA Cartilage 12, no. 4 (2004): 277–283, 10.1016/j.joca.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 26. Golightly Y. M., Marshall S. W., Kraus V. B., et al., “Biomarkers of Incident Radiographic Knee OA: Do They Vary by Chronic Knee Symptoms?,” Arthritis & Rheumatism 63, no. 8 (2011): 2276–2283, 10.1002/art.30412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kumm J., Tamm A., Lintrop M., and Tamm A., “The Value of Cartilage Biomarkers in Progressive Knee OA: Cross‐Sectional and 6‐Year Follow‐Up Study in Middle‐Aged Subjects,” Rheumatology International 33, no. 4 (2013): 903–911, 10.1007/s00296-012-2463-8. [DOI] [PubMed] [Google Scholar]
- 28. Bondeson J., Lauder S., Wainwright S., et al., “Adenoviral Gene Transfer of the Endogenous Inhibitor IkappaBalpha into Human Osteoarthritis Synovial Fibroblasts Demonstrates that Several Matrix Metalloproteinases and Aggrecanases are Nuclear Factor‐kappaB‐Dependent,” Journal of Rheumatology 34, no. 3 (2007): 523–533. [PubMed] [Google Scholar]
- 29. Li W., Du C., Wang H., and Zhang C., “Increased Serum ADAMTS‐4 in Knee OA: A Potential Indicator for the Diagnosis of OA in Early Stages,” Genetics and Molecular Research 13, no. 4 (2014): 9642–9649, 10.4238/2014.November.14.9. [DOI] [PubMed] [Google Scholar]
- 30. Ishibashi K., Sasaki E., Ota S., et al., “Detection of Synovitis in Early Knee OA by MRI and Serum Biomarkers in Japanese General Population,” Scientific Reports 10, no. 1 (2020): 12310, 10.1038/s41598-020-69328-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sasaki E., Chiba D., Ota S., et al., “Reduced Serum Levels of Anti‐Mullerian Hormone is a Putative Biomarker of Early Knee OA in Middle‐Aged Females at Menopausal Transition,” Scientific Reports 11, no. 1 (2021): 4931, 10.1038/s41598-021-84584-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van Spil W. E., Welsing P. M., Kloppenburg M., et al., “Cross‐Sectional and Predictive Associations Between Plasma Adipokines and Radiographic Signs of Early‐Stage Knee OA: Data From CHECK,” OA Cartilage 20, no. 11 (2012): 1278–1285, 10.1016/j.joca.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 33. Daghestani H. N., Jordan J. M., Renner J. B., Doherty M., Wilson A. G., and Kraus V. B., “Serum N‐Propeptide of Collagen IIA (PIIANP) as a Marker of Radiographic OA Burden,” PLoS One 12, no. 12 (2017): e0190251, 10.1371/journal.pone.0190251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garnero P., Ayral X., Rousseau J. C., et al., “Uncoupling of Type II Collagen Synthesis and Degradation Predicts Progression of Joint Damage in Patients With Knee OA,” Arthritis & Rheumatism 46, no. 10 (2002): 2613–2624, 10.1002/art.10576. [DOI] [PubMed] [Google Scholar]
- 35. Fasanello D. C., Su J., Deng S., et al., “Hyaluronic Acid Synthesis, Degradation, and Crosslinking in Equine Osteoarthritis: TNF‐α‐TSG‐6‐Mediated HC‐HA Formation,” Arthritis Research & Therapy 23, no. 1 (2021): 218, 10.1186/s13075-021-02588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ishijima M., Watari T., Naito K., et al., “Relationships Between Biomarkers of Cartilage, Bone, Synovial Metabolism and Knee Pain Provide Insights Into the Origins of Pain in Early Knee OA,” Arthritis Research & Therapy 13, no. 1 (2011): R22, 10.1186/ar3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weick J. W., Bawa H. S., and Dirschl D. R., “Hyaluronic Acid Injections for Treatment of Advanced OA of the Knee: Utilization and Cost in a National Population Sample,” Journal of Bone and Joint Surgery (American Volume) 98, no. 17 (2016): 1429–1435, 10.2106/JBJS.15.01358. [DOI] [PubMed] [Google Scholar]
- 38. Goldring M. B. and Otero M., “Inflammation in Osteoarthritis,” Current Opinion in Rheumatology 23, no. 5 (2011): 471–478, 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scanzello C. R. and Goldring S. R., “The Role of Synovitis in Osteoarthritis Pathogenesis,” Bone 51, no. 2 (2012): 249–257, 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jay G. D., Torres J. R., Warman M. L., Laderer M. C., and Breuer K. S., “The Role of Lubricin in the Mechanical Behavior of Synovial Fluid,” Proceedings of the National Academy of Sciences of the United States of America 104, no. 15 (2007): 6194–6199, 10.1073/pnas.0608558104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ahmed U., Anwar A., Savage R. S., Thornalley P. J., and Rabbani N., “Protein Oxidation, Nitration and Glycation Biomarkers for Early‐Stage Diagnosis of OA of the Knee and Typing and Progression of Arthritic Disease,” Arthritis Research & Therapy 18, no. 1 (2016): 250, 10.1186/s13075-016-1154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Farì G., Santagati D., Pignatelli G., et al., “Collagen Peptides, in Association with Vitamin C, Sodium Hyaluronate, Manganese and Copper, as Part of the Rehabilitation Project in the Treatment of Chronic Low Back Pain,” Endocrine, Metabolic & Immune Disorders Drug Targets 22, no. 1 (2022): 108–115, 10.2174/1871530321666210210153619. [DOI] [PubMed] [Google Scholar]
- 43. Skrzypa M., Szala D., Gablo N., et al., “miRNA‐146a‐5p is Upregulated in Serum and Cartilage Samples of Patients With OA,” Polski Przeglad Chirurgiczny 91, no. 3 (2019): 1–5, 10.5604/01.3001.0013.0135. [DOI] [PubMed] [Google Scholar]


