Abstract
Background:
The aim of this study is to assess the effectiveness of the Early Warning Score [ANDC (age (A), neutrophil-to-lymphocyte ratio (NLR (N)), D-dimer (D), and CRP (C)] in predicting the treatment response in patients receiving tocilizumab for Coronavirus Disease 2019 (COVID-19)-related cytokine storm.
Methods:
A retrospective review of medical records was conducted for patients treated with tocilizumab for a cytokine storm related to COVID-19 between April 1, 2020, and April 1, 2021. Patient demographics, clinical characteristics, and laboratory parameters within 24 hours before tocilizumab were recorded. 1.14 × (age − 20) (years) + 1.63 × NLR + 5.00 × D-dimer (mg/L) + 0.14 × C-reactive protein (CRP) (mg/L) was used as the formula for the ANDC score. The study population was divided into 2 groups: those who died within 28 days of receiving tocilizumab and those who recovered. A comparative analysis was conducted.
Results:
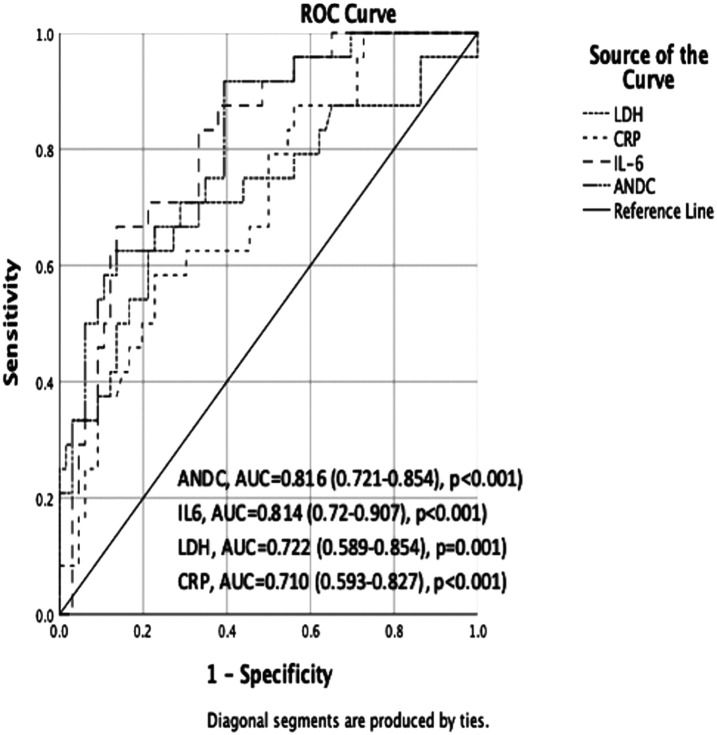
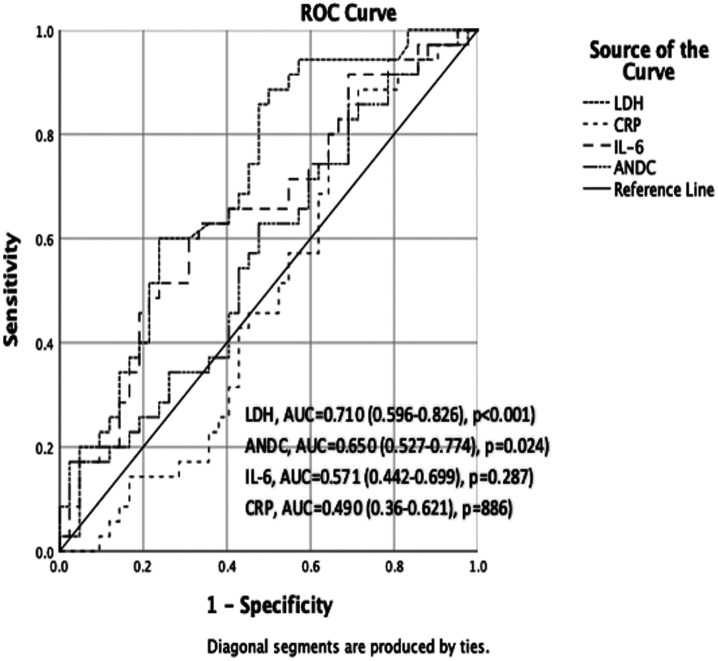
Within 28 days of tocilizumab treatment, 59 (35.32%) patients died. In comparison with living patients, deceased patients exhibited considerably higher levels of interleukin (IL)-6, lactate dehydrogenase (LDH), ANDC score, and CRP (P < .05). Lactate dehydrogenase was an independent predictor of response to tocilizumab treatment (P < .001) in a multivariate logistic regression analysis. In patients who did not receive steroid therapy before tocilizumab treatment, the ANDC score had the highest area under the curve (AUC). The optimal cut-off value was determined to be 92.56, with a sensitivity of 91.67% and a specificity of 60.61% (P < .001). In patients receiving steroids before tocilizumab, LDH had the highest AUC. The optimal cut-off value was 484.5 U/L (P < .001).
Conclusion:
Lactate dehydrogenase was identified as an independent predictor of response to tocilizumab treatment. The ANDC score showed the highest AUC value in steroid-naïve patients before tocilizumab, whereas LDH showed the highest AUC value in patients receiving steroids before tocilizumab. Both the ANDC score and LDH levels show potential as valuable tools to guide treatment decisions.
Keywords: Coronavirus-2019, tocilizumab, ANDC score, interleukin-6, lactate dehydrogenase
Main Points
Within 28 days of tocilizumab treatment, 35.32% of patients died. IL-6, LDH, ANDC score, and CRP levels were higher in patients who died (P < .05).
Lactate dehydrogenase was found to be an independent predictor of response to tocilizumab treatment (P < .001).
The ANDC score had the highest AUC value (0.816) in steroid-naïve patients before tocilizumab, while LDH had the highest AUC value (0.710) in patients receiving steroids before tocilizumab.
Introduction
Coronavirus Disease 2019 (COVID-19) emerged in December 2019. It has spread globally and has become a pandemic. It can cause acute respiratory distress syndrome and is fatal in approximately 1% of patients. However, the disease is mostly asymptomatic or mild. Additionally, some patients develop macrophage activation syndrome (MAS). A cytokine storm results from the overproduction of pro-inflammatory cytokines, including interleukin (IL)-1 and IL-6. Macrophage activation syndrome is defined by a cytokine storm resulting from a hyperinflammatory response. The presence of specific findings, including elevated temperatures, elevated ferritin, C-reactive protein (CRP), D-dimer, triglycerides, thrombocytopenia, lymphopenia, hypofibrinogenemia, and impaired liver function tests, indicates the development of MAS. 1-4 However, the efficacy of these findings in predicting the development of MAS has been variable. It has been postulated that a cytokine storm is the underlying cause of secondary hemophagocytic lymphohistiocytosis and fatal lung injury observed in patients with severe COVID-19. 5-7 In response, the therapeutic armamentarium has been expanded to stop or reverse this detrimental process, including steroids, IL-1, and IL-6 blockers. However, these agents are a double-edged sword, as they may leave patients susceptible to infections due to the immunosuppression they induce. Consequently, the timing of treatment has emerged as a crucial aspect in the management of patients with COVID-19.
Tocilizumab is a monoclonal antibody that antagonizes IL-6 and is used to treat autoimmune diseases such as rheumatoid arthritis and giant cell arteritis. 8 It has recently been shown to be beneficial in severe COVID-19 pneumonia. However, the appropriate group of patients and the timing for the best outcome of treatment with tocilizumab are still not known. Identifying patient populations likely to benefit from tocilizumab is therefore important. The ANDC score, which stands for age (A), neutrophil-to-lymphocyte ratio (NLR (N)), D-dimer (D), and CRP (C), attracted attention in COVID-19 patients and was first proposed in the literature as an early warning score to predict mortality.9 In another study, it was found to be associated with mortality despite lower performance.10 Hsu et al11 reported that ANDC predicts COVID-19 disease severity and length of hospital stay. Al-Shami et al12 reported that ANDC can be used as a predictor of disease severity and nutritional status in hospitalized COVID-19 patients. It was reported that ANDC was a stronger score than the systemic immune-inflammation index in predicting in-hospital mortality in COVID-19 patients with malignancy.13 When the available literature data are examined, it is noteworthy that COVID-19 is also used to evaluate systemic inflammation and outcomes, especially in hospitalized patients. No studies have evaluated the relationship between ANDC score and tocilizumab response to our knowledge. The objective of this study was to assess the efficacy of ANDC in predicting the response to tocilizumab treatment in patients with COVID-19-related cytokine storms.
Material and Methods
The study was conducted at Gülhane Training and Research Hospital. The initial protocol was first recorded by the Scientific Research Council of the Ministry of Health and later approved by the Ethics Committee of Health Sciences Gulhane University of Health Sciences Scientific Research (date: March 2, 2022, number: 2022/20). A retrospective analysis was conducted on the electronic medical records of patients diagnosed with COVID-19 who were treated at our clinic between April 1, 2020, and April 1, 2021, due to clinical deterioration. The data collection period was planned as March 2, 2022 to May 2, 2022.
The following were considered to be inclusion criteria: positive SARS-CoV-2 RNA nasopharyngeal swab reverse transcriptase-polymerase chain reaction, patients over 18 years of age, and chest CT findings compatible with COVID-19. Patients under 18 years of age and patients with incomplete data were excluded.
The study included a total of 167 patients meeting the criteria for tocilizumab treatment. In addition to the demographic and clinical characteristics, the medical treatments of the patients were recorded. Additionally, serum levels of alanine transaminase (ALT), aspartate transaminase (AST), creatinine, urea, CRP, lactate dehydrogenase (LDH), ferritin, albumin, and IL-6 were measured and recorded for all patients in the last 24 hours prior to tocilizumab administration. The NLR is obtained by dividing the absolute count of neutrophils by the absolute count of lymphocytes. The formula for the ANDC score was calculated as follows: [1.14 × (age − 20) (years) + 1.63 × NLR + 5.00 × D-dimer (mg/L) + 0.14 × CRP (mg/L)].9 The effectiveness of tocilizumab treatment was assessed by calculating the percentage of patients who died within 28 days of the infusion. The study population was split into 2 groups: patients who died within 28 days of receiving tocilizumab and those who recovered. The demographic, clinical, and laboratory data of these groups were then compared. Additionally, since steroid administration affected laboratory parameters, patients were classified into 2 groups based on whether they had received steroid treatment before tocilizumab therapy.
Treatment Protocol
The Turkish Ministry of Health’s recommendations were followed for the entirety of the patients’ treatment regimens. The recommendations included the administration of favipiravir in conjunction with hydroxychloroquine and azithromycin for a period of 5-10 days. Venous thromboembolic prophylaxis was recommended for all patients without contraindications. In the event that the laboratory and clinical findings of the patients were compatible with a cytokine storm, tocilizumab at a dose of 8 mg/kg (maximum dose: 800 mg) was administered intravenously as a single dose after bacterial and fungal co-infection had been excluded. If no improvement was observed following the initial dose, a second dose was administered within 24 hours in accordance with national guidelines.14 Due to the recent change in treatment guidelines in Türkiye, tocilizumab was administered to some patients following steroid treatment and to others prior to this.
Statistical Analysis
SPSS version 26 (IBM SPSS Corp.; Armonk, NY, USA) software was used for statistical analyses. The distribution of variables was analyzed by both visual and analytical methods. The former included the use of histograms and probability graphs, while the latter employed the Kolmogorov–Smirnov test. In descriptive analyses, data with a normal distribution were summarized using the mean and SD, whereas data without a normal distribution were described using the median and interquartile range. Categorical variables were quantified in numeric and percentage form. The Mann–Whitney U-test was used to compare the medians of non-normally distributed numerical variables between groups. The Pearson chi-squared test and Fisher’s exact test were utilized to evaluate differences between groups for categorical variables. Variables with a P-value less than .250 were included in the regression analysis to identify independent predictors of mortality after tocilizumab treatment. The univariate logistic regression analysis identified statistically significant parameters, and these were subsequently evaluated in a multivariate logistic regression analysis in order to ascertain the independent mortality risk factors. To assess the diagnostic decision-making capabilities of LDH, CRP, IL-6, and ANDC scores in predicting the response to tocilizumab treatment, the receiver operating characteristic (ROC) curve was employed. The specificity, negative predictive value, sensitivity, and positive predictive value were presented when a significant cut-off value was found. In the evaluation of the area under the curve (AUC), a type I error level below 5% was taken as a criterion for the statistically significant interpretation of the diagnostic value of the test. To determine the optimal cut-off value for these biomarkers, the Youden index was employed. The P-value, which is considered to be statistically significant, is less than .05.
Results
A total of 167 patients with COVID-19 who received tocilizumab due to clinical progression and cytokine storm despite standard guideline therapy were included in the study. The patients were predominantly male (72.4%), and the mean ± standard deviation (SD) age was 62.05 ± 13.11 years. A total of 59 patients (35.32%) died following tocilizumab treatment. The majority of patients who died were male (67.8%). The mean ± SD was 67.53 ± 10.4 years. A total of 139 patients (67.1%) had at least one comorbidity. Hypertension was the most common (38.6%), followed by diabetes mellitus (29.5%). Patients who recovered from tocilizumab were significantly younger than those who died (P = .001), and their IL-6, CRP, ANDC score, NLR and LDH levels were significantly lower. A higher proportion of patients who died were administered steroids before tocilizumab (P = .011). The clinical characteristics and laboratory findings of the study groups are shown in Table 1.
Table 1.
Clinical Characteristics and Laboratory Parameters of the Study Groups
| Patients Who Improved (n : 108) | Patients Who Died (n : 59) | P | |
|---|---|---|---|
| Age (years)* | 60.80 ± 13.08 | 67.53 ± 10.46 | .001 |
| Female/male** | 27(25)/81 (75) | 19 (32.2)/40(67.8) | .319 |
| The interval between the initial onset of symptoms and the administration of tocilizumab (days) | 9.00 (6.00-11.00) | 9.50 (5.00-12.5) | .821 |
| Presence of comorbidities** | |||
| Hypertension | 30 (27.8) | 30 (50.8) | .003 |
| Diabetes mellitus | 27 (25) | 21 (35.6 ) | .148 |
| Intermediate or advanced chronic kidney disease | 5 (4.6) | 9 (15.3) | .037 |
| Coronary artery disease | 20 (18.5) | 13 (22) | .586 |
| Congestive heart failure | 3 (2.8) | 9 (15.3) | .005 |
| Hyperlipidemia | 2 (1.9) | 2 (3.4) | .615 |
| Treatment** | |||
| Antibiotic treatment | 83 (76.9) | 53 (89.8) | .039 |
| Steroid before tocilizumab | 42 (38.9) | 35 (59.4 ) | .011 |
| Laboratory parameters at tocilizumab administration*** | |||
| WBC (109/L) | 7.15 (5.82-9.77) | 9.7 (8-12) | <.001 |
| Lymphocyte (109/L) | 0.7 (0.425-1) | 0.4 (0.3-0.8) | .001 |
| Neutrophil (109/L) | 5.8 (4.32-8.6) | 8.6 (6.6-10.8) | <.001 |
| Platelet (109/L) | 236 (191.5-292) | 221 (154-284) | .081 |
| LDH (U/L) | 433.5 (330.5-578.5) | 609 (489-770) | <.001 |
| IL-6 (pg/mL) | 75.99 (47.41-132.23) | 154.13 (75.6-263.47) | <.001 |
| CRP (mg/L) | 154.18 (108.1-226.1) | 185.3(135.4-253) | .039 |
| Fibrinogen (mg/dL) | 653 (540-802.25) | 688 (550-867) | .484 |
| D-dimer (mg/L) | 0.93 (0.56-1.7) | 1.82 (1.03-4.12) | <.001 |
| Albumin (g/dL) | 3 (2.73-3.32) | 2.83 (2.57-3.13) | .002 |
| NLR | 9 (4.51-15.43) | 20.5 (9.56-28) | <.001 |
| ANDC score | 101.28 (80.27-122.39) | 129.58 (107.22-144.84) | <.001 |
Bold values indicate statistical significance. CRP, C-reactive protein; LDH, lactate dehydrogenase; IL-6, interleukin-6; NLR, neutrophils-to-lymphocytes ratio; WBC, white blood cell.
*Mean ± SD.
**Number (%).
***Median (IQR).
A multivariate logistic regression analysis was employed to identify independent predictors of mortality following tocilizumab administration. Parameters that demonstrated a significant association with mortality in univariate analyses, including age, hypertension, intermediate or advanced chronic kidney disease, steroid treatment, NLR, D-dimer, congestive heart failure, IL-6, LDH, and ANDC score, were subjected to multivariate analysis. LDH was identified as an independent predictor of mortality in patients who had received tocilizumab treatment (Table 2).
Table 2.
Independent Predictors of Mortality After Tocilizumab Therapy in Multivariate Logistic Regression Analysis
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age | 1.048 | 1.018-1.078 | .001 | 1.053 | 0.992-1.117 | .09 |
| Hypertension | 2.69 | 1.388 -5.213 | .003 | 2.190 | 0.897-5.348 | .085 |
| Diabetes mellitus | 1.658 | 0.833-3.3 | .15 | |||
| Intermediate or advanced chronic kidney disease | 3.708 | 1.181-11.643 | .025 | 3.631 | 0.791-16.661 | .097 |
| Congestive heart failure | 6.300 | 1.634-24.286 | .008 | 3.507 | 0.71-17.319 | .124 |
| Steroid before tocilizumab | 2.292 | 1.199-4.38 | .012 | 1.753 | 0.666-4.615 | .256 |
| LDH (U/L) | 1.005 | 1.003-1.007 | <.001 | 1.006 | 1.003-1.01 | <.001 |
| IL-6 (pg/mL) | 1.004 | 1.001-1.006 | .006 | 1.003 | 1.000 -1.005 | .05 |
| NLR | 1.049 | 1.022 -1.077 | <.001 | 1.026 | 0.955-1.103 | .48 |
| D-dimer (mg/L) | 1.123 | 1.012 -1.248 | .029 | 1.076 | 0.852 -1.358 | .54 |
| CRP (mg/L) | 1.004 | 0.999 -1.008 | .089 | |||
| ANDC | 1.023 | 1.012 -1.034 | <.001 | 1.002 | 0.963-1.043 | .928 |
Bold values indicate statistical significance. CRP, C-reactive protein; LDH, lactate dehydrogenase; IL-6, interleukin-6; NLR, neutrophils-to-lymphocytes ratio.
The results of the ROC analysis in patients with COVID-19 who did not receive steroid treatment prior to tocilizumab treatment indicated that the ANDC score exhibited the highest diagnostic value in predicting mortality. The ANDC score exhibited the greatest AUC (AUC: 0.816, 95% CI: 0.721-0.912), followed by IL-6, LDH, and CRP. In patients with COVID-19 who had received steroid therapy before tocilizumab therapy, the LDH exhibited the highest AUC (AUC: 0.710, 95% CI: 0.596-0.826, P < .001), followed by ANDC, IL-6, and CRP (Figures 1 and 2). The optimal cut-off value for the ANDC score was 92.56, with a sensitivity of 91.67% and a specificity of 60.61% (P < .001) in patients with COVID-19 who had not received steroid therapy prior to tocilizumab treatment. In patients with COVID-19 who had received steroid therapy before tocilizumab therapy, the optimal cut-off value for LDH was 484.5, with a sensitivity of 85.71% and a specificity of 52.38% (P < .001). The predictive performance of these markers in relation to mortality following tocilizumab is presented in Table 3.
Figure 1.
Receiver operating characteristic curve of steroid non-received patients before tocilizumab (125 × 94 mm (300 × 300 DPI)).
Figure 2.
Receiver operating characteristic curve of steroid received patients before tocilizumab (119 × 97 mm (300 × 300 DPI)).
Table 3.
Predictive Performance of Inflammatory Biomarkers in the Prediction of Mortality after Tocilizumab Treatment
| Sensitivity | Specificity | Cut-off | PLR | NLR | PPV | NPV | |
|---|---|---|---|---|---|---|---|
| Steroid non-received patients | |||||||
| ANDC | 91.67% | 60.61% | 92.56 | 2.33 | 0.14 | 45.83% | 95.24% |
| IL-6 | 83.33% | 66.67% | 104.74 | 2.50 | 0.25 | 47.62 % | 91.67% |
| LDH | 70.83% | 71.21% | 480.5 | 2.46 | 0.41 | 47.22% | 87.04% |
| CRP | 62.50% | 69.70% | 187.75 | 2.06 | 0.54 | 42.86% | 83.64% |
| Steroid received patients | |||||||
| ANDC | 62.86% | 52.38% | 125.37 | 1.32 | 0.71 | 52.38% | 62.86% |
| IL-6 | 62.86% | 66.67% | 99.25 | 1.89 | 0.56 | 61.11% | 68.29% |
| LDH | 85.71% | 52.38% | 484.5 | 1.80 | 0.27 | 60.00% | 81.48% |
| CRP | 80.00% | 35.71% | 127.05 | 1.24 | 0.56 | 50.91% | 68.18% |
CRP, C-reactive protein; IL-6, interleukin-6; LDH, lactate dehydrogenase; NLR, negative likelihood ratio; NPV, negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value.
Discussion
Tocilizumab, an IL-6 receptor-targeting monoclonal antibody, has been proposed as a potential treatment for cytokine storms, a hyperinflammatory response seen in COVID-19 patients.15-20 The results of this study highlight the association between inflammatory markers and therapeutic response in patients experiencing cytokine storms due to COVID-19 and treated with tocilizumab. The results of our study indicate that the ANDC score exhibited the highest AUC value (0.816) in steroid-naïve patients before tocilizumab administration, while LDH demonstrated the highest AUC value (0.710) in patients receiving steroids before tocilizumab. However, in the group that received steroid treatment before tocilizumab treatment, the predictive power of the ANDC score was compromised, and its sensitivity and specificity for predicting treatment response decreased. A multivariate regression analysis demonstrated that serum LDH was an independent predictor of response to tocilizumab treatment.
In studies evaluating the reliability of IL-6 as a biomarker, Li et al21 found that pretreatment serum IL-6 levels ≥100 pg/mL were predictive of worse outcomes in patients receiving tocilizumab. Flisiak et al22 reported that tocilizumab administration decreased mortality and accelerated clinical recovery in patients with pretreatment serum IL-6 levels >100 pg/mL who required oxygen support. The IL-6-related findings of our study support the literature. However, steroid treatment prior to tocilizumab lowered the cut-off. This difference is probably due to the fact that steroids decrease IL-6 synthesis and release.
Studies of the COVID-19 pandemic have reported that gender is associated with disease incidence and clinical severity. Although there are conflicting results reported from China regarding the effect of gender on the incidence of disease, it has been suggested that the incidence is slightly higher in males and that they are more susceptible to the disease.3,5 In older individuals, the disease is more likely to be severe and fatal.7 In our study, 72.4% of patients who received tocilizumab due to non-response to standard treatment were male. In addition, patients who did not respond to tocilizumab were significantly older. As in previous studies on COVID-19, we can define advanced age and male gender as indicators of poor prognosis in our study.
Decreased oxygenation, multiple organ damage, and hypercoagulopathy may contribute to elevated LDH levels in COVID-19 patients. In COVID-19 patients, high LDH levels have been associated with an increased risk of serious illness and death.23,24 Similar to our findings, Li et al21 observed that a comparison of baseline LDH levels in patients who died after treatment with tocilizumab and those who recovered showed a statistically significant difference between the 2 groups. They also showed that while no significant improvement in LDH levels was observed in patients who died after treatment, LDH levels decreased significantly in those who recovered. In our study, the higher AUC of LDH in steroid non-responders serves to reinforce its role as a robust marker in this context. The optimal cut-off value for LDH identified in the study (484.5 U/L) may help clinicians stratify patients according to their likelihood of responding to tocilizumab, thereby facilitating more personalized treatment approaches.
Weng et al9 developed a quantitative tool, the ANDC score, using 4 variables—CRP, age, NLR, and D-dimer—to predict the risk of premature death in COVID-19 patients. To divide the patients into 3 groups, they suggested cut-off values of 59 and 101: low-risk (ANDC <59) with a probability of death below 5%, intermediate-risk (59 ≤ ANDC ≤ 101) with a probability of death between 5% and 50%, and high-risk (ANDC >101) with a probability of death above 50%. This nomogram, based on these risk factors, showed good calibration in predicting the prognosis of COVID-19 patients. A correlation between the presence of shortness of breath in the absence of hypoxemia and elevated laboratory values for D-dimer, leukocyte, and CRP and the development of severe COVID-19 pneumonia was observed in a study by Hamidi and Ulu et al.25 The ANDC score was derived by combining these parameters with age. Recent studies in the literature have confirmed that COVID-19 can also be used to assess systemic inflammation and outcomes, particularly in hospitalized patients.10-13 Due to changes in local treatment protocols regarding the timing of steroid treatment during the study period, analyses were performed according to whether patients received steroid treatment prior to tocilizumab. The results of our study highlight the role of the ANDC score as a potential tool to assess systemic inflammation and predict mortality outcomes, particularly in COVID-19 patients who are hospitalized and receiving tocilizumab treatment. This is consistent with recent studies suggesting that the ANDC score may provide insight into disease severity and patient prognosis in COVID-19. Steroid administration appears to affect the sensitivity and specificity of the ANDC score for predicting mortality. As steroids have known immunomodulatory effects, their use prior to tocilizumab likely altered inflammatory markers and potentially reduced the ability of the score to predict mortality with the same accuracy. This reduction in sensitivity and specificity highlights a potential limitation of the ANDC score in steroid-treated patients prior to tocilizumab. Thus, our results suggest that the predictive ability of the ANDC score may need to be interpreted with caution or adjusted for this confounding effect in contexts where steroids are administered. Given these findings, future research could explore the standardization of ANDC score cut-offs for different therapeutic scenarios or develop adjusted scoring metrics that account for pretreatment effects. In conclusion, the ANDC score remains a promising indicator for the assessment of mortality risk in COVID-19.
The majority of COVID-19 patients only have mild-to-moderate symptoms, according to medical literature, and do not need anti-cytokine medication.26 However, a subset of patients may progress to severe disease requiring anti-cytokine therapy due to the risk of death. Delayed efforts to suppress the inflammatory cascade do not reduce mortality. In addition, early use of anti-cytokine therapy may result in increased treatment costs and various risks and side effects, including iatrogenic immunosuppression. The use of various biomarkers, including those proposed in this study, may help determine the optimal timing and identify patients who would benefit from anti-cytokine therapy. In conclusion, determining the optimal timing of tocilizumab treatment in COVID-19 patients is important but remains uncertain.
This study had several limitations. First, the fact that it was a retrospective and single-center study caused selection bias. Second, there was heterogeneity between groups due to changes in local treatment protocols during the study period. This limitation was mitigated by calculating the predictive performance of these markers separately according to whether or not steroid treatment was received prior to tocilizumab. The fact that this is the first study conducted for the stated purpose is a strength of the study. Prospective comprehensive studies are needed to overcome the current limitations.
This study provides valuable information on predictive markers of tocilizumab efficacy in patients with COVID-19 and cytokine storm. In particular, ANDC score and LDH predicted mortality prior to tocilizumab and appear to be promising tools to guide treatment decisions. However, the impact of prior steroid treatment on these markers highlights the urgent need for a more sophisticated approach to patient management that takes into account the sequence of therapeutic interventions and the timeframe in which they are administered. Future research should focus on prospective validation and investigation of combined biomarker models to improve the accuracy of therapeutic predictions in the management of cytokine storms.
Funding Statement
The authors declared that this study has received no financial support.
Footnotes
Ethics Committee Approval: This study was approved by the Ethics Committee of Health Sciences University of Health Sciences Scientific Research (date: March 2, 2022, number: 2022/20).
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – Ö.K., D.T.; Design – Ö.K., D.T.; Supervision – M.Ç., S.Y.; Resources – MN.K., Ö.K.; Materials – MN.K., Ö.K.; Data Collection and/or Processing – MN.K., D.T.; Analysis and/or Interpretation – Ö.K., D.T.; Literature Search – Ö.K., MN.K.; Writing – Ö.K., D.T.; Critical Review – M.Ç., S.Y.
Declaration of Interests: The authors have no conflict of interest to declare.
Data Availability Statement:
The data that support the findings of this study are available on request from the corresponding author.
References
- 1. Antinori S, Bonazzetti C, Gubertini G, et al. Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: an increased risk for candidemia? Autoimmun Rev. 2020;19(7):102564. ( 10.1016/j.autrev.2020.102564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(5):105954. ( 10.1016/j.ijantimicag.2020.105954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033 1034. ( 10.1016/S0140-6736(20)30628-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaya MN. Mental status of healthcare professionals according to the level of exposure to COVID-19 patient during the pandemic. J Health Sci Med JHSM. 2022;5(4):1081 1085. ( 10.32322/jhsm.1112460) [DOI] [Google Scholar]
- 5. Uğraş Dikmen A, Kına M, Özkan S, İlhan MN. COVID-19 Epidemiyolojisi: Pandemiden ne öğrendik. J Biotechnol Strateg Health Res. 2020;4:29 36. ( 10.34084/bshr.715153) [DOI] [Google Scholar]
- 6. Ritchie AI, Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword?. Lancet. 2020;395(10230):1111. ( 10.1016/S0140-6736(20)30691-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soy M, Keser G, Atagündüz P, Tabak F, Atagündüz I, Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol. 2020;39(7):2085 2094. ( 10.1007/s10067-020-05190-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tufan A, Avanoğlu Güler A, Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk J Med Sci. 2020;50(SI-1):620 632. ( 10.3906/sag-2004-168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weng Z, Chen Q, Li S, et al. ANDC: an early warning score to predict mortality risk for patients with coronavirus disease 2019. J Transl Med. 2020;18(1):328. ( 10.1186/s12967-020-02505-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wolfisberg S, Gregoriano C, Struja T, et al. Call, chosen, HA2T2, ANDC: validation of four severity scores in COVID-19 patients. Infection. 2022;50(3):651 659. ( 10.1007/s15010-021-01728-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hsu JL, Liu MC, Tsau PW, et al. Clinical presentations, systemic inflammation response and ANDC scores in hospitalized patients with COVID-19. Sci Rep. 2024;14(1):22480. ( 10.1038/s41598-024-73001-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Al-Shami I, Hourani HMA, Alkhatib B. The use of prognostic nutritional index (PNI) and selected inflammatory indicators for predicting malnutrition in COVID-19 patients: a retrospective study. J Infect Public Health. 2023;16(2):280 285. ( 10.1016/j.jiph.2022.12.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bilge M, Akilli IK, Karaayvaz EB, Yesilova A, Kart Yasar K. Comparison of systemic immune-inflammation index (SII), early warning score (ANDC) and prognostic nutritional index (PNI) in hospitalized patients with malignancy, and their influence on mortality from COVID-19. Infect Agent Cancer. 2021;16(1):60. ( 10.1186/s13027-021-00400-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Turkey Ministry of Health. 2020. Anticytokin-anti-inflammatory therapies, management of coagulopathy. Available at: https://covid19bilgi.saglik.gov.tr/tr/covid-19-rehberi.html. Accessed 2020 June 3. Available from: [Google Scholar] [Google Scholar]
- 15. Uciechowski P, Dempke WCM. Interleukin-6: a Masterplayer in the cytokine network. Oncology. 2020;98(3):131 137. ( 10.1159/000505099) [DOI] [PubMed] [Google Scholar]
- 16. Rutgers A, Westerweel PE, van der Holt B, et al. Timely administration of tocilizumab improves outcome of hospitalized COVID-19 patients. PLoS One. 2022;17(8):e0271807. ( 10.1371/journal.pone.0271807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117(20):10970 10975. ( 10.1073/pnas.2005615117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study [published correction appears in Lancet Rheumatol. 2020;2(10):e591]. Lancet Rheumatol. 2020;2(8): e474 e484. ( 10.1016/S2665-9913(20)30173-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637 1645. ( 10.1016/S0140-6736(21)00676-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503 1516. ( 10.1056/NEJMoa2028700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li P, Lu Z, Li Q, et al. Administration timing and efficacy of tocilizumab in patients with COVID-19 and elevated IL-6. Front Mol Biosci. 2021;8:651662. ( 10.3389/fmolb.2021.651662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Flisiak R, Jaroszewicz J, Rogalska M, et al. Tocilizumab improves the prognosis of COVID-19 in patients with high IL-6. J Clin Med. 2021;10(8):1583. ( 10.3390/jcm10081583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Henry BM, Aggarwal G, Wong J, et al. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am J Emerg Med. 2020;38(9):1722 1726. ( 10.1016/j.ajem.2020.05.073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kermali M, Khalsa RK, Pillai K, Ismail Z, Harky A. The role of biomarkers in diagnosis of COVID-19 - A systematic review. Life Sci. 2020;254:117788. ( 10.1016/j.lfs.2020.117788) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hamidi AA, Ulu Y. Mild/moderate and severe COVID-19 pneumonia: Can the clinical course be predicted ?. Maltepe tıp derg. 2021;13(3):92 96. [Google Scholar]
- 26. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239 1242. ( 10.1001/jama.2020.2648) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.



 Content of this journal is licensed under a
Content of this journal is licensed under a