Abstract
Background
Drug-induced second tumors (DIST) refer to new primary cancers that develop during or after the treatment of an initial cancer due to the long-term effects of medications. As a severe long-term adverse event, DIST has gained widespread attention globally in recent years. With the increasing prevalence of cancer treatments and the prolonged survival of patients, drug-induced second tumors have become more prominent and pose a significant public health challenge. However, most existing studies have focused on individual drugs or small patient cohorts, lacking large-scale, real-world data evaluations. Particularly, the potential second-tumor risk of new drugs remains underexplored.
Objective
This study aims to systematically assess the adverse event signals between drugs and second tumors using the U.S. FDA Adverse Event Reporting System (FAERS) database, employing disproportionality analysis (DPA) methods. It particularly focuses on uncovering drugs that have not clearly labeled second-tumor risks.
Methods
Data from the FDA Adverse Event Reporting System (FAERS), covering reports from its inception to the third quarter of 2024, was retrieved. After data standardization, four disproportionality methods were used: Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Multi-item Gamma Poisson Shrinker (MGPS). These methods assessed the correlation between azacitidine and adverse drug events (ADEs). Additionally, the Weibull Shape Parameter (WSP) was used to analyze the characteristic patterns of time-to-onset curves. Newly discovered signals were verified against FDA drug labels to confirm their novelty. The Weibull analysis was conducted to examine the temporal aspects of adverse event occurrences.
Results
Since 2004, drug-induced tumor events have been increasing annually, with a total of 7597 drug-related tumor adverse events recorded. A total of 250 drugs were identified as having potential risk signals. High-incidence populations were primarily aged between 65 and 85 years, with a higher proportion of individuals with a body weight ≥ 90 kg. The most frequent occurrence was observed in patients with Chronic Myeloid Leukemia (13.36%). Among the top 5 drugs with the highest number of reported drug-induced second tumor adverse events, IMATINIB (906 reports), RUXOLITINIB (554 reports), PALBOCICLIB (552 reports), OCTREOTIDE (399 reports), and DOXORUBICIN (380 reports) were identified. Among these, PALBOCICLIB, OCTREOTIDE, and DOXORUBICIN are drugs for which the risk of drug-induced second tumors is not explicitly mentioned in their labels. A total of 76 drugs were identified through four disproportionality algorithms (ROR, PRR, MGPS, BCPNN), with a minimum time to drug-induced tumor occurrence of 5 years, exhibiting an early failure-type curve.
Conclusion
This study, based on large-scale real-world data, reveals the potential associations between drugs and second tumors, especially highlighting the risks of some new drugs. The findings provide valuable insights for drug safety monitoring and have significant public health implications. By uncovering previously unrecognized potential risks, this research lays the groundwork for further advancements in pharmacovigilance.
Keywords: Second tumor, FAERS, Pharmacovigilance, Proportional imbalance analysis, Real world research
Introduction
Drug-induced second tumors (DIST) refer to new primary cancers that develop during or after the treatment of an initial tumor due to the long-term effects of medications [1, 2]. This phenomenon, as a serious long-term adverse event, has gradually attracted global attention from both academic circles and clinical practice in recent years. The occurrence of second tumors not only severely impacts the quality of life of patients but may also become a major cause of treatment failure and disease progression [3]. Worldwide, with the increasing prevalence of cancer treatments and the prolonged survival of patients, the issue of drug-induced second tumors has become more prominent, presenting a significant public health challenge. Existing studies have shown that various therapeutic agents, including chemotherapy drugs, targeted therapies, and immunosuppressants, may be associated with the occurrence of second tumors. Drugs such as cyclophosphamide, doxorubicin, and imatinib have been widely reported to carry a risk of secondary cancers [4–6]. The mechanisms underlying drug-induced second tumors are complex and may involve abnormal DNA damage repair, gene mutations, dysregulation of epigenetic controls, and alterations in the immune microenvironment [7, 8]. However, these studies are mostly limited to individual drugs or small patient cohorts, lacking large-scale, real-world data-based systematic evaluations.
With the rapid advancement of new drug development and the continuous expansion of the pharmaceutical market, many novel drugs lack long-term safety data after their market approval, and the potential risk of second tumors may be underestimated or not detected in a timely manner [9]. Previous signal detection studies have mainly focused on specific classes of drugs or short-term adverse events, without conducting comprehensive signal mining and evaluation of drug-induced second tumors. This research gap has led to a delay in recognizing the risk of second tumors, which increases potential clinical hazards.
The FAERS (FDA Adverse Event Reporting System) database provides a wealth of real-world spontaneous reporting data, offering critical support for drug safety signal detection. In recent years, FAERS-based drug safety research has become a hotspot in the field of pharmacovigilance, especially in identifying potential adverse events of new drugs [10]. However, there is still a lack of research on signal analysis for drug-induced second tumors, particularly in areas such as signal strength analysis, exploring unreported new signals, and verifying existing research findings.
This study, based on the FAERS database and covering a 20-year period (2004 to 2024) of large-scale adverse event reports, aims to systematically assess the adverse event signals related to drugs and second tumors through disproportionality analysis (DPA) methods, with a particular focus on uncovering drugs that do not explicitly label second tumor risks [11]. The ultimate goal of the study is to reveal potentially underestimated drug-induced second tumor risks, improve drug safety monitoring systems, provide data support for clinical rational drug use, and offer scientific evidence for future related research. Through this approach, we aim to fill existing research gaps and provide a more solid scientific foundation for drug safety regulation and clinical decision-making, thereby protecting patients from the potential long-term risks.
Materials and methods
Data source and process
The adverse event data for this study were sourced from the FAERS (FDA Adverse Event Reporting System) database (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html). This database has been publicly available since 2004, and this study downloaded adverse event report data from the FAERS database, spanning from the first quarter of 2004 to the third quarter of 2024.
For the data downloaded from the FAERS database that share the same “caseid” (report code), only the most recent report based on the date was retained, and duplicate reports were excluded. Drug names were standardized according to the RxNorm drug standardization naming system to standardize the drug names in the FAERS data. The MedDRA (Medical Dictionary for Regulatory Activities) version 26.1 was used to match the primary term (PT) for “SECOND PRIMARY MALIGNANCY” adverse events.
After standardizing the drug names and adverse event terms, reports related to second tumors and the primary suspected (PS) drugs were collected. These adverse event reports were characterized based on sex, age, weight, indication, reporting country, and outcomes.
Signal analysis algorithms
In this study, disproportionality analysis (DPA) algorithms, commonly used in pharmacovigilance research, were applied to detect potential signals of adverse drug events (ADEs) related to second tumors. Disproportionality analysis is a widely used data mining method that analyzes the correlation between drugs and adverse reactions by comparing the observed frequency ratios in exposed versus unexposed populations using a 2 × 2 contingency table (Table 1).
Table 1.
. Methods and thresholds for ROR, PRR, BCPNN and EBGM
| Target AEs | Other AEs | |
|---|---|---|
| a | b | |
| Other drugs | c | d |
| Total | a + c | b + d |
| Algorithms | Equation | Criteria |
| ROR | ROR = ad/b/c | lower limit of 95% CI > 1, N ≥ 3 |
| 95%CI = eln(ROR)±1.96(1/a+1/b+1/c+1/d)^0.5 | ||
| PRR | PRR = a(c + d)/c/(a + b) | PRR ≥ 2, χ2 ≥ 4, N ≥ 3 |
| χ2 = [(ad-bc)^2](a + b + c + d)/[(a + b)(c + d)(a + c)(b + d)] | ||
| BCPNN | IC = log2a(a + b + c + d)(a + c)(a + b) | IC025 > 0 |
| 95%CI = E(IC) ± 2 V(IC)^0.5 | ||
| EBMG | EBGM = a(a + b + c + d)/(a + c)/(a + b) | EBGM05 > 2 |
| 95%CI = eln(EBGM)±1.96(1/a+1/b+1/c+1/d)^0.5 |
AEs: Adverse events. a: Number of reports containing both the target drug and the target adverse drug reaction. b: Number of reports containing other adverse drug reactions of the target drug. c: Number of reports containing the target adverse drug reaction associated with other drugs. d: Number of reports containing other drugs and other adverse drug reactions. 95% CI: 95% confidence interval. N: Number of reports. χ2: Chi-squared statistic. IC: Information component, a Bayesian measure used in disproportionality analysis. IC025: Lower limit of the 95% confidence interval for the IC. E(IC): Expected value of the information component. V(IC): Variance of the information component. EBGM: Empirical Bayesian geometric mean, a Bayesian measure used for signal detection. EBGM05: Lower limit of the 95% confidence interval for the EBGM
In this study, we utilized several signal strength calculation methods, including the Reporting Odds Ratio (ROR) [12], Proportional Reporting Ratio (PRR) [13], Bayesian Confidence Propagation Neural Network (BCPNN) [14], and Empirical Bayesian Geometric Mean (EBGM) [15]. These algorithms are employed to evaluate the strength of the adverse event signals. To identify valid signals, the results from the four algorithms must meet the positive signal criteria (Table 1). Once potential signals are identified, those not mentioned in the FDA drug labels (https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm) are further examined and classified as new adverse event signals.
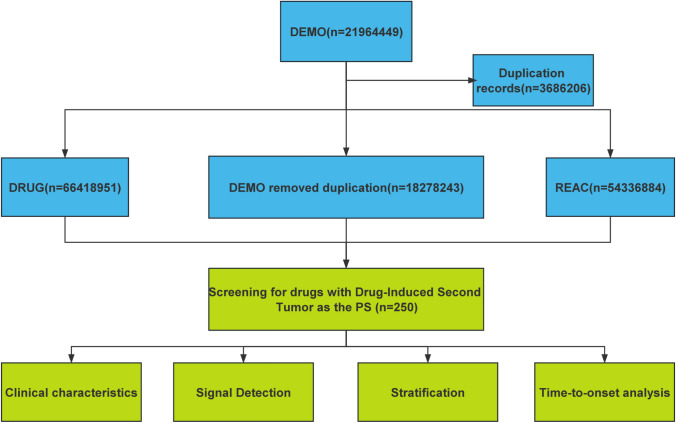
All data in this study were processed and statistically analyzed using R4.4.0 and MS Excel software. The data extraction and analysis workflow is depicted in Fig. 1.
Fig. 1.
Analysis process of the second tumor signal caused by drugs
Results
Basic characteristics of adverse events associated with second tumors
By the third quarter of 2024, the number of adverse events related to the second tumor in the faers database was 7597, and the number of adverse events reported with the second primary malignant tumor as the preferred term was 55,837. From the first quarter of 2004 to the third quarter of 2024, the number of adverse event reports for second tumors increased annually, with the highest number recorded in 2019, reaching 884 reports (Fig. 2). The polynomial fit curve shows a slow upward trend, with a coefficient of determination (R2 = 0.8931), indicating that this model explains 89.31% of the data variability, suggesting the trend is of high reference value.
Fig. 2.
Reporting trend of adverse events in drug induced second tumors
Table 2 presents the characteristics of the population associated with second tumor adverse events. Among the reports, 43.68% were female, 36.07% were male, and 20.26% had an unknown gender. Age-wise, the incidence showed a gradual increase, with the highest proportion of reported adverse events found in the 65–85 age group (27.91%) among the known age reports. The highest proportion of second tumor adverse events was observed in individuals with higher body weights (≥ 90 kg), accounting for 8.58%, although 82.16% of the reports had unknown weight data.
Table 2.
Baseline characteristics of drug-induced second tumor population
| Characteristics | Case numbers | Case proportion (%) |
|---|---|---|
| Number of events | 7597 | – |
| Gender | ||
| Male | 2740 | 36.07 |
| Female | 3318 | 43.68 |
| Miss | 1539 | 20.26 |
| Age | ||
| Median (IQR) | 63 | |
| < 18 | 293 | 3.86 |
| 18–65 | 2381 | 31.34 |
| 65–85 | 2120 | 27.91 |
| > 85 | 100 | 1.32 |
| Miss | 2703 | 35.58 |
| Weight (KG) | ||
| < 50 | 76 | 1.00 |
| 50–69 | 326 | 4.29 |
| 70–89 | 301 | 3.96 |
| ≥ 90 | 652 | 8.58 |
| Miss | 6242 | 82.16 |
| Top 5 indication | ||
| Chronic myeloid leukaemia | 1015 | 13.36 |
| Breast cancer | 800 | 10.53 |
| Myelofibrosis | 331 | 4.36 |
| Plasma cell myeloma | 267 | 3.51 |
| Product used for unknown indication | 265 | 3.49 |
| Top 5 reported countries | ||
| United States | 1758 | 23.14 |
| Japan | 970 | 12.77 |
| Canada | 793 | 10.44 |
| France | 497 | 6.54 |
| Germany | 414 | 5.45 |
IQR: Interquartile range
It is noteworthy that Chronic Myeloid Leukaemia (13.36%) was the condition with the highest frequency of second tumors, followed by Breast Cancer (10.53%) and Myelofibrosis (4.36%). Most second tumor adverse events were reported from developed countries, with the United States (23.14%), Japan (12.77%), and Canada (10.44%) showing the highest frequencies.
Drug analysis
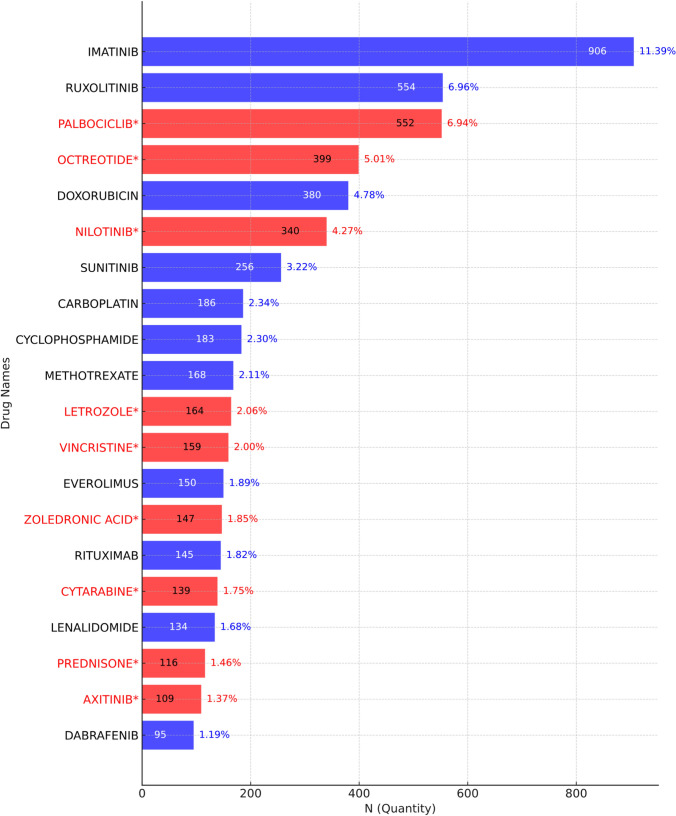
The top 20 drugs with the highest number of drug-induced second tumor adverse events are shown in Fig. 3. The five drugs with the highest number of reports are IMATINIB (906 reports), RUXOLITINIB (554 reports), PALBOCICLIB (552 reports), OCTREOTIDE (399 reports), and DOXORUBICIN (380 reports). Among these, PALBOCICLIB, OCTREOTIDE, and DOXORUBICIN are drugs for which the risk of drug-induced second tumors is not explicitly mentioned in their labels. Among the top 20 drugs associated with the highest number of drug-induced second tumor adverse events, 9 drugs do not mention the risk of second tumors in their labels.
Fig. 3.
The top 20 drugs with the highest reported number of drug-related second tumors
Signal detection of drug-induced second tumor adverse events
The statistical results of disproportionate analysis show that among the top 20 drugs ranked by signal strength for adverse events, 27 drugs had their drug labels not mentioning the risk of fat metabolism disorders, which is a new adverse event signal. The top five drugs with the strongest new signal strength are as follows: BLINDED THERAPY [n = 1, ROR 133.63 (17.84, 1001.14), PRR 126.65 (124.69), Chi sq 124.69, EBGM 126.63 (23.48), IC 6.98 (4.84)], IDARUBICIN [n = 34, ROR 75.14 (53.37, 105.79), PRR 72.91 (2401.51), Chi sq 2401.51, EBGM 72.59 (54.52), IC 6.18 (5.69)], TISAGENLECLEUCEL [n = 76, ROR 66.83 (53.15, 84.04), PRR 65.07 (4748.63), Chi sq 4748.63, EBGM 64.43 (53.2), IC 6.01 (5.67)], TALAZOPARIB [n = 21, ROR 55.23 (35.81, 85.17), PRR 54.01 (1090.07), Chi sq 1090.07, EBGM 53.87 (37.49), IC 5.75 (5.13)], VINCRISTINE [n = 159, ROR 53.72 (45.83, 62.96), PRR 52.59 (7881.36), Chi sq 7881.36, EBGM 51.51 (45.10), IC 5.69 (5.45)].
Additionally, among the top 5 drugs with the highest number of reports but no mention of this adverse event in their labels, we find: OCTREOTIDE [n = 399, ROR 41.85 (37.80, 46.32), PRR 41.18 (14,827.83), Chi sq 14,827.83, EBGM 39.07 (35.89), IC 5.29 (5.14)], NILOTINIB [n = 340, ROR 33.86 (30.35, 37.77), PRR 33.42 (10,219.21), Chi sq 10,219.21, EBGM 31.97 (29.17), IC 5.00 (4.84)], VINCRISTINE [n = 159, ROR 53.72 (45.83, 62.96), PRR 52.59 (7881.36), Chi sq 7881.36, EBGM 51.51 (45.10), IC 5.69 (5.45)]、CYTARABINE [n = 139, ROR 29.40 (24.83, 34.80), PRR 29.06 (3698.85), Chi sq 3698.85, EBGM 28.55 (24.79), IC 4.84 (4.59)], BOSUTINIB [n = 82, ROR 30.85 (24.78, 38.40), PRR 30.48 (2313.42), Chi sq 2313.42, EBGM 30.16 (25.11), IC 4.91 (4.59)].
Adverse events related to drug-induced secondary cancers for drugs such as Imatinib, Octreotide, Doxorubicin, Nilotinib, Vincristine, and Cytarabine were reported more than 100 times (Table 3). Notably, Vincristine, Octreotide, Nilotinib, and Cytarabine are among the top 20 drugs in both total report count and signal strength, yet none of these drugs are mentioned in their product labeling as having tumor-related adverse events.
Table 3.
Top 20 Drugs in Signal Strength Ranking
| Drug name | Case reports | ROR (95% CI) | PRR (95% CI) | Chi sq | EBGM(EBGM05) | IC(IC025) |
|---|---|---|---|---|---|---|
| BLINDED THERAPY* | 1 | 133.63 (17.84–1001.14) | 126.65 (124.69) | 124.69 | 126.63 (23.48) | 6.98 (4.84) |
| IDARUBICIN | 34 | 75.14 (53.37–105.79) | 72.91 (2401.51) | 2401.51 | 72.59 (54.52) | 6.18 (5.69) |
| TISAGENLECLEUCEL | 76 | 66.83 (53.15–84.04) | 65.07 (4748.63) | 4748.63 | 64.43 (53.2) | 6.01 (5.67) |
| TALAZOPARIB | 21 | 55.23 (35.81–85.17) | 54.01 (1090.07) | 1090.07 | 53.87 (37.49) | 5.75 (5.13) |
| VINCRISTINE* | 159 | 53.72 (45.83–62.96) | 52.59 (7881.36) | 7881.36 | 51.51 (45.10) | 5.69 (5.45) |
| BLEOMYCIN* | 32 | 51.8 (36.47–73.57) | 50.73 (1554.03) | 1554.03 | 50.52 (37.67) | 5.66 (5.15) |
| VINBLASTINE* | 4 | 49.61 (18.43–133.56) | 48.63 (186.58) | 186.58 | 48.61 (21.22) | 5.6 (4.3) |
| ENCORAFENIB | 84 | 47.66 (38.36–59.22) | 46.76 (3721.95) | 3721.95 | 46.26 (38.57) | 5.53 (5.21) |
| IMATINIB | 906 | 44.9 (41.87–48.15) | 44.19 (33,697.42) | 33,697.42 | 39.04 (36.82) | 5.29 (5.18) |
| OCTREOTIDE* | 399 | 41.85 (37.80–46.32) | 41.18 (14,827.83) | 14,827.83 | 39.07 (35.89) | 5.29 (5.14) |
| PENTOSTATIN* | 6 | 39.24 (17.51–87.95) | 38.63 (219.84) | 219.84 | 38.6 (19.65) | 5.27 (4.17) |
| PAMIDRONIC ACID* | 63 | 38.96 (30.34–50.02) | 38.36 (2274.02) | 2274.02 | 38.05 (30.87) | 5.25 (4.88) |
| NILOTINIB* | 340 | 33.86 (30.35–37.77) | 33.42 (10,219.21) | 10,219.21 | 31.97 (29.17) | 5.00 (4.84) |
| ESTRAMUSTINE* | 5 | 33.61 (13.90–81.27) | 33.16 (155.92) | 155.92 | 33.14 (15.83) | 5.05 (3.86) |
| DACTINOMYCIN | 11 | 32.67 (18.01–59.25) | 32.24 (332.66) | 332.66 | 32.2 (19.56) | 5.01 (4.17) |
| BOSUTINIB* | 82 | 30.85 (24.78–38.40) | 30.48 (2313.42) | 2313.42 | 30.16 (25.11) | 4.91 (4.59) |
| DOXORUBICIN | 380 | 30.5 (27.50–33.84) | 30.15 (10,179.23) | 10,179.23 | 28.69 (26.31) | 4.84 (4.69) |
| CYTARABINE* | 139 | 29.4 (24.83–34.80) | 29.06 (3698.85) | 3698.85 | 28.55 (24.79) | 4.84 (4.59) |
| LESTAURTINIB* | 1 | 26.43 (3.68–189.70) | 26.16 (24.20) | 24.20 | 26.15 (5.03) | 4.71 (2.65) |
| FLUDARABINE | 85 | 26.4 (21.30–32.73) | 26.13 (2032.08) | 2032.08 | 25.85 (21.59) | 4.69 (4.38) |
*Indicates new signals not mentioned in the manual
Figure 4 illustrates the Venn diagram for the four algorithms used (ROR, PRR, MGPS, BCPNN), highlighting 76 drugs that meet the criteria for a positive signal according to all four algorithms. Figure 5 further highlights a forest plot of drugs that meet the positive signal criteria from all four algorithms, with the following drugs in particular showing no mention in their labels regarding drug-induced second tumors: VINCRISTINE, BLEOMYCIN, VINBLASTINE, OCTREOTIDE, PENTOSTATIN, PAMIDRONIC ACID, NILOTINIB, ESTRAMUSTINE, BOSUTINIB, CYTARABINE, DACOMITINIB, and several others. This information emphasizes the need for further evaluation and awareness of potential drug-induced second tumors in these medications.
Fig. 4.

Venn diagrams of drugs under four algorithms: ROR, PRR, MGPS, and BCPNN
Fig. 5.
Forest map of positive drugs with the top 20 signal strengths under the ROR algorithm. * Indicates new signals not mentioned in the manual
Induction event analysis
The analysis of the induction time of adverse drug reactions (ADRs) plays a crucial role in drug safety monitoring, clinical medication guidance, regulatory decision-making, and drug development improvements. In this study, the median induction time for adverse events associated with PALBOCICLIB was found to be as low as 1807 days, approximately 5 years. The Weibull distribution shape parameter (β) was less than 1, with a 95% confidence interval (CI) also less than 1, indicating that the incidence of adverse events is considered to decrease over time (early failure-type curve). Specifically, the induction times for second tumors associated with eight drugs, including IMATINIB, RUXOLITINIB, PALBOCICLIB, OCTREOTIDE, and DOXORUBICIN, followed the early failure-type curve. Conversely, when the shape parameter (β) is greater than 1 and the 95% CI does not include 1, the incidence of ADRs is considered to increase over time (wear-out failure-type curve). The induction times for drugs such as CYCLOPHOSPHAMIDE and LETROZOLE followed the wear-out failure-type curve, as shown in Table 4.
Table 4.
Analysis of the occurrence time and Weibull distribution of drug-induced second tumor adverse events
| PT | Date of onset (days) | Weibull distribution | |||
|---|---|---|---|---|---|
| Case reports | Median(d)(IQR) | Scale parameter: α(95%CI) | Shape parameter: β(95%CI) | Type | |
| IMATINIB | 906 | 2712 | 1182.01 (876.16–1484.86) | 0.80 (0.67–0.93) | Early failure |
| RUXOLITINIB | 554 | 1962 | 501.34 (383.28–619.39) | 0.74 (0.64–0.84) | Early failure |
| PALBOCICLIB | 552 | 1807 | 525.75 (325.25–526.26) | 0.83 (0.71–0.96) | Early failure |
| OCTREOTIDE | 399 | 2584 | 756.95 (589.07–924.82) | 0.68 (0.61–0.76) | Early failure |
| DOXORUBICIN | 380 | 3941.5 | 1143.75 (620.37–1667.14) | 0.84 (0.61–1.61) | Early failure |
| NILOTINIB | 340 | 1838 | 521.19 (357.58–684.81) | 0.73 (0.61–0.86) | Early failure |
| SUNITINIB | 256 | 2883 | 348.82 (163.17–534.48) | 0.64 (0.48–0.81) | Early failure |
| CARBOPLATIN | 186 | 3788 | 1148.41 (631.34–1665.46) | 0.82 (0.59–1.06) | Early failure |
| CYCLOPHOSPHAMIDE | 183 | 3089.5 | 1200.80 (757.01–1644.60) | 1.04 (0.75–1.32) | Wear-out failure |
| LETROZOLE | 164 | 2856 | 830.60 (621.74–1039.46) | 1.11 (0.87–1.35) | Wear-out failure |
CI: Confidence interval; IQR: Interquartile range
Discussion
In 1895, Röntgen discovered X-rays, and in 1896, they were first used for the treatment of tumors, marking the beginning of a new era in radiotherapy for cancer [16]. In the 1940s, nitrogen mustard emerged as the first anticancer drug, signifying the birth of chemotherapy [17]. In 1891, Coley's toxin therapy pioneered immunotherapy, which is now considered a powerful weapon in the fight against cancer [18]. However, radiotherapy is limited by its damage to normal tissues [19], chemotherapy faces challenges such as drug resistance and ineffectiveness against dormant tumor cells [20], and immunotherapy still needs to overcome issues such as antigen escape and immune-suppressive microenvironments in the treatment of solid tumors [21]. Notably, the occurrence of secondary cancers remains a major challenge for these therapies.
This study, based on the FAERS database, systematically analyzed adverse events related to drug-induced secondary cancers from the first quarter of 2004 to the third quarter of 2024. Using disproportionate reporting analysis (DPA), we identified potential signals from 250 drugs, revealing a year-on-year increase in the number of reports of drug-induced secondary cancers, particularly concentrated in countries such as the United States (23.14%), Japan (12.77%), and Canada (10.44%). These findings suggest that drug-induced secondary cancers have become an important public health issue that requires attention.
Susceptible populations and clinical management
This study, based on data from the FAERS database, reveals that elderly individuals (≥ 65 years), those with a higher body weight (≥ 90 kg), and patients with chronic myeloid leukemia (CML) are at higher risk for drug-induced secondary cancers. These findings are closely linked to the unique physiological characteristics of these populations: elderly individuals exhibit reduced metabolic and repair capacities, while obese individuals may experience chronic inflammation and metabolic disorders [22]. These characteristics not only influence drug metabolism and distribution but may also contribute to tumorigenesis and cancer progression [23].
The high risk associated with CML may be related to commonly used treatment drugs, such as imatinib and other tyrosine kinase inhibitors (TKIs). Although these drugs are highly effective in controlling the disease, they may possess mutagenic properties. These drugs could increase the risk of secondary cancers by affecting DNA repair mechanisms, inducing genetic mutations, or altering cell cycle regulation [24].
Given the specific vulnerabilities of these susceptible populations, personalized treatment becomes crucial. When prescribing drugs with potential tumorigenic risks to these high-risk groups, a comprehensive evaluation of the patient's overall health is essential, including liver and kidney function, metabolic status, and inflammatory biomarkers.
Major high-risk drug categories
The study found that IMATINIB, RUXOLITINIB, and PALBOCICLIB were the drugs most frequently associated with reports of secondary cancer-related adverse events. Although some drugs, such as IMATINIB, have been reported in existing literature as potential triggers for tumor-related adverse events [25], drugs like RUXOLITINIB and PALBOCICLIB show new potential signals, the specific mechanisms of which require further investigation.
Among the top five drugs ranked by signal strength, VINCRISTINE, OCTREOTIDE, and DOXORUBICIN, which are not explicitly listed as having secondary cancer risks on their labels, exhibited significant associations. VINCRISTINE is a mitotic inhibitor that prevents cell division by disrupting microtubule polymerization. Its potential mechanism for inducing secondary cancers may involve interference with DNA damage repair processes and disruption of cell cycle regulation [26]. Long-term OCTREOTIDE therapy could affect cellular differentiation and apoptosis, thus increasing the risk of secondary tumors [27]. The cumulative dose of DOXORUBICIN is associated with an increased risk of cardiotoxicity and secondary leukemia, which may be related to its mutagenic properties [28].
Therefore, a comprehensive risk assessment is essential when clinically using these drugs, including factors such as patient age, sex, body weight, medical history, and family history. Close monitoring of patient responses and adverse effects during treatment is crucial to mitigate the risk of secondary cancers and enhance treatment outcomes.
Drug-induced onset time analysis and characteristics
This study incorporated Weibull distribution analysis to examine the onset time characteristics of major high-risk drugs. The results indicated that PALBOCICLIB had the shortest median onset time for adverse events, which was 1807 days (approximately 5 years). The carcinogenic effects of these drugs were predominantly concentrated in the early phase of use, with a median onset time of around 5 years, possibly related to early immune suppression, the accumulation of genetic mutations, or metabolic disturbances.
In contrast, CYCLOPHOSPHAMIDE and LETROZOLE exhibited a wear-out failure curve in their onset time distribution, suggesting that the incidence of adverse events increased gradually over time with prolonged use. This cumulative risk of drug-induced secondary cancers may be associated with chronic DNA damage, a decline in cellular repair capacity, and increased metabolic burden on tissues, particularly in patients undergoing long-term treatment.
Genotoxicity assays, which can detect DNA damage and its fixation, are crucial for predicting the carcinogenic potential of drugs [29]. Furthermore, long-term toxicity studies provide valuable information regarding drug accumulation, tolerance, and the maximum non-toxic dose, all of which are essential for assessing the safety of drugs for prolonged use [30]. Drug-induced onset time analysis not only reveals the risks associated with long-term drug use but also provides important insights for drug safety research and clinical prescribing guidance.
Exploration of potential mechanisms
In exploring the potential mechanisms underlying drug-induced secondary cancers, we identified several biological processes that may be involved, including gene mutations, epigenetic alterations, immune microenvironment modulation, and metabolic reprogramming. Specifically, DOXORUBICIN induces cellular mutations primarily by causing DNA damage and inhibiting the activity of topoisomerase II. Research on its antitumor molecular mechanisms suggests that the drug activates caspase-1/3/8 and FAS, inducing apoptosis in tumor cells [31].
Moreover, RUXOLITINIB, a selective JAK1/2 kinase inhibitor, may influence tumorigenesis and progression by modulating the JAK-STAT pathway within the immune-suppressive tumor microenvironment [32]. OCTREOTIDE, a somatostatin analog, may indirectly affect tumor development by regulating endocrine hormones. Experimental evidence supporting this hypothesis includes its sensitizing effect on paclitaxel-resistant DU145 tumor cells and the underlying mechanisms [33].
These drugs, targeting different systems within the body, underscore the importance of systemic balance in maintaining cellular homeostasis. The body’s ability to regulate and coordinate multiple pathways is essential for preventing tumorigenesis. Disruption of this balance—whether through DNA damage, immune suppression, or metabolic reprogramming—can lead to the emergence of secondary cancers. For instance, while DOXORUBICIN directly causes genetic mutations, its effects are exacerbated in an environment where immune surveillance or metabolic processes are also altered, as seen with drugs like RUXOLITINIB and OCTREOTIDE. These drugs disrupt critical regulatory systems, leading to an increased risk of tumor formation.
The body’s regulatory mechanisms, which depend on the intricate collaboration between multiple biological systems, play a critical role in defending against cancer. When this systemic balance is disrupted—whether through immune modulation or DNA damage—the likelihood of secondary tumors increases. These hypotheses provide a theoretical basis for studying drug-induced secondary cancers, but further molecular and animal studies are needed to confirm the pathogenic pathways of different drugs. Such research will be essential for developing targeted risk intervention strategies based on scientific evidence.
Limitations of the study and future directions
Although this study utilized large-scale real-world data from the FAERS database to reveal the potential risks and onset time patterns of drug-induced secondary cancers, several limitations should be considered.
First, as a spontaneous reporting system, FAERS is subject to reporting bias. High-risk drugs may report more adverse events due to heightened attention, while certain drugs’ potential signals may be underestimated due to insufficient reporting. This can lead to both underreporting and overreporting of adverse events, which may impact the accuracy of the findings. Furthermore, newly approved drugs may not have enough reported cases to detect significant signals, and the lack of long-term data for these drugs can introduce uncertainty in evaluating their risks.
Second, the calculation of onset time relies on the completeness and accuracy of the reported data, which may be affected by incomplete medication records or missing time information. Additionally, the FAERS database does not include precise dosage information or treatment regimens, making it difficult to assess the impact of drug dosages on the risk of secondary tumors. The lack of detailed patient data, such as age, weight, medical history, and genetic predispositions, also limits the ability to evaluate how these factors may contribute to the risk of drug-induced second tumors. Moreover, this study only filtered data based on the MedDRA preferred terms, which may have overlooked other relevant adverse events not explicitly labeled or categorized under these terms.
While this study provides important insights, the findings should be considered preliminary and require further validation. Future research should integrate multiple data sources, such as clinical trial data, electronic health records, and molecular studies, to confirm the associations observed in this study and enhance the applicability and accuracy of the results. Clinical trial data would provide more detailed information on patient characteristics, treatment regimens, and dosages, while molecular studies could elucidate the underlying mechanisms contributing to drug-induced secondary cancers.
Additionally, prospective cohort studies or clinical trials should be conducted to establish a clearer understanding of the causal relationship between drug exposure and second tumors. These studies should include control populations and more precise data on drug exposure and patient follow-up to improve the accuracy of risk assessments. Moreover, incorporating molecular biomarkers and genetic information could enhance the understanding of how individual patient factors influence drug-related risks.
Finally, the development of dynamic risk prediction models is recommended, incorporating onset time, patient characteristics, and drug usage patterns into an evaluative framework. This will facilitate more precise identification of high-risk patients, optimize personalized treatment plans, and enhance drug safety monitoring. Regulatory agencies should also strengthen post-marketing surveillance of high-signal drugs, improve safety label update mechanisms, and promptly communicate potential risk information to ensure the safety of patients using these medications.
Clinical and regulatory implications
The findings of this study provide critical insights into the identification and management of drug-induced secondary cancers. The potential signals identified for certain drugs highlight the need for more comprehensive risk assessment and monitoring, especially for high-risk groups such as elderly, obese, and CML patients, who may be more susceptible to the development of second tumors. Healthcare providers are encouraged to strengthen patient follow-up and monitoring, particularly when administering drugs with potential tumorigenic effects. This includes regular assessment of tumor-related markers, close monitoring for early signs of secondary malignancies, and timely interventions when necessary.
However, it is important to note that the results of this study are based on real-world data and should be viewed as preliminary signals rather than definitive conclusions. Further validation through prospective cohort studies, clinical trials, and molecular research is needed to confirm these associations and better understand the underlying mechanisms of drug-induced second tumors. In light of these uncertainties, healthcare providers should remain vigilant and consider individual patient factors when prescribing medications associated with these potential risks.
Regulatory agencies should play a key role in enhancing post-market surveillance of high-signal drugs. Given the potential under-recognition of drug-induced secondary cancers, regulatory bodies should ensure that safety monitoring systems are robust and that drug labeling is updated promptly to reflect emerging evidence of risks. Furthermore, it is crucial for regulatory agencies to integrate data from diverse sources, including clinical trial outcomes, electronic health records, and pharmacovigilance reports, to refine risk assessments and ensure that patients receive the safest possible care.
Conclusion
Based on data from the FAERS database, this study identified potential signals of adverse events related to secondary cancers for multiple drugs. These findings provide valuable insights for the identification and management of drug-related adverse reactions, particularly highlighting the potential risks for high-risk groups, such as elderly and obese patients. While these signals offer important preliminary evidence, it is essential to emphasize that further validation is needed through prospective studies, clinical trials, and molecular research to confirm these associations and better understand the underlying mechanisms.
The results underscore the importance of strengthening post-marketing safety research and improving pharmacovigilance systems to ensure patient safety. Moreover, they call for a deeper exploration of the molecular pathways underlying drug-induced second tumors, which will help optimize clinical drug use and inform regulatory decision-making. Future studies integrating clinical trial data, electronic health records, and other real-world evidence are crucial to refine risk assessments and provide a more comprehensive understanding of drug safety.
Acknowledgements
This study was performed using open-source data provided by the FAERS database, and we thank all those who provided information for this database.
Author contributions
ShuPeng Chen: writing original draft and Methodology. Yuzhe Zhang: methodology, data curation, conceptualization. Xiaojian Li: methodology, data curation, conceptualization. Nana Tang: methodology, formal analysis, data curation. Yinjian Zeng: writing—review and editing. All authors contributed to manuscript revision, and read and approved the submitted version.
Funding
National Natural Science Foundation of China (82260914); General Project of Jiangxi Provincial Natural Science Foundation (20192BAB205100); Traditional Chinese Medicine Advantageous Disease Cultivation Project of Jiangxi Provincial Administration of Traditional Chinese Medicine (Gan Cai She Zhi [2023] No. 70); Innovation and Entrepreneurship Training Program for College Students at Jiangxi University of Traditional Chinese Medicine (202410412256).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Ethical approval and consent were not required as this study was based on publicly available data.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shupeng Chen, Yuzhe Zhang and Xiaojian Li have contributed equally to this work, are co-first authors.
References
- 1.Hamilton MP, Miklos DB, Alizadeh AA. Risk of second tumors and t-cell lymphoma after car t-cell therapy: reply. N Engl J Med. 2024;391:870–1. 10.1056/NEJMc2408733. [DOI] [PubMed] [Google Scholar]
- 2.Papavassiliou AG, Delle Cave D. Novel therapeutic approaches for colorectal cancer treatment. Int J Mol Sci. 2024. 10.3390/ijms25042228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Massironi S, Campana D, Pusceddu S, et al. Second primary neoplasms in patients with lung and gastroenteropancreatic neuroendocrine neoplasms: data from a retrospective multi-centric study. Dig Liver Dis. 2021;53:367–74. 10.1016/j.dld.2020.09.031. [DOI] [PubMed] [Google Scholar]
- 4.Katsimigas A, Pedersen LB, Haunstrup T, et al. Excellent clinical outcomes in deeply mutated IGHV chronic lymphocytic leukemia upon fludarabine and cyclophosphamide plus rituximab. Blood. 2024;144:1860. 10.1182/blood-2024-203046. [Google Scholar]
- 5.Corazzelli G, Cuccaro A, Morelli E, et al. Long-term efficacy and safety of dose-dense and dose-intense ABVD without consolidation radiotherapy in patients with advanced hodgkin lymphoma: a 15-year follow-up of the ABVD(DD-DI) phase ii study. Br J Haematol. 2024;205:1383–8. 10.1111/bjh.19646. [DOI] [PubMed] [Google Scholar]
- 6.Blay J, Schiffler C, Bouché O, et al. A randomized study of 6 versus 3 years of adjuvant imatinib in patients with localized gist at high risk of relapse. Ann Oncol. 2024;35:1157–68. 10.1016/j.annonc.2024.08.2343. [DOI] [PubMed] [Google Scholar]
- 7.Mani N, Daiya A, Chowdhury R, et al. Epigenetic adaptations in drug-tolerant tumor cells. In: Landry JW, Das SK, Fisher PB, editors., et al., Advances in cancer research. Cambridge: Academic Press; 2023. p. 293–335. [DOI] [PubMed] [Google Scholar]
- 8.Drew Y, Zenke FT, Curtin NJ. Dna damage response inhibitors in cancer therapy: lessons from the past, current status and future implications. Nat Rev Drug Discov. 2024. 10.1038/s41573-024-01060-w. [DOI] [PubMed] [Google Scholar]
- 9.Davids MS, Burke JM, Woyach JA, et al. Brave: a phase 2 trial evaluating the efficacy and safety of venetoclax in combination with Bruton’s tyrosine kinase inhibitors in patients with first-line chronic lymphocytic leukemia. Blood. 2024;144:3252–7. 10.1182/blood-2024-201555. [Google Scholar]
- 10.Li D, Wang H, Qin C, et al. Drug-induced acute pancreatitis: a real-world pharmacovigilance study using the fda adverse event reporting system database. Clin Pharmacol Ther. 2024;115:535–44. 10.1002/cpt.3139. [DOI] [PubMed] [Google Scholar]
- 11.Li B, Hu X, Yue Z. Drug-induced hearing disorders: a disproportionality analysis of the faers database. Front Pharmacol. 2024;15:1480994. 10.3389/fphar.2024.1480994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 2004;13:519–23. 10.1002/pds.1001. [DOI] [PubMed] [Google Scholar]
- 13.Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (prrs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 2001;10:483–6. 10.1002/pds.677. [DOI] [PubMed] [Google Scholar]
- 14.Bate A, Lindquist M, Edwards IR, et al. A bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998;54:315–21. 10.1007/s002280050466. [DOI] [PubMed] [Google Scholar]
- 15.Fan Y, Wu T, Xu P, et al. Neratinib safety evaluation: real-world adverse event analysis from the faers database. Front Pharmacol. 2024;15:1425171. 10.3389/fphar.2024.1425171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliveira AM, Akkerman HB, Braccini S, et al. A high-resolution large-area detector for quality assurance in radiotherapy. Sci Rep. 2024;14:10637. 10.1038/s41598-024-61095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith SL. War! What is it good for? Mustard gas medicine. CMAJ. 2017;189:E321–2. 10.1503/cmaj.161032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan RJ. The evolutionary nature of the cancer immunotherapy revolution. Future Oncol. 2017;13:1565–7. 10.2217/fon-2017-0232. [DOI] [PubMed] [Google Scholar]
- 19.Zhou L, Zhu J, Liu Y, et al. Mechanisms of radiation-induced tissue damage and response. Medcomm. 2024;5: e725. 10.1002/mco2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A, Saha L. Colorectal cancer cell dormancy: an insight into pathways. World J Gastroenterol. 2024;30:3810–7. 10.3748/wjg.v30.i33.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen S, Liu J, He G, et al. Research hotspots and trends in global cancer immunometabolism:a bibliometric analysis from 2000 to 2023. J Multidiscip Healthc. 2024;17:5117–37. 10.2147/JMDH.S495330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao G, Lin M, Gu W, et al. The rules and regulatory mechanisms of foxo3 on inflammation, metabolism, cell death and aging in hosts. Life Sci. 2023;328:121877. 10.1016/j.lfs.2023.121877. [DOI] [PubMed] [Google Scholar]
- 23.Chen S, He G, Zhang M, et al. Causal relationship between branched-chain amino acids and leukemia risk: insights from a two-sample mendelian randomization study. Hematology. 2024;29:2433904. 10.1080/16078454.2024.2433904. [DOI] [PubMed] [Google Scholar]
- 24.Ciccia A, Elledge SJ. The dna damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miranda MB, Lauseker M, Kraus M, et al. Secondary malignancies in chronic myeloid leukemia patients after imatinib-based treatment: long-term observation in cml study iv. Leukemia. 2016;30:1255–62. 10.1038/leu.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poruchynsky MS, Komlodi-Pasztor E, Trostel S, et al. Microtubule-targeting agents augment the toxicity of dna-damaging agents by disrupting intracellular trafficking of dna repair proteins. Proc Natl Acad Sci USA. 2015;112:1571–6. 10.1073/pnas.1416418112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrante E, Pellegrini C, Bondioni S, et al. Octreotide promotes apoptosis in human somatotroph tumor cells by activating somatostatin receptor type 2. Endocr Relat Cancer. 2006;13:955–62. 10.1677/erc.1.01191. [DOI] [PubMed] [Google Scholar]
- 28.Babudri N, Pani B, Tamaro M, et al. Mutagenic and cytotoxic activity of doxorubicin and daunorubicin derivatives on prokaryotic and eukaryotic cells. Br J Cancer. 1984;50:91–6. 10.1038/bjc.1984.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uzu S, Sekine S, Asano J, et al. Assessment of the impact of japanese-specific long-term safety data on new drug approval. Clin Transl Sci. 2021;14:2339–47. 10.1111/cts.13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer-Massetti C, Cheng CM, Schwappach DLB, et al. Systematic review of medication safety assessment methods. Am J Health Syst Pharm. 2011;68:227–40. 10.2146/ajhp100019. [DOI] [PubMed] [Google Scholar]
- 31.Mizutani H, Tada-Oikawa S, Hiraku Y, et al. Mechanism of apoptosis induced by doxorubicin through the generation of hydrogen peroxide. Life Sci. 2005;76:1439–53. 10.1016/j.lfs.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 32.Qureshy Z, Li H, Zeng Y, et al. Stat3 activation as a predictive biomarker for ruxolitinib response in head and neck cancer. Clin Cancer Res. 2022;28:4737–46. 10.1158/1078-0432.CCR-22-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen Y, Zhang X, Chen X, et al. Octreotide reverses the resistance of a2780/pacliaxel ovarian cancer cell line to paclitaxel chemotherapy in vitro. J Cancer Res Ther. 2016;12:657–62. 10.4103/0973-1482.151861. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.